Highlights
-
•
Autistics with a Block Design peak were faster at a mental rotation task.
-
•
Distinct cognitive profiles in autism modulate brain activation and functional connectivity.
-
•
Greater parietal and occipital functioning is specific to autistics with visuospatial strengths.
-
•
Increased fronto-occipital connectivity characterized autistics with visuospatial peaks.
Keywords: Autism, Cognitive strength, Block Design peak, Mental rotation, fMRI, Functional connectivity
Abstract
Enhanced visuospatial abilities characterize the cognitive profile of a subgroup of autistics. However, the neural correlates underlying such cognitive strengths are largely unknown. Using functional magnetic resonance imaging (fMRI), we investigated the neural underpinnings of superior visuospatial functioning in different autistic subgroups. Twenty-seven autistic adults, including 13 with a Wechsler’s Block Design peak (AUTp) and 14 without (AUTnp), and 23 typically developed adults (TYP) performed a classic mental rotation task. As expected, AUTp participants were faster at the task compared to TYP. At the neural level, AUTp participants showed enhanced bilateral parietal and occipital activation, stronger occipito-parietal and fronto-occipital connectivity, and diminished fronto-parietal connectivity compared to TYP. On the other hand, AUTnp participants presented greater activation in right and anterior regions compared to AUTp. In addition, reduced connectivity between occipital and parietal regions was observed in AUTnp compared to AUTp and TYP participants. A greater reliance on posterior regions is typically reported in the autism literature. Our results suggest that this commonly reported finding may be specific to a subgroup of autistic individuals with enhanced visuospatial functioning. Moreover, this study demonstrated that increased occipito-frontal synchronization was associated with superior visuospatial abilities in autism. This finding contradicts the long-range under-connectivity hypothesis in autism. Finally, given the relationship between distinct cognitive profiles in autism and our observed differences in brain functioning, future studies should provide an adequate characterization of the autistic subgroups in their research. The main limitations are small sample sizes and the inclusion of male-only participants.
1. Introduction
Beyond the diagnostic socio-communicative atypicalities, autism is characterized by the presence of enhanced visuospatial abilities. Indeed, a wide range of studies has reported superior visuospatial abilities in autistic individuals compared to neurotypical peers (Caron et al., 2006, Constable et al., 2020, Falter et al., 2008, Jolliffe and Baron-Cohen, 1997 Kuschner et al., 2007, McGrath et al., 2012, Mottron et al., 2003, O’Riordan, 2004, O'Riordan et al., 2001 O'Riordan and Plaisted, 2001, Pearson et al., 2016, Pellicano et al., 2005, Pellicano et al., 2006, Plaisted et al., 1998, Ropar and Mitchell, 2001, Shah and Frith, 1983, Soulières et al., 2011). However, discordant results regarding visuospatial abilities in autism have also been observed in the literature. For instance, some studies have reported that autistic individuals display similar (Damarla et al., 2010, Edgin and Pennington, 2005, Kana et al., 2013, Planche and Lemonnier, 2010) or weaker performance (Nejati et al., 2021, Pearson et al., 2014) in some visuospatial tasks compared to neurotypicals. Given the heterogeneity of cognitive profiles of individuals on the autism spectrum, this may in part explain the mixed findings in the literature (Audras‐Torrent et al., 2020, Nader et al., 2015, Silleresi et al., 2020).
One of the most documented cognitive superiorities in autism is the relative visuospatial strength on the Block Design (BD) subtest from the Wechsler’s intelligence scales (Asarnow et al., 1987, Audras-Torrent et al., 2020, Caron et al., 2006, Happé, 1994, Koyama et al., 2009, Koyama and Kurita, 2008, Nader et al., 2015, Nader et al., 2016, Shah and Frith, 1993, Siegel et al., 1996, Silleresi et al., 2020). This task requires the individual to reproduce a target model figure using geometric blocks within a time limit. A strikingly higher proportion of autistic individuals (33 % to 47 %) present a strength on the BD task compared to the general population (<5 %; Caron et al., 2006, Nader et al., 2015). Moreover, these individuals tend to show a general enhancement in perceptual functioning and possess significant strengths in many visual and visuospatial tasks including the creation and manipulation of mental images (Falter et al., 2008, McGrath et al., 2012, Pearson et al., 2016, Soulières et al., 2011), visual search for a target among distractors (Joseph et al., 2009, Kemner et al., 2008, O’Riordan, 2004, O'Riordan et al., 2001), perceptual extraction of a visual figure from a complex background (Jarrold et al., 2005, Jolliffe and Baron-Cohen, 1997, Mottron et al., 2003, Pellicano et al., 2005, Pellicano et al., 2006, Shah and Frith, 1983, Ropar and Mitchell, 2001), among others. Furthermore, evidence suggests that superior visuospatial abilities are typically observed in individuals who presented a speech onset delay during infancy (Mottron et al., 2008). In addition, autistic toddlers with language delays already manifest a preference for geometric figures over social images (Pierce et al., 2011). These toddlers also demonstrate enhanced visual search abilities (Kaldy et al., 2011) and atypical visual explorations (Mottron et al., 2007). Taken alongside studies investigating the various intellectual profiles in autism (Audras-Torrent et al., 2020, Nader et al., 2015, Silleresi et al., 2020), these findings suggest that different cognitive phenotypes exist within the autism population. Specifically, one of these phenotypes would be characterized by the development of visuospatial expertise.
The unique superiority in visuospatial cognition observed in this specific autism subgroup may be concomitant with atypical functional resource allocation in regions associated with visual processing (Jassim et al., 2021, Samson et al., 2012) and an altered pattern of functional connectivity (Belmonte et al., 2004, Courchesne and Pierce, 2005, Minshew and Keller, 2010, O’Reilly et al., 2017, Picci et al., 2016). In concert with decreased activation in certain frontal areas, stronger activation in parietal and occipital regions has been observed in autistics during tasks involving different types of stimuli (e.g., objects, faces, words; Samson et al., 2012) and even during complex cognitive tasks such as fluid reasoning (Sahyoun et al., 2010, Simard et al., 2015, Soulières et al., 2009, Yamada et al., 2012). Patterns of functional connectivity have also revealed an under-connectivity between frontal and posterior regions, as well as an over-connectivity between parietal and occipital regions (Cherkassky et al., 2006, Chien et al., 2015, Just et al., 2004, Just et al., 2007, Kana et al., 2006, Kana et al., 2009, Kennedy and Courchesne, 2008, Koshino et al., 2008, McGrath et al., 2012, O’Reilly et al., 2017, Sahyoun et al., 2010, Solomon et al., 2009, Villalobos et al., 2005). However, local over-connectivity has been less consistently reported in the literature (O’Reilly et al., 2017, Picci et al., 2016). This pattern of brain activation and functional connectivity gives support to the idea that enhanced visual processing may be less influenced by top-down processes (Caron et al., 2006, Hong et al., 2019, Loth et al., 2010, Mottron et al., 2006) and/or reflects a more prominent implication of bottom-up processing in autistic individuals (Cook et al., 2012, Hong et al., 2019, Takarae et al., 2014, Takesaki et al., 2016). However, other studies have also demonstrated similar (Kana et al., 2013, Keehn et al., 2013, Tyszka et al., 2014) or even stronger long-range functional connectivity between frontal and perceptual areas in autistic compared to neurotypical participants (Keehn et al., 2013, Léveillé et al., 2010, Noonan et al., 2009, Simard et al., 2015). Of note, the discovery of over- or under-connectivity is dependent on various factors, including the task being performed and methodological choices, among others (Chung and Son, 2020, Philip et al., 2012). As suggested by recent findings and literature reviews, there is an increasing acknowledgement of the presence of over- and under-connectivity in the autistic brain (Nair et al., 2020, O’Reilly et al., 2017, Müller et al., 2011, Müller and Fishman, 2018, Picci et al., 2016). Discrepancies in results can be largely attributed to methodological decisions made by the research group (e.g., resting-state vs task-related functional magnetic resonance imaging [fMRI] connectivity, whole-brain vs ROI analyses), the age range of participants, as well as diagnostic, neurological, psychological (e.g., self-regulation) and cognitive heterogeneity among participants (Lin et al., 2020, O’Reilly et al., 2017, Müller et al., 2018, Picci et al., 2016). Differences in observed connectivity also vary according to the anatomical and/or functional cerebral areas being targeted (e.g., primary sensory vs higher-order processing areas; Kana et al., 2014, Keown et al., 2017).
Yet, most studies in cognition and neuroimaging fail to distinguish autism subgroups according to their cognitive profile. To gain a better understanding of the cognitive functions (e.g., visuospatial processing) and their underlying cerebral mechanisms, future studies must make this distinction. An increasing number of authors have supported this idea in recent years; insisting that autism research should be conducted based on the different brain or behavioural phenotypes (Crippa et al., 2016, Duffy et al., 2013, Hong et al., 2022, Lombardo et al., 2019, O’Reilly et al., 2017, Rødgaard et al., 2019, Yao et al., 2021).
Mental rotation is a paradigmatic task that is used to evaluate high-level visuospatial abilities. The neurofunctional correlates associated with mental rotation tasks have been extensively studied in the general population. Several meta-analyses on this topic have consistently identified a large network of brain regions including the middle and inferior occipital gyri, fusiform gyrus, intraparietal sulcus, superior and inferior parietal lobules, insula, some frontal regions (e.g., precentral gyrus, middle and inferior frontal gyri, supplementary motor area), as well as the cerebellum (Cona and Scarpazza, 2019, Hawes et al., 2019, Tomasino and Gremese, 2016, Zacks, 2008). Other regions such as the calcarine areas and precuneus have also been identified as being implicated in mental rotation (Cona and Scarpazza, 2019, Hawes et al., 2019, Zacks, 2008). Although mental rotation processes largely involve the activation of bilateral regions throughout this network, a slight increase in activation was observed in the right portion of the parietal cortex (Harris and Miniussi, 2003, Tomasino and Gremese, 2016, Zacks, 2008). This increase was observed notably for stimuli unrelated to body parts, such as geometric figures and letters, among others (Tomasino and Gremese, 2016). Moreover, the activation of the right superior parietal region around the intraparietal sulcus was found to be modulated by the degree of mental rotation performed (Zacks, 2008). This speaks to the region’s pivotal role in treating visuospatial image transformation.
Contradictory results have been observed regarding the behavioural performance of autistic individuals (Muth et al., 2014, Nejati et al., 2021). With that said, a few studies have shown relative strength in mental rotation and visual imagery tasks and this, regardless of the type of stimuli (e.g., concrete objects, geometric figures, letters; Falter et al., 2008, Hamilton et al., 2009, Happé et al., 2006, McGrath et al., 2012, Pearson et al., 2016, Soulières et al., 2011). Notably, Soulières et al. (2011) investigated mental rotation processes in autistic participants with different cognitive profiles (with or without a BD strength) versus non-autistic participants. Results of this study revealed overall better performances (accuracy rate and response times) in autistics with a BD strength. These group differences were even more distinct for complex stimuli (e.g., 3D shapes) compared to other stimuli (e.g. 2D shapes, letters, hand positions). A few studies have also investigated the mechanisms of brain functioning that underly mental rotation processes in autism. They have reported greater activation in parietal regions (Beacher et al., 2012) coupled with diminished activation in some frontal regions (McGrath et al., 2012, Silk et al., 2006). The literature on occipital regions is less conclusive, such that some studies have observed either increased (Beacher et al., 2012), decreased (McGrath et al., 2012), or similar (Silk et al., 2006) brain activation in autistic individuals compared to their neurotypical counterparts. Only one study has further investigated functional connectivity related to mental rotation. It reported under-connectivity between frontal and parietal regions, along with increased functional connectivity in the occipital lobe in autistic people (McGrath et al. 2012). Importantly, none of these studies on mental rotation in autism have quantified brain activation and functional connectivity as a function of different autistic cognitive profiles.
The goal of this study was to uncover the neural underpinnings of visuospatial expertise in autism by comparing autistic individuals with enhanced visuospatial abilities (measured using a BD performance peak), autistic individuals without a BD peak, and a neurotypical control group. Therefore, our choice of sample allowed us to investigate the relationship between enhanced visuospatial abilities and functional brain organization in autistic individuals with distinct cognitive profiles. Using fMRI, we aimed to (1) identify the neural networks involved in autistic visuospatial expertise by comparing the manipulation of mental images during a classic three-dimensional mental rotation task in autistic individuals (with versus without enhanced visuospatial abilities) and neurotypical individuals, (2) inquire into the synchronization of neural activation among the regions constituting this mental rotation network and its modulation as a function of task complexity, and (3) quantify the associations between behavioural performance, brain activation, and functional connectivity within the mental rotation neural network.
Previous research has observed greater functional resource allocation in posterior brain regions in autism (Samson et al., 2012). Thus, we predicted greater occipital and parietal activation along with a concomitant increase in functional connectivity in these posterior regions in both autistic subgroups compared to the neurotypical group. Decreased activation in certain frontal regions, paired with reduced functional connectivity between frontal and posterior regions, was also expected in autistic individuals (O’Reilly et al., 2017, Picci et al., 2016). Importantly, when comparing the different autistic subgroups amongst each other and to the neurotypical group, we hypothesized that these differences would be more prominent in autistic individuals with visuospatial strengths.
2. Material and methods
2.1. Participants
Thirty-one autistic adults (AUT) and 28 adults with typical development (TYP) participated in the study (all males, 18 to 41 years old). Autistic participants were recruited from the database of the specialized autism clinic at Rivière-des-Prairies Hospital (Montreal, Canada). Participants with typical development were recruited from the same database and from the community through online advertisements. All participants were screened with a semi-structured interview assessing any personal or familial neurological and psychiatric conditions. They gave written informed consent and received a financial compensation for their participation. The study was approved by the ethics committees of Rivière-des-Prairies Hospital and the Regroupement Neuroimagerie Québec.
The diagnosis of autism spectrum disorder was established by experienced clinicians based on a multidisciplinary evaluation, including both the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994) and the Autism Diagnosis Observation Schedule (ADOS-G or ADOS-2; Lord et al., 2000, Lord et al., 2012) for 26 participants, and ADI-R alone for four participants. Participants had a diagnosis of autistic disorder (with language delay in infancy) based on the Diagnostic and Statistical Manual of Mental Disorders IV-TR (American Psychiatric Association, 2000), except for two who had a diagnosis of Asperger syndrome. Exclusion criteria for all participants were a Full-Scale Intellectual Quotient inferior to 70 or superior to 130, uncorrected visual impairment, the use of drugs or alcohol (exceeding two drinks per day) or MRI contraindications. Autistic and non-autistic participants who presented any personal or familial history of genetic, neurologic or psychiatric condition were also excluded, except for Attention deficit and hyperactivity disorder (ADHD) in autistic participants. As such, 10 autistic participants had a diagnosis of ADHD (five in each subgroup). Seven of them took psychostimulants (three: amphetamine, four: methylphenidate), though they were instructed to not take it the day of the MRI session.
The autistic group was further divided in two subgroups based on the presence/absence of relative visuospatial strengths, as assessed prior to scanning session by the Block Design (BD) subtest of the Wechsler’s Intelligence Scales (WISC-III, WISC-IV, WAIS-III or WAIS-IV). For a given participant, a significantly higher performance on this subtest compared with their performance on other verbal and perceptual reasoning Wechsler subtests is considered to be a relative strength. According to the definitions provided by the different versions of the test (Wechsler, 1991, Wechsler, 1997, Wechsler, 2003, Wechsler, 2008), a performance peak refers to a difference seen in <5 % of the population between the standard score of BD subtest and the average scores on all other Wechsler subtests. The minimum difference required to be considered a strength varies between 2.84 and 3.61 depending on the version of the tests and the number of subtests completed (Wechsler, 1991, Wechsler, 1997, Wechsler, 2003, Wechsler, 2008). Based on that definition, 15 autistic participants presented a significant BD performance peak (AUTp group) and 16 did not (AUTnp group). Only three participants in the TYP group presented a BD peak. They were excluded from analyses comparing the TYP group with autistic subgroups (AUTp vs TYP and AUTnp vs TYP).
Due to very poor performance defined as <60 % accuracy rate (accuracy near chance level) on the mental rotation task administered in the scanner, one participant from each AUT subgroup and three participants from the TYP group were excluded. In addition, one participant from each AUT subgroup was also excluded due to excessive head motion (translation displacement range > 3.5 mm and rotation displacement range > 3.5°). In the TYP group, two additional participants were excluded due to a misunderstanding of task instructions and poor fMRI data quality. The final sample therefore consisted of 27 autistic participants from the AUTc group (combined), including 13 from the AUTp subgroup (four with ADHD) and 14 from the AUTnp subgroup (four with ADHD), and 23 participants from the TYP group. As expected, the groups differed significantly on the BD peak, F (2, 47) = 26.55, p <.001, with the AUTp presenting a superior peak compared to the other groups (all ps < 0.001). Participant characteristics are shown in Table 1.
Table 1.
