Highlights
-
•
Menopause involves decreased oestrogen and higher risk of obesity, which can impact brain health.
-
•
Body composition, oestrogen exposure, and brain health post-menopause is largely unexplored.
-
•
Higher adipose tissue post-menopause is linked to older brain age and WM hyperintensities.
-
•
Associations were stronger in females with longer reproductive spans.
-
•
Body-brain associations post-menopause may partly depend on lifetime oestrogen exposure.
Keywords: Brain age, White matter hyperintensities, Adipose tissue, Cardiometabolic health, Body MRI, Menopause, Reproductive span, Polygenic scores, UK Biobank
Abstract
The menopause transition involves changes in oestrogens and adipose tissue distribution, which may influence female brain health post-menopause. Although increased central fat accumulation is linked to risk of cardiometabolic diseases, adipose tissue also serves as the primary biosynthesis site of oestrogens post-menopause. It is unclear whether different types of adipose tissue play diverging roles in female brain health post-menopause, and whether this depends on lifetime oestrogen exposure, which can have lasting effects on the brain and body even after menopause. Using the UK Biobank sample, we investigated associations between brain characteristics and visceral adipose tissue (VAT) and abdominal subcutaneous adipose tissue (ASAT) in 10,251 post-menopausal females, and assessed whether the relationships varied depending on length of reproductive span (age at menarche to age at menopause). To parse the effects of common genetic variation, we computed polygenic scores for reproductive span. The results showed that higher VAT and ASAT were both associated with higher grey and white matter brain age, and greater white matter hyperintensity load. The associations varied positively with reproductive span, indicating more prominent associations between adipose tissue and brain measures in females with a longer reproductive span. The effects were in general small, but could not be fully explained by genetic variation or relevant confounders. Our findings indicate that associations between abdominal adipose tissue and brain health post-menopause may partly depend on individual differences in cumulative oestrogen exposure during reproductive years, emphasising the complexity of neural and endocrine ageing processes in females.
1. Introduction
The menopause transition is characterised by decreased circulating oestradiol levels and the cessation of menstrual cycles, marking the end of the reproductive phase (Hall, 2015, Jett et al., 2022, Marlatt et al., 2022). Although many individuals transition through menopause without long-term health issues, this life phase involves higher risk of obesity (Khoudary et al., 2015, Leeners et al., 2017, Lovejoy et al., 2008, Lizcano and Guzmán, 2014) and cardiometabolic diseases (Carr, 2003, Janssen et al., 2008, Pu et al., 2017), which may contribute to the observed post-menopausal risk for neurodegeneration and dementia (Brinton et al., 2015, Jett et al., 2022, Rahman et al., 2019).
The relationships between oestrogen exposure, body composition, and brain health in females are complex and largely unexplored. The menopause transition is linked to an accelerated increase of central fat accumulation (Lizcano and Guzmán, 2014), and abdominal adipose tissue has been associated with higher grey matter (GM) and white matter (WM) brain age (Beck et al., 2022, Beck et al., 2022, Subramaniapillai et al., 2022), WM hyperintensities (WMH) (Arnoldussen et al., 2019, Han et al., 2021, Lampe et al., 2019, Pasha et al., 2017, Park et al., 2018, Vuorinen et al., 2014), and dementia risk (Kiliaan et al., 2014, Tang et al., 2021, Razay et al., 2006, Whitmer et al., 2008). However, in females, adipose tissue also serves as the primary biosynthesis site of oestrogens post-menopause (Steiner and Berry, 2022, Bhardwaj et al., 2019, Kershaw and Flier, 2004, Siiteri, 1987, Simpson, 2003). Since oestradiol is consistently found to exert neuroprotective effects on the pre-menopausal female brain across preclinical and clinical studies (Azcoitia et al., 2019, Barth et al., 2016, Galea et al., 2017, Jacobs and Goldstein, 2018, Merlo et al., 2017, Scott et al., 2012, Zárate et al., 2017), changes in adipose tissue distribution could also involve mechanisms that foster a protective source of oestrogens after menopause (Klosinski et al., 2015, Subramaniapillai et al., 2022). Although the oestrogen levels produced via adipose tissue do not fully compensate for the loss of ovarian oestrogen production (Steiner and Berry, 2022), it is possible that different types of adipose tissue may play diverging roles in female brain health post-menopause.
Studies utilising magnetic resonance imaging (MRI) of the body, which allows for more precise measures of fat distribution than conventional anthropomorphic methods (Borga et al., 2018), demonstrate that visceral adipose tissue (VAT; the fat surrounding the abdominal organs) increases more following menopause than abdominal subcutaneous adipose tissue (ASAT; the fat below the skin) (Lee et al., 2009, Leeners et al., 2017, Lovejoy et al., 2008, Samargandy et al., 2021). Consistent evidence shows that higher midlife VAT in both males and females is associated with lower cortical and total brain volume (Debette et al., 2010, Isaac et al., 2011, Veit et al., 2014), higher WMH load (Anand et al., 2022, Kim et al., 2017, Pasha et al., 2017), and accelerated brain ageing (Zsido et al., 2019), while some studies indicate that ASAT may be significantly less detrimental or even protective for brain volume (Debette et al., 2010, Isaac et al., 2011, Widya et al., 2015, Qi et al., 2021) and WMH load (Kim et al., 2017, Yamashiro et al., 2014), especially in females (Nam et al., 2019). Although midlife adipose tissue levels relate to brain health in both males and females, the known menopause-related changes in body composition (Lizcano and Guzmán, 2014) highlight the need for targeted research into VAT, ASAT, and brain characteristics in post-menopausal females, which have not been examined previously.
In females, levels of oestrogen exposure pre-menopause may influence both brain health and body composition post-menopause, emphasising the complex interactions between neuroendocrine and metabolic processes across the female lifespan. For example, levels of cumulative oestrogen exposure, often assessed by reproductive span (age at menarche to age at menopause; (Fu et al., 2022, Gilsanz et al., 2019, Jett et al., 2022), have been linked to larger GM volumes (Schelbaum et al., 2021), lower WM brain age (Subramaniapillai et al., 2022), and lower dementia risk in older-age samples (Fox et al., 2013, Gilsanz et al., 2019, Gong et al., 2022), although contrasting results have linked a longer reproductive span to increased risk of Alzheimer’s disease (Najar et al., 2020, Geerlings et al., 2001). Age at menarche and menopause are also known to have genetic components (Fernández-Rhodes et al., 2018, Wang et al., 2019, Ruth et al., 2021), but it is unclear how the genetics underlying reproductive span relate to body composition and brain structure (Roa-Díaz et al., 2021). A later age at natural menopause has also been associated with lower risk for post-menopausal abdominal obesity (Zsakai et al., 2015), smaller post-menopausal increase of BMI (Montazeri et al., 2019), and decreased risk for cardiometabolic diseases (Muka et al., 2016, Roa-Díaz et al., 2021, Yang et al., 2017). However, the relationship between these are likely to be bidirectional, as pre-menopausal body composition can influence the timing of natural menopause (Dorjgochoo et al., 2008, Roa-Díaz et al., 2021, Tao et al., 2015, Zhu et al., 2019). Although increasing evidence points to greater lifetime exposure to oestrogens as beneficial for neural and cardiometabolic health, the mechanisms of these long-lasting actions of oestrogens are poorly understood. It is also unclear how cumulative oestrogen exposure during reproductive years interacts with adipose tissue and its post-menopausal oestrogen production to influence brain health at later life stages.
In this study, we investigated associations between different types of abdominal adipose tissue and brain characteristics in 10,251 post-menopausal females, and assessed whether the relationships varied depending on length of reproductive span (age at menarche to age at menopause). Measures of VAT and ASAT were extracted based on body MRI (Linge et al., 2018), and GM- and WM-specific brain age estimates were generated using T1- and diffusion-weighted MRI (dMRI) data, respectively (Voldsbekk et al., 2021). Brain age prediction has emerged as a useful tool for combining a rich variety of brain characteristics into single estimates per individual, providing a reliable proxy of brain integrity and health (Franke et al., 2010, Cole et al., 2019, Beck et al., 2021). Based on recent studies suggesting that tissue-specific age prediction can provide further detail (Beck et al., 2022, de Lange et al., 2020a, Eavani et al., 2018, Voldsbekk et al., 2021), we estimated GM and WM brain age separately. WMH volume derived from T2 fluid-attenuated inversion recovery (FLAIR) images was examined as an additional measure, as a number of studies indicate higher WMH prevalence in females compared to males (Alqarni et al., 2021, Jorgensen et al., 2018, Lohner et al., 2022, Sachdev et al., 2009, Than et al., 2021, Van Den Heuvel et al., 2004) and recent evidence points to sex-specific associations between cardiometabolic risk factors and WMH pathology (Alqarni et al., 2021). Sex differences in WMH prevalence have been observed to primarily emerge after the age of 50 (Wen et al., 2009), which is close to the average age of menopause (51 years, (InterLACE, 2019)), or specifically after the menopause (Jorgensen et al., 2018, Lohner et al., 2022, Than et al., 2021), indicating a link between WMHs and female endocrine ageing processes (Thurston et al., 2016). We used Bayesian linear models to assess relationships between the brain measures and VAT and ASAT, and included interaction terms to test if associations varied depending on reproductive span. To parse the effects of common genetic variation, we also tested for associations between the brain measures and polygenic scores (PGS) for the phenotype reproductive span.
We hypothesised that i) greater levels of abdominal adipose tissue, particularly VAT, would be associated with higher brain age and WMH load, ii) a shorter reproductive span would be associated with higher brain age and WMH load, and iii) the associations between abdominal adipose tissue and brain measures would vary depending on reproductive span, possibly reflecting a protective effect of adipose tissue in females with a shorter reproductive span.
2. Methods and materials
2.1. Sample characteristics
The sample was drawn from the UK Biobank cohort (www.ukbiobank.ac.uk), and included 20,540 female participants with both T1- and diffusion-weighted MRI data. To ensure a neurologically healthy sample, 1,759 participants with disorders known to affect the brain, including stroke, dementia, and neurodegenerative and psychiatric disorders, were excluded based on ICD10 diagnoses in line with earlier work (Voldsbekk et al., 2021, de Lange et al., 2020) (details are provided in the UK Biobank online resources: http://biobank.ndph.ox.ac.uk/showcase/field.cgi?id=41270). In addition, 160 participants were excluded based on poor-quality MRI data likely due to motion (see Section 2.2), yielding a total of 18,621 participants with T1- and diffusion-weighted MRI data. Out of these, 16,542 participants had data entries across demographic factors, WMH volume, ASAT, VAT, age at menopause, age at menarche, hysterectomy, and bilateral oophorectomy. After removing missing values (NaN, ‘prefer not to answer’, ‘do not know’), 11,381 were included in the subsequent analyses (missing data = 271 for demographic factors, 629 for WMH volume, 4,329 for age at menopause/menarche, and 1,806 for hysterectomy/oophorectomy, with some participants having missing values across several variables). Participants who had undergone a hysterectomy and/or oophorectomy were excluded (N = 1,010) in order to focus on variation in natural menopause. To avoid outlier-driven results, participants with age at menarche 9 and 17 and age at menopause 39 and 63 were excluded (N = 120, see Section 2.5), yielding a final sample of 10,251. As a cross-check, we also conducted the analyses including all ages at menarche/menopause as well as participants with hysterectomy and/or oophorectomy. Sample demographics are provided in Table 1.
