Highlights
-
•
FDG-PET reveals heterogenous patterns of brain hypometabolism in familial ALS.
-
•
Single-subject analysis reveals hypometabolism in motor, prefrontal and limbic areas.
-
•
Patients with fast-progressing C9orf72-ALS have extensive brain hypometabolism.
-
•
Regional brain metabolism correlates with specific neuropsychological profiles.
-
•
FDG-PET single-subject analysis supports the diagnostic workup in genetic ALS.
Keywords: Neurodegenerative diseases, Amyotrophic lateral sclerosis, Genetic, Brain metabolism, Positron emission tomography, 18F-FDG-PET
Abstract
Background and objectives: The ALS diagnosis requires an integrative approach, combining the clinical examination and supporting tests. Nevertheless, in several cases, the diagnosis proves to be suboptimal, and for this reason, new diagnostic methods and novel biomarkers are catching on. The 18F-fluorodeoxyglucose (18F-FDG)-PET could be a helpful method, but it still requires additional research for sensitivity and specificity. We performed an 18F-FDG-PET single-subject analysis in a sample of familial ALS patients carrying different gene mutations, investigating the genotype-phenotype correlations and exploring metabolism correlations with clinical and neuropsychological data. Methods: We included ten ALS patients with pathogenic gene mutation who underwent a complete clinical and neuropsychological evaluation and an 18F-FDG-PET scan at baseline. Patients were recruited between 2018 and 2022 at the ALS Tertiary Centre in Novara, Italy. Patients were selected based on the presence of ALS gene mutation (C9orf72, SOD1, TBK1, and KIF5A). Following a validated voxel-based Statistical Parametric Mapping (SPM) procedure, we obtained hypometabolism maps at single-subject level. We extracted regional hypometabolism from the SPM maps, grouping significant hypometabolism regions into three meta-ROIs (motor, prefrontal association and limbic). Then, the corresponding 18F-FDG-PET regional hypometabolism was correlated with clinical and neuropsychological features. Results: Classifying the patients with C9orf72-ALS based on the rate of disease progression from symptoms onset to the time of scan, we observed two different patterns of brain hypometabolism: an extensive motor and prefrontal hypometabolism in patients classified as fast progressors, and a more limited brain hypometabolism in patients grouped as slow progressors. Patients with SOD1-ALS showed a hypometabolic pattern involving the motor cortex and prefrontal association regions, with a minor involvement of the limbic regions. The patient with TBK1-ALS showed an extended hypometabolism, in limbic systems, along with typical motor involvement, while the hypometabolism in the patient with KIF5A-ALS involved almost exclusively the motor regions, supporting the predominantly motor impairment linked to this gene mutation. Additionally, we observed strong correlations between the hypometabolism in the motor, prefrontal association and limbic meta-ROI and the specific neuropsychological performances. Conclusions: To our knowledge, this is the first study investigating brain hypometabolism at the single-subject level in genetic ALS patients carrying different mutations. Our results show high heterogeneity in the hypometabolism maps and some commonalities in groups sharing the same mutation. Specifically, in patients with C9orf72-ALS the brain hypometabolism was larger in patients classified as fast progressors than slow progressors. In addition, in the whole group, the brain metabolism showed specific correlations with clinical and neuropsychological impairment, confirming the ability of 18F-FDG-PET in revealing pattern of neuronal dysfunction, aiding the diagnostic workup in genetic ALS patients.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly fatal neurodegenerative disorder characterized by the degeneration of the upper and lower motor neurons. The European incidence is 2–3/100.000 by the year, although the number of affected patients is increasing due to the aging population (Chiò et al., 2009). Although the disease is primarily classified as sporadic, roughly 10–15% of patients with ALS have a significant history of motor neuron disease in the family. Overall, patients aged between 50 and 75 years at diagnosis but generally the familial disease forms have a lower age of onset (Chia et al., 2018). Until 2014, about 20 genes were discovered in ALS familial forms, accounting for about 70% of all familial cases and 10% of sporadic cases. The most frequent genetic alterations in familial ALS regard the pathogenic variants in SOD1, C9orf72, FUS, and TARDBP (Mejzini et al., 2019). In recent years, related to a significant improvement in the sequencing technologies, novel causative ALS genes (e,g., MATR3, CHCHD10, TBK1, TUBA4A, and KIF5A) have been identified. From a clinical point of view, most genetic forms are related to different motor and cognitive phenotypes, thus accentuating the phenotypic disease variability and its complexity (Chiò et al., 2020).
According to the El Escorial revised criteria (Agosta et al., 2015) and the Gold Coast criteria (Hannaford et al., 2021), the diagnosis of ALS requires an integrative approach, combining the clinical history, physical examination, exclusion of other potential causes of motor neuron suffering, and supporting tests (mainly, the electromyography). Genetic testing is becoming more common in clinical practice, even not necessary for the diagnosis. Nevertheless, in several cases, the diagnosis proves to be suboptimal, and for this reason, several available methods and novel biomarkers are catching on. Among them, advanced brain and spinal cord imaging such as brain MRI, together with 18F-fluorodeoxyglucose (18F-FDG)-PET are helpful methods to be integrated with clinical data. However, to date, they still require additional research for sensitivity and specificity (Goutman et al., 2022).
Due to non-univocal published results, the role of 18F-FDG-PET in ALS diagnostic and prognostic workup and characterization of clinical phenotypes is debated (Agosta et al., 2018). Several groups reported brain metabolism abnormalities in ALS patients when compared with controls, with the most consistent findings revealing cortical frontal and occipital hypometabolism and relative hypermetabolism in the brainstem, cerebellum, and temporal lobe (Cistaro et al., 2014, Cistaro et al., 2012, Liao et al., 2020, Rajagopalan and Pioro, 2015). The metabolism changes in specific regions of interest have been used to distinguish patients and controls with an overall accuracy of over 90% (Pagani et al., 2014). In addition, some studies analyzed the relations between brain hypometabolism and clinical and neuropsychological outcomes. For example, hypometabolism involving frontal regions has been related to the evidence of impaired performances in tests evaluating attention and executive functions, such as the word fluency test (Boeve et al., 2012, Canosa et al., 2016, Ludolph et al., 1992). One study showed that the cognitive theory of mind alterations correlated with the prefrontal cortices and supplementary motor area metabolism (Carluer et al., 2015). The studies exploring the ability of 18F-FDG-PET to discriminate ALS clinical phenotypes, including patients with bulbar or spinal onset, reported inconsistent or negative results (Cistaro et al., 2012, Pagani et al., 2014, Van Laere et al., 2014, Sala et al., 2019).
18F-FDG-PET studies in genetic ALS patients are fewer. The patients with C9orf72-ALS showed significant hypometabolism in the cingulate cortex, insula, and subcortical structures (Cistaro et al., 2014). SOD1-mutation carriers showed relative hypermetabolism in the precentral frontal gyrus and the paracentral lobule compared with sporadic patients (Canosa et al., 2022b). Lastly, the TARDBP-ALS patients showed a significant relative hypometabolism in the right precentral and postcentral gyrus, superior and middle temporal gyrus and insula compared with sporadic ALS (Canosa et al., 2022a). Further ALS-associated mutations have not been extensively investigated.
Here, we perform the 18F-FDG-PET single-subject analysis of brain metabolism in a sample of familial ALS patients carrying different gene mutations. The study aims to investigate genotype-endophenotype-phenotype correlations in single genetic ALS patients, exploring brain metabolism correlations with clinical and neuropsychological data.
2. Methods
2.1. Study design
The study cohort included ten ALS patients referred to the ALS Tertiary Center at the “Maggiore della Carità” University Hospital (Novara, Italy) from January 2018 to February 2022. We retrospectively selected the ALS patients carrying a pathogenic genetic mutation, who underwent a complete baseline neuropsychological evaluation and an 18F-FDG-PET scan during the diagnostic process. Specifically, the criteria for the retrospective inclusion were as follows: 1) a diagnosis of ALS (definite or probable with laboratory) according to the revised El Escorial criteria (Agosta et al., 2015); 2) detailed demographic, clinical and electromyographic assessment; 3) neuropsychological evaluation within three months from the first neurological evaluation at Our Center; 4) 18F-FDG-PET imaging performed within three months from the first neurological evaluation at Our Center; 5) clinical follow-up for all the disease courses or until the censoring data (30 April 2022) in the same Center. We excluded 18F-FDG-PET imaging acquired from patients with: 1) neoplastic or significant cerebrovascular lesions; 2) neurosurgery or other neurological conditions, including epilepsy, encephalitis, or stroke; 3) not-ALS behavioral disorders or history of drug or alcohol abuse/dependence. Before the exam, the 18F-FDG-PET was explained to all patients in detail, and they provided written informed consent for the exam.
