PURPOSE
Despite an increasing number of survivors of childhood cancer (CCS) in low- and middle-income countries, survivorship care is in its nascent stages. We describe the spectrum of late effects seen, challenges faced, and lessons learnt over three decades of a late effects program in India.
METHODS
We describe the demographics and profile of late effects of all CCS survivors enrolled in our After Completion of Treatment Clinic from February 5, 1991 (inception) to February 4, 2021. We analyzed the trends by the decade of diagnosis.
RESULTS
There were 3,067 CCS survivors, the median age was 18 years (range, 3-57 years), and the median follow-up was 11 years (range, 2-46 years). Two thirds (62.4%) had either no or mild late effects, 480 (15.6%), 497 (16.2%), and 162 (5.3%) had grades 2, 3, and 4 late effects, with 67 deaths reported. Notable late effects were chronic viral hepatitis (7.8%), thyroid dysfunction (7.5%), other endocrine issues (13.6%), psychosocial issues (57%), neurocognitive impairment (4.1%), and metabolic syndrome (4%). The cumulative incidence and severity of late effects showed a consistent decline by the decade of diagnosis. Twenty-two percent of survivors are lost to follow-up.
CONCLUSION
Survivors of childhood cancer treated on contemporary treatment protocols have a significantly lower side-effect profile. Attrition to long-term follow-up and psychosocial issues are significant concerns. Understanding the unique spectrum of late effects and establishing a holistic support system go a long way in ensuring the long-term physical and mental health and psychosocial concerns of childhood cancer survivors in low- and middle-income countries.
BACKGROUND
An estimated 52,366 children (0-14 years) and 76,805 children and adolescents (0-19 years) develop cancer every year in India.1 Although the great leaps in childhood cancer survival from the West have not been replicated in India and other low-middle income countries (LMICs), there has been a modest improvement in survival.2 With the focus being improvement in cure rates, survivorship has not been a priority until recently.3 This is unfortunate since it is well recognized that survivors of childhood cancer might have a high and varying risk of developing long-term health conditions.4-6 The prevalence of late effects increases as time from cancer diagnosis elapses (beyond the fourth decade of life) such that by age 50 years, survivors experience an average of 4.7 chronic health conditions of grade 3-5, double that of age-matched controls.4-6
CONTEXT
Key Objective
Does the profile of late toxicities in long-term survivors of childhood cancer in India differ from the West? Have there been any notable changes over the decades?
Knowledge Generated
In 3,067 survivors of childhood cancer, more than one third had late effects requiring intervention. Notable late effects were transfusion-transmitted infections, endocrinopathies, psychosocial issues, and metabolic syndrome. Attrition to follow-up is a major concern. There was a consistent decline in the late effect profile by the decade of diagnosis.
Relevance
Understanding the unique spectrum of late effects and multifactorial etiology helps ensure holistic and sustainable support for survivors of childhood cancer.
Although there is increasing interest in survivorship care in India in the past few years, this field is still in its nascent stages.3,7 A recent comprehensive review described how several centers have initiated late effects services, predominantly within larger pediatric oncology units, often catering to children and adolescents with a relatively short duration of follow-up. The past decade has also seen advocacy by nonprofit organizations and the emergence of an active late effects subcommittee in the Indian Paediatric Oncology Group.3
The After Completion Treatment (ACT) Clinic at Tata Memorial Hospital, Mumbai, is the oldest and largest survivorship clinic in India, established in 1991 after a similar initiative at the St Jude Research Hospital, USA. The clinic coordinates the care of a large proportion of survivors with late effects requiring intervention. As of 2021, the Clinic caters to more than 3,000 long-term survivors of childhood cancer.3,7-9
Importantly, as the first such survivorship clinic in India, the ACT Clinic has been instrumental in mentoring several centers in starting late effects services. We hypothesized that the burden of late effects in our population is different from that in the West and aimed to assess the burden of late effects in childhood cancer survivors registered at our clinic and to identify the population at a higher risk of developing late effects. This article also describes the stages of development of the Clinic.
METHODS
Setting and Participants
The Pediatric Oncology Unit at the Tata Memorial Centre is the largest such unit in India, and sees close to 2,500 children (age < 18 years) annually. The ACT Clinic is part of the pediatric oncology unit and is currently situated in a separate area within the routine clinic. Only children (age at diagnosis < 18 years) who have received complete treatment at our center are eligible. Until 2013, children 2 years after completion of treatment and in remission were eligible. In 2013, because of the large volume of patients, the inclusion criteria were amended to make only children age 5 years from initial diagnosis of cancer eligible. Patients who had relapsed previously need to be in remission for at least 2 years after salvage treatment.
