Abstract
Obinutuzumab (G) has become part of front-line treatment of follicular lymphoma (FL) based on results of a large randomized study. Data on patients treated outside of clinical trials are lacking. We have retrospectively investigated efficacy and safety of G-based immunochemotherapy regimens in 114 patients treated in a real-life setting during a period of 2 years, largely coinciding with the COVID-19 pandemic. The response rate was 93.8%; 18-months overall (OS) and progression-free survival (PFS) were 88% and 84%, respectively. Patients treated with G-cyclophosphamide, vincristine and glucocorticoid + doxorubicine (CHOP) had statistically significantly superior OS and PFS compared to patients treated with G-bendamustine (G-B) (P = 0.002 and P = 0.006, respectively) due to an increase in lethal infections, most notably COVID-19, in the latter group. A total of 12 patients died during follow-up; 9 of 61 treated with G-B, 1 of 49 treated with G-CHOP and 2 of 4 treated with G-cyclophosphamide, vincristine and glucocorticoid (CVP). SARS-CoV-2 infection was diagnosed in 20 (17.5%) patients. All of the 7 treated with G-CHOP recovered, while 4 of 12 treated with G-B died. Immunoglobulin levels and severity of neutropenia were similar between the groups. In multivariate analysis, G-B in comparison to G-CHOP was an independent prognostic factor (P = 0.044, hazard ratio = 9.81) after adjustment for age, sex and Follicular Lymphoma International Prognostic Index (FLIPI). Based on our experience G has excellent antilymphoma activity in patients receiving front-line treatment for FL in real-life setting, but during the COVID-19 pandemic, it should be preferentially combined with CHOP, at least in patients younger than 65.
INTRODUCTION
Follicular lymphoma (FL) is the most frequent indolent non-Hodgkin lymphoma (NHL) in Europe and North America comprising approximately 20% of all NHLs and 70% of indolent lymphomas.1 Four types of systemic FL are recognized histologically based on the number and distribution of centroblasts in a high-power microscopic field: grade (gr) 1, 2, 3A, and 3B. The former 3 have similar prognosis and are usually treated similarly. The latter resembles genetically B-large cell lymphoma and carries an inferior prognosis. Even though median age at the time of diagnosis is around 60 years, median survival for newly diagnosed patients with FL gr. 1–3A might be as high as 20 years.2 Until recently, standard front-line therapy for FL was a combination of rituximab (R) with either CVP (cyclophosphamide, vincristine and glucocorticoid), CHOP (CVP + doxorubicine) or bendamustine (B) followed by R maintenance. Two randomized studies showed improved progression-free survival (PFS), but not overall survival (OS), in patients with FL gr. 1 and 2 treated with BR in comparison to R-CHOP.3,4 Only retrospective analyses are available for FL gr. 3A patients; in one, patients treated with BR had inferior PFS5; outcomes were similar in the other two.6,7 In 2017 results of GALLIUM, a large randomized study, were published indicating that the combination of obinutuzumab (G) with any of the three standard chemotherapy regimens followed by G maintenance improved PFS in comparison to R-based combinations followed by R maintenance.8 There was no difference in OS between G- and R-treated patients.9 Based on this study, G was registered and has been widely accepted as the first choice in front-line treatment of FL. However, data on the efficacy and safety of these combinations in real-life setting to our knowledge have so far not been published.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for coronavirus disease 2019 (COVID-19), is causing a serious worldwide impact on public health organization. Knowledge of the COVID-19 disease process improved with time and is now well documented in patients with hematologic malignancies.10,11 More cases and increased mortality of COVID-19 were confirmed among patients with lymphoproliferative disorders compared with the general population.12–14 Furthermore, some recent studies raise specific concerns in terms of morbidity and mortality for patients with FL because of their immunocompromised status induced by recent exposure to cytotoxic chemotherapy, especially bendamustine and anti-CD20 immunotherapy.14,15
G became reimbursable for front-line treatment of FL in Croatia in 2019. We performed this retrospective noninterventional real-life study to investigate efficacy and safety of combinations of G with standard immunochemotherapy protocols in patients with newly diagnosed FL gr. 1–3A, which in a large part coincided with the COVID-19 pandemic.
