Abstract
Objective:
To evaluate associations between use of seven progestogens and incident acute venous thromboembolism (VTE) among women of reproductive age.
Methods:
This nested matched case-control study identified women aged 15–49 years old from January 1, 2010, through October 8, 2018, in the IBM® MarketScan® Databases, a nationwide sample of private insurance claims in the U.S. After exclusions, 21,405 cases with incident acute VTE identified by diagnosis codes were matched 1:5 by year of birth and index date through risk set sampling to 107,025 controls without prior VTE. From lowest to highest systemic dose based on a modified hierarchy, progestogens studied were levonorgestrel intrauterine device (LNG-IUD), oral norethindrone (NET), etonogestrel implant (Implant), oral progesterone (Prog), oral medroxyprogesterone acetate (MPA), oral norethindrone acetate (NETA), and depot medroxyprogesterone acetate (DMPA). Conditional logistic regression models adjusted for 16 VTE risk factors were used to estimate odds ratios (OR) and 99% confidence intervals (99% CI) for incident acute VTE associated with current progestogen use compared with non-use. The primary analysis treated each progestogen as a binary exposure. Dose, which varied for oral formulations, and chronicity were explored separately. Significance was set at P<.01 to allow for multiple comparisons.
Results:
Current use of higher dose progestogens was significantly associated with increased odds of VTE compared with non-use: NETA (adjusted OR, 3.00 [99% CI, 1.96–4.59]), DMPA (adjusted OR, 2.37 [99% CI, 1.95–2.88]), and MPA (adjusted OR, 1.98 [99% CI, 1.41–2.80]). Current use of other progestogens was not significantly different from non-use (LNG-IUD, Implant, and Prog) or had reduced odds of VTE (NET). Sensitivity analyses that assessed misclassification bias supported the primary findings.
Conclusion:
Among reproductive-aged women using one of seven progestogens, only use of norethindrone acetate and medroxyprogesterone acetate – considered higher dose progestogens – was significantly associated with increased odds of incident acute VTE. The roles of progestogen type, dose, and indication for use warrant further study.
Precis:
Higher-dose progestogens were associated with increased odds of venous thromboembolism, but lower dose progestogens demonstrated reduced odds of venous thromboembolism or null associations.
Introduction
Venous thromboembolism (VTE), defined as deep venous thrombosis, pulmonary embolism, or both, is associated with severe morbidity and mortality and is increasing in the U.S.1 For women with significant VTE risk factors in which estrogen use is contraindicated, progestogen-only medications (progestogens) have been recommended as alternative forms of contraception and treatments for benign menstrual disorders.2,3 However, the assumption that progestogens are safer may not hold for all types and doses.
Twenty-two studies have assessed the association between progestogen use and VTE, but most were judged to be of poor to fair quality and limited by small sample sizes.4–12 In their systematic review, Tepper et al. described a potential dose-response relationship based on a hierarchy by Bergendal et al. that reflected systemic effects (e.g., ovulation inhibition).4,13 The available evidence suggests that use of the very low dose levonorgestrel intrauterine device (LNG-IUD), low dose norethindrone (NET), or the moderate dose etonogestrel subcutaneous contraceptive implant (Implant) does not increase VTE risk. However, a recent meta-analysis found that use of the high dose injectable contraceptive depot medroxyprogesterone acetate (DMPA) was associated with a 2.6-fold increase in odds of VTE.7,12,14,15 Non-contraceptive progestogens (e.g., oral norethindrone acetate [NETA], oral medroxyprogesterone acetate [MPA], and oral progesterone [Prog]), have also been associated with a 2.4- to 5.9-fold increase in odds of VTE in four small studies.10,16–18
In contrast to clinical guidelines that have questioned studies on DMPA use, some authors have urged caution when prescribing higher dose progestogens to individuals at increased VTE risk.2,4,10,16,19–25 This nested matched case-control study aimed to evaluate associations between current use of seven progestogens and incident acute VTE, compared with non-use of any progestogen.
Methods
The IBM® MarketScan® Commercial Claims and Encounters Databases contain routinely collected, de-identified data for individuals and their dependents with employer-based insurance and some non-group commercial health plans in the U.S.26 Databases include enrollment records, inpatient and outpatient adjudicated medical claims, and pharmacy claims for filled prescriptions.
Case-defining VTE diagnoses (DVT, PE, or both) were identified by International Classification of Diseases, Ninth Revision (ICD-9) or Tenth Revision (ICD-10) diagnosis codes from inpatient and outpatient claims (Appendix 1, available online at http://links.lww.com/AOG/C813). Cases were identified as women of reproductive age, defined as 15–49 years old, who were newly diagnosed with VTE from January 1, 2010 (when diagnosis codes first differentiated acute and chronic VTE) through October 8, 2018 (the most recently available data).9,11,27,28 The index date for cases was defined as the inpatient date of admission or outpatient date of service. Inpatient diagnoses adjudicated at the time of discharge have been shown to be more accurate than outpatient diagnoses.29 Outpatient diagnoses required corroboration with a new anticoagulation prescription within 42 days before or after the index date.7,30,31 Finally, continuous enrollment in medical and pharmacy benefit coverage was required from one year before the index date through 84 days after the index date, to ascertain conditions for exclusion.
Exclusions were applied to create a sample representative of cases with incident acute VTE who could have been exposed to progestogens at typical doses for contraception or treatment of benign menstrual disorders (e.g., endometriosis, abnormal uterine bleeding). We excluded cases with non-qualifying VTE diagnoses (e.g., chronic, pregnancy-related) or with any earlier anticoagulation prescription that could indicate recurrent VTE. Superficial venous thrombosis was evaluated as a covariate rather than an exclusion criterion.2,19 We then excluded women diagnosed with conditions that can be treated with ultra-high dose progestogens (e.g., breast cancer, ductal carcinoma in situ, endometrial cancer, endometrial intraepithelial neoplasia or hyperplasia, and cachexia or acquired immune deficiency syndrome) or who had a megestrol acetate prescription within one year before the index date. Prior studies have already demonstrated increased odds of VTE and mortality in these settings.16,32,33 Cases who were determined to be pregnant or postpartum based on claims within 42–84 days before or after the index date were then excluded to allow for an adequate washout period and for new progestogen exposure (Appendix 1, available online at http://links.lww.com/AOG/C813). Similarly, we excluded cases with exposure within 84 days before the index date to a medication containing estrogen.