2.2. Clinical features
Clinical data, including age at onset, age at diagnosis, sex, site of onset, phenotype, time from symptoms onset to diagnosis (in months), time of death/tracheostomy, and related survival time considering months from symptoms onset to death or last available follow-up (at the censored date of 30 April 2022), were available for all patients. As clinical scale, the ALS Functional Rating Scale-Revised Score (ALSFRS-R) (Cedarbaum et al., 1999) was used. The scale represents the functional status of ALS patients, scoring from 0 to 48, where higher score indicates better clinical status. The scale includes 12 items evaluating bulbar (item 1-2-3), fine motor (item 4-5-6), gross motor (item 7-8-9) and respiratory functions (item 10-11-12). Additionally, we estimated disease progression at the date of scan calculating the ALSFRS-R rate of progression as: 48 - ALSFRS-R score at PET scan / months between symptoms’ onset and scan. Based on this value, we classified patients into “fast progressors” (monthly decrease of ALSFRS-R score ≥ 0.9 from the symptoms onset to the PET scan) and “slow progressors” (monthly decrease of ALSFRS-R score < 0.9 from the symptoms onset to the PET scan). Patients were named as “ALS” plus a progressive number from 1 to 10.
2.3. Genetic analysis
After informed consent, all patients underwent blood sampling. The gene analyses were performed at the Laboratory of Genetics, Department of Health Sciences, at the University of Piemonte Orientale, Italy. DNA of the patients was extracted from peripheral blood by a standard procedure. Analysis of the GGGGCC repeat tract in the C9orf72 gene was carried out using the “PCR repeat” technique and of “STR genotyping” both followed by analysis on the ABI PRISM 3130XL sequencer (DeJesus-Hernandez et al., 2011, Renton et al., 2011). Sequences analysis of patients was performed using Next Generation Sequencing (NGS) technology with platform MiSeq Illumina with target sequencing methodology (Library obtained with SureSelect QXT custom probesTarget Enrichment for Illumina Multiplexed Sequencing (Agilent; ID 3227281). The NGS panel included the following genes for neurodegenerative diseases: SOD1, TBK1, KIF5A, ABCA7, ADAR, ALS2, ANO3, APP, ATL1, ATP13A2, CHCHD2, CHCHD10, CHMP2B, DCTN1, DNAJC6, FBX07, FIG4, FUS, GARS, GBA, GCH1, GDAP1, GJB1, GNAL, GRN, KTM2B, LRRK2, MAPT, MATR3, MFN2, MPZ, NEK1, OPTN, PARK2, PARK7, PFN1, PINK1, PLA2G6, PMP22, PNKD, PNP22, PRKRA, PRRT2, PSEN1, PSEN2, REEP1, SETX, SGCE, SIGMAR1, SLC2A1, SNCA, SPAST, SPG11, SPG7, SQSTM1, SYNJ1, TAF1, THAP1, TOR1A, TREM2, TUB4A, UBQLN2, VAPB, VCP, VPS13C, VPS35. The pathogenic DNA variants were confirmed by Sanger sequencing using standard protocols and using an automated 3130 XL DNA analyzer (Applied Biosystems, Foster City, CA, USA). The interpretation of the pathogenicity of the variants was carried out according to the ACMG guidelines (Richards et al., 2015). We selected patients carrying ALS genes mutation with available 18F-FDG-PET scan; in detail: six were carriers of the C9orf72 GGGGCC expansion, two were carriers of the SOD1 mutation (p.L145F), one was a carrier of the TBK1 mutation (exon4:c.358 + 3 A > G) and another one of the KIF5A mutation (exon27:c.3020 + 1G > A).
2.4. Neuropsychological evaluation
All patients underwent a complete neuropsychological battery and behavioral evaluation, including tests to evaluate attention and executive functions, visuospatial abilities, language, memory, and standard behavior questionnaires to evaluate anxiety and depression. The whole cognitive and neuropsychological battery included the following tests: Mini-Mental State Examination, Clock Drawing Test, Frontal Assessment Battery, Trail-Making Test A and B, Cognitive Estimation Test, letter fluency test, Raven’s Progressive Colored Matrices, Digit Span Test, and Logical memory test. The presence of neurobehavioral dysfunctions was assessed using the Neuropsychiatric Inventory. The battery was administered following the same sequence in each patient. Subsequently, patients were classified based on the Strong criteria in ALS-normal, ALS with cognitive dysfunctions, ALS with behavioral dysfunctions, ALS with both behavioral and cognitive dysfunctions, and ALS with frontotemporal dementia (Strong et al., 2017).
2.5. 18F-FDG-PET image acquisition, pre-processing and single-subject analysis
Brain 18F-FDG-PET acquisition was performed at the “Maggiore della Carità” University Hospital (Novara, Italy) following standardized procedures, in compliance with the European Association of Nuclear Medicine guidelines (Guedj et al., 2022). PET/CT images were acquired by the Philips Ingenuity TF 64 PET/CT (Philips Healthcare, Cleveland, OH, USA). Patients were fasted for at least six hours before radiopharmaceutical injection, hence the blood glucose level was < 120 mg/dl. Static emission images were acquired 40–50 min after injecting 175–210 MBq of 18F-FDG via a venous cannula. The mean static acquisition scan duration was 10 min. PET images were reconstructed using a Time-Of-Flight (TOF), list mode, blob based, ordered subsets maximum likelihood expectation maximization algorithm (OSEM). Attenuation, scatter, random, detector normalization, isotope decay, system dead time, and crystal timing corrections were applied.
Image analysis was performed according to a method optimized at San Raffaele Hospital, Milan, Italy, following a voxel-based Statistical Parametric Mapping (SPM) procedure previously validated (Della Rosa et al., 2014, Perani et al., 2014). The procedure allows obtaining hypometabolism maps at the single-subject level by comparing each patient scan with a large dataset of healthy controls using a two-sample t-test. SPM12 software was used for the image analysis, through the Matlab platform (Mathworks, Natick, MA, US). In detail, the CT scans contextually acquired with the 18F-FDG-PET were visually inspected to exclude patients carrying severe vascular leukoencephalopathy, major strokes or severe brain atrophy, which can result in artifacts. All the ten single-subject images were then normalized using the 18F-FDG-PET specific template as previously described, which allows an accurate estimation of metabolic abnormalities for single-subject analysis (Della Rosa et al., 2014). The normalized and subsequently smoothed images were tested for relative hypometabolism by entering a two-sample t-test in which the single-subject image was compared with a large normal control (n = 112) FDG-PET dataset. The resulting hypometabolism SPM map was used for the single-subject disease-specific, pattern description. The single-subject method has been previously used in ALS patients (Castelnovo et al., 2018; Sala et al., 2019). The procedure, using a large dataset of healthy controls, provides reliable maps of brain hypometabolism in the single individuals also when less conservative statistical thresholds are applied, e.g., 0.05 uncorrected, that we used in the present study (Caminiti et al., 2021). In addition, all imaging analyses included age and sex as covariates, thus correcting for possible confounding factors.
2.6. Regional brain metabolism changes and clinical correlations
18F-FDG-PET regional hypometabolism was extracted from regions of interest (ROIs) defined by using the automated anatomical labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) to extract ROIs and the toolbox REX (http://web.mit.edu/swg/software.htm) to extract values from the hypometabolism maps. To underline significant correlations between peaks of hypometabolism and clinical data, the regions presenting with hypometabolism values greater than two standard deviations of the average group values were selected for further analyses and grouped into functional meta-ROI (namely macro-ROIs including areas with the same function). The meta-ROI hypometabolism was entered in Spearman's correlation analysis together with clinical and neuropsychological features. Specifically, the extracted metabolic rate from the three meta-ROI in each patient was used to operate correlations with the following variables: the corrected score for each neuropsychological test (described in Method, 2.4), the ALSFRS-R at diagnosis, and the rate of progression from symptoms onset to the PET scan (as measured in monthly ALSFRS-R decline). Lastly, to test whether clinical variables can affect the hypometabolism pattern, we calculated multivariate regression analyses considering the metabolic rate in the three meta-ROI (motor, premotor association and limbic) as dependent variable and the following as independent variables: time from onset to diagnosis (months), phenotype (spinal vs bulbar), genetic group (C9orf72, SOD1, TBK1, KIF5A) and cognitive status (ALS-no, ALS-ci, ALS-bi, ALS-cibi, ALS-FTD).
