Abstract
Culture filtrate proteins (CFP) of Mycobacterium tuberculosis have been shown to contain immunogenic components that elicit at least partial protective immunity against Mycobacterium infection. To clone genes encoding some of the immunogenic proteins, we made a high-titer rabbit anti-CFP serum and used it to screen an M. tuberculosis genomic expression library in Escherichia coli. In this paper, we describe the molecular cloning of two new protein components of CFP and identified them as members of the serine protease gene family. Their open reading frames contain N-terminal hydrophobic secretory signals consistent with their detection in CFP. The predicted molecular masses of the mature, fully processed forms of both antigens are ∼32 kDa, in agreement with their observed sizes on immunoblots of CFP probed with polyclonal rabbit antisera made to the recombinant proteins. Thus, these proteins have been designated MTB32A and MTB32B. Interestingly, and despite 66% amino acid sequence homology between the two proteins, polyclonal rabbit antisera made to each of the recombinant proteins were found to be specific for the respective immunizing antigens. The recombinant proteins were also evaluated in in vitro assays with donor peripheral blood mononuclear cells (PBMC) from healthy purified protein derivative (PPD)-positive individuals of diverse ethnic backgrounds. MTB32A but not MTB32B stimulated PBMC from healthy PPD-positive donors but not from PPD-negative donors to proliferate and secrete gamma interferon. MTB32A is encoded by a single-copy gene which is present in both virulent and avirulent strains of the M. tuberculosis complex and the BCG strain of Mycobacterium bovis but absent in the environmental mycobacterial species tested. In addition, nucleotide sequence comparison of mtb32a of the avirulent H37Ra strain and the virulent Erdman strain, as well as with the corresponding sequences (identified in the databases) of strain H37Rv and the clinical isolate CSU93, revealed 100% identity. MTB32A, therefore, represents a candidate for inclusion in subunit vaccine development. Finally, the possible role of MTB32 serine proteases as a virulence factor(s) during Mycobacterium spp. infection is discussed.
Tuberculosis remains one of the world’s most serious health threats not only in developing countries but also in industrialized countries, where a resurgence, particularly with human immunodeficiency virus infection and the emergence of drug-resistant strains, underscores the need for an effective vaccine against this important disease (15, 23, 41). Approximately 2 billion people are infected worldwide, and an estimated 2.9 million deaths due to tuberculosis occur annually (25, 38). The only vaccine currently in use is the live, attenuated strain of Mycobacterium bovis, bacillus Calmette-Guérin (BCG) (7, 9). Although vaccination with BCG is widely practiced, its efficacy is reported to vary considerably among different clinical trials and geographically distinct populations (10).
Recently, there has been increased interest in the secreted antigens of mycobacteria as candidates for a subunit-based vaccine. This stems from the observation that immunization of mice with live but not killed preparations of Mycobacterium tuberculosis resulted in the generation of a partially protective response (31). In addition, culture filtrate proteins (CFP) obtained from in vitro-cultivated M. tuberculosis have been shown to offer some degree of protection when used as vaccines in animal models of tuberculosis (1, 19, 20, 32, 33). These findings, combined with the ability of CFP to stimulate proliferation and cytokine production from T cells of infected mice, guinea pigs, and purified protein derivative-positive (PPD+) human donors (2, 5, 12, 18, 19, 21, 31, 33, 39, 43, 44) have led to the conclusion that CFP are an important source of candidate antigens for a subunit vaccine against tuberculosis. Thus, several laboratories are currently working toward the identification of proteins that are secreted or shed by M. tuberculosis. In this paper, we describe the molecular cloning and immunological evaluation of two new protein components of CFP, MTB32A and MTB32B, and identify them as being encoded by members of the serine protease family.
MATERIALS AND METHODS
Mycobacterial strains.
M. tuberculosis H37Ra, H37Rv, and Erdman were provided by Sean Skerritt (Seattle VA Hospital). M. tuberculosis C is a clinical isolate provided by Lee Riley (University of California, Berkeley). Pelleted samples of M. bovis (BCG) and Mycobacterium leprae were kindly provided by Paul Tan (Genesis Corp., Auckland, New Zealand). Other species of mycobacteria were obtained from the American Type Culture Collection (Manassas, Va.): M. tuberculosis H37Ra (ATCC 25177), M. tuberculosis Erdman (ATCC 35801) Mycobacterium scrofulaceum (ATCC 19981), Mycobacterium smegmatis (ATCC 19420), Mycobacterium fortuitum (ATCC 6841), Mycobacterium malmoense (ATCC 29571), and Mycobacterium gordonae (ATCC 14470). Mycobacterial genomic DNA was prepared as previously described (12).
Secreted proteins.
M. tuberculosis H37Rv was grown in 90 ml of Sauton’s medium (3) for 18 days at 37°C in 5% CO2. The medium (containing CFP) was harvested after 18 days of growth by centrifugation at 2,000 × g for 20 min, and the supernatant was sterilized by passage through a 0.2-μm-pore-size filter. The filtrate was concentrated with an Amicon 3 Centriprep concentrator (Amicon, Beverly, Mass.) to 1/100 of the original volume, and the protein content was determined with a bicinchoninic acid protein assay (Pierce, Rockford, Ill.). Secreted proteins from M. tuberculosis Erdman and H37Rv were also provided by John Belisle, Colorado State University, produced through National Institute of Allergy and Infectious Diseases-National Institutes of Health Tuberculosis Research Materials contract N01-AI-25147.
Generation of rabbit antisera.
Rabbit antisera to M. tuberculosis (Erdman and H37Rv strains)-secreted proteins and the recombinant antigens were prepared by injecting 200 μg of protein with 1 ml of incomplete Freunds adjuvant (Life Technologies, Grand Island, N.Y.) and 100 μg of muramyl dipeptide subcutaneously followed with a boost of 100 μg of protein incomplete Freunds adjuvant given subcutaneously 6 weeks later and a final boost of 50 μg administered intravenously 1 month later. The animals were sacrificed 1 week after the second boost, and sera were stored in aliquots at −20°C.
Library preparation and serological expression screening.