Participant characteristics.
| AUTc (n = 27) Mean ± SD |
AUTp (n = 13) Mean ± SD |
AUTnp (n = 14) Mean ± SD |
TYP (n = 23) Mean ± SD |
p-value (AUTc vs TYP) |
p-value (AUTp vs AUTnp vs TYP) |
|
|---|---|---|---|---|---|---|
| Age in years Range |
25.2 ± 5.5 18–36 |
24.5 ± 5.4 18–35 |
25.9 ± 5.8 18–36 |
24.4 ± 3.6 18–32 |
0.50 | 0.59 |
| Full Scale IQ Range |
97.8 ± 11.8 77–118 |
99.4 ± 12.1 81–118 |
96.3 ± 11.7 77–117 |
105.9 ± 9.1 87–125 |
0.00 | 0.03 |
| Performance IQ Range |
106.0 ± 11.7a 89–134 |
110.7 ± 13.6 90–134 |
101.2 ± 7.3a 89–114 |
106.7 ± 9.7 87–120 |
0.80 | 0.07 |
| Verbal IQ Range |
92.6 ± 14.9a 63–123 |
91.2 ± 14.4 69–121 |
94.0 ± 15.7a 63–123 |
103.7 ± 8.7 90–123 |
0.00 | 0.01 |
| BD peak Range |
2.9 ± 3.1 −1.5–9.4 |
5.4 ± 2.1 3.1–9.4 |
0.6 ± 1.7 −1.5–3.5 |
0.2 ± 2.5 −4.4–4.5 |
0.05 | 0.00 |
| RSPM (percentile) Range |
75.2 ± 22.9a 13–98 |
80.2 ± 21.8 20–98 |
70.3 ± 23.8a 13–98 |
77.4 ± 21.0 28–97.5 |
0.73 | 0.49 |
| Manual preference Range |
60.2 ± 67.2 −100 to + 100 |
67.2 ± 53.9 −100 to + 100 |
53.8 ± 79.1 −100 to + 100 |
54.7 ± 69.2 −100 to + 100 |
0.78 | 0.85 |
| ADI-R scores Social Communication Repetitive behaviour |
20.4a 16.6a 7.1a |
22.4 16.4 6.1 |
0.41 0.92 0.22 |
|||
| ADOS scores Social Communication Imagination/play Repetitive behaviour |
8.8c 4.5c 1.0c 3.1c |
9.3b 4.4b 1.3b 3.3b |
0.71 0.90 0.42 0.82 |
AUTc: autistic group combined. AUTp: autistic group with a Block Design peak. AUTnp: autistic group with no Block Design peak. TYP: group with typical development. BD: Block Design subtest. RSPM: Raven’s Standard Progressive Matrices. ADI-R: Autism Diagnostic Interview-Revised. ADOS: Autism Diagnostic Observation Schedule.
a. Data missing for one subject.
b. Data missing for 2 subjects.
c. Data missing for 3 subjects.
2.2. Behavioural assessment
Prior to the scanning session, participants underwent a behavioural assessment. They completed one of the Wechsler Intelligence scales at the time of their enrolment in the database (either Wechsler Adult Intelligence Scale – WAIS-III (Wechsler, 1997) in 10 participants, WAIS-IV (Wechsler, 2008) in 20 participants, Wechsler Intelligence Scale for Children – WISC-III (Wechsler, 1991) in 14 participants or WISC-IV (Wechsler, 2003) in six participants). As the Wechsler Scales often underestimate autistic intelligence when assessed uniquely with this tool (Barbeau et al., 2013, Dawson et al., 2007), the Raven’s Standard Progressive Matrices (RSPM) (Raven et al., 1998) were also administered as this test is thought to be more representative of general intelligence in autism (Barbeau et al., 2013, Dawson et al., 2007). Manual preference was assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). All participants had normal or corrected-to-normal visual acuity, estimated using a Snellen chart. Although the three groups had mean full-scale IQ scores within the average range, they differed significantly, F(2, 47) = 3.84, p =.03, with the TYP group having a significantly higher mean score than the AUTnp group (p =.03). The three groups were also different in verbal IQ, F(2, 47) = 5.02, p =.01, with a higher mean score in the TYP group compared to the AUTp group (p =.02). However, groups did not differ significantly in terms of age, performance IQ, RSPM performance and manual preference, all ps > 0.05 (Table 1). Even if performance IQ and RSPM scores probably provide better indices of intellectual functioning in autism, we still controlled for full-scale IQ differences in all behavioural and brain imaging analyses to remove any potential confounding effects.
2.3. FMRI mental rotation task and procedure
The testing session lasted approximately two hours, including preparation time, the scanning session, and a quick debriefing at the end. Prior to scanning, procedure and task instructions were explained to each participant. They were trained with a set of practice trials for 6 min on a computer to become familiar with the task and response boxes. The training was repeated if the instructions were poorly understood.
In the scanner, participants completed a classic mental rotation task with three-dimensional figures inspired by Shepard and Metzler (1971). The stimuli were taken from Peters and Battista (2008)’s stimulus library. A trial consisted of two figures each composed of 10 cubes with black contours on a white background that were presented on the screen by a computer-projector (see Fig. 1). The task was created and performed with E-prime software Version 2.0 (Psychology Software Tools Inc.). In total, there were 104 trials presented randomly. The angle disparity between the two figures presented in each trial was either 0°, 70°, 140° or 180° along the y axis. For half of the trials, the two figures were identical (except for angle disparity) and for the other half of the trials, they were mirror images. There were then 13 trials per angle (four angles) per inversion type (two: identical or mirror). Participants were instructed “to visualize one of the objects rotating until it mentally appears in the same orientation as the second object”. They were asked to respond as quickly and accurately as possible. Each pair of stimuli was presented on the screen until a response was made, up to a maximum of 10.0 s. The inter-trial interval varied randomly between 3.0 and 5.0 s (mean 4.0 s), during which a fixation cross was displayed in the center of the screen. Participants held a response box in each hand and indicated whether the figures were identical by pressing a key with the left index or mirror images by pressing a key with the right index. Accuracy and response time were measured.
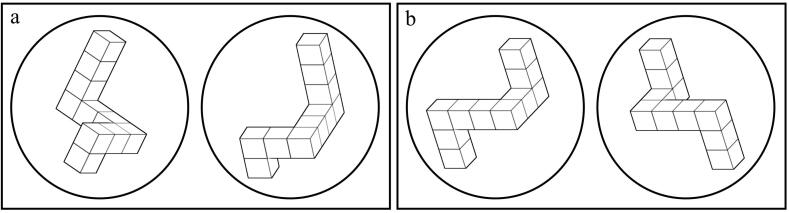
Fig. 1.
Two examples of trials. The left rectangle (a) displays identical figures at 70-degree. The right rectangle (b) displays mirror figures at 0-degree.
2.4. MRI data acquisition
Images were acquired on a Siemens TRIO 3.0T MRI system with a 32-channel phased-array head coil at the Functional Neuroimaging Unit in Montreal. Functional data were recorded using a T2* weighted gradient echo-planar imaging (EPI) sequence [repetition time (TR) = 2650 ms, echo time (TE) = 30.0 ms, flip angle = 90°, matrix size = 64 × 64]. Gradient echo phase and magnitude field maps were then acquired (45 slices, matrix size = 64 × 64, slice thickness = 3 mm, TR = 476 ms, TE short = 4.92 ms, TE long = 7.38 ms, flip angle = 60°) for the correction of image distortions and the improvement of co-registration accuracy. A T1-weighted structural scan was then acquired with an MPRAGE sequence (three-dimensional, spoiled gradient echo sequence; 176 slices, slice thickness = 1.00 mm, TR = 2300.0 ms, TE = 2.98 ms, flip angle = 9°). There was an upgrade to the MRI system during the study (MRI Siemens 3T Prisma fit). An independent-sample t-test comparing all participants in the scanner before the update (n = 40; 23 in the AUTc group and 17 in the TYP group) to all participants in the scanner after the update (n = 10; four in the AUTc group and six in the TYP group) did not show any significant difference in brain activation (visualised using p <.001, unc., k = 30 as a threshold). Nevertheless, we still controlled for the update with a covariable added through all fMRI analyses.
2.5. Preprocessing
SPM12 was used for preprocessing and statistical modeling. During preprocessing, all images were realigned and unwrapped. Fieldmaps were used for distortion correction. Images were then corrected for slice timing using the first slice as reference, coregistered onto their T1 image, segmented into gray matter, white matter and cerebrospinal fluid, and then spatially normalized into the ICBM152MNI space. Normalized images were finally smoothed using a 6-mm full-width half-maximum isotropic Gaussian kernel. No difference in head motion (translation and rotation displacement ranges) was found between the three groups (rotation max p =.879, rotation min p =.148, translation max p =.599, translation min p =.322).
2.6. Plan of analyses
For reasons of clarity, we subdivided the presentation of the analysis and the results in three parts following the three aims: (1) the behavioural performance on the mental rotation task administered in the scanner, using SPSS 26.0 (SPSS Inc., Chicago, IL, USA), and task-related brain activation using SPM12 (Statistical Parametric Mapping software, Wellcome Centre for Human Neuroimaging, London, UK), (2) the task-related correlated brain activation (or functional connectivity) computed with CONN functional connectivity toolbox (20.b) (https://www.nitrc.org/projects/conn) in MATLAB R2017b, and (3) the associations between visuospatial performances, brain activation and functional connectivity through SPM12 and CONN (20.b).
3. Aim 1: Behavioural performance and task-related activation
3.1. Analyses
3.1.1. Behavioural analyses
Three-way mixed ANOVAs with Group (AUTc and TYP) as a between-subject factor, Angle of rotation (0°, 70°, 140°, 180°) and Inversion type (Identical, Mirror) as within-subject factors, were conducted separately for the two dependent variables: accuracy measured as the percentage of correct answers and mean response time. The same analyses were conducted afterward with the three groups (AUTp, AUTnp and TYP) as a between-subject factor. Because the groups differed on full-scale IQ, we controlled for this variable in all the analyses. Full-scale IQ was found to be correlated with the accuracy (r = 0.317, p =.03), but not with the mean response time (r = −0.111, p =.44). Alpha level was set at 0.05 with Bonferroni corrections applied when needed. Pearson correlation coefficients between total accuracy and total mean response time were also computed for each group separately (AUTc, AUTp, AUTnp, TYP).
3.1.2. Statistical modeling: task-related activation
Functional images were analyzed for each participant separately on a voxel-by-voxel basis, according to the general linear model. Separate regressors were included for each angle and inversion conditions (0° identical, 0° mirror, 70° identical, 70° mirror, 140° identical, 140° mirror, 180° identical, 180° mirror). Regressors of non-interest included the outlier trials (response time higher than 3 standard deviations from the subject’s mean response time) as well as the six movement parameters (three translations, three rotations). Low-frequency noise was removed with a high-pass temporal filter with a cut-off of 128 s. Group analyses were then performed on parameter estimates through a full factorial model with Group, Angle, and Inversion as factors in two sets of analyses. The first set compared the AUT group combined (AUTc) to the TYP group. The second set of analyses compared brain activation differences between the TYP group and each AUT subgroup separately, and between the two AUT subgroups. Contrasts were computed to examine within-group and between-group effects on hemodynamic response. A first contrast, referring to mental rotation processes, was created to isolate the activation specific to the cognitive processes of mental rotation by combining the 70°, 140° and 180° conditions, subtracting the baseline (fixation cross). A second contrast, referring to complexity, was also computed to examine regions in which activation increased as the amount of mental rotation to be performed increased for identical trials only (0° < 70° < 140° < 180°), subtracting the baseline (fixation cross). Uncorrected voxel-wise cluster-forming threshold p <.001 and FDR-corrected cluster-level p <.05 were used for all fMRI analyses, with an extent threshold of 100 voxels (within-group) or 50 voxels (between-group). SPM12 Anatomy toolbox and the MNI2TAL application from the Yale BioImage Suite Package (https://bioimagesuiteweb.github.io/webapp/mni2tal.html) were used to locate cortical activation peaks. Visualization of brain activation results was achieved through MRIcroGL with SPM thresholded maps superimposed on the anatomical MNI152 template (https://www.mccauslandcenter.sc.edu/mricrogl) (Rorden and Brett, 2000). Scan update and full-scale IQ were controlled for in all analyses described above.
3.2. Results
3.2.1. Behavioural performance
3.2.1.1. Accuracy
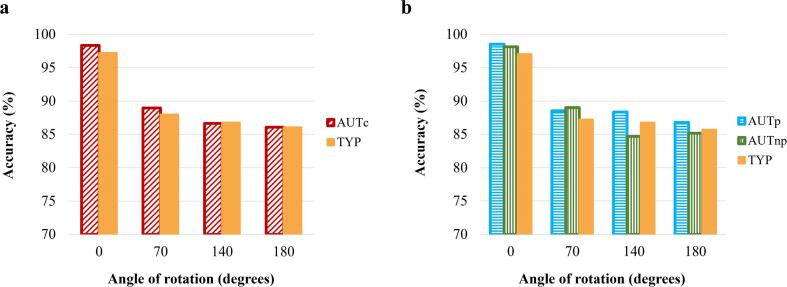
As the majority of accuracy variables among the three groups and the eight experimental conditions were non-normally distributed (kurtosis and skewness values > 2.0), winsorizing technique was applied by replacing extreme scores (≥3 standard deviations from the mean) with the next higher score that was not an outlier. Frequency of extreme scores were similar in all the groups (two in the TYP group and three in the AUTc group, with one and two in the AUTp and the AUTnp groups respectively). Three-way repeated measures ANOVA (Group [AUTc vs TYP] × Angles × Inversion type) showed no difference between the groups in accuracy F(1,47) = 0.082, p =.776 (see Fig. 2.a). Similar results were obtained for the three-way repeated measures comparing AUTp vs AUTnp vs TYP groups, with no main effect of group, F(2, 43) = 0.282, p =.755 and no main effect of Inversion type, F(1,43) = 0.045, p =.833. However, a significant main effect of angle F(3,129) = 2.728, p =.047, η2 = 0.060 was obtained. Post-hoc analyses revealed a significantly higher proportion of correct answers for the 0-degree condition, compared to all other conditions, all ps < 0.001, and there were no differences between 70, 140 and 180-degrees conditions (see Fig. 2.b).
Fig. 2.
Proportion of correct responses for (a) the AUTc and TYP groups and for (b) the AUTp, AUTnp and TYP groups at 0, 70, 140 and 180 degrees of rotation.
3.2.1.2. Mean response time
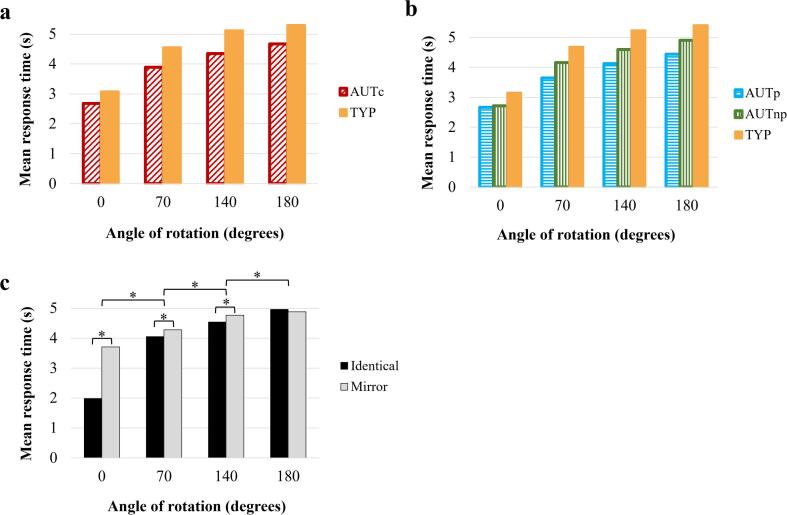
A main effect of group was found for mean response time following a three-way repeated measures ANOVA (Group [AUTc vs TYP] × Angles × Inversion type), F(1,47) = 4.839, p =.033 η2 = 0.093, with autistic participants showing significantly faster mean response time (see Fig. 3.a). When comparing AUTp vs AUTnp vs TYP groups, a main effect of group was also revealed, F(1,43) = 3.633, p =.035 η2 = 0.145 (see Fig. 3.b). Post-hoc analyses revealed that only autistic individuals with visuospatial strength had significantly faster mean response time compared to the TYP group (p =.032). A significant main effect of angle with a Greenhouse-Geisser correction, F(2.319, 99.721) = 3.111, p =.042, η2 = 0.067, and an interaction effect between angle and inversion, F(3,129) = 6.412, p <.001, η2 = 0.130, were obtained (see Fig. 3.c). Post-hoc analyses revealed a significant increase in mean response time as degrees of rotation increased for all groups, all ps < 0.005. All participants were also faster to respond to identical images compared to mirror images at 0 (p <.001), 70 (p =.014) and 140-degrees (p =.004), while they took a similar amount of time to respond to identical and mirror figures at 180-degrees rotation (p =.469).
Fig. 3.
Mean response time for (a) the AUTc vs TYP, (b) AUTp vs AUTnp vs TYP groups at 0, 70, 140 and 180 degrees of rotation, and for (c) all participants together for the Identical versus Mirror conditions at 0, 70, 140 and 180 degrees of rotation (*ps < 0.01).
3.2.1.3. Correlations between accuracy and mean response time
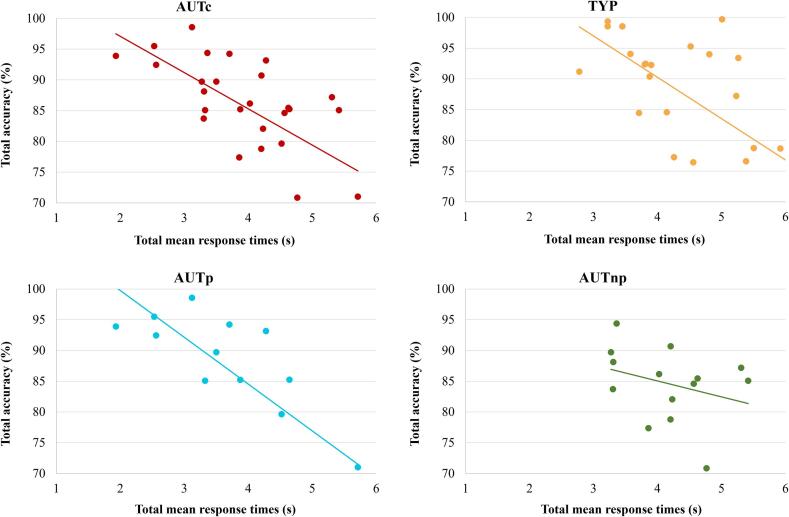
Considering all angles and condition types, higher accuracy (percentage of correct answers) was significantly associated with faster mean response time for the AUTc (r = −0.632, p <.001). This correlation was specific to the AUTp subgroup (r = −0.756, p =.003), but was not significant in the AUTnp group (r = -0.309, p =.282) (see Fig. 4). For TYP participants, accuracy and mean response time were also negatively correlated (r = −0.625, p =.001).
