Abstract
Chemical overexposures and war-related stress during the 1990–1991 Gulf War (GW) are implicated in the persisting pathological symptoms that many GW veterans continue to endure. These symptoms culminate into a disease known as Gulf War Illness (GWI) and affect about a third of the GW veteran population. Currently, comprehensive effective GWI treatment options are unavailable. Here, an established GWI mouse model was utilized to explore the (1) long-term behavioral and neuroinflammatory effects of deployment-related GWI chemicals exposure and (2) ability of the immunotherapeutic lacto-N-fucopentaose III (LNFPIII) to improve deficits when given months after the end of exposure. Male C57BL6/J mice (8–9 weeks old) were administered pyridostigmine bromide (PB) and DEET for 14 days along with corticosterone (CORT; latter 7 days) to emulate wartime stress. On day 15, a single injection of the nerve agent surrogate diisopropylfluorophosphate (DFP) was given. LNFPIII treatment began 7 months post GWI chemicals exposure and continued until study completion. A battery of behavioral tests for assessment of cognition/memory, mood, and motor function in rodents was performed beginning 8 months after exposure termination and was then followed by immunohistochemcal evaluation of neuroinflammation and neurogenesis. Within tests of motor function, prior GWI chemical exposure led to hyperactivity, impaired sensorimotor function, and altered gait. LNFPIII attenuated these motor-related deficits and improved overall grip strength. GWI mice also exhibited more anxiety-like behavior that was reduced by LNFPIII; this was test-specific. Short-term, but not long-term memory, was impaired by prior GWI exposure; LNFPIII improved this measure. In the brains of GWI mice, but not in mice treated with LNFPIII, glial activation was increased. Overall, it appears that months after exposure to GWI chemicals, behavioral deficits and neuroinflammation are present. Many of these deficits were attenuated by LNFPIII when treatment began long after GWI chemical exposure termination, highlighting its therapeutic potential for veterans with GWI.
Keywords: Gulf war illness, LNFPIII, Pesticides, Behavior, Neuroinflammation, Neurogenesis
Highlights
-
•
Neurobehavioral alterations were present months post GWI chemicals exposure.
-
•
Prior GWI exposure produced increased hippocampal glial activation.
-
•
Delayed LNFPIII treatment improved multiple neurobehavioral measures.
-
•
Delayed LNFPIII treatment reduced hippocampal glial activation and improved neurogenesis.
1. Introduction
Thirty years after the 1990–1991 Gulf War (GW), approximately 30% of the United States personnel that served continue to suffer from Gulf War Illness (GWI; White et al., 2016). GWI veterans experience a multitude of symptoms, including neurological and immunological aberrations. Over the past three decades, several etiological factors have been investigated, and a consensus emerged linking GWI pathogenesis to wartime chemical overexposures like pesticides, nerve agents, and a nerve agent prophylactic superimposed with war-related psychological and environmental stressors (White et al., 2016). These combinatorial chemical overexposures putatively culminate in the symptom presentation of GWI, as associations between GW deployment-related exposures and GWI symptomology are well-documented (Janulewicz et al., 2017; Proctor et al., 1998, 2006; Toomey et al., 2009; Zundel et al., 2019).
GWI veterans undergoing neuropsychological evaluations report deficits in motor (Anger et al., 1999b; Hom et al., 1997; Proctor et al., 2006; Toomey et al., 2009) and cognitive (Anger et al., 1999a; Goldstein et al., 1996; Hom et al., 1997; Janulewicz et al., 2017; Toomey et al., 2009) tasks. Veterans with GWI also have higher rates of mood dysfunction (Binder et al., 1999; Hom et al., 1997; Lindem et al., 2003; Sillanpaa et al., 1997) that might impact other reported symptoms (i.e., cognitive and memory complaints) and performance in neuropsychological tests of sustained attention, motor coordination, and executive functioning (Binder et al., 1999; Lindem et al., 2003; Sillanpaa et al., 1997). These behavioral deficits may be linked to altered brain architecture, as clinical MRI studies point to abnormalities in brain areas, such as the hippocampus and cerebellum, that govern these behaviors (Calley et al., 2010; Chao, 2020; Chao et al., 2010, 2011, 2015, 2016; Christova et al., 2017; Gopinath et al., 2012; Hubbard et al., 2014; James et al., 2017; Van Riper et al., 2017; Washington et al., 2020; Wylie et al., 2019; Zhang et al., 2020, 2021).
Accumulating clinical evidence highlights immune system dysregulation, inflammation in particular, at the core of GWI (Alshelh et al., 2020; Broderick et al., 2011, 2013; Khaiboullina et al., 2015; Parkitny et al., 2015). Due to the innate difficulties in assessing the neuroimmune state in the brains of living GWI veterans, the majority of these clinical data rely on peripheral measures of inflammation. However, a recent MRI study observed widespread cortical neuroinflammation in the brains of veterans with GWI; the primary motor and somatosensory cortices were among the affected areas (Alshelh et al., 2020).
Current GWI preclinical research focuses on utilizing various paradigms of GWI chemical combinations and exposure paradigms to elucidate the underlying disease mechanisms. Relevant GWI chemicals include insecticides/repellants (i.e., permethrin (PM), DEET, chlorpyrifos), a nerve agent prophylactic (pyridostigmine bromide, PB), and an organophosphate nerve agent exposure mimic (diisopropylfluorophosphate, DFP) (Ribeiro and Deshpande, 2021). The translational value of GWI animal models has been established by recapitulating key features of GWI pathophysiology like behavioral performance aberrations (i.e., cognitive, motor, mood dysfunction, and pain) and numerous biological alterations (i.e., gut dysbiosis, elevated oxidative stress, glial activation, neurochemical alterations, chronic neuroinflammation, and leaky blood-brain barrier (reviewed in: Dickey et al., 2021; Ribeiro and Deshpande, 2021; White et al., 2016). In addition to neuroinflammation, decreases in hippocampal neurogenesis have been observed in GWI models that correspond to behavioral impairments (i.e., cognition and memory; Iannucci et al., 2022; Kodali et al., 2018a; Parihar et al., 2013; Shetty et al., 2020).
One established GWI model utilizes exposure to the GWI-relevant chemicals PB, the insect repellent DEET, corticosterone (CORT) to emulate war-related stress, and DFP (O'Callaghan et al., 2015). Acutely, the PB/DEET/CORT/DFP model produced widespread neuroinflammation in multiple areas of the brain including the prefrontal cortex, hippocampus, and striatum (O'Callaghan et al., 2015). In our studies, we found similar acute neuroinflammatory effects (i.e., increases in TNF-α, IL-1β, IL-6, CCL-2, YM-1, and HMGB1) within the hippocampus (Carpenter et al., 2020). Additionally, acute widespread monoaminergic disbalance, i.e., increases in the utilization of serotonin (nucleus accumbens and ventral hippocampus) and dopamine (nucleus accumbens, dorsal hippocampus, and ventral hippocampus), was evident (Carpenter et al., 2020). Further, detailed time-course characterization of this model at 48 h, 7-, and 11-months post GWI chemicals exposure termination provided insights into the progression of hippocampal impairments, particularly in regard to synaptic plasticity and transmission (Brown et al., 2021a). In this study (Brown et al., 2021a), we found that basal synaptic transmission was increased at 48 h, had no change at 7 months, and decreased at 11 months post-PB/DEET/CORT/DFP exposure. Similar results were seen at 11 months when long-term potentiation was evaluated; these delayed reductions were consistent with a delayed onset of hippocampal neuroinflammation (IL-6) at this time point (Brown et al., 2021a). However, the long-term behavioral consequences in this GWI model remain unknown.
Curative treatment modalities for GWI do not exist. Nevertheless, several disease-modifying therapeutic interventions have been explored including cognitive behavioral therapy (Chao et al., 2021; Donta et al., 2003; Mathersul et al., 2020), mindfulness-based stress reduction (Kearney et al., 2016), dietary changes (Holton et al., 2020, 2021), and various pharmacological supplement treatments (i.e., Baraniuk et al., 2013; Donovan et al., 2021; Donta et al., 2004; Golier et al., 2016; Golomb et al., 2014; Helmer et al., 2020; Holodniy and Kaiser, 2019). Although these therapies have provided some symptomatic relief, they have limited efficacy in resolving the underlying causes of GWI (Dickey et al., 2021). Thus, preclinical GWI research aims at broader therapeutic target identification and testing. Considering the immune dysfunction in GWI, recent preclinical studies have explored the efficacy of several immune-targeted compounds that have shown some promising results (Joshi et al., 2018; Kodali et al., 2018a; Ribeiro et al., 2020; Shetty et al., 2020). In our studies, we have focused on one potent immunomodulatory and anti-inflammatory treatment, Lacto-N-fucopentaose III (LNFPIII), that may be an advantageous treatment option for GWI as demonstrated in two, chemically distinct GWI models (Brown et al., 2021a, 2021b; Carpenter et al., 2020, 2021; Mote et al., 2020). LNFPIII is a Lewis(X)-containing immunomodulatory glycan found in human breast milk and on parasitic helminths that, to date, has had no documented adverse effects (Atochina et al., 2008; Bhargava et al., 2012; Srivastava et al., 2014; Tundup et al., 2015; Zhu et al., 2012). LNFPIII, when conjugated to a dextran carrier, activates CD14/TLR-4 signaling for extracellular signal-regulated kinase (ERK)-dependent production of anti-inflammatory mediators to skew the inflammatory balance of the innate immune system in an anti-inflammatory direction (Atochina et al., 2008; Bhargava et al., 2012; Carpenter et al., 2020, 2021; Srivastava et al., 2014; Tundup et al., 2015; Zhu et al., 2012). Of note, in utilizing the PB/DEET/CORT/DFP and the pyridostigmine bromide/permethrin (PB/PM) GWI models, we demonstrated that LNFPIII was beneficial in restoring acute monoaminergic disbalance and neuroinflammation (both models) as well as long-term behavioral deficits (PB/PM model), neuroinflammation, and hippocampal synaptic plasticity impairments after these chemical exposures (Brown et al., 2021a, 2021b; Carpenter et al., 2020, 2021). In non-immune cells, ERK-activation (LNFPIII target) is important for neurogenesis (Hao et al., 2004; Ma et al., 2013).
Our earlier studies utilizing the PB/DEET/CORT/DFP GWI exposure paradigm determined the acute neuroinflammatory and monoaminergic effects (Carpenter et al., 2020), as well as neuroinflammatory effects and hippocampal functional alterations months post GWI treatment termination (Brown et al., 2021a), with neuroinflammation evaluation being limited to inflammatory cytokine mRNA in the hippocampus. However, the long-term impact of these GWI exposures on behavioral measures or glial activation has not been characterized. Moreover, whether LNFPIII treatment will be beneficial in this setting is unknown. Thus, the present study sought to evaluate the long-term implications of prior GWI exposure on neurobehavioral function, glial cell status, and hippocampal neurogenesis post GWI symptomatology induction. Additionally, we examined whether delayed treatment with the immunotherapeutic LNFPIII will be beneficial.
2. Materials and methods
2.1. Materials
The following chemicals were used for animal treatments: pyridostigmine bromide (PB; Sigma Aldrich, St. Louis, MO), N-Diethyl-3-methylbenzamide (DEET; Sigma Aldrich), corticosterone (CORT; Steraloids, Newport, RI), and diisopropylfluorophosphate (DFP; Sigma Aldrich). Lacto-N-fucopentaose III (LNFPIII) dextran conjugate was produced as in Carpenter et al. (2021). All additional chemicals and reagents, unless otherwise noted, were of analytical or higher grade and obtained from Sigma Aldrich or Fisher Scientific (Hampton, NH).
2.2. Animals
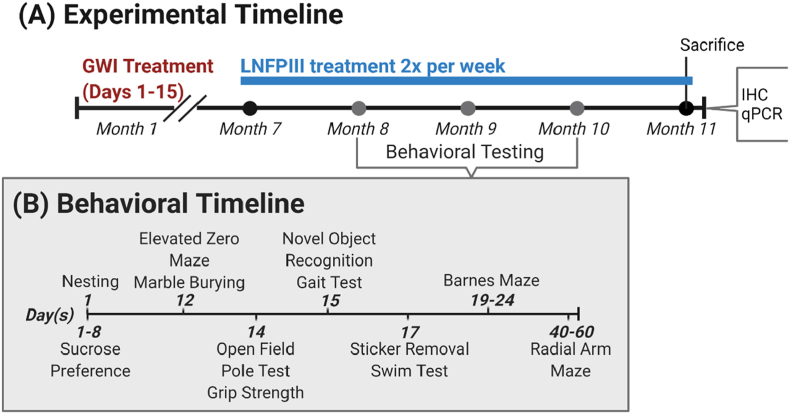
Male C57BL/6J mice (8–9 weeks old; N = 54, Jackson Laboratories, Bar Harbor, ME) were housed 4 per cage in an environmentally controlled room (22–24 °C) and maintained on a 12 h light/dark cycle (0700–1900 lights on) for one week of acclimation and throughout the study. Mice were handled daily for one week prior to the start of the study to minimize experimenter-induced stress. Food and water were available ad libitum. All procedures were approved in advance by the University of Georgia Institutional Animal Care and Use Committee and were in accordance with the latest National Institutes of Health guidelines. Body weights were measured daily during GWI chemicals exposure and biweekly afterward. During the radial arm maze (RAM), in which mice underwent food restriction, weight measurements were taken daily for monitoring as described under the RAM testing protocol. Refer to Fig. 1A for a detailed experimental timeline.
