Highlights
-
•
There is a discrepancy between self-report and objective memory performance in SCD.
-
•
A subtype of SCD tended to be overconfident on memory performance.
-
•
Metamemory capacity is affected by altered neuroimaging in one subtype of SCD.
Abbreviations: AD, Alzheimer’s disease; AFT, Animal Verbal Fluency Test; ALFF, amplitude of the low frequency fluctuation; AVLT, Auditory Verbal Learning Test; BA, Brodmann area; BNT, Boston Naming Test; DOC, degree of confidence; fALFF, fractional low-frequency fluctuation amplitude; HAMA, Hamilton Anxiety Rating Scale; HAMD, Hamilton Depression Rating Scale; IR, immediate recall; EOP, estimation of performance; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; MoCA-B, Montreal Cognitive Assessment Basic Version; ROI, region of interest; ROJ, relative accuracy of judgment; S, slight cognitive impairment; SCD, subjective cognitive decline; STT, Shape Trails Test Parts; P, peer-related self-evaluation of cognition; FWE, Family-wise error correction
Keywords: Subjective cognitive decline, Metamemory, fMRI, Cortical thickness, Slight cognitive impairment
Abstract
Backgrounds
Subjective cognitive decline (SCD), one of the important clinical indicators for preclinical Alzheimer's disease (AD), is primarily defined as self-perceived cognitive decline without objective evidence for cognitive impairment. However, the accuracy of their self-evaluation of cognition is unclear. This study sought to investigate the capacity for self-evaluation of own cognitive performance in SCD by applying an objective metamemory paradigm.
Methods
147 individuals with SCD were classified into four subgroups by their subjective feeling of worse performance than peers or not (P+/-) and whether they have objectively slight cognitive impairment compared to normative data (S+/-). Metamemory scores, the amplitude of the low-frequency fluctuation (ALFF), fractional low-frequency fluctuation amplitude (fALFF), and cortical thickness were compared among four subgroups. Partial correlations between neuropsychological scores and neuroimaging measures were examined, controlling for age, sex, and education years.
Results
SCD S+P- showed the worst performance in short-term delayed recall and the worst metamemory performance, indicated by the highest value in the degree of confidence of short-term delayed recall (DOC-N4) and long-term cued recall (DOC-N6) and the worst value in relative accuracy of judgments of short-term delayed recall (ROJ-N4). ALFF values in the bilateral superior medial frontal and olfactory cortices and the left superior orbitofrontal gyrus cortex were significantly higher in SCD P- compared with SCD P+ groups (all P < 0.05, FWE-corrected, cluster-wise level). A significant S × P interaction effect in the left hippocampus and middle cingulate cortex was found for the fALFF signals (all P < 0.05, FWE-corrected, cluster-wise level). Significant interaction and main effects on cortical thickness were reported. The parahippocampal and posterior cingulate cortices were significantly decreased in SCD S+P- (all P < 0.05).
Conclusion
SCD S+P- showed the worst episodic memory performance, altered metamemory capacity (overconfidence and less accuracy of judgment), and altered neuroimaging measures, though they had feelings of similar performance with peers. Our results indicate that metamemory capacity is affected in a subtype of SCD with reduced cortical thickness and intensity of regional spontaneous activity in key areas for metamemory processing.
1. Introduction
Self-report of cognitive decline is an essential clinical feature throughout the Alzheimer's continuum, including the clinical and preclinical stages. (Albert et al., 2011, Jessen et al., 2014) International consensus on the biological definition of AD has recognized subjective cognitive decline (SCD) as an indicator of the cognitive transition stage (stage 2), which lies between completely unimpaired cognition (stage 1) and objectively impaired cognition (stage 3). (Jack et al., 2018) In cognitively normal older adults, two broad criteria can define SCD: (1) a self-experienced persistent decline in cognitive capacity compared to previously normal status and unrelated to an acute event, and (2) normal performance on standardized cognitive tests, which are used to classify MCI. (Jessen et al., 2014) In addition, an open set of specific features has been proposed (SCD-plus), which has been previously found to indicate an increased association with AD pathology or a particular risk of objective decline. (Jessen et al., 2014, Jessen et al., 2020) These features include but are not limited to (1) subjective decline in the memory domain, (Mitchell et al., 2014, Jessen et al., 2010, Amieva et al., 2008) (2) onset within the past five years, (Amieva et al., 2008, Jessen et al., 2020, Mitchell et al., 2014) (3) concerns associated with SCD. (Jessen et al., 2010, Verfaillie et al., 2019) The SCD-plus criteria are subjective to ongoing validation and refinement and may be adapted in the future. (Jessen et al., 2020) One of the SCD-plus features, the feeling of worse cognitive performance than peers, has become a matter of debate. (Jessen et al., 2014, Jessen et al., 2020, Verfaillie et al., 2019) It was included in the first proposal of the SCD-plus criteria (Jessen et al., 2014) based on the evidence that individuals with this feature had a greater amyloid burden in the brain. (Perrotin et al., 2021) However, this feature was excluded from the recent proposal for the SCD plus criteria due to no further supporting evidence. (Jessen et al., 2020) In our clinical practice, many SCD patients report having worse cognitive capacity than the same age group. There is an essential need to understand this feature and its relationship to SCD better.
The feeling of worse cognitive performance than peers requires the ongoing appraisal and monitoring of one's cognition in daily life and comparing with others. This cognitive process partially overlaps with the metamemory, defined as our ability to monitor, regulate and predict one's own memory performance. (Bertrand et al., 2019, Souchay, 2007) Metamemory capacity can be objectively assessed by requiring participants to evaluate their memory performance during real-time cognitive tests and inferred by the discrepancy between the subjective evaluation and the actual objective performance. (Souchay, 2007, Bertrand et al., 2018) Nearly half of the patients with AD dementia were impaired in the metamemory capacity. (Leicht et al., 2010, Souchay et al., 2003, Starkstein et al., 2006, Rosen et al., 2014, Starkstein, 2014) Patients with MCI and mild AD dementia tend to overestimate their memory performance. (Souchay, 2007, Starkstein et al., 2006, Fragkiadaki et al., 2016, Wadley et al., 2003, Stewart et al., 2010) Moreover, the severity of the metamemory impairment increases with AD progression. (Starkstein et al., 2006) It is unclear whether the metamemory capacity may be affected already in the risk condition for AD such as SCD. More importantly, SCD is conceptually not defined by objective cognitive performance but refers to subjective evaluation. (Jessen et al., 2020) Metamemory capacity has been shown to largely influence the accuracy of self-evaluation of cognitive performance. (Bahrami et al., 2020, Saenz et al., 2017) Therefore, it is essential to investigate the potential alteration in metamemory capacity in SCD.
Previous studies on the neural substrates of metamemory revealed a network of midline cortical regions, such as the medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC), and medial temporal lobe (MTL) regions, such as the entorhinal cortex, hippocampus, and parahippocampus, as well as insular cortex. (Cosentino et al., 2015, D’Oleire Uquillas et al., 2020, Fleming and Dolan, 2012, Hallam et al., 2020, Huntley et al., 2021) These key regions are considered to underlie impaired self-awareness of cognitive processes in AD. (Huntley et al., 2021) Impaired metamemory capacity was found in patients with mPFC lesions. (Modirrousta and Fellows, 2008) Less accurate metamemory was associated with reduced thickness of mPFC and PCC in patients with AD dementia. (Bertrand et al., 2018) In a group of cognitively healthy old subjects, overestimation of memory performance was associated with lower hemodynamic response to the learning prediction success in midline cortical areas. (D’Oleire Uquillas et al., 2020) The ventral mPFC was also involved during metamemory judgment in a group of young and healthy subjects. (Jessen et al., 2022, Tondelli et al., 2018) Severer anosognosia scores and less accurate metamemory performance have been previously associated with reduced right insular volume. (Cosentino et al., 2015, Hallam et al., 2020) Within the MTL regions, decreased gray matter volume of the hippocampus and parahippocampus were associated with poorer metamemory performance in patients with AD dementia and MCI. (Tondelli et al., 2018) Interestingly, overestimation of memory performance has been associated with tau deposition in the entorhinal cortex in cognitively unimpaired older adults. (D’Oleire Uquillas et al., 2020) Note that the tau burden in the MTL regions is one of the initial pathological hallmarks of preclinical AD. (Jack et al., 2018) Likely, the metamemory deficits may already occur at the very early cognitive transition stage (stage 2), which can be indicated by SCD. Nevertheless, there is a lack of neuroimaging studies on metamemory capacity in SCD.