Fig. 4.
Pearson correlations between total accuracy and total mean response time for the AUTc (p <.001), the TYP (p =.001), the AUTp (p =.003) and the AUTnp (p =.282) groups.
3.2.2. Brain correlates of mental rotation processes
3.2.2.1. Within-group activation network underlying mental rotation processes
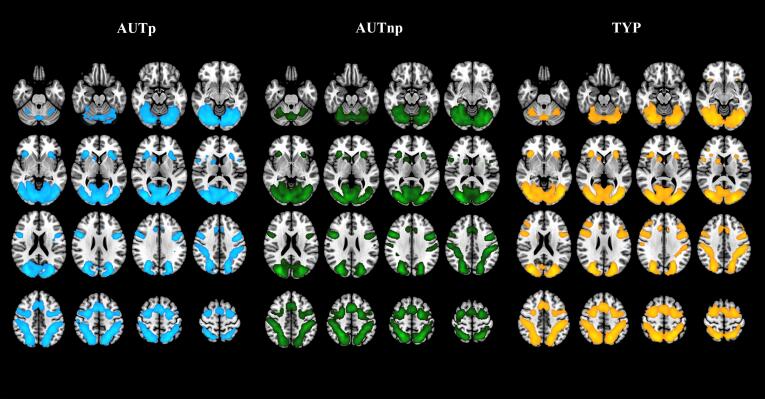
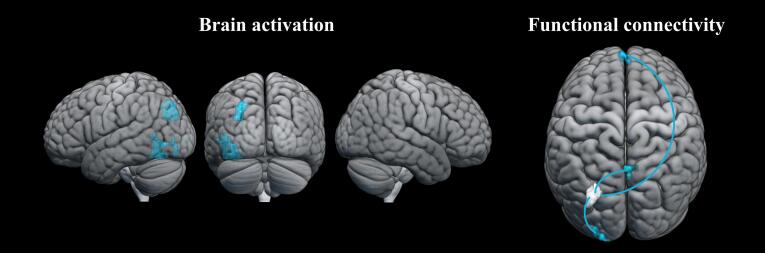
In the three groups, the network solicited by the mental rotation task revealed activations that match the brain network consistently reported in previous imaging studies (Hawes et al., 2019, Tomasino and Gremese, 2016, Zacks, 2008). Recruited brain regions were located mainly in occipital (inferior, middle and superior occipital gyri), parietal (inferior and superior parietal lobule), frontal (precentral gyrus, inferior frontal gyrus pars opercularis, middle frontal gyrus, superior frontal gyrus and posterior-medial frontal) and insula lobes (see Fig. 5).
Fig. 5.
Within-group activation network for average activation across all angle conditions (70°, 140° and 180°) for the three groups, AUTp (blue), AUTnp (green) and TYP (yellow) (uncorrected voxel-wise cluster-forming threshold p <.001 and FDR-corrected cluster-level p <.05, k = 100). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
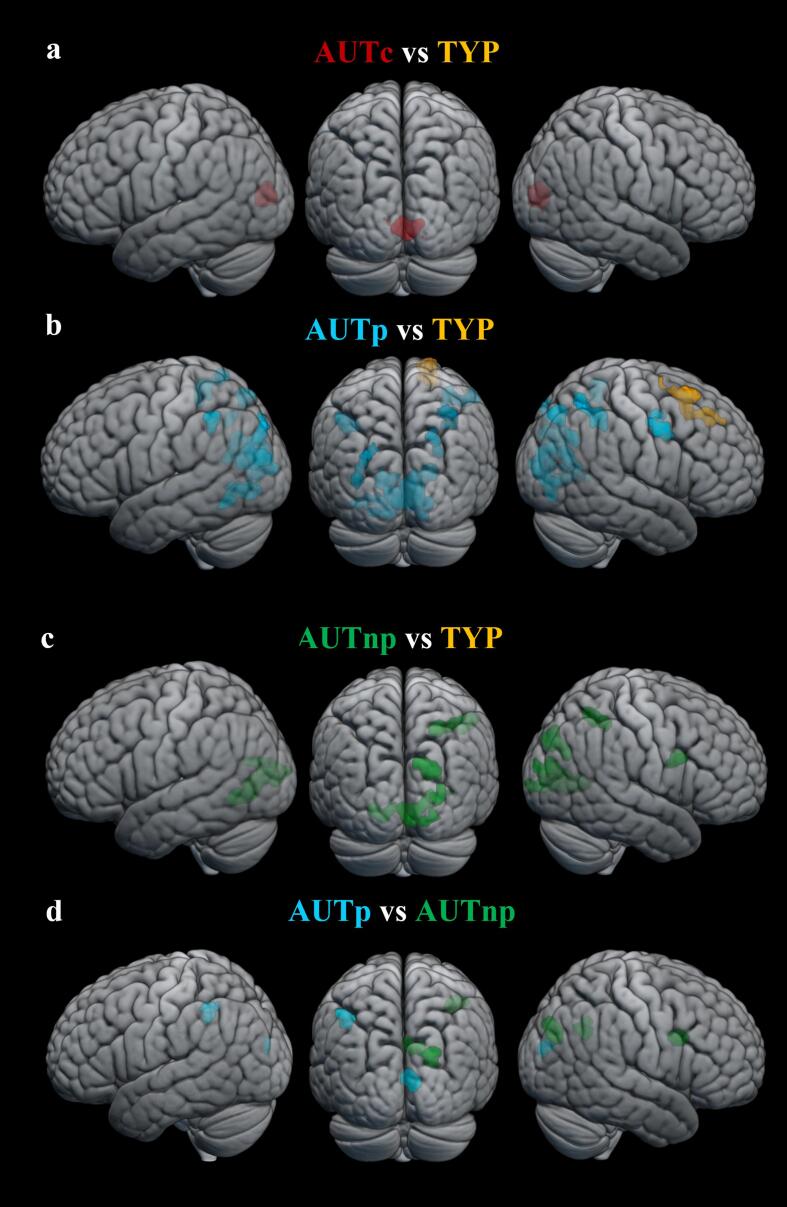
3.2.2.2. Between-group effects of brain activation underlying mental rotation processes
Relative to the TYP group, the AUTc group showed greater brain activation in occipital regions while performing mental rotation. No region was found to be more activated in the TYP group compared to the AUTc group (see Table 2 and Fig. 6.a). Higher activation was observed in occipital, parietal and frontal regions in the AUTp group compared to the TYP group, whereas the latter showed greater activation in the right superior frontal gyrus (see Fig. 6.b). The AUTnp group showed greater activation in occipital, parietal and frontal regions relative to the TYP group, whereas no region was more activated for the TYP group (see Fig. 6.c). Compared to the AUTnp group, the AUTp group showed greater activation mostly in left parietal (intraparietal sulcus) and right occipital areas. The AUTnp group showed greater activation in the right hemisphere in frontal, parietal and occipital regions (all ps < 0.05, FDR-corrected at the cluster level) (see Fig. 6.d).
Table 2.
MNI coordinates of brain areas showing significant between-group differences on BOLD response during mental rotation task (uncorrected voxel-wise cluster-forming threshold p <.001 and FDR-corrected cluster-level p <.05, k = 50).
| Region | Location (Anatomy toolbox & MNI2TAL) | BA | k | T value | x | y | z | ||
|---|---|---|---|---|---|---|---|---|---|
| AUTc > TYP | Occipital | B | Calcarine gyrus | 17/18 | 104 | 4.85 | 6 | −85 | −1 |
| 0 | −88 | 2 | |||||||
| −9 | −85 | 8 | |||||||
| R | Lingual gyrus | 15 | −82 | −7 | |||||
| AUTp > TYP | Occipital | B | Calcarine gyrus | 17/18 | 401 | 5.07 | −3 | −91 | 5 |
| −12 | −70 | 11 | |||||||
| −9 | −82 | 8 | |||||||
| 3 | −82 | 8 | |||||||
| 21 | −67 | 17 | |||||||
| 12 | −88 | 5 | |||||||
| R | Lingual gyrus | 18 | −79 | −4 | |||||
| 15 | −79 | −13 | |||||||
| 6 | −82 | −1 | |||||||
| 15 | −61 | 5 | |||||||
| R | Cuneus | 9 | −82 | 17 | |||||
| L | Lingual gyrus | 18/19/37 | 122 | 4.57 | −15 | −67 | −10 | ||
| −24 | −61 | −10 | |||||||
| L | Inferior occipital gyrus | −21 | −76 | −13 | |||||
| L | Middle occipital gyrus | −27 | −70 | −4 | |||||
| L | Fusiform gyrus | –33 | −61 | −13 | |||||
| −36 | −55 | −16 | |||||||
| Occipito-parietal | L | Middle occipital gyrus | 7/19/39 | 78 | 5.74 | –33 | −82 | 17 | |
| −30 | −73 | 23 | |||||||
| L | Superior parietal lobule | −21 | −82 | 45 | |||||
| L | Angular gyrus | −27 | −82 | 35 | |||||
| R | Superior occipital gyrus (*) | 19 | 56 | 4.33 | 27 | −61 | 29 | ||
| R | Superior parietal lobule | 7 | 24 | −73 | 44 | ||||
| Parietal | R | Inferior and superior parietal lobule (*) | 7 | 79 | 4.87 | 36 | −43 | 53 | |
| 33 | −58 | 62 | |||||||
| L | Inferior parietal lobule (intraparietal sulcus) | 40 | 51 | 5.02 | −45 | −46 | 50 | ||
| Frontal | R | Precentral gyrus/Supplementary motor area | 6 | 94 | 5.40 | 33 | −4 | 50 | |
| 45 | −4 | 44 | |||||||
| 51 | 2 | 44 | |||||||
| 51 | −4 | 50 | |||||||
| TYP > AUTp | Frontal | R | Superior frontal gyrus/Supplementary motor area | 6/8 | 145 | 5.13 | 18 | 17 | 62 |
| 21 | 32 | 47 | |||||||
| 21 | 17 | 50 | |||||||
| 18 | −1 | 74 | |||||||
| 18 | 44 | 41 | |||||||
| AUTnp > TYP | Occipital | B | Calcarine gyrus | 17/18/19 | 285 | 5.88 | 9 | −85 | −1 |
| 18 | −76 | 5 | |||||||
| 6 | −91 | −4 | |||||||
| 21 | −91 | 2 | |||||||
| L | Lingual gyrus | −18 | −64 | −10 | |||||
| −12 | −73 | −7 | |||||||
| −9 | −70 | −4 | |||||||
| R | Middle occipital gyrus | 27 | −82 | 17 | |||||
| R | Superior occipital gyrus | 24 | −91 | 14 | |||||
| R | Superior occipital gyrus | 19 | 90 | 6.01 | 21 | −82 | 35 | ||
| Parietal | R | Inferior and superior parietal lobule | 7 | 68 | 6.06 | 24 | −55 | 53 | |
| 33 | −46 | 50 | |||||||
| Frontal | R | Inferior frontal gyrus (p. Opercularis) | 44 | 59 | 5.56 | 45 | 8 | 29 | |
| TYP > AUTnp | no significant result | ||||||||
| AUTp > AUTnp | Parietal | L | Inferior parietal lobule (intraparietal sulcus) | 40 | 50 | 4.43 | −45 | −49 | 50 |
| Occipital | R | Cuneus | 18 | 50 | 3.95 | 9 | −85 | 14 | |
| 3 | −82 | 17 | |||||||
| AUTnp > AUTp | Occipital | R | Superior occipital gyrus | 19 | 58 | 5.83 | 24 | −82 | 35 |
| 24 | −76 | 26 | |||||||
| Frontal | R | Inferior frontal gyrus (p. Opercularis) | 44 | 51 | 5.78 | 39 | 11 | 29 | |
| Parietal | R | Precuneus | 7/31 | 50 | 4.55 | 12 | −62 | 32 | |
| 6 | −69 | 36 | |||||||
Fig. 6.
Results of between-group differences on BOLD response during mental rotation are shown in (a) AUTc > TYP, (b) AUTp vs TYP, (c) AUTnp > TYP and (d) AUTp vs AUTnp (uncorrected voxel-wise cluster-forming threshold p <.001 and FDR-corrected cluster-level p <.05). Higher brain activation is shown for the AUTc group (red), the AUTp group (blue), the AUTnp group (green) and the TYP group (orange). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2.3. Brain correlates associated with mental rotation complexity
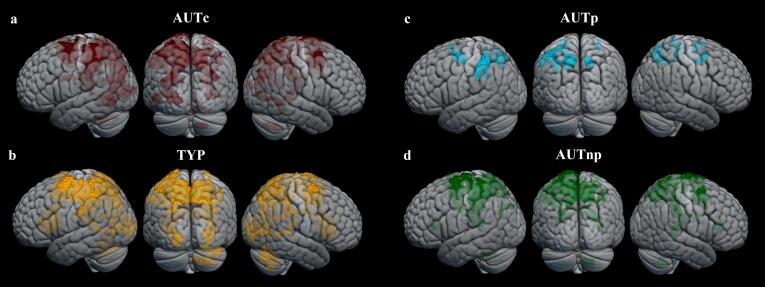
3.2.3.1. Within-group activation network underlying mental rotation complexity
A linear contrast examining mental rotation complexity for identical items (0° < 70° < 140° < 180°) revealed an extensive network in frontal, parietal, occipital, cerebellum (only for TYP) and subcortical regions for AUTc and TYP groups (see Table 3 and Fig. 7.a and b). When looking at the specific brain network associated with mental rotation complexity in AUT subgroups, the results showed increasing brain activation in left parietal regions in the AUTp group (see Table 3 and Fig. 7.c) whereas the AUTnp group presented a more extensive network similar to that found in the TYP group (see Fig. 7.d).
Table 3.
MNI coordinates of brain areas showing within- and between-group BOLD responses underlying mental rotation complexity (uncorrected voxel-wise cluster-forming threshold p <.001 and FDR-corrected cluster-level p <.05, k = 100 for within-group effects and k = 50 for between-group effects).
| Region | Location (Anatomy toolbox & MNI2TAL) |
BA | k | T value | x | y | z | ||
|---|---|---|---|---|---|---|---|---|---|
| AUTc | Frontal | R | Superior frontal gyrus | 6 | 244 | 8.49 | 27 | 2 | 62 |
| L | Superior frontal gyrus | 1/6/7/40 | 2935 | 9.67 | −24 | −4 | 59 | ||
| Parietal | L | Inferior parietal lobule | −36 | −37 | 44 | ||||
| L | Postcentral gyrus | −39 | −43 | 62 | |||||
| −39 | −40 | 56 | |||||||
| B | Precuneus | 12 | −61 | 59 | |||||
| −12 | −61 | 59 | |||||||
| −12 | −70 | 56 | |||||||
| −12 | −49 | 62 | |||||||
| −9 | −52 | 65 | |||||||
| R | Inferior parietal lobule | 1/40 | 218 | 6.04 | 36 | −34 | 44 | ||
| R | Postcentral gyrus | 39 | −40 | 56 | |||||
| Subcortical | L | Thalamus | 161 | 5.88 | −24 | −31 | 14 | ||
| −12 | −28 | 17 | |||||||
| −27 | −34 | 2 | |||||||
| R | Thalamus | 123 | 6.38 | 21 | −28 | 14 | |||
| 30 | −34 | 2 | |||||||
| 12 | −16 | 17 | |||||||
| AUTp | Parietal | L | Postcentral gyrus | 1/7/40 | 271 | 5.22 | −39 | −43 | 62 |
| −48 | −34 | 50 | |||||||
| L | Inferior parietal lobule (intraparietal sulcus) | −39 | −43 | 53 | |||||
| −45 | −46 | 53 | |||||||
| –33 | −40 | 44 | |||||||
| −54 | −31 | 41 | |||||||
| L | Superior parietal lobule | −24 | −45 | 71 | |||||
| −27 | −49 | 68 | |||||||
| L | Superior parietal lobule | 7 | 145 | 4.96 | −15 | −64 | 59 | ||
| L | Precuneus | −9 | −67 | 53 | |||||
| AUTnp | Frontal | R | Superior frontal gyrus | 6 | 227 | 7.96 | 27 | 2 | 62 |
| L | Superior frontal gyrus | 1/6/7 | 2211 | 8.54 | −24 | −7 | 56 | ||
| L | Precentral gyrus | −18 | −7 | 68 | |||||
| Parietal | L | Postcentral gyrus | –33 | −34 | 41 | ||||
| −39 | −37 | 53 | |||||||
| −36 | −40 | 65 | |||||||
| L | Superior parietal lobule | −21 | −49 | 68 | |||||
| B | Precuneus | 12 | −58 | 59 | |||||
| −6 | −64 | 65 | |||||||
| −12 | −49 | 62 | |||||||
| 9 | −52 | 68 | |||||||
| R | Supramarginal gyrus (intraparietal sulcus) | 1/40 | 126 | 5.61 | 39 | −34 | 44 | ||
| R | Postcentral gyrus | 36 | −40 | 56 | |||||
| Subcortical | R | Thalamus | 151 | 6.44 | 24 | −31 | 14 | ||
| TYP | Frontal | R | Superior frontal gyrus | 6/8 | 323 | 7.89 | 24 | −4 | 62 |
| 24 | −7 | 56 | |||||||
| R | Middle frontal gyrus | 24 | 8 | 44 | |||||
| L | Superior frontal gyrus | 1/6/7/40 | 3714 | 10.85 | −27 | −7 | 59 | ||
| −24 | −1 | 59 | |||||||
| −27 | −4 | 68 | |||||||
| Parietal | L | Postcentral gyrus | −39 | −37 | 53 | ||||
| L | Superior parietal lobule | −27 | −55 | 62 | |||||
| −18 | −61 | 59 | |||||||
| L | Supramarginal gyrus (intraparietal sulcus) | −48 | −28 | 35 | |||||
| B | Precuneus | 12 | −64 | 59 | |||||
| −6 | −64 | 59 | |||||||
| Occipital | L | Middle occipital gyrus | 18 | 238 | 5.72 | −18 | −94 | −1 | |
| Cerebellum | R | VIII | 295 | 6.59 | 24 | −55 | −52 | ||
| 27 | −61 | −46 | |||||||
| 33 | −58 | −49 | |||||||
| 18 | −73 | −46 | |||||||
| 12 | −61 | −46 | |||||||
| R | VII | 39 | −64 | −49 | |||||
| R | VI | 21 | −70 | –22 | |||||
| 27 | −55 | −34 | |||||||
| 33 | −58 | −34 | |||||||
| R | Crus 1 | 42 | −67 | −31 | |||||
| R | Crus 2 | 6 | −76 | −34 | |||||
| Subcortical | B | Caudate nucleus | 479 | 6.70 | −9 | 20 | −4 | ||
| 9 | 17 | −1 | |||||||
| 9 | 8 | −1 | |||||||
| −3 | 11 | −1 | |||||||
| −18 | 11 | 17 | |||||||
| AUTnp > TYP | Parietal | L | Paracentral lobule | 1/5/6/7 | 72 | 4.71 | −12 | −13 | 76 |
| −3 | −19 | 75 | |||||||
| −6 | −31 | 68 | |||||||
| L | Precuneus | −12 | −40 | 68 | |||||
| −12 | −49 | 68 | |||||||
Fig. 7.
