Abstract
Orthostatic hypotension affects roughly 10% of individuals with hypertension and is associated with a number of adverse health outcomes, including dementia, cardiovascular disease, stroke, and death. Among adults with hypertension, orthostatic hypotension has also been shown to predict patterns of blood pressure dysregulation that may not be appreciated in the office setting, including nocturnal non-dipping. Individuals with uncontrolled hypertension are at particular risk of orthostatic hypotension and may meet diagnostic criteria for the condition with a smaller relative reduction in blood pressure compared with normotensive individuals. Antihypertensive medications are commonly de-prescribed to address orthostatic hypotension; however, this approach may worsen supine or seated hypertension, which may be an important driver of adverse events in this population. There is significant variability between guidelines for the diagnosis of orthostatic hypotension with regards to timing and position of blood pressure measurements. Clinically relevant orthostatic hypotension may be missed when standing measurements are delayed or when taken after a seated rather than supine position. The treatment of orthostatic hypotension in patients with hypertension poses a significant management challenge for clinicians; however, recent evidence suggests that intensive blood pressure control may reduce the risk of orthostatic hypotension. A detailed characterization of blood pressure variability is essential to tailoring a treatment plan and can be accomplished using both in-office and out-of-office monitoring.
I. Introduction
Orthostatic hypotension (OH) is relatively common among individuals with hypertension, affecting about 10% of hypertensive adults.1–3 Individuals with OH and hypertension face a number of important health concerns related to both conditions, including increased risk of falls, syncope, dementia, cardiovascular disease (CVD), stroke, and mortality.4–6 The treatment of patients with concurrent OH and hypertension is a particular challenge as treatments for OH may exacerbate hypertension. In this review, we discuss the physiology of OH in hypertensive patients, subtypes and causes of OH, and management approaches to treat OH in the hypertensive patient.
II. Hypertension and Blood Pressure Dysregulation
Blood pressure (BP) fluctuates in response to environmental (i.e., altitude, ambient temperature), physical (i.e., posture, volume status, diet), and emotional (i.e., stress) factors.7 BP variability (BPV) is a term used to describe the magnitude and patterns of BP changes over time, from the short-term (between beats, minutes, hours, or day-to-night changes) to the long-term (over days, weeks, clinic visits, or years).7,8 Greater long-term variability in systolic BP (SBP) is associated with higher risk of all-cause mortality, fatal and nonfatal CVD, and stroke independent of mean BP.9 BPV is greater in patients with hypertension than those without hypertension and increases with higher BP.10 Orthostatic hypotension, also called postural hypotension, may be considered a form of BP dysregulation and a manifestation of BPV.11 Along these lines, OH has been shown to be useful in predicting hypotensive events12 as well as patterns of BPV outside of the clinic among adults with hypertension.13–16
III. Orthostatic Hypotension as a Clinical Assessment Predicting Hypotensive Events and Ambulatory Blood Pressure Phenotypes
OH is defined by various guidelines as a decrease in SBP by ≥20 mm Hg or a decrease in diastolic BP (DBP) by ≥10 mm Hg when an individual stands from a seated or supine position.17–19 During the normal physiological response to standing, baroreceptors in the carotid sinus and the aortic arch sense the sudden decrease in BP due to venous pooling and respond by increasing sympathetic tone and decreasing parasympathetic tone. The influx of sympathetic tone results in increased peripheral vascular resistance leading to an increase in venous return and BP augmentation.20 The withdrawal of parasympathetic tone results in augmentation of heart rate.20 OH results from an insufficient physiologic response to the decreased venous return upon standing, and can be broadly classified as neurogenic or non-neurogenic. Non-neurogenic OH is far more common and may be caused by various etiologies, including intravascular volume depletion, medications, CVD, and prolonged recumbency or deconditioning.20 While rare in the general population, neurogenic OH is relatively common among patients with neurodegenerative disorders (i.e., Parkinson disease, multiple system atrophy, Lewy body dementia, and pure autonomic failure) or small-fiber neuropathies, including diabetes mellitus or B12 deficiency.21 A less common etiology of OH is baroreceptor failure which occurs due to bilateral disruption of baroreceptor function and can result from neck radiation, surgery, or trauma.22
OH is a predictor of hypotensive events among adults with treated hypertension. In a secondary analysis of the Systolic Blood Pressure Intervention Trial (SPRINT), adults with OH had nearly a two-fold higher risk of hypotension-related hospitalizations or emergency department visits, which was independent of assigned treatment goal (i.e. intensive BP control [< 120 mm Hg] versus standard BP control [<140 mm Hg]).12
OH also associates with distinct ambulatory BP phenotypes.14,16 In a subgroup analysis of 849 adults from SPRINT, OH was found to be associated with white coat hypertension (a state where BP measured in the clinic are higher than BP measured at home) and nocturnal elevations in BP.13
IV. Orthostatic Hypotension and Hypertension
The term OH may itself be a misnomer, implying hypotension upon standing. However, given that OH is derived from two BP measurements, a supine/seated measurement and a standing measurement, having an elevated supine/seated BP can meet criterion for OH even in absence of standing hypotension.
It should thus come as little surprise that OH is more frequently observed among those with hypertension.23,24 In observational studies, approximately 1 in 10 individuals with hypertension have OH1–3 and those with uncontrolled hypertension are at higher risk for OH.2,25 There may be multiple reasons for this observation. First, altered BP regulatory mechanisms and autonomic dysfunction underpin both OH and hypertension.20,26 Second, hypertension itself may blunt sympathetic nervous system regulation of BP.27 Third, certain antihypertensive drug classes are associated with OH.28 Fourth, increased age and clinical comorbidities such as heart failure, diabetes mellitus, and chronic kidney disease are associated with both conditions. Fifth, those with hypertension may meet diagnostic criteria for OH based on absolute reductions in BP (i.e. mm Hg) with smaller relative reduction (i.e. percent decrease) in BP. Sixth, cerebral autoregulation which normally protects the brain from transient hypotension is impaired by hypertension and associated cerebrovascular disease.29 As such, hypertensive individuals may develop symptoms of cerebral hypoperfusion during standing at blood pressure values typically considered normal.30 Last, vascular remodeling, CVD, and stroke are all adverse complications of hypertension that are related to OH.31
Individuals with both hypertension and OH pose a particular treatment challenge, as OH carries an increased risk of falls, syncope, and dementia while under-treatment of hypertension is associated with CVD, stroke, and all-cause mortality.4–6 A common approach to treating OH is to recommend increased salt and fluid consumption, deprescribe antihypertensives, and initiate mineralocorticoids for BP augmentation. These approaches operate under the assumption that the adverse events caused by OH are secondary to organ hypoperfusion and injury. However, it is possible that the impetus of adverse events in hypertensive patients with OH is supine/seated hypertension, not the standing BP or even the change between the two.