MATERIALS AND METHODS
Data on patients starting front-line treatment for FL gr. 1, 2, or 3A with G-chemotherapy combination regimens from April 2019 to March 2021 were collected by retrospective chart review. FL was diagnosed according to standard criteria.1 Patients with FL grade 3B or composite lymphoma were excluded from this analysis. G-B, G-CHOP, and G-CVP were administered as usual. Response to therapy and outcomes were evaluated using common lymphoma response criteria, usually CT-based.16,17 Patients with disease responding to treatment were scheduled to continue with G maintenance, q 2 months for 2 years. During maintenance and follow-up radiological examinations were performed only if clinically indicated. Toxicity was graded according to CTCAE v.5.18 COVID-19 was diagnosed by PCR testing. Patients dying with COVID-19 were considered as dying from COVID-19.
For some analyses, 65 years of age was chosen as the cutoff between younger and elderly to correspond to accepted age limits and be close to the median age of our cohort. Normality of distribution of numerical variables was tested using the Shapiro–Wilk test. Normally distributed variables are presented as arithmetic mean ± standard deviation and were compared using the t-test, whereas nonnormally distributed variables were presented as median and interquartile range (IQR) and were compared using the Mann–Whitney U test. Categorical variables were presented as proportions and percentages and were compared using the χ2 test or the Fisher exact test where appropriate. Survival analyses were based on the Kaplan–Meier method. Screening of survival associations was performed using the custom made MS Excel workbook.19 Survival curves were compared using the log-rank test. The Cox regression analysis was used for multivariate survival analysis. All analyses were performed using the MedCalc statistical software version 20.014 (MedCalc Software Ltd, Ostend, Belgium).
The study was performed in accordance to all applicable Croatian, EU and international rules and regulations and with the approval of the relevant Ethical Committee.
RESULTS
We identified a total of 114 FL patients fulfilling the inclusion criteria (Table 1). Median age was 63, range 33-86 years. Majority of patients were female (71, 62.3%). FL gr. 1 was present in 45 (39.5%), gr. 2 in 28 (24.6%), gr. 3A in 27 (23.7%), and indolent unspecified in 14 (12.3%) patients. Median FLIPI score was 2.5 points, IQR (2–3). A total of 61 (53.5%) patients were treated with G-B, 49 (43%) with G-CHOP, and 4 (3.5%) with G-CVP.
Table 1.
Patients’ Characteristics and Treatment
| Overall | G-B | G-CHOP | P | |
|---|---|---|---|---|
| Total number | 114 | 61 | 49 | – |
| Age (y) (median/range) | 63/33–86 | 66/36–83 | 59/33–75 | 0.001a |
| Sex | ||||
| Male | 43 (37.7%) | 23/61 (37.7%) | 19/49 (38.8%) | |
| Female | 71 (62.3%) | 38/61 (62.3%) | 30/49 (61.2%) | 0.909 |
| FL type | <0.001a | |||
| 1 | 45 (39.5%) | 33 (54.1%) | 9 (18.4%) | |
| 2 | 28 (24.6%) | 15 (24.6%) | 12 (24.5%) | |
| 3A | 27 (23.7%) | 3 (4.9%) | 24 (49%) | |
| Indolent unspecified | 14 (12.3%) | 10 (16.4%) | 4 (8.2%) | |
| FLIPI | 2.5 IQR (2–3) | 3 IQR (2–3) | 2 IQR (2–3) | 0.159 |
| IPI | 2 IQR (1–3) | 2 IQR (2–3) | 2 IQR (1–2) | 0.116 |
| IgG levels prior therapy (g/L) | 10.1 ± 2.9 | 10.6 ± 3.2 | 9.8 ± 2.5 | 0.155 |
| Hosp. for first G application | 49 (43.0%) | 21 (34.4%) | 26 (53.1%) | 0.050a |
| No. cycles | 6 IQR (6–6) | 6 IQR (6–6) | 6 IQR (6–8) | 0.350 |
| Total G dose | 8 IQR (8–10) | 8 IQR (8–10) | 9 IQR (8–10) | 0.277 |
| Interruption due to AE | 41 (36.0%) | 19 (31.1%) | 19 (38.8%) | 0.403 |
| G-CSF prophylaxis | 0.135 | |||
| None | 35 (30.7%) | 23 (37.7%) | 10 (20.4%) | |
| Primary | 66 (57.9%) | 31 (50.8%) | 33 (67.3%) | |
| Secondary | 13 (11.4%) | 7 (11.5%) | 6 (12.2%) | |
| Pts starting maintenance/ Pts. responding to induction | 90 (84.1%) | 43 (78.2%) | 44 (89.8%) | 0.122 |
| No. cycles of maintenance | 3 IQR (1–6) | 2 IQR (0–5) | 5 IQR (2–8) | <0.001a |
| Pts. interrupting maintenance/pts. starting maintenance | 34 (37.8%) | 16 (37.2%) | 17 (38.6%) | 0.891 |
aStatistically significant at level P < 0.05.