Controls were initially individually matched 50:1 to cases by risk set sampling with replacement based on year of birth and index date. The index date for controls was set to the index date of the matched case so that the surveillance periods overlapped exactly within each stratum. Additional confounders were incorporated as covariates instead of matching criteria as recommended by “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE).34 Propensity score matching may have reduced bias from differences in measurable VTE risk factors between cases and controls. However, it would have prevented use of a single study population for multiple progestogen exposures and reduced statistical efficiency without eliminating residual confounding. Controls could not have any VTE diagnosis or anticoagulation prescriptions in the available records prior to the index date. This initial larger pool ensured adequate eligible controls remained after exclusions that were time dependent. After applying enrollment and exclusion criteria, excess controls for each case were randomly removed to reach a 5:1 ratio. Cases with inadequate matched controls were excluded from the sample.
Exposures to four oral progestogens (NET, Prog, MPA, and NETA) were defined by filled outpatient prescriptions as proxies for actual use as prescribed. Exposures to three non-oral progestogens (LNG-IUD, Implant, and DMPA) were defined by pharmacy and medical claims (Appendix 1, available online at http://links.lww.com/AOG/C813). Claims data did not allow for direct ascertainment of indication for progestogen use or for identification of all contraindications for alternatives. Some progestogens were more likely used for contraception only (NET and Implant), treatment only (Prog, MPA, and NETA), or both (LNG-IUD and DMPA). Definitions of exposure characteristics (Table 1) were generated for additional analyses of recency, dose, and chronicity. Categories reflected common prescribing practices, assumed that progestogen use nearest the index date was most important, and aligned with prior studies.14,28,30,35 Users with more than one current or recent progestogen exposure were classified as having mixed use. The primary progestogen for analysis was selected as that with the highest systemic dose using a dose hierarchy by Bergendal et al. modified to incorporate non-contraceptive progestogens (from lowest to highest: LNG-IUD, NET, Implant, Prog, MPA, NETA, DMPA).13,36,37 The hierarchy did not account for potential pharmacological differences in oral and non-oral administration or for dosing patterns (e.g., cyclic, continuous). Current and recent users of more than two progestogens were excluded from analyses as outliers.
Table 1.
Exposure Characteristics
| Category | Term | Definition |
|---|---|---|
| Exposure Windows * | Current Use | Exposure within 28 days before the index date (primary analysis) |
| Recent Use | Exposure within 84 days before the index date | |
| Prior Use | Exposure within 85 through 365 days before the index date | |
| Non-current Use | Cannot have any progestogen exposure within 28 days before the index date (reference for primary analysis) | |
| Dose | Weighted Average Daily Dose | The cumulative prescribed dose within recent use averaged over the interval from start of exposure until the index date (maximum of 84 days) |
| Low Category | Weighted average daily dose less than half the standard daily dose of each oral progestogen | |
| Standard Category | Weighted average daily dose between half to less than double the standard daily dose of each oral progestogen | |
| High Category | Weighted average daily dose greater than or equal to double the standard daily dose of each oral progestogen | |
| Chronicity | New Users | All exposure occurs within no more than 84 days before the index date (i.e., no prior use) and meets current use criteria |
| Chronic Users | Exposure meets both prior use and current use criteria | |
| Former Users | Exposure meets prior use but not current use criteria | |
| Non-users | Cannot have any progestogen exposure that meets criteria for current use or prior use (reference only for chronicity analysis) |
Current, recent, and prior use definitions are not affected by progestogen exposure or absence of exposure outside of its time window (e.g., exposures may continue beyond the index date).
Covariates were identified using diagnosis and procedure codes from inpatient and outpatient claims during the one year prior to the index date (Appendix 1, available online at http://links.lww.com/AOG/C813). We included 16 VTE risk factors as pre-specified confounders based on prior literature: obesity (body mass index ≥30 mg/kg2), history of smoking, atherosclerotic cardiovascular disease, hypertension, congestive heart failure, chronic kidney disease, rheumatoid arthritis, systemic lupus erythematous, inflammatory bowel disease, cancer (excluding non-melanoma skin cancer, and breast and uterine cancers based on study exclusion criteria), hemiplegia or paraplegia, hypercoagulable state, superficial venous thrombosis, varicose veins, history of surgery or lower extremity fracture within 180 days before the index date, and history of hospitalization within 180 days before the index date.1–3,9,11,27,38
Descriptive statistics were calculated for case and control characteristics. Qualifying VTE diagnoses, pregnancy exclusions, and progestogen use were assessed by year to evaluate secular trends and similar ascertainment between ICD-9 and ICD-10 codes. For each of the seven progestogens of interest, the primary outcome was the odds of incident acute VTE comparing current users with non-current users of any progestogen (Table 1).28,30,35 Crude odds ratios were calculated by conditional logistic regression, adjusted for year of birth and index date by study design. The final multivariable model for each progestogen additionally adjusted for all 16 VTE risk factors previously described. Other studies have variably adjusted for VTE risk factors even when individual covariates fail to significantly modify the measure of association.8,9,14,16,28 To aid in clinical application of the findings, estimates of the absolute risks of VTE were calculated as excess VTEs per 10,000 exposed women at both ends of the reproductive age range for all associations with an adjusted odds ratio of at least 2.0.1,39,40
Secondary analyses evaluated the effect of varying dose and chronicity within exposure to individual progestogens (Table 1), adjusted for the same covariates as the primary analysis. For each oral progestogen, weighted average daily dose was assessed as a categorical and continuous variable in conditional logistic regression models. The categorical model determined odds of VTE for low, standard, and high dose users compared with non-current users of any progestogen (Table 1). The continuous model determined the change in odds of VTE for increasing weighted average daily dose. Units were set at half of the most commonly prescribed tablet of that progestogen per day. Users with more than one recent progestogen exposure and prescriptions with missing dose information or with quantities less than one were excluded from this analysis. Chronicity was assessed for all progestogens by operationalizing exposure as a four-level categorical variable combining current use and prior use variables (Table 1) in conditional logistic regression models. For each progestogen, comparisons were made between new, chronic, former, and non-users.