In this study, we classify our SCD samples into four subgroups according to subjects' self-evaluation of their cognitive performance in comparison to peers (P+/-: worse than peers or not) and the actual cognitive performance on standardized neuropsychological tests in comparison to normative data (S+/-: with or without slight cognitive impairment). Slight cognitive impairment also potentially indicates the transition stage of the AD continuum. (Jack et al., 2018) Though this feature is not required for the broad definition of SCD, it frequently exists in the SCD population. (Jessen et al., 2022, Jessen et al., 2014) The current study applied the Jak/Bondi criteria for excluding subjects with MCI. (Bondi et al., 2014) SCD subjects who did not meet the Jak/Bondi criteria for MCI but did not show normal cognition in all subtests (>1 SD below demographically adjusted mean) are defined with slight cognitive impairment. Metamemory capacity was determined by comparing subjects' self-evaluation of their cognitive performance with the actual performance during the neuropsychological test on episodic memory. Meanwhile, all SCD subjects received structural magnetic resonance imaging (MRI) and resting-state functional MRI (rs-fMRI) measurements. Cortical thickness and spontaneous fluctuations of hemodynamic signals at wakeful rest were measured and compared among the four subtypes of SCD (S-P--, S-P++, S+P-, S+P+). We aim to assess the influence of peer-related self-evaluation of cognition (P-/+) and slight cognitive impairment (S-/+) variables on the metamemory capacity and the imaging outcomes in SCD. We hypothesize that SCD with slight cognitive impairment but without feeling worse performance than peers (S+P-) would overestimate their actual memory performance and show alterations in cortical thickness and spontaneous brain activity in the key metamemory processing areas, including midline cortical areas, medial temporal lobe, and the insula cortex.
The current study critically evaluated the self-evaluation of cognitive performance as a core research definition for SCD by investigating the metamemory process and comparing the metamemory capacity in different subtypes of SCD classified by the peer-related self-evaluation of cognition and slight cognitive impairment. We further evaluated the neuroimaging correlates potentially associated with the alterations in metamemory capacity. Our results will deepen our understandings of the clinical symptoms of SCD, which may potentially contribute to the early identification and prevention of AD.
2. Materials and methods
2.1. Participants
A total of 147 individuals with SCD (77.55 % females; Mean age = 64.16; range: 50–84) were recruited from April 2019 to April 2021, including 33 SCD S-P-, 47 SCD S-P+, 29 SCD S+P-, and 38 SCD S+P+, from the Department of Geriatrics, Shanghai Sixth People's Hospital, Shanghai, China. Consistent with the broad definition of SCD, (Jessen et al., 2014) all participants enrolled in the current study were individuals who experienced cognitive decline, sought medical help in our memory clinic, and performed normal cognitive performance on the comprehensive neuropsychological test battery (see section 2.2). We applied a structured SCD interview (SCD-I) translated from German to Chinese by XH, (Miebach et al., 2019) to evaluate the self-perceived cognitive decline of different cognitive domains and associated SCD-plus questions. The current study enrolled individuals who met the following SCD-plus criteria based on the SCD-I: (1) self-perceived decline in the memory domain, (2) onset of SCD in the last five years, and (3) concerns associated with SCD. Further inclusion criteria were more than six years of education; nearly normal eyesight after correction and hearing. Exclusion criteria were: MCI, dementia of AD type, history of alcoholism, drug abuse, head trauma, or other neuropsychiatric diseases, such as depression and anxiety; apparent abnormalities in folic acid, vitamin B12, rapid plasma regain, thyroid function, and Treponema pallidum particle agglutination. The study was approved by the ethics committee of the Shanghai Sixth People's Hospital. All participants signed informed consent.
2.2. Neuropsychological assessments
All participants received a comprehensive neuropsychological assessment on global cognitive function, memory, attention, language, executive function, and metamemory. The tests included Auditory Verbal Learning Test-Huashan (AVLT-H) for episodic memory measure, (Zhao et al., 2013, Zhao et al., 2013, Zhao et al., 2015) Boston Naming Test (BNT) and Animal Verbal Fluency Test (AFT) (Mack et al., 1992, Zhao et al., 2013) for language measure, Shape Trails Test Parts A (STT-A) and B (STT-B) (Zhao et al., 2013) for executive function measure. The AVLT-H contains a learning phase and a delayed recall phase. During the learning phase, 12 two-syllable words of three different types were orally presented three times, and subjects were asked to recall the word list after each trial. The values of three free recalls were summarized as the immediate recall score (AVLT-IR). During the delayed recall phase, additional indices for episodic memory were probed, including free recall over short-term (AVLT-N4) and long-term delays (AVLT-N5), long-term cued recall (AVLT-N6), and recognition memory (AVLT-N7). Short and long-term delayed recalls are separated by the time intervals of other tests, approximately 5 and 20 min, respectively, in which the other nonverbal neuropsychological tests are administered. Global cognitive screening tests, including Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment Basic Version (MoCA-B). (Chen et al., 2016) In addition, Hamilton Depression Rating Scale (HAMD) and Hamilton Anxiety Rating Scale (HAMA) were used to assess psychiatric symptoms.
2.3. Classification of SCD subtypes
In the current study, the Jak/Bondi neuropsychological criteria were applied to define the MCI, (Bondi et al., 2014) for which six subtests of the neuropsychological battery were considered, including two subtests for episodic memory (AVLT-N5, N7), (Zhao et al., 2013, Zhao et al., 2015) two subtests for language (BNT, AFT), (Mack et al., 1992, Zhao et al., 2013) and two subtests for executive function (STT-A, STT-B). (Chen et al., 2016, Zhao et al., 2013) Impaired scores were defined as lower than 1 SD below the demographically adjusted mean. Participants who had impaired scores in two subtests within one cognitive domain or impaired scores in at least one subtest of all three cognitive domains were diagnosed as MCI. All SCD subjects did not fulfill the neuropsychological criteria of MCI.
SCD subjects who had entirely normal cognition (unimpaired scores on all subtests: higher than 1 SD below the demographically adjusted mean) were defined as having a slight cognitive impairment (SCD S-). SCD subjects without entirely normal cognition did not meet Jak/Bondi criteria for MCI and were defined as having a slight cognitive impairment (SCD S+). (Bondi et al., 2014) More specifically, they may have one impaired score in only one or two cognitive domains.
Based on whether individuals have a feeling of worse performance than peers or not (derived from the SCD-I, (Miebach et al., 2019) memory domain), participants were further divided into four subgroups. SCD S-P- meet the criteria of SCD S- and have a feeling of similar performance with peers; SCD S-P+ meet the criteria of SCD S-, at the same time, they have a feeling of worse performance than peers. SCD S+P- meet the criteria of SCD S+ and have a feeling of similar performance with peers; SCD S+P+ meet the criteria of SCD S+, at the same time, they have a feeling of worse performance than peers.
2.4. Metamemory capacity
The metamemory capacity was measured by comparing subjects' self-estimation (prediction or judgment) of their cognitive performance with their actual cognitive performance. The self-estimation process was embedded in the standardized neuropsychological test for episodic memory, the AVLT-H. (Zhao et al., 2013, Zhao et al., 2013, Zhao et al., 2015) Following the learning phase and prior to the delayed recall phase of AVLT-H, subjects were asked to predict the number of words they could remember after 5 min (N4) and 20 min (N5), respectively. During the delayed recall phase after the long-term cued recall (N6), subjects were required to estimate how many words they had correctly remembered. Three types of metamemory indices (relative accuracy of judgment, ROJ; estimation of performance, EOP; and degree of confidence, DOC) were calculated by comparing self-predicted (N4, N5) or self-estimated (N6) performance with the actual performance of the corresponding trial, yielding a metrics of 9 metacognitive scores.
