Abstract
Decellularization approaches have been commonly used as alternative techniques to reconstruct tissues. However, due to the complex tissue compartmentation of the larynx, the decellularization process may not retain the characteristics necessary for the successful recreation of the larynx. The aim of this study was to assess the effect of the decellularization process on the framework of the human cadaveric larynx generally and the cricoarytenoid joint specifically. In this work, five freshly frozen human cadaveric larynges were decellularized utilizing a protocol that was previously demonstrated to be effective in decellularizing a porcine larynx. The decellularization protocol included: biological, chemical, and physical decellularization methods. Each specimen served as its own control to assess changes after decellularization. Studies and measurements included: histological, using Hematoxylin and Eosin (H&E) and Live/Dead™ stains; DNA quantification; micro-computed tomography (μ-CT) imaging; and biomechanical testing of the cricoarytenoid joints. The decellularization protocol took 12 days for each specimen. Microscopy of H&E stained samples demonstrated substantial removal of cells with preservation of the extracellular matrix that was more evident in cartilage than muscle specimens. Confocal microscope images of Live/Dead™ stained specimens also demonstrated almost complete removal of cells. Pre-decellularization cartilage-DNA quantity range was 27.0 to 336.8 ng/mg while post-decellularization DNA quantity range was 0 to 30.4 ng/mg (p=0.031). For muscles, pre-decellularization DNA quantity range was 150.0 to 3,384.6 ng/mg, while post-decellularization DNA quantity range was 0 to 45.5 ng/mg (p=0.031). μ-CT demonstrated preservation of the cartilaginous framework with a slight reduction of cricoarytenoid joint space. Furthermore, μ-CT demonstrated no significant reduction in the Housefield unit (p=0.25) and mineral density (p=0.25) after decellularization. Biomechanical testing demonstrated a non-significant reduction of forces required for anterior displacement of the arytenoid (mean reduction of forces, 0.1±0.2 N, p=0.16) and forces required for posterior displacement of the arytenoid (mean reduction of forces, 0.2±0.3 N, p=0.05). This study demonstrates effective decellularization of human larynges as evidenced by significant DNA depletion and preservation of extracellular matrix, which are outcomes that are required for a biological scaffold to regenerate a non-immunogenic larynx. The decellularization process caused minimal weakness in the cricoarytenoid joints due to treatment with multiple detergents and enzymes in the decellularization protocol.
Keywords: Tissue engineering, regenerative medicine, larynx, decellularization, biological scaffold, recellularization, otolaryngology, head and neck surgery
Graphical Abstract
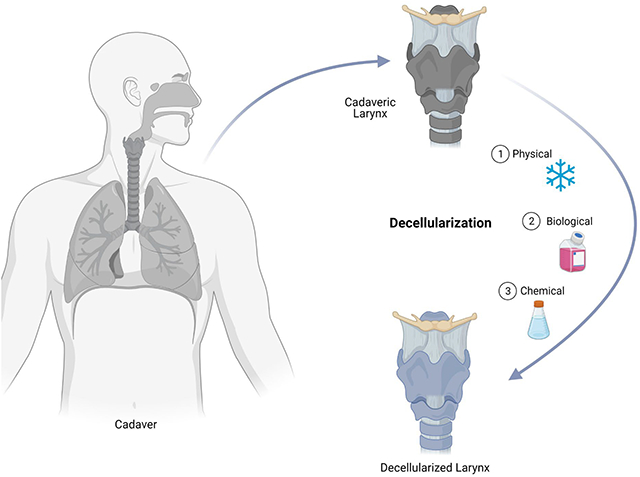
1. INTRODUCTION
The first reported larynx transplantation in a human was performed by Kluyskens and Ringoir in Belgium on February 10, 1969.1 They transplanted the larynx of a 40-year-old man into a 62-year-old man who was indicated for a total laryngectomy because of laryngeal cancer.1 The transplantation preserved the recipient’s laryngeal perichondrium, hence, the procedure was considered partial larynx transplantation.1 Despite the technical success of the surgery and voice restoration, cancer recurred after eight months, likely attributed to the use of immunosuppressant medications. The recurrence culminated in mortality despite explanting the graft and administering radiotherapy and chemotherapy.1 This dire consequence raised ethical concerns regarding the justification of larynx transplantation and its risk-benefit ratio. It was not until 1998 that Strome et al. performed the first total laryngeal transplantation in Cleveland Clinic, Ohio.2 This was followed by two reported total laryngeal transplantations: one was in 2010 by Farwell et al. of the University of California, Davis3 while the other transplantation was in 2015 by Grajek et al. of the Cancer Center IMSC, Gliwice, Poland.4 The latter was more complex, and it was performed in a patient who had lost the larynx due to cancer as opposed to the former in whom the larynx was lost due to trauma. However, the patient in whom Grajek et al. performed the transplantation was already on lifelong immunosuppression because of previous kidney transplantation.4 In all attempts at laryngeal transplantation, one major argument continues to resurface which is the ethical justification of using immunosuppression following a non-life saving procedure.5
Recent progress in tissue-engineering has ushered the field to increasingly expanding clinical applications. One main advantage of transplanting tissue-engineered organs is there is a reduced requirement for immunosuppression.6 Tissue-engineering is increasingly being recognized for its potential role in the future of medicine as a solution to overcome shortages of donated organs. Additionally, one of its most appealing advantages is negating the need for rendering the recipient of a tissue-engineered organ immunosuppressed.6 It is particularly this feature that makes tissue-engineering the whole larynx a better alternative to allogenic larynx transplantation.6 Laryngectomy is mostly performed for oncological indications.7 Although it is surgically achievable, the utilization of immunosuppressant medications is fraught with clinical concerns of cancer recurrence, as well as ethical concerns regarding whether the risk-benefit ratio is justifiable.8
Success in tissue-engineering the larynx has been remarkable, however, it is limited by the inability to regenerate a functioning glottis. Therefore, all previous attempts focused on regenerating no more than half of the larynx and relied on the contralateral native half for glottic compensation. Birchall et al. of the College University of London have demonstrated successful decellularization of a pig larynx and reimplanted a half larynx after recellularization.9 The purpose of this study was to explore the impact of decellularization on the integrity and mobility of the cricoarytenoid joint.
2. MATERIALS AND METHODS
2.1. Specimens acquisition and characterization
Five adult-cadaveric larynges (two adult-male larynges and three adult-female larynges), labeled larynges A-E, were obtained from the University of Iowa deeded body program. The specimens were freshly frozen and had not been treated with any chemicals. The donors’ information including age and gender were not provided. According to the University of Iowa Institutional Review Board, research on cadaveric specimens does not meet the criteria of human subject research.10 Morphological analysis of nine selected measurements as described by Tayama et al. were recorded.11 Those measurements included: (i) laryngeal prominence to the posterior edge, (ii) superior to superior cornu, (iii) inferior to inferior cornu, (iv) inferior to superior cornu – right side, (v) inferior to superior cornu – left side, (vi) anterior thyroid notch to the inferior border of the thyroid cartilage, (vii) superior thyroid cartilage seam thickness, (viii) anterior gap between thyroid and cricoid cartilage, and (ix) angle between thyroid laminae. The obtained measurements were compared to previously published normative data on specimens obtained through the University of Iowa Hospitals and Clinics.11 The comparison revealed the following: (i) Larynx A has 67% masculine features, (ii) Larynx B has 11% masculine features, (iii) Larynx C has 78% masculine features, (iv) Larynx D has 0% masculine features, and (v) Larynx E has 11% masculine features.
