Abstract
The application of three-dimensional (3D) printing has been increasing and we invented cost-effective and time-saving 3D printed model of intra-abdominal cavity which was utilized in liver transplantation (LT) to prevent large-for-size syndrome. 3D printings were performed on potential adult recipients with small cavity and pediatric patients scheduled for transplantation during July 2020 – September 2021. Based on the computed tomography of the recipient, the inner surface of the abdominal cavity was outlined. The line was marked with a distance of 1 – 3 cm. Then, the outlined data were reconstructed as a 3D model and printed by a fused deposition modeling type 3D printer with a thickness of 2 mm. Pillars and footings for holding the lines were printed and assembled altogether. During deceased donor organ procurement, the size of the graft was compared to that of the printed model. For living donor LT, preoperatively planned liver graft was printed and was physically placed into the 3D printed abdominal cavity. All the 16 cases with 3D printed abdominal cavity showed appropriate fitting of the donor’s liver graft to both the 3D printed model and actual recipient’s abdominal cavity with no large-for-size syndrome after LT. Median time for manufacturing the model was 576 min (IQR 434 – 680) and estimated median cost for the filament was US$ 1.6 (IQR 1.2 – 1.7). The 3D printed abdominal cavity model can be manufactured in <10 h and was useful for preventing large-for-size syndrome in small-sized recipients.
Keywords: 3D printing, Large-for-size syndrome, Liver transplantation, 3D printer
1. Introduction
Three-dimensional (3D) printing, also known as rapid prototyping or additive manufacturing, is one of the rapidly developing technologies. At present, diverse techniques for 3D printing have been developed which were outlined by the ISO/ASTM standard: Binder jetting, material jetting, material extrusion, powder bed fusion, directed energy deposition, sheet lamination, and vat photopolymerization[1-5]. The application of 3D printing has also been gradually increasing in the medical field due to its potential as a personalized medical tool, especially in maxillofacial and craniofacial surgery[6,7]. Recently, patient-specific 3D models of liver were utilized to investigate the relationship between liver tumors and anatomical structures including hepatic vasculatures and bile ducts[8]. Despite its benefits on enabling a better anatomical understanding and more precise preoperative planning[9], 3D printing is expensive and time-consuming, which limits commercialization of the technology.
During liver transplantation (LT), using large-sized grafts may result in difficult abdominal closure as well as graft compression, followed by poor oxygen supply and graft dysfunction[10]. Large-for-size syndrome is not common but can occur with fatal outcome in pediatric LT from living donors and in whole LT from deceased donor in adults with small abdominal cavity. Therefore, we created a 3D printed model of abdominal cavity of recipients based on the idea that large-for-size syndrome could be prevented in advance through size comparison between the recipient’s abdominal cavity and the donor liver graft. The most important features of techniques and biomaterials to have for 3D printing of intra-abdominal cavity are: (i) Harmless to human body since it is used during surgery, (ii) strong and rigid enough to maintain its shape while fitting the liver graft to the 3D printed model, and (iii) can be readily utilized in emergency operation. Therefore, we decided to use fused deposition modeling (FDM)-based 3D printing technique and polylactic acid material, and created 3D printed models of LT recipients’ abdominal cavity and utilized them during LT with a potential for large-for size syndrome. Our 3D model has advantages of low cost and fast production time compared to previous 3-D printed models used in LT. This study is designed to describe methods for manufacturing 3D printed abdominal cavity model and evaluate the usefulness of our 3D printed model in actual practice.
2. Methods
2.1. Patient selection for 3D printing of abdominal cavity
A total of 16 patients who underwent LT at Samsung Medical Center using 3D printed abdominal cavity model between July 2020 and September 2021 were enrolled in this study. In living donor LT (LDLT), expected graft-recipient weight ratio (GRWR) exceeding 2% was selected for 3D printing. For patients on the waiting list for deceased donor matching, criteria for printing a 3D model were as follows: (i) Female recipient who is not 10 cm taller than allocated male donor, (ii) male recipient who is ≥10 cm shorter than allocated female donor, (iii) same sex between donor and recipient while recipient is ≥10 cm shorter than the donor, and (iv) small right liver fossa which can be measured as anteroposterior (AP) length of ≤13 cm or lateral space from inferior vena cava to be ≤10 cm. Besides these criteria, the selection was based on the transplant surgeon’s judgment especially when the recipient had deformity of the abdominal cavity of which the above-mentioned criteria cannot be applied.
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2020-07-118). Informed consent was acquired from the recipients who were enrolled prospectively after approval of the Institutional Review Board; for minors, informed consent was obtained from their parents or legal guardians. The research was performed in accordance with relevant guidelines/regulations which are in accordance with the Declaration of Helsinki. Both recipients and donors were not recruited from prisons.
2.2. 3D modeling of the recipient’s abdominal cavity
Based on the computed tomography (CT) of the recipient, the abdominal cavity where the graft liver would be placed was masked using Mimics Medical 21.0 (Materialise, Leuven, Belgium). For efficient modeling and printing, the inner surface of the abdominal cavity was outlined with a 1 – 3 cm distance between slices. While the distance between the printed lines was longer in adult recipients, the distance was relatively shorter in pediatric recipients. The anterior wall of the abdominal cavity was marked on the peritoneal side of the anterior abdominal wall. The line was continued to the lateral wall outlining the peritoneal surface. The posterior wall of the abdominal cavity consisted of perirenal fat surrounding the kidney. The midline of the abdominal cavity was outlined along the inferior vena cava and abdominal aorta. The medial two-third of the anterior wall outline was removed with a vertical marking that pointed out the anterior limit of the abdominal cavity. While only the right hemi-abdomen was outlined in adult recipients, both right and left hemi-abdomen were outlined in pediatric recipients and adult recipient who were planned for left LT (Figure 1A and B). The outline was designed to be printed with 2 mm thickness.
