Abstract
Pediatric thyroid carcinomas (TC) are rare and mainly approached based on data extrapolated from adults. We retrospectively reviewed 222 pediatric TCs (patient age ≤21 years). Lymph node (LN) disease volume at presentation was considered high if the largest positive LN measured ≥ 1 cm and/or more than 5 LNs were positive. High-grade follicular cell-derived TCs (HGFCTC) were defined by the presence of marked mitotic count and/or tumor necrosis and considered as high-risk histology along with papillary thyroid carcinomas (PTC) diffuse sclerosing variant (DSV). Disease free survival (DFS) was analyzed. LN involvement at presentation was significantly associated with male sex, larger tumor size, lymphatic invasion, positive surgical margins, and distant metastases at presentation. Five and ten-year DFS was 84% and 77% respectively. Only one patient with HGFCTC died of disease. Within PTC variants, PTC-DSV was associated with adverse histopathologic parameters and higher regional disease spread, unlike PTC tall cell variant (TCV) which did not portend worse behavior. The presence of necrosis conferred worse DFS (p=0.006) while increased mitotic activity did not. While the entire HGFCTC group did not correlate with outcome (p=0.071), HGFCTC with necrosis imparted worse DFS (p=0.006). When restricted to PTC-DSV and HGFCTC with necrosis, high-risk histologic classification emerged as an independent prognostic parameter of DFS (p=0.020). The excellent prognosis of pediatric TCs differs from that of adult TCs showing similar histologic features. While neither increased mitotic activity nor PTC-TCV histology predict adverse outcome, PTC-DSV and tumors with necrosis constitute high-risk histologic variants with an increased risk of protracted disease.
Keywords: Pediatric carcinomas, thyroid neoplasms, papillary thyroid carcinoma
Introduction:
Older age has consistently been demonstrated to be associated with lower rates of survival in patients with follicular cell-derived thyroid carcinomas (TCs).(1) The 55 year age mark serves as the cut-off for risk stratification in the American Joint Committee on Cancer (AJCC) staging system.(2) Whereas older age has been shown to be an independent prognostic parameter in thyroid neoplasms, there is no concrete data to support differential consideration of TCs that arise in children and young adults.(3) Previous studies have noted that pediatric TCs differ from adult carcinomas in multiple aspects.(4) Clinically, children and adolescent tend to have more advanced disease at presentation, with a higher rate of regional lymph node involvement and distant metastases than adult onset TC.(4, 5) Despite a presumably greater risk for disease recurrence, pediatric TCs are nonetheless characterized by an excellent prognosis and very low mortality rate.(4, 5) This combination-with its adverse prognostic parameters, but excellent survival prospects- has been termed paradoxical, and marks a break with the lessons learned from adult thyroid pathology.(4) It places children and young adolescents with TC at risk of potential complications from overtreatment, particularly that of secondary malignancy among patient treated with radiation.(6) As risk stratification models in thyroid pathology grow more precise and the field continues its path towards more conservative treatment planning, there is a growing need to look into pediatric TCs to evaluate them as a separate population to which data extrapolated from adult cohorts cannot be applied.(2) In this study, we report the clinicopathologic and prognostic features of 222 pediatric TCS in the aim of characterizing pediatric TCs and identifying which tumor parameters confer a higher risk of adverse events.
Materials and Methods:
Patient selection and IRB approval
A retrospective cohort of 222 cases of follicular cell-derived TCs diagnosed in patients aged 21 years or younger were selected from the archives of Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY, USA) from 1994 to 2018 and reviewed. The study was approved by the institutional review board of MSKCC.
Histopathologic review
Primary data extracted from pathology reports included the extent of surgery and lymph node dissection. Hematoxylin and eosin-stained slides from all cases were reviewed by two pathologists with interest in thyroid neoplasia (MS, RG). The specific histologic features documented included the lateralization of the tumor; tumor size; tumor encapsulation; tumor histologic subtype; extrathyroidal tumor extension and invaded perithyroidal structures (e.g. skeletal muscle); vascular invasion; lymphatic invasion; capsular invasion; mitotic activity per 10 high power fields (HPF)/400 x corresponding to 2.4 mm2; tumor necrosis; margin status; presence of lymph node metastases (number and greatest size of affected lymph nodes) and extranodal extension. Vascular invasion was defined as the presence of tumor thrombus attached to the vessel wall and hanging in the lumen, covered by endothelium or associated with fibrin. If the tumor is encapsulated, invasion of intratumoral or subcapsular vessels was not considered as vascular invasion.(7) Capsular and vascular invasion extent were defined as focal (1-3 foci) or extensive (≥4 foci). The TCs were classified according to the latest 2017 World Health Organization classification of endocrine tumors, except for high-grade follicular cell–derived thyroid carcinomas (HGFCTC) and cribriform morular thyroid carcinoma. The latter two entities are an emanation of the upcoming WHO classification of endocrine tumors (8). The new term HGFCTC encompasses poorly differentiated TC (PDTC) defined based on the Turin proposal, and differentiated high-grade TC (DHGTC) defined by tumors that are still differentiated i.e. retaining the cytoarchitectural features of well differentiated carcinoma such as papillae and papillary thyroid carcinoma (PTC) nuclei, but having ≥5 mitoses/2 mm2 and/or tumor necrosis (the Memorial Sloan-Kettering Cancer Center criteria).(8-10). A tumor was diagnosed as HGFCTC if the primary or metastatic tumor (i.e. tumor in the lymph nodes) displayed the above mentioned criteria and lacked anaplastic component.(11) The entity known as cribriform morular variant of PTC in the latest 2017 WHO classification is now renamed cribriform morular thyroid carcinoma in the upcoming WHO classification, and that new nomenclature is used in this study.(8) PTCs were subtyped as solid variants if ≧70% of the tumor harbored a solid, insular and/or trabecular growth pattern.(12) Amongst PTCs, classic and follicular variants were considered low-risk PTC subtypes. Solid variant PTC was excluded from this risk categorization for reasons detailed in the discussion. Amongst all included histologic subtypes, two different, tentative sets of criteria were used to define high-risk histology. The first “adult” criteria included all HGFCTC and PTC diffuse sclerosing variant (DSV) cases based on studies largely performed on adult patient populations.(11, 13) The second “pediatric” criteria included only HGFCTCs with tumor necrosis in addition to PTC-DSVs.
Patients with no histologic and/or clinical evidence of regional lymph nodes metastasis at presentation were staged as N0. The volume of lymph node disease at primary resection was considered as high if the largest positive lymph node measured ≥ 1 cm and/or more than 5 lymph nodes were positive.
Tumor size was divided into less than 4 cm and 4 cm or more based on the largest dimension of the dominant tumor nodule. Cases of PTC-DSV with multifocal tumor over the span of one or two lobes were considered as > 4 cm in size regardless of the dominant nodules’ measurements in order to better represent tumor volume.