2.2. Study design
Each specimen served as its own control comparing characteristics before and after decellularization. The Student’s t-test was used for comparing the parameters of interest among the study groups. The significance level was set as α=0.05. Statistical analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
2.3. Decellularization
All larynges were decellularized by applying a modified protocol by Ansari et al. that demonstrated successful decellularization of porcine larynges.12 The protocol involved biological, chemical, and physical decellularization methods. All decellularization steps were performed while the specimens were kept under constant agitation (100 rpm) and all solutions contained 1% penicillin/streptomycin (Gibco®, Life Technologies Corporation™, New York, USA). Between every decellularization step, the larynges were washed for 15 minutes twice with autoclaved nanopure water. The decellularization of each larynx took 12 days. In detail, the decellularization process was as follows: first, the larynges were thawed at room temperature for approximately 1 hr. Then, the larynges were placed in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco®, Life Technologies Corporation™, New York, USA) containing 50 nmol/L Latrunculin B (Tocris bioscience, Bristol, UK) for 2 hr at 37°C. After this, the larynges were washed and placed in 0.6 mol/L KCl (Research Products International Corporation, Illinois, USA) solution for 2 hr at room temperature. This was followed by another washing step, then larynges were placed in 1 mol/L KI (Fisher Scientific, New Jersey, USA) for 2 hr at room temperature. Next, the larynges were left to soak overnight at room temperature. On the second day, the immersion in KCl and KI steps were repeated and followed by incubation in a solution containing 2 units/mL DNase I (New England BioLabs® inc, Massachusetts, USA) in distilled water for 2 hr at 37°C. Subsequently, the larynges were washed and frozen overnight. On the following day, larynges were left to thaw at room temperature for 24 hr. On the fourth day, the larynges were incubated in 0.25% Triton X-100 (Sigma-Aldrich Inc, Steinheim, Germany) and 0.25% Na deoxycholate (Sigma-Aldrich Inc, Steinheim, Germany) in phosphate-buffered saline (PBS) (Sigma-Aldrich Inc, Steinheim, Germany) for 24 hr at 37°C. For the following two days, the larynges were left for 24 hr at 4°C. On day 7, the larynges were incubated in 2 units/mL DNase I and 0.1g/L RNase in distilled water for 24 hr at 37°C. On the following day, the larynges were washed for 24 hr at 4°C. The incubation in DNase I and RNase I solution was repeated. Subsequently, over the following three days, the larynges were left to wash for 24 hr at 4°C. This concluded the decellularization process and the specimens were frozen at −20°C.
2.4. Histology
Specimens from thyroid and cricoid cartilages, and posterior cricoarytenoid muscle were collected from Larynx A before and after decellularization. The specimens were stained using Hematoxylin & Eosin (H&E) stain and examined under a light microscope to evaluate changes in cells and extracellular matrix. The specimen was also stained with LIVE/DEAD™ stain (Life Technologies Corporation, Oregon, USA) and examined under a confocal microscope to examine the distribution and visual prevalence of dead cells. The LIVE/DEAD™ stain contains two molecular probes: calcein AM and ethidium homodimer (EthD-1). Calcein AM is a cell-permeant that becomes intensely green fluorescent after undergoing enzymatic conversion by intracellular esterase in a living cell (i.e., calcein AM is used to identify viable cells which are not within the scope of this project). The other probe is EthD-1, which enters cells with damaged membranes and undergoes a 40-fold enhancement of fluorescence upon binding to nucleic acids, producing a bright red fluorescence in dead cells when examined with confocal microscopy. This particular feature is of interest in this project as the goals of decellularization are in two folds; first, disrupt and damage the cells, and secondly wash out the cellular material. Therefore, to visualize the specimens (i.e., EthD-1), confocal images were captured at excitation/emission of 495/635 nm using Leica TCS SP8 STED confocal laser scanning microscope (Leica Microsystems Inc., Buffalo Grove, IL).
2.5. Mechanical properties
Anterior and posterior displacements of cricoarytenoid movements were assessed before and after decellularization. Since the objective was to detect stiffness in the cricoarytenoid joint that could result from decellularization treatment, the minimum force required for anterior and posterior displacements was recorded. Force measurement setup was developed as illustrated in Figure 1. The larynx and force transducer (Mark-10, Series 3, New York, USA) were both stabilized during the examination of force vectors. An arytenoid repositioning device (ARD) provided by COOK® Medical (Illinois, USA) was inserted using fiberoptic illumination guidance through the cricothyroid membrane and inserted into the muscular process of the arytenoid cartilage. The free end of the ARD was secured to the force transducer. The manual force was applied until a movement of the arytenoid process was detected visually. For each vector measurement, 5 readings were obtained, and their mean was used to estimate the minimum force required for anterior and posterior displacement of the arytenoid.
Figure 1:
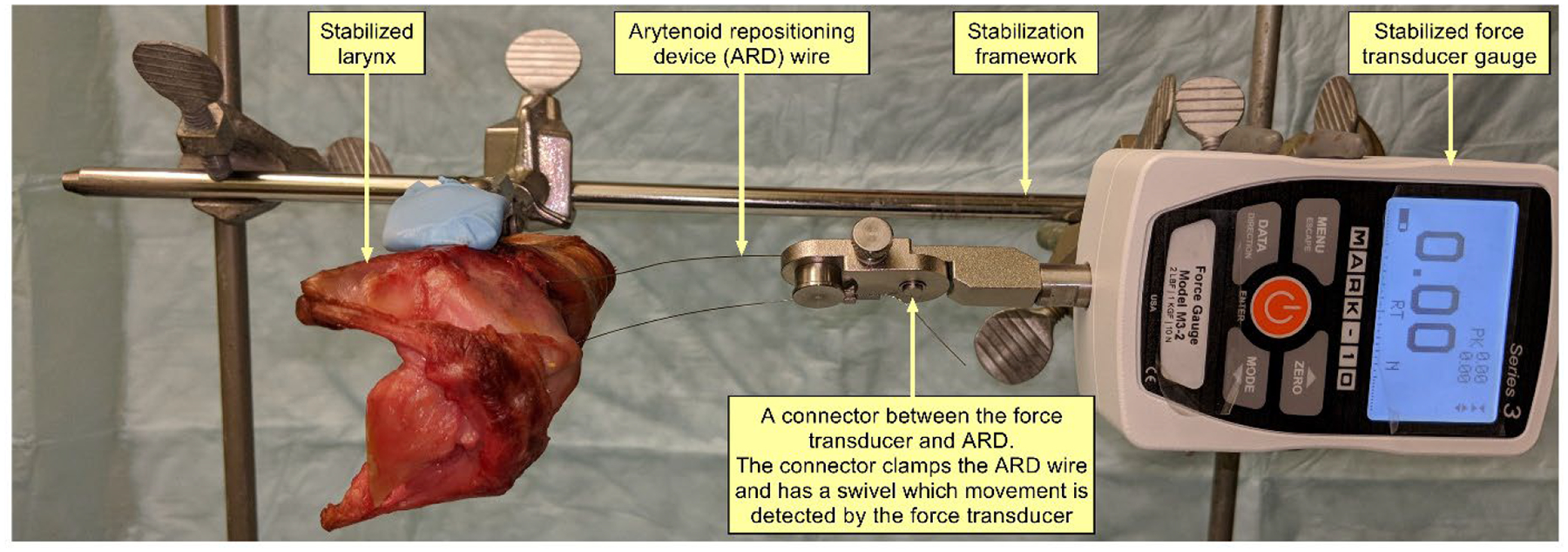
Setup for measuring arytenoid displacement force vector demonstrating stabilized larynx, force transducer gauge, and arytenoid repositioning device connecting the force transducer to the muscular process of each arytenoid.
