Abstract
Growing evidence suggests that sustained concentrated urine contributes to chronic metabolic and kidney diseases. Recent results indicate that a daily urinary concentration of 500 mOsm/kg reflects optimal hydration. This study aims at providing personalized advice for daily water intake considering personal intrinsic (age, sex, height, weight) and extrinsic (food and fluid intakes) characteristics to achieve a target urine osmolality (UOsm) of 500 mOsm/kg using machine learning and optimization algorithms. Data from clinical trials on hydration (four randomized and three non-randomized trials) were analyzed. Several machine learning methods were tested to predict UOsm. The predictive performance of the developed algorithm was evaluated against current dietary guidelines. Features linked to urine production and fluid consumption were listed among the most important features with relative importance values ranging from 0.10 to 0.95. XGBoost appeared the most performing approach (Mean Absolute Error (MAE) = 124.99) to predict UOsm. The developed algorithm exhibited the highest overall correct classification rate (85.5%) versus that of dietary guidelines (77.8%). This machine learning application provides personalized advice for daily water intake to achieve optimal hydration and may be considered as a primary prevention tool to counteract the increased incidence of chronic metabolic and kidney diseases.
Subject terms: Physiology, Kidney, Metabolism, Biomarkers, Nutrition, Machine learning, Scientific data
Introduction
In recent years a sharp increase in the prevalence of metabolic and kidney diseases across the general population has been observed1,2. Growing evidence shows that body fluid imbalances, which result in elevated hydration biomarkers such as concentrated urine, contribute to negative health outcomes or life-threatening situations3–7. In particular, low water intake and concentrated urine have been adversely linked to chronic kidney disease progression2,8–10, kidney stones incidence11 and glucose dysregulation12,13. Additionally, interventional studies showed that increased fluid intake improved renal functions14,15 in the general population, and greatly decreased urinary tract infection (UTI) recurrence rate16 in adult women. Low water intake is also a global public health challenge as recent research assessing fluid intake habits across different countries worldwide highlights that about 50% of the study adult population and more than 50% of the child and adolescent study population did not comply with the European Food Safety Authority (EFSA) Adequate Intake of water from fluids17,18. Despite existing recommendations for water intake and scientific evidence on the role of water for health, today, a considerable portion of the population is at risk of hydration-related health consequences such as metabolic and kidney disease.
It was previously proposed that a threshold value of daily urinary concentration may identify optimal fluid balance and provide a target to aim for19,20. Several recent intervention studies, including randomized controlled trials, have now demonstrated that lowering 24 h urine osmolality (UOsm) to 500 mOsm/kg or below, can reduce a predictive marker of cardiovascular risk, namely arginine vasopressin as measured by copeptin16,21–23, as well as reduce UTI incidence16. Collectively, these experimental results suggest that optimal hydration may be achieved by drinking sufficient water to reach this daily target for urine concentration. While current government-issued dietary guidelines consider the average need of a population, evidence sheds light on the opportunity for implementing novel, individual-centric interventions to improve hydration among the general population24. In this context, personalized hydration approach aims to develop specific and comprehensive water advice. This methodology accounts for the physiological requirements of an individual25,26 based on phenotypic characteristics, analysis of current behavior, preferences, barriers, and objectives.
Physiological requirements for fluids are the result of a continuous and complex interplay between an individual’s intrinsic and extrinsic factors which challenge homeostasis. Consequently, fluid balance, as a key player in our ability to maintain homeostasis, should be regarded as one of the main components for the provision of a personalized hydration intervention. In general terms, factors that may affect fluid balance are sex, body mass composition27, physical activity, thermoregulatory processes28, and medical conditions29. As human beings constantly lose water through urine and insensible water losses, the only way to replenish total body water is to drink water from an external source. Considering the dynamic nature of the homeostatic processes that ultimately determine fluid balance, we considered machine learning (ML) statistical analysis, a relevant and novel approach, to reflect these dynamics. Datasets from multiple clinical studies on hydration were used as a valid source of data for this investigation. The aim was to provide personalized advice on daily water intake considering personal intrinsic and extrinsic variability to achieve a target 24 h UOsm of 500 mOsm/kg in a subset of healthy adults excluding athletes and pregnant and lactating women.
