Abstract
Background
Osteoarthritis (OA) is a common degenerative joint disease with significant negative impact on the quality of life. It has been reported that abnormal upregulation of β-catenin signaling could lead to OA development; however, the upstream regulatory mechanisms of β-catenin signaling have not been determined.
Methods
Primary rat chondrocytes and ATDC5 chondrocyte cell line were stimulated with AKT2 and treated with or without metformin, an adenosine 5′-monophosphate-activated protein kinase (AMPK) activator. Westerrn blot analysis, luciferase reporter assay and immunofluorescent (IF) staining were performed to examine changes in β-cateninS552 phosphorylation and β-catenin nuclear translocation in ATDC5 cells and in primary chondrocytes.
Results
We found that metformin inhibited β-cateninS552 phosphorylation in ATDC5 cells and in primary chondrocytes in a time-dependent manner. Metformin inhibited β-catenin nuclear translocation and β-catenin reporter activity. In addition, metformin also attenuated the expression of β-catenin downstream target genes. We also demonstrated that metformin inhibited β-cateninS552 phosphorylation in articular cartilage in mice.
Conclusion
These findings suggest that metformin may exert its chondro-protective effect at least in part through the inhibition of β-catenin signaling in chondrocytes.
The translational potential of this article
This study demonstrated the interaction between AMPK and β-catenin signaling in chondrocytes and defined novel molecular targets for the treatment of OA disease.
Keywords: Osteoarthritis, AMPK, β-catenin, Phosphorylation, Metformin, Chondrocyte
Abbreviations: Osteoarthritis, OA
1. Introduction
Osteoarthritis (OA) is a degenerative disease affecting entire joint [1,2]. Although the pathological mechanisms of OA development are not fully defined, it has been demonstrated that patients with the FrezB mutation had increased risk in OA development [[3], [4], [5]]. Frezb (sFRP3) protein is a decoy receptor of Wnt signaling and in vitro studies showed that FrezB mutation could activate β-catenin signaling [6]. In animal studies, FrezB deletion or β-catenin activation in chondrocytes leads to OA initiation and progression in knee joint [[7], [8], [9]]. Additional evidence also demonstrated that β-catenin activation leads to OA development in hip joint [10] and temporomandibular joint (TMJ) [11]. Although these studies demonstrated the importance of β-catenin signaling in OA development, the upstream regulatory mechenism and downstream target genes of β-catenin signaling in articular chondrocytes have not been defined. In this study, we investigated the signaling interaction between adenosine 5′-monophosphate-activated protein kinase (AMPK) with β-catenin during OA development.
It has been reported that activation of AMPK signaling has chondro-protective effect [12]. Metformin and berberine are known AMPK activators [[13], [14], [15]]. We have previously shown that metformin and berberine protected OA development and relieved OA associated pain [16,17]. However, detail molecular mechanisms have not been determined. It has been shown that AMPK activator inhibited β-cateninS552 phosphorylation and prevented β-catenin nuclear translocation in cancer cells [18,19]. In the present studies, we have determined the effects of AMPK activator metformin on β-catenin phosphorylation in chondrocytes. We found that metformin inhibited β-cateninS552 phosphorylation and nuclear translocation in chondrocytes. Metformin also inhibited β-catenin reporter activity and the expression of β-catenin downstream target genes in chondrocytes.
2. Materials and methods
2.1. Chondrocyte culture and chemicals
Primary mouse knee chondrocytes were isolated from articular cartilage of 4-day-old neonatal mice [20]. Articular cartilage fragments were digested with 0.2% type II collagenase at 37 °C for 4 h. Then released articular chondrocytes were cultured in Dulbecco's Modified Eagle Media (DMEM)/F12 (1:1) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin maintained at 37 °C in a humidified 5% carbon dioxide (CO2). The ATDC5 cell line was purchased from the American-type culture collection (ATCC). Metformin and Acadesine (AICAR) were purchased from Selleck (No.S5958 & No.S1802, Selleck Chemicals LLC, USA) was dissolved in DMSO.
2.2. Animal studies
All animal studies were approved by our Institutional Animal Care and Use Committee. Eight-week-old male wild mice with a mean weight of 20 ± 2g were used in the present study. Mice were housed in the standard cages in a temperature-controlled room with a 12 h light/dark cycle. Caging, food, and water bottles were changed weekly. After anesthesia and standard aseptic surgical procedures destabilization of medial meniscus (DMM) was performed in WT mice (n = 3 in each group) right knee to establish an OA model. Sham-operated mice were used as controls (n = 3). The mice were administered metformin 2 weeks after (Met group) DMM surgery.
2.3. Plasmid and small interfering RNA (siRNA) transfection
AK mouse plus Transforming or Thymoma (AKT) is a frequent oncogene expressed in most tissues which includes three isoforms AKT1, AKT2, and AKT3. In this study we amplified mouse AKT2 and Stk11 (or liver kinase B1, LKB1) cDNA with PCR and cloned into a GV657 lentiviral vector (GeneChem, Shanghai, China). Oligos of AKT2 and LKB1 shRNAs were synthesized and inserted into a GV657 vector (GeneChem Co., Ltd, Shanghai, China). Small interfering RNA targeting Adenosine 5‘-monophosphate (AMP)-activated protein kinase α1 (AMPKα1) and negative control (NC) siRNA were chemically synthesized by Tsingke Biotech (Tsingke Biotech, China). Transfection was performed using Lipofectamine 3000 (Invitrogen, CA, USA) according to the manufacturer's instructions.