Relative accuracy of judgment (ROJ): This index is calculated from the differences between self-estimated performance (Nj) and actual performance (Nr) without taking into account the direction of the discrepancies (Formula (1)). It ranges from zero to one, with a higher value indicating higher accuracy of judgment. It reflects how judgments/predictions vary with actual performance.
| (1) |
Estimation of performance (EOP): This index is calculated by the self-estimated performance divided by the total number of items (Formula (2)). It indicates the rate of self-estimated scores. A higher value indicates a higher estimated score.
| (2) |
Degree of Confidence (DOC): This index is calculated from the deviations between self-estimated performance (Nj) and actual performance (Nr), taking into account the direction of the deviations (Formula (3)). It reflects the extent to which participants were overconfident or unconfident about their performance. A score of 1 reflects adequate confidence; a score below 1 indicates underconfidence; a score above 1 indicates overconfidence.
| (3) |
2.5. MRI data acquisition and processing
Data were collected using a 3.0 Tesla scanner (SIEMENS MAGNETOM, Prisma 3.0 T, Siemens, Erlangen, Germany). Resting-State fMRI images were collected by the following parameters: echo-planar imaging (EPI) sequence, transverse plane, repetition time = 800 ms, echo time = 37 ms, flip angle = 52°, matrix size = 112×112, field of view = 224 mm × 224 mm, slice number = 66 slices, slice thickness = 2 mm, and voxel size = 2 mm × 2 mm × 2 mm. The scan obtained 488 volumes and took a total of 390.4 s. Three-dimensional T1-weighted images were acquired by using magnetization-prepared rapid gradient-echo sequence in the sagittal plane with the following parameters: matrix = 320×320, field of view = 256 mm × 256 mm, slice thickness = 0.8 mm, voxel size = 0.8 mm × 0.8 mm × 0.8 mm, repetition time = 3000 ms, echo time = 2.56 ms, inversion time = 1100 ms, flip angle = 7°, and number of slices = 208.
Topup (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/topup) was applied to the EPI distortion correction for the fMRI data, (Andersson et al., 2003, Smith et al., 2004) which was based on the FSL 5.0.9 (https://fsl.fmrib.ox.ac.uk/fsl). (Jia et al., 2019, Smith et al., 2004) After the preprocessing, the images were further processed using the toolbox of Statistic Parametric Mapping 12 (SPM12, https://www.fil.ion.ucl.ac.uk/spm/software/spm12) and the RESTplus toolkit (https://restfmri.net/forum/restplus). (Jia et al., 2019) The first 10 time points were discarded. Then, slice timing and realignment were also conducted to correct head motion (head movement: ≥ 3 mm or 3° were excluded). In the next step, normalization was performed with isotropic voxel size (2 × 2 × 2 mm3). Then, spatial smoothing was used by convolution the three-dimensional image with a three-dimensional Gaussian kernel with a full width at half maximum (FWHM) of 6 mm linear. After that, detrending processing and regression analyses were carried out to eliminate linear drift and minimize the effects of head movement, white matter signal noise, and cerebrospinal fluid signal noise. Finally, the previously generated images were filtered between 0.01 and 0.08 Hz to control noise interferences, and the fractional low-frequency fluctuation amplitude (fALFF) in the normal band was calculated. After standardization, we used the mean value of the amplitude of the low frequency fluctuation (mALFF) and the fractional low-frequency fluctuation amplitude (mfALFF) map for further analysis.
FreeSurfer (v.6.0.0. https://surfer.nmr.mgh.harvard.edu) was used to estimate brain regional cortical thickness. Structural images were automatically processed to reconstruct cortical surfaces and to segment using the Freesurfer recon-all procedures (https://surfer.nmr.mgh.harvard.edu/); the procedures have been described in prior publications. (Desikan et al., 2006, Fischl et al., 1999, Fischl and Dale, 2000, Fischl et al., 2002) Briefly, the processing includes motion correction, Talairach transformation, segmentation, topology correction, and normalization. Cortical thickness was obtained by measuring the distance between the pial surface and the white matter boundary. Quality control was conducted by visual assessment and overlapping the parcellations on the FreeSurfer's template. Eight regions of interest (ROIs) previously implicated in studies were derived. (D’Oleire Uquillas et al., 2020, Meiberth et al., 2015, Bertrand et al., 2018) Based on the Desikan-Killiany atlas, (Desikan et al., 2006, Meiberth et al., 2015) including the cortices of the middle temporal, parahippocampal, entorhinal, insula, posterior cingulate, medial orbitofrontal, rostral middle frontal, and frontal pole. We chose the mean value of bilateral cortical thickness.
2.6. Statistical analysis
Statistical analyses were conducted using SPSS statistic 23 (IBM, New York, U.S.A.), including demographic and general cognitive scores. The Shapiro–Wilk test was used to investigate the distribution of data. ANOVA was used on normally distributed variables to test group differences, and Kruskal–Wallis H test was used if variables were not normally distributed. Pearson Chi-Square test was used to calculate the differences in sex. Post hoc comparisons with the method of the Tukey HSD test or Games-Howell test were used to investigate the between-group differences. We did not evaluate the effect of handedness in the current study due to the small number of left-handed participants in the current database (6 %).
Two-way between-groups multivariate analysis of covariance (MANCOVA) models was performed to investigate the influence of the S factor and P factor on the metamemory capacity and cortical thickness. The independent variables were the S-/+ and P-/+ groups. Nine dependent variables were used in the analyses of metamemory scores: ROJ-N4/N5/N6, EOP-N4/N5/N6, and DOC-N4/N5/N6. Eight dependent variables were used in the analyses of cortical thickness: eight ROIs of cortical thickness. Age and sex were used as covariates to control for demographic differences. The effect size assessment for each of the results was obtained using the partial eta squared (η2) proposed by Cohen, (Cohen, 1988) where 0.01 to 0.06 = small effect; 0.06 to 0.14 = moderate effect, and a value>0.14 = large effect. P values of < 0.05 (two-tailed) were considered statistically significant for the interaction effect. If there was an acceptable size of an effect, the marginally significant interaction effect could be observed at p < 0.1, (Armitage et al., 2019, Chang et al., 2010, Huang et al., 2021, Lochner et al., 2020) and the significance of the main effect was indicated by p < 0.05 (two-tailed). Partial correlation analysis was performed to calculate the correlations between imaging and neuropsychological scores, controlling for age, gender, and education, the level of significance was set at P < 0.05/n, and n was the number of imaging ROIs. The general linear model (GLM) of SPM12 was used to analyze the effect of two factors (S-/+, P-/+) on ALFF or fALFF, controlling for age, sex, and education. Results were reported at a statistical threshold of p < 0.05 (whole brain family-wise error (FWE) corrected at the cluster level, with a primary height threshold of p < 0.001).
3. Results
3.1. Demographics and cognitive performance scores
Demographic characteristics and cognitive performances were summarized in Table 1. 147 participants were included in the analysis, including 33 SCD S-P-, 47 SCD S-P+, 29 SCD S+P-, and 38 SCD S+P+. There were no significant differences among the four groups with regard to age, sex, education years, HAMA, HAMD, STT-A, and BNT. Bonferroni corrected post hoc analyses revealed the following results. SCD S+P+ had significantly lower MMSE scores than SCD S-P- (P = 0.021). Compared with SCD S-P- group, SCD S+P- and SCD S+P+ groups showed significantly lower MoCA-B performance (P = 0.029 and 0.023, respectively). Compared with SCD S-P-, SCD S+P- showed significantly higher scores in STT-B (P = 0.045). SCD S+P+ had significantly lower AFT performance than SCD S-P- and SCD S-P+ (P = 0.018 and 0.002, respectively). Regarding the AVLT-IR, SCD S+P- and SCD S+P+ had significantly lower scores than SCD S-P- (all P < 0.001) and SCD S-P+ (P = 0.001 and 0.006, respectively). Regarding the AVLT-N4, SCD S+P- and SCD S+P+ had significantly lower scores than SCD S-P- (P < 0.001 and P = 0.003) and SCD S-P+ (P < 0.001 and P = 0.017). Both SCD S+P- and SCD S+P+ had significantly lower AVLT-N5 scores than SCD S-P- and SCD S-P+ (all P < 0.001) and SCD S-P+ (all P < 0.001), respectively. SCD S+P- and SCD S+P+ showed significantly reduced AVLT-N6 scores than SCD S-P- (all P < 0.001) and SCD S-P+ (P < 0.001 and P = 0.010), respectively.
Table 1.