Fig. 1.
Experimental and behavioral timelines. (A) Depicts the experimental timeline in which mice received GWI-related chemicals: pyridostigmine bromide (PB) and DEET (days 1–14, SC), corticosterone (days 8–14, via drinking water), and diisopropylfluorophosphate (DFP, day 15, IP). Lacto-N-fucopentaose III (LNFPIII, SC) treatment began 7 months after GWI exposure. (B) Is a detailed timeline of behavioral tests that were performed during months 8–10.
2.3. Experimental design: GWI exposure and LNFPIII treatment
Following O'Callaghan et al. (2015) and our earlier work (Brown et al., 2021a; Carpenter et al., 2020), mice were treated for 14 days with a combination of PB and DEET (2 and 30 mg/kg, respectively; SC), or saline vehicle after randomization. On days 8–14, these mice were also administered CORT via drinking water (200 mg/L in 1.5% EtOH tap water) ad libitum; control mice were given the 1.5% EtOH vehicle water during this period. On day 15, mice received a single IP injection of DFP (3.75 mg/kg) or saline vehicle.
At 7 months post GWI exposure, mice within each of the two original treatments were randomly subdivided into LNFPIII or dextran vehicle groups. Mice were treated with LNFPIII or dextran vehicle (both 35 μg/mouse diluted in sterile saline; SC) twice a week until study completion as in (Carpenter et al., 2021). At this stage of the study, treatment groups were as follows: Vehicle-Vehicle (Saline/water/saline-Dextran, n = 16), Vehicle-LNFPIII (Saline/water/saline-LNFPIII, n = 12), GWI-Vehicle (PB/DEET/CORT/DFP-Dextran, n = 13), and GWI-LNFPIII (PB/DEET/CORT/DFP-LNFPIII, n = 13).
Behavioral testing began 8 months post GWI exposure and 1 month post LNFPIII treatment initiation. After behavioral testing completion, a subset of mice underwent hippocampal electrophysiology assessment (Brown et al., 2021a, Brown et al., 2021b) while the remaining mice received bromodeoxyuridine 3 weeks prior to euthanasia for post-mortem evaluation of hippocampal neurogenesis. Additionally, the brains from these mice were processed for immunohistochemistry and qPCR, for which some results have been published elsewhere (Brown et al., 2021a, Brown et al., 2021b).
2.4. Behavioral tests
One month after LNFPIII treatment initiation, a battery of behavioral tests for assessment of cognition/memory, mood, and motor function were performed as done previously (Carpenter et al., 2021). All behavioral testing, scoring, and analysis were done in a randomized, treatment-blind manner. Refer to Fig. 1B for a detailed behavioral testing timeline.
2.4.1. Nest building
Nest building was used to measure cognitive and motor functions as severe hippocampal (Deacon, 2012) and/or striatal (Hofele et al., 2001) damage leads to nest building deficits independent of sex. Briefly, mice were individually housed with a fresh paper nestlet (Bed-r’Nest, The Anderson's Inc., Maumee, OH). Nest pictures were taken from above at different time points (30 min, 60 min, 90 min, 120 min, 180 min, 240 min, 300 min, 360 min, and 24 h) after nestlet placement and scored based on a modified 4-point scale (1 = untouched, 4 = nest completed) as in (Carpenter et al., 2021; Deacon, 2012).
2.4.2. Sucrose preference (SP)
The SP test, used to measure anhedonia in mice (two-bottle choice paradigm; Eagle et al., 2016), was performed during behavioral days 1–8. Individually housed mice were first given access to two bottles filled with tap water for 4 days to establish a baseline and then to one bottle of tap water and one bottle of 1.5% sucrose water for 4 additional days. Solution intake was measured daily by weighing bottles, and bottle position was switched after each measurement to prevent side preference. Sucrose preference (%) was determined using the following equation: [(total sucrose intake/(sucrose + water intake)) x 100].
2.4.3. Elevated zero maze (EZM)
The EZM is a test frequently used to assess anxiety-like behaviors in mice but can also be expanded to assess certain motor function-related parameters (Braun et al., 2011; Conrad et al., 2011), including in a GWI context (Carpenter et al., 2021). On behavioral day 12, an EZM apparatus (Stoelting Co., Wood Dale, IL) was used under dim illumination (8W red bulb). Lighting conditions, measured with an URCERI light meter, were 30–40 lux and 10–15 lux in the open and closed quadrants, respectively. Starting at a central, open quadrant position facing a closed quadrant, mice were allowed to freely explore the maze for 5 min. All behaviors were tracked and scored using ANY-Maze software (Stoelting). Mice were in a quadrant when 70% of their body was in the area. Scored parameters of interest included time spent in the open and closed areas, number of entries into the open and closed areas, and the latency to enter the closed area at the start of the test as in Carpenter et al. (2021). Additionally, stretch attends from the closed arm were scored as in Bell et al. (2014).
2.4.4. Marble burying (MB)
Anxiety-like behaviors were also assessed using the MB test as previously described 3 h after the EZM (Carpenter et al., 2021). Briefly, clean testing cages were filled with 5 cm of corncob bedding (Bed-o’Cobs ¼ inch, The Anderson's Inc., Maumee, OH), and mice were individually placed in these cages for 10 min (habituation phase). After a 1 h resting period, mice were returned to the bedding-leveled testing cages with 20 marbles (diameter 10 mm, Panacea Products Corp., Columbus, OH) aligned 4 × 5 in the cage for 20 min (testing phase). Baseline and post-test pictures were captured, and the number of marbles buried (>50%) was counted for statistical analysis as in Carpenter et al. (2021).
2.4.5. Open field (OF)
Locomotor activity was assessed in an OF test as previously described (Carpenter et al., 2021) on behavior day 14. Briefly, mice were individually placed into an open field arena (25 cm × 25 cm x 40 cm; Coulbourn Instruments, Whitehall, PA) and allowed to freely explore for 30 min. The distance traveled, time spent in the center and periphery, and the number of vertical rearings were tracked and scored with the Limelight software (Actimetrics, Wilmette, IL) for both the total 30 min and per 5 min intervals.
2.4.6. Pole test (PT)
Following a 5-min resting period after the OF, a PT for motor coordination was used as in Carpenter et al. (2021). Mice were placed upright on a vertical pole (1 × 55 cm; d x h) and the times to turn and complete the test by reaching the bottom (total time) of the pole were recorded. Each mouse underwent 4 trials (5 min inter-trial interval) with the last 3 used for statistical analysis.
2.4.7. Grip strength (GS)
After a resting period following the PT, forelimb GS (in newtons, N) was measured over 4 trials (1-min inter-trial interval) using a gauge attached to a mouse-specific wire grid (6 cm × 6 cm; Bioseb, France) as detailed in Carpenter et al. (2021). Briefly, mice were carefully placed in front of the wire grid and allowed to grab hold with both forepaws. Once grip was established, mice were gently lifted to induce a gripping reflex. Average and max grip strengths were used for statistical analysis.
2.4.8. Novel object recognition (NOR)
The NOR test (Carpenter et al., 2021) was performed on day 15. Briefly, the previous day's OF test served as the NOR habituation phase. During the identical phase of the NOR, mice were placed in the open field with two identical objects and allowed to freely explore them for 5 min. Mice were then returned to their home cage for 1 h. During the novel phase, one identical object was replaced with a novel object. Mice were placed in the open field and allowed to explore both objects. The number of novel (n) or familiar (f) object approaches (N) and time spent (T) were scored for the first 20 s and the total 5 min of the novel phase with the Limelight software (Actimetrics) as in Carpenter et al. (2021). The novelty preference index (NPI) was calculated as described in Lin et al. (2013) using the following equations: NPI = (Nn − Nf)/(Nn + Nf) or NPI = (Tn − Tf)/(Tn + Tf).
2.4.9. Gait test
Following a 3-h home cage resting period after the NOR, gait was evaluated using the gait test (Carpenter et al., 2021). Briefly, a runway (82 cm long, 5.5 cm wide, with 8 cm high walls) lined with paper was used; an empty cage with home cage bedding was placed at the end of the runway during testing for escape. Prior to testing, the front and hind paws of the mice were painted with non-toxic red and black ink, respectively (Office Depot, item: #839–994 and #839–967). Two trials were conducted (5 min apart), a pre-trial and the test trial that was used for subsequent statistical analyses. Between each trial, the runway was cleaned with 70% EtOH and a new paper strip was placed in it. Gait stride length, base width, inter/intra-step distance, stride variability, total steps, cadence, and velocity were analyzed (Carpenter et al., 2021; Mulherkar et al., 2013; Wang et al., 2017).
2.4.10. Sticker removal (SR)
The SR test was used to determine sensorimotor function on behavioral day 17 as in (Carpenter et al., 2021; Fleming et al., 2013). Food was removed from the rodents’ home cage and ¾ of the bedding was transferred to a clean holding cage where the mice acclimated for 1 h. During the testing phase, a small circular sticker (Avery ¼” round label 5793, Office Depot item: # 113019) was applied using forceps to the nose of the mouse, and the mouse was placed into the home cage. This test was performed over three 90 s trials with a 5 min inter-trial rest. Parameters used for statistical analysis included the average and fastest times for contact and removal of the sticker (Carpenter et al., 2021)2.4.11 Swim Test (ST).
Following a 3 h rest after the SR, depressive-like behaviors were assessed using the ST as previously described (Carpenter et al., 2021). Briefly, mice were placed in a large beaker filled with 3 L of water (29 ± 2 °C) for 15 min. After each test, the beaker was sanitized thoroughly. Total climbing counts and times spent climbing, mobile, and immobile were manually scored using Limelight software (Actimetrics). Additionally, the time to the first immobile bout was measured using ANY-Maze (Stoelting).
2.4.11. Barnes Maze (BM)
The BM test was used to assess spatial learning and memory on behavioral testing days 19–24 (Carpenter et al., 2021), using a 20-hole circular maze (Stoelting) in which one hole is equipped with an escape box (target hole; TH). The maze is brightly illuminated to promote anxiogenic escape motivation (∼1000 lux measured by URCERI light meter). During the acquisition phase (days 1–4), mice were trained to learn and escape into the TH over four 3-min trials, and after trial completion, mice remained or were manually placed into the TH for 1 min. For the probe trial, the escape box was removed, and mice explored the maze for 90 s. Between mice, the maze was rotated and sanitized to remove any residual olfactory cues. The latency, distance, and number of errors to reach the TH were tracked and scored with the ANY-Maze software (Stoelting).
2.4.12. Radial arm maze (RAM)
An 8-arm RAM (Med Associates, St. Albans, VT) was used to assess learning and memory function 9 months post-GWI exposure as in (Carpenter et al., 2021). Prior to RAM testing, mice were single-housed and underwent a 14-day food restriction protocol until they reached 85% of their free-fed body weight; this was used to motivate learning of the RAM apparatus. During the last 3 days of the food restriction period, mice were behaviorally acclimated to the RAM as described previously (Carpenter et al., 2021; Preston et al., 2019).
Spatial short-term working memory was assessed by an 8-arm RAM foraging task over 10 consecutive days. Following a 20 min room acclimation period, each mouse was placed in the central RAM hub for 1 min before all 8 doors to each arm opened to allow free all-arm access. In the foraging task, each arm is “baited” so that one food reward was dispensed upon the first head poke into the food trough of each arm. An error in spatial short-term working memory was defined as an animal revisiting a trough after the initial visit, and the total number of errors for each animal was recorded. Animal performance was assessed by the average total number of errors of the first three days of testing, the last three days of testing, and comparing the two (Carpenter et al., 2021).
Spatial long-term working memory was evaluated over 10 consecutive days using a modified spatial Win-Shift task after the last day of the foraging task (Carpenter et al., 2021; Clark et al., 2015; Furgerson et al., 2014). This test incorporates a 2-phase (a study phase and a test phase) procedure with an interposed delay. During the study phase, 4 arms were randomly opened, allowing the animal to receive a reward from 4 baited arms. Following the completion of the study phase, the animal was returned to the home cage for a 4 min retention interval, and the maze was cleaned. Then, the animal was returned to the central hub for 1 min (total retention interval of 5 min) before initiation of the test phase in which all 8 doors were opened, allowing free access to all arms. However, only the 4 arms that were unopened in the study phase dispensed food reward. An error in spatial long-term working memory was defined as a visit to an arm that was baited in the study phase and the total number of errors was recorded. Animal performance was determined by assessing the mean errors from the first three days of testing, the last three days of testing, and comparing the two as in Carpenter et al. (2021).