Genomic DNA from M. tuberculosis H37Ra and Erdman was fragmented for library generation by sonication, yielding DNA fragments in a size range of 300 to 4,000 bp. The ends were blunted with Klenow polymerase, ligated to EcoRI adapters, and subcloned into EcoRI predigested λZAP bacteriophage arms according to the manufacturer’s protocols (Stratagene, La Jolla, Calif.). Phage were packaged with Gigapack II packaging extracts (Stratagene) as recommended. Rabbit anti-CFP sera were preadsorbed against total Escherichia coli proteins and were used to screen the M. tuberculosis H37Ra genomic expression libraries (10,000 PFU/plate; 60,000 PFU total) following a 1:250 dilution. Reactivity was assessed as previously described (25) with 125I-protein A, followed by autoradiography to detect immunoreactive plaques. Following plaque purification, excision of the pBSK(−) phagemid was carried out according to the manufacturer’s protocol (Stratagene). Plasmid DNA was purified with an anion-exchange resin (Qiagen, Chatsworth, Calif.) as recommended by the manufacturer. DNA was sequenced by the dye terminator technique with an ABI 373-A Stretch DNA sequencer (Applied Biosystems, Inc., Foster City, Calif.).
High-level expression and affinity purification of recombinant mycobacterial antigens.
Oligonucleotide PCR primers were designed to amplify the mature (devoid of secretory sequence) forms or overlapping amino- and carboxy-terminal portions of MTB32A and MTB32B. The secreted form of MTB32A was amplified with the oligonucleotide primers 5′ (5′-TTA CAT ATG GCT AGC CAT CAC CAT CAC CAT CAC AGC AAT TCG CGC CGC CGC TCA C-3′) and 3′ (5′-AAA GGG GGA TGT GCT GCA AGG CG-3′) (underlining, boldface, and italics are defined below). The amino and carboxy termini of MTB32A (N- and C-terminal halves, respectively) were cloned with the oligonucleotide primers 5′ (5′-CAA TTA CAT ATG CAT CAC CAT CAC CAT CAC GCC CCG CCG GCC TTG TCG CAG GAC-3′) and 3′ (5′-GAT TAG GAA TTC CTA GGA CGC GGC CGT GTT CAT AC-3′) for the MTB32A N-terminal part and 5′ (5′-TTA CAT ATG GCT AGC CAT CAC CAT CAC CAT CAC ACG GCC GCG TCC GAT AAC TTC-3′) and 3′ (5′-GTA CGG AAT TCG TAA AAC GAC GGC CAG T-3′) for the MTB32A C-terminal part.
Fragments comprising the full-length and amino- and carboxy-terminal portions of MTB32B were PCR amplified with the following primer pairs: 5′ (5′-CAA TTA CAT ATG CAT CAC CAT CAC CAT CAC GGT TTT ACC GGT CGG CAT CGG CAC-3′) and 3′ (5′-GTA CGG AAT TCG ACC TTC ATC ACT GCT CCG CCT TG-3′) for the mature form of MTB32B, 5′ (5′-CAA TTA CAT ATG CAT CAC CAT CAC CAT CAC GCG CCA AGC ATC CCC GCA GCA AAC ATG C-3′) and 3′ (5′-GTA CGG AAT TCC TAC GTG GCA ATG GCC GAG TTG ACT C-3′) for the amino-terminal half, and 3′ (5′-CAA TTA CAT ATG CAT CAC CAT CAC CAT CAC GTC CGT GTT CAG GGC GTC TCC G-3′) and 3′ (5′-GTA CGG AAT TCG TAA AAC GAC GGC CAG T-3′) for the carboxy-terminal half.
Sequence comprising antigen 85B (13, 29) was cloned by PCR amplification (of genomic DNA isolated from the H37Ra strain) with primers designed to amplify the entire mature-secreted portion. For 85B, the oligonucleotides used for PCR amplification contained the following sequences: 5′-CAA TTA CAT ATG CAT CAC CAT CAC CAT CAC TTC TCC CGG CCG GGG CTG C-3′ (5′) and 5′-GTA CGG AAT TCC CTT CGG TTG ATC CCG TCA GC-3′ (3′).
The 5′ oligonucleotides contain an NdeI restriction site preceding the ATG initiation codons (underlined) followed by nucleotide sequences encoding six histidines (boldface) and sequences derived from the gene (italics). The resultant PCR products were digested with NdeI and EcoRI and subcloned into the pET17b vector similarly digested with NdeI and EcoRI for directional cloning. Ligation products were initially transformed into E. coli XL1-Blue competent cells (Stratagene) and were subsequently transformed into E. coli BL-21(pLysE) host cells (Novagen, Madison, Wis.) for expression.
Production and purification of recombinant proteins.
The recombinant proteins were expressed in E. coli with six histidine residues at the amino-terminal portion with the pET plasmid vector (pET17b) and a T7 RNA polymerase expression system (Novagen). E. coli BL-21(DE3)pLysE (Novagen) was used for high-level expression. Recombinant (His-tagged) antigens were purified from the soluble supernatant or the insoluble inclusion body of 500 ml of isopropyl-β-d-thiogalactopyranoside (IPTG)-induced batch cultures by affinity chromatography with the one-step QIAexpress Ni-nitrilotriacetic acid (NTA) agarose matrix (Qiagen) in the presence of 8 M urea. Briefly, 20 ml of an overnight saturated culture of BL-21 containing the pET construct was added to 500 ml of 2× yeast extract-tryptone medium containing 50 μg of ampicillin per ml and 34 μg of chloramphenicol per ml and grown at 37°C with shaking. The bacterial cultures were induced with 2 mM IPTG at an optical density (OD) at 560 nm of 0.3 and grown for an additional 3 h (OD = 1.3 to 1.9). Cells were harvested from 500-ml batch cultures by centrifugation and resuspended in 20 ml of binding buffer (0.1 M sodium phosphate [pH 8.0], 10 mM Tris-HCl [pH 8.0]) containing 2 mM phenylmethylsulfonyl fluoride and 20 μg of leupeptin per ml plus one Complete protease inhibitor tablet (Boehringer Mannheim) per 25 ml. E. coli was lysed by freeze-thaw followed by brief sonication and then spun at 12,000 rpm for 30 min to pellet the inclusion bodies.