Demographics and cognitive performance scores.
| SCD S-P- |
SCD S-P+ |
SCD S+P- |
SCD S+P+ |
|
|---|---|---|---|---|
| n = 33 | n = 47 | n = 29 | n = 38 | |
| Age | 62.82 (8.12) | 63.15 (7.97) | 66.29 (8.58) | 64.39 (9.02) |
| Sex (M/F) | 5/28 | 8/39 | 10/19 | 10/28 |
| Education (year) | 13.88 (2.92) | 12.36 (2.94) | 12.68 (3.48) | 12.21 (2.76) |
| Mean cortical thickness (mm) | 2.33 (0.12) | 2.37 (0.15) | 2.30 (0.10) | 2.37 (0.11) |
| General cognitive function | ||||
| MMSE*§ | 28.61 (1.25) | 28.15 (1.53) | 27.72 (1.51) | 27.53 (1.83)b |
| MoCA-B**§ | 26.70 (2.54) | 26.26 (2.52) | 24.55 (3.29)a | 24.65(3.68)b |
| HAMA | 5.26 (2.55) | 7.33 (5.18) | 7.13 (5.13) | 8.23 (5.69) |
| HAMD§ | 3.25 (3.22) | 6.78 (5.62) | 5.43 (4.34) | 5.30 (5.12) |
| Executive function | ||||
| STT-A total time (second) | 41.21 (10.70) | 46.11 (14.49) | 45.50 (11.63) | 49.89 (17.62) |
| STT-B total time (second)* | 110.33 (37.04) | 110.17 (33.99) | 132.76 (42.62)c | 123.24 (32.72) |
| Language function | ||||
| AFT** | 18.06 (3.06) | 18.45 (4.11) | 16.76 (4.58) | 15.39 (3.15)b,d |
| BNT | 25.06 (3.21) | 25.45 (2.23) | 24.93 (2.31) | 23.95 (2.24) |
| Memory function | ||||
| Immediate recall (IR)*** | 20.42 (5.10) | 19.38 (4.83) | 15.03 (3.67)a,c | 15.95 (5.05)b,d |
| Short-term delay recall (N4)*** | 7.58 (2.11) | 7.13 (2.03) | 4.00 (1.98)a,c | 5.65 (2.92)b,d,e |
| Long-term delay recall (N5)***§ | 7.15 (2.15) | 6.98 (1.97) | 3.55 (2.13)a,c | 4.87 (3.11)b,d |
| Long-term cued recall (N6)*** | 7.39 (2.44) | 6.62 (2.27) | 3.61 (2.28)a,c | 4.89 (2.91)b,d |
*: P < 0.05; **: P < 0.01; ***: P < 0.001. §: Games-Howell test, rest of items with Tukey HSD test. P-/+: peer-related self-evaluation of cognition; S-/+:slight cognitive impairment; MMSE, Mini-Mental State Examination; MoCA-B, Montreal Cognitive Assessment Basic Version; HAMA, Hamilton Anxiety Rating Scale; HAMD, Hamilton Depression Rating Scale; STT, Shape Trails Test Parts; AFT, Animal Verbal Fluency Test; BNT, Boston Naming Test.
Significant difference between SCD S-P- and SCD S+P-, P < 0.05.
Significant difference between SCD S-P- and SCD S+P+, P < 0.05.
Significant difference between SCD S-P+ and SCD S+P-, P < 0.05.
Significant difference between SCD S-P+ and SCD S+P+, P < 0.05.
Significant difference between SCD S+P- and SCD S+P+, P < 0.05.
3.2. Metamemory capacity scores
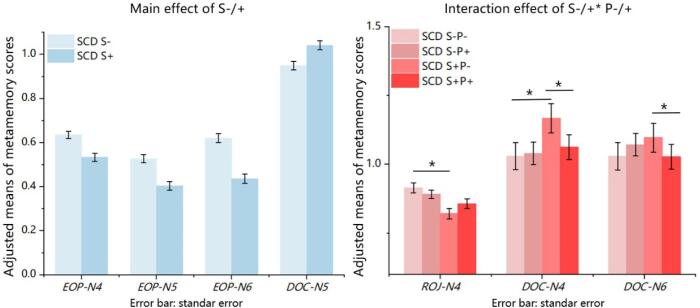
The two-way MANCOVA analysis revealed a significant main effect for the S factor (F (1, 141) = 8.748, P < 0.001, Wilks' Lambda 0.688, large effect size of partial η2 = 0.312), and a marginally significant interaction between the S and P factors (F (1, 141) = 2.057, p = 0.052, Wilks' Lambda 0.904, moderate effect size of partial η2 = 0.096). Further analyses of independent variables revealed a significant main effect for the S factor on EOP-N4 (F = 17.394, P < 0.001), EOP-N5 (F = 20.536, P < 0.001), EOP-N6 (F = 38.052, P < 0.001), DOC-N5 (F = 10.062, P = 0.002). The values of EOP-N4, N5, and N6 in SCD S+ were significantly decreased compared with SCD S- (all P < 0.001), while the DOC-N5 in SCD S+ was significantly increased compared with SCD S- (P = 0.002). Moreover, there was a significant interaction effect on DOC-N4 (F = 5.798, P = 0.017), DOC-N6 (F = 5.452, P = 0.021), and a marginally significant S × P interaction effect on ROJ-N4 (F = 3.035, P = 0.084) were reported. (Fig. 1; Supplementary materials, Tables 1 and 2). Bonferroni corrected post hoc analyses revealed the following results. The mean value of ROJ-N4 in SCD S+P- was significantly lower than SCD S-P- (P < 0.001). The DOC-N4 in SCD S+P- was significantly higher than SCD S-P- (P < 0.001) and SCD S+P+ (P = 0.003). The value of DOC-N6 in SCD S+P- was significantly higher than SCD S+P+ (P = 0.048) (Fig. 1; Supplementary materials, Table 3).
Fig. 1.
Main effect and interaction effect on metamemory scores. After controlling for the influence of age and sex. Bonferroni's adjusted group comparisons showed the significant difference between SCD S- and SCD S+ on EOP-N4, N5, N6, and DOC-N5, all with P < 0.05, and post hoc analyses for interaction effect on ROJ-N4, DOC-N4, and DOC-N6.*: P < 0.05.
3.3. Functional brain imaging analyses
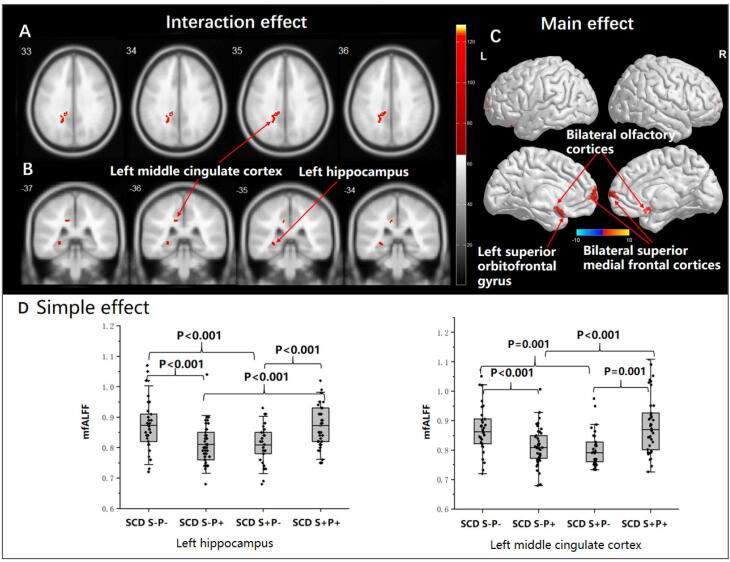
The full factorial ANOVA revealed a significant main effect for the P factor. Compared with SCD P- groups, decreased ALFF signals were found in three clusters in the SCD P+ groups (P < 0.05, FWE-corrected, cluster-wise level), including the left superior orbitofrontal gyrus, bilateral olfactory cortices, and bilateral superior medial frontal cortices (See Table 2 and Fig. 2). No main effect for the S factor and no interaction effect was found for the ALFF signals.
Table 2.
Functional brain imaging results.
| Clusters | BA | Volumes | x, y, z | T | Z | |
|---|---|---|---|---|---|---|
| ALFF | ||||||
| Main effect for the P-/+ (P- > P + ) | ||||||
| Cluster 1 | BA11 | 100 | -12 22 -28 | 4.98 | 4.77 | |
| Left superior orbitofrontal gyrus, extending to left inferior orbitofrontal gyrus | ||||||
| Cluster 2 | BA25 | 121 | -10 10 -16 | 4.19 | 4.06 | |
| Bilateral olfactory cortices, extending to left rectus | ||||||
| Cluster 3 | BA10 | 140 | 0 68 10 | 4.62 | 4.45 | |
| Bilateral superior medial frontal cortices | ||||||
| fALFF | ||||||
| Interaction effect of S-/+*P-/+ | ||||||
| Cluster 1 | BA23 | 81 | -14 -38 34 | 4.43 | 4.28 | |
| Left middle cingulate cortex | ||||||
| Cluster 2 | BA37 | 74 | -28 -24 2 | 4.20 | 4.07 | |
| Left hippocampus | ||||||
P-/+: peer-related self-evaluation of cognition; S-/+:slight cognitive impairment; ALFF = the amplitude of the low frequency fluctuation; BA = Brodmann area; fALFF = fractional low-frequency fluctuation amplitude.