2.5. Tissue processing and immunohistochemistry
Following methods from Littlefield et al. (2015), 5 days after completing the RAM, mice received 4 consecutive daily bromodeoxyuridine (BrdU; 75 mg/kg IP) treatments for evaluation of hippocampal neurogenesis. Twenty-five days after the last BrdU injection (11 months post GWI chemicals exposure), mice were euthanized, brains were removed, and split into two halves by a sagittal cut. One half was fresh frozen on dry ice and the other half was immersion fixed in 4% paraformaldehyde and transferred to 30% sucrose before flash freezing in isopentane as in (Coban and Filipov, 2007). All tissues were stored at −80 °C until analysis.
Fixed half-brains were coronally sectioned into 40-μm thick sections and placed in phosphate buffer at 4 °C until staining. To determine inflammation, free-floating sections containing the hippocampus were stained for microglia (IBA-1), astrocytes (GFAP), and an inflammasome marker, the apoptosis-associated spec-like protein (ASC; Yang et al. (2019). Additionally, free-floating hippocampal sections were stained for BrDU and doublecortin (DCX), an immature neuron marker, for determination of neurogenesis. Sections were incubated, as appropriate, with the following primary antibodies: 1) 1:1000 chicken anti-IBA-1 microglia marker (Aves Lab, Davis, CA) and 1:1000 rabbit anti-ASC (AdipoGen, San Diego, CA); 2) 1:2000 chicken anti-glial fibrillary acidic protein (GFAP) astrocyte marker (Aves Labs) and 1:1000 rabbit anti-ASC (AdipoGen); or 3) 1:1000 rat anti-BrDU (Abcam) and 1:1000 chicken anti-DCX (Aves Labs), all diluted in 0.1% TritonX-100 in PBS for 48 h at 4 °C. Following primary antibody incubation, sections were thoroughly washed and incubated with the appropriate secondary fluorescent antibodies (GFAP: 1:1000 goat anti-chicken 488, Abcam; IBA-1: 1:1000 goat anti-chicken 488, Abcam; ASC: 1:1000 goat anti-rabbit 594, Abcam; BrDU: goat anti-rat 488, Abcam; DCX: goat anti-chicken 594, Abcam) for 2 h at RT in the dark, followed by incubation with a nuclear stain for 5 min (Hoechst 33258, Invitrogen). Following the final washes, sections were mounted to slides and coverslipped with VectaMount (Vector Labs, Newark, CA).
Images were taken on a Zeiss Axioscope A1 microscope. The dentate gyrus of the hippocampus was captured at 5x-40x. Signal intensity (IBA-1, GFAP, and ASC staining) and cell counts (all staining) were analyzed using ImageJ software. For statistical analysis, the images’ signal intensity (3–4 images/section; 5–6 sections/animal) and/or cell counts (2–3 images/section; 3 sections/animal) were averaged per animal, and data were analyzed as in Carpenter et al. (2021).
2.6. qPCR for IL-10 and 18S
In addition to analyses already completed on tissues from animals described in (Brown et al., 2021a), mRNA levels of the anti-inflammatory cytokine IL-10 in the dorsal and ventral hippocampus were analyzed in this study. Briefly, total RNA from a single hippocampal brain punch (1.5 mm diameter, 500 μm thick section) was isolated by an E.Z.N.A total RNA isolation kit (Omega Bio-Tek, Norcross, GA) according to the manufacturer's directions. RNA was quantified with a Take3 micro-volume plate and Epoch microplate spectrophotometer (BioTek, Winooski, VT). Seventy-five ng RNA/sample was used to synthesize cDNA with a Maxima first-strand cDNA synthesis kit for RT-qPCR (Thermo Fisher Scientific, Waltham, MA) and a PTC-200 Peltier thermal cycler (Bio-Rad, Hercules, CA; 10 min at 25 °C, 15 min at 50 °C, 5 min at 85 °C). Using one (18S) or two (IL-10) ng of cDNA per sample, the expression of IL-10 and 18S as a house-keeping gene was determined by a qPCR with mouse-specific primers (RealTimePrimers, Elkins Park, PA; Supplemental Table 1) and Maxima SYBR Green/lowRox qPCR Master Mix (2x) (Thermo Fisher Scientific, Waltham, MA). Amplifications were performed on a Mx3005 P qPCR machine (Stratagene, San Diego, CA) programmed for an initial warming cycle (95 °C, 10 min) followed by 45 cycles (95 °C, 15 s, 60 °C, 1 min) with each sample run in duplicate.
2.7. Statistical analyses
A two-way analysis of variance (ANOVA) or two-way repeated analysis of variance (RM-ANOVA), as appropriate, were used to determine main effects of treatments or treatment interactions. If an ANOVA was significant (p ≤ 0.05), treatment means were separated by Student-Newman-Keuls (SNK) post hoc test or by pre-planned pairwise comparisons (Student's t-test, as appropriate). All data, except the RAM, were analyzed using SigmaPlot 12.5 (San Jose, CA), and all graphs were generated using GraphPad Prism (San Diego, CA). Statistical analyses for RAM data were done using R version 3.3.2 (R Development Core Team, 2016; https://www.r-project.org/) via aov() function for analysis of variance (ANOVA) and the t.test function for the Student's t-test and paired t-test.
3. Results
3.1. Body weights
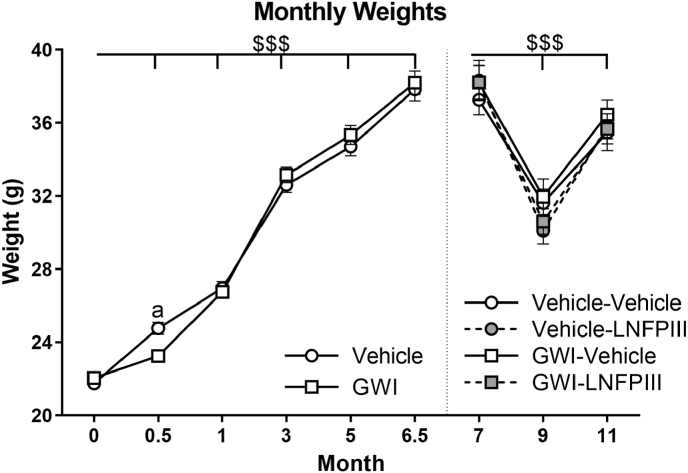
Body weights of the vehicle and GWI groups were not different at the study start (21.8 ± 0.21 g vs. 22.0 ± 0.24 g, p > 0.65), but significantly decreased in the GWI group immediately after end of treatment (0.5 months, p ≤ 0.05; Fig. 2), an acute DFP effect. Both groups gained weight over time (F(13,688) = 903.42, p ≤ 0.001) and were not different (p's > 0.23) at all subsequent time points up to 6.5 months post exposure (Fig. 2). At 7 months, these two groups were randomly subdivided into a LNFPIII or vehicle group for a total of 4 groups. No significant group differences in weight occurred during months 7–11 (Fig. 2). However, due to RAM food restriction during months 8 and 9, weights were significantly affected (F(8,361) = 214.51, p ≤ 0.001; Fig. 2), but recovered by the end of the study (9 vs. 11 months, p ≤ 0.001; Fig. 2); treatment was not a significant factor (p > 0.76).
Fig. 2.
Monthly Weights. Monthly weights were monitored and are presented for the start of study (0), post GWI exposure (0.5), start of LNFPIII treatment (6.5), prior to Radial Arm Maze (RAM) food restriction (7), during RAM food restriction (9), and end of study (11). Data are presented as mean ± SEM (n = 12–16/group). $$$ indicates significant main effect of time, p < 0.001. a indicates p < 0.05 from Vehicle.
3.2. Motor alterations after prior GWI exposure and delayed LNFPIII treatment
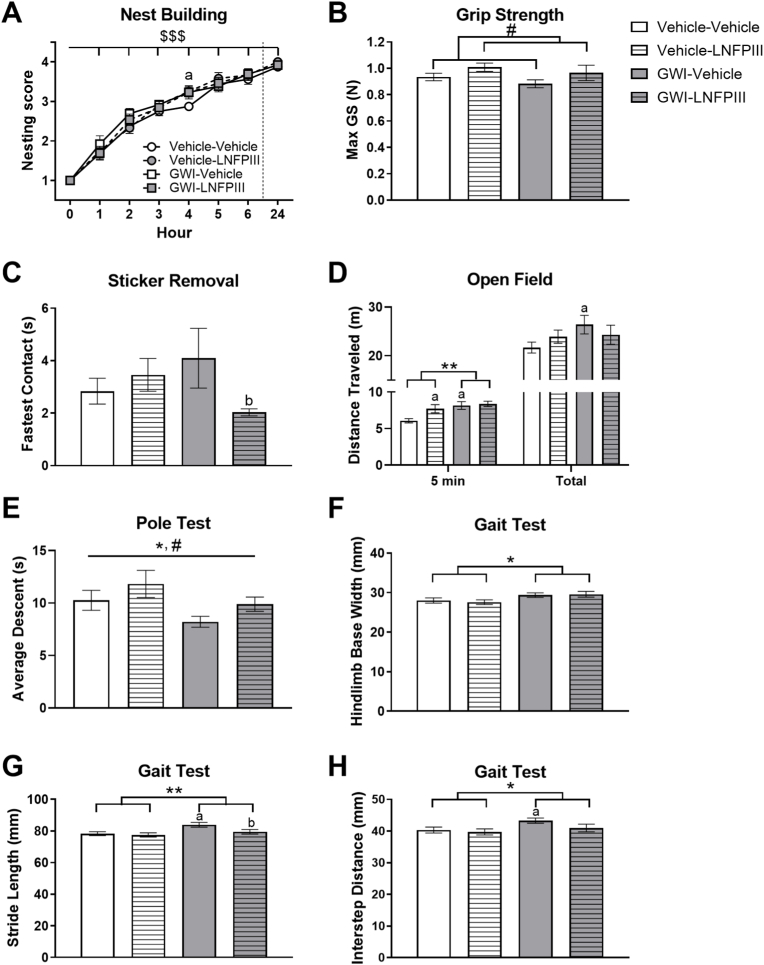
3.2.1. Nest building
Overall, nest building was not affected by any treatments as all groups built full nests over the course of 24 h (F(7,350) = 334.25, p ≤ 0.001; Fig. 3A). Post hoc analysis revealed a significant difference in nesting scores between the Vehicle-Vehicle and GWI-Vehicle groups (p ≤ 0.05) at 4 h, but this effect was transient, as there were no group differences at subsequent time points. This suggests that prior GWI exposure did not cause marked hippocampal or striatal deficits associated with decreased performance in this test.
Fig. 3.
Long-term motor effects of prior GWI exposure and delayed LNFPIII treatment. Several motor effects of prior GWI exposure and LNFPIII treatment were evaluated by nest building (A); grip strength (B); sticker contact (C) in the sticker removal test; distance traveled (D) in the open field; descent time (E) in the pole test; and hindlimb base width (F), stride length (G), and interstep distance (H) in the gait test. Data are presented as mean ± SEM (n = 12–16/group). * and ** indicate significant main effect of GWI treatment, p < 0.05 and 0.01, respectively. $$$ indicates significant main effect of time, p < 0.001. # indicates significant main effect of LNFPIII treatment, p < 0.05. a and b indicate p < 0.05 from Vehicle-Vehicle and GWI-Vehicle, respectively.
3.2.2. Grip strength (GS)
Grip strength (GS), a measure of neuromuscular function, was unaffected by prior GWI exposure for average and max GS (p's > 0.15). However, LNFPIII increased both and max (F(1,50) = 4.13, p ≤ 0.05; Fig. 3B) and the average (F(1,50) = 3.65, p = 0.06; Supplemental Fig. 1A) GS, suggesting LNFPIII might improve neuromuscular function irrespective of GWI treatment.
3.2.3. Sticker removal (SR)
Sensorimotor function, measured by SR, was affected by prior GWI chemical exposure; a significant treatment interaction was present for fastest contact (F(1,48) = 4.67, p ≤ 0.05; Fig. 3C). Here, GWI-Vehicle mice took longer to contact the sticker, an effect significantly prevented by LNFPIII treatment (p ≤ 0.05; Fig. 3C). Similar numerical increases were observed in the GWI-Vehicle group for the average contact, average removal, and fastest removal, but were not significant or altered by LNFPIII treatment (p's > 0.47). However, LNFPIII treatment tended to reduce the latency overall for sticker removal (F(1,48) = 3.86, p = 0.06; Supplemental Fig. 1B).
3.2.4. Open field (OF)
As expected, all mice habituated to the OF arena as evident by decreases in distance traveled (F(5,250) = 175.86, p ≤ 0.001) and line crossings (F(5,250) = 139.94, p ≤ 0.001, Supplemental Figs. 1C and 1D) over time. All mice, compared to the Vehicle-Vehicle group (p's ≤ 0.01; Fig. 3D), exhibited hyperactivity during the first 5 min of exploration, i.e., the distance traveled (F(1,50) = 7.50, p ≤ 0.01, Fig. 3D) and the line crossings (F(1,50) = 5.89, p ≤ 0.05, Supplemental Fig. 1E) were increased. Notably, only GWI mice remained hyperactive for the duration of the test (total distance: GWI-Vehicle vs Vehicle-Vehicle, t(27) = 2.21, p ≤ 0.05, Fig. 3D); this was not observed in mice treated with LNFPIII.
