Summary
The inflammatory caspases, such as caspase-1, caspase-4, or caspase-11, are key enzymes in mammalian innate immunity as they control inflammasome-dependent inflammation. Assessing the specific proteolytic activities of these caspases in the context of a cell remains challenging, which is why in vitro studies of their catalytic activity have proven useful. Herein, we describe a detailed protocol for the purification of recombinant inflammatory caspases after heterologous expression in bacteria and how to assess and quantify cleavage of full-length protein substrates.
For complete details on the use and execution of this protocol, please refer to Devant et al. (2021).1
Subject areas: Cell Biology, Immunology, Molecular Biology, Protein expression and purification
Graphical abstract

Highlights
-
•
Production of recombinant inflammatory caspases (e.g., caspase-4) in E. coli
-
•
Streamlined purification protocol without the need for specialized equipment
-
•
In vitro cleavage assay allows for quantitation of specific catalytic activity
-
•
Simple experimental strategy enables screening of multiple proteins side-by-side
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The inflammatory caspases, such as caspase-1, caspase-4, or caspase-11, are key enzymes in mammalian innate immunity as they control inflammasome-dependent inflammation. Assessing the specific proteolytic activities of these caspases in the context of a cell remains challenging, which is why in vitro studies of their catalytic activity have proven useful. Herein, we describe a detailed protocol for the purification of recombinant inflammatory caspases after heterologous expression in bacteria and how to assess and quantify cleavage of full-length protein substrates.
Before you begin
All mammalian inflammatory caspases have a conserved domain structure comprising an N-terminal Caspase Activation and Recruitment Domain (CARD) and a C-terminal catalytic domain. The catalytic domain consists of two subunits (p20 and p10) connected by a cleavable interdomain linker (Figure 1). The CARD is critical for the activation of the caspases in the cell but is dispensable for its activity in vitro. Presence of the CARD hampers expression and purification of full-length caspases due to its propensity to aggregate. The protocol below describes the purification of the readily soluble catalytic domain (lacking the CARD) of human caspase-4 and an in vitro cleavage assay to quantify cleavage of the cytokine pro-interleukin (IL)-1β. Pro-IL-1β is a stereotypical substrate for many inflammatory caspases but it has been unclear whether it can be efficiently processed by human caspase-4 or other caspase-4 homologs. We have also used this protocol to purify other inflammatory caspases such as murine caspase-1 and caspase-11, several other mammalian caspase-4 homologs (including canine caspase-1/4) and have adapted the in vitro cleavage assay to other full-length protein substrates, such as gasdermin D (GSDMD) and pro-IL-18. This procedure therefore allows us to compare how well certain caspases cleave a protein substrate and assess the effect of specific mutations in the caspase on its catalytic activity.
Figure 1.
Schematic representation of conserved inflammatory caspase domain structure
Domain architecture shown is for human caspase-4 and caspase-5, murine caspase-11 and caspase-1. Caspase activation and recruitment domain (CARD), large catalytic subunit (p20) and small catalytic subunit (p10) are labeled accordingly. Internal cleavage sites and catalytic cysteine are indicated by black and green arrows, respectively.
The general methodology was originally developed for the study of apoptotic caspases2,3,4 and can be broadly applied to obtain enzyme kinetic parameters for any protease-substrate relationship.
Preparation of plasmids encoding caspase(s) and substrate(s)
Timing: 1+ days
-
1.Design construct for expression of caspase of interest (in this case, caspase-4). The construct should include (from 5′ to 3′):
-
a.Start codon (ATG).
-
b.Poly-histidine (His)-tag consisting of at least six consecutive His residues.
-
c.Sequence encoding the entire catalytic domain, but not the CARD of the caspase (residues 94–377 for human caspase-4).
-
d.Stop codon.
-
a.
-
2.Design construct for expression of substrate of interest (pro-IL-1β). The construct should include (from 5′ to 3′):
-
a.Start codon (ATG).
-
b.His-tag.
-
c.Sequence encoding the full-length substrate.
-
d.Stop codon.
-
a.
Note: Coding sequences for the caspase or substrate of interest can be obtained by performing a gene search on the National Center for Biotechnology Information (NCBI) website (https://www.ncbi.nlm.nih.gov/gene). In most cases, we recommend downloading the mRNA sequence marked as ‘canonical’.
Note: We most commonly use the stop codon TGA, but the identity of the stop codon is inconsequential in most cases.
Note: If desired, sequences can be codon-optimized for expression in E. coli. Since in this protocol we use a bacterial strain encoding rare tRNAs (described in more detail below), we have not found this to be necessary and it might limit the ability to reuse the sequences for experiments involving expression in mammalian cells.
-
3.
Synthesize cDNA fragments. We typically use the gBlock synthesis service by Integrated DNA Technologies to obtain our cDNAs of interest.
Alternatives: Gene sequences can also be obtained by amplifying the coding sequence of interest by PCR from mammalian total cDNA using appropriate primers.
-
4.
Subclone caspase and substrate of interest into a vector suitable for IPTG-induced overexpression in E. coli. We routinely use a vector based on the pET28a backbone, but most other vectors of the pET line should work as well.
Note: Short flexible linker or protease cleavage sites can be added between the caspase/substrate and the His-tag. Our most used design includes a tobacco etch virus (TEV) protease and a Thrombin cleavage site followed by a BamHI restriction site for in-frame cloning of a caspase or substrate of interest. These protease sites are not present in the original pET28a vector, but we have made plasmids encoding human caspase-4, pro-IL-1β and several other relevant caspases and substrates available on Addgene (Addgene IDs: 183381 and 183386), which follow this design strategy.