2.4. Western blot analysis
Cells were lysed in cold RIPA buffer containing phosphatase and protease inhibitor, then centrifuged at 13000 g at 4 °C for 15 min. The total protein concentration was then determined with a Bicinchoninic Acid (BCA) kit (Solelybio, Beijing, China). The equivalent quantity of protein (30 μg) in each group was separated by 10–12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, MA, USA). After blocking with 5% skimmed milk, membranes were probed with primary antibodies against Phospho-β-Catenin (Ser552) (CST, MA, USA), β-catenin (ABclonal, Wuhan, China), Axin2 (A17021, ABclonal, Wuhan, China), Cyclin D1 (CST, MA, USA), AMPKα (CST, MA, USA), Phospho-AMPKα (Thr172) (CST, MA, USA), β-Actin (ABclonal, Wuhan, China) overnight at 4 °C. Horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG (Abcam, MA, USA) was used as secondary antibodies. Enhanced Chemiluminescence (ECL, Thermo Fisher Scientific, MA, USA) detection system was used to detect the protein bands on the membrane. The intensity of the protein bands was analyzed by Image J software, using β-Actin as reference protein.
2.5. Immunofluorescent staining
Cells were washed with PBS and fixed with 4% paraformaldehyde (PFA) for 10 min. Next, cells were permeabilized with 0.3% Triton X-100 (Sigma, MO,USA) for 10 min and blocked with bovine serum albumin (Sigma, MO,USA) for 1 h at room temperature, followed by the incubation with the primary antibodies overnight at 4 °C. Samples were washed and further incubated with the secondary antibodies, Cy3 Goat Anti-Rabbit IgG (H + L) (ABclonal, Wuhan, China) for 1 h at room temperature. Nuclei were stained with DAPI (Beyotime, Shanghai, China). Images were taken and observed by fluorescence microscopy.
2.6. Luciferase reporter assay
Mouse embryonic tumor cells (ATDC5) cells were transfected with the TOP/FOP-flash reporter plasmid using Lipofectamine 3000 (Invitrogen, USA) and treated with metformin. After transfection for 24 h, the cells were harvested for analysis with the Dual-Luciferase Reporter Assay System (Beyotime, Shanghai, China). Luciferase activity was measured using the Thermo Scientific Fluoroskan FL (Thermo Fisher Scientific, MA, USA). The luciferase activity was normalized to the Renilla luciferase activity.
2.7. Immunohistochemistry (IHC) analysis
Mice at 6 weeks after DMM surgery were euthanized and knee joints were fixed in 4% paraformaldehyde overnight followed by decalcification in 0.5 M EDTA (pH 7.4) for 4 weeks before paraffin embedding. For IHC staining, 5 μm-thick coronal sections were heated at 95 °C in Antigen Unmasking Solution (Vector Laboratories) for 15 min, and then sequentially treated with 3% H2O2, 0.5% Triton X-100, Avidin/Biotin Blocking Kit (Invitrogen). After blocking with 10% normal goat serum (Vector Laboratories) for 1h, sections were treated with primary antibodies, including anti-β-catenin (1:100, Abcam, ab32572) and anti- β-catenin (phosphor S552) (1:100, Abcam, ab277785) antibodies overnight at 4 °C and incubated with secondary biotinylated goat anti-rabbit antibody (1:200, Vector Laboratories) for 30 min, followed by the treatment with VECTASTAIN Elite ABC Kit (Vector Laboratories). IHC signals were revealed by ImmPACT DAB Peroxidase Substrate (Vector Laboratories). Images were captured under a light microscope (Eclipse 90i, Nikon) and analyzed by Image J.