Supine Hypertension
Supine hypertension is defined by the American Autonomic Society and the European Federation of Autonomic Societies as an SBP of 140 mm Hg or greater and DBP of 90 mm Hg or greater while in the supine position, and is physiologically linked to OH.32,33 Orthostatic episodes during the day can activate the renin-angiotensin-aldosterone pathway and exacerbate supine hypertension.34 Meanwhile, supine hypertension causes significant nocturnal diuresis, which can worsen daytime orthostasis.32 Non-pharmacologic strategies to decrease supine hypertension include avoiding the supine position during the day and elevating the head of the bed at night;35 although evidence in support of these maneuvers is limited. Pharmacologic treatment of supine hypertension is challenging and should be balanced against the potential exacerbation of morning hypotensive episodes, which can have immediate consequences such as orthostatic symptoms and falls.36 Certain antihypertensives including captopril, clonidine, overnight nitroglycerine patches, and short-acting nifedipine have been trialed to reduce nocturnal hypertension.37–39
While there is uncertainty whether supine hypertension without seated hypertension requires treatment, particularly in cases with OH in which treatment can exacerbate orthostasis, supine hypertension is associated with adverse outcomes. The Irish Longitudinal Study on Ageing (TILDA) of 1500 community-dwelling adults found that orthostasis with co-existing hypertension either in the seated/supine position was significantly associated with a higher risk of falls and syncope, though the effect size was notably larger for seated compared to supine hypertension.40 Studies of patients with autonomic dysfunction have found that those with supine hypertension had worse renal function, higher rates of left ventricular hypertrophy, and earlier incidence of CVD and all-cause mortality than those without supine hypertension.41,42
V. Measurement Considerations
Measurement Timing
While guidelines generally recommend measuring BP in the seated or recumbent position after a period of 5 minutes,17,43 there is substantial heterogeneity with respect to the timing of standing OH measurements across guidelines with the majority focused on delayed measurements within 1–3 minutes after standing (Table).17,18,43–48 Despite these recommendations, hypotension is more frequently detected when measured at earlier intervals upon standing. One study of 8,908 individuals found a prevalence of OH immediately after standing of 12.3% and at 2 minutes of standing of 2.9%.49 ‘Initial OH,’ i.e., a transient decrease in SBP of >40 mm Hg within 15 s of standing regardless of symptoms of orthostatic intolerance,50,51 is often missed by traditional automated or auscultatory BP measurements and may be more readily captured by self-reported symptoms of light-headedness, visual disturbances, and presyncope.52 ‘Delayed recovery OH’, OH that resolves by 2–3 minutes after standing, is yet another OH phenotype often missed with delayed assessments.35 One observational study found that OH measurements performed within 1 minute of standing were associated with dizziness and adverse outcomes including fall, fracture, syncope, mortality, and motor vehicle accidents,53 suggesting that delayed OH assessments may miss important clinical information with respect to concurrent symptoms and long-term risks. Furthermore, an analysis of data from the African American Study of Kidney Disease and Hypertension (AASK) trial found that consensus definitions of OH yielded high specificity for OH symptoms but low sensitivity.54
Table. Guideline Definitions of Orthostatic Hypotension.
| Guideline | Blood Pressure Change Cutoffs | Time Cutoffs | Position |
|---|---|---|---|
| American Academy of Family Physicians 2022 | Decrease in SBP ≥20 mm Hg or DBP ≥10 mmHg | 3 minutes | Supine to standing or tilt-table testing |
| American Autonomic Society Consensus Definition 1996 and 2011 | Sustained decrease in SBP ≥20 mm Hg or DBP ≥10mmHg. Consider SBP ≥30 mm Hg for adults with supine hypertension | Two measurements <3 minutes | Recumbent to standing or tilt-table testing. |
| American College of Cardiology / American Heart Association 2017 | Decrease in SBP >20 mm Hg or DBP >10 mmHg | 1 minute | Seated to standing |
| American Diabetes Association 2017 | Decrease in SBP >20 mm Hg or DBP >10 mmHg | 3 minutes | Seated or supine to standing |
| Centers for Disease Control and Prevention 2017 | Decrease in SBP ≥20 mm Hg or DBP ≥10 mm Hg | 1 minute and 3 minutes | Supine to standing |
| European Society of Hypertension/ European Society of Cardiology 2018 | Decrease in SBP ≥20 mm Hg or DBP ≥10 mmHg | 1 minute and 3 minutes | Seated to standing as initial screen, can consider supine to standing in subsequent visits for high risk patients |
| National Heart Foundation of Australia 2016 | Not specified | 2 minutes | Seated to standing |
| National Institute for Health and Care Excellence 2019 (United Kingdom) | Decrease in SBP ≥20 mmHg | 1 minute | Seated or supine to standing |
Starting Position: Supine Versus Seated
Guidelines have conflicted with regards to the starting position for OH assessments (Table). The American College of Cardiology /American Heart Association (ACC/AHA) guidelines describe OH being performed from the seated to standing position.17 In contrast, the autonomic society consensus recommends recumbent to standing or tilt table testing with respect to OH assessments.45 Utilization of seated versus supine initial positions can alter the detection of OH. In a study of older adults age 70 years and older, where nearly two thirds had hypertension, OH was diagnosed more frequently when using supine assessments (14.8%) compared to seated assessments (2.2%) and the two protocols were not interchangeable based on both their observed physiologic responses and their associations with clinical symptoms and falls.55 Similarly, in study of 831 patients presenting to a neurology clinic, Shaw et al found that using a lower cutoff point (i.e., SBP drop of >15 mm Hg or a DBP of >7 mm Hg) to define OH from a seated position yielded a comparable sensitivity relative to standard cutoff points (i.e. SBP drop ≥20 mmHg or DBP drop ≥10 mmHg) to define OH from a supine position.56 These findings have important clinical implications, as detection of OH using seated assessments can result in an underestimation of the prevalence of clinically relevant OH compared to supine assessments.