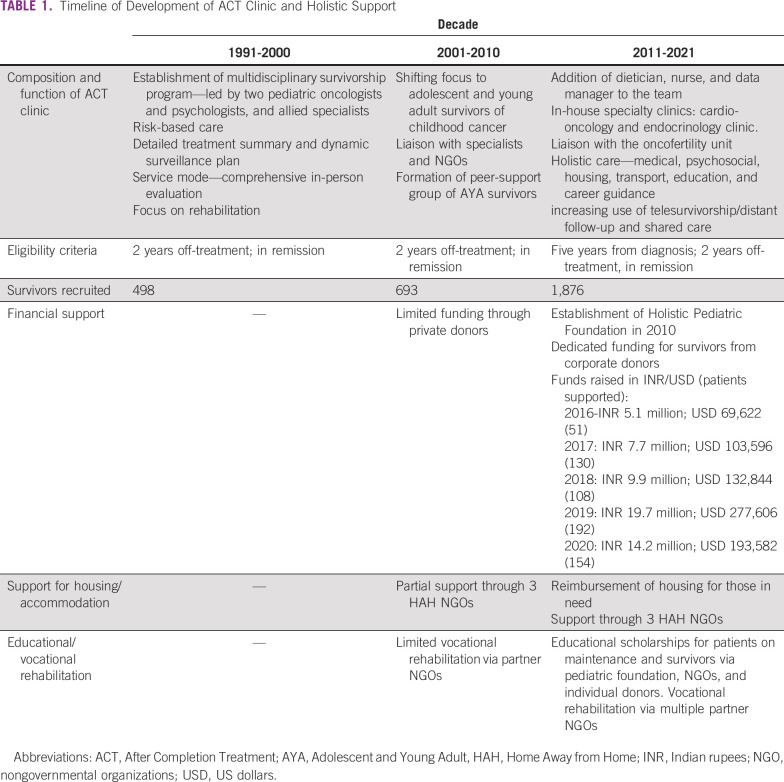
Although the clinic was initially conducted once weekly, since 2017, the clinic is conducted twice weekly, and walk-in appointments are seen on all days. The clinic is coordinated by pediatric oncologists; in addition, all survivors are seen by psychologists, dieticians, and social workers attached to the clinic. Selected survivors are assessed by radiation oncologists, surgical oncologists, and ophthalmologists.8,9 Since 2016, dedicated clinics for cardio-oncology and endocrinology follow-up of survivors of childhood cancer are functional within the hospital.10 The ACT Clinic co-ordinates the care of a large proportion of survivors with late effects requiring intervention and is part of the successful holistic support group at Tata Memorial Hospital.11 Collaborations with nonprofit organizations and other donors have helped the ACT Clinic expand its rehabilitation-focused services to offer financial, psychosocial, educational, and vocational support, crucial to our population of survivors (Table 1).
TABLE 1.
Timeline of Development of ACT Clinic and Holistic Support
Evaluation of Late Effects, Data Collection, and Extraction
Survivors are evaluated using a modified version of the international guidelines (Data Supplement).12 Late effects are graded using an adapted version of the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE).13 The grading is as follows: grade 0: Normal; grade 1: mild symptoms; clinical or diagnostic observations only; intervention not indicated; grade 2: Moderate; minimal, local or noninvasive intervention indicated; grade 3: Severe or medically significant but not life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care; grade 4: life-threatening consequences; urgent intervention indication; and grade 5: Death related to AE.13 Although grades 1-3 and 5 may refer to late effects involving any organ or system, grade 4 in our context predominantly involves late recurrences and second malignant neoplasms. Cumulative doses of anthracyclines and alkylating agents are calculated as per standard recommendations .14,15 These data are recorded and updated at every visit in the Clinic Database, which is maintained on SPSS. Data of all survivors registered in ACT Clinic between February 1991 and February 2021 were retrieved retrospectively from case files, the database of ACT Clinic and Electronic Medical records. Data included clinical, demographic and treatment details (including cumulative doses), investigations, and details of late effects. History of any health problems attributable to cancer diagnosis or treatment or because of other causes was noted. For the purpose of this article, the survivors were analyzed by the decade of diagnosis (1971-1980, 1981-1990, 1991-2000, 2001-2010, and 2011 onwards), and the cumulative incidence of the commonly encountered late effects was compared. Survivors were considered lost to follow-up if a time period of at least 5 years had elapsed since their last clinic visit or virtual/online consultation. A cohort of 625 survivors from our center was previously described in 2003.8,9 The late effect profile of this cohort was updated as of 2021 and compared with the original description.