Note: When studying a previously uncharacterized caspase, it might be necessary to screen several constructs of varying length to find a version that is well-expressed. Domain prediction software (such as InterPro https://www.ebi.ac.uk/interpro/) can help to define the boundary between CARD and catalytic domain.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-pro-IL-1β | Genetex | GTX74034 |
| HRP-conjugated goat anti-rabbit IgG (H+L) | Jackson Immunolabs | 111-035003 |
| Bacterial and virus strains | ||
| Rosetta (DE3) pLysS chemically competent E. coli | EMD Millipore | 70956-4 |
| Chemicals, peptides, and recombinant proteins | ||
| Tris | Sigma | T1503-5kg |
| Glycine | Sigma | G7126-5kg |
| Imidazole | Sigma | I2399-500G |
| HEPES | Sigma | H3375-1KG |
| Yeast extract | BD | 212750 |
| Tryptone | BD | 211699 |
| Agar | BD | 214010 |
| Kanamycin sulfate | vwr | 97061-600 |
| Chloramphenicol | Sigma | C0378 |
| Potassium chloride | vwr | 97061-562 |
| Potassium phosphate monobasic (KH2PO4) | Fisher Scientific | P285-500 |
| Sodium phosphate dibasic heptahydrate (Na2HPO4 · 7 H2O) | Sigma | S9390 |
| Methanol | Fisher Chemical | A412-4 |
| IPTG | RPI | 367-93-1 |
| SDS | Bio-Rad | 1610302 |
| Glycerol | Fisher Chemical | BP-2291 |
| Bromophenol blue | Sigma | B0126-25G |
| 2-Mercaptoethanol | Sigma | M6250-500ML |
| Dithiothreitol (DTT) | Sigma | D9779-10G |
| PIPES | Sigma | P6757-100G |
| Sucrose | Sigma | S0389-1KG |
| 0.5 M EDTA pH 8.0 | Thermo Fisher Scientific | 15575020 |
| CHAPS hydrate | Sigma | C9426-5G |
| Tween 20 | Fisher Scientific | BP337-500 |
| Protogel 30% acrylamide | National Diagnostics | EC809 |
| 4× Protogel stacking buffer | National Diagnostics | EC-893 |
| 4× Protogel resolving buffer | National Diagnostics | EC-892 |
| TEMED | Sigma | T9281-25ML |
| Ammonium persulfate | Thermo Fisher Scientific | 17874 |
| Nonfat dry milk | Cell Signaling Technologies | 9999S |
| SOC outgrowth media | NEB | B9020 |
| NaCl | Sigma | S9625-5KG |
| Critical commercial assays | ||
| SuperSignal™ West Pico PLUS Chemiluminescent Substrate | Thermo Fisher Scientific | 34580 |
| PageRuler prestained protein ladder | Thermo Fisher Scientific | 26616 |
| InstantBlue Coomassie stain | Expedeon | ISB1L |
| Recombinant DNA | ||
| pET28_mouse-pro-IL-1 | Devant et al.1 | Addgene #183386 |
| pET28_caspase-4-catalytic-domain | Devant et al.1 | Addgene #183381 |
| Software and algorithms | ||
| ImageJ v.1.53 | Schneider et al.5 | https://imagej.nih.gov/ij/ |
| GraphPad Prism v.6 | GraphPad | https://www.graphpad.com/ |
| ProtParam | Expasy | https://web.expasy.org/protparam/ |
| Other | ||
| PVDF membrane | EMD Millipore | IPVH00010 |
| 0.22 μM PES syringe filter | EMD Millipore | SLGP033RS |
| 12-tube PCR strips | Corning | PCR-0212-FCP-C |
| Amicon Ultra Centrifugal filter units, 4 mL, 10 kDa cutoff | EMD Millipore | UFC801024 |
| 50 mL plastic syringes | Fisher Scientific | 13-689-8 |
| Polyprep chromatography columns | Bio-Rad | 7311550 |
| ChemiDoc MP Imaging system | Bio-Rad | 12003154 |
| PD-10 Desalting column | Cytiva | 17085101 |
| Square plastic dishes for western blot | Fisher Scientific | FB0875711A |
| Petri dishes | Fisher Scientific | FB0875713 |
| T100 Thermal Cycler | Bio-Rad | 1861096 |
| Whatman filter paper | Fisher Scientific | 10427806 |
| Mini Trans-Blot transfer cell | Bio-Rad | 1703930 |
| PowerPac HC power supply | Bio-Rad | 1645052 |
| Mini Protean Tetra cell electrophoresis system | Bio-Rad | 1658003 |
| Sonicator | Qsonica | Q125-110 |
| Innova 44 incubator shaker | New Brunswick | M1282-0000 |
| 50 mL conicals for fast spins | VWR | 89401-572 |
| Ni-NTA Agarose | Qiagen | 30230 |
Materials and equipment
2×YT media (Dissolve components in water, then autoclave)
| Reagent | Final concentration | Amount |
|---|---|---|
| Yeast extract | 1% (w/v) | 10 g |
| Tryptone | 2% (w/v) | 20 g |
| NaCl | 1% (w/v) | 10 g |
| ddH2O | N/A | Up to 1,000 mL |
| Total | N/A | 1,000 mL |
Store at 20°C–25°C.
LB agar plates with kanamycin and chloramphenicol (Dissolve yeast extract, tryptone, agar and NaCl in water, then autoclave. Add antibiotics when media cools down below 55°C before pouring plates)
| Reagent | Final concentration | Amount |
|---|---|---|
| Yeast extract | 0.5% (w/v) | 2.5 g |
| Tryptone | 1% (w/v) | 5 g |
| Agar | 1.5% (w/v) | 7.5 g |
| NaCl | 1% (w/v) | 5 g |
| 25 mg/mL Kanamycin | 25 μg/mL | 0.5 mL |
| 25 mg/mL Chloramphenicol | 25 μg/mL | 0.5 mL |
| ddH2O | N/A | Up to 500 mL |
| Total | N/A | 500 mL |
Store plates at 4°C for up to 3 months.
500 mM IPTG (Dissolve IPTG in water, then sterilize using 0.22 μm syringe filter)
| Reagent | Final concentration | Amount |
|---|---|---|
| IPTG | 500 mM | 1.19 g |
| ddH2O | N/A | Up to 10 mL |
| Total | N/A | 10 mL |
Make 1 mL aliquots and store at −20°C for several months.
Protein lysis buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M HEPES (pH 7.4) | 25 mM | 25 mL |
| 5 M NaCl | 150 mM | 30 mL |
| 2 M Imidazole (pH 8.0) | 10 mM | 5 mL |
| ddH2O | N/A | Up to 1,000 mL |
| Total | N/A | 1,000 mL |
Store at 4°C for several weeks to months.
Protein wash buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M HEPES (pH 7.4) | 25 mM | 25 mL |
| 5 M NaCl | 400 mM | 80 mL |
| 2 M Imidazole (pH 8.0) | 25 mM | 12.5 mL |
| ddH2O | N/A | Up to 1,000 mL |
| Total | N/A | 1,000 mL |
Store at 4°C for several weeks to months.