Table 1.
Sample demographics. Percentage in each group for ethnic background, education, and assessment location. Mean ± standard deviation (SD) and ranges for age, visceral adipose tissue (VAT), abdominal subcutaneous adipose tissue (ASAT), reproductive span, age at menarche, and age at menopause. GCSE = General Certificate of Secondary Education, NVQ = National Vocational Qualification.
| Sample N | 10,251 | |
| Age | Mean ± SD | 63.99 ± 6.63 |
| Range [years] | 48.09–81.49 | |
| Ethnic background | % White | 97.55 |
| % Black | 0.52 | |
| % Mixed | 0.42 | |
| % Asian | 0.65 | |
| % Chinese | 0.34 | |
| % Other | 0.52 | |
| Education | % University/college degree | 47.25 |
| % A levels or equivalent | 14.52 | |
| % O levels/GCSE or equivalent | 20.26 | |
| % NVQ or equivalent | 6.96 | |
| % Professional qualification | 5.62 | |
| % None of the above | 5.39 | |
| Assessment location | % Newcastle | 27.25 |
| % Cheadle | 58.94 | |
| % Reading | 13.81 | |
| VAT | Mean ± SD | 0.95 ± 0.54 |
| Range | 0.04–4.11 | |
| ASAT | Mean ± SD | 2.90 ± 1.24 |
| Range | 0.19–9.60 | |
| Reproductive span [years] | Mean ± SD | 37.95 ± 4.28 |
| Range | 23–51 | |
| Age at menarche [years] | Mean ± SD | 12.99 ± 1.50 |
| Range | 9–17 | |
| Age at menopause [years] | Mean ± SD | 50.94 ± 4.02 |
| Range | 39–63 | |
2.2. MRI data acquisition and processing
Information about the UK Biobank data acquisition protocols is available in (Alfaro-Almagro et al., 2018, Miller et al., 2016). Raw T1-weighted MRI data were processed using a harmonised analysis pipeline, including the FreeSurfer (version 5.3) automated surface-based morphometry and subcortical segmentation (Fischl et al., 2002). In line with recent brain age studies (Kaufmann et al., 2019, de Lange et al., 2019, Voldsbekk et al., 2021), we used the standard set of subcortical and cortical summary statistics from FreeSurfer (Fischl et al., 2002), as well as a fine-grained cortical parcellation scheme (Glasser et al., 2016), to extract cortical thickness, area, and volume for 180 regions of interest per hemisphere. This yielded a total set of 1,118 structural brain imaging features (360/360/360 for cortical thickness/area/volume respectively, and 38 for cerebellar/subcortical and cortical summary statistics). All 1,118 features were used as input features in the GM-specific age prediction model (Section 2.3). The brain morphometric data obtained from Freesurfer were residualised with respect to scanning site and intracranial volume using linear models. To remove poor-quality MRI data likely due to motion, participants with Euler numbers (Rosen et al., 2018) ± 4 standard deviations from the mean were excluded (N = 160).
The dMRI data were processed using an optimised diffusion pipeline as described in detail in (Maximov et al., 2019). Metrics derived from diffusion tensor imaging (DTI) (Basser et al., 1994), diffusion kurtosis imaging (DKI) (Jensen et al., 2005), WM tract integrity (WMTI) (Fieremans et al., 2011), and spherical mean technique (SMT) (Kaden et al., 2016, Kaden et al., 2016) were used as input features in the WM-specific age prediction model (Section 2.3), as described in Voldsbekk et al., 2021. The metrics for each diffusion model are listed in Supplementary Information (SI) Section 1. For each metric, WM features were extracted based on John Hopkins University (JHU) atlases for white matter tracts and labels (with 0 thresholding) (Mori et al., 2005), including global mean values and regional measures (Voldsbekk et al., 2021, Beck et al., 2021). The dMRI data were residualised with respect to scanning site using linear models, and passed tract-based spatial statistics (TBSS) post-processing quality control using the YTTRIUM algorithm (Maximov et al., 2021).
Total volume of WMH was derived for each participant based on T2 FLAIR images in combination with T1-weighted data (https://biobank.ndph.ox.ac.uk/showcase/field.cgi?id=25781) using the Brain Intensity Abnormality Classification Algorithm (BIANCA) (Griffanti et al., 2016), which is part of the FMRIB Software Library FSL (Jenkinson et al., 2012). BIANCA is a fully automated tool for segmentation of WMH based on the k-nearest neighbour algorithm, and is documented as a reliable method for WMH segmentation in large cross-sectional cohort studies (Griffanti et al., 2016). The WMH volume measures were log transformed to normalise and stabilise the variance (Veldsman et al., 2020, Wartolowska and Webb, 2021). WMH volume was examined separately, as we were specifically interested in this measure due to the known female prevalence and links to oestradiol levels and adipose tissue. Hence, this feature was not included in the WM brain age estimate.
2.3. Brain age prediction
GM- and WM-specific age prediction models were run using the XGBoost regression algorithm (eXtreme Gradient Boosting; https://github.com/dmlc/xgboost). XGBoost includes advanced regularisation to reduce overfitting, and has shown superior performance in machine learning competitions (Chen and Guestrin, 2016). Parameters were tuned in a nested cross-validation using 5 inner folds for randomised search, and 10 outer folds for model validation (see https://github.com/amdelange/brainage_women/blob/main/python/run_prediction_model.py for general model setup). Brain age gap (BAG) values were calculated by subtracting chronological age from predicted brain age, providing an estimate of each participant’s brain age relative to their chronological age (Cole and Franke, 2017). To ensure that any associations with the variables of interest were not driven by age-dependence in the predictions (Liang et al., 2019, Smith et al., 2019), chronological age was included as a covariate in all subsequent analyses (de and Cole, 2020, Le et al., 2018).
2.4. Abdominal adipose tissue measures
Abdominal adipose tissue measures derived from body MRI were processed by AMRA medical, and accessed via UK Biobank Returned Datasets (Return ID 3666; https://biobank.ndph.ox.ac.uk/ukb/app.cgi?id=6569). The extracted measures included VAT volume, measured within the abdominal cavity, and ASAT volume, measured from the top of the femoral head to the top of the thoracic vertebrae T9, both measured in litres and divided by height squared.
2.5. Reproductive span
To calculate reproductive span (age at menopause – age at menarche), we first removed extreme outliers for age at menarche and age at menopause in the sample used for the PGS (see Section 2.6), using median absolute deviation (Leys et al., 2013) with a threshold of 4. The same cut-offs were used in the MRI sample, resulting in a mean reproductive span of 37.95 years ± 4.28 (SD) in the final sample (see Section 2.1 for ages of menarche/menopause removed, and Section 2.8 for cross-checks including all ages at menarche/menopause).
2.6. PGS calculations
A genome-wide association study (GWAS) was run on the UK Biobank female cohort (N = 121,620, excluding the MRI sample), using PLINK 2.0 (Chang et al., 2015) with the default additive model, making use of the UK Biobank v3 imputed genetic data, filtering out single nucleotide polymorphisms (SNPs) with a minor allele frequency below 0.001 or failing the Hardy–Weinberg equilibrium test at . Individuals with known brain disorders as indicated by ICD10 diagnoses (see Section 2.1), previous hysterectomy, and/or oophorectomy, and non-white Europeans were excluded. Linear regressions were run on the variable reproductive span, covarying for age and the first 20 genetic principal components (https://biobank.ndph.ox.ac.uk/showcase/field.cgi?id=22009). PRSice v2 (Choi and O’Reilly, 2019, Euesden et al., 2015) was used to calculate PGS of reproductive span at a p-value threshold of 0.05 for each European individual in the MRI subsample, using PRSice default settings. This includes the removal of the major histocompatibility complex (MHC) (chromosome 6, 26 to 33 Mb) and thinning of SNPs based on linkage disequilibrium and p-value.
2.7. Statistical analyses
In line with guidelines for reporting Bayesian analyses (Sung et al., 2005, Kruschke, 2021), we list the statistical software used, define our priors, describe the statistical models used, and quote the results using central tendencies (mean, median, mode) and credible intervals. To test for associations between the brain measures and adipose tissue (VAT and ASAT) and their interaction with reproductive span, we ran Bayesian multiple linear models using the Bayesian Model-Building Interface (Bambi) package (Capretto et al., 2022) in Python 3.7.6 (https://pypi.org/project/bambi). All variables were standardised (subtracting the mean and dividing by the standard deviation) prior to analyses. Four chains, with 2000 samples each, were estimated. In the sampling process, the first 1000 samples served as burn-ins to identify the region of best-fitting values in the parameter space. Weakly informative normal priors with and were generated for all model terms by loosely scaling them to the standardised data (Bambi default) (Capretto et al., 2022). The model results for each association are described by the mean and the 95% highest density interval (HDI) of its posterior distributions. The mean represents the central tendency of the association, while there is a 95% probability that the true value lies within the HDI (see e.g. Hespanhol et al., 2019). Median and mode central tendencies are also provided.
Models were run with VAT and ASAT separately due to high correlation between the variables (r = 0.77). The models included brain measure (GM BAG, WM BAG, or WMH vol) as the dependent variable, adipose tissue volume (VAT or ASAT) × reproductive span as independent variables, and age as a covariate:
| (1) |
To test for effects of common genetic variation, we calculated the phenotypic variance explained by the reproductive span PGS, tested for main effects of PGS on the brain measures, and re-ran the main analyses including the PGS as a covariate.
2.8. Sensitivity analyses
To account for potential confounding factors that could influence brain structure, adipose tissue levels, and/or reproductive span, the models were rerun with the following covariates (in addition to age): health factors including diabetes status (Cole, 2020, Peters et al., 2014), hypertension (Fuchs and Whelton, 2020, Newby et al., 2022) and a lifestyle score which was computed by adding one point per unhealthy lifestyle factor (physical activity level, intake of fruits, vegetables, oily fish and red meats, sleep duration, television viewing time, current and past smoking status, and alcohol use) (Foster et al., 2018), female-specific factors including number of previous childbirths (de Lange et al., 2019), hormone replacement therapy use (user versus never user) (Hogervorst et al., 2000, Maki et al., 2011), and oral contraceptive use (user versus never user) (De Bondt et al., 2013), and socioeconomic factors including educational level (Fotenos et al., 2008, Meng and D’arcy, 2012, Walhovd et al., 2022) and ethnic background (Goff, 2019). We first included all covariates in one model, and then in three separate models including i) the health factors, ii) the female-specific factors, and iii) the socioeconomic factors, to test for specific influences of these potential covariates on the results. Furthermore, we repeated the analyses excluding subjects with a BMI 40 (N excluded = 117), as these values may indicate morbid obesity and risk for serious health complications (Jarolimova et al., 2013). To account for potential uncertainties related to self-reporting of age at menarche decades later (Cooper et al., 2006, Must et al., 2002), the models were also conducted using age at menopause instead of reproductive span. Finally, we repeated the analyses without excluding any participants with outlier values for ages at menarche/menopause or hysterectomy and/or oophorectomy.
3. Results
3.1. Associations with GM/WM BAG and WMH volume
Table 2 shows the age prediction accuracy of the GM- and WM-based models, respectively.
Table 2.