Statistical Analysis
Results for continuous variables are expressed as median with range or mean (± standard deviation), and categorical variables are expressed using frequencies and percentages. For comparison of trends in cumulative incidence of late effects, a P value < .05 was considered significant, and all P values were two-sided. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 24.0 (IBM Corp, Armonk, NY).
This study was approved by the Institutional Ethics Committee at the Tata Memorial Centre, and waiver of informed patient consent was obtained.
RESULTS
Demographic Profile and Spectrum of Late Effects
From February 5, 1991, to February 4, 2021, 3,067 long-term survivors of childhood cancer were enrolled into the ACT Clinic. A large proportion of survivors use the clinic as their primary point of late effects care, with an average of 400-500 new enrolments annually and 40 follow-up visits/week.
The demographic profile and outcomes of these survivors are detailed in Table 2. The cohort has a median current age of 18 years (range, 3-57 years), with a median follow-up of 11 years (range, 2-46 years). The gender ratio is skewed with 2.5 times more males. The most common diagnoses included Acute Lymphoblastic Leukemia (ALL, 26.5%), Hodgkin Lymphoma (HL, 18.5%), and Wilms' Tumor (8%). More than one third had received chemotherapy and radiation, whereas approximately a fifth received chemotherapy alone or in combination with surgery (Table 2). Overall, half the cohort had received radiation therapy (any site) and 11% had received cranial irradiation (Table 3). The survivors belong to all regions across India; 35% are from Maharashtra state (where the clinic is situated), and the rest come from all parts of India (mainly Northern and Eastern India).
TABLE 2.
Demographics and Outcome of Survivors (N = 3,067) Enrolled From February 1991 to February 2021
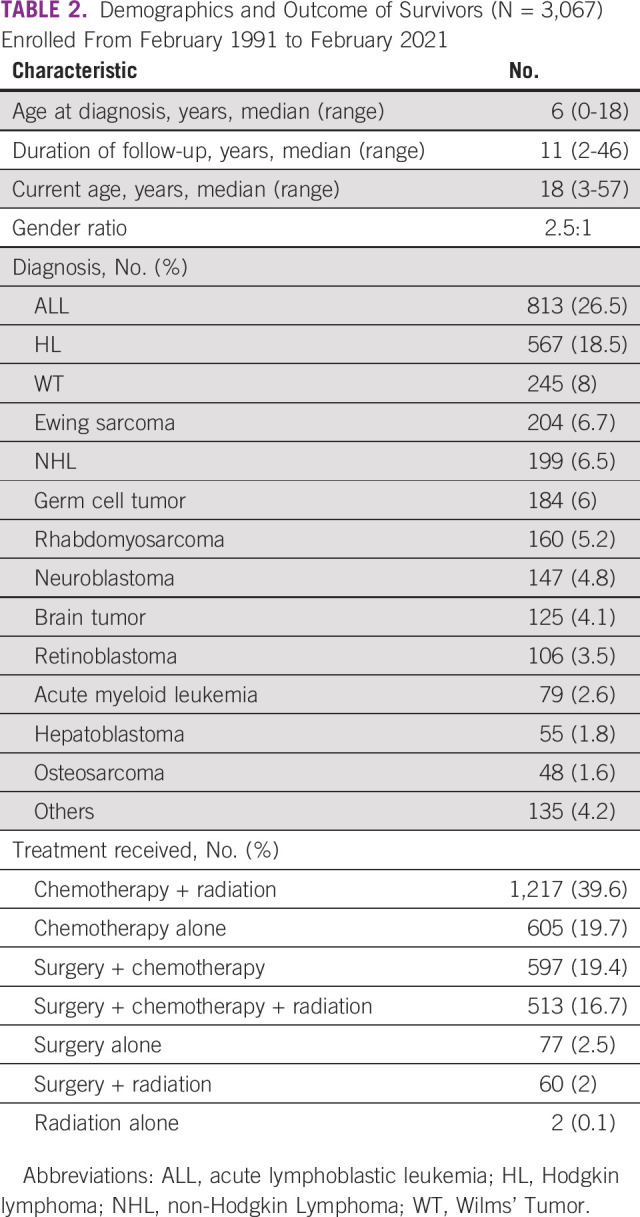
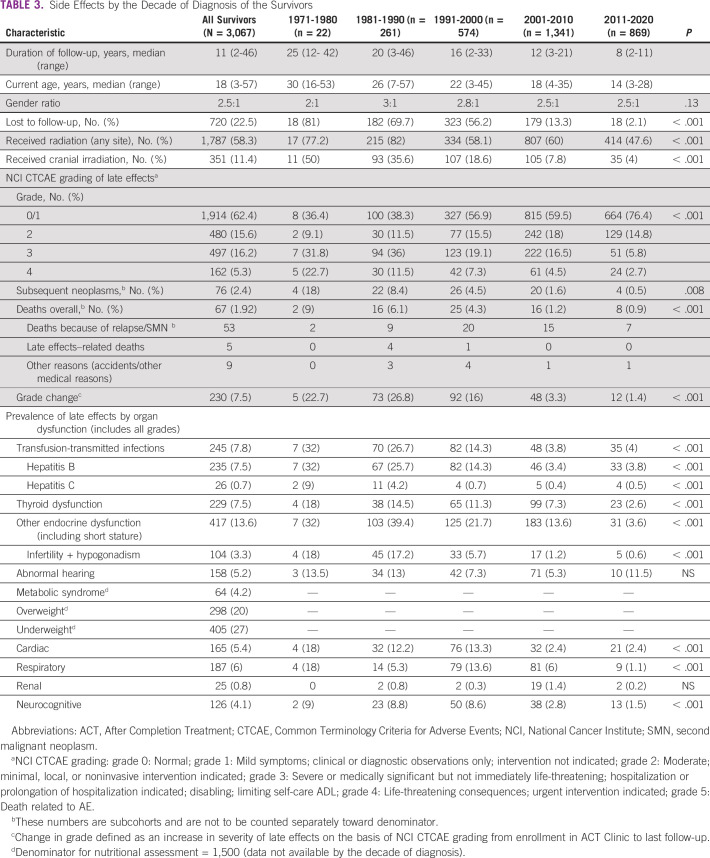
TABLE 3.
Side Effects by the Decade of Diagnosis of the Survivors
Nearly two thirds (1,914 [62.4%]) of survivors had either no or mild late effects, and 480 (15.6%), 497 (16.2%), and 162 (5.3%) had grades 2, 3, and 4 late effects. Sixty-seven deaths were recorded overall, 53 attributable to recurrence/second neoplasm. Among the common late effects (all grades) in this cohort were chronic viral hepatitis (7.8%); thyroid dysfunction (7.5%); other