2.8. Statistical analysis
All data sets were compared using GraphPad Prism (GraphPad Software, La Jolla, CA) version 9.0. Data are shown as mean ± standard deviation (SD). For in vivo and vitro studies, Unpaired Student's t test (for two groups), one-way ANOVA (for multiple groups) were used followed by the Tukey–Kramer test. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. AMPK activators inhibit β-catenin phosphorylation
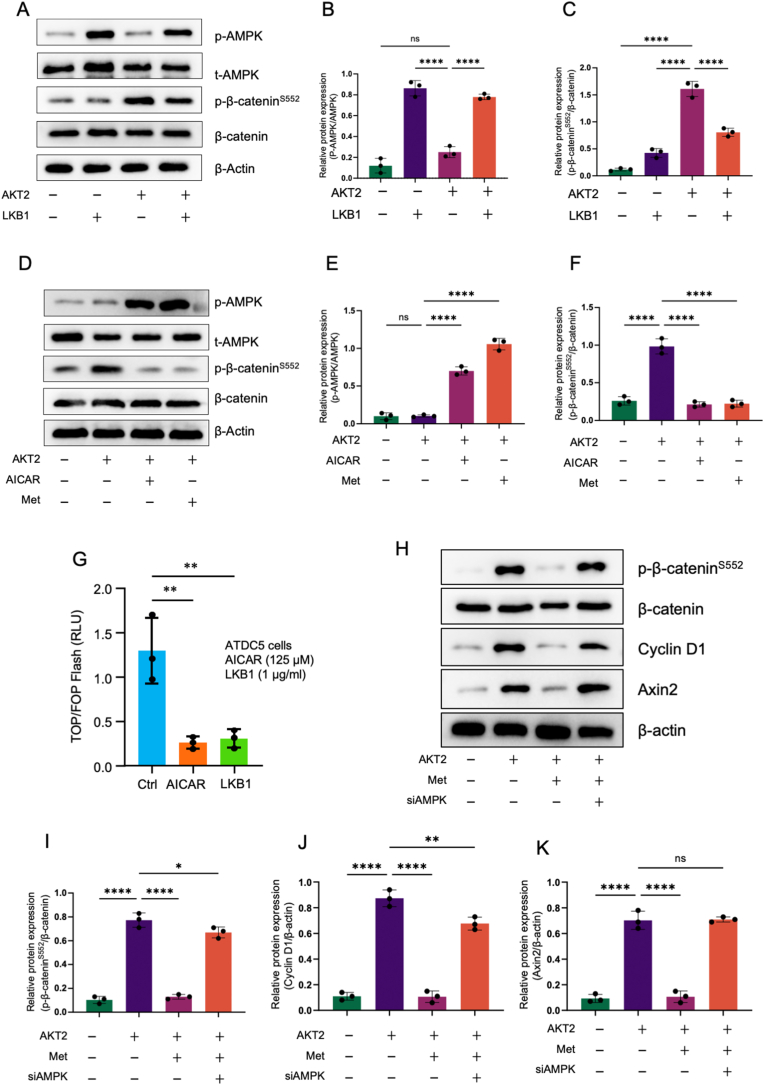
To determine the interaction of AMPK and β-catenin signaling, we examined the effects of liver kinase B1 (LKB1) on β-cateninS552 phosphorylation. We found that LKB1 induced AMPKα1 phosphorylation (Thr172) and LKB1 also slightly increased total AMPK protein levels in ATDC5 chondrogenic cells (Fig. 1A and B). AKT2 upregulated β-cateninS552 phosphorylation and LKB1 inhibited AKT2-induced β-cateninS552 phosphorylation in ATDC5 cells (Fig.1A). Metformin has been known as an AMPK activator. In this study, we determined the effect of metformin on β-cateninS552 phosphorylation. We found significant changes in β-cateninS552 phosphorylation after AKT2 transfection in mouse primary chondrocytes, and metformin can significantly inhibited β-cateninS552 phosphorylation (Fig. 1A,C). In contract, AICAR, a specific AMPK activator, only had minor effect on inhibition of β-cateninS552 phosphorylation (Fig. 1D–F). In β-catenin reporter assay, we found that both AICAR and LKB1 significantly inhibited β-catenin reporter activity in ATDC5 cells (Fig. 1G). In addition, we silenced AMPK in cultured ATDC5 cells and measured β-cateninS552 phosphorylation. As shown in Fig. 1H–K, treatment with AMPK siRNA significantly reversed the inhibitory effect of metformin on β-cateninS552 phosphorylation and the inhibitory effects of metformin on the expression of β-catenin downstream target genes, Axin2 and cyclin D1. These findings demonstrate that AMPK activators inhibits β-catenin phosphorylation in chondrocytes.
Figure 1.
Activation of AMPK signaling inhibits β-cateninSer552phosphorylation (A–C) ATDC5 cells were transfected with LKB1 or AKT2 or both. Western blot (WB) analysis for detecting AMPK, p-AMPK, β-catenin and p-β-cateninS552 protein expression in different groups, and β-Actin was used as reference protein (n = 3) (D–F) ATDC5 cells were transfected with AKT2 or AKT2 plus AICAR or treatment with metformin. WB analysis for detecting AMPK, p-AMPK, β-catenin and p-β-cateninS552 protein expression in different groups, and β-Actin was used as reference protein (n = 3). Metformin significantly inhibited β-cateninSer552 phosphorylation (G) ATDC5 cells were transfected with TOP-flash reporter and LKB1 (1 μg/ml) or treated with AICAR (125 μM) and then incubated for 24 h. Both LKB1 and AICAR inhibited luciferase activity (H–K) ATDC5 cells were transfected with empty vector (group 1) or transfected with AKT2 without (group 2) or with metformin treatment (group 3) or co-transfected with AKT2 and AMPK siRNA (siAMPK) in the presence of metformin (group 4) (n = 3). The data were expressed as mean ± SD, ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001, ∗∗∗∗P<0.0001,and ns, not significant.