2.7. Ethical considerations
The Ethics Committee approved the study at Maggiore della Carità Hospital, Novara, Italy (number CE 065/2022). All the procedures involving human participants performed in this study were in accordance with the Declaration of Helsinki. There were no clinical/therapeutic and management changes for the patients included in the study. The corresponding author will share data upon reasonable request.
3. Results
3.1. Patients’ features
Of the ten included patients, six were women (60%). The mean age at onset was 55.8 years (SD = 7.99). At the time of inclusion in this study the mean age was 56.3 years (SD = 8.07). Six patients (60%) had a spinal onset, and four (40%) had a bulbar one. The median time from symptoms onset to diagnosis was 10.3 months (IQR: 6.25–15). The median disease duration was 28.9 months (IQR: 14.25–42.75). The ALSFRS-R median score at diagnosis was 41/48 (IQR: 40–4.75). Based on the Strong classification, four patients (40%) had a normal neurocognitive status, three patients (30%) were classified as ALS-ci, two (20%) as ALS-bi, and one (10%) as ALS-ci-bi. No participant had co-occurring FTD at the time of the scan. Based on disease progression, we considered seven patients as slow progressors (70%) and three patients (30%) as fast progressors. According to the disease progression, the C9orf72 cohort were classified into two subgroups, specifically:
-
1)
a cluster including three patients (named ASL1, ALS2, and ALS3, mean follow-up time 8.33 months, SD: 5.03) characterized by older age, lower ALSFRS-R score at baseline, and faster disease progression measured from the symptoms’ onset to the 18F-FDG-PET date of scan;
-
2)
a second cluster, included three patients (named ALS4, ALS5, and ALS6, mean follow-up time 36.33 months, SD: 8.05) characterized by a slower clinical progression, younger age, higher ALSFRS-R score at baseline - meaning a lower motor and functional involvement -, and longer survival time. Single patients’ clinical and neuropsychological characteristics are shown in Table 1.
Table 1.
Patients’ demographic and clinical features. ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Scale – Revised; ALS-cn: normal cognition; ALS-ci: cognitive impairment; ALS-bi: behavioural impairment; ALS-FTD: frontotemporal dementia; LMN: lower motor neuron; UMN: upper motor neuro; n/a: not applicable. In bold: pathological scores for the neuropsychological tests. Age is presented as 5-year range.
| ALS1 | ALS2 | ALS3 | ALS4 | ALS5 | ALS6 | ALS7 | ALS8 | ALS9 | ALS10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age at onset (range) | 60–65 | 60–65 | 60–65 | 45–50 | 45–50 | 55–60 | 45–50 | 40–45 | 60–65 | 60–65 |
| Time from onset to diagnosis, months | 15 | 6 | 3 | 8 | 7 | 5 | 16 | 8 | 15 | 20 |
| Phenotype (spinal/bulbar) | spinal (classic) |
bulbar | bulbar | bulbar | spinal (classic) |
bulbar | spinal (>LMN) |
spinal (>LMN) |
spinal (>UMN) |
spinal (>UMN) |
| Gene | C9orf72 | C9orf72 | C9orf72 | C9orf72 | C9orf72 | C9orf72 | SOD1 | SOD1 | TBK1 | KIF5A |
|
ALSFRS-R at diagnosis |
29 | 35 | 44 | 46 | 44 | 45 | 40 | 46 | 40 | 41 |
|
ALSFRS-R subscore (bulbar/fine motor/ gross motor/respiratory) |
10/6/ 6/7 |
7/8/ 8/12 |
8/12/ 12/12 |
10/12/ 12/12 |
12/12/ 9/12 |
9/12/ 12/12 |
12/9/ 7/12 |
12/12/ 10/12 |
10/11/ 7/12 |
11/9/ 9/12 |
| ALSFRS-R rate of progression* | 1.25 | 2.00 | 1.25 | 0.33 | 0.62 | 0.66 | 0.53 | 0.33 | 0.55 | 0.31 |
| Survival, months (from diagnosis to death or last available follow-up) | 3 (dead) |
9 (dead) |
13 (dead) |
45 (dead) |
28 (dead) |
36 (dead) |
54 (alive) |
50 (alive) |
33 (dead) |
18 (alive) |
| Progression (slow/fast)** | fast | fast | fast | slow | slow | slow | slow | slow | slow | slow |
| Cognitive status | ALS- ci |
ALS- ci-bi |
ALS- ci |
ALS- bi |
ALS- cn |
ALS- cn |
ALS- cn |
ALS- cn |
ALS- bi |
ALS- ci |
| Raven’s Progressive Matrices | 25.5 | 15 | 32.5 | 28.5 | 33.5 | 33.5 | 34 | 35 | 36 | 27.5 |
| Digit Span - forward | 3.75 | 5.25 | 6.25 | 5 | 6 | 6 | 6 | 6.5 | 6.75 | 6.5 |
| Digit Span - backward | 2 | 2 | 4 | 4 | 3 | 3 | 3 | 4 | 7 | 2 |
| Logical memory test | 16 | 10 | 18 | 16.5 | 13 | 8.5 | 10 | 11 | 4.5 | 7.5 |
| Phonological verbal fluency | 6 | 18 | 36 | 45 | 37 | 30 | 20 | 21 | 42 | 28 |
| Frontal Assessment Battery | 6.7 | 8.4 | 14 | 18 | 18 | 16.1 | 15 | 16 | 16 | 13.8 |
| Clock Drawing Test | 1 | 4 | 3 | 5 | 5 | 3 | 4 | 5 | 5 | 4 |
| Cogntive estimation Test − 1 | 16 | 21.2 | 12.8 | 12.2 | 16.9 | 16.2 | 16 | 18 | 18.2 | 20.97 |
| Cogntive estimation Test − 2 | 4 | 9 | 2 | 3 | 2 | 5 | 4 | 5 | 2 | 5 |
| Trail making test - A | 92 | 51 | 32 | 31 | 37 | 24 | 34 | 30 | 26 | 54 |
| Trail making test - B | 161 | n/a | 209 | 57 | 91 | n/a | 150 | 130 | 113 | 92 |
| Trail making test - B-A | 69 | n/a | 177 | 26 | 54 | n/a | 116 | 100 | 86 | 38 |
The ALSFRS-R rate progression is calculated as: 48-ALSFRS-R score at PET scan/months between symptoms’ onset and scan.
Patients were classified as slow progression if the monthly decrease of ALSFRS-R score was <0.9/month; as fast progressors if ≥0.9.
3.2. 18F-FDG-PET single-subject maps
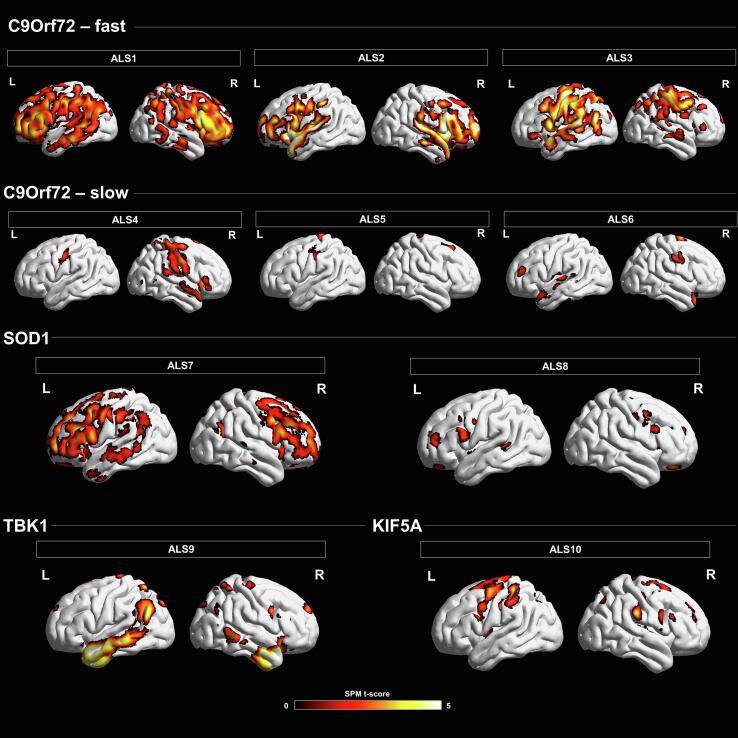
The 18F-FDG-PET single-subject analysis showed a variable pattern of brain hypometabolism in each ALS patient, with some commonalities emerging in the largest group (the C9orf72 group) (see Fig. 1).