The inclusion bodies were washed three times in 1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) in 10 mM Tris-HCl (pH 8.0). This step greatly reduced the level of contaminating lipopolysaccharide. The inclusion body was finally solubilized in 20 ml of binding buffer containing 8 M urea, or 8 M urea was added directly into the soluble supernatant. Recombinant antigens with His-tagged residues were batch bound to Ni-NTA agarose resin (5 ml of resin per 500-ml induction) by rocking at room temperature for 1 h, and the complex was passed over a column. The flowthrough was passed twice over the same column, and the column was washed three times with 30 ml each of wash buffer (0.1 M sodium phosphate and 10 mM Tris-HCl, pH 6.3) also containing 8 M urea. Bound protein was eluted with 30 ml of 150 mM imidazole in wash buffer, and 5-ml fractions were collected. Fractions containing the recombinant antigen were pooled, dialyzed against 10 mM Tris-HCl (pH 8.0) bound one more time to the Ni-NTA matrix, eluted, and dialyzed in 10 mM Tris-HCl (pH 7.8). The yield of recombinant protein varied from 25 to 150 mg per liter of induced bacterial culture with greater than 98% purity. Recombinant proteins were assayed for endotoxin contamination by the Limulus amebocyte assay (BioWhittaker) and were shown to contain <100 endotoxin units/mg of protein.
Immunoblot analysis of recombinant MTB32A (rMTB32A) and rMTB32B.
M. tuberculosis (strain H37Rv) total lysate or CFP (2.5 μg each) as well as 50 ng of the indicated recombinant proteins were separated by electrophoresis on sodium dodecyl sulfate (SDS)–15% polyacrylamide gels and transferred to nitrocellulose with a semidry transfer apparatus (Bio-Rad). Blots, in triplicate, were blocked for a minimum of 1 h with phosphate-buffered saline (PBS)–1.0% Tween and were probed with anti-CFP, anti-MTB32A, anti-MTB32B, and anti-85B polyclonal rabbit antisera diluted 1:500 in PBS–0.1% Tween 20 as indicated. Reactivity was assessed as previously described (37) with 125I-protein A, followed by autoradiography.
Southern analysis.
Genomic DNA was prepared by standard techniques (36) and was digested with PstI. One microgram of each digest was run on a 1% agarose gel and stained with ethidium bromide to confirm equivalent loading of each lane prior to overnight transfer to a Nytran membrane. Random hexamer 32P-radiolabeled insert DNA was prepared by the random priming method (17) and hybridized overnight at 65°C. Blots were washed at 65°C twice for 15 min each with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1× SSC containing 0.1% SDS.
Immune responses of donor PBMC to rMTB32 antigens.
The recombinant antigens were evaluated in vitro for their ability to induce T-cell proliferation and gamma interferon (IFN-γ) production with a panel of peripheral blood mononuclear cells (PBMC) obtained from healthy PPD+ (indurations of >10 mm) and PPD− individuals of diverse ethnic backgrounds (African, Middle Eastern, Hispanic, European, and Asian). PBMC were obtained from heparanized blood by Ficoll gradient centrifugation or by leukapheresis. PBMC (2 × 105/well) were incubated in 96-well round-bottomed plates (Costar) in medium only (RPMI with 10% pooled human serum and gentamicin at 50 μg/ml) or in medium containing specific antigens at the indicated concentrations. Plates were cultured for 5 days at 37°C in 5% CO2 and were pulsed with 1 μCi of [3H]thymidine (Amersham) for an additional 18 h. Cells were harvested onto filter mats and counted with a Matrix 9600 Direct Beta gas scintillation counter (Packard).
Cytokine ELISA.
The levels of supernatant IFN-γ were analyzed by sandwich enzyme-linked immunosorbent assay (ELISA), with antibody pairs and procedures available from PharMingen. Standard curves were generated with recombinant mouse cytokines. ELISA plates (Corning) were coated with 50 μl (1 μg/ml, in 0.1 M bicarbonate coating buffer [pH 9.6]) of a cytokine capture monoclonal antibody (rat anti-mouse IFN-γ; PharMingen; catalog no. 18181D) per well and incubated for 4 h at room temperature. Liquid was removed, and plates were blocked with PBS–0.05% Tween–1.0% bovine serum albumin (BSA) (200 μl/well) overnight at 4°C and washed six times in PBS–0.1% Tween. Standards (recombinant mouse IFN-γ) and supernatant samples diluted in PBS–0.05% Tween–0.1% BSA were then added for 2 h at room temperature. The plates were washed as described above and then incubated for 2 h at room temperature with 100 μl of a second antibody (biotin–rat anti-mouse IFN-γ ([catalog no. 18112D; PharMingen]) per well at 0.5 μg/ml diluted in PBS–0.05% Tween–0.1% BSA. After washing, plates were incubated with 100 μl of streptavidin-horseradish peroxidase (Zymed) per well at a 1:2,500 dilution in PBS–0.05% Tween–0.1% BSA at room temperature for 1 h. The plates were washed one last time and developed with 100 μl of TMB substrate (3,3′,5,5′-tetramethylbenzidine; Kirkegaard & Perry Laboratories, Gaithersburg, Md.) per well, and the reaction was stopped after the color developed with H2SO4, 50 μl/well. Absorbance (OD) was determined at 450 nm with 570 nm as a reference wavelength, and the cytokine concentration was evaluated with the standard curve.
RESULTS
Expression cloning and molecular characterization of MTB32A and MTB32B.
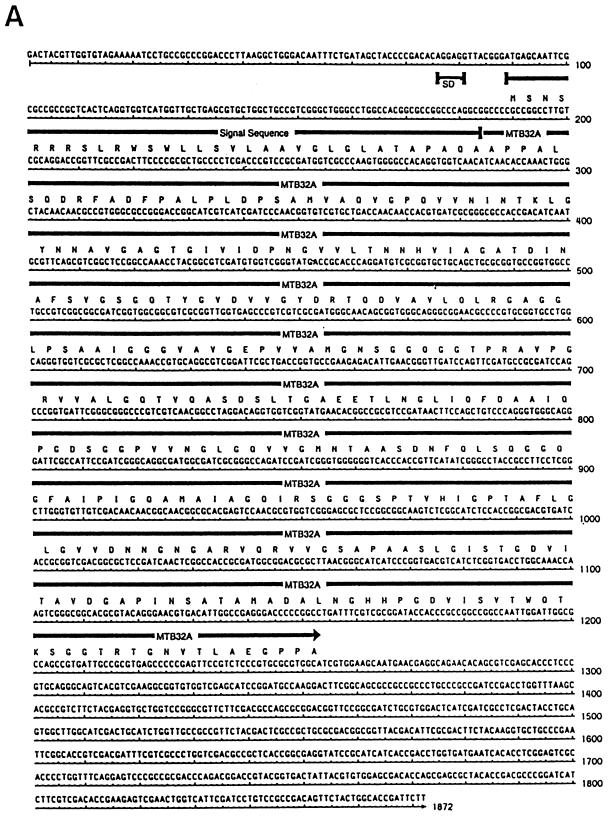
A genomic expression library of the M. tuberculosis avirulent strain H37Ra was screened with a rabbit antiserum made against CFP of virulent M. tuberculosis Erdman. Several clones were identified, and two of these, mtb32a and mtb32b, were pursued further because (i) MTB32A was found to be antigenic in healthy, PPD+ individuals and (ii) both proteins have sequence similarities with proteins identified from other species implicated in constitution of virulence factors. Figures 1A and B show the nucleotide sequences and predicted open reading frames (ORFs) of mtb32a and mtb32b, respectively.