Chart review
Patients’ charts were reviewed for age at diagnosis, sex, AJCC stage, gross extrathyroidal extension (ETE), radioactive iodine administration, and outcome. Structural tumor progression and/or disease recurrence, both local and metastatic, was established based on clinical and radiologic examination. Distant metastases at initial presentation were defined as metastases to distant sites identified prior to or within 2 months of the primary thyroid resection.
Statistical analysis
Statistical analysis was performed using SPSS software (IBM SPSS Statistics for Windows, Version 28.0. IBM Corp, Armonk, NY, USA). The risk of nodal metastases at presentation was compared using the chi-square test for categorical variables and the two-tailed Student’s t test for continuous variables. The primary end point of the study was disease-free survival (DFS), calculated from the time of thyroidectomy. Locoregional-recurrence-free survival (LRFS) and distant metastases-free survival (DMFS) were also calculated. Univariate survival analysis was performed using the Kaplan-Meier method and the log-rank test. Subsequent multivariate survival analysis was performed using the Cox proportional hazards model and the significant covariates identified on univariate analysis. P<0.05 was considered statistically significant.
Results:
Clinicopathologic characteristics (table 1, figures 1 and 2)
Table 1.
Clinico-pathologic characteristics of the cohort
| Characteristic | Median (Range) | n | % of total |
|---|---|---|---|
| Age at diagnosis in years, n=222 | 16.5 (2.7-21.8) | ||
| Follow-up in years, n=215 | 6.6 (0.2-29.6) | ||
| Sex, n=222 | |||
| Female | 172 | 77.5% | |
| Male | 50 | 22.5% | |
| Surgery type, n=219 | |||
| Total thyroidectomy | 174 | 78.4% | |
| Other (lobectomy/ isthmusectomy/ hemithyroidectomy/ subtotal thyroidectomy) | 45 | 20.3% | |
| Extent of lymph node surgery, n=222 | |||
| None | 30 | 13.5% | |
| Central compartment | 93 | 41.9% | |
| Unilateral neck | 65 | 29.3% | |
| Bilateral neck | 34 | 15.3% | |
| Regional lymph node status at presentation, n=222 | |||
| N0 | 79 | 35.6% | |
| N1 | 143 | 64.4% | |
| Stage at presentation, n=222 | |||
| I | 208 | 93.7% | |
| II | 14 | 6.3% | |
| Radioiodine (131I) treatment, n=220 | |||
| No | 82 | 36.9% | |
| Yes | 138 | 62.2% | |
| Tumor size in cm, n=222 | 1.9 (0.1-11.0) | ||
| ≤ 4 cm | 178 | 80.2% | |
| > 4 cm | 44 | 19.8% | |
| Encapsulation, n=221 | |||
| Partial/none | 171 | 77.4% | |
| Complete | 50 | 22.6% | |
| Capsular invasion*, n=50 | |||
| None | 23 | 46.0% | |
| Focal | 20 | 40.0% | |
| Extensive | 7 | 14.0% | |
| Mitoses (per 10 HPFs), n=222 | 0 (0-17) | ||
| <5 | 200 | 90.1% | |
| ≥5 | 22 | 9.9% | |
| Necrosis, n=222 | |||
| Absent | 195 | 87.8% | |
| Present | 27 | 12.2% | |
| Lymphatic invasion, n=222 | |||
| Absent | 181 | 81.5% | |
| Present | 41 | 18.5% | |
| Vascular invasion, n=222 | |||
| Absent | 161 | 72.5% | |
| Present | 61 | 27.5% | |
| Gross extrathyroidal extension, n=222 | |||
| Absent | 208 | 93.7% | |
| Present | 14 | 6.3% | |
| Surgical margins, n=218 | |||
| Negative | 174 | 79.8% | |
| Positive | 44 | 20.2% | |
| Histology, n=222 | |||
| Follicular carcinoma | 5 | 2.3% | |
| Hurthle cell carcinoma | 1 | 0.5% | |
| Cribriform morular thyroid carcinoma | 1 | 0.5% | |
| Papillary thyroid carcinoma | 177 | 79.7% | |
| Follicular variant | 12 | 5.4% | |
| Infiltrative | 2 | 0.9% | |
| Encapsulated | 10 | 4.5% | |
| Classical | 108 | 48.6% | |
| Solid variant | 3 | 1.4% | |
| Tall-cell variant | 32 | 14.4% | |
| Diffuse sclerosing variant | 22 | 9.9% | |
| High-grade follicular cell-derived thyroid carcinoma | 38 | 17.1% |
For completely encapsulated tumors
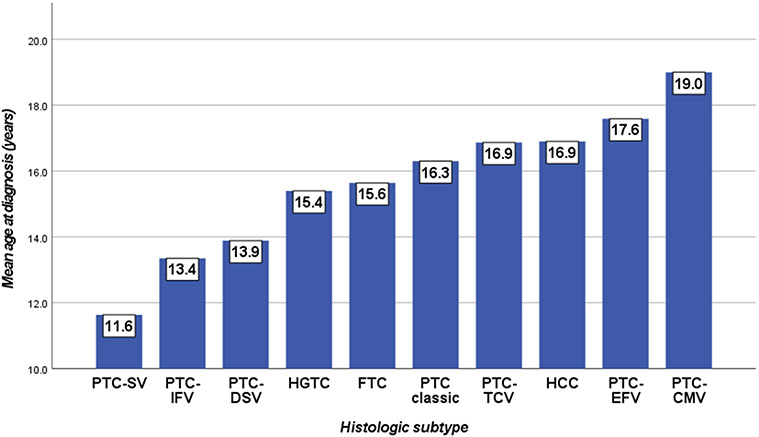
Figure 1. Age at presentation per histologic subtype.
Abbreviations: PTC= papillary thyroid carcinoma; SV= solid variant; IFV= infiltrative follicular variant; DSV= diffuse sclerosing variant; HGFCTC= High-grade follicular cell-derived thyroid carcinoma; FTC= follicular thyroid carcinoma; TCV= tall cell variant; HCC= Hurthle cell carcinoma; EFV= encapsulated follicular variant; CMTC= cribriform morular thyroid carcinoma.
