Abstract
Fetal growth restriction is one of the leading causes of perinatal mortality and morbidity and has consequences that extend well beyond the neonatal period. Current management relies on timely delivery rather than improving placental function. Several prenatal strategies have failed to show benefit in clinical trials after promising results in animal models. Most of these animal models have important developmental and structural differences compared to the human and/or are insufficiently characterized. We aimed to describe placental function and structure in an FGR rabbit model, and to characterize the early brain and lung developmental morbidity using a multimodal approach. FGR was induced in time-mated rabbits at gestational day 25 by partial uteroplacental vessel ligation in one horn. Umbilical artery Doppler was measured before caesarean delivery at gestational day 30, and placentas were harvested for computed microtomography and histology. Neonates underwent neurobehavioral or pulmonary functional assessment the day after delivery, followed by brain or lung harvesting, respectively. Neuropathological assessment included multiregional quantification of neuron density, apoptosis, astrogliosis, cellular proliferation, and oligodendrocyte progenitors. Brain region volumes and diffusion metrics were obtained from ex-vivo brain magnetic resonance imaging. Lung assessment included biomechanical tests and pulmonary histology. Fetal growth restriction was associated with labyrinth alterations in the placenta, driven by fetal capillary reduction, and overall reduced vessels volume. FGR caused altered neurobehavior paralleled by regional neuropathological deficits and reduced fractional anisotropy in the cortex, white matter, and hippocampus. In addition, FGR kittens presented functional alterations in the peripheral lung and structurally underdeveloped alveoli. In conclusion, in a uteroplacental insufficiency FGR rabbit model, placental vascular alterations coincide with neurodevelopmental and pulmonary disruption.
Subject terms: Intrauterine growth, Preclinical research, Experimental models of disease, Paediatric research
Introduction
Fetal Growth Restriction (FGR) refers to the inability of a fetus to achieve its genetic growth potential1. It is one of the main contributors to perinatal mortality and morbidity, and it is associated with almost 50% of stillbirth cases worldwide2–4. Beyond the neonatal period, it causes cognitive and behavioral impediment in childhood5 and metabolic, pulmonary, and cardiovascular disease in adult life6,7. The main cause of FGR is uteroplacental insufficiency (UPI), a condition whereby a defective placental development leads to an insufficient supply of oxygen and nutrients to support the developing fetus, resulting in acidosis, hypoxemia, and in more severe cases ultimately intrauterine fetal demise. There is currently no effective prenatal treatment for FGR: clinical trials have failed to deliver a safe and effective therapy, even after successful preclinical studies8–10. Well characterized animal models with developmental, functional, and structural similarities to the human are fundamental to understand the consequences of FGR and to design new therapeutic strategies aimed at ameliorating them.
Several animal models have been described but so far no single animal model mimics the entire spectrum of human FGR11. In recent years, the rabbit has been established as a well-rounded, versatile model for perinatal conditions12–18. Like humans and rodents, they have discoid, haemochorial placentas, in which maternal blood is in direct contact with the trophoblast. Furthermore, only two trophoblast layers separate maternal and fetal blood spaces (hemo-dichorial placenta), closing the gap between the murine hemo-trichorial and the human hemo-monochorial placentas19. Trophoblast volumes, placental glucose transporters, and other pregnancy-specific changes in the rabbit have also been shown to mimic the clinical scenario20.
In addition to the morphological and functional placental similarities, rabbits resemble humans closer than other species in terms of neurodevelopment. Neurogenesis starts in the first trimester, and white matter maturation begins around birth to continue through the first year of life21,22. Pulmonary development is also a perinatal process, with alveolarization beginning prior to birth, so that at term rabbit lungs are in the terminal air sac stage23–25. In addition to that, rabbits have several practical benefits; they are non-seasonal in mating habits, have a short gestation period with a large litter size, and are suitable for foster care and follow-up assessment.
Our group has utilized and refined a robust neurodevelopmental and pulmonary multimodal evaluation platform to assess newborn and preadolescent rabbits and phenotype the effects of bronchopulmonary dysplasia26–29, prematurity30,31, and congenital diaphragmatic hernia32,33. Herein, we characterize the consequences of FGR induced by uteroplacental vessel ligation (UPVL) in placental microvasculature, and early brain and lung development in the rabbit model.
Results
UPVL alters labyrinth zone vascularization, increases mortality, and induces FGR
Twenty-two does delivered 65 live kittens from the ligated horns, and 86 from the control horns. UPVL resulted in higher intrauterine mortality (p < 0.0001) and a significant reduction in body weight (p < 0.0001), placental weight (p < 0.0001), and fetal/placental weight ratio (p < 0.0001; Table 1). Umbilical artery doppler parameters (PSV, EDV, VTI, MV, PR and RI) were not significantly different between groups (Supplementary Table S1).
Table 1.
Survival and biometrics.
| n = 22 does | FGR | Control | P-value |
|---|---|---|---|
| Survival | 65/130 (50%) | 86/97 (89%) | < 0.0001 |
| Birth weight (g) | 32.93 ± 0.93 | 44.29 ± 1.05 | < 0.0001 |
| Placental weight (g) | 5.33 ± 0.15 | 6.15 ± 0.15 | < 0.0001 |
| Brain weight (g) | 1.46 ± 0.04 | 1.74 ± 0.05 | < 0.0001 |
| Brain/body weight ratio | 0.031 ± 0.002 | 0.027 ± 0.004 | 0.0036 |
| Fetal/placental weight ratio | 6.41 ± 1.07 | 7.30 ± 1.07 | < 0.0001 |
Survival and birth weight were assessed 4 h after caesarian delivery. Brain weight was measured at PND1. Data were analysed with Fischer’s exact test or using a linear mixed-effects model. Values are displayed as mean ± SE. FGR: fetal growth restriction.
At histological assessment placentas from FGR kittens had a significantly smaller labyrinth (p < 0.0001) and junction zone (p = 0.003) when compared to controls (Fig. 1A). Within the labyrinth zone, the proportion of fetal capillaries (FC) was significantly reduced (p < 0.0001) in FGR placentas, and the proportion of maternal blood spaces (MBS) significantly increased (p < 0.0001) (Supplementary Table S2). FC (p < 0.0001) and trophoblast volumes (p = 0.0001) were significantly decreased in FGR placentas, while MBS volumes were not significantly different when compared to controls (Fig. 1A). The area of fibrosis in the decidua was not significantly different between groups (Supplementary materials, Fig. S1). Microvasculature assessment by computerized tomography showed FGR placentas had significantly reduced vessel volume after correction for placental weight (p = 0.015) (Fig. 1B). The vessel surface area and the distribution of vessels according to their diameter was not significantly different between groups.
Figure 1.