endocrine issues including growth and hypogonadism (13.6%); abnormal hearing (5.2%); and cardiac (5%), respiratory (6%), and metabolic syndrome (4%; Table 3). Survivors with diagnoses of retinoblastoma, brain tumor, and nasopharyngeal carcinoma had the highest cumulative incidence of late effects grade 3 and above (76%, 56%, and 55% respectively), followed by bone tumors (Ewing sarcoma 41.2% and osteosarcoma 57.5%), Rhabdomyosarcoma (43.7%), HL (15.7%), non-HL (14.5%), and ALL (10.8%).
Transfusion-Transmitted Infections
These were noted in 245 (7.8%), with hepatitis B in 235 (7.5%) and hepatitis C in 26 (0.7%). Of these, 133 did not receive any antiviral treatment and 112 were receiving/had received antivirals in the past. One patient died of chronic liver disease and subsequent hepatocellular carcinoma.
Fertility Outcomes
Sixty-two females and 135 males were documented as married, and four males and three females as divorced. In 35 instances, spouses were unaware of the survivor's previous cancer diagnosis (unrelated to sex of the survivor). Sixty-three (53%) males and seven (16.6%) females were infertile (P < .001). Seventy males had offspring—49 normally conceived, 15 after intervention, and six adopted. Thirty-three females had offspring, all normally conceived. None of the offspring has medical concerns, including cancer. In addition, 34 of the unmarried survivors were documented to have hypogonadism (Table 3).
Neurocognition and Psychosocial Issues
One hundred and twenty-six (4.1%) of the cohort were found to have moderate-severe neurocognitive impairment. The prevalence was highest in brain tumors (17.4%), ALL (4.4%), and Hodgkin Lymphoma (3.7%). Age at diagnosis and sex were not significantly associated with neurocognitive impairment. Formal neurocognitive assessment of 261 survivors referred on the basis of clinical concerns and assessed using the Wechsler Intelligence Scale for Children showed moderate-severe intellectual disability in 22 (8.4%), mild intellectual disability in 37 (14.4%), average in 145 (55.5%), borderline in 38 (14.6%), and superior/very superior intelligence in five (1.9%).
Our cohort had a high prevalence of scholastic problems (43%), school dropouts (13%), and other psychosocial issues (overall 57%). Eighty-five (2.7%) of survivors were documented to have either a psychiatric issue or significant psychological issue needing intervention.