Age prediction accuracy for the models based on grey matter (GM) and white matter (WM) features, respectively. Model accuracy is measured by R2 (proportion of variance in age explained), root mean square error (RMSE), mean absolute error (MAE), and Pearson’s correlations (r) between predicted and chronological age. R2, RMSE, and MAE are averaged across folds, providing the mean ± standard error of each performance measure. CI = confidence interval.
| Model | R2 | RMSE | MAE | r [95% CI] | p |
|---|---|---|---|---|---|
| GM | 0.0001 | ||||
| WM | 0.0001 |
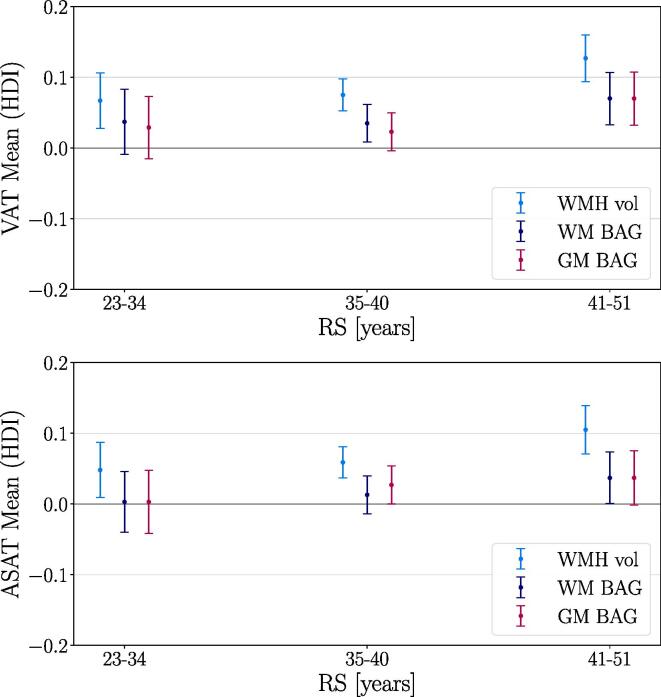
Fig. 1 and Table 3 show the associations of the brain measures with VAT, ASAT, reproductive span, and the interaction terms (see Eq. 1), as described by the mean and the 95% HDI of their posterior distributions. Table 3 also includes mode and median values, which were highly similar to the means across associations. SI Fig. 1 shows the posterior distributions from the model including VAT, RS, and GM BAG for illustration. Higher VAT and ASAT were associated with higher BAG (i.e., older brain age relative to chronological age) and higher WMH volume. A shorter reproductive span was related to higher GM/WM BAG and WMH volume. This is indicative of a negative relationship between reproductive span and the brain measures, but the results are not fully conclusive as the upper HDIs approach or overlap with zero (see Table 3). As a cross-check, we measured the main effects of ASAT, VAT, and reproductive span on the brain measures in separate models that did not include the interaction term. The associations were consistent, as shown in SI Fig. 2. The relationships between VAT and ASAT and the brain measures varied positively with reproductive span based on the HDI mean values, such that longer reproductive spans and higher VAT and ASAT were associated with higher BAG and WMH load. However, these interactions are not fully conclusive as the lower HDIs approach or overlap with zero (see Table 3). To illustrate the interaction effects, Fig. 2 shows the VAT and ASAT associations with the brain measures in bins of reproductive span length. Fig. 3 shows the correlations between VAT and ASAT, reproductive span, and the brain measures.
Fig. 1.
Associations between visceral adipose tissue (VAT), abdominal subcutaneous adipose tissue (ASAT), reproductive span (RS) and brain measures. The points show the means of the posterior distributions for the associations, with error bars indicating the 95% highest density intervals (HDI). Higher VAT and ASAT were associated with higher GM/WM BAG and WMH volume. Shorter RS was associated with higher GM/WM BAG and WMH volume, and the relationships between VAT and ASAT and the brain measures varied positively with RS as indicated by the interaction terms. As the HDIs for the RS associations and interactions approach or overlap with zero, these results are not fully conclusive. GM = grey matter, WM = white matter, BAG = brain age gap, WMH vol = white matter hyperintensity volume.
Table 3.
Means and highest density intervals (HDIs) of the posterior distributions for each Bayesian regression model, in addition to mode and median values for each association. VAT = visceral adipose tissue, ASAT = abdominal subcutaneous adipose tissue, RS = reproductive span, GM = grey matter, WM = white matter, BAG = brain age gap, WMH vol = white matter hyperintensity volume.
| Brain measures | Term | Mean | Mode | Median | HDI 2.5% | HDI 97.5% |
|---|---|---|---|---|---|---|
| GM BAG | VAT | 0.036 | 0.036 | 0.036 | 0.017 | 0.056 |
| RS | −0.011 | −0.013 | −0.011 | −0.032 | 0.007 | |
| VAT × RS | 0.018 | 0.018 | 0.018 | −0.002 | 0.037 | |
| WM BAG | VAT | 0.045 | 0.044 | 0.045 | 0.025 | 0.064 |
| RS | −0.018 | −0.017 | −0.018 | −0.038 | 0.002 | |
| VAT × RS | 0.011 | 0.012 | 0.012 | −0.008 | 0.030 | |
| WMH vol | VAT | 0.087 | 0.086 | 0.087 | 0.071 | 0.105 |
| RS | −0.018 | −0.017 | −0.018 | −0.033 | 0.000 | |
| VAT × RS | 0.020 | 0.020 | 0.020 | 0.004 | 0.036 | |
| GM BAG | ASAT | 0.025 | 0.022 | 0.025 | 0.005 | 0.044 |
| RS | −0.011 | −0.012 | −0.011 | −0.030 | 0.008 | |
| ASAT × RS | 0.014 | 0.015 | 0.014 | −0.005 | 0.033 | |
| WM BAG | ASAT | 0.018 | 0.019 | 0.018 | −0.003 | 0.036 |
| RS | −0.019 | −0.018 | −0.018 | −0.038 | 0.001 | |
| ASAT × RS | 0.017 | 0.016 | 0.017 | −0.002 | 0.036 | |
| WMH vol | ASAT | 0.069 | 0.069 | 0.069 | 0.053 | 0.086 |
| RS | −0.020 | −0.018 | −0.019 | −0.036 | −0.002 | |
| ASAT × RS | 0.021 | 0.021 | 0.021 | 0.005 | 0.037 | |
Fig. 2.
Associations between visceral adipose tissue (VAT) and abdominal subcutaneous adipose tissue (ASAT) and brain measures, estimated in bins of reproductive span (RS) to illustrate the interaction effects observed in Fig. 1. In females with a longer RS, higher VAT and ASAT was slightly more positively associated with GM/WM BAG and WMH vol than in subjects with a shorter RS. Note that the continuous RS variable was used in the analyses (Eq. 1), and the bins are created only to visualise the direction of the interaction. The points show the means of the posterior distributions for the associations, with error bars indicating the 95% highest density intervals (HDI). The mean ± SD for RS was 37.95 ± 4.28 years, with bins including 1,914, 5,564, and 2,772 participants with a RS between 23–34, 35–40, and 41–51 years, respectively. VAT = visceral adipose tissue, ASAT = abdominal subcutaneous adipose tissue, GM = grey matter, WM = white matter, BAG = brain age gap, WMH vol = white matter hyperintensity volume.
Fig. 3.
Correlations between visceral adipose tissue (VAT), abdominal subcutaneous adipose tissue (ASAT), reproductive span (RS), age, grey matter (GM) and white matter (WM) brain age gap (BAG), and white matter hyperintensity volume (WMH vol).
3.2. Reproductive span PGS
To measure the phenotypic variance explained by the reproductive span PGS, we ran a linear regression for PGS and reproductive span in years to calculate , adjusting for age (Choi et al., 2020). The adjusted value was 0.045, with a value of (standard error) for the PGS, as shown in Fig. 4. The PGS scores showed no associations with the brain measures (SI Fig. 3), and the correlations of RS PGS with VAT and ASAT were r = −0.02 and −0.01, respectively (see SI Fig. 4 for correlation matrix). The associations between VAT and ASAT, reproductive span, and the brain measures persisted when partialling out polygenic scores, as shown in SI Fig. 5 and SI Table 1.
Fig. 4.
Reproductive span in years (x-axis) versus polygenic score (PGS) for reproductive span (y-axis), based on a linear regression adjusting for age. The adjusted value was 0.045, with a (slope) value of (standard error).
3.3. Sensitivity analyses
The sensitivity analyses showed that when including the additional covariates specified in Section 2.8 in a single model, the associations showed a pattern consistent with the main results, but with minor shifts towards zero for the main effects of both adipose tissue types and reproductive span on brain measures. The three separate covariate models (SI Fig. 7) showed that the positive associations between adipose tissue and the brain measures were slightly weaker when including the health factors (diabetes, hypertension, and the lifestyle score), and the negative associations between reproductive span and the brain measures were slightly weaker when including the female-specific factors (number of previous childbirths, hormone replacement therapy use and oral contraceptive use). When including only the socioeconomic factors (ethnic background and educational level), the associations were highly consistent with the main results. The results remained consistent with the main results when excluding subjects with a BMI above 40 (SI Fig. 8), when repeating the models using age at menopause instead of reproductive span (SI Fig. 9), and when including all ages at menarche/menopause as well as participants with hysterectomy and/or oophorectomy (SI Fig. 10).
4. Discussion
This study examined the associations between different types of abdominal adipose tissue (VAT and ASAT), reproductive span, and brain characteristics (GM/WM BAG and WMH volume) in a large sample of post-menopausal females. In summary, greater VAT and ASAT were both associated with higher GM/WM BAG (older brain age relative to chronological age) and higher WMH volume. Based on the HDI mean values, a shorter reproductive span was related to higher GM/WM BAG and WMH volume, and the associations between abdominal adipose tissue and brain measures varied positively with reproductive span, potentially indicating more prominent associations in females with greater levels of lifetime oestrogen exposure. The effects were in general small, but could not be fully explained by genetic variation or relevant confounders, and further studies are needed to draw conclusions.
The associations between abdominal adipose tissue and brain characteristics are consistent with previous studies linking elevated adipose tissue to older brain age (Beck et al., 2022, Subramaniapillai et al., 2022), lower brain volume (Cho et al., 2021, Debette and Markus, 2010, Gurholt et al., 2021, Isaac et al., 2011, Veit et al., 2014), and higher WMH load (Arnoldussen et al., 2019, Lampe et al., 2019, Park et al., 2018, Vuorinen et al., 2014). The relationships were more prominent for WMH volume compared to GM and WM BAG (about 3 standard deviations higher, see Fig. 1), indicating that the WMH volume measures from FLAIR images may represent a particularly sensitive measure. Although WMH are likely to also influence WM diffusion measures (Raghavan et al., 2021), this finding may suggest an increased risk for white matter lesions in post-menopausal females with elevated abdominal adipose tissue. Inflammation has been proposed as a key factor linking central adiposity and WMH load (Lampe et al., 2019), and weight gain during the menopause transition involves a heightened inflammatory state (Lee et al., 2009, McCarthy and Raval, 2020). Inflammation linked to changes in hormone levels and body composition may thus be a mechanistic explanation for the higher risk of WMH in post-menopausal females (Fatemi et al., 2018, Sachdev et al., 2009, Than et al., 2022, Wen et al., 2009). However, the relationship between changes in adipose tissue and WM lesions within the shorter perimenopausal time window remains to be investigated.