Placental histology and microcomputed tomography. (A) Placentas with cytokeratin/lectin double staining, divided by placental zones (left images). Within the labyrinth (zoomed, right), fetal capillaries (arrow), maternal blood spaces (arrowhead) and trophoblast (star) were grid counted to calculate relative and absolute volume densities. For placental volumes, 51 FGR and 59 control placentas were used from 20 litters. For labyrinth structures, 10 FGR and 10 control placentas were used from 10 litters (1 placenta per horn, per litter). (B) Representative image of a placental microcomputed tomography after color coding according to vessel diameter. Data were analysed using a linear mixed-effects model and displayed as mean ± SD with significance as *0.05 ≥ p > 0.01; **0.01 ≥ p > 0.001.; ***0.001 > p > 0.0001; ****p < 0.0001.
FGR is associated with early neurodevelopmental impairment
FGR neonates had a significant reduction in brain weight (p < 0.0001) and increased brain to body weight ratio (p = 0.0036) at birth (Table 1). FGR was also associated with motor and sensory deficits on neurobehavioral assessment (NBA), as displayed in Fig. 2A. FGR kittens had abnormal posture, gait, and locomotion, but activity duration was similar to controls. Additionally, FGR led to hampered cranial nerves activity, pain response, and righting reflex.
Figure 2.
Brain assessment in postnatal day 1 rabbits. (A) Neurobehavioral tests (left) and grouped scores (right) from 43 FGR and 61 control subjects from 16 litters. (B) Neuron density was assessed in cresyl violet stained slides in the depicted areas (top images). Bottom images show neuron density in the frontal cortex of FGR and control brains. To the right, neuron density from 27 FGR and 29 control subjects from 16 litters. (C) Representative images of apoptosis (TUNEL), and astrogliosis (GFAP) in hippocampus and corpus callosum, respectively. Data from 14 FGR and 19 control subjects from 11 litters. CA1 cornu ammonis 1, CA3 cornu ammonis 3, DG dentate gyrus, AVTN anteroventral thalamic nuclei. Data were analysed using a linear mixed-effects model and displayed as mean ± SD with significance as *0.05 ≥ p > 0.01; **0.01 ≥ p > 0.001.; ***0.001 > p > 0.0001; ****p < 0.0001.
FGR kittens have lower multiregional neuron density, associated with increased apoptosis and astrogliosis, and reduced oligodendrocyte precursor cells
As shown in Fig. 2B, FGR led to a significant reduction in neuron density in the frontal cortex (FCx, p < 0.0001), corpus callosum (CC, p = 0.0059), caudate nucleus (CN, p < 0.0001), putamen (p = 0.0018), cornu ammonis 1 (CA1; p < 0.0001), cornu ammonis 3 (CA3; p < 0.0001), dentate gyrus (DG, p < 0.0001), and anteroventral thalamic nuclei (AVTN, p = 0.0170). This coincided with an increased proportion of TUNEL-positive cells in the CC (p = 0.0127), CA1 (p = 0.0005), CA3 (p = 0.0004), AVTN (p = 0.002) and whole hippocampus (p = 0.0003) in FGR brains (Fig. 2C). Additionally, rabbits from the FGR group had a higher proportion of GFAP-positive cells in the CC (p < 0.0001), CN (p = 0.0305) and DG (p = 0.0168) (Fig. 2C). NG-2-positive cells were significantly decreased in the FCx (p = 0.0093), CC (p = 0.0423) and CN (p = 0.0245) (Supplementary materials, Fig. S2, Table S3). No significant differences were found in Ki67-positive cells in the same regions (Fig. S3, Table S3).
Regional neuropathological changes coincide with MRI volumetric and diffusion weighted imaging
T1-derived volumetric data from magnetic resonance imaging (MRI) showed that FGR rabbits had an overall lower absolute brain volume. However, after normalizing the regional volumes to their respective total brain volume, no differences were present between the two groups in the volume of specific brain regions.
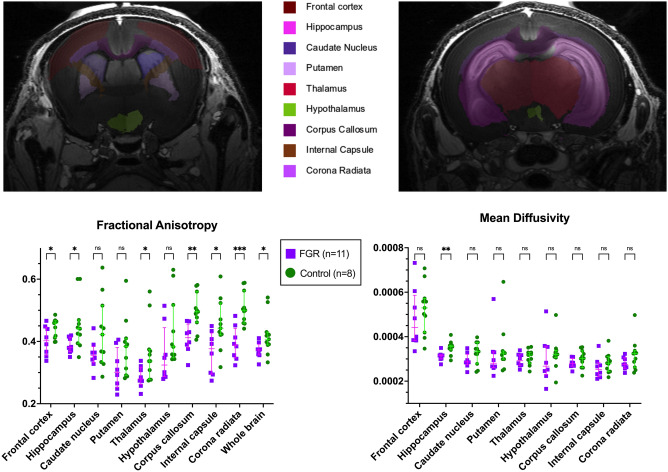
Differences on diffusion tensor metrics also paralleled histological findings. Fractional anisotropy (FA) was globally reduced (p = 0.0444), and additionally significantly lower in the FCx (p = 0.0374), CC (p = 0.0032), internal capsule (IC, p = 0.0202), corona radiata (CR, p = 0.0003), hippocampus (p = 0.0259), thalamus (p = 0.0326) in FGR rabbits, while there was no significant difference in the CN, putamen, and hypothalamus (Fig. 3, Table S4). Mean diffusivity (MD) was only found significantly reduced in the hippocampus (p = 0.004) (Fig. 3, Table S4).
Figure 3.
Ex vivo brain diffusion tensor metrics at postnatal day 1 from 11 FGR and 8 control brains, from 6 litters. Data were analysed using a linear mixed-effects model and displayed as mean ± SD with significance as *0.05 ≥ p > 0.01; **0.01 ≥ p > 0.001.; ***0.001 > p > 0.0001; ****p < 0.0001.
FGR leads to significant alteration of peripheral lung mechanics and compliance
Lung function was significantly reduced in FGR animals. Forced oscillation tests showed increased tissue damping (p < 0.0001), tissue elastance (p < 0.0001), respiratory system resistance (p < 0.0001) and decreased dynamic compliance (p < 0.0001) in FGR lungs, while central airway resistance was not significantly different between groups (Fig. 4A, Table S5). In pressure–volume perturbations, FGR lungs had significantly reduced hysteresis (p = 0.0004) and static compliance (p = 0.0018) compared to controls (Fig. 4B, Table S5).
Figure 4.
Pulmonary assessment in postnatal day 1. (A, B) Pulmonary function tests results from 19 FGR and 21 control subjects from 6 litters. (C) Representative images of histological H&E-stained slides (left) and alveolar architecture data (right) from 16 FGR and 21 control subjects from 6 litters. Data were analysed using a linear mixed-effects model and displayed as mean ± SD with significance as *0.05 ≥ p > 0.01; **0.01 ≥ p > 0.001.; ***0.001 > p > 0.0001; ****p < 0.0001.