3.2. Metformin suppresses β-catenin signaling through AMPK activation
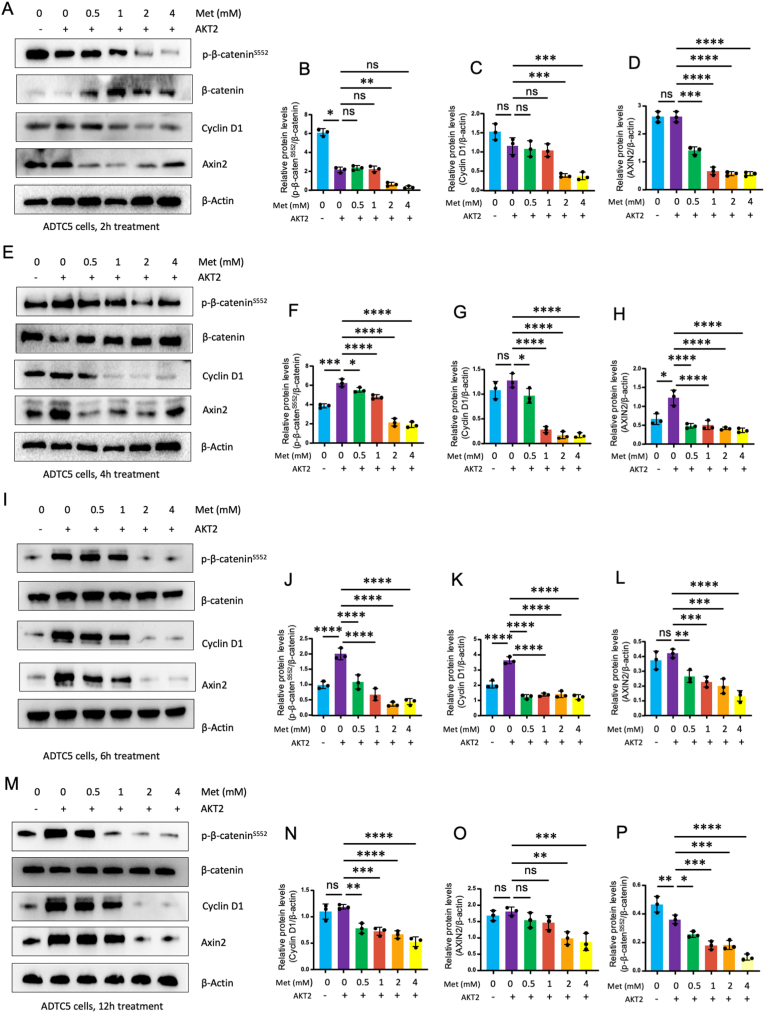
We then determined the effects of metformin (Met) on β-catenin phosphorylation and on the expression of β-catenin downstream target genes, Axis inhibition protein 2 (Axin2) and cyclin D1. We found that treatment of ATDC5 cells with metformin (0.5, 1, 2 and 4 mM) for different times (2, 4, 6 and 12 h) significantly inhibited β-cateninS552 phosphorylation and reduced the expression of β-catenin downstream target genes, Axin2 and cyclin D1 (Fig. 2A-M). In contrast, metformin had no significant effect on the expression of total β-catenin, except upregulation of total β-catenin protein levels by metformin at 2h treatment group (Fig. 2A).
Figure 2.
Metformin inhibits phosphorylation of β-cateninSer552in ATDC5 cells. ATDC5 cells were transfected with AKT2 or treated with metformin (0.5, 1, 2, and 4 mM) and incubated for 2 (A–D), 4 (E–H), 6 (I–L), 12 (M–P) hours. At the end of the experiment, cell lysates were collected and Western blot analysis was performed (n = 3). Metformin significantly inhibits β-cateninS552 phosphorylation, cyclin D1 and Axin2 expression. Cyclin D1 and Axin2 are downstream target genes of β-catenin. Metformin inhibited β-cateninS552 phosphorylation but had no significant effect on total β-catenin levels. The data are expressed as mean ± SD, ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001, ∗∗∗∗P<0.0001 and ns, not significant.
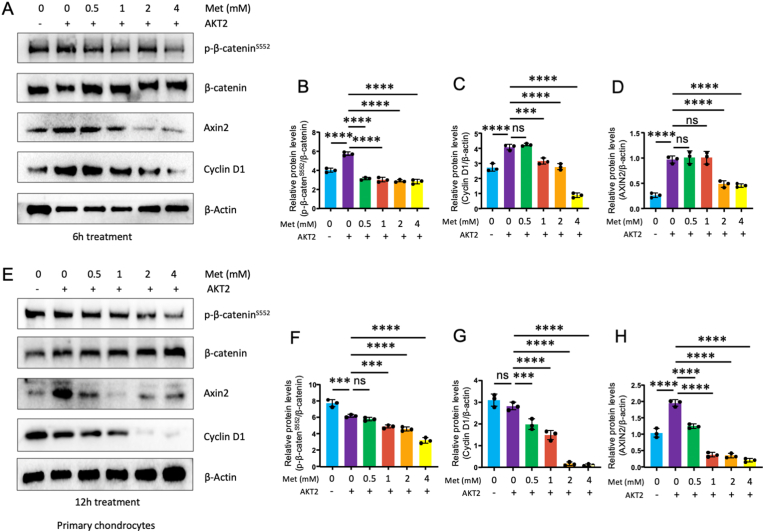
We were also curious about the effect of metformin on primary chondrocytes. Therefore, we extracted mouse knee cartilage cells for the experiment. After 6 or 12 h treatment with different concentrations (0.5, 1, 2, 4 mM) of metformin (Fig. 3A–H), we observed the similar results which inhibited the phosphorylation of β-catenin and decreased the expression of the downstream target genes, Axin2 and cyclin D1, in Wingless/Int (Wnt)/β-catenin signaling pathway.