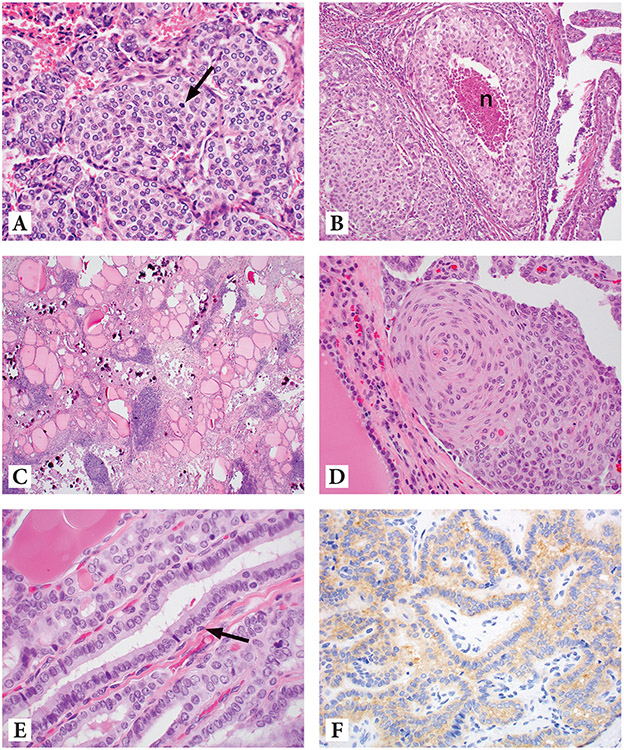
Figure 2.
Various histologic subtypes seen in pediatric thyroid carcinomas. Figure 2A: High-grade follicular cell-derived thyroid carcinoma showing increased mitotic activity (arrow) with no necrosis seen (H&E, 400X). Figure 2B: High-grade follicular cell-derived thyroid carcinoma showing foci (n) of tumor necrosis (H&E, 200X). 2C: Papillary thyroid carcinoma, diffuse sclerosing variant (H&E, 100X). 2D. Squamous metaplasia in papillary thyroid carcinoma, diffuse sclerosing variant (same case as 2C) (H&E, 200X). 2E. Papillary thyroid carcinoma, tall-cell variant with elongated follicle and tall cells (H&E, 600X). Figure 2F: Positive BRAFV600E immunostain in papillary thyroid carcinoma, tall-cell variant (same case as 2E) (400X)
The distribution of clinical and histopathologic variables is summarized in table 1. Age at presentation was significantly (p=0.05) associated with the histologic subtype (figure 1). Most patients (78.4%) underwent total thyroidectomy. PTCs constituted 79.7% of cases and HGFCTC 17.1%. According to the AJCC 8th edition, 14 patients presented as stage II as they had distant metastases at presentation.(14)
Nodal disease at presentation (table 2)
Table 2.
Lymph node status at presentation in relation to clinicopathologic features
| N0 (n=79) | N1 (n=143) | p values | ||||
|---|---|---|---|---|---|---|
| Age | Mean (range) | 16.3 (5.0-21.6) | 15.8 (2.7-21.8) | NS | ||
| N | % | N | % | |||
| Sex | Male | 11 | 22.0% | 39 | 78.0% | 0.023 |
| Female | 68 | 39.5% | 104 | 60.5% | ||
| Tumor Size | < or equal to 4 cm | 74 | 41.6% | 104 | 58.4% | <0.001 |
| > 4 cm | 5 | 11.4% | 39 | 88.6% | ||
| Histology | Follicular carcinoma | 5 | 100.0% | 0 | 0.0% | <0.001 |
| Hurthle cell carcinoma | 1 | 100.0% | 0 | 0.0% | ||
| Cribriform morular thyroid carcinoma | 0 | 0.0% | 1 | 100.0% | ||
| PTC, encapsulated follicular variant | 10 | 100.0% | 0 | 0.0% | ||
| PTC, infiltrative follicular variant | 1 | 50.0% | 1 | 50.0% | ||
| PTC, classical | 31 | 28.7% | 77 | 71.3% | ||
| PTC, solid variant | 0 | 0.0% | 3 | 100.0% | ||
| PTC, tall cell variant | 16 | 50.0% | 16 | 50.0% | ||
| PTC, diffuse sclerosing variant | 0 | 0.0% | 22 | 100.0% | ||
| HGFCTC | 15 | 39.5% | 23 | 60.5% | ||
| Mitosis | Less than 5 per 10 HPF | 65 | 32.5% | 135 | 67.5% | 0.004 |
| 5 or more per 10 HPF | 14 | 63.6% | 8 | 36.4% | ||
| Necrosis | Absent | 70 | 35.9% | 125 | 64.1% | NS |
| Present | 9 | 33.3% | 18 | 66.7% | ||
| Encapsulation | Completely encapsulated | 43 | 86.0% | 7 | 14.0% | <0.001 |
| Infiltrative | 36 | 21.1% | 135 | 78.9% | ||
| Capsular invasion * | None | 21 | 91.3% | 2 | 8.7% | NS |
| Focal | 17 | 85.0% | 3 | 15.0% | ||
| Extensive | 5 | 71.4% | 2 | 28.6% | ||
| Lymphatic invasion | Absent | 78 | 43.1% | 103 | 56.9% | <0.001 |
| Present | 1 | 2.4% | 40 | 97.6% | ||
| Vascular Invasion | Absent | 57 | 35.4% | 104 | 64.6% | NS |
| Present | 22 | 36.1% | 39 | 63.9% | ||
| Gross extrathyroidal extension | Absent | 77 | 37.0% | 131 | 63.0% | NS |
| Present | 2 | 14.3% | 12 | 85.7% | ||
| Margins status | Negative | 72 | 41.4% | 102 | 58.6% | <0.001 |
| Positive | 6 | 13.6% | 38 | 86.4% | ||
| Stage at presentation | Stage 1 | 79 | 38.0% | 129 | 62.0% | 0.004 |
| Stage 2 | 0 | 0.0% | 14 | 100.0% | ||
For completely encapsulated tumors
Continuous p values for age obtained using two-tailed Student’s t test.
Categorical p values obtained using chi square or Fisher’s exact test.
Abbreviations: PTC= Papillary thyroid carcinoma, HPF= High power fields, NS= Not significant, HGFCTC= High-grade follicular cell-derived thyroid carcinoma.
Of the 222 patients, 143 (64.4%) presented with metastatic carcinoma to regional lymph nodes, with 82 of 143 patients (57.3%) presenting with more than 5 positive regional lymph nodes and 96 of 140 patients (68.6%) presenting with at least one positive lymph node that is 1 cm in size or more. Overall, 106 of 141 (75.2%) patients with nodal metastases presented with high volume nodal disease.
Patients with lymph node metastases at presentation (N1) were significantly more likely to be male, have tumors larger than 4 cm, a low mitotic count (less than 5 mitoses per 10 HPF/400 x), lymphatic invasion, positive surgical margins, and distant metastases at presentation than N0 patients. Completely encapsulated tumors were strongly associated with N0 disease. There were no significant differences between N1 and N0 patients with respect to age, necrosis, capsular invasion, vascular invasion and gross ETE (p>0.05).
PTC-tall cell variant (TCV, table 3)
Table 3.