Finally, we planned two sensitivity analyses. To assess for misclassification bias from inclusion of false positive VTE diagnoses, the primary analysis was repeated after excluding inpatient cases without a corroborating anticoagulation prescription. The primary analysis was also repeated with an extended exposure time window comparing recent progestogen use to non-recent use to assess for misclassification from delayed use and to allow for comparisons to prior literature.14
For all models, significance was set at P<.01 to allow for multiple comparisons in the primary analysis. Adjusted odds ratios were calculated with 99% confidence intervals. We considered secondary analyses to be hypothesis-generating. A pre-specified sample size calculation was performed with 90% power to detect an association with an odds ratio of at least 2.0 at a frequency of current use of each progestogen of at least 0.1% among controls. Reliable estimates of frequencies for non-contraceptive progestogens were not available. The entire available sample was used since it was less than the target of 24,252 cases. All analyses were performed using SAS version 9.4. This study using de-identified data was exempted from review by the University of Chicago Biological Sciences Division Institutional Review Board.
Results
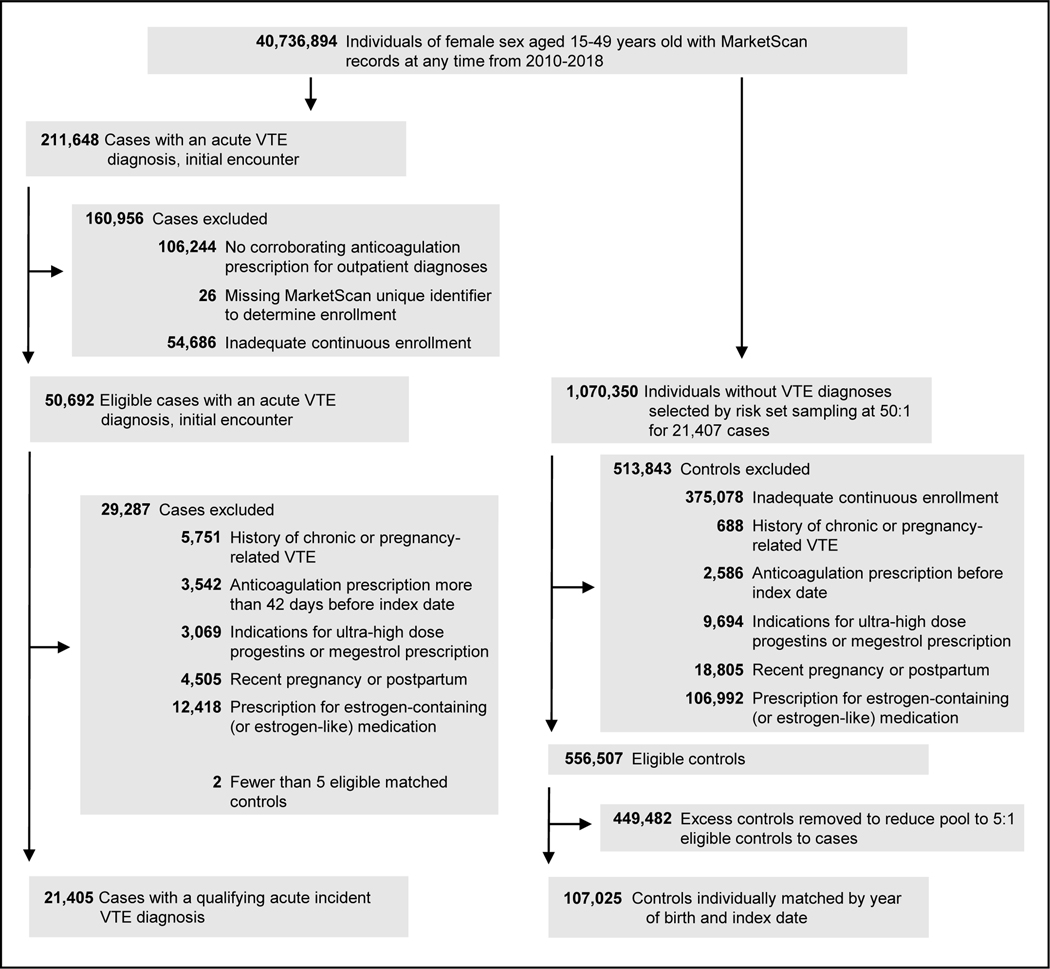
Out of 50,692 individuals with a qualifying VTE diagnosis and adequate continuous enrollment, 21,405 cases matched 1:5 by year of birth and index date to 107,025 controls remained after exclusions (Figure 1). Characteristics of all qualifying acute VTE diagnoses by type and source are provided in Appendix 2, available online at http://links.lww.com/AOG/C813. We did not see abrupt changes in ascertainment of VTE or pregnancy diagnoses at the transition from ICD-9 to ICD-10 codes (Appendix 3, available online at http://links.lww.com/AOG/C813). Characteristics of cases and controls are provided in Table 2. Sixty percent of the study population were aged 40–49 years old at the index date. Cases and controls were significantly different in all other covariates with 66.3% of cases having one or more VTE risk factors compared with 25.4% of controls (P<.001). Frequency of current use of any progestogen was 4.1% and 2.4% for cases and controls, respectively (P<.001), when combining single and mixed use. While current use of LNG-IUD, NET, and Implant increased during the study period, current use of other progestogens remained stable (Appendix 3, available online at http://links.lww.com/AOG/C813).
Figure 1.
Case and matched control selection flow diagram. VTE, venous thromboembolism.
Table 2.