The associations with brain characteristics showed similar patterns for the two abdominal adipose tissue types (see Fig. 1). Although VAT and ASAT may have distinct anatomical, cellular, molecular, physiological, clinical, and prognostic correlates (Ibrahim, 2010, Kwok et al., 2016), the high correlation between them () in the current study indicates that these measures shared a relatively large degree of overlapping information. However, other studies have specifically linked midlife VAT to more adverse effects on brain structure compared to ASAT (Debette et al., 2010, Isaac et al., 2011, Widya et al., 2015, Qi et al., 2021). One possible explanation for this arises from a biopsy study, which showed changes in adipose tissue phenotypes across the menopause transition (Abildgaard et al., 2021). Specifically, these changes were linked to metabolic dysfunction in both VAT and ASAT post-menopause (Abildgaard et al., 2021), which could potentially contribute to detrimental effects of both tissue types on brain structure. However, longitudinal studies assessing changes in adipose tissue phenotype and brain health across the menopause transition and beyond are needed to draw causal conclusions.
Although adipose tissue is the primary biosynthesis site of oestrogens post-menopause (Bhardwaj et al., 2019), we found no direct evidence towards neuroprotective effects of certain adipose tissue types. We did however observe that the associations of VAT and ASAT with the brain measures were more prominent in females with a longer reproductive span. Although these results were not fully conclusive (see Section 3.1), this finding could indicate that a combination of higher levels of adipose tissue and greater exposure to oestrogens may constitute a risk of adverse brain health (see e.g. Brinton, 2005, Brinton, 2008). Alternatively, these findings could indicate that with earlier decline of ovarian hormone production, higher levels of adipose tissue may be less detrimental due to beneficial oestrogen production via adipose tissue. However, it is unclear how individual variation in oestrogen exposure pre-menopause may influence associations between adipose tissue and brain health post-menopause, and how this may relate to circulating oestrogen levels, which were not available at the time of the brain and body MRI scans. Although oestradiol measures were available from baseline in a smaller subsample, it can take several years for oestradiol levels to stabilise following menopause (Randolph Jr et al., 2011), and these measurements may be influenced by certain types of hormone replacement therapy (Waaseth et al., 2008) as well as lifestyle factors (Wiggs et al., 2021). Due to the time window between assessments, changes in both oestradiol and adipose tissue levels could occur, limiting any firm conclusions based on these measures. Future studies should target both current and previous oestrogen levels to clarify the links between adipose tissue, oestrogen exposure, and brain characteristics, and ideally measure how changes in hormones and body fat link to brain health across the menopause transition.
Our results further indicated associations between a shorter reproductive span and higher GM/WM BAG and WMH volume independent of abdominal adipose tissue levels. Although these effects were not fully conclusive, the directions of the associations align with previous studies showing beneficial effects of a longer reproductive span on a number of brain health markers (Georgakis et al., 2016, Schelbaum et al., 2021, Subramaniapillai et al., 2022, Zeydan et al., 2019) and dementia risk (Gong et al., 2022). In line with previous studies (Day et al., 2015, Day et al., 2017, Zhao et al., 2021) we found an association between polygenic and phenotypic variance in reproductive span, but we found no notable associations between PGS, adipose tissue measures and brain measures, nor did PGS alter the interactions when included as a covariate. This suggests that the observed associations may be driven by factors such as oestrogen exposure rather than common genetic variation. While it is plausible that longer-term exposure to oestrogens pre-menopause may have lasting effects on brain health beyond the menopause transition (Schelbaum et al., 2021), the observed effects were small, which may explain why other studies have found no association between reproductive span length and brain characteristics (Prince et al., 2022). Proxies of lifetime oestrogen exposure also vary across studies (de Lange et al., 2020, Fox et al., 2013, Zhao et al., 2021), and factors such as duration of hormone replacement/contraceptive use and time spent breastfeeding are likely to influence cumulative oestrogen exposure across the female lifespan (Barth and de Lange, 2020). When including available covariates (hormone replacement therapy and oral contraceptive use, and previous pregnancies), we observed these to slightly moderate the associations between reproductive span and the brain measures, indicating that a range of female-specific factors relate to brain health in line with previous studies (Gong et al., 2022, de Lange et al., 2019).
Whether brain characteristics are influenced by higher abdominal adipose tissue generally or by its typical increase during the menopause transition is unclear. For example, a longitudinal study found that it was the change in BMI over a 20-year period spanning from pre- to post-menopause that predicted GM volume in 48 females (Soreca et al., 2009). Recent studies also point towards a biphasic association between BMI and dementia (Kivimäki et al., 2018, Pedditizi et al., 2016). For example, while midlife obesity predicts risk for dementia (Albanese et al., 2017, Floud et al., 2020, Pedditizi et al., 2016), the prevalence of dementia has been found to be higher in underweight than in normal weight or overweight females (Dong et al., 2022). Prodromal stages of neurodegenerative diseases can involve weight loss as a result of disrupted brain function and dietary changes (Floud et al., 2020, Gu et al., 2014), and longitudinal studies targeting early markers of neurodegeneration may further the understanding of changes in body weight, brain health, and dementia risk in females.
Also yet to be elucidated are the mechanisms underlying the associations between adipose tissue, oestrogen exposure, and brain characteristics, which are likely multifactorial and interactive. Factors such as elevated inflammatory markers have been associated with increased adipose tissue levels (Aguilar-Valles et al., 2015, Miller and Spencer, 2014), even specifically during the menopause transition (Lee et al., 2009), as well as decreasing oestrogens (McCarthy and Raval, 2020), brain atrophy (Luo et al., 2022), and dementia risk (Heneka et al., 2015, Ransohoff, 2016). Biological markers of obesity, such as lipid profile (Anstey et al., 2017, Reitz, 2012), glucose (Crane et al., 2013), HbA1c (Ramirez et al., 2015), leptin (Zeki Al Hazzouri et al., 2013), and Vitamin B12 (Lauer et al., 2022), may also influence associations between adipose tissue and brain health, and contribute to risk of comorbidities such as type II diabetes and hypertension, which are known to impact neural and cardiometabolic health (Cole, 2020, Fuchs and Whelton, 2020, Newby et al., 2022, Peters et al., 2014).
Importantly, sex differences have been observed for the aetiology and progression of cardiometabolic risk factors (Gerdts and Regitz-Zagrosek, 2019, Yoshida et al., 2022) and their relation to the brain (Alqarni et al., 2021, Subramaniapillai et al., 2021, Subramaniapillai et al., 2022, Than et al., 2022), illustrating the critical need for sex-specific studies (Miller and Spencer, 2014, Shansky and Murphy, 2021, Taylor et al., 2019). Further research is necessary to understand the complex interplay of mechanisms that contribute to risk for cardiometabolic and neurodegenerative diseases in post-menopausal females (Christensen and Pike, 2015), and how preventive measures such as physical exercise and hormone replacement therapy can be optimised to moderate risk.
In conclusion, our findings indicate that higher levels of both visceral and abdominal subcutaneous adipose tissue are associated with higher brain age and WMH volume in post-menopausal females. These associations may partly depend on individual differences in cumulative oestrogen exposure, and future studies should aim to disentangle the complex relationships between oestrogen exposure, adipose tissue, and brain health both across the menopause transition and beyond. As the menopause transition involves an accelerated increase of central fat accumulation, further research into mechanisms and risks is pertinent to facilitate preventive interventions that can reduce the risk of adverse brain health post-menopause.
5. Data availability statement
The data that support the findings of this study are available through the UK Biobank data access procedures (https://www.ukbiobank.ac.uk/researchers).
CRediT authorship contribution statement
Louise S. Schindler: Conceptualization, Methodology, Formal analysis, Visualization, Writing – original draft. Sivaniya Subramaniapillai: Conceptualization, Methodology, Writing – review & editing. Claudia Barth: Methodology, Writing – review & editing. Dennis van der Meer: Methodology, Formal analysis, Writing – review & editing. Mads L. Pedersen: Methodology, Visualization, Writing – review & editing. Tobias Kaufmann: Methodology, Writing – review & editing. Ivan I. Maximov: Writing – review & editing. Jennifer Linge: Methodology, Writing – review & editing. Olof Dahlqvist Leinhard: Methodology, Writing – review & editing. Dani Beck: Writing – review & editing. Tiril P. Gurholt: Writing – review & editing. Irene Voldsbekk: Writing – review & editing. Sana Suri: Writing – review & editing. Klaus P. Ebmeier: Writing – review & editing, Project administration. Bogdan Draganski: Writing – review & editing, Project administration. Ole A. Andreassen: Writing – review & editing, Project administration. Lars T. Westlye: Methodology, Writing – review & editing, Project administration. Ann-Marie G. de Lange: Conceptualization, Methodology, Formal analysis, Visualization, Writing – original draft, Project administration.
Acknowledgements
This research has been conducted using the UK Biobank data under Application 27412. UK Biobank has received ethics approval from the National Health Service National Research Ethics Service (ref 11/NW/0382). The work was performed on the Service for Sensitive Data (TSD) platform, owned by the University of Oslo, operated and developed by the TSD service group at the University of Oslo IT-Department (USIT). Computations were also performed using resources provided by UNINETT Sigma2 - the National Infrastructure for High Performance Computing and Data Storage in Norway. While working on this study, the authors received funding from the Swiss National Science Foundation (PZ00P3_193658; NCCR Synapsy, project grants Nr 32003B_135679, 32003B_159780, 324730_192755, and CRSK-3_190185), the Leenaards Foundation, the Natural Sciences and Engineering Research Council of Canada, the Research Council of Norway (276082, 323961, 273345, 249795, 298646, 300768, 250358, 223273, 283799, 283798), the South-Eastern Norway Regional Health Authority (2018076, 2019101, 2017112, 2022080), the European Research Council under the European Union’s Horizon 2020 research and innovation programme (802998, 732592, 847776), the HDH Wills 1965 Charitable Trust (1117747), the Alzheimer’s Society (Grant Ref 441), and the Academy of Medical Sciences/the Wellcome Trust/the Government Department of Business, Energy and Industrial Strategy/the British Heart Foundation/Diabetes UK Springboard Award (SBF006/1078). The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z).
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.nicl.2022.103239.