Protein elution buffer(s) (Prepare 10 different elution buffers with imidazole concentrations of 40 mM, 50 mM, 60 mM, 70 mM, 80 mM, 90 mM, 100 mM, 125 mM, 150 mM, 250 mM)
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M HEPES (pH 7.4) | 25 mM | 1.25 mL |
| 5 M NaCl | 150 mM | 1.5 mL |
| 2 M Imidazole (pH 8.0) | 40–250 mM | 1–6.25 mL |
| ddH2O | N/A | Up to 50 mL |
| Total | N/A | 50 mL |
Store at 4°C for several weeks to months.
Protein storage buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M HEPES (pH 7.4) | 25 mM | 6.25 mL |
| 5 M NaCl | 150 mM | 7.5 mL |
| Glycerol | 10% (v/v) | 25 mL |
| ddH2O | N/A | Up to 250 mL |
| Total | N/A | 250 mL |
Store at 4°C for several weeks to months.
Caspase assay buffer (Dissolve all components except DTT in water before filtering through a 0.22 μm syringe filter. DTT should be added fresh before each experiment)
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M PIPES (pH 7.2) | 10 mM | 1 mL |
| Sucrose | 10% (w/v) | 10 g |
| 5 M NaCl | 100 mM | 2 mL |
| CHAPS hydrate | 0.1% (w/v) | 0.1 g |
| 500 mM EDTA (pH 8.0) | 1 mM | 0.2 mL |
| 1 M DTT | 10 mM | 1 mL |
| ddH2O | N/A | Up to 100 mL |
| Total | N/A | 100 mL |
Store at 20°C–25°C. 1 M DTT stock solution should be stored at −20°C and added to the buffer right before use.
5× SDS loading buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M Tris (pH 6.8) | 250 mM | 25 mL |
| SDS | 10% (w/v) | 10 g |
| Glycerol | 30% (v/v) | 30 mL |
| Bromophenol blue | 0.25% (w/v) | 0.25 g |
| 2-Mercaptoethanol | 5% (v/v) | 5 mL |
| ddH2O | N/A | Up to 100 mL |
| Total | N/A | 100 mL |
Aliquot and store at −20°C for several months.
10× SDS-PAGE running buffer (pH 8.3)
| Reagent | Final concentration | Amount |
|---|---|---|
| Tris | 250 mM | 30 g |
| Glycine | 1.9 M | 144 g |
| SDS | 1% (w/v) | 10 g |
| ddH2O | N/A | Up to 1,000 mL |
| Total | N/A | 1,000 mL |
Store at 20°C–25°C for up to 6 months.
1× SDS-PAGE running buffer (pH 8.3)
| Reagent | Final concentration | Amount |
|---|---|---|
| 10× SDS running buffer (pH 8.3) | 1× | 100 mL |
| ddH2O | N/A | 900 mL |
| Total | N/A | 100 mL |
Store at 20°C–25°C for up to 6 months.
Transfer buffer (pH 8.3)
| Reagent | Final concentration | Amount |
|---|---|---|
| Tris | 25 mM | 15 g |
| Glycine | 190 mM | 72 g |
| SDS | 0.04% (w/v) | 2 g |
| ddH2O | N/A | Up to 4,000 mL |
| Methanol | 20% | 1,000 mL |
| Total | N/A | 5,000 mL |
Store at 4°C or 20°C–25°C for up to 3 months.
10× PBS (pH 7.4)
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl | 1.37 M | 400 g |
| KCl | 27 mM | 10 g |
| KH2PO4 | 18 mM | 12 g |
| Na2HPO4 · 7 H2O | 100 mM | 134 g |
| ddH2O | N/A | Up to 5,000 mL |
| Total | N/A | 5,000 mL |
Store at 20°C–25°C for several weeks.
1× PBST (pH 7.4)
| Reagent | Final concentration | Amount |
|---|---|---|
| 10× PBS (pH 7.4) | 1× | 200 mL |
| Tween 20 | 0.02% | 0.4 mL |
| ddH2O | N/A | Up to 2,000 mL |
| Total | N/A | 2,000 mL |
Store at 20°C–25°C for several weeks.
Step-by-step method details
Overexpression of caspase-4 in E. coli
Timing: 4 days
An inherent challenge when expressing inflammatory caspases is that their proteolytic activity can lead to severe growth defects and even cell death of the producing cells, including bacteria. In addition, genes of mammalian origin might contain codons that are rarely used in E. coli and might therefore hamper overexpression. We therefore recommend using bacterial strains encoding T7 lysozyme (reduces basal expression of T7 polymerase and therefore leaky expression of the gene of interest) and tRNA genes for rare codons, such as Rosetta (DE3) pLysS. Rare tRNA genes and T7 lysozyme are encoded on a plasmid conferring chloramphenicol resistance.
Alternatives: If sequences are codon-optimized for bacterial expression it might not be necessary to use an expression strain encoding rare tRNAs.
Alternatives: Regular LB media can be used for the expression cultures, but we have achieved better yields using the richer 2×YT media.
-
1.Transform E. coli with the pET28a plasmid encoding caspase-4 (strain: Rosetta (DE3) pLysS).
-
a.Remove a frozen aliquot of chemically competent cells from −80°C and thaw on ice.
-
b.Add 1 μL of plasmid (concentration between 10 and 100 ng/μL) to the competent cells and gently flick the tube to mix.
-
c.Incubate on ice for 30 min.
-
d.Heat-shock bacteria for 30 s at 42°C using a temperature-controlled water bath or heat block.
-
e.Incubate on ice for 5 min.
-
f.Add 1 mL of SOC media to cells.
-
g.Incubate for 1 h at 37°C while shaking at 250 rpm in a shaking incubator.
-
h.Plate 100 μL of bacteria on LB agar plates containing 25 μg/mL chloramphenicol and 25 μg/mL kanamycin using sterile plating beads.
-
i.Incubate for 18–24 h at 37°C.
-
a.
-
2.Pick a single colony off the plate using a sterile pipette tip.
-
a.Inoculate 10 mL of 2×YT media containing 25 μg/mL chloramphenicol and 25 μg/mL kanamycin in a sterile 50 mL conical tube.
-
b.Leave lid of the tube loosely attached to allow for sufficient aeration of the culture.