2.6. DNA quantification
Quantification of DNA amount in thyroid cartilage and posterior cricoarytenoid muscle was assessed for each larynx using DNAeasy® (Qiagen, Hilden, Germany). The assay was performed following the manufacturer’s protocol. In summary, a full-thickness piece of cartilage was obtained from three pairs (a total of 6, 3 from each study group). The pairs were randomly selected from the total pairs included in the study. The weight of each cartilage specimen was recorded. The specimen was lysed using proteinase K while incubated at 56°C followed by multiple steps of centrifugation using manufacturer-provided buffer solution aimed at isolating the DNA content using manufacturer-provided spin tubes. Then the DNA content in the isolates was estimated using spectrophotometry.
2.7. Sulfated glycosaminoglycan (sGAG) quantification
sGAG content of cricothyroid muscle and cricoid cartilage from three randomly selected larynges was quantified using Blyscan™ sGAG Assay (Biocolor, Northern Irland, UK). This assay is widely used to measure sGAG content in cartilages, organs/tissues (such as heart, lungs, kidney, larynx, and trachea), and solid tumors.12–14 The assay was performed following the manufacturer’s protocol. In summary, the weight of each of the muscle and cartilage specimens (3 each) was recorded. The sGAG was extracted from the tissue using a Papain extraction reagent. Then, a dye was added that forms a complex with sGAG. The dye-bound sGAG was precipitated and drained. Subsequently, a dissociation agent was added to unbind the sGAG from the dye. The recovered sGAG content was measured at 656 nm using spectrophotometry and estimated by plotting on a standardized curve.
2.8. Micro-computed tomography (μCT) scan
μCT scan of whole Larynx B, with a focus on both cricoarytenoid joints specifically, was performed before and after decellularization using the Xradia 520 Versa Submicron X-ray imaging system (ZEISS, Oberkochen, Germany). Radiodensity and mineral density of thyroid and cricoid cartilage was estimated before and after decellularization.
3. RESULTS
3.1. Gross changes
Decellularization of all larynges was completed per design. Gross examination of the specimens demonstrated a change in color from a shade of red to light yellow (Figure 2–3). The cartilages were pale yellow, the muscles were light brown, and the mucosa was pale yellow. The tactile examination did not reveal perceptible changes in the integrity of the cartilaginous scaffold.
Figure 2:
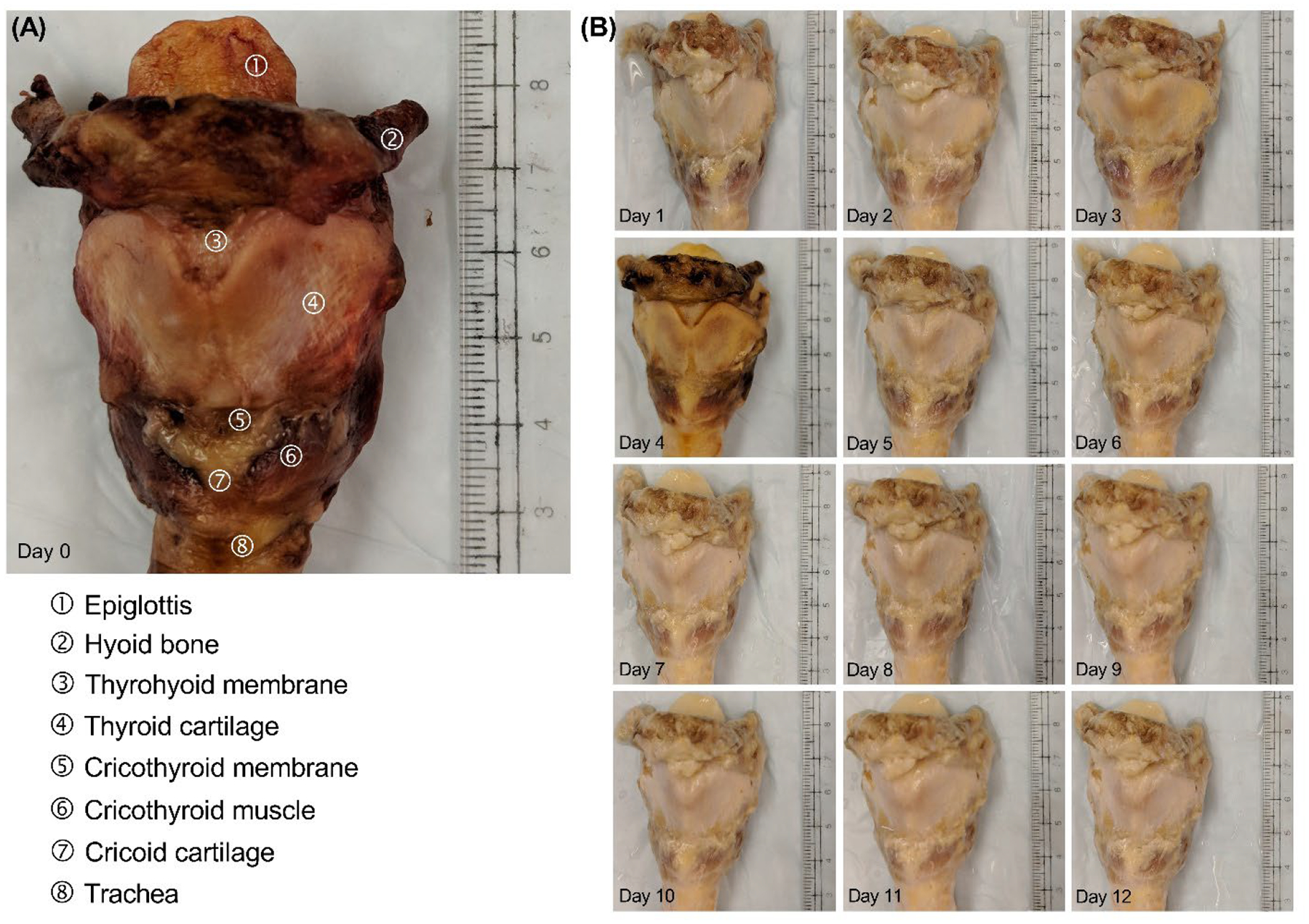
Decellularization process of Larynx B. (A) Image of the larynx before decellularization (i.e., day 0). (B) Gross changes throughout the decellularization process.