Features of PTC-TCV and PTC-DSV as compared with low-risk PTC histologic subtypes
| Follicular and classic PTC |
PTC-TCV | PTC-DSV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | P value | n | % | P value | ||
| Tumor size | ≤ 4 cm | 107 | 89.2% | 28 | 87.5% | NS | 6 | 27.3% | <0.001 |
| > 4 cm | 13 | 10.8% | 4 | 12.5% | 16 | 72.7% | |||
| Lymphatic invasion | Absent | 113 | 94.2% | 29 | 90.6% | NS | 2 | 9.1% | 0.000 |
| Present | 7 | 5.8% | 3 | 9.4% | 20 | 90.9% | |||
| Vascular invasion | Absent | 91 | 75.8% | 28 | 87.5% | NS | 18 | 81.8% | NS |
| Present | 29 | 24.2% | 4 | 12.5% | 4 | 18.2% | |||
| Gross extrathyroidal extension | Absent | 113 | 94.2% | 32 | 100.0% | NS | 21 | 95.5% | NS |
| Present | 7 | 5.8% | 0 | 0.0% | 1 | 4.5% | |||
| Margin status | Negative | 98 | 82.4% | 27 | 84.4% | NS | 13 | 65.0% | NS |
| Positive | 21 | 17.6% | 5 | 15.6% | 7 | 35.0% | |||
| Lymph node status at presentation | N0 | 42 | 35.0% | 16 | 50.0% | NS | 0 | 0.0% | 0.001 |
| N1 | 78 | 65.0% | 16 | 50.0% | 22 | 100.0% | |||
| Volume of nodal disease | Low | 23 | 29.9% | 3 | 18.8% | NS | 2 | 9.5% | NS (0.058) |
| High | 54 | 70.1% | 13 | 81.3% | 19 | 90.5% | |||
| Stage at presentation | Stage 1 | 112 | 93.3% | 32 | 100.0% | NS | 19 | 86.4% | NS |
| Stage 2 | 8 | 6.7% | 0 | 0.0% | 3 | 13.6% | |||
P values obtained using chi square test with follicular and classic PTCs as baseline comparison group.
Abbreviations: PTC= Papillary thyroid carcinoma; TCV= Tall cell variant; DSV= Diffuse sclerosing variant; NS=Not significant.
There were no significant differences (p> 0.05) between PTC-TCV and low-risk PTC variants in regard to tumor size, presence of lymphatic or vascular invasion, gross ETE, margin status and lymph node status at presentation.
PTC-DSV (table 3)
When compared with low-risk PTC histologic subtypes, PTC-DSV had significantly higher rates of lymphatic invasion (90.9% vs. 5.8%), positive surgical margins (35.0% vs. 17.6%) and higher rates of nodal metastases at presentation (100% vs 65.0%). The rate of high-volume nodal disease was also markedly higher in PTC-DSVs (90.9 vs 9.1%), coming close to but not reaching statistical significance (p=0.058). There was no difference in the rate of vascular invasion or gross ETE (p>0.05).
HGFCTC
The 38 cases of HGFCTCs consisted of 27 DHGTCs and 11 PDTCs. The DHGTCs were composed of one high-grade cribriform morular TC, and 26 high-grade PTCs. The latter were subdivided as follows: 10 high-grade PTC-DSVs, 7 high-grade classic PTCs, 6 high-grade encapsulated follicular variant PTCs, 2 high-grade infiltrative follicular variant PTCs, and 1 high-grade solid variant PTC. Of the 38 cases (17.1%) of HGFCTCs in our cohort, 34 cases were subtyped as such based on diagnostic features noted in the primary tumor. The remaining 4 were classified as HGFCTC for showing tumor necrosis in a cervical nodal metastasis while the primary tumor showed features of PTC-DSV in 3 cases and features of PTC classic type in another. HGFCTCs were significantly more likely to be completely encapsulated (47.4%) than other TCs (17.5%, p<0.001). Completely encapsulated HGFCTCs were significantly less likely (16.7%) to present with N1 nodal disease than partially encapsulated or unencapsulated HGFCTC (100%, p<0.001).
High-risk histology group (table 4)
Table 4.
Features of high-risk versus low-risk histologic groups pediatric TCs
| Histologic risk group (Adult criteria) |
Histologic risk group (Proposed pediatric criteria) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low-risk histology | High-risk histology | P value | Low-risk histology | High-risk histology | P value | ||||||
| n | % | n | % | n | % | n | % | ||||
| Overall | 162 | 73.0% | 60 | 27.0% | 173 | 77.9% | 49 | 22.1% | |||
| Tumor size | ≤ 4 cm | 145 | 89.5% | 33 | 55.0% | <0.001 | 154 | 89.0% | 24 | 49.0% | <0.001 |
| > 4 cm | 17 | 10.5% | 27 | 45.0% | 19 | 11.0% | 25 | 51.0% | |||
| Lymphatic invasion | Absent | 151 | 93.2% | 30 | 50.0% | <0.001 | 162 | 93.6% | 19 | 38.8% | <0.001 |
| Present | 11 | 6.8% | 30 | 50.0% | 11 | 6.4% | 30 | 61.2% | |||
| Vascular invasion | Absent | 126 | 77.8% | 35 | 58.3% | 0.004 | 131 | 75.7% | 30 | 61.2% | 0.045 |
| Present | 36 | 22.2% | 25 | 41.7% | 42 | 24.3% | 19 | 38.8% | |||
| Gross extrathyroidal extension | Absent | 153 | 94.4% | 55 | 91.7% | NS | 163 | 94.2% | 45 | 91.8% | NS |
| Present | 9 | 5.6% | 5 | 8.3% | 10 | 5.8% | 4 | 8.2% | |||
| Margin status | Negative | 134 | 83.2% | 40 | 70.2% | 0.035 | 144 | 83.7% | 30 | 65.2% | 0.005 |
| Positive | 27 | 16.8% | 17 | 29.8% | 28 | 16.3% | 16 | 34.8% | |||
| Lymph node status at presentation | N0 | 64 | 39.5% | 15 | 25.0% | 0.045 | 70 | 40.5% | 9 | 18.4% | 0.004 |
| N1 | 98 | 60.5% | 45 | 75.0% | 103 | 59.5% | 40 | 81.6% | |||
| Volume of nodal disease | Low | 28 | 28.9% | 7 | 15.9% | NS | 33 | 32.4% | 2 | 5.1% | <0.001 |
| High | 69 | 71.1% | 37 | 84.0% | 69 | 67.6% | 37 | 94.9% | |||
| Stage at presentation | Stage 1 | 153 | 94.4% | 55 | 91.7% | NS | 164 | 94.8% | 44 | 89.8% | NS |
| Stage 2 | 9 | 5.6% | 5 | 8.3% | 9 | 5.2% | 5 | 10.2% | |||
P values obtained using chi square test
Abbreviation: NS=Not significant.