CHOP = CVP + doxorubicine; CVP = cyclophosphamide, vincristine and glucocorticoid; FL = follicular lymphoma; IQR = interquartile range.
Due to the small number of patients receiving G-CVP, comparisons were only made between the G-B and G-CHOP-treated groups. Patients receiving G-B were significantly older, more likely to have lower FL grade and less likely to be hospitalized for the first obinutuzumab application (P < 0.05 for all analyses). There was no significant difference in FLIPI, sex and pretreatment IgG levels between the 2 groups. Severe hypogammaglobulinemia was present in 3% of patients. Both groups received similar number of chemotherapy cycles, similar total obinutuzumab dose in the induction phase of treatment and were similarly exposed to primary and secondary neutropenia prophylaxis with G-CSF. In approximately one-third of patients was the treatment delayed or prematurely stopped due to side effects. Thirteen patients (11%) received less than 6 cycles of chemotherapy; the same number of patients received less than 8 doses of G. Patients treated with G-B and G-CHOP had similar rates of neutropenia, anemia, thrombocytopenia, infections and hospitalizations due to adverse events (P > 0.05 for all analyses) (Table 2). IgG levels at end of induction were comparably reduced in both treatment groups; 11% of patients had severe hypogammaglobulinemia.
Table 2.
Toxicity and Efficacy
| Overall | G-B | G-CHOP | P | |
|---|---|---|---|---|
| Induction therapy | ||||
| Thrombocytopenia any grade | 29 (25.4%) | 19 (31.1%) | 10 (20.4%) | 0.204 |
| Thrombocytopenia gr. 3–4– | 2 (1.8%) | 1 (1.6%) | 1 (2%) | 1.000 |
| Anemia any grade | 33 (29.5%) | 17 (28.8%) | 16 (32.7%) | 0.666 |
| Anemia gr. 3 –4 | 1 (0.9%) | 1 (1.7%) | 0 (0%) | 1.000 |
| Neutropenia any grade | 53 (46.5%) | 27 (44.3%) | 24 (49%) | 0.622 |
| Neutropenia gr. 3–4– | 33 (28.9%) | 15 (24.6%) | 17 (34.7%) | 0.246 |
| Infections any grade | 34 (31.2%) | 15 (26.3%) | 18 (37.5%) | 0.219 |
| Infections gr. 3–4– | 15 (13.8%) | 8 (14%) | 6 (12.5%) | 0.818 |
| Hospitalization due to AE | 20 (17.5%) | 10 (16.4%) | 9 (18.4%) | 0.785 |
| IgG levels after induction (g/L) | 7.3 ± 2.1 | 7.6 ± 2.5 | 7.1 ± 1.6 | 0.261 |
| Response after induction | 0.046a | |||
| CR | 78 (68.4%) | 39 (63.9%) | 39 (79.6%) | |
| PR | 29 (25.4%) | 16 (26.2%) | 10 (20.4%) | |
| PD | 2 (1.8%) | 1 (1.6%) | 0 | |
| Nonrelapse death | 3 (2.6%) | 3 (4.9%) | 0 | |
| Response not evaluated | 2 (1.8%) | 2 (3.3%) | 0 | |
| Infection at any time any grade | 47/114 (19.1%) | 23/61 (26.2%) | 22/49 (13.6%) | 0.559 |
| Infection at any time gr. 3–4 | 24/114 (10.1%) | 13/61 (14.3%) | 9/49 (6.8%) | 0.812 |
| Response at last follow-up | ||||
| Remission | 99 (86.8%) | 50 (82.0%) | 47 (95.9%) | |
| Progression | 6 (5.3%) | 3 (4.9%) | 2 (4.1%) | 1.000 |
| Nonrelapse death | 9 (7.9%) | 8 (13.1%) | 0 | 0.008a |
aStatistically significant at level P < 0.05.