VI. Management
Traditional Approaches
Management of OH generally begins with an appraisal of potential precipitants or exacerbators of OH. Patients should be screened for etiologies of OH such as neurodegenerative diseases, diabetes mellitus, B12 deficiency, renal failure, rheumatologic or autoimmune conditions, dehydration, prolonged recumbency, deconditioning, and medications.19,57 Evaluation for whether medications that contribute to orthostasis can be withdrawn or replaced is warranted. The medications most strongly associated with OH include alpha blockers, beta blockers, tricyclic antidepressants, nitrates, phosphodiesterase-5 inhibitors, and antipsychotics.19,23,58,59 Several non-pharmacologic interventions may improve orthostatic symptoms, including advising patients to change position slowly, maintaining adequate hydration, and avoiding alcohol and large meals.19 Countermeasure maneuvers such as crossing legs while standing have evidence for improving orthostasis in a placebo-controlled crossover study.60 Abdominal compression binders have also been shown to increase upright blood pressure in small studies.61,62
When non-pharmacologic therapies are ineffective, pharmacologic therapies such as midodrine, droxidopa, or fludrocortisone may be trialed, with the goal being to relieve symptoms rather than focusing on a specific blood pressure target. Midodrine is a prodrug of a sympathomimetic agent and is approved by the Food & Drug Administration (FDA) for symptomatic OH.63 Midodrine should be used with caution in patients with heart failure or renal disease and should also be avoided within 3–4 hours of lying down to prevent supine hypertension.32 Droxidopa, a prodrug of noradrenaline, is approved by the FDA for patients with neurogenic OH. While droxidopa appears to be less likely to cause supine hypertension than midodrine, the use of droxidopa in patients with non-neurogenic OH is less well-established.64 Fludrocortisone is a synthetic adrenocortical steroid that is often used off-label for OH but its effects on positional blood pressure changes and orthostatic symptoms is uncertain.65 Fludrocortisone poses particular risk to patients with CVD and is generally avoided in patients with heart failure or supine hypertension.66 The addition of pressor agents in patients with concomitant OH and hypertension should be avoided to prevent exacerbating hypertensive episodes; however, if pharmacologic therapy is necessary to improve hypotensive symptoms, midodrine is preferred due to its short half-life as well as the stronger evidence base for symptomatic improvement compared with the other available medications.
Pharmacologic Treatment of Hypertension and OH
While some observational data support the traditional view that antihypertensive therapies increase the risk of the OH,23,67 a number of clinical studies have demonstrated that targeting lower BP goals does not increase risk of OH. In a study of 702 adults aged 70 and older, OH prevalence was found to be higher in adults with uncontrolled rather than controlled hypertension (19% vs 5%; P <0.001).25 In 4266 patients of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, the incidence of OH was unrelated to randomization into the intensive (<120 mm Hg) vs standard (<140 mm Hg) blood pressure arm.68 Furthermore, in a follow-up study of ACCORD, the prevalence of OH did not differ between either arm at baseline, 12 months, or at 48 months.69 Similarly, the SPRINT trial found comparable rates of OH in patients randomized to intensive blood pressure (<120 mmHg) and standard blood pressure (<140 mmHg) goals.70 Finally, an individual participant level meta-analysis of 9 randomized controlled trials found that among 18,466 adults, more aggressive antihypertensive treatment lowered OH risk.71
A common critique of the above trials (e.g., SPRINT) is the exclusion of adults with more severe forms of OH thereby excluding a proportion of patients who could develop more severe orthostasis from antihypertensive treatment. However, in subgroups by low standing BP or pre-randomization OH in the meta-analysis above, there was no evidence of greater risk of OH with treatment. It should be noted that the antihypertensive agents used in hypertension trials may differ from those used in clinical practice, which may account for the discrepancy between hypertension trials and some observational studies. For example, in the AASK trial, initiating hypertension therapy with metoprolol was associated with a greater risk of OH compared with ramipril or amlodipine.72 Nevertheless, evidence does not support the routine down-titration or discontinuation of antihypertensives in asytablemptomatic patients with hypertension and orthostasis. Rather, a more nuanced approach with selective discontinuation of medications associated with OH, while maintaining appropriate hypertension treatment goals is warranted.
Antihypertensive Classes Selection and OH
Antihypertensive classes that interfere with sympathetic compensatory responses to positional changes are most strongly associated with OH.26 These classes include alpha blockers, beta blockers, and central sympatholytics.73 In contrast, numerous studies suggest that dihydropyridine calcium channel blockers, ACE inhibitors, and angiotensin receptor blockers (ARBs) have either a neutral or protective effect on OH.26,73 A recent meta-analysis of randomized trials provides further evidence that drugs causing sympathetic inhibition are associated with a significantly increased odds of OH, whereas medications with a predominantly vasodilator mechanism of action are not associated with OH.28 Observational studies have found that diuretics and, in particular, loop diuretics, are associated with OH.2,23 Chlorthalidone, however, has not clearly been found to increase OH risk compared to other drug classes as seen in a secondary analysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) trial.74
While most available data suggest that calcium channel blockers are not associated with OH, there are notable exceptions. In a secondary analysis of ALLHAT there was a short-term increased risk of falls among patients taking amlodipine compared with either lisinopril or chlorthalidone.74 A cross-sectional analysis of the SPRINT trial examining patterns of orthostatic SBP changes found that greater postural reductions in SBP were associated with calcium channel blockers as well as alpha and beta blockers.75 However, this study did not differentiate between dihydropyridine and non-dihydropyridine calcium channel blockers.
Short-term Risks of Antihypertensive Changes
Several studies suggest that the risk of adverse events related to OH is highest in the short-term period surrounding BP medication changes, particularly among older adults. A case cross-over study of over 90,000 adults aged 65 or older found a significantly higher risk of serious fall injuries within the 15 days after antihypertensive medication initiation or intensification.76 A similar finding in relationship to antihypertensive withdrawal was observed in a secondary analysis of the Trial of Nonpharmacologic Interventions in the Elderly (TONE), in which 975 older adults were followed for 36 months after randomization to lifestyle interventions or usual care in the context of antihypertensive medication withdrawal. This study found that the majority of symptomatic adverse events (i.e., dizziness, blacking out, or presyncope) occurred in the 3 months after drug withdrawal.77 Together, these data suggest increased vulnerability to orthostatic symptoms and falls in the short-term period following either the addition or withdrawal of antihypertensive medications, likely related to adaptations in BP regulation. As such, vigilance is warranted during the short-term period after antihypertensive medication changes.
Dietary sodium modulation
Volume expansion through increased dietary sodium intake, in some cases as high as 10 g daily, is recommended in clinical practice guidelines to improve orthostatic tolerance.78 There is evidence from small observational studies to suggest sodium restriction worsens OH in patients with neurogenic OH79,80 and other forms of orthostatic intolerance;81 however, the quality of the evidence for improvement in symptoms is lacking.82 Moreover, it is unclear that the above recommendations are applicable to hypertensive adults with OH, given the effects of sodium on supine hypertension. On the contrary, reduced dietary sodium intake of less than 1.5 g/day is recommended for the prevention and treatment of hypertension.17 There is a paucity of data on the effects of increased sodium intake in older adults with OH and cardiovascular comorbidities both with respect to clinical outcomes as well as symptoms. In fact, in a secondary analysis of the Dietary Approaches to Stop Hypertension (DASH) trial, higher sodium intake increased symptoms of postural light-headedness among participants assigned the DASH diet.83
Meanwhile contrary to traditional recommendations, there is physiologic evidence to support sodium restriction as a therapeutic approach for adults with nocturnal/supine hypertension and OH. Sodium homeostasis and BP regulation are linked: dietary sodium intake is a determinant of urinary sodium excretion and increased renal perfusion in the presence of hypertension induces a pressure natriuresis to lower total body sodium and reduce BP. The pressure natriuresis associated with nocturnal hypertension may be as high as 2 liters, which can exacerbate early morning symptoms of OH.26 Dietary sodium restriction has been shown to reduce BP with sustained effects over a 24-hour period.84 This raises the hypothesis that improved nocturnal BP through sodium restriction could mitigate nocturnal pressure diuresis, therefore preserving intravascular volume while optimizing cardiovascular risk in patients with hypertension.85 Nevertheless, this would require formal testing and may be specifically relevant for adults with hypertensive forms of OH.