Figure 3.
Metformin inhibits phosphorylation of β-cateninS552in primary mouse chondrocytes (A–D) Primary chondrocytes were transfected with AKT2 or treated with metformin (0.5, 1, 2, and 4 mM) and incubated for 6 h. At the end of the experiment, cell lysates were collected and Western blot analysis was performed (n = 3). Metformin significantly inhibits β-cateninS552 phosphorylation and cyclin D1 expression. Cyclin D1 is a downstream target gene of β-catenin (E–H) Primary chondrocytes were transfected with AKT2 or treated with metformin (0.5, 1, 2 and 4 mM) and incubated for 12 h (n = 3). Metformin inhibited β-cateninS552 phosphorylation but had no significant effect on total β-catenin levels. The data are expressed as mean ± SD, ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001, ∗∗∗∗P<0.0001 and ns, not significant.
3.3. Metformin inhibits β-catenin activity and β-catenin nuclear translocation
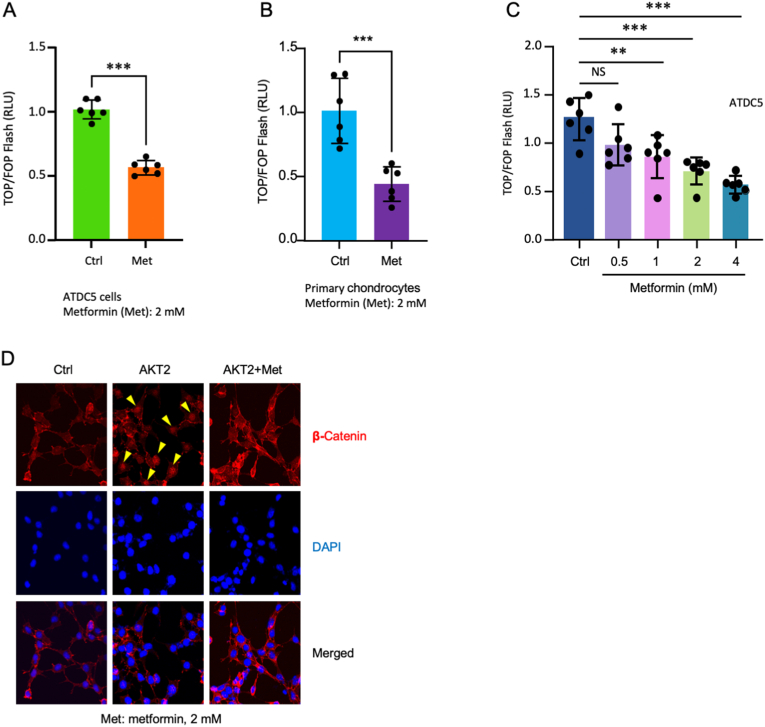
To determine the effect of metformin on β-catenin signaling, we evaluated TCF transcriptional activity by transiently transfection of ATDC5 cells and mouse primary chondrocytes with the T cell factor (TCF) reporter plasmid (TOP)/FOP-Flash luciferase reporter genes. We found that metformin inhibited β-catenin reporter activity in both primary chondrocytes and in ATDC5 cells (Fig. 4A and B). Especially we found that metformin inhibited β-catenin reporter activity in a dose-dependent manner in ATDC5 cells (Fig. 4C). We also determined β-catenin nuclear translocation in ATDC5 cells and found that transfection of ATDC5 cells with AKT2 increased β-catenin nuclear translocation. Treatment of these cells with metformin inhibited AKT2-induced β-catenin nuclear translocation (Fig. 4D). These findings indicate that metformin prevents β-catenin phosphorylation and nuclear translocation and inhibits β-catenin downstream target gene expression in chondrocytes.
Figure 4.
Metformin inhibits β-catenin signaling in vitro (A & B) ATDC5 cells (A) or primary mouse chondrocytes (B) were transfected with TOP-flash reporter and treated with metformin (2 mM) for 24 h (n = 5) (C) ATDC5 cells were transfected with TOP-flash reporter and treated with metformin (0.5, 1, 2, and 4 mM) for 24 h (n = 5). Changes in luciferase activities were analyzed by dual-luciferase assay kit. Metformin significantly inhibited luciferase activity (D) ATDC5 cells were transfected with AKT2 or treated with metformin and β-catenin nuclear translocation was detected by immunofluorescence (IF), DAPI was used to staining cell nucleus, scale bar: 50 μm. The data are expressed as mean ± SD, ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001, ∗∗∗∗P<0.0001and ns, not significant.