Subsequent Neoplasms
There were overall 162 survivors who developed subsequent neoplasms (malignant = 50 and benign = 26) or late recurrences (n = 86) in this cohort. The commonest subsequent malignant neoplasms (SMNs) included papillary carcinoma thyroid (n = 10), Ewing sarcoma (n = 7), and glioblastoma (n = 5); benign neoplasms included benign thyroid nodules (n = 6), fibroadenoma (n = 5), and meningioma (n = 5). The median time to develop SMN was 14 years (range 2-29 years), and late recurrence was 7 years (2-27 years).
Deaths
The documented late mortality in this cohort was 67 (1.92%), 53 deaths because of SMN and relapse, five because of other late effects (two cardiomyopathy, one renal failure, one uncontrolled diabetes mellitus, and one complicated pancreatitis), and nine because of unrelated causes. There was a significant decline in late mortality in the most recently treated cohort (Table 3).
Attrition to Follow-Up
Nearly one fourth (22.5%) of the entire cohort is lost to follow-up, with 60% of survivors treated before 2000 being lost to follow-up. Older age of survivors, longer time from diagnosis, and residence outside of Mumbai were significantly associated with attrition to follow-up (P = .004, .01, and < 0.001 respectively).
Analysis of Temporal Trends by the Decade of Diagnosis
Table 3 details the profile of late toxicities by the decade of cancer diagnosis and treatment. There was a consistent decrease in the cumulative incidence of grade 2 and higher late effects in subsequent decades, including transfusion-transmitted infections; thyroid and other endocrine dysfunction; cardiac, respiratory, and neurocognitive dysfunction; and subsequent neoplasms and deaths (Table 3). There were a slight improvement in the gender ratio and decreased attrition to follow-up in recently treated survivors.
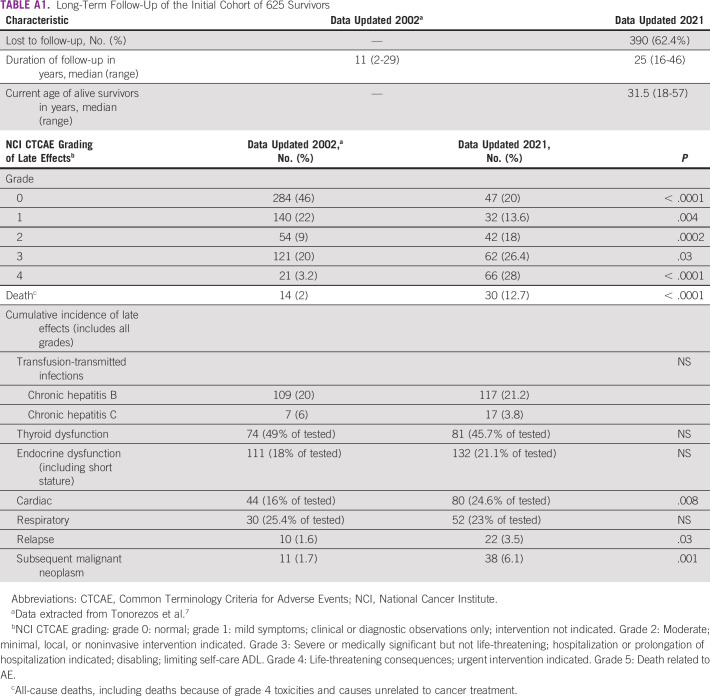
The updated follow-up of the original cohort of 625 patients confirmed the increase in cumulative incidence and severity of late toxicities with longer follow-up (25 years v 11 years). Cardiac late effects and subsequent malignant neoplasms/relapse were significantly increased. Although the absolute numbers of chronic hepatitis B/C and thyroid/endocrine dysfunction increased with increased follow-up, the proportion among patients tested remained constant (Appendix Table A1).
DISCUSSION
From its inception in 1991, the ACT clinic has been focused on service delivery, expanding over the past three decades to offer multidisciplinary care and holistic rehabilitation. An early report from the ACT Clinic (n = 625) was the first of its kind from India and possibly a LMIC. In this cohort, 32% had late effects requiring any form of intervention with growth disturbances (16%), endocrine dysfunction (18%), chronic viral hepatitis (20%), and cardiac toxicity (16%) being issues of concern.8,9
In the current, expanded cohort of more than 3,000 survivors, 37.6% had late effects of grade 2 and higher. The gender ratio is skewed with 2.5 times more males, reflecting the gender ratio at initial cancer diagnosis. The most common diagnoses included those with the best long-term survival outcomes—ALL (26.5%), HL (18.5%), and WT (8%). Notably, more than half of the cohort had received radiation therapy (any site) and 11% had received cranial irradiation although there has been a significant reduction in the use of radiation in recent years. Although not analyzed for this article, chemotherapy protocols at our center in the past two decades have incorporated risk stratification and either omitted or reduced the dose of alkylating agents and anthracyclines. The late effect profile with reductions in anticancer therapy has shown a decrease in severity, including late mortality.
