Abstract
Since the 1930s, new methods of drug delivery, such as implantable devices with drug release control, have been developed. However, manufacturing techniques require bulk due to high initial production costs. Three-dimensional (3D) printing, also known as additive manufacturing or rapid prototyping, allows the fabrication of personalized drug delivery that uses different materials and complex geometries with multiple release profiles, thereby eradicating high initial costs. Different studies have been developed showing the extensive potential of 3D printing for the pharmaceutical industry, and despite in-depth discussions that have been published, there is no comprehensive review of processes, materials, and effects in drug delivery applications thus far. This review aims to fill this gap by presenting the use of 3D printing technology for drug delivery, exposing the different variations of the technique according to the characteristics, material, and dosage form sought. There are seven main categories of 3D printing according to the standards jointly developed by International Organization for Standardization and American Society for Testing and Materials: material jetting, binder jetting, material extrusion, vat photopolymerization, powder bed fusion, sheet lamination, and directed energy deposition. There are different 3D fabrication processes used for drug delivery applications depending on the dosage form and material applied. In this context, polymers, glasses, and hydrogels represent the most frequent materials used. 3D printing allows different forms of drug dosage. Oral, topical, rectal and vaginal, parental and implantable are discussed in this paper, presenting the identification of the type of 3D printing technology, the active pharmaceutical ingredient, formulation, and pharmaceutical effect. The main aim of this paper is to offer insights to people from academy and industry who are interested in the advancement of drug delivery and in knowing the future directions in the development of 3D printing applications in this area.
Keywords: Three-dimensional printing, Drug delivery, Pharmaceutical applications, Additive manufacturing
1. Introduction
The introduction of conventional drug delivery devices (DDDs) in the form of solid oral dosage forms began in the early 18th century, whereby the active pharmaceutical ingredient (API) was swallowed into the gastrointestinal (GI) tract, dissolved and absorbed into the gut prior to circulating through the body in the blood stream[1]. By the late 1800s, formulations began to be designed to better suit a particular condition, such as those with an enteric and hydrophilic polymer coating exhibiting resilience to the acidic conditions in the stomach, and produce delayed release (DR) of the API[2]. This widened the possible drugs that could be ingested orally, with APIs which otherwise degraded in the stomach acid now being able to be released into the GI tract instead[3].
The concept of delivering drugs over a longer time span was introduced in the 1930s when Deansely and Parkes subcutaneously implanted pellets constructed from compressed powder into male rats, achieving the sustained release of a testosterone API over a 2-week period[4].
Since this discovery, many new methods of drug administration have been formulated varying from implantable devices using permeable membranes to control the release of drug, to injectable microspheres. Despite this, the majority of DDD manufacturing techniques require bulk manufacturing of identical products due to high initial production costs[2]. As a result, traditional DDDs fit a “one-dose-fits-all” paradigm, and as such, between 4% and 25% of the ten top-grossing drugs in the U.S. were rendered unsuccessful in their intended treatment[5], due to variances in the patients’ age, weight, medical history, and environment, among others[6]. In addition, many manufacturing techniques, for example, injection molding which is commonly used to create implantable DDDs, often require the heating of the polymer and API to above the polymer’s melting temperature, risking damages to the drug in the process[4].
The introduction of additively manufacturing pharmaceuticals eradicates the high initial input costs seen in traditional manufacturing techniques, opening the scope for DDDs with drug doses tailored for each individual patient. In addition, the creation of parts with multiple materials and highly complex geometries vastly widens the design scope of each device type to create drug delivery systems with multiple release profiles[2]. While the 3D printed drug Spritam gained U.S. Food and Drug Administration (FDA) approval in 2016, its potential is still largely unchartered[7]. The following sections in this paper detail the potential uses for 3D printing in a range of pharmaceutical applications and its current limitations.
The origins of 3D printing can be split into the two sub-fields of photo sculpture and topography:
1.1. Photo sculpture
In the 1800s, the process of using multiple photographs from differing angles of a 3D object was introduced. These early technologies required the artist to carve the photographed silhouettes of each object or person from each angle to create a completed 3D sculpture[3]. In the 1900s, Carlo Baese patented a simplified technique, implementing light to a photo-sensitive gelatine to create a replica of the original model[8].
1.2. Topography
The concept of combining multiple layers with differing geometries was suggested by Blanther in the 1890s, who layered wax sections on top of one another and smoothed them together to make a 3D structure[8]. Numerous variations of this concept ensued, such as the use of layered cardboard contours and the photo curing of photo-polymer resins onto powder particles[2].
The first technique for 3D printing was developed in 1951 by Otto John Munz, who detailed a method of producing 3D objects through the use of surface maps (topoglyphs) and curing the dimensions each of these maps into incremental layers of a vat of clear, photo-curable polymer resin[8]. Since the success of this initial 3D printing technology, now widely known as vat polymerization, many other methods of building up a model in a layer-by-layer approach have been developed[7].
2. 3D printing technologies
According to the standards jointly developed by International Organization for Standardization and American Society for Standards, 3D printing technology, also known in a technical context as additive manufacturing or rapid prototyping, is divided into seven categories: material jetting, binder jetting, material extrusion, vat photopolymerization, powder bed fusion (PBF), sheet lamination, and directed energy deposition[9]. The processes that have been investigated for use in drug delivery applications are shown in Figure 1 and are detailed in this section. Printing techniques, printing characteristics, and applicable materials are discussed with the aim of helping distinguish the applicability of each process to the various DDDs and studies detailed in section 4.
Figure 1.
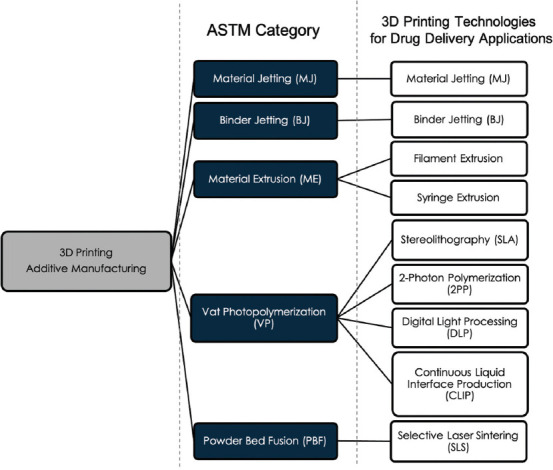
Three dimentional printing technologies for drug delivery applications.
2.1. Inkjet printing
Originating from the initial concept of inkjet printing detailed by Lord Raleigh in 1878, traditional two-dimensional inkjet printing to produce documents and photographs was introduced by Siemens in 1951[7]. The deposition of droplets on top of one another to build a 3D part was later developed in the 1980s. Inkjet printing can be split into two classifications: material jetting and binder jetting[7].
(1) Material jetting (MJ)
MJ can be defined as the process in which droplets of build material are selectively deposited onto a substrate[9] and can be split into two main techniques: drop on demand (DoD) and continuous inkjet (CIJ) (Figure 2)[7,10].
Figure 2.
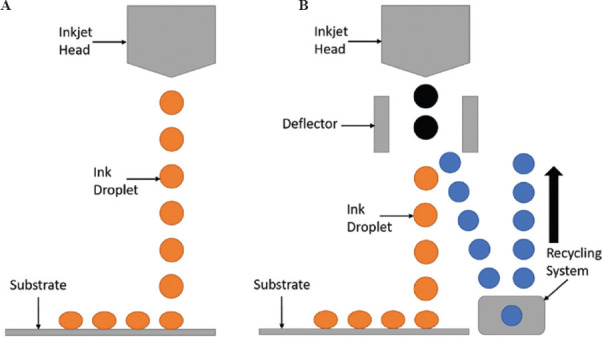
(A) Drop on demand (DoD); (B) continuous inkjet (CIJ).
DoD technique includes the use of either a vapor bubble or piezoelectric crystal which are subject to an increase in heat or voltage, respectively, to enlarge and force the ink from the nozzle, following which the input force is removed, allowing the nozzle to refill. In contrast, CIJ technique charge droplets upon ejection, following which deflector plates deflect them either onto the substrate or away as waste to be recirculated (Table 1)[7,10].
Table 1.
Characteristics and challenges of MJ
| Characteristics | Challenges | |
|---|---|---|
| Method | Droplets of ink are deposited from the nozzle into thin layers, then cured with cooling air and in the presence of high-energy light, such as ultraviolet (UV)[10]. The droplet flight path, droplet impact, and surface wetting can change the geometry of the product[11]. MJ can tune the drug composition during the printing process[12]. MJ can work with thermal or piezoelectric nozzle[13]. |
In some cases, MJ needs support structure[14]. It is necessary to perform a post-processing for removing the support structure[15]. |
| Material | MJ works with melted polymers and waxes, UV curable resins, solutions, suspensions, and complex multicomponent fluids[11]. MJ used waxes as the first materials[10]. This process can use photosensitive polymers but requires further processing to remove the photoinitiator[10]. This process can use multimaterials and full color[10]. |
The viscosity can affect the optimization requirement[12]. The materials are photosensitive (e.g., sensitive to daylight), and the mechanical properties degrade over time[14]. |
| Quality | The quality and the curing method depend on material properties[10]. The final product has homogeneous mechanical and thermal properties[14]. |
The final product has poor mechanical properties[14]. |
MJ: Material jetting, UV: Ultraviolet
(2) Binder jetting
Binder jetting (BJ) uses the DoD approach as shown in Figure 1A to selectively deposit the liquid bonding agent to join powder materials located upon the print bed beneath[9]. This inkjet printing process allows for the deposition of inks made primarily of solvent binders with low viscosities, with the bulk of the material typically presented in the spherical powder particles situated on the print bed, widening the scope of potential materials (Table 2)[10,11,16].
Table 2.
Characteristics and challenges of binder jetting (BJ)
| Characteristics | Challenges | |
|---|---|---|
| Method | Droplets of liquid binder bind the particles of the powder bed layer[10-12]. The drug can be found in the ink or in the powder bed[10]. BJ is a simple, versatile, low-cost and high-speed process that provides the personalized drug delivery system as well as customizing the composition and properties of drug-eluting implants[11,16]. The physicochemical properties of the dosage forms, and the drug-release profiles could be adjusted using different polymers and/or ratios between the bulk material, API (s), binding agents, and other excipients[16]. |
Pre-processing of the powdered materials is necessary to ensure proper distribution of the particle size and flow capacity of the powder for uniform filling of the power bed[16]. Clogging of the print head can occur during printing of non-homogeneous binder solutions[11,16]. The porosity of products is poorly controllable[16]. One risk in the process is the inconsistency or less homogeneity between the layers, which can be controlled by mediating the temperature and drying time between layers or jetting rate[11,16]. |
| Material | The process works with ambient temperature that is suitable for thermolabile drugs and excipients[16]. | The availability of suitable non-toxic solvents to increase the capacity of the binder solution is limited[16]. |
| Quality | BJ has lower printing resolution and poorer surface quality that can result in imprecise printing and low dimensional tolerance, compared to other AM processes[11,16]. | This could be improved by the additional post-processing[16]. |
2.2. Material extrusion
Arguably the most recognized process of 3D printing, extrusion-based technologies can be defined as the process in which material is selectively dispensed through a nozzle or orifice[9]. Extrusion-based printing can be split into three key categories: hot melt extrusion (HME), filament extrusion, and syringe extrusion. In all three techniques, the material undergoes a change in physical state between ejection from the nozzle and solidification upon the substrate either by cooling or solvent evaporation, with printing processes (Figure 3)[10,12].
Figure 3.
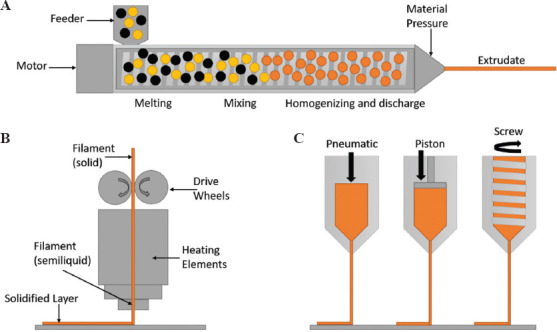
(A) Hot melt extrusion; (B) filament extrusion; (C) syringe extrusion.
(1) HME
HME ejects semi-molten material from the nozzle tip; however, it additionally incorporates heated screws, which melt, mix and eject the polymer from the nozzle[16]. This technique is regularly used for gels and pastes containing APIs at room or elevated temperatures, allowing for solid dispersions to be printed[7]. Where filament extrusion requires impregnation of the filament with an API prior to printing, HME offers the addition of the API in the melting stage, whereby it either melts alongside the polymer, dissolves within it, or disperses across the polymer mix[17].
(2) Filament extrusion
Filament extrusion is the technique of using rollers to feed a solid polymer filament through a chamber with heating elements which melt the polymer filament into a semi-molten state, following which it is ejected from the end of a nozzle or orifice. Following ejection, the polymer cools and solidifies on the substrate, allowing further layers to be deposited on top (Table 3)[10,16].
Table 3.