Figure 3:
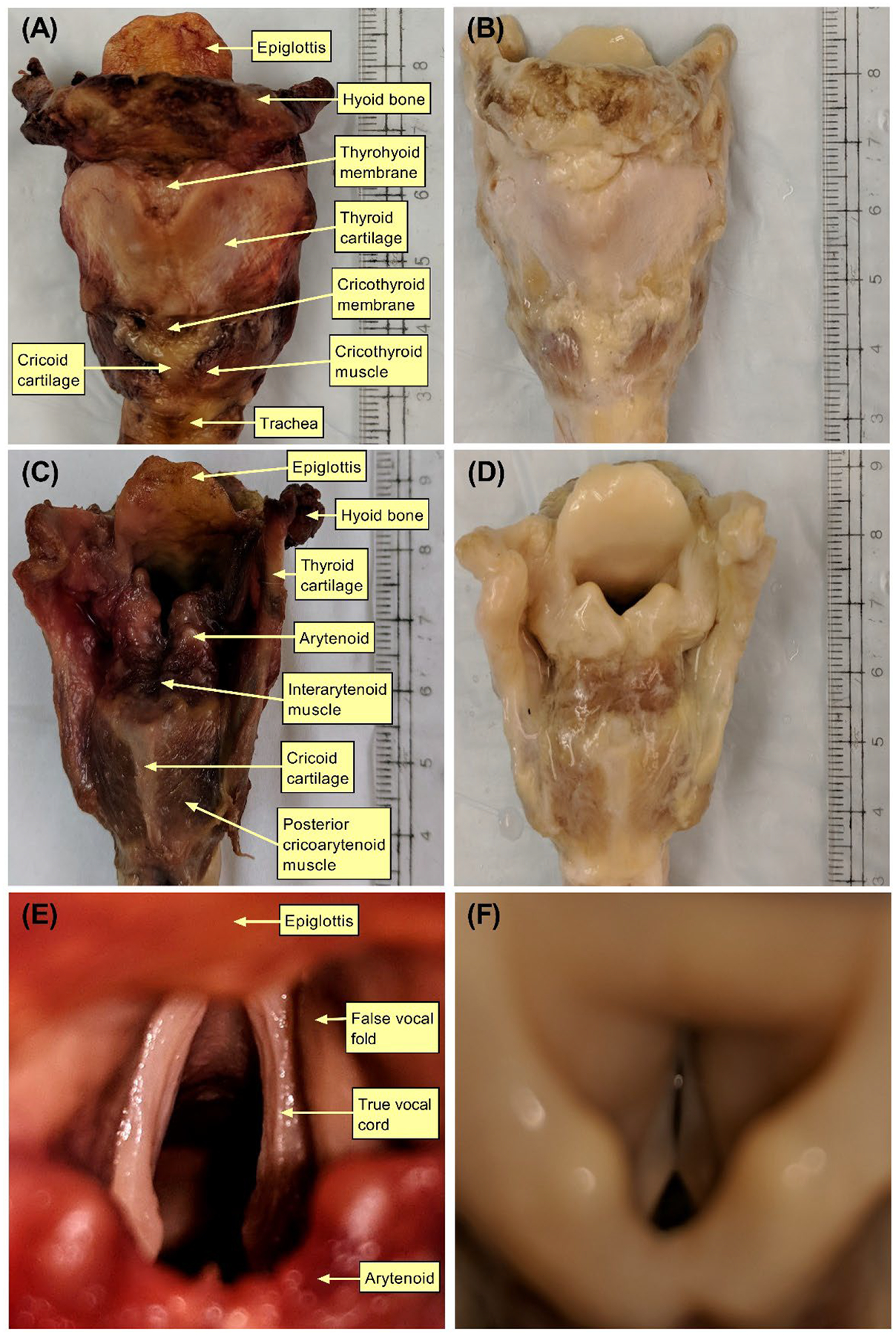
Gross changes before and after decellularization of Larynx B. Images (A) and (B) show the anterior view before and after decellularization, respectively. Images (C) and (D) show the posterior view before and after decellularization, respectively. Images (E) and (F) show the supraglottic view before and after decellularization.
3.2. Histological changes
Microscopy of H&E stained specimens demonstrated substantial removal of cells from both cartilaginous and muscular tissues (Figure 4). However, there is a visible substantial disruption of the extracellular matrix that was more evident in muscle than cartilage specimens. Confocal microscopy of Live/Dead™ stained specimens also visually demonstrated almost complete removal of intracellular nucleic acid (Figure 5).
Figure 4:
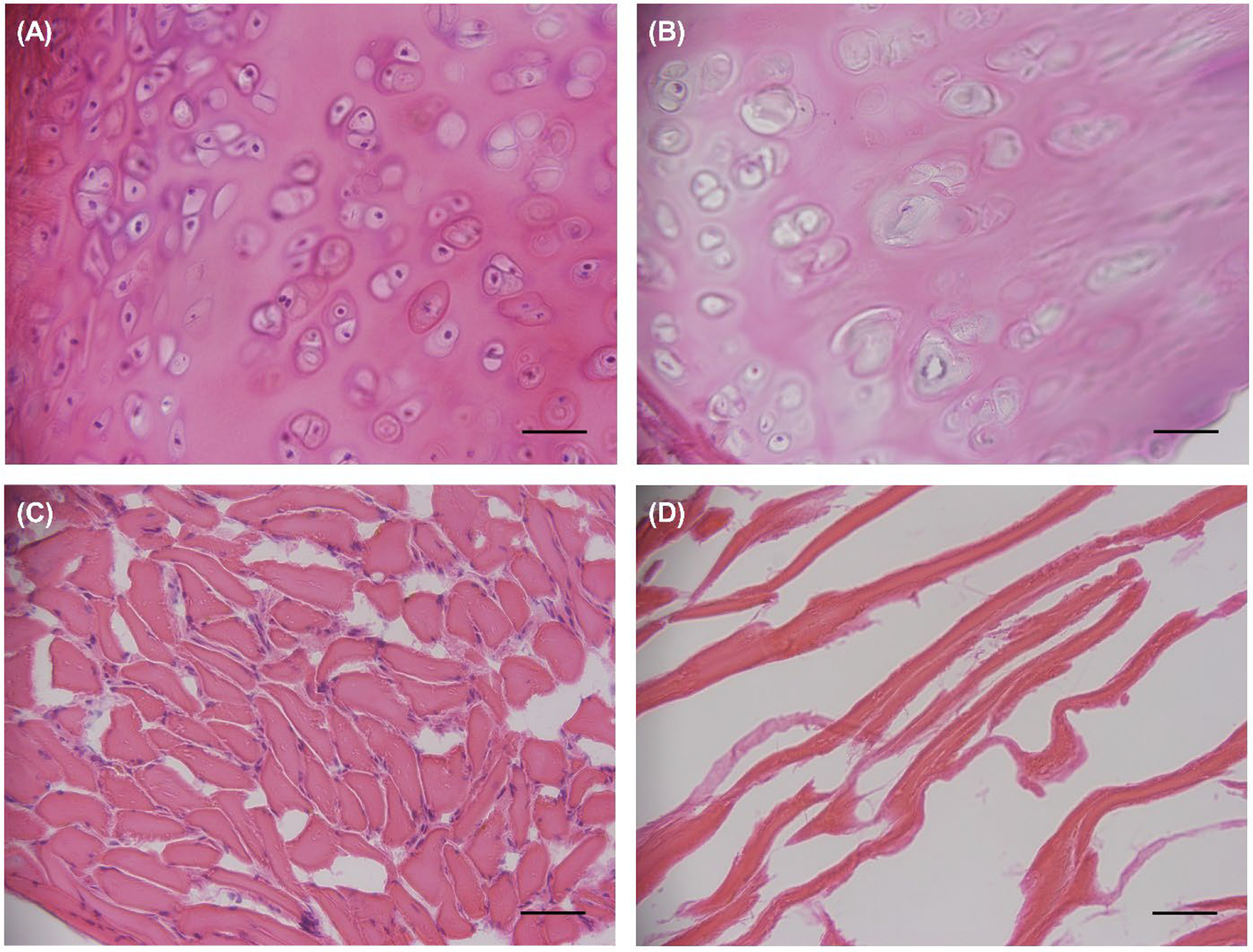
Light microscopy of Hematoxylin & Eosin-stained samples of Larynx A. Images (A) and (B) show the cartilage before and after decellularization, respectively. Images (C) and (D) display the muscle before and after decellularization, respectively. Images were captured at 40x magnification. Scale bar = 1 inch.