The two largest reports of outcomes in childhood cancer survivors from other centers in India consist of 300 and 155 survivors.16,17 In the first report (n = 300, median follow-up 8.5 years), 23% had a minimal disability and 13% had moderate disabilities needing medical attention; 11 relapses, 2 second malignancies, and 5 deaths were reported.16 The second report (n = 155, median follow-up 8 years, median age 24 years) noted impaired fertility (24.5%), impaired growth pattern (4.5%), endocrine dysfunction (4.5%), and second malignancy (1.2%) to be major concerns.17 Other studies have focused on specific subsets of survivors.3
The spectrum of late effects in India (including at our center) is similar to those reported in the world literature, with certain issues such as transfusion-transmitted viral infections, metabolic syndrome, and psychosocial issues being specific concerns.3,18 Of note, in our cohort is the large proportion of survivors with neurocognitive and psychosocial issues. Although the conventional risk factors for neurocognitive impairment in survivors of childhood cancer are well recognized, scholastic problems and school dropout in India are multifactorial.19,20 A controlled comparison with the sibling cohort is essential for a meaningful root-cause analysis and sustainable intervention. Although only 4% of the cohort had metabolic syndrome, this was concerning in view of the young median age of 18 years and 20% prevalence of overweight. Metabolic syndrome and altered body composition are emerging chronic health issues among Indian survivors of childhood cancer.10,21
In our cohort, survivors with diagnoses of retinoblastoma, brain tumor, and nasopharyngeal carcinoma had the highest cumulative incidence of late effects grade 3 and above, possibly because of the large proportion of patients who underwent radiation and enucleation (in retinoblastoma) and use of cranial-directed radiotherapy in the others. Similarly, a high proportion of bone tumors underwent surgeries such as amputation and chemotherapy with alkylating agent and anthracyclines. A varied late toxicity profile in survivors of retinoblastoma, including a poorer quality of life, has been documented in other reports from North America and India.22-24
The current article reports the late effect profile of an expanded cohort with the longest follow-up from India. Although the median follow-up of the entire cohort is 11 years, the median age of survivors is 18 years; 300 and 600 survivors have been followed up for more than 20 and 15 years, respectively. Moreover, all toxicities are clinically ascertained in line with standard recommendations.
The subcohort of survivors with a relatively long follow-up allows for reporting of outcomes such as subsequent neoplasms and fertility in our population, and attrition of large numbers of longer-term survivors (up to 60% in those treated before 2000) has led to gaps in the data. Older age of survivors, longer time from diagnosis, long distance, gaps in awareness, financial toxicity, and social stigma are causes of attrition.18 The recently updated data of our initially published cohort of 625 survivors showed that there was a 62% attrition to follow-up.9 The remaining cohort had a 66.4% prevalence of late effects requiring intervention, double from 32% at 10 years, including a 6-fold increase in mortality (Appendix Table A1). The cumulative incidence of cardiac and respiratory toxicities as well as subsequent neoplasms showed an increase with a longer duration of follow-up, but transfusion-transmitted infections and thyroid dysfunction remained relatively stable. Notably, more survivors received targeted testing between 2002 and 2021. It could be hypothesized that a large proportion of the lost to follow-up survivors have suffered severe late effects including death, with a small proportion leading completely normal lives, far removed from their history of cancer. These findings are consistent with the most literature and appears to be a universal challenge.2,25,26 We have tried to tackle this major problem by incorporating counseling regarding the need for continuous follow-up and proactively reaching out by postal letters and telephone calls in the case of missed follow-up appointments. Possibly the most effective strategy in ensuring follow-up has been the establishment of a holistic support model (Table 1), which combines substantial financial assistance for treatment and educational/vocational guidance. The increasing use of telesurvivorship has emerged as a preferred mode of consultation for many survivors who might not have followed up otherwise.27
Our analysis found a consistent decrease in the cumulative incidence of severe late effects including mortality in recent decades, as described in other, larger cohorts.28 In our cohort, however, these findings might be confounded by the shorter duration of follow-up. In a separate analysis, an additional 14 years of follow-up data showed an increase in the cumulative incidence of cardiac late effects and subsequent malignant neoplasms/relapse in the original cohort of 625 patients. Detailed analysis of medical and psychosocial late effects in this cohort has been presented at various conferences and is the subject of a separate manuscript in preparation.29-36
With an ever-increasing cohort of childhood cancer survivors and a limited capacity to expand further, we are attempting to decentralize care by developing a strong multicentric network of late effects clinics at our allied centers providing holistic, standardized care. Incorporation of technology to facilitate survivorship care in our cohort of largely adolescent and young adults is another priority. There is a definite need to improve communication, build rapport, and improve education of patients/survivors, families, and health care professionals regarding potential late toxicities. Several research projects at our center attempt to minimize and alleviate late toxicities, especially neurocognitive issues in brain tumors, and azoospermia.29,37-39
In conclusion, it is both feasible and crucial to establish and sustain a survivorship program in centers treating children with cancer in LMICs, where the late effects differ from those described in the Western literature. Understanding the unique spectrum of late effects and multifactorial etiology helps ensure holistic and sustainable support. Although survivors of childhood cancer treated on contemporary treatment protocols have a significantly lower side-effect profile, several gaps need to be bridge to ensure the long-term physical and mental health and psychosocial support of childhood cancer survivors.