Fig. 1.
18F-FDG-PET imaging finding in ALS genetic patients. Brain hypometabolism maps are presented in single cases. Patients ALS1, ALS2 and ALS3 are considered as C9orf72 fast progressors. Patients ALS4, ALS5 and ALS6 are considered as C9orf72 slow progressors. Patient ALS7 and ALS8 were carriers of the SOD1 mutation. The bottom row shows the results of the single-subject analysis in TBK1 (ALS9) and KIF5A (ALS10) patient. The hypometabolism regions are overlaid on a 3D brain template using Brainnet toolbox. The statistical threshold was set at a p-value < 0.05 uncorrected, minimal cluster extent = 100.
3.2.1. Clinical and 18F-FDG-PET imaging findings in patients with C9orf72-ALS
In the C9orf72 group, patients considered as fast progressors (monthly decrease of ALSFRS-R score from symptoms’ onset to 18F-FDG-PET scan > 0.9/month), showed moderate to severe cognitive impairment involving mainly executive functions and language. Analyzing each patient individually, ALS1 had a bulbar onset but a rapid subsequent spinal involvement, and a simultaneous severe non-motor decline. 18F-FDG-PET maps showed widely bilateral involvement of the precentral gyrus, supplementary motor area, superior, middle and inferior frontal gyrus, superior and middle temporal gyrus, angular and fusiform gyrus, insula and precuneus. ALS2 had a predominant bulbar form, with a fast respiratory decline. Similar to ALS1, ALS2 had a severe neurocognitive involvement. 18F-FDG-PET analysis showed hypometabolism in the precentral gyrus, supplementary motor area, superior, middle and inferior frontal gyrus, superior temporal gyrus, anterior cingulate cortex, fusiform gyrus, and orbitofrontal cortex. ALS3 had a predominant bulbar involvement, with moderate executive function deficit. 18F-FDG-PET analysis showed hypometabolism in the precentral gyrus, supplementary motor area, superior, middle and inferior frontal gyrus, superior temporal gyrus, angular gyrus, anterior cingulate cortex and postcentral gyrus.
Patients considered as slow progressors (monthly decrease of ALSFRS-R score from symptoms’ onset to 18F-FDG-PET scan > 0.9/month), had no consistent cognitive impairment. Specifically, ALS4 had a bulbar onset started only with mild dysarthria, associated with behavioral features, mainly apathy, fatuous attitude, and little disease awareness. 18F-FDG-PET maps showed involvement of precentral gyrus, supplementary motor areas, orbitofrontal cortex, and cerebellum. On the contrary, ALS5 had a spinal onset, without bulbar and cognitive involvement over the disease course. 18F-FDG-PET analysis underlined hypometabolism in the precentral gyrus, supplementary motor area, superior frontal gyrus, and postcentral gyrus. ALS6 had a bulbar onset, minimal motor symptoms, and limited to the cranial region, slight executive deficits, albeit not falling ALS-ci within the Strong classification. 18F-FDG-PET analysis showed hypometabolism in the precentral gyrus, superior and middle frontal gyrus, anterior cingulate cortex, insula and superior, middle and inferior temporal gyrus. See Fig. 1 for all details.
3.2.2. Clinical and 18F-FDG-PET imaging findings in patients with SOD1 p.L145F-ALS
The patients with SOD1-ALS (ALS7 and ALS8) had a spinal onset, mainly characterized by the involvement of the lower motor neuron, without bulbar signs. Both subjects had a normal cognitive status, without neuropsychological deficits. Both had a prolonged disease course (>50 months from diagnosis) with upper and lower limbs involved, and mild to moderate respiratory deficits. ALS7 had an unusual clinical feature in ALS patients, with the occurrence of severe urge incontinence both for urine and stools over the course of the disease. 18F-FDG-PET in both patients showed involvement of the precentral gyrus, superior, middle, and inferior frontal gyrus, anterior cingulate, and the medial part of the superior gyrus (Fig. 1). ALS7 expressed a larger extension of frontal dysfunction than ALS8.
3.2.3. Clinical and 18F-FDG-PET imaging findings in patients with TBK1-ALS
The main clinical feature of the patient who carried the TBK1 mutation (ALS9) was the presence of persistent compulsive and repetitive disorder without therapy positive response, before motor symptoms onset. A high level of anxiety was evident. The baseline neurocognitive evaluation showed intact cognitive functioning, but with a worsening in long-term memory deficit and executive functions over the disease course. The motor symptoms, characterized by a prevalent impairment of the upper motor neuron, appeared a few months after the behavioral disturbances and involved both the upper and lower limbs, with a slowly worsening course. In this patient, the SPM t-map showed marked bilateral hypometabolism involving the precentral gyrus, superior frontal gyrus (including the medial part), supplementary motor area, temporal pole, middle and inferior temporal gyrus, fusiform gyrus, angular gyrus, orbitofrontal cortex and cerebellum (Fig. 1).
3.2.4. Clinical and 18F-FDG-PET imaging findings in patient with KIF5A-ALS
The patient carrying the KIF5A mutation (ALS10) was clinically characterized by a prevalent upper motor neuron involvement, mild spastic dysarthria, and indolent disease progression. The neurocognitive evaluation showed a primary involvement of working memory (Digit span test) and a mild involvement of the executive functions, classifying the patient as ALS-ci following the Strong criteria. In this patient, the SPM t-map showed marked bilateral hypometabolism involving the precentral gyrus, superior, middle, and inferior frontal gyrus, supplementary motor area, anterior cingulate, postcentral gyrus, and precuneus (Fig. 1).
3.3. Regional brain metabolism changes and clinical correlations
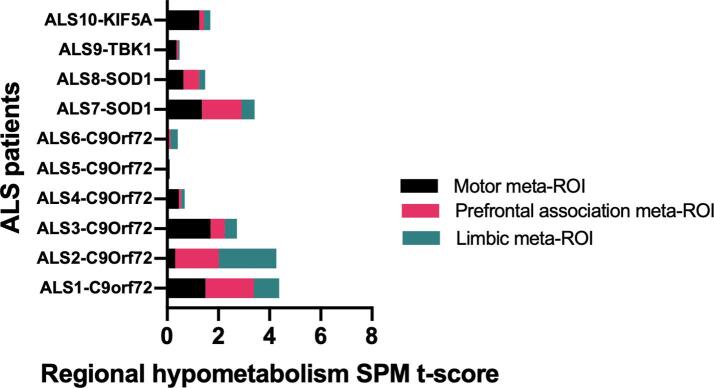
By extracting regional metabolism data from the hypometabolism SPM-maps, the following ROIs resulted as the highest hypometabolic regions: precentral motor, supplementary motor region, superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus, insula, temporal superior gyrus and temporal pole. According to the specific function, we combined the ROIs into three meta-ROIs, grouping motor-related regions into the motor meta-ROI (including the precentral gyrus and the supplementary motor area); regions related to prefrontal activities into the prefrontal association meta-ROI (including the middle frontal gyrus, superior frontal gyrus – orbital part, superior frontal gyrus – medial part and inferior frontal gyrus); regions related to the medial temporal lobe functions into the limbic meta-ROI (including the insula, superior temporal gyrus and temporal pole).
By comparing the single-subject meta-ROI pattern expression, the motor meta-ROI hypometabolism was moderate-to-high in all patients, except for ALS6, a patient with C9orf72-ALS showing only a slight bulbar impairment. The prefrontal associative meta-ROI involvement was highly present in ALS1 and ALS2, the two patients with C9orf72-ALS with the worst cognitive performances at baseline. ALS1 and ALS2, together with ALS6, had the most severe hypometabolism in the limbic meta-ROIs. ALS5, clinically characterized by a pure and slight spinal involvement (without cognitive impairment), had pure motor meta-ROI hypometabolism (Fig. 2).
Fig. 2.