-
a.
-
3.
Grow pre-culture for 18–24 h at 30°C while shaking at 250 rpm.
-
4.
Inoculate the expression culture by adding 5 mL of the pre-culture to 500 mL of 2×YT media in a 2 L baffled flask.
Note: For caspase-4, an expression culture of 500 mL usually yields ample amounts of protein. For other inflammatory caspases or caspase mutants, it might be advised to scale up culture size in order to obtain sufficient yields. In such a case, make sure to also increase pre-culture size to allow for inoculation at a 1:100 ratio (v/v).
-
5.
Grow culture at 37°C while shaking at 250 rpm while regularly checking optical density of the culture at 600 nm (OD600).
-
6.
Once the culture reaches an OD600 of 0.6–0.8 (this usually takes 3–5 h), remove flask from shaking incubator and place on ice for 15 min.
-
7.
While culture cools, turn down temperature of shaking incubator to 18°C.
-
8.
Add IPTG to a final concentration of 0.25 mM (250 μL of 500 mM stock for 500 mL culture) to induce expression of the caspase-4 transgene.
-
9.
Place culture flask back in the incubator and allow expression to proceed for 18–24 h at 18°C while shaking at 250 rpm.
-
10.Harvest bacteria.
-
a.Transfer cultures into large centrifuge buckets and centrifuge for 15 min at 5,000 × g.
-
b.Discard supernatant and resuspend cell pellets in 50 mL 1× PBS and collect in a single 50 mL tube.
-
c.Spin down again (10 min at 5,000 × g). Discard supernatants.
-
d.Freeze down pellets in the bottom of the tube at −20°C.
-
a.
Pause point: Bacterial pellets can be stored at −20°C for several weeks to months before proceeding with purification.
Purification of recombinant caspase-4
Timing: 1 day
We employ a straightforward purification protocol of His-tagged caspases by immobilized metal affinity chromatography (IMAC). Protein is eluted in a stepwise imidazole gradient, which separates the protein of interest from other contaminants that non-specifically bind to the resin. This strategy eliminates the necessity for further purification steps requiring specialized, expensive equipment such as an FPLC system. Therefore, this protocol can be performed in any standard molecular biology laboratory. The absence of time- and labor-intensive steps such as size-exclusion chromatography also allows for the purification of multiple proteins side-by-side, which makes this protocol amenable to screening of multiple caspase variants or mutants.
-
11.
Resuspend bacterial pellet in 50 mL protein lysis buffer (∼1 mL of lysis buffer per 10 mL of expression culture).
Note: Most purification protocols for recombinant protein recommend adding protease inhibitors during lysis. Since caspases themselves are proteases, we advise against the use of protease inhibitors (particularly commercial protease inhibitor cocktails of proprietary composition). It is possible to add single inhibitors (e.g., phenylmethylsulfonyl fluoride or benzamidine hydrochloride for serine proteases), but we have not found this to be necessary.
CRITICAL: From this step forward, all steps should be performed on ice and with chilled reagents.
-
12.
Lyse cells by sonication (100% intensity, 2 s pulses on/off, 5 min) on ice.
-
13.
Spin down intact cells and debris by ultracentrifugation (20,000 × g, 45 min, 4°C).
CRITICAL: Make sure to use 50 mL tubes suitable for high-speed centrifugation to prevent damage to the tubes and centrifuge and sample loss.
-
14.
Filter clarified lysates through a 0.22 μm syringe filter and collect in a fresh 50 mL tube.
-
15.
Transfer 0.5 mL of Ni-NTA agarose beads (1 mL of slurry) into a 15 mL Poly-prep gravity flow column.
-
16.
Equilibrate beads with 5 mL of protein lysis buffer.
CRITICAL: Carefully add buffers/protein solutions to the column to not disturb the bead bed.
-
17.
Stepwise add the clarified lysate to the column until entire volume has passed through the beads. The His-tagged target protein will bind to the Ni-NTA agarose beads; discard the flowthrough.
-
18.
Wash beads with 10 mL of protein wash buffer.
-
19.
Label ten 15 mL tubes for collection of individual elution fraction and place on ice.
-
20.Elute the bound proteins from the beads.
-
a.Sequentially add 1 mL of the protein elution buffers with increasing imidazole concentrations (40–250 mM).
-
b.Collect eluates (each eluate fraction should be 1 mL) in individual, pre-labeled tubes and store on ice or in the fridge.
-
a.
Note: This stepwise gradient elution protocol ensures the efficient separation of common E. coli-derived protein contaminants from the His-tagged target protein, which is eluted at markedly higher imidazole concentrations.
Note: The Ni-NTA resin may be regenerated and reused multiple times. A stepwise protocol for how to wash, strip and recharge the beads with fresh Ni2+ ions is available in Kielkopf et al., 2020.6 We recommend collecting and storing the used resin in 20% EtOH to prevent contaminations and regenerating them in batch.
-
21.Run SDS-PAGE gel to identify fractions containing protein of interest. Due to the small size of caspase subunits, we use homemade gels (Biorad Mini-Protean system) with a high percentage of acrylamide (15%).
-
a.Take 40 μL of each fraction and add 10 μL of 5× SDS loading buffer. Heat samples for 10 min at 65°C.
-
b.Assemble Mini-Protean gel running chamber.
-
c.Load 5 μL of PageRuler prestained protein ladder (Thermo Fisher) into the first well of the gel.
-
d.Load 20 μL of each sample into residual wells of the gel.
-
e.Run gel at a constant voltage of 180 V for 45 min to 1 h. The lowest band of the prestained protein ladder should have reached the bottom third of the gel but not run out of the gel to ensure that the small subunit of the caspase (∼10 kDa in size) is still detectable.
-
a.
-
22.Stain gel using Coomassie to visualize proteins.
-
a.Remove gel from chamber and place in a small plastic tray containing 25 mL of InstantBlue Coomassie protein stain (Expedeon Protein Solutions).
-
b.Incubate for at least 15 min at 20°C–25°C while shaking gently.
-
a.
Note: Inflammatory caspases autocatalytically cleave themselves during overexpression/purification into the large and small catalytic subunit, which stay associated with each other in solution via non-covalent interactions. Therefore, it is expected to see two bands at around 20 kDa and 10 kDa in the SDS-PAGE gel.
-
23.