Figure 5:
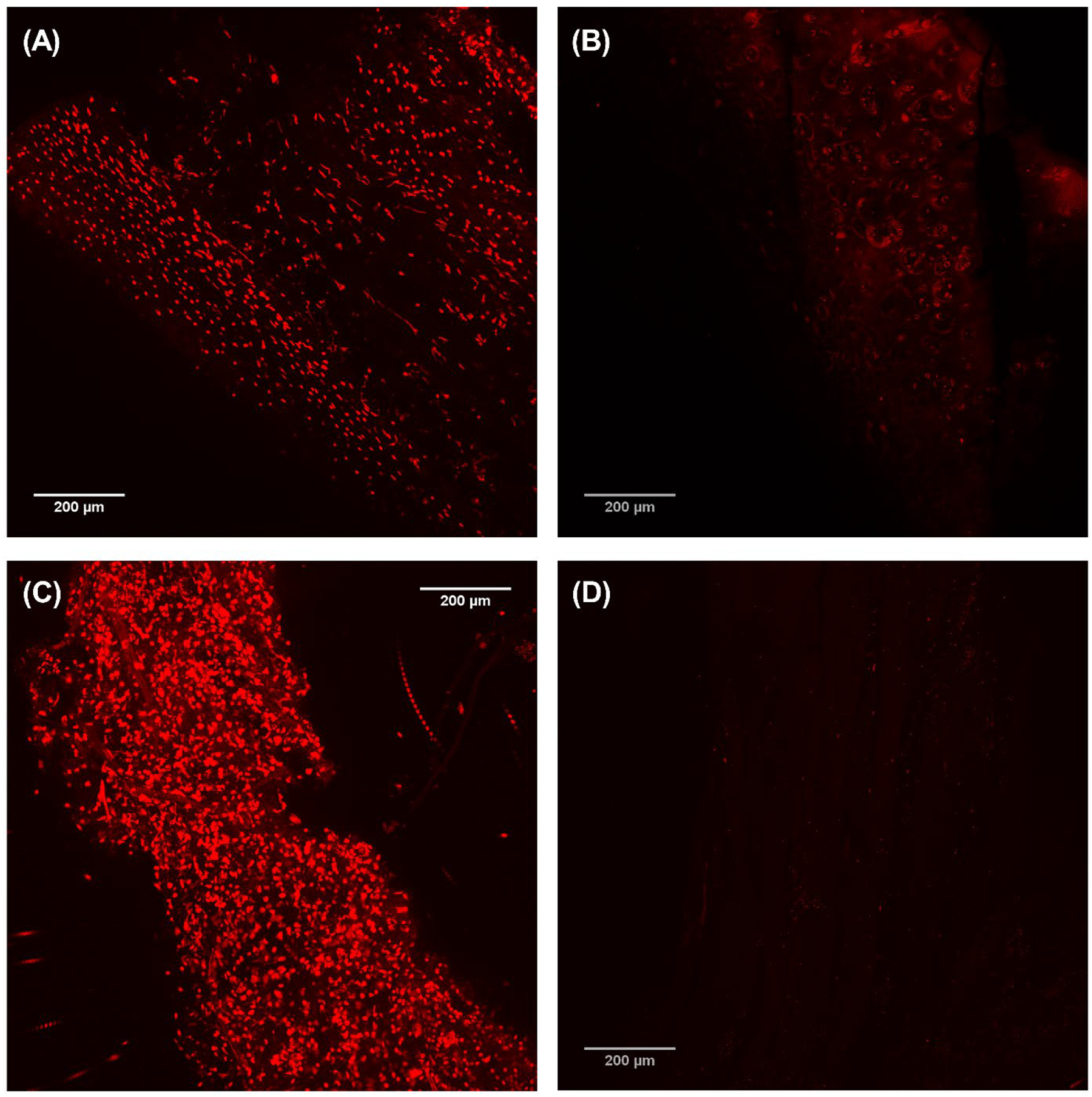
Confocal microscopy of LIVE/DEAD™ stained samples of Larynx A. Images (A) and (B) show the cartilage before and after decellularization, respectively. Images (C) and (D) show the muscle before and after decellularization, respectively. Scale bar = 200 μm.
3.3. DNA and sGAG content
Pre-decellularization cartilage-DNA quantity range was 27.0 to 336.8 ng/mg while post-decellularization DNA quantity range was 0 to 30.4 ng/mg (p=0.031). For muscles, pre-decellularization DNA quantity range was 150.0 to 3,384.6 ng/mg while post-decellularization DNA quantity range was 0 to 45.5 ng/mg (p=0.031). Pre-decellularization cartilage-sGAG quantity range was 65.33 to 244.71 μg/mg while post-decellularization sGAG quantity range was 46.61 to 175.132 μg/mg (p=0.47). For muscles, pre-decellularization sGAG quantity range was 2.30 to 10.95 μg/mg while post-decellularization sGAG quantity range was 0 to 4.96 μg/mg (p=0.19).
3.4. Micro-computed tomography
μ-CT demonstrated preservation of the cartilaginous framework with a slight reduction of the cricoarytenoid joint space (Figures 6–8). Prior to decellularization, cricoarytenoid joint space was 1,946.34 μm. Upon decellularization, the joint space marginally decreased to 1,098.42 μm. cμ-CT also demonstrated no significant reduction in Housefield unit (−103.7 ± 165.8, p=0.25) and mineral density (−68.0 ± 108.7 gm/cm3, p=0.25) after decellularization.
Figure 6:
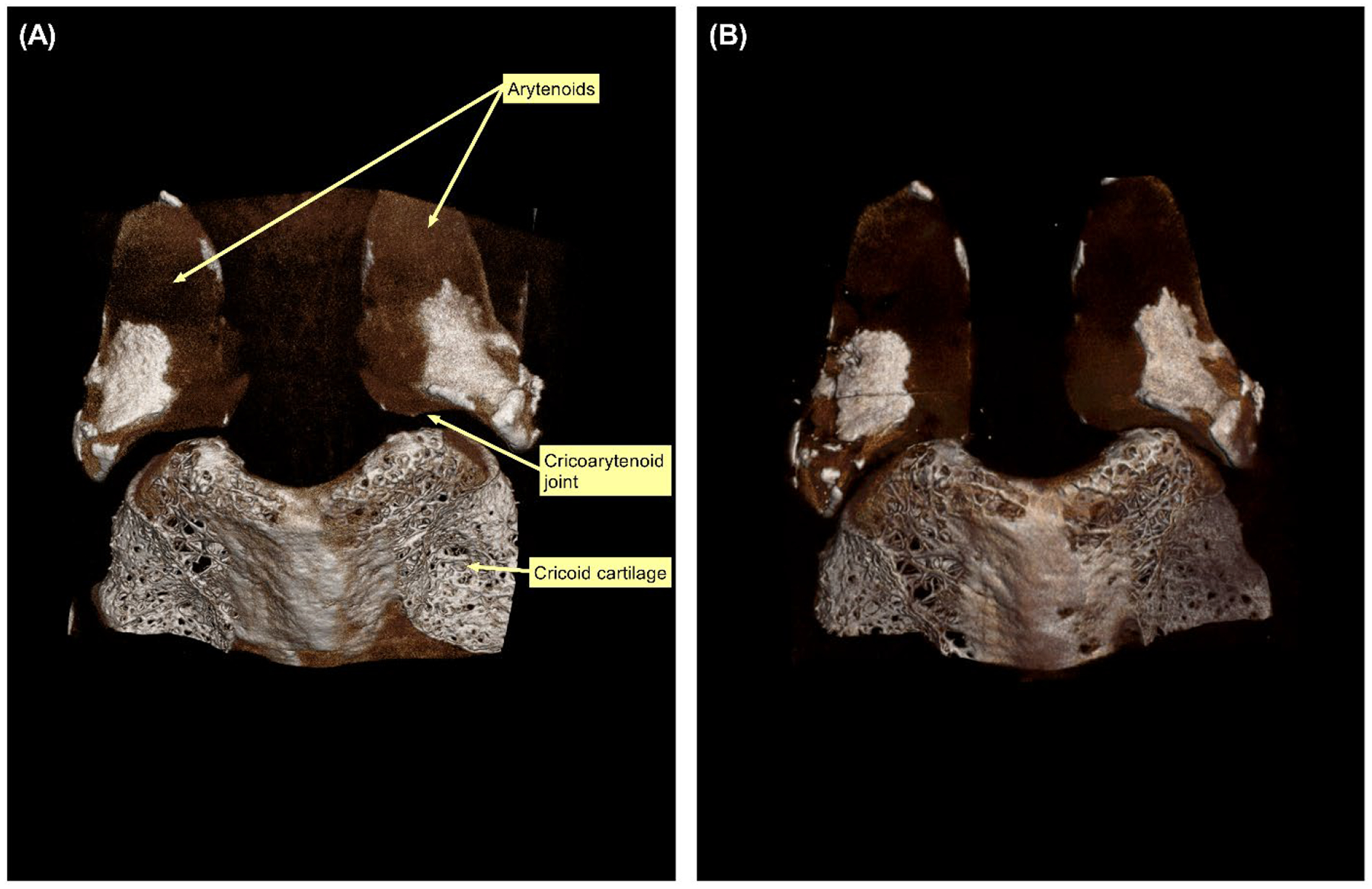
Micro-computed tomography (μCT) scan demonstrating the posterior surface of cricoid and arytenoid cartilages of Larynx B. (A) μCT scan before decellularization. (B) μCT scan after decellularization.