Supplementary data
The following are the Supplementary data to this article:
References
- Abildgaard J., Ploug T., Al-Saoudi E., Wagner T., Thomsen C., Ewertsen C., Bzorek M., Pedersen B.K., Pedersen A.T., Lindegaard B. Changes in abdominal subcutaneous adipose tissue phenotype following menopause is associated with increased visceral fat mass. Sci. Rep. 2021;11:14750. doi: 10.1038/s41598-021-94189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Valles A., Inoue W., Rummel C., Luheshi G.N. Obesity, adipokines and neuroinflammation. Neuropharmacology. 2015;96:124–134. doi: 10.1016/j.neuropharm.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Albanese E., Launer L.J., Egger M., Prince M.J., Giannakopoulos P., Wolters F.J., Egan K. Body mass index in midlife and dementia: Systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst.) 2017;8:165–178. doi: 10.1016/j.dadm.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro-Almagro F., Jenkinson M., Bangerter N.K., Andersson J.L., Griffanti L., Douaud G., Sotiropoulos S.N., Jbabdi S., Hernandez-Fernandez M., Vallee E., et al. Image processing and quality control for the first 10,000 brain imaging datasets from uk biobank. Neuroimage. 2018;166:400–424. doi: 10.1016/j.neuroimage.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqarni A., Jiang J., Crawford J.D., Koch F., Brodaty H., Sachdev P., Wen W. Sex differences in risk factors for white matter hyperintensities in non-demented older individuals. Neurobiol. Aging. 2021;98:197–204. doi: 10.1016/j.neurobiolaging.2020.11.001. [DOI] [PubMed] [Google Scholar]
- Anand S.S., Friedrich M.G., Lee D.S., Awadalla P., Després J.P., Desai D., de Souza R.J., Dummer T., Parraga G., Larose E., Lear S.A. Evaluation of Adiposity and Cognitive Function in Adults. JAMA network open. 2022;5(2):e2146324. doi: 10.1001/jamanetworkopen.2021.46324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey K.J., Ashby-Mitchell K., Peters R. Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. J. Alzheimer’s Disease. 2017;56:215–228. doi: 10.3233/JAD-160826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoldussen I.A., Gustafson D.R., Leijsen E.M., de Leeuw F.-E., Kiliaan A.J. Adiposity is related to cerebrovascular and brain volumetry outcomes in the run dmc study. Neurology. 2019;93:e864–e878. doi: 10.1212/WNL.0000000000008002. [DOI] [PubMed] [Google Scholar]
- Azcoitia I., Barreto G.E., Garcia-Segura L.M. Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. Analysis of sex differences. Front. Neuroendocrinol. 2019;55 doi: 10.1016/j.yfrne.2019.100787. [DOI] [PubMed] [Google Scholar]
- Barth C., de Lange A.M.G. Towards an understanding of women’s brain aging: the immunology of pregnancy and menopause. Frontiers in Neuroendocrinology. 2020;58:100850. doi: 10.1016/j.yfrne.2020.100850. [DOI] [PubMed] [Google Scholar]
- Barth C., Steele C.J., Mueller K., Rekkas V.P., Arélin K., Pampel A., Burmann I., Kratzsch J., Villringer A., Sacher J. In-vivo Dynamics of the Human Hippocampus across the Menstrual Cycle. Sci. Rep. 2016;6:32833. doi: 10.1038/srep32833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D., De Lange A.-M., Maximov I.I., Richard G., Andreassen O.A., Nordvik J.E., Westlye L.T. White matter microstructure across the adult lifespan: A mixed longitudinal and cross-sectional study using advanced diffusion models and brain-age prediction. NeuroImage. 2021;224 doi: 10.1016/j.neuroimage.2020.117441. [DOI] [PubMed] [Google Scholar]
- Beck D., de Lange A.-M.G., Alnæs D., Maximov I.I., Pedersen M.L., Leinhard O.D., Linge J., Simon R., Richard G., Ulrichsen K.M., Dørum E.S., Kolskår K.K., Sanders A.-M., Winterton A., Gurholt T.P., Kaufmann T., Steen N.E., Nordvik J.E., Andreassen O.A., Westlye L.T. Adipose tissue distribution from body MRI is associated with cross-sectional and longitudinal brain age in adults. NeuroImage: Clinical. 2022;33 doi: 10.1016/j.nicl.2022.102949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D., de Lange A.M.G., Pedersen M.L., Alnæs D., Maximov I.I.., Voldsbekk I., Richard G., Sanders A.M., Ulrichsen K.M., Dørum E.S., Kolskår K.K. Cardiometabolic risk factors associated with brain age and accelerate brain ageing. Human brain mapping. 2019;43(2):700–720. doi: 10.1002/hbm.25680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj P., Au C.C., Benito-Martin A., Ladumor H., Oshchepkova S., Moges R., Brown K.A. Estrogens and breast cancer: Mechanisms involved in obesity-related development, growth and progression. J. Steroid Biochem. Mol. Biol. 2019;189:161–170. doi: 10.1016/j.jsbmb.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borga M., West J., Bell J.D., Harvey N.C., Romu T., Heymsfield S.B., Leinhard O.D. Advanced body composition assessment: from body mass index to body composition profiling. Journal of Investigative Medicine. 2018;66(5):1–9. doi: 10.1136/jim-2018-000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton R., Yao J., Yin F., Mack W., Cadenas E. Perimenopause as a neurological transition state. Nature Rev. Endocrinol. 2015:11. doi: 10.1038/nrendo.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton R.D. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann. N. Y. Acad. Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Brinton R.D. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capretto T., Piho C., Kumar R., Westfall J., Yarkoni T., Martin Bambi: A Simple Interface for Fitting Bayesian Linear Models in Python. Journal of Statistical Software. 2022;15:1–29. [Google Scholar]
- Carr M.C. The Emergence of the Metabolic Syndrome with Menopause. J. Clin. Endocrinol. Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- Chang C.C., Chow C.C., Tellier L.C., Vattikuti S., Purcell S.M., Lee J.J. Second-generation plink: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:s13742–015. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Guestrin C. Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining. 2016. Xgboost: A scalable tree boosting system; pp. 785–794. [Google Scholar]
- Cho J., Seo S., Kim W.-R., Kim C., Noh Y. Association Between Visceral Fat and Brain Cortical Thickness in the Elderly: A Neuroimaging Study. Frontiers in Aging. Neuroscience. 2021;13 doi: 10.3389/fnagi.2021.694629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.W., Mak T.S.-H., O’Reilly P.F. Tutorial: a guide to performing polygenic risk score analyses. Nature Protocols. 2020;15:2759–2772. doi: 10.1038/s41596-020-0353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.W., O’Reilly P.F. Prsice-2: Polygenic risk score software for biobank-scale data. Gigascience. 2019;8:giz082. doi: 10.1093/gigascience/giz082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A., Pike C.J. Menopause, obesity and inflammation: interactive risk factors for alzheimer’s disease. Front. Aging Neurosci. 2015;7:130. doi: 10.3389/fnagi.2015.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J.H. Multimodality neuroimaging brain-age in uk biobank: relationship to biomedical, lifestyle, and cognitive factors. Neurobiol. Aging. 2020;92:34–42. doi: 10.1016/j.neurobiolaging.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J.H., Franke K. Predicting age using neuroimaging: innovative brain ageing biomarkers. Trends Neurosci. 2017;40:681–690. doi: 10.1016/j.tins.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Cole J.H., Marioni R.E., Harris S.E., Deary I.J. Brain age and other bodily ‘ages’: implications for neuropsychiatry. Mol. Psychiatry. 2019;24:266–281. doi: 10.1038/s41380-018-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R., Blell M., Hardy R., Black S., Pollard T., Wadsworth M., Pearce M., Kuh D. Validity of age at menarche self-reported in adulthood. J. Epidemiol. Commun. Health. 2006;60:993–997. doi: 10.1136/jech.2005.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane P.K., Walker R., Hubbard R.A., Li G., Nathan D.M., Zheng H., Haneuse S., Craft S., Montine T.J., Kahn S.E., et al. Glucose levels and risk of dementia. N. Engl. J. Med. 2013;369:540–548. doi: 10.1056/NEJMoa1215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day F.R., Ruth K.S., Thompson D.J., Lunetta K.L., Pervjakova N., Chasman D.I., Stolk L., Finucane H.K., Sulem P., Bulik-Sullivan B., Esko T., et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nature genetics. 2015;47(11):1294–1303. doi: 10.1038/ng.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day F.R., Thompson D.J., Helgason H., Chasman D.I., Finucane H., Sulem P., Ruth K.S., Whalen S., Sarkar A.K., Albrecht E., et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nature Genet. 2017;49:834–841. doi: 10.1038/ng.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bondt T., Jacquemyn Y., Van Hecke W., Sijbers J., Sunaert S., Parizel P. Regional gray matter volume differences and sex-hormone correlations as a function of menstrual cycle phase and hormonal contraceptives use. Brain Res. 2013;1530:22–31. doi: 10.1016/j.brainres.2013.07.034. [DOI] [PubMed] [Google Scholar]
- Debette S., Beiser A., Hoffmann U., Decarli C., O’Donnell C.J., Massaro J.M., Au R., Himali J.J., Wolf P.A., Fox C.S., Seshadri S. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann. Neurol. 2010;68:136–144. doi: 10.1002/ana.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S., Markus H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C., Zhou C., Fu C., Hao W., Ozaki A., Shrestha N., Virani S.S., Mishra S.R., Zhu D. Sex differences in the association between cardiovascular diseases and dementia subtypes: a prospective analysis of 464,616 uk biobank participants. Biol. Sex Differences. 2022;13:1–11. doi: 10.1186/s13293-022-00431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorjgochoo T., Kallianpur A., Gao Y.-T., Cai H., Yang G., Li H., Zheng W., Shu X.O. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the shanghai women’s health study. Menopause (New York, NY) 2008;15:924. doi: 10.1097/gme.0b013e3181786adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eavani H., Habes M., Satterthwaite T.D., An Y., Hsieh M.-K., Honnorat N., Erus G., Doshi J., Ferrucci L., Beason-Held L.L., et al. Heterogeneity of structural and functional imaging patterns of advanced brain aging revealed via machine learning methods. Neurobiol. Aging. 2018;71:41–50. doi: 10.1016/j.neurobiolaging.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoudary S.R., Shields K.J., Janssen I., Hanley C., Budoff M.J., Barinas-Mitchell E., Everson-Rose S.A., Powell L.H., Matthews K.A. Cardiovascular Fat, Menopause, and Sex Hormones in Women: The SWAN Cardiovascular Fat Ancillary Study. J. Clin. Endocrinol. Metab. 2015;100:3304–3312. doi: 10.1210/JC.2015-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euesden J., Lewis C.M., O’Reilly P.F. Prsice: polygenic risk score software. Bioinformatics. 2015;31:1466–1468. doi: 10.1093/bioinformatics/btu848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi F., Kantarci K., Graff-Radford J., Preboske G.M., Weigand S.D., Przybelski S.A., Knopman D.S., Machulda M.M., Roberts R.O., Mielke M.M., et al. Sex differences in cerebrovascular pathologies on flair in cognitively unimpaired elderly. Neurology. 2018;90:e466–e473. doi: 10.1212/WNL.0000000000004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Rhodes L., Malinowski J.R., Wang Y., Tao R., Pankratz N., Jeff J.M., Yoneyama S., Carty C.L., Setiawan V.W., Le Marchand, Haiman C. The genetic underpinnings of variation in ages at menarche and natural menopause among women from the multi-ethnic Population Architecture using Genomics and Epidemiology (PAGE) Study: A trans-ethnic meta-analysis. PloS one. 2018;13(7) doi: 10.1371/journal.pone.0200486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieremans E., Jensen J.H., Helpern J.A. White matter characterization with diffusional kurtosis imaging. Neuroimage. 2011;58:177–188. doi: 10.1016/j.neuroimage.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., Van Der Kouwe A., Killiany R., Kennedy D., Klaveness S., et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Floud S., Simpson R.F., Balkwill A., Brown A., Goodill A., Gallacher J., Sudlow C., Harris P., Hofman A., Parish S., et al. Body mass index, diet, physical inactivity, and the incidence of dementia in 1 million uk women. Neurology. 2020;94:e123–e132. doi: 10.1212/WNL.0000000000008779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster H.M., Celis-Morales C.A., Nicholl B.I., Petermann-Rocha F., Pell J.P., Gill J.M., O’Donnell C.A., Mair F.S. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: a prospective analysis of the uk biobank cohort. Lancet Public Health. 2018;3:e576–e585. doi: 10.1016/S2468-2667(18)30200-7. [DOI] [PubMed] [Google Scholar]