Hypometabolism cluster extraction according to the limbic, prefrontal association and motor meta-ROIs. Mean regional hypometabolism in the three meta-ROI is reported in each single patient. The figure underlines the large variability among the whole sample in the hypometabolism patterns and some commonalities. ALS1, ALS2 and ALS3 are patients with C9orf72-ALS showing widespread hypometabolism involving all the three meta-ROIs and worse clinical functional status. ALS4 ALS5 and ALS6 are patients with C9orf72-ALS showing slight clinical impairment and minor hypometabolism (sole motor involvement has present in ALS5). ALS7 and ALS8 share the same metabolism meta-ROI pattern, even with a different rate of involvement.
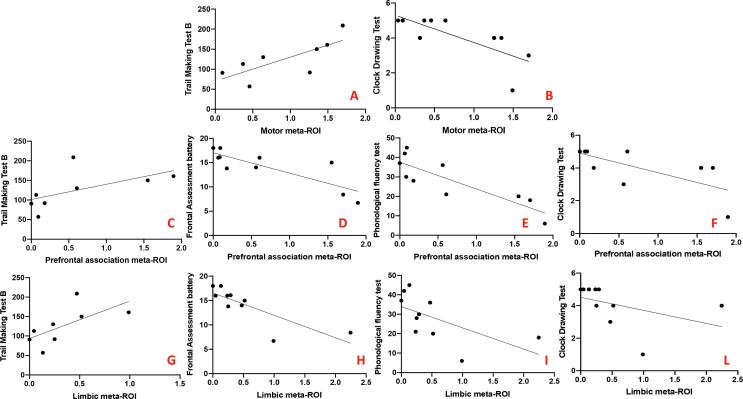
We observed direct correlations between the hypometabolism in the motor meta-ROI and the scores in the Trail-Making Test B (r = 0.83, p = 0.01) and an inverse correlation with scores in the Clock Drawing Test (r = -0.75, p = 0.01). The hypometabolism in the prefrontal association meta-ROI directly correlated with the Trail-Making Test B score (r = 0.74, p = 0.04) and inversely correlated with the scores in the Frontal Assessment battery (r = -0.80, p = 0.006), the phonological fluency test (r = -0.87, p = 0.001) and the Clock Drawing test (r = -0.74, p = 0.01). The hypometabolism in the limbic meta-ROI correlated with scores in the Trail-Making Test B (r = 0.76, p = 0.03), the Frontal Assessment Battery (r = -0.80, p = 0.006), the phonological fluency test (r = -0.82, p = 0.004) and the Clock Drawing Test (r = -0.78, p = 0.008) (Fig. 3).
Fig. 3.
Significant correlation between hypometabolism peak expression and neuropsychological scores. From Panel A and Panel B: correlations between the hypometabolism in the motor meta-ROI and the neuropsychological tests; from Panel C to Panel F: correlations between the hypometabolism in the prefrontal association meta-ROI and the neuropsychological tests; from Panel G to Panel L: correlations between the hypometabolism in the limbic meta-ROI and the neuropsychological tests. X-axis: hypometabolism values; Y-axis: neuropsychological scores.
We did not obtain any correlation between metabolism and both the functional status, evaluated with the ALSFRS-R score (at diagnosis – total and sub-scores, and the rate of disease progression), and the time from symptoms onset to diagnosis. In the multivariate regression analysis, the metabolic rate showed to be unaffected by the considered regressor variables (time from onset to diagnosis, phenotype, genetic group and cognitive status) within the motor (F[5, 4] = 0.354, p = 0.857), prefrontal association (F[5, 4] = 1.633, p = 0.328), and limbic meta-ROIs (F[5, 4] = 2.662, p = 0.182), meaning that none of the cited variables predicted the hypometabolism rate.
4. Discussion
This study, involving ALS patients with known gene mutations, revealed both individual-specific maps of brain hypometabolism and crucial pattern commonalities in patients sharing the same mutation and specific clinical features. Analyzing the clinical and neuropsychological correlates of brain metabolic changes in single mutation carrier patient, we demonstrated a large variability in the genotype-endophenotype-phenotype correlations, and some commonalities. Firstly, we observed an extensive motor and prefrontal hypometabolism in patients with C9orf72-ALS classified as fast progressors. Conversely, the 18F-FDG-PET analysis revealed more limited hypometabolism in patients showing the same mutations, but grouped as slow progressors. Clinical and neuropsychological characteristics were related to regional brain hypometabolism also in ALS patients carrying other gene mutations, including SOD1, TBK1, and KIF5A, supporting the importance of 18F-FDG-PET in revealing patterns of altered neuronal function.
Most literature regarding the role of 18F-FDG-PET in ALS patients reports results at the group level but, crucially, only two research works evaluated single-subject brain hypometabolism maps in sporadic (Sala et al., 2019) and genetic (Castelnovo et al., 2019) ALS. A previous study investigated the brain metabolism differences between a group of patients with C9orf72-ALS and sporadic ALS (Cistaro et al., 2014). The C9orf72 expressed a more severe hypometabolism in the anterior and posterior cingulate cortex, frontotemporal cortices, insula, and subcortical structures (caudate and thalamus), suggesting that genetic ALS may manifest a specific pattern of neuronal dysfunction compared with sporadic ALS. The only previous study using the 18F-FDG-PET single-subject analysis in patients with C9orf72-ALS included three ALS patients and showed heterogeneous patterns of hypometabolism associated with various cognitive and neuropsychological features (but comparable clinical disease onset, which was bulbar in all patients) (Castelnovo et al., 2019). Our study, including a larger sample of C9orf72 mutated ALS patients, allows further evidence and considerations. By grouping patients according to the rate of disease progression (ALSFRS-R worsening from the symptoms onset to the PET scan), commonalities in the hypometabolism patterns emerged. Specifically, C9orf72 fast progressor patients showed a common hypometabolism pattern widely involving both motor and prefrontal association cortices, along with limbic structures, temporal and parietal regions, and the cerebellum. The fast progressor participants were older and showed at the diagnosis greater clinical and cognitive impairment than the other patients with C9orf72-ALS. Conversely, the group of C9orf72 slow progressors showed a common, more restricted hypometabolism pattern distribution involving the same regions. The relatively spared hypometabolism in these patients reflects the relatively slow disease progression and the longer survival time, along with the absence of relevant cognitive impairment. Our results are in line with the previous literature findings, confirming that hypometabolism can also involve limbic regions in patients with C9orf72-ALS, but adding the relevant result of two identifiable patterns associated with different disease progression. Coherently with the evidence derived from autoptic and imaging studies, the ALS pathogenic process can spread from the spinal cord and brainstem to cortical and subcortical regions (Agosta et al., 2012, Brettschneider et al., 2013), and the degree of cortical alterations has been correlated to the clinical impairment (Walhout et al., 2015). We can hypothesize a similar disease spreading in the SOD1 group. In our two patients with SOD1-ALS, we observed a hypometabolic pattern mainly in the motor and prefrontal association regions, e.g., the mesial frontal regions, with a relative saving of the limbic areas, more evident in patient ALS7 than patient ALS8, likely related to a longer disease duration and a relative more advanced disease at the scan time. Additionally, patient ALS7 reported incontinence for urine and stools, described as associated with frontal lobe dysfunctions, especially with the involvement of the superior frontal gyrus and anterior cingulate gyrus (Tish and Geerling, 2020, Woessner et al., 2012). This finding can further justify the presence of a more evident hypometabolic pattern in these areas compared to ALS8. Only one previous FDG-PET study analyzed the metabolic pattern of patients with SOD1-ALS at the group level, showing in the SOD1-ALS group relative hypermetabolism in the motor cortex compared to sporadic ALS and healthy controls (Canosa et al., 2022a, Canosa et al., 2022b). It has been recently suggested that these findings may be due to the glial cells’ activity, namely the neuroinflammatory responses, which have been related to the disease spread in SOD1 mutated asymptomatic and symptomatic subjects (Tondo et al., 2020).
The clinical-metabolic relationship was of particular interest in patients carrying the rarely described mutations TBK1 and KIF5A. ALS9 carried a loss-of-function mutation, of which was recently demonstrated that causes and aberrant pre-mRNA processing leading TBK1 haploinsufficiency (Pozzi et al, 2017). This patient, along with typical motor involvement, showed an extended limbic hypometabolism. Clinically, the patient showed an unusual early behavioral disturbance (obsessive–compulsive disorder), representing the dominant feature in the early disease phase. The KIF5A is a novel gene associated with ALS (Nicolas et al., 2018). Mutations in this gene are also causative of two other neurodegenerative diseases: the hereditary spastic paraplegia (SPG10) and the Charcot-Marie-Tooth type 2 (CMT2), characterized by a predominant motor involvement. There are no published papers describing neuroimaging findings in patient with KIF5A-ALS. Only one previous work using functional brain MRI described the involvement of the supplementary motor area in SPG-KIF5A patients (Tomberg et al., 2012). The hypometabolism described in ALS10, restricted primarily to the motor region, supports the hypothesis of a predominantly motor involvement related to mutations in this gene, associated with a clinically evident upper motor neuron phenotype.