Characteristics of Cases with Acute Incident Venous Thromboembolism and Matched General Population Controls.
| Characteristics | Cases (n = 21,405) |
Controls (n = 107,025) |
|---|---|---|
| Age, median (IQR), y | 42 (35 – 46) | 42 (35 – 46) |
| Age group, No. (%) | ||
| 15 – 24 years old | 1,806 (8.4) | 9,049 (8.5) |
| 25 – 34 years old | 3,118 (14.6) | 15,555 (14.5) |
| 35 – 39 years old | 3,647 (17.0) | 18,277 (17.1) |
| 40 – 44 years old | 5,692 (26.6) | 28,436 (26.6) |
| 45 – 49 years old | 7,142 (33.4) | 35,708 (33.4) |
| Comorbidities, No. (%) | ||
| Obesity (BMI ≥ 30 kg/m2) | 4,451 (20.8) | 7,882 (7.4) |
| History of smoking | 1,789 (8.4) | 3,059 (2.9) |
| Atherosclerotic cardiovascular disease | 1,772 (8.3) | 1,496 (1.4) |
| Hypertension | 5,740 (26.8) | 13,068 (12.2) |
| Congestive heart failure | 729 (3.4) | 333 (0.3) |
| Chronic kidney disease | 806 (3.8) | 432 (0.4) |
| Rheumatoid arthritis | 363 (1.7) | 706 (0.7) |
| Systemic lupus erythematous | 641 (3.0) | 655 (0.6) |
| Inflammatory bowel disease | 464 (2.2) | 574 (0.5) |
| Cancera (with exclusions) | 1,007 (4.7) | 406 (0.4) |
| Hemiplegia or paraplegia | 337 (1.6) | 143 (0.1) |
| Hypercoagulable state | 332 (1.6) | 101 (0.1) |
| Superficial venous thrombosis | 359 (1.7) | 57 (0.1) |
| Varicose veins | 610 (2.9) | 807 (0.8) |
| Exposures | ||
| History of surgery or lower extremity fracture (within 180 days before index) | 6,919 (32.3) | 6,056 (5.7) |
| History of hospitalization, excluding index encounter (within 180 days before index) | 4,894 (22.9) | 2,313 (2.2) |
| Current user of a single progestogen* | 836 (3.9) | 2,533 (2.4) |
| Current user of multiple progestogens* | 15 (0.1) | 19 (0.02) |
| Recent user of a single progestogen† | 990 (4.6) | 2,983 (2.8) |
| Recent user of multiple progestogens† | 27 (0.1) | 34 (0.03) |
IQR, interquartile range; BMI, Body Mass Index.
Current use is defined as exposure that occurs within 28 days before the index date.
Recent use is defined as exposure that occurs within 84 days before the index date.
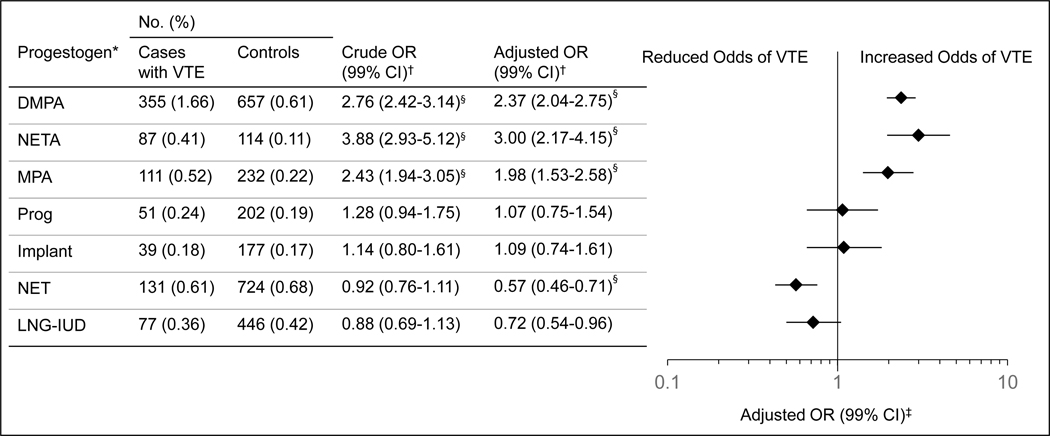
Odds of VTE associated with current use of each progestogen compared with non-current use of any progestogen are provided in Figure 2. Use of the higher dose progestogens demonstrated significantly increased adjusted odds of VTE (MPA, adjusted OR, 1.98 [99% CI,1.41–2.80]; NETA, adjusted OR, 3.00 [99% CI, 1.96–4.59]; DMPA, adjusted OR, 2.37 [99% CI, 1.95–2.88]), whereas low dose NET use was associated with significantly reduced adjusted odds of VTE (adjusted OR, 0.57 [99% CI, 0.43–0.76]). Use of other progestogens was not significantly different from non-use (LNG-IUD, adjusted OR, 0.72 [99% CI, 0.50–1.05]; Implant, adjusted OR, 1.09 [99% CI, 0.66–1.82]; Prog, adjusted OR, 1.07 [99% CI, 0.66–1.73]). All 16 VTE risk factors included in the multivariable models were independently associated with VTE. The conditional logistic regression model for NETA is provided as a representative example (Appendix 2, available online at http://links.lww.com/AOG/C813). Odds ratios for covariates included for adjustment differed by less than 10% between all models.
Figure 2.
Current use of progestogens and odds of venous thromboembolism (VTE). *Exposures are listed using a progestogen dose hierarchy by Bergendal et al, modified to incorporate noncontraceptive progestogens (lowest to highest: levonorgestrel intrauterine device [LNG-IUD], oral norethindrone [NET], etonogestrel implant [implant], oral progesterone [prog], oral medroxyprogesterone acetate [MPA], oral norethindrone acetate [NETA], DMPA, depot medroxyprogesterone acetate [DMPA]). †Referent group for each conditional logistic regression model is noncurrent users of any progestogen (ie, no progestogen use within 28 days before index date), n=125,027 (cases: n=20,554; controls: n=104,473). The crude model was adjusted for year of birth and index date by study design. The fully adjusted model additionally included 16 VTE risk factors: obesity (body mass index >30 mg/kg2), history of smoking, atherosclerotic cardiovascular disease, hypertension, congestive heart failure, chronic kidney disease, rheumatoid arthritis, systemic lupus erythematous, inflammatory bowel disease, cancer (with exclusions), hemiplegia or paraplegia, hypercoagulable state, superficial venous thrombosis, varicose veins, history of surgery or lower extremity fracture within 180 days before index date, and history of hospitalization within 180 days of index date. ‡P<.01. §P<.001. OR, odds ratio.
In estimates of absolute risk (99% CI) for women aged 20–24 years old, current use of NETA was associated with 7 (3–13) excess VTEs per 10,000 exposed women, and current use of DMPA was associated with 5 (3–7) excess VTEs per 10,000 exposed women. For women aged 45–49 years old with a higher baseline VTE incidence, current use of NETA was associated with 12 (6–22) excess VTEs per 10,000 exposed women, and current use of DMPA was associated with 8 (6–11) excess VTEs per 10,000 exposed women.
