Highlights
-
•
MS white matter lesions are perivenular. The central vein sign (CVS) is a promising diagnostic biomarker with excellent sensitivity and specificity.
-
•
A subset of MS lesions, termed “chronic active,” shows persistent inflammatory demyelinating activity at the lesion edges. Their imaging correlate, “paramagnetic rim lesions” (PRL), can identify MS cases at risk for early disability accrual and progression.
-
•
PRL are specific for MS, however their diagnostic role requires more investigation. Pilot proof-of-concept clinical trials are now implementing PRL as outcome measures.
-
•
Imaging remyelination remains challenging, and no method has been accepted as a gold standard. At 7T, T1 times correlate with the degree of lesion myelination.
-
•
Cortical lesions are specific for MS and included in the current criteria for MS dissemination in space. Although cortical lesions have been associated with disease progression, their use is limited due to their difficult visualization in vivo, even with 7T MRI.
Keywords: Paramagnetic rim lesions, Central vein sign, Remyelination, Cortical lesions, MRI-pathology correlations, Multiple sclerosis
Abstract
Focal lesions in both white and gray matter are characteristic of multiple sclerosis (MS). Histopathological studies have helped define the main underlying pathological processes involved in lesion formation and evolution, serving as a gold standard for many years. However, histopathology suffers from an intrinsic bias resulting from over-reliance on tissue samples from late stages of the disease or atypical cases and is inadequate for routine patient assessment. Pathological-radiological correlative studies have established advanced MRI’s sensitivity to several relevant MS-pathological substrates and its practicality for assessing dynamic changes and following lesions over time. This review focuses on novel imaging techniques that serve as biomarkers of critical pathological substrates of MS lesions: the central vein, chronic inflammation, remyelination and repair, and cortical lesions. For each pathological process, we address the correlative value of MRI to MS pathology, its contribution in elucidating MS pathology in vivo, and the clinical utility of the imaging biomarker.
1. Introduction
The visualization of focal areas of demyelination with magnetic resonance imaging (MRI) is the most important biomarker for diagnosis and monitoring of multiple sclerosis (MS) disease activity as well as for evaluating the efficacy of all currently approved disease-modifying treatments, which can halt the waves of peripheral inflammation that lead to central nervous system damage. In the last decade, despite their limited availability, 7-tesla (T) research MRI scanners sparked tremendous progress, especially in MS, thanks to their higher resolution and signal-to-noise ratio and the development of new pulse sequences sensitive to important aspects of disease pathology. As MRI is now approaching a resolution relevant to neuropathology, imaging data can clarify and discover new and important information about the longitudinal evolution of critical pathological processes, variously confirming or refuting prior knowledge (Fig. 1).
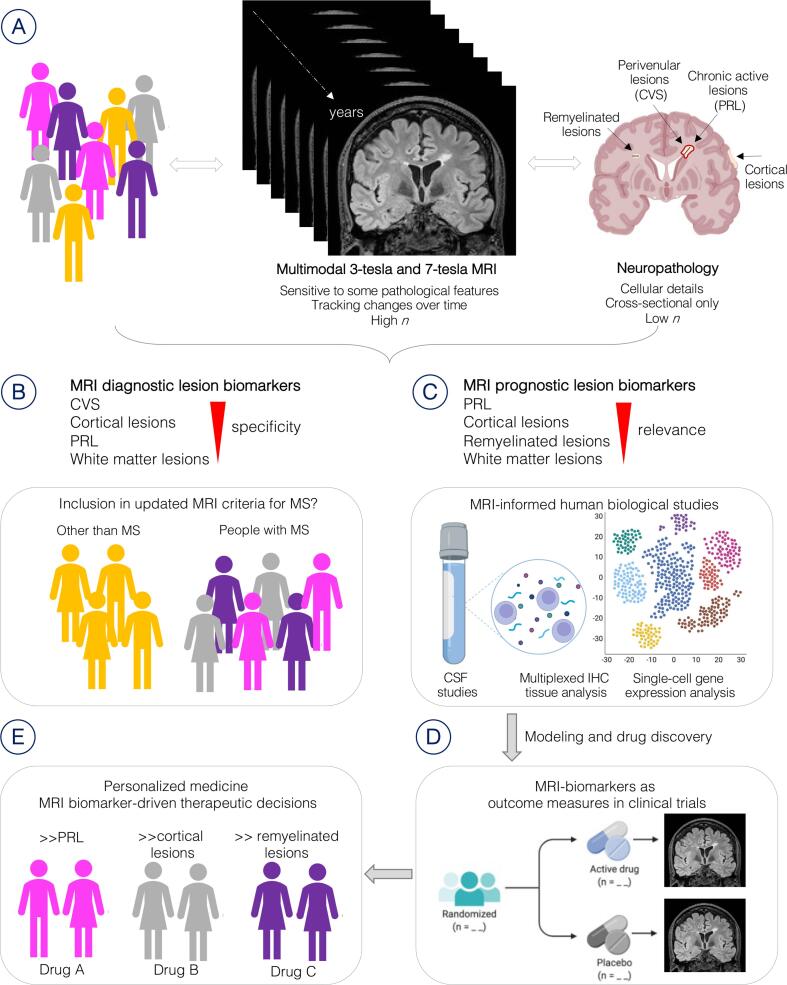
Fig. 1.
Overview of the roles of advanced MRI in multiples sclerosis. (A) Imaging and neuropathology have complementary value in expanding the understanding of MS lesion pathobiology and evolution. (B) Specific pathological features of MS lesions have been translated recently into novel imaging biomarkers. Some of these biomarkers, especially the CVS, have a prominent diagnostic role and may be included in the MRI criteria for MS in the future. (C) The newly discovered prognostic role of other biomarkers, such as the PRL, has prompted further study of their immunobiology, guiding cutting-edge single-cell gene expression analysis as well as multiplexed tissue immunostaining (IHC) and cerebrospinal fluid (CSF) analysis, all with the ultimate aim of finding new treatments to halt disease progression. (D) The same MRI biomarkers can then be implemented to test the efficacy of treatments in clinical trials and (E) guide patient selection for therapeutic decisions. For example, MS cases with high PRL burden may require a specific and different class of drugs than those with high lesion remyelination capacity. Abbreviations: MRI = magnetic resonance imaging; CVS = central vein sign; PRL = paramagnetic rim lesions; IHC = multiplex immunohistochemistry; MS = multiple sclerosis. Created with BioRender.com.
Some of these 7T MRI studies have implemented innovative, customized, 3D-printed, MRI-guided cutting boxes for formalin-fixed brains to guide brain cutting, thereby facilitating more precise MRI-pathological correlations (Absinta et al., 2014). Imaging-inspired approaches have also guided the discovery of novel imaging biomarkers of MS progression via cutting-edge single-cell gene-expression analysis2 and multiplexed cerebrospinal fluid analysis, reinforcing the important pathological and clinical role of neuroimaging. Indeed, with respect to the pathobiology of MS lesions, radiology and pathology have become so intertwined that it is often difficult to keep their terminology separate, leading to some confusion in the literature. The success of these approaches makes it inevitable that knowledge gleaned will prove vital in the near future for diagnosis, as outcome measures of newly designed clinical trials and, ultimately, for guiding patient selection for targeted therapeutic decisions (Fig. 1).
In this context, knowledge of the main MS-related immunobiological processes is now a prerequisite for neuroimagers, as their work will inspire and drive further neuropathological and biological studies and, eventually, treatment testing and approval. This review summarizes the main pathological processes occurring at the MS lesion level in both white and gray matter, highlighting how neuroimaging has been able, in the last decade, to approach the neuropathological level and provide novel, specific biomarkers of the disease and its evolution. With the knowledge gained from the initial 7T MRI studies, most of these features are now also visible using 3T MRI scanners, opening the possibility of their use in clinical practice for diagnosis and prognosis and in newly designed clinical trials for halting clinical progression.
2. Immunopathology of white matter lesions
Focal white matter lesions have historically been identified as a major culprit of MS pathobiology (JM, 1880). A complex interaction between several pathological processes — inflammation, demyelination, and neurodegeneration — contributes to the evolution of white matter lesions in MS (Lassmann, 2018). The prominence and timing of each process varies across lesions and patients and over time (Frischer et al., 2015). Consequently, staging white matter lesions based on onset (early/new vs late/chronic) or key pathological processes depends on the critical question at hand (Kuhlmann et al., 2017). Most pathological classifications of MS lesions focus on cellularity and demyelination, classifying lesions into acute, chronic active, and inactive lesions (Lassmann et al., 1998, Lucchinetti et al., 2000, Trapp et al., 1998). Interestingly, despite remyelination's documented importance in MS, it is missing from most histological classification systems, primarily because of difficulties in capturing the dynamic nature of this process with histological “snapshot” from biopsy material. An exception is the classification of Kuhlmann et al., which included remyelination as a feature of the chronic lesion (Kuhlmann et al., 2017).
The appearance of a new focal area of inflammatory demyelination in the white matter, known as a “lesion” or “plaque,” is the hallmark of MS. It is also one of the first signs easily depicted in vivo using.
MRI. In the white matter, the initial inflammatory reaction occurs mainly at the blood–brain barrier (BBB) level and involves postcapillary venules. BBB disruption is essential for initiating the inflammatory response in the nascent white matter lesion regardless of the triggering events (Balasa et al., 2021). Lymphocytic venulitis is reported in 60 % of acute lesions (Adams et al., 1985) and spreads along a portion of the course of the lesion’s central vein, resulting in the characteristic pattern of perivenular inflammatory demyelination and explaining lesion topography in the brain (Fig. 2, Panels A–B) (Absinta et al., 2016, JW, 1916).