Figure 1.
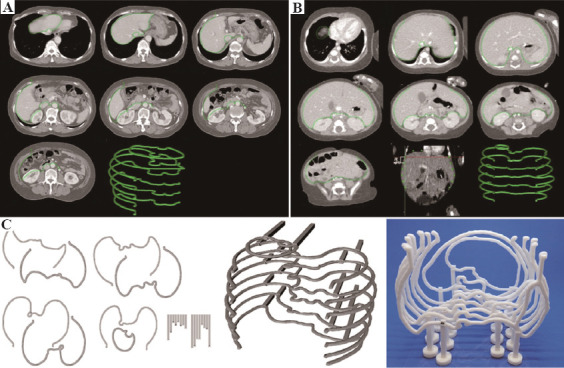
Manufacturing flow for the 3D printed abdominal cavity. (A) For adult patients, only the intra-abdominal cavity of the right hemi-abdomen is outlined with a slice distance of 2–3 cm based on the computed tomography. (B) For pediatric patients and adult patients who are planned to receive left liver, the whole intra-abdominal cavity is outlined based on the computed tomography. The slice distance was 1–2 cm in pediatric patients. (C) The outlines were modeled and printed with a thickness of 2 mm and assembled with a pillar and footing to manufacture a cost-effective and time-saving model of intra-abdominal cavity.
After marking the outline of the intra-abdominal cavity, the data were manipulated using Cinema 4D (Maxon, Friedrichsdorf, Germany). Two pillars for supporting each object were designed to fit on the pre-printed footing that looks like a Korean chess piece with a square hole in the middle. The 3D model was printed using Cubicreator software and Cubicon Single Plus (Cubicon, Seong nam, Republic of Korea), which is a FDM Type 3D printer. After printing the models, post-printing procedure for assembling the parts was performed. Based on the blueprint where the exact marking of each footing’s location was printed, the 3D model of the intra-abdominal cavity was reassembled on a transparent acrylic panel (Figure 1C).
2.3. Data acquisition and statistical analysis
Demographical data of the donor and recipient were collected. In addition, data of graft, recipients’ abdominal cavity, and 3D model were collected. The graft data include the type of graft, weight, and GRWR. We measured AP and lateral length of the right liver fossa as well as AP length of midline of recipients to roughly estimate the abdominal cavity data based on CT of recipients before LT. 3D model data include amount of materials, cost, and manufacturing time needed for 3D modeling.
The clinical decision made by the surgeons, whether the team maintained or changed the initial plan, was collected. The clinical course before and after using the 3D printed model was collected. Whether the actual graft fit appropriately inside the 3D printed model was checked by putting the graft after back-table procedure. For putting the graft inside the 3D model with a sterile measure, the printed model was covered twice with a Steri-Drape™ (3M Science, Saint Paul, MN). After placing the graft inside the sterile plastic bag-covered 3D model, whether the graft fit into the right hemi-abdomen was evaluated with AP diameter of the right hemi-abdomen and lateral distance between the peritoneum and the inferior vena cava. The location of the inferior vena cava of the graft and the 3D model was also evaluated. Finally, the anterior side of the graft and the anterior peritoneal wall was evaluated for fitness (Figure 2). The time spent during modeling, printing, and assembling the 3D printed model was collected. The amount of filaments used for 3D printing and the estimated cost of the filaments used were calculated.
Figure 2.
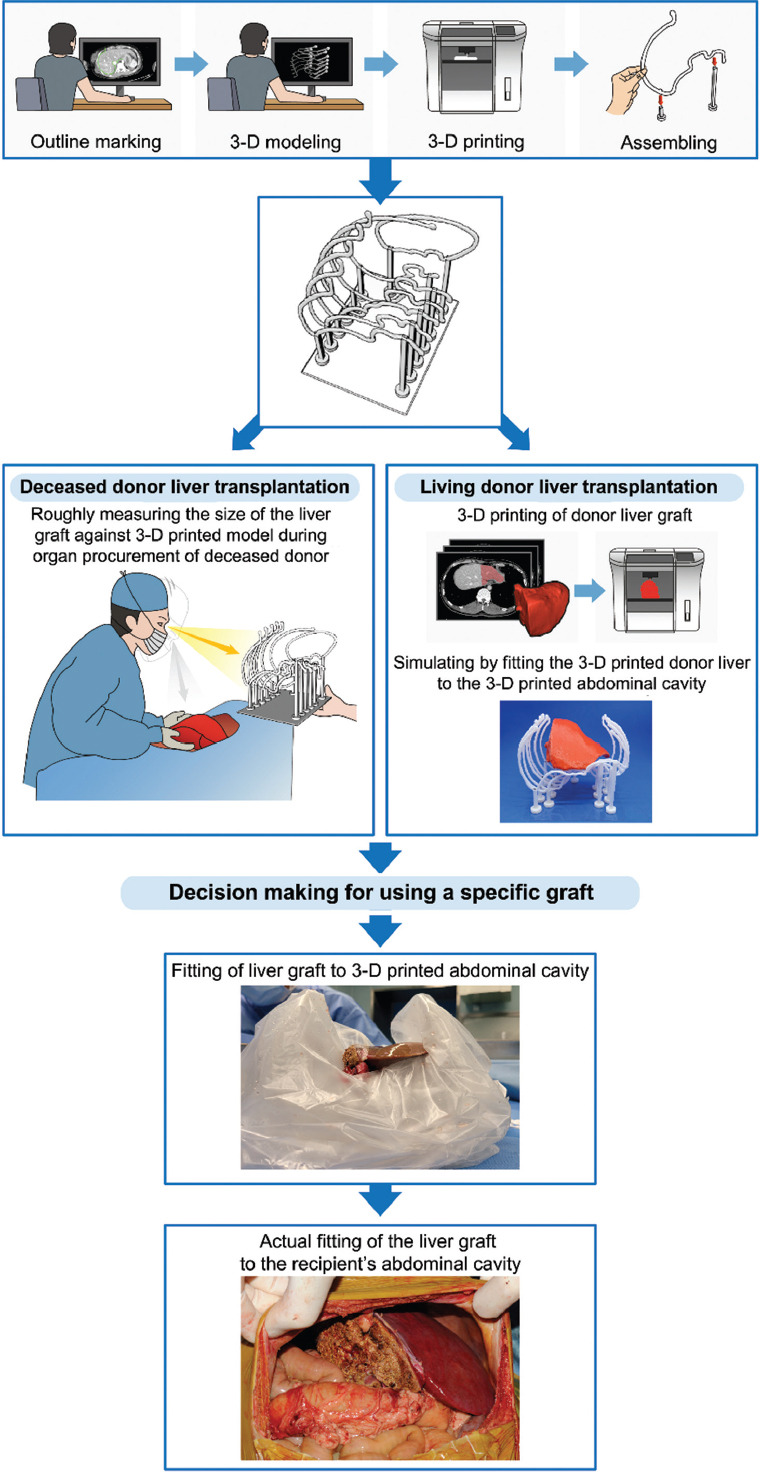
Workflow of manufacturing the 3D printed abdominal cavity model and its application to clinical practice in both living donor and deceased donor liver transplantation.