As defined in Table 1, most oral progestogen users were exposed to low or standard weighted average daily dose (median, interquartile range): 97% of NET users (0.34 mg/day, 0.28–0.35), 83% of Prog users (94.1 mg/day, 53.3–168.6), 95% of MPA users (3.45 mg/day, 1.71–7.63), and 86% of NETA users (4.68 mg/day, 2.51–6.33). In models where progestogen exposure was represented by weighted average daily dose as a continuous variable (Appendix 2, available online at http://links.lww.com/AOG/C813), increasing NETA and MPA doses were directly proportional to adjusted odds of VTE: +22% (adjusted OR, 1.22 [99% CI, 1.05–1.41]) for each 2.5 mg/day increase in NETA and +19% (adjusted OR, 1.19 [99% CI, 1.05–1.34]) for each 5 mg/day increase in MPA. Increasing NET dose was inversely proportional to adjusted odds of VTE. We did not find an association between increasing Prog dose and VTE. Models of weighted average daily dose as a categorical variable (Table 3) were limited by small sample sizes but reflected the results of the continuous model. Comparisons of chronicity by a 4-level categorical variable broadly reflected the associations in the primary analysis (Appendix 2, available online at http://links.lww.com/AOG/C813).
Table 3.
Oral Progestogen Exposure (Categorical) Modeled by Weighted Average Daily Dose.
| Progestogen | Dose Category | Recent Users* | Odds of VTE with Recent Progestogen Use Compared with Non-Recent Use of Any Progestogen | |
|---|---|---|---|---|
| No. (%) | Crude OR (99% CI)† | Adjusted OR (99% CI)‡ | ||
| NETA | Non-user | 124,392 (99.80) | Reference | Reference |
| Low (< 2.5 mg/d) | 58 (0.05) | 2.68 (1.32–5.47)‖ | 2.27 (1.04–4.97)§ | |
| Standard (2.5 to < 10 mg/d) | 152 (0.12) | 3.71 (2.43–5.67)‖ | 2.78 (1.69–4.57)‖ | |
| High (≥ 10 mg/d) | 34 (0.03) | 4.00 (1.64–9.74)‖ | 2.76 (0.98–7.81) | |
| MPA | Non-user | 124,392 (99.57) | Reference | Reference |
| Low (< 5 mg/d) | 342 (0.27) | 1.64 (1.18–2.27)‖ | 1.37 (0.95–1.98) | |
| Standard (5 to < 20 mg/d) | 169 (0.14) | 3.28 (2.18–4.92)‖ | 2.66 (1.67–4.26)‖ | |
| High (≥ 20mg/d) | 25 (0.02) | 4.00 (1.42–11.3)‖ | 2.27 (0.67–7.67) | |
| Prog | Non-user | 124,392 (99.72) | Reference | Reference |
| Low (< 50 mg/d) | 74 (0.06) | 1.52 (0.75–3.10) | 1.56 (0.71–3.46) | |
| Standard (50 to < 200 mg/d) | 217 (0.17) | 1.12 (0.71–1.76) | 0.92 (0.54–1.58) | |
| High (≥ 200 mg/d) | 60 (0.05) | 2.01 (0.96–4.21) | 1.54 (0.63–3.79) | |
| NET | Non-user | 124,392 (99.22) | Reference | Reference |
| Low (< 0.175 mg/d) | 107 (0.09) | 0.90 (0.45–1.80) | 0.50 (0.22–1.12) | |
| Standard (0.175 to < 0.7 mg/d) | 837 (0.67) | 0.94 (0.73–1.20) | 0.58 (0.44–0.77)‖ | |
| High (≥ 0.7 mg/d) | 22 (0.02) | 0.24 (0.02–3.40) | 0.31 (0.02–4.38) | |
NETA, oral norethindrone acetate; MPA, oral medroxyprogesterone acetate; Prog, oral progesterone; NET, oral norethindrone; VTE, venous thromboembolism; OR, odds ratio; CI, confidence interval; IQR, interquartile range.
Sample sizes were smaller than in binary progestogen exposure models due to additional exclusions for multiple progestogen use, missing dose information, or prescribed metric quantities less than 1 (NETA, n=124,636; MPA, n=124,928; Prog, n=124,743; NET, n=125,358).
Adjusted for year of birth and index date by study design.
Adjusted for year of birth and index date by study design and for 16 VTE risk factors: obesity, history of smoking, atherosclerotic cardiovascular disease, hypertension, congestive heart failure, chronic kidney disease, rheumatoid arthritis, systemic lupus erythematous, inflammatory bowel disease, cancer (with exclusions), hemiplegia or paraplegia, hypercoagulable state, superficial venous thrombosis, varicose veins, history of surgery or lower extremity fracture within 180 days before index date, and history of hospitalization within 180 days of index date.
P<.01
P<.001
In the sensitivity analysis excluding potentially false positive VTE diagnoses, 4,374 (35.6%) of 12,276 inpatient cases did not have a corroborating new anticoagulation prescription (Appendix 2, available online at http://links.lww.com/AOG/C813). Only the adjusted odds ratios for current use of NETA and DMPA were changed by more than 10% and both increased (adjusted OR, 3.34 [99% CI, 2.08–5.35], and adjusted OR, 2.66 [99% CI, 2.15–3.31], respectively). In the sensitivity analysis using the extended exposure window of recent use (Appendix 2, available online at http://links.lww.com/AOG/C813), all adjusted odds ratios decreased, but only the estimate for NETA decreased by more than 10% (adjusted OR, 2.65 [99% CI, 1.82–3.87]).
Discussion
We found that the association of current use of progestogens and VTE was dependent on the type and dose of progestogen. Use of higher dose progestogens (MPA, NETA, and DMPA) was significantly associated with increased odds of VTE compared with non-use, whereas use of lower dose progestogens (LNG-IUD, NET, Implant, and Prog) was not significantly different from non-use or had reduced odds of VTE. The importance of progestogen type and dose is supported by the associations for LNG-IUD and DMPA at the extremes of systemic dose because both can be used for contraception and to treat menstrual disorders.19,20 This study provides the first adequate assessments for use of the contraceptive Implant and non-contraceptive progestogens (Prog, MPA, and NETA). To do so, it used a larger sample size than the sum of all relevant studies identified in recent systematic reivews.4,6
Compared with those studies, we performed the first exploration of varying progestogen dose and chronicity.4,6 Modeling oral progestogen exposure by weighted average daily dose for NETA and MPA suggested a dose-response relationship with VTE, though the categorical dose model was limited by wide confidence intervals. In the chronicity model, we expected new users to demonstrate a stronger association with VTE than chronic users due to depletion of susceptibles, but these groups were only significant different for NET.41 The effect size was as expected for NETA but much smaller for DMPA and MPA. We also expected associations with VTE would not persist after progestogen discontinuation. Former users and non-users were not significantly different for NETA and MPA, but they were significantly different for DMPA (possibly due to its long duration) and NET. Future research could be powered to evaluate these associations and incorporate dosing pattern.