AE = adverse event; CHOP = CVP + doxorubicine; CR = complete response; CVP = cyclophosphamide, vincristine and glucocorticoid; PD = progressive disease; PR = partial response.
In 107 (94.8%) patients disease responded to induction treatment, two patients had progressive disease, three died without progression, and two were not evaluated.
Median follow-up of surviving patients was 17 months. G maintenance was started in 90 (84% of those eligible), but was interrupted in 34 (37.8%). The percentage of patients starting and prematurely stopping maintenance was similar between G-B and G-CHOP-treated groups, but less cycles were administered in the former (2 versus 5, P < 0.001).
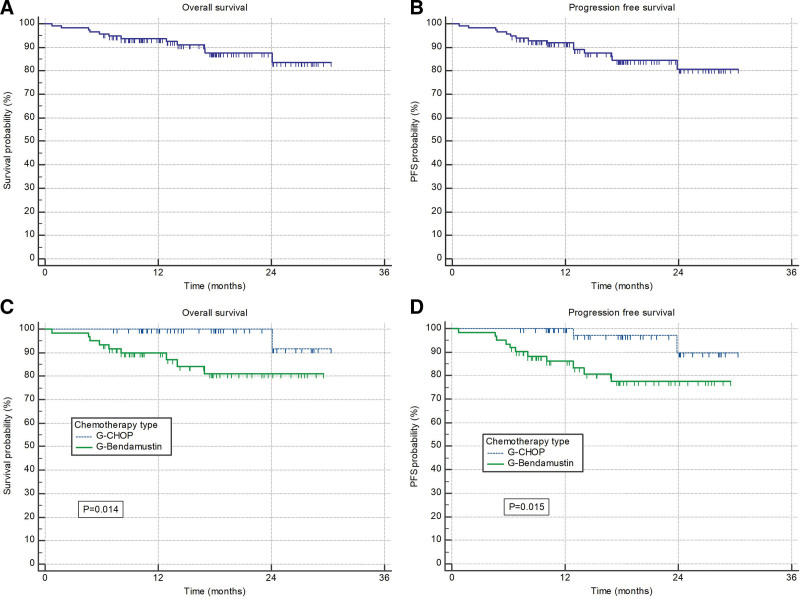
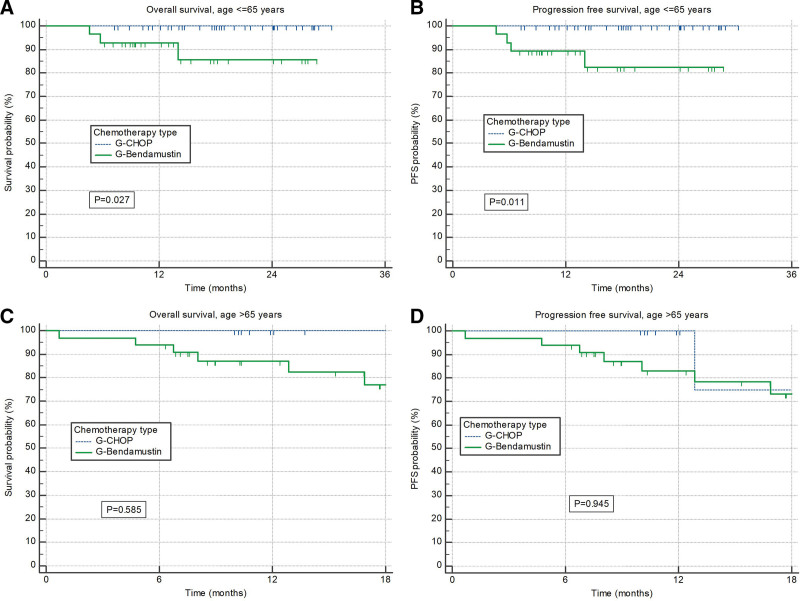
At time of data cutoff a total of 6 patients had progressive disease and 12 died. Median OS and PFS were not reached; 18-months rates were 88% and 84%, respectively (Figure 1A and B). G-CHOP treated patients had significantly better OS and PFS than those treated with G-B (P = 0.014 and P = 0.015, respectively) (Figure 1C and D). The differences were significant in patients younger than 65 years (P = 0.027 and P = 0.011, respectively) (Figure 2A and B), while older patients had similar outcomes irrespective of type of chemotherapy (Figure 2C and D). Difference in survival was driven mostly by nonrelapse mortality; probability of disease progression was comparable between 2 groups of patients (4.9% with G-B versus 4.1% with G-CHOP).