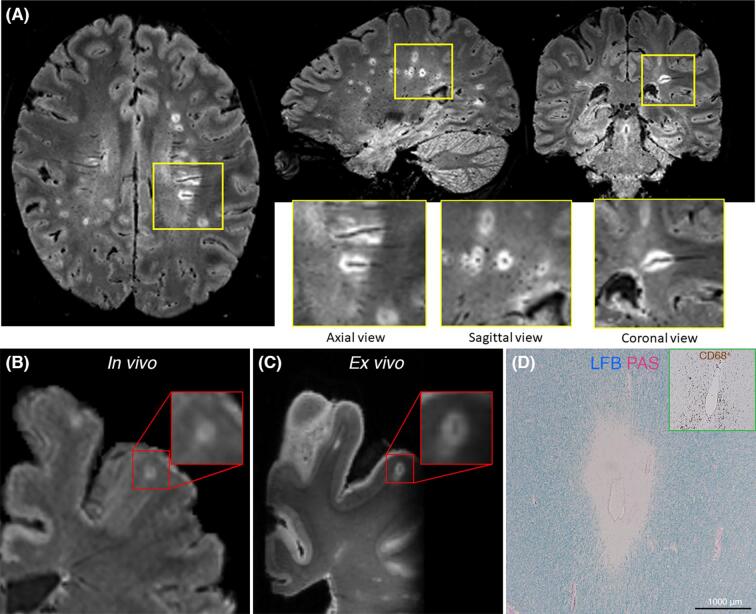
Fig. 2.
In vivo MRI, postmortem MRI, and histopathological visualization of the central vein sign in MS. (A) Representative case with multiple CVS-positive brain lesions, forming radially around the lateral ventricles (along the axis of medullary veins) and in other areas of the cerebral white matter. The MRI technique is FLAIR*, an MRI contrast created after the scan by combining FLAIR and T2*w images separately acquired at 3T, with which it is possible to visualize both lesions and CVS on the same image. A central vein can be detected in all three image planes (Panel A, yellow insets) as a linear hypointensity running centrally through a hyperintense lesion on axial and coronal views and a central hypointense dot within a hyperintense lesion on the sagittal view. (B) In vivo 3T FLAIR* images, slightly motion degraded, and (C) high-resolution, postmortem, 7TT2*-weighted images showing evidence of a CVS + leukocortical lesion in a 60-year-old woman with primary progressive multiple sclerosis. (D) Targeted histopathological analysis revealed perivenular demyelination on Luxol fast blue – periodic acid Schiff staining (LFB–PAS) surrounding a central vein, along with venular wall and perivenular CD68 + macrophage infiltration (green inset). Abbreviations: CVS = central vein sign; FLAIR = fluid-attenuated inversion recovery; LFB–PAS = Luxol Fast Blue – Periodic Acid Schiff; MRI = magnetic resonance imaging; MS = multiple sclerosis. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Animal studies suggest that focal perivascular and parenchymal infiltrates, comprised of activated monocytes and lymphocytes, can be seen even before demyelination and are associated with a gradual opening of the BBB (Maggi et al., 2014). Following early BBB opening, there is an influx of blood-derived inflammatory cells, including monocytes, T-cells, and B-cells, into the CNS, together with a local inflammatory response characterized by glial activation and oligodendrocyte loss (Ortiz et al., 2014). Myelin is rapidly stripped from axons throughout the newly formed lesion. In the early stages, the active inflammatory demyelinating MS lesion is hypercellular throughout, fully demyelinated with relatively preserved axons. Venular remodeling occurs early in the course of lesion formation and is characterized by luminal enlargement and eccentric thickening of the perivascular space with fibrillary collagen type I deposition (Absinta et al., 2019). Interestingly, these changes predominantly affect white matter lesions and are less frequently seen in cortical lesions (Absinta et al., 2019).
3. The central vein sign
Advanced susceptibility-based MRI techniques have made it possible to visualize small veins in vivo within the human brain. T2* contrast is particularly well-suited for this application given its sensitivity to paramagnetic deoxyhemoglobin, which shortens T2* relaxation and causes veins to appear hypointense (Chavhan et al., 2009, Reichenbach et al., 1997, Sati et al., 2014). Combination with fluid-attenuated inversion recovery (FLAIR) images allows the simultaneous visualization of MS lesions and their central veins (Sati et al., 2012), a radiological feature coined the “central vein sign” (CVS; Fig. 2, Panels A–D). The CVS is more common in periventricular and deep white matter lesions compared to cortical lesions (Kilsdonk et al., 2014). This topographical predilection of the CVS for the white matter may relate to reduced venous visibility in cortical gray matter or to disparate pathobiological mechanisms. Chronological variation is also evident, with a more significant reduction in luminal diameter seen when the BBB is breached, although subtle changes persist into the chronic stage (Eisele et al., 2018).
An early study employing MR venography techniques at 1.5T clearly illustrated the perivenous nature of MS lesions in vivo (Tan et al., 2000). Interestingly, the proportion of CVS + lesions was similar in relapsing-remitting and progressive MS (Kilsdonk et al., 2014, Kuchling et al., 2014), suggesting a similar prevalence of perivenular pathology. Multiple 7T and 3T MRI studies found a significantly higher proportion of CVS + lesions in MS compared to its mimics, including cerebral small vessel disease, CNS inflammatory vasculopathies, myelin oligodendrocyte glycoprotein antibody disease (MOGAD) (Ciotti et al., 2022), and neuromyelitis optica spectrum disorders (NMOSD). Proposed thresholds range from 35 to 60 % for the diagnostic use of the CVS in MS (Campion et al., 2017, Ciotti et al., 2021, Cortese et al., 2018, Maggi et al., 2018, Mistry et al., 2016, Sati et al., 2016, Sinnecker et al., 2012). The CVS is particularly helpful for reducing the incidence of MS misdiagnosis in patients with atypical features for MS (Kaisey et al., 2021, Maggi et al., 2020). Three recent meta-analyses have confirmed the clinical relevance of the CVS in improving MS diagnostic accuracy (pooled sensitivity and specificity of 0.95 and 0.92, respectively) (Bhandari et al., 2020, Castellaro et al., 2020, Suh et al., 2019) as compared to 2017 McDonald Criteria (sensitivity 0.83 and specificity 0.39) (Filippi et al., 2021). These meta-analyses also highlighted important differences in CVS detection depending on field strength (lower at 1.5T compared to 3T or 7T), as well as the type of susceptibility-based MR sequence used (high-isotropic-resolution T2* versus SWI) (Castellaro et al., 2020).
4. Practical considerations and current limitations for the use of CVS in clinical workflow
Rating all lesions to compute CVS percentage can be a tedious and time-consuming task, particularly in patients with high lesion load. Therefore, more recent investigations have developed simplified approaches. One study examined the utility of either pre-selecting three lesions on FLAIR and then evaluating them for CVS on FLAIR* (select3), or directly evaluating the FLAIR* image for three or more CVS + lesions (select3*), and showed that both methods yielded excellent specificity for MS diagnosis (Solomon et al., 2018). This approach effectively reduces the number of lesions that require a manual rating, thereby saving time when using the CVS in clinical practice. Similarly, another study evaluated the 6-lesion rule (the presence of at least six morphologically characteristic perivenous lesions) (Mistry et al., 2016). By applying these rules in a small cohort of MS patients and patients diagnosed with small vessel ischemia, all patients were correctly classified, and the classification process took less than two minutes per case (Mistry et al., 2016).
A different approach to this problem has been to develop innovative automated approaches for CVS detection either using a probabilistic Frangi vesselness filtering (Dworkin et al., 2018), or 3D convolutional neural network designs (Maggi et al., 2020). Fully automated CVS analysis approaches from MRI data are indeed promising alternatives to manual rating, especially for clinicians or institutions that lack the time or expertise needed for manual CVS assessment. However, most studies have been conducted with single-center datasets. Further validation is needed to integrate post-acquisition fully automated CVS detection and quantification methods in multicenter settings. Importantly, variation in the optimal CVS percentage cutoff observed between studies likely relates to several technical factors, such as heterogeneity in scanner hardware, field strength, and type of susceptibility-based sequence used (Castellaro et al., 2020), as well as pathobiological factors, such as the diagnostic composition of the non-MS comparator groups. These effects include intra-rater, inter-rater, scan-rescan, and inter-scanner reliability of central vein detection and require further study. A large, well-powered, prospective, multicenter study (CAVS-MS, NCT04495556) is currently underway to examine the CVS as a diagnostic biomarker in typical and atypical presentations of suspected MS and to define simplified diagnostic criteria amenable to routine clinical practice (Ontaneda et al., 2021). Results of this and similar studies will help establish the utility of the CVS as a diagnostic imaging biomarker in MS and pave the way toward standardization and streamlined use in clinical practice.
5. Imaging new inflammatory white matter lesions
White matter lesions in MS are easily identified by their hyperintense signal on T2-weighted and FLAIR sequences. The fact that new MS lesions are not always associated with neurological symptoms makes MRI a more sensitive tool than clinical history and exam for capturing new inflammation in MS. The radiological hallmark of an early MS lesion is the appearance of new parenchymal enhancement on T1-weighted images after intravenous administration of gadolinium-based contrast material, although subtle tissue changes have also been detected with quantitative and qualitative MRI techniques in the months preceding enhancement (Absinta et al., 2015, Fazekas et al., 2002, Ontaneda et al., 2014).
Two different enhancement patterns have been described using dynamic contrast-enhancement (DCE) acquisitions at 7T MRI. In the centrifugal pattern, contrast material leaks out from the disrupted BBB of or around the central vein, whereas in the centripetal pattern, contrast material leaks predominantly from the actively demyelinating edge, eventually filling the lesion (Gaitán et al., 2011). The centripetal DCE pattern, seen only in a percentage of new lesions, temporally follows the centrifugal pattern, but not vice versa (Gaitán et al., 2011). Interestingly, a recent study using an intravenous manganese-based contrast agent, mangafodipir, found that manganese ions enable visualization of acute white matter lesions and their surrounding white matter, with persistent enhancement for an extended period relative to gadolinium-based contrast material (Suto et al., 2020). This persistent enhancement is thought to be due to the intracellular uptake of manganese ions released from mangafodipir, compared to the gadolinium agents, which are restricted to the interstitial and intravascular compartments.