Using adult high-risk criteria for high-risk histology, 27.0% of cases (n=60) were classified as high-risk subgroup, compared to 22.1% (n=49) when using pediatric high-risk criteria. As per both criteria sets, histologically high risk carcinomas were significantly more likely to have larger tumors, lymphatic and vascular invasion, positive surgical margins and lymph node involvement at presentation. In contrast, histologically high risk carcinomas were only associated with high-volume nodal disease when using the proposed pediatric criteria (p<0.05).
Clinical Outcome
In our cohort, 215 patients had a follow-up of at least three months. The median follow-up was 6.6 years in duration (range 0.2-29.6 years) with 79.1% of patients followed for ≥10 years. Only one patient died of disease. The deceased was a 17-year-old female patient with infiltrative, angioinvasive HGFCTC, extensive tumor necrosis, 5 mitoses per 10 HPFs, gross ETE, positive lymph nodes and metastasis to the lung at presentation who died one year after being diagnosed. Twenty-five of 215 patients (11.6%) developed locoregional recurrence, three of which recurred in the thyroid surgical bed. Distant metastasis were found in 20 of 215 patients (9.3%), with 14 of these patients having had distant metastases at presentation. The cases that metastasized consisted of 8 classic PTCs, 6 HGFCTCs, 5 PTC-DSVs, and 1 PTC solid variant. Overall, the cohort was found to have a 5 and 10-year DFS rate of 84% and 77%, respectively.
Univariate survival analysis (supplementary table 1, figure 3)
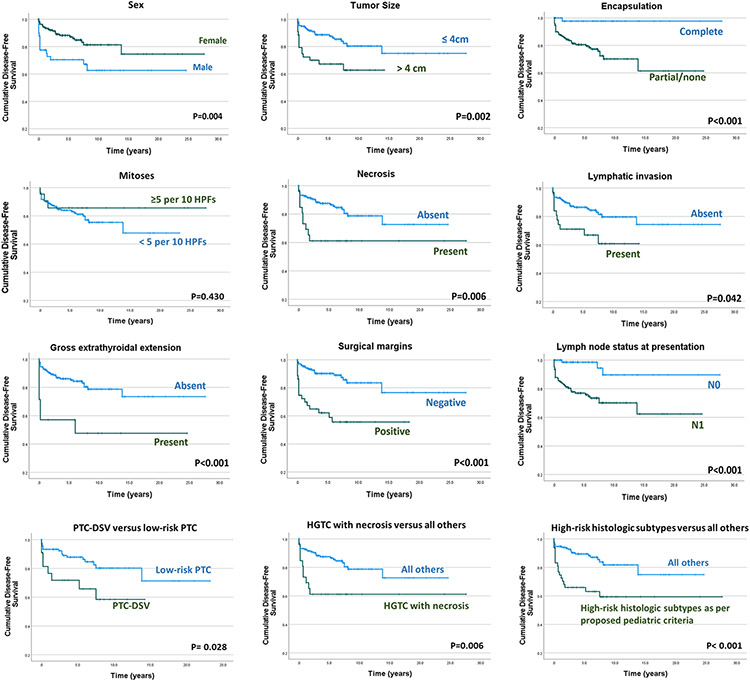
Figure 3. Disease free survival according to various clinicopathologic parameters, histologic subtypes and risk categories.
Abbreviations: PTC= papillary thyroid carcinoma; DSV= diffuse sclerosing variant; HGFCTC= High-grade follicular cell-derived thyroid carcinoma.
Supplementary table 1 lists the prognostic significance of various variables on DFS by univariate analysis. Male patients had worse DFS than female patients (p=0.004). High-risk histology (as per both adult and the proposed pediatric criteria), tumor size ≥4 cm, gross ETE and positive surgical resection margins also conveyed worse DFS (p<0.05). While completely encapsulated tumors were found to have superior DFS (p<0.001), the presence or absence of capsular invasion did not significantly affect DFS in these tumors. The presence of necrosis portended significantly worse DFS (p=0.006). Increased mitotic activity, however, had no significant effect on disease recurrence. As opposed to vascular invasion, lymphatic invasion conveyed worse DFS (p=0.042). The presence of lymph node metastases at presentation was found to be a significant adverse factor for DFS (p<0.001). Patient age was not found to have a significant effect on the DFS.
While PTC-TCV failed to emerge as a significant predictor of disease recurrence, PTC-DSV portended worse DFS (p=0.028). Whereas the HGFCTC group as a whole did not impart shorter DFS (p=0.071), HGFCTCs with necrosis had significantly worse DFS as compared with all other histologic subtypes (p=0.006).
Multivariate analysis and proposed constituents of high-risk pediatric TCs subgroup (table 5)
Table 5.
Multivariate survival analyses with each of the adult and the proposed pediatric high-risk criteria sets using cox proportional hazards model.
| P value | Hazard ratio | 95.0% CI |
||
|---|---|---|---|---|
| Lower | Upper | |||
| Disease-free survival | ||||
| Sex | 0.060 | 0.500 | 0.243 | 1.029 |
| Low versus high-risk histologic group as per adult criteria | 0.066 | 2.773 | 0.935 | 8.226 |
| Tumor size | 0.134 | 1.788 | 0.836 | 3.825 |
| Encapsulation | 0.075 | 8.007 | 0.809 | 79.235 |
| Necrosis | 0.313 | 1.680 | 0.613 | 4.603 |
| Lymphatic invasion | 0.026 | 0.320 | 0.117 | 0.875 |
| Gross extrathyroidal extension | 0.562 | 1.303 | 0.533 | 3.181 |
| Surgical margins | 0.022 | 2.368 | 1.131 | 4.958 |
| Lymph node status at presentation | 0.183 | 2.492 | 0.649 | 9.566 |
| P value | Hazard ratio | 95.0% CI |
||
| Lower | Upper | |||
| Disease-free survival | ||||
| Sex | 0.063 | 0.510 | 0.251 | 1.038 |
| Low versus high-risk histologic group as per proposed pediatric criteria | 0.020 | 4.379 | 1.259 | 15.232 |
| Tumor size | 0.199 | 1.652 | 0.768 | 3.555 |
| Encapsulation | 0.131 | 6.059 | 0.586 | 62.682 |
| Necrosis | 0.750 | 1.196 | 0.398 | 3.591 |
| Lymphatic invasion | 0.010 | 0.246 | 0.084 | 0.719 |
| Gross extrathyroidal extension | 0.286 | 1.637 | 0.662 | 4.044 |
| Surgical margins | 0.027 | 2.262 | 1.097 | 4.667 |
| Lymph node status at presentation | 0.154 | 2.724 | 0.686 | 10.819 |
Significant p values in bold
CI: confidence interval.
There were insufficient events to perform multivariate survival analysis for distant metastasis-free survival and locoregional recurrence-free survival. Therefore, multivariate survival analysis was performed for DFS only. On multivariate analysis, lymphatic invasion and surgical margin status emerged as significant variables in predicting DFS. This was true when using both the adult and the proposed pediatric criteria for high-risk histology.