Characteristics and challenges of filament extrusion
| Characteristics | Challenges | |
|---|---|---|
| Method | The filament guided by gears is moved, then it is melted and pushed forward through the nozzle orifice[10]. HME is the main method for creating good quality filaments containing APIs[10]. Another method of filament preparation is the incorporation of model drugs by filament swelling in volatile solvent solution of API and drying[10,12,16]. |
For complex geometries, it requires printing support structures, which must be removed during post-processing[16]. The preparation of the filament is tedious because the quality of the final piece depends on this[10,12]. |
| Material | The filaments are made of thermoplastic polymers, such as acrylonitrile butadiene styrene (ABS), poly lactic acid (PLA), high-impact polystyrene (HIPS), and nylon[10]. This process can produce drug delivery systems with multiple APIs[16]. |
Thermoplastic polymers are only used due to the heating step[12,16]. Filament extrusion process is not suitable for the thermolabile APIs[12,16]. |
| Quality | The diameter of the nozzle orifice has an impact on the resolutionA. The filament has a big impact in the quality by its attributes, such as constant dimension, elasticity stiffness, and homogenous drug distribution[10]. As mentioned previously, the resolution depends on different factors. For example, Stratasys Company (US) has the Fortus Printer that works with a layer thickness of 178 or 254 mm, which can achieve a resolution of 250 mm[18]. |
The rheology of raw materials can produce inconsistent extrusion patterns[10,11]. |
(3) Syringe extrusion
Syringe extrusion uses a plunger-type system to push semi-molten materials, such as gels and pastes, through the print nozzle, following which they are dried[10]. Pressure-assisted microsyringes (PAM) are capable of producing DDDs with a combination of materials of drugs using multi-head extruders (Table 4)[16].
Table 4.
Characteristics and challenges of syringe extrusion
| Characteristics | Challenges | |
|---|---|---|
| Method | This process prints semisolid or semi-molten materials, such as gels and pastes, through orifice by syringe plunger[10]. After printing, the drying step is required[10,16]. HME is the main method for creating good quality gels or pastes containing APIs at room or elevated temperature[10]. |
The extrusion forces depend on the viscosity of the material[16]. |
| Material | Polymers with crosslinking mechanism or shear thinning properties are preferred[16]. Syringe extrusion is the main printing technique for many biocompatible materials[16]. |
APIs are required to be uniformly dispersed in the printing material[16]. |
| Quality | The nozzle size of the syringe defines the print resolution[16]. To improve the properties, a post-treatment can be used, such as crosslinking[16]. |
The rheology of raw materials can produce inconsistent extrusion patterns[10,11,16]. The mechanical strength and durability are low[16]. |
2.3. Vat photopolymerization
Vat photopolymerization can be defined as the process in which liquid photopolymer in a vat is selectively cured by light-activated polymerization[9] (Figure 4). It comes in four main forms: stereolithography (SLA), 2-photon polymerization (2PP), digital light processing (DLP), and continuous liquid interface production (CLIP)[10,12,16].
Figure 4.
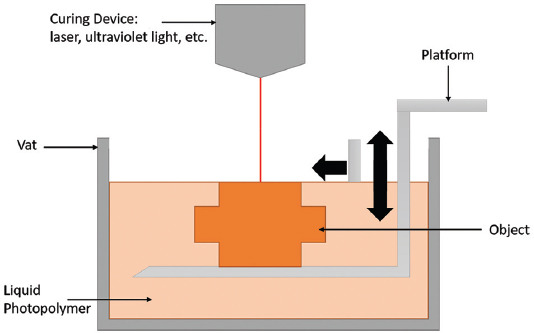
Vat photopolymerization.
(1) Stereolithography
Stereolithography (SLA) uses a vat of UV-cross linkable polymer resin paired with a UV light source which scans along the X and Y axes of the surface of the resin in a defined geometry[10]. A single layer of resin is cross-linked, and the build plate lowered a specified layer thickness between each curing layer to allow for the next layer to be cured on top (Table 5)[12,16].
Table 5.
Characteristics and challenges of stereolithography (SLA)
| Characteristics | Challenges | |
|---|---|---|
| Method | The resin vat contains a build platform. A single point laser located inside the machine maps selectively a light solidifying the liquid material[10,16]. Usually, the products are post-cured by UV light to improve their mechanical properties[16]. Inverted SLA uses the light under transparent bottom of a resin tank[10]. SLA can print porous infill, such as QuickCast, from 3D systems. To modify the porosity of some tablets, it is necessary to change their geometry, because the objects that are printed with this method have a solid filling[10]. |
Post-processing with UV-curing could be unsuitable for the APIs[16]. The instruments are expensive[16]. The post-curing steps for render biocompatible material can result in a loss of drug loaded and subsequent imprecise dosing[12]. The residual resin can represent a toxicity risk[11]. |
| Material | SLA uses photoreactive and photocurable materials[12]. The drugs can be loaded directly into the liquid pre-polymer solution depending on its solubility[12]. |
SLA has been limited by photopolymerizable materials that would be biocompatible and approved for human use[16]. SLA that works with multiples materials, such as polymer mixtures and drug loaded structures, is limited[16]. |
| Quality | High accuracy and resolution that allows fabrication of personalized organic shapes for controlling drugs release kinetics[12,16]. An example is ProX/Project printer made by 3D Systems Company (US). This printer has a layer thickness of 20 – 150 mm and has a resolution of 50 µm[18]. |
(2) 2PP
2PP follows a similar technique. 2PP is a non-linear near infrared (NIR) light process in which two photons are simultaneously absorbed with short laser pulses in a photosensitive material. 2PP, along with DLP and CLIP in sections 2.3.3 and 2.3.4, may allow for the pre-loading of APIs directly into the liquid prepolymer solution, but may suffer from a loss of drug loaded and precision on printing (Table 6)[12].
Table 6.
Characteristics and challenges of 2-photon polymerization (2PP)
| Characteristics | Challenges | |
|---|---|---|
| Method | The lasers provide femtosecond pulses, which induce a highly localized chemical reaction, leading to polymerization of the photosensitive material[19]. 2PP requires multiple post-curing steps to make it biocompatible[12]. This method can print highly personalized organic shapes, which are critical for implants and transdermal delivery for controlling drug release kinetics[12]. |
The post-curing steps may result in a loss of drug loaded and imprecise dosing[12]. |
| Material | The medicine can be loaded directly into the liquid prepolymer solution, depending on its solubility[12]. 2PP uses biocompatible photoinitiations or monomers[12]. |
This process uses only photoreactive and photocurable materials[12]. 2PP is not suitable for medications with antioxidant properties, such as Vitamins A, C and E, due to potential residual free radicals or monomers[12]. |
| Quality | 2PP has a resolution in the nanometer range, and it is the vat photopolymerization process with the best resolution[12]. Nanoscribe (Germany) created the Photonic Professional GT printer, which works with a layer thickness of <1 mm and has a resolution of 0.15 µm[18]. |
(3) DLP
DLP projects UV light onto a digital micro-mirror device (DMD) which projects the light waves onto the top or bottom surface of the vat resin[12]. The use of the DMD means that the UV light can cure larger areas of resin per unit time than that seen in traditional SLA, while maintaining its high dimensional accuracy (Table 7)[12,14].
Table 7.
Characteristics and challenges of digital light processing (DLP)
| Characteristics | Challenges | |
|---|---|---|
| Method | DLP uses UV light and micro-mirror device for projected digital light into the vat of photopolymer[20]. The projector system can be bottom-up or top-bottom[21]. This process is faster in printing time and more efficient as well as allows operating at a wider range of wavelengths, compared to SLA[22]. DLP can work with customized resin reservoirs and small volumes of photoreactive polymers[22]. |
The post-curing steps may result in a loss of drug loaded and imprecise dosing[12]. The residual resin can represent a toxicity risk[11]. |
| Material | DLP uses photoreactive and photocurable materials[12]. The medicine can be loaded directly into the liquid prepolymer solution, depending on its solubility[12]. DLP uses standard and castable resins[14]. |
This process only works with photoreactive and photocurable materials[12]. |
| Quality | Like the others processes of vat photopolymerization, DLP prints with high resolution[21]. Carbon 3D (US) has several Carbon Digital Light Synthesis printers and the resolution are 25, 50 or 100 µm[18,23]. |
(4) Continuous liquid interface production
Similarly, to DLP, continuous liquid interface production (CLIP) utilizes a DMD to project digital light into the polymer vat through an O2 window, which inhibits the cross-linking of the layer of resin closest to the window, called the dead-zone, allowing the solidified resin not to adhere to the window[12].
2.4. PBF
PBF is defined as the process in which thermal energy selectively fuses regions of a powder bed[9], (Figure 5). Similarly, to the binder jetting processes detailed in section 2.1.2, once a print layer is completed, the print bed is lowered by a specified layer thickness, another layer of powder deposited and spread through a roller, and the next layer fused to the previous. PBF comes in two main forms: selective laser sintering (SLS) and selective laser melting (SLM)[10,16].
Figure 5.
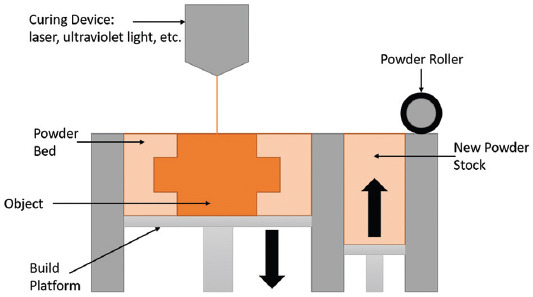
Powder bed fusion.
(1) Selective laser sintering
Selective laser sintering (SLS) technique uses a focused layer to selectively scan polymer powder material slightly below its melting temperature, while selective laser melting (SLM) uses a laser, which fully melts the powder, fusing it to the layer below[10,14]. As SLS is mainly used for polymers, it has a wide range of applications for DDDs purposes; conversely, since SLM is mainly used for metals, it is not applied for DDDs. These similar techniques have comparable properties with respect to quality and macroscale resolution; however, SLS techniques are capable of producing parts with lower layer thicknesses and higher flexibility (Table 8)[10,16].
Table 8.
Characteristics and challenges of selective laser sintering (SLS)
| Characteristics | Challenges | |
|---|---|---|
| Method | The powder bed is controlled by a leveling system that moves in from one part to another. Then, a focused laser beam scans selectively, which heats the powder just below the melting temperature[10,16]. Only a few researchers work with this process in pharmaceutical field[16]. The printing product does not need supporting structures because the unsintered powder provides all the necessary support[14]. |
The printing speed of SLS is limited to 1–5 cm/h approximately, which impacts the time of production[16]. SLS produces significant powder waste after printing process[16]. |
| Material | Drug delivery device worked with nylon, polyamide, PLLA, and PCL as the polymeric base material for customized porosity and microstructures[16]. | SLS works with high levels of energy; therefore, there are few pharmaceutical excipients and API that can resist these temperatures[10,16]. |
| Quality | The porosity of the drug delivery device could be controlled by varying the powder bed temperature, the length between the dense walls and the SLS laser power[16]. The sPro EOS P 396 printer from 3D Systems (US) and EOS (Germany), has a layer thickness of 100 mm and can achieve a resolution of 500 µm[18]. |
On the final structure, a post-treatment is required due to non-sintered powder residue[11,16]. |
Previous tables have shown main characteristics of material jetting (MJ), binder jetting (BJ), filament extrusion, syringe extrusion, stereolithography (SLA), 2PP, DLP, and selective laser sintering (SLS), including advantages and disadvantages of methods as well as materials and product quality of each technique. In the next section, specific materials for 3D printing drug delivery will be discussed.
3. Materials for 3D printing in pharmaceutical manufacturing
There are a wide range of polymeric, glass, and hydrogel materials which have been explored to act as drug-eluting devices, many of which exhibit biodegradable characteristics that allow for single administration into the body[11].
3.1. Polymers
Polymers can be divided into those which are biodegradable and those which are not. Biodegradable polymers degrade into the body over a specified time period by either surface erosion, whereby the material degrades at the outermost surface of the polymer via hydrolysis, or bulk erosion, whereby the polymer degrades evenly throughout the entire polymer bulk. In contrast, non-biodegradable polymers retain their structural and chemical integrity throughout the intended life cycle. Examples of biodegradable polymers used in DDDs include poly(caprolactone), poly (trimethylene carbonate), poly(lactide), poly (vinyl alcohol), and triethyl citrate (TEC), among others. Non-biodegradable polymers include poly (ethylene glycol) and ethylene vinyl acetate (EVA). Each polymer exhibits a particular degradation rate, and therefore drug release profile, with an alteration to the polymers molecular weight throughout the synthesis process able to tailor this further to suit a particular printing technology (e.g., material jetting which requires low-viscosity polymer inks, or extrusion-based methods which require more paste-like consistencies) or intended treatment dosage or administration time period[16,17].
Polymers are quite attractive for 3D printed drug delivery due to their distinctive capabilities for drug loading, drug release, biocompatibility, and biodegradability. In particular, smart polymers have attracted attention of the industry, as they are able to deliver the drug at specific moments and places as a response to physiological stimuli. Their main advantages lie in their versatility and tunable sensitivity while their main drawback is their slow response time. Despite this disadvantage, they have a huge potential to deliver oral drugs sensitive to both gastric acid and enteric enzymes as well as to make smart diagnostics[24]. Polymers can be applied to both hydrophilic and hydrophobic drugs, which allow drug-controlled release in constant doses even over long periods[25]. There are different types of polymers. One of the most common polymers is poly (vinyl alcohol), also designated as PVAL, which has good solubility in water but not in ethanol nor in various organic diluents. PVAL can be used to produce polymeric multiple-layered material for 3D printing through IP technique, and by varying the molecular weight of PVAL, it is possible to generate specific viscosity rates in combination with 3D models[26].
3.2. Glasses
Glasses have shown potential in pharmaceutical applications, with their potential bioactivity allowing for interactions with living cells. Similar to polymeric materials, glasses can be biodegradable or non-biodegradable, more or less brittle, and can be tailored to exhibit customizable degradation rates. As an example, mesoporous bioactive glass (Sr-MBG) containing strontium has shown sustained drug release due to its mesoporous structure, along with good bone-forming bioactivity and enhanced mechanical strength in comparison to polyurethane foams previously used[27]. For drug delivery purposes, bioceramic carriers are increasing its popularity. In fact, they have been considered a good replacement for polymers, particularly for bone local drug applications and tissue regeneration. Bioceramic materials for drug delivery include tricalcium phosphate, hydroxyapatite, and bioactive glass, among others. They exhibit unique characteristics; for example, bioactive glass is bioactive, osteoconductive and osteoinductive, and has a good degradation rate[6,7,28]. Moreover, due to the unique characteristics of mesoporous bioactive glass, such as large surface area, nanopore volume and nano-channel structure, it is frequently used for drug delivery as powders, fibers, disks, microspheres, MBG-polymer composites, and 3D scaffolds[29].