This new MRI-based insight into the late stages of active MS lesions may help to refine not only their classification but also those variables associated with lesion fate, such as remyelination or persistent chronic inflammation. In this context, the presence of a paramagnetic rim in formerly centripetally enhancing lesions after closure of the BBB, approximately 3 months after lesion onset, is prognostic of remyelination failure, as suggested by larger lesions and longer T1 relaxation times at one year (Absinta et al., 2016). Another predictor of persistent perilesional chronic inflammation at the time of lesion formation is reduced diffusivity at the lesion rim (Absinta et al., 2016, Wenzel et al., 2022).
6. Pathological staging of white matter lesions
Following the initial wave of inflammatory demyelination, the fate of MS lesions is variable. In most lesions, remyelination fails. In the so-called “chronic inactive lesions,” the tissue remains demyelinated without a relevant inflammatory infiltrate but with a variable degree of axon loss and gliosis. Inflammation may persist in some other white matter lesions, consisting of a mononuclear infiltrate (a subset of which is iron-enriched) concentrated at the lesion border, with or without ongoing demyelination (Kuhlmann et al., 2017, Matthews, 2019 Oct). When demyelination is present, the lesions have been called “smoldering” (though the use of this term is inconsistent in the literature) (Frischer et al., 2009). The presence and type of myelin breakdown products in macrophages and microglia at the lesion border is the histological marker of this ongoing demyelination (Kuhlmann et al., 2017). A variable degree of axon loss is present in chronic lesions, roughly inversely associated with the degree of remyelination. Neurodegeneration most likely reflects the long-term consequence of a persistent inflammatory milieu, demyelination, other cellular processes such as metabolic failure and loss of trophic support, as well as aging (Trapp et al., 1998, Irvine and Blakemore, 2008, Kornek et al., 2000, Schultz et al., 2017). Alternatively, MS lesions can undergo spontaneous complete or partial remyelination following the initial inflammatory phase. Despite the clinical importance of remyelination, it has also not consistently been adopted into the routine pathological classification, possibly because differentiating between lesions according to the extent of repair and degree of neuroaxonal loss is challenging even histopathologically.
7. Imaging chronic active lesions
Although chronic active/smoldering lesions have long been recognized on neuropathology, their visualization on MRI is a far more recent development, since the typical inflammatory activity at their edges is not associated with opening of the BBB and is thus not visible using conventional postcontrast images. Initial postmortem MRI-pathology correlation studies at 7T MRI used high-resolution susceptibility-based sequences and focused on the validation of paramagnetic rim detection as the MRI biomarker for the characteristic accumulation of iron-laden microglia/macrophage at the chronic active lesion border (Fig. 3) (Absinta et al., 2016, Absinta et al., 2019, Bagnato et al., 2011, Dal-Bianco et al., 2017). These lesions have since been termed “paramagnetic rim lesions” (PRL) and can now be visualized on a variety of susceptibility-based MR images (gradient echo, T2*-weighted segmented echo-planar, and susceptibility-weighted imaging) at both 7T and 3T. The susceptibility changes are also quantifiable using quantitative susceptibility mapping (QSM) (Chen et al., 2014, Barquero et al., 2020, Lou et al., 2021, Zhang et al., 2022). Additional imaging strategies have been developed to characterize various features of these lesions. For example, due to smoldering demyelination at the lesion edge, some of these lesions expand over years, a process that can be identified by analysis of the Jacobian determinant, a sensitive method for calculating volume change from nonlinear registration of T1- and T2-weighted images collected ideally over at least 2 years follow up. Such lesions have also been called “slowly evolving lesions” in the neuroimaging literature (Elliott et al., 2019), however their neuropathological validation is lacking. Also, translocator protein (TSPO) radiotracers for positron emission tomography (PET) can identify lesions with high inflammatory activity of microglia and astrocytes. Innate immune system activation within and around MS lesions as measured by TSPO PET has been shown to correlate with clinical disability and progressive course of the disease (Bodini et al., 2016, Nylund et al., 2022). So far, only one study has directly confirmed TSPO PET signal correlation with PRL (Kaunzner et al., 2019), but TSPO PET's ability to directly detect innate immune system activation may be particularly beneficial in identifying iron-unassociated chronic inflammation as well as chronically inflammed tissue outside the white matter, such as cortex (Herranz et al., 2020).
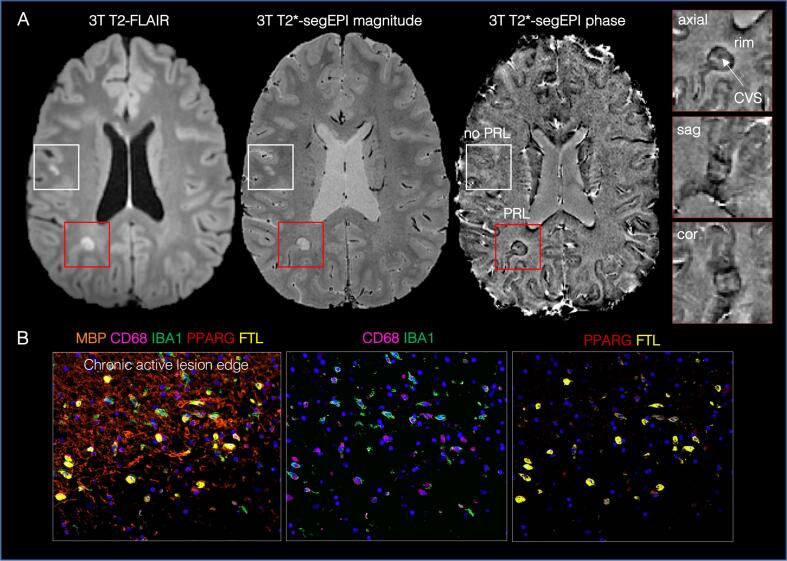
Fig. 3.
Imaging of chronic active white matter lesions: the paramagnetic rim. (A) Representative axial 3T images of a relapsing-remitting MS case showing a PRL (red box) and a non-PRL (white box). PRL are defined as lesions with a paramagnetic hypointense phase rim surrounding an isointense or slightly hypointense lesion core. Triplanar views of the T2*-segEPI phase image (0.55 mm isotropic) can help visualize both the PRL and the CVS. (B) The pathological correlate of PRL is the chronic active/smoldering lesion characterized by a dense iron-laden inflammatory infiltrate at the lesion edge. Multiplex immunostaining is shown in the lower panel from a chronic active lesion of a progressive MS case. Most IBA1-positive microglia/macrophages at the edge are phagocytic (high expression of the lysosomal marker CD68) with upregulation of iron-storage proteins (ferritin light chain, FTL). Fragmentation of the myelin (MBP) is seen at the edge. Some of the IBA1-positive microglia/macrophages activate pathways involved in lipid uptake (PPARG), important for clearance of damaged myelin. Abbreviations: 3T = 3T; FLAIR = fluid-attenuated inversion recovery; segEPI = segmented echo planar imaging; PRL = paramagnetic rim lesions; CVS = central vein sign; MBP = myelin basic protein; CD68 = cluster of Differentiation 68; IBA1 = Ionized calcium binding adapter molecule; PPARG = peroxisome proliferator activated receptor-gamma; FTL = feriritin light chain. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
At both 7T and 3T, PRL are frequent and appear to be specific to MS, only rarely being found in a few other conditions, such as Susac disease (Maggi et al., 2020 Nov, Wuerfel et al., 2012). Overall, about 50 % of relapsing-remitting patients, and about 60 % of progressive patients, have at least one PRL (Absinta et al., 2019, Maggi et al., 2020 Nov). This frequency is similar to what has been found in autopsy cohort studies and was confirmed in a recent meta-analysis of the 20 7T and 11 3T MRI studies performed to date. Recent studies have also looked at the combination of PRL and CVS as valuable diagnostic tools to increase specificity (>90 %) of the MS diagnosis in CIS, RIS, and early relapsing-remitting MS cases (Blindenbacher et al., 2020, Clarke et al., 2020, Micheletti et al., 2021, Suthiphosuwan et al., 2020).
With respect to prognosis, relapsing-remitting patients with high PRL burden reach motor and cognitive disability milestones at an earlier age on average, suggesting that PRL might be one of the factors driving progression in MS (Absinta et al., 2019). There are several possible mechanisms by which PRL may contribute to disability. First, PRL are destructive and do not remyelinate well, if at all. When lesions with a persistent paramagnetic rim are compared to lesions with only a transient or absent rim one year after they arise, PRL have higher lesion volumes and longer T1 values, indicating absence of normal neuropil (Absinta et al., 2016). Detailed analysis shows that this early repair failure occurs within the first three months of lesion development, after the peripheral immune attack subsides and inflammation remains trapped behind a partially intact BBB (Absinta et al., 2016). In addition, PRL have a propensity to expand over time, demyelinating the surrounding periplaque white matter (Absinta et al., 2019, Dal-Bianco et al., 2017). This process is often associated with subtle ongoing axon damage, seen in tissue by accumulation of amyloid precursor protein (APP) ovoids and nonphosphorylated neurofilaments (SMI32 + ) that are markers of acute axon injury and transection (Absinta et al., 2019, Maggi et al., 2021). Degenerating axons release neurofilament light chains (NfL) that can be measured, in vivo, in both the CSF and the serum of MS individuals. In a cohort of 137 non-active MS cases, those with high PRL burden had a significant elevation of the serum NfL age-corrected percentile, supporting in vivo the role of PRL in accelerating axon loss in MS (Maggi et al., 2021).