Progestogens as a class have not been associated with acute prothrombotic changes, but effects may vary by type.33,42–45 NET and NETA are uniquely metabolized to ethinyl estradiol at 0.2–1% of the orally administered dose.46–48 Approved dosing for NETA at 5–15 mg/day approximates at least 10–30 μg/day of oral ethinyl estradiol, typical contraceptive doses. Medroxyprogesterone acetate (MPA and DMPA) upregulates thrombin receptor expression through its potent glucocorticoid activity.49 Clinical studies have found dose-dependent increases in VTE risk associated with glucocorticoid use.50,51 Progesterone (Prog) and etonogestrel (Implant) also potentiate thrombin-induced procoagulant activity but have much lower affinity and activity at the glucocorticoid receptor.37,52,53 Additional research is needed on progestogen-mediated prothrombotic effects at relevant doses and with novel types (e.g., dienogest).
This study has several limitations. First, 35.6% of inpatient VTE diagnoses did not have a corroborating anticoagulation prescription, similar to other studies.9,11,28,29 The sensitivity analysis confirmed that such misclassification bias shifted the associations towards unity, supporting the primary findings. Second, exposure to oral progestogens was determined by filled prescriptions without confirmation of actual use. Third, though using a nationwide sample with inpatient and outpatient cases improved generalizability, associations in this study may not apply to individuals without private insurance or outside of the U.S. Fourth, the data source did not contain objective clinical data (e.g., weight, family history), and under-ascertainment of covariates cannot be differentiated from absence of a condition. However, obesity and smoking status have not demonstrated large confounding effects in the association of hormone use and VTE.9,14,28 Fifth, these data were not collected for research purposes and were subject to misclassification, such as through conversion of validated ICD-9 codes to ICD-10 codes.
Finally, these associations may have been confounded by indication due to contraindications for alternatives (i.e., estrogen) or conditions being treated.4,19 Because progestogens are used by women with VTE risk factors, the associations for MPA, NETA, and DMPA may remain inflated after adjustment for measured confounders. Future studies on individual progestogens could construct more balanced populations using propensity score matching. This study design also did not permit direct comparisons of VTE risk with users of combined hormonal contraception because of differential bias in those populations (e.g., selection bias, healthy user bias). Our findings for LNG-IUD, NET, and DMPA aligned closely with prior studies that controlled for indications for use and other confounders not available in the data source, adding to the external validity of the study.7,11,14,15,54 We also assumed that benign conditions treated by progestogens were not causally linked to VTE. Menstrual disorders were not found to have confounding effects in one of the largest studies on hormone use and VTE in women of reproductive age.9 Acknowledging that residual confounding cannot be excluded, our study design benefited from a large sample size, a well-suited data source, rigorous classification, and sensitivity analyses of bias.
While a dose-response relationship between progestogens and VTE remains uncertain, we recommend that clinicians consider progestogen type and dose to balance benefits, risks, and alternatives, which differ for contraceptive and non-contraceptive indications.
Supplementary Material
Acknowledgements
This project was supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) through Grant Number 5UL1TR002389-02 that funds the Institute for Translational Medicine (ITM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial Disclosure
Kenneth S. Cohen received travel reimbursement from the Japan Cancer Society for an awards ceremony in 2019. He received payment from the American Medical Forum for independent CME talks in 2019 and honoraria for streaming lectures in 2020. He also received honoraria from Bioascend for a lecture at the International Ultmann Chicago Lymphoma Symposium 2022.
Each author has confirmed compliance with the journal’s requirements for authorship.
Source data and raw data of the sample used in this study are not publicly available in accordance with data-sharing limits by IBM MarketScan Research Databases but may be accessed under licensure with the company.
Footnotes
The other authors did not report any potential conflicts of interest.