Special Considerations in Older Adults
Both OH and hypertension are particularly prevalent and morbid in older adults due to age-related dysfunction in BP regulation mechanisms. Physiologic changes which predispose older adults to OH include vascular stiffness and endothelial dysfunction,86 impaired diastolic ventricular filling which causes pre-load dependent cardiac output reductions,87 impaired salt and water conservation with reduced renin, angiotensin, and aldosterone levels, and reduced thirst.88 Older adults also have a decline in baroreflex sensitivity characterized by a beta-receptor defect which manifests as a reduction in the chronotropic cardiac response to sympathetic stimulation.89 One consequence of this age-related baroreflex impairment is an increased sensitivity to alpha blockers which effectively diminish the remaining compensatory mechanism against OH. Another consequence is that postural heart rate change is not a reliable measure in the diagnosis of OH in older adults, as the typical beta receptor mediated tachycardia response is blunted.
Clinical Approach to OH in Hypertensive Adults
Clinical practice guidelines for OH in hypertensive adults are not well-established. A suggested diagnostic approach is presented in the Figure. The initial assessment should begin with a detailed characterization of OH symptoms. This should be followed by an in-office evaluation of OH through physical exam and BP assessments with repeat measurements over serial visits. Out-of-office monitoring of OH should be performed in conjunction with in-office assessments and may include a symptom diary to elucidate the context and triggers of OH and 24-hr ABPM to identify BP phenotypes. Patients who present with symptoms of OH that are not corroborated by BP measurements should prompt an investigation of mimics of OH or other BP-related conditions that can provoke similar symptoms. For patients with confirmed OH, further work-up and treatment should be tailored to the suspected subgroup of OH (i.e., neurogenic, cardiovascular, pharmacologic, or other) and should emphasize improving orthostatic symptoms as well as controlling BP to avoid supine/seated hypertension.
Figure. Diagnostic Approach for Patients with Hypertension and Orthostatic Hypotension.
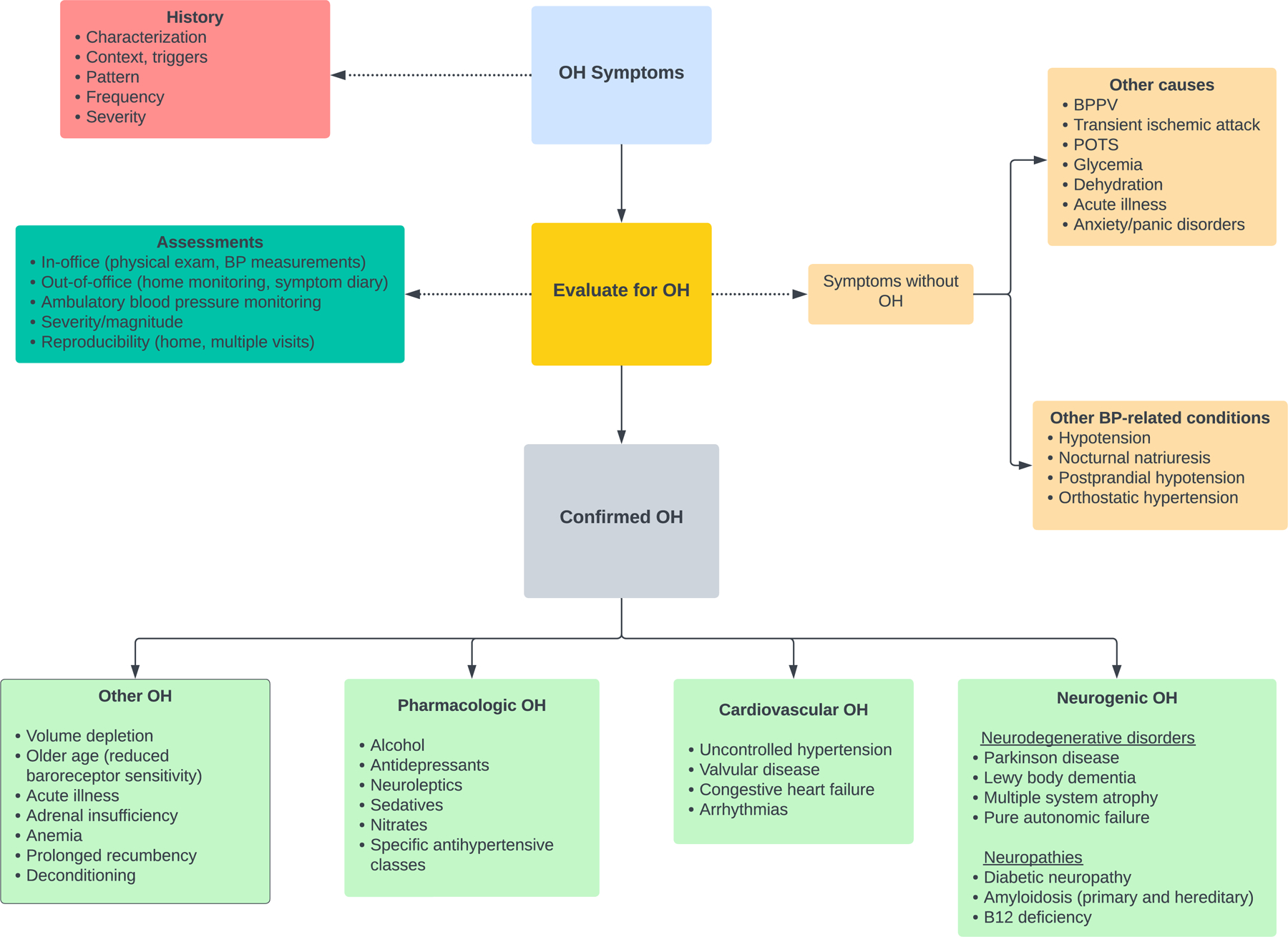
Abbreviations: BPPV, benign paroxysmal positional vertigo; BP, blood pressure; OH, orthostatic hypotension; POTS, postural orthostatic tachycardia syndrome.