Although the paramagnetic rims of PRL appear stable in short-to-medium term MRI follow-up (Zhang et al., 2019), recent evidence shows that in a subset of lesions, the rim can vanish over time, after a median of 7 years (Absinta et al., 2021, Dal-Bianco et al., 2021). This result suggests that the rim of PRL could be a pharmacodynamic biomarker for use as an outcome measure in clinical trials designed to halt ongoing damage at the lesion edge. More research is needed to understand better how to use this biomarker for patient stratification and clinical trials. Notably, although the effect of treatment on rim lesions has not been extensively evaluated, preliminary data suggest that currently approved therapies for MS have poor efficacy in controlling this type of inflammation (Absinta et al., 2019), urging development of new drugs that can modulate the inflammatory and glial response within these lesions. A recent retrospective study of 34 cases showed a significant decrease of susceptibility signal in PRL, measured by 3T QSM, in patients on dimethyl fumarate (n = 50 PRL analyzed) compared to those treated with glatiramer acetate (n = 41 PRL analyzed) (Zinger et al., 2022). To date, two clinical trials are implementing this biomarker as primary (phase I/II studies, clinicaltrials.gov identifiers: NCT04025554; NCT04742400) outcome measures, but other trials have incorporated it for secondary or exploratory analysis (Reich et al., 2021). An international consensus on the definition of PRL and the most suitable MRI sequences for its visualization is the next critical task to achieve. Meanwhile, the importance and utility of PRL have prompted great interest in developing automatic PRL detection methods (using machine learning and radiomic features approaches) (Barquero et al., 2020, Lou et al., 2021, Zhang et al., 2022), potentially allowing larger-scale, multicenter, and longitudinal PRL evaluation in the future. Finally, the recent visualization of PRL also at 1.5T MRI paves the way for their future clinical application in patient monitoring (Hemond et al., 2022).
The absence of in vitro or animal models that recapitulate the chronic inflammation seen in PRL prompted additional MRI-informed biological studies using human MS autopsy brain tissue, such as those implementing single nucleus RNA-seq and highly-multiplexed immunostaining (Absinta et al., 2021). The recently-obtained blueprint at the single-cell level notably identified, at the chronic active edge, the presence of few tissue resident T-cells and plasmablasts accompanied by expanded populations of activated microglia with a TREM2-APOE signature, reactive and A1-like inflamed astrocytes (AIMS) which are most abundant around chronic active lesions, stressed oligodendrocytes and immune-like antigen-presenting oligodendrocyte precursor cells (Absinta et al., 2021). The accompanying insights into the crosstalk among these critical cell populations is a first step to better understand the underlying immunological mechanisms and identify new therapeutic targets (Absinta et al., 2021). Recent advances in PET imaging allow quantification of astrocytes in MS patients (Ng et al., 2017), and more specifically, reactive astrocytes (Ishibashi et al., 2020). Imaging glial response in vivo could provide new insights into MS pathophysiology, particularly the driving processes of disease progression, and help identify new therapeutic targets (Healy et al., 2022).
8. Differentiating chronic demyelinated from remyelinated lesions in vivo
While current MS therapies effectively target acute inflammation and lesion formation, they do not meaningfully promote neuroprotection and remyelination, and there is a great need for new therapies to target these processes (Kremer et al., 2019). As remyelination approaches continue to evolve, the need for in vivo markers that can accurately pinpoint the underlying pathophysiological changes has become pressing (Oh et al., 2019). Unfortunately, in contrast to the extensive and detailed description of remyelination in experimental animal models, capturing remyelination in patients is more challenging.
Several advanced imaging techniques have been proposed as in vivo biomarkers to discriminate remyelinated from demyelinated lesions, but most have modest specificity to myelin (Oh et al., 2019), and to date, no method has been widely accepted as the gold standard. Assessing remyelination with magnetization transfer (MT) imaging is supported by MRI-histopathology and in vivo studies, despite only moderate specificity (Barkhof et al., 2003, Moccia et al., 2020, Schmierer et al., 2004). Recovery of the MT ratio as a marker of remyelination is well established in the context of acute lesion repair (Silver et al., 1998, van Waesberghe et al., 1998), which lasts for several months, suggesting a narrow temporal window of spontaneous remyelination (Chen et al., 2008). Recent technical advances implementing new techniques for myelin water imaging (Vavasour et al., 2021, Lee et al., 2018, Ma et al., 2020) and multi-shell diffusion imaging hold promise for better quantifying injury to myelin and axons, respectively (Rahmanzadeh et al., 2021). However, while these new and improved approaches address many historic limitations such as large voxel size, long scanning time, and sensitive post-processing, thus partially closing the gap between research and clinical implementation, they still require histological validation (Oh et al., 2019). Myelin PET imaging offers a complementary approach, with the advantage of direct molecular specificity to myelin pathology (Stankoff et al., 2011, Stankoff et al., 2006, Brugarolas et al., 2018). It has provided corroborating evidence of the highly variable nature of remyelination, raising the intriguing possibility, as yet unverified, that different portions of individual chronic lesions may remyelinate on short time scales while other portions continue to demyelinate (Bodini et al., 2016). Remyelination assessed by PET is correlated with relevant outcomes, including brain atrophy and neurological disability (Bodini et al., 2016, Lubetzki et al., 2020).
A recent study showed the utility of the T1 relaxation time at high magnetic field strength for characterization and qualitative and quantitative discrimination of fully and partially remyelinated from fully demyelinated lesions (Kolb et al., 2021). Histological and 7TT1 map characteristics of remyelinated lesions were similar: a well-demarcated border with remyelination present homogeneously throughout the lesions or in discrete areas. PRL exhibited almost exclusively long-T1 times (Fig. 4), reinforcing the destructive nature of these lesions, as seen with other imaging modalities and histological studies (Absinta et al., 2016, Maggi et al., 2021, Rahmanzadeh et al., 2021). Additionally, several clinical and radiological predictors of lesional remyelination were identified (e.g., patient age at the time of lesion formation (Absinta et al., 2016, Neumann et al., 2019) and lesion location within the brain (Cunniffe and Coles, 2019, Goldschmidt et al., 2009, Patrikios et al., 2006), supporting the potential relevance of T1 time as a marker of remyelination for research and clinical purposes.
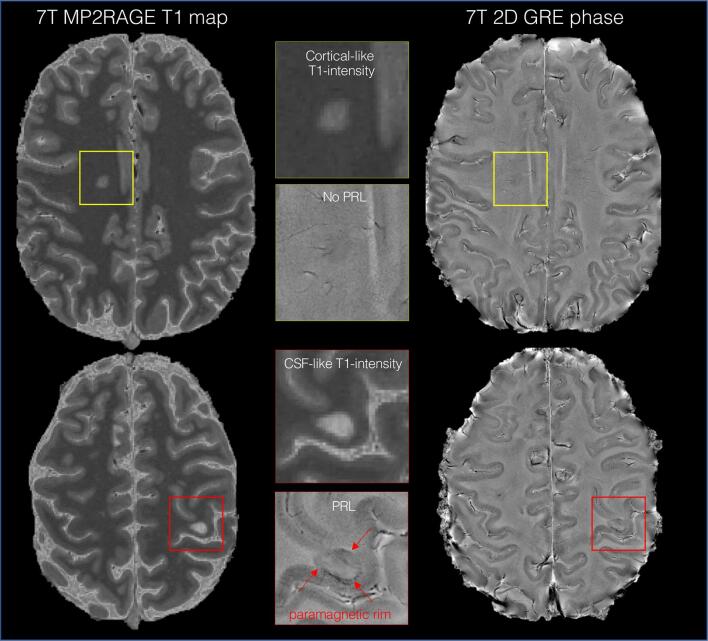
Fig. 4.
Imaging chronic active and potentially remyelinated white matter lesions. Representative axial 7-tesla images of a relapsing-remitting MS case (woman, 40 years old, EDSS 2.5) showing a PRL (red box) and a non-PRL (yellow box) on unwrapped, filtered phase images. As in this case, on 7-tesla T1 maps, long-T1 times (CSF-like T1-intensity appearance) is often seen in the destructive PRL, whereas non-PRL have relatively shorter T1 times (cortical-like T1-intensity appearance), potentially suggesting reduced tissue loss and remyelination. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Aggregated data suggest that QSM-based imaging analysis can be extended to reconstruct not only the sum of susceptibilities but also to separate the influence of diamagnetic (e.g., myelin) and paramagnetic (e.g., iron) susceptibility sources within the same voxel (Shin et al., 2021, Chen et al., 2021). In the context of MS, the ability to identify chronic inflammation (i.e., the paramagnetic rim) and estimate myelin content with the same image sequence makes the QSM-source separation approach an attractive potential myelin biomarker (Shin et al., 2021, Dimov et al., 2022, Emmerich et al., 2021). Qualitative assessment of susceptibility changes on QSM-based imaging was recently suggested as a straightforward approach to distinguish remyelinated from demyelinated MS lesions (Rahmanzadeh et al., 2022). Overall, advances in QSM analysis reinforce its potential as a tool for interrogating microstructural damage, and in particular myelin content in MS, though further improvements in image quality may be required.
It is important to note that the optic nerve and spinal cord are often affected in MS. Identification of spinal cord lesions is currently implemented in routine clinical activities for both diagnosis and disease monitoring. Standardized guidelines for MRI protocol acquisition and detailed indications for use are available (Wattjes et al., 2021). As such, imaging of both optic nerve and cord lesions are potentially important outcome measures of repair. However, imaging these structures is technically challenging, particularly with respect to quantification of myelin content and neuroaxonal integrity, and thus the path to clinical implementation is more remote than other structures discussed in this review.