Results of within-group effects on BOLD response associated with mental rotation complexity are shown in (a) AUTc, (b) TYP, (c) AUTp and (d) AUTnp (uncorrected voxel-wise cluster-forming threshold p <.001 and FDR-corrected cluster-level p <.05, k = 100). Brain activation is shown for the AUTc group (red), the TYP group (yellow), the AUTp group (blue) and the AUTnp group (green). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2.3.2. Between-group effects of brain activation underlying mental rotation complexity
Between-group analysis revealed greater activation only in the AUTnp group relative to the TYP group in left superior parietal areas (paracentral lobule and precuneus) (see Table 3). The AUTc and AUTp groups did not significantly differ from the TYP group as task complexity increased, nor did the autistic subgroups between one another. As the intraparietal sulcus (IPS) has been reported to have increased activation with increasing angle of rotation in previous studies (Papadopoulos et al., 2018, Zacks, 2008) and in our within-group results (see Table 3), we further explored if any group differences may potentially arise in this region by increasing the threshold at p <.005 uncorrected at the voxel-level and applying a mask around the IPS. Interestingly, the AUTp showed greater activation in the left IPS compared to the TYP group (T = 3.46, k = 14, mni coordinates xyz: −51, −46, 53) and in the right and left IPS compared to the the AUTnp group (k = 55, T = 4.06, mni coordinates xyz: −48, −46, 53; k = 15, T = 3.10, mni coordinates xyz: 45, −58, 53). No difference was observed between the AUTc and the TYP and between the AUTnp and the TYP groups (voxel-level p <.005 uncorrected).
4. Aim 2: task-related correlated brain activation (functional connectivity)
4.1. Statistical modeling: Functional connectivity – Generalized psychophysiological interactions (gPPI)
Task-related correlated brain activation (or functional connectivity) was assessed using gPPI measures implemented in the CONN functional connectivity toolbox (20.b) (https://www.nitrc.org/projects/conn) in MATLAB R2017b. These measures are well suited for investigating functional connectivity patterns in the context of task event-related designs (Nieto-Castanon, 2020). We conducted a denoising method on the same preprocessed functional data used in SPM12 for brain activation analyses (see section 3.1.2. Statistical modeling: task-related activation for details). Outlier scans were removed based on motion (subject-motion threshold = 1.5 mm) and global signal (z-value threshold = 3) deviations using the Artifact Detection and Repair toolbox implemented in CONN. Linear regressions were used to remove the following confounding effects from the BOLD signal: five principal components from white matter, five principal components from cerebrospinal fluid, one principal component from grey matter, twelve principal components from subject-motion parameters, twelve principal components from scrubbing and two principal main task effects per condition, with linear detrending. After the denoising step, we also removed slowly fluctuating signal such as scanner drift by performing a high-pass filter of 0.008 Hz. Seed-to-voxel analyses were then conducted by correlating the average time-series within the selected seed regions of interest with the time-series from all other voxels in the brain. Fifteen bilateral seeds were selected based on the regions that showed between-group differences in brain activation underlying mental rotation processes (see Table 2). The seeds were located in the frontal (inferior frontal gyrus pars opercularis, precentral gyrus, superior frontal gyrus and supplementary motor area to be more specific since group differences were observed in those areas), parietal (superior parietal lobule, supramarginal gyrus anterior and posterior, angular gyrus and precuneus) and occipital regions (superior and inferior lateral occipital cortex, intracalcarine cortex, cuneal cortex, lingual gyrus and occipital fusiform gyrus). The seeds were taken from the atlas implemented in the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012). Selecting these whole anatomical-defined ROIs rather than the specific clusters showing between-group differences in brain activation may help prevent biasing the results towards one group comparison over another. Moreover, as increased variability of task-related brain activation in perceptive associative regions across autistic individuals has been reported (Poulin-Lord et al., 2014), using whole anatomically-based ROIs may reduce the impact of this within-group variability in the functional connectivity findings. Moreover, using the whole anatomically-based ROIs provides good coverage of the mental rotation network of interest in the current study, which comprises occipital, parietal and frontal regions identified in Table 2. It also has sufficient anatomical specificity and boundaries that make results easy to interpret. Note that as the superior lateral occipital cortex ROI from the atlas provided in CONN extends superior to the parieto-occipital sulcus and also includes part of the inferior (angular gyrus) and superior parietal lobule (see https://web.conn-toolbox.org/conn-in-pictures), we refer to this region as a posterior parieto-occipital area. As group differences in brain activation were found exclusively within these 15 ROIs (see Table 2, Table 3), only significant clusters of functional connectivity measures located within this network were considered, to concentrate on patterns of connectivity within this specific occipital-parietal-frontal mental rotation network. Bivariate correlations were measured for within-group effects (AUTc, AUTp, AUTnp, TYP) and between-group effects (AUTc vs TYP, AUTp vs TYP, AUTnp vs TYP, AUTp vs AUTnp) for the contrasts of mental rotation processes and complexity. Significant clusters were thresholded at p <.05 FDR-corrected with a voxel-wise cluster-forming threshold at p <.001 uncorrected and an extent cluster threshold of k = 50 voxels.
4.2. Results
4.2.1. Connectivity pattern for within-group effects underlying mental rotation processes
Within-group analyses in the AUTc group revealed positive connectivity in posterior regions between occipital and parietal areas and within the occipital cortex (see Table 4). Looking at the AUT subgroups separately revealed that the AUTp group showed positive connectivity in posterior regions as well, within the parietal cortex, between parietal and posterior parieto-occipital regions and within the occipital cortex. Long-range negative connectivity was found between frontal and parietal regions and between frontal and occipital regions. The AUTnp group displayed positive connectivity between frontal and parietal regions and within the occipital cortex. For the TYP group, within-group analyses showed significant positive connectivity in posterior regions between parietal and occipital areas and within the occipital cortex.
Table 4.
MNI coordinates of brain areas showing within-group functional connectivity during mental rotation processes within the fronto-parieto-occipital network (uncorrected voxel-wise cluster-forming threshold p <.001 and FDR-corrected cluster-level p <.05, k = 50).
| Regions | Effect |
Seed (CONN toolbox) |
Cluster location (CONN toolbox & MNI2TAL) |
BA | k | T value | x | y | z | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUTc | |||||||||||
| Parietal-Parietooccipital | + | R | Superior lateral occipital cortex | B | Precuneus | 7 | 81 | 5.43 | 4 | −70 | 40 |
| + | R | Lingual gyrus | L | Angular gyrus | 39 | 55 | 5.03 | −44 | −62 | 44 | |
| Occipital-Occipital | + | L | Cuneal cortex | R | Occipital fusiform gyrus | 37 | 96 | 6.46 | 58 | −64 | −16 |
| AUTp | |||||||||||
| Parietal-Parietal | + | L | Superior parietal lobule | L | Angular gyrus | 39 | 130 | 9.84 | –32 | −68 | 44 |
| + | L | Superior parietal lobule | R | Angular gyrus | 39 | 116 | 10.22 | 36 | −70 | 52 | |
| Parietal-Parietooccipital | + | L | Superior lateral occipital cortex | L | Precuneus | 7 | 103 | 8.24 | −8 | −70 | 52 |
| + | L | Superior lateral occipital cortex | L | Angular gyrus | 39 | 53 | 8.20 | –32 | −56 | 50 | |
| Occipital-Occipital | + | L | Cuneal cortex | R | Inferior lateral occipital cortex | 19 | 100 | 8.32 | 52 | −76 | −10 |
| Frontal-Parietal | – | L | Angular gyrus | R | Precentral gyrus | 4 | 55 | −7.41 | 60 | −4 | 20 |
| – | R | Superior frontal gyrus | L | Superior parietal lobule | 7 | 90 | −8.81 | −20 | −68 | 44 | |
| Frontal-Occipital | – | R | Cuneal cortex | R | Precentral gyrus | 6 | 56 | −7.09 | 46 | −10 | 44 |
| AUTnp | |||||||||||
| Frontal-Parietal | + | R | Precentral gyrus | L | Superior parietal lobule | 7 | 79 | 9.69 | −26 | −50 | 46 |
| Occipital-Occipital | + | L | Inferior lateral occipital cortex | R | Fusiform gyrus | 37 | 54 | 6.28 | 48 | −68 | 2 |
| TYP | |||||||||||
| Parietal-Occipital | + | R | Occipital fusiform gyrus | R | Angular gyrus | 39 | 102 | 5.77 | 52 | −50 | 32 |
| + | L | Inferior lateral occipital cortex | R | Precuneus | 7 | 69 | 5.64 | 12 | −70 | 44 | |
| Occipital-Occipital | + | L | Inferior lateral occipital cortex | R | Cuneal cortex | 18 | 96 | 6.97 | 16 | −64 | 28 |
4.2.2. Connectivity pattern for between-group effects underlying mental rotation processes
Between-group differences in task-related connectivity associated with mental rotation processes were found in several areas (see Table 5). First, the AUTc group showed significantly higher connectivity between frontal and parietal regions (between the right inferior frontal gyrus pars opercularis and the precuneus), between frontal and occipital areas (between the right precentral gyrus and the left lingual gyrus) and between posterior parieto-occipital and parietal regions (between the right superior lateral occipital cortex and the left angular gyrus) compared to the TYP group. On the contrary, compared to the AUTc group, the TYP group showed significantly higher functional connectivity in the left hemisphere between frontal and parietal regions (between the supplementary motor area and the angular gyrus). The AUTp and TYP groups also differed, with significantly higher functional connectivity in the AUTp group between the frontal cortex and the left occipital lobe (between the right precentral gyrus as a seed and three occipital regions, namely the lingual gyrus, the cuneus and the fusiform gyrus) and between parietal and occipital areas (between the right supramarginal gyrus and the left lingual gyrus). The TYP group, compared to the AUTp group, showed a significant increase in functional connectivity between frontal and parietal regions in the left hemisphere (between the supplementary motor area and the angular gyrus). Compared to the TYP group, the AUTnp group showed no significant increase in connectivity, whereas participants with typical development showed an increase in functional connectivity between frontal and parietal areas (between the right supplementary motor area and the right supramarginal gyrus, between the left supplementary motor area and the right angular gyrus, and between the left angular gyrus and the left supplementary motor area) and within the parietal cortex (between the left angular gyrus as a seed and the left superior parietal lobule and the left supramarginal gyrus, and within the precuneus). Finally, when comparing autistic subgroups, the AUTp group showed an increase in fronto-parietal functional connectivity compared to the AUTnp group specifically between the left inferior frontal gyrus pars opercularis and the right superior parietal lobule and between the right supplementary motor area and the right angular gyrus. The AUTnp group showed an increase in functional connectivity within the frontal cortex (between the right precentral and the right superior frontal gyri and between the left precentral and the left middle frontal gyri) relative to the AUTp group.
Table 5.
MNI coordinates of brain areas showing between-group differences on functional connectivity underlying mental rotation processes within the fronto-parieto-occipital network (uncorrected voxel-wise cluster-forming threshold p <.001 and FDR-corrected cluster-level p <.05, k = 50).
| Regions | Group effect |
Seed (CONN toolbox) |
Cluster location (CONN toolbox & MNI2TAL) |
BA | k | T value | x | y | z | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUTc > TYP | |||||||||||
| Frontal-Parietal | AUTc + TYP- | R | Inferior frontal gyrus (p. operc.) | B | Precuneus | 7 | 406 | 5.93 | −2 | −62 | 46 |
| Frontal-Occipital | AUTc + TYP- | R | Precentral gyrus | L | Lingual gyrus | 18 | 198 | 5.83 | −6 | −58 | 8 |
| Parietal-Parieto-occipital | AUTc + TYP- | R | Superior lateral occipital cortex | L | Angular gyrus | 39 | 124 | 4.63 | −50 | −50 | 40 |
| L | Supramarginal gyrus | ||||||||||
| TYP > AUTc | |||||||||||
| Frontal-Parietal | AUTc- TYP+ | L | Supplementary motor area | L | Angular gyrus | 39 | 190 | −5.45 | −56 | −50 | 52 |
| L | Supramarginal gyrus | ||||||||||
| AUTp > TYP | |||||||||||
| Frontal-Occipital | AUTp + TYP- | R | Precentral gyrus | L | Lingual gyrus | 18 | 322 | 8.04 | −8 | −52 | 4 |
| AUTp + TYP- | R | Precentral gyrus | L | Cuneal cortex | 18 | 65 | 4.41 | −8 | −68 | 22 | |
| AUTp + TYP- | R | Precentral gyrus | L | Occipital fusiform gyrus | 37 | 56 | 5.57 | −20 | −44 | −14 | |
| Parietal-Occipital | AUTp + TYP- | R | Supramarginal gyrus | L | Lingual gyrus | 18 | 90 | 6.91 | −6 | −58 | 2 |
| TYP > AUTp | |||||||||||
| Frontal-Parietal | AUTp- TYP+ | L | Supplementary motor area | L | Angular gyrus | 39 | 157 | −4.95 | −50 | −56 | 56 |
| L | Supramarginal gyrus | ||||||||||
| AUTnp > TYP | no significant results | ||||||||||
| TYP > AUTnp | |||||||||||
| Frontal-Parietal | AUTnp- TYP+ | R | Supplementary motor area | R | Supramarginal gyrus | 40 | 235 | −5.67 | 52 | −46 | 38 |
| R | Angular gyrus | ||||||||||
| AUTnp- TYP+ | L | Supplementary motor area | R | Angular gyrus | 39 | 250 | −5.41 | 54 | −58 | 32 | |
| AUTnp- TYP+ | L | Angular gyrus | L | Supplementary motor area | 6 | 157 | −5.80 | −8 | 2 | 50 | |
| Parietal-Parietal | AUTnp- TYP+ | L | Angular gyrus | L | Superior parietal lobule | 7 | 91 | −6.54 | −18 | −46 | 74 |
| AUTnp- TYP+ | L | Angular gyrus | L | Supramarginal gyrus | 40 | 70 | −5.44 | −60 | −26 | 26 | |
| AUTnp- TYP+ | B | Precuneus | B | Precuneus | 7 | 82 | −5.26 | 0 | −68 | 38 | |
| AUTp > AUTnp | |||||||||||
| Frontal-Parietal | AUTp + AUTnp- | L | Inferior frontal gyrus (p. operc.) | R | Superior parietal lobule | 7 | 104 | 5.74 | 24 | −52 | 50 |
| AUTp + AUTnp- | L | Inferior frontal gyrus (p. operc.) | R | Superior parietal lobule | 7 | 51 | 4.89 | 30 | −68 | 58 | |
| AUTp + AUTnp- | R | Supplementary motor area | R | Angular gyrus | 39 | 116 | 5.56 | 42 | −62 | 28 | |
| AUTnp > AUTp | |||||||||||
| Frontal-Frontal | AUTp- AUTnp+ | R | Precentral gyrus | R | Superior frontal gyrus | 6 | 86 | −5.61 | 22 | −10 | 50 |
| R | Precentral gyrus | ||||||||||
| AUTp- AUTnp+ | L | Precentral gyrus | L | Middle frontal gyrus | 6 | 85 | −7.59 | −30 | −2 | 52 | |
| L | Precentral gyrus | ||||||||||
4.2.3. Connectivity pattern for within-group effect underlying mental rotation complexity
In order to explore specific group patterns of connectivity in relation to mental rotation complexity, within-group seed-to-voxels gPPI analyses were performed to identify the key areas that are unique to each group as the angle of mental rotation increases. For the AUTc group, increased task complexity was associated with negative functional connectivity between a frontal seed (right inferior frontal gyrus pars opercularis) and the left inferior lateral occipital cortex and between a parietal seed (left supramarginal gyrus) and the right intracalcarine cortex (see Table 6). Autistic individuals with strengths in visuospatial abilities (AUTp) exhibited complexity-related positive connectivity between frontal and parietal regions (between the right supramarginal gyrus and the left frontal eye fields) and between frontal and occipital regions in the right hemisphere (between the precentral gyrus and the inferior lateral occipital cortex). On the contrary, the AUTnp group showed negative functional connectivity within the occipital cortex (between the right and the left lingual gyrus) as task complexity increased. Finally, the TYP group showed significantly negative functional connectivity within the parietal cortex between the left supramarginal gyrus and the right angular gyrus.