3.2.5. Pole test (PT)
Motor coordination during the PT was largely unaffected by GWI exposure or LNFPIII treatment as the average time to turn (p's > 0.87) and total time (p's > 0.15) were not significantly different. However, GWI mice descended the pole significantly faster (F(1,50) = 4.86, p ≤ 0.05, Fig. 3E), perhaps a reflection of their hyperactivity, while LNFPIII treated mice were slower to do so (F(1,50) = 4.05, p ≤ 0.05, Fig. 3E).
3.2.6. Gait test
Prior GWI exposure led to specific gait alterations. GWI mice exhibited an increase in hindlimb base width (F(1,50) = 6.59, p ≤ 0.05, Fig. 3F), stride length (F(1,50) = 7.47, p ≤ 0.01, Fig. 3G), and interstep distance (F(1,50) = 4.46, p ≤ 0.05, Fig. 3H), which, together, are indicative of hyperactivity. LNFPIII prevented the increase in stride length (p ≤ 0.05, Fig. 3G) and this preventative effect was numerical for interstep distance (Fig. 3H). Additionally, cadence was decreased by the LNFPIII treatment independent of GWI exposure, an effect that approached significance (F(1,50) = 3.78, p = 0.06, Supplemental Fig. 1F).
3.3. Mood effects after prior GWI exposure and delayed LNFPIII treatment
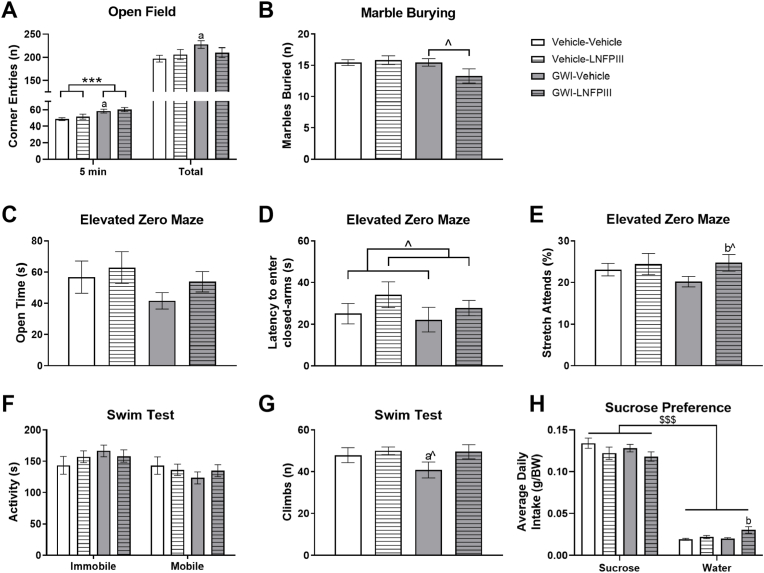
3.3.1. Open field (mood parameters)
Prior GWI exposure led to more anxiety-like behavior in the OF. During the 1st 5 min of the OF test, GWI mice made significantly more corner entries (F(1,50) = 15.29, p ≤ 0.001; Fig. 4A), and this was not altered by LNFPIII treatment. This effect persisted for only the GWI-Vehicle group as they entered (t(27) = 2.79, p ≤ 0.01; Fig. 4A) and spent more time (t(27) = 2.39, p ≤ 0.05; Supplementary Fig. 2B) in the corners than Vehicle-Vehicle mice for the 30 min test duration; this was not seen in GWI-LNFPIII mice.
Fig. 4.
Long-term mood effects of prior GWI exposure and delayed LNFPIII treatment. Mood disturbances of prior GWI exposure and LNFPIII treatment were evaluated by several tests for anxiety-like and depressive-like behaviors: corner entries (A) in the open field; number of marbles buried (B) in the marble burying test; open arm time (C), latency to enter closed-arm (D), and stretch attends while in the closed arm (E) of the elevated zero maze; immobile and mobile time (F) and climbing attempts (G) in the swim test; average daily fluid intake (H) in the sucrose preference test. Data are presented as mean ± SEM (n = 12–16/group). *** indicates significant main effect of treatment, p < 0.001. $$$ indicates significant main effect of drink in the SP test, p < 0.001. ˆ indicates p < 0.10. a and aˆ indicate p < 0.05 or 0.10, respectively, from Vehicle-Vehicle. b and bˆ indicate p < 0.05 or 0.10, respectively, from GWI-Vehicle.
3.3.2. Marble burying (MB)
The average number of marbles buried (≥50%) during the test, irrespective of treatment, was 15 (75%), and there were no significant main effects of GWI (p = 0.10) or LNFPIII (p ≥ 0.24). Numerically, the GWI-LNFPIII mice buried less than all other groups, with the difference compared GWI-Vehicle being greatest (t(24) = −1.70, p = 0.10, Fig. 4B); although not significant, this hints towards a subtle, LNFPIII anxiolytic benefit in the presence of GWI.
3.3.3. Elevated zero maze (EZM)
No significant treatment differences were observed when overall time spent in the open and closed arms of the EZM was analyzed (p's > 0.17). No significance differences were also observed when the number of entries into either EZM segments was analyzed (Supplementary Fig. 2A). Interestingly, prior to the first closed arm entry, LNFPIII treated mice tended to spend longer periods of time in the starting open arm (F(1,50) = 3.66, p = 0.06; Fig. 4D), suggesting LNFPIII may have mild initial anxiolytic effects. Moreover, when the percentage of stretch attends from the closed arm was analyzed for the time spent in the closed arm, there was a numerical overall increase with LNFPIII treatment (p = 0.11). Here, LNFPIII treated GWI mice had a trending increase in the percentage of time stretching out from the closed arm (t(24) = −1.94, p = 0.06; Fig. 4E).
3.3.4. Swim test (ST)
Times spent immobile or mobile were not significantly affected by GWI or LNFPIII during the 1st 5 min of the test (p's > 0.29; Fig. 4F), although a numerical increase and decrease were present for immobile and mobile times, respectively, in the GWI-Vehicle group. In line with this observation, there was a slight decrease for climbing attempts in GWI-Vehicle mice that was absent with LNFPIII treatment (t(24) = −1.71, p = 0.09; Fig. 4G). There were no other effects observed among treatment groups.
3.3.5. Sucrose preference
All mice displayed a strong sucrose preference (F(4,98) = 349.38, p ≤ 0.001; Fig. 4H), but there were no significant differences among treatment groups for sucrose consumption. However, for average water intake, GWI mice receiving LNFPIII treatment consumed significantly more water (p ≤ 0.001; Fig. 4H).
3.4. Cognition and memory effects after prior GWI and delayed LNFPIII treatment
3.4.1. Novel object recognition (NOR)
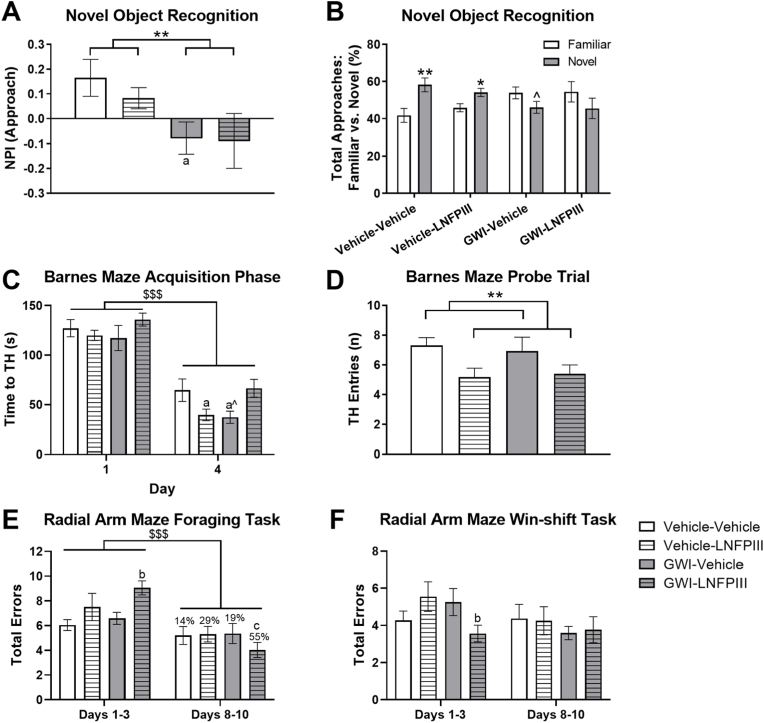
Prior GWI chemical exposure led to impairments in short-term object recognition memory in the NOR test. A significant GWI effect was present in the approach novelty preference index (NPI) for the first 20 s (F(1,50) = 6.25, p ≤ 0.05; Supplementary Fig. 3A) and total time (F(1,50) = 6.95, p ≤ 0.01; Fig. 5A) of the test. Post hoc analysis revealed that GWI-Vehicle mice had a lower NPI than Vehicle-Vehicle (p ≤ 0.05; Fig. 5A) overall. Vehicle-Vehicle and Vehicle-LNFPIII mice approached, as expected, the novel object more than the familiar one (p's ≤ 0.05), while the GWI-Vehicle numerically approached the novel object less (p = 0.09; Fig. 5B). LNFPIII treatment did not modulate these GWI effects.
Fig. 5.
Long-term cognition and memory effects of prior GWI exposure and delayed LNFPIII treatment. Cognition and memory were evaluated by several tests: novel preference index (NPI) (A) and total approaches % (B) in the novel object recognition test; acquisition phase time to target hole (TH) (C) and probe trial TH entries (D) in the Barnes Maze; and total errors in the Radial Arm Maze (RAM) foraging task (E) and Win-shift task (F). Additionally, improvement percentages from days 1–3 were calculated for the RAM foraging task (E). Data are presented as mean ± SEM (n = 12–16/group). * and ** indicate significance, p < 0.05 and 0.01, respectively. $$$ indicates significant main effect of time, p < 0.001. ˆ indicates p < 0.10. a and aˆ indicate p < 0.05 or 0.10, respectively, from Vehicle-Vehicle. b indicates p < 0.05 from GWI-Vehicle. c indicates p < 0.05 from days 1–3 in the RAM.
3.4.2. Barnes Maze (BM)
During the acquisition phase of the Barnes Maze (BM), all mice learned the target hole (TH) location as they made fewer errors (F(1,50) = 174.79, p ≤ 0.001), entered the TH faster (F(1,50) = 241.94, p ≤ 0.001, Fig. 5C), and traveled less distance (F(1,50) = 183.70, p ≤ 0.001) over the 4 training days. Post hoc analysis revealed that, within day 4, the Vehicle-Vehicle and GWI-LNFPIII groups tended to take longer to enter the TH (p's ≤ 0.05 and 0.07, respectively; Fig. 5C), make more errors (p's > 0.06; data not shown), and travel more distance (p's > 0.05; data not shown) than Vehicle-LNFPIII and GWI-Vehicle groups, respectively. However, the average improvement from day 1 to day 4 was 60% for time to TH, errors, and distance; there were no significant main effects of treatment (p's > 0.62; Supplementary Figs. 3B, 3C, 3D). In the BM probe trial, there were no significant main effects for the number of errors before reaching the TH, latency to TH, or distance to TH (p's > 0.15; Supplementary Figs. 3E, 3F, 3G). Of note, mice treated with LNFPIII, irrespective of GWI, made significantly fewer entries into the TH (F(1, 50) = 7.42, p ≤ 0.01, Fig. 5D) and tended to travel less distance (F(1,50) = 3.58, p = 0.07; Supplementary Fig. 3H).
3.4.3. Radial arm maze (RAM)
Following 10 consecutive days of training in a dorsal hippocampus (dH)-dependent 8-arm radial arm maze foraging task, there was a significant main effect of training (F(1,49) = 23.9, p ≤ 0.001; Fig. 5E), a significant LNFPIII treatment × training interaction effect (F(1,49) = 7.54, p ≤ 0.01), and a numerical GWI × training interaction effect (F(1,49) = 3.29, p = 0.08) on performance in the foraging task. Post hoc analyses revealed that initially, during days 1–3, GWI-LNFPIII mice made significantly more errors than GWI-Vehicle mice (p ≤ 0.05, Fig. 5E). However, GWI-LNFPIII treated mice reduced their average total errors on days 8–10 compared to days 1–3 significantly (p's < 0.01, Fig. 5E). Improved performance in Vehicle-LNFPIII treated mice also approached significance (p = 0.06). These data suggest that delayed LNFPIII treatment improves learning rate in dH-dependent short-term spatial working memory in and, possibly, outside of a GWI context.