Figure 1.
(A) Overall survival and (B) progression-free survival of all patients. (C) Overall survival and (D) progression-free survival stratified according to the chemotherapy type (G-CHOP and G-Bendamustine). CHOP = CVP + doxorubicine.
Figure 2.
Overall survival and progression-free survival according to chemotherapy type. (A) Overall survival and (B) progression-free survival according to the chemotherapy type (G-CHOP and G-Bendamustine) in patients younger than 65 years. (C) Overall survival and (D) progression-free survival according to the chemotherapy type (G-CHOP and G-Bendamustine) in patients older than 65 years. CHOP = CVP + doxorubicine.
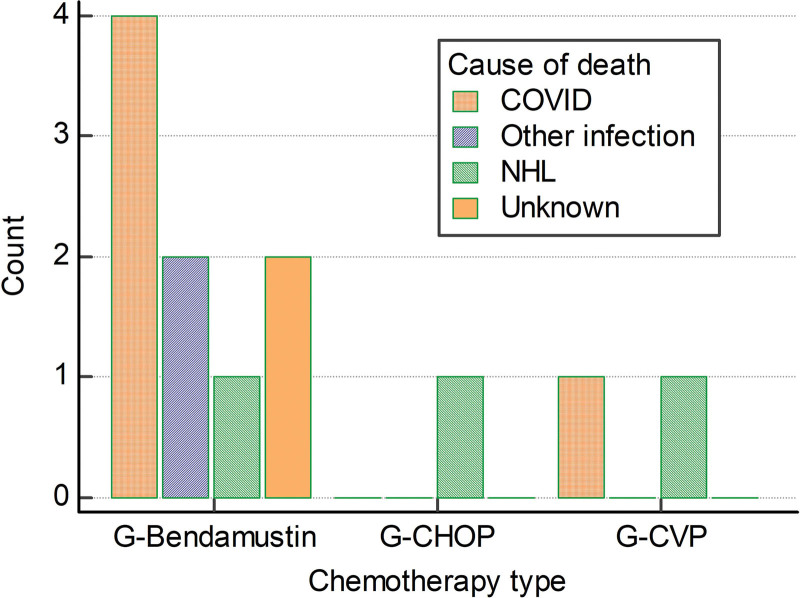
A total of 20 (17.5%) patients had SARS-CoV-2 infection before lymphoma progression, 5 died; 4 of 12 treated with G-B, 1 of 1 treated with G-CVP, and 0 of 7 treated with G-CHOP. Only 3 patients were fully vaccinated before infection; 18 received remdesivir and 9 additionally received convalescent plasma; data on treatment were unknown for 2 patients. Treatment of COVID-19 was not significantly different between patients treated with G-B and G-CHOP. Causes of death are depicted in Figure 3. Two additional patients died of other infections, both were treated with G-B; 3 died after FL progression, 1 in each chemotherapy group. In 2 patients, the cause of death is unknown, both were treated with G-B. Five patients died during induction, 5 during maintenance and 2 while off therapy.
Figure 3.
Causes od death stratified according to the chemotherapy type (G-Bendamustine, G-CHOP and G-CVP). CHOP = CVP + doxorubicine; CVP = cyclophosphamide, vincristine and glucocorticoid.
In the multivariate Cox regression model (Table 3), therapy with G-B in comparison to G-CHOP (P = 0.044, hazard ratio [HR] = 9.81) and FLIPI >3 (P = 0.008, HR = 8.17) were independent negative predictors of survival; age, sex, and grade were not.
Table 3.
Cox Regression Analysis of Factors Predictive for Overall Survival
| Variable | P Value | Hazard Ratio | HR 95% CI |
|---|---|---|---|
| Chemotherapy CHOP vs Bendamustine | 0.044a | 9.81 | 1.06-90.37 |
| Age >65 y | 0.643 | 1.46 | 0.29-7.35 |
| Male sex | 0.551 | 1.61 | 0.34-7.62 |
| Grade 3 FL | 0.775 | 1.41 | 0.13-15.54 |
| FLIPI > 3 | 0.008a | 8.17 | 1.72-38.67 |
aStatistically significant at level P < 0.05.