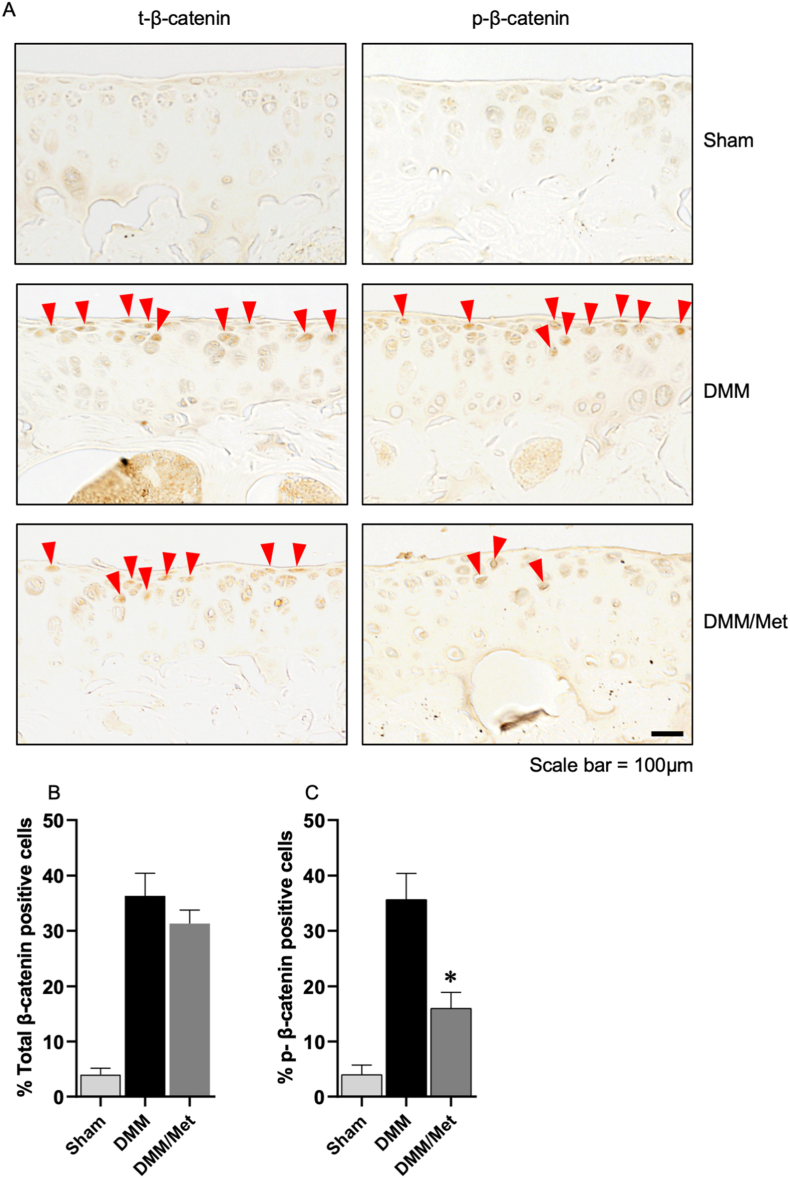
3.4. Metformin inhibits β-cateninS552 phosphorylation in vivo
To further confirm the effect of metformin on β-cateninS552 phosphorylation, we examined the effects of metformin on expression of β-cateninS552 phosphorylation in articular cartilage in wild-type and OA mouse model. OA model was created by the surgery of destabilization of medial meniscus (DMM) and expression of phosphorylated β-cateninS552 was examined by immunohistochemistry (IHC) assay. Total and phosphorylated β-cateninS552 levels were significantly increased in articular cartilage of the mice with DMM surgery. Treatment of metformin caused a significant decrease in β-cateninS552 levels in the mice with DMM surgery. In contrast, metformin had no significant effect on total β-catenin level (Fig. 5).
Figure 5.
Metformin inhibits phosphorylation of β-cateninS552in vivo (A) The effect of metformin in DMM model on β-catenin was detected by immunohistochemistry (IHC), scale bar: 200 μm (B–C) Cells with positive staining for p-β-cateninS552 and total β-catenin were quantified (n = 3). Statistical analysis was conducted using two-way analysis of variance (ANOVA) followed by the Tukey–Kramer test. The data are expressed as mean ± SD, ∗P<0.05 and ns, not significant.
4. Discussion
In this study, we determined the interaction of AMPK signaling with β-catenin in chondrocytes and found that AMPK activator metformin inhibited β-cateninS552 phosphorylation and β-catenin nuclear translocation. We also found that metformin inhibited β-catenin reporter activity and expression of β-catenin downstream target genes in chondrocytes. AMPK agonist 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) is a specific AMPK activator [21] and liver kinase B1 (LKB1) has been shown to induce AMPK phosphorylation and activates AMPK signaling [22,23]. In this study, we used both AICAR and LKB1 as positive controls and we found that both AICAR and LKB1 inhibited β-cateninS552 phosphorylation in chondrocytes, further demonstrating the interaction between AMPK and β-catenin signaling.