Table 6.
MNI coordinates of brain areas showing within-group functional connectivity underlying mental rotation complexity within the fronto-parieto-occipital network (uncorrected voxel-wise cluster-forming threshold p <.001 and FDR-corrected cluster-level p <.05, k = 50).
| Regions | Effect |
Seed (CONN toolbox) |
Cluster location (CONN toolbox & MNI2TAL) |
BA | k | T value | x | y | z | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUTc | |||||||||||
| Frontal-Occipital | – | R | Inferior frontal gyrus (p. operc.) | L | Inferior lateral occipital cortex | 19 | 75 | −6.45 | −50 | −74 | −2 |
| Parietal-Occipital | – | L | Supramarginal gyrus | R | Intracalcarine cortex | 18 | 282 | −5.07 | 4 | −86 | −4 |
| R | Lingual gyrus | ||||||||||
| AUTp | |||||||||||
| Frontal-Parietal | + | R | Supramarginal gyrus | L | Frontal eye fields | 8 | 55 | 7.45 | −24 | 22 | 46 |
| Frontal-Occipital | + | R | Inferior lateral occipital cortex | R | Precentral gyrus | 6 | 56 | 7.02 | 16 | −20 | 70 |
| AUTnp | |||||||||||
| Occipital-Occipital | – | R | Lingual gyrus | R | Lingual gyrus | 18 | 53 | −7.99 | −2 | −68 | 2 |
| TYP | |||||||||||
| Parietal-Parietal | – | L | Supramarginal gyrus | R | Angular gyrus | 39 | 79 | −5.07 | 54 | −64 | 40 |
4.2.4. Connectivity patterns for between-group effect underlying mental rotation complexity
Between-group seed-to-voxels gPPI analyses were also performed to examine group differences associated with complexity of the mental rotation task. As task complexity increased, the AUTc group showed significant increase in functional connectivity between parietal and occipital regions (between the right superior parietal lobule and the left lingual gyrus) compared to the TYP group (see Table 7). The latter, on the contrary, showed a significant increase in functional connectivity between frontal and parietal areas (between the right precentral gyrus and the left angular gyrus).
Table 7.
MNI coordinates of brain areas showing between-group differences on functional connectivity associated with mental rotation complexity during the task within the fronto-parieto-occipital network (uncorrected voxel-wise cluster-forming threshold p <.001 and FDR-corrected cluster-level p <.05, k = 50).
| Regions | Group effect | Seed(CONN toolbox) | Cluster location (CONN toolbox) | BA | k | T value | x | y | z | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUTc > TYP | |||||||||||
| Parietal-Occipital | AUTc + TYP- | R | Superior parietal lobule | L | Lingual gyrus | 18 | 67 | 5.00 | −12 | −50 | 4 |
| TYP > AUTc | |||||||||||
| Frontal-Parietal | AUTc- TYP+ | R | Precentral gyrus | R | Angular gyrus | 39 | 280 | −6.25 | 46 | −50 | 40 |
| AUTp > TYP | |||||||||||
| Frontal-Parieto-occipital | AUTp + TYP- | R | Superior lateral occipital cortex | L | Superior frontal gyrus | 9 | 115 | 5.07 | −8 | 46 | 34 |
| TYP > AUTp | |||||||||||
| Frontal-Parietal | AUTp- TYP+ | R | Precentral gyrus | R | Angular gyrus | 39 | 169 | −6.52 | 48 | −44 | 38 |
| Parietal-Parietal | AUTp- TYP+ | L | Supramarginal gyrus | R | Angular gyrus | 39 | 126 | −5.61 | 40 | −70 | 40 |
| AUTnp > TYP | |||||||||||
| Parietal-Parietal | AUTnp + TYP- | B | Precuneus | R | Superior parietal lobule | 7 | 84 | 6.14 | 22 | −46 | 58 |
| TYP > AUTnp | |||||||||||
| Parietal-Parietal | AUTnp- TYP+ | R | Superior parietal lobule | R | Supramarginal gyrus | 40 | 259 | −5.35 | 52 | −40 | 40 |
| AUTnp- TYP+ | R | Angular gyrus | R | Superior parietal lobule | 7 | 123 | −5.54 | 18 | −52 | 62 | |
| Occipital-Occipital | AUTnp- TYP+ | L | Cuneal cortex | L | Lingual gyrus | 18 | 92 | −5.82 | −14 | −76 | −8 |
| AUTp > AUTnp | |||||||||||
| Parietal-Parietal | AUTp + AUTnp- | L | Supramarginal gyrus | L | Superior parietal lobule | 7 | 93 | 8.09 | −24 | −44 | 74 |
| AUTp + AUTnp- | L | Supramarginal gyrus | R | Supramarginal gyrus | 40 | 76 | 5.13 | 46 | −40 | 58 | |
| AUTp + AUTnp- | L | Superior parietal lobule | L | Angular gyrus | 39 | 147 | 6.81 | −50 | −50 | 34 | |
| AUTp + AUTnp- | R | Supramarginal gyrus | R | Superior parietal lobule | 7 | 77 | 5.92 | 16 | −58 | 68 | |
| Occipital-Occipital | AUTp + AUTnp- | R | Inferior lateral occipital cortex | B | Lingual gyrus | 18 | 130 | 7.34 | 4 | −82 | −11 |
| AUTnp > AUTp | |||||||||||
| Frontal-Parietal | AUTp- AUTnp+ | R | Supplementary motor area | B | Precuneus | 7 | 143 | −5.84 | −6 | −74 | 38 |
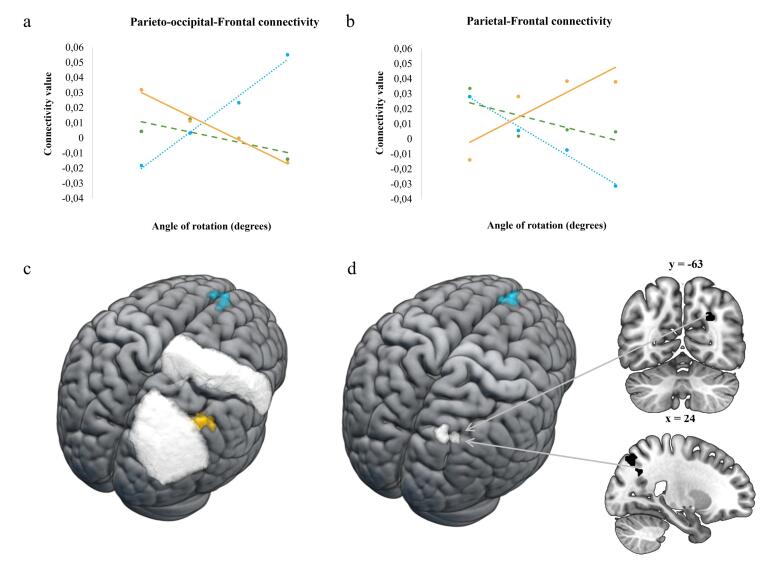
Functional connectivity increased significantly more between frontal and posterior parieto-occipital regions (between the right superior lateral occipital cortex and the left superior frontal gyrus) in the AUTp group compared to the TYP group as the complexity of the task increased (see Fig. 8). The TYP group showed a significant increase in functional connectivity (as rotation complexity increased) between frontal and parietal regions (between the right precentral gyrus and the right angular gyrus; see Fig. 8) and within the parietal cortex (between the left supramarginal gyrus and the right angular gyrus), relative to the AUTp group.
Fig. 8.
Results of between-group effects in functional connectivity changes in association with increasing mental rotation complexity (angles of rotation) are shown for AUTp (blue), AUTnp (green) and TYP (yellow). Regions in white are ROIs corresponding to (c) the right superior lateral occipital and the right precentral regions from the CONN atlas and (d) the parieto-occipital cluster (k = 56) extracted from Table 2 results. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Compared to the TYP group, the AUTnp group showed significant increase in functional connectivity within the parietal cortex (between the bilateral precuneus and the right superior parietal lobule), whereas the TYP group showed a higher increase in functional connectivity within the right parietal lobe (between the superior parietal lobule and the supramarginal gyrus/angular gyrus) and within the left occipital cortex (between the cuneal cortex and the lingual gyrus) (see Table 7).
Finally, comparing autistic subgroups, the AUTp showed significantly more functional connectivity than AUTnp as task complexity increased within the parietal cortex (between the left supramarginal gyrus and the left superior parietal lobule, between the left supramarginal gyrus and the right supramarginal gyrus, between the left superior parietal lobule and the left angular gyrus and between the right supramarginal gyrus and the right superior parietal lobule) and within the occipital lobe (between the right inferior lateral occipital cortex and the lingual gyrus bilaterally) (see Table 7). On the contrary, the AUTnp group showed significantly more functional connectivity than the AUTp group between the frontal and the parietal cortex (between the right supplementary motor area and the precuneus bilateral) as mental rotation complexity increased.
In order to better understand the group differences in functional connectivity patterns with increasing mental rotation complexity, we extracted ROI-to-ROI connectivity values for each participant for the two main pairs of ROIs where connectivity differed between AUTp and TYP. The first ROI pair (parieto-occipital-frontal) was from the AUTp > TYP results (right superior lateral occipital cortex and left superior frontal gyrus) and the second pair of ROIs (parieto-frontal) was from the TYP > AUTp results (right precentral gyrus and right angular gyrus) (see Table 7). As can be observed in Fig. 8.a and 8.b for the AUTp group, as the level of task complexity increased, the parieto-occipital-frontal connectivity increased but the parieto-frontal connectivity decreased, and the opposite pattern was observed in typically developed individuals. In the AUTnp group, smaller variations of connectivity between those two pairs of ROIs were observed. Furthermore, as previously mentioned, given the large size of the superior lateral occipital cortex (sLOC) ROI implemented in CONN (see Fig. 8.c) that extends over both the occipital and the parietal lobes, we further investigated the connectivity patterns involving this region by using smaller ROIs. These smaller ROIs were the clusters extracted from between-group differences (AUTp > TYP; see the two clusters with a * in Table 2) that were identified as part of this larger right sLOC ROI. Only the right parieto-occipital cluster (k = 56, peak: x = 27, y = −61, z = 29) showed significant between-group differences in connectivity (AUTp > TYP) with the superior frontal gyrus (k = 108, T = 5.11, x = −6, y = 52, z = 28), a cluster overlapping with the one observed in the previous analysis (see Fig. 8.d). This suggests that within the large sLOC ROI, a specific region near the parieto-occipital junction was driving the observed connectivity finding.
5. Aim 3: Associations between visuospatial performance, brain activation and functional connectivity
5.1. Analyses
To better understand the association between visuospatial abilities and brain functioning, we first investigated the associations between brain activation within the fronto-parieto-occipital network identified earlier and behavioural performances (BD peak amplitude and mean response time at the mental rotation task) for AUTc, AUTp, AUTnp and TYP groups. As only mean response time was associated with brain activation in the AUTc group, we increased the voxel-wise cluster-forming threshold from p <.001 to p <.005 in autistic subgroups (AUTp and AUTnp) to better understand the relationship found within the AUTc group. This exploratory analysis aimed at determining if only one or the two subgroups differentially or equally contributed to the findings revealed in the AUTc group. Then, from the regions being associated with behavioural performances, we further examined the associations with task-related functional connectivity.
More specifically, regressions were further conducted between functional connectivity and mean response time in the AUTp group to clarify the association found between brain activation and behavioural performance at the task. The two ROIs selected as seeds for this regression were the two clusters where the brain activation showed significant association with mean response time in the AUTp group (left inferior occipital, k = 153 and left angular gyrus, k = 100, see Table 8).
Table 8.
MNI coordinates of brain areas showing significant associations between BOLD response related to mental rotation processes and faster mean response time within the fronto-parieto-occipital network (uncorrected voxel-wise cluster-forming threshold p <.001 and FDR-corrected cluster-level p <.05 FDR-corrected, k = 50).
| Region |
Location (Anatomy toolbox & MNI2TAL) |
BA | k | T value | x | y | z | ||
|---|---|---|---|---|---|---|---|---|---|
| AUTc | Occipital | L | Inferior occipital gyrus | 19/37 | 126 | 6.82 | –33 | −67 | −19 |
| −48 | −76 | −4 | |||||||
| −51 | −67 | −4 | |||||||
| Fusiform gyrus (occipito-temporal) | −45 | −67 | −19 | ||||||
| −45 | −55 | −10 | |||||||
| L | Middle occipital gyrus | 19/39 | 55 | 6.19 | −27 | −85 | 35 | ||
| Parietal | L | Angular gyrus | −30 | −79 | 29 | ||||
| TYP | no significant result | ||||||||
| Exploration with higher voxel-level threshold (uncorrected voxel-wise cluster-forming threshold p <.005 and FDR-corrected cluster-level p <.05) | |||||||||
| AUTp | Occipital | L | Inferior occipital gyrus | 18/19/37 | 153 | 15.60 | −48 | −76 | −4 |
| L | Fusiform gyrus | −45 | −69 | −19 | |||||
| L | Lingual gyrus | −36 | −88 | −13 | |||||
| L | Middle occipital gyrus | −48 | −67 | −1 | |||||
| −39 | −88 | −4 | |||||||
| −57 | −67 | 2 | |||||||
| −48 | −64 | −16 | |||||||
| −42 | −85 | 2 | |||||||
| Parietal | L | Angular gyrus | 19/7/39 | 100 | 9.80 | −30 | −76 | 32 | |
| –33 | −73 | 29 | |||||||
| L | Inferior parietal lobule | −27 | −73 | 41 | |||||
| Occipital | L | Middle occipital gyrus | −27 | −85 | 35 | ||||
| −30 | −88 | 32 | |||||||
| AUTnp | no significant result | ||||||||
5.2. Results
No significant associations between brain activation and BD peak amplitude were found for all groups (AUTc, AUTp, AUTnp and TYP). However, faster mean response time at the mental rotation task was associated with increasing brain activation in the left hemisphere in occipital/temporal (fusiform gyrus) and parietal regions in the AUTc group (see Table 8), while no regional activation was significantly related to mean response time in TYP participants. No significant association was found in each autistic subgroup (AUTp and AUTnp). In order to determine whether the associations found in the AUTc group were mainly driven by one autistic subgroup or the two, we further conducted an exploratory analysis with higher uncorrected cluster-forming threshold (p <.005). Interestingly, we found that the association obtained in the AUTc group was entirely explained by the AUTp group (see Table 8 and Fig. 9). No region was found to be associated with faster mean response time in the AUTnp even with this more liberal threshold.
Fig. 9.
Results of regressions between BOLD signal and faster mean response time and between functional connectivity and faster mean response time for the left middle occipital cluster as a seed are shown for the AUTp group (blue) (uncorrected voxel-wise cluster-forming threshold p <.001 and FDR-corrected cluster-level p <.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Regressions between mean response time and functional connectivity from these same occipital and parietal regions revealed that faster mean response time was associated with a significant increase in functional connectivity between the left middle occipital gyrus and the superior frontal gyrus, the right posterior cingulate and the left occipital pole in autistic participants with enhanced visuospatial abilities (AUTp) (see Table 9 and Fig. 9).
Table 9.
MNI coordinates of brain areas showing significant associations between positive functional connectivity related to mental rotation processes and faster mean response time for the AUTp group for the four regions of interest located in occipital and parietal regions (uncorrected voxel-wise cluster-forming threshold p <.001 and FDR-corrected cluster-level p <.05, k = 50).
| Regions |
Seed (CONN toolbox) |
Cluster location (CONN toolbox & MNI2TAL) |
BA | k | T value | x | y | z | ||
|---|---|---|---|---|---|---|---|---|---|---|
| AUTp | ||||||||||
| Frontal-Parieto-occipital | L | Middle occipital gyrus | B | Superior frontal gyrus | 9 | 79 | −8.15 | 0 | 50 | 38 |
| R | Posterior cingulate | 31 | 44 | −6.64 | 4 | −52 | 32 | |||
| L | Occipital pole | 18 | 49 | −6.00 | −18 | −106 | −8 |
|||
6. Discussion
The present study aimed to identify the neural networks involved in mental rotation processes in subgroups of autistic adults with and without visuospatial strengths (i.e., with a Block Design (BD) peak) compared to a sample of typically developed adults. We used fMRI to examine task-related brain activation and functional connectivity during a mental rotation task, as well as their modulation by task performance and increasing levels of mental rotation complexity.
6.1. Behavioural performance
While the three groups showed similar accuracy when solving mental rotation problems, our findings revealed that autistic participants displayed superior performance (measured as faster mean response times) compared to their neurotypical counterparts. When dividing autistic participants into subgroups based on their visuospatial abilities (though all groups had equivalent performance IQs), only those with a strength on the BD task (AUTp) were found to be significantly faster than typically developed participants (TYP). This finding represents behavioural evidence of more efficient visuospatial processing in this subgroup. Moreover, faster mean response times on the mental rotation task were associated with increased accuracy in these AUTp participants. This relationship was also observed for TYP participants but not for autistic individuals without superior visuospatial abilities (AUTnp).