By multivariate analysis, the proposed pediatric criteria for histologic risk stratification (table 5) showed high-risk histology to be an independent adverse prognostic factor for DFS (p=0.020, hazard ratio=4.379, 95% confidence interval: 1.259–15.232). The same was not true for adult high-risk histology criteria (table 5). Based on the results of the two alternate multivariate survival analyses, we herein suggest that high-risk pediatric thyroid carcinomas be limited to PTC-DSVs and HGFCTCs exhibiting necrosis.
Discussion:
Through this cohort, we profile the clinicopathologic and prognostic features of a large cohort of pediatric TCs treated in a single institution.
Clinical features and their relevance
Sex
In congruence with previous studies, female patients represent the majority of patients in our pediatric TC cohort, with a F/M ratio approaching 2/1.(4) As shown by others, male patients presented with more advanced disease and were significantly more likely to have nodal involvement at presentation.(4, 15)
Age
We found age at presentation to be significantly associated with the tumor histologic subtype, with infiltrative follicular variant PTC and PTC-DSV presenting at a younger average age (13.4 and 13.9 years, respectively), encapsulated follicular variant PTC presenting in older patients (17.6 years average), and both classic and PTC-TCV presenting in between (16.3 and 16.9 years, respectively). At variance with prior studies, we did not find age to be a significant prognostic parameter. In an earlier account, Alessandri et al. had concluded that older age (>10 years) was the major determinant of recurrence in pediatric differentiated TC. In contrast, Galuppini et al. noted younger patients had more extensive disease with more frequent lymph node and distant metastases.(16) Within our cohort, we did not find age to be associated with neither extent of disease at presentation nor with DFS. We thereby suggest that TCs diagnosed in patients aged 21 or younger be regarded as a homogeneous group in regard to age as a risk stratifier.
Nodal disease at presentation
Compared to adults, children are more likely than adults to present with locoregional and distant disease spread.(4) The present cohort confirms the latter statement as 64.4% of our patients had lymph node involvement at presentation, a figure similar to the ones reported by prior series.(4, 17) In addition to male sex, we found large tumor size, lack of complete encapsulation, lymphatic invasion and positive resection margins to be significantly associated with nodal disease at presentation, echoing previous published results.(17) The observation that completely encapsulated pediatric TCs have a significantly lower risk of lymph node involvement at presentation can be attributed to the high prevalence of encapsulated follicular variant PTCs in this histologic subgroup. In contrast with classic and infiltrative follicular variant PTCs, these tumors are commonly associated with NRAS mutations that confer a significantly lower rate of lymph node metastases.(18, 19) Finally, consistent with the histologic features of the PTC-DSV, lymphatic spread was omnipresent in the PTC-DSVs of our cohort, a finding reflected by the invariable presence of nodal involvement in these cases.
Histologic variants and relevance
Recent efforts in the field of thyroid pathology have aimed at de-escalating therapy by elaborating detailed risk stratification models that identify which patients would benefit most from aggressive adjuvant treatments.(20) In adult patients, tumor histologic subtype is an important factor when placing a carcinoma into either a low-risk or a high-risk category.(17)
Classic and solid PTC
As predicted, PTC represents the bulk of our pediatric follicular cell-derived TC cohort, whilst classic PTC dominates amongst the assessed PTC subtypes.(6, 17, 21) By following our diagnostic criteria, only three of our cases were subtyped as solid variant PTC.(9) Previous studies, however, had reported a much more frequent occurrence of solid and trabecular growth patterns in childhood PTCs.(21, 22) The WHO also reports this pattern to be more common in pediatric patients.(9) The discrepant results between investigators could be related to variations in the cut-off values used for the minimal proportion of solid architecture required for diagnosing solid PTC. In fact, the literature disagrees on the exact cut-off value needed whilst the WHO classification does not provide a precise percentage.(9, 23, 24) Interestingly, our three patients with solid PTC cases presented at an average age of 11.6 years, the youngest average for any of our included histologic subgroups. Collini et al had, in fact, theorized that solid and trabecular childhood PTCs recapitulate the first stages of development of the thyroid gland, in which the formation of follicles is not well developed.(21) Of note, solid PTCs were excluded from both risk categorization in our analyses following the ongoing controversy on whether they truly represent high-risk tumors.(9, 22, 25)
PTC-TCV
It is now well established that in the general population, PTC-TCV should be placed under a high-risk histology category in view of its aggressive biologic behavior.(9, 17) PTC-TCV arising in adult patients are known to be more aggressive than classical PTC and to have a higher risk of metastasis and high-grade transformation.(26) The present pediatric cohort, however, shows PTC-TCV to have similar clinicopathologic features as other PTC subtypes that are traditionally regarded as low-risk variants e.g. classic PTC and follicular variant PTC. We did not find PTC-TCV to differ from traditionally low-risk subtypes in terms of tumor size, lymphovascular invasion, ETE, nodal or distant metastasis at presentation. Of the 32 PTC-TCV cases in our series, only two patients experienced locoregional recurrences, one of which was alive with disease at last follow-up, and the other free of disease. None of the PTC-TCVs in our cohort developed distant metastases, in striking contrast with what is expected for adult patients. (26) This paucity in adverse events was reflected in our univariate survival analysis, which showed no significant effect of PTC-TCV histology on any of our assessed survival endpoints. Therefore, our study supports the previously put forth opinion that a tall cell morphology in childhood and adolescent PTC does not carry the same poor prognostic implications as in adults.(21) This difference in biologic behavior between pediatric and adult PTC-TCV may be explained by the underlying differences in their respective genetic make-up. While PTC-TCVs occurring in adult patients are enriched with BRAFV600E mutations, the same does not appear to be true for pediatric patients, whose tumors predominantly exhibit RET mutations/rearrangements.(9, 17) Gallupini et al. found a BRAFV600E mutation in only 1 of 8 tested cases of pediatric PTC-TCV.(4)
PTC-DSV
PTC-DSV, an uncommon variant of PTC with a predilection to the pediatric population, stood out as one of two high-risk histologic variants in our cohort. As a distinct PTC variant, this tumor is characterized by a mixture of clinical (i.e. young patients with diffuse lobe involvement) and histologic features (squamous metaplasia, stromal sclerosis, and psammoma bodies).(9, 27) PTC-DSV are known to carry an increased risk of locoregional and distant metastases and recurrence.(9, 17) Our cohort showed PTC-DSV histologic subtype to be strongly associated with nodal involvement at presentation, with up to 90% of cases presenting with high volume of nodal disease. Out of the 21 cases of PTC-DSV with follow-up in the series, 3 experienced locoregional recurrence while 3 experienced distant metastases and 2 experienced both. PTC-DSV histology was significantly associated with decreased DFS and emerged as an independent adverse prognosticator when placed under the heading of high-risk pediatric histologic groups in multivariate analysis.