FGR does not influence overall lung volume, but disrupts alveolar development
Both groups had similar lung sizes when corrected by body weight, as measured by water displacement (37.21 ± 7.02 vs. 39.12 ± 5.45 mL; p = 0.1333) and inspiratory capacity (31.60 ± 5.8 vs. 34.13 ± 4.55 mL; p = 0.0823). In contrast, on histological assessment FGR lungs had significantly decreased alveolar surface area (S; p = 0.0004), increased alveolar size (Lm; p = 0.0049), and increased alveolar airspace (Lma; p = 0.0191), while septal thickness (Lmw) was similar between groups (Fig. 4C). The medial thickness of the peripheral pulmonary arteries was also comparable between groups (Supplementary materials, Fig. S4).
Discussion
This study expands the characterization of the rabbit FGR model as previously described12,16 by utilizing a multimodal assessment approach, adding a description of placental microvascular changes, and subsequent early functional and structural alterations in the brain and lungs. In this model the exchange zone area -the labyrinth- and overall vessel volume in FGR placentas are significantly reduced. Upon neuropathological review, lower neuron density and higher apoptosis coincide with reduced fractional anisotropy in grey and white matter structures and are associated with neurobehavioral deficits. Furthermore, lung parenchymal functional and structural changes are demonstrated for the first time in this FGR model. Herein, the reduction in alveolar surface area accompanies the increased resistance and damping in the peripheric lungs.
Underdevelopment of the labyrinth is the most notable finding in the placenta. Decidual volume was similar between FGR and control placentas and did not present significantly higher fibrosis after UPVL, in line with previous reports using this model34. Labyrinth volume reduction was driven by a reduction in fetal capillaries at histological level and coincided with the reduction in fetoplacental vessel volume on microcomputed tomography. Although this is the first published experience using this technique in the rabbit, labyrinth alterations have been previously described in several other FGR models, including those induced by maternal undernutrition35,36, exogenous37,38 and endogenous39 glucocorticoid excess, heavy metal exposure40, NO synthetase inhibition41,42, and systemic inflammation43. Furthermore, labyrinth underdevelopment, and particularly fetal vessel reduction, has been associated with reduced levels of different placental vascular endothelial growth factor (VEGF) mRNAs38, and glucose transport capacity39,44. Future placental characterization in this model to include the expression of these and other key genes, as well a more in-depth description of the decidua and junction zone, might help unravel the complex molecular machinery behind placental development and function in uteroplacental insufficiency.
Interestingly, histological findings in the placenta were not associated with an alteration in umbilical artery doppler, in accordance with the work by Eixarch et al.45. Anesthesia-induced hemodynamic changes at the time of evaluation might have masked true differences between subjects. Indeed, the umbilical artery PI and RI in our study are considerably higher than those observed in normally grown, non-sedated animals at the same gestational age46.
The pulmonary morbidity in this study is likely the result of uteroplacental insufficiency and not directly caused by toxins or drugs, as in other models47,48. Moreover, the pulmonary impairment was not influenced by prematurity or postnatal lung injury, commonly present in perinatal lung assessment. The rabbit mimics human pulmonary developmental closer than other species as alveolarization is a perinatal process49, and not entirely prenatal as in sheep and guinea pigs, or postnatal as in rats and mice. Nonetheless, our results are consistent with findings in murine and ovine models50–53, and suggest FGR disrupts alveolar development and leads to biomechanical dysfunction, but does not affect lung volume. The functional impairment was observed in both forced oscillation and pressure–volume maneuvers. On the former, increased tissue damping, tissue elastance and respiratory system resistance indicate peripheral remodeling. The similar central airway resistance suggests comparable large airways caliber and is in line with the timing of the placental insult after the formation of the central airways. The decreased hysteresis and poorer compliance found on pressure–volume maneuvers indicate that FGR results in stiffer lungs and that the work of breathing is higher in this group. Structural data is in line with the biomechanical findings and coincides in a peripheral lung alteration, showing a simplified alveolar morphology, with increased airspace size and lower alveolar surface area available for gas exchange.
The molecular and cellular drivers underlying these changes are likely multiple and complex, and their description was not the purpose on this model characterization. They will, however, need to be approached and compared against findings in other models, which include dysregulation of VEGF50,52, retinoic acid receptors54,55, and other pathways involved in hypoxia signaling56,57, tissue repair, and cellular communication regulation58. Additionally, it will be of interest to determine if these functional and structural alterations are sustained in the longer term, or if any new pulmonary findings develop overtime.
Regarding neurodevelopmental assessment, the neurobehavioral deficit we describe is in line with previous reports in the rabbit59,60 and other animal models61–63, highlighting the cross-species nature of these findings. Furthermore, the neurobehavioral deficit correlated with lower neuron density at neuropathology and altered structural integrity at MRI. Interestingly, the latter was only significant in the frontal cortex, white matter regions, and hippocampus, yet the caudate nucleus and putamen seem to be less affected. This coincides with regional variations in brain perfusion described in the context of brain sparing in severe FGR64 with preferential supply of blood flow to the deep grey matter of the basal ganglia in detriment of the frontal cortex. Reduced fractional anisotropy, lower neuron density, and increased apoptosis in these regions suggest structural integrity is compromised in FGR brains even before myelination takes place65. Organization in neuronal population, followed by axonal and dendritic growth, increase density in these areas, thus an insult to neuronal organization might reduce FA independent of myelination. Recent work describing the significant correlation between birth weight and preoligodendrocyte density in this model34 suggest a disruption in the oligodendrocyte maturation process. Although the difference in immunohistochemical techniques does not allow for a direct comparison, these results are in accordance with the reduced density of NG2+-cells found in the corpus callosum in our FGR subjects. Moreover, the same group has recently described an impairment in the oligodendrocyte differentiation capacity of neural progenitor cells incubated in a novel neurosphere culture66.
We acknowledge our study has limitations. First, the acute induction of UPI does not mimic the slow progressive nature of FGR. However, most models with an earlier and/or more persistent induction, like maternal undernutrition or glucocorticoid excess, either fail to recreate the severity of FGR, or expose the fetus to insults not related to placental function. Similarly, attempts to reproduce the progressiveness of UPI in surgical models have failed to mimic the severity on the disease by increased fetal mortality67,68. Secondly, this model describes the effects of FGR in near-term neonates, even though most severe clinical cases require preterm delivery. Pulmonary and neurodevelopment are highly dependent on GA at delivery, and thus assessing near-term neonates allows us to unmask the effects of FGR from those of prematurity. Lastly, only immediate neonatal consequences of FGR are reported in the current study. We are aware that our findings need to be supplemented with observations on the mid and long-term, and that further assessment is a necessary next step into the robust characterization of the rabbit model, and we are currently working towards such characterization.
In conclusion, in the rabbit UPVL FGR model we demonstrated that placental vascular alterations lead not only to neurodevelopmental but also pulmonary deficits in the early neonatal period. A longitudinal FGR model characterization of placental, neurocognitive, and pulmonary function and structure could greatly aid in assessing the safety and efficacy of novel therapeutic strategies.