3.3. Hydrogels
Hydrogels consist of water-soluble polymers that are cross-linked in a 3D network[10,30]. The potential to create a hydrogel out of any water-soluble polymer results in them being considered an attractive alternative to polymeric materials in drug delivery applications as they encompass a wide range of chemical compositions and, as a result, physical properties. These physical properties can be tailored in terms of porosity and material swelling, which, in turn, allows the opportunity to control drug diffusion out of the polymer matrix. Some examples of hydrogels used in drug delivery include alginates, fibrins, gelatine, and polyacrylamide[30]. Of these, one of the most cost-effective biomaterials is gelatin methacrylamide (GelMA)[26]. In fact, gelatines have particular attributes for drug delivery applications, which include higher drug encapsulation efficiency, stable carrier and drug complexation, fewer side effects, lower systemic cytotoxicity, reduced immunogenicity, and prolonged circulatory time[31].
4. 3D printing in pharmaceutical manufacturing
The potential of parts with high geometric complexity, precise dimensional accuracy, and multi-material capabilities exhibited by various 3D printing processes has seen a rapidly expanding surge of research over the past two decades, with oral, topical, rectal and vaginal, parenteral, and implantable DDDs among those reviewed to target a range of conditions. Some examples are shown in Figure 6.
Figure 6.
Examples of drug delivery devices.
4.1. Oral drug dosage form
Oral DDDs (ODDDs) such as tablets and capsules are arguably the most widely accepted method of drug administration, regularly exhibiting near-immediate release profiles[32].
Modern ODDDs can be designed to exhibit a range of release speeds and manufactured with multiple drugs. Despite this, traditional powder compaction methods largely restrict the design freedom and therefore hinder the therapeutic efficacy of the dosage form. In addition, the high initial investment costs for the compression mold and high input energy requires the production of large volumes of pills per cycle to reduce processing costs[12]. As such, tablet variance is not possible and results in all produced pills falling under the “one-dose-fits-all” paradigm. Other problems include the even dispersion of the API within the polymer excipient, and therefore in the pills, along with the restriction on producing pills with multiple drugs due to the potential of interactions between the differing drugs[32].
Printing of ODDDs was first investigated into a 3D part in 1999, when Kastra et al. began to use binder jetting to tailor release mechanisms via the use of different binder inks. Binder inks containing either Eudragit® E-100 with ethanol or Eudragit® RLPO onto cellulose powder to produce tablets exhibiting either erosion or diffusion-based drug release. In addition, the ability to tailor the release profile by varying of quantity of polymer in the ink was demonstrated, with lower polymer concentrations exhibiting faster dissolution rates[33]. In their further studies, Rowe et al. utilized the pH dependency of excipients to control drug release in correlation to the ingested ODDDs location in the body, and achieved immediate release, DR, break-away devices capable of exhibiting two pulses of drug release through the incorporation of multiple material and drugs[34]. Binder jetting has since been investigated to print a range of dissolution profiles, including those exhibiting zero-order release, fast-dissolving tablets, and extended release in addition to fast-disintegrating oral films, which led to the first 3D printed drug Spritam® that showed drug release within the therapeutic window within 9 min of administration being given approval by the U.S. FDA in 2016 for the treatment of epilepsy[35].
BJ of ODDDs usually includes the drug in the polymer powder. Unlike BJ process, the API is situated in the injectable ink for MJ printing and solidified by either polymer cross-linking or solvent evaporation, so fewer studies have been conducted using this inkjet method. The first use of MJ in pharmaceutical printing was by Hsu et al. in 2015 who printed multi-layer tablets using naproxen (NAP)/polyethylene glycol (PEG) solid dispersions with various PEG barriers to control the release rate of the NAP, with higher dissolution rates being evident with the increasing PEG molecular weight[36]. Later studies investigated the effect of geometry on drug release, and Kyobula et al. detailed faster release rates with higher surface areas, with the highlighted limitation being the factor of wettability of the inner honeycomb structure of smaller cell sizes[37].
Extrusion-based 3D printing techniques have also been explored to manufacture ODDDs with tuneable release profiles. Filament extrusion has been used to print a range of immediate, extended and modified release profiles through the use of polymers include poly (lactic acid) (PLA), poly (vinyl alcohol) (PVA), PEG, and its diacrylates (PEGDA). Although a range of biodegradable and biocompatible materials are able to extrude filament, the generally high molecular weights required to retain its form upon printing tend to correlate to slow degradation rates; to deal with this, a number of studies have been explored. Alhijjaj et al. performed an investigation into the blending of multiple polymers to widen the material base for extrusion-printing in pharmaceutics, and to control drug release rate through the polymer blend[38]. Arafat et al. incorporated “caplets” into the print, thereby achieving faster degradation rates due to an increase in fluid flow throughout the pill[39], and Sadia et al. included perforating channels[40], whereas Goyanes et al. created similar pores in the pill structure by reducing the % infill of the pill in the printing process while investigating the effect of external geometry on drug release, and concluded that an increase in surface area/volume ratio corresponds to an increase in release rate[41]. Alternatively, Goyanes et al. investigated the filament extrusion printing of caplets to achieve a fast pulse of drug release upon the dissolution of the outer shell[42]. To achieve sustained release, filament extrusion has been shown to print tablets with hollow or lattice internal structures in order to keep the ingested pills within the stomach for a sustained period[43,44]. 3D printing technologies for oral drug dosage form are shown in Table 9.
Table 9.
3D printing technologies for oral drug dosage form
| 3D printing technology | API | Formulation | Effect | References |
|---|---|---|---|---|
| Tablet Material jetting |
Naproxen | Polyethylene glycol (PEG) | Controlled release | [36] |
| Fenofibrate | Beeswax, potassium phosphate monobasic, sodium phosphate dibasic, sodium lauryl sulfate | Controlled release | [37] | |
| Thiamine hydrochloride | Polyvinyl pyrrolidone (PVP), polysorbate 20 (Tween 20), glycerol, deionized water | Immediate release | [45] | |
| Ropinirole hydrochloride | Polyethylene glycol diacrylate (PEGDA), Irgacure 2959 photoinitator (BASF) | Release mechanism | [46] | |
| Binder jetting | Chlorphenamine maleate, fluorescein disodium salt | Eudragit E-100, Eudragit RLPO, lactose, ethanol, acetone, polyvinyl pyrrolidone (PVP), Tween 20 in deionized water | Multiple mechanism Delayed release |
[33] |
| Chlorpheniramine maleate, diclofenac | Microcrystalline cellulose (MCC), Eudragit E-100, Eudragit RLPO, Eudragit L100, ethanol, acetone | Multiple mechanism | [34] | |
| Captopril | Polyvinyl pyrrolidone (PVP), maltitol, maltodextrin, water | Rapidly dispersing tablet | [47] | |
| Paracetamol, alizarin yellow | Colloidal silicon dioxide (SiO2), polyvinyl pyrrolidone (PVP) K30, mannitol, lactose | Fast-dissolving drug | [48] | |
| Levetiracetam | Microcrystalline cellulose (MCC), glycerin, Tween 80, povidone, sucralose | Rapidly dispersing dosage form | [49] | |
| Acetaminophen | Hydroxypropyl methylcellulose E100 (HPMC), ethyl cellulose (EC), polyvinyl pyrrolidone K30 (PVP), colloidal silicon dioxide | Zero-order release kinetics | [50] | |
| Acetaminophen | Hydroxypropyl methylcellulose E50 (HPMC), ethyl cellulose (EC), sodium lauryl sulfate, stearic acid, Eudragit RS-100, fluorescein, polyvinyl pyrrolidone K30 (PVP), colloidal silicon dioxide | Zero-order release kinetics | [51] | |
| Filament extrusion | Felodipine | Polyethylene glycol (PEG), polysorbate (Tween 80), polyethylene oxide, Eudragit EPO, soluplus, polyvinyl alcohol (PVA) | Controlled release | [38] |
| Theophylline | Hydroxypropyl cellulose (HPC), triacetin, sodium starch glycolate, croscarmellose sodium, crospovidone | Immediate release | [39] | |
| Hydrochlorothiazide | Triethyl citrate (TEC), tri-Calcium phosphate (TCP), Eudragit E | Design with perforating channels of increasing width | [40] | |
| 4-aminosalicylic acid (4-ASA), 5-aminosalicylic acid (5-ASA) | Polyvinyl alcohol (PVA). | Modified release | [41] | |
| Dipyridamole | Hydroxypropyl methyl cellulose (HPMC), microcrystalline cellulose (MCC), lactose, polyvinyl pyrrolidone (PVP), ethanol | Intragastric floating tablet, sustained release | [43] | |
| Domperidone | Hydroxy propyl cellulose (HPC), BaSo4 | Intragastric floating tablet | [44] | |
| Theophylline | Eudragit RL100, Eudragit RS100, hydroxypropyl cellulose (HPC), triethyl citrate (TEC), triacetin | Immediate and extended release | [52] | |
| Prednisolone | Polyvinyl alcohol (PVA), glycerol, acetonitrile, methanol | Extended release | [53] | |
| Hydrochlorothiazide | Polyvinyl alcohol (PVA), mannitol, polylactic acid (PLA) | Controlled release | [54] | |
| Nitrofurantoin | Polylactic acid (PLA), hydroxypropyl methylcellulose (HPMC) | Controlled release | [55] | |
| Nitrofurantoin | Hydroxyapatite, polylactic acid (PLA) | Controlled release | [56] | |
| Paracetamol | Hypromellose acetate succinate (HPMCAS): grades LG, MG and HG, methylparaben, magnesium stearate | Modified release | [57] | |
| Acetaminophen | Hydroxy propyl cellulose (HPC), hydroxypropyl methylcellulose (HPMC), ethyl cellulose (EC), Soluplus, Eudragit L100 | Controlled release | [58] | |
| 5-ASA, captopril, theophylline, prednisolone | Eudragit, triethyl citrate (TEC), tri-calcium phosphate (TCP), talc, microcrystalline cellulose (MMC) | Immediate release | [59] | |
| Glipizide | Polyvinyl alcohol (PVA) | Controlled release | [60] | |
| Cinnarizine | Hydroxypropyl cellulose (HPC), vinylpyrrolidone vinyl acetate copolymer (PVP VA 64) | Controlled release | [61] | |
| Haloperidol | Acid-base supersolubilization (ABS), Kollidon VA64, Affinisol 15cP | Slow release | [62] | |
| Isoniazid, rifampicin | Hydroxypropyl cellulose (HPC), hypromellose acetate succinate (HPMC – AS) | Controlled release | [63] | |
| Syringe extrusion | Guaifenesin | Hydroxypropyl methylcellulose (HPMC), poly acrylic acid (PAA), microcrystalline cellulose (MCC), sodium starch glycolate | Bi-layers tablets for respiratory tract infections | [64] |
| Nifedipine, captopril, glipizide | Hydroxypropyl methylcellulose (HPMC), microcrystalline cellulose (MCC), lactose, NaCl, polyethylene glycol 6000, tromethamine, D-mannitol, cellulose acetate | Multi-active (Polypill) | [65] | |
| Hydrochlorothiazide, aspirin, pravastatin, atenolol, ramipril | Polyethylene glycol (PEG) 600, D-mannitol, cellulose acetate, hydroxypropyl methylcellulose (HPMC), lactose, sodium starch glycolate, polyvinyl pyrrolidine | Multi-active (Polypill) | [66] | |
| Curcumin, chloramphenicol | Sodium alginate-cellulose nanofibers (SA- CNF) | Controlled release | [67] | |
| Stereolithography | Paracetamol, 4-ASA | Polyethylene glycol (PEG), polyethylene glycol diacrylate (PEGDA) | Modified release | [68] |
| Ibuprofen, riboflavin | Polyethylene glycol (PEG), polyethylene glycol diacrylate (PEGDA), triethanolamine (TEA), diphenyl 2,4,6-trimethylbenzoyl phosphine oxide (DPPO), water | Controlled release | [69] | |
| Digital light processing | 5-fluorouracil | Acrylic acid (AA), polyethylene glycol dimethacrylate (PEGDMA), acrylated hyperbranched polyester (AHBPE) | Controlled release | [70] |
| Selective laser sintering | Paracetamol | Hydroxypropyl methylcellulose (HPMC), vinylpyrrolidone vinyl acetate copolymer, candurin | Fast drug release | [71] |
| Paracetamol | Kollicoat IR, Eudragit L100–55, candurin | Immediate/modified release | [72] | |
| Caplet Filament extrusion |
Budesonide | Polyvinyl alcohol (PVA) | Controlled release | [42] |
| Paracetamol or caffeine | Polyvinyl alcohol (PVA) | Drug release of PVA based caplets | [73] | |
| Acetaminophen | Polyvinyl alcohol (PVA), polyvinyl alcohol-polyethylene glycol graft copolymer (KIR), glycerol, hydroxypropyl methylcellulose (HPMC), polyethylene glycol (PEG), hypromellose acetate succinate (HPMCAS) | Two-pulse oral drug delivery | [74] | |
| Oral film Material jetting |
Rasagiline mesylate | Propylene glycol (PG), hydroxypropyl methylcellulose (HPMC), crospovidone, glycerol, water | Fast disintegration and dissolution | [75] |
| Loperamide or caffeine | Propylene glycol (PG), ethanol, water | Fast disintegration and dissolution | [76] | |
| Prednisolone | Ethanol, water, glycerol | Fast disintegration and dissolution | [77] | |
| Riboflavin sodium phosphate | Glycerol, water | Modified release | [78] | |
| Sodium picosulfate | Tesa, microcrystalline cellulose (MCC), hydroxypropyl methylcellulose (HPMC), gelatin, Listerine, hydroxypropyl methyl cellulose with 2% TiO2 (HPMCT), gelatin with 2% TiO2, hydrophilic microcrystalline cellulose (pMCC) | Modified release | [79] | |
| Enalapril maleate | Macrogol 400, water, methanol | Modified release | [80] | |
| Binder jetting | Levetiracetam | Undisclosed formula | Fast disintegration and dissolution | [81] |
| Filament extrusion | Aripiprazole | Polyvinyl alcohol (PVA) | Fast disintegration and dissolution | [82] |
| Syringe extrusion | Warfarin sodium | Polyvinyl alcohol (PVA), hydroxypropyl cellulose (HPC), ethanol, water | Modified release | [83] |
| Saquinavir | Hydroxypropyl methylcellulose (HPMC), malic acid, glycerol, water | Controlled release | [84] |
4.2. Topical dosage form
Topical delivery of drugs, also known as transdermal drug delivery, is the process of administering drugs on to the surface of the skin. Due to the high permeability of the skin, this often requires the assistance of a rate-controlling barrier layer with lower permeability to prevent over-dosing[16]. Transdermal DDDs may come in the form of patches, masks, wound dressing, etc. Goyanes et al. compared the use of filament extrusion and SLA to incorporate anti-acne drug, salicylic acid, into a mask of the intended patients’ nose attained through 3D scanning[85]. Drug diffusion tests showed SLA to produce masks with slower degradation, higher drug loading (1.9% w/w compared to 0.4–1.2% w/w for FDM) and higher dimensional accuracy[85]. Later, the same research group continued to print 3D-scanned masks as drug-delivering wound dressings, adding antimicrobial metals including zinc, copper, and silver into polycaprolactone to better aid wound healing[86].