Subjects and methods
Study population
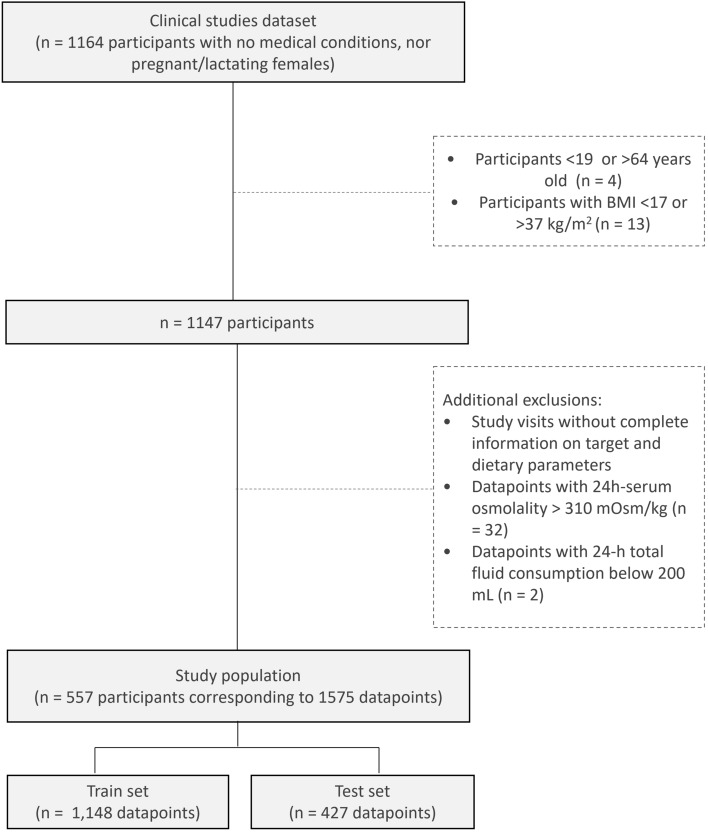
All research was conducted according to the ethical principles stated in the Declaration of Helsinki. All subjects provided written informed consent. Each study was approved by a local Ethics Committee (Comité Etico De Investigacion Clinica (CEIC) del Hospital Universitario La Princesa, Madrid, Spain; CEIC Hospital Universitario de La Paz, Madrid, Spain; Comité de Ética e Investigación para Estudios en Humanos (CEIEH), Mexico, Mexico; Comité de Bioética Para la Investigacion Clinica, Mexico, Mexico; Comité de Protection des Personnes (CPP) of Ile de France XI, Paris, France; Ethics Committee CPP Sud Méditerranée III, Nîmes, France; Ethics Committee CPP Est IV, Strasbourg, France; Ethics Committee CPP Est III, Nancy, France; Ethics Committee of COMAC Medical, Sofia, Bulgaria; Supplemental Methods). Multiple datasets from previous clinical trials on hydration and fluid balance (4 open label randomized controlled trials, and 3 open label non randomized trials in parallel groups) were merged into one single dataset (n = 1164 participants) (Fig. 1). Each subject had 107 possible variables collected during the clinical studies at each single time point. Some participants had measurements of their biological variables performed on multiple timepoints during the same clinical study, consequently their information was arranged on multiple lines for homogeneity within the dataset. Participants not meeting the aforementioned criteria on age (n = 4) and BMI (n = 13) were filtered out. Datapoints from study visits without complete information on biological and dietary parameters were filtered out. Additionally, subjects' datapoints were further filtered out if Plasma Osmolality (POsm) was above 310 mOsm/kg (n = 32), a threshold for dehydration which corresponds to a ~ 5% body weight loss, a condition not generally met by the general population30. Also, participants with a 24 h total fluid consumption below 200 mL (n = 2) were excluded on the basis that such fluid intake cannot meet fluid balance physiological requirements in general population. This resulted in a dataset of 1575 rows reporting information of 557 subjects. Within the resulting dataset, some participants had missing fluid intake data (n = 107). Nonetheless, to maintain a fair representation of the subject population and to not potentially undermine statistical analysis capability, these participants were retained in the dataset. This choice was driven by the capability that the ML approach has to deal with missing values, and it was worth considering the wealth of information these participants recorded for other variables. Following this process, participants were randomly split according to their participant ID number with a ratio of 75:25, ensuring that each participant was assigned to either the training set or the test set. The final dataset consisted in 1,148 rows for the train test and 427 rows for the test set.
Figure 1.
Participant flowchart.
Feature selection
Extreme Gradient Boosting (XGBoost) was adopted to achieve the preliminary identification of the most relevant features linked to urine production in the dataset (Hyperparameters tuning in Supplemental Methods).
Machine learning algorithms
Once the most relevant features were identified in the dataset, several ML methods were used to predict UOsm, including Random Forest (RF)31, Gradient Boosting Machines (GBM)32, Automatic Machine Learning (AutoML)33 using h2o package34, and XGBoost35 using XGBoost package36 (Supplemental Methods; Supplemental Fig. 1). Cross-validation and hyperparameters tuning were used to optimize the ML models (Supplemental Methods). These methods were selected due to their capability of automatically handling missing values. For example, XGBoost supports missing values by default, with branch directions for missing values are learned during training. In h2o package, missing values are interpreted as containing information and in tree-based techniques split decisions for every node are found by treating missing values as a separate category.
The highest performing methodology was identified by applying the aforementioned ML techniques on the train set. Once the algorithm was obtained by the application of these techniques, predictions of UOsm were generated on the test set. Once the predictions on the test set were calculated, Mean Absolute Error (MAE) was used to compare the predicted values of UOsm to the actual ones recorded in the dataset. In particular, MAE is defined as , where yi and indicate the ith actual value and the related predicted value of the response variable respectively (i.e., estimates the conditional mean of UOsm given subject-specific information). It was chosen as it is in the same scale of the outcome and does not assign large weight to infrequent large errors. The highest performing methodology, chosen on the lowest prediction error was selected (XGBoost). The reliability of the model was tested via repeating five times the whole train-test split and model estimation. Then, the performances on the test set were re-assessed to confirm matching results (Supplemental Table 3). The XGBoost is a black box model, where the importance of each feature on UOsm is not disclosed. To elucidate the relationships among these variables, several simulations were performed using Partial Dependence Plots (PDP)32,37 and Accumulated Local Effects (ALE)38 plots (Supplemental Methods).