Animals then underwent training in a ventral hippocampus (vH)-dependent modified Win-shift task, a task that assesses long-term spatial working memory. There were no significant effects of training, GWI chemical exposure, or LNFPIII treatment on performance in the Win-shift task (p's > 0.13; Fig. 5F). No significant treatment × training interaction effects were observed; however, a significant three-way interaction between GWI chemical exposure x LNFPIII treatment x training was detected (F(1,49) = 4.09, p ≤ 0.05; Fig. 5F). Notably, the performance of GWI-LNFPIII mice was significantly better than the GWI-Vehicle mice on days 1–3 (p ≤ 0.05; Fig. 5F), indicating that delayed LNFPIII treatment improved performance in the acquisition component of the Win-shift task months after exposure to GWI chemicals.
3.5. Evaluation of hippocampal inflammation and neurogenesis post GWI exposure and delayed LNFPIII treatment
3.5.1. GFAP, IBA-1, and ASC immunoreactivity
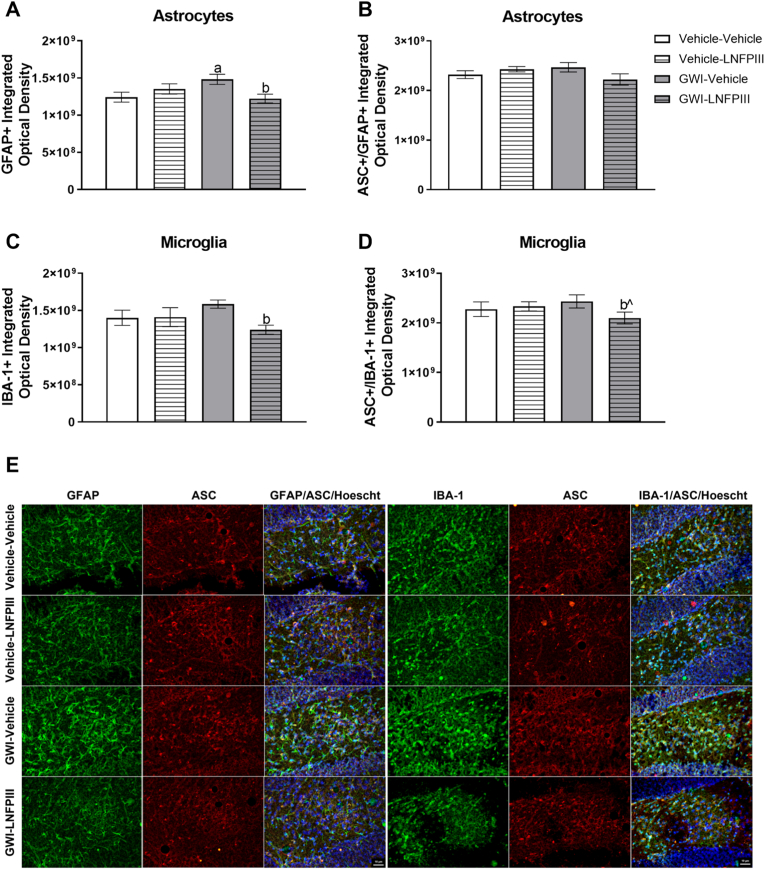
Prior exposure to GWI chemicals led to hippocampal increases in glial activation that were attenuated by delayed LNFPIII treatment. Intensity analysis for astrocytes (GFAP+) revealed a significant GWI × LNFPIII interaction (F(1,18) = 7.50, p ≤ 0.05, Fig. 6A) in which prior exposure to GWI increased GFAP staining compared to Vehicle-Vehicle (p ≤ 0.05) and LNFPIII eliminated this effect (p ≤ 0.01, Fig. 6A). Similar results were found for microglia (IBA-1+), in which there were trending LNFPIII treatment (F(1,18) = 3.79, p = 0.07; Fig. 6C) and a GWI × LNFPIII interaction (F(1,18) = 4.21, p = 0.06; Fig. 6C) effects. These effects were driven primarily by a reduction in the IBA-1 intensity staining by LNFPIII in the GWI group (t(10) = −4.20, p ≤ 0.01, Fig. 6C), suggesting that LNFPIII is beneficial in reducing glial activation.
Fig. 6.
GFAP and IBA-1 immunoreactivity in the hilus region of the hippocampus 11 months post-GWI exposure and delayed LNFPIII treatment. Immunoreactivity of (A) GFAP+ and (B) GFAP+/ASC + astrocytes and (C) IBA-1+ and (D) ASC+/IBA-1+ microglia were analyzed in ImageJ. Representative images taken at 40x are shown in panel (E). Data are presented as mean ± SEM, n = 4–6 mice per group. a and b indicate p ≤ 0.05 for Vehicle-Vehicle vs GWI-Vehicle and GWI-Vehicle vs GWI-LNFPIII, respectively. bˆ indicates p ≤ 0.10 for GWI-Vehicle vs GWI-LNFPIII.
Further, to identify possible neuroinflammatory mechanisms, the inflammasome marker ASC was examined in both astrocytes and microglia. No significant differences among treatments were found for the number of GFAP/ASC + or IBA-1/ASC + cells (p's > 0.52). However, intensity analysis indicated that LNFPIII treatment numerically reduced staining in GWI ASC + microglia (t(10) = −1.88, p = 0.09; Fig. 6D), suggesting that LNFPIII subtly attenuates microglial inflammasome activation. A similar numerical reduction with LNFPIII was observed for ASC intensity in astrocytes, but was less robust (Fig. 6B). Refer to Fig. 6E for representative images.
3.5.2. qPCR
The expression of the anti-inflammatory cytokine IL-10 was assessed in the dorsal and ventral hippocampus 11 months post GWI chemical exposures and 4 months post LNFPIII treatment initiation. There were no significant treatment differences within the dorsal hippocampus, but analysis within the ventral hippocampus revealed increased IL-10 expression in the GWI-LNFPIII group compared to Vehicle-Vehicle (p ≤ 0.05) and GWI-Vehicle (p = 0.08) groups (Table 1).
Table 1.
Hippocampal mRNA levels of IL-10 11 months post-GWI exposure and delayed LNFPIII treatment. The effect of GWI exposure and delayed LNFPIII treatment on the expression of anti-inflammatory cytokine IL-10 in the dorsal and ventral hippocampus measured by qPCR. Hippocampal samples from mice (n = 4–6/treatment) were used for RNA isolation and subsequent qPCR analyses. Data were analyzed by the 2−ΔΔCt method with 18S as the housekeeping gene and are expressed as a mean fold change from the Vehicle-Vehicle group ±SE. * indicates significant fold change from Vehicle-Vehicle, p < 0.05, and values are bolded. ˆ indicates p < 0.10.
|
Table 1. Hippocampal IL-10 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle-LNFPIII |
GWI-Vehicle |
GWI-LNFPIII |
GWI-Vehicle vs GWI-LNFPIII |
|||||||
| Region | fold change | se | p value | fold change | se | p value | fold change | se | p value | p value |
| Dorsal | 0.95 | 0.43 | 0.89 | 1.15 | 0.73 | 0.74 | 1.04 | 0.26 | 0.90 | 0.83 |
| Ventral | 1.41 | 0.38 | 0.48 | 1.11 | 0.34 | 0.84 | 3.41 | 4.15 | 0.03* | 0.08 ˆ |
3.5.3. Hippocampal neurogenesis
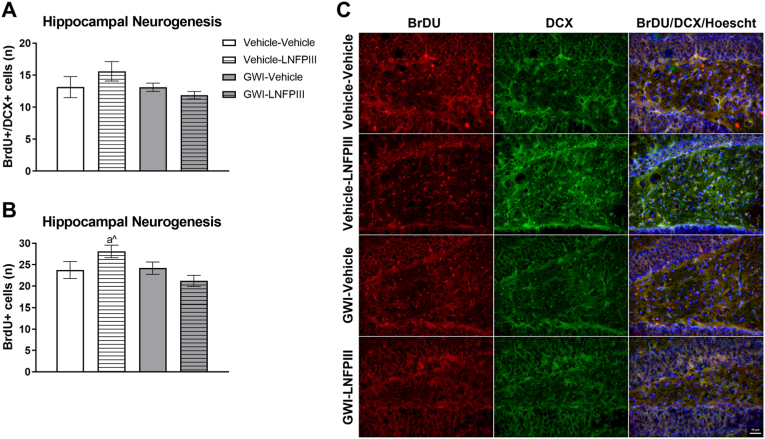
A trend for a reduction of BrDU + cells in the GWI mice (F(1,16) = 4.01, p = 0.06, Fig. 7B) and significant GWI x LNFPIII treatment interaction (F(1,16) = 5.20, p ≤ 0.05, Fig. 7B) effect were observed. LNFPIII, irrespective of GWI exposure, tended to numerically increase the number of BrDU + cells in the hippocampus (p = 0.09) compared to control (Fig. 7B). A similar numerical (p ≥ 0.12) reduction in BrDU+/DCX + cells in GWI mice and an increase by LNFPIII was observed (Fig. 7A), but was not significant. Refer to Fig. 7C for representative images.
Fig. 7.
BrDU and DCX immunoreactivity in the hippocampus 11 months post-GWI exposure and delayed LNFPIII treatment. The number of (A) BrDU+/DCX+ and (B) BrDU + cells were counted in the hippocampus. Representative images taken at 40x are shown in panel (C). Data are presented as mean ± SEM, n = 4–6 mice per group. aˆ indicates p ≤ 0.10 from Vehicle-Vehicle.
4. Discussion
Epidemiological evidence suggests that exposures to multiple toxicants during the Gulf War contributed to the development of GWI (White et al., 2016) and that these overexposures have accelerated age-related chronic conditions (Zundel et al., 2019). Because of the persistent and progressive nature of GWI, investigations into therapeutic interventions are imperative. The present GWI model (PB/DEET/CORT/DFP) has been primarily characterized at acute time points post GWI chemical exposure (Carpenter et al., 2020; O'Callaghan et al., 2015), with limited studies examining the model's chronic effects (Brown et al., 2021a). As the war occurred more than 30 years ago, it is crucial that GWI preclinical investigations align with the aging GWI veteran population. Thus, the current study aimed to further characterize the long-term behavioral and neuroinflammatory effects of PB/DEET/CORT/DFP exposure and to examine whether delayed treatment with LNFPIII was beneficial.
Sensorimotor function was impacted by prior GWI exposure, as GWI mice tended to exhibit higher latencies to contact the sticker in the sticker removal task; this sensorimotor deficit was eliminated by LNFPIII. These results align with sensorimotor deficits observed previously in GWI veterans (Axelrod and Milner, 1997; Proctor et al., 2006; Toomey et al., 2009) as well as in animal GWI models (Abou-Donia et al., 2002; Carpenter et al., 2021). This suggests LNFPIII may be beneficial in alleviating sensorimotor deficits like it did in another GWI model (Carpenter et al., 2021). Neuromuscular function remained unaltered by prior GWI exposure as measured by grip strength, but LNFPIII treatment improved overall strength within this test. Further, all mice were able to build nests indicating that there were not any marked striatal or hippocampal deficits that are normally associated with the nest test.
Interestingly, in this study, prior exposure to GWI-related chemicals led to hyperactivity in several tests. This was notable in the open field (OF), as GWI treated mice traveled further distances and made more line crossings within the first 5 min of the test; however, this hyperactivity did not persist for the 30 min test duration in GWI mice receiving LNFPIII treatment, suggesting LNFPIII may reduce the persistence of this deficit. This increased activity was in line with the observed GWI gait alterations in this study as GWI mice exhibited increased hindlimb base widths, stride lengths, and interstep distance; the latter two were prevented by LNFPIII treatment. Hyperactivity was also evident in the pole test, as GWI mice descended the pole faster. These increases in motor activity might be due to persisting effects of the AChE inhibitors PB (reversible) and DFP (irreversible) that were components of the GWI chemical exposure mixture. In fact, it has been shown that following sarin vapor exposure, sustained increases in locomotor activity were apparent 1 and 6 months post exposure in rats (Allon et al., 2011). Further investigations into the mechanisms underlying this increase in motor activity are warranted. Notably, LNFPIII treatment was beneficial in this GWI model as it was in the PB/PM GWI model (Carpenter et al., 2021).
Affect symptoms have been widely self-reported by GWI veterans and present as increases in depression and anxiety (Binder et al., 1999; Sullivan et al., 2018); these effects have been recapitulated in multiple GWI exposure paradigms (Carreras et al., 2018; Phillips and Deshpande, 2016; Zhu et al., 2020), especially those including a stress component (Abdullah et al., 2012; Parihar et al., 2013). Here, GWI mice exhibited mild mood impairments, driven mostly by anxiety-like behaviors. Subtle, anxiety-like behavior was observed in the EZM as GWI-Vehicle mice spent less time in the open areas of the arena. Additionally, these mice spent less time exploring the open arms from the comforts of the closed arms (stretch attends). In line with these EZM results, GWI treated mice also spent numerically less time in the anxiogenic center of the OF arena. Moreover, in the OF, GWI mice entered and spent more time in the arena's corners, suggestive of anxiety-like behavior. Notably, LNFPIII treatment increased the time spent exploring the open area of the EZM before entering and while occupying the closed areas, reduced the number of marbles buried in the marble burying test, and decreased the overall amount of corner entries and time in the OF. These effects suggest that LNFPIII has some anxiolytic properties on multiple anxiety-like parameters. Anhedonia, a behavior associated with depression, was not present in the sucrose preference test, as all mice preferred the sucrose solution to normal tap water. Depressive-like behavior was also evaluated using the swim test, and GWI mice tended to exhibit more depressive-like behavior within this test; they spent numerically more time immobile (e.g., behavior despair) and had fewer climbing attempts (e.g., escape behavior). These effects were not seen with control or LNFPIII treated groups. Clinical studies have shown that mood deficits experienced by GWI veterans can impact the reporting of other GWI symptoms and impact the results of neuropsychological evaluations like motor coordination and executive function (Binder et al., 1999; Lindem et al., 2003; Sillanpaa et al., 1997). Thus, the affect results observed here, albeit subtle, might impact other behavioral domains examined in this study.