P < 0.05, FWE-corrected, cluster-wise level.
Fig. 2.
Interaction effect, main effect, and simple effect analyses in fMRI. Interaction effect, main effect, and simple effect analyses in fMRI, controlling with the influence of age, sex, and education. A-B: The interaction effect between the S and P factors was found for fALFF signals in the left middle cingulate cortex and the left hippocampus (all P < 0.05, FWE-corrected, cluster-wise level); C: The main effect of the P factor was found for decreased ALFF signals in the SCD P+ groups, including the left superior orbitofrontal gyrus, bilateral olfactory cortices, and bilateral superior medial frontal cortices (all P < 0.05, FWE-corrected, luster-wise level); D: Mean fALFF (mfALFF) values were extracted from the left hippocampus and left middle cingulate cortex, simple effect analyses were performed in two regions.
The interaction effect between the S and P factors was found for fALFF signals in the left middle cingulate cortex and the left hippocampus (P < 0.05, FWE-corrected, cluster-wise level). No significant main effect for S or P factor was found for the fALFF signals (See Table 2 and Fig. 2).
Mean fALFF values were extracted from the significant voxels within the clusters of the left middle cingulate cortex and the left hippocampus to characterize the direction and magnitude of the interaction effects. The simple effect analyses revealed the following results. Compared with the SCD S-P-, significantly decreased fALFF signals in SCD S-P+ and SCD S+P- were reported in the left hippocampus (all P < 0.001). Compared with the SCD S+P+, the left hippocampus reported significantly reduced fALFF signals in SCD S-P+ and SCD S+P- (all P < 0.001). Moreover, significantly increased fALFF signals in the left middle cingulate cortex were found in the SCD S-P- subgroup compared with SCD S+P- (P = 0.001) and SCD S-P+ (P < 0.001), and in the SCD S+P+ subgroup compared with S+P- (P = 0.001) and SCD S-P+ (P < 0.001) (Fig. 2).
3.4. Interaction, main effects on cortical thickness
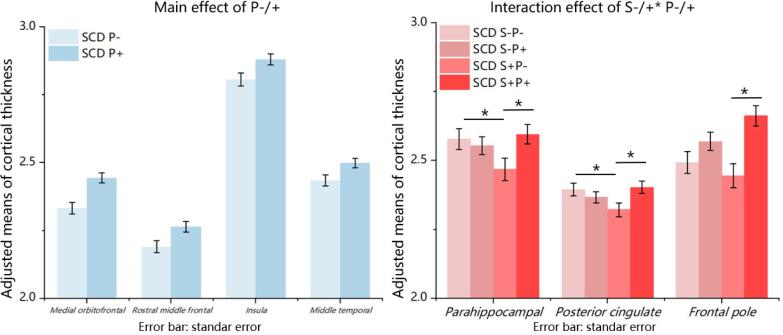
The two-way MANCOVA analysis showed a significant main effect for the S factor (F (1, 141) = 2.363, P = 0.021, Wilks' Lambda 0.876, moderate effect size of partial η2 = 0.124), a significant main effect for the P factor (F (1, 141) = 2.77, P = 0.007, Wilks' Lambda 0.858, large effect size of partial η2 = 0.142), and a significant interaction effect between the two factors (F (1, 141) = 2.782, P = 0.007; Wilks' Lambda 0.858, large effect size of partial η2 = 0.142), after controlling for age and sex (supplementary materials, Table 4). Further analyses of the independent variables revealed a significant main effect for the P factor. The SCD P- subgroups showed significantly decreased cortical thickness in the medial orbitofrontal (F = 16.466, P < 0.001), rostral middle frontal (F = 6.654, P = 0.011), insular (F = 5.578, P = 0.020), and middle temporal cortical regions (F = 5.771, P = 0.018), compared with the SCD P+ subgroups. Moreover, significant S × P interactions were found for cortical thickness in the parahippocampal cortex (F = 4.28, P = 0.040) and posterior cingulate cortex (F = 6.011, P = 0.015). A marginally significant S × P interaction effect was reported on the frontal pole cortex (F = 3.455, P = 0.065). No significant main effect for the S factor was found for the cortical thickness measure. (Fig. 3; supplementary materials, Table 5). Bonferroni corrected post hoc analyses revealed the following results. The cortical thickness in parahippocampal in SCD S+P- subgroup was significantly thinner than SCD S-P- (P = 0.048) and SCD S+P+ (P = 0.018) subgroups. The posterior cingulate cortices in SCD S+P- subgroup was significantly thinner than SCD S-P- (P = 0.033) and SCD S+P+ (P = 0.015) subgroups. The frontal pole cortex in the SCD S+P- was significantly thinner than the SCD S+P+ subgroup (P < 0.001) (Fig. 3; supplementary materials, Table 6). The entorhinal cortex was not significant with any effect. In addition, we demonstrated the patterns of all cognitive and neuroimaging results among all four SCD subgroups (Fig. 4).
Fig. 3.
Main effect and interaction effect on cortical thickness. Left: Bonferroni's adjusted group comparisons showed the significant difference between SCD P- and SCD P+ on the cortical thickness of medial orbitofrontal, rostral middle frontal, insula, and middle temporal cortices, all with P < 0.05; Right: Bonferroni corrected post hoc analyses for interaction effect on the cortical thickness of parahippocampal and posterior cingulate cortices and frontal pole, *: P < 0.05; All results were controlled for age and sex.
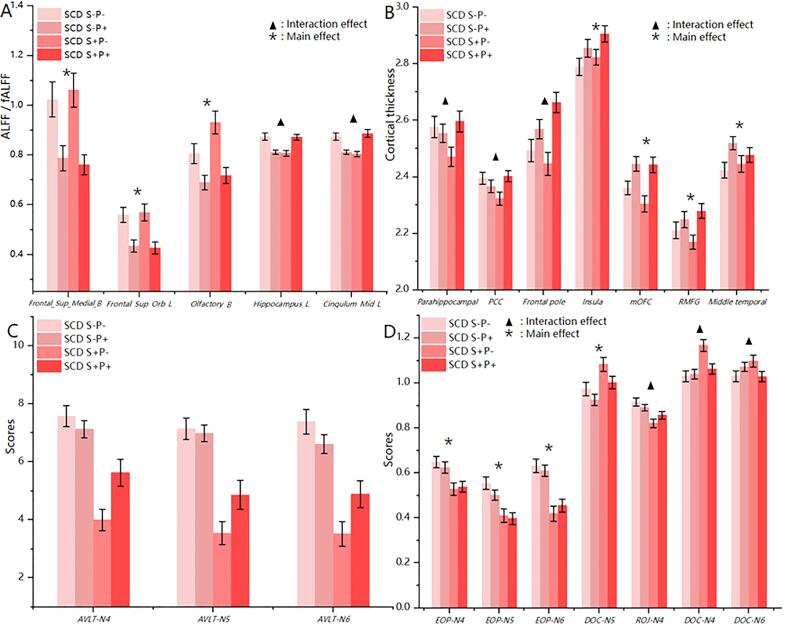
Fig. 4.
The alternations of neuroimaging and cognitive scores among four groups. ALFF/fALFF: the mean ALFF value of Frontal_Sup_Medial_B, Frontal_Sup_Orb_L and Olfactory_B/ the mean fALFF value of Hippocampus_L and Cingulum_Mid_L. Frontal_Sup_Medial_B, Bilateral superior medial frontal cortices; Frontal_Sup_Orb_L, Left superior orbitofrontal gyrus; Olfactory_B, Bilateral olfactory cortices; Hippocampus_L, Left hippocampus; Cingulum_Mid_L, Left middle cingulate cortex; PCC, Posterior cingulate cortex; mOFC, Medial orbitofrontal cortex; RMFG, Rostral middle frontal gyus.