Data with normal distribution are expressed as mean ± standard deviation, while data that do not show normal distribution are expressed as median and interquartile range (IQR). Comparison in 3D modeling and printing data between the adult and the pediatric recipients was performed using Mann–Whitney U test and Fisher’s exact test. Statistical analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Patient characteristics
During the study period, a total of 16 cases of LT were performed using a 3D printed abdominal cavity model. These recipients included ten adult recipients and six pediatric recipients. The median height of adult recipients was 161.0 cm (IQR 158.0 – 163.8) and that of pediatric recipients was 70.0 cm (IQR 60.8 – 116.8). The median weights of adult and pediatric recipients were 56.5 kg (IQR 49.9 – 62.5) and 7.6 kg (IQR 7.0 – 22.0), respectively.
3.2. Data related to 3D printing
The mean total time for manufacturing the 3D printed model was 576 min (IQR 434 – 680). Mean times for modeling, printing, and assembling were 105 min (IQR 90 – 142), 418 min (IQR 276 – 488), and 60 min (IQR 50 – 70), respectively. Median amount and cost of filaments used for single case of printing were 62.5 g (IQR 46.2 – 65.8) and US$ 1.6 (IQR 1.2 – 1.7), respectively.
3.3. Comparison between adult and pediatric recipients
The comparisons between adult and pediatric patients are summarized in Table 1. Eight out of 10 adult recipients (80.0%) and 4 out of 6 pediatric recipients (66.7%) were female. The median age of adult recipients was 43.5 years (IQR 32.8 – 58.2) and that of pediatric recipients was 1.1 year (IQR 0.5 – 6.4, P = 0.001). The types of liver grafts transplanted for the ten adult recipients were whole liver grafts (n = 7) and reduced extended right hemi- liver (n = 1) from deceased donors while right hemi-liver (n = 1) and extended left hemi-liver (n = 1) were donated from living liver donors who were family and relative of the recipients. All pediatric recipients received partial livers and five of them received graft from living donors who were their parents while one received graft from deceased donor (P = 0.011). The types of liver grafts were extended left lateral grafts (n = 2), reduced extended left lateral grafts (n = 2), reduced S2 mono-segment graft (n = 1), and left hemi-liver (n = 1). The median GRWR of adult recipients and pediatric recipients was 2.40 (IQR 1.90 – 2.60) and 2.90 (IQR 2.40 – 3.30), respectively (P = 0.104).
Table 1.
Comparison of donor, recipient, graft, and printing-related data between adult and pediatric group
| Variables | Adult group (n=10) | Pediatric group (n=6) | P-value | |
|---|---|---|---|---|
| Recipient - related | Recipient sex (M/F, male%) | 2/8 (20.0) | 2/4 (33.3) | 0.604 |
| Median age of recipients (years) | 43.5 (32.8–58.2) | 1.1 (0.5–6.4) | 0.001 | |
| Median height of recipients (cm) | 161.0 (158.0–163.8) | 70.0 (60.8–116.8) | 0.001 | |
| Median weight of recipients (kg) | 56.5 (49.9–62.5) | 7.6 (7.0–22.0) | 0.001 | |
| Median length of (mm) | ||||
| AP right liver fossa | 148.4 (136.3–169.3) | 109.6 (86.9–122.8) | 0.003 | |
| Lateral right liver fossa | 86.0 (80.1–96.9) | 51.1 (48.2–65.2) | 0.002 | |
| AP midline | 96.4 (74.1–114.3) | 75.8 (62.5–79.9) | 0.083 | |
| Donor - related | Donor type (Living/deceased, living%) | 2/8 (20.0) | 5/1 (83.3) | 0.035 |
| Donor sex (M/F, male %) | 5/5 (50.0) | 5/1 (80.0) | 0.307 | |
| Median age of donors (years) | 51.5 (35.5–52.0) | 32.5 (29.5–40.8) | 0.064 | |
| Median height of donors (cm) | 161.0 (158.2–169.5) | 172.5 (167.5–179.0) | 0.057 | |
| Median weight of donors (kg) | 62.5 (60.2–68.0) | 76.0 (56.5–86.5) | 0.277 | |
| Graft - related | Graft type (Whole/partial, whole %) | 7/3 (70.0) | 0/6 (0.0) | 0.011 |
| Median graft weight (g) | 1320 (1001–1444) | 256 (211–318) | 0.002 | |
| Median graft-recipient weight ratio (%) | 2.4 (1.9–2.6) | 2.9 (2.4–3.3) | 0.104 | |
| Printing - related | Printing of | |||
| Whole abdomen | 1 (10.0) | 6 (100.0) | 0.001 | |
| Right hemi-abdomen | 9 (90.0) | 0 (0) | ||
| Median slice thickness of 3D model (mm) | 20.0 (18.5–20.0) | 15.0 (12.2–15.0) | 0.003 | |
| Median amount of materials used (g) | 62.8 (47.9–65.2) | 60.8 (56.8–64.8) | 0.664 | |
| Median cost for material used (US Dollars) | 1.58 (1.20–1.64) | 1.53 (1.43–1.63) | 0.664 | |
| Median modeling time of 3D model (min) | 90.0 (90.0–112.5) | 150.0 (127.5–150.0) | 0.033 | |
| Median printing time of 3D model (min) | 418.0 (249.8–488.2) | 382.5 (321.5–435.2) | 0.744 | |
| Median assembling time of 3D model (min) | 60.0 (51.2–63.8) | 65.0 (52.5–77.5) | 0.476 | |
| Median total time for manufacturing (min) | 568.0 (407.2–630.8) | 649.0 (589.2–686.2) | 0.103 |
3D, Three-dimensional; AP, Antero-posterior