Optimization
The ML approach allowed to establish a relationship between the UOsm of each participant with their anthropometric characteristics, food and fluid intakes. Once this relationship was described, an optimization procedure was implemented to generate personalized advice on the daily amount of water needed to reach the target UOsm for optimal hydration (i.e. a UOsm < 500 mOsm/kg). For each individual, all anthropometric and food parameters remain unchanged. The only variable that was modified in the reverse engineering phase is the amount of plain water, which indirectly affects total fluid intake. The augmented Lagrangian was used as optimization algorithm39, because it also offers the opportunity to consider eventual constraints of interest which could be useful to avoid unreliable suggestions for particular individuals. This method integrates eventual non-linear constraints into the objective function (UOsm < 500 mOsm/kg), so that a penalty is added for any violated constraint. The following constraints were used: the minimum and maximum acceptable values were set to 375 mOsm/kg and 625 mOsm/kg, respectively, and the target value was 500 mOsm/kg; the lower and upper bounds for the plain water intake were set to the observed minimum and maximum values in the dataset (i.e. 0 and 4050 mL); all other variables remained constant during the optimization process. This objective function was then optimized by a local solver without non-linear constraints using Sequential Quadratic Programming (SLSQP) algorithm40. The optimization procedure was applied on all the datapoints included in the test set.
Evaluation of classification performance
Contingency tables were used to evaluate the classification rate of the developed algorithm (Table 1). The difference between the actual Total Fluid Intake (TFI) and the predicted TFI were calculated and checked against the actual UOsm reported in the dataset, either above or below the 500 mOsm/kg threshold. This Uosm threshold is the target value of a 24 h urinary concentration and reflective of optimal fluid balance. This allowed to classify datapoints into four categories. True positives were those both with a higher TFI than the predicted TFI and being well hydrated (UOsm < 500 mOsm/kg). True negatives were those both with a lower TFI than the predicted TFI and being underhydrated (UOsm ≥ 500 mOsm/kg). The false positives were those with a higher TFI than the predicted TFI but who were underhydrated (UOsm < 500 mOsm/kg). Which means that the predicted TFI is not high enough to ensure optimal hydration. Finally, the false negatives were those with a lower TFI than the predicted TFI but who were still considered well hydrated (UOsm < 500 mOsm/kg). This means that the predicted TFI could have been lower.
Table 1.
Evaluation of the algorithm classification.
| UOsm < 500 mOsm/kg | UOsm ≥ 500 mOsm/kg | |
|---|---|---|
|
∆[Actual-Predicted] Total fluid intake ≥ 0 |
True positive (TP) | False positive (FP) |
|
∆[Actual-Predicted] Total fluid intake < 0 |
False negative (FN) | True negative (TN) |
Overall percent classification by the algorithm was calculated using two metrics as follows:
The performance of the algorithm was further evaluated against values deriving from current European dietary guidelines for water intake. EFSA has set daily Adequate Intakes (AIs) for total water for the adult population of 2.5 L for men and 2.0 L for women41. These values include water that comes both from consumed fluids and food. It is estimated that the contribution of food and fluids to total water intake represent about 20% and 80%, respectively, in adults. This means male adults should drink 2.0 L per day, and female adults 1.6 L. These sex-specific thresholds were used to evaluate the difference between the actual TFI and the predicted TFI in relation to UOsm. The difference between the actual TFI and the EFSA AI was then checked against the actual UOsm on the two sides of the 500 mOsm/kg threshold to generate contingency tables.
Results
Demographic characteristics
Data are presented as mean (min; max) or as % of the respective dataset, unless specified otherwise.
Both the train and test set showed an average age of 30 (19; 51) years (Supplemental Fig. 2) with a higher presence of female participants (64% and 62%, respectively). BMI was of 23 (18;30) kg/m2 in both sets. The range of UOsm was [109–1252] mOsm/kg, with a mean value of 554 mOsm/kg in the train set, while in the test set it was [141–1343] mOsm/kg with a mean value of 546 mOsm/kg. The total fluid consumption showed an average value of about 1889 mL and a minimum value of 215 mL and 329 mL in the train and test sets, respectively. The maximum values were equal to 6109 mL and 6742 mL in the train set and test set, respectively. The total plain water consumption could vary between 0 and 4050 mL in the train set and between 100 and 3500 mL in the test set. The average values were about 1301 mL and 1049 mL, in the train and test sets, respectively.
Feature selection
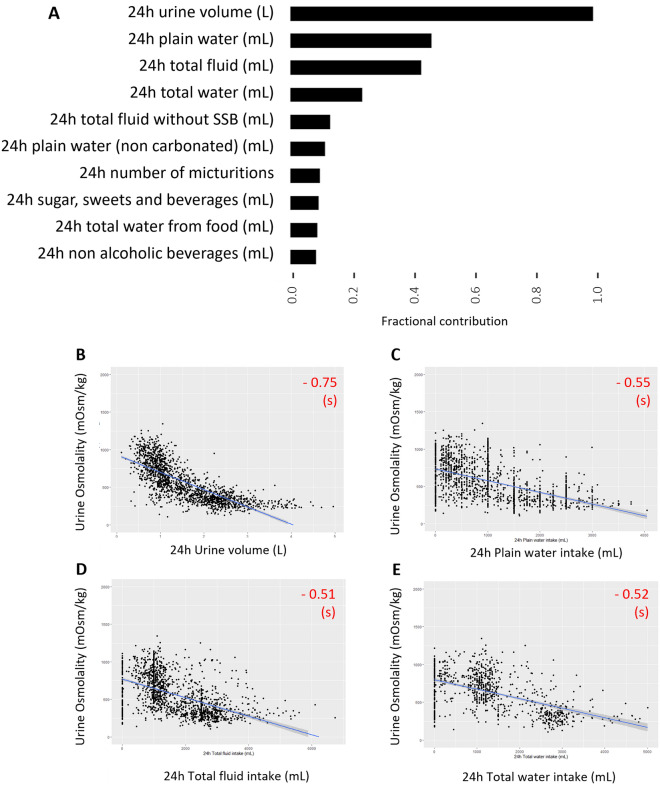
The preliminary identification of the most relevant features linked to urine production is shown in Fig. 2. The features are ranked based on their fractional contribution to the model, i.e. the total gain of each feature's splits (x-axis of Fig. 2A). The adopted configuration of the model is reported in Supplemental Methods. Features linked to urine production (volume and number of micturitions) as well as fluid consumption were listed among the most important features with relative importance values ranging from 0.10 to 0.95. At this stage, some key features were purposely excluded before generating predictions of urine osmolality such as Urine volume and Number of micturition, to only utilize information easily accessible to the general population. As a result, 23 variables out of 107 were kept in the dataset to generate predictions. These included Age, Sex, Weight, Height and some food and fluid intake variables (See Supplemental Table 2 for the full list of features).