The correlations between brain glucose metabolism and clinical data confirm the correspondence between neuronal dysfunctions and neurological and neuropsychological impairment, using the meta-ROI method in a wider perspective. Hypometabolism in all the three meta-ROIs, namely the motor, prefrontal association and limbic meta-ROI was inversely correlated with performances in the Clock Drawing Task. This represent a multifaced neurocognitive assessment test evaluating several cognitive functions, including visuomotor abilities, executive functions, planning, spatial and semantic memory, and associated with activation in medial frontal, precentral and prefrontal cortex, parietal, temporal and occipital regions, as showed by molecular imaging and functional MRI studies (Matsuoka et al., 2013, Talwar et al., 2019). Coherently, the Trail Making Test scores, evaluating attention and executive functions, throughout the execution of visuo-motor abilities, directly correlated with hypometabolism in the motor, prefrontal association and limbic meta-ROIs, explaining the association with altered attention and procession speed performances (MacPherson et al., 2017). Additionally, the prefrontal association meta-ROI hypometabolism correlated with impaired performances in the fluency verbal test, a test considered as a frontal lobe dysfunction clue (Ghanavati et al., 2019, Riello et al., 2021). Intriguingly, the fluency verbal test also correlated with the limbic meta-ROI hypometabolism. This test is usually considered as a frontal lobe dysfunction clue, but the neural substrates have been recently extended to other brain regions, including temporal and posterior parietal occipital cortices (Cipolotti et al., 2018). Similarly, the Frontal Assessment Battery global score inversely correlated with the prefrontal association and limbic hypometabolism. The Frontal Assessment Battery is a complex battery designed to test the frontal lobe functions, especially sensitive to damage of the middle frontal gyrus, inferior frontal gyrus (prefrontal association) and insula (limbic) (Kopp et al., 2013). Regarding the correlation between the functional status and meta-ROI involvement, truly related to the limited sample size, we did not observe an association between hypometabolism and clinical impairment. Lastly, in the multivariate regression model, none of the regressor variables predicted the hypometabolism in the three meta-ROIs, Again, the group size, could have limited the power of this analysis.
The pathophysiological understanding of neurodegenerative diseases, including ALS, goes through the integration of clinical, genetic, and biological mechanisms. Over the last two decades, the advances in biomarkers research based on pathology and neurodegeneration fluid and imaging measures have paved new opportunities for early diagnosis (Tondo and De Marchi, 2022). On the other hand, the development of brain banks and post-mortem tissue analyses provided new insight into epidemiological aspects, clinic-pathological correlations, and neuronal and extra-neuronal pathology (Mazumder et al., 2022). The higher aim of the convergence of different research lines relies on identifying promising therapeutic targets and developing potential treatment strategies. In our study, we proposed an accurate integration of clinical, neuropsychological, genetic, and imaging data to provide a comprehensive description, at the single-subject level, of the phenotype characteristics of genetic ALS patients. To our knowledge, this is the first study investigating the relationship between the single-subject brain hypometabolism maps, the clinical features, and neuropsychological performances in participants with a definite or laboratory-supported (i.e., electromyographic findings of motoneuron suffering) ALS diagnosis based on the revised El Escorial criteria, carrying pathogenic gene mutations. Due to the relative rarity of the condition, the main limitation of this study regards the small sample, which allows for an accurate single-subject description but hampers conclusive considerations and generalization at a larger level.
Furthermore, the limited sample size does not allow us to disentangle whether the metabolism patterns are linked to the mutational status or are simply expressions of the known clinical heterogeneity of ALS. Likewise, the results described in the patients with mutations in C9orf72, SOD1, TBK1, and KIF5A genes should be considered a presentation of cases rather than a definite genotype-phenotype grouping. Nevertheless, our findings confirm the crucial role of 18F-FDG-PET in revealing specific brain metabolic patterns associated with corresponding clinical and neuropsychological impairment and suggest a multimodal approach integrating clinical, neuropsychological, genetic, and imaging data is recommended for the early characterization and staging in single ALS patients.
5. Conclusions
This study supports the importance of investigating different patterns of brain hypometabolism in patients with motor neuron diseases. The current work brings some evidence in patients with a mendelian form of ALS, where however the genetic testing is - and will - remain the most sensitive supporting parameter, both for diagnosis and prognosis. Our next goal will be the reproduction of this imaging approach on a large case series of ALS sporadic patients, where the current absence of such supportive test forces us to persevere in the disease biomarkers search.
Funding
The study was supported by the AGING Project for Department of Excellence at the Department of Translational Medicine (DIMET), Università del Piemonte Orientale, Novara, Italy.
CRediT authorship contribution statement
Giacomo Tondo: Conceptualization, Methodology, Software, Writing – original draft, Writing – review & editing. Letizia Mazzini: Conceptualization, Writing – review & editing. Silvia Paola Caminiti: Conceptualization, Methodology, Software, Writing – original draft, Writing – review & editing. Maria Francesca Sarnelli: Data curation, Writing – review & editing. Lucia Corrado: Investigation, Writing – review & editing. Roberta Matheoud: Methodology, Software, Writing – review & editing. Sandra D'Alfonso: Methodology, Writing – review & editing. Roberto Cantello: Supervision, Writing – review & editing. Gian Mauro Sacchetti: Methodology, Software, Validation, Writing – review & editing. Daniela Perani: Methodology, Software, Writing – review & editing. Cristoforo Comi: Supervision, Writing – review & editing. Fabiola De Marchi: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank all the individuals with ALS and their caregivers who participated and support this research.
Data availability
Data will be made available on request.