6.2. Patterns of brain activity during mental rotation
In general, the fMRI task revealed enhanced activity in posterior occipital and parietal regions, as well as in some frontal areas of the mental rotation network in AUTp and AUTnp compared to TYP individuals. These results oppose findings from earlier neuroimaging studies that have shown similar or decreased brain activation in occipital regions, along with decreased frontal involvement in autistic participants (not selected based on their visuospatial abilities) while performing a mental rotation task (McGrath et al., 2012, Silk et al., 2006). However, the increased frontal activation we observed in the AUTp subgroup was in an area associated with motor control (precentral gyrus), rather than with an area used for higher-order cognitive functions (attention/executive control). On the other hand, the AUTp subgroup had decreased activation in more anterior frontal regions (e.g., a region involved with attentional control: BA8) compared to the TYP group. In an attempt to understand the crucial differences between the two autistic subgroups and TYP individuals, we discovered an interesting pattern of lateralization of neural recruitment. Compared to the TYP group, the observed brain activation within the network in the AUTp subgroup was more bilateral (mainly in occipito-parietal areas), while increased parieto-occipital activation was restricted to the right hemisphere in the AUTnp subgroup. Interestingly, a direct comparison of the two AUT subgroups revealed significant differences in brain activation. This suggests that lateralization of activation varied across the two different cognitive profiles. Indeed, the task-related brain activation was more left-lateralized in the inferior parietal lobule and more posterior (increased bilateral occipital activation) for autistic participants with a BD peak. For autistic participants in the AUTnp subgroup, we observed restricted activation patterns within the right hemisphere and more largely distributed activity across parietal and frontal areas. Further investigating into the modulating role of task complexity (i.e., degrees of rotation) on the mental rotation network revealed that increasing task complexity was mirrored by increased brain activation in the left parietal cortex in the AUTp subgroup. Conversely, complexity modulated brain activation in a larger bilateral network of cortical, subcortical, and cerebellar (only in TYP) regions in AUTnp and TYP individuals. Also, higher activation in left occipital regions was correlated with faster response times on the mental rotation task in AUTp individuals only. Taken together, these results suggest that individuals in the AUTp subgroup displayed a more efficient and specialized brain network underlying mental rotation processes (involving the recruitment of occipital and parietal regions).
Mental rotation processes are known to elicit brain activation mainly in bilateral areas of the occipital, parietal, and frontal networks (Cona and Scarpazza, 2019, Tomasino and Gremese, 2016, Zacks, 2008). In addition, these processes normally engender a slight right hemispheric dominance in the parietal cortex (Harris and Miniussi, 2003, Tomasino and Gremese, 2016, Zacks, 2008), notably for geometric figures (Tomasino and Gremese, 2016). Here we found that the AUTp subgroup showed increased bilateral activation during the mental rotation task compared to the TYP group. Our findings align with those in the literature, such that O'Boyle et al. (2005) found that mathematically gifted individuals showed more bilateral activation of the frontal and parietal areas compared to individuals with average mathematical abilities. This suggests a more integrated interhemispheric network underlying mental rotation processes in the AUTp subgroup compared to participants with typical development. Moreover, several studies have observed a reduced or atypical lateralization in autism for many domains of cognition such as language, executive control, attention, working memory, and different networks (visual, auditory, motor, and default mode networks; Cardinale et al., 2013, Floris et al., 2016, Koshino et al., 2005, Lindell and Hudry, 2013, Nielsen et al., 2014, Philip et al., 2012, Samson et al., 2012). In analyzing a large data set including >800 autistic individuals, Floris et al. (2021) recently reported a more pronounced leftward lateralization in the visuospatial network in autism. In our study, we found that autistic participants with enhanced visuospatial abilities presented significant correlations between enhanced recruitment of left posterior (occipital and parietal) regions and faster mean response times. In addition, these individuals had greater left parietal activation compared to autistic individuals with no BD peak. Taken together, this suggests that neural activation of left posterior regions specifically may contribute to the enhanced visuospatial abilities in autism.
6.3. Importance of the intraparietal sulcus
Increased parietal activation in the areas surrounding the intraparietal sulcus (IPS) found in both autistic subgroups is consistent with the literature that has shown highly robust activation of this region in visuospatial processing involved in mental rotation (Jordan et al., 2001, Papadopoulos et al., 2018, Zacks, 2008). Of note, the IPS is important for spatial processing (e.g., object-based visuospatial transformations). Indeed, many studies have reported that the increased complexity of mental rotation tasks is accompanied by increased activity in the IPS (Papadopoulos et al., 2018, Zacks, 2008). In general, it is interesting to note that autistic individuals in our study showed greater activity in the IPS compared to TYP individuals. Compared to TYP individuals, this difference was observed in both hemispheres for the AUTp group and restricted to the right hemisphere for the AUTnp group. Again, this speaks to the aforementioned differential patterns of lateralization. Between the two autistic subgroups, individuals with a BD performance peak presented enhanced activation of the left IPS compared to those with no BD peak. No group differences were observed in the IPS as task complexity increased. However, increasing the significance threshold (p <.005 uncorrected) revealed group differences in the IPS, with the AUTp group displaying increased bilateral activation compared to the AUTnp group and increased left activation compared to the TYP group. Given that the IPS is known to play a crucial role in object-based visuospatial transformation, our results regarding the differential patterns of lateralization and these subthreshold findings in the IPS strongly suggest that this specialized visuospatial area may play a crucial role in the enhanced visuospatial abilities in autism.
6.4. Connectivity within the mental rotation network
Our study revealed three main findings regarding task-related functional connectivity. First, when examining within-group patterns, we observed greater overall connectivity within the occipito-parieto-frontal mental rotation network in AUTp compared to other groups (AUTnp and TYP), with a marked synchronization between and within occipital and parietal regions while solving mental rotation problems. Moreover, when investigating group differences during mental rotation processes, a significant increase in functional connectivity within bilateral posterior regions involved in visual perception was found for the AUTp group compared to the TYP group. The opposite was found for the AUTnp group such that these individuals displayed reduced intraparietal connectivity on the left side compared to the TYP group. Also, with increasing task complexity (i.e., angle of mental rotation), autistics with enhanced visuospatial abilities had significantly stronger synchronization of brain activation within parietal and occipital regions compared to AUTnp participants. Lastly, better performance at the task was associated with increased connectivity within the occipital lobe and between occipital areas and posterior cingulate cortex. Thus, consistent with findings on brain activation, increased synchronization of activity in occipital and parietal regions underlying mental rotation processes were associated with superior visuospatial abilities in autism (Samson et al., 2012).
Second, the occipital cortex and its functional synchronization with frontal areas seem to play a particularly important role in supporting mental rotation processes and enhanced visuospatial functioning in autism. As task complexity increased, AUTp individuals had positive connectivity between the right precentral gyrus and inferior occipital areas, as well as increased connectivity between the superior frontal gyrus (SFG) and posterior occipito-parietal areas compared to TYP individuals. In other words, as the AUTp participants performed increasing magnitudes of mental rotation, their posterior occipito-parietal regions became increasingly synchronized with the SFG compared to that of TYP individuals. This was not observed in the AUTnp group. These latter findings mirror those found by McGrath et al. (2012) who observed decreased fronto-occipital connectivity in autistics (not selected based on their visuospatial abilities). In contrast to the AUTp participants, the TYP group showed patterns of decreased connectivity between SFG and occipital regions as task complexity increased. In the AUTnp group, we found no relationship between task complexity and connectivity between these regions (within- and between-group results). Moreover, enhanced integration of activation between the SFG and the occipital lobe was correlated with task performance in the AUTp group. The SFG resides in the dorsolateral prefrontal cortex (dlPFC), an area known to be involved in attentional and executive control (Jones and Graff-Radford, 2021). In addition, the SFG is involved in monitoring information in working memory (Petrides, 2005), notably spatial working memory (Courtney et al., 1998, Haxby et al., 2000). Greater long-range connectivity between these frontal and occipital areas in the AUTp group suggests a better synchronization of the network involved in mental rotation top-down processes in autistic individuals with superior visuospatial abilities.
Finally, our functional connectivity results revealed group differences in the synchronization of fronto-parietal brain activation (mainly between the supplementary motor area/precentral gyrus and inferior parietal lobule). Consistent with the literature, the TYP group showed greater fronto-parietal connectivity while solving mental rotation problems than both autistic subgroups (Damarla et al., 2010, Just et al., 2004, Just et al., 2007, McGrath et al., 2012, O’Reilly et al., 2017). On the other hand, autistics with enhanced visuospatial abilities presented a significant decrease in fronto-parietal connectivity as task complexity increased compared to AUTnp and TYP participants. In sum, these results suggest that the synchronization of activation between frontal regions (SMA and precentral gyrus) and the inferior parietal lobule seems to play a less crucial role in mental rotation processes, and perhaps even in superior visuospatial abilities, in autism.
The second and third main findings revealed inverse patterns of long-range functional connectivity associated with task complexity across AUTp and TYP groups. For the AUTp group, we found increased (decreased in TYP) fronto-parieto-occipital and decreased (increased in TYP) fronto-parietal connectivity (between motor areas and the angular region). These results are consistent with the findings by Simard et al. (2015). Using a fluid reasoning task, they showed higher fronto-occipital and lesser fronto-parietal modulation of activation as task complexity increased in autistic individuals. Although long-range under-connectivity between visual associative areas and other parts of the brain has been well documented in autism research (Hong et al., 2019, O’Reilly et al., 2017, Picci et al., 2016, Rane et al., 2015), some authors have noted that this pattern of brain connectivity may be modulated by task requirements and cognitive processes (O’Reilly et al., 2017, Sharda et al., 2015). This statement is supported by our findings and those in the literature. For instance, studies examining cognitive strengths in autism, such as visual search (Keehn et al., 2013) and fluid reasoning (Simard et al., 2015), have also observed increased connectivity between the occipital cortex and multiple frontal regions in autistic individuals compared to neurotypicals. These reports contradict previous research that found under-connectivity between fronto-posterior areas (Belmonte et al., 2004, Just et al., 2004). However, most studies documenting under-connectivity between regions focused on impairment domains in autism or resting-state connectivity. As previously mentioned, group differences between autistic subgroups have been documented in our study and others using different methodologies and modalities (auditory/visual/motor; Barbeau et al., 2020, Duret et al., 2018, Samson et al., 2015). Thus, in addition to the importance of including well-defined autistic groups with varying levels of cognitive abilities, our results highlight the importance of task type and its consideration when interpreting results (Chung and Son, 2020). Moreover, as a function of the specific cognitive processes that are required, under- and over-connectivity in autism can be observed in similar brain areas. Thus, our findings also emphasize that connectivity models or the directionality of findings (under- versus over-connectivity) cannot be generalized to specific brain areas.
6.5. Neural basis of mental rotation processes in autistics without a BD peak
A different pattern of brain functioning underlying mental rotation processes was found in the AUTnp group compared to the other two groups. Specifically, in the AUTnp group, we found increased right parietal activation and connectivity, less connectivity between multiple parietal and occipital regions, and a greater reliance on the precuneus compared to TYP and AUTp groups. The precuneus is typically involved in higher-order cognitive functions such as maintaining and updating visuospatial information in working memory, integration of perception information from the environment (gestalt), and visuospatial mental imagery strategies (Al-Ramadhani et al., 2021, Cavanna and Trimble, 2006, Müller et al., 2018, Owen et al., 2005, Yeh et al., 2007). Compared to the TYP group, this region was increasingly recruited as task complexity became more challenging in AUTnp individuals. Interestingly, better visuospatial skills are correlated with lower activation of the precuneus during reasoning tasks in neurotypicals (Ruff et al., 2003). Enhanced perceptual functioning has also been associated with lower recruitment of the precuneus in autistics during reasoning (Soulières et al., 2009). Moreover, with increasing mental rotation complexity, the AUTnp group had increased right intraparietal connectivity involving the precuneus compared to typically developed individuals, as well as increased connectivity between the right precuneus and frontal cortex compared to the AUTp group. In addition, the AUTnp group showed increased connectivity within frontal regions during mental rotation processes compared to AUTp. Furthermore, compared to AUTp and TYP groups, AUTnp individuals expressed largely reduced connectivity between many other posterior regions involving the superior and inferior (angular and supramarginal gyri) parietal lobule, lingual gyrus, cuneal cortex, and right inferior lateral occipital cortex. They also showed negative connectivity within the occipital lobe (lingual gyrus) as task complexity increased. These atypical patterns of task-related brain activation (more right-lateralized) and functional connectivity (decreased between posterior regions) in the AUTnp group suggest a less efficient, less inter-connected, and less specialized mental rotation network in comparison to the other two groups (AUTp and TYP).
6.6. Enhanced perceptual functioning in autism
Here, we showed that enhanced functional resource allocation in more posterior visuoperceptual and visuospatial regions specific to the autism subtype with superior visuospatial abilities reflects hyper-specialization of visuospatial processes. This aligns with the Enhanced Perceptual Functioning Model (Mottron et al., 2006) which predicts superior perceptual performance and stronger engagement of visuospatial processes in autistic cognition. This model has been further supported by neuroimaging findings showing that enhanced perception in autistic individuals was associated with an overall stronger reliance on posterior regions related to visual processing for tasks involving different types of stimuli and levels of complexity (Samson et al., 2012, Simard et al., 2015). Our findings directly contribute to this model by showing that enhanced functioning of visuoperceptual areas applies more particularly to a subgroup of autistic individuals who present cognitive strengths in these domains. Our results specific to this subgroup are also consistent with studies showing a stronger involvement of perceptual processes in more complex cognitive tasks in autism such as matrix reasoning, working memory, and mental rotation. Indeed, these studies have found a heavier reliance on occipital and posterior parietal regions in autistic individuals compared to neurotypicals (Koshino et al., 2005, Soulières et al., 2009, Simard et al., 2015). However, although the model suggests that increased independence of these posterior regions is less influenced by top-down processes, our study has shown that peaks in visuospatial cognitive abilities may be related to greater fronto-posterior synchronization of activation during mental rotation processes.
6.7. Heterogeneity in autism and the importance of improved group characterization
As we observed clear differences in brain functioning in the visuospatial network between different autistic cognitive profiles, this is convincing evidence of the importance of adequate characterization of autistic individuals in research. Autism is characterized by a substantial phenotypical and biological heterogeneity (Feczko et al., 2018, Happé et al., 2006, Lenroot and Yeung, 2013, Lombardo et al., 2019). Many studies have failed to account for this heterogeneity by pooling together all individuals on the autistic spectrum and thus, potentially diluting the intensity of the observed effects (Hong et al., 2022, Lombardo et al., 2019, Rødgaard et al., 2019). In consequence, these methodological choices prevent us from having a better understanding of how the autistic brain works and may be one of the contributing factors to the mixed and inconsistent results frequently seen in behavioural and neuroimaging studies. Thereby, the present study supports a large body of literature arguing for the distinction between different etiological and phenotypical subtypes in autism research (Feczko et al., 2018, Floris et al., 2021, Hong et al., 2020, Lombardo et al., 2019). Beyond solely studying the impairments associated with the condition, this study also highlights the importance of studying the strengths of autistic individuals. Doing so may generate novel findings and contribute to a better understanding of how the autistic brain functions.
6.8. Limitations and future directions
The current study had a few limitations. First, our autistic sample was composed of men who presented initial language delays and with normal ranging levels of intelligence. Hence, our results may not apply to individuals who fall elsewhere on the autism intelligence continuum, notably those with intellectual disabilities and enhanced perceptual functioning (Wilkinson and McIlvane, 2013). Further, our results may not be representative of the brain functioning of autistic women with a BD peak (Meilleur et al., 2015). Finally, as differences in functional connectivity patterns have been observed throughout development, our results may not be generalizable to autistic children (O’Reilly et al., 2017, Picci et al., 2016, Uddin et al., 2013, Wiggins et al., 2011). Future neuroimaging studies in autistic children targeting the brain development of enhanced visuospatial abilities would be necessary to examine when and how the differences observed in our study emerge in a pediatric population. As gender differences in the behavioural and brain functioning underlying mental rotation processes have been documented in the general population (Maeda and Yoon, 2013, Semrud-Clikeman et al., 2012), including autistic women in our study would have required a minimum of two additional groups of women (autistics and non-autistics). Future larger studies exploring gender effects related to superior visuospatial abilities would be important to have a more complete understanding of these abilities in autism. In addition, our sample sizes were relatively small. However, the use of meaningful cognitive markers to build more homogenous autistic subgroups helped to increase the specificity of our findings. Finally, we noted a co-occurrence of attention deficit hyperactivity disorder (ADHD) in a certain number of autistic individuals included in this study. Although our autistic subgroups were equivalent in the incidence of ADHD, this may have influenced the brain responsiveness and behavioural performances of these subgroups.
6.9. Conclusion
Different neuroimaging correlates of brain activation and functional connectivity were observed in autistic individuals with distinct cognitive profiles. A BD peak on the Wechsler’s intelligence scales was associated with faster response times, enhanced recruitment of posterior visuospatial regions, and a reverse pattern of long-range functional connectivity between frontal and occipital/parietal areas. Autistics with no BD peak showed less efficient and inter-connected recruitment of the mental rotation network. In sum, beyond solely investigating the impairments associated with autism, this study highlights the importance of studying the strengths of autistic individuals. In addition, it emphasizes the relevance of studying different autism subgroups by creating well-defined distinct cognitive profiles. If these methodological suggestions are integrated into future research, this may contribute to the discovery of novel findings, a better understanding of underlying brain mechanisms and organization, and ultimately, the development of more effective support services for autistic individuals.