ACKNOWLEDGMENT
The authors acknowledge Dr Aruna Dhir, Dr Sheela Desai, Dr Anuprita Daddi, Dr Sudha Rao, Dr Vandana Dhamankar, Dr Hari Sankaran, Dr Ram Mohan, and various health care professionals involved in the care of survivors over the decades. Ms Shalini Jatia has been instrumental in securing financial and other support, and Mr Shreyas Chunekar and Mr Kiran Gowda were involved in data management and co-ordination of the ACT Clinic. Most importantly, the authors acknowledge the role of survivors and families over the decades who have placed their trust in us and been part of an extended family.
APPENDIX
TABLE A1.
Long-Term Follow-Up of the Initial Cohort of 625 Survivors
Girish Chinnaswamy
Consulting or Advisory Role: AstraZeneca
No other potential conflicts of interest were reported.
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Maya Prasad, Girish Chinnaswamy, Purna A. Kurkure
Provision of study materials or patients: Maya Prasad, Shripad D. Banavali, Purna A. Kurkure
Collection and assembly of data: All authors
Data analysis and interpretation: Maya Prasad, Girish Chinnaswamy, Shripad D. Banavali
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Girish Chinnaswamy
Consulting or Advisory Role: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.Arora RS, Bagai P, Bhakta N: Estimated national and state level incidence of childhood and adolescent cancer in India. Indian Pediatr 58:417-423, 2021 [PubMed] [Google Scholar]
- 2.Atun R, Bhakta N, Denburg A, et al. : Sustainable care for children with cancer: A Lancet Oncology Commission. Lancet Oncol 21:e185-e224, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Arora RS, Arora PR, Seth R, et al. : Childhood cancer survivorship and late effects: The landscape in India in 2020. Pediatr Blood Cancer 67:e28556, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Hudson MM, Ness KK, Gurney JG, et al. : Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 309:2371-2381, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GT, Kawashima T, Leisenring W, et al. : Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clinoncol 32:1218-1227, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhakta N, Liu Q, Ness KK, et al. : The cumulative burden of surviving childhood cancer: An initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 390:2569-2582, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonorezos ES, Barnea D, Cohn RJ, et al. : Models of care for survivors of childhood cancer from across the globe: Advancing survivorship care in the next decade. J Clin Oncol 36:2223-2230, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurkure PA, Achrekar S, Uparkar U, et al. : Surviving childhood cancer: What next? Issues under consideration at the After Completion of Therapy (ACT) clinic in India. Med Pediatr Oncol 41:588-589, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Kurkure P, Achrekar S, Dalvi N, Goswami S: Childhood cancer survivors—living beyond cure. Indian J Pediatr 70:825-828, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Prasad M, Sawant S, Dhir AA: Comment on: Nutritional concerns of survivors of childhood cancer: A “first world” perspective; perspective from a low/middle-income country. Pediatr Blood Cancer:e28362, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Jatia S, Prasad M, Paradkar A, et al. : Holistic support coupled with prospective tracking reduces abandonment in childhood cancers: A report from India. Pediatr Blood Cancer 66:e27716, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Children's Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers Version 5.0 (2018). http://www.survivorshipguidelines.org/ [Google Scholar]
- 13.National Cancer Institute : Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 [Google Scholar]
- 14.Green DM, Nolan VG, Goodman PJ, et al. : The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 61:53-67, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armenian SH, Hudson MM, Mulder RL, et al. : Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 16:e123-e136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seth R, Singh A, Seth S, Sapra S: Late effects of treatment in survivors of childhood cancers: A single-centre experience. Indian J Med Res 146:216, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajendranath R, Veeraiah S, Ramesh A, Sagar TG: Late effects of treatment in survivors of childhood cancer from a tertiary cancer center in South India. South Asian J Cancer 3:60-65, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad M, Goswami S: Barriers to long-term follow-up in adolescent and young adult survivors of childhood cancer: Perspectives from a low-middle income setting. Pediatr Blood Cancer 