Materials and methods
Animal model
All animals were treated according to current guidelines for animal well-being, and experiments were approved by the Ethics Committee for Animal Experimentation of the Faculty of Medicine (P080/2019). Experiments are reported according to ARRIVE guidelines69. Time-mated rabbits (hybrid of Dendermonde and New Zealand White) were housed in individual cages at 21 °C, 42% humidity, with a 12-h day/night cycle and free access to food and water. Conception day was considered day 0 of pregnancy (GA = 0). At GA 25 (full term 31.5 days), does underwent induction of FGR. Briefly, rabbits were administered induction anesthesia with IM ketamine (35 mg/kg Nimatek®, Eurovet Animal Health BV, Bladel, The Netherlands) and xylazine (5 mg/kg XYL-M® 2%, VMD, Arendonk, Belgium), and antibiotic prophylaxis (10 mg/kg Baytril® 2.5% SC, Bayer, Diegem, Belgium), tocolysis (10 mg/kg Depo-Provera® SC, Pfizer, Puurs, Belgium), and analgesia (0.03 mg/kg Vetergesic® SC, Ceva Animal Health, Brussels, Belgium) prior to surgery. Anesthesia was maintained with a continuous IV infusion of ketamine (8–16 mg/kg/h) and xylazine (2.4–4.8 mg/kg/h), while monitoring vital signs. Following laparotomy, 30–40% of the vessels going to each placenta were ligated in one random horn with Vicryl® 5-0 (Ethicon®, Diegem, Belgium), leaving the contralateral unligated horn as internal control. The abdomen was closed with Vicryl® 2-0 and Monocryl® 3-0 (Ethicon®, Diegem, Belgium) for fascia and skin, respectively. The surgical wound was infiltrated with levobupivacaine (2 mg/kg Chirocaine®, Abbvie, Wavre, Belgium) and sprayed with aluminium (Kela®, Hoogstraten, Belgium).
Rabbits were monitored daily until delivery by caesarian section at GA 30. Following delivery, does were euthanized using IV phenytoin/pentobarbital (140 mg/kg Euthasol®, Kela, Bladel, The Netherlands). Kittens were pet dried, numbered with a permanent marker, and kept in a warmed (34 °C) and humidified (55% RH) incubator. Later that day they were stimulated to urinate, weighed, and fed a commercial milk substitute (Day One, protein 30%, fat 50%; Fox Valley, Lakemoor, IL) with added probiotics (Bio-Lapis; Probiotics International, Somerset, UK) and immunoglobulins (Col-o-Cat; SanoBest, Hertogenbosch, The Netherlands). The following morning litters were allocated for brain or lung assessment.
Placental ultrasound
At the time of caesarean delivery rabbits were anesthetized with the same protocol as for FGR induction, put on a warming pad under a warming light, and uterine horns were exposed and continuously rinsed with warm saline (37 °C). The VisualSonics VEVO 2100 (Toronto, Ontario, Canada) high-resolution micro ultrasound platform and a VisualSonics MS-250 transducer were used for image acquisition (center frequency 21 MHz; bandwidth 13–24 MHz; geometric focus 15 mm; maximum image width 23 mm; maximum image depth 30 mm; footprint 28 × 5.75 mm). Uterine artery doppler waveforms were obtained from the fetuses located closest to the ovarian end of each uterine horn. The umbilical vessels were located using color Doppler and the Doppler sample gate was placed over the umbilical artery, keeping the angle to a minimum. Peak systolic velocity (PSV), end diastolic velocity (EDV), velocity time integral (VTI), mean velocity (MV), pulsatility index (PI), and resistance index (RI) were calculated offline using the VisualSonics analysis software.
Placental histology
After delivery, placentas (until and including the decidua) were carefully separated from the implantation sites in the uterine wall, washed in PBS, trimmed from umbilical cord and membranes, blotted dry, weighed, and immerse-fixed in 4% paraformaldehyde (PFA) for 72 h. The smallest lobe was sectioned in 2 coronal portions, embedded in paraffin blocks, and cut in 4 µm slides. 2 slides per placenta were then double stained for cytokeratin and lectin to assess trophoblast, FC, and MBS. The detailed staining protocol is added in the Supplementary materials. Another 2 slides per placenta were subjected to standard Masson’s trichrome staining to visualize collagen deposition in the decidua.
All slides were digitally scanned with the Zeiss AxioScan Z1 imaging platform (AxioScan® Slide Scanner, Carl Zeiss MicroImaging GmbH, Munich, Germany).
Placental zones (labyrinth, junction, decidua) were manually delineated using QuPath software70 and their area relative to the total placental area was calculated. The structures within the labyrinth zone were manually quantified as described before71. Briefly, using a 4 × 4 point counting grid on 16 random fields at 20× magnification, the number of MBS, FC, and trophoblast cells was quantified and expressed as a percentage of the total count. Placental zone volumes and labyrinth structures volumes were calculated multiplying the relative volumes and the placental weight. To calculate the area of fibrosis in the decidua, images were manually delineated, and the selection was transferred to ImageJ (version 1.53e, Fiji, USA)72. Blue-stained collagen fibers were used to calculate the ratio of signal-positive area/total decidual area, expressed as percentage. A blinded observer (I.V.) performed all histological evaluations.
Placental computed microtomography
A subset of rabbits was used for fetoplacental perfusion with a contrast agent under the same anesthesia protocol at GA30. Following laparotomy, both uterine horns were removed and immediately placed on ice. Fetuses and placentas were exposed, and a 24G cannula was inserted in the umbilical vein of each fetus, while an umbilical artery was cut open to serve as vent. Initially, warm heparinized saline was perfused, followed by a barium-based contrast solution consisting of 0.9% sodium chloride solution, barium sulfate (E-Z PAQUE® High Density Barium Sulfate 98.75%, Bracco imaging, Milan, Italy), and porcine skin gelatin (Gelatin 48723, Sigma-Aldrich, Darmstadt, Germany), previously filtered and warmed to 60 °C. Perfusion was stopped when the contrast solution was clearly seen in the capillary bed of the placenta. Thereafter, placentas were separated from the conceptuses and immersed in 4% PFA for 24 h, washed, and stored in PBS. A total of 16 placentas (eight FGR and eight controls) from four different litters were included for analysis.
X-ray computed microtomography scans were performed using SkyScan 1172 micro-CT system (Bruker microCT, Kontich, Belgium) with X-ray energy of 80 keV. The image pixel size was set to 8.98 µm and a 0.5 mm Al filter was applied. Projection images were captured between the rotation steps of 0.2°. Each projection was merged from 6 or 8 images averaged from 5 frames to minimize the image noise and captured with different field of view to cover the entire sample, resulting in 3872 by 3872–4956 pixels. The total scan duration was up to 15 h.