Cognitive and memory aberrations remain a consistent feature among veterans with GWI and numerous studies have shown poorer performance in cognitive/memory tasks (Goldstein et al., 1996; Hom et al., 1997; Janulewicz et al., 2017; Toomey et al., 2009). GWI preclinical investigations also report deficits in tests of learning and memory (Carpenter et al., 2021; Hattiangady et al., 2014; Madhu et al., 2019; Parihar et al., 2013; Phillips and Deshpande, 2016). Here, no profound GWI-induced cognitive and memory impairments were observed in hippocampal dependent tasks such as nesting, the BM, and RAM; all animals performed at similar rates in these tasks. However, GWI treatment impaired short-term recognition memory in the NOR task. These results align with GWI veterans performing worse in visual memory tasks (both immediate and delayed recall; Sullivan et al. (2018)), and NOR performance in another GWI model we reported on (Carpenter et al., 2021). In the BM, all mice significantly improved their performance (∼60%) over the course of the 4-day acquisition period indicative of learning. There were no significant GWI induced deficiencies within the BM probe trial, but LNFPIII treatment decreased the number of TH entries and distance traveled; this suggests that LNFPIII improved memory performance, as these mice traveled less after first approaching the target hole. Similarly, in the RAM foraging task, which measures dorsal hippocampal dependent short-term working spatial memory, all mice improved their performance over time. While early on in this task the GWI-LNFPIII group made more errors, this group improved the most overall with a 55% decrease in errors and this was significant compared to GWI-Vehicle, indicating LNFPIII treatment improved performance rate. There were no observable long-term memory deficits in the ventral hippocampus spatial working memory RAM Win-Shift task by the end of training, but it is notable that GWI-LNFPIII mice performed better early on within the acquisition component of this task. These results align with a separate study on these mice investigating hippocampal synaptic plasticity and transmission at 11 months (Brown et al., 2021a) where prior PB/DEET/CORT/DFP exposure produced decreases in hippocampal basal synaptic plasticity and long-term potentiation (LTP) that were restored by LNFPIII treatment. Taken together, these data suggest that LNFPIII may benefit some domains of cognition and memory in this model as it did in another one (Carpenter et al., 2021).
Prior investigations utilizing this GWI treatment paradigm revealed widespread acute neuroinflammation (O'Callaghan et al., 2015). We also found similar acute neuroinflammatory (i.e., increases in TNF-α, IL-1β, IL-6, CCL-2, YM-1, and HMGB1) effects within the hippocampus (Carpenter et al., 2020), and some neuroinflammation was also apparent at 11 months post exposure in this cohort of mice (IL-6; Brown et al., 2021a). Notably, at 11 months post GWI exposure, the increase in hippocampal IL-6 mRNA levels was lessened with delayed LNFPIII treatment (Brown et al., 2021a). To determine if the LNFPIII-induced dampening of IL-6 was due to an increase in anti-inflammatory signaling, we measured IL-10, a cytokine already known to be affected by LNFPIII in a different setting (Tundup et al., 2015). Indeed, in this cohort of mice, hippocampal (ventral) levels of IL-10 were increased in mice exposed to GWI-LNFPIII treatment. Further, because enhanced inflammatory cytokine production can lead to glial activation and resulting behavioral deficits (Habbas et al., 2015; Miller, 2009; Minogue et al., 2012), immunohistochemical analysis of GFAP (astrocytes) and IBA-1 (microglia) was done in the hippocampus. Prior GWI treatment led to increases in GFAP and IBA-1 immunoreactivity that were more pronounced for GFAP. Notably, LNFPIII significantly reduced these effects indicative of its ability to counteract neuroinflammation. Additionally, inflammasome activation by ASC co-localization with GFAP+ and IBA-1+ cells was evaluated. Briefly, upon activation, ASC functions to form the NLRP3 inflammasome which in turn mediates caspase-1 activation of inflammatory IL-1β and IL-18 release (Yang et al., 2019). There were no differences in the number of ASC positive GFAP or IBA-1 cells. However, LNFPIII reduced the intensity of ASC+/IBA-1+ cells within GWI mice, suggesting that inflammasome activation occurs more so in microglia and LNFPIII can curb this activation.
Numerous studies have indicated that hippocampal neurogenesis occurs throughout the lifespan in both humans and rodents (Eriksson et al., 1998; Moreno-Jiménez et al., 2021; Spalding et al., 2013). Behaviorally, in rodents, neurogenesis has been linked to cognitive performance in hippocampal-dependent tasks (Bolz et al., 2015; Drapeau et al., 2003; van Praag et al., 2005) as well as mood (Hill et al., 2015). In this study, a slight reduction in BrDU-positive cells in GWI mice was observed at 11 months, suggesting subtly decreased neurogenesis. These results were milder, but in general agreement with other GWI studies indicating reductions in neurogenesis, albeit with different GWI chemical exposures and timelines (Iannucci et al., 2022; Kodali et al., 2018b; Parihar et al., 2013; Shetty et al., 2020). Additionally, the GWI reduction in hippocampal neurogenesis could be contributory to the cognitive impairments in the NOR. Notably, apart from GWI exposure, LNFPIII increased neurogenesis, indicative of a potential novel beneficial property of this treatment. Investigating whether a higher dose of LNFPIII can mitigate GWI neurogenesis impairments in multiple models is warranted in future studies.
Overall, this study provides valuable insights into the long-term neurological alterations of prior GWI-related chemical exposures along a translationally relevant time course for current GWI veterans. Evaluating the neurological adaptions in this model has shown that prior exposure leads to deficits in behavioral domains (e.g., motor, mood, and cognitive function), neuroinflammation (glial activation), and reduced neurogenesis, all of which are prominent symptoms in the GWI veteran population. Moreover, the translational relevance of this model allows for the evaluation of therapeutic interventions such as LNFPIII. Importantly, the data in this study indicate that LNFPIII, when initiated months after GWI exposure, ameliorated many of the observed deficits including restoring locomotor and sensorimotor function, improving cognitive abilities, alleviating affect changes, and reducing hippocampal neuroinflammation. These data highlight that LNFPIII has broad beneficial properties and suggests that it might be an efficacious, multi-symptom therapy for GWI veterans.
Author contributions
NMF conceived and designed the study. TN and DAH synthesized, characterized, and provided LNFPIII used in the study. NMF, JMC, KAB, HDL, and KBC assisted in the investigation and sample collection. JMC, LV, and KAB performed sample and data analysis. JMC and NMF wrote the manuscript with input from KAB, JJW, and DAH. All authors read/contributed editorially and approved the final manuscript version.
Conflict of Interest
None of the authors have conflict of interest.
Acknowledgements
This research was supported by Department of Defense grant numbers W81XWH1610586 and W81XWH2110661 to NMF. We also acknowledge support by the Sharma Distinguished Professorship in Toxicology endowment to NMF.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2022.100553.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abdullah L., Evans J.E., Bishop A., Reed J.M., Crynen G., Phillips J., Pelot R., Mullan M.A., Ferro A., Mullan C.M., Mullan M.J., Ait-Ghezala G., Crawford F.C. Lipidomic profiling of phosphocholine-containing brain lipids in mice with sensorimotor deficits and anxiety-like features after exposure to Gulf War agents. NeuroMolecular Med. 2012;14:349–361. doi: 10.1007/s12017-012-8192-z. [DOI] [PubMed] [Google Scholar]
- Abou-Donia M.B., Dechkovskaia A.M., Goldstein L.B., Bullman S.L., Khan W.A. Sensorimotor deficit and cholinergic changes following coexposure with pyridostigmine bromide and sarin in rats. Toxicol. Sci. 2002;66:148–158. doi: 10.1093/toxsci/66.1.148. [DOI] [PubMed] [Google Scholar]
- Allon N., Chapman S., Egoz I., Rabinovitz I., Kapon J., Weissman B.A., Yacov G., Bloch-Shilderman E., Grauer E. Deterioration in brain and heart functions following a single sub-lethal (0.8 LCt50) inhalation exposure of rats to sarin vapor:: a putative mechanism of the long term toxicity. Toxicol. Appl. Pharmacol. 2011;253:31–37. doi: 10.1016/j.taap.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Alshelh Z., Albrecht D.S., Bergan C., Akeju O., Clauw D.J., Conboy L., Edwards R.R., Kim M., Lee Y.C., Protsenko E., Napadow V., Sullivan K., Loggia M.L. In-vivo imaging of neuroinflammation in veterans with Gulf War illness. Brain Behav. Immun. 2020;87:498–507. doi: 10.1016/j.bbi.2020.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anger W., Storzbach D., Binder L., Campbell K., Rohlman D., McCauley L. Evidence of cognitive deficits in Persian Gulf War veterans: interim report from a population-based study. J. Int. Neuropsychol. Soc. 1999;5:203–212. doi: 10.1017/s1355617799533031. [DOI] [PubMed] [Google Scholar]
- Anger W.K., Storzbach D., Binder L.M., Campbell K.A., Rohlman D.S., McCauley L., Kovera C.A., Davis K.L. Neurobehavioral deficits in Persian Gulf veterans: evidence from a population-based study. J. Int. Neuropsychol. Soc. 1999;5:203–212. doi: 10.1017/s1355617799533031. [DOI] [PubMed] [Google Scholar]
- Atochina O., Da'dara A.A., Walker M., Harn D.A. The immunomodulatory glycan LNFPIII initiates alternative activation of murine macrophages in vivo. Immunology. 2008;125:111–121. doi: 10.1111/j.1365-2567.2008.02826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod B.N., Milner I.B. Neuropsychological findings in a sample of operation desert storm veterans. J. Neuropsychiatry Clin. Neurosci. 1997;9:23–28. doi: 10.1176/jnp.9.1.23. [DOI] [PubMed] [Google Scholar]
- Baraniuk J.N., El-Amin S., Corey R., Rayhan R., Timbol C. Carnosine treatment for gulf war illness: a randomized controlled trial. Global J. Health Sci. 2013;5:69–81. doi: 10.5539/gjhs.v5n3p69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R., Duke A.A., Gilmore P.E., Page D., Bègue L. Anxiolytic-like effects observed in rats exposed to the elevated zero-maze following treatment with 5-HT2/5-HT3/5-HT4 ligands. Sci. Rep. 2014;4:3881. doi: 10.1038/srep03881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava P., Li C., Stanya K.J., Jacobi D., Dai L., Liu S., Gangl M.R., Harn D.A., Lee C.H. Immunomodulatory glycan LNFPIII alleviates hepatosteatosis and insulin resistance through direct and indirect control of metabolic pathways. Nat. Med. 2012;18:1665–1672. doi: 10.1038/nm.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder L.M., Storzbach D., Anger W.K., Campbell K.A., Rohlman D.S., of the Portland Environmental O.M., Center H.R. Subjective cognitive complaints, affective distress, and objective cognitive performance in Persian gulf war veterans. Arch. Clin. Neuropsychol. 1999;14:531–536. [PubMed] [Google Scholar]
- Bolz L., Heigele S., Bischofberger J. Running improves pattern separation during novel object recognition. Brain Plast. 2015;1:129–141. doi: 10.3233/BPL-150010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A.A., Skelton M.R., Vorhees C.V., Williams M.T. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague–Dawley rats: effects of anxiolytic and anxiogenic agents. Pharmacol. Biochem. Behav. 2011;97:406–415. doi: 10.1016/j.pbb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick G., Ben-Hamo R., Vashishtha S., Efroni S., Nathanson L., Barnes Z., Fletcher M.A., Klimas N. Altered immune pathway activity under exercise challenge in Gulf War Illness: an exploratory analysis. Brain Behav. Immun. 2013;28:159–169. doi: 10.1016/j.bbi.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Broderick G., Kreitz A., Fuite J., Fletcher M.A., Vernon S.D., Klimas N. A pilot study of immune network remodeling under challenge in Gulf War Illness. Brain Behav. Immun. 2011;25:302–313. doi: 10.1016/j.bbi.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Brown K.A., Carpenter J.M., Preston C.J., Ludwig H.D., Clay K.B., Harn D.A., Norberg T., Wagner J.J., Filipov N.M. Lacto-N-fucopentaose-III ameliorates acute and persisting hippocampal synaptic plasticity and transmission deficits in a Gulf War Illness mouse model. Life Sci. 2021;279 doi: 10.1016/j.lfs.2021.119707. [DOI] [PubMed] [Google Scholar]