Among the ten adult patients, 9 patients (90.0%) were printed with the right hemi-abdomen while 1 patient (10.0%) who received extended left liver was printed with both hemi-abdomens. In contrast, all the pediatric recipients were printed with whole abdomen 3D model (P = 0.001). The median amount of materials used to create the 3D printed abdominal cavity model was 62.8 g (IQR 47.9 – 65.2) and 60.8 g (IQR 56.8 – 64.8) for adults and pediatrics, respectively (P = 0.664). The median cost of materials required to manufacture the 3D model was US$ 1.58 and US$ 1.53 for adult and pediatric patients, respectively (P = 0.664). The total median time spent to create 3D abdominal cavity model was 568.0 min (IQR 407.2 – 630.8, P = 0.103) in adult patients and 649.0 min (IQR 589.2 – 686.2, P = 0.103) in pediatric patients.
3.4. Cases of 3D printed model proven to be beneficial
The characteristics and the outcome after using 3-D printed abdominal cavity model of each patient are shown in Table 2. Figure 3 summarizes specific situations when the 3D abdominal cavity model can be helpful for the surgeon to make a decision.
Table 2.
Case information of 3D printed abdominal cavity including the recipient’s and donor’s characteristics and clinical outcome
| Recipient | Donor | Outcome | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| No. | Sex/age | Height/weight (cm/kg) | Abdominal cavity (cm) (AP of right liver fossa/lateral of right liver fossa/AP of midline) | Cause of liver disease and brief history | MELD/PELD | Sex/age | Height/Weight (cm/kg) | Donor type | Graft type | Graft weight (g)/GRWR (%) | Aborted trans plantation | Fitting (3D - printed model/patient) | Clinical course |
| 1 | F/44 | 158/43 | 13.5/9.6/7.0 | Alcoholic cirrhosis and graft failure due to noncompliance after LDLT | 39 | M/66 | 173/60 | DD | Reduced extended right | 947/2.20 | +/+ | Expired due to invasive aspergillus pneumonia | |
| 2 | F/41 | 158/63 | 16.0/8.8/11.7 | HCC-autoimmune; graft failure due to hepatic artery occlusion after LDLT | 40 | F/31 | 158/64 | DD | Whole | 1580/2.51 | First matched deceased donor due to large size | +/+ | Successful transplantation from second matched deceased donor |
| 3 | F/6m | 63/7 | 9.7/4.8/7.6 | Liver failure although Kasai operation due to biliary atresia has been performed | 34 | M/44 | 181/82 | LD | Reduced extended left lateral | 240/3.43 | +/+ | Successful transplantation of reduction graft (240 g from 305 g) | |
| 4 | F/8m | 56/5.4 | 8.3/4.8/5.8 | Fulminant hepatitis of unknown origin; preterm (GA 24+5wks) with bronchopulmonary dysplasia | 59 | F/31 | 166/52 | LD | Reduced extended left lateral | 151/2.80 | +/+ | Successful transplantation of reduction graft (151 g from 236 g) | |
| 5 | F/43 | 164/58 | 14.0/8.0/7.9 | Alcoholic cirrhosis | 33 | F/26 | 159/69 | DD | Whole | 1450/2.50 | First matched deceased donor due to large size | +/+ | Successful transplantation |
| 6 | F/50 | 166/70 | 14.2/8.3/9.3 | Toxic hepatitis due to drug intoxication | 37 | M/34 | 158/65 | DD | Whole | 1281/1.83 | +/+ | Successful transplantation | |
| 7 | F/71 | 155/61 | 17.2/9.8/12.3 | HCC, hepatitis B | 27 | F/52 | 156/61 | DD | Whole | 1584/2.60 | +/+ | Successful transplantation | |
| 8 | M/30 | 163/49 | 17.7/9.7/10.5 | Hepatitis B; chronic graft failure after LDLT leading to portal vein thrombosis | 40 | M/51 | 170/81 | DD | Whole | 1425/2.91 | +/+ | Successful transplantation (initial plan was to use a reduction graft) | |
| 9 | F/8 | 132/27 | 12.3/7.5/7.4 | Wilson’s disease | 26 | M/43 | 173/52 | LD | Extended left lateral | 333/1.23 | +/+ | Successful transplantation | |
| 10 | M/4m | 59/6.8 | 8.2/4.6/5.8 | Ornithine transcarbamylase deficiency | 25 | M/29 | 166/88 | LD | Reduced from S2 graft | 201/2.96 | +/+ | Successful transplantation | |
| 11 | M/19m | 77/8.1 | 12.2/5.3/8.4 | Alagille syndrome | 22 | M/34 | 186/70 | LD | Extended left lateral | 272/3.36 | +/+ | Successful transplantation | |
| 12 | F/25 | 162/55 | 12.5/7.3/6.7 | Drug intoxication of chronic alcoholic | 40 | F/52 | 162/61 | LD | Right | 680/1.17 | +/+ | Successful transplantation | |
| 13 | F/61 | 160/44 | 11.1/7.9/7.2 | Hepatitis C; contracture of right liver fossa due to chronic empyema of the right hemithorax | 28 | F/67 | 160/58 | LD | Extended left | 399/0.97 | First matched deceased donor due to large size | +/+ | Successful transplantation |
| 14 | M/25 | 176/67 | 19.2/7.7/12.4 | Chronic liver failure after LDLT; Kasai operation has been previously done due to biliary atresia | 40 | M/52 | 185/77 | DD | Whole | 1358/2.88 | +/+ | Successful transplantation | |
| 15 | F/8 | 130/28.8 | 12.3/6.9/8.1 | Wilson’s disease | 22 | M/16 | 172/94 | DD | Left hemiliver | 657/2.28 | +/+ | Successful transplantation | |
| 16 | F/62 | 152/52.5 | 15.4/9.7/9.9 | Wilson’s disease | 40 | M/40 | 168/50 | DD | Whole | 1164/2.22 | +/+ | Successful transplantation | |
AP, Antero-posterior; DD, Deceased donor; HCC, Hepatocellular carcinoma; LD, Living donor; LDLT, Living donor liver transplantation
Figure 3.