VII. Conclusions
OH is common among adults with hypertension and is associated with numerous adverse outcomes, including hypotensive events, falls, dementia, CVD, and death. The diagnostic approach for detecting OH can miss pathologic phenotypes when standing assessments are delayed or occur after a seated (versus supine) position. Since OH may be observed in the setting of hypertension without standing hypotension, thorough characterization of patterns of BP variability is critical for tailoring a treatment plan. For hypertensive adults, there is strong evidence that more aggressive BP lowering, may reduce risk of OH. Nevertheless, further research on optimal approaches to screening, diagnosis, and treatment of OH in hypertensive adults is needed to inform evidence-based guidelines for this heterogeneous and complex condition.
Sources of Funding:
SPJ is supported by NIH/NHLBI grants K23HL135273, R01HL158622, and R01HL153191.
Abbreviations used:
- AASK
African American Study of Kidney Disease and Hypertension
- ABPM
Ambulatory blood pressure monitoring
- ACC/AHA
American College of Cardiology/ American Heart Association
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- ALLHAT
Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial
- BPV
Blood pressure variability
- CI
Confidence Interval
- CVD
Cardiovascular disease
- DASH
Dietary Approaches to Stop Hypertension
- DBP
Diastolic blood pressure
- FDA
Food & Drug Administration
- OH
Orthostatic Hypotension
- SBP
Systolic blood pressure
- SPRINT
Systolic Blood Pressure Intervention Trial
- STURDY
Study To Understand Fall Reduction and Vitamin D in You
- TONE
Trial of Nonpharmacologic Interventions in the Elderly
Footnotes
Disclosures: None
References
- 1.Fedorowski A, Burri P, Melander O. Orthostatic hypotension in genetically related hypertensive and normotensive individuals. J Hypertens 2009;27:976–982. doi: 10.1097/hjh.0b013e3283279860 [DOI] [PubMed] [Google Scholar]
- 2.Di Stefano C, Milazzo V, Totaro S, Sobrero G, Ravera A, Milan A, Maule S, Veglio F. Orthostatic hypotension in a cohort of hypertensive patients referring to a hypertension clinic. J Hum Hypertens 2015;29:599–603. doi: 10.1038/jhh.2014.130 [DOI] [PubMed] [Google Scholar]
- 3.Applegate WB, Davis BR, Black HR, Smith WM, Miller ST, Burlando AJ. Prevalence of postural hypotension at baseline in the Systolic Hypertension in the Elderly Program (SHEP) cohort. J Am Geriatr Soc 1991;39:1057–1064. doi: 10.1111/j.1532-5415.1991.tb02869.x [DOI] [PubMed] [Google Scholar]
- 4.Lipsitz LA. Orthostatic Hypotension and Falls. Journal of the American Geriatrics Society 2017;65:470–471. doi: 10.1111/jgs.14745 [DOI] [PubMed] [Google Scholar]
- 5.Rawlings AM, Juraschek SP, Heiss G, Hughes T, Meyer ML, Selvin E, Sharrett AR, Windham BG, Gottesman RF. Association of orthostatic hypotension with incident dementia, stroke, and cognitive decline. Neurology 2018;91:e759–e768. doi: 10.1212/wnl.0000000000006027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parati G, Stergiou GS, Dolan E, Bilo G. Blood pressure variability: clinical relevance and application. J Clin Hypertens (Greenwich) 2018;20:1133–1137. doi: 10.1111/jch.13304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol 2013;10:143–155. doi: 10.1038/nrcardio.2013.1 [DOI] [PubMed] [Google Scholar]
- 9.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 2016;354:i4098. doi: 10.1136/bmj.i4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parati G Blood pressure variability: its measurement and significance in hypertension. J Hypertens Suppl 2005;23:S19–25. doi: 10.1097/01.hjh.0000165624.79933.d3 [DOI] [PubMed] [Google Scholar]
- 11.Cremer A, Boutouyrie P, Laurent S, Gosse P, Tzourio C. Orthostatic hypotension: a marker of blood pressure variability and arterial stiffness: a cross-sectional study on an elderly population: the 3-City study. J Hypertens 2020;38:1103–1109. doi: 10.1097/hjh.0000000000002374 [DOI] [PubMed] [Google Scholar]
- 12.Juraschek SP, Taylor AA, Wright JT Jr., Evans GW, Miller ER 3rd, Plante TB, Cushman WC, Gure TR, Haley WE, Moinuddin I, et al. Orthostatic Hypotension, Cardiovascular Outcomes, and Adverse Events: Results From SPRINT. Hypertension 2020;75:660–667. doi: 10.1161/hypertensionaha.119.14309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghazi L, Drawz PE, Pajewski NM, Juraschek SP. The Association of Orthostatic Hypotension With Ambulatory Blood Pressure Phenotypes in SPRINT. Am J Hypertens 2021;34:511–520. doi: 10.1093/ajh/hpaa184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuasa S, Yamamoto H, Suzuki Y, Chin K, Ukai H, Kobayashi Y, Yano Y, Mori H. White-coat effect on orthostatic hypotension: a nationwide survey of Japanese general practitioners. Blood Pressure Monitoring 2. [DOI] [PubMed] [Google Scholar]
- 15.Kario K, Eguchi K, Hoshide S, Hoshide Y, Umeda Y, Mitsuhashi T, Shimada K. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: Orthostatic hypertension as a new cardiovascular risk factor. Journal of the American College of Cardiology 2002;40:133–141. doi: 10.1016/S0735-1097(02)01923-X [DOI] [PubMed] [Google Scholar]
- 16.Patetta LMA, Reffo A, Trevisan C, Curreri C, Giantin V, Franchin A, Sergi G. Orthostatic hypotension and night-time dipper patterns in geriatric outpatients. Hypertension Research 2022. doi: 10.1038/s41440-022-00950-z [DOI] [PubMed] [Google Scholar]
- 17.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:e13–e115. doi: 10.1161/hyp.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 18.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 19.Gilani A, Juraschek SP, Belanger MJ, Vowles JE, Wannamethee SG. Postural hypotension. BMJ 2021;373:n922. doi: 10.1136/bmj.n922 [DOI] [PubMed] [Google Scholar]
- 20.Gupta V, Lipsitz LA. Orthostatic Hypotension in the Elderly: Diagnosis and Treatment. The American Journal of Medicine 2007;120:841–847. doi: 10.1016/j.amjmed.2007.02.023 [DOI] [PubMed] [Google Scholar]