Figure 2.
Variable importance to determine UOsm and data distribution. (A) Feature importance plot showing the 10 most important features obtained with XGBoost. (B–E) Data distribution of Urine osmolality against 24 h Urine volume (n = 1575), Plain water intake (n = 1404), Total fluid intake (n = 1468) and Total water intake (n = 1448). Pearson’s correlation coefficient; (s) significant, (ns) non-significant based on linear regression p-value (< 0.05). The R ggplot2 package was used to generate the figures https://ggplot2.tidyverse.org. R Statistical Software version 3.6.3 (R Core Team https://cran.r-project.org/bin/windows/base/old/3.6.3/).
Mean Absolute Error (MAE) of machine learning algorithms
Several ML approaches were compared to generate predictions of urine osmolality. XGBoost was the most performing approach (MAE = 124.99), returning the minimum test error as compared to AutoML (MAE = 126.86), RF (MAE = 129.03) and GBM (MAE = 132.15). The results of the multiple evaluations of our models are reported in Supplemental Table 3. The average MAEs were 124.47, 126.88, 128.8, and 130.11 and the related standard deviations were 2.65, 1.38, 1.93, and 1.48, respectively.
Since XGBoost is a black box model, several simulations were performed using PDP and ALE plots. Both methods showed that increasing water intake led to a progressive reduction of UOsm; the greater reduction was observed when moving from 1200 to 1500 mL (Fig. 3). The observed relationship between plain water intake and UOsm reflected homeostatic fluid balance physiological processes.
Figure 3.
Partial dependence plots (PDP) and accumulated local effects (ALE) plots for 24 h Plain water intake. (A) PDP plot for 24 h plain water intake. The large white curve represents the PDP plot. The black lines represent the individual conditional expectation (ICE) curves, i.e. the equivalent to a PDP for individual data instances. (B) ALE plots for 24 h plain water intake. The R pdp package was used to generate the figures https://journal.r-project.org/archive/2017/RJ-2017-016/index.html. R Statistical Software version 3.6.3 (R Core Team https://cran.r-project.org/bin/windows/base/old/3.6.3/).
An optimization algorithm was generated to frame the final advice for water intake, using constraints on 24 h UOsm and 24 h plain water intake. The minimum and maximum acceptable values were set to 375 mOsm/kg and 625 mOsm/kg, respectively, and the target value was set to 500 mOsm/kg. The lower and upper bounds for the plain water intake were set to the observed minimum and maximum values from the dataset (i.e. 0 and 4050 mL). This allowed to estimate personalized advice for the individuals in the test set (Table 2). When the original urine osmolality was higher than 500 mOsm/kg, the tool calculated increased volumes of water intake. Conversely, when urine osmolality was low, (i.e. when the urine is diluted), the tool showed that the individual could reduce the water intake and still be optimally hydrated.
Table 2.
Personalized advice for some individuals of the test set.
| Age (years) | Height (cm) | Weight (kg) | Sex | Urine osmolality (mOsm/kg) | Plain water intake (mL) | Total fluid intake (mL) | |||
|---|---|---|---|---|---|---|---|---|---|
| Original | Original | Original | Original | Original | Optimized | Original | Optimized | Original | Optimized |
| 20 | 161 | 67 | Female | 1139 | 492 | 500 | 1298 | 1200 | 1998 |
| 42 | 160 | 67 | Female | 1020 | 505 | 250 | 1313 | 1000 | 2063 |
| 20 | 178 | 68.3 | Male | 750 | 501 | 1500 | 1750 | 2172 | 2422 |
| 26 | 181 | 75.2 | Male | 330 | 498 | 2000 | 1298 | 4115 | 3413 |
The algorithm developed in this study consisted of the combination of two main elements. Firstly, a ML algorithm, which generates a prediction for UOsm starting from anthropometric characteristics, food, and fluid intake data. Secondly an optimization algorithm that generates personalized advice for optimal water intake based on the predicted UOsm and actual fluid intake. To compare the performance of this combination of algorithms against current dietary guidelines, the relevance of the classification rates was calculated using contingency tables (Tables 3 and 4).
Table 3.
Contingency table of the machine learning/optimization algorithm prediction of total fluid intake.
| UOsm | Accuracy | Acceptable classification | |||
|---|---|---|---|---|---|
| < 500 mOsm/kg | ≥ 500 mOsm/kg | ||||
| ∆[Actual-predicted] Total fluid intake | ≥ 0 | 185 | 22 | 85.5% | 94.8% |
| < 0 | 40 | 180 | |||
Values are number of datapoints per category. Total sample size is n = 427 datapoints.
Table 4.