9. Immunopathology of cortical lesions
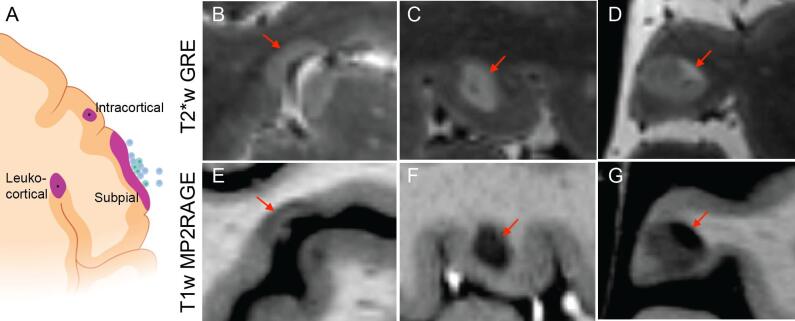
Although traditionally thought of as a disease of white matter, MS lesions in the cerebral cortex are common and can be extensive (Bø et al., 2003, JM, 1868). Cortical lesions have been divided into subtypes: leukocortical, which involve both cortex and white matter; intracortical, which are contained entirely within the cortex; and subpial, which touch the pial surface of the brain and may extend through the entire cortical thickness (Fig. 5). In postmortem samples, cortical lesions have fewer lymphocytes and microglia/macrophages than white matter lesions and have evidence of neuronal damage (Lagumersindez-Denis et al., 2017, Peterson et al., 2001). Inflammation is most pronounced in acute cortical lesions from biopsy samples (Lucchinetti et al., 2011). Subpial cortical lesions are often found underlying meningeal aggregates of inflammatory cells (Haider et al., 2016, Howell et al., 2011, Magliozzi et al., 2007, Serafini et al., 2004), and it is hypothesized that subpial lesions may form due to inflammation originating in the meninges, in contrast to white matter lesions and other cortical lesion subtypes, which are thought to originate from inflammation emanating from parenchymal veins. In support of this hypothesis, high cortical lesion burden was associated with elevated levels of cytokines in the cerebrospinal fluid, including tumor necrosis factor (TNF), C-X-C motif chemokine ligand 13 (CXCL13), and IL10, whereas white matter lesion burden was not (Magliozzi et al., 2018). Subpial lesions are also of interest as they may be specific to MS (Sinnecker et al., 2012, Fischer et al., 2013, Junker et al., 2020), which may have diagnostic and mechanistic implications. Finally, there is evidence that remyelination is more common in cortical lesions than in white matter lesions (Albert et al., 2007, Strijbis et al., 2017), which may be due to differences in intrinsic myelination in the cortex compared to white matter or to differences in inflammation or recovery from inflammation in the two tissue compartments.
Fig. 5.
Topography and imaging appearance of cortical lesions. (A) Cortical lesions are commonly divided into leukocortical, intracortical, and subpial subtypes. Leptomeningeal aggregates of inflammatory cells are associated with subpial lesions, whereas inflammation in leukocortical and intracortical lesions is thought to originate from central parenchymal veins. The panel was created with BioRender.com. 7-tesla T2*-weighted (B–D) and T1-weighted (E–G) scans are the most sensitive known methods for cortical lesion detection on in vivo MRI. These high resolution (0.5 mm isotropic) images also allow visualization of cortical lesion morphology, including lesion borders at the cortex-white matter junction (B, C, E, F) and lesions with different signal intensity in the cortical vs white matter portions of the lesion (D, G).
10. Imaging cortical lesions
Until recently, it was difficult to visualize cortical lesions on in vivo MRI due to small lesion size and low levels of myelination in the normal cortex. Even “advanced” methods at 1.5 and 3T, such as double inversion recovery and magnetization prepared 2 rapid acquisition gradient echo (MP2RAGE), are only ∼ 10 % sensitive for the detection of subpial lesions (Beck et al., 2020, Kilsdonk et al., 2016, Maranzano et al., 2019 Jul, Seewann et al., 2012). Over the last decade, 7TT1 (especially MP2RAGE) and T2*-weighted methods have dramatically improved the ability to see these lesions in vivo (Abdel-Fahim et al., 2014, Beck et al., 2018, Mainero et al., 2009, Nielsen et al., 2012). Use of these methods has demonstrated that cortical lesions are prevalent, even in early disease, and are associated with disability and its progression (Harrison et al., 2015, Nielsen et al., 2013, Datta et al., 2017, Beck et al., 2022). How cortical lesions change and repair over time is largely unknown, as are the relationships between cortical lesion formation and lesion formation in other parts of the central nervous system. For example, there is evidence that the rate of cortical lesion formation may be higher in progressive than relapsing MS (Sethi et al., 2016, Treaba et al., 2019), in contrast to white matter lesions, which form mostly during the relapsing phase of the disease. Whether formation of new cortical lesions explains the accumulation of disability in progressive MS or whether the association between cortical lesion burden and progressive disease is driven by long-term effects of lesions that form early in disease is an open question. It is also unknown if and to what extent currently available disease-modifying therapies effectively prevent cortical lesion formation.
High-resolution MR images acquired at 7T not only improve sensitivity for cortical lesions but also allow an unprecedented view of lesion characteristics (Fig. 5). Some lesions stop abruptly on either side of the cortex-white matter junction, whereas others cross the junction but have different intensities in the cortex vs white matter, suggesting differences in susceptibility to lesion formation, inflammation, and/or repair processes in the two tissue compartments (Beck et al., 2022). BBB opening also appears to be different in the cortex, with gadolinium enhancement only very rarely seen in cortical lesions (Maranzano et al., 2017). Chronic inflammation may also differ, as iron-laden macrophages at the lesion edge (visualized on pathology or by MRI) stop abruptly at the cortical border within leukocortical lesions (Absinta et al., 2016). On the other hand, TSPO-PET might help the identification of microglia-related but iron-unassociated inflammation within the cortex (Herranz et al., 2020).
Our understanding of the natural history and clinical implications of cortical lesions is still limited by the relatively small sample size of the studies that have been done to date. The generalizability of individual centers’ results is also limited by varying MRI sequences and lesion definitions. Larger, multicenter studies and standardization of imaging approaches, some of which are ongoing, will be essential for advancing cortical lesions as a useful biomarker. Cortical lesion segmentation is time-intensive and requires expertise, so the development of automated segmentation methods (reviewed separately in this issue) will also be key for a wider-spread analysis of these lesions. New 3T methods under development may also offer the opportunity for larger-scale studies and eventually integration of cortical lesion imaging into clinical trials and clinical practice (Beck et al., 2020). Finally, use of ultra-high-field MRI methods to guide histopathologic and cerebrospinal fluid analysis, including next-generation transcriptomic methods, offers the exciting potential to understand the mechanisms underlying cortical lesion formation and repair, and in so doing, develop targets for treatment.
11. Conclusions
This review of the last decade’s literature highlights relevant MS lesion-pathological features and their imaging correlates. Their use in clinical practice or clinical trials is limited by the availability and standardization of dedicated high-resolution MRI sequences at 3T and 7T. We envision that the introduction of new computational approaches, e.g., artificial intelligence, will be pivotal for faster readout in multicenter cohorts in both research and clinical care, with implications for diagnosis and prognostic stratification. In the meantime, some of the MRI biomarkers discussed here are already being used in clinical trials of new compounds that may potentially modify or even halt clinical progression.
12. Review design
This review focuses on PubMed-available literature published between January 2011 and December 2021 related to the following imaging terms: central vein sign, paramagnetic rim lesions, remyelinated lesions, and cortical lesions, at both 3T and 7T MRI. For each term, an immunopathological context is provided.
Author's contribution
All authors performed the conceptualization, data curation, and literature search, drafted the manuscript, critically edited, reviewed, approved, and submitted the final draft of the manuscript. HK, OAL, ESB, and MA drafted the figures.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work is primarily supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health, with partial support from the Myelin Repair Foundation. OA is supported by a National Multiple Sclerosis Society-American Brain Foundation Clinician Scientist Development Award (FAN-1807-32163). ESB is supported by a National Multiple Sclerosis Society Career Transition Fellowship (TA-1805-31038). MA is supported by the Conrad N. Hilton Foundation (Marylin Hilton Bridging Award for Physician-Scientists, grant #17313), the International Progressive MS alliance (21NS037), the Roche Foundation for Independent Research, the Cariplo Foundation is a swiss foundation (established in Milan, Italy) (grant #1677), and the FRRB Early Career Award (grant #1750327).
Contributor Information
Hadar Kolb, Email: hadarko@tlvmc.gov.il.
Omar Al-Louzi, Email: omar.al-louzi@cshs.org.
Erin S. Beck, Email: erin.beck@mssm.edu.
Pascal Sati, Email: pascal.sati@cshs.org.
Martina Absinta, Email: absinta.martina@hsr.it.
Daniel S. Reich, Email: daniel.reich@nih.gov.
Data availability
No data was used for the research described in the article.