- Fotenos A.F., Mintun M.A., Snyder A.Z., Morris J.C., Buckner R.L. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical alzheimer disease, and reserve. Arch. Neurol. 2008;65:113–120. doi: 10.1001/archneurol.2007.27. [DOI] [PubMed] [Google Scholar]
- Fox M., Berzuini C., Knapp L.A. Cumulative estrogen exposure, number of menstrual cycles, and Alzheimer’s risk in a cohort of British women. Psychoneuroendocrinology. 2013;38:2973–2982. doi: 10.1016/j.psyneuen.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Franke K., Ziegler G., Klöppel S., Gaser C., Initiative A.D.N., et al. Estimating the age of healthy subjects from t1-weighted mri scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010;50:883–892. doi: 10.1016/j.neuroimage.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Fu C., Hao W., Shrestha N., Virani S.S., Mishra S.R., Zhu D. Association of reproductive factors with dementia: A systematic review and dose-response meta-analyses of observational studies. eClinicalMedicine. 2022;43 doi: 10.1016/j.eclinm.2021.101236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs F.D., Whelton P.K. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285–292. doi: 10.1161/HYPERTENSIONAHA.119.14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea L.A., Frick K.M., Hampson E., Sohrabji F., Choleris E. Why estrogens matter for behavior and brain health. Neurosci. Biobehav. Rev. 2017;76:363–379. doi: 10.1016/j.neubiorev.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings M.I., Ruitenberg A., Witteman J.C., van Swieten J.C., Hofman A., van Duijn C.M., Breteler M.M., Launer L.J. Reproductive period and risk of dementia in postmenopausal women. Jama. 2001;285:1475–1481. doi: 10.1001/jama.285.11.1475. [DOI] [PubMed] [Google Scholar]
- Georgakis M.K., Kalogirou E.I., Diamantaras A.-A., Daskalopoulou S.S., Munro C.A., Lyketsos C.G., Skalkidou A., Petridou E.T. Age at menopause and duration of reproductive period in association with dementia and cognitive function: A systematic review and meta-analysis. Psychoneuroendocrinology. 2016;73:224–243. doi: 10.1016/j.psyneuen.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Gerdts E., Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nature Med. 2019;25:1657–1666. doi: 10.1038/s41591-019-0643-8. [DOI] [PubMed] [Google Scholar]
- Gilsanz P., Lee C., Corrada M.M., Kawas C.H., Quesenberry C.P., Whitmer R.A. Reproductive period and risk of dementia in a diverse cohort of health care members. Neurology. 2019;92:e2005–e2014. doi: 10.1212/WNL.0000000000007326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Coalson T.S., Robinson E.C., Hacker C.D., Harwell J., Yacoub E., Ugurbil K., Andersson J., Beckmann C.F., Jenkinson M., et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff L.M. Ethnicity and type 2 diabetes in the uk. Diabet. Med. 2019;36:927–938. doi: 10.1111/dme.13895. [DOI] [PubMed] [Google Scholar]
- Gong J., Harris K., Peters S.A.E., Woodward M. Reproductive factors and the risk of incident dementia: A cohort study of UK Biobank participants. PLOS Med. 2022;19 doi: 10.1371/journal.pmed.1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L., Zamboni G., Khan A., Li L., Bonifacio G., Sundaresan V., Schulz U.G., Kuker W., Battaglini M., Rothwell P.M., Jenkinson M. BIANCA (Brain Intensity AbNormality Classification Algorithm): A new tool for automated segmentation of white matter hyperintensities. NeuroImage. 2016;141:191–205. doi: 10.1016/j.neuroimage.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Scarmeas N., Cosentino S., Brandt J., Albert M., Blacker D., Dubois B., Stern Y. Change in body mass index before and after alzheimer’s disease onset. Curr. Alzheimer Res. 2014;11:349–356. doi: 10.2174/1567205010666131120110930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurholt T.P., Kaufmann T., Frei O., Alnæs D., Haukvik U.K., van der Meer D., Moberget T., O’Connell K.S., Leinhard O.D., Linge J., et al. Population-based body–brain mapping links brain morphology with anthropometrics and body composition. Transl. Psychiatry. 2021;11:1–12. doi: 10.1038/s41398-021-01414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.E. Endocrinology of the Menopause. Endocrinol. Metab. Clin. North Am. 2015;44:485–496. doi: 10.1016/j.ecl.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.-P., Tang X., Han M., Yang J., Cardoso M.A., Zhou J., Simo R. Relationship between obesity and structural brain abnormality: Accumulated evidence from observational studies. Ageing Res. Rev. 2021;71 doi: 10.1016/j.arr.2021.101445. [DOI] [PubMed] [Google Scholar]
- Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., et al. Neuroinflammation in alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespanhol L., Vallio C.S., Costa L.M., Saragiotto B.T. Understanding and interpreting confidence and credible intervals around effect estimates. Braz. J Phys. Therapy. 2019;23:290–301. doi: 10.1016/j.bjpt.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E., Williams J., Budge M., Riedel W., Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101:485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- Ibrahim M.M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obesity Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- InterLACE Study Team Variations in reproductive events across life: a pooled analysis of data from 505 147 women across 10 countries. Hum. Reprod. 2019;34:881–893. doi: 10.1093/humrep/dez015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac V., Sim S., Zheng H., Zagorodnov V., Tai E.-S., Chee M. Adverse Associations between Visceral Adiposity, Brain Structure, and Cognitive Performance in Healthy Elderly. Front. Aging Neurosci. 2011;3 doi: 10.3389/fnagi.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E.G., Goldstein J.M. The middle-aged brain: biological sex and sex hormones shape memory circuitry. Curr. Opin. Behav. Sci. 2018;23:84–91. doi: 10.1016/j.cobeha.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I., Powell L.H., Crawford S., Lasley B., Sutton-Tyrrell K. Menopause and the Metabolic Syndrome: The Study of Women’s Health Across the Nation. Arch. Intern. Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolimova J., Tagoni J., Stern T.A. Obesity: its epidemiology, comorbidities, and management. The primary care companion for CNS disorders. 2013;15:27045. doi: 10.4088/PCC.12f01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jensen J.H., Helpern J.A., Ramani A., Lu H., Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Resonance Med.: Official J. Int. Soc. Magn. Resonance Med. 2005;53:1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- Jett S., Malviya N., Schelbaum E., Jang G., Jahan E., Clancy K., Hristov H., Pahlajani S., Niotis K., Loeb-Zeitlin S., Havryliuk Y., Isaacson R., Brinton R.D., Mosconi L. 2022. Endogenous and Exogenous Estrogen Exposures: How Women’s Reproductive Health Can Drive Brain Aging and Inform Alzheimer’s Prevention. Frontiers in Aging Neuroscience; p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen D.R., Shaaban C.E., Wiley C.A., Gianaros P.J., Mettenburg J., Rosano C. A population neuroscience approach to the study of cerebral small vessel disease in midlife and late life: an invited review. Am. J. Physiol.-Heart Circulat. Physiol. 2018;314:H1117–H1136. doi: 10.1152/ajpheart.00535.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaden E., Kelm N.D., Carson R.P., Does M.D., Alexander D.C. Multi-compartment microscopic diffusion imaging. NeuroImage. 2016;139:346–359. doi: 10.1016/j.neuroimage.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaden E., Kruggel F., Alexander D.C. Quantitative mapping of the per-axon diffusion coefficients in brain white matter. Magn. Resonance Med. 2016;75:1752–1763. doi: 10.1002/mrm.25734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T., van der Meer D., Doan N.T., Schwarz E., Lund M.J., Agartz I., Alnæs D., Barch D.M., Baur-Streubel R., Bertolino A., et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat. Neurosci. 2019;22:1617–1623. doi: 10.1038/s41593-019-0471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw E.E., Flier J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Kiliaan A.J., Arnoldussen I.A., Gustafson D.R. Adipokines: a link between obesity and dementia? Lancet Neurol. 2014;13:913–923. doi: 10.1016/S1474-4422(14)70085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.W., Seo H., Kwak M.-S., Kim D. Visceral obesity is associated with white matter hyperintensity and lacunar infarct. Int. J. Obes. (Lond.) 2017;41:683–688. doi: 10.1038/ijo.2017.13. [DOI] [PubMed] [Google Scholar]
- Kivimäki M., Luukkonen R., Batty G.D., Ferrie J.E., Pentti J., Nyberg S.T., Shipley M.J., Alfredsson L., Fransson E.I., Goldberg M., et al. Body mass index and risk of dementia: analysis of individual-level data from 1.3 million individuals. Alzheimer’s Dementia. 2018;14:601–609. doi: 10.1016/j.jalz.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosinski L.P., Yao J., Yin F., Fonteh A.N., Harrington M.G., Christensen T.A., Trushina E., Brinton R.D. White matter lipids as a ketogenic fuel supply in aging female brain: implications for alzheimer’s disease. EBioMedicine. 2015;2:1888–1904. doi: 10.1016/j.ebiom.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschke J.K. Bayesian analysis reporting guidelines. Nature Human Behaviour. 2021;5:1282–1291. doi: 10.1038/s41562-021-01177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok K.H., Lam K.S., Xu A. Heterogeneity of white adipose tissue: molecular basis and clinical implications. Exp. Mol. Med. 2016;48:e215. doi: 10.1038/emm.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe L., Zhang R., Beyer F., Huhn S., Kharabian Masouleh S., Preusser S., Bazin P.-L., Schroeter M.L., Villringer A., Witte A.V. Visceral obesity relates to deep white matter hyperintensities via inflammation. Ann. Neurol. 2019;85:194–203. doi: 10.1002/ana.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange A.M.G., Anatürk M., Suri S., Kaufmann T., Cole J.H., Griffanti L., Zsoldos E., Jensen D.E., Filippini N., Singh-Manoux A., Kivimäki M. Multimodal brain-age prediction and cardiovascular risk: The Whitehall II MRI sub-study. NeuroImage. 2020;222:117292. doi: 10.1016/j.neuroimage.2020.117292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange A.-M.G., Barth C., Kaufmann T., Anatürk M., Suri S., Ebmeier K.P., Westlye L.T. The maternal brain: Region-specific patterns of brain aging are traceable decades after childbirth. Human Brain Mapping. 2020 doi: 10.1002/hbm.25152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange A.