Figure 8:
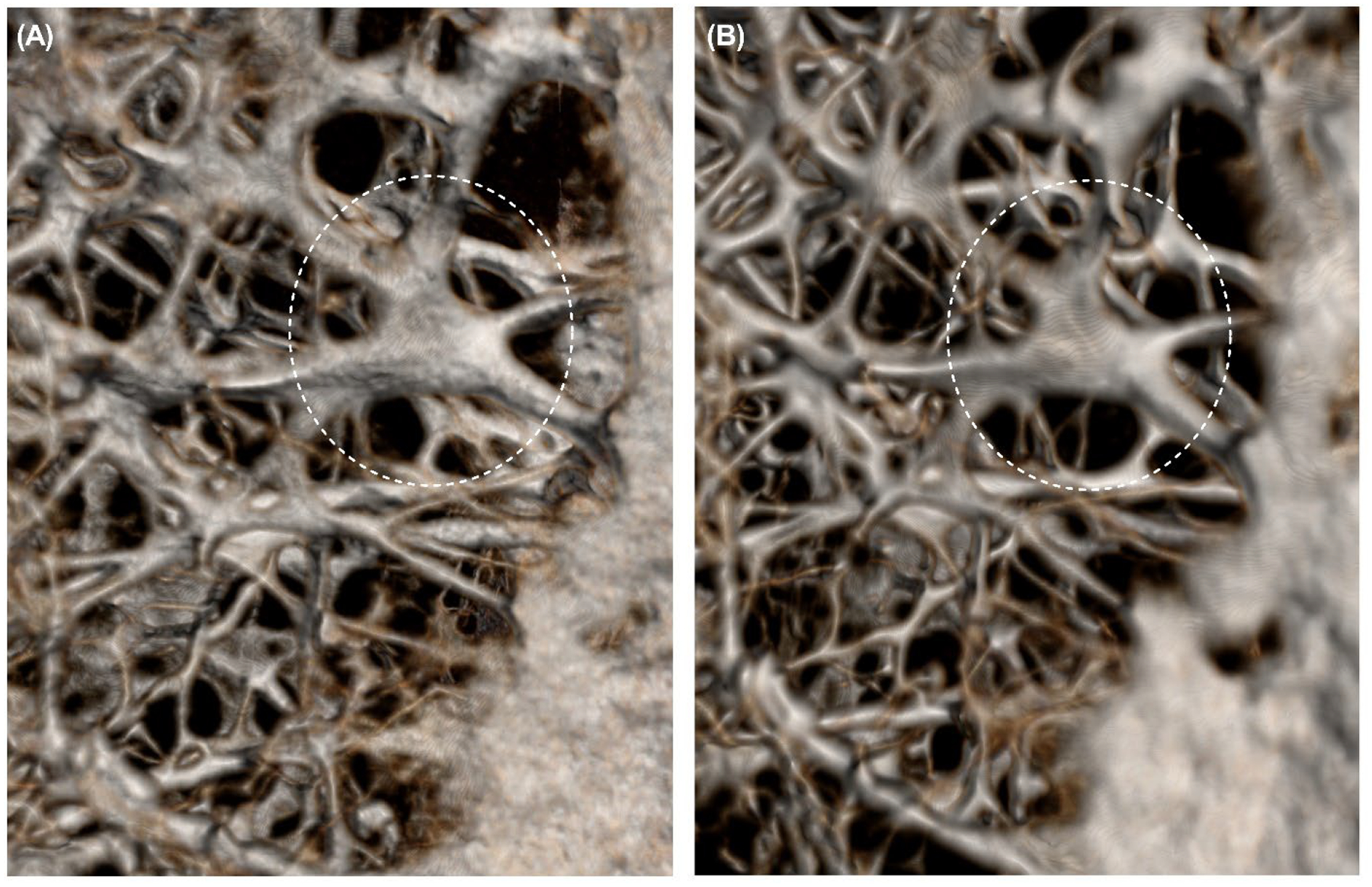
Micro-computed tomography (μCT) scan demonstrating the unchanged microstructure of the cricoid intracartilaginous calcification network before and after decellularization of Larynx B. (A) μCT scan before decellularization. (B) μCT scan after decellularization. Dashed circles highlight the region where changes were observed after decellularization.
3.5. Mechanical properties
Biomechanical testing demonstrated a non-significant reduction of forces required for anterior displacement of the arytenoid (mean reduction of forces, 0.1±0.2 N, p=0.16) and forces required for posterior displacement of the arytenoid (mean reduction of forces, 0.2±0.3 N, p=0.05).