A similar concept of using 3D scans of an individual to tailor transdermal DDDs was exhibited by Wei et al., who demonstrated the ability to produce a face mask based on a pre-scanned file of the patient’s face, a mask was created using a medical-grade silicone gel and a transparent biocompatible material, for a 20-h/day treatment of facial hypertrophic scars[87]. More information of studies about topical dosage form using 3D printing technology is presented in Table 10.
Table 10.
3D printing technologies for topical dosage form
| 3D printing technology | API | Formulation | Effect | References |
|---|---|---|---|---|
| Facial mask | ||||
| Filament extrusion | Salicylic acid | Flex EcoPLA (FPLA), polycaprolactone (PCL) | Personalized anti-acne facial masks | [85] |
| Stereolithography | Salicylic acid | Polyethylene glycol diacrylate (PEGDA), polyethylene glycol (PEG) | Personalized anti-acne facial masks | [85] |
| Polyjet | Silicone gel | OBJET MED610 | Treatment of facial hypertrophic scars | [87] |
| Patch | ||||
| Filament extrusion | Copper sulphate, zinc oxide | Polycaprolactone (PCL) | Antimicrobial wound dressing | [86] |
| Montelukast sodium | Kollidon 12PF, polyethylene glycol (PEG), and Polyethylene oxide (PEO) | Personalized patches | [88] | |
| Syringe extrusion | Lidocaine hydrochloride, levofloxacin | Chitosan methacrylate hydrogels | Personalized wound dressing | [89] |
4.3. Rectal and vaginal dosage form
Similarly, to topical dosage form, rectal and vaginal DDDs are administered in direct contact with the rectal mucosa or a vaginal epithelium, respectively, due to their permeability to a range of substances[12]. As with the 3D scanned masks detailed in section 4.2, Sun et al. utilized the ability of 3D printing to produce customizable geometries by using SLA technology, DLP, to print molds of the rectal and vaginal suppository in which silicon polymers loaded with analgesics were adhered[90]. Numerous studies have demonstrated the use of filament extrusion techniques to 3D print T-shaped intrauterine system (IUS) devices, which are regularly used to administer long-lasting contraceptives, with materials such as polycaprolactone and ethylene vinyl acetate. Details are shown in Table 11.
Table 11.
3D printing technologies for rectal and vaginal dosage form
| 3D printing technology | API | Formulation | Effect | References |
|---|---|---|---|---|
| Suppository | ||||
| Syringe extrusion | Lidocaine | Kolliphor RH40, Gelucire 48/16, Geloil | Personalized delivery system | [91] |
| Digital light processing | Lidocaine, ibuprofen sodium, diclofenac sodium, ketoprofen | Suppositories/silastic1 Q-4720 & MED-4901 Mold/3DM resin | Sustained release | [90] |
| T-shape IUS | ||||
| Filament extrusion | Indomethacin | Polycaprolactone (PCL) | Controlled release | [92] |
| Indomethacin | Ethylene vinyl acetate (EVA), polycaprolactone (PCL) | Controlled release | [93] | |
| Estrogen, progesterone | Polycaprolactone (PCL) | Extended release | [94] | |
| Vaginal Pessaries | ||||
| Filament extrusion | Acyclovir | Thermoplastic polyurethanes (TPU) | Controlled release | [95] |
4.4. Parenteral dosage form
Parenteral dosage form is the injection of drugs through subcutaneous, intramuscular, intravenous, or intra-arterial routes. This dosage form allows the rapid action of the administered drug[96].
To enhance the powerful delivery capabilities of needles, smaller devices were created known as microneedles, which are large enough to contain the drug but small enough to avoid pain and fear[97]. Taking advantage of 3D printing, Pere et al. used stereolithography technology to create pyramid and cone microneedles with a coat of insulin formulations[98]. Furthermore, Lim et al. developed microneedles with non-steroidal anti-inflammatory drugs (NSAIDs) that are useful to relieve finger pain, this device was fabricated with DLP[99]. Table 12 shows information of studies of parenteral dosage form applying 3D printing.
Table 12.
3D printing technologies for parenteral dosage form
| 3D printing technology | API | Formulation | Effect | References |
|---|---|---|---|---|
| Microneedle Material jetting |
5-fluorouracil, curcumin, cisplatin | Soluplus, sodium fluorescein, methanol, ethanol, acetonitrile, acetic acid, phosphoric acid, hydrochloric | Anticancer agent coated metal | [100] |
| Stereolithography | Insulin | Dental SG resin, xylitol, mannitol, trehalose | Insulin skin delivery | [98] |
| 2-photon polymerization | Gentamicin sulfate | Polyethylene glycol diacrylate (PEGDA), polyethylene glycol (PEG) | Antimicrobial loaded | [101] |
| Digital light processing | Diclofenac sodium | 3DM-Cast | Splint for trigger finger | [99] |
| Silver, zinc oxide coating | eShell 200, envisiontec GmbH | Antimicrobial loaded | [102] | |
| Riboflavin | Silk fibroin (SF) | Safe protein-based microneedle | [103] | |
| Continuous liquid interface production | Rhodamine, fluorescein | Polycaprolactone (PCL), polyethylene glycol (PEG), polyacrylic acid (PAA), trimethylolpropane triacrylate (TMPTA) | Varying geometries | [104] |
4.5. Implants
Implantable DDDs (IDDDs) offer numerous advantages over oral and parenteral administration methods, which often require frequent re-administration of one or multiple drug(s). First, the issue of patient compliance can lead to variations in dosing frequencies, and therefore fluctuations in plasma concentrations[10,96]. The administration of IDDDs can either require a single administration, which can release drugs in two main ways: diffusion or dissolution. Diffusion-based administration, also known as membrane systems, requires a secondary procedure to remove the implant on completion of delivery, and tends to use a semi-permeable membrane through which drug molecules diffuse slowly over time. Dissolution-based administration, also known as matrix systems, requires a single invasive procedure upon administration, and breaks up the polymer chain to release the drug molecules either by surface or bulk erosion[2].
Several studies have been conducted on BJ of IDDDs, with Wu et al. in 2009 showing the successful printing of a concentric cylinder with alternating isoniazid and rifampicin layers to create a pulsatile release of the two drugs for long-term tuberculosis treatments[105]. Later that year, they printed an implant containing both a reservoir system containing rifampicin and a matrix system containing levofloxacin aimed at treating conditions with combined bone infections in the same device[106], demonstrating the ability to print an implant with multiple drug release systems within a single IDDD. Wu et al. also investigated the use of BJ processing to build columnar-shaped tablets (CST), doughnut-shaped tablets (DST), and multilayer-shaped tablets (MLST) from PLLA, which contained a barrier layer without drug on the upper and lower surfaces of the implant[107]. Dynamic soaking of the implants displayed the MLST to provide improved consistency of drug release characteristics due to smaller fluctuations in surface area of the device. Years after, Wu et al. replaced the drugs with levofloxacin and tobramycin in the layers to demonstrate its applicability to treat osteomyelitis[108].
Extrusion-based printing has been used to print IDDDs, including implant, stents, catheters and hernia meshes. Sandler et al. produced PLA antimicrobial medical devices, whereby HME technique allows 5% loading of the anti-microbial drug to be mixed into the material in the printing process, showing 89.56% reduction of biofilm formation[109]. Other studies, which used filament extrusion instead, loaded the API by either coating of the polymer pellets with the API[110,111] or mixing before the creation of the final filament[112,113]. Boetker et al. co-extruded polylactic acid and either 20 or 40% hydroxypropyl methylcellulose (HE) (Metolose®) into disks, and determined an increase in degradation rates associated with higher amount of ME. This study shows the potential to customize the degradation rates of materials by altering the flow properties of the polymer blend[55].
Syringe extrusion has predominantly been utilized to validate the ability to extrude magnetic composite scaffolds and silica nanoparticulate composites and hydrogels, which would be unsuitable to print under heated conditions seen in filament extrusion and HME. Unlike heated extrusion techniques, these materials do not solidify on printing, with the gel-like structure providing enough support for the following layers to be printed. Instead, they require post-printing drying processes to evaporate any remaining solvents[27,114-119].
SLM techniques have also been established as a method for 3D printing IDDDs, with prominence in producing parts with good structural integrity. For instance, Maher et al. used SLM to print titanium bone replacement implants enriched with anticancer drugs doxorubicin (DOX) with particles and tubular arrays on the surface of the implant in order to promote cell attachment[120]. A similar concept was detailed by Parry et al. who used SLA to produce poly (propylene fumarate) scaffolds with integrated pores to encourage cell attachment, whilst the printing of carbonate hydroxyapatite mineral coatings and polymer microspheres promoted DR of the drug rhBMP-2[121]. Implant studies that use 3D printing technology are presented in Table 13.
Table 13.
3D printing technologies for implants
| 3D printing technology | API | Formulation | Effect | References |
|---|---|---|---|---|
| Implant | ||||
| Binder jetting | Isoniazid, rifampicin | Poly D, L - lactic acid (PDLLA) | Multiactive, sustained release | [105] |
| Levofloxacin, rifampicin | Poly L - lactic acid (PLLA) | Multiactive, controlled release | [106] | |
| Isoniazid | Poly L-lactic acid (PLLA), acetone, ethanol, water | Sustained release | [107] | |
| Levofloxacin, tobramycin | Poly D, L - lactic acid (PDLLA) | Multiactive, sustained release | [108] | |
| Levofloxacin | Poly L-lactic acid (PLLA), ethanol, acetone | Pulsed release profile | [122] | |
| Filament extrusion | Nitrofurantoin | Polylactic acid (PLA), hydroxypropyl methylcellulose (HPMC) | Flexible dosing and precision medication | [55] |
| Nitrofurantoin | Polylactic acid (PLA) | Biofilm inhibition | [109] | |
| Gentamicin | Polylactic acid (PLA) | Hernia meshes | [110] | |
| Gentamicin, methotrexate | Polylactic acid (PLA) | Drugs eluting product | [111] | |
| Niclosamide, inositol phosphate (IP6) | Polycaprolactone (PCL), graphene nanoplatelets (GR) | Vascular stent | [112] | |
| Ciprofloxacin hydrochloride | Polylactic acid (PLA), nano-hydroxyapatite | Bone defect diseases | [113] | |
| Syringe extrusion | Dexamethasone | Strontium containing mesoporous bioactive glass (Sr-MBG) | Controlled ion release | [27] |
| Isoniazid, rifampicin | Mesoporous silica nanoparticles (MSN), beta-tricalcium phosphate (B-TCP) | Multi-drug, osteoarticular tuberculosis therapy | [114] | |
| Doxorubicin | Polycaprolactone (PCL), mesoporous bioactive glass (MBG), Fe3 O4 | Local anticancer and enhanced osteogenic activity, and magnetic hyperthermia | [115] | |
| Dimethyloxallyl glycine | Mesoporous bioactive glass (MBG), poly (3-hydroxybutyrate-co-3- hydroxyhexanoate) | Composite scaffold | [116] | |
| Vascular endothelial growth factor | Calcium phosphate cement (CPC), alginate, alginate-gellan gum | Bone defect healing | [117] | |
| Ciprofloxacin | Polylactic acid (PLA), nano-hydroxyapatite (n-HA), Hydroxypropyl cellulose (HPC-M), Microcrys- talline cellulose Pharmacel 101 (MCC PH 101). | Controlled antibacterial release | [118] | |
| Amikacin sulfate | Polylactic acid (PLA), nano-hydroxyapatite (n-HA) | Local drug delivery | [119] | |
| Stereolithography | Recombinant human bone morphogenetic protein 2 (rhBMP-2) | Polypropylene fumarate (PPF), carbonate, hydroxyapatite, polylactic-co-glycolic acid (PLGA) microspheres, collagen | Delayed release | [121] |
| Lidocaine hydrochloride | Gelucire | Sustained and localized delivery | [123] | |
| Selective laser melting | Doxorubicin, apoptosis- inducing ligand (Apo2L/TRAIL) | Ti6Al4V, ethylene glycol | Bone cancer therapy | [120] |
Seemingly, most drugs are tissue growth factors and antibiotics. There are limited works on 3D printing of immunoregulatory drugs, which are needed in the recent development in tissue engineering[124].