CRediT authorship contribution statement
Véronique D. Thérien: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing - original draft. Janie Degré-Pelletier: . Elise B. Barbeau: . Fabienne Samson: . Isabelle Soulières: Conceptualization, Supervision, Funding acquisition, Methodology, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank all participants for their time and commitment to this project. We also thank Éliane Danis for her help with data collection and Rebecca Cernik for her help with the linguistic revision and editing of the manuscript. This work was supported by grants from the Brain and Behavior Research Foundation (grant numbers 19633) and the Canadian Institutes of Health Research (grant numbers PJT-178310) to I.S., as well as a graduate award from Quebec Autism Research Training Program (QART) to V.D.T. In addition, I.S. also wishes to acknowledge the Fonds de recherche du Quebec – Santé (Quebec Health Research Fund) for their support via a career award.
Data availability
Data will be made available on request.
References
- Al-Ramadhani R.R., Shivamurthy V.K.N., Elkins K., Gedela S., Pedersen N.P., Kheder A. The precuneal cortex: anatomy and seizure semiology. Epileptic Disorders. 2021;23(2):218–227. doi: 10.1684/epd.2021.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . 4th-Revised ed. American Psychiatric Association; Washington: 2000. Diagnostic and statistical manual of mental disorders DSM-IV-TR. [Google Scholar]
- Asarnow R.F., Tanguay P.E., Bott L., Freeman B.J. Patterns of intellectual functioning in non-retarded autistic and schizophrenic children. J. Child Psychol. Psychiatry. 1987;28(2):273–280. doi: 10.1111/j.1469-7610.1987.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Audras-Torrent L., Miniarikova E., Couty F., Dellapiazza F., Berard M., Michelon C., Baghdadli A. WISC-V profiles and their correlates in children with autism spectrum disorder without intellectual developmental disorder: report from the ELENA cohort. Autism Res. 2020 doi: 10.1002/aur.2444. [DOI] [PubMed] [Google Scholar]
- Barbeau E.B., Soulières I., Dawson M., Zeffiro T.A., Mottron L. The level and nature of autistic intelligence III: Inspection time. J. Abnorm. Psychol. 2013;122(1):295. doi: 10.1037/a0029984. [DOI] [PubMed] [Google Scholar]
- Barbeau E.B., Klein D., Soulières I., Petrides M., Bernhardt B., Mottron L. Age of Speech Onset in AutismRelates to structural connectivity in the language network. Cerebral Cortex Commun. 2020;1(1):tgaa077. doi: 10.1093/texcom/tgaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacher F.D., Radulescu E., Minati L., Baron-Cohen S., Lombardo M.V., Lai M.C., Critchley H.D. Sex differences and autism: brain function during verbal fluency and mental rotation. PLoS ONE. 2012;7(6):e38355. doi: 10.1371/journal.pone.0038355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte M.K., Allen G., Beckel-Mitchener A., Boulanger L.M., Carper R.A., et al. Autism and abnormal development of brain connectivity. J. Neurosci. 2004;24(42):9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale R.C., Shih P., Fishman I., Ford L.M., Müller R.A. Pervasive rightward asymmetry shifts of functional networks in autism spectrum disorder. JAMA Psychiatry. 2013;70(9):975–982. doi: 10.1001/jamapsychiatry.2013.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron M.J., Mottron L., Berthiaume C., Dawson M. Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain. 2006;129(7):1789–1802. doi: 10.1093/brain/awl072. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cherkassky V.L., Kana R.K., Keller T.A., Just M.A. Functional connectivity in a baseline resting-state network in autism. NeuroReport. 2006;17(16):1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Chien H.Y., Lin H.Y., Lai M.C., Gau S.S.F., Tseng W.Y.I. Hyperconnectivity of the right posterior temporo-parietal junction predicts social difficulties in boys with autism spectrum disorder. Autism Res. 2015;8(4):427–441. doi: 10.1002/aur.1457. [DOI] [PubMed] [Google Scholar]
- Chung S., Son J.W. Visual perception in autism spectrum disorder: A review of neuroimaging studies. J. Korean Acad. Child Adolescent Psychiatry. 2020;31(3):105. doi: 10.5765/jkacap.200018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona G., Scarpazza C. Where is the “where” in the brain? A meta-analysis of neuroimaging studies on spatial cognition. Hum. Brain Mapp. 2019;40(6):1867–1886. doi: 10.1002/hbm.24496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable P.A., Bailey K., Beck A., Borrello D., Kozman M., Schneider K. Effect size of search superiority in autism spectrum disorder. Clin. Exp. Optometry. 2020;103(3):296–306. doi: 10.1111/cxo.12940. [DOI] [PubMed] [Google Scholar]
- Cook J.L., Barbalat G., Blakemore S.J. Top-down modulation of the perception of other people in schizophrenia and autism. Front. Hum. Neurosci. 2012;6:175. doi: 10.3389/fnhum.2012.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E., Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr. Opin. Neurobiol. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Courtney S.M., Petit L., Maisog J.M., Ungerleider L.G., Haxby J.V. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279(5355):1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Crippa A., Del Vecchio G., Busti Ceccarelli S., Nobile M., Arrigoni F., Brambilla P. Cortico-cerebellar connectivity in autism spectrum disorder: what do we know so far? Front. Psychiatry. 2016;7:20. doi: 10.3389/fpsyt.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damarla S.R., Keller T.A., Kana R.K., Cherkassky V.L., Williams D.L., Minshew N.J., Just M.A. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism Res. 2010;3(5):273–279. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M., Soulières I., Ann Gernsbacher M., Mottron L. The level and nature of autistic intelligence. Psychol. Sci. 2007;18(8):657–662. doi: 10.1111/j.1467-9280.2007.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy F.H., Shankardass A., McAnulty G.B., Als H. The relationship of Asperger’s syndrome to autism: a preliminary EEG coherence study. BMC Med. 2013;11(1):1–13. doi: 10.1186/1741-7015-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duret P., Samson F., Pinsard B., Barbeau E.B., Boré A., Soulières I., Mottron L. Gyrification changes are related to cognitive strengths in autism. NeuroImage: Clinical. 2018;20:415–423. doi: 10.1016/j.nicl.2018.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgin J.O., Pennington B.F. Spatial cognition in autism spectrum disorders: Superior, impaired, or just intact? J. Autism Dev. Disord. 2005;35(6):729–745. doi: 10.1007/s10803-005-0020-y. [DOI] [PubMed] [Google Scholar]
- Falter C.M., Plaisted K.C., Davis G. Visuo-spatial processing in autism—testing the predictions of extreme male brain theory. J. Autism Dev. Disord. 2008;38(3):507–515. doi: 10.1007/s10803-007-0419-8. [DOI] [PubMed] [Google Scholar]
- Feczko E., Balba N.M., Miranda-Dominguez O., Cordova M., Karalunas S.L., Irwin L., Demeter D.V., Hill A.P., Langhorst B.H., Grieser Painter J., Van Santen J., Fombonne E.J., Nigg J.T., Fair D.A. Subtyping cognitive profiles in autism spectrum disorder using a functional random forest algorithm. Neuroimage. 2018;172:674–688. doi: 10.1016/j.neuroimage.2017.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris D.L., Lai M.-C., Auer T., Lombardo M.V., Ecker C., Chakrabarti B., Wheelwright S.J., Bullmore E.T., Murphy D.G.M., Baron‐Cohen S., Suckling J. Atypically rightward cerebral asymmetry in male adults with autism stratifies individuals with and without language delay. Hum. Brain Mapp. 2016;37(1):230–253. doi: 10.1002/hbm.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris D.L., Wolfers T., Zabihi M., Holz N.E., Zwiers M.P., Charman T., Tillmann J., Ecker C., Dell’Acqua F., Banaschewski T., Moessnang C., Baron-Cohen S., Holt R., Durston S., Loth E., Murphy D.G.M., Marquand A., Buitelaar J.K., Beckmann C.F., Ahmad J., Ambrosino S., Auyeung B., Banaschewski T., Baron-Cohen S., Baumeister S., Beckmann C.F., Bölte S., Bourgeron T., Bours C., Brammer M., Brandeis D., Brogna C., de Bruijn Y., Buitelaar J.K., Chakrabarti B., Charman T., Cornelissen I., Crawley D., Dell’Acqua F., Dumas G., Durston S., Ecker C., Faulkner J., Frouin V., Garcés P., Goyard D., Ham L., Hayward H., Hipp J., Holt R., Johnson M.H., Jones E.J.H., Kundu P., Lai M.-C., Liogier d’Ardhuy X., Lombardo M.V., Loth E., Lythgoe D.J., Mandl R., Marquand A., Mason L., Mennes M., Meyer-Lindenberg A., Moessnang C., Mueller N., Murphy D.G.M., Oakley B., O’Dwyer L., Oldehinkel M., Oranje B., Pandina G., Persico A.M., Ruggeri B., Ruigrok A., Sabet J., Sacco R., San José Cáceres A., Simonoff E., Spooren W., Tillmann J., Toro R., Tost H., Waldman J., Williams S.C.R., Wooldridge C., Zwiers M.P. Atypical brain asymmetry in autism—a candidate for clinically meaningful stratification. Biol. Psychiatry: Cognit. Neurosci. Neuroimag. 2021;6(8):802–812. doi: 10.1016/j.bpsc.2020.08.008. [DOI] [PubMed] [Google Scholar]
- Hamilton A.F.D.C., Brindley R., Frith U. Visual perspective taking impairment in children with autistic spectrum disorder. Cognition. 2009;113(1):37–44. doi: 10.1016/j.cognition.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Happé F.G. Wechsler IQ profile and theory of mind in autism: A research note. J. Child Psychol. Psychiatry. 1994;35(8):1461–1471. doi: 10.1111/j.1469-7610.1994.tb01287.x. [DOI] [PubMed] [Google Scholar]
- Happé F., Ronald A., Plomin R. Time to give up on a single explanation for autism. Nat. Neurosci. 2006;9(10):1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- Harris I.M., Miniussi C. Parietal lobe contribution to mental rotation demonstrated with rTMS. J. Cognit. Neurosci. 2003;15(3):315–323. doi: 10.1162/089892903321593054. [DOI] [PubMed] [Google Scholar]
- Hawes Z., Sokolowski H.M., Ononye C.B., Ansari D. Neural underpinnings of numerical and spatial cognition: An fMRI meta-analysis of brain regions associated with symbolic number, arithmetic, and mental rotation. Neurosci. Biobehav. Rev. 2019;103:316–336. doi: 10.1016/j.neubiorev.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Petit L., Ungerleider L.G., Courtney S.M. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage. 2000;11(2):145–156. doi: 10.1006/nimg.2000.0592. [DOI] [PubMed] [Google Scholar]
- Hong S.-J., Vos de Wael R., Bethlehem R.A.I., Lariviere S., Paquola C., Valk S.L., Milham M.P., Di Martino A., Margulies D.S., Smallwood J., Bernhardt B.C. Atypical functional connectome hierarchy in autism. Nat. Commun. 2019;10(1) doi: 10.1038/s41467-019-08944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.-J., Mottron L., Park B.-Y., Benkarim O., Valk S.L., Paquola C., Larivière S., Vos de Wael R., Degré-Pelletier J., Soulieres I., Ramphal B., Margolis A., Milham M., Di Martino A., Bernhardt B.C. A convergent structure-function substrate of cognitive imbalances in autism. Cereb. Cortex. 2022 doi: 10.1093/cercor/bhac156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.J., Vogelstein J.T., Gozzi A., Bernhardt B.C., Yeo B.T., Milham M.P., Di Martino A. Toward neurosubtypes in autism. Biol. Psychiatry. 2020;88(1):111–128. doi: 10.1016/j.biopsych.2020.03.022. [DOI] [PubMed] [Google Scholar]
- Jarrold C., Gilchrist I.D., Bender A. Embedded figures detection in autism and typical development: Preliminary evidence of a double dissociation in relationships with visual search. Develop. Sci. 2005;8(4):344–351. doi: 10.1111/j.1467-7687.2005.00422.x. [DOI] [PubMed] [Google Scholar]
- Jassim N., Baron-Cohen S., Suckling J. Meta-analytic evidence of differential prefrontal and early sensory cortex activity during non-social sensory perception in autism. Neurosci. Biobehav. Rev. 2021;127:146–157. doi: 10.1016/j.neubiorev.2021.04.014. [DOI] [PubMed] [Google Scholar]
- Jolliffe T., Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? J. Child Psychol. Psychiatry. 1997;38(5):527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Jones D.T., Graff-Radford J. Executive dysfunction and the prefrontal cortex. CONTINUUM: lifelong learning. Neurology. 2021;27(6):1586–1601. doi: 10.1212/CON.0000000000001009. [DOI] [PubMed] [Google Scholar]
- Jordan K., Heinze H.J., Lutz K., Kanowski M., Jäncke L. Cortical activations during the mental rotation of different visual objects. Neuroimage. 2001;13(1):143–152. doi: 10.1006/nimg.2000.0677. [DOI] [PubMed] [Google Scholar]
- Joseph R.M., Keehn B., Connolly C., Wolfe J.M., Horowitz T.S. Why is visual search superior in autism spectrum disorder? Develop. Sci. 2009;12(6):1083–1096. doi: 10.1111/j.1467-7687.2009.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just M.A., Cherkassky V.L., Keller T.A., Minshew N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just M.A., Cherkassky V.L., Keller T.A., Kana R.K., Minshew N.J. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb. Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldy Z., Kraper C., Carter A.S., Blaser E. Toddlers with autism spectrum disorder are more successful at visual search than typically developing toddlers. Develop. Sci. 2011;14(5):980–988. doi: 10.1111/j.1467-7687.2011.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana R.K., Keller T.A., Cherkassky V.L., Minshew N.J., Just M.A. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(9):2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana R.K., Keller T.A., Cherkassky V.L., Minshew N.J., Just M.A. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc. Neurosci. 2009;4(2):135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana R.K., Liu Y., Williams D.L., Keller T.A., Schipul S.E., Minshew N.J., Just M.A. The local, global, and neural aspects of visuospatial processing in autism spectrum disorders. Neuropsychologia. 2013;51(14):2995–3003. doi: 10.1016/j.neuropsychologia.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana R.K., Uddin L.Q., Kenet T., Chugani D., Müller R.A. Brain connectivity in autism. Front. Hum. Neurosci. 2014;8:349. doi: 10.3389/fnhum.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B., Shih P., Brenner L.A., Townsend J., Müller R.A. Functional connectivity for an “island of sparing” in autism spectrum disorder: An fMRI study of visual search. Hum. Brain Mapp. 2013;34(10):2524–2537. doi: 10.1002/hbm.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemner C., Van Ewijk L., Van Engeland H., Hooge I. Brief report: Eye movements during visual search tasks indicate enhanced stimulus discriminability in subjects with PDD. J. Autism Dev. Disord. 2008;38(3):553–557. doi: 10.1007/s10803-007-0406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.P., Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39(4):1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Keown C.L., Datko M.C., Chen C.P., Maximo J.O., Jahedi A., Müller R.A. Network organization is globally atypical in autism: a graph theory study of intrinsic functional connectivity. Biol. Psychiatry: Cognitive Neurosci. Neuroimag. 2017;2(1):66–75. doi: 10.1016/j.bpsc.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H., Carpenter P.A., Minshew N.J., Cherkassky V.L., Keller T.A., Just M.A. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24(3):810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Koshino H., Kana R.K., Keller T.A., Cherkassky V.L., Minshew N.J., Just M.A. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb. Cortex. 2008;18(2):289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T., Kamio Y., Inada N., Kurita H. Sex differences in WISC-III profiles of children with high-functioning pervasive developmental disorders. J. Autism Dev. Disord. 2009;39(1):135–141. doi: 10.1007/s10803-008-0610-6. [DOI] [PubMed] [Google Scholar]
- Koyama T., Kurita H. Cognitive profile difference between normally intelligent children with Asperger's disorder and those with pervasive developmental disorder not otherwise specified. Psychiatry Clin. Neurosci. 2008;62(6):691–696. doi: 10.1111/j.1440-1819.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- Kuschner E.S., Bennetto L., Yost K. Patterns of nonverbal cognitive functioning in young children with autism spectrum disorders. J. Autism Dev. Disord. 2007;37(5):795–807. doi: 10.1007/s10803-006-0209-8. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Yeung P.K. Heterogeneity within autism spectrum disorders: what have we learned from neuroimaging studies? Front. Hum. Neurosci. 2013;7:733. doi: 10.3389/fnhum.2013.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léveillé C., Barbeau E.B., Bolduc C., Limoges É., Berthiaume C., Chevrier É., Mottron L., Godbout R. Enhanced connectivity between visual cortex and other regions of the brain in autism: a REM sleep EEG coherence study. Autism Res. 2010;3(5):280–285. doi: 10.1002/aur.155. [DOI] [PubMed] [Google Scholar]
- Lin H.Y., Ni H.C., Tseng W.Y.I., Gau S.S.F. Characterizing intrinsic functional connectivity in relation to impaired self-regulation in intellectually able male youth with autism spectrum disorder. Autism. 2020;24(5):1201–1216. doi: 10.1177/1362361319888104. [DOI] [PubMed] [Google Scholar]
- Lindell A.K., Hudry K. Atypicalities in cortical structure, handedness, and functional lateralization for language in autism spectrum disorders. Neuropsychol. Rev. 2013;23(3):257–270. doi: 10.1007/s11065-013-9234-5. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Lai M.C., Baron-Cohen S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol. Psychiatry. 2019;24(10):1435–1450. doi: 10.1038/s41380-018-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Jr., Leventhal B.L., DiLavore P.C., et al. The autism diagnostic observation schedule, generic: A standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P., Risi S., Gotham K., Bishop S. Western Psychological Corporation; Los Angeles, CA: 2012. Autism Diagnostic Observation Schedule–2nd edition (ADOS-2) p. 284. [Google Scholar]