In previous studies, we found that metformin increased AMPK phosphorylation in articular chondrocytes and protected cartilage from OA development [16]. In the present studies, we found that treatment of mice with same concentrations of metformin also inhibited β-cateninS552 phosphorylation in vivo. These findings suggest that metformin may exert its chondro-protective effect by inhibition of β-cateninS552 phosphorylation and nuclear translocation in chondrocytes.
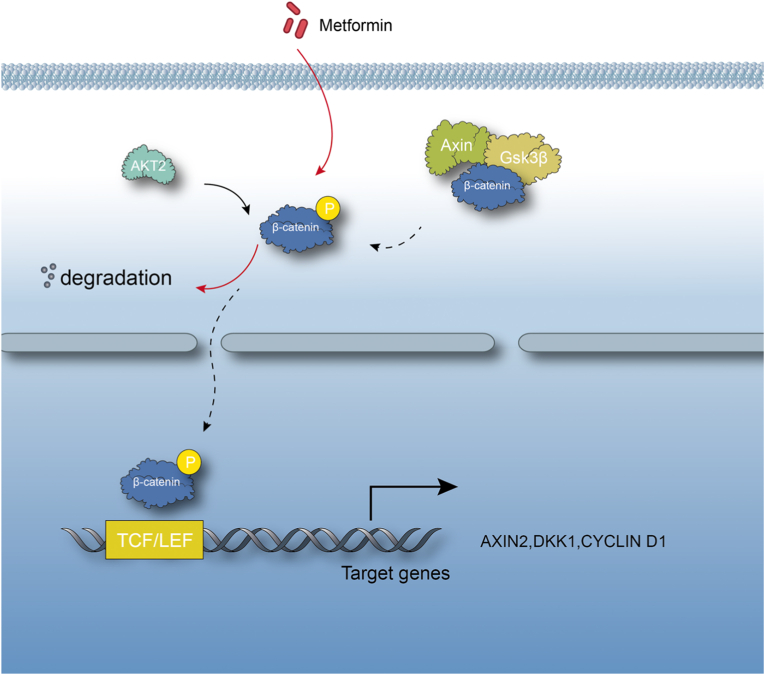
In recent years, significant progresses have been made in OA research and it has been demonstrated that disruption of several signaling pathways in chondrocytes caused OA development. The key issue is what is the signaling interaction among different signaling pathways and what is the key and common signaling pathway or signaling molecule critically contributed to OA development. The current study is the first attempt to investigate signaling interaction between AMPK and β-catenin signaling pathways. More intensive and in-depth studies are still required. In previous studies, we demonstrated that Runx2 and MMP13 are two critical downstream target genes of β-catenin signaling in chondrocytes [9,24]. Chondrocyte-specific deletion of Runx2 or Mmp13 decelerates OA progression [25,26]. In contrast, the upstream regulatory mechanisms of β-catenin signaling during OA development have not been fully characterized. In the present studies, we demonstrated that activation of AMPK could inhibit β-catenin signaling in chondrocytes (Fig. 6), suggesting that one potential mechanism for β-catenin upregulation could be the reduction or inhibition of AMPK signaling under pathological conditions in chondrocytes. This possibility needs to be further explored.
Figure 6.
A proposed model of action. AMPK activator decelerates osteoarthritis development by inhibition of β-catenin signaling in chondrocytes.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This project has been supported by the National Natural Science Foundation of China (NSFC) grants (82030067, 82161160342 and 82172397) to D.C and L.T. and the grant from Youth Innovation Promotion Association of Chinese Academy of Sciences (2020353) to L.T. This work was also supported by National Key Research and Development Program of China (2021YFB3800800) to L.T. and D.C. D.Y. was supported by the grant of China Postdoctoral Research Foundation (2021M703376).
Contributor Information
Liping Tong, Email: lp.tong@siat.ac.cn.
Wei Huang, Email: huangwei68@263.net.
Chen Di, Email: di.chen@siat.ac.cn.