References
- Abdel-Fahim R., Mistry N., Mougin O., et al. Improved detection of focal cortical lesions using 7T magnetisation transfer imaging in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2014;3(2):258–265. doi: 10.1016/j.msard.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Absinta M., Nair G., Filippi M., et al. Postmortem magnetic resonance imaging to guide the pathologic cut: individualized, 3-dimensionally printed cutting boxes for fixed brains. J. Neuropathol. Exp. Neurol. 2014;73(8):780–788. doi: 10.1097/NEN.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absinta M., Nair G., Sati P., Cortese I.C., Filippi M., Reich D.S. Direct MRI detection of impending plaque development in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2015;2(5):e145. doi: 10.1212/NXI.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absinta M., Sati P., Schindler M., et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J. Clin. Invest. 2016;126(7):2597–2609. doi: 10.1172/JCI86198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absinta M., Sati P., Reich D.S. Advanced MRI and staging of multiple sclerosis lesions. Nat. Rev. Neurol. 2016;12(6):358–368. doi: 10.1038/nrneurol.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absinta M., Nair G., Monaco M.C.G., et al. The “central vein sign” in inflammatory demyelination: the role of fibrillar collagen type I. Ann. Neurol. 2019;85(6):934–942. doi: 10.1002/ana.25461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absinta M., Sati P., Masuzzo F., et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol. 2019;76(12):1474–1483. doi: 10.1001/jamaneurol.2019.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absinta M., Sati P., Masuzzo F., et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol. 2019;76(12):1474–1483. doi: 10.1001/jamaneurol.2019.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absinta M., Maric D., Gharagozloo M., et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature. 2021;597(7878):709–714. doi: 10.1038/s41586-021-03892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams C.W., Poston R.N., Buk S.J., Sidhu Y.S., Vipond H. Inflammatory vasculitis in multiple sclerosis. J. Neurol. Sci. 1985;69(3):269–283. doi: 10.1016/0022-510x(85)90139-x. [DOI] [PubMed] [Google Scholar]
- Albert M., Antel J., Brück W., Stadelmann C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007;17(2):129–138. doi: 10.1111/j.1750-3639.2006.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato F., Hametner S., Yao B., et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134(Pt 12):3602–3615. doi: 10.1093/brain/awr278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasa R., Barcutean L., Mosora O., Manu D. Reviewing the significance of blood-brain barrier disruption in multiple sclerosis pathology and treatment. Int. J. Mol. Sci. 2021;22(16) doi: 10.3390/ijms22168370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhof F., Bruck W., De Groot C.J., et al. Remyelinated lesions in multiple sclerosis: magnetic resonance image appearance. Arch. Neurol. 2003;60(8):1073–1081. doi: 10.1001/archneur.60.8.1073. [DOI] [PubMed] [Google Scholar]
- Barquero G., La Rosa F., Kebiri H., et al. RimNet: a deep 3D multimodal MRI architecture for paramagnetic rim lesion assessment in multiple sclerosis. Neuroimage Clin. 2020;28 doi: 10.1016/j.nicl.2020.102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, E.S., Maranzano, J., Luciano, N.J., et al. 2022. Cortical lesion hotspots and association of subpial lesions with disability in multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England). 10:13524585211069167. [DOI] [PMC free article] [PubMed]
- Beck E.S., Sati P., Sethi V., et al. Improved visualization of cortical lesions in multiple sclerosis using 7T MP2RAGE. AJNR Am. J. Neuroradiol. 2018;39(3):459–466. doi: 10.3174/ajnr.A5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E.S., Gai N., Filippini S., Maranzano J., Nair G., Reich D.S. Inversion recovery susceptibility weighted imaging with enhanced T2 weighting at 3 T improves visualization of subpial cortical multiple sclerosis lesions. Invest. Radiol. 2020;55(11):727–735. doi: 10.1097/RLI.0000000000000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari, A., Xiang, H., Lechner-Scott, J., Agzarian, M. 2020. Central vein sign for multiple sclerosis: a systematic review and meta-analysis. Clin Radiol. 75(6), 479.e9-.e15. [DOI] [PubMed]
- Blindenbacher N., Brunner E., Asseyer S., et al. Evaluation of the ‘ring sign’ and the ‘core sign’ as a magnetic resonance imaging marker of disease activity and progression in clinically isolated syndrome and early multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2020;6(1) doi: 10.1177/2055217320915480. 2055217320915480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bø L., Vedeler C.A., Nyland H.I., Trapp B.D., Mørk S.J. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J. Neuropathol. Exp. Neurol. 2003;62(7):723–732. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- Bodini B., Veronese M., García-Lorenzo D., et al. Dynamic imaging of individual Remyelination profiles in multiple sclerosis. Ann. Neurol. 2016;79(5):726–738. doi: 10.1002/ana.24620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugarolas P., Reich D.S., Popko B. Detecting demyelination by PET: the lesion as imaging target. Mol. Imaging. 2018;17 doi: 10.1177/1536012118785471. 1536012118785471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion T., Smith R.J.P., Altmann D.R., et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur. Radiol. 2017;27(10):4257–4263. doi: 10.1007/s00330-017-4822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellaro M., Tamanti A., Pisani A.I., Pizzini F.B., Crescenzo F., Calabrese M. The use of the central vein sign in the diagnosis of multiple sclerosis: a systematic review and meta-analysis. Diagnostics (Basel) 2020;10(12) doi: 10.3390/diagnostics10121025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavhan G.B., Babyn P.S., Thomas B., Shroff M.M., Haacke E.M. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009;29(5):1433–1449. doi: 10.1148/rg.295095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.T., Collins D.L., Atkins H.L., Freedman M.S., Arnold D.L. Magnetization transfer ratio evolution with demyelination and remyelination in multiple sclerosis lesions. Ann. Neurol. 2008;63(2):254–262. doi: 10.1002/ana.21302. [DOI] [PubMed] [Google Scholar]
- Chen W., Gauthier S.A., Gupta A., et al. Quantitative susceptibility mapping of multiple sclerosis lesions at various ages. Radiology. 2014;271(1):183–192. doi: 10.1148/radiol.13130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Gong N.J., Chaim K.T., Otaduy M.C.G., Liu C. Decompose quantitative susceptibility mapping (QSM) to sub-voxel diamagnetic and paramagnetic components based on gradient-echo MRI data. Neuroimage. 2021;15(242) doi: 10.1016/j.neuroimage.2021.118477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciotti J.R., Eby N.S., Brier M.R., et al. Central vein sign and other radiographic features distinguishing myelin oligodendrocyte glycoprotein antibody disease from multiple sclerosis and aquaporin-4 antibody-positive neuromyelitis optica. Multiple Sclerosis (Houndmills, Basingstoke, England). 2022;28(1):49–60. doi: 10.1177/13524585211007086. [DOI] [PubMed] [Google Scholar]
- Ciotti J.R., Eby N.S., Brier M.R., et al. Central vein sign and other radiographic features distinguishing myelin oligodendrocyte glycoprotein antibody disease from multiple sclerosis and aquaporin-4 antibody-positive neuromyelitis optica. Multiple Sclerosis (Houndmills, Basingstoke, England) 2021;19 doi: 10.1177/13524585211007086. 13524585211007086. [DOI] [PubMed] [Google Scholar]
- Clarke M.A., Pareto D., Pessini-Ferreira L., et al. Value of 3T susceptibility-weighted imaging in the diagnosis of multiple sclerosis. AJNR Am. J. Neuroradiol. 2020;41(6):1001–1008. doi: 10.3174/ajnr.A6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese R., Magnollay L., Tur C., et al. Value of the central vein sign at 3T to differentiate MS from seropositive NMOSD. Neurology. 2018;90(14):e1183–e1190. doi: 10.1212/WNL.0000000000005256. [DOI] [PubMed] [Google Scholar]
- Cunniffe N., Coles A. Promoting remyelination in multiple sclerosis. J. Neurol. 2019;12 doi: 10.1007/s00415-019-09421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal-Bianco A., Grabner G., Kronnerwetter C., et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133(1):25–42. doi: 10.1007/s00401-016-1636-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal-Bianco A., Grabner G., Kronnerwetter C., et al. Long-term evolution of multiple sclerosis iron rim lesions in 7 T MRI. Brain. 2021;144(3):833–847. doi: 10.1093/brain/awaa436. [DOI] [PubMed] [Google Scholar]
- Datta R., Sethi V., Ly S., et al. 7T MRI visualization of cortical lesions in adolescents and young adults with pediatric-onset multiple sclerosis. J. Neuroimaging. 2017;27(5):447–452. doi: 10.1111/jon.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimov A.V., Gillen K.M., Nguyen T.D., et al. Magnetic susceptibility source separation solely from gradient echo data: histological validation. Tomography. 2022;8(3):1544–1551. doi: 10.3390/tomography8030127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin J.D., Sati P., Solomon A., et al. Automated integration of multimodal MRI for the probabilistic detection of the central vein sign in white matter lesions. AJNR Am. J. Neuroradiol. 2018;39(10):1806–1813. doi: 10.3174/ajnr.A5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele P., Szabo K., Ebert A., Brueck W., Platten M., Gass A. Spatiotemporal evolution of venous narrowing in acute MS lesions. Neurol. Neuroimmunol. Neuroinflamm. 2018;5(2):e440. doi: 10.1212/NXI.0000000000000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott C., Wolinsky J.S., Hauser S.L., et al. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Multiple Sclerosis (Houndmills, Basingstoke, England) 2019;25(14):1915–1925. doi: 10.1177/1352458518814117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerich J., Bachert P., Ladd M.E., Straub S. On the separation of susceptibility sources in quantitative susceptibility mapping: theory and phantom validation with an in vivo application to multiple sclerosis lesions of different age. J. Magn. Reson. 2021;330 doi: 10.1016/j.jmr.2021.107033. [DOI] [PubMed] [Google Scholar]
- Fazekas F., Ropele S., Enzinger C., Seifert T., Strasser-Fuchs S. Quantitative magnetization transfer imaging of pre-lesional white-matter changes in multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2002;8(6):479–484. doi: 10.1191/1352458502ms860oa. [DOI] [PubMed] [Google Scholar]
- Filippi M., Preziosa P., Meani A., et al. Performance of the 2017 and 2010 revised McDonald criteria in predicting MS diagnosis after a clinically isolated syndrome: a MAGNIMS study. Neurology. 2021 doi: 10.1212/WNL.0000000000013016. [DOI] [PubMed] [Google Scholar]
- Fischer M.T., Wimmer I., Höftberger R., et al. Disease-specific molecular events in cortical multiple sclerosis lesions. Brain. 2013;136(Pt 6):1799–1815. doi: 10.1093/brain/awt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischer J.M., Bramow S., Dal-Bianco A., et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(Pt 5):1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischer J.M., Weigand S.D., Guo Y., et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 2015;78(5):710–721. doi: 10.1002/ana.24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitán M.I., Shea C.D., Evangelou I.E., et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Ann. Neurol. 2011;70(1):22–29. doi: 10.1002/ana.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt T., Antel J., Konig F.B., Bruck W., Kuhlmann T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology. 2009;72(22):1914–1921. doi: 10.1212/WNL.0b013e3181a8260a. [DOI] [PubMed] [Google Scholar]
- Haider L., Zrzavy T., Hametner S., et al. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain. 2016;139(Pt 3):807–815. doi: 10.1093/brain/awv398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D.M., Roy S., Oh J., et al. Association of cortical lesion burden on 7-T magnetic resonance imaging with cognition and disability in multiple sclerosis. JAMA Neurol. 2015;72(9):1004–1012. doi: 10.1001/jamaneurol.2015.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy L.M., Stratton J.A., Kuhlmann T., Antel J. The role of glial cells in multiple sclerosis disease progression. Nat. Rev. Neurol. 2022;18(4):237–248. doi: 10.1038/s41582-022-00624-x. [DOI] [PubMed] [Google Scholar]
- Hemond C.C., Reich D.S., Dundamadappa S.K. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am. J. Roentgenol. 2022;219(1):120–131. doi: 10.2214/AJR.21.26777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz E., Louapre C., Treaba C.A., et al. Profiles of cortical inflammation in multiple sclerosis by (11)C-PBR28 MR-PET and 7 Tesla imaging. Multiple Sclerosis (Houndmills, Basingstoke, England). 2020;26(12):1497–1509. doi: 10.1177/1352458519867320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell O.W., Reeves C.A., Nicholas R., et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134(Pt 9):2755–2771. doi: 10.1093/brain/awr182. [DOI] [PubMed] [Google Scholar]
- Irvine K.A., Blakemore W.F. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131(Pt 6):1464–1477. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- Ishibashi K., Miura Y., Hirata K., Toyohara J., Ishii K. 18F-THK5351 PET can identify Astrogliosis in multiple sclerosis plaques. Clin. Nucl. Med. 2020;45(2):e98–e100. doi: 10.1097/RLU.0000000000002751. [DOI] [PubMed] [Google Scholar]
- Jm C. Histologie de la sclerose en plaques. Gaz Hop (Paris) 1868:554–566. [Google Scholar]
- JM C. Lecons sur les maladies du systeme nerveux faites a la Salpetriere [Lectures about the diseases of the nervous system done at the Salpetriere]. 4th ed1880.