References
- Agosta F., Al-Chalabi A., Filippi M., Hardiman O., Kaji R., Meininger V., Nakano I., Shaw P., Shefner J., Van Den Berg L.H. The El Escorial criteria: strengths and weaknesses. Amyotroph. Lateral Scler. Front. Degener. 2015;16:1–7. doi: 10.3109/21678421.2014.964258. [DOI] [PubMed] [Google Scholar]
- Agosta F., Altomare D., Festari C., Orini S., Gandolfo F., Boccardi M., Arbizu J., Bouwman F., Drzezga A., Nestor P. Clinical utility of FDG-PET in amyotrophic lateral sclerosis and Huntington’s disease. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:1546–1556. doi: 10.1007/s00259-018-4033-0. [DOI] [PubMed] [Google Scholar]
- Agosta, F., Valsasina, P., Riva, N., Copetti, M., Messina, M.J., Prelle, A., Comi, G., Filippi, M., 2012. The cortical signature of amyotrophic lateral sclerosis. [DOI] [PMC free article] [PubMed]
- Boeve B.F., Boylan K.B., Graff-Radford N.R., DeJesus-Hernandez M., Knopman D.S., Pedraza O., Vemuri P., Jones D., Lowe V., Murray M.E., Dickson D.W., Josephs K.A., Rush B.K., Machulda M.M., Fields J.A., Ferman T.J., Baker M., Rutherford N.J., Adamson J., Wszolek Z.K., Adeli A., Savica R., Boot B., Kuntz K.M., Gavrilova R., Reeves A., Whitwell J., Kantarci K., Jack C.R.J., Parisi J.E., Lucas J.A., Petersen R.C., Rademakers R. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain. 2012;135:765–783. doi: 10.1093/brain/aws004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J., Del Tredici K., Toledo J.B., Robinson J.L., Irwin D.J., Grossman M., Suh E., Van Deerlin V.M., Wood E.M., Baek Y. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013;74:20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminiti S.P., Sala A., Presotto L., Chincarini A., Sestini S., Perani D., Schillaci O., Berti V., Calcagni M.L., Cistaro A. Validation of FDG-PET datasets of normal controls for the extraction of SPM-based brain metabolism maps. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:2486–2499. doi: 10.1007/s00259-020-05175-1. [DOI] [PubMed] [Google Scholar]
- Canosa A., Pagani M., Cistaro A., Montuschi A., Iazzolino B., Fania P., Cammarosano S., Ilardi A., Moglia C., Calvo A., Chiò A. 18 F-FDG-PET correlates of cognitive impairment in ALS. Neurology. 2016;86:44–49. doi: 10.1212/WNL.0000000000002242. [DOI] [PubMed] [Google Scholar]
- Canosa A., Calvo A., Moglia C., Vasta R., Palumbo F., Fuda G., Di Pede F., Cabras S., Arena V., Novara A. Brain 18fluorodeoxyglucose-positron emission tomography changes in amyotrophic lateral sclerosis with TARDBP mutations. J. Neurol. Neurosurg. Psychiatry. 2022 doi: 10.1136/jnnp-2021-328296. [DOI] [PubMed] [Google Scholar]
- Canosa A., Calvo A., Moglia C., Vasta R., Palumbo F., Solero L., Di Pede F., Cabras S., Arena V., Zocco G. Amyotrophic lateral sclerosis with SOD1 mutations shows distinct brain metabolic changes. Eur. J. Nucl. Med. Mol. Imaging. 2022:1–9. doi: 10.1007/s00259-021-05668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carluer L., Mondou A., Buhour M.-S., Laisney M., Pélerin A., Eustache F., Viader F., Desgranges B. Neural substrate of cognitive theory of mind impairment in amyotrophic lateral sclerosis. Cortex. 2015;65:19–30. doi: 10.1016/j.cortex.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Castelnovo V., Caminiti S.P., Riva N., Magnani G., Silani V., Perani D. Heterogeneous brain FDG-PET metabolic patterns in patients with C9orf72 mutation. Neurol. Sci. 2019;40:515–521. doi: 10.1007/s10072-018-3685-7. [DOI] [PubMed] [Google Scholar]
- Cedarbaum J.M., Stambler N., Malta E., Fuller C., Hilt D., Thurmond B., Nakanishi A., Group, B.A.S., Group, 1A complete listing of the BDNF Study The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J. Neurol. Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Chia R., Chiò A., Traynor B.J. Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol. 2018;17:94–102. doi: 10.1016/S1474-4422(17)30401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiò A., Mora G., Calvo A., Mazzini L., Bottacchi E., Mutani R. Epidemiology of ALS in Italy: a 10-year prospective population-based study. Neurology. 2009;72:725–731. doi: 10.1212/01.wnl.0000343008.26874.d1. [DOI] [PubMed] [Google Scholar]
- Chiò A., Moglia C., Canosa A., Manera U., D’Ovidio F., Vasta R., Grassano M., Brunetti M., Barberis M., Corrado L., D’Alfonso S., Iazzolino B., Peotta L., Sarnelli M.F., Solara V., Zucchetti J.P., De Marchi F., Mazzini L., Mora G., Calvo A. ALS phenotype is influenced by age, sex, and genetics: A population-based study. Neurology. 2020;94 doi: 10.1212/WNL.0000000000008869. [DOI] [PubMed] [Google Scholar]
- Cipolotti L., MacPherson S.E., Gharooni S., van-Harskamp N., Shallice T., Chan E., Nachev P. Cognitive estimation: Performance of patients with focal frontal and posterior lesions. Neuropsychologia. 2018;1(115):70–77. doi: 10.1016/j.neuropsychologia.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cistaro A., Valentini M.C., Chiò A., Nobili F., Calvo A., Moglia C., Montuschi A., Morbelli S., Salmaso D., Fania P. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:251–259. doi: 10.1007/s00259-011-1979-6. [DOI] [PubMed] [Google Scholar]
- Cistaro A., Pagani M., Montuschi A., Calvo A., Moglia C., Canosa A., Restagno G., Brunetti M., Traynor B.J., Nobili F. The metabolic signature of C9ORF72-related ALS: FDG PET comparison with nonmutated patients. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:844–852. doi: 10.1007/s00259-013-2667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Rosa P.A., Cerami C., Gallivanone F., Prestia A., Caroli A., Castiglioni I., Gilardi M.C., Frisoni G., Friston K., Ashburner J. A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics. 2014;12:575–593. doi: 10.1007/s12021-014-9235-4. [DOI] [PubMed] [Google Scholar]
- Ghanavati E., Salehinejad M.A., Nejati V., Nitsche M.A. Differential role of prefrontal, temporal and parietal cortices in verbal and figural fluency: Implications for the supramodal contribution of executive functions. Sci. Rep. 2019;9(1):3700. doi: 10.1038/s41598-019-40273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman S.A., Hardiman O., Al-Chalabi A., Chió A., Savelieff M.G., Kiernan M.C., Feldman E.L. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 2022 doi: 10.1016/S1474-4422(21)00465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj E., Varrone A., Boellaard R., Albert N.L., Barthel H., van Berckel B., Brendel M., Cecchin D., Ekmekcioglu O., Garibotto V., Lammertsma A.A., Law I., Peñuelas I., Semah F., Traub-Weidinger T., van de Giessen E., Van Weehaeghe D., Morbelli S. EANM procedure guidelines for brain PET imaging using [18F]FDG, version 3. Eur. J. Nucl. Med. Mol. Imaging. 2022;49(2):632–651. doi: 10.1007/s00259-021-05603-w. Epub 2021 Dec 9. Erratum in: Eur J Nucl Med Mol Imaging. 2022 Mar 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannaford A., Pavey N., van den Bos M., Geevasinga N., Menon P., Shefner J.M., Kiernan M.C., Vucic S. Diagnostic utility of gold coast criteria in amyotrophic lateral sclerosis. Ann. Neurol. 2021;89:979–986. doi: 10.1002/ana.26045. [DOI] [PubMed] [Google Scholar]
- Kopp B., Rösser N., Tabeling S., Stürenburg H.J., de Haan B., Karnath H.O., Wessel K. Performance on the Frontal Assessment Battery is sensitive to frontal lobe damage in stroke patients. BMC Neurol. 2013;16(13):179. doi: 10.1186/1471-2377-13-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, G., Tang, Y., Yu, J., Hu, S., 2020. The metabolism pattern of brain FDG-PET in amyotrophic lateral sclerosis.
