Abstract
Background:
Circadian rhythms play an essential role in physiological function. The molecular clock that underlies circadian physiological function consists of a core group of transcription factors, including the protein Period1 (PER1). Studies in mice show that PER1 plays a role in the regulation of blood pressure and renal sodium handling; however, the results are dependent on the strain being studied. Using male Dahl salt-sensitive (SS) rats with global knockout of PER1 (SSPer1−/−), we aim to test the hypothesis that PER1 plays a key role in the regulation of salt-sensitive blood pressure.
Methods:
The model was generated using CRISPR/Cas9 and was characterized using radiotelemetry and measures of renal function and circadian rhythm.
Results:
SSPer1−/− rats had similar mean arterial pressure (MAP) when fed a normal 0.4% NaCl diet but developed augmented hypertension after three weeks on a high salt (4% NaCl) diet. Despite being maintained on a normal 12:12 light:dark cycle, SSPer1−/− rats exhibited desynchrony MAP rhythms on a high salt diet, as evidenced by increased variability in the time of peak MAP. SSPer1−/− rats excrete less sodium after three weeks on the high salt diet. Furthermore, SSPer1−/− rats exhibited decreased creatinine clearance, a measurement of renal function, as well as increased signs of kidney tissue damage. SSPer1−/− rats also exhibited higher plasma aldosterone levels.
Conclusions:
Altogether, our findings demonstrate that loss of PER1 in Dahl S rats causes an array of deleterious effects, including exacerbation of the development of salt-sensitive hypertension and renal damage.
Keywords: Circadian rhythm, Circadian genes, Dahl salt-sensitive rat, Period1, sodium, aldosterone
Graphical Abstract
Introduction
Nearly every organism exhibits synchronization to the 24-hour day and night cycle, influencing their behavioral and physiological functions (1). This phenomenon, known as circadian rhythm, plays an important role in nearly all physiological processes, including blood pressure and kidney function; disruption of this rhythm can have drastic effects on the aforementioned processes, which can lead to or aggravate health issues, especially so in the cardiovascular system (2, 3). The mechanisms that control the circadian rhythm involve a pacemaker, or central clock, located in the suprachiasmatic nucleus, and four clock proteins, CLOCK (Circadian Locomotor Output Cycles Kaput), BMAL1 (brain and muscle ARNT-like 1), PER1 (period circadian regulator), and CRY (circadian clock component CRYPTOCHROME), which constitute the peripheral clocks and are found in approximately every cell in the body (4). The clock proteins act as transcription factors, modifying the expression of genes in their respective cells and organ systems (5).
It is well established that blood pressure and kidney function follow diurnal patterns, with increasing evidence that these processes are in part modulated by the circadian clock proteins (6). An individual with a healthy circadian rhythm exhibits a 10-20% dip in blood pressure at night. Individuals that do not experience this decrease are characterized as “non-dippers,” and when combined with salt-sensitivity, where an individual’s blood pressure directly correlates to sodium intake, have greatly increased risk of cardiovascular disease (3). Among the clock proteins, PER1 has been associated with both blood pressure and renal handling of sodium, particularly through interactions with the mineralocorticoid hormone aldosterone and the epithelial sodium channel (ENaC) (reviewed in (7)). Studies performed on the salt-sensitive 129/sv mouse model showed that knockout of PER1 was protective in these animals, lowering blood pressure, likely due to decreased ENaC activity (8, 9). In contrast, deletion of PER1 in male salt-resistant C57BL/6 mice resulted in a non-dipping, hypertensive phenotype in response to a high salt diet plus mineralocorticoid treatment (10). Global female PER1 knockout C57BL/6 mice were protected from this phenotype (11). Recently, we showed that kidney-specific knockout of PER1 in the distal nephron and collecting duct resulted in increased urinary sodium retention and increased blood pressure in response to a high salt diet plus mineralocorticoid in mice on a C57BL/6 background (12). These studies demonstrate that PER1 regulates renal sodium handling and blood pressure, but the strain-specific differences in phenotypes show that additional studies are needed to understand the mechanism by which PER1 and the circadian clock affect kidney function and blood pressure control.
The mouse strain-specific information from previous investigations indicates that PER1 action is mechanistically complex, and may depend on the state of salt-sensitivity. Therefore, other models of study could provide vital information. As it was reported by Speed et al., high dietary sodium causes desynchrony of the renal molecular clock in rats, mediated, in part, through activation of endothelin B receptors (13). Recently, Johnston et al. showed that global BMAL1 knockout rats, on the Sprague Dawley background, exhibit lower blood pressure compared to control rats, but maintain their circadian rhythm of blood pressure (14). In contrast, global BMAL1 knockout mice have lower blood pressure than WT mice and display a “non-riser” pattern in which the circadian blood pressure pattern is lost (15). These interesting findings support the need for diverse rodent models in order to better approximate the heterogeneity of human phenotypes. Clearly, in order to fully understand the connection between the molecular clock and blood pressure control, diverse animal models are necessary. The Dahl S rat is an important model for understanding salt-sensitive hypertension be it recapitulates the human condition in which salt-sensitive hypertension drives end-organ damage, including kidney injury (16). Here, we aim to use a salt-sensitive rat model to study the effect of PER1 knockout on the development of salt-sensitive hypertension and kidney function. For the first time, we show that male Dahl SSPer1−/− rats exhibit exacerbated hypertension in response to a high salt diet, and this effect is associated with loss of circadian synchrony in mean arterial pressure and increased kidney damage.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals.