For image reconstruction, GPU powered NRecon software (v.1.7.3.2, Bruker micro-CT) was used. Image processing and analysis was done by CT analyser software (v.1.20.3.0, Bruker micro-CT,). The reconstructed image stacks were median filtered with 2 pixels circular kernel, and histogram based manual global threshold was applied. The distribution of vessels thickness, and parameters of volume and surface of vascular tree were calculated. Additionally, color-coding visualization of the local thickness was applied. An observer blinded to group assignment (B.L.) performed all evaluations.
Neurobehavioral assessment
On postnatal day 1 (PND1), kittens underwent a validated NBA protocol18,73. Short-term motor assessment comprised scoring of gait, posture, locomotion, head and limb activity, and activity duration. Afterwards, the cranial nerves, pain response, and righting reflex were tested for sensory evaluation. All assessments were filmed and later scored by an observer blinded to the groups (I.V.). A full description of NBA protocols can be found in the Supplementary materials.
Brain harvesting
Immediately after NBA, animals were deeply sedated with IM ketamine (35 mg/kg) and xylazine (6 mg/kg), and transcardially perfused with 0.9% saline + heparin (100 u/mL; 3 min at 30 mL/min) followed by 4% PFA (5 min at 30 ml/min). Their brains were removed from the skull and further immerse fixed in 4% PFA for 48 h. A subgroup of kittens (n = 23) was perfused with heparinized saline, followed by 4% PFA and 2% (10 mM) gadoteridol (ProHance® 1 mL of 279.3 mg/mL solution). Their brains were kept inside their craniums for a maximum of 7 days to undergo MRI, after which they were removed and processed for histological assessment.
Brain MRI
Ex vivo MRI was performed on perfused fixed brains using the active staining technique. Briefly, a Bruker Biospec 9.4 Tesla small animal MR scanner (Bruker Biospin, Ettlingen, Germany; horizontal bore, 20 cm) equipped with actively shielded gradients (600 mT/m) was used. Data was acquired using a 72 mm internal diameter quadrature volume coil for transmission decoupled with a rat brain quadrature shaped surface coil for signal reception (volume resonator, Rapid Biomedical, Rimpar, Germany). Data was acquired with a high-resolution 3D Flash sequence (TE/TR 5.5/50 ms; flip angle 70 degrees; slice thickness 0.35 mm with no interslice gap, data matrix 392 × 392 × 392; isotropic resolution 89 μm; 4 averages, acquisition time 1h15min) and a SE-EPI sequence (8 segments; TE/TR: 25/150 ms; slice thickness 0.4 mm with no interslice gap, data matrix 192 × 160 × 160; isotropic spatial resolution of 208 μm; 64 directions and 3 b-values per direction of 800, 1000, 1500 s/mm2, acquisition time 11 h 43 min).
MRI volumes were processed to quantify microstructural properties in the following brain regions: frontal cortex, corpus callosum, caudate nucleus, putamen, internal capsule, corona radiata, hippocampus, thalamus, and hypothalamus. Automatic atlas-based parcellation was obtained by diffeomorphic registration of the atlas template in Ferraris et al. 74 to the acquired structural images, and then translated to the diffusion space. Fractional anisotropy (FA) and mean diffusivity (MD) were estimated using dipy python libraries75,76, and their mean values in regions of interest were computed. An observer blinded to group assignment (E.M.) performed all evaluations. A complete description of MRI processing can be found in Supplementary materials.
Of 23 acquisitions (13 controls 10 FGR), 4 (2 in each group) were excluded from analysis due to important artifacts. Statistical comparison between groups was performed in one random cerebral hemisphere. An interim power calculation, based on the FA data in the hippocampus, confirmed a power of 95% to detect these differences.
Brain histology
Following fixation, brains were paraffin embedded and serially sectioned at 4 µm. Three sets of four serial coronal sections every 100 µm were taken at each of the following two levels, as previously described18: level 1 started at the medial septal nucleus and level 2 at the hippocampal formation.
Six slides per brain (three slides per level) were stained with Cresyl Violet (CV; C5042-10G; Sigma-Aldrich, Overijse, Belgium), and two slides per brain (one slide per level, eight slides in total) were incubated with each of the following four primary antibodies: mouse monoclonal anti-human Ki67 (M724001-2; Agilent, Diegem, Belgium), mouse monoclonal anti-glial fibrillary acidic protein antibody (GFAP) (G6171, Sigma-Aldrich, St Louis, MO, USA), anti-NG2 chondroitin sulfate proteoglycan antibody (MAB5384, Millipore, Billerica, MA, USA), or a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) method for fluorescent in situ end labeling of double- stranded DNA fragmentation (Apoptag S7110; Millipore). The secondary antibody was Alexa Fluor® 488 goat anti-mouse conjugate (Invitrogen) or Alexa Fluor® 647 goat anti-mouse conjugate. Sections were counterstained with Hoechst 33342 (Sigma-Aldrich, Bornem, Belgium). Eight brain areas were assessed, namely, the frontal cortex, corpus callosum, caudate nucleus, putamen, hippocampus (CA1, CA3, dentate gyrus), and thalamus (anteroventral nucleus).
Details on image acquisition and quantification can be found in Supplementary materials.
Pulmonary function testing
Pressure–volume and forced oscillation maneuvers were performed via a tracheostomy on PND1 using the FlexiVent system (SciReq; FlexiVent, Montreal, QC, Canada). Animals were sedated with ketamine (35 mg/kg) and xylazine (6 mg/kg) before tracheostomy. An 18-gauge metal cannula was inserted into the trachea and secured with an airtight suture. Kittens were ventilated with a tidal volume of 10 mL/kg and positive end-expiratory pressure of 3 cmH2O at a rate of 120 breaths/min. Before lung function tests, two deep inflation maneuvers were performed until reaching a pressure of 30 cmH2O to maximally inflate the lungs and standardize lung volume. Both pressure–volume (inspiratory capacity, static compliance, and static elastance) and forced oscillation tests (tissue damping, tissue elastance, central airway resistance, respiratory system resistance, dynamic compliance, and dynamic elastance) were performed as previously described77. The mean of three separate measurements for each maneuver, with a coefficient of determination > 95%, was calculated and used as a single data point for analysis.