- Brown K.A., Preston C.J., Carpenter J.M., Ludwig H.D., Norberg T., Harn D.A., Filipov N.M., Wagner J.J. Lacto-N-fucopentaose-III (LNFPIII) ameliorates acute aberrations in hippocampal synaptic transmission in a Gulf War Illness animal model. Brain Res. 2021;1766 doi: 10.1016/j.brainres.2021.147513. [DOI] [PubMed] [Google Scholar]
- Calley C.S., Kraut M.A., Spence J.S., Briggs R.W., Haley R.W., Hart J. The neuroanatomic correlates of semantic memory deficits in patients with Gulf War illnesses: a pilot study. Brain Imag. Behav. 2010;4:248–255. doi: 10.1007/s11682-010-9103-2. [DOI] [PubMed] [Google Scholar]
- Carpenter J.M., Brown K.A., Diaz A.N., Dockman R.L., Benbow R.A., Harn D.A., Norberg T., Wagner J.J., Filipov N.M. Delayed treatment with the immunotherapeutic LNFPIII ameliorates multiple neurological deficits in a pesticide-nerve agent prophylactic mouse model of Gulf War Illness. Neurotoxicol. Teratol. 2021;87 doi: 10.1016/j.ntt.2021.107012. [DOI] [PubMed] [Google Scholar]
- Carpenter J.M., Gordon H.E., Ludwig H.D., Wagner J.J., Harn D.A., Norberg T., Filipov N.M. Neurochemical and neuroinflammatory perturbations in two Gulf War Illness models: modulation by the immunotherapeutic LNFPIII. Neurotoxicology. 2020;77:40–50. doi: 10.1016/j.neuro.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras I., Aytan N., Mellott T., Choi J.K., Lehar M., Crabtree L., Leite-Morris K., Jenkins B.G., Blusztajn J.K., Dedeoglu A. Anxiety, neuroinflammation, cholinergic and GABAergic abnormalities are early markers of Gulf War illness in a mouse model of the disease. Brain Res. 2018;1681:34–43. doi: 10.1016/j.brainres.2017.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L.L. The prevalence of mild cognitive impairment in a convenience sample of 202 gulf war veterans. Int. J. Environ. Res. Publ. Health. 2020;17:7158. doi: 10.3390/ijerph17197158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L.L., Abadjian L., Hlavin J., Meyerhoff D.J., Weiner M.W. Effects of low-level sarin and cyclosarin exposure and gulf war illness on brain structure and function: a study at 4T. Neurotoxicology. 2011;32:814–822. doi: 10.1016/j.neuro.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Chao L.L., Kanady J.C., Crocker N., Straus L.D., Hlavin J., Metzler T.J., Maguen S., Neylan T.C. Cognitive behavioral therapy for insomnia in veterans with gulf war illness: results from a randomized controlled trial. Life Sci. 2021;279 doi: 10.1016/j.lfs.2021.119147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L.L., Reeb R., Esparza I.L., Abadjian L.R. Associations between the self-reported frequency of hearing chemical alarms in theater and regional brain volume in Gulf War Veterans. Neurotoxicology. 2016;53:246–256. doi: 10.1016/j.neuro.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L.L., Rothlind J.C., Cardenas V.A., Meyerhoff D.J., Weiner M.W. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in US veterans. Neurotoxicology. 2010;31:493–501. doi: 10.1016/j.neuro.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L.L., Zhang Y., Buckley S. Effects of low-level sarin and cyclosarin exposure on white matter integrity in Gulf War Veterans. Neurotoxicology. 2015;48:239–248. doi: 10.1016/j.neuro.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christova P., James L.M., Engdahl B.E., Lewis S.M., Carpenter A.F., Georgopoulos A.P. Subcortical brain atrophy in gulf war illness. Exp. Brain Res. 2017;235:2777–2786. doi: 10.1007/s00221-017-5010-8. [DOI] [PubMed] [Google Scholar]
- Clark J.K., Furgerson M., Crystal J.D., Fechheimer M., Furukawa R., Wagner J.J. Alterations in synaptic plasticity coincide with deficits in spatial working memory in presymptomatic 3xTg-AD mice. Neurobiol. Learn. Mem. 2015;125:152–162. doi: 10.1016/j.nlm.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban A., Filipov N.M. Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. J. Neurochem. 2007;100:1177–1187. doi: 10.1111/j.1471-4159.2006.04294.x. [DOI] [PubMed] [Google Scholar]
- Conrad K.L., Louderback K.M., Gessner C.P., Winder D.G. Stress-induced alterations in anxiety-like behavior and adaptations in plasticity in the bed nucleus of the stria terminalis. Physiol. Behav. 2011;104:248–256. doi: 10.1016/j.physbeh.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon R. Assessing burrowing, nest construction, and hoarding in mice. JoVE. 2012 doi: 10.3791/2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey B., Madhu L.N., Shetty A.K. Gulf war illness: mechanisms underlying brain dysfunction and promising therapeutic strategies. Pharmacol. Therapeut. 2021;220 doi: 10.1016/j.pharmthera.2020.107716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan E.K., Kekes-Szabo S., Lin J.C., Massey R.L., Cobb J.D., Hodgin K.S., Ness T.J., Hangee-Bauer C., Younger J.W. A placebo-controlled, pseudo-randomized, crossover trial of botanical agents for gulf war illness: curcumin (curcuma longa), Boswellia (Boswellia serrata), and French maritime pine bark (Pinus pinaster) Int. J. Environ. Res. Publ. Health. 2021;18:2468. doi: 10.3390/ijerph18052468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donta S.T., Clauw D.J., Engel J., Charles C., Guarino P., Peduzzi P., Williams D.A., Skinner J.S., Barkhuizen A., Taylor T., Kazis L.E., Sogg S., Hunt S.C., Dougherty C.M., Richardson R.D., Kunkel C., Rodriguez W., Alicea E., Chiliade P., Ryan M., Gray G.C., Lutwick L., Norwood D., Smith S., Everson M., Blackburn W., Martin W., Griffiss J.M., Cooper R., Renner E., Schmitt J., McMurtry C., Thakore M., Mori D., Kerns R., Park M., Pullman-Mooar S., Bernstein J., Hershberger P., Salisbury D.C., Feussner J.R., Group, f.t.V.C.S.S Cognitive behavioral therapy and aerobic exercise for gulf war veterans' IllnessesA randomized controlled trial. JAMA. 2003;289:1396–1404. doi: 10.1001/jama.289.11.1396. [DOI] [PubMed] [Google Scholar]
- Donta S.T., Engel C.C., Jr., Collins J.F., Baseman J.B., Dever L.L., Taylor T., Boardman K.D., Kazis L.E., Martin S.E., Horney R.A., Wiseman A.L., Kernodle D.S., Smith R.P., Baltch A.L., Handanos C., Catto B., Montalvo L., Everson M., Blackburn W., Thakore M., Brown S.T., Lutwick L., Norwood D., Bernstein J., Bacheller C., Ribner B., Church L.W., Wilson K.H., Guduru P., Cooper R., Lentino J., Hamill R.J., Gorin A.B., Gordan V., Wagner D., Robinson C., DeJace P., Greenfield R., Beck L., Bittner M., Schumacher H.R., Silverblatt F., Schmitt J., Wong E., Ryan M.A., Figueroa J., Nice C., Feussner J.R., Group V.A.C. Benefits and harms of doxycycline treatment for Gulf War veterans' illnesses: a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2004;141:85–94. doi: 10.7326/0003-4819-141-2-200407200-00006. [DOI] [PubMed] [Google Scholar]
- Drapeau E., Mayo W., Aurousseau C., Le Moal M., Piazza P.-V., Abrous D.N. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle A.L., Mazei-Robison M., Robison A.J. Sucrose preference test to measure stress-induced anhedonia. Bio-protocol. 2016;6 [Google Scholar]
- Eriksson P.S., Perfilieva E., Björk-Eriksson T., Alborn A.-M., Nordborg C., Peterson D.A., Gage F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fleming S.M., Ekhator O.R., Ghisays V. Assessment of sensorimotor function in mouse models of Parkinson's disease. JoVE : JoVE. 2013 doi: 10.3791/50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furgerson M., Clark J.K., Crystal J.D., Wagner J.J., Fechheimer M., Furukawa R. Hirano body expression impairs spatial working memory in a novel mouse model. Acta Neuropathol. Commun. 2014;2:131. doi: 10.1186/s40478-014-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G., Beers S.R., Morrow L.A., Shemansky W.J., Steinhauer S.R. A preliminary neuropsychological study of Persian Gulf veterans. J. Int. Neuropsychol. Soc. 1996;2:368–371. doi: 10.1017/s1355617700001399. [DOI] [PubMed] [Google Scholar]
- Golier J.A., Caramanica K., Michaelides A.C., Makotkine I., Schmeidler J., Harvey P.D., Yehuda R. A randomized, double-blind, placebo-controlled, crossover trial of mifepristone in Gulf War veterans with chronic multisymptom illness. Psychoneuroendocrinology. 2016;64:22–30. doi: 10.1016/j.psyneuen.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Golomb B.A., Allison M., Koperski S., Koslik H.J., Devaraj S., Ritchie J.B. Coenzyme Q10 benefits symptoms in Gulf War veterans: results of a randomized double-blind study. Neural Comput. 2014;26:2594–2651. doi: 10.1162/NECO_a_00659. [DOI] [PubMed] [Google Scholar]
- Gopinath K., Gandhi P., Goyal A., Jiang L., Fang Y., Ouyang L., Ganji S., Buhner D., Ringe W., Spence J., Biggs M., Briggs R., Haley R. FMRI reveals abnormal central processing of sensory and pain stimuli in ill Gulf War veterans. Neurotoxicology. 2012;33:261–271. doi: 10.1016/j.neuro.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbas S., Santello M., Becker D., Stubbe H., Zappia G., Liaudet N., Klaus F.R., Kollias G., Fontana A., Pryce C.R. Neuroinflammatory TNFα impairs memory via astrocyte signaling. Cell. 2015;163:1730–1741. doi: 10.1016/j.cell.2015.11.023. [DOI] [PubMed] [Google Scholar]
- Hao Y., Creson T., Zhang L., Li P., Du F., Yuan P., Gould T.D., Manji H.K., Chen G. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J. Neurosci. 2004;24:6590. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B., Mishra V., Kodali M., Shuai B., Rao X., Shetty A.K. Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War illness. Front. Behav. Neurosci. 2014;8:78. doi: 10.3389/fnbeh.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmer D.A., Van Doren W.W., Litke D.R., Tseng C.L., Ho L., Osinubi O., Pasinetti G.M. Safety, tolerability and efficacy of dietary supplementation with concord grape juice in gulf war veterans with gulf war illness: a phase I/IIA, randomized, double-blind, placebo-controlled trial. Int. J. Environ. Res. Publ. Health. 2020;17 doi: 10.3390/ijerph17103546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.S., Sahay A., Hen R. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology. 2015;40:2368–2378. doi: 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofele K., Sedelis M., Auburger G.W., Morgan S., Huston J.P., Schwarting R.K.W. Evidence for a dissociation between MPTP toxicity and tyrosinase activity based on congenic mouse strain susceptibility. Exp. Neurol. 2001;168:116–122. doi: 10.1006/exnr.2000.7588. [DOI] [PubMed] [Google Scholar]
- Holodniy M., Kaiser J.D. Treatment for Gulf War Illness (GWI) with KPAX002 (methylphenidate hydrochloride + GWI nutrient formula) in subjects meeting the Kansas case definition: a prospective, open-label trial. J. Psychiatr. Res. 2019;118:14–20. doi: 10.1016/j.jpsychires.2019.08.003. [DOI] [PubMed] [Google Scholar]
- Holton K.F., Kirkland A.E., Baron M., Ramachandra S.S., Langan M.T., Brandley E.T., Baraniuk J.N. The low glutamate diet effectively improves pain and other symptoms of gulf war illness. Int. J. Environ. Res. Publ. Health. 2020;12:2593. doi: 10.3390/nu12092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton K.F., Ramachandra S.S., Murray S.L., Baron M., Baraniuk J.N. Effect of the low glutamate diet on inflammatory cytokines in veterans with Gulf War Illness (GWI): a pilot study. Life Sci. 2021;280 doi: 10.1016/j.lfs.2021.119637. [DOI] [PubMed] [Google Scholar]
- Hom J., Haley R.W., Kurt T.L. Neuropsychological correlates of gulf war syndrome. Arch. Clin. Neuropsychol. 1997;12:531–544. [PubMed] [Google Scholar]
- Hubbard N.A., Hutchison J.L., Motes M.A., Shokri-Kojori E., Bennett I.J., Brigante R.M., Haley R.W., Rypma B. Central executive dysfunction and deferred prefrontal processing in veterans with gulf war illness. Clin. Psychol. Sci. 2014;2:319–327. doi: 10.1177/2167702613506580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannucci J., Nizamutdinov D., Shapiro L.A. Neurogenesis and chronic neurobehavioral outcomes are partially improved by vagus nerve stimulation in a mouse model of Gulf War illness. Neurotoxicology. 2022;90:205–215. doi: 10.1016/j.neuro.2022.04.001. [DOI] [PubMed] [Google Scholar]