FIG. 1.
Nucleotide and deduced amino acid sequences of the predicted ORF of mtb32a (A) and mtb32b (B) genes. Residues corresponding to the signal peptide and the mature (secreted) proteins are indicated. The potential ribosome binding site (SD sequence) for mtb32a is shown. The ORF of mtb32a encodes 355 amino acid residues with the first 32 residues comprising the putative hydrophobic leader sequence. Unlike mtb32a, mtb32b does not have an obvious ribosome binding site. A putative hydrophobic signal sequence as well as the mature secreted form (322 amino acids) is indicated and underlined.
The insert of the clone containing gene mtb32a (Ra35) is 1,872 bp and contains an ATG start codon preceded by a Shine-Dalgarno (SD) sequence (AGGAGG), a predicted ORF coding for a ∼35-kDa protein (355 amino acids) followed by 3′ untranslated sequences. The first 32 amino acids of MTB32A are highly hydrophobic with a potential signal peptidase cleavage site predicted to result in the secretion of a 32-kDa mature protein with an estimated isoelectric point of 4.34 and a net charge of −9.41 at pH 7.0.
The clone comprising gene mtb32b (Ra29) contains an insert of 1,771 bp but lacks an apparent SD sequence, thus making it difficult to predict the initiator amino acid residue. However, based on sequence homology with MTB32A and the apparent molecular weight of the mature form of MTB32B detected in CFP (see below), clone mtb32b appears to comprise sequences coding for the entire secreted protein. Figure 1B shows the location of the putative secreted (mature) form of MTB32B with a predicted molecular mass of 32 kDa (322 amino acids), an isoelectric point of 4.72, and a net charge of −6.73 at pH 7.0. The secreted form of MTB32B is in frame with an extended upstream ORF that presumably comprises the signal sequence. However, given that many of the CFP thus far identified have short (25 to 50 residues) leader sequences, we predict that the hydrophobic 42-amino-acid upstream sequence represents the signal sequence (Fig. 1B). Thus, the predicted molecular mass of the unprocessed form of MTB32B within the mycobacterium is similar to that of MTB32A (∼35 kDa).
MTB32A and MTB32B are trypsin-like serine proteases.
Comparison of the amino acid sequences of the mature forms of MTB32A and MTB32B with protein databases (Swiss, PIR, and translated release 104) revealed that these proteins resemble trypsin-like serine proteases with the classical trypsin active-site triad (His, Asp, and Ser) comprising not only serine but also histidine and aspartate residues (16, 30). MTB32A shows a higher degree of homology (90%; 72% identity and 18% conservative substitution) to a previously described 34-kDa serine protease antigen of Mycobacterium paratuberculosis (8) than to MTB32B (66% overall homology; 35% identity and 31% conservative substitution) (Fig. 2). A putative serine protease antigen was also identified on an M. leprae cosmid clone (accession no. U15180). The translated sequence of the M. leprae serine protease homologue shows 57% homology (31% identity and 26% conservative substitution) with MTB32A and 61% homology (33% identity and 28% conservative substitution) with MTB32B.
FIG. 2.
Comparison of amino acid sequences of MTB32A and MTB32B with the homologous sequence of M. paratuberculosis (M.para) (GenBank accession no. S47170) and a putative M. leprae serine protease antigen (GenBank accession no. U15180). Identical amino acid residues are shaded solid black, and conservative substitutions are boxed. All four proteins contain the classical trypsin active-site triad, His, Asp, and Ser (indicated by asterisks), a feature characteristic of trypsin-like serine proteases.
Expression and Western blot analysis of MTB32A and MTB32B.
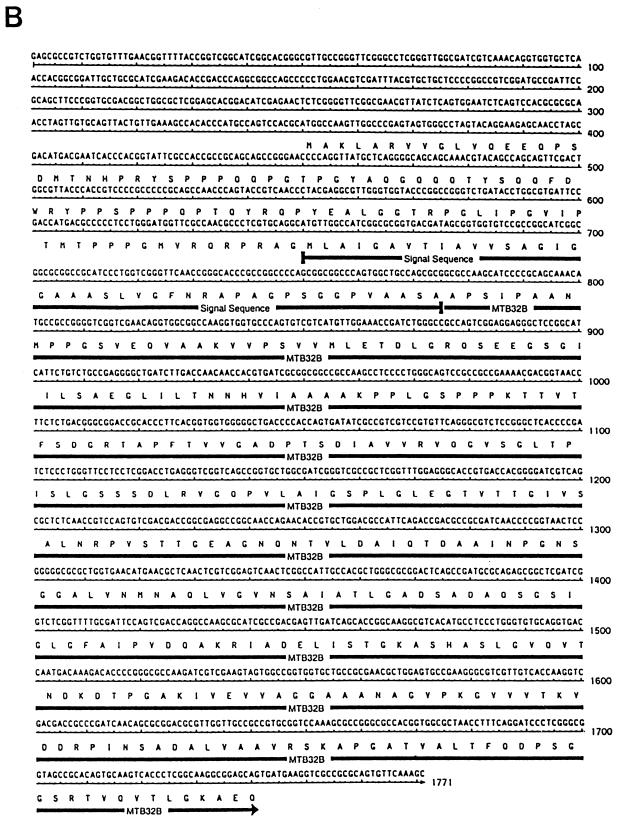
Several attempts were made to express MTB32A and MTB32B as either full-length (unprocessed) or mature (devoid of their leader sequences) proteins with the pET17b expression vector. In the presence of their leader sequences, the expressing bacterial host grew very slowly, and upon IPTG induction, growth of the culture was halted and lysis of the E. coli host was frequently observed. When the secreted forms were engineered for expression, growth of the cultures was found to be in the normal range but the expression levels of MTB32A and MTB32B were low and detectable only by immunoblotting (data not shown). In addition, the expressed proteins were found to be rapidly cleaved into several species following induction with IPTG at either 37 or 30°C. In contrast, the mature form of 85B (an established secretory protein) was rapidly and stably expressed at high levels (∼50 mg of purified protein per liter) with similar conditions (Fig. 3). This suggested that the difficulties encountered with the expression of the putative serine protease antigens may be inherent in their proteolytic properties.