Pool fractions containing the pure protein of interest (Figure 2A).
-
24.
Concentrate pooled eluates using Amicon Ultra centrifugal filter unit with a 10 kDa size cutoff (5,000 × g, several 5–10 min spins, 4°C) to a volume smaller than 2.5 mL.
CRITICAL: Even though the assembled caspase complex is markedly larger (>30 kDa), using a filter unit with a 10 kDa size cutoff minimizes sample loss. Additionally, gentle mixing of the sample in the filter unit with a pipette between spins maximizes yield and prevents protein aggregation.
-
25.Perform buffer exchange into protein storage buffer using PD-10 desalting columns.
-
a.Place PD-10 column in holder for gravity flow protocol provided by manufacturer.
-
b.Equilibrate columns five times with 5 mL of protein storage buffer. Discard flowthrough.
-
c.Add protein solution to column. Discard flowthrough.Note: If volume after ultrafiltration is below 2.5 mL, add appropriate amount of protein storage buffer to column, so total volume added in this step is 2.5 mL.
-
d.Elute protein by adding 3.5 mL of protein storage buffer to the column. Collect flowthrough in this step.
-
a.
-
26.
Determine protein concentration in the eluate by measuring absorbance at 280 nm using a Nanodrop spectrophotometer.
Note: Raw absorbance values are converted into protein concentrations by correcting for the protein-specific extinction coefficient, which depends on the amino acid composition of the protein (Edelhoch relationship). This coefficient can be obtained using the Expasy ProtParam online tool (https://web.expasy.org/protparam/). For caspase-4, the raw absorbance value is to be divided by 0.796. For the in vitro cleavage assay below it is useful to also convert into a molar concentration by dividing by the molecular weight.
-
27.
Concentrate protein to the desired concentration using Amicon Ultra centrifugal filter unit with a 10 kDa size cutoff as described above (we usually aim for a molar concentration of at least 50–100 μM).
-
28.
Aliquot concentrated protein into PCR strip tubes, snap-freeze in liquid nitrogen and store at −80°C.
Pause point: Purified recombinant caspases are stable at −80°C for multiple months.
Figure 2.
Purification of recombinant caspase-4 and pro-IL-1β
(A and B) SDS-PAGE analysis of fractions from stepwise gradient elution of (A) caspase-4 and (B) pro-IL-1β. Fractions containing pure protein of interest are indicated in red.
Overexpression and purification of substrate protein
Timing: 1+ days
The in vitro cleavage assay described below requires purified recombinant protein as a substrate; in this example protocol, full-length murine pro-IL-1β. Pro-IL-1β can be produced and purified using the same procedure described above for caspase-4 (Figure 2B). In the case of pro-IL-1β, purification should result in a single protein of ∼35 kDa (Figure 2B). For protein concentration, we recommend the use of Amicon Ultra centrifugal filter unit with a 10 kDa size cutoff. As described for caspase-4, we discourage the use of protease inhibitors to rule out any interference with downstream assays. If desired, expression and purification of the caspase and the substrate can also be performed simultaneously.
Note: The expression and purification strategy described above has been successfully utilized for a range of substrate proteins including pro-IL-1β, pro-IL-18 or GSDMD. If substrates of interest include a biochemically uncharacterized protein, we recommend using our streamlined protocol described here as a framework, but additional quality control measures might be advised when adapting the protocol to other targets. These include (but are not limited to): Small scale expression cultures to determine if the target protein is soluble or found in inclusion bodies; optimization of temperature and duration of expression culture to increase yields; sampling of flow-through and wash fractions after every step of the purification and checking for presence of protein by SDS-PAGE and Coomassie staining or western blot; performing size-exclusion chromatography of purified product to assess oligomeric state/aggregation.
Perform in vitro cleavage assay
Timing: 1–2 h
Here we describe how to perform an in vitro cleavage assay to quantitatively assess the cleavage of a specific protein substrate (pro-IL-1β) by recombinant caspase-4.
-
29.
Switch on thermocycler instrument and pre-warm to 37°C.
-
30.
Add 10 mM of DTT to an appropriate amount of caspase assay buffer (usually 1–2 mL of buffer per assay).
CRITICAL: DTT should be added fresh to an aliquot of caspase assay buffer right before each assay. Addition of the reducing agent DTT maintains proteolytic activity of the caspase over time by keeping the cysteine residue in the active site in a reduced state.
-
31.
Remove one aliquot of caspase-4 and pro-IL-1β from the −80°C freezer and thaw on ice.
CRITICAL: Avoid freeze/thawing of the recombinant caspase as it will impair its activity. Use a fresh aliquot each time to guarantee consistent results.
CRITICAL: Keep protein solutions and buffers on ice to ensure maintenance of protein activity during assay preparation.
-
32.
Pre-set a PCR cycler to a constant temperature of 37°C.
-
33.
Dilute substrate to 100 nM in caspase assay buffer. Prepare at least ∼250 μL of diluted substrate for each assay.
-
34.
In the first tube of an appropriately labeled 12-tube PCR strip, dilute caspase-4 to 45 μM in caspase assay buffer in a total volume of 60 μL.
Note: Diluted caspase-4 and pro-IL-1β will be mixed at a 1:1 (v/v) ratio, resulting in final concentrations of up to 22.5 μM and 50 nM, respectively. We have found that this range of concentrations works well for this specific caspase/substrate couple. If this assay is adapted to other caspases and/or substrates, caspase concentrations in a higher or lower range might be required for best results.
-
35.
Add 30 μL of caspase assay buffer to residual 11 tubes in the same PCR strip.
-
36.
Perform ten 2-fold serial dilutions in the PCR strip (mix 30 μL of buffer with 30 μL of protein solution from previous tube). The last tube should contain buffer only.
Note: We recommend using a fresh pipette tip for each step of the serial dilution.
-
37.
Add 20 μL of diluted pro-IL-1β (100 nM) to each tube of a second PCR strip.
-
38.
Using a multichannel pipette transfer 20 μL of each caspase-4 dilution to the tubes containing pro-IL-1β. Mix gently by pipetting up and down.
CRITICAL: Adding caspase-4 (and later the SDS loading dye; see below) to all tubes simultaneously with a multichannel allows for synchronized starting and stopping of the reaction and therefore most consistent results.
-
39.