5. Future directions and challenges
3D printing technology will transform disease treatment, enabling more advanced high-resolution DDDs, with suitable substrates and more controlled release profiles. This technology offers unique advantages in terms of product consistency, customization of drug administration, and combinations of different APIs, making the treatment more accurate for the benefit of the patient[125]. To this end, challenges to bed addressed in 3D printing technology as well as the efforts to adapt to or benefit from new technologies are inevitable.
In pharmaceutical applications, many variables regarding processes, printers, compounds, formulations, type of dosages, post-treatments, and final distribution contribute to the drug delivery success, and compounds with the highest quality, accuracy and efficacy as well as safety to patients are paramount. Management and care of all compounds involved represent a critical factor not only when formulations are created, but also when type of dosage is selected and the drug is printed. In addition, even though the printed product complies with all desired characteristics, it may also need a post-treatment, a stage that should be carefully monitored to avoid any alteration to the effect of the drug[126]. Therefore, quality control and safety are fundamental throughout the fabrication process. Assuring quality and safety already represents a challenge and even more so when it comes to 3D printing in large-scale manufacturing[127,128].
While it is clear that very strict parameters should be met to avoid any problem for patients, clear guidance and regulations regarding the materials, processes, as well as printers almost do not exist due to the novelty of the technology. Even for the post-manufacture quality assessment of 3D-printed devices, standard guidance has not yet been published. Current regulations of traditional manufacturing are not applicable to the flexibility that 3D printing techniques would need; 3D printing allows the manufacture of personalized and multi-drug medicines, and there is still no standard guidance in this regard[129-131]. In 2017, the U.S. FDA published a guidance on 3D printed medical devices and prosthetics, which does not apply to DDDs. Spritam, by Aprecia Pharmaceuticals, is the only product fabricated by 3D printing that has been approved for commercialization[127,130].
Regulatory guidance is needed for materials, processes and products, and for this, there are different elements to consider, as detailed in Table 14.
Table 14.
Regulatory considerations for materials, processes and products when applying 3D printing technologies
| Features | |
|---|---|
| Materials | The ink should go through a strict quality control evaluation to guarantee its homogeneity and traceability as with any raw material in manufacturing processes[129]. In case of powder, a standardized procedure is required to ensure a uniform particle size and polydispersity index[129]. As in any manufacturing process, quality control is carried out to evaluate impurities, among other elements. It is necessary to have a procedure to evaluate unreacted photoinitiators, free radicals or even degradation[132]. Furthermore, it is necessary to evaluate the level of toxicity and to verify if it is acceptable or not[132]. |
| Processes | Proper documentation of all printing parameters and procedures will reduce the risk of inaccuracies in the manufacturing process[129]. Laser beam energy density, scanning speed, deposition velocity, and humidity are parameters that need to be measured to guarantee the consistency of the manufacturing process. As it is known, these parameters have a great impact on the physical characteristics of the final product[129]. |
| Products | Specialized quality control tests of the final product should be performed to obtain an accurate result of the design. Some examples for quality control tests are surface laser scanning, micro-CT, and various printer monitoring strategies[129]. A cleaning process of the finished product should be carried out regardless the 3D printing process that was used to remove, for example, support material, residual monomers, etc.[129]. |
Furthermore, 3D printing techniques require a unique production environment and/or the use of some specific resources, such as a highly specialized laser[127]. Current challenges of 3D printing technologies for drug delivery applications can lead to a long trial and error process before transforming it from a laboratory to a revolutionary manufacturing process[127,133]. Large-scale manufacturing represents a big challenge as explained in previous sections; different techniques and processes have emerged and the evolution to mass production and further commercialization will also require an entire ecosystem where academy, industry, and government participate in to facilitate all the essential conditions[128,131,133].
Despite the challenges presented to date, 3D printed drug delivery system has a promising future that will change the course of current healthcare. Synergic efforts in different fields are required. They include a sustainability focus to produce eco-friendly and physiologically safe excipients and filaments, research to reduce waste of 3D printing processes, and studies for the improvement of the dosage accuracy until the incursion to digitalization[131].
Machine learning (ML), which is an application of artificial intelligence (AI) to enable pattern recognition from large and complex datasets, is gaining presence in the 3D printing field[134-141]. This tool contributes to product quality and productivity by in situ monitoring, optimizing design and process parameters, and speeding up the microstructure evolution prediction[142].
In this context, ML has been applied in different 3D printing techniques to estimate performance and quality indicators. Recently in 2022, an integration of ML and 3D printing through a graphical user interface for printing parameter optimization was published. While the majority of 3D printing research considers orthogonal designs, authors employed nine different computer-aided design (CAD) images to allow ML algorithms to identify the difference among designs, calculating their complexity[143]. Also in 2022, a study working on ML to predict 3D printing performance parameters of different formulations, such as processing temperatures (extrusion and printing temperatures), feedstock characteristics, and printability, was published. Ong et al. mined data on hot-melt extrusion (HME) and fused deposition modeling (FDM), and an extensive range of different 3DP formulations to optimize product design without having it physically. Through this research, it was discovered that the simulated drugs had accurate release profiles; this represents a strong advantage in terms of time saving because each iteration would take days[127].
ML has also been used in decision trees for HME and artificial neural networks (ANNs) to enhance the quality of drug products throughout the pharmaceutical workflow. In addition, ANNs have correctly predicted the dissolution profiles of ibuprofen-loaded Printlets™ fabricated using DLP[144,145]. Moreover, ML has contributed to predicting the required force for penetration of 3D printed microneedle arrays (MLA) as well as the capabilities for their insertion into the skin[146].
Considering the advances worldwide, a strong emphasis on collaborative work in the digital era is expected to happen. In the future, automatization and robotics will be a reality, giving rise to more innovative and efficient 3D printed drugs. In a more distant future, a big transformation from 3D to 4D printed drugs is foreseen, and this next generation of drugs will come true thanks to shape memory materials[10,147]. This new technology enables adaptability and dynamic response to the structures according to the desired effect or shape[147]. 4D printing will bring unique characteristics in bio-robotics for medical purposes, such as drug delivery, tissue engineering, and medical devices[10]. Future trends envision a revolution in pharmacy in the next years, and science and technology advances will enable 3D printing of innovative drug delivery systems.
6. Conclusions
3D printing technology is evolving quickly providing a new way to develop attractive solutions for medical applications[148]. Medication administration is being revolutionized, and researchers, doctors and patients become increasingly interested in having alternatives that are more efficient and friendly. At present, standardized doses of medicines predominate but each patient requires unique treatments with tailored dosages. The DDDs fabricated by 3D printing enable the production of personalized drugs for the patients with specific needs. Moreover, this technology has unique capabilities to work with complex geometries, high precision, and multiple APIs.
In this review, the potential uses of 3D printing technology are detailed, as well as the different techniques that have been developed along with their challenges and application in drug delivery is identified. Materials used were also determined through three principal categories: polymers, glasses and hydrogels. In addition, five dosage forms were identified: (i) Oral drug dosage, (ii) topical dosage form, (iii) rectal and vaginal, (iv) parenteral dosage form, and (v) implants. The API, formulation and effect are discussed for all cases.
3D printing will revolutionize the concept of traditional manufacturing by innovatively adding value to health applications, such as drug delivery. A radical change in medical treatments will happen in the coming years.
Acknowledgments
We acknowledge Tecnologico de Monterrey and CONACYT for their support.
Funding
The authors acknowledge institutional funding received from Tecnologico de Monterrey and Consejo Nacional de Ciencia y Tecnología (CONACyT) through a Graduate Studies Scholarship and an Academic Scholarship as member of the National System of Researchers (Sistema Nacional de Investigadores).
Conflict of interest
The authors declare that they have no conflicts of interest.
Author contributions
Conceptualization, Methodology, Supervision, writing – review & editing: Marisela Rodriguez-Salvador
Information collection, data analysis, graphics and writing – original draft: Jessica Mancilla-De-la-Cruz
Review, vetting and editing: Jia An, Chee Kai Chua
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data
Not applicable.
References
- 1.Florence A, Attwood D. London: Pharmaceutical Press; 2015. Physiochemical Principles of Pharmacy. [Google Scholar]
- 2.Kleiner L, Wright J, Wang Y. Evolution of Implantable and Insertable Drug Delivery Systems. J Control Release. 2014;181:1–10. doi: 10.1016/j.jconrel.2014.02.006. https://doi.org/10.1016/j.jconrel.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman A, Hillery A. Historical Introduction to the Field of Controlled Drug Delivery. Drug Delivery Fundamental Appllications. United States: CRC Press; 2017. pp. 1–21. [Google Scholar]
- 4.Deanesly R, Parkes A. Biological Properties of Some New Derivatives of Testosterone. Biochem J. 1937;31:1161–4. doi: 10.1042/bj0311161. https://doi.org/10.1042/bj0311161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schork NJ. Personalized Medicine:Time for one-Person Trials. Nature. 2015;520:609–11. doi: 10.1038/520609a. https://doi.org/10.1038/520609a. [DOI] [PubMed] [Google Scholar]
- 6.Peak R. Precision Medicine is not Just Genomics:The right dose for every patient. Ann Rev Pharmacol Toxicol. 2018;58:105–22. doi: 10.1146/annurev-pharmtox-010617-052446. https://doi.org/10.1146/annurev-pharmtox-010617-052446. [DOI] [PubMed] [Google Scholar]
- 7.Prasad L, Smyth H. 3D Printing Technologies for Drug Delivery:A Review. Drug Dev Ind Pharm. 2016;42:1019–31. doi: 10.3109/03639045.2015.1120743. https://doi.org/10.3109/03639045.2015.1120743. [DOI] [PubMed] [Google Scholar]
- 8.Syrkel M. A Brief History of Additive Manufacturing. Arizona: Nexus 3 Manufacturing and Engineering; 2021. [Last accessed on 2021 Apr 06]. Available from: https://www.nexus3mfg.com/3d-printing . [Google Scholar]
- 9.International Organization for Standardization/ASTM 52900. Additive Manufacturing-General Principles-Fundamentals and Vocabulary. Switzerland: International Organization for Standardization; 2021. Available from: https://Www.Iso.Org/Obp/Ui/Iso:Std:Iso-Astm:52900:Ed-2:V1:En . [Google Scholar]
- 10.Jamroz W, Szafraniec J, Kurek M, et al. 3D Printing in Pharmaceutical and Medical Applications-Recent Achievements and Challenges. Pharm Res. 2018;35:176. doi: 10.1007/s11095-018-2454-x. https://doi.org/10.1007/s11095-018-2454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norman J, Madurawe R, Moore C, et al. A New Chapter in Pharmaceutical Manufacturing:3D-printed Drug Products. Adv Drug Deliv Rev. 2017;108:39–50. doi: 10.1016/j.addr.2016.03.001. https://doi.org/10.1016/j.addr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Lim SH, Kathuria H, Yao J, et al. 3D Printed Drug Delivery and Testing Systems-a Passing Fad or the Future? Adv Drug Deliv Rev. 2018;132:139–68. doi: 10.1016/j.addr.2018.05.006. https://doi.org/10.1016/j.addr.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Rossignol F, Macdonald J. Inkjet Printing for Biosensor Fabrication:Combining Chemistry and Technology for Advanced Manufacturing. Lab Chip. 2015;15:2538–58. doi: 10.1039/c5lc00235d. https://doi.org/10.1039/c5lc00235d. [DOI] [PubMed] [Google Scholar]
- 14.Redwood B. Types of 3D Printing. HUBS, Netherlands. 2022. [Last accessed on 2022 Apr 03]. Available from: https://www.hubs.com/knowledge-base/types-of-3d-printing .
- 15.Loughborough University. The 7 Categories of Additive Manufacturing. England: Loughborough University; 2021. [Last accessed on 2022 Apr 03]. Available from: https://www.lboro.ac.uk/research/amrg/about/the7categoriesofadditivemanufacturing . [Google Scholar]
- 16.Palo M, Holländer J, Suominen J, et al. 3D Printed Drug Delivery Devices:Perspectives and Technical Challenges. Expert Rev Med Dev. 2017;14:685–96. doi: 10.1080/17434440.2017.1363647. https://doi.org/10.1080/17434440.2017.1363647. [DOI] [PubMed] [Google Scholar]
- 17.Moulton S, Wallace G. 3-Dimensional (3D) Fabricated Polymer Based Drug Delivery Systems. J Controlled Release. 2014;193:27–34. doi: 10.1016/j.jconrel.2014.07.005. https://doi.org/10.1016/j.jconrel.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Lee JY, Tan WS, An J, et al. The Potential to Enhance Membrane Module Design with 3D Printing Technology. J Membr Sci. 2016;499:480–90. https://doi.org/10.1016/j.memsci.2015.11.008. [Google Scholar]
- 19.Ostendorf A, Chichkov B. Two-Photon Polymerization:A New Approach to Micromachining. United States: Photonics Spectra; 2006. [Last accessed on 2022 Sep 18]. Available from: https://www.photonics.com/Articles/Two-Photon_Polymerization_A_New_Approach_to/a26907 . [Google Scholar]
- 20.Lee J, Sing S, Zhou M, et al. 3D Bioprinting Processes:A Perspective on Classification and Terminology. Int J Bioprint. 2018;4:151. doi: 10.18063/IJB.v4i2.151. https://doi.org/10.18063/IJB.v4i2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge L, Dong L, Wang D, et al. A Digital Light Processing 3D Printer for Fast and High-Precision Fabrication of Soft Pneumatic Actuators. Sens Actuators A Phys. 2018;273:285–92. https://doi.org/10.1016/j.sna.2018.02.041. [Google Scholar]
- 22.Kadry H, Wadnap S, Xu C, et al. Digital Light Processing (DLP) 3D-Printing Technology and Photoreactive Polymers in Fabrication of Modified-Release Tablets. Eur J Pharm Sci. 2019;135:60–7. doi: 10.1016/j.ejps.2019.05.008. https://doi.org/10.1016/j.ejps.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Carbon 3D. A Carbon 3D Printer for Every Budget. 2022. [Last accessed on 2022 Sep 17]. Available from:https://www.carbon3d.com/products .