HGFCTC
Although less common than PTC, HGFCTCs as defined by the upcoming WHO classification represented a minor but significant proportion of tumors in our pediatric TC cohort, contrasting with previous statement that they are exceptional in children.(8, 21) In a previous publication, our group reported that patients aged 21 years or younger accounted for 5.7% of our HGFCTC cohort.(10) On the other hand, no cases of anaplastic thyroid carcinomas were seen in our cohort of 222 patients. Therefore, to date, we agree with the statement that anaplastic carcinomas are virtually absent in children.(21) Considering that HGFCTCs can be encountered in the pediatric population, our next step was to establish whether the HGFCTC histologic subtype and its diagnostic high-grade features carry the same prognostic significance in children as they do in adult patients. In 2005, Hiltzik et al found patients with HGFCTC defined by a high mitotic rate and/or tumor necrosis to have an overall survival outcome that was inferior to that of well-differentiated papillary and follicular carcinomas, however still superior to that of anaplastic TC.(11) The same does not appear to be applicable for pediatric TCs, for whom the definition of HGFCTC may need to be revisited. In fact, our analysis shows that increased mitotic activity and HGFCTC (defined on the basis of high mitotic count and/or tumor necrosis) are not significant predictors of DFS. Furthermore, tumors with increased mitotic counts were significantly associated with a decreased risk of nodal disease. This unexpected and paradoxical observation could be explained by the fact that 47.4% of our HGFCTCs consist of encapsulated tumors that would thereby have less propensity for nodal metastases.(28) On the other hand, the N1 tumor group of our cohort is populated by cases of classic PTCs and PTC-DSVs, all of which have less than 5 mitoses per 10 high-power fields (400x). It is not unprecedented for increased mitotic activity not to be an adverse prognosticator in the tumoral processes of young children. Nevi exhibiting mitotic figures are significantly more frequent in patients younger than 20 years of age than in patients older than 50 years.(29) In pituitary adenomas of the pediatric and adolescent population, mitotic activity does not appear to correlate with recurrence.(30) Increased mitotic figures and atypical mitotic figures have been described in pediatric adrenal cortical neoplasms that did not go on to recur or develop metastatic disease.(31) Tumor necrosis, on the other hand, emerged as a powerful parameter in predicting worse DFS. Our cohort’s only death from disease stemmed from a HGFCTC with extensive tumor necrosis. The presence of necrosis does not, however predict nodal involvement, perhaps for the same reason detailed for high mitotic count above. We therefore hypothesize that while the presence of necrosis is a universal adverse prognostic parameter in patients with TC of all ages, increased mitotic activity is not an adverse prognostic parameter in pediatric TCs. In contrast, recognizing and communicating the presence of even focal necrosis in the diagnostic pathology report is prognostically important even in carcinomas with papillary or follicular architecture, supporting the new concept of DHGTC developed in the upcoming WHO classification.(8)
Having established that the prognostic value of the diagnostic elements of HGFCTC differs between adult and pediatric TC patients, differences in the prognostic effect of HGFCTC as a histologic subtype can be anticipated. Of the 37 HGFCTCs in our cohort with follow-up, 6 (16%) experienced distant metastases. This contrasts sharply with Xu et al.’s study on HGFCTCs in the general population, in which 62.4% of patients manifested distant metastases.(10) In fact, our previous affiliated studies have already demonstrated how pediatric HGFCTC does not carry as poor of a prognosis as HGFCTC occurring in adults. Xu et al. reported the pediatric and young adult age group afflicted with HGFCTC to have improved disease-specific survival as compared with other age groups.(10) Of interest, Xu et al. also reported HGFCTC to have a high propensity for late distant metastasis with a significant number of patients having developed distant metastases more than 10 years following thyroid resection.(10) Late metastases were not seen in our pediatric cohort and constitute another contrasting feature with the adult population. In fact, of our 5 HGFCTC patients that experienced distant metastases, two were detected upon presentation while the others were discovered in an interval of up to 1.6 years following initial diagnosis. It therefore appears that late onset metastases may not be as major of a concern in pediatric patients as it is in adults.(10)
Outcome
Overall, we agree with the literature in that childhood TCs have an excellent prognosis, as only one of the patients in our series died of disease with 79.1% of patients followed for ≥10 years.(6, 17, 21) This demonstrates that, although possible, death from childhood TC is a highly unlikely eventuality. It is in line with the previously reported 30-year survival rate of 90–99%, and disease-specific mortality of less than 3%.(5, 17) Accurate recognition of adverse clinical and histopathologic prognostic elements allows for timely selection of the patients that would benefit from aggressive treatment options. In variance with the literature, we report a lower rate of distant metastases (9.3%) in pediatric patients than the 25.0% rate put forth by the literature.(4) We also provide a lower rate of locoregional recurrence (11.6%) than that provided by both pediatric and adult series.(4, 17, 32, 33) The different results between investigators could be related to variation in sample size and risk factors in addition to the various outcome measures used to detect recurrence. While we relied on structural evidence of recurrence only, other studies evaluated recurrence using not only clinical but also biochemical parameters.(4)
A major strength of this study was that all tumor slides were reviewed by the same panel of two pathologists in the light of the most up-to-date criteria for thyroid neoplasia classification. Despite the large size of our series, overall survival and disease-free survival analyses were limited by the paucity of events. The data from this single institution cohort can be verified by performing future larger multicenter series.
This study reaffirms that pediatric TCs have an overall excellent prognosis.(17) Though death from disease is possible, it is nevertheless a very rare event that should be most considered in patients with advanced stage and large tumors showing necrosis. In addition, patients with PTC-DSV, and not PTC-TCV, are at increased risk of protracted disease course and adverse events. In contrast, increased mitotic activity does not predict aggressive behavior nor adverse outcome in pediatric TC patients. Therefore, the definition of pediatric HGFCTC needs to be revisited and restricted to tumors with necrosis only. In addition, the group of histologically high-risk pediatric carcinomas should be limited to PTC-DSV and HGFCTC with necrosis. The next step in the characterization of childhood and young adulthood TCs will be large-scale genomic analysis to investigate the prognostic value of the molecular alterations found in these tumors.
Supplementary Material
Supplementary Table 1: Univariate analysis of disease-free, locoregional recurrence-free and distant metastasis-free survival in pediatric thyroid carcinomas
Conflicts of Interest and Source of Funding:
No competing financial interests exist for all contributory authors. No competing financial interests exist for all contributory authors.