- James L.M., Christova P., Engdahl B.E., Lewis S.M., Carpenter A.F., Georgopoulos A.P. Human leukocyte antigen (HLA) and gulf war illness (GWI): HLA-DRB1*13:02 spares subcortical atrophy in gulf war veterans. EBioMedicine. 2017;26:126–131. doi: 10.1016/j.ebiom.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janulewicz P.A., Krengel M.H., Maule A., White R.F., Cirillo J., Sisson E., Heeren T., Sullivan K. Neuropsychological characteristics of Gulf War illness: a meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177121. e0177121-e0177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi U., Evans J.E., Joseph R., Emmerich T., Saltiel N., Lungmus C., Oberlin S., Langlois H., Ojo J., Mouzon B. Oleoylethanolamide treatment reduces neurobehavioral deficits and brain pathology in a mouse model of Gulf War Illness. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-31242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney D.J., Simpson T.L., Malte C.A., Felleman B., Martinez M.E., Hunt S.C. Mindfulness-based stress reduction in addition to usual Care is associated with improvements in pain, fatigue, and cognitive failures among veterans with gulf war illness. Am. J. Med. 2016;129:204–214. doi: 10.1016/j.amjmed.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Khaiboullina S.F., DeMeirleir K.L., Rawat S., Berk G.S., Gaynor-Berk R.S., Mijatovic T., Blatt N., Rizvanov A.A., Young S.G., Lombardi V.C. Cytokine expression provides clues to the pathophysiology of Gulf War illness and myalgic encephalomyelitis. Cytokine. 2015;72:1–8. doi: 10.1016/j.cyto.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M., Hattiangady B., Shetty G., Bates A., Shuai B., Shetty A. Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav. Immun. 2018;69:499–514. doi: 10.1016/j.bbi.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M., Hattiangady B., Shetty G.A., Bates A., Shuai B., Shetty A.K. Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav. Immun. 2018;69:499–514. doi: 10.1016/j.bbi.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z., Dodd C.A., Filipov N.M. Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice. Neurotoxicol. Teratol. 2013;39:26–35. doi: 10.1016/j.ntt.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Lindem K., Proctor S.P., Heeren T., Krengel M., Vasterling J., Sutker P.B., Wolfe J., Keane T.M., White R.F. Neuropsychological performance in gulf war era veterans: neuropsychological symptom reporting. J. Psychopathol. Behav. Assess. 2003;25:121–127. [Google Scholar]
- Littlefield A.M., Setti S.E., Priester C., Kohman R.A. Voluntary exercise attenuates LPS-induced reductions in neurogenesis and increases microglia expression of a proneurogenic phenotype in aged mice. J. Neuroinflammation. 2015;12:138. doi: 10.1186/s12974-015-0362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Gong K., Yan Y., Zhang L., Tang P., Zhang X., Gong Y. Huperzine A promotes hippocampal neurogenesis in vitro and in vivo. Brain Res. 2013;1506:35–43. doi: 10.1016/j.brainres.2013.02.026. [DOI] [PubMed] [Google Scholar]
- Madhu L.N., Attaluri S., Kodali M., Shuai B., Upadhya R., Gitai D., Shetty A.K. Neuroinflammation in Gulf War Illness is linked with HMGB1 and complement activation, which can be discerned from brain-derived extracellular vesicles in the blood. Brain Behav. Immun. 2019;81:430–443. doi: 10.1016/j.bbi.2019.06.040. [DOI] [PubMed] [Google Scholar]
- Mathersul D.C., Eising C.M., DeSouza D.D., Spiegel D., Bayley P.J. Brain and physiological markers of autonomic function are associated with treatment-related improvements in self-reported autonomic dysfunction in veterans with gulf war illness: an exploratory pilot study. Glob. Adv. Health Med. 2020;9 doi: 10.1177/2164956120922812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface. Brain Behav. Immun. 2009;23:149–158. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minogue A.M., Barrett J.P., Lynch M.A. LPS-induced release of IL-6 from glia modulates production of IL-1β in a JAK2-dependent manner. J. Neuroinflammation. 2012;9:126. doi: 10.1186/1742-2094-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Jiménez E.P., Terreros-Roncal J., Flor-García M., Rábano A., Llorens-Martín M. Evidences for adult hippocampal neurogenesis in humans. J. Neurosci. 2021;41:2541. doi: 10.1523/JNEUROSCI.0675-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote R.S., Carpenter J.M., Dockman R.L., Steinberger A.J., Suen G., Norberg T., Harn D.A., Wagner J.J., Filipov N.M. Assessing the beneficial effects of the immunomodulatory glycan LNFPIII on gut microbiota and health in a mouse model of gulf war illness. Int. J. Environ. Res. Publ. Health. 2020;17:7081. doi: 10.3390/ijerph17197081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulherkar S., Liu F., Chen Q., Narayanan A., Couvillon A.D., Shine H.D., Tolias K.F. The small GTPase RhoA is required for proper locomotor circuit assembly. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan J.P., Kelly K.A., Locker A.R., Miller D.B., Lasley S.M. Corticosterone primes the neuroinflammatory response to DFP in mice: potential animal model of Gulf War Illness. J. Neurochem. 2015;133:708–721. doi: 10.1111/jnc.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar V.K., Hattiangady B., Shuai B., Shetty A.K. Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacology. 2013;38:2348–2362. doi: 10.1038/npp.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkitny L., Middleton S., Baker K., Younger J. Evidence for abnormal cytokine expression in Gulf War Illness: a preliminary analysis of daily immune monitoring data. BMC Immunol. 2015;16:57. doi: 10.1186/s12865-015-0122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips K.F., Deshpande L.S. Repeated low-dose organophosphate DFP exposure leads to the development of depression and cognitive impairment in a rat model of Gulf War Illness. Neurotoxicology. 2016;52:127–133. doi: 10.1016/j.neuro.2015.11.014. [DOI] [PubMed] [Google Scholar]
- Preston C.J., Brown K.A., Wagner J.J. Cocaine conditioning induces persisting changes in ventral hippocampus synaptic transmission, long-term potentiation, and radial arm maze performance in the mouse. Neuropharmacology. 2019;150:27–37. doi: 10.1016/j.neuropharm.2019.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor S.P., Heaton K.J., Heeren T., White R.F. Effects of sarin and cyclosarin exposure during the 1991 Gulf War on neurobehavioral functioning in US army veterans. Neurotoxicology. 2006;27:931–939. doi: 10.1016/j.neuro.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Proctor S.P., Heeren T., White R.F., Wolfe J., Borgos M.S., Davis J.D., Pepper L., Clapp R., Sutker P.B., Vasterling J.J., Oznoff D. Health status of Persian Gulf War veterans: self-reported symptoms, environmental exposures and the effect of stress. Int. J. Epidemiol. 1998;27:1000–1010. doi: 10.1093/ije/27.6.1000. [DOI] [PubMed] [Google Scholar]
- Ribeiro A.C., Zhu J., Kronfol M.M., Jahr F.M., Younis R.M., Hawkins E., McClay J.L., Deshpande L.S. Molecular mechanisms for the antidepressant-like effects of a low-dose ketamine treatment in a DFP-based rat model for Gulf War Illness. Neurotoxicology. 2020;80:52–59. doi: 10.1016/j.neuro.2020.06.011. [DOI] [PubMed] [Google Scholar]
- Ribeiro A.C.R., Deshpande L.S. A review of pre-clinical models for Gulf War Illness. Pharmacol. Therapeut. 2021;228 doi: 10.1016/j.pharmthera.2021.107936. [DOI] [PubMed] [Google Scholar]
- Shetty A.K., Attaluri S., Kodali M., Shuai B., Shetty G.A., Upadhya D., Hattiangady B., Madhu L.N., Upadhya R., Bates A. Monosodium luminol reinstates redox homeostasis, improves cognition, mood and neurogenesis, and alleviates neuro-and systemic inflammation in a model of Gulf War Illness. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpaa M.C., Agar L.M., Milner I.B., Podany E.C., Axelrod B.N., Brown G.G. Gulf War veterans: a neuropsychological examination. J. Clin. Exp. Neuropsychol. 1997;19:211–219. doi: 10.1080/01688639708403852. [DOI] [PubMed] [Google Scholar]
- Spalding K.L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H.B., Boström E., Westerlund I., Vial C., Buchholz B.A., Possnert G., Mash D.C., Druid H., Frisén J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava L., Tundup S., Choi B.S., Norberg T., Harn D. Immunomodulatory glycan lacto-N-fucopentaose III requires clathrin-mediated endocytosis to induce alternative activation of antigen-presenting cells. Infect. Immun. 2014;82:1891–1903. doi: 10.1128/IAI.01293-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K., Krengel M., Bradford W., Stone C., Thompson T.A., Heeren T., White R.F. Neuropsychological functioning in military pesticide applicators from the Gulf War: effects on information processing speed, attention and visual memory. Neurotoxicol. Teratol. 2018;65:1–13. doi: 10.1016/j.ntt.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Toomey R., Alpern R., Vasterling J.J., Baker D.G., Reda D.J., Lyons M.J., Henderson W.G., Kang H.K., Eisen S.A., Murphy F.M. Neuropsychological functioning of U.S. Gulf War veterans 10 years after the war. J. Int. Neuropsychol. Soc. 2009;15:717–729. doi: 10.1017/S1355617709990294. [DOI] [PubMed] [Google Scholar]
- Tundup S., Srivastava L., Norberg T., Watford W., Harn D. A neoglycoconjugate containing the human milk sugar LNFPIII drives anti-inflammatory activation of antigen presenting cells in a CD14 dependent pathway. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Shubert T., Zhao C., Gage F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Riper S.M., Alexander A.L., Koltyn K.F., Stegner A.J., Ellingson L.D., Destiche D.J., Dougherty R.J., Lindheimer J.B., Cook D.B. Cerebral white matter structure is disrupted in Gulf War Veterans with chronic musculoskeletal pain. Pain. 2017;158 doi: 10.1097/j.pain.0000000000001038. [DOI] [PubMed] [Google Scholar]
- Wang X., Wang Q.M., Meng Z., Yin Z., Luo X., Yu D. Gait disorder as a predictor of spatial learning and memory impairment in aged mice. PeerJ. 2017;5 doi: 10.7717/peerj.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington S.D., Rayhan R.U., Garner R., Provenzano D., Zajur K., Addiego F.M., VanMeter J.W., Baraniuk J.N. Exercise alters cerebellar and cortical activity related to working memory in phenotypes of Gulf War Illness. Brain Commun. 2020;2 doi: 10.1093/braincomms/fcz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.F., Steele L., O'Callaghan J.P., Sullivan K., Binns J.H., Golomb B.A., Bloom F.E., Bunker J.A., Crawford F., Graves J.C., Hardie A., Klimas N., Knox M., Meggs W.J., Melling J., Philbert M.A., Grashow R. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: effects of toxicant exposures during deployment. Cortex. 2016;74:449–475. doi: 10.1016/j.cortex.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie G.R., Genova H., Dobryakova E., DeLuca J., Chiaravalloti N., Falvo M., Cook D. Fatigue in Gulf War Illness is associated with tonically high activation in the executive control network. Neuroimage Clin. 2019;21 doi: 10.1016/j.nicl.2018.101641. 101641-101641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wang H., Kouadir M., Song H., Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Avery T., Vakhtin A.A., Mathersul D.C., Tranvinh E., Wintermark M., Massaband P., Ashford J.W., Bayley P.J., Furst A.J. Brainstem atrophy in gulf war illness. Neurotoxicology. 2020;78:71–79. doi: 10.1016/j.neuro.2020.02.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Vakhtin A.A., Dietch J., Jennings J.S., Yesavage J.A., Clark J.D., Bayley P.J., Ashford J.W., Furst A.J. Brainstem damage is associated with poorer sleep quality and increased pain in gulf war illness veterans. Life Sci. 2021;280 doi: 10.1016/j.lfs.2021.119724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B., Trikudanathan S., Zozulya A.L., Sandoval-Garcia C., Kennedy J.K., Atochina O., Norberg T., Castagner B., Seeberger P., Fabry Z., Harn D., Khoury S.J., Guleria I. Immune modulation by Lacto-N-fucopentaose III in experimental autoimmune encephalomyelitis. Clin. Immunol. 2012;142:351–361. doi: 10.1016/j.clim.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Hawkins E., Phillips K., Deshpande L.S. Assessment of ketamine and its enantiomers in an organophosphate-based rat model for features of gulf war illness. Int. J. Environ. Res. Publ. Health. 2020;17:4710. doi: 10.3390/ijerph17134710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zundel C.G., Krengel M.H., Heeren T., Yee M.K., Grasso C.M., Janulewicz Lloyd P.A., Coughlin S.S., Sullivan K. Rates of chronic medical conditions in 1991 gulf war veterans compared to the general population. Int. J. Environ. Res. Publ. Health. 2019;16 doi: 10.3390/ijerph16060949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.