Contingency table of values for total water intakes coming from fluids deriving from the European Food Safety Authority (EFSA) dietary guidelines.
| UOsm | Accuracy | Acceptable classification | |||
|---|---|---|---|---|---|
| < 500 mOsm/kg | ≥ 500 mOsm/kg | ||||
| ∆[Actual-EFSA AI] Total fluid intake | ≥ 0 | 161 | 31 | 77.8% | 92.7% |
| < 0 | 64 | 171 | |||
Values are number of datapoints per category. Total sample size is n = 427 datapoints.
EFSA AI European Food Safety Authority Adequate Intake.
The developed algorithm exhibited the highest overall correct classification rates with an accuracy rate of 85.5% compared to an accuracy rate of 77.8% derived from dietary guidelines. When considering the proportion of false negatives, (i.e. participants with a lower fluid intake than the predicted one and showing a urine concentration below the threshold for optimal hydration) the rate of acceptable classifications was still higher for the algorithm developed in the current study (94.8%) than that derived from dietary guidelines (92.7%).
Discussion
In this study, we generated a ML algorithm which in combination with an optimization algorithm provides personalized advice for daily water intake to achieve optimal hydration, as defined by a target 24 h UOsm of 500 mOsm/kg in healthy adults. This target has been previously proposed to decrease risks for long term diseases while ensuring optimal fluid balance processes19. It is worth mentioning that we initially considered analyzing a subsample of data from the National Health and Nutrition Examination Survey (NHANES), a broad, validated and publicly available dataset of the United States population. Urine osmolality measures are available from NHANES 2009–2010 and 2011–2012. However, the large number of missing values and the low fractional contribution of the most relevant features to the model impaired the ability of generating predictions with an adequate degree of precision. All details about this analysis can be found in the Supplemental Data, NHANES section. Therefore, in the investigation here presented, we performed a post-hoc analysis of data pooled from multiple clinical studies on hydration and fluid balance in general adult population. This allowed to gather higher quality data from trustworthy and reliable sources in regards of data quality. Hence, we could highlight existing relationships between the observed features and UOsm. With no similar ML application reported in the literature, we evaluated the performance of the prediction against values deriving from current EFSA dietary guidelines for water intake. Our prediction model proved to generate advice for 24 h optimal water intake for healthy adults with an excellent degree of fitting compared to current adequate intakes (AIs) for water.
Representing 40–60% of body mass, water is the largest constituent of the human body. Like any other human physiological processes, water homeostasis is continuously challenged; in particular trans-epidermal, respiratory, fecal and urinary represent the main fluid losses and therefore threats to normal body functioning6,42. The human body has a limited capacity to store water and previous reports highlight that there is a mean daily water turnover of 3.6 ± 1.2 L/day or 2.8–3.3 and 3.4–3.8 L/day in women, and men, respectively43. Being between 0.25 to 0.35 L/day, only a fraction of water is produced in the body as result of metabolic processes, consequently, water losses must be replaced by ingestion of fluids44. The body puts in place highly refined responses to maintain the volume of the body water within a narrow range independently to the conditions that it may be facing45. Under this light, water has been called the ‘most essential’ nutrient45.
Despite its primary role, water is under-researched and often referred to as a forgotten, neglected nutrient. As an example, a review from Perrier and colleagues points to the discrepancies between regional water intake recommendations, and the fact that these reference values represent AIs41,46,47. The AIs derive from observational or experimental data that provide estimates of average water intake while lacking scientific evidence to associate a consumption threshold with positive or negative health outcomes4,7. From a practical point of view, general population currently does not benefit from recommendations for plain water intake in the dietary guidelines that are based on the health outcomes associated with the consumption of such nutrient. While thirst is generally considered enough of a warning for fluid replenishment48,49, guidelines are mostly generated for those who face physical exertion or extreme environmental conditions50. Implicitly, the message spread is that there is no need to pay attention to water intake except for those conditions.
On the contrary, a large and growing body of evidence suggests that maintaining an optimal water intake which avoids urine supersaturation and reduces excessive arginine vasopressin (AVP) secretion may be greatly beneficial for the kidney and reduce metabolic risk47. If we consider the astonishing increase in incidence of these diseases in the general population, this should be regarded as a form of primary prevention for public health. Ultimately, this optimal intake is reflected by the cut-off value of 500 mOsm/kg for 24 h UOsm51–53. Initially, this target was based on retrospective analyses of existing clinical data. Currently, evidence deriving from several randomized control trials have showed that reaching such a target for UOsm can reduce circulating copeptin, as a proxy for AVP, as well as improve metabolic markers and reduce UTI incidence3,16,22.
In this investigation, we showed that a ML algorithm which integrates clinical features in combination with an optimization algorithm can accurately predict personalized water intake to achieve a target UOsm of 500 mOsm/kg in healthy adults. More in detail, the algorithm holistically considers different variables spanning from anthropometric characteristics, biological, nutritional and beverage intake data. These variables may not be related to fluid-balance processes directly but comprehensively describe individuals from a physiological point of view. From there, it employs a data-driven unbiased approach to infer the main factors predictive of optimal water intake. To this instance, the algorithm identifies multiple functional pathways in respect to optimal water intake. As examples, individuals with low water intakes are associated with a higher UOsm which results in advice to increase plain water consumption. Oppositely, individuals with low UOsm are advised an amount of water which is lower compared to the reported data contained in the dataset. Therefore, when an individual shows high UOsm, the algorithm is essentially capable to advise an increase of plain water consumption, while when opposite scenario appears, a decrease in water consumption is proposed.