- 24.James HP, John R, Alex A, et al. Smart Polymers for the Controlled Delivery of Drugs-a Concise Overview. Acta Pharm Sin B. 2014;4:120–7. doi: 10.1016/j.apsb.2014.02.005. https://doi.org/10.1016/j.apsb.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liechty WB, Kryscio DR, Slaughter BV, et al. Polymers for Drug Delivery Systems. Ann Rev Chem Biomol Eng. 2010;1:149–73. doi: 10.1146/annurev-chembioeng-073009-100847. https://doi.org/10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmood M. 3D Printing in Drug Delivery and Biomedical Applications:A State-of-the-art Review. Compounds. 2021;1:94–115. https://doi.org/10.3390/compounds1030009. [Google Scholar]
- 27.Zhang J, Zhao S, Zhu Y, et al. Three-dimensional Printing of Strontium-Containing Mesoporous Bioactive Glass Scaffolds for Bone Regeneration. Acta Biomater. 2014;10:2269–81. doi: 10.1016/j.actbio.2014.01.001. https://doi.org/10.1016/j.actbio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Soundrapandian C, Datta S, Kundu B, et al. Porous Bioactive Glass Scaffolds for Local Drug Delivery in Osteomyelitis:Development and in Vitro Characterization. AAPS PharmaSciTech. 2010;11:1675–83. doi: 10.1208/s12249-010-9550-5. https://doi.org/10.1208/s12249-010-9550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu C, Chang J, Xiao Y. Mesoporous Bioactive Glasses as Drug Delivery and Bone Tissue Regeneration Platforms. Ther Deliv. 2011;2:1189–98. doi: 10.4155/tde.11.84. https://doi.org/10.4155/tde.11.84. [DOI] [PubMed] [Google Scholar]
- 30.Hoare TR, Kohane DS. Hydrogels in Drug Delivery:Progress and Challenges. Polymer. 2008;49:1993–2007. https://doi.org/10.1016/j.polymer.2008.01.027. [Google Scholar]
- 31.Santoro M, Tatara AM, Mikos AG. Gelatin Carriers for Drug and Cell Delivery in Tissue Engineering. J Controlled Release. 2014;190:210–8. doi: 10.1016/j.jconrel.2014.04.014. https://doi.org/10.1016/j.jconrel.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trenfield S, Awad A, Goyanes A, et al. 3D Printing Pharmaceuticals:Drug Development to Frontline Care. Trends Pharmacol Sci. 2018;39:440–51. doi: 10.1016/j.tips.2018.02.006. https://doi.org/10.1016/j.tips.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Katstra W, Palazzolo R, Rowe C, et al. Oral Dosage Forms Fabricated by Three Dimensional Printing. J Controlled Release. 2000;66:1–9. doi: 10.1016/s0168-3659(99)00225-4. https://doi.org/10.1016/S0168-3659(99)00225-4. [DOI] [PubMed] [Google Scholar]
- 34.Rowe CW, Katstra WE, Palazzolo RD, et al. Multimechanism Oral Dosage forms Fabricated by Three Dimensional Printing. J Controlled Release. 2000;66:11–7. doi: 10.1016/s0168-3659(99)00224-2. https://doi.org/10.1016/S0168-3659(99)00224-2. [DOI] [PubMed] [Google Scholar]
- 35.Groll J, Burdick J, Cho D, et al. A Definition of Bioinks and their Distinction from Biomaterials Inks. Biofabrication. 2018;11:013001. doi: 10.1088/1758-5090/aaec52. [DOI] [PubMed] [Google Scholar]
- 36.Hsu H, Harris M, Toth S, et al. Drop Printing of Pharmaceuticals:Effect of Molecular Weight on PEG Coated-naproxen/PEG 3350 Solid Dispersions. AIChE J. 2015;61:4502–8. doi: 10.1002/aic.14979. https://doi.org/10.1002/aic.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kyobula M, Adedeji A, Alexander MR, et al. 3D Inkjet Printing of Tablets Exploiting Bespoke Complex Geometries for Controlled and Tuneable Drug Release. J Controlled Release. (2017);261:207–15. doi: 10.1016/j.jconrel.2017.06.025. https://doi.org/10.1016/j.jconrel.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 38.Alhijjaj M, Belton P, Qi S. An Investigation into the use of Polymer Blends to Improve the Printability of and Regulate Drug Release from Pharmaceutical Solid Dispersions Prepared Via Fused Deposition Modeling (FDM) 3D Printing. Eur J Pharm Biopharm. 2016;108:111–25. doi: 10.1016/j.ejpb.2016.08.016. https://doi.org/10.1016/j.ejpb.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Arafat B, Wojsz M, Isreb A, et al. Tablet Fragmentation without a Disintegrant:A Novel Design Approach for Accelerating Disintegration and Drug Release from 3D Printed Cellulosic Tablets. Eur J Pharm Sci. 2018;118:191–9. doi: 10.1016/j.ejps.2018.03.019. https://doi.org/10.1016/j.ejps.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Sadia M, Arafat B, Ahmed W, et al. Channelled Tablets:An Innovative Approach to Accelerating Drug Release from 3D Printed Tablets. J Controlled Release. 2018;269:355–63. doi: 10.1016/j.jconrel.2017.11.022. https://doi.org/10.1016/j.jconrel.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Goyanes A, Buanz AB, Hatton GB, et al. 3D Printing of Modified-release Aminosalicylate (4-ASA and 5-ASA) Tablets. Eur J Pharm Biopharm. 2015;89:157–62. doi: 10.1016/j.ejpb.2014.12.003. https://doi.org/10.1016/j.ejpb.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Goyanes A, Chang H, Sedough D, et al. Fabrication of Controlled-release Budesonide Tablets via Desktop (FDM) 3D Printing. Int J Pharm. 2015;496:414–20. doi: 10.1016/j.ijpharm.2015.10.039. https://doi.org/10.1016/j.ijpharm.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Guan X, Cui M, et al. Preparation and Investigation of Novel Gastro-Floating Tablets with 3D Extrusion-based Printing. Int J Pharm. 2018;535:325–32. doi: 10.1016/j.ijpharm.2017.10.037. https://doi.org/10.1016/j.ijpharm.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 44.Chai X, Chai H, Wang X, et al. Fused Deposition Modeling (FDM) 3D Printed Tablets for Intragastric Floating Delivery of Domperidone. Sci Rep. 2017;7:2829. doi: 10.1038/s41598-017-03097-x. https://doi.org/10.1038/s41598-017-03097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cader HK, Rance GA, Alexander MR, et al. Water-based 3D Inkjet Printing of an Oral Pharmaceutical Dosage form. Int J Pharm. 2019;564:359–68. doi: 10.1016/j.ijpharm.2019.04.026. https://doi.org/10.1016/j.ijpharm.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 46.Clark E, Alexander M, Irvine D, et al. 3D Printing of Tablets using Inkjet with UV Photoinitiation. Int J Pharm. 2017;529:523–30. doi: 10.1016/j.ijpharm.2017.06.085. https://doi.org/10.1016/j.ijpharm.2017.06.085. [DOI] [PubMed] [Google Scholar]
- 47.Lee K, Kang A, Delfino JJ, et al. Evaluation of Critical Formulation Factors in the Development of a Rapidly Dispersing Captopril Oral Dosage Form. Drug Dev Ind Pharm. 2003;29:967–79. doi: 10.1081/ddc-120025454. https://doi.org/10.1081/DDC-120025454. [DOI] [PubMed] [Google Scholar]
- 48.Yu D, Shen X, Branford-White C, et al. Novel Oral Fast-disintegrating Drug Delivery Devices with Predefined Inner Structure Fabricated by Three-dimensional Printing. J Pharm Pharmacol. 2009;61:323–9. doi: 10.1211/jpp/61.03.0006. https://doi.org/10.1211/jpp.61.03.0006. [DOI] [PubMed] [Google Scholar]
- 49.Jacob J, Coyle N, West TG, et al. Aprecia Pharmaceuticals LLC. Rapid Disperse Dosage form Containing Levetiracetam. US9339489B2, Abstract 2014 [Google Scholar]
- 50.Yu DG, Branford-White C, Ma ZH, et al. Novel Drug Delivery Devices for Providing Linear Release Profiles Fabricated by 3DP. Int J Pharm. 2009;370:160–6. doi: 10.1016/j.ijpharm.2008.12.008. https://doi.org/10.1016/j.ijpharm.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Yu DG, Yang X, Huang W, et al. Tablets with Material Gradients Fabricated by Three-dimensional Printing. J Pharm Sci. 2007;96:2446–56. doi: 10.1002/jps.20864. https://doi.org/10.1002/jps.20864. [DOI] [PubMed] [Google Scholar]
- 52.Pietrzak K, Isreb A, Alhnan M. A Flexible-dose Dispenser for Immediate and Extended Release 3D Printed Tablets. Eur J Pharm Biopharm. 2015;96:380–7. doi: 10.1016/j.ejpb.2015.07.027. https://doi.org/10.1016/j.ejpb.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 53.Skowyra J, Pietrzak K, Alhnan MA. Fabrication of Extended-release patient-Tailored Prednisolone Tablets via Fused Deposition Modelling (FDM) 3D Printing. Eur J Pharm Sci. 2015;68:11–7. doi: 10.1016/j.ejps.2014.11.009. https://doi.org/10.1016/j.ejps.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Gioumouxouzis C, Katsamenis O, Bouropoulos N, et al. 3D Printed Oral Solid Dosage forms Containing Hydrochlorothiazide for Controlled Drug Delivery. J Drug Deliv Sci Technol. 2017;40:164–71. https://doi.org/10.1016/j.jddst.2017.06.008. [Google Scholar]
- 55.Boetker J, Water JJ, Aho J, et al. Modifying Release Characteristics from 3D Printed Drug-eluting Products. Eur J Pharm Sci. 2016;90:47–52. doi: 10.1016/j.ejps.2016.03.013. https://doi.org/10.1016/j.ejps.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 56.Water JJ, Bohr A, Boetker J, et al. Three-dimensional Printing of Drug-Eluting Implants:Preparation of an Antimicrobial Polylactide Feedstock Material. J Pharm Sci. 2015;104:1099–107. doi: 10.1002/jps.24305. https://doi.org/10.1002/jps.24305. [DOI] [PubMed] [Google Scholar]
- 57.Goyanes A, Fina F, Martorana A, et al. Development of Modified Release 3D Printed Tablets (printlets) with Pharmaceutical Excipients using Additive Manufacturing. Int J Pharm. 2017;527:21–30. doi: 10.1016/j.ijpharm.2017.05.021. https://doi.org/10.1016/j.ijpharm.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Feng X, Patil H, et al. Coupling 3D Printing with Hot-melt Extrusion to Produce Controlled-release Tablets. Int J Pharm. 2017;519:186–97. doi: 10.1016/j.ijpharm.2016.12.049. https://doi.org/10.1016/j.ijpharm.2016.12.049. [DOI] [PubMed] [Google Scholar]
- 59.Sadia M, Sośnicka A, Arafat B, et al. Adaptation of Pharmaceutical Excipients to FDM 3D Printing for the Fabrication of Patient-Tailored Immediate Release Tablets. Int J Pharm. 2016;513:659–68. doi: 10.1016/j.ijpharm.2016.09.050. https://doi.org/10.1016/j.ijpharm.2016.09.050. [DOI] [PubMed] [Google Scholar]
- 60.Li Q, Wen H, Jia D, et al. Preparation and Investigation of Controlled-release Glipizide Novel Oral Device with Three-dimensional Printing. Int J Pharm. 2017;525:5–11. doi: 10.1016/j.ijpharm.2017.03.066. https://doi.org/10.1016/j.ijpharm.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 61.Vo AQ, Zhang J, Nyavanandi D, et al. Hot Melt Extrusion Paired Fused Deposition Modeling 3D Printing to Develop Hydroxypropyl Cellulose Based Floating Tablets of Cinnarizine. Carbohydr Polym. 2020;246:116519. doi: 10.1016/j.carbpol.2020.116519. https://doi.org/10.1016/j.carbpol.2020.116519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel NG, Serajuddin AT. Development of FDM 3D-Printed Tablets with Rapid Drug Release, High Drug-polymer Miscibility and Reduced Printing Temperature by Applying the Acid-base Supersolubilization (ABS) Principle. Int J Pharm. 2021;600:120524. doi: 10.1016/j.ijpharm.2021.120524. https://doi.org/10.1016/j.ijpharm.2021.120524. [DOI] [PubMed] [Google Scholar]
- 63.Tabriz AG, Nandi U, Hurt AP, et al. 3D Printed Bilayer Tablet with Dual Controlled Drug Release for Tuberculosis Treatment. Int J Pharm. 2021;593:120147. doi: 10.1016/j.ijpharm.2020.120147. https://doi.org/10.1016/j.ijpharm.2020.120147. [DOI] [PubMed] [Google Scholar]