Histological lung assessment
Lungs were processed and assessed in accordance with international guidelines78. Briefly, after PFT, lungs were removed en bloc via thoracotomy, the trachea was cannulated with a 20-gauge catheter, and the left lung was pressure fixed for 24 h at a constant hydrostatic pressure of 25 cmH2O in 4% PFA77. After fixation, left lung volume was calculated using water displacement before paraffin embedding. Alveolar morphology was measured on digitally scanned 5 µm hematoxylin and eosin (H&E)-stained slides using a semi-automated, validated Fiji-plugin (ImageJ) (http://fiji.sc/Fiji) that randomly selected 20 fields per lung79, according to stereological principles. Calculations of mean linear intercept (Lm), alveolar air space (Lma), and interalveolar septal thickness (Lmw) were made as previously described29. Vascular morphology was evaluated using immunohistochemistry. A primary α-smooth muscle actin (α-SMA) antibody (mouse anti-human, M0851; DakoCytomation, Glostrup, Denmark) was used in combination with a horseradish peroxidase-conjugated secondary antibody (goat anti-mouse, 115-035-044; Jackson ImmunoResearch, Ely, UK). Aminoethyl carbazole was used as a chromogen. A minimum of 10 pulmonary arteries with an external diameter of 30–100 μm per lung slide were examined, measuring both internal and external diameter of the media to calculate the vascular medial thickness27. An observer blinded to group assignment (D.B.) performed all pulmonary histological evaluations.
Statistical analysis
Data were analysed and graphed using RStudio (RStudio: Integrated Development for R. RStudio, PBC, Boston, MA, USA) and Prism 9 for MacOS (GraphPad Prism, San Diego, CA, USA). Data distribution was checked for normality and presented as mean with standard deviation or median with interquartile range, as appropriate. Survival data was assessed by Fisher’s exact; all other parameters were analysed using a linear mixed-effects model, considering the mother as a random effect and the presence of FGR as a fixed effect. A p value of < 0.05 was considered significant.
Supplementary Information
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Grant agreement No. 765274. We would like to thank the technical assistance from the G-Pure laboratory at KU Leuven, namely Katrien Luyten and Sofie Jannes, and the valuable advice from Dr. Miriam Illa from the University of Barcelona. We would also like to thank Dr. Colin Murdoch and Mirren Augustin from Dundee University and iPLACENTA for their continued support.
Author contributions
All individuals listed as authors have significantly contributed to this study. I.V. performed all the surgical procedures, placental histology assessment, and brain assessment. D.B., L.V. and S.V. assisted all the surgical procedures. D.B., Y.R., and A.G., performed the pulmonary functional tests and lung histological analysis. E.M. analysed the MRI data, and B.L. processed and analysed the CT data. B.T. and G.VV. set-up the protocols for placental CT. J.M. and J.D. guided the experiments and corrected the work.
Data availability
The datasets analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-22895-6.
References
- 1.Resnik R. Intrauterine growth restriction. Obstet. Gynecol. 2002;99:490–496. doi: 10.1016/s0029-7844(01)01780-x. [DOI] [PubMed] [Google Scholar]
- 2.Flenady V, et al. Major risk factors for stillbirth in high-income countries: A systematic review and meta-analysis. Lancet. 2011;377:1331–1340. doi: 10.1016/s0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- 3.Lawn JE, et al. Stillbirths: Rates, risk factors, and acceleration towards 2030. Lancet. 2016;387:587–603. doi: 10.1016/s0140-6736(15)00837-5. [DOI] [PubMed] [Google Scholar]
- 4.Allanson ER, et al. The WHO application of ICD-10 to deaths during the perinatal period (ICD-PM): Results from pilot database testing in South Africa and United Kingdom. BJOG. 2016;123:2019–2028. doi: 10.1111/1471-0528.14244. [DOI] [PubMed] [Google Scholar]
- 5.von Beckerath AK, et al. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am. J. Obstet. Gynecol. 2013;208(130):e131–136. doi: 10.1016/j.ajog.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ. Adult consequences of fetal growth restriction. Clin. Obstet. Gynecol. 2006;49:270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Pike K, Jane Pillow J, Lucas JS. Long term respiratory consequences of intrauterine growth restriction. Seminars Fetal Neonatal Med. 2012;17:92–98. doi: 10.1016/j.siny.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Pels A, et al. Maternal sildenafil vs placebo in pregnant women with severe early-onset fetal growth restriction: A randomized clinical trial. JAMA Netw. Open. 2020;3:e205323. doi: 10.1001/jamanetworkopen.2020.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satterfield MCB, Spencer TE, Wu G. Sildenafil citrate treatment enhances amino acid availability in the conceptus and fetal growth in an ovine model of intrauterine growth restriction. J. Nutr. 2010;140:251–258. doi: 10.3945/jn.109.114678. [DOI] [PubMed] [Google Scholar]
- 10.Dilworth MRA, Renshall LJ, Cowley E, Baker P, Greenwood S, Sibley CP, Wareing M. Sildenafil citrate increases fetal weight in a mouse model of fetal growth restriction with a normal vascular phenotype. PLoS ONE. 2013;8:e77748. doi: 10.1371/journal.pone.0077748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanson AM, David AL. Animal models of fetal growth restriction: Considerations for translational medicine. Placenta. 2015;36:623–630. doi: 10.1016/j.placenta.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Bassan H, et al. Experimental intrauterine growth retardation alters renal development. Pediatr. Nephrol. (Berlin, Germany) 2000;15:192–195. doi: 10.1007/s004670000457. [DOI] [PubMed] [Google Scholar]
- 13.Bleeser T, et al. Effects of maternal abdominal surgery on fetal brain development in the rabbit model. Fetal Diagn. Ther. 2021;48:189–200. doi: 10.1159/000512489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derrick M, Drobyshevsky A, Ji X, Tan S. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731–735. doi: 10.1161/01.STR.0000251445.94697.64. [DOI] [PubMed] [Google Scholar]
- 15.Eastwood MP, et al. A growing animal model for neonatal repair of large diaphragmatic defects to evaluate patch function and outcome. PLoS ONE. 2017;12:e0174332. doi: 10.1371/journal.pone.0174332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eixarch E, et al. An experimental model of fetal growth restriction based on selective ligature of uteroplacental vessels in the pregnant rabbit. Fetal Diagn. Ther. 2009;26:203–211. doi: 10.1159/000264063. [DOI] [PubMed] [Google Scholar]
- 17.Fischer B, Chavatte-Palmer P, Viebahn C, Navarrete Santos A, Duranthon V. Rabbit as a reproductive model for human health. Reproduction (Cambridge, England). 2012;144:1–10. doi: 10.1530/REP-12-0091. [DOI] [PubMed] [Google Scholar]