FIG. 3.
Overexpression of rMTB32 antigens in E. coli. The figure shows expression and purification of two overlapping constructs comprising a 20-kDa N-terminal half (residues 1 to 195) (A) and an overlapping 14-kDa C-terminal fragment (residues 192 to 323) (B) of the mature secreted MTB32A antigen and the expression and purification of the mature form of antigen 85B (∼30 kDa) (C). The recombinant antigens were purified by affinity chromatography with an Ni-NTA agarose matrix. The gels shown are Coomassie blue-stained SDS–15% polyacrylamide gels containing 10 μg (A and B) or 5 μg (C) of E. coli lysates from uninduced (lanes 1) and IPTG-induced (lanes 2) cultures. Lanes 3 show loadings of the purified protein (5 μg [A and B] and 2.5 μg [C]). Numbers at left of each panel indicate molecular masses in kilodaltons.
To circumvent these problems, the secreted forms of the MTB32 antigens were engineered for expression of E. coli as two overlapping constructs. MTB32A was expressed as an ∼20-kDa N-terminal half comprising residues 1 to 195 and an overlapping ∼14-kDa C-terminal fragment (132 amino acids; residues 192 to 323). Both constructs were designed to contain six N-terminal histidine residues for ease of purification by affinity chromatography over an Ni-NTA matrix (Fig. 3A and B). The expression levels of both fragments were in the range of 50 to 150 mg (for the N- and C-terminal halves) of purified protein per liter of induced culture. Similarly, MTB32B was expressed as an ∼20-kDa fragment (amino acid residues 1 to 194) and an overlapping 22-kDa C-terminal fragment (225 amino acids; residues 97 to 322) (data not shown).
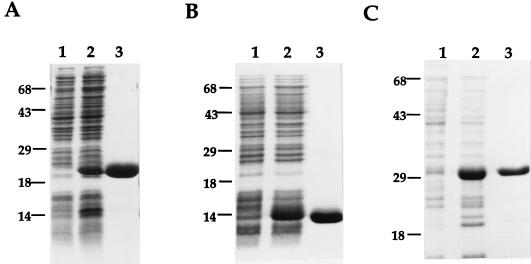
The N-terminal halves of the purified recombinant antigens were also used to raise high-titer rabbit antisera. Immunoblot analysis was subsequently performed on M. tuberculosis lysate and M. tuberculosis CFP to determine their secretory nature. In parallel, to have an estimate on the abundance of MTB32A relative to that of 85B, varying amounts of both recombinant antigens were also run on the same gel. In the case of MTB32A, the recombinant 20-kDa N-terminal half was used. The anti-MTB32A antiserum revealed a dominant species of ∼32 kDa in the CFP lane in agreement with the predicted molecular mass of the mature form of MTB32A (Fig. 4A). Interestingly, for both MTB32A and 85B (Fig. 4B), the sizes of the proteins detected in the lysate were similar to those found in CFP, suggesting that they are rapidly processed and exported from the bacilli after synthesis. In addition, the intensity of the signal in the Western blot of M. tuberculosis CFP probed with anti-rMTB32A indicated that, relative to 85B, MTB32A is also an abundant protein in CFP. Probing of a similar blot with anti-MTB32B serum revealed a ∼32-kDa dominant species in the CFP lane which is also in agreement with the predicted size of the mature and secreted form of this protein. Two higher-molecular-mass species with sizes of ∼35 and 70 kDa were identified in the lysate lane. To gain an estimate of the relative abundance of MTB32 antigens in CFP, immunoblots containing 2.5 μg of CFP were run alongside 50 ng each of the recombinant antigens (internal control) and the blots were probed with their respective antisera. The reactivities of the rabbit anti-MTB32A and MTB32B sera to the corresponding recombinant antigens were comparable (Fig. 4A and C, lanes 1). However, the immunoreactivity of the rabbit anti-MTB32A sera to native MTB32A found in CFP was significantly more intense than that observed on a duplicate blot probed with the rabbit anti-MTB32B serum (compare lanes CFP and 1 of panels A and C). Finally, and despite 66% overall homology between the two proteins, the reactivities of the polyclonal rabbit antisera were found to be specific to the respective immunizing proteins (data not shown).
FIG. 4.
Immunoblot analysis of MTB32A and MTB32B. The localization and abundance of MTB32A and MTB32B relative to one another and to a known secreted M. tuberculosis antigen (antigen 85b) were evaluated by immunoblot analysis of 2.5 μg of M. tuberculosis H37Rv lysate (Lysate) and CFP and 50 ng (lanes 1) of rMTB32A, recombinant antigen 85B, and rMTB32B (A, B, and C, respectively). For a quantitative estimate of the abundance of MTB32B in CFP relative to antigen 85B, serial dilutions of both recombinant antigens (25, 10, and 2.5 ng [lanes 2 to 4, respectively]) were also run alongside M. tuberculosis lysate and CFP. The blots were probed with the corresponding rabbit antisera made against the recombinant antigens MTB32A (A), antigen 85B (B), and MTB32B (C). Numbers at left of each panel indicate molecular masses in kilodaltons.
Sequence conservation and genomic organization of MTB32A in mycobacterial species.