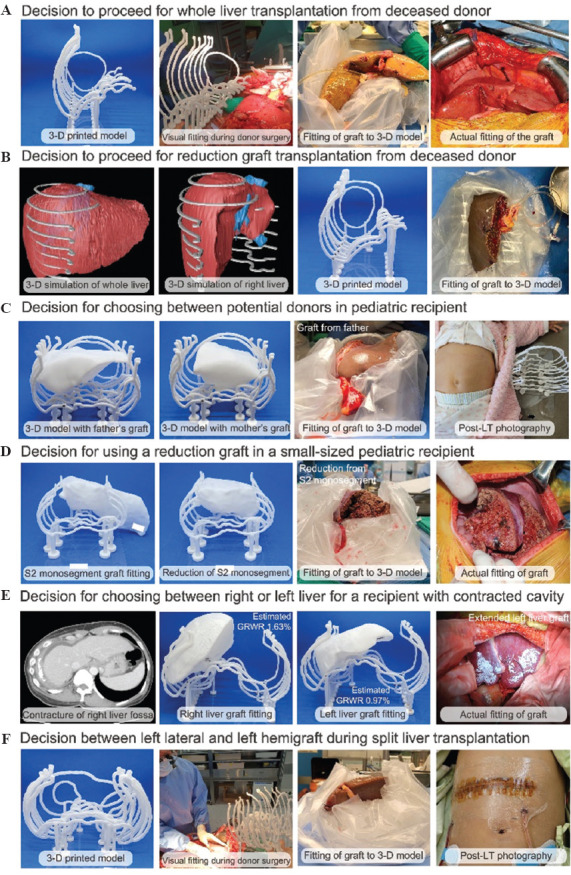
Specific situations where 3D printed abdominal cavity model can be clinically beneficial during liver transplantation and some example cases. (A) Decision to proceed with whole liver transplantation from deceased donor in a patient with small abdominal cavity. (B) Decision to proceed with reduction graft transplantation from deceased donor when the whole liver graft is too large for the recipient. (C) Decision for choosing between different donors in pediatric recipient. (D) Decision for using a reduction graft in a pediatric recipient. (E) Decision for selecting a donor and graft for a recipient with a spatial deformity in the liver fossa. (F) Decision for choosing between left lateral or left hemigraft during deceased donor liver procurement for split liver transplantation.
A 71-year-old female of 155 cm and 61 kg received a whole liver graft from a female deceased donor (height: 156 cm and weight: 61 kg). During the donor organ procurement, the donor liver graft and the patient’s 3D abdominal cavity model were visually compared to confirm whether the size of the graft was appropriate, then the liver graft was placed inside the 3D model after back-table procedure. The graft was actually well-fitted to the patient’s abdominal cavity and the LT was successfully completed (Figure 3A, Case No. 7 in Table 2). While the body size of the donor and recipient was similar in this case, female recipients with a small body size are always under the risk of large-for-size syndrome since allocation can be matched to male donor. Therefore, in our center, female patients with high model for end-stage liver disease (MELD) scores with a potential for being matched to a deceased donor were prepared for 3D model printing in case of donor match.
A 44-year-old female of 158 cm and 43 kg had alcoholic liver cirrhosis and underwent graft failure due to non-compliance after LDLT. The initial plan was for her to receive a whole liver graft from a male donor (height: 173 cm and weight: 66 kg). However, after visual comparison to the 3D printed model, the size of the graft seemed too large to fit into the recipient’s abdominal cavity. Therefore, we decided to use reduced extended right hemi-liver graft, and the graft fitted well with no large-for-size syndrome (Figure 3B, Case No. 1 in Table 2).
A 6-month-old boy who underwent Kasai operation due to biliary atresia eventually had liver failure; therefore, his father and mother were examined to see if they could serve as living donors. The estimated liver volumes of father and mother were 232 cm3 and 201 cm3, respectively, and GRWRs were 3.32 and 2.87, respectively. After comparing the 3D printed liver grafts of both candidates with the recipient’s 3D printed abdominal cavity, we finally decided to use liver graft from the recipient’s father. During the back-table procedure, actual liver graft was placed in the 3D abdominal cavity model and reduction of the graft liver was done so that it fits perfectly in the recipient’s actual abdominal cavity (Figure 3C, Case No. 3 in Table 2).