CHOP = CVP + doxorubicine; CVP = cyclophosphamide, vincristine and glucocorticoid; FL = follicular lymphoma.
DISCUSSION
To our knowledge, this is the first report on outcomes of patients with FL receiving front-line treatment with G-chemotherapy regimens outside of clinical trials. The Croatian Society for Hematology and KROHEM has since 2006 published and regularly updated recommendations for the diagnosis and treatment of lymphomas.20 G has been the recommended anti-CD20 monoclonal antibody since GALLIUM data were published. It became fully reimbursed by the Croatian National Health Insurance in March 2019. Due to the administrative requirements of the reimbursement procedure, patients receiving G can easily be identified. All Croatian hematology centers treating lymphoma participated in this study, so we believe that our results represent unbiased real-life data on the effects of G-chemotherapy combinations in front-line FL.
Treatment was generally well tolerated; few patients stopped treatment prematurely for toxicity. Contrary to most other studies was the incidence of neutropenia and gr. 3–4 neutropenia comparable between patients treated with B and CHOP.3,4,8,9 This might be related to the high frequency of primary G-CSF prophylaxis in our series. Infections, all and gr. 3–4, occurred in both groups with similar frequency, but lethal infections were more common in patients treated with bendamustine. This was related neither to age (since the difference in outcomes was more pronounced in younger patients) nor to hypogammaglobulinemia (which was similar between the groups).
The efficacy of G-based combinations was excellent and comparable to those from the GALLIUM study.8,9 Only in 2 patients disease progressed during induction, in 2 during maintenance and in 2, who did not start maintenance, during follow-up. However, PFS and OS are approximately 10% lower than reported in the seminal study. This difference is driven almost exclusively by nonrelapse mortality. The major cause of death was COVID-19. Four of 5 patients who died of this infection before lymphoma progression had been treated with bendamustine. All of the 7 patients treated with CHOP recovered from COVID-19 infection, while the mortality was 33% in patients treated with bendamustine. This translated into a significantly inferior PFS and OS of patients treated with G-B in comparison to those treated with G-CHOP.
Despite the fact that bendamustine is an old drug, the immunological consequences of its use are still incompletely understood, but include prolonged B- and T-lymphocyte depletion.21 The latter is probably the most important cause of increased mortality due to infections seen in our series and in the GALLIUM study, since neutropenia and hypogammaglobulinemia were similar in patients treated with G-CHOP and G-B. The consequences of T-cell depletion became more deleterious during the COVID-19 pandemic. Multiple studies, including those from our group, have shown that treatment with purine analogues, such as fludarabine, bendamustine, and cladribine, increases the risk of prolonged viral shedding and dying of infection.14,15,22 While anti-CD20 monoclonal antibodies increase the risk of prolonged COVID-19, their effect on mortality seems less than that of chemotherapy.
Our study has significant limitations. The main are short follow-up for this type of lymphoma, retrospective design and non-randomized nature of treatment allocation resulting in the possibility that different outcomes between G-B and G-CHOP could be caused by comorbidities and some other unmeasured parameters outside conventional risk scores. Other liabilities include relatively small sample size and single country experience. Small sample size could also be reason for nonsignificant differences between protocols found in the older population. All our patients started treatment before full vaccination against COVID-19 could be performed. It remains unclear whether full vaccination or administration of protective monoclonal antibodies before the start of bendamustine reduces the risk of lethal infection during treatment.23
Despite these shortcomings, we believe that, based on these results, in patients with FL younger than 65 without significant cardiac comorbidities, CHOP should be considered as the preferred front-line chemotherapy while the risk of COVID-19 infection persists. In those older than 65 the choice should be made based on individual risk factors and current epidemiological situation. In either case, obinutuzumab remains an excellent part of front-line therapy for FL.
ACKNOWLEDGMENTS
List of participating institutions and principal investigators not qualifying for authorship: Ivan Krecak, Department of Internal Medicine, General Hospital of Sibenik - Knin County, Sibenik, Croatia; Ivan Zekanovic, Department of Internal Medicine, General Hospital Zadar, Zadar, Croatia; Nika Popovic, Department of Haematology, Oncology and Clinical Immunology, General Hospital Varazdin, Varazdin, Croatia; Petra Berneš, Department of Internal Medicine, General Hospital Pula, Pula, Croatia.