- 64.Khaled SA, Burley JC, Alexander MR, et al. Desktop 3D Printing of Controlled Release Pharmaceutical Bilayer Tablets. Int J Pharm. 2014;461:105–11. doi: 10.1016/j.ijpharm.2013.11.021. https://doi.org/10.1016/j.ijpharm.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 65.Khaled SA, Burley JC, Alexander MR, et al. 3D Printing of Tablets Containing Multiple Drugs with Defined Release Profiles. Int J Pharm. 2015;494:643–50. doi: 10.1016/j.ijpharm.2015.07.067. https://doi.org/10.1016/j.ijpharm.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 66.Khaled S, Burley J, Alexander M, et al. 3D Printing of Five-in-one dose Combination Polypill with Defined Immediate and Sustained Release Profiles. J Controlled Release. 2015;217:308–14. doi: 10.1016/j.jconrel.2015.09.028. https://doi.org/10.1016/j.jconrel.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 67.Olmos-Juste R, Guaresti O, Calvo-Correas T, et al. Design of Drug-loaded 3D Printing Biomaterial Inks and Tailor-made Pharmaceutical forms for Controlled Release. Int J Pharm. 2021;609:121124. doi: 10.1016/j.ijpharm.2021.121124. https://doi.org/10.1016/j.ijpharm.2021.121124. [DOI] [PubMed] [Google Scholar]
- 68.Wang J, Goyanes A, Gaisford S, et al. Stereolithographic (SLA) 3D Printing of Oral Modified-release Dosage forms. Int J Pharm. 2016;503:207–12. doi: 10.1016/j.ijpharm.2016.03.016. https://doi.org/10.1016/j.ijpharm.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Martinez PR, Goyanes A, Basit AW, et al. Fabrication of Drug-loaded Hydrogels with Stereolithographic 3D Printing. Int J Pharm. 2017;532:313–7. doi: 10.1016/j.ijpharm.2017.09.003. https://doi.org/10.1016/j.ijpharm.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Chen K, Zeng J, Lin G. Fabrication of 5-Fluorouracil-loaded Tablets with Hyperbranched Polyester by Digital Light Processing 3D Printing Technology. Eur Polym J. 2022;171:111190. https://doi.org/10.1016/j.eurpolymj.2022.111190. [Google Scholar]
- 71.Fina F, Madla C, Goyanes A, et al. Fabricating 3D Printed Orally Disintegrating Printlets using Selective Laser Sintering. Int J Pharm. 2018;541:101–7. doi: 10.1016/j.ijpharm.2018.02.015. https://doi.org/10.1016/j.ijpharm.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 72.Fina F, Goyanes A, Gaisford S, et al. Selective Laser Sintering (SLS) 3D Printing of Medicines. Int J Pharm. 2017;529:285–93. doi: 10.1016/j.ijpharm.2017.06.082. https://doi.org/10.1016/j.ijpharm.2017.06.082. [DOI] [PubMed] [Google Scholar]
- 73.Goyanes A, Kobayashi M, Martínez-Pacheco R, et al. Fused-filament 3D Printing of Drug Products:Microstructure Analysis and Drug Release Characteristics of PVA-based Caplets. Int J Pharm. 2016;514:290–5. doi: 10.1016/j.ijpharm.2016.06.021. https://doi.org/10.1016/j.ijpharm.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 74.Maroni A, Melocchi A, Parietti F, et al. 3D Printed Multi-compartment Capsular Devices for Two-pulse Oral Drug Delivery. J Controlled Release. 2017;268:10–8. doi: 10.1016/j.jconrel.2017.10.008. https://doi.org/10.1016/j.jconrel.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Genina N, Janßen E, Breitenbach A, et al. Evaluation of Different Substrates for Inkjet Printing of Rasagiline Mesylate. Eur J Pharm Biopharm. 2013;85:1075–83. doi: 10.1016/j.ejpb.2013.03.017. https://doi.org/10.1016/j.ejpb.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 76.Genina N, Fors D, Palo M, et al. Behavior of Printable Formulations of Loperamide and Caffeine on Different Substrates-Effect of Print Density in Inkjet Printing. Int J Pharm. 2013;453:488–97. doi: 10.1016/j.ijpharm.2013.06.003. https://doi.org/10.1016/j.ijpharm.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Meléndez P, Kane K, Ashvar C, et al. Thermal Inkjet Application in the Preparation of Oral Dosage Forms:Dispensing of Prednisolone Solutions and Polymorphic Characterization by Solid-State Spectroscopic Techniques. J Pharm Sci. 2008;97:2619–36. doi: 10.1002/jps.21189. https://doi.org/10.1002/jps.21189. [DOI] [PubMed] [Google Scholar]
- 78.Takala M, Helkiö H, Sundholm J, et al. Ink-jet Printing of Pharmaceuticals. In:8th International DAAAM Baltic Conference. 2012:233–9. [Google Scholar]
- 79.Wimmer-Teubenbacher M, Planchette C, Pichler H, et al. Pharmaceutical-grade Oral Films as Substrates for Printed Medicine. Int J Pharm. 2018;547:169–80. doi: 10.1016/j.ijpharm.2018.05.041. https://doi.org/10.1016/j.ijpharm.2018.05.041. [DOI] [PubMed] [Google Scholar]
- 80.Thabet Y, Lunter D, Breitkreutz J. Continuous Inkjet Printing of Enalapril Maleate onto Orodispersible Film Formulations. Int J Pharm. 2018;546:180–7. doi: 10.1016/j.ijpharm.2018.04.064. https://doi.org/10.1016/j.ijpharm.2018.04.064. [DOI] [PubMed] [Google Scholar]
- 81.Boudriau S, Hanzel C, Massicotte J, et al. Randomized Comparative Bioavailability of a Novel Three-dimensional Printed Fast-Melt Formulation of Levetiracetam Following the Administration of a Single 1000-mg Dose to Healthy Human Volunteers Under Fasting and Fed Conditions. Drugs R D. 2016;16:229–38. doi: 10.1007/s40268-016-0132-1. https://doi.org/10.1007/s40268-016-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jamróz W, Kurek M, Łyszczarz E, et al. 3D Printed Orodispersible Films with Aripiprazole. Int J Pharm. 2017;533:413–20. doi: 10.1016/j.ijpharm.2017.05.052. https://doi.org/10.1016/j.ijpharm.2017.05.052. [DOI] [PubMed] [Google Scholar]
- 83.Sjöholm E, Sandler N. Additive Manufacturing of Personalized Orodispersible Warfarin Films. Int J Pharm. 2019;564:117–23. doi: 10.1016/j.ijpharm.2019.04.018. https://doi.org/10.1016/j.ijpharm.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 84.He S, Radeke C, Jacobsen J, et al. Multi-material 3D Printing of Programmable and Stretchable Oromucosal Patches for Delivery of Saquinavir. Int J Pharm. 2021;610:121236. doi: 10.1016/j.ijpharm.2021.121236. https://doi.org/10.1016/j.ijpharm.2021.121236. [DOI] [PubMed] [Google Scholar]
- 85.Goyanes A, Det-Amornrat U, Wang J, et al. 3D Scanning and 3D Printing as Innovative Technologies for Fabricating Personalized Topical Drug Delivery Systems. J Controlled Release. 2016;234:41–8. doi: 10.1016/j.jconrel.2016.05.034. https://doi.org/10.1016/j.jconrel.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 86.Muwaffak Z, Goyanes A, Clark V, et al. Patient-specific 3D Scanned and 3D Printed Antimicrobial Polycaprolactone Wound Dressings. Int J Pharm. 2017;527:161–70. doi: 10.1016/j.ijpharm.2017.04.077. https://doi.org/10.1016/j.ijpharm.2017.04.077. [DOI] [PubMed] [Google Scholar]
- 87.Wei Y, Li-Tsang CW, Liu J, et al. 3D-Printed Transparent Facemasks in the Treatment of Facial Hypertrophic Scars of Young Children with Burns. Burns. 2017;43:e19–26. doi: 10.1016/j.burns.2016.08.034. https://doi.org/10.1016/j.burns.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 88.Azizoglu E, Ozer O. Fabrication of Montelukast Sodium Loaded Filaments and 3D Printing Transdermal Patches onto Packaging Material. Int J Pharm. 2020;587:119588. doi: 10.1016/j.ijpharm.2020.119588. https://doi.org/10.1016/j.ijpharm.2020.119588. [DOI] [PubMed] [Google Scholar]
- 89.Teoh JH, Tay SM, Fuh J, et al. Fabricating Scalable, Personalized Wound Dressings with Customizable Drug Loadings via 3D Printing. J Controlled Release. 2022;341:80–94. doi: 10.1016/j.jconrel.2021.11.017. https://doi.org/10.1016/j.jconrel.2021.11.017. [DOI] [PubMed] [Google Scholar]
- 90.Sun Y, Ruan X, Li H, et al. Fabrication of Non-dissolving Analgesic Suppositories using 3D Printed Moulds. Int J Pharm. 2016;513:717–24. doi: 10.1016/j.ijpharm.2016.09.073. https://doi.org/10.1016/j.ijpharm.2016.09.073. [DOI] [PubMed] [Google Scholar]
- 91.Chatzitaki AT, Tsongas K, Tzimtzimis EK, et al. 3D Printing of Patient-Tailored SNEDDS-based Suppositories of Lidocaine. J Drug Deliv Sci Technol. 2021;61:102292. https://doi.org/10.1016/j.jddst.2020.102292. [Google Scholar]
- 92.Holländer J, Genina N, Jukarainen H, et al. Three-Dimensional Printed PCL-Based Implantable Prototypes of Medical Devices for Controlled Drug Delivery. J Pharm Sci. 2016;105:2665–76. doi: 10.1016/j.xphs.2015.12.012. https://doi.org/10.1016/j.xphs.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 93.Genina N, Holländer J, Jukarainen H, et al. Ethylene Vinyl Acetate (EVA) as a New Drug Carrier for 3D Printed Medical Drug Delivery Devices. Eur J Pharm Sci. 2016;90:53–63. doi: 10.1016/j.ejps.2015.11.005. https://doi.org/10.1016/j.ejps.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 94.Tappa K, Jammalamadaka U, Ballard D, et al. Medication Eluting Devices for the Field of OBGYN (MEDOBGYN):3D Printed Biodegradable Hormone Eluting Constructs, a Proof of Concept Study. PLoS One. 2017;12:e0182929. doi: 10.1371/journal.pone.0182929. https://doi.org/10.1371/journal.pone.0182929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eder S, Wiltschko L, Koutsamanis I, et al. Toward a New Generation of Vaginal Pessaries via 3D-printing:Concomitant Mechanical Support and Drug Delivery. Eur J Pharm Biopharm. 2022;174:77–89. doi: 10.1016/j.ejpb.2022.04.001. https://doi.org/10.1016/j.ejpb.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 96.Indurkhya A, Patel M, Sharma P, et al. Chapter 6 Influence of Drug Properties and Routes of Drug Administration on the Design of Controlled Release System. Adv Pharm Prod Dev Res. 2018:i179–223. https://doi.org/10.1016/B978-0-12-814423-7.00006-X. [Google Scholar]
- 97.Kim YC, Park JH, Prausnitz MR. Microneedles for Drug and Vaccine Delivery. Adv Drug Deliv Rev. 2012;64:1547–68. doi: 10.1016/j.addr.2012.04.005. https://doi.org/10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pere CP, Economidou SN, Lall G, et al. 3D Printed Microneedles for Insulin Skin Delivery. Int J Pharm. 2018;544:425–32. doi: 10.1016/j.ijpharm.2018.03.031. https://doi.org/10.1016/j.ijpharm.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 99.Lim S, Ng J, Kang L. Three-dimensional Printing of a Microneedle Array on Personalized Curved Surfaces for Dual-pronged Treatment of Trigger Finger. Biofabrication. 2017;9:015010. doi: 10.1088/1758-5090/9/1/015010. https://doi.org/10.1088/1758-5090/9/1/015010. [DOI] [PubMed] [Google Scholar]
- 100.Uddin M, Scoutaris N, Klepetsanis P, et al. Inkjet Printing of Transdermal Microneedles for the Delivery of Anticancer Agents. Int J Pharm. 2015;494:593–602. doi: 10.1016/j.ijpharm.2015.01.038. https://doi.org/10.1016/j.ijpharm.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 101.Gittard S, Ovsianikov A, Akar H, et al. Two Photon Polymerization-Micromolding of Polyethylene Glycol-Gentamicin Sulfate Microneedles. Adv Eng Mater. 2010;12:B77–82. doi: 10.1002/adem.200980012. https://doi.org/10.1002/adem.200980012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gittard S, Miller P, Jin C, et al. Deposition of Antimicrobial Coatings on Microstereolithography-fabricated Microneedles. JOM. 2011;63:59–68. https://doi.org/10.1007/s11837-011-0093-3. [Google Scholar]
- 103.Shin D, Hyun J. Silk Fibroin Microneedles Fabricated by Digital Light Processing 3D Printing. J Ind Eng Chem. 2021;95:126–33. https://doi.org/10.1016/j.jiec.2020.12.011. [Google Scholar]
- 104.Johnson A, Caudill C, Tumbleston J, et al. Single-Step Fabrication of Computationally Designed Microneedles by Continuous Liquid Interface Production. PLoS One. 2016;11:e0162518. doi: 10.1371/journal.pone.0162518. https://doi.org/10.1371/journal.pone.0162518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu W, Zheng Q, Guo X, et al. A programmed Release Multi-drug Implant Fabricated by Three-dimensional Printing Technology for Bone Tuberculosis Therapy. Biomed Mater. 2009;4:065005. doi: 10.1088/1748-6041/4/6/065005. https://doi.org/10.1088/1748-6041/4/6/065005. [DOI] [PubMed] [Google Scholar]