A 4-month-old boy with acute liver failure due to ornithine transcarbamylase deficiency was initially planned to receive S2 monosegment graft from his father. The estimated S2 monosegment graft volume and GRWR were 194 cm3 and 3.03 cm3, respectively. As shown in Figure 3D, 3D printed liver graft was too large to fit into the 3D printed abdominal cavity. The newly produced 3D printed reduced graft seemed suitable, and the actual reduced liver graft fit perfectly into the 3D abdominal cavity model, leading to successful transplantation to the recipient (Figure 3D, Case No. 10 in Table 2).
A 61-year-old female with liver cirrhosis due to hepatitis C had chronic empyema of the right hemi-thorax, which was followed by contracture of the right liver fossa. Because of the extremely small abdominal cavity, the first matched deceased donor graft was aborted after comparing it with 3D printed abdominal cavity model. LDLT was planned, and 3D printed right liver graft and left liver graft model were printed to choose a proper graft for the recipient’s abdominal cavity. The GRWRs of the right and left hemi-livers were 1.63 and 0.97, respectively. When we fit the printed graft to the printed abdominal cavity, the right hemi-liver graft seemed too large to be transplanted. Therefore, we decided to use extended left liver graft for transplantation and the operation was successfully carried out (Figure 3E, Case No. 13 in Table 2).
An 8-year-old female with Wilson’s disease was allocated for split LT from a 16-year-old male donor who had a weight of 94 kg. The 3D printed model was used during procurement to guide the surgeon to choose between using left lateral graft and left hemigraft. The team decided to use a left hemiliver which weighted 657 g and GRWR was measured to be 2.28%. Split LT for pediatric recipients is considered to be the most valuable circumstance that the model can be useful (Figure 3F, Case No 15. in Table 2).
4. Discussion
Large-for-size syndrome is a rare but devastating condition that can interfere with the survival of both the graft and the recipient[11]. During deceased donor LT (DDLT), the size mismatch between the donor’s liver and recipient’s abdominal cavity can occur due to the limited evaluation of both the donor and recipient[12]. Although the chance is low, since CT scan is not a routine evaluation procedure for deceased donors in Republic of Korea for protecting the kidney from contrast-induced kidney injury, there is a risk for the occurrence of large-for-size syndrome especially in small female recipients matched to male donors. The decision to perform LT using the graft or to reduce the graft should be decided based on the understanding of the size of the recipient’s abdominal cavity. However, in most cases, the donor and recipient are operated in different hospitals. Therefore, the donor surgeon should decide whether the graft is in adequate size based on the visual examination of the graft liver without actual visual comparison of the recipient’s abdominal cavity. The rarity of large-for-size syndrome justifies the limited number of published studies[13]. By calculating the diameter of the right hemi-abdomen where the liver will be placed can be helpful for the decision. However, while experienced surgeons can manage to perform adequate decision making with limited information, surgeons in their learning curve need more assistance not to make a mistake during the decision process. Therefore, we managed to utilize 3D printing technology to build a 3D printed model of the intra-abdominal cavity to its actual size.
3D printing technology has been applied in the field of liver surgery and several studies have been published[8,14]. However, most studies focused on liver malignancy to print the cancer on its actual location in relation to the adjacent anatomical structures[15-17]. These approaches seem valuable as it can print the liver and cancer mass with the exact size. However, since 3D reconstruction without 3D printing can also give an advanced view to the surgeon, whether 3D printing significantly enhances the surgeon’s insight is questionable, especially when time and cost of the 3D printing are taken into account. 3D printing in LT was first introduced in the literature of Zein et al.[18]. In the study, the surgical team printed the graft liver as well as the recipient’s original liver and compared them to the actual livers. The study showed high level of resemblance of the 3D printed model to the graft. However, how to use the technology was up to the clinicians. The study published by Wang et al.[19] showed that 3D printing technology can be used in pediatric LT for surgical planning. The surgical team printed the abdominal cavity and planned liver graft and evaluated whether the surgery can be performed safely. The outcome showed that 3D printing can be practically used during clinical practice. However, the study focused on recreating the abdominal cavity as realistic as possible and the reported time for manufacturing was about two days in printing the model half the size of the actual abdominal cavity.
Our 3D printed model was originally planned to be used for small donors who might accept large liver graft during DDLT. Therefore, the key to success was to print the model as fast as possible with low cost. We prepared 3D printed model of the intra-abdominal cavity in advance for the patients with high MELD scores, who are expected to undergo DDLT. Nevertheless, there are times when allocation and transplantation occur abruptly. Case No. 14 (Table 2) was an example of which the transplant surgeon requested for a 3D printed model 6 h before surgery. Therefore, we planned a 3D model with a wider slice distance to reduce printing time. This case showed the possibility that our model could be much more time-saving in the future.
Three cases were performed successfully with modification of the 3D printed model. During the three cases, one case required a reduction graft which fit perfectly to the small abdominal cavity. After the three cases, we designed a prospective study to use the 3D printed model for potential adult recipients in the waiting list with small intra-abdominal cavity and pediatric LT recipients. The goal of our 3D printed model was not to mimic every detail of the human body or liver graft, but only to focus on giving the surgeon the idea of the actual size of cavity and graft. The 3D printed model was used for comparing the size of the graft to the size of the recipient’s intra-abdominal cavity during organ harvest operation from deceased donor. Decision to either receive whole liver or just part of the liver or to withdraw the chance was made by the donor surgeon with the assistance of the 3D printed model. After back-table procedure, evaluation for the fitness was carried out, and every case fitted properly to the 3D model as well as the actual recipient’s cavity.
Pediatric LT cases were also good candidates for 3D printing. Unlike adult recipients, 3D printing was performed on both hemiabdomens. Liver grafts, whether they were left hemiliver, extended left lateral liver, or reduced liver graft of extended left lateral liver, were printed as per the surgeon’s plan.