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Gilliland FD, Hunt WC, Morris DM, et al. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973-1991. Cancer. 1997;79:564–573. [DOI] [PubMed] [Google Scholar]
- 2.Nixon IJ, Wang LY, Migliacci JC, et al. An International Multi-Institutional Validation of Age 55 Years as a Cutoff for Risk Stratification in the AJCC/UICC Staging System for Well-Differentiated Thyroid Cancer. Thyroid : official journal of the American Thyroid Association. 2016;26:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haymart MR. Understanding the Relationship Between Age and Thyroid Cancer. The Oncologist. 2009;14:216–221. [DOI] [PubMed] [Google Scholar]
- 4.Galuppini F, Vianello F, Censi S, et al. Differentiated Thyroid Carcinoma in Pediatric Age: Genetic and Clinical Scenario. Frontiers in endocrinology. 2019;10:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christison-Lagay ER, Baertschiger RM, Dinauer C, et al. Pediatric differentiated thyroid carcinoma: An update from the APSA Cancer Committee. Journal of pediatric surgery. 2020;55:2273–2283. [DOI] [PubMed] [Google Scholar]
- 6.Dermody S, Walls A, Harley EH. Pediatric thyroid cancer: An update from the SEER database 2007–2012. International journal of pediatric otorhinolaryngology. 2016;89:121–126. [DOI] [PubMed] [Google Scholar]
- 7.Ghossein R, Barletta JA, Bullock M, et al. Data set for reporting carcinoma of the thyroid: recommendations from the International Collaboration on Cancer Reporting. Human pathology. 2021;110:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baloch ZW, Asa SL, Barletta JA, et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocrine pathology. 2022;33:27–63. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd RV, Osamura RY, Klöppel G, et al. WHO Classification of Tumours of Endocrine Organs. International Agency for Research on Cancer; 2017. [Google Scholar]
- 10.Xu B, David J, Dogan S, et al. Primary high-grade non-anaplastic thyroid carcinoma: a retrospective study of 364 cases. Histopathology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiltzik D, Carlson DL, Tuttle RM, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006;106:1286–1295. [DOI] [PubMed] [Google Scholar]
- 12.Nikiforov YE, Erickson LA, Nikiforova MN, et al. Solid variant of papillary thyroid carcinoma: incidence, clinical-pathologic characteristics, molecular analysis, and biologic behavior. The American journal of surgical pathology. 2001;25:1478–1484. [DOI] [PubMed] [Google Scholar]
- 13.Malandrino P, Russo M, Regalbuto C, et al. Outcome of the Diffuse Sclerosing Variant of Papillary Thyroid Cancer: A Meta-Analysis. Thyroid : official journal of the American Thyroid Association. 2016;26:1285–1292. [DOI] [PubMed] [Google Scholar]
- 14.Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid : official journal of the American Thyroid Association. 2017;27:751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Wang Y, Zhao K, et al. Lymph node metastasis in young and middle-aged papillary thyroid carcinoma patients: a SEER-based cohort study. BMC Cancer. 2020;20:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alessandri AJ, Goddard KJ, Blair GK, et al. Age is the major determinant of recurrence in pediatric differentiated thyroid carcinoma. Medical and pediatric oncology. 2000;35:41–46. [DOI] [PubMed] [Google Scholar]
- 17.Balachandar S, La Quaglia M, Tuttle RM, et al. Pediatric Differentiated Thyroid Carcinoma of Follicular Cell Origin: Prognostic Significance of Histologic Subtypes. Thyroid : official journal of the American Thyroid Association. 2016;26:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera M, Ricarte-Filho J, Knauf J, et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23:1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franco AT, Ricarte-Filho JC, Isaza A, et al. Fusion Oncogenes Are Associated With Increased Metastatic Capacity and Persistent Disease in Pediatric Thyroid Cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2022:Jco2101861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu B, Ghossein R. Evolution of the histologic classification of thyroid neoplasms and its impact on clinical management. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2018;44:338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collini P, Mattavelli F, Pellegrinelli A, et al. Papillary carcinoma of the thyroid gland of childhood and adolescence: Morphologic subtypes, biologic behavior and prognosis: a clinicopathologic study of 42 sporadic cases treated at a single institution during a 30-year period. The American journal of surgical pathology. 2006;30:1420–1426. [DOI] [PubMed] [Google Scholar]
- 22.Vural Ç, Kiraz U, Turan G, et al. Solid variant of papillary thyroid carcinoma: An analysis of 28 cases with current literature. Annals of Diagnostic Pathology. 2021;52:151737. [DOI] [PubMed] [Google Scholar]
- 23.Baloch ZW, LiVolsi VA. Special types of thyroid carcinoma. Histopathology. 2018;72:40–52. [DOI] [PubMed] [Google Scholar]
- 24.Ohashi R. Solid variant of papillary thyroid carcinoma: an under-recognized entity. Endocrine journal. 2020;67:241–248. [DOI] [PubMed] [Google Scholar]
- 25.Nath MC, Erickson LA. Aggressive Variants of Papillary Thyroid Carcinoma: Hobnail, Tall Cell, Columnar, and Solid. Advances in anatomic pathology. 2018;25:172–179. [DOI] [PubMed] [Google Scholar]
- 26.Ganly I, Ibrahimpasic T, Rivera M, et al. Prognostic implications of papillary thyroid carcinoma with tall-cell features. Thyroid : official journal of the American Thyroid Association. 2014;24:662–670. [DOI] [PubMed] [Google Scholar]
- 27.Thompson LD, Wieneke JA, Heffess CS. Diffuse sclerosing variant of papillary thyroid carcinoma: a clinicopathologic and immunophenotypic analysis of 22 cases. Endocrine pathology. 2005;16:331–348. [DOI] [PubMed] [Google Scholar]
- 28.Rivera M, Ricarte-Filho J, Patel S, et al. Encapsulated thyroid tumors of follicular cell origin with high grade features (high mitotic rate/tumor necrosis): a clinicopathologic and molecular study. Human pathology. 2010;41:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glatz K, Hartmann C, Antic M, et al. Frequent mitotic activity in banal melanocytic nevi uncovered by immunohistochemical analysis. The American Journal of dermatopathology. 2010;32:643–649. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Schmidt RE, Dahiya S. Pituitary Adenoma in Pediatric and Adolescent Populations. Journal of Neuropathology & Experimental Neurology. 2019;78:626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wieneke JA, Thompson LD, Heffess CS. Adrenal cortical neoplasms in the pediatric population: a clinicopathologic and immunophenotypic analysis of 83 patients. The American journal of surgical pathology. 2003;27:867–881. [DOI] [PubMed] [Google Scholar]
- 32.Cordioli MI, Moraes L, Cury AN, et al. Are we really at the dawn of understanding sporadic pediatric thyroid carcinoma? Endocrine-related cancer. 2015;22:R311–324. [DOI] [PubMed] [Google Scholar]
- 33.Tuttle RM, Vaisman F, Tronko MD. Clinical presentation and clinical outcomes in Chernobyl-related paediatric thyroid cancers: what do we know now? What can we expect in the future? Clinical oncology (Royal College of Radiologists (Great Britain)). 2011;23:268–275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Univariate analysis of disease-free, locoregional recurrence-free and distant metastasis-free survival in pediatric thyroid carcinomas