Immediately after mixing the caspase and the substrate, place PCR strip into pre-warmed thermocycler and incubate at 37°C for exactly 30 min.
CRITICAL: Set a timer to ensure proceeding of the reaction for exactly 30 min. If several assays are performed side-by-side, space them out by a regular time interval (e.g., 1 min) and start/stop them sequentially.
-
40.
While reactions are incubating, prepare another PCR strip by adding an appropriate amount of 5× SDS loading buffer into each tube.
Note: You can reuse one PCR strip filled with 5× SDS loading buffer for all your assays. When finished, store PCR strip with residual buffer at −20°C. When performing more assays another time, simply place PCR strip in pre-warmed thermocycler for a few minutes to thaw.
-
41.
To stop the reactions, add 15 μL of 5× SDS loading dye into each tube with a multichannel pipette.
-
42.
Set thermocycler to 65°C for 10 min to denature proteins.
Pause point: Prepared western blot samples can be stored at −20°C for several weeks to months.
Analyze substrate cleavage by western blot
Timing: 2 days
Alternatives: We use western blot analysis to analyze the processing of the substrate by the protease. Alternatively, for example if no specific antibody is available, the protein can also be detected by Coomassie or silver staining (e.g., see Bibo-Verdugo et al., 2020 or Ramirez et al., 20187,8). Detection by western blot, although more laborious, has the advantage that we can use very low amounts of substrate (nanomolar range) and yet achieve good signal to noise images. Using the lowest possible substrate concentration is not only beneficial due to lower sample consumption, but also for accurate quantification. Calculation of Km/kcat from the EC50 (see below) are only accurate if we can assume pseudo-first order conditions, which means that the substrate concentration is negligible compared to Km. Additionally, Coomassie staining visualizes not only the substrate, but also the protease, which complicates data interpretation.
-
43.Separate proteins by SDS-PAGE. We routinely use homemade gels with 12% or 15% acrylamide.
 CRITICAL: When processing of other substrates is investigated, acrylamide concentration of the gels might have to be optimized based on the size of the cleavage products.
CRITICAL: When processing of other substrates is investigated, acrylamide concentration of the gels might have to be optimized based on the size of the cleavage products.-
a.Assemble Mini-Protean gel running chamber.
-
b.Load 5 μL of PageRuler prestained protein ladder (Thermo Fisher) into the first well of the gel.
-
c.Load 5 μL of each sample into residual wells of the gel.
-
d.Run gel at a constant voltage of 130 V for ∼1.5 h.
-
a.
-
44.Transfer proteins onto PVDF membrane. We routinely use a wet transfer protocol in a Mini Trans-Blot cell (Biorad).
-
a.Take gel out of electrophoresis chamber.
-
b.Prepare PVDF membrane by cutting it to the appropriate size and wet with 100% methanol for 30 s.
-
c.Assemble transfer sandwich in a tray filled with transfer buffer in the following order:
-
i.Foam pad.
-
ii.2 layers of filter paper.
-
iii.SDS-PAGE gel.
-
iv.Methanol-soaked PVDF membrane.
-
v.2 layers of filter paper.
-
vi.Foam pad.
-
i.
-
d.Remove air bubbles that may be present between the layers with a roller.
-
e.Place sandwich in wet transfer apparatus.
-
f.Fill wet transfer chamber with transfer buffer.
-
g.Run transfer for 1 h at a constant voltage of 110 V.
-
a.
-
45.
Place the membrane in a small plastic tray and block the membrane by incubating in PBST + 5% milk for 1 h at 20°C–25°C while gently shaking.
-
46.
Rinse the membrane 3 times with PBST.
-
47.
Incubate membrane in 10 mL of primary antibody against pro-IL-1β diluted 1:1000 in PBST + 5% BSA for 18–24 h at 4°C while gently shaking.
-
48.
Remove primary antibody solution and wash membrane 3–5 times with PBST. Incubate for 5 min per wash while gently shaking at 20°C–25°C.
Note: Diluted primary antibody can be collected and reused multiple times with minimal loss of sensitivity. Low concentration (0.02%) of sodium azide can be added to avoid contamination of the stock solution during storage at 4°C.
-
49.
Incubate membrane in 15 mL of HRP-conjugated secondary antibody (here we use goat anti-rabbit IgG (H+L)) diluted 1:5000 in PBST + 5% milk for 1 h at 20°C–25°C while gently shaking.
CRITICAL: Make sure the secondary antibody recognizes the host species of the primary antibody.
-
50.
Remove secondary antibody solution and wash membrane 3–5 times with PBST. Incubate for 5 min per wash while gently shaking at 20°C–25°C.
Note: Unlike the primary antibody solution, we do not routinely reuse the secondary antibody solution to ensure maximum activity of the conjugated HRP enzyme.
-
51.Expose membrane and acquire image.
-
a.Prepare HRP substrate solution by mixing equal volumes of the two components provided.
-
b.Add HRP substrate solution to the membrane (around 1 mL needed for a membrane the size of a regular mini gel; 8.5 cm x 6.5 cm).
-
c.Incubate at 20°C–25°C for 1–5 min.
 CRITICAL: Make sure all parts of the membrane are equally covered with substrate solution.
CRITICAL: Make sure all parts of the membrane are equally covered with substrate solution. -
d.Acquire image using a Chemidoc MP imaging system (BioRad).
 CRITICAL: Exposure time and settings may require optimization to achieve best results. We recommend using the ‘rapid auto exposure’ mode on the ChemiDoc MP system.
CRITICAL: Exposure time and settings may require optimization to achieve best results. We recommend using the ‘rapid auto exposure’ mode on the ChemiDoc MP system.
-
a.
Quantification of western blot images and calculation of kinetic parameters
Timing: 30 min
Here we describe how to perform a densitometric analysis of the western blot images obtained from the in vitro cleavage assay and how to estimate the EC50 and catalytic efficiency kcat/Km of the reaction. As briefly mentioned above, these calculations are only reliable under pseudo-first order conditions. For details on how the equation is derived, see publications by Boucher et al., 2014 or Stennicke and Salvesen, 2000.2,4
Alternatives: This protocol describes how to quantify the obtained western blot images using ImageJ image processing software on a computer with a Windows operating system. Commands may vary slightly for Mac users.
-
52.
Export images from internal hard drive of ChemiDoc system in a .tif format.
-
53.
Open ImageJ image processing software.