- Junker A., Wozniak J., Voigt D., et al. Extensive subpial cortical demyelination is specific to multiple sclerosis. Brain Pathol. 2020;30(3):641–652. doi: 10.1111/bpa.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JW D. The histology of disseminated sclerosis. Edinb. Med. J. 1916;17(4):229. [Google Scholar]
- Kaisey M., Solomon A.J., Guerrero B.L., et al. Preventing multiple sclerosis misdiagnosis using the “central vein sign”: a real-world study. Mult. Scler. Relat. Disord. 2021;48 doi: 10.1016/j.msard.2020.102671. [DOI] [PubMed] [Google Scholar]
- Kaunzner U.W., Kang Y., Zhang S., et al. Quantitative susceptibility mapping identifies inflammation in a subset of chronic multiple sclerosis lesions. Brain. 2019;142(1):133–145. doi: 10.1093/brain/awy296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilsdonk I.D., Lopez-Soriano A., Kuijer J.P., et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J. Neurol. 2014;261(7):1356–1364. doi: 10.1007/s00415-014-7351-6. [DOI] [PubMed] [Google Scholar]
- Kilsdonk I.D., Jonkman L.E., Klaver R., et al. Increased cortical grey matter lesion detection in multiple sclerosis with 7 T MRI: a post-mortem verification study. Brain. 2016;139(Pt 5):1472–1481. doi: 10.1093/brain/aww037. [DOI] [PubMed] [Google Scholar]
- Kolb H., Absinta M., Beck E.S., et al. 7T MRI Differentiates Remyelinated from Demyelinated Multiple Sclerosis Lesions. Ann. Neurol. 2021;90(4):612–626. doi: 10.1002/ana.26194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornek B., Storch M.K., Weissert R., et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 2000;157(1):267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer D., Göttle P., Flores-Rivera J., Hartung H.P., Küry P. Remyelination in multiple sclerosis: from concept to clinical trials. Curr. Opin. Neurol. 2019;32(3):378–384. doi: 10.1097/WCO.0000000000000692. [DOI] [PubMed] [Google Scholar]
- Kuchling J., Ramien C., Bozin I., et al. Identical lesion morphology in primary progressive and relapsing-remitting MS–an ultrahigh field MRI study. Multiple Sclerosis (Houndmills, Basingstoke, England) 2014;20(14):1866–1871. doi: 10.1177/1352458514531084. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T., Ludwin S., Prat A., Antel J., Bruck W., Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133(1):13–24. doi: 10.1007/s00401-016-1653-y. [DOI] [PubMed] [Google Scholar]
- Lagumersindez-Denis N., Wrzos C., Mack M., et al. Differential contribution of immune effector mechanisms to cortical demyelination in multiple sclerosis. Acta Neuropathol. 2017;134(1):15–34. doi: 10.1007/s00401-017-1706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H., Raine C.S., Antel J., Prineas J.W. Immunopathology of multiple sclerosis: report on an international meeting held at the Institute of Neurology of the University of Vienna. J. Neuroimmunol. 1998;86(2):213–217. doi: 10.1016/s0165-5728(98)00031-9. [DOI] [PubMed] [Google Scholar]
- Lee L.E., Ljungberg E., Shin D., et al. Inter-vendor reproducibility of myelin water imaging using a 3D gradient and spin echo sequence. Front. Neurosci. 2018;12:854. doi: 10.3389/fnins.2018.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou C., Sati P., Absinta M., et al. Fully automated detection of paramagnetic rims in multiple sclerosis lesions on 3T susceptibility-based MR imaging. Neuroimage Clin. 2021;32 doi: 10.1016/j.nicl.2021.102796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubetzki C., Zalc B., Williams A., Stadelmann C., Stankoff B. Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol. 2020;19(8):678–688. doi: 10.1016/S1474-4422(20)30140-X. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C., Brück W., Parisi J., Scheithauer B., Rodriguez M., Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 2000;47(6):707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C.F., Popescu B.F., Bunyan R.F., et al. Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 2011;365(23):2188–2197. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.J., Jang H., Wei Z., et al. Myelin imaging in human brain using a Short Repetition Time Adiabatic Inversion Recovery Prepared Ultrashort Echo Time (STAIR-UTE) MRI sequence in multiple sclerosis. Radiology. 2020;297(2):392–404. doi: 10.1148/radiol.2020200425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi P., Macri S.M., Gaitán M.I., et al. The formation of inflammatory demyelinated lesions in cerebral white matter. Ann. Neurol. 2014;76(4):594–608. doi: 10.1002/ana.24242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi P., Absinta M., Grammatico M., et al. Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies. Ann. Neurol. 2018;83(2):283–294. doi: 10.1002/ana.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi P., Sati P., Nair G., et al. Paramagnetic rim lesions are specific to multiple sclerosis: an international Multicenter 3T MRI study. Ann. Neurol. 2020;88(5):1034–1042. doi: 10.1002/ana.25877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi P., Fartaria M.J., Jorge J., et al. CVSnet: a machine learning approach for automated central vein sign assessment in multiple sclerosis. NMR Biomed. 2020;33(5):e4283. doi: 10.1002/nbm.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi P., Absinta M., Sati P., et al. The “central vein sign” in patients with diagnostic “red flags” for multiple sclerosis: a prospective multicenter 3T study. Multiple sclerosis (Houndmills, Basingstoke, England). 2020;26(4):421–432. doi: 10.1177/1352458519876031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi P., Kuhle J., Schädelin S., et al. Chronic White Matter Inflammation and Serum Neurofilament Levels in Multiple Sclerosis. Neurology. 2021;97(6):e543–e553. doi: 10.1212/WNL.0000000000012326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliozzi R., Howell O., Vora A., et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130(Pt 4):1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- Magliozzi R., Howell O.W., Nicholas R., et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann. Neurol. 2018;83(4):739–755. doi: 10.1002/ana.25197. [DOI] [PubMed] [Google Scholar]
- Mainero C., Benner T., Radding A., et al. In vivo imaging of cortical pathology in multiple sclerosis using ultra-high field MRI. Neurology. 2009;73(12):941–948. doi: 10.1212/WNL.0b013e3181b64bf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranzano J., Rudko D.A., Nakamura K., et al. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology. 2017;89(7):714–721. doi: 10.1212/WNL.0000000000004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranzano J., Dadar M., Rudko D.A., et al. Comparison of multiple sclerosis cortical lesion types detected by multicontrast 3T and 7T MRI. AJNR Am. J. Neuroradiol. 2019;40(7):1162–1169. doi: 10.3174/ajnr.A6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P.M. Chronic inflammation in multiple sclerosis - seeing what was always there. Nat. Rev. Neurol. 2019;15(10):582–593. doi: 10.1038/s41582-019-0240-y. [DOI] [PubMed] [Google Scholar]
- Micheletti, L., Maldonado, F.R., Watal, P., et al. 2021. Utility of paramagnetic rim lesions on 1.5-T susceptibility phase imaging for the diagnosis of pediatric multiple sclerosis. Pediatr. Radiol. [DOI] [PubMed]
- Mistry N., Abdel-Fahim R., Samaraweera A., et al. Imaging central veins in brain lesions with 3-T T2*-weighted magnetic resonance imaging differentiates multiple sclerosis from microangiopathic brain lesions. Multiple Sclerosis (Houndmills, Basingstoke, England). 2016;22(10):1289–1296. doi: 10.1177/1352458515616700. [DOI] [PubMed] [Google Scholar]
- Moccia M., van de Pavert S., Eshaghi A., et al. Pathologic correlates of the magnetization transfer ratio in multiple sclerosis. Neurology. 2020;95(22):e2965–e2976. doi: 10.1212/WNL.0000000000010909. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Multiple sclerosis pathology. Cold Spring Harb. Perspect Med. 2018;8:3. doi: 10.1101/cshperspect.a028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann B., Segel M., Chalut K.J., Franklin R.J. Remyelination and ageing: reversing the ravages of time. Multiple sclerosis (Houndmills, Basingstoke, England). 2019;25(14):1835–1841. doi: 10.1177/1352458519884006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K.P., Pascoal T.A., Mathotaarachchi S., et al. Monoamine oxidase B inhibitor, selegiline, reduces (18)F-THK5351 uptake in the human brain. Alzheimers Res. Ther. 2017;9(1):25. doi: 10.1186/s13195-017-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen A.S., Kinkel R.P., Tinelli E., Benner T., Cohen-Adad J., Mainero C. Focal cortical lesion detection in multiple sclerosis: 3 Tesla DIR versus 7 Tesla FLASH-T2. J. Magn. Reson. Imaging. 2012;35(3):537–542. doi: 10.1002/jmri.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen A.S., Kinkel R.P., Madigan N., Tinelli E., Benner T., Mainero C. Contribution of cortical lesion subtypes at 7T MRI to physical and cognitive performance in MS. Neurology. 2013;81(7):641–649. doi: 10.1212/WNL.0b013e3182a08ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund M., Sucksdorff M., Matilainen M., Polvinen E., Tuisku J., Airas L. Phenotyping of multiple sclerosis lesions according to innate immune cell activation using 18 kDa translocator protein-PET. Brain Commun. 