One of the first steps of our approach consisted in the ranking of the most important features related to urine osmolality. This revealed that the most important features were related to hydration physiology (i.e., urine volume, and the consumption of plain water and fluids in general). This shows that the ML approach is a suitable approach to model the fluids that come in and out of the human body. Urine volume and concentration are regulated by the same hormonal mechanisms and were shown to be highly correlated3,54,55. Given that the end goal of this study was to provide any human being with advice for water intake to be optimally hydrated, we had to consider the accessibility of the data that individuals could provide. The number of features was reduced to a minimum to allow for a minimalist need of information from the general population while maintaining and excellent output quality. As an example, outside of any clinical context, people would generally be able to provide anthropometric variables such as age, sex, weight, and height. Also, food and fluid intake data can be easily recollected. Oppositely, the volume of urine collected throughout a 24 h period may pose some challenges and therefore prevent usage from the general population. For this reason, the algorithms developed do not ask for such information to generate a prediction for optimal water intake.
Our investigation represents a first exploration on the application of ML techniques on fluid balance physiological processes. Nonetheless, the study here presented comes with several limitations. Currently, the overall weight in determining the prediction for water intake between the algorithm and the optimization process is undetermined. Future research should address this aspect to determine whether more effective methodologies in regards of the optimization algorithm could be implemented. However, it appears that with current optimization process the overall performance of the algorithm in providing a prediction for optimal intake remains adequate. For this initial investigation, the data used to generate predictions rely uniquely on a subset of healthy clinical trials’ participants. Athletes, or subject taking part in strenuous physical activity, pregnant and lactating women were excluded from these trials on purpose. Therefore, it should be regarded as a priority to integrate data from more diverse and vulnerable populations such as aging population56,57, pregnant and lactating women58,59, and people regularly drinking extremely low or high volumes of water, especially when their UOsm still shows reasonable values regardless of the expectations. The latter would allow to test the method at the two-ends of the physiological spectrum. Integrating additional extrinsic data on seasonality, ambient temperature, physical activity should be regarded as an additional next step to further personalize the advice on water intake. Additionally, while considering different beverages consumed by individuals, the current algorithm only modulates plain water intake in the prediction generated. While water consumption is associated with positive health outcomes, the same cannot be currently supported for other beverages. Therefore, future developments could integrate advice for different beverages in combination with plain water intake. Finally, a clinical validation of the proposed ML algorithm is warranted to validate the predicted amount water to achieve UOsm of 500 mOsm/kg with real world evidence.
An additional learning we would like to share is about the importance of blending high-quality, context-specific data deriving from clinical studies into ML. This may allow to fix the reliability issues often reported by healthcare recipients when using artificial intelligence derived applications in decision-making processes.
Conclusions
Employing personalized advice for optimal water intake may contribute to decrease disease development and progression and may also be valuable in rationally designing nutritional interventions in a variety of kidney and metabolic disorders. More broadly, accurate personalized water intake predictions in these scenarios may be of great practical value, as they will integrate nutritional modifications more extensively into the clinical decision-making scheme. Indeed, we contributed to the demonstration that artificial intelligence and ML adoption can be implemented for taking advantage of digital algorithmic evidence to improve healthcare in general population. Here, we present an application of ML as primary prevention providing personalized advice on daily water intake considering personal intrinsic and extrinsic variability to achieve an optimal target for UOsm of 500 mOsm/kg.
Supplementary Information
Abbreviations
- AIs
Adequate intakes
- ALE
Accumulated local effects
- AUC
Area under the curve
- AutoML
Automatic machine learning
- AVP
Arginine vasopressin
- BMI
Body mass index
- EFSA
European Food Safety Authority
- FDA
Federal Drug Administration
- GBM
Gradient boosting machines
- IOM
Institute of Medicine
- MAE
Mean absolute error
- MEC
Mobile Examination Center
- ML
Machine learning
- NHANES
National Health and Nutrition Examination Survey
- PDP
Partial dependence plots
- POsm
Plasma osmolality
- RF
Random forest
- SLSQP
Sequential quadratic programming
- TFI
Total fluid intake
- UOsm
Urine osmolality
- US
United States
- USG
Urine specific gravity
- UTI
Urinary tract infections
- WHO
World Health Organisation
- XGBoost
Extreme gradient boosting
Author contributions
A.D. designed research; A.D., T.V., J.Q., L.S. conducted research; A.D. and T.V. provided essential materials; A.D., T.V., J.Q., R.C., R.A., L.S. analyzed data or performed statistical analyses; A.D. and T.V. wrote paper; A.D., T.V. and L.S. had primary responsibility for final content. All authors: read, critically revised, and approved the final manuscript.
Funding
The study was funded by Danone Research.
Data availability
The source code and datasets generated and analyzed during the current study may be made available from the corresponding author with prior agreement of legal and compliance Danone Research offices on reasonable request.
Competing interests
AD was a full time Danone Research employee at the time of analysis. TV and JQ are full time Danone Research employees. The authors report no other competing interests in this work.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alberto Dolci and Tiphaine Vanhaecke.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-21869-y.