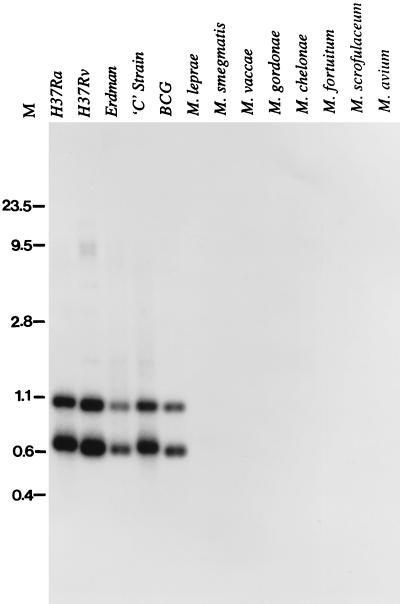
Since MTB32A was cloned from the avirulent H37Ra strain, we wanted to determine its presence in clinical isolates as well as in environmental isolates other than those of the M. tuberculosis complex. We used as a probe the N-terminal ∼600-bp fragment which spans the single PstI site within the protein coding region of mtb32a in Southern hybridization of genomic DNA from several mycobacterial species digested with PstI. As shown in Fig. 5, mtb32a is specific to the M. tuberculosis complex. The DNA probe hybridized to two species with sizes of ∼1.0 and 0.62 kb. The hybridizing fragments were indistinguishable among the strains H37Ra, H37Rv, Erdman, and BCG and the clinical isolate C. Sequence analysis of cosmid clone MTCI418B.07 of the H37Rv strain revealed the presence of two flanking PstI sites located ∼1.0 and 0.62 kb from the internal PstI site of mtb32a, thus suggesting that mtb32a is a single-copy gene. Similarly, a single copy of the mtb32a gene was also found in the genome database of the clinical isolate CSU 93. Finally, nucleotide sequence comparison of mtb32a genes of the avirulent H37Ra and virulent Erdman strains, as well as with the corresponding sequences (identified in the databases) of the H37Rv strain and the CSU93 clinical isolate, revealed 100% identity.
FIG. 5.
Southern blot analysis of mtb32a genomic sequences. One microgram each of genomic DNAs from several strains of the M. tuberculosis complex (H37Ra, H37Rv, Erdman, and C) and BCG and from other environmental mycobacterial species (M. scrofulaceum, M. smegmatis, M. fortuitum, M. malmoense, and M. gordonae) was digested with PstI and probed with a 600-bp coding fragment of MTB32A. Numbers at left are the sizes, in kilobase pairs, of HindIII-HincII-digested DNAs.
Immune responses of human PBMC to rMTB32 antigens.
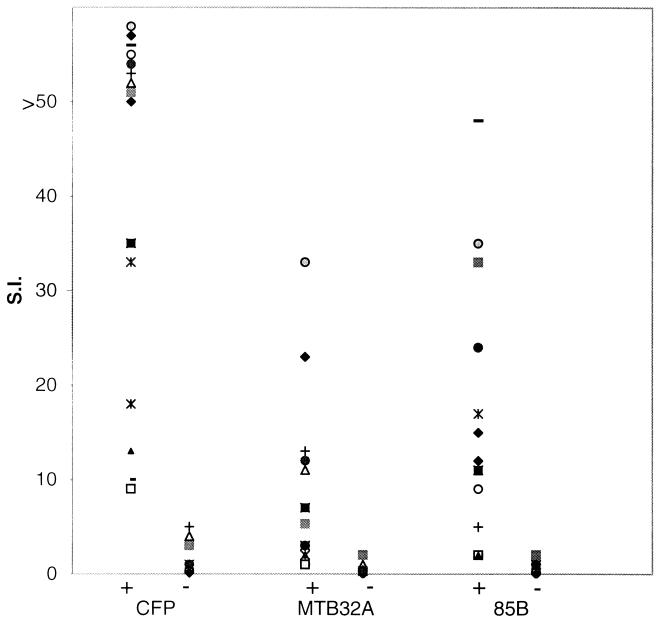
The recombinant antigens were evaluated in vitro for their ability to induce T-cell proliferation and IFN-γ production with a panel of PBMC obtained from healthy PPD+ (indurations of 10 to 20 mm) and PPD− individuals of diverse ethnic backgrounds. Both the N- and C-terminal portions of rMTB32A and rMTB32B were evaluated alongside r85B and total CFP on a panel of 14 PPD+ and 7 PPD− donors. With a stimulation index (SI) cutoff of >5 as a positive response, 7 of the 14 (50%) PPD+ donors responded to the N-terminal half of rMTB32A with SI values ranging from 5.3 to 33 (Fig. 6) while only one of the 14 donors proliferated in response to stimulation with the C-terminal half of rMTB32A (data not shown). Interestingly, and despite 66% homology between MTB32A and MTB32B, none of the PPD+ donors evaluated responded to either the N- or the C-terminal half of MTB32B, suggesting that the T-cell epitopes of PPD+ individuals recognized by PBMC reside within sequences that are specific to MTB32A. None of the seven healthy PPD− donors responded to either rMTB32A or rMTB32B. The viability of all donor PBMC was confirmed by proliferation and the secretion of IFN-γ in response to tetanus toxoid and phytohemagglutinin (data not shown). In parallel, an aliquot of the supernatant was removed from the same culture of donor PBMC prior to pulsing with [3H]thymidine, and the levels of secreted IFN-γ were measured. A direct correlation was observed between a positive proliferative response and the production of IFN-γ. Thus, donor PBMC that proliferated following stimulation with rMTB32A secreted IFN-γ, while no IFN-γ was detected in the culture supernatant of PBMC (of both PPD+ and PPD− donors) that did not proliferate.
FIG. 6.
Proliferative responses (symbols) of PBMC from PPD+ (+) and PPD− (−) healthy donors following stimulation with CFP (10 μg/ml) or rMTB32A and r85B (5 μg/ml). In vitro proliferation was measured by [3H]thymidine incorporation and is presented as SI: mean counts per minute of test antigen/mean counts per minute of medium alone. Identical symbols represent the same donor PBMC evaluated with multiple antigens.