References
- 1.Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464–474. doi: 10.1038/nrcardio.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtis KM. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(3):1–103. doi: 10.15585/mmwr.rr6503a1 [DOI] [PubMed] [Google Scholar]
- 3.Guideline FSRH (January 2019) Combined Hormonal Contraception (Revision due by January 2024). BMJ Sex Reprod Health. 2019;45:1–93. doi: 10.1136/bmjsrh-2018-CHC [DOI] [PubMed] [Google Scholar]
- 4.Tepper NK, Whiteman MK, Marchbanks PA, James AH, Curtis KM. Progestin-only contraception and thromboembolism: A systematic review. Contraception. 2016;94(6):678–700. doi: 10.1016/j.contraception.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantha S, Karp R, Raghavan V, Terrin N, Bauer KA, Zwicker JI. Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis. BMJ. 2012;345:e4944. doi: 10.1136/bmj.e4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glisic M, Shahzad S, Tsoli S, et al. Association between progestin-only contraceptive use and cardiometabolic outcomes: A systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25(10):1042–1052. doi: 10.1177/2047487318774847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tepper NK, Jeng G, Curtis KM, Boutot ME, Boulet SL, Whiteman MK. Venous Thromboembolism Among Women Initiating Depot Medroxyprogesterone Acetate Immediately Postpartum. Obstet Gynecol. 2019;133(3):533–540. doi: 10.1097/AOG.0000000000003135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floyd JL, Beasley AD, Swaim LS, Turrentine MA, Nijjar JB. Association of Immediate Postpartum Etonogestrel Implant Insertion and Venous Thromboembolism. Obstet Gynecol. 2020;135(6):1275–1280. doi: 10.1097/AOG.0000000000003760 [DOI] [PubMed] [Google Scholar]
- 9.Vinogradova Y, Coupland C, Hippisley-Cox J. Use of combined oral contraceptives and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2015;350:h2135. doi: 10.1136/bmj.h2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundström A, Seaman H, Kieler H, Alfredsson L. The risk of venous thromboembolism associated with the use of tranexamic acid and other drugs used to treat menorrhagia: a case–control study using the General Practice Research Database. BJOG. 2009;116(1):91–97. doi: 10.1111/j.1471-0528.2008.01926.x [DOI] [PubMed] [Google Scholar]
- 11.Lidegaard Ø, Nielsen LH, Skovlund CW, Løkkegaard E. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001–10. BMJ. 2012;344:e2990. doi: 10.1136/bmj.e2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien SH, Koch T, Vesely SK, Schwarz EB. Hormonal Contraception and Risk of Thromboembolism in Women With Diabetes. Diabetes Care. 2017;40(2):233–238. doi: 10.2337/dc16-1534 [DOI] [PubMed] [Google Scholar]
- 13.Bergendal A, Odlind V, Persson I, Kieler H. Limited knowledge on progestogen-only contraception and risk of venous thromboembolism. Acta Obstet Gynecol Scand. 2009;88(3):261–266. doi: 10.1080/00016340902730375 [DOI] [PubMed] [Google Scholar]
- 14.Bergendal A, Persson I, Odeberg J, et al. Association of Venous Thromboembolism With Hormonal Contraception and Thrombophilic Genotypes. Obstet Gynecol. 2014;124(3):600–609. doi: 10.1097/AOG.0000000000000411 [DOI] [PubMed] [Google Scholar]
- 15.van Hylckama Vlieg Astrid, Helmerhorst Frans M, Rosendaal Frits R. The Risk of Deep Venous Thrombosis Associated With Injectable Depot–Medroxyprogesterone Acetate Contraceptives or a Levonorgestrel Intrauterine Device. Arterioscler Thromb Vasc Biol. 2010;30(11):2297–2300. doi: 10.1161/ATVBAHA.110.211482 [DOI] [PubMed] [Google Scholar]
- 16.Barsoum MK, Heit JA, Ashrani AA, Leibson CL, Petterson TM, Bailey KR. Is progestin an independent risk factor for incident venous thromboembolism? A population-based case-control study. Thromb Res. 2010;126(5):373–378. doi: 10.1016/j.thromres.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poulter NR, Chang CL, Farley TM, Meirik O. Risk of cardiovascular diseases associated with oral progestagen preparations with therapeutic indications. Lancet Lond Engl. 1999;354(9190):1610–1610. doi: 10.1016/s0140-6736(99)03132-3 [DOI] [PubMed] [Google Scholar]
- 18.Vasilakis C, Jick H, del Mar Melero-Montes M. Risk of idiopathic venous thromboembolism in users of progestagens alone. Lancet Lond Engl. 1999;354(9190):1610–1611. doi: 10.1016/S0140-6736(99)04394-9 [DOI] [PubMed] [Google Scholar]
- 19.ACOG Practice Bulletin No. 206: Use of Hormonal Contraception in Women With Coexisting Medical Conditions. Obstet Gynecol. 2019;133(2):e128–e150. doi: 10.1097/AOG.0000000000003072 [DOI] [PubMed] [Google Scholar]
- 20.Bradley LD, Gueye NA. The medical management of abnormal uterine bleeding in reproductive-aged women. Am J Obstet Gynecol. 2016;214(1):31–44. doi: 10.1016/j.ajog.2015.07.044 [DOI] [PubMed] [Google Scholar]
- 21.Bonnington A, Autry AM. Abnormal Vaginal Bleeding in Women Desiring Contraception Who Are Taking Anticoagulation Therapy. Obstet Gynecol. 2018;131(4):632–634. doi: 10.1097/AOG.0000000000002518 [DOI] [PubMed] [Google Scholar]
- 22.Khialani D, Rosendaal F, Vlieg A van H. Hormonal Contraceptives and the Risk of Venous Thrombosis. Semin Thromb Hemost. 2020;46(08):865–871. doi: 10.1055/s-0040-1715793 [DOI] [PubMed] [Google Scholar]
- 23.Mansour D. Safer prescribing of therapeutic norethisterone for women at risk of venous thromboembolism. J Fam Plann Reprod Health Care. 2012;38(3):148–149. doi: 10.1136/jfprhc-2012-100345 [DOI] [PubMed] [Google Scholar]
- 24.Huvinen E, Holopainen E, Heikinheimo O. Norethisterone and its acetate – what’s so special about them? BMJ Sex Reprod Health. 2021;47(2):102–109. doi: 10.1136/bmjsrh-2020-200619 [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 5th ed. World Health Organization; 2015. https://apps.who.int/iris/handle/10665/181468 [PubMed] [Google Scholar]
- 26.Butler AM, Nickel KB, Overman RA, Brookhart MA. IBM MarketScan Research Databases. In: Sturkenboom M, Schink T, eds. Databases for Pharmacoepidemiological Research. Springer Series on Epidemiology and Public Health. Springer International Publishing; 2021:243–251. doi: 10.1007/978-3-030-51455-6_20 [DOI] [Google Scholar]
- 27.Heinemann LAJ, Lewis MA, Assmann A, Thiel C. Case-control studies on venous thromboembolism: bias due to design? A methodological study on venous thromboembolism and steroid hormone use. Contraception. 2002;65(3):207–214. doi: 10.1016/S0010-7824(01)00309–2 [DOI] [PubMed] [Google Scholar]