- 21.Freeman R Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med 2008;358:615–624. doi: 10.1056/NEJMcp074189 [DOI] [PubMed] [Google Scholar]
- 22.Heusser K, Tank J, Luft FC, Jordan J. Baroreflex Failure. Hypertension (Dallas, Tex : 1979) 2005;45:834–839. doi: doi: 10.1161/01.HYP.0000160355.93303.72 [DOI] [PubMed] [Google Scholar]
- 23.Kamaruzzaman S, Watt H, Carson C, Ebrahim S. The association between orthostatic hypotension and medication use in the British Women’s Heart and Health Study. Age Ageing 2010;39:51–56. doi: 10.1093/ageing/afp192 [DOI] [PubMed] [Google Scholar]
- 24.Luukinen H, Koski K, Laippala P, Kivelä SL. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med 1999;159:273–280. doi: 10.1001/archinte.159.3.273 [DOI] [PubMed] [Google Scholar]
- 25.Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc 2011;59:383–389. doi: 10.1111/j.1532-5415.2011.03317.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biaggioni I Orthostatic Hypotension in the Hypertensive Patient. Am J Hypertens 2018;31:1255–1259. doi: 10.1093/ajh/hpy089 [DOI] [PubMed] [Google Scholar]
- 27.Masuo K, Mikami H, Ogihara T, Tuck ML. Changes in frequency of orthostatic hypotension in elderly hypertensive patients under medications. Am J Hypertens 1996;9:263–268. doi: 10.1016/0895-7061(95)00348-7 [DOI] [PubMed] [Google Scholar]
- 28.Bhanu C, Nimmons D, Petersen I, Orlu M, Davis D, Hussain H, Magammanage S, Walters K. Drug-induced orthostatic hypotension: A systematic review and meta-analysis of randomised controlled trials. PLoS Med 2021;18:e1003821. doi: 10.1371/journal.pmed.1003821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruland S, Aiyagari V. Cerebral Autoregulation and Blood Pressure Lowering. Hypertension (Dallas, Tex : 1979) 2007;49:977–978. doi: doi: 10.1161/HYPERTENSIONAHA.107.087502 [DOI] [PubMed] [Google Scholar]
- 30.Tryambake D, He J, Firbank MJ, O’Brien JT, Blamire AM, Ford GA. Intensive Blood Pressure Lowering Increases Cerebral Blood Flow in Older Subjects With Hypertension. Hypertension (Dallas, Tex : 1979) 2013;61:1309–1315. doi: doi: 10.1161/HYPERTENSIONAHA.112.200972 [DOI] [PubMed] [Google Scholar]
- 31.Juraschek SP, Daya N, Appel LJ, Miller ER 3rd, McEvoy JW, Matsushita K, Ballantyne CM, Selvin E. Orthostatic Hypotension and Risk of Clinical and Subclinical Cardiovascular Disease in Middle-Aged Adults. J Am Heart Assoc 2018;7. doi: 10.1161/jaha.118.008884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, Karabin B, Kuritzky L, Lew M, Low P, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol 2017;264:1567–1582. doi: 10.1007/s00415-016-8375-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fanciulli A, Jordan J, Biaggioni I, Calandra-Buonaura G, Cheshire WP, Cortelli P, Eschlboeck S, Grassi G, Hilz MJ, Kaufmann H, et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS) : Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res 2018;28:355–362. doi: 10.1007/s10286-018-0529-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnold AC, Okamoto LE, Gamboa A, Black BK, Raj SR, Elijovich F, Robertson D, Shibao CA, Biaggioni I. Mineralocorticoid Receptor Activation Contributes to the Supine Hypertension of Autonomic Failure. Hypertension (Dallas, Tex : 1979) 2016;67:424–429. doi: 10.1161/hypertensionaha.115.06617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wieling W, van Lieshout JJ, van Leeuwen AM. Physical manoeuvres that reduce postural hypotension in autonomic failure. Clin Auton Res 1993;3:57–65. doi: 10.1007/bf01819146 [DOI] [PubMed] [Google Scholar]
- 36.Jordan J, Biaggioni I. Diagnosis and treatment of supine hypertension in autonomic failure patients with orthostatic hypotension. J Clin Hypertens (Greenwich) 2002;4:139–145. doi: 10.1111/j.1524-6175.2001.00516.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan J, Shannon JR, Pohar B, Paranjape SY, Robertson D, Robertson RM, Biaggioni I. Contrasting effects of vasodilators on blood pressure and sodium balance in the hypertension of autonomic failure. J Am Soc Nephrol 1999;10:35–42. doi: 10.1681/asn.V10135 [DOI] [PubMed] [Google Scholar]
- 38.Qiu Y-G, Zhu J-H, Tao Q-M, Zheng P, Chen J-Z, Hu S-J, Zhang F-R, Zheng L-R, Zhao L-L, Yao X-Y. Captopril Administered at Night Restores the Diurnal Blood Pessure Rhythm in Adequately Controlled, Nondipping Hypertensives. Cardiovascular Drugs and Therapy 2005;19:189–195. doi: 10.1007/s10557-005-1376-0 [DOI] [PubMed] [Google Scholar]
- 39.Shibao C, Gamboa A, Abraham R, Raj SR, Diedrich A, Black B, Robertson D, Biaggioni I. Clonidine for the Treatment of Supine Hypertension and Pressure Natriuresis in Autonomic Failure. Hypertension (Dallas, Tex : 1979) 2006;47:522–526. doi: doi: 10.1161/01.HYP.0000199982.71858.11 [DOI] [PubMed] [Google Scholar]
- 40.Donoghue OA, O’Connell MDL, Bourke R, Kenny RA. Is orthostatic hypotension and co-existing supine and seated hypertension associated with future falls in community-dwelling older adults? Results from The Irish Longitudinal Study on Ageing (TILDA). PLoS One 2021;16:e0252212. doi: 10.1371/journal.pone.0252212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garland EM, Gamboa A, Okamoto L, Raj SR, Black BK, Davis TL, Biaggioni I, Robertson D. Renal Impairment of Pure Autonomic Failure. Hypertension (Dallas, Tex : 1979) 2009;54:1057–1061. doi: doi: 10.1161/HYPERTENSIONAHA.109.136853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palma JA, Redel-Traub G, Porciuncula A, Samaniego-Toro D, Millar Vernetti P, Lui YW, Norcliffe-Kaufmann L, Kaufmann H. The impact of supine hypertension on target organ damage and survival in patients with synucleinopathies and neurogenic orthostatic hypotension. Parkinsonism Relat Disord 2020;75:97–104. doi: 10.1016/j.parkreldis.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Measuring Orthostatic Blood Pressure Centers for Disease Control and Prevention. https://www.cdc.gov/steadi/pdf/Measuring_Orthostatic_Blood_Pressure-print.pdf. 2017.