The case presented in Figure 3E was a perfect situation where 3D printed model can be beneficial. In a patient with a distorted space, well-matched GRWR can be a misleading factor that can lead to devastating situation of large-for-size syndrome. The space of the patient’s right liver fossa which underwent contracture after chronic empyema was somewhat similar to the 8-year-old female recipient’s liver fossa (Table 2). The GRWR of the donor’s right liver was 1.63 % while the actual printed right liver far exceeded the spatial boundaries of the 3D printed abdominal cavity.
Based on Table 1, adult recipients and pediatric recipients have distinct characteristics, which require different management from the surgical team. 3D printing for the patients was also different between adult and pediatric patients. While adult recipients mostly required printing of the right hemi-abdomen, pediatric patients required printing of the entire abdomen. This is basically due to the type of graft used. When whole liver graft or right liver graft is used, the main place will be placed is right liver fossa. However, the left liver graft is placed on the midline of the abdominal cavity, which points to the need to design both hemi-abdomens. Slice distance was also significantly narrower in the pediatric recipients. However, the fundamental process for manufacturing the 3D printed model is similar, and the time and the amount of filaments required are also similar between the two groups. This shows that our 3D printed model can be utilized properly for both adult and pediatric recipients with minimal error.
The limitation of this study is that our study only showed descriptive data of our patients whose abdominal cavity was 3D printed. This study did not compare the impact of using 3D printed model between an experimental group and a control group. The reason for not showing such comparative data is that the patients who were expected to benefit from 3D printing were apparently patients whose abdominal cavity has a potential to be small and who were selected to be prepared in our 3D imaging and printing laboratory. Even though our 3D model simulates the patient’s intra-abdominal space, the model does not reflect the actual elasticity of the abdominal wall and the diaphragm. This could lead to a practical question on whether the real graft could fit into the recipient, and as a result, surgeons could be conservative while making decision. The surgeon should take into account the elasticity of the muscular structures comprising the liver fossa when making a decision to proceed with transplantation based on our 3D printed model.
In general, when height and weight are not so different between the donor and recipient, the graft liver usually fits into the recipient’s abdominal cavity. However, there lies a risk of large-for-size syndrome when small-sized patients, particularly female, are matched to deceased donor. Among seven adult patients of our study, five patients were managed to pivot from the initial plan established before deceased donor operation; two patients were transplanted with organ from another donor due to large-sized graft; one patient decided to undergo reduction of the original graft; one patient decided to receive the whole liver while the initial plan was to perform a reduction hepatectomy; and one patient decided to receive liver graft from living donor after deciding not to use a pre-allocated deceased donor graft; The other two recipients received whole liver as initially planned after comparing their 3D printed intra-abdominal cavity model with those grafts.
In pediatric LT, large-for-size syndrome can occur, especially in younger patients with lower weight. Among five pediatric patients, two patients decided to undergo reduction of the original graft after actual fitting of donor graft to 3D printed model of recipients. The other three patients also underwent LT as initially planned after fitting the donor graft to the 3D printed model. All donor grafts suited perfectly to the recipients’ abdominal cavity and were successfully transplanted without having the difficulty in closing abdominal wall of recipients.
Our 3D printed model enabled fast printing with low cost, which is essential for emergency operation such as DDLT. The median manufacturing times were 568 min and 601 min for adult and pediatric recipients, respectively. Since most of time is consumed during 3D printing, the time can be shortened if multiple 3D printers are used simultaneously or number of slices are lowered. The time consumed for printing the expected liver graft was 25 – 40 h according to the study of Zein et al.[18] From the study of Wang et al.[19], the cost for printing the half-sized model of abdominal cavity and liver graft was US$ 929.6 and the time required for printing was approximately 48 h. Compared to the previous studies, our simplified 3D printing model showed superior feasibility in time and cost. Another strength of our model is that it only requires an FDM type 3D printer, which is cheaper and does not require special facility. The strength can allow many transplantation centers to utilize the model in emergency cases.
5. Conclusion
The 3D abdominal cavity model, which was made using PLA filament and printed by FDM type printer, was manufactured in <10 h with minimal amount and cost for filaments used. The 3D printed model was successfully used in 16 cases of LTs in small-sized recipients. The 3D printed model can be utilized in real-world practice as a guide for LT in small-sized recipients to prevent large-for-size syndrome.
Acknowledgments
This research was supported by the Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2020R1C1C1010525).
Conflict of interest
The 3D printing protocol of intra-abdominal cavity for LT is under the process of patent registration.
Author contributions
Conceptualization: Jinsoo Rhu, Gyu-Seong Choi, Jae-Won Joh
Investigation: Jinsoo Rhu, Sunghae Park, Gyu-Seong Choi, Sanghoon Lee, Jae-Won Joh
Methodology: Jinsoo Rhu, Sunghae Park, Jong Man Kim, Sanghoon Lee
Formal analysis: Jinsoo Rhu, Sunghae Park
Writing – original draft: Jinsoo Rhu, Sunghae Park
Writing – review & editing: Jinsoo Rhu, Sunghae Park, Jong Man Kim, Jae-Won Joh.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2020-07-118). Informed consent was acquired from the recipients who were enrolled prospectively after approval of the Institutional Review Board; for minors, informed consent was obtained from their parents or legal guardians.
Availability of data
The datasets used and analyzed during the present study can be obtained from the corresponding author on request.
Further disclosure
The paper has been uploaded in a preprint server (https://assets.researchsquare.com/files/rs-1294880/v1/090dd421-ee52-429f-81bd-397dc2c0b049.pdf?c=1652850853).