3.5. Partial correlations
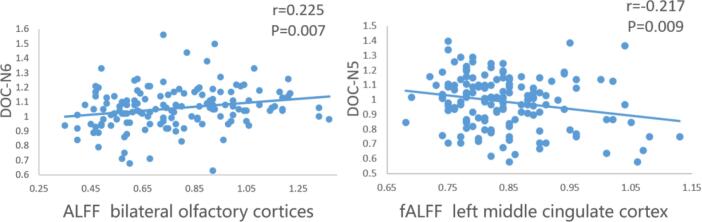
In the entire group, partial correlations between five clusters of fMRI indices (see Table 2) and neuropsychological performances were performed, controlling for age, sex, and education years. (See Fig. 5). The ALFF value of the bilateral olfactory cortex was positively correlated with DOC-N6 (r = 0.225, P = 0.007). The fALFF value of the left middle cingulate cortex was negatively correlated with DOC-N5 (r = -0.217, P = 0.009). There was no significant correlation between cortical thickness and neuropsychological performances (P > 0.05/8 = 0.006).
Fig. 5.
Partial correlations between ROIs and neuropsychological performances. Partial correlation analyses were conducted between five clusters of fMRI and neuropsychological performances among all participants, controlling for age, sex, and education years. The level of significance was set at P < 0.05/5 = 0.01.
4. Discussion
The main findings of this study were decreased cognitive performance and altered metamemory performance (overconfidence and less accuracy of judgment) in a subtype of SCD with slight cognitive impairment and no feeling of worse performance than the peers. We also found decreased cortical thickness and altered intensity of spontaneous brain activity in the medial temporal lobe and midline cortical areas in this subtype of SCD individuals. These results suggested that SCD subjects are heterogeneous with respect to metamemory capacity, which may be related to structural and functional alterations in key brain areas of metamemory processing.
We found decreased general cognition in SCD S+P- (MoCA) and SCD S+P+ (MMSE, MoCA) compared with SCD S-P-. Interestingly, SCD S+P- exhibited the worst short-term delay recall (AVLT-N4) among the four groups. We also found the individuals with SCD S+P- showed the worst metamemory performance, indicated by the highest value in DOC-N4, N6, and worst value of ROJ-N4. DOC scores reflect the degree of overconfidence (value above 1) or underconfidence (value below 1). Our results showed that individuals with SCD S+P- tend to be overconfident in their performance on the short-term (5 min) delay recall and long-term cued recall. There was a significant deviation between self-estimated performance and actual performance on AVLT-N4 in SCD S+P-.
To our knowledge, this is the first study to associate the metamemory capacity with the feature of a feeling of worse performance relative to peers in SCD. We used objective methods to measure metamemory under the influence of with and without a slight cognitive impairment in individuals with SCD. We detected a subtype of SCD with reduced metamemory capacity. Previous studies showed that reduced metamemory capacity might potentially influence the accuracy of self-judgment of cognitive performance (Saenz et al., 2017, Bahrami et al., 2020). Our findings indicate that at least a subtype of SCD may have distorted self-awareness of own cognition, which may interfere with the self-monitoring, self-judgment, and self-report of one's cognition in the SCD stage. The results obtained in our study were consistent with those of the previous studies on metamemory in MCI and AD dementia, (Edmonds et al., 2018, Rosen et al., 2014) such that impaired cognitive state is often accompanied by disturbed self-awareness of memory deficits. Significant patient/caregiver discrepancies were found in MCI on objective scales (Everyday Cognition Questionnaire, ECog) or impaired performance on Feeling of Knowing a metacognition task). (Edmonds et al., 2018, Rosen et al., 2014) There were discrepancies between self and objective (informant or scales) reports in patients with MCI and AD. (Fragkiadaki et al., 2016, Souchay, 2007, Steward et al., 2019a, Wadley et al., 2003).
In our study, the supporting evidence for the discrepancies between subjective feelings and objective performance in a subgroup of SCD (S+P-) was the significantly reduced cognitive performance and the worst metamemory capacity (the highest value in DOC-N4, N6, and worst value of ROJ-N4) compared to other SCD subgroups. These results suggested that they had lost some of the accuracies on judgments and tended to be overconfident, though they did not feel any worse off than their peers. The feature of overconfidence on cognitive performance was reported in mild AD dementia and MCI in previous studies; older people with cognitive impairment may lack sufficient self-awareness of their deficits—leading to underreporting of their difficulties. (Fragkiadaki et al., 2016, Souchay, 2007, Steward et al., 2019a) The inability to recognize cognitive, behavioral, or functional impairment occurring as a complex phenomenon of a dementing illness is indicated as “anosognosia”, “lack of insight”, or “unawareness of disease”. (Tondelli et al., 2018) Previous studies indicated that memory dysfunction might affect the immediate ability to judge cognitive performance, (Ansell and Bucks, 2006, Hannesdottir and Morris, 2007, Martyr et al., 2014, Tondelli et al., 2018) and anosognosia in AD can in part be explained by a loss of mnemonic ability in which knowledge about self-ability is degraded. (Robin and Daniel, 2013).
We found significant differences between SCD P- and SCD P+ in functional and structural imaging. Compared with SCD P+, SCD P- subjects showed decreased cortical thickness in the medial orbitofrontal, rostral middle frontal, insular, and middle temporal regions. This finding is consistent with previous studies showing associations between impaired metacognition or anosognosia and AD pathophysiology (tauopathy, (Vannini et al., 2019) atrophy, (Cosentino et al., 2015, Genon et al., 2016, Steward et al., 2019b) abnormal glucose metabolism, (Perrotin et al., 2015) or dysfunction (Zamboni et al., 2013, Perrotin et al., 2015) in the middle temporal lobe, (Genon et al., 2016, Vannini et al., 2019) orbital frontal lobe, (Lak et al., 2014, Hallam et al., 2020, Perrotin et al., 2015) insula, (Cosentino et al., 2015, Steward et al., 2019b) and middle frontal cortex. (Genon et al., 2016, Zamboni et al., 2013, Hallam et al., 2020) The lack of insight of worse cognition than peers may reflect a reduced ability of ongoing appraisal and monitoring of their cognitive performance in everyday life, probably related to the altered structure in the key areas of metamemory processing. We also found a heightened intensity of spontaneous brain activity (ALFF) in the medial frontal regions (left superior orbitofrontal, bilateral superior medial frontal, and bilateral olfactory areas) in the SCD P- than in the SCD P+ subjects. Previous findings on the association between the spontaneous brain activity value and anosognosia are heterogeneous. Both increased and decreased intrinsic functional connectivity values associated with patients with disturbed self-awareness/consciousness have been observed. (Perrotin et al., 2015, Yao et al., 2015) Therefore, our findings on the association between spontaneous fluctuation measured by ALFF and the P factor remain to be investigated in more detail in the future.
In addition, we found that the parahippocampal, posterior cingulate, and frontal pole cortices were correlated with both the P and S factors. Previous neuroimaging studies have implicated that the frontal cortex, (Hu et al., 2017) posterior cingulate cortex (PCC) were the neural basis of self-awareness. (Hallam et al., 2020) There was intrinsic connectivity between PCC and hippocampus areas belonging to the default mode network. (Perrotin et al., 2015) A personal database (memory storage) plays a crucial role in memory awareness, and the parahippocampal plays a key role in memory encoding and episodic memory. (Hu et al., 2017) Long-term memory impairment can cause a deterioration in self-awareness. (Tondelli et al., 2018) A previous study mentioned that disruption of the cingulum bundle is related to perturbation of the hippocampus. (Villain et al., 2008) Our partial correlation analyses also revealed a significant positive correlation between bilateral olfactory cortices ALFF value and the degree of confidence for long-term cued recall (DOC-N6) and a negative correlation between left middle cingulate cortex fALFF value and the degree of confidence for long-term delayed recall (DOC-N5). Both the olfactory cortex (i.e., a subregion of the ventromedial prefrontal cortex) and the cingulate cortex are crucial regions for self-awareness. (Bertrand et al., 2018, Huntley et al., 2021) It is not clear why activations in these two regions showed opposite associations with the metamemory performance and should be investigated in more detail in the future.
SCD has been accepted as a risk condition for AD. Nevertheless, it is also recognized as a heterogeneous construct. Efforts have been made to identify additional features that may increase the likelihood of preclinical AD in this population, which is known as SCD-plus criteria. In the current set of SCD-plus features suggested by the international working group, (Jessen et al., 2020) the feeling of worse performance than others of the same age group has been removed due to a lack of further supporting evidence. However, a tremendous amount of SCD individuals in the clinical practice in east china complain of have worse cognitive states when compared with peers. Indeed, all existing pieces of evidence for SCD-plus criteria are based on research findings from western SCD cohorts. Our study with the SCD subjects from the Asian population provided additional supporting evidence for the importance of this SCD feature (together with the feature of subtle cognitive decline). Future studies need to quantify the likelihood of AD pathology and longitudinal cognitive changes in this specific subtype of SCD.