-
54.
Upload image in ImageJ by dragging and dropping them into ImageJ window.
-
55.
Select rectangle tool and create a rectangular region of interest (ROI) around band of interest (uncleaved substrate) in the first lane (no caspase control lane).
CRITICAL: Catalytic activity of the caspase can be measured by assessing disappearance of the band for the full-length substrate or by assessing the appearance of the cleavage product band. We generally recommend quantifying disappearance of the full-length band.
CRITICAL: Make sure that ROI is adequately sized to fit all the bands of interest. Width of bands might vary slightly from lane to lane.
-
56.
Save first ROI position using command ‘1’.
-
57.
Move rectangular ROI to the next lane and save second ROI position using command ‘2’.
CRITICAL: Only use sideways arrow keys to move rectangle, as only ROIs with identical y-coordinates can be saved. Also do not change size of rectangle, as all ROIs must have the same size.
-
58.
Repeat for all lanes until all bands of interest are surrounded by rectangular ROIs.
-
59.
Once the last ROI is in place, press ‘3’ to generate mean intensity plots for each ROI in a new window (intensity for each ROI plotted left to right).
-
60.
For each peak, generate a new ROI covering its entire area using the polygon or freehand tool. Measure area of each individual ROI by pressing ‘M’.
CRITICAL: If image has considerable background, only include the area of the peak above the background level when drawing the ROI for this measurement.
-
61.
Plot area values in dependency of the log of the respective caspase concentration in GraphPad prism.
-
62.
Perform a nonlinear regression using the function ‘log(agonist) vs. response (three parameters)’ to obtain EC50 values. This EC50 corresponds to the caspase concentration at which 50% of the substrate has been consumed.
-
63.
Calculate catalytic efficiency of the reaction by using the following equation, where kcat/Km is the catalytic efficiency, EC50 is the value derived from the nonlinear regression and t is the duration of the reaction (in this case 30 min):
Expected outcomes
Using this protocol, we usually obtain 1–5 mg of pure, recombinant human caspase-4 protein from a 500 mL expression culture, which can be used in the described in vitro cleavage assay or for other purposes. The catalytic domain of the caspase is expected to be fully processed into its p10 and p20 subunit, indicating an active caspase species (Figure 3A). When this protocol is applied to other inflammatory caspases or caspase mutants, yields typically vary between a few hundred μg to several mg.
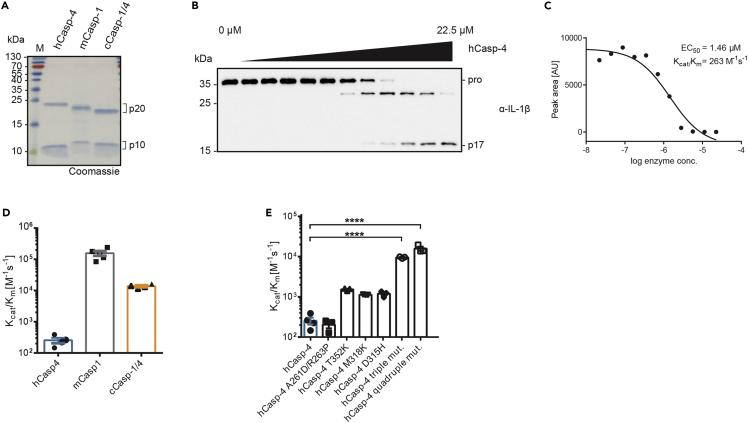
Figure 3.
Analysis of pro-IL-1β cleavage by human caspase-4 in vitro
(A) Recombinant catalytic domains of human caspase-4 (hCasp-4), murine caspase-1 (mCasp-1) and canine caspase-1/4 (cCasp-1/4) were separated by SDS-PAGE and visualized by Coomassie staining.
(B) Representative western blot result for in vitro cleavage of pro-IL-1β by hCasp-4.
(C) Result of quantitative analysis of western blot results displayed in (B).
(D and E) Catalytic efficiencies of pro-IL-1β cleavage for indicated caspases and caspase mutants. Panels (A, D and E) and data used to generate panels B and C of this figure were previously published.1
Data are represented as mean ± SEM of at least three independent experiments. Immunoblot and gel images are representative of at least three independent repeats. Statistical significance was determined one-way ANOVA: ∗∗∗∗p < 0.0001.
The in vitro cleavage assay determines whether a caspase is able to proteolytically cleave a substrate protein within a given range of concentrations and time frame. Image analysis and quantification allows for the estimate of the apparent kinetic efficiency kcat/Km of the reaction. In the case of pro-IL-1β, we observe the appearance of two cleavage products, one transient product at around 30 kDa and a stable fragment at 17 kDa. This pattern indicates sequential cleavage at two sites within the pro-domain of pro-IL-1β (Figure 3B). Our in vitro cleavage assay suggests that caspase-4 performs poorly at processing pro-IL-1β, shown clearly by its EC50 value in the μM range and a low kcat/Km when compared to other inflammatory caspases, such as caspase-1 or canine caspase-1/4 (Figures 3C and 3D). These results validate previous observations suggesting that caspase-4 does not process pro-IL-1β in the context of a cell.9,10,11 This specific example of pro-IL-1β cleavage by caspase-4 also highlights the advantages of our quantitative assay over simpler assays using a single fixed concentration of enzyme and substrate. While such assays provide a binary response (‘cleavage’ vs ‘no cleavage’) our quantitative assay allows the benchmarking of catalytic efficiencies against other known enzymes to estimate physiological relevance of the in vitro results.
In a previous publication, our comparably fast purification and assay protocol, which allows for the testing of several proteins simultaneously, enabled us to screen a large number of caspase-4 mutants. Employing this strategy, we were able to engineer a caspase-4 mutant with considerably higher IL-1β processing activity, providing insights into the molecular determinants of inflammatory caspase substrate specificity (Figure 3E).1
Limitations
We cannot guarantee that our purification protocol will be successful for all inflammatory caspases or caspase mutants of interest. We have had, for example, problems reaching sufficient yields for human caspase-1. In such a case, optimization of the expression system or purification procedure may be necessary (e.g., purification from inclusion bodies).
The in vitro cleavage assay is simple and straightforward, but only provides kcat/Km as an estimate of enzyme kinetics. No information about the exact kcat or Km can be obtained since determination of rate constants requires more laborious measurements of proteolysis at several time points and enzyme concentrations.