DISCUSSION
This report describes the molecular cloning, characterization, and immunological evaluation of two secreted serine protease antigens (MTB32A and MTB32B) found in CFP of M. tuberculosis. The ORFs of these antigens correspond to “pepA” (Rv0125) and a “probable serine protease” (Rv0983), respectively, as defined in the sequenced M. tuberculosis H37Rv genome (11). These two genes were identified on separate loci in the M. tuberculosis genome sequence and are not closely linked; they are separated by ∼1 Mb on the circular genome. MTB32A is rapidly processed and exported from the bacilli after synthesis; only the mature, not the precursor, form was detected in whole-cell lysate. In contrast, antibody made against MTB32B identified two precursor forms of MTB32B in the whole-cell lysate with molecular masses of 35 and 70 kDa. The 35-kDa species could be accounted for as the precursor molecule to the 32-kDa secreted protein (assuming a translation initiation site as predicted in Fig. 1B). Thus, the 70-kDa band may represent a dimer of the 35-kDa precursor molecule of MTB32A. Alternatively, it is possible that the 35- and 70-kDa species detected in the lysate resulted from the presence of antigens that share a cross-reactive B-cell epitope(s) with MTB32B. Finally, the results of the Western blots also demonstrated the specificity of the rabbit antibodies to the immunizing antigen despite 66% homology between MTB32A and MTB32B.
The presence of MTB32 homologies in only mycobacterial species most strongly associated with human disease suggests that these genes may confer virulence properties upon these organisms. Sequence comparison revealed that MTB32A and MTB32B resemble trypsin-like serine proteases with the classical trypsin active-site triad (His, Asp, and Ser) comprising not only serine but also histidine and aspartate residues (16, 30). There is ample precedent in the literature associating the surface expression or secretion of serine proteases with various diseases caused by infectious agents. Bacterial proteases have the potential to destroy the structural and functional proteins that constitute the host as well as proteins important in host defense. For example, the HtrA serine protease of Salmonella typhimurium has been associated with virulence of this intracellular pathogen (24). Similarly, Plasmodium falciparum is thought to use secretory forms of serine proteases in erythrocyte invasion during the blood-borne stage of malaria (4, 6). A secreted serine proteinase identified in the culture supernatant of virulent strains of Dichelobacter nodosus has also been associated with virulent foot-rot disease (26). Perhaps of most significance for a potential role of M. tuberculosis serine protease as a virulence factor is the recent demonstration that, in Aspergillus-related lung disease, a secreted serine proteinase was shown to be capable of hydrolyzing the major structural barriers of the lung (22).
CFP from M. tuberculosis have been identified as a rich source of antigens that elicit protective responses in various animal models of tuberculosis (1, 19, 20, 32, 33). In addition, the active synthesis and secretion of CFP components are apparently responsible for the greater efficacy of vaccination with live attenuated mycobacteria than of that with killed organisms. Similarly, individual components of the secreted proteins of M. tuberculosis including members of the antigen 85 complex (28, 34, 35, 42), the APA (Tb45/47) protein (14, 27), ESAT6 (40), MTB8.4 (12), and MTB12 (43) have been shown to induce cellular immune responses. In this study, we have shown that rMTB32A protein is recognized by PBMC of healthy, disease-free, PPD+ donors, suggesting that this protein may play a role during the development of protective immune responses against M. tuberculosis. Interestingly, and despite 66% homology between MTB32A and MTB32B, none of the PPD+ donors evaluated responded to MTB32B. Thus, the immunogenic T-cell epitopes of PPD+ individuals recognized by PBMC reside within sequences that are specific to MTB32A and are located predominantly within the amino-terminal half of the molecule. We are currently assessing MTB32A-specific immune responses in a broader array of donor types including patients in varying states of disease progression, healthy household contacts, and BCG recipients.
MTB32A is encoded by a single-copy gene which is present in both virulent and avirulent strains of the M. tuberculosis complex and the BCG strain of M. bovis but absent in the environmental mycobacterial species. In addition, nucleotide sequence comparison of mtb32a genes of the avirulent H37Ra strain and the virulent Erdman strain, as well as with the corresponding sequences (identified in the databases) of H37Rv and the clinical isolate CSU93, revealed 100% identity. It would be expected that, for an antigen to be considered for inclusion in the design of a subunit vaccine, it should be highly conserved among clinical isolates.
Thus, given that MTB32A (i) is a relatively abundant protein in culture supernatant, (ii) is recognized by PBMC from healthy PPD+ donors, and (iii) is conserved among different strains of Mycobacterium spp., we suggest its inclusion as a candidate component in the development of a subunit-based vaccine against M. tuberculosis infection. We are currently assessing the protective capability of MTB32 vaccination in murine models of tuberculosis with both recombinant proteins in conjunction with specific adjuvants and DNA vaccine approaches.
ACKNOWLEDGMENTS
We thank John Belisle for providing M. tuberculosis CFP (produced through NIAID/NIH Tuberculosis Research Materials contract N01-AI-25147), Stephan Johnson and Rhea Coler for providing some of the genomic DNA used in the Southern blot assay, Pamela Ovendale for help in performing human PBMC assays, and Shyian Jen for performing the immunoblotting.
Y.A.W.S. was supported in part by a Centennial Fellowship from the Medical Research Council of Canada.