- 28.Lidegaard Ø, Nielsen LH, Skovlund CW, Skjeldestad FE, Løkkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001–9. BMJ. 2011;343:d6423. doi: 10.1136/bmj.d6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang MC, Fan D, Sung SH, et al. Validity of Using Inpatient and Outpatient Administrative Codes to Identify Acute Venous Thromboembolism: The CVRN VTE Study. Med Care. 2017;55(12):e137–e143. doi: 10.1097/MLR.0000000000000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to combined oral contraceptives and risk of venous thromboembolism: a protocol for nested case–control studies using the QResearch and the CPRD databases. BMJ Open. 2014;4(4):e004499. doi: 10.1136/bmjopen-2013-004499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okoroh EM, Boulet SL, George MG, Hooper WC. Assessing the intersection of cardiovascular disease, venous thromboembolism, and polycystic ovary syndrome. Thromb Res. 2015;136(6):1165–1168. doi: 10.1016/j.thromres.2015.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia VR, López-Briz E, Sanchis RC, Perales JLG, Bort-Marti S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;(3):CD004310. doi: 10.1002/14651858.CD004310.pub3 [DOI] [PMC free article] [PubMed]
- 33.Schindler AE. Differential effects of progestins on hemostasis. Maturitas. 2003;46(Suppl 1):31–37. doi: 10.1016/j.maturitas.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 34.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinemann LAJ, Assmann A, Dominh T, Garbe E. Oral progestogen-only contraceptives and cardiovascular risk: Results from the Transnational Study on Oral Contraceptives and the Health of Young Women. Eur J Contracept Reprod Health Care. 1999;4(2):67–73. doi: 10.3109/13625189909064007 [DOI] [PubMed] [Google Scholar]
- 36.Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Düsterberg B. Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide. Contraception. 2011;84(6):549–557. doi: 10.1016/j.contraception.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 37.Sitruk-Ware R. Pharmacological profile of progestins. Maturitas. 2004;47(4):277–283. doi: 10.1016/j.maturitas.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 38.Bergendal A, Bremme K, Hedenmalm K, et al. Risk factors for venous thromboembolism in pre-and postmenopausal women. Thromb Res. 2012;130(4):596–601. doi: 10.1016/j.thromres.2012.05.024 [DOI] [PubMed] [Google Scholar]
- 39.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ III. Trends in the Incidence of Deep Vein Thrombosis and Pulmonary Embolism: A 25-Year Population-Based Study. Arch Intern Med. 1998;158(6):585–593. doi: 10.1001/archinte.158.6.585 [DOI] [PubMed] [Google Scholar]
- 40.Bjerre LM, LeLorier J. Expressing the magnitude of adverse effects in case-control studies: “the number of patients needed to be treated for one additional patient to be harmed.” BMJ. 2000;320(7233):503–506. doi: 10.1136/bmj.320.7233.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renoux C, Dell’Aniello S, Brenner B, Suissa S. Bias from depletion of susceptibles: the example of hormone replacement therapy and the risk of venous thromboembolism. Pharmacoepidemiol Drug Saf. 2017;26(5):554–560. doi: 10.1002/pds.4197 [DOI] [PubMed] [Google Scholar]
- 42.Blanco-Molina MA, Lozano M, Cano A, Cristobal I, Pallardo LP, Lete I. Progestin-only contraception and venous thromboembolism. Thromb Res. 2012;129(5):e257–e262. doi: 10.1016/j.thromres.2012.02.042 [DOI] [PubMed] [Google Scholar]
- 43.Algra A(1), Kemmeren J m., Meijers J c. m., et al. Effect of second- and third-generation oral contraceptives on the protein C system in the absence or presence of the factor VLeiden mutation: A randomized trial. Blood. 2004;103(3):927–933. doi: 10.1182/blood-2003-04-1285 [DOI] [PubMed] [Google Scholar]
- 44.Melhado-Kimura V, Bizzacchi JMA, Quaino SKP, Montalvao S, Bahamondes L, Fernandes A. Effect of the injectable contraceptive depot-medroxyprogesterone acetate on coagulation parameters in new users. J Obstet Gynaecol Res. 2017;43(6):1054–1060. doi: 10.1111/jog.13306 [DOI] [PubMed] [Google Scholar]
- 45.Vieira CS, Ferriani RA, Garcia AA, et al. Use of the etonogestrel-releasing implant is associated with hypoactivation of the coagulation cascade. Hum Reprod. 2007;22(8):2196–2201. doi: 10.1093/humrep/dem153 [DOI] [PubMed] [Google Scholar]
- 46.Chu MC, Zhang X, Gentzschein E, Stanczyk FZ, Lobo RA. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 2007;92(6):2205–2207. doi: 10.1210/jc.2007-0044 [DOI] [PubMed] [Google Scholar]
- 47.Friedrich C, Berse M, Klein S, Rohde B, Höchel J. In Vivo Formation of Ethinylestradiol After Intramuscular Administration of Norethisterone Enantate. J Clin Pharmacol. 2018;58(6):781–789. doi: 10.1002/jcph.1079 [DOI] [PubMed] [Google Scholar]
- 48.Kuhnz W, Heuner A, Hümpel M, Seifert W, Michaelis K. In vivo conversion of norethisterone and norethisterone acetate to ethinyl etradiol in postmenopausal women. Contraception. 1997;56(6):379–385. [DOI] [PubMed] [Google Scholar]
- 49.Olaf Herkert, Herbert Kuhl, Jurgen Sandow, Rudi Busse, Schini-Kerth Valérie B. Sex Steroids Used in Hormonal Treatment Increase Vascular Procoagulant Activity by Inducing Thrombin Receptor (PAR-1) Expression. Circulation. 2001;104(23):2826–2831. doi: 10.1161/hc4801.099737 [DOI] [PubMed] [Google Scholar]
- 50.Johannesdottir SA, Horváth-Puhó E, Dekkers OM, et al. Use of Glucocorticoids and Risk of Venous Thromboembolism: A Nationwide Population-Based Case-Control Study. JAMA Intern Med. 2013;173(9):743. doi: 10.1001/jamainternmed.2013.122 [DOI] [PubMed] [Google Scholar]
- 51.Higgins PDR, Skup M, Mulani PM, Lin J, Chao J. Increased Risk of Venous Thromboembolic Events With Corticosteroid vs Biologic Therapy for Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2015;13(2):316–321. doi: 10.1016/j.cgh.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 52.Koubovec D, Ronacher K, Stubsrud E, Louw A, Hapgood JP. Synthetic progestins used in HRT have different glucocorticoid agonist properties. Mol Cell Endocrinol. 2005;242(1–2):23–32. doi: 10.1016/j.mce.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 53.Huijbregts RPH, Michel KG, Hel Z. Effect of progestins on immunity: medroxyprogesterone but not norethisterone or levonorgestrel suppresses the function of T cells and pDCs. Contraception. 2014;90(2):123–129. doi: 10.1016/j.contraception.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cardiovascular disease and use of oral and injectable progestogen-only contraceptives and combined injectable contraceptives: Results of an international, multicenter, case-control study. Contraception. 1998;57(5):315–324. doi: 10.1016/S0010-7824(98)00041-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.