- Loth E., Gómez J.C., Happé F. When seeing depends on knowing: adults with autism spectrum conditions show diminished top-down processes in the visual perception of degraded faces but not degraded objects. Neuropsychologia. 2010;48(5):1227–1236. doi: 10.1016/j.neuropsychologia.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Maeda Y., Yoon S.Y. A meta-analysis on gender differences in mental rotation ability measured by the Purdue spatial visualization tests: Visualization of rotations (PSVT: R) Educat. Psychol. Rev. 2013;25(1):69–94. doi: 10.1007/s10648-012-9215-x. [DOI] [Google Scholar]
- McGrath J., Johnson K., Ecker C., O'Hanlon E., Gill M., Gallagher L., Garavan H. Atypical visuospatial processing in autism: insights from functional connectivity analysis. Autism Res. 2012;5(5):314–330. doi: 10.1002/aur.1245. [DOI] [PubMed] [Google Scholar]
- Meilleur A.A.S., Jelenic P., Mottron L. Prevalence of clinically and empirically defined talents and strengths in autism. J. Autism Dev. Disord. 2015;45(5):1354–1367. doi: 10.1007/s10803-014-2296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew N.J., Keller T.A. The nature of brain dysfunction in autism: functional brain imaging studies. Curr. Opin. Neurol. 2010;23(2):124. doi: 10.1097/WCO.0b013e32833782d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L., Burack J.A., Iarocci G., Belleville S., Enns J.T. Locally oriented perception with intact global processing among adolescents with high-functioning autism: evidence from multiple paradigms. J. Child Psychol. Psychiatry. 2003;44(6):904–913. doi: 10.1111/1469-7610.00174. [DOI] [PubMed] [Google Scholar]
- Mottron L., Dawson M., Soulières I., Hubert B., Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J. Autism Dev. Disord. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Mottron L., Mineau S., Martel G., Bernier C.S., Berthiaume C., Dawson M., Lemay M., Palardy S., Charman T., Faubert J. Lateral glances toward moving stimuli among young children with autism: Early regulation of locally oriented perception? Dev. Psychopathol. 2007;19(01) doi: 10.1017/s0954579407070022. [DOI] [PubMed] [Google Scholar]
- Mottron L., Soulières I., Simard-Meilleur A.A., Dawson M. International Meeting for Autism Research. 2008. Peaks of ability as a subtyping tool for autism. [Google Scholar]
- Müller R.A., Fishman I. Brain connectivity and neuroimaging of social networks in autism. Trends Cognitive Sci. 2018;22(12):1103–1116. doi: 10.1016/j.tics.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N.G., Riemer M., Brandt L., Wolbers T. Repetitive transcranial magnetic stimulation reveals a causal role of the human precuneus in spatial updating. Sci. Rep. 2018;8(1):1–8. doi: 10.1038/s41598-018-28487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R.A., Shih P., Keehn B., Deyoe J.R., Leyden K.M., Shukla D.K. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb. Cortex. 2011;21(10):2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth A., Hönekopp J., Falter C.M. Visuo-spatial performance in autism: a meta-analysis. J. Autism Dev. Disord. 2014;44(12):3245–3263. doi: 10.1007/s10803-014-2188-5. [DOI] [PubMed] [Google Scholar]
- Nader A.M., Courchesne V., Dawson M., Soulières I. Does WISC-IV underestimate the intelligence of autistic children? J. Autism Dev. Disord. 2016;46(5):1582–1589. doi: 10.1007/s10803-014-2270-z. [DOI] [PubMed] [Google Scholar]
- Nader, A. M., Jelenic, P., & Soulières, I. (2015). Discrepancy between WISC-III and WISC-IV cognitive profile in autism spectrum: what does it reveal about autistic cognition?. PloS one, 10(12), e0144645. https://doi.org/10.1371/journal.pone.0144645. [DOI] [PMC free article] [PubMed]
- Nair A., Jolliffe M., Lograsso Y.S.S., Bearden C.E. A review of default mode network connectivity and its association with social cognition in adolescents with autism spectrum disorder and early-onset psychosis. Front. Psychiatry. 2020;11:614. doi: 10.3389/fpsyt.2020.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejati V., Moradkhani L., Suggate S., Jansen P. The impact of visual-spatial abilities on theory of mind in children and adolescents with autism spectrum disorder. Res. Dev. Disabil. 2021;114 doi: 10.1016/j.ridd.2021.103960. [DOI] [PubMed] [Google Scholar]
- Nielsen J.A., Zielinski B.A., Fletcher P.T., Alexander A.L., Lange N., Bigler E.D., Anderson J.S. Abnormal lateralization of functional connectivity between language and default mode regions in autism. Mol. Autism. 2014;5(1):1–11. doi: 10.1186/2040-2392-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Castanon A., editor. Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN. Hilbert Press; 2020. [Google Scholar]
- Noonan S.K., Haist F., Müller R.A. Aberrant functional connectivity in autism: evidence from low-frequency BOLD signal fluctuations. Brain Res. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly, C., Lewis, J. D., & Elsabbagh, M. (2017). Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PloS one, 12(5), e0175870. https://doi.org/10.1371/journal.pone.0175870. [DOI] [PMC free article] [PubMed]
- O'Boyle M.W., Cunnington R., Silk T.J., Vaughan D., Jackson G., Syngeniotis A., Egan G.F. Mathematically gifted male adolescents activate a unique brain network during mental rotation. Cognitive Brain Res. 2005;25(2):583–587. doi: 10.1016/j.cogbrainres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O’Riordan M.A. Superior visual search in adults with autism. Autism. 2004;8(3):229–248. doi: 10.1177/1362361304045219. [DOI] [PubMed] [Google Scholar]
- O'Riordan M., Plaisted K. Enhanced discrimination in autism. Quarterly J. Exp. Psychol.: Section A. 2001;54(4):961–979. doi: 10.1080/713756000. [DOI] [PubMed] [Google Scholar]
- O'Riordan M.A., Plaisted K.C., Driver J., Baron-Cohen S. Superior visual search in autism. J. Exp. Psychol. Hum. Percept. Perform. 2001;27(3):719. doi: 10.1177/1362361304045219. [DOI] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos A., Sforazzini F., Egan G., Jamadar S. Functional subdivisions within the human intraparietal sulcus are involved in visuospatial transformation in a non-context-dependent manner. Hum. Brain Mapp. 2018;39(1):354–368. doi: 10.1002/hbm.23847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson A., Marsh L., Hamilton A., Ropar D. Spatial transformations of bodies and objects in adults with autism spectrum disorder. J. Autism Dev. Disord. 2014;44(9):2277–2289. doi: 10.1007/s10803-014-2098-6. [DOI] [PubMed] [Google Scholar]
- Pearson A., Marsh L., Ropar D., Hamilton A. Cognitive Mechanisms underlying visual perspective taking in typical and ASC children. Autism Res. 2016;9(1):121–130. doi: 10.1002/aur.1501. [DOI] [PubMed] [Google Scholar]
- Pellicano E., Gibson L., Maybery M., Durkin K., Badcock D.R. Abnormal global processing along the dorsal visual pathway in autism: a possible mechanism for weak visuospatial coherence? Neuropsychologia. 2005;43(7):1044–1053. doi: 10.1016/j.neuropsychologia.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pellicano E., Maybery M., Durkin K., Maley A. Multiple cognitive capabilities/deficits in children with an autism spectrum disorder: 'Weak' central coherence and its relationship to theory of mind and executive control. Dev. Psychopathol. 2006;18(1):77–98. doi: 10.1017/S0954579406060056. [DOI] [PubMed] [Google Scholar]
- Peters M., Battista C. Applications of mental rotation figures of the Shepard and Metzler type and description of a mental rotation stimulus library. Brain Cogn. 2008;66(3):260–264. doi: 10.1016/j.bandc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos. Trans. R. Soc. B: Biol. Sci. 2005;360(1456):781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip R.C., Dauvermann M.R., Whalley H.C., Baynham K., Lawrie S.M., Stanfield A.C. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neurosci. Biobehav. Rev. 2012;36(2):901–942. doi: 10.1016/j.neubiorev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Picci G., Gotts S.J., Scherf K.S. A theoretical rut: revisiting and critically evaluating the generalized under/over-connectivity hypothesis of autism. Develop. Sci. 2016;19(4):524–549. doi: 10.1111/desc.12467. [DOI] [PubMed] [Google Scholar]
- Pierce K., Conant D., Hazin R., Stoner R., Desmond J. Preference for geometric patterns early in life as a risk factor for autism. Arch. Gen. Psychiatry. 2011;68(1):101–109. doi: 10.1001/archgenpsychiatry.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisted K., O'Riordan M., Baron-Cohen S. Enhanced discrimination of novel, highly similar stimuli by adults with autism during a perceptual learning task. J. Child Psychol. Psychiatry. 1998;39(5):765–775. doi: 10.1017/S0021963098002601. [DOI] [PubMed] [Google Scholar]
- Planche P., Lemonnier E. Does the islet of ability on visuospatial tasks in children with high-functioning autism really indicate a deficit in global processing? L'encephale. 2010;37(1):10–17. doi: 10.1016/j.encep.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Poulin-Lord M.P., Barbeau E.B., Soulières I., Monchi O., Doyon J., Benali H., Mottron L. Increased topographical variability of task-related activation in perceptive and motor associative regions in adult autistics. NeuroImage: Clinical. 2014;4:444–453. doi: 10.1016/j.nicl.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane P., Cochran D., Hodge S.M., Haselgrove C., Kennedy D., Frazier J.A. Connectivity in autism: a review of MRI connectivity studies. Harvard Rev. Psychiatry. 2015;23(4):223. doi: 10.1097/HRP.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J., Raven J.C., Court J.H. Oxford Psychologists Press; Oxford: 1998. Standard Progressive Matrices Raven Manual. [Google Scholar]
- Rødgaard E.M., Jensen K., Vergnes J.N., Soulières I., Mottron L. Temporal changes in effect sizes of studies comparing individuals with and without autism: a meta-analysis. JAMA Psychiatry. 2019;76(11):1124–1132. doi: 10.1001/jamapsychiatry.2019.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropar D., Mitchell P. Susceptibility to illusions and performance on visuospatial tasks in individuals with autism. J. Child Psychol. Psychiatry. 2001;42(4):539–549. doi: 10.1017/S002196300100717X. [DOI] [PubMed] [Google Scholar]
- Rorden C., Brett M. Stereotaxic display of brain lesions. Behav. Neurol. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Ruff C.C., Knauff M., Fangmeier T., Spreer J. Reasoning and working memory: common and distinct neuronal processes. Neuropsychologia. 2003;41(9):1241–1253. doi: 10.1016/S0028-3932(03)00016-2. [DOI] [PubMed] [Google Scholar]
- Sahyoun C.P., Belliveau J.W., Soulières I., Schwartz S., Mody M. Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia. 2010;48(1):86–95. doi: 10.1016/j.neuropsychologia.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F., Mottron L., Soulières I., Zeffiro T.A. Enhanced visual functioning in autism: An ALE meta-analysis. Hum. Brain Mapp. 2012;33(7):1553–1581. doi: 10.1002/hbm.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F., Zeffiro T.A., Doyon J., Benali H., Mottron L. Speech acquisition predicts regions of enhanced cortical response to auditory stimulation in autism spectrum individuals. J. Psychiatr. Res. 2015;68:285–292. doi: 10.1016/j.jpsychires.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M., Fine J.G., Bledsoe J., Zhu D.C. Gender differences in brain activation on a mental rotation task. Int. J. Neurosci. 2012;122(10):590–597. doi: 10.3109/00207454.2012.693999. [DOI] [PubMed] [Google Scholar]
- Shah A., Frith U. An islet of ability in autistic children: A research note. J. Child Psychol. Psychiatry. 1983;24(4):613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Shah A., Frith U. Why do autistic individuals show superior performance on the block design task? J. Child Psychol. Psychiatry. 1993;34(8):1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Sharda M., Midha R., Malik S., Mukerji S., Singh N.C. Fronto-temporal connectivity is preserved during sung but not spoken word listening, across the autism spectrum. Autism Res. 2015;8(2):174–186. doi: 10.1002/aur.1437. [DOI] [PubMed] [Google Scholar]
- Shepard R.N., Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171(3972):701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- Siegel D.J., Minshew N.J., Goldstein G. Wechsler IQ profiles in diagnosis of high-functioning autism. J. Autism Dev. Disord. 1996;26(4):389–406. doi: 10.1007/BF02172825. [DOI] [PubMed] [Google Scholar]
- Silk T.J., Rinehart N., Bradshaw J.L., Tonge B., Egan G., O’Boyle M.W., Cunnington R. Visuospatial processing and the function of prefrontal-parietal networks in autism spectrum disorders: a functional MRI study. Am. J. Psychiatry. 2006;163(8):1440–1443. doi: 10.1176/ajp.2006.163.8.1440. [DOI] [PubMed] [Google Scholar]
- Silleresi S., Prevost P., Zebib R., Bonnet-Brilhault F., Conte D., Tuller L. Identifying language and cognitive profiles in children with ASD via a cluster analysis exploration: Implications for the new ICD-11. Autism Research. 2020;13(7):1155–1167. doi: 10.1002/aur.2268. [DOI] [PubMed] [Google Scholar]
- Simard I., Luck D., Mottron L., Zeffiro T.A., Soulières I. Autistic fluid intelligence: Increased reliance on visual functional connectivity with diminished modulation of coupling by task difficulty. NeuroImage: Clin. 2015;9:467–478. doi: 10.1016/j.nicl.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M., Ozonoff S.J., Ursu S., Ravizza S., Cummings N., Ly S., Carter C.S. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47(12):2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulières I., Dawson M., Samson F., Barbeau E.B., Sahyoun C.P., Strangman G.E., Zeffiro T.A., Mottron L. Enhanced visual processing contributes to matrix reasoning in autism. Hum. Brain Mapp. 2009;30(12):4082–4107. doi: 10.1002/hbm.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulières I., Zeffiro T.A., Girard M.L., Mottron L. Enhanced mental image mapping in autism. Neuropsychologia. 2011;49(5):848–857. doi: 10.1016/j.neuropsychologia.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Takarae Y., Luna B., Minshew N.J., Sweeney J.A. Visual motion processing and visual sensorimotor control in autism. J. Int. Neuropsychol. Soc.: JINS. 2014;20(1):113. doi: 10.1017/S1355617713001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesaki, N., Kikuchi, M., Yoshimura, Y., Hiraishi, H., Hasegawa, C., Kaneda, R., ... & Minabe, Y. (2016). The contribution of increased gamma band connectivity to visual non-verbal reasoning in Autistic children: a MEG study. PloS one, 11(9), e0163133. https://doi.org/10.1371/journal.pone.0163133. [DOI] [PMC free article] [PubMed]
- Tomasino B., Gremese M. Effects of stimulus type and strategy on mental rotation network: an activation likelihood estimation meta-analysis. Front. Hum. Neurosci. 2016;9:693. doi: 10.3389/fnhum.2015.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyszka J.M., Kennedy D.P., Paul L.K., Adolphs R. Largely typical patterns of resting-state functional connectivity in high-functioning adults with autism. Cereb. Cortex. 2014;24(7):1894–1905. doi: 10.1093/cercor/bht040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front. Hum. Neurosci. 2013;7:458. doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos M.E., Mizuno A., Dahl B.C., Kemmotsu N., Müller R.A. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25(3):916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; 1991. WISC-III: Wechsler Intelligence Scale for Children: Manual. [Google Scholar]
- Wechsler D. Psychological Corporation; 1997. WAIS-III: Administration and Scoring Manual: Wechsler Adult Intelligence Scale. [Google Scholar]
- Wechsler D. 4th ed. Pearson; TX, San Antonio: 2008. Wechsler Adult Intelligence Scale—Fourth Edition (WAIS–IV) [Google Scholar]
- Wechsler, D., (2003). Wechsler Intelligence Scale for Children-WISC-IV. Psychological Corporation. https://doi.org/.
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wiggins J.L., Peltier S.J., Ashinoff S., Weng S.-J., Carrasco M., Welsh R.C., Lord C., Monk C.S. Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum disorders. Brain Res. 2011;1380:187–197. doi: 10.1016/j.brainres.2010.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson K.M., McIlvane W.J. Perceptual factors influence visual search for meaningful symbols in individuals with intellectual disabilities and Down syndrome or autism spectrum disorders. Am. J. Intellectual Develop. Disabilities. 2013;118(5):353–364. doi: 10.1352/1944-7558-118.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Ohta H., Watanabe H., Kanai C., Tani M., Ohno T., Takayama Y., Iwanami A., Kato N., Hashimoto R., Hashimoto R. Functional alterations in neural substrates of geometric reasoning in adults with high-functioning autism. PLoS ONE. 2012;7(8):e43220. doi: 10.1371/journal.pone.0043220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S., Becker B., Kendrick K.M. Reduced inter-hemispheric resting state functional connectivity and its association with social deficits in autism. Front. Psychiatry. 2021;12:244. doi: 10.3389/fpsyt.2021.629870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y.Y., Kuo B.C., Liu H.L. The neural correlates of attention orienting in visuospatial working memory for detecting feature and conjunction changes. Brain Res. 2007;1130:146–157. doi: 10.1016/j.brainres.2006.10.065. [DOI] [PubMed] [Google Scholar]
- Zacks J.M. Neuroimaging studies of mental rotation: a meta-analysis and review. J. Cognit. Neurosci. 2008;20(1):1–19. doi: 10.1162/jocn.2008.20013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.