- 18.van der Merwe J, et al. Early neuropathological and neurobehavioral consequences of preterm birth in a rabbit model. Sci. Rep. 2019;9:3506. doi: 10.1038/s41598-019-39922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furukawa S, Kuroda Y, Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 2014;27:11–18. doi: 10.1293/tox.2013-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Tello J, Arias-Alvarez M, Gonzalez-Bulnes A, Sferuzzi-Perri AN. Models of Intrauterine growth restriction and fetal programming in rabbits. Mol. Reprod. Dev. 2019;86:1781–1809. doi: 10.1002/mrd.23271. [DOI] [PubMed] [Google Scholar]
- 21.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter DS, et al. Programming the brain: Common outcomes and gaps in knowledge from animal studies of IUGR. Physiol. Behav. 2016;164:233–248. doi: 10.1016/j.physbeh.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Pringle KC. Human fetal lung development and related animal models. Clin. Obstet. Gynecol. 1986;29:502–513. doi: 10.1097/00003081-198609000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Salaets T, Gie A, Tack B, Deprest J, Toelen J. Modelling bronchopulmonary dysplasia in animals: Arguments for the preterm rabbit model. Curr. Pharm. Des. 2017;23:5887–5901. doi: 10.2174/1381612823666170926123550. [DOI] [PubMed] [Google Scholar]
- 25.Toelen J, et al. The fetal respiratory system as target for antenatal therapy. Facts Views Vision ObGyn. 2011;3:22–35. [PMC free article] [PubMed] [Google Scholar]
- 26.Arigliani M, Spinelli AM, Liguoro I, Cogo P. Nutrition and lung growth. Nutrients. 2018 doi: 10.3390/nu10070919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gie AG, et al. Intermittent CPAP limits hyperoxia-induced lung damage in a rabbit model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cellular Mol. Physiol. 2020;318:L976–L987. doi: 10.1152/ajplung.00465.2019. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez J, et al. Progressive vascular functional and structural damage in a bronchopulmonary dysplasia model in preterm rabbits exposed to hyperoxia. Int. J. Mol. Sci. 2016 doi: 10.3390/ijms17101776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salaets T, et al. Preterm birth impairs postnatal lung development in the neonatal rabbit model. Respir. Res. 2020;21:59. doi: 10.1186/s12931-020-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Merwe J, et al. Neurocognitive sequelae of antenatal corticosteroids in a late preterm rabbit model. Am. J. Obstet. Gynecol. 2021 doi: 10.1016/j.ajog.2021.11.1370. [DOI] [PubMed] [Google Scholar]
- 31.van der Merwe J, et al. Earlier preterm birth is associated with a worse neurocognitive outcome in a rabbit model. PLoS ONE. 2021;16:e0246008. doi: 10.1371/journal.pone.0246008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo FM, et al. Transplacental sildenafil rescues lung abnormalities in the rabbit model of diaphragmatic hernia. Thorax. 2016;71:517–525. doi: 10.1136/thoraxjnl-2015-207949. [DOI] [PubMed] [Google Scholar]
- 33.Roubliova XI, et al. Morphologic changes and methodological issues in the rabbit experimental model for diaphragmatic hernia. Histol. Histopathol. 2010;25:1105–1116. doi: 10.14670/hh-25.1105. [DOI] [PubMed] [Google Scholar]
- 34.Pla L, et al. Structural brain changes during the neonatal period in a rabbit model of intrauterine growth restriction. Dev. Neurosci. 2020;42:217–229. doi: 10.1159/000512948. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Tello JA-A, Jimenez-Martinez MA, Barbero-Fernandez A, Garcia-Garcia RM, Rodriguez M, Lorenzo PL, Torres-Rovira L, Astiz S, Gonzalez-Bulnes A, Rebollar PG. The effects of sildenafil citrate on feto-placental development and haemodynamics in a rabbit model of intrauterine growth restriction. Reproduct. Fertility Development. 2017;29:1239–1248. doi: 10.1071/rd15330. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Tello J, et al. Competition for materno-fetal resource partitioning in a rabbit model of undernourished pregnancy. PLoS ONE. 2017;12:e0169194. doi: 10.1371/journal.pone.0169194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang AZ, Xiang XL, Wang K, Zhou Q, Duan T. Folic acid attenuates dexamethasone-induced placental growth restriction. Eur. Rev. Med. Pharmacol. Sci. 2015;19:1130–1140. [PubMed] [Google Scholar]
- 38.Hewitt DP, Mark PJ, Waddell BJ. Glucocorticoids prevent the normal increase in placental vascular endothelial growth factor expression and placental vascularity during late pregnancy in the rat. Endocrinology. 2006;147:5568–5574. doi: 10.1210/en.2006-0825. [DOI] [PubMed] [Google Scholar]
- 39.Wyrwoll CS, Seckl JR, Holmes MC. Altered placental function of 11beta-hydroxysteroid dehydrogenase 2 knockout mice. Endocrinology. 2009;150:1287–1293. doi: 10.1210/en.2008-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo MYW, Chen YH, Xia MZ, Zhang C, Xu DX. N-Acetylcysteine alleviates cadmium-induced placental endoplasmic reticulum stress and fetal growth restriction in mice. PLoS ONE. 2018;13:e0191667. doi: 10.1371/journal.pone.0191667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohta HK, Li H, Sakai H, Okamura K, Yaegashi N. Potential new non-invasive therapy using artificial oxygen carriers for pre-eclampsia. J. Funct. Biomater. 2017 doi: 10.3390/jfb8030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarrade A, et al. Analysis of placental vascularization in a pharmacological rabbit model of IUGR induced by L-NAME, a nitric oxide synthase inhibitor. Placenta. 2014;35:254–259. doi: 10.1016/j.placenta.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Chen YHZ, Chen X, Zhang Y, Wang H, Huang YY, Wang Z, Zhang ZH, Zhang C, Xu DX. Zinc supplementation during pregnancy protects against lipopolysaccharide-induced fetal growth restriction and demise through its anti-inflammatory effect. J. Immunol. (Baltimore, Md.: 1950) 2012;189:454–463. doi: 10.4049/jimmunol.1103579. [DOI] [PubMed] [Google Scholar]
- 44.Jones HN, Crombleholme T, Habli M. Adenoviral-mediated placental gene transfer of IGF-1 corrects placental insufficiency via enhanced placental glucose transport mechanisms. PLoS ONE. 2013;8:e74632. doi: 10.1371/journal.pone.0074632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eixarch E, et al. Impact on fetal mortality and cardiovascular Doppler of selective ligature of uteroplacental vessels compared with undernutrition in a rabbit model of intrauterine growth restriction. Placenta. 2011;32:304–309. doi: 10.1016/j.placenta.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Polisca A, Scotti L, Orlandi R, Brecchia G, Boiti C. Doppler evaluation of maternal and fetal vessels during normal gestation in rabbits. Theriogenology. 2010;73:358–366. doi: 10.1016/j.theriogenology.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Diaz V, Lebras-Isabet MN, Denjean A. Effect of Nomega-nitro-L-arginine methyl ester-induced intrauterine growth restriction on postnatal lung growth in rats. Pediatr. Res. 2005;58:557–561. doi: 10.1203/01.PDR.0000179398.62365.43. [DOI] [PubMed] [Google Scholar]
- 48.Velten MB, Heyob KM, Tipple TE, Rogers LK. Maternal dietary docosahexaenoic acid supplementation attenuates fetal growth restriction and enhances pulmonary function in a newborn mouse model of perinatal inflammation. J. Nutr. 2014;144:258–266. doi: 10.3945/jn.113.179259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karnak I, Muftuoglu S, Cakar N, Tanyel FC. Organ growth and lung maturation in rabbit fetuses. Res. Exp. Med. (Berl.) 1999;198:277–287. doi: 10.1007/s004330050111. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, et al. Maternal protein restriction alters VEGF signaling and decreases pulmonary alveolar in fetal rats. Int. J. Clin. Exp. Pathol. 2014;7:3101–3111. [PMC free article] [PubMed] [Google Scholar]