- 44.Arnold AC, Raj SR. Orthostatic Hypotension: A Practical Approach to Investigation and Management. Can J Cardiol 2017;33:1725–1728. doi: 10.1016/j.cjca.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011;21:69–72. doi: 10.1007/s10286-011-0119-5 [DOI] [PubMed] [Google Scholar]
- 46.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology 1996;46:1470. doi: 10.1212/wnl.46.5.1470 [DOI] [PubMed] [Google Scholar]
- 47.de Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P, Rossing P, Zoungas S, Bakris G. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care 2017;40:1273–1284. doi: 10.2337/dci17-0026 [DOI] [PubMed] [Google Scholar]
- 48.Guideline for the diagnosis and management of hypertension in adults National Heart Foundation of Australia https://www.heartfoundation.org.au/getmedia/c83511ab-835a-4fcf-96f5-88d770582ddc/PRO-167_Hypertension-guideline-2016_WEB.pdf. 2016.
- 49.Shin C, Abbott RD, Lee H, Kim J, Kimm K. Prevalence and correlates of orthostatic hypotension in middle-aged men and women in Korea: the Korean Health and Genome Study. Journal of Human Hypertension 2004;18:717–723. doi: 10.1038/sj.jhh.1001732 [DOI] [PubMed] [Google Scholar]
- 50.Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond) 2007;112:157–165. doi: 10.1042/cs20060091 [DOI] [PubMed] [Google Scholar]
- 51.van Wijnen VK, Harms MP, Go-Schön IK, Westerhof BE, Krediet CT, Stewart J, Wieling W. Initial orthostatic hypotension in teenagers and young adults. Clin Auton Res 2016;26:441–449. doi: 10.1007/s10286-016-0382-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Wijnen VK, Harms MPM, Wieling W. Orthostatic Hypotension in the First Minute After Standing Up: What Is the Clinical Relevance and Do Symptoms Matter? Hypertension 2018;71:816–818. doi: 10.1161/hypertensionaha.118.10609 [DOI] [PubMed] [Google Scholar]
- 53.Juraschek SP, Daya N, Rawlings AM, Appel LJ, Miller ER 3rd, Windham BG, Griswold ME, Heiss G, Selvin E. Association of History of Dizziness and Long-term Adverse Outcomes With Early vs Later Orthostatic Hypotension Assessment Times in Middle-aged Adults. JAMA Intern Med 2017;177:1316–1323. doi: 10.1001/jamainternmed.2017.2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juraschek SP, Miller ER 3rd, Appel LJ. Orthostatic Hypotension and Symptoms in the AASK Trial. American journal of hypertension 2018;31:665–671. doi: 10.1093/ajh/hpy010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juraschek SP, Miller ER, Wanigatunga AA, Schrack JA, Michos ED, Mitchell CM, Kalyani RR, Appel LJ. Effects of Vitamin D Supplementation on Orthostatic Hypotension: Results From the STURDY Trial. American journal of hypertension 2022;35:192–199. doi: 10.1093/ajh/hpab147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaw BH, Garland EM, Black BK, Paranjape SY, Shibao CA, Okamoto LE, Gamboa A, Diedrich A, Plummer WD, Dupont WD, et al. Optimal diagnostic thresholds for diagnosis of orthostatic hypotension with a ‘sit-to-stand test’. Journal of hypertension 2017;35:1019–1025. doi: 10.1097/HJH.0000000000001265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lipsitz LA. Orthostatic Hypotension in the Elderly. New England Journal of Medicine 1989;321:952–957. doi: 10.1056/nejm198910053211407 [DOI] [PubMed] [Google Scholar]
- 58.Poon IO, Braun U. High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther 2005;30:173–178. doi: 10.1111/j.1365-2710.2005.00629.x [DOI] [PubMed] [Google Scholar]
- 59.Bird ST, Delaney JA, Brophy JM, Etminan M, Skeldon SC, Hartzema AG. Tamsulosin treatment for benign prostatic hyperplasia and risk of severe hypotension in men aged 40–85 years in the United States: risk window analyses using between and within patient methodology. Bmj 2013;347:f6320. doi: 10.1136/bmj.f6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krediet CT, van Lieshout JJ, Bogert LW, Immink RV, Kim YS, Wieling W. Leg crossing improves orthostatic tolerance in healthy subjects: a placebo-controlled crossover study. Am J Physiol Heart Circ Physiol 2006;291:H1768–1772. doi: 10.1152/ajpheart.00287.2006 [DOI] [PubMed] [Google Scholar]
- 61.Smit AA, Wieling W, Fujimura J, Denq JC, Opfer-Gehrking TL, Akarriou M, Karemaker JM, Low PA. Use of lower abdominal compression to combat orthostatic hypotension in patients with autonomic dysfunction. Clin Auton Res 2004;14:167–175. doi: 10.1007/s10286-004-0187-x [DOI] [PubMed] [Google Scholar]
- 62.Okamoto LE, Diedrich A, Baudenbacher FJ, Harder R, Whitfield JS, Iqbal F, Gamboa A, Shibao CA, Black BK, Raj SR, et al. Efficacy of Servo-Controlled Splanchnic Venous Compression in the Treatment of Orthostatic Hypotension: A Randomized Comparison With Midodrine. Hypertension (Dallas, Tex : 1979) 2016;68:418–426. doi: 10.1161/hypertensionaha.116.07199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Izcovich A, González Malla C, Manzotti M, Catalano HN, Guyatt G. Midodrine for orthostatic hypotension and recurrent reflex syncope: A systematic review. Neurology 2014;83:1170–1177. doi: 10.1212/wnl.0000000000000815 [DOI] [PubMed] [Google Scholar]
- 64.Chen JJ, Han Y, Tang J, Portillo I, Hauser RA, Dashtipour K. Standing and Supine Blood Pressure Outcomes Associated With Droxidopa and Midodrine in Patients With Neurogenic Orthostatic Hypotension: A Bayesian Meta-analysis and Mixed Treatment Comparison of Randomized Trials. Ann Pharmacother 2018;52:1182–1194. doi: 10.1177/1060028018786954 [DOI] [PubMed] [Google Scholar]
- 65.Veazie S, Peterson K, Ansari Y, Chung KA, Gibbons CH, Raj SR, Helfand M. Fludrocortisone for orthostatic hypotension. Cochrane Database Syst Rev 2021;5:Cd012868. doi: 10.1002/14651858.CD012868.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olshansky B, Muldowney J. Cardiovascular Safety Considerations in the Treatment of Neurogenic Orthostatic Hypotension. American Journal of Cardiology 2020;125:1582–1593. doi: 10.1016/j.amjcard.2020.01.037 [DOI] [PubMed] [Google Scholar]