- Ludolph A.C., Langen K.J., Regard M., Herzog H., Kemper B., Kuwert T., Böttger I.G., Feinendegen L. Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta Neurol. Scand. 1992;85:81–89. doi: 10.1111/j.1600-0404.1992.tb04003.x. [DOI] [PubMed] [Google Scholar]
- MacPherson S.E., Cox S.R., Dickie D.A., Karama S., Starr J.M., Evans A.C., Bastin M.E., Wardlaw J.M., Deary I.J. Processing speed and the relationship between Trail Making Test-B performance, cortical thinning and white matter microstructure in older adults. Cortex. 2017;95:92–103. doi: 10.1016/j.cortex.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T., Narumoto J., Okamura A., Taniguchi S., Kato Y., Shibata K., Nakamura K., Okuyama C., Yamada K., Fukui K. Neural correlates of the components of the clock drawing test. Int. Psychogeriatr. 2013;25(8):1317–1323. doi: 10.1017/S1041610213000690. [DOI] [PubMed] [Google Scholar]
- Mazumder S., Kiernan M.C., Halliday G.M., Timmins H.C., Mahoney C.J. The contribution of brain banks to knowledge discovery in amyotrophic lateral sclerosis: a systematic review. Neuropathol. Appl. Neurobiol. 2022;3:e12845. doi: 10.1111/nan.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejzini R., Flynn L.L., Pitout I.L., Fletcher S., Wilton S.D., Akkari P.A. ALS genetics, mechanisms, and therapeutics: Where are we now? Front. Neurosci. 2019;6(13):1310. doi: 10.3389/fnins.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas A., Kenna K.P., Renton A.E., Ticozzi N., Faghri F., Chia R., Dominov J.A., Kenna B.J., Nalls M.A., Keagle P., Rivera A.M., van Rheenen W., Murphy N.A., van Vugt J.J.F.A., Geiger J.T., Van der Spek R.A., Pliner H.A., Shankaracharya, Smith B.N., Marangi G., Topp S.D., Abramzon Y., Gkazi A.S., Eicher J.D., Kenna A., ITALSGEN Consortium, Mora G., Calvo A., Mazzini L., Riva N., Mandrioli J., Caponnetto C., Battistini S., Volanti P., La Bella V., Conforti F.L., Borghero G., Messina S., Simone I.L., Trojsi F., Salvi F., Logullo F.O., D'Alfonso S., Corrado L., Capasso M., Ferrucci L., Genomic Translation for ALS Care (GTAC) Consortium, Moreno CAM, Kamalakaran S., Goldstein D.B., ALS Sequencing Consortium, Gitler A.D., Harris T., Myers R.M., NYGC ALS Consortium, Phatnani H., Musunuri R.L., Evani U.S., Abhyankar A., Zody M.C., Answer ALS Foundation, Kaye J., Finkbeiner S., Wyman S.K., LeNail A., Lima L., Fraenkel E., Svendsen C.N., Thompson L.M., Van Eyk J.E., Berry J.D., Miller T.M., Kolb S.J., Cudkowicz M., Baxi E., Clinical Research in ALS and Related Disorders for Therapeutic Development (CReATe) Consortium, Benatar M., Taylor J.P., Rampersaud E., Wu G., Wuu J., SLAGEN Consortium, Lauria G., Verde F., Fogh I., Tiloca C., Comi G.P., Sorarù G., Cereda C., French ALS Consortium, Corcia P., Laaksovirta H., Myllykangas L., Jansson L., Valori M., Ealing J., Hamdalla H., Rollinson S., Pickering-Brown S., Orrell R.W., Sidle K.C., Malaspina A., Hardy J., Singleton A.B., Johnson J.O., Arepalli S., Sapp P.C., McKenna-Yasek D., Polak M., Asress S., Al-Sarraj S., King A., Troakes C., Vance C., de Belleroche J., Baas F., Ten Asbroek A.L.M.A., Muñoz-Blanco J.L., Hernandez D.G., Ding J., Gibbs J.R., Scholz S.W., Floeter M.K., Campbell R.H., Landi F., Bowser R., Pulst S.M., Ravits J.M., MacGowan D.J.L., Kirby J., Pioro E.P., Pamphlett R., Broach J., Gerhard G., Dunckley T.L., Brady C.B., Kowall N.W., Troncoso J.C., Le Ber I., Mouzat K., Lumbroso S., Heiman-Patterson T.D., Kamel F., Van Den Bosch L., Baloh R.H., Strom T.M., Meitinger T., Shatunov A., Van Eijk K.R., de Carvalho M., Kooyman M., Middelkoop B., Moisse M., McLaughlin R.L., Van Es M.A., Weber M., Boylan K.B., Van Blitterswijk M., Rademakers R., Morrison K.E., Basak A.N., Mora J.S., Drory V.E., Shaw P.J., Turner M.R., Talbot K., Hardiman O., Williams K.L., Fifita J.A., Nicholson G.A., Blair I.P., Rouleau G.A., Esteban-Pérez J., García-Redondo A., Al-Chalabi A., Project MinE ALS Sequencing Consortium, Rogaeva E., Zinman L., Ostrow L.W., Maragakis N.J., Rothstein J.D., Simmons Z., Cooper-Knock J., Brice A., Goutman S.A., Feldman E.L., Gibson S.B., Taroni F., Ratti A., Gellera C., Van Damme P., Robberecht W., Fratta P., Sabatelli M., Lunetta C., Ludolph A.C., Andersen P.M., Weishaupt J.H., Camu W., Trojanowski J.Q., Van Deerlin V.M., Brown R.H., Jr, van den Berg L.H., Veldink J.H., Harms M.B., Glass J.D., Stone D.J., Tienari P., Silani V., Chiò A., Shaw C.E., Traynor B.J., Landers J.E. Genome-wide Analyses Identify KIF5A as a Novel ALS Gene. Neuron. 2018;97(6) doi: 10.1016/j.neuron.2018.02.027. 1268-1283.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M., Chiò A., Valentini M.C., Öberg J., Nobili F., Calvo A., Moglia C., Bertuzzo D., Morbelli S., De Carli F. Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology. 2014;83:1067–1074. doi: 10.1212/WNL.0000000000000792. [DOI] [PubMed] [Google Scholar]
- Perani D., Della Rosa P.A., Cerami C., Gallivanone F., Fallanca F., Vanoli E.G., Panzacchi A., Nobili F., Pappatà S., Marcone A. Validation of an optimized SPM procedure for FDG-PET in dementia diagnosis in a clinical setting. NeuroImage Clin. 2014;6:445–454. doi: 10.1016/j.nicl.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi L., Valenza F., Mosca L., et al. TBK1 mutations in Italian patients with amyotrophic lateral sclerosis: genetic and functional characterisation. J. Neurol. Neurosurg. Psychiatry. 2017;88(10):869–875. doi: 10.1136/jnnp-2017-316174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan V., Pioro E.P. Comparing brain structural MRI and metabolic FDG-PET changes in patients with ALS-FTD:‘the chicken or the egg?’question. J. Neurol. Neurosurg. Psychiatry. 2015;86:952–958. doi: 10.1136/jnnp-2014-308239. [DOI] [PubMed] [Google Scholar]
- Renton A.E., Majounie E., Waite A., et al. A hexanucleotide repeat expansion in C9ORF72 Is the cause of chromosome 9p21-Linked ALS-FTD. Neuron. 2011;72(2) doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riello M., Frangakis C.E., Ficek B., Webster K.T., Desmond J.E., Faria A.V., Hillis A.E., Tsapkini K. Neural correlates of letter and semantic fluency in primary progressive aphasia. Brain Sci. 2021;12(1):1. doi: 10.3390/brainsci12010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala A., Iaccarino L., Fania P., Vanoli E.G., Fallanca F., Pagnini C., Cerami C., Calvo A., Canosa A., Pagani M. Testing the diagnostic accuracy of [18F] FDG-PET in discriminating spinal-and bulbar-onset amyotrophic lateral sclerosis. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:1117–1131. doi: 10.1007/s00259-018-4246-2. [DOI] [PubMed] [Google Scholar]
- Strong M.J., Abrahams S., Goldstein L.H., Woolley S., Mclaughlin P., Snowden J., Mioshi E., Roberts-South A., Benatar M., HortobáGyi T. Amyotrophic lateral sclerosis-frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph. lateral Scler. Front. Degener. 2017;18:153–174. doi: 10.1080/21678421.2016.1267768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar N.A., Churchill N.W., Hird M.A., Pshonyak I., Tam F., Fischer C.E., Graham S.J., Schweizer T.A. The neural correlates of the clock-drawing test in healthy aging. Front. Hum. Neurosci. 2019;5(13):25. doi: 10.3389/fnhum.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tish M.M., Geerling J.C. The brain and the bladder: forebrain control of urinary (in)continence. Front. Physiol. 2020;3(11):658. doi: 10.3389/fphys.2020.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomberg T., Braschinsky M., Rannikmäe K., Kepler J., Kepler K., Kõrv J., Linnamägi Ü., Asser T. Functional MRI of the cortical sensorimotor system in patients with hereditary spastic paraplegia. Spinal Cord. 2012;50(12):885–890. doi: 10.1038/sc.2012.70. [DOI] [PubMed] [Google Scholar]
- Tondo G., De Marchi F. From biomarkers to precision medicine in neurodegenerative diseases: Where are we? J Clin Med. 2022;11(15):4515. doi: 10.3390/jcm11154515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondo G., Iaccarino L., Cerami C., Vanoli G.E., Presotto L., Masiello V., Coliva A., Salvi F., Bartolomei I., Mosca L., Lunetta C., Perani D. 11 C-PK11195 PET-based molecular study of microglia activation in SOD1 amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2020;7(9):1513–1523. doi: 10.1002/acn3.51112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Laere K., Vanhee A., Verschueren J., De Coster L., Driesen A., Dupont P., Robberecht W., Van Damme P. Value of 18fluorodeoxyglucose–positron-emission tomography in amyotrophic lateral sclerosis: a prospective study. JAMA Neurol. 2014;71:553–561. doi: 10.1001/jamaneurol.2014.62. [DOI] [PubMed] [Google Scholar]
- Walhout R., Westeneng H.-J., Verstraete E., Hendrikse J., Veldink J.H., Van Den Heuvel M.P., Van Den Berg L.H. Cortical thickness in ALS: towards a marker for upper motor neuron involvement. J. Neurol. Neurosurg. Psychiatry. 2015;86:288–294. doi: 10.1136/jnnp-2013-306839. [DOI] [PubMed] [Google Scholar]
- Woessner H., Vibhute P., Barrett K. Acute loss of bladder control in a stroke of the frontal cortex. Neurohospitalist. 2012;2(4):129–131. doi: 10.1177/1941874412450715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.