5. Conclusion
In the current study, we found that a subtype of SCD (SCD S+P-) with slight cognitive impairment had the poorest memory performance and biased metamemory performance (lack of judgment accuracy and overconfidence in their own memory performance) compared to other SCD individuals, despite subjectively feeling similar performance compared to peers. Meanwhile, SCD S+P- showed decreases in regional cortical thickness and altered intensity of spontaneous brain activity in the midline brain areas and medial temporal lobe, which may underlie the alterations in the metamemory capacity in these subjects. This is the first study on metamemory performance in SCD with different subtypes. The current analysis is a part of an ongoing large-scale multimodal imaging study. Future investigations will include more participants and incorporate other modalities such as molecular imaging and blood-based biomarkers.
Ethics approval
The study was approved by the ethics committee of Shanghai sixth people's hospital; ID: 2019–041.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We want to thank all the subjects that participated in this study.
Funding
This work was supported by the National Key R&D Program of China(2016YFC1306305、2018YFE0203600), the Guangdong Provincial Key S&T Program (2018B030336001), and the Hirnliga fond (Germany) and Cologne.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103255.
Contributor Information
Xiaochen Hu, Email: xiaochen.hu@uk-koeln.de.
Qihao Guo, Email: qhguo@sjtu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data are not publicly available due to privacy issues of clinical data.
References
- Albert M.S., DeKosky S.T., Dickson D., et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amieva H., Le Goff M., Millet X., et al. Prodromal Alzheimer's disease: Successive emergence of the clinical symptoms. Ann. Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Skare S., Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage (Orlando, Fla.) 2003;20:870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Ansell Eleanor L., Bucks Romola S. Mnemonic anosognosia in Alzheimer’s disease: A test of Agnew and Morris (1998) Neuropsychologia. 2006;44:1095–1102. doi: 10.1016/j.neuropsychologia.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Armitage J., Baigent C., Barnes E., et al. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. The Lancet. 2019;393:407–415. doi: 10.1016/S0140-6736(18)31942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahador Bahrami, Hertz U and Kanai R, et al. The effect of feedback valence and source on perception and metacognition: An fMRI investigation. Cogn Neurosci 2020: 1-09. Journal Article. DOI: 10.1080/17588928.2020.1828323. [DOI] [PubMed]
- Bertrand E, Azar M and Rizvi B, et al. Cortical thickness and metacognition in cognitively diverse older adults. Neuropsychology 2018; 32: 700-10. Journal Article. DOI: 10.1037/neu0000458. [DOI] [PMC free article] [PubMed]
- Bertrand JM, Mazancieux A and Moulin C, et al. In the here and now: Short term memory predictions are preserved in Alzheimer's disease. Cortex 2019; 119: 158-64. Journal Article; Research Support, Non-U.S. Gov't. DOI: 10.1016/j.cortex.2019.03.027. [DOI] [PubMed]
- Bondi M.W., Edmonds E.C., Jak A.J., et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimer's disease. 2014;42:275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang V.W., Asch D.A., Werner R.M. Quality of care among obese patients. JAMA. 2010;303(13):1274–1281. doi: 10.1001/jama.2010.339. [DOI] [PubMed] [Google Scholar]
- Chen K., Xu Y., Chu A., et al. Validation of the Chinese Version of Montreal Cognitive Assessment Basic for Screening Mild Cognitive Impairment. Journal of the American Geriatrics Society (JAGS) 2016;64:e285. doi: 10.1111/jgs.14530. e290. [DOI] [PubMed] [Google Scholar]
- Cohen Jacob. Statistical power analysis for the behavioral sciences. NJ: Hillsdale. 1988 doi: 10.4324/9780203771587. [DOI] [Google Scholar]
- Cosentino S., Brickman A.M., Griffith E., et al. The right insula contributes to memory awareness in cognitively diverse older adults. Neuropsychologia. 2015;75:163–169. doi: 10.1016/j.neuropsychologia.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. 2006;31::968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- D’Oleire Uquillas F., Jacobs H.I.L., Schultz A.P., et al. Functional and Pathological Correlates of Judgments of Learning in Cognitively Unimpaired Older Adults. Cerebral cortex (New York, N.Y. 2020;30:1974–1983. doi: 10.1093/cercor/bhz217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds E.C., Weigand A.J., Thomas K.R., et al. Increasing Inaccuracy of Self-Reported Subjective Cognitive Complaints Over 24 Months in Empirically Derived Subtypes of Mild Cognitive Impairment. J. Int. Neuropsych. Soc. 2018;24:842–853. doi: 10.1017/S1355617718000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH and Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341-55. Comparative Study; Journal Article; Research Support, U.S. Gov't, P.H.S. DOI: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed]
- Fischl B., Dale A.M. Measuring the Thickness of the Human Cerebral Cortex from Magnetic Resonance Images. Proceedings of the National Academy of Sciences - PNAS. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Tootell R.B.H., et al. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM and Dolan RJ. The neural basis of metacognitive ability. Philos Trans R Soc Lond B Biol Sci 2012; 367: 1338-49. Journal Article; Research Support, Non-U.S. Gov't; Review. DOI: 10.1098/rstb.2011.0417. [DOI] [PMC free article] [PubMed]
- Fragkiadaki S., Kontaxopoulou D., Beratis I.N., et al. Self-awareness of cognitive efficiency: Differences between healthy elderly and patients with mild cognitive impairment (MCI) J. Clin. Exp. Neuropsyc. 2016;38:1144–1157. doi: 10.1080/13803395.2016.1198469. [DOI] [PubMed] [Google Scholar]
- Genon S., Simon J., Bahri M.A., et al. Relating pessimistic memory predictions to Alzheimer's disease brain structure. Cortex. 2016;85:151–164. doi: 10.1016/j.cortex.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Hallam B, Chan J and Gonzalez Costafreda S, et al. What are the neural correlates of meta-cognition and anosognosia in Alzheimer's disease? A systematic review. Neurobiol. Aging 2020; 94: 250-64. Journal Article; Research Support, Non-U.S. Gov't; Systematic Review. DOI: 10.1016/j.neurobiolaging.2020.06.011. [DOI] [PMC free article] [PubMed]
- Hannesdottir K., Morris R.G. Primary and Secondary Anosognosia for Memory Impairment in Patients with Alzheimer's Disease. Cortex. 2007;43:1020–1030. doi: 10.1016/S0010-9452(08)70698-1. [DOI] [PubMed] [Google Scholar]
- Hu X., Liu Z., Chen W., et al. Individual Differences in the Accuracy of Judgments of Learning Are Related to the Gray Matter Volume and Functional Connectivity of the Left Mid-Insula. Front Hum Neurosci. 2017;11:399. doi: 10.3389/fnhum.2017.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Hung C., Hsu S., et al. Effects of aerobic walking on cognitive function in patients with schizophrenia: A randomized controlled trial. J. Psychiatr. Res. 2021;134:173–180. doi: 10.1016/j.jpsychires.2020.12.062. [DOI] [PubMed] [Google Scholar]
- Huntley JD, Fleming SM and Mograbi DC, et al. Understanding Alzheimer's disease as a disorder of consciousness. Alzheimers Dement (N Y) 2021; 7: e12203. Journal Article. DOI: 10.1002/trc2.12203. [DOI] [PMC free article] [PubMed]
- Jack C.R., Bennett D.A., Blennow K., et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & Dementia. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F., Wolfsgruber S., Kleineindam L., et al. Subjective cognitive decline and stage 2 of Alzheimer disease in patients from memory centers. Alzheimer's & dementia. 2022 doi: 10.1002/alz.12674. [DOI] [PubMed] [Google Scholar]
- Jessen F., Amariglio R.E., Buckley R.F., et al. The characterisation of subjective cognitive decline. The Lancet Neurology. 2020;19:271–278. doi: 10.1016/S1474-4422(19)30368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE and van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 2014; 10: 844-52. Journal Article; Review. DOI: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed]