References
- 1.Saklayen MG. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018;20(2):12–12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sontrop JM, et al. Association between water intake, chronic kidney disease, and cardiovascular disease: A cross-sectional analysis of NHANES data. Am. J. Nephrol. 2013;37(5):434–442. doi: 10.1159/000350377. [DOI] [PubMed] [Google Scholar]
- 3.Brunkwall L, et al. High water intake and low urine osmolality are associated with favorable metabolic profile at a population level: Low vasopressin secretion as a possible explanation. Eur. J. Nutr. 2020;59:3715–3722. doi: 10.1007/s00394-020-02202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stookey JD, et al. Underhydration is associated with obesity, chronic diseases, and death within 3 to 6 years in the U.S. population aged 51–70 years. Nutrients. 2020;12(4):905. doi: 10.3390/nu12040905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horswill CA, Janas LM. Hydration and health. Am. J. Lifestyle Med. 2011;5(4):304–315. [Google Scholar]
- 6.Manz F. Hydration and disease. J. Am. Coll. Nutr. 2007;26(5 Suppl):535s–541s. doi: 10.1080/07315724.2007.10719655. [DOI] [PubMed] [Google Scholar]
- 7.Manz F, Wentz A. The importance of good hydration for the prevention of chronic diseases. Nutr. Rev. 2005;63(6 Pt 2):S2–S5. doi: 10.1111/j.1753-4887.2005.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 8.Tasevska I, et al. Increased levels of copeptin, a surrogate marker of arginine vasopressin, are associated with an increased risk of chronic kidney disease in a general population. Am. J. Nephrol. 2016;44(1):22–28. doi: 10.1159/000447522. [DOI] [PubMed] [Google Scholar]
- 9.Wang CJ, Grantham JJ, Wetmore JB. The medicinal use of water in renal disease. Kidney. Int. 2013;84(1):45–53. doi: 10.1038/ki.2013.23. [DOI] [PubMed] [Google Scholar]
- 10.Strippoli GF, et al. Fluid and nutrient intake and risk of chronic kidney disease. Nephrology (Carlton) 2011;16(3):326–334. doi: 10.1111/j.1440-1797.2010.01415.x. [DOI] [PubMed] [Google Scholar]
- 11.Curhan GC, et al. Dietary factors and the risk of incident kidney stones in younger women: Nurses' Health Study II. Arch. Intern. Med. 2004;164(8):885–891. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 12.Roussel R, et al. Low water intake and risk for new-onset hyperglycemia. Diabetes Care. 2011;34(12):2551–2554. doi: 10.2337/dc11-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson EC, et al. Reduced water intake deteriorates glucose regulation in patients with type 2 diabetes. Nutr. Res. (New York, NY) 2017;43:25–32. doi: 10.1016/j.nutres.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Borghi L, et al. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J. Urol. 1996;155(3):839–843. [PubMed] [Google Scholar]
- 15.Lotan Y, et al. Increased water intake as a prevention strategy for recurrent urolithiasis: Major impact of compliance on cost-effectiveness. J. Urol. 2012;12:10. doi: 10.1016/j.juro.2012.08.254. [DOI] [PubMed] [Google Scholar]
- 16.Hooton TM, Vecchio M, Iroz A, Tack I, Dornic Q, Seksek I, Lotan Y. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: A randomized clinical trial. JAMA Intern. Med. 2018;178:1509–1515. doi: 10.1001/jamainternmed.2018.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira-Pêgo C, et al. Total fluid intake and its determinants: Cross-sectional surveys among adults in 13 countries worldwide. Eur. J. Nutr. 2015;54(Suppl 2):35–43. doi: 10.1007/s00394-015-0943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guelinckx I, et al. Intake of water and beverages of children and adolescents in 13 countries. Eur. J. Nutr. 2015;54(Suppl 2):69–79. doi: 10.1007/s00394-015-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrier ET, et al. Twenty-four-hour urine osmolality as a physiological index of adequate water intake. Dis. Mark. 2015;2015:231063. doi: 10.1155/2015/231063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrier ET, et al. From state to process: Defining hydration. Obes. Facts. 2014;7(Suppl 2):6–12. doi: 10.1159/000360611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemetais G, et al. Effect of increased water intake on plasma copeptin in healthy adults. Eur. J. Nutr. 2018;57(5):1883–1890. doi: 10.1007/s00394-017-1471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enhörning S, et al. Water supplementation reduces copeptin and plasma glucose in adults with high copeptin: The H2O metabolism pilot study. J. Clin. Endocrinol. Metab. 2019;104(6):1917–1925. doi: 10.1210/jc.2018-02195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enhorning S, et al. Copeptin is an independent predictor of diabetic heart disease and death. Am. Heart. J. 2015;169(4):549–556. doi: 10.1016/j.ahj.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seal A, et al. Effectiveness of total water intake guidelines in maintaining lowered urine osmolality. FASEB J. 2018;32(1_supplement):6222. [Google Scholar]
- 25.Strange K. Cellular volume homeostasis. Adv. Physiol. Educ. 2004;28(1–4):155–159. doi: 10.1152/advan.00034.2004. [DOI] [PubMed] [Google Scholar]
- 26.van Ommen B, et al. Systems biology of personalized nutrition. Nutr. Rev. 2017;75(8):579–599. doi: 10.1093/nutrit/nux029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimamoto H, Komiya S. The turnover of body water as an indicator of health. J. Physiol. Anthropol. Appl. Hum. Sci. 2000;19(5):207–212. doi: 10.2114/jpa.19.207. [DOI] [PubMed] [Google Scholar]
- 28.Sawka MN, Montain SJ, Latzka WA. Hydration effects on thermoregulation and performance in the heat. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001;128(4):679–690. doi: 10.1016/s1095-6433(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 29.El-Sharkawy AM, Sahota O, Lobo DN. Acute and chronic effects of hydration status on health. Nutr. Rev. 2015;73(Suppl 2):97–109. doi: 10.1093/nutrit/nuv038. [DOI] [PubMed] [Google Scholar]
- 30.Popowski LA, et al. Blood and urinary measures of hydration status during progressive acute dehydration. Med. Sci. Sports Exerc. 2001;33(5):747–753. doi: 10.1097/00005768-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Genuer R, Poggi J-M, Tuleau-Malot C. Variable selection using Random Forests. Pattern Recogn. Lett. 2010;31(14):2225–2236. [Google Scholar]
- 32.Friedman JH. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001;29(5):1189–1232. [Google Scholar]
- 33.LeDell, E. & Poirier, S. H2O automl: Scalable automatic machine learning. In Proceedings of the AutoML Workshop at ICML. (2020).