Troubleshooting
Problem 1
Purified caspase is not completely processed into p20 and p10 subunit, additional higher molecular weight band is visible in SDS-PAGE gel suggesting presence of uncleaved, inactive caspase catalytic domain (step 22 of step-by-step method details section).
Potential solution
Processing of the caspase usually occurs during the overexpression step of the protocol and is either mediated by the caspase itself or endogenous E. coli proteases. Overexpression conditions (prolonged duration, higher temperature, higher concentration of IPTG) may need to be optimized. If complete processing of the caspase cannot be achieved, exact concentration of active caspase species in the mixture can be determined by active site titration with the caspase inhibitor zVAD-fmk as described previously.3
Problem 2
Protein yield is low (step 26 of step-by-step method details section).
Potential solution
Scale up culture size or optimize overexpression conditions. Make sure cells are properly lysed to release protein of interest; possibly optimize sonication conditions or use alternative lysis method (e.g., a French press).
Problem 3
All (or none) of the substrate is cleaved in all the tested conditions (step 51 of step-by-step method details).
Potential solution
Optimize range of caspase concentrations used in the assay. If substrate is cleaved in all condition (except substrate only control), choose lower caspase concentrations. If no cleavage is observed even at very high caspase concentrations, it may be that the tested protein is a poor substrate for this specific caspase. Check literature for evidence that protein is a bona fide substrate for your caspase of interest.
Problem 4
Substrate of interest cannot be purified (‘Overexpression and purification of substrate protein’ in step-by-step method details section).
Potential solution
This type of in vitro cleavage assay can be adapted for the use with unpurified substrates present in crude cell lysates.4,12 This eliminates the requirement for purification but complicates the interpretation and analysis of the data, as other caspase substrates might be present in the lysates.
Problem 5
Nonlinear fit does not accurately represent the data (step 62 in step-by-step method details section).
Potential solution
This type of fit requires plateaus before and after the EC50. Ensure that several data points are available before and after the EC50. In some cases, the fit can be improved by including constraints on the bottom and top parameters (e.g., constraining bottom value to 0, or top value to the value observed in the no caspase control lane). Nevertheless, constraining the data may also introduce analysis artifacts. We recommend to always check that the calculated EC50 accurately represents the data (compare EC50 estimate to raw image).
Possibly repeat the assay with optimized range of concentrations around the estimated EC50.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Jonathan C. Kagan (Jonathan.kagan@childrens.harvard.edu).
Materials availability
Plasmids generated in this study will be made available on request or were deposited to Addgene (Addgene IDs: 183381, 183386).
Acknowledgments
We thank the members of the Kagan Laboratory for helpful discussions. This work was supported by NIH grants AI133524, AI093589, AI116550, and P30DK34854 to J.C.K. P.D. was supported by a fellowship by the Boehringer Ingelheim Fonds.
Author contributions
P.D. conceived the study, performed experiments, and wrote the manuscript. J.C.K. conceived the study, supervised the work, and edited the manuscript.
Declaration of interests
J.C.K. consults and holds equity in Corner Therapeutics, Larkspur Biosciences, and Neumora Therapeutics. None of these relationships influenced the manuscript.
Contributor Information
Pascal Devant, Email: pdevant@g.harvard.edu.
Jonathan C. Kagan, Email: jonathan.kagan@childrens.harvard.edu.
Data and code availability
This study did not generate any datasets or code.
References
- 1.Devant P., Cao A., Kagan J.C. Evolution-inspired redesign of the LPS receptor caspase-4 into an interleukin-1β–converting enzyme. Sci. Immunol. 2021;6:eabh3567. doi: 10.1126/sciimmunol.abh3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stennicke H.R., Salvesen G.S. Caspase assays. Methods Enzymol. 2000;322:91–100. doi: 10.1016/S0076-6879(00)22010-7. [DOI] [PubMed] [Google Scholar]
- 3.Denault J.B., Salvesen G.S. Expression, purification, and characterization of caspases. Curr. Protoc. Protein Sci. 2002;Chapter 21 doi: 10.1002/0471140864.ps2113s30. Unit 21.13. [DOI] [PubMed] [Google Scholar]
- 4.Boucher D., Duclos C., Denault J.-B. General in vitro caspase assay procedures. Methods Mol. Biol. 2014;1133:3–39. doi: 10.1007/978-1-4939-0357-3_1. [DOI] [PubMed] [Google Scholar]
- 5.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kielkopf C.L., Bauer W., Urbatsch I.L. Purification of polyhistidine-tagged proteins by immobilized metal affinity chromatography. Cold Spring Harb. Protoc. 2020;2020 doi: 10.1101/pdb.prot102194. pdb.prot102194. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez M.L.G., Poreba M., Snipas S.J., Groborz K., Drag M., Salvesen G.S. Extensive peptide and natural protein substrate screens reveal that mouse caspase-11 has much narrower substrate specificity than caspase-1. J. Biol. Chem. 2018;293:7058–7067. doi: 10.1074/jbc.RA117.001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibo-Verdugo B., Snipas S.J., Kolt S., Poreba M., Salvesen G.S. Extended subsite profiling of the pyroptosis effector protein gasdermin D reveals a region recognized by inflammatory caspase-11. J. Biol. Chem. 2020;295:11292–11302. doi: 10.1074/jbc.RA120.014259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker P.J., Boucher D., Bierschenk D., Tebartz C., Whitney P.G., D’Silva D.B., Tanzer M.C., Monteleone M., Robertson A.A.B., Cooper M.A., et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur. J. Immunol. 2015;45:2918–2926. doi: 10.1002/eji.201545655. [DOI] [PubMed] [Google Scholar]
- 10.Rühl S., Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K + efflux. Eur. J. Immunol. 2015;45:2927–2936. doi: 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- 11.Schmid-Burgk J.L., Gaidt M.M., Schmidt T., Ebert T.S., Bartok E., Hornung V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur. J. Immunol. 2015;45:2911–2917. doi: 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- 12.Boucher D., Blais V., Denault J.-B. Caspase-7 uses an exosite to promote poly(ADP ribose) polymerase 1 proteolysis. Proc. Natl. Acad. Sci. USA. 2012;109:5669–5674. doi: 10.1073/pnas.1200934109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any datasets or code.