68:e29248, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Brinkman TM, Krasin MJ, Liu W, et al. : Long-term neurocognitive functioning and social attainment in adult survivors of pediatric CNS tumors: Results from the St Jude Lifetime Cohort Study. J Clin Oncol 34:1358-1367, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krull KR, Brinkman TM, Li C, et al. : Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: A report from the St Jude Lifetime Cohort Study. J Clin Oncol 31:4407-4415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malhotra P, Kapoor G, Jain S, et al. : Obesity and sarcopenia in survivors of childhood acute lymphoblastic leukemia. Indian Pediatr 58:436-440, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seth R, Singh A, Guru V, et al. : Long-term follow-up of retinoblastoma survivors: Experience from India. South Asian J Cancer 6:176-179, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batra A, Kumari M, Paul R, et al. : Quality of life assessment in retinoblastoma: A cross-sectional study of 122 survivors from India. Pediatr Blood Cancer 63:313-317, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Friedman DN, Chou JF, Oeffinger KC, et al. : Chronic medical conditions in adult survivors of retinoblastoma: Results of the Retinoblastoma Survivor Study. Cancer 122:773-781, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kagramanov D, Sutradhar R, Lau C, et al. : Impact of the model of long-term follow-up care on adherence to guideline-recommended surveillance among survivors of adolescent and young adult cancers. Cancer Med 10:5078-5087, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson TO, Oeffinger KC: Enhancing health care of survivors of childhood cancer with tailored education. J Clin Oncol 33:3849-3850, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Prasad M, Goswami S, Deodhar J, Chinnaswamy G: Impact of the COVID pandemic on survivors of childhood cancer and survivorship care: Lessons for the future. Support Care Cancer 30:3303-3311, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong GT, Chen Y, Yasui Y, et al. : Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 374:833-842, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurkure P, Prasad M, Dhamankar V, Bakshi G: Very small embryonic-like stem cells (VSELs) detected in azoospermic testicular biopsies of adult survivors of childhood cancer. Reprod Biol Endocrinol 13:122, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad M, Chinnaswamy G, Vora T, Goswami S: Burden of late effects and challenges faced in the long-term follow-up of paediatric germ cell tumour survivors: A report from India. Eur Urol Suppl 18:31, 2019 [Google Scholar]
- 31.Kurkure PA, Panda SP, Prasad M, Goswami S: Adolescent and young adult (AYA) survivors of childhood cancers: A challenge in after completion of therapy (ACT) clinic in resource-constrained country. J Clin Oncol 34 (suppl 3; abstr 19) [Google Scholar]
- 32.Dhir AA, Sawant S, Daddi A, Kurkure P: Anthracycline induced cardiotoxicity in childhood cancer survivors. Asia Pac J Clin Oncol 10:246, 2014. 24673966 [Google Scholar]
- 33.Kurkure P, Dhamankar V, Arora B, et al. : Long term follow up of Incidentally Detected Asymptomatic HBSAg Positive (IDAHP) survivors of childhood cancer. Pediatr Blood Cancer 53:859-860, 2009 [Google Scholar]
- 34.Mohan Gollamudi R, Chinnaswamy G, Vora T, et al. : Late toxicities in longterm survivors of non-syndromic unilateral Wilms tumour treated with doxorubicin based chemotherapy. Pediatr Hematol Oncol 3:S14, 2018 [Google Scholar]
- 35.Phad P, Sanadhya B, Goswami S, et al. : Audit of pyschosocial problems faced by childhood cancer survivors. Pediatr Blood Cancer 63:S232, 2016 [Google Scholar]
- 36.Prasad M, Nair K, Krishnatry R, et al. : LINC-07. Prevalence and spectrum of early endocrine disorders in survivors of paediatric embryonal brain tumors (PEBT): An experience from India. Neurooncology 22:iii379, 2020. (suppl 3) [Google Scholar]
- 37.Jalali R, Mallick I, Dutta D, et al. : Factors influencing neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. Int J Radiat Oncol Biol Phys 77:974-979, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Gupta T, Jalali R, Goswami S, et al. : Early clinical outcomes demonstrate preserved cognitive function in children with average-risk medulloblastoma when treated with hyperfractionated radiation therapy. Int J Radiat Oncol Biol Phys 83:1534-1540, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Goda JS, Dutta D, Krishna U, et al. : Hippocampal radiotherapy dose-constraints for predicting long-term neurocognitive outcomes: Mature data from a prospective trial in young patients with brain tumors. Neuro Oncol 22:1677-1685, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.