- Jessen F., Wiese B., Bachmann C., et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Archives of general psychiatry. 2010;67:414. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- Jia X., Wang J., Sun H., et al. RESTplus: an improved toolkit for resting-state functional magnetic resonance imaging data processing. Science bulletin (Beijing) 2019;64:953–954. doi: 10.1016/j.scib.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Lak A., Costa G.M., Romberg E., et al. Orbitofrontal cortex is required for optimal waiting based on decision confidence. Neuron (Cambridge, Mass.) 2014;84:190–201. doi: 10.1016/j.neuron.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner C, Chamberlain SR and Kidd M, et al. The effects of acute serotonin challenge on executive planning in patients with obsessive-compulsive disorder (OCD), their first-degree relatives, and healthy controls. Psychopharmacology (Berl) 2020; 237: 3117-23. Journal Article; Randomized Controlled Trial. DOI: 10.1007/s00213-020-05597-7. [DOI] [PMC free article] [PubMed]
- Leicht Hanna, Berwig Martin, Gertz Hermann-Josef. Anosognosia in Alzheimer’s disease: The role of impairment levels in assessment of insight across domains. J. Int. Neuropsych. Soc. 2010;16:463–473. doi: 10.1017/S1355617710000056. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Freed DM and Williams BW, et al. Boston Naming Test: shortened versions for use in Alzheimer's disease. J Gerontol 1992; 47: P154-58. Journal Article; Research Support, U.S. Gov't, P.H.S. DOI: 10.1093/geronj/47.3.p154. [DOI] [PubMed]
- Martyr A., Nelis S.M., Clare L. Predictors of perceived functional ability in early-stage dementia: self-ratings, informant ratings and discrepancy scores. Int. J. Geriatr. Psych. 2014;29:852–862. doi: 10.1002/gps.4071. [DOI] [PubMed] [Google Scholar]
- Meiberth D., Scheef L., Wolfsgruber S., et al. Cortical thinning in individuals with subjective memory impairment. Journal of Alzheimer's disease. 2015;45:139–146. doi: 10.3233/JAD-142322. [DOI] [PubMed] [Google Scholar]
- Miebach L., Wolfsgruber S., Polcher A., et al. Which features of subjective cognitive decline are related to amyloid pathology? Findings from the DELCODE study. Alzheimer's research & therapy. 2019;11:66. doi: 10.1186/s13195-019-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A.J., Beaumont H., Ferguson D., et al. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiat. Scand. 2014;130:439–451. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- Modirrousta M., Fellows L.K. Medial prefrontal cortex plays a critical and selective role in ‘feeling of knowing’ meta-memory judgments. Neuropsychologia. 2008;46:2958–2965. doi: 10.1016/j.neuropsychologia.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Perrotin A, Desgranges B and Landeau B, et al. Anosognosia in Alzheimer disease: Disconnection between memory and self-related brain networks. Ann. Neurol. 2015; 78: 477-86. Journal Article; Research Support, Non-U.S. Gov't. DOI: 10.1002/ana.24462. [DOI] [PubMed]
- Perrotin Audrey, Mormino Elizabeth, Madison Cindee, et al. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch. Neurol. 2021;69:223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Alcantar O and Zakrzewski J, et al. Metacognition in the behavioral variant of frontotemporal dementia and Alzheimer's disease. Neuropsychology 2014; 28: 436-47. Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't. DOI: 10.1037/neu0000012. [DOI] [PMC free article] [PubMed]
- Robin, G. Morris, Daniel, C., 2013. Anosognosia, autobiographical memory and self knowledge in Alzheimer’s disease. Cortex 49, 1553–1564. 10.1016/j.cortex.2012.09.006. [DOI] [PubMed]
- Saenz G.D., Geraci L., Miller T.M., et al. Metacognition in the classroom: The association between students’ exam predictions and their desired grades. Conscious. Cogn. 2017;51:125–139. doi: 10.1016/j.concog.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage (Orlando, Fla.) 2004;23:S208. doi: 10.1016/j.neuroimage.2004.07.051. S219. [DOI] [PubMed] [Google Scholar]
- Souchay C. Metamemory in Alzheimer's Disease. Cortex. 2007;43:987–1003. doi: 10.1016/S0010-9452(08)70696-8. [DOI] [PubMed] [Google Scholar]
- Souchay C., Isingrini M., Pillon B., et al. Metamemory Accuracy in Alzheimer's Disease and Frontotemporal Lobe Dementia. Neurocase. 2003;9:482–492. doi: 10.1076/neur.9.6.482.29376. [DOI] [PubMed] [Google Scholar]
- Starkstein S.E., Jorge R., Mizrahi R., et al. A diagnostic formulation for anosognosia in Alzheimer’s disease. Journal of neurology, neurosurgery and psychiatry. 2006;77:719–725. doi: 10.1136/jnnp.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE. Anosognosia in Alzheimer's disease: diagnosis, frequency, mechanism and clinical correlates. Cortex 2014; 61: 64-73. Journal Article; Research Support, Non-U.S. Gov't; Review. DOI: 10.1016/j.cortex.2014.07.019. [DOI] [PubMed]
- Steward K.A., Bull T.P., Wadley V.G. Differences in self-awareness of functional deficits between amnestic single- and multidomain mild cognitive impairment. J. Clin. Exp. Neuropsyc. 2019;41:544–553. doi: 10.1080/13803395.2019.1586839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward K.A., Kennedy R., Erus G., et al. Poor awareness of IADL deficits is associated with reduced regional brain volume in older adults with cognitive impairment. Neuropsychologia. 2019;129:372–378. doi: 10.1016/j.neuropsychologia.2019.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G., McGeown W.J., Shanks M.F., et al. Anosognosia for memory impairment in Alzheimer's disease. Acta Neuropsychiatr. 2010;22:180–187. doi: 10.1111/j.1601-5215.2010.00463.x. [DOI] [PubMed] [Google Scholar]
- Tondelli M., Barbarulo A.M., Vinceti G., et al. Neural Correlates of Anosognosia in Alzheimer's Disease and Mild Cognitive Impairment: A Multi-Method Assessment. Front Behav Neurosci. 2018;12:100. doi: 10.3389/fnbeh.2018.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, D'Oleire UF and Jacobs H, et al. Decreased meta-memory is associated with early tauopathy in cognitively unimpaired older adults. Neuroimage Clin 2019; 24: 102097. Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't. DOI: 10.1016/j.nicl.2019.102097. [DOI] [PMC free article] [PubMed]
- Verfaillie S, Timmers T and Slot R, et al. Amyloid-beta Load Is Related to Worries, but Not to Severity of Cognitive Complaints in Individuals With Subjective Cognitive Decline: The SCIENCe Project. Front Aging Neurosci 2019; 11: 7. Journal Article. DOI: 10.3389/fnagi.2019.00007. [DOI] [PMC free article] [PubMed]
- Villain N, Desgranges B and Viader F, et al. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer's disease. J. Neurosci. 2008; 28: 6174-81. Journal Article; Research Support, Non-U.S. Gov't. DOI: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed]
- Wadley VG, Harrell LE and Marson DC. Self- and informant report of financial abilities in patients with Alzheimer's disease: reliable and valid? J. Am. Geriatr. Soc. 2003; 51: 1621-26. Journal Article; Research Support, U.S. Gov't, P.H.S. DOI: 10.1046/j.1532-5415.2003.51514.x. [DOI] [PubMed]
- Yao S, Song J and Gao L, et al. Thalamocortical Sensorimotor Circuit Damage Associated with Disorders of Consciousness for Diffuse Axonal Injury Patients. J. Neurol. Sci. 2015; 356: 168-74. Journal Article; Research Support, Non-U.S. Gov't. DOI: 10.1016/j.jns.2015.06.044. [DOI] [PubMed]
- Zamboni G., Drazich E., McCulloch E., et al. Neuroanatomy of impaired self-awareness in Alzheimer's disease and mild cognitive impairment. Cortex. 2013;49:668–678. doi: 10.1016/j.cortex.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Guo Q and Hong Z. Clustering and switching during a semantic verbal fluency test contribute to differential diagnosis of cognitive impairment. Neurosci Bull 2013; 29: 75-82. Comparative Study; Journal Article; Research Support, Non-U.S. Gov't. DOI: 10.1007/s12264-013-1301-7. [DOI] [PMC free article] [PubMed]
- Zhao Q, Guo Q and Li F, et al. The Shape Trail Test: application of a new variant of the Trail making test. Plos One 2013; 8: e57333. Journal Article; Research Support, Non-U.S. Gov't. DOI: 10.1371/journal.pone.0057333. [DOI] [PMC free article] [PubMed]
- Zhao Q, Guo Q and Liang X, et al. Auditory Verbal Learning Test is Superior to Rey-Osterrieth Complex Figure Memory for Predicting Mild Cognitive Impairment to Alzheimer's Disease. Curr Alzheimer Res 2015; 12: 520-26. Journal Article; Research Support, Non-U.S. Gov't. DOI: 10.2174/1567205012666150530202729. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are not publicly available due to privacy issues of clinical data.