References
- 1.Ahangar P, Cooke ME, Weber MH, et al. Current Biomedical Applications of 3D Printing and Additive Manufacturing. Appl Sci. 2019;9:1713. https://doi.org/10.3390/app9081713. [Google Scholar]
- 2.Daminabo SC, Goel S, Grammatikos SA, et al. Fused Deposition Modeling-based Additive Manufacturing (3D printing):Techniques for Polymer Material Systems. Mater Today Chem. 2020;16:10. https://doi.org/10.1016/j.mtchem.2020.100248. [Google Scholar]
- 3.Gudapati H, Dey M, Ozbolat I. A Comprehensive Review on Droplet-based Bioprinting:Past, Present and Future. Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. https://doi.org/10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Kingsley DM, Roberge CL, Rudkouskaya A, et al. Laser-based 3D Bioprinting for Spatial and Size Control of Tumor Spheroids and Embryoid Bodies. Acta Biomater. 2019;95:357–70. doi: 10.1016/j.actbio.2019.02.014. https://doi.org/10.1016/j.actbio.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaeri A, Cao K, Zhang F, et al. A Review of the Structural and Physical Properties that Govern Cell Interactions with Structured Biomaterials Enabled by Additive Manufacturing. Bioprinting. 2022;26:e00201. https://doi.org/10.1016/j.bprint.2022.e00201. [Google Scholar]
- 6.Marro A, Bandukwala T, Mak W. Three-dimensional Printing and Medical Imaging:A Review of the Methods and Applications. Curr Probl Diagn Radiol. 2016;45:2–9. doi: 10.1067/j.cpradiol.2015.07.009. https://doi.org/10.1067/j.cpradiol.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Rengier F, Mehndiratta A, Von Tengg-Kobligk H, et al. 3D Printing Based on Imaging Data:Review of Medical Applications. Int J Comput Assist Radiol Surg. 2010;5:335–41. doi: 10.1007/s11548-010-0476-x. https://doi.org/10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 8.Madurska MJ, Poyade M, Eason D, et al. Development of a Patient-specific 3D-printed Liver Model for Preoperative Planning. Surg Innov. 2017;24:145–50. doi: 10.1177/1553350616689414. https://doi.org/10.1177/1553350616689414. [DOI] [PubMed] [Google Scholar]
- 9.Marconi S, Pugliese L, Botti M, et al. Value of 3D Printing for the Comprehension of Surgical Anatomy. Surg Endosc. 2017;31:4102–10. doi: 10.1007/s00464-017-5457-5. https://doi.org/10.1007/s00464-017-5457-5. [DOI] [PubMed] [Google Scholar]
- 10.Kiuchi T, Kasahara M, Uryuhara K, et al. Impact of Graft Size Mismatching on Graft Prognosis in Liver Transplantation from Living Donors1, 2. Transplantation. 1999;67:321–7. doi: 10.1097/00007890-199901270-00024. https://doi.org/10.1097/00007890-199901270-00024. [DOI] [PubMed] [Google Scholar]
- 11.Fukazawa K, Nishida S, Pretto EA, Jr, et al. Detrimental Graft Survival of Size-mismatched Graft for High Model for End-stage Liver Disease Recipients in Liver Transplantation. J Hepatobiliary Pancreat Sci. 2016;23:406–13. doi: 10.1002/jhbp.355. https://doi.org/10.1002/jhbp.355. [DOI] [PubMed] [Google Scholar]
- 12.Fukazawa K, Yamada Y, Nishida S, et al. Determination of the Safe Range of Graft Size Mismatch Using Body Surface Area Index in Deceased Liver Transplantation. Transpl Int. 2013;26:724–33. doi: 10.1111/tri.12111. https://doi.org/10.1111/tri.12111. [DOI] [PubMed] [Google Scholar]
- 13.Addeo P, Noblet V, Naegel B, et al. Large-for-size Orthotopic Liver Transplantation:A Systematic Review of Definitions, Outcomes, and Solutions. J Gastrointest Surg. 2020;24:1192–200. doi: 10.1007/s11605-019-04505-5. https://doi.org/10.1007/s11605-019-04505-5. [DOI] [PubMed] [Google Scholar]
- 14.Blanco AM, Krauel L, Artés FF. Development of a Patients-specific 3D-printed Preoperative Planning and Training Tool, with Functionalized Internal Surfaces, for Complex Oncologic Cases. Rapid Prototyp J. 2019;25:363–77. https://doi.org/10.1108/rpj-03-2018-0063. [Google Scholar]
- 15.Igami T, Nakamura Y, Hirose T, et al. Application of a Three-dimensional Print of a Liver in Hepatectomy for Small Tumors Invisible by Intraoperative Ultrasonography:Preliminary Experience. World J Surg. 2014;38:3163–6. doi: 10.1007/s00268-014-2740-7. https://doi.org/10.1007/s00268-014-2740-7. [DOI] [PubMed] [Google Scholar]
- 16.Oshiro Y, Mitani J, Okada T, et al. A Novel Three-dimensional Print of Liver Vessels and Tumors in Hepatectomy. Surg Today. 2017;47:521–4. doi: 10.1007/s00595-016-1383-8. https://doi.org/10.1007/s00595-016-1383-8. [DOI] [PubMed] [Google Scholar]
- 17.Rhu J, Kim MS, Kim S, et al. Application of three-dimensional Printing for Intraoperative Guidance during Liver Resection of a Hepatocellular Carcinoma with Sophisticated Location. Ann Hepatobiliary Pancreat Surg. 2021;25:265–69. doi: 10.14701/ahbps.2021.25.2.265. https://doi.org/10.14701/ahbps.2021.25.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional Print of a Liver for Preoperative Planning in Living Donor Liver Transplantation. Liver Transpl. 2013;19:1304–10. doi: 10.1002/lt.23729. https://doi.org/10.1002/lt.23729. [DOI] [PubMed] [Google Scholar]
- 19.Wang P, Que W, Zhang M, et al. Application of 3-dimensional Printing in Pediatric Living Donor Liver Transplantation:A Single-center Experience. Liver Transpl. 2019;25:831–40. doi: 10.1002/lt.25435. https://doi.org/10.1002/lt.25435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the present study can be obtained from the corresponding author on request.