REFERENCES
- 1.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59:1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atlas R M. In: Handbook of microbiological media. Parks L C, editor. Boca Raton, Fla: CRC Press, Inc.; 1993. p. 791. [Google Scholar]
- 4.Blackman M J, Fujioka H, Stafford W H, Sajid M, Clough B, Fleck S L, Aikawa M, Grainger M, Hackett F. A subtilisin-like protein in secretory organelles of Plasmodium falciparum merozoites. J Biol Chem. 1998;273:23398–23409. doi: 10.1074/jbc.273.36.23398. [DOI] [PubMed] [Google Scholar]
- 5.Boesen H, Jensen B N, Wilcke T, Andersen P. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 1995;63:1491–1497. doi: 10.1128/iai.63.4.1491-1497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun Breton C, Pereia de Silva L H. Malarial proteases and red blood cell invasion. Parasitol Today. 1993;9:92–96. doi: 10.1016/0169-4758(93)90212-x. [DOI] [PubMed] [Google Scholar]
- 7.Brewer T F, Colditz G A. Relationship between bacille Calmette-Guerin (BCG) strains and the efficacy of BCG vaccine in the prevention of tuberculosis. Clin Infect Dis. 1995;20:126–135. doi: 10.1093/clinids/20.1.126. [DOI] [PubMed] [Google Scholar]
- 8.Cameron R M, Stevenson K, Inglis N F, Klausen J, Sharp J M. Identification and characterization of a serine protease expressed in vivo by Mycobacterium avium subsp. paratuberculosis. Microbiology. 1994;140:1977–1982. doi: 10.1099/13500872-140-8-1977. [DOI] [PubMed] [Google Scholar]
- 9.Colditz G A, Berkey C S, Mosteller F, Brewer T F, Wilson M E, Burdick E, Fineberg H V. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analysis of the published literature. Pediatrics. 1995;96:29–35. [PubMed] [Google Scholar]
- 10.Colditz G A, Brewer T F, Berkey C S, Wilson M E, Burdick E, Fineberg H V, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 11.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Conner R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 12.Coler R N, Skeiky Y A W, Vedvick T, Bement T, Ovendale P, Campos-Neto A, Alderson M R, Reed S G. Molecular cloning and immunological reactivity of a novel low molecular mass antigen of Mycobacterium tuberculosis. J Immunol. 1998;161:2356–2364. [PubMed] [Google Scholar]
- 13.Content J, de la Cuvellerie A, De Wit L, Vincent-Levy-Frebault V, Ooms J, De Bruyn J. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination, and genomic organization of the gene coding for antigen 85-C of M. tuberculosis. Infect Immun. 1991;59:3205–3212. doi: 10.1128/iai.59.9.3205-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobos K M, Swiderek K, Khoo K H, Brennan P J, Belisle J T. Evidence for glycosylation sites on the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. Infect Immun. 1995;63:2846–2853. doi: 10.1128/iai.63.8.2846-2853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolin P J, Raviglione M C, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bull WHO. 1994;72:213–220. [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn B M. Determination of protease mechanism. In: Beynon R J, Bond J S, editors. Proteolytic enzymes: a practical approach. Oxford, United Kingdom: Oxford University Press; 1989. pp. 57–81. [Google Scholar]
- 17.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restricted endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 18.Garbe T, Harris D, Vordermeier M, Lathigra R, Ivanyi J, Young D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun. 1993;61:260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz M A, Lee B E, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubbard R D, Flory C M, Collins F M. Immunization of mice with mycobacterial culture filtrate proteins. Clin Exp Immunol. 1992;87:94–98. doi: 10.1111/j.1365-2249.1992.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D’Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 22.Iadarola P, Lungarella G, Martorana P A, Viglio S, Guglielminetti M, Korzus E, Gorrini M, Cavarra E, Rossi A, Travis J, Luisetti M. Lung injury and degradation of extracellular matrix components by Aspergillus fumigatus serine proteinase. Exp Lung Res. 1998;24:233–251. doi: 10.3109/01902149809041532. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs R F. Multiple-drug-resistant tuberculosis. Clin Infect Dis. 1994;19:1–10. doi: 10.1093/clinids/19.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Johnson K, Charles I, Dougan G, Pickard D, O’Gaora P, Costa G, Ali T, Miller I, Hormaeche C. The role of a stress response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991;5:401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 25.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 26.Kortt A A, Caldwell J B, Lilley G G, Edwards R, Vaughan J, Stewart D J. Characterization of a basic serine proteinase (pI approximately 9.5) secreted by virulent strains of Dichelobacter nodosus and identification of a distinct, but closely related, proteinase secreted by benign strains. Biochem J. 1994;299:521–525. doi: 10.1042/bj2990521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laqueyrerie A, Militzer P, Romain F, Eiglmeier K, Cole S, Marchal G. Cloning, sequencing, and expression of the apa gene coding for the Mycobacterium tuberculosis 45/47-kilodalton secreted antigen complex. Infect Immun. 1995;63:4003–4010. doi: 10.1128/iai.63.10.4003-4010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Launois P, De Leys R, Niang M N, Drowart A, Andrien M, Dierckx P, Cartel J L, Sarthou J L, Van Vooren J P, Huygen K. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect Immun. 1994;62:3679–3687. doi: 10.1128/iai.62.9.3679-3687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuo K, Yamaguchi R, Yamazaki A, Tasaka H, Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol. 1988;170:3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neurath H. The diversity of proteolytic enzymes. In: Beynon R J, Bond J S, editors. Proteolytic enzymes: a practical approach. Oxford, United Kingdom: Oxford University Press; 1989. pp. 1–13. [Google Scholar]
- 31.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 32.Pal P G, Horwitz M A. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts A D, Sonnenberg M G, Ordway D J, Furney S K, Brennan P J, Belisle J T, Orme I M. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 34.Roche P W, Triccas J A, Avery D T, Fifis T, Billman-Jacobe H, Britton W J. Differential T cell responses to mycobacteria-secreted proteins distinguish vaccination with bacille Calmette-Guerin from infection with Mycobacterium tuberculosis. J Infect Dis. 1994;170:1326–1330. doi: 10.1093/infdis/170.5.1326. [DOI] [PubMed] [Google Scholar]
- 35.Roche P W, Peake P W, Billman-Jacobe H, Doran T, Britton W J. T-cell determinants and antibody binding sites on the major mycobacterial secretory protein MPB59 of Mycobacterium bovis. Infect Immun. 1994;62:5319–5326. doi: 10.1128/iai.62.12.5319-5326.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Skeiky Y A, Benson D R, Parsons M, Elkon K B, Reed S G. Cloning and expression of Trypanosoma cruzi ribosomal protein P0 and epitope analysis of anti-P0 autoantibodies in Chagas’ disease patients. J Exp Med. 1992;176:201–211. doi: 10.1084/jem.176.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snider D E, Raviglione M, Kochi A. Global burden of tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: American Society for Microbiology; 1994. pp. 3–11. [Google Scholar]
- 39.Sonnenberg M G, Belisle J T. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect Immun. 1997;65:4515–4524. doi: 10.1128/iai.65.11.4515-4524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorenson A L, Nagai S, Houen G, Anderson P, Anderson A B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sudre P, ten Dam G, Kochi A. Tuberculosis: a global overview of the situation today. Bull WHO. 1992;70:149–159. [PMC free article] [PubMed] [Google Scholar]
- 42.Torres M, Herrera T, Villareal H, Rich E A, Sada E. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun. 1998;66:176–180. doi: 10.1128/iai.66.1.176-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webb J R, Vedvick T S, Alderson M R, Guderian J A, Jen S S, Ovendale P J, Johnson S M, Reed S G, Skeiky Y A W. Molecular cloning, expression, and immunogenicity of MTB12, a novel low-molecular-weight antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1998;66:4208–4214. doi: 10.1128/iai.66.9.4208-4214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young D B, Kaufmann S H, Hermans P W, Thole J E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992;6:133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]