- 106.Wu W, Zheng Q, Guo X, et al. The Controlled-releasing Drug Implant Based on the Three Dimensional Printing Technology:Fabrication and Properties of Drug Releasing. in Vivo J Wuhan Univ Technol Mater Sci Ed. 2009;24:977–81. https://doi.org/10.1007/s11595-009-6977-1. [Google Scholar]
- 107.Wu G, Wu W, Zheng Q, et al. Experimental Study of PLLA/INH Slow Release Implant Fabricated by Three Dimensional Printing Technique and Drug Release Characteristics. in vitro BioMed Eng Online. 2014;13:97. doi: 10.1186/1475-925X-13-97. https://doi.org/10.1186/1475-925X-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu W, Ye C, Zheng Q, et al. A Therapeutic Delivery System for Chronic Osteomyelitis via a Multi-drug Implant Based on Three-dimensional Printing Technology. J Biomater Appl. 2016;31:250–60. doi: 10.1177/0885328216640660. https://doi.org/10.1177/0885328216640660. [DOI] [PubMed] [Google Scholar]
- 109.Sandler N, Salmela I, Fallarero A, et al. Towards Fabrication of 3D Printed Medical Devices to Prevent Biofilm Formation. Int J Pharm. 2014;459:62–4. doi: 10.1016/j.ijpharm.2013.11.001. https://doi.org/10.1016/j.ijpharm.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 110.Ballard D, Weisman J, Jammalamadaka U, et al. Three-dimensional Printing of Bioactive Hernia Meshes:In vitro Proof of Principle. Surgery. 2017;161:1479–81. doi: 10.1016/j.surg.2016.08.033. https://doi.org/10.1016/j.surg.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 111.Weisman JA, Jammalamadaka U, Tappa K, et al. 3D Printing Antibiotic and Chemotherapeutic Eluting Catheters and Constructs. J Vasc Interv Radiol. 2015;26:S12. https://doi.org/10.1016/j.jvir.2014.12.040. [Google Scholar]
- 112.Misra SK, Ostadhossein F, Babu R, et al. 3D-Printed Multidrug-Eluting Stent from Graphene-Nanoplatelet-Doped Biodegradable Polymer Composite. Adv Healthc Mater. 2017;6:1700008. doi: 10.1002/adhm.201700008. https://doi.org/10.1002/adhm.201700008. [DOI] [PubMed] [Google Scholar]
- 113.Cui M, Hu N, Fang D, et al. Fabrication and Evaluation of Customized Implantable Drug Delivery System for Orthopedic Therapy Based on 3D Printing Technologies. Int J Pharm. 2022;618:121679. doi: 10.1016/j.ijpharm.2022.121679. https://doi.org/10.1016/j.ijpharm.2022.121679. [DOI] [PubMed] [Google Scholar]
- 114.Zhu M, Wang H, Liu J, et al. A Mesoporous Silica Nanoparticulate/b-TCP/BG Composite Drug Delivery System for Osteoarticular Tuberculosis Therapy. Biomaterials. 2011;32((7)):1986–95. doi: 10.1016/j.biomaterials.2010.11.025. https://doi.org/10.1016/j.biomaterials.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 115.Zhang J, Zhao S, Zhu M, et al. 3D-printed Magnetic Fe3O4/MBG/PCL Composite Scaffolds with Multifunctionality of Bone Regeneration, Local Anticancer Drug Delivery and Hyperthermia. J Mater Chem B. 2014;2:7583–95. doi: 10.1039/c4tb01063a. https://doi.org/10.1039/C4TB01063A. [DOI] [PubMed] [Google Scholar]
- 116.Min Z, Shichang Z, Chen X, et al. 3D-printed Dimethyloxallyl Glycine Delivery Scaffolds to Improve Angiogenesis and Osteogenesis. Biomater Sci. 2015;3:1236–44. doi: 10.1039/c5bm00132c. https://doi.org/10.1039/C5BM00132C. [DOI] [PubMed] [Google Scholar]
- 117.Ahlfeld T, Akkineni A, Förster Y, et al. Design and Fabrication of Complex Scaffolds for Bone Defect Healing:Combined 3D Plotting of a Calcium Phosphate Cement and a Growth Factor-Loaded Hydrogel. Ann Biomed Eng. 2016;45:224–36. doi: 10.1007/s10439-016-1685-4. https://doi.org/10.1007/s10439-016-1685-4. [DOI] [PubMed] [Google Scholar]
- 118.Cui M, Pan H, Li L, et al. Exploration and Preparation of Patient-specific Ciprofloxacin Implants Drug Delivery System Via 3D Printing Technologies. J Pharm Sci. 2021;110:3678–89. doi: 10.1016/j.xphs.2021.08.004. https://doi.org/10.1016/j.xphs.2021.08.004. [DOI] [PubMed] [Google Scholar]
- 119.Cui M, Pan H, Fang D, et al. 3D Printed Personalized Amikacin Sulfate Local Drug Delivery System for Bone Defect Therapy. J Drug Deliv Sci Technol. 2022;70:103208. https://doi.org/10.1016/j.jddst.2022.103208. [Google Scholar]
- 120.Maher S, Kaur G, Lima-Marques L, et al. Engineering of Micro-to Nanostructured 3D-Printed Drug-Releasing Titanium Implants for Enhanced Osseointegration and Localized Delivery of Anticancer Drugs. ACS Appl Mater Interfaces. 2017;9:29562–70. doi: 10.1021/acsami.7b09916. https://doi.org/10.1021/acsami.7b09916. [DOI] [PubMed] [Google Scholar]
- 121.Parry JA, Olthof MG, Shogren KL, et al. Three-Dimension-Printed Porous Poly (Propylene Fumarate) Scaffolds with Delayed rhBMP-2 Release for Anterior Cruciate Ligament Graft Fixation. Tissue Eng Part A. 2017;23:359–65. doi: 10.1089/ten.TEA.2016.0343. https://doi.org/10.1089/ten.tea.2016.0343. [DOI] [PubMed] [Google Scholar]
- 122.Huang W, Zheng Q, Sun W, et al. Levofloxacin Implants with Predefined Microstructure Fabricated by Three-dimensional Printing Technique. Int J Pharm. 2007;339:33–8. doi: 10.1016/j.ijpharm.2007.02.021. https://doi.org/10.1016/j.ijpharm.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 123.Xu X, Goyanes A, Trenfield SJ, et al. Stereolithography (SLA) 3D Printing of a Bladder Device for Intravesical Drug Delivery. Mater Sci Eng C Mater Biol Appl. 2021;120:111773. doi: 10.1016/j.msec.2020.111773. https://doi.org/10.1016/j.msec.2020.111773. [DOI] [PubMed] [Google Scholar]
- 124.Soetedjo AA, Lee JM, Lau HH, et al. Tissue Engineering and 3D Printing of Bioartificial Pancreas for Regenerative Medicine in Diabetes. Trends Endocrinol Metab. 2021;32:609–22. doi: 10.1016/j.tem.2021.05.007. https://doi.org/10.1016/j.tem.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 125.Dhavalikar P, Lan Z, Kar R, et al. 1.4.8-Biomedical Applications of Additive Manufacturing. Biomater Sci (Fourth Ed) 2020:623–39. https://doi.org/10.1016/B978-0-12-816137-1.00040-4. [Google Scholar]
- 126.Barua R, Datta S, Roychowdhury A, et al. “Importance of 3D printing technology in medical fields”. In: Zindani D, Paulo J, Kumar DK, editors. Additive Manufacturing Technologies From an Optimization Perspective. United States: IGI Global; 2019. pp. 21–40. https://doi.org/10.4018/978-1-5225-9167-2.ch002. [Google Scholar]
- 127.Seoane-Viano I, Trenfield SJ, Basit AW, et al. Translating 3D Printed Pharmaceuticals:From Hype to Real-world Clinical Applications. Adv Drug Deliv Rev. 2021;174:553–75. doi: 10.1016/j.addr.2021.05.003. https://doi.org/10.1016/j.addr.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 128.Kalyan BG, Kumar L. 3D Printing:Applications in Tissue Engineering, Medical Devices, and Drug Delivery. AAPS PharmSciTech. 2022;23:92. doi: 10.1208/s12249-022-02242-8. https://doi.org/10.1208/s12249-022-02242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morrison RJ, Kashlan KN, Flanangan CL, et al. Regulatory Considerations in the Design and Manufacturing of Implantable 3D-Printed Medical Devices. Clin Transl Sci. 2015;8:594–600. doi: 10.1111/cts.12315. https://doi.org/10.1111/cts.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mohapatra S, Kar RK, Biswal PK, et al. Approaches of 3D Printing in Current Drug Delivery. Sensors Int. 2022;3:100146. https://doi.org/10.1016/j.sintl.2021.100146. [Google Scholar]
- 131.Varghese R, Sood P, Salvi S, et al. 3D Printing in the Pharmaceutical Sector:Advances and Evidences. Sensors Int. 2022;3:100177. https://doi.org/10.1016/j.sintl.2022.100177. [Google Scholar]
- 132.Ng WL, Lee JM, Zhou M, et al. Vat polymerization Based Bioprinting-process, Materials, Applications and Regulatory Challenges. Biofabrication. 2020;12:022001. doi: 10.1088/1758-5090/ab6034. https://doi.org/10.1088/1758-5090/ab6034. [DOI] [PubMed] [Google Scholar]
- 133.Smith J, Shanler M. Stamford: Gartner Research; 2021. Hype Cycle for Life Science Research and Development 2021; pp. 1–128. [Google Scholar]
- 134.Ong JJ, Muniz B, Gaisford S, et al. Accelerating 3D Printing of Pharmaceutical Products using Machine Learning. Int J Pharm. 2022;4:100120. doi: 10.1016/j.ijpx.2022.100120. https://doi.org/10.1016/j.ijpx.2022.100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yu C, Jiang J. A Perspective on using Machine Learning in 3D Bioprinting. Int J Bioprint. 2020;6:253. doi: 10.18063/ijb.v6i1.253. https://doi.org/10.18063/ijb.v6i1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lao W, Li M, Wong TN, et al. Improving Surface Finish Quality in Extrusion-based 3D Concrete Printing using Machine Learning-based Extrudate Geometry Control. Virtual Phys Prototyp. 2020;15:178–93. [Google Scholar]
- 137.Ng WL, Chan A, Ong YS, et al. Deep Learning for Fabrication and Maturation of 3D Bioprinted Tissues and Organs. Virtual Phys Prototyp. 2020;15:340–58. [Google Scholar]
- 138.Sing SL, Kuo CN, Shih CT, et al. Perspectives of using Machine Learning in Laser Powder Bed Fusion for Metal Additive Manufacturing. Virtual Phys Prototyp. 2021;16:372–86. [Google Scholar]
- 139.Lyu J, Manoochehri S. Online Convolutional Neural Network-based Anomaly Detection and Quality Control for Fused Filament Fabrication Process. Virtual Phys Prototyp. 2021;16:160–77. [Google Scholar]
- 140.An J, Chua CK, Mironov V. Application of Machine Learning in 3D Bioprinting:Focus on Development of Big Data and Digital Twin. Int J Bioprint. 2021;7:342. doi: 10.18063/ijb.v7i1.342. https://doi.org/10.18063/ijb.v7i1.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fu Z, Angeline V, Sun W. Evaluation of Printing Parameters on 3D Extrusion Printing of Pluronic Hydrogels and Machine Learning Guided Parameter Recommendation. Int J Bioprint. 2021;7:434. doi: 10.18063/ijb.v7i4.434. https://doi.org/10.18063/ijb.v7i4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dong G, Yeong WY. Applications of Machine Learning in 3D Printing. Mater Today Proceed. 2022;54:2214–7853. https://doi.org/10.1016/j.matpr.2022.08.551. [Google Scholar]
- 143.Rahmani S, Ozcan O, Tasoglu S. Machine Learning-Enabled Optimization of Extrusion-Based 3D printing. Methods. 2022;206:27–40. doi: 10.1016/j.ymeth.2022.08.002. https://doi.org/10.1016/j.ymeth.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 144.Elbadawi M, McCoubrey LE, Gavins FK, et al. Disrupting 3D Printing of Medicines with Machine Learning. Trends Pharmacol Sci. 2021;42:745–57. doi: 10.1016/j.tips.2021.06.002. https://doi.org/10.1016/j.tips.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 145.Simões MF, Silva G, Pinto AC, et al. Artificial Neural Networks Applied to Quality-by-Design:From Formulation Development to Clinical Outcome. Eur J Pharm Biopharm. 2020;152:282–95. doi: 10.1016/j.ejpb.2020.05.012. https://doi.org/10.1016/j.ejpb.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 146.Sarabi MR, Alseed MM, Karagoz AA, et al. Machine Learning-enabled Prediction of 3D-printed Microneedle Features. Biosensors. 2022;12:491. doi: 10.3390/bios12070491. https://doi.org/10.3390/bios12070491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ahmed A, Arya S, Gupta V, et al. 4D Printing:Fundamentals, Materials, Applications and Challenges. Polymer. 2021;228:123926. https://doi.org/10.1016/j.polymer.2021.123926. [Google Scholar]
- 148.Chua CK. Publication Trends in 3D Bioprinting and 3D Food Printing. Int J Bioprint. 2020;6:257. doi: 10.18063/ijb.v6i1.257. https://doi.org/10.18063/ijb.v6i1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