References
- 1.Chen D., Shen J., Zhao W., Wang T., Han L., Hamilton J.L., et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5 doi: 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao L., Zhang S., Zhao L., Chang X., Han L., Huang J., et al. Acute synovitis after trauma precedes and is associated with osteoarthritis onset and progression. Int J Biol Sci. 2020;16:970–980. doi: 10.7150/ijbs.39015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane N.E., Lian K., Nevitt M.C., Zmuda J.M., Lui L., Li J., et al. Frizzled-related protein variants are risk factors for hip osteoarthritis. Arthritis Rheum. 2006;54:1246–1254. doi: 10.1002/art.21673. [DOI] [PubMed] [Google Scholar]
- 4.Loughlin J. The genetic epidemiology of human primary osteoarthritis: current status. Expet Rev Mol Med. 2005;7(9):1–12. doi: 10.1017/S1462399405009257. [DOI] [PubMed] [Google Scholar]
- 5.Min J.L., Meulenbelt I., Riyazi N., Kloppenburg M., Houwing-Duistermaat J.J., Seymour A.B., et al. Association of the Frizzled-related protein gene with symptomatic osteoarthritis at multiple sites. Arthritis Rheum. 2005;52:1077–1080. doi: 10.1002/art.20993. [DOI] [PubMed] [Google Scholar]
- 6.Corr M., Lane N.E. FRZB: a bone and joint connection. Arthritis Rheum. 2007;56:3881–3883. doi: 10.1002/art.23139. [DOI] [PubMed] [Google Scholar]
- 7.Lories R.J.U., Peeters J., Bakker A., Tylzanowski P., Derese I., Schrooten J., et al. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 2007;56:4095–4103. doi: 10.1002/art.23137. [DOI] [PubMed] [Google Scholar]
- 8.Thysen S., Luyten F.P., Lories R.J. Loss of Frzb and Sfrp1 differentially affects joint homeostasis in instability-induced osteoarthritis. Osteoarthritis Cartilage. 2015;23:275–279. doi: 10.1016/j.joca.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Zhu M., Tang D., Wu Q., Hao S., Chen M., Xie C., et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/jbmr.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia C., Wang P., Fang L., Ge Q., Zou Z., Dong R., et al. Activation of β-catenin in Col2-expressing chondrocytes leads to osteoarthritis-like defects in hip joint. J Cell Physiol. 2019;234:18535–18543. doi: 10.1002/jcp.28491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui T., Zhou Y., Wang T., Li J., Zhang S., Liao L., et al. Activation of β-catenin signaling in aggrecan-expressing cells in temporomandibular joint causes osteoarthritis-like defects. Int J Oral Sci. 2018;10:13. doi: 10.1038/s41368-018-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng X., Pan J., Li J., Zeng C., Qi W., Shao Y., et al. Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR. Aging (Albany NY) 2020;12:1087–1103. doi: 10.18632/aging.102635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foretz M., Guigas B., Bertrand L., Pollak M., Viollet B. Metformin: from mechanisms of action to therapies. Cell Metabol. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y.S., Kim W.S., Kim K.H., Yoon M.J., Cho H.J., Shen Y., et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 15.Jeong H.W., Hsu K.C., Lee J.-W., Ham M., Huh J.Y., Shin H.J., et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–E964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 16.Li J., Zhang B., Liu W.-X., Lu K., Pan H., Wang T., et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann Rheum Dis. 2020;79:635–645. doi: 10.1136/annrheumdis-2019-216713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J., Wang Y., Chen D., Liu-Bryan R. Oral administration of berberine limits post-traumatic osteoarthritis development and associated pain via AMP-activated protein kinase (AMPK) in mice. Osteoarthritis Cartilage. 2022;30:160–171. doi: 10.1016/j.joca.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amable G., Martínez-León E., Picco M.E., Di Siervi N., Davio C., Rozengurt E., et al. Metformin inhibits β-catenin phosphorylation on Ser-552 through an AMPK/PI3K/Akt pathway in colorectal cancer cells. Int J Biochem Cell Biol. 2019;112:88–94. doi: 10.1016/j.biocel.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Park S.Y., Kim D., Kee S.-H. Metformin-activated AMPK regulates β-catenin to reduce cell proliferation in colon carcinoma RKO cells. Oncol Lett. 2019;17:2695–2702. doi: 10.3892/ol.2019.9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gosset M., Berenbaum F., Thirion S., Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3:1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 21.Hall D.T., Griss T., Ma J.F., Sanchez B.J., Sadek J., Tremblay A.M.K., et al. The AMPK agonist 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), but not metformin, prevents inflammation-associated cachectic muscle wasting. EMBO Mol Med. 2018;10 doi: 10.15252/emmm.201708307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciccarese F., Zulato E., Indraccolo S. LKB1/AMPK pathway and drug response in cancer: a therapeutic perspective. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/8730816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou S., Li Y., Qiao L., Ge Y., Huang X., Gao X., et al. Inactivation of in postnatal chondrocytes leads to epiphyseal growth-plate abnormalities and promotes enchondroma-like formation. Faseb J. 2019;33:9476–9488. doi: 10.1096/fj.201900294RR. [DOI] [PubMed] [Google Scholar]
- 24.Wang M., Tang D., Shu B., Wang B., Jin H., Hao S., et al. Conditional activation of β-catenin signaling in mice leads to severe defects in intervertebral disc tissue. Arthritis Rheum. 2012;64:2611–2623. doi: 10.1002/art.34469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M., Sampson E.R., Jin H., Li J., Ke Q.H., Im H.-J., et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013:15. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao L., Zhang S., Gu J., Takarada T., Yoneda Y., Huang J., et al. Deletion of Runx2 in articular chondrocytes decelerates the progression of DMM-induced osteoarthritis in adult mice. Sci Rep. 2017;7:2371. doi: 10.1038/s41598-017-02490-w. [DOI] [PMC free article] [PubMed] [Google Scholar]