4. DISCUSSION
The larynx is a segment of the respiratory system and responsible for swallowing, protecting the airway as well as producing speech.15 In addition, the larynx contains different components such as cartilage, bone, muscle, etc. (i.e., complex immune environment), which make larynx transplantation a much more complicated procedure in comparison to the transplantation of other organs/tissues.16 To date, only three successful total larynx transplantation have been performed in humans despite the extensive studies that have been performed to explore the feasibility of larynx transplantation.2–4 One of the major factors that has reduced the interest of otolaryngologists in larynx transplantation is that previous attempts had met with limited success, especially since this procedure requires a lifelong regimen of immunosuppressant treatments to prevent larynx rejection.17 In the United States alone, approximately 60 thousand people are living without larynx due to either a disease (laryngeal cancer) or trauma,18 and need to take careful consideration about their daily activities due to decreased ability to smell/taste and inability to blow their nose or hold their breath. To avoid sudden shortness of breath, people who have had their entire larynx removed also need to plan for frequent rest periods.19 To date, there is no efficient reconstructive strategy to restore the vital larynx functions, and excision of the larynx is a serious yet necessary treatment for people who have either laryngeal cancer or severe laryngeal injury.20 People who have had their larynx removed can breathe via a small hole or opening (stoma) directly into their neck/lungs and can communicate with others through using a special implantable prosthesis. However, laryngectomy stomas are associated with serious risks and complications (e.g. pharyngocutaneous fistula, stomal crusting, and acute airway obstruction) that are reported in more than 60% of cases.21 To help improve the quality of life for these patients, our team has initiated studies to establish a protocol to decellularize a human larynx in an attempt to facilitate the development of an implantable larynx and minimize/avoid the need for post-transplant immunosuppressive medications. In this study, we demonstrated the outcomes of decellularizing human laryngeal cartilage and muscle, and the study goal was to examine the impact of decellularization on the integrity of the laryngeal framework and cricoarytenoid joint. We adopted a decellularization protocol, with modifications, that was used by Ansari et al. to decellularize porcine larynges.8 The main modification was that we did not perform the entire decellularization process while placing the specimens in a vacuumed desiccator.12
H&E staining experiment revealed that there was a substantial disruption of muscle extracellular matrix compared to cartilage matrix. This is likely attributed to the physical characteristics of those two types of tissue as laryngeal cartilages are firmer and thicker compared to muscles, making muscular tissue more susceptible to the diffusion of decellularization agents and their effect. It is also a reflection of the different compositions of the muscular and cartilaginous extracellular matrix. It should be noted it is not within the scope of this project to compare the effect of decellularization on different types of tissue, rather the focus is on changes before and after decellularization in each tissue type. The microscopic findings on the H&E stain are consistent with the sGAG quantifications test. At the baseline, cartilage sGAG content was more than that of muscle reflecting the difference in their matrices. Quantification of sGAG content also demonstrated that sGAG has dropped substantially for muscle after decellularization. Extracellular matrix disruption was an expected outcome after decellularization, and an important next step for this project is to test whether the residual muscular scaffold is sufficient for recellularization and production of aligned muscular fiber using myogenic cells. Overall, the results from this study indicated there was no significant damage to the laryngeal cartilage and cricoarytenoid joint. Results also suggested the potential for success in developing a non-immunogenic human laryngeal framework similar to the Ansari et al. porcine model.
Larynx transplantation, although demonstrated to be technically possible,2–4 is not a life-saving procedure that comes with the cost of requiring life-long immunosuppression.8,22 The concept behind generating a tissue-engineered larynx is to improve quality of life and to avoid using immunosuppressants that would be required in the case of allogenic laryngeal transplantation. The larynx is a dynamic mucosal-lined conduit that consists of articulating cartilage and a neuromuscular system.16 Multiple studies in the past have demonstrated effective decellularization of animal or human partial laryngeal frameworks.6 Additionally, there has been a success with the implantation of the tissue-engineered partial larynx in animal models.12,23 In those previous studies, there was reliance on preserving the function of one of the two native cricoarytenoid joints in order to compensate for a lack of functional glottis. Fishman et al. reported decellularization of rabbit cricoarytenoid dorsalis muscle, which is anatomically equivalent to the posterior cricoarytenoid muscle in humans.9 They demonstrated effective decellularization of the muscle that was eventually transplanted into Sprague-Dawley rats and studied for two weeks with no signs of rejection.9 The study presented here is a continuation of previous efforts. First, we have adopted a protocol used by Ansari et al. for porcine larynges: instead applying it to human larynges. Secondly, this is the first study focused on the characterization of the cricoarytenoid joint before and after decellularization. We demonstrated that the joint did not sustain significant damage or become stiff and likely could be rehabilitated upon recellularization of the neuromuscular unit.
Opportunities to improve future studies include increasing sample size, measurement of the in vivo immunogenicity of any generated tissue, and increasing refinement in the force vector measurements. The force vector measurements were intended to rule out joint stiffness rather than measure laxity. This study is the first work to report on whole human larynx decellularization with a focus on the cricoarytenoid joint and confirms the potential of protocols demonstrated in animal models. Furthermore, the study employed μCT in quantifying the cricoarytenoid joint space, measured the Housefield unit, and visualized the microstructure of the laryngeal cartilage.
5. CONCLUSIONS
This study demonstrated effective decellularization of human larynges as evidenced by significant DNA depletion and preservation of the extracellular matrix, properties that are required in a biological scaffold used to regenerate a non-immunogenic larynx. The decellularization process caused minimal weakness in the cricoarytenoid joints and this is attributed to an effective protocol that utilizes multiple detergents and enzymes.
Figure 7:
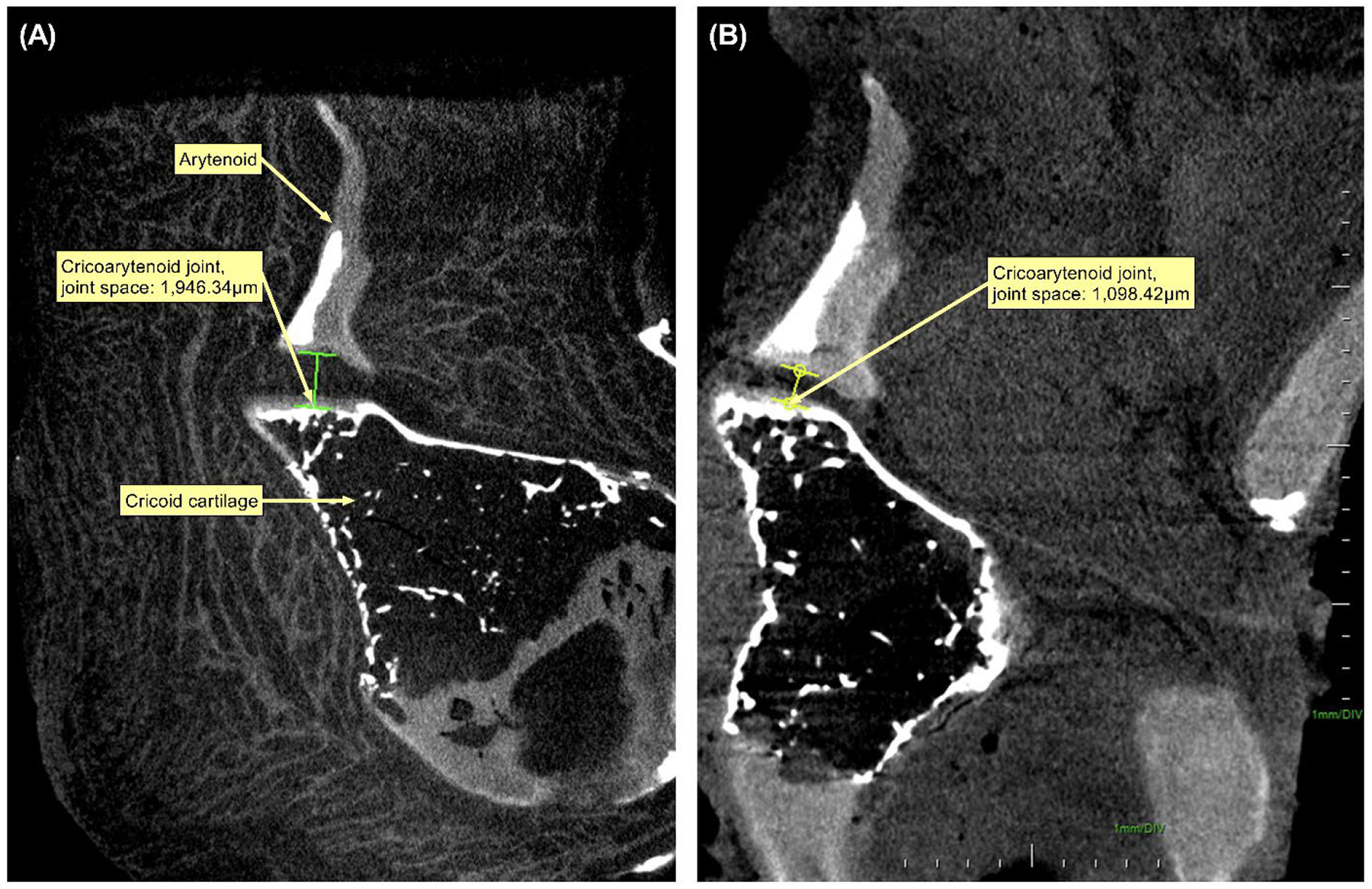
Micro-computed tomography (μCT) scan demonstrating the cricoarytenoid joint of Larynx B. (A) μCT scan before decellularization. (B) μCT scan after decellularization.