- 34.Landry, M., Machine Learning with R and H2O. (H2O, 2016).
- 35.Chen, T. et al., Xgboost: Extreme Gradient Boosting. R package version 0.4-2, 2015. 1(4).
- 36.Chen, T. & Guestrin, C. XGBoost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 785–794 (Association for Computing Machinery, 2016).
- 37.Krause, J., Perer, A., & Ng, K. Interacting with predictions: Visual inspection of black-box machine learning models. In Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems. (2016).
- 38.Apley DW, Zhu J. Visualizing the effects of predictor variables in black box supervised learning models. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2020;82(4):1059–1086. [Google Scholar]
- 39.Conn AR, Gould NIM, Toint P. A globally convergent augmented lagrangian algorithm for optimization with general constraints and simple bounds. SIAM J. Numer. Anal. 1991;28(2):545–572. [Google Scholar]
- 40.Kraft, D. A Software Package for Sequential Quadratic Programming. (1988).
- 41.EFSA Scientific opinion on dietary reference values for water. EFSA J. 2010;8(3):1459–1506. [Google Scholar]
- 42.Manz F, Wentz A. 24-h hydration status: Parameters, epidemiology and recommendations. Eur. J. Clin. Nutr. 2003;57(Suppl 2):S10–S18. doi: 10.1038/sj.ejcn.1601896. [DOI] [PubMed] [Google Scholar]
- 43.Johnson EC, et al. Urinary markers of hydration during 3-day water restriction and graded rehydration. Eur. J. Nutr. 2019 doi: 10.1007/s00394-019-02065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raman A, et al. Water turnover in 458 American adults 40–79 year of age. Am. J. Physiol. Ren. Physiol. 2004;286(2):F394–F401. doi: 10.1152/ajprenal.00295.2003. [DOI] [PubMed] [Google Scholar]
- 45.Manz F, Wentz A, Sichert-Hellert W. The most essential nutrient: defining the adequate intake of water. J. Pediatr. 2002;141(4):587–592. doi: 10.1067/mpd.2002.128031. [DOI] [PubMed] [Google Scholar]
- 46.IOM . Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. National Academies Press; 2004. [Google Scholar]
- 47.Perrier ET, et al. Hydration for health hypothesis: A narrative review of supporting evidence. Eur. J. Nutr. 2020;60:1–14. doi: 10.1007/s00394-020-02296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noakes TD. Is drinking to thirst optimum? Ann. Nutr. Metab. 2010;57(Suppl 2):9–17. doi: 10.1159/000322697. [DOI] [PubMed] [Google Scholar]
- 49.Thornton SN. Thirst and hydration: Physiology and consequences of dysfunction. Physiol. Behav. 2010;100(1):15–21. doi: 10.1016/j.physbeh.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 50.Casa DJ, et al. National athletic trainers' association position statement: Fluid replacement for athletes. J. Athl. Train. 2000;35(2):212–224. [PMC free article] [PubMed] [Google Scholar]
- 51.Armstrong LE. Assessing hydration status: The elusive gold standard. J. Am. Coll. Nutr. 2007;26(5 Suppl):575S–584S. doi: 10.1080/07315724.2007.10719661. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong LE, et al. Interpreting common hydration biomarkers on the basis of solute and water excretion. Eur. J. Clin. Nutr. 2013;67(3):249–253. doi: 10.1038/ejcn.2012.214. [DOI] [PubMed] [Google Scholar]
- 53.Armstrong LE, et al. Evaluation of Uosm: Posm ratio as a hydration biomarker in free-living, healthy young women. Eur. J. Clin. Nutr. 2013;67(9):934–938. doi: 10.1038/ejcn.2013.79. [DOI] [PubMed] [Google Scholar]
- 54.Enhörning S, et al. Increasing water intake reduces high copeptin in healthy adults. FASEB J. 2018;32(1_supplement):597.3. [Google Scholar]
- 55.Francesconi RP, et al. Plasma hormonal responses at graded hypohydration levels during exercise-heat stress. J. Appl. Physiol. 1985;59(6):1855–1860. doi: 10.1152/jappl.1985.59.6.1855. [DOI] [PubMed] [Google Scholar]
- 56.Abdallah L, et al. Dehydration reduction in community-dwelling older adults: Perspectives of community health care providers. Res. Gerontol. Nurs. 2009;2(1):49–57. doi: 10.3928/19404921-20090101-01. [DOI] [PubMed] [Google Scholar]
- 57.Hooper L, et al. Water-loss dehydration and aging. Mech. Ageing Dev. 2014;136:50–58. doi: 10.1016/j.mad.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 58.Anderson SC, Grant JF. Pregnant women and alcohol: Implications for social work. Soc. Casework. 1984;65(1):3–10. [Google Scholar]
- 59.Bardosono S, et al. Pregnant and breastfeeding women: Drinking for two? Ann. Nutr. Metab. 2017;70(Suppl. 1):13–17. doi: 10.1159/000462998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The source code and datasets generated and analyzed during the current study may be made available from the corresponding author with prior agreement of legal and compliance Danone Research offices on reasonable request.