-M.G., Barth C., Kaufmann T., Maximov I.I., van der Meer D., Agartz I., Westlye L.T. Women's brain aging: Effects of sex‐hormone exposure, pregnancies, and genetic risk for Alzheimer's disease. Human Brain Mapping. 2020;41(18):5141–5150. doi: 10.1002/hbm.25180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange A.-M.G., Cole J.H. Commentary: Correction procedures in brain-age prediction. NeuroImage: Clinical. 2020;26 doi: 10.1016/j.nicl.2020.102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange A.-M.G., Kaufmann T., van der Meer D., Maglanoc L.A., Alnæs D., Moberget T., Douaud G., Andreassen O.A., Westlye L.T. Population-based neuroimaging reveals traces of childbirth in the maternal brain. Proc. Natl. Acad. Sci. U.S.A. 2019;116:22341–22346. doi: 10.1073/pnas.1910666116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer A.A., Grimm H.S., Apel B., Golobrodska N., Kruse L., Ratanski E., Schulten N., Schwarze L., Slawik T., Sperlich S., et al. Mechanistic link between vitamin b12 and alzheimer’s disease. Biomolecules. 2022;12:129. doi: 10.3390/biom12010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.T., Kuplicki R.T., McKinney B.A., Yeh H.-W., Thompson W.K., Paulus M.P., Investigators T., et al. Front. Aging Neurosci. 2018. A nonlinear simulation framework supports adjusting for age when analyzing brainage; p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.G., Carr M.C., Murdoch S.J., Mitchell E., Woods N.F., Wener M.H., Chandler W.L., Boyko E.J., Brunzell J.D. Adipokines, Inflammation, and Visceral Adiposity across the Menopausal Transition: A Prospective Study. J. Clin. Endocrinol. Metab. 2009;94:1104–1110. doi: 10.1210/jc.2008-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeners B., Geary N., Tobler P.N., Asarian L. Ovarian hormones and obesity. Human Reprod. Update. 2017;23:300–321. doi: 10.1093/humupd/dmw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys C., Ley C., Klein O., Bernard P., Licata L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013;49:764–766. [Google Scholar]
- Liang H., Zhang F., Niu X. Investigating systematic bias in brain age estimation with application to post-traumatic stress disorders. Human Brain Mapp. 2019 doi: 10.1002/hbm.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linge J., Borga M., West J., Tuthill T., Miller M.R., Dumitriu A., Thomas E.L., Romu T., Tunón P., Bell J.D., et al. Body composition profiling in the uk biobank imaging study. Obesity. 2018;26:1785–1795. doi: 10.1002/oby.22210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano F., Guzmán G. Estrogen Deficiency and the Origin of Obesity during Menopause. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/757461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohner V., Pehlivan G., Sanroma G., Miloschewski A., Schirmer M.D., Stöcker T., Reuter M., Breteler M.M. Relation between sex, menopause, and white matter hyperintensities: The rhineland study. Neurology. 2022;99:e935–e943. doi: 10.1212/WNL.0000000000200782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy J., Champagne C., de Jonge L., Xie H., Smith S. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. (Lond.) 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., le Cessie S., Blauw G.J., Franceschi C., Noordam R., van Heemst D. Systemic inflammatory markers in relation to cognitive function and measures of brain atrophy: a Mendelian randomization study. GeroScience. 2022:1–12. doi: 10.1007/s11357-022-00602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki P.M., Dennerstein L., Clark M., Guthrie J., LaMontagne P., Fornelli D., Little D., Henderson V.W., Resnick S.M. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res. 2011;1379:232–243. doi: 10.1016/j.brainres.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt K.L., Pitynski-Miller D.R., Gavin K.M., Moreau K.L., Melanson E.L., Santoro N., Kohrt W.M. Body composition and cardiometabolic health across the menopause transition. Obesity. 2022;30:14–27. doi: 10.1002/oby.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov I.I., Alnaes D., Westlye L.T. Towards an optimised processing pipeline for diffusion magnetic resonance imaging data: Effects of artefact corrections on diffusion metrics and their age associations in UK biobank. Hum. Brain Mapp. 2019;40:4146–4162. doi: 10.1002/hbm.24691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov I.I., van der Meer D., de Lange A.-M.G., Kaufmann T., Shadrin A., Frei O., Wolfers T., Westlye L.T. Fast quality control method for derived diffusion metrics (YTTRIUM) in big data analysis: UK biobank 18,608 example. Human Brain Mapp. 2021;42:3141–3155. doi: 10.1002/hbm.25424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M., Raval A.P. The peri-menopause in a woman’s life: a systemic inflammatory phase that enables later neurodegenerative disease. J. Neuroinflamm. 2020;17:1–14. doi: 10.1186/s12974-020-01998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., D’arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PloS one. 2012;7 doi: 10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo S., Spampinato S.F., Sortino M.A. Estrogen and Alzheimer’s disease: Still an attractive topic despite disappointment from early clinical results. Eur. J. Pharmacol. 2017;817:51–58. doi: 10.1016/j.ejphar.2017.05.059. [DOI] [PubMed] [Google Scholar]
- Miller A.A., Spencer S.J. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav. Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Miller K.L., Alfaro-Almagro F., Bangerter N.K., Thomas D.L., Yacoub E., Xu J., Bartsch A.J., Jbabdi S., Sotiropoulos S.N., Andersson J.L., et al. Multimodal population brain imaging in the uk biobank prospective epidemiological study. Nature Neurosci. 2016;19:1523. doi: 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri S.A., Tehrani F.R., Yarandi R.B., Erfani H., Mansournia M.A., Azizi F. Effect of aging, menopause, and age at natural menopause on the trend in body mass index: a 15-year population-based cohort. Fertility Sterility. 2019;111:780–786. doi: 10.1016/j.fertnstert.2018.12.017. [DOI] [PubMed] [Google Scholar]
- Mori S., Wakana S., Van Zijl P.C., Nagae-Poetscher L. Elsevier; 2005. MRI atlas of human white matter. [DOI] [PubMed] [Google Scholar]
- Muka T., Oliver-Williams C., Kunutsor S., Laven J.S., Fauser B.C., Chowdhury R., Kavousi M., Franco O.H. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016;1:767–776. doi: 10.1001/jamacardio.2016.2415. [DOI] [PubMed] [Google Scholar]
- Must A., Phillips S., Naumova E., Blum M., Harris S., Dawson-Hughes B., Rand W. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am. J. Epidemiol. 2002;155:672–679. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]
- Najar J., Östling S., Waern M., Zettergren A., Kern S., Wetterberg H., Hällström T., Skoog I. Reproductive period and dementia: A 44-year longitudinal population study of Swedish women. Alzheimer’s Dementia. 2020;16:1153–1163. doi: 10.1002/alz.12118. [DOI] [PubMed] [Google Scholar]
- Nam K.-W., Kwon H., Kwon H.-M., Park J.-H., Jeong H.-Y., Kim S.H., Jeong S.-M., Kim H.J., Hwang S.-S. Abdominal fatness and cerebral white matter hyperintensity. J. Neurol. Sci. 2019;404:52–57. doi: 10.1016/j.jns.2019.07.016. [DOI] [PubMed] [Google Scholar]
- Newby D., Winchester L., Sproviero W., Fernandes M., Ghose U., Lyall D., Launer L.J., Nevado‐Holgado A.J. The relationship between isolated hypertension with brain volumes in UK Biobank. Brain and. 2022;behavior,:e2525. doi: 10.1002/brb3.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.C., Lee T.S., Kang H.-T., Cho E.S., Kim J.S., Hwang Y.J., Kim J.-K. Association Between Duration of Reproductive Years and Metabolic Syndrome. J. Women’s Health. 2018;27:271–277. doi: 10.1089/jwh.2017.6364. [DOI] [PubMed] [Google Scholar]
- Pasha E.P., Birdsill A., Parker P., Elmenshawy A., Tanaka H., Haley A.P. Visceral adiposity predicts subclinical white matter hyperintensities in middle-aged adults. Obes. Res. Clin. Pract. 2017;11:177–187. doi: 10.1016/j.orcp.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Pedditizi E., Peters R., Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. 2016;45:14–21. doi: 10.1093/ageing/afv151. [DOI] [PubMed] [Google Scholar]
- Peters S.A., Huxley R.R., Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551. doi: 10.1007/s00125-014-3260-6. [DOI] [PubMed] [Google Scholar]
- Prince C., Sharp G.C., Howe L.D., Fraser A., Richmond R.C. The relationships between women’s reproductive factors: a Mendelian randomisation analysis. BMC Med. 2022;20:103. doi: 10.1186/s12916-022-02293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu D., Tan R., Yu Q., Wu J. Metabolic syndrome in menopause and associated factors: a meta-analysis. Climacteric. 2017;20:583–591. doi: 10.1080/13697137.2017.1386649. [DOI] [PubMed] [Google Scholar]
- Qi Y., Lin M., Yang Y., Li Y. Relationship of visceral adipose tissue with dilated perivascular spaces. Front. Neurosci. 2021;14 doi: 10.3389/fnins.2020.583557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S., Reid R.I., Przybelski S.A., Lesnick T.G., Graff-Radford J., Schwarz C.G., Knopman D.S., Mielke M.M., Machulda M.M., Petersen R.C., et al. Diffusion models reveal white matter microstructural changes with ageing, pathology and cognition. Brain Commun. 2021;3:fcab106. doi: 10.1093/braincomms/fcab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A., Jackson H., Hristov H., Isaacson R.S., Saif N., Shetty T., Etingin O., Henchcliffe C., Brinton R.D., Mosconi L. Sex and Gender Driven Modifiers of Alzheimer’s: The Role for Estrogenic Control Across Age, Race, Medical, and Lifestyle Risks. Front. Aging Neurosci. 2019;11 doi: 10.3389/fnagi.2019.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez A., Wolfsgruber S., Lange C., Kaduszkiewicz H., Weyerer S., Werle J., Pentzek M., Fuchs A., Riedel-Heller S.G., Luck T., et al. Elevated hba 1c is associated with increased risk of incident dementia in primary care patients. J. Alzheimer’s Disease. 2015;44:1203–1212. doi: 10.3233/JAD-141521. [DOI] [PubMed] [Google Scholar]
- Randolph Jr J.F., Zheng H., Sowers M.R., Crandall C., Crawford S., Gold E.B., Vuga M. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J. Clin. Endocrinol. Metab. 2011;96:746–754. doi: 10.1210/jc.2010-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff R.M. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- Razay G., Vreugdenhil A., Wilcock G. Obesity, abdominal obesity and alzheimer disease. Dementia and geriatric cognitive disorders. 2006;22:173–176. doi: 10.1159/000094586. [DOI] [PubMed] [Google Scholar]
- Reitz C. Dyslipidemia and dementia: current epidemiology, genetic evidence, and mechanisms behind the associations. J. Alzheimer’s Dis. 2012;30:S127–S145. doi: 10.3233/JAD-2011-110599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa-Díaz Z.M., Raguindin P.F., Bano A., Laine J.E., Muka T., Glisic M. Menopause and cardiometabolic diseases: What we (don’t) know and why it matters. Maturitas. 2021;152:48–56. doi: 10.1016/j.maturitas.2021.06.013. [DOI] [PubMed] [Google Scholar]