2022;4(1):fcab301. doi: 10.1093/braincomms/fcab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J., Ontaneda D., Azevedo C., et al. Imaging outcome measures of neuroprotection and repair in MS: a consensus statement from NAIMS. Neurology. 2019;92(11):519–533. doi: 10.1212/WNL.0000000000007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontaneda D., Sakaie K., Lin J., et al. Identifying the start of multiple sclerosis injury: a serial DTI study. J. Neuroimaging. 2014;24(6):569–576. doi: 10.1111/jon.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontaneda D., Sati P., Raza P., et al. Central vein sign: a diagnostic biomarker in multiple sclerosis (CAVS-MS) study protocol for a prospective multicenter trial. Neuroimage Clin. 2021;32 doi: 10.1016/j.nicl.2021.102834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz G.G., Pacheco-Moisés F.P., Macías-Islas M., et al. Role of the blood-brain barrier in multiple sclerosis. Arch. Med. Res. 2014;45(8):687–697. doi: 10.1016/j.arcmed.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Patrikios P., Stadelmann C., Kutzelnigg A., et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129(Pt 12):3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- Peterson J.W., Bö L., Mörk S., Chang A., Trapp B.D. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann. Neurol. 2001;50(3):389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- Rahmanzadeh R., Lu P.J., Barakovic M., et al. Myelin and axon pathology in multiple sclerosis assessed by myelin water and multi-shell diffusion imaging. Brain. 2021;144(6):1684–1696. doi: 10.1093/brain/awab088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanzadeh R., Galbusera R., Lu P.J., et al. A new advanced MRI Biomarker for remyelinated lesions in multiple sclerosis. Ann. Neurol. 2022 doi: 10.1002/ana.26441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich D.S., Arnold D.L., Vermersch P., et al. Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: a phase 2b, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2021;20(9):729–738. doi: 10.1016/S1474-4422(21)00237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach J.R., Venkatesan R., Schillinger D.J., Kido D.K., Haacke E.M. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997;204(1):272–277. doi: 10.1148/radiology.204.1.9205259. [DOI] [PubMed] [Google Scholar]
- Sati P., George I.C., Shea C.D., Gaitán M.I., Reich D.S. FLAIR*: a combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology. 2012;265(3):926–932. doi: 10.1148/radiol.12120208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sati P., Thomasson D.M., Li N., et al. Rapid, high-resolution, whole-brain, susceptibility-based MRI of multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England). 2014;20(11):1464–1470. doi: 10.1177/1352458514525868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sati P., Oh J., Constable R.T., et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12(12):714–722. doi: 10.1038/nrneurol.2016.166. [DOI] [PubMed] [Google Scholar]
- Schmierer K., Scaravilli F., Altmann D.R., Barker G.J., Miller D.H. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann. Neurol. 2004;56(3):407–415. doi: 10.1002/ana.20202. [DOI] [PubMed] [Google Scholar]
- Schultz V., van der Meer F., Wrzos C., et al. Acutely damaged axons are remyelinated in multiple sclerosis and experimental models of demyelination. Glia. 2017;65(8):1350–1360. doi: 10.1002/glia.23167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seewann A., Kooi E.J., Roosendaal S.D., et al. Postmortem verification of MS cortical lesion detection with 3D DIR. Neurology. 2012;78(5):302–308. doi: 10.1212/WNL.0b013e31824528a0. [DOI] [PubMed] [Google Scholar]
- Serafini B., Rosicarelli B., Magliozzi R., Stigliano E., Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14(2):164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi V., Yousry T., Muhlert N., et al. A longitudinal study of cortical grey matter lesion subtypes in relapse-onset multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2016;87(7):750–753. doi: 10.1136/jnnp-2015-311102. [DOI] [PubMed] [Google Scholar]
- Shin H.G., Lee J., Yun Y.H., et al. χ-separation: Magnetic susceptibility source separation toward iron and myelin mapping in the brain. Neuroimage. 2021;15(240) doi: 10.1016/j.neuroimage.2021.118371. [DOI] [PubMed] [Google Scholar]
- Silver N.C., Lai M., Symms M.R., Barker G.J., McDonald W.I., Miller D.H. Serial magnetization transfer imaging to characterize the early evolution of new MS lesions. Neurology. 1998;51(3):758–764. doi: 10.1212/wnl.51.3.758. [DOI] [PubMed] [Google Scholar]
- Sinnecker T., Dörr J., Pfueller C.F., et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79(7):708–714. doi: 10.1212/WNL.0b013e3182648bc8. [DOI] [PubMed] [Google Scholar]
- Solomon A.J., Watts R., Ontaneda D., Absinta M., Sati P., Reich D.S. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Multiple Sclerosis (Houndmills, Basingstoke, England). 2018;24(6):750–757. doi: 10.1177/1352458517726383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankoff B., Wang Y., Bottlaender M., et al. Imaging of CNS myelin by positron-emission tomography. Proc. Natl. Acad. Sci. USA. 2006;103(24):9304–9309. doi: 10.1073/pnas.0600769103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankoff B., Freeman L., Aigrot M.S., et al. Imaging central nervous system myelin by positron emission tomography in multiple sclerosis using [methyl-11C]-2-(4'-methylaminophenyl)- 6-hydroxybenzothiazole. Ann. Neurol. 2011;69(4):673–680. doi: 10.1002/ana.22320. [DOI] [PubMed] [Google Scholar]
- Strijbis E.M.M., Kooi E.J., van der Valk P., Geurts J.J.G. Cortical remyelination is heterogeneous in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2017;76(5):390–401. doi: 10.1093/jnen/nlx023. [DOI] [PubMed] [Google Scholar]
- Suh C.H., Kim S.J., Jung S.C., Choi C.G., Kim H.S. The “Central Vein Sign” on T2*-weighted images as a diagnostic tool in multiple sclerosis: a systematic review and meta-analysis using individual patient data. Sci. Rep. 2019;9(1):18188. doi: 10.1038/s41598-019-54583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthiphosuwan S., Sati P., Absinta M., et al. Paramagnetic rim sign in radiologically isolated syndrome. JAMA Neurol. 2020;77(5):653–655. doi: 10.1001/jamaneurol.2020.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto D.J., Nair G., Sudarshana D.M., et al. Manganese-enhanced MRI in patients with multiple sclerosis. AJNR Am. J. Neuroradiol. 2020;41(9):1569–1576. doi: 10.3174/ajnr.A6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan I.L., van Schijndel R.A., Pouwels P.J., et al. MR venography of multiple sclerosis. AJNR Am. J. Neuroradiol. 2000;21(6):1039–1042. [PMC free article] [PubMed] [Google Scholar]
- Trapp B.D., Peterson J., Ransohoff R.M., Rudick R., Mörk S., Bö L. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998;338(5):278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Treaba C.A., Granberg T.E., Sormani M.P., et al. Longitudinal characterization of cortical lesion development and evolution in multiple sclerosis with 7.0-T MRI. Radiology. 2019;291(3):740–749. doi: 10.1148/radiol.2019181719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Waesberghe J.H., van Walderveen M.A., Castelijns J.A., et al. Patterns of lesion development in multiple sclerosis: longitudinal observations with T1-weighted spin-echo and magnetization transfer MR. AJNR Am. J. Neuroradiol. 1998;19(4):675–683. [PMC free article] [PubMed] [Google Scholar]
- Vavasour I.M., Chang K.L., Combes A.J.E., et al. Water content changes in new multiple sclerosis lesions have a minimal effect on the determination of myelin water fraction values. J. Neuroimaging. 2021;31(6):1119–1125. doi: 10.1111/jon.12908. [DOI] [PubMed] [Google Scholar]
- Wattjes M.P., Ciccarelli O., Reich D.S., et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20(8):653–670. doi: 10.1016/S1474-4422(21)00095-8. [DOI] [PubMed] [Google Scholar]
- Wenzel N., Wittayer M., Weber C.E., et al. MRI predictors for the conversion from contrast-enhancing to iron rim multiple sclerosis lesions. J. Neurol. 2022;269(8):4414–4420. doi: 10.1007/s00415-022-11082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuerfel J., Sinnecker T., Ringelstein E.B., et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England). 2012;18(11):1592–1599. doi: 10.1177/1352458512441270. [DOI] [PubMed] [Google Scholar]
- Zhang S., Nguyen T.D., Hurtado Rúa S.M., et al. Quantitative susceptibility mapping of time-dependent susceptibility changes in multiple sclerosis lesions. AJNR Am. J. Neuroradiol. 2019;40(6):987–993. doi: 10.3174/ajnr.A6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Nguyen T.D., Zhang J., et al. QSMRim-Net: Imbalance-aware learning for identification of chronic active multiple sclerosis lesions on quantitative susceptibility maps. Neuroimage Clin. 2022;34 doi: 10.1016/j.nicl.2022.102979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinger N., Ponath G., Sweeney E., et al. Dimethyl fumarate reduces inflammation in chronic active multiple sclerosis lesions. Neurol. Neuroimmunol. Neuroinflamm. 2022;9(2) doi: 10.1212/NXI.0000000000001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.