AUTHOR CONTRIBUTIONS
DG and IA: Conceptualization, Methodology, Investigation, Resources, Formal analysis, Validation, Writing original draft, Writing – review & editing. SB-K, AP, VM, BD, NF, BC, JS-P, PG, and VP: Investigation, Validation, Writing – review & editing. ML: Data curation, Formal analysis, Investigation, Validation, Writing original draft. All authors approved the final version of article.
DISCLOSURES
The authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
Supported in part by an unrestricted grant from Roche.
Footnotes
Presented in part at the annual European Hematology Association meeting, Vienna, Austria, June 10–12, 2022.
REFERENCES
- 1.Jaffe ES, Harris NL, Swerdlow SH, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2017:266–273. [Google Scholar]
- 2.Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: the Stanford University experience. Blood. 2013;122:981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. [DOI] [PubMed] [Google Scholar]
- 4.Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123:2944–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pouyiourou M, Meyer A, Stroux A, et al. First-line treatment with R-CHOP or rituximab-bendamustine in patients with follicular lymphoma grade 3A-results of a retrospective analysis. Ann Hematol. 2020;99:2821–2829. [DOI] [PubMed] [Google Scholar]
- 6.Mondello P, Steiner N, Willenbacher W, et al. Bendamustine plus rituximab versus R-CHOP as first-line treatment for patients with follicular lymphoma grade 3A: evidence from a multicenter, retrospective study. Oncologist. 2018;23:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah NN, Szabo A, Saba R, et al. Multicenter analysis of advanced stage grade 3A follicular lymphoma outcomes by frontline treatment regimen. Clin Lymphoma Myeloma Leuk. 2019;19:95–102. [DOI] [PubMed] [Google Scholar]
- 8.Marcus R, Davies A, Ando K, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377:1331–1344. [DOI] [PubMed] [Google Scholar]
- 9.Hiddemann W, Barbui AM, Canales MA, et al. Immunochemotherapy with obinutuzumab or rituximab for previously untreated follicular lymphoma in the GALLIUM study: influence of chemotherapy on efficacy and safety. J Clin Oncol. 2018;36:2395–2404. [DOI] [PubMed] [Google Scholar]
- 10.Paul S, Rausch CR, Jain N, et al. Treating leukemia in the time of COVID-19. Acta Haematol. 2021;144:132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pagano L, Salmanton-Garcia J, Marchesi F, et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA). J Hematol Oncol. 2021;14:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regalado-Artamendi I, Jimenez-Ubieto A, Hernandez-Rivas JA, et al. Risk factors and mortality of COVID-19 in patients with lymphoma: a multicenter study. HemaSphere. 2021;5:e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura S, Kanemasa Y, Atsuta Y, et al. Characteristics and outcomes of coronavirus disease 2019 (COVID-19) patients with cancer: a single-center retrospective observational study in Tokyo, Japan. Int J Clin Oncol. 2021;26:485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamure S, Dulery R, Di Blasi R, et al. Determinants of outcome in Covid-19 hospitalized patients with lymphoma: a retrospective multicentric cohort study. EClinicalMedicine. 2020;27:100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aurer I, Jaksic O, Basic-Kinda S, et al. Purine analogues increase the risk of lethal and/or prolonged COVID19 while obinutuzumab increases the risk of prolonged but not lethal infection in patients treated for lymphoid malignancies -a study of Krohem, the Croatian group for hematologic diseases. Blood. 2021;138(Supplement 1):3553–3553. [Google Scholar]
- 16.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Younes A, Hilden P, Coiffier B, et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol. 2017;28:1436–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events. Version 5.0. Published November 27, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed November 3, 2022.
- 19.Lucijanic M. An update to the custom-made MS Excel workbook performing the log-rank test with extended functionality and a new original COVID-19 training data set. Croat Med J. 2021;62:531–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aurer I, Gasparov S, Kralik M, et al. DIJAGNOSTIKA I LIJECENJE LIMFOMA – DRUGI HRVATSKI KONSENZUS. [In Croatian]. Lijecnicki vjesnik. 2013;135:63–76. [PubMed] [Google Scholar]
- 21.Lalic H, Aurer I, Batinic D, et al. Bendamustine: a review of pharmacology, clinical use and immunological effects (review). Oncol Rep. 2022;47:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima Y, Ogai A, Furukawa K, et al. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J Infect Chemother. 2021;27:387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of COVID-19. N Engl J Med. 2022;386:2188–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]