Acknowledgments
Keerthi Atluri, Ph.D., and Zach Laird of the College of Pharmacy at the University of Iowa provided support in the experiments. The University of Iowa Central Microscopy Research Facility and Pathology Department helped in processing tissue samples.
Funding
ZA acknowledges support by the National Institutes of Health – Institutional National Research Award: T32 (#5T32DC000040). AKS acknowledges support from the Lyle and Sharon Bighley Chair of Pharmaceutical Sciences and the National Cancer Institute at the National Institutes of Health P30 CA086862 Cancer Center support grant. COOK® Medical provided the Arytenoid Repositioning Device and did not have any other role in the study.
Footnotes
Conflict of interest
The authors declare no potential conflict of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Kluyskens P, Ringoir S. Follow-up of a human larynx transplantation. Laryngoscope 1970;80(8):1244–50. [DOI] [PubMed] [Google Scholar]
- 2.Strome M, Stein J, Esclamado R, Hicks D, Lorenz RR, Braun W, Yetman R, Eliachar I, Mayes J. Laryngeal transplantation and 40-month follow-up. N Engl J Med 2001;344(22):1676–9. [DOI] [PubMed] [Google Scholar]
- 3.Farwell DG, Birchall MA, Macchiarini P, Luu QC, de Mattos AM, Gallay BJ, Perez RV, Grow MP, Ramsamooj R, Salgado MD and others. Laryngotracheal transplantation: technical modifications and functional outcomes. Laryngoscope 2013;123(10):2502–8. [DOI] [PubMed] [Google Scholar]
- 4.Grajek M, Maciejewski A, Giebel S, Krakowczyk L, Ulczok R, Szymczyk C, Wierzgon J, Szumniak R, Dobrut M, Oles K and others. First Complex Allotransplantation of Neck Organs: Larynx, Trachea, Pharynx, Esophagus, Thyroid, Parathyroid Glands, and Anterior Cervical Wall: A Case Report. Ann Surg 2017;266(2):e19–e24. [DOI] [PubMed] [Google Scholar]
- 5.Birchall M Human laryngeal allograft: shift of emphasis in transplantation. Lancet 1998;351(9102):539–40. [DOI] [PubMed] [Google Scholar]
- 6.Baiguera S, Gonfiotti A, Jaus M, Comin CE, Paglierani M, Del Gaudio C, Bianco A, Ribatti D, Macchiarini P. Development of bioengineered human larynx. Biomaterials 2011;32(19):4433–42. [DOI] [PubMed] [Google Scholar]
- 7.Bozec A, Culie D, Poissonnet G, Dassonville O. Current Role of Total Laryngectomy in the Era of Organ Preservation. Cancers (Basel) 2020;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakallioglu O Laryngeal Transplantation. Turk Arch Otorhinolaryngol 2015;53(3):128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fishman JM, Ansari T, Sibbons P, De Coppi P, Birchall MA. Decellularized rabbit cricoarytenoid dorsalis muscle for laryngeal regeneration. Ann Otol Rhinol Laryngol 2012;121(2):129–38. [DOI] [PubMed] [Google Scholar]
- 10.Studies that are not human subjects research. Human Subjects Office, The University of Iowa; 2020. [Google Scholar]
- 11.Tayama N, Chan RW, Kaga K, Titze IR. Geometric characterization of the laryngeal cartilage framework for the purpose of biomechanical modeling. Ann Otol Rhinol Laryngol 2001;110(12):1154–61. [DOI] [PubMed] [Google Scholar]
- 12.Ansari T, Lange P, Southgate A, Greco K, Carvalho C, Partington L, Bullock A, MacNeil S, Lowdell MW, Sibbons PD and others. Stem Cell-Based Tissue-Engineered Laryngeal Replacement. Stem Cells Transl Med 2017;6(2):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He M, Callanan A, Lagaras K, Steele JAM, Stevens MM. Optimization of SDS exposure on preservation of ECM characteristics in whole organ decellularization of rat kidneys. J Biomed Mater Res B Appl Biomater 2017;105(6):1352–1360. [DOI] [PubMed] [Google Scholar]
- 14.Moser PT, Gerli M, Diercks GR, Evangelista-Leite D, Charest JM, Gershlak JR, Ren X, Gilpin SE, Jank BJ, Gaudette GR and others. Creation of Laryngeal Grafts from Primary Human Cells and Decellularized Laryngeal Scaffolds. Tissue Eng Part A 2020;26(9–10):543–555. [DOI] [PubMed] [Google Scholar]
- 15.Wadie M, Adam SI, Sasaki CT. Development, Anatomy, and Physiology of the Larynx. In: Shaker R, Belafsky PC, Postma GN, Easterling C, editors. Principles of Deglutition: A Multidisciplinary Text for Swallowing and its Disorders. New York, NY: Springer New York; 2013. p 175–197. [Google Scholar]
- 16.Noordzij JP, Ossoff RH. Anatomy and physiology of the larynx. Otolaryngol Clin North Am 2006;39(1):1–10. [DOI] [PubMed] [Google Scholar]
- 17.Birchall MA, Lorenz RR, Berke GS, Genden EM, Haughey BH, Siemionow M, Strome M. Laryngeal transplantation in 2005: a review. Am J Transplant 2006;6(1):20–6. [DOI] [PubMed] [Google Scholar]
- 18.Restoring function with larynx transplantation. Mayo Clinic Center for Regenerative Medicine; 2019. [Google Scholar]
- 19.Sanchez LD, Ban KM, Bramwell K, Davis D, Rosen P. Acute shortness of breath 10 years post laryngectomy. Intern Emerg Med 2008;3(2):145–9. [DOI] [PubMed] [Google Scholar]
- 20.Muller A Reconstructive procedures for impaired upper airway function: laryngeal respiration. GMS Curr Top Otorhinolaryngol Head Neck Surg 2005;4:Doc09. [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson C, Grigg C, Green M, Grigg R. Care of laryngectomy stomas. Australian Journal for General Practitioners 2019;48:373–377. [DOI] [PubMed] [Google Scholar]
- 22.Luu Q, Farwell DG. Laryngeal Transplantation. Current Otorhinolaryngology Reports 2014;2(3):192–195. [Google Scholar]
- 23.Kitamura M, Hirano S, Kanemaru SI, Kitani Y, Ohno S, Kojima T, Nakamura T, Ito J, Rosen CA, Gilbert TW. Glottic regeneration with a tissue-engineering technique, using acellular extracellular matrix scaffold in a canine model. J Tissue Eng Regen Med 2016;10(10):825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