- 51.Maritz GS, et al. Effects of fetal growth restriction on lung development before and after birth: A morphometric analysis. Pediatr. Pulmonol. 2001;32:201–210. doi: 10.1002/ppul.1109. [DOI] [PubMed] [Google Scholar]
- 52.Zana-Taieb E, et al. Effect of two models of intrauterine growth restriction on alveolarization in rat lungs: Morphometric and gene expression analysis. PLoS ONE. 2013;8:e78326. doi: 10.1371/journal.pone.0078326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khazaee R, McCaig LA, Yamashita C, Hardy DB, Veldhuizen RAW. Maternal protein restriction during perinatal life affects lung mechanics and the surfactant system during early postnatal life in female rats. PLoS ONE. 2019;14:e0215611. doi: 10.1371/journal.pone.0215611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang LT, Chou HC, Lin CM, Chen CM. Uteroplacental insufficiency alters the retinoid pathway and lung development in newborn rats. Pediatr. Neonatol. 2016;57:508–514. doi: 10.1016/j.pedneo.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Joss-Moore L, et al. Intrauterine growth restriction transiently delays alveolar formation and disrupts retinoic acid receptor expression in the lung of female rat pups. Pediatr. Res. 2013;73:612–620. doi: 10.1038/pr.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGillick EV, Orgeig S, Morrison JL. Regulation of lung maturation by prolyl hydroxylase domain inhibition in the lung of the normally grown and placentally restricted fetus in late gestation. Am. J. Physiol. Regulat. Integr. Comparat. Physiol. 2016;310:R1226–1243. doi: 10.1152/ajpregu.00469.2015. [DOI] [PubMed] [Google Scholar]
- 57.Orgeig S, et al. Increased lung prolyl hydroxylase and decreased glucocorticoid receptor are related to decreased surfactant protein in the growth-restricted sheep fetus. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;309:L84–97. doi: 10.1152/ajplung.00275.2014. [DOI] [PubMed] [Google Scholar]
- 58.Dravet-Gounot P, et al. Lung microRNA deregulation associated with impaired alveolarization in rats after intrauterine growth restriction. PLoS ONE. 2017;12:e0190445. doi: 10.1371/journal.pone.0190445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eixarch E, et al. Neonatal neurobehavior and diffusion MRI changes in brain reorganization due to intrauterine growth restriction in a rabbit model. PLoS ONE. 2012;7:e31497. doi: 10.1371/journal.pone.0031497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Illa M, et al. Early environmental enrichment enhances abnormal brain connectivity in a rabbit model of intrauterine growth restriction. Fetal. Diagn. Ther. 2018;44:184–193. doi: 10.1159/000481171. [DOI] [PubMed] [Google Scholar]
- 61.Saito A, et al. Behavioral abnormalities of fetal growth retardation model rats with reduced amounts of brain proteoglycans. Exp. Neurol. 2009;219:81–92. doi: 10.1016/j.expneurol.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 62.Villescas R, Van Marthens E, Hammer RP., Jr Prenatal undernutrition: Effects on behavior, brain chemistry and neuroanatomy in rats. Pharmacol. Biochem. Behav. 1981;14:455–462. doi: 10.1016/0091-3057(81)90302-6. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Li N, Yang J, Zhang T, Yang Z. Effects of maternal food restriction on physical growth and neurobehavior in newborn Wistar rats. Brain Res. Bull. 2010;83:1–8. doi: 10.1016/j.brainresbull.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 64.Hernandez-Andrade E, Figueroa-Diesel H, Jansson T, Rangel-Nava H, Gratacos E. Changes in regional fetal cerebral blood flow perfusion in relation to hemodynamic deterioration in severely growth-restricted fetuses. Ultrasound Obstetr. Gynecol. 2008;32:71–76. doi: 10.1002/uog.5377. [DOI] [PubMed] [Google Scholar]
- 65.Kalusa M, Heinrich MD, Sauerland C, Morawski M, Fietz SA. Developmental differences in neocortex neurogenesis and maturation between the altricial dwarf rabbit and precocial guinea pig. Front. Neuroanat. 2021;15:678385. doi: 10.3389/fnana.2021.678385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barenys M, et al. Rabbit neurospheres as a novel in vitro tool for studying neurodevelopmental effects induced by intrauterine growth restriction. Stem Cells Transl. Med. 2021;10:209–221. doi: 10.1002/sctm.20-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herrera EA, et al. Assessment of in vivo fetal growth and placental vascular function in a novel intrauterine growth restriction model of progressive uterine artery occlusion in guinea pigs. J. Physiol. 2016;594:1553–1561. doi: 10.1113/JP271467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsuji M, Coq JO, Ogawa Y, Yamamoto Y, Ohshima M. A rat model of mild intrauterine hypoperfusion with microcoil stenosis. J. Vis. Exp. 2018 doi: 10.3791/56723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.PercieduSert N, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bankhead P, et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017;7:16878. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Clercq K, Lopez-Tello J, Vriens J, Sferruzzi-Perri AN. Double-label immunohistochemistry to assess labyrinth structure of the mouse placenta with stereology. Placenta. 2020;94:44–47. doi: 10.1016/j.placenta.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 72.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Derrick M, et al. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: A model for human cerebral palsy? J. Neurosci. 2004;24:24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferraris S, et al. A magnetic resonance multi-atlas for the neonatal rabbit brain. Neuroimage. 2018;179:187–198. doi: 10.1016/j.neuroimage.2018.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fick RHJ, Wassermann D, Deriche R. The Dmipy Toolbox: Diffusion MRI multi-compartment modeling and microstructure recovery made easy. Front. Neuroinform. 2019;13:64. doi: 10.3389/fninf.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garyfallidis E, et al. Dipy, a library for the analysis of diffusion MRI data. Front. Neuroinform. 2014;8:8. doi: 10.3389/fninf.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Richter J, et al. Functional assessment of hyperoxia-induced lung injury after preterm birth in the rabbit. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;306:L277–283. doi: 10.1152/ajplung.00315.2013. [DOI] [PubMed] [Google Scholar]
- 78.Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: Standards for quantitative assessment of lung structure. Am. J. Respir. Crit. Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salaets T, et al. A semi-automated method for unbiased alveolar morphometry: Validation in a bronchopulmonary dysplasia model. PLoS ONE. 2020;15:e0239562. doi: 10.1371/journal.pone.0239562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study available from the corresponding author on reasonable request.