- Rosen A.F., Roalf D.R., Ruparel K., Blake J., Seelaus K., Villa L.P., Ciric R., Cook P.A., Davatzikos C., Elliott M.A., et al. Quantitative assessment of structural image quality. Neuroimage. 2018;169:407–418. doi: 10.1016/j.neuroimage.2017.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth K.S., Day F.R., Hussain J., Martínez-Marchal A., Aiken C.E., Azad A., Thompson D.J., Knoblochova L., Abe H., Tarry-Adkins J.L., et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature. 2021;596:393–397. doi: 10.1038/s41586-021-03779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P.S., Parslow R., Wen W., Anstey K., Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol. Aging. 2009;30:946–956. doi: 10.1016/j.neurobiolaging.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Samargandy S., Matthews K.A., Brooks M.M., Barinas-Mitchell E., Magnani J.W., Janssen I., Kazlauskaite R., El Khoudary S.R. Abdominal visceral adipose tissue over the menopause transition and carotid atherosclerosis: the SWAN heart study. Menopause. 2021;28:626–633. doi: 10.1097/GME.0000000000001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelbaum E., Loughlin L., Jett S., Zhang C., Jang G., Malviya N., Hristov H., Pahlajani S., Isaacson R., Dyke J.P., Kamel H., Brinton R.D., Mosconi L. Association of Reproductive History With Brain MRI Biomarkers of Dementia Risk in Midlife. Neurology. 2021;97:e2328–e2339. doi: 10.1212/WNL.0000000000012941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E., Zhang Q.-G., Wang R., Vadlamudi R., Brann D. Estrogen neuroprotection and the critical period hypothesis. Front. Neuroendocrinol. 2012;33:85. doi: 10.1016/j.yfrne.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky R.M., Murphy A.Z. Considering sex as a biological variable will require a global shift in science culture. Nature Neurosci. 2021;24:457–464. doi: 10.1038/s41593-021-00806-8. [DOI] [PubMed] [Google Scholar]
- Siiteri P.K. Adipose tissue as a source of hormones. Am. J. Clin. Nutr. 1987;45:277–282. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]
- Simpson E.R. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Vidaurre D., Alfaro-Almagro F., Nichols T.E., Miller K.L. Estimation of brain age delta from brain imaging. NeuroImage. 2019 doi: 10.1016/j.neuroimage.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreca I., Rosano C., Jennings J.R., Sheu L.K., Kuller L.H., Matthews K.A., Aizenstein H.J., Gianaros P.J. Gain in adiposity across 15 years is associated with reduced gray matter volume in healthy women. Psychosom. Med. 2009;71:485. doi: 10.1097/PSY.0b013e3181a5429d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B., Berry D. The regulation of adipose tissue health by estrogens. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.889923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniapillai S., Rajagopal S., Snytte J., Otto A.R., Einstein G., Rajah M.N. Sex differences in brain aging among adults with family history of Alzheimer’s disease and APOE4 genetic risk. NeuroImage: Clinical. 2021;30 doi: 10.1016/j.nicl.2021.102620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniapillai S., Suri S., Barth C., Maximov I.I., Voldsbekk I., van der Meer D., Gurholt T.P., Beck D., Draganski B., Andreassen O.A., Ebmeier K.P. Sex‐and age‐specific associations between cardiometabolic risk and white matter brain age in the UK Biobank cohort. Human brain mapping. 2022;43(12):3759–3774. doi: 10.1002/hbm.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung L., Hayden J., Greenberg M.L., Koren G., Feldman B.M., Tomlinson G.A. Seven items were identified for inclusion when reporting a bayesian analysis of a clinical study. J. Clin. Epidemiol. 2005;58:261–268. doi: 10.1016/j.jclinepi.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Tang X., Zhao W., Lu M., Zhang X., Zhang P., Xin Z., Sun R., Tian W., Cardoso M.A., Yang J., et al. Relationship between central obesity and the incidence of cognitive impairment and dementia from cohort studies involving 5,060,687 participants. Neurosci. Biobehav. Rev. 2021;130:301–313. doi: 10.1016/j.neubiorev.2021.08.028. [DOI] [PubMed] [Google Scholar]
- Tao X., Jiang A., Yin L., Li Y., Tao F., Hu H. Body mass index and age at natural menopause: a meta-analysis. Menopause. 2015;22:469–474. doi: 10.1097/GME.0000000000000324. [DOI] [PubMed] [Google Scholar]
- Taylor C., Pritschet L., Yu S., Jacobs E.G. Applying a women’s health lens to the study of the aging brain. Front. Human Neurosci. 2019;13:224. doi: 10.3389/fnhum.2019.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than S., Moran C., Beare R., Vincent A.J., Collyer T.A., Wang W., Callisaya M.L., Thomson R., Phan T.G., Fornito A., et al. Interactions between age, sex, menopause, and brain structure at midlife: A uk biobank study. J. Clin. Endocrinol. Metab. 2021;106:410–420. doi: 10.1210/clinem/dgaa847. [DOI] [PubMed] [Google Scholar]
- Than S., Moran C., Collyer T.A., Beare R.J., Lane E.M., Vincent A.J., Wang W., Callisaya M.L., Thomson R., Phan T.G., et al. Associations of sex, age, and cardiometabolic risk profiles with brain structure and cognition: A uk biobank latent class analysis. Neurology. 2022 doi: 10.1212/WNL.0000000000201028. [DOI] [PubMed] [Google Scholar]
- Thurston R.C., Aizenstein H.J., Derby C.A., Sejdić E., Maki P.M. Menopausal hot flashes and white matter hyperintensities. Menopause (New York, NY) 2016;23:27. doi: 10.1097/GME.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel D., Admiraal-Behloul F., Ten Dam V., Olofsen H., Bollen E., Murray H., Blauw G., Westendorp R., De Craen A., Van Buchem M., et al. Different progression rates for deep white matter hyperintensities in elderly men and women. Neurology. 2004;63:1699–1701. doi: 10.1212/01.wnl.0000143058.40388.44. [DOI] [PubMed] [Google Scholar]
- Veit R., Kullmann S., Heni M., Machann J., Häring H.-U., Fritsche A., Preissl H. Reduced cortical thickness associated with visceral fat and BMI. NeuroImage: Clinical. 2014;6:307–311. doi: 10.1016/j.nicl.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldsman M., Kindalova P., Husain M., Kosmidis I., Nichols T.E. Spatial distribution and cognitive impact of cerebrovascular risk-related white matter hyperintensities. NeuroImage: Clinical. 2020;28 doi: 10.1016/j.nicl.2020.102405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voldsbekk I., Barth C., Maximov I.I., Kaufmann T., Beck D., Richard G., Moberget T., Westlye L.T., de Lange A.-M.G. A history of previous childbirths is linked to women’s white matter brain age in midlife and older age. Hum. Brain Mapp. 2021;42:4372–4386. doi: 10.1002/hbm.25553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorinen M., Damangir S., Niskanen E., Miralbell J., Rusanen M., Spulber G., Soininen H., Kivipelto M., Solomon A. Coronary Heart Disease and Cortical Thickness, Gray Matter and White Matter Lesion Volumes on MRI. PLOS ONE. 2014;9 doi: 10.1371/journal.pone.0109250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waaseth M., Bakken K., Dumeaux V., Olsen K.S., Rylander C., Figenschau Y., Lund E. Hormone replacement therapy use and plasma levels of sex hormones in the norwegian women and cancer postgenome cohort–a cross-sectional analysis. BMC Women’s Health. 2008;8:1–11. doi: 10.1186/1472-6874-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M., Wang Y., Amlien I.K., Mowinckel A.M., Lindenberger U., Düzel S., Bartrés-Faz D., Ebmeier K.P., Drevon C.A., Baaré W.F. Education and income show heterogeneous relationships to lifespan brain and cognitive differences across European and US cohorts. Cerebral cortex. 2022;32(4):839–854. doi: 10.1093/cercor/bhab248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Lv J., Qiu X., An Y. Integrating genome-wide association and eQTLs studies identifies the genes associated with age at menarche and age at natural menopause. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0213953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartolowska K.A., Webb A.J.S. Midlife blood pressure is associated with the severity of white matter hyperintensities: analysis of the uk biobank cohort study. Eur. Heart J. 2021;42:750–757. doi: 10.1093/eurheartj/ehaa756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W., Sachdev P.S., Li J.J., Chen X., Anstey K.J. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44–48. Human Brain Mapp. 2009;30:1155–1167. doi: 10.1002/hbm.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer R., Gustafson D., Barrett-Connor E., Haan M., Gunderson E., Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- Widya R.L., Kroft L.J., Altmann-Schneider I., van den Berg-Huysmans A.A., van der Bijl N., de Roos A., Lamb H.J., van Buchem M.A., Slagboom P.E., van Heemst D., et al. Visceral adipose tissue is associated with microstructural brain tissue damage. Obesity. 2015;23:1092–1096. doi: 10.1002/oby.21048. [DOI] [PubMed] [Google Scholar]
- Wiggs A.G., Chandler J.K., Aktas A., Sumner S.J., Stewart D.A. The Effects of Diet and Exercise on Endogenous Estrogens and Subsequent Breast Cancer Risk in Postmenopausal Women. Frontiers in Endocrinology. 2021;12:1140. doi: 10.3389/fendo.2021.732255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro K., Tanaka R., Tanaka Y., Miyamoto N., Shimada Y., Ueno Y., Urabe T., Hattori N. Visceral fat accumulation is associated with cerebral small vessel disease. Eur. J. Neurol. 2014;21:667–673. doi: 10.1111/ene.12374. [DOI] [PubMed] [Google Scholar]
- Yang L., Lin L., Kartsonaki C., Guo Y., Chen Y., Bian Z., Xie K., Jin D., Li L., Lv J., et al. Menopause characteristics, total reproductive years, and risk of cardiovascular disease among chinese women. Circulation: Cardiovasc. Q. Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.117.004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Chen Z., Baudier R.L., Krousel-Wood M., Anderson A.H., Fonseca V.A., Mauvais-Jarvis F. Sex differences in the progression of metabolic risk factors in diabetes development. JAMA Network Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.22070. e2222070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zárate S., Stevnsner T., Gredilla R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Frontiers in Aging. Neuroscience. 2017;9 doi: 10.3389/fnagi.2017.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki Al Hazzouri A., Stone K.L., Haan M.N., Yaffe K. Leptin, mild cognitive impairment, and dementia among elderly women. Journals of Gerontology Series A Biomedical Sciences and Medical Sciences. 2013;68(2):175–180. doi: 10.1093/gerona/gls155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeydan B., Tosakulwong N., Schwarz C.G., Senjem M.L., Gunter J.L., Reid R.I., Rocca L.G., Lesnick T.G., Smith C.Y., Bailey K.R., et al. Association of bilateral salpingo-oophorectomy before menopause onset with medial temporal lobe neurodegeneration. JAMA Neurol. 2019;76:95–100. doi: 10.1001/jamaneurol.2018.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Smith J.A., Bielak L.F., Ruiz-Narvaez E.A., Yu M., Hood M.M., Peyser P.A., Kardia S.L., Harlow S.D. Associations between polygenic risk scores for age at menarche and menopause, reproductive timing, and serum hormone levels in multiple race/ethnic groups. Menopause. 2021;28:819–828. doi: 10.1097/GME.0000000000001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Chung H.F., Pandeya N., Dobson A.J., Hardy R., Kuh D., Brunner E.J., Bruinsma F., Giles G.G., Demakakos P., Lee J.S. Premenopausal cardiovascular disease and age at natural menopause: a pooled analysis of over 170,000 women. European journal of epidemiology. 2019;34(3):235–246. doi: 10.1007/s10654-019-00490-w. [DOI] [PubMed] [Google Scholar]
- Zsakai A., Mascie-Taylor N., Bodzsar E.B. Relationship between some indicators of reproductive history, body fatness and the menopausal transition in Hungarian women. J. Physiol. Anthropol. 2015;34:35. doi: 10.1186/s40101-015-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsido R.G., Heinrich M., Slavich G.M., Beyer F., Kharabian Masouleh S., Kratzsch J., Raschpichler M., Mueller K., Scharrer U., Löffler M., Schroeter M.L., Stumvoll M., Villringer A., Witte A.V., Sacher J. Association of Estradiol and Visceral Fat With Structural Brain Networks and Memory Performance in Adults. JAMA Network Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available through the UK Biobank data access procedures (https://www.ukbiobank.ac.uk/researchers).