- 67.Fotherby MD, Potter JF. Orthostatic hypotension and anti-hypertensive therapy in the elderly. Postgrad Med J 1994;70:878–881. doi: 10.1136/pgmj.70.830.878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fleg JL, Evans GW, Margolis KL, Barzilay J, Basile JN, Bigger JT, Cutler JA, Grimm R, Pedley C, Peterson K, et al. Orthostatic Hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) Blood Pressure Trial: Prevalence, Incidence, and Prognostic Significance. Hypertension (Dallas, Tex : 1979) 2016;68:888–895. doi: 10.1161/hypertensionaha.116.07474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aronow WS. Orthostatic Hypotension in Diabetics in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) Blood Pressure Trial. Hypertension 2016;68:851–852. doi: 10.1161/hypertensionaha.116.07605 [DOI] [PubMed] [Google Scholar]
- 70.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. JAMA 2016;315:2673–2682. doi: 10.1001/jama.2016.7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Juraschek SP, Hu JR, Cluett JL, Ishak A, Mita C, Lipsitz LA, Appel LJ, Beckett NS, Coleman RL, Cushman WC, et al. Effects of Intensive Blood Pressure Treatment on Orthostatic Hypotension : A Systematic Review and Individual Participant-based Meta-analysis. Ann Intern Med 2020. doi: 10.7326/m20-4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Juraschek SP, Appel LJ, Miller ER, 3rd, Mukamal KJ, Lipsitz LA. Hypertension Treatment Effects on Orthostatic Hypotension and Its Relationship With Cardiovascular Disease. Hypertension (Dallas, Tex : 1979) 2018;72:986–993. doi: 10.1161/HYPERTENSIONAHA.118.11337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rivasi G, Rafanelli M, Mossello E, Brignole M, Ungar A. Drug-Related Orthostatic Hypotension: Beyond Anti-Hypertensive Medications. Drugs Aging 2020;37:725–738. doi: 10.1007/s40266-020-00796-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Juraschek SP, Simpson LM, Davis BR, Beach JL, Ishak A, Mukamal KJ. Effects of Antihypertensive Class on Falls, Syncope, and Orthostatic Hypotension in Older Adults. Hypertension (Dallas, Tex : 1979) 2019;74:1033–1040. doi: doi: 10.1161/HYPERTENSIONAHA.119.13445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Townsend RR, Chang TI, Cohen DL, Cushman WC, Evans GW, Glasser SP, Haley WE, Olney C, Oparil S, Del Pinto R, et al. Orthostatic changes in systolic blood pressure among SPRINT participants at baseline. J Am Soc Hypertens 2016;10:847–856. doi: 10.1016/j.jash.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimbo D, Barrett Bowling C, Levitan EB, Deng L, Sim JJ, Huang L, Reynolds K, Muntner P. Short-Term Risk of Serious Fall Injuries in Older Adults Initiating and Intensifying Treatment With Antihypertensive Medication. Circ Cardiovasc Qual Outcomes 2016;9:222–229. doi: 10.1161/circoutcomes.115.002524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Juraschek SP, Cluett JL, Belanger MJ, Anderson TS, Ishak A, Sahni S, Millar C, Appel LJ, Miller Iii ER, Lipsitz LA, et al. Effects of Antihypertensive De-prescribing Strategies on Blood Pressure, Adverse Events, and Orthostatic Symptoms in Older Adults: Results from TONE. Am J Hypertens 2021. doi: 10.1093/ajh/hpab171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Freeman R, Abuzinadah AR, Gibbons C, Jones P, Miglis MG, Sinn DI. Orthostatic Hypotension: JACC State-of-the-Art Review. J Am Coll Cardiol 2018;72:1294–1309. doi: 10.1016/j.jacc.2018.05.079 [DOI] [PubMed] [Google Scholar]
- 79.Wilcox CS, Aminoff MJ, Slater JD. Sodium homeostasis in patients with autonomic failure. Clin Sci Mol Med 1977;53:321–328. doi: 10.1042/cs0530321 [DOI] [PubMed] [Google Scholar]
- 80.Shear L Orthostatic hypotension. Treatment with sodium chloride and sodium retaining steroid hormones. Arch Intern Med 1968;122:467–471. doi: 10.1001/archinte.122.6.467 [DOI] [PubMed] [Google Scholar]
- 81.Williams EL, Raj SR, Schondorf R, Shen WK, Wieling W, Claydon VE. Salt supplementation in the management of orthostatic intolerance: Vasovagal syncope and postural orthostatic tachycardia syndrome. Auton Neurosci 2022;237:102906. doi: 10.1016/j.autneu.2021.102906 [DOI] [PubMed] [Google Scholar]
- 82.Loughlin EA, Judge CS, Gorey SE, Costello MM, Murphy RP, Waters RF, Hughes DS, Kenny RA, O’Donnell MJ, Canavan MD. Increased Salt Intake for Orthostatic Intolerance Syndromes: A Systematic Review and Meta-Analysis. Am J Med 2020;133:1471–1478.e1474. doi: 10.1016/j.amjmed.2020.05.028 [DOI] [PubMed] [Google Scholar]
- 83.Peng AW, Appel LJ, Mueller NT, Tang O, Miller ER 3rd, Juraschek SP. Effects of sodium intake on postural lightheadedness: Results from the DASH-sodium trial. J Clin Hypertens (Greenwich) 2019;21:355–362. doi: 10.1111/jch.13487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell’Italia LJ, Calhoun DA. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension 2009;54:475–481. doi: 10.1161/hypertensionaha.109.131235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Biaggioni I Blood pressure regulation in autonomic failure by dietary sodium, blood volume and posture. Auton Neurosci 2021;236:102891. doi: 10.1016/j.autneu.2021.102891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mattace-Raso FU, van der Cammen TJ, Knetsch AM, van den Meiracker AH, Schalekamp MA, Hofman A, Witteman JC. Arterial stiffness as the candidate underlying mechanism for postural blood pressure changes and orthostatic hypotension in older adults: the Rotterdam Study. J Hypertens 2006;24:339–344. doi: 10.1097/01.hjh.0000202816.25706.64 [DOI] [PubMed] [Google Scholar]
- 87.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jacobsen SJ, Rodeheffer RJ. Progression of Left Ventricular Diastolic Dysfunction and Risk of Heart Failure. JAMA 2011;306:856–863. doi: 10.1001/jama.2011.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luckey AE, Parsa CJ. Fluid and Electrolytes in the Aged. Archives of Surgery 2003;138:1055–1060. doi: 10.1001/archsurg.138.10.1055 [DOI] [PubMed] [Google Scholar]
- 89.Davy KP, Seals DR, Tanaka H. Augmented Cardiopulmonary and Integrative Sympathetic Baroreflexes but Attenuated Peripheral Vasoconstriction With Age. Hypertension (Dallas, Tex : 1979) 1998;32:298–304. doi: doi: 10.1161/01.HYP.32.2.298 [DOI] [PubMed] [Google Scholar]