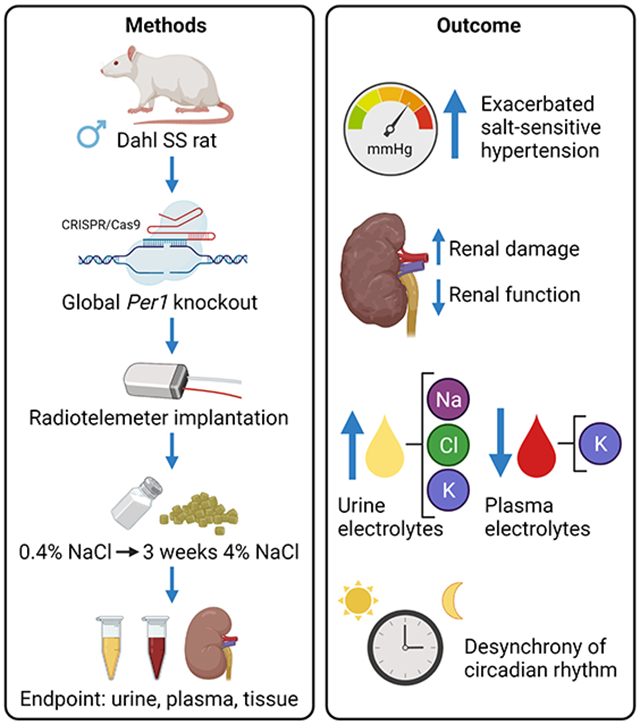
All animal experiments adhered to the National Institute of Health Guide for the Care and Use of Laboratory Animals, and all protocols were reviewed and approved by the Medical College of Wisconsin IACUC. All rats were kept under a normal 12:12 light:dark cycle with lights on at 6 am and lights off at 6 pm. Animal bedding was Sani-Chips®, a hardwood chipped based bedding. The SSPer1−/− rat was created on the Dahl salt-sensitive (SS) rat background (SS/JrHsdMcwi; RRID: RGD_61499) at the MCW Gene Editing Rat Resource Center using CRISPR/Cas9. One base pair of the Per1 gene was deleted in exon 1 (CTCCTCCAGGACAAAAAGGTTCTCCGGgCCTGGGGTCTCCTCCCCCATCAGCCCCT)(confirmed by genomic DNA and cDNA sequencing (Figure S1)), resulting in a truncated protein consisting of a predicted 109 amino acids (Figure S2A). Loss of PER 1 protein was confirmed by Western blot (Figure S2B-D). Animals for these studies were generated from homozygous x homozygous breeding pairs as well as heterozygous x heterozygous breeding pairs. Previously, blood pressure phenotypes were observed in male, but not female, PER1 knockout mice; for this reason, the current study focused on male animals. Power analysis was performed using the SigmaPlot ANOVA sample size function based on previous experimental designs. Male control Dahl SS and SSPer1−/− were weaned at 3 weeks of age and placed on a normal salt 0.4% NaCl diet (Dyets, Inc.; D113755). At around 8 weeks of age, male rats were anesthetized with isoflurane and implanted with a radio telemeter (PA-C40; DSI) in the femoral artery, as described previously (17). Once the animals’ blood pressures stabilized, diets were switched to high salt 4% NaCl (Dyets, Inc.; D113756). After three weeks on the 4% NaCl diet, between 1:00-4:00 pm, animals were euthanized under inhalant isoflurane, kidneys were flushed with PBS and collected along with other tissues which were fixed in 10% formalin and snap-frozen in liquid nitrogen. For circadian rhythm evaluation, telemetry data were analyzed using Cosinor software (circadian.org) and Rayleigh test statistics and plots were generated using Oriana software (https://www.kovcomp.co.uk/oriana/).
Plasma and urine measurements.
On the last day of the 0.4% NaCl diet, and the seventh and twenty-first days of the 4% NaCl diet, animals were placed in metabolic cages overnight for 24-hr urine collection. Upon completion of the dietary protocol, blood was collected by canulating the abdominal descending aorta and spun at 6000 RCF for five minutes to separate and collect plasma. A radiometer (ABL800 FLEX, Radiometer America Inc.) was utilized to measure electrolyte (K+, Na+, Cl−, Ca2+) concentration in urine. Urinary electrolyte values were normalized to 24-hr excretion. Urine and plasma samples were shipped to Marshfield Labs (Marshfield Labs, Waukesha, WI) for analysis of plasma electrolytes as well as renal damage indicators, including creatinine, albumin, and blood urea nitrogen (BUN). These values were used to calculate creatinine clearance using the following equation: Creatinine clearance = (UCr/PCr)(V/24) where UCr = urine creatinine concentration, PCr = plasma creatinine concentration, and V = 24 hr urine volume.
Immunohistochemical analysis.
Kidneys were collected as described previously (18), fixed in 10% formalin, and embedded in paraffin blocks. Tissue blocks were sliced at 4 μm thick slices, deparaffinized, and stained with Masson’s Trichrome. Stained kidney slides were analyzed for cortical fibrosis and medullary protein casts under blinded conditions. Image J software was used to color threshold the respective areas to determine the area percentage of cortical fibrosis or medullary protein casts. Four random non-overlapping fields (400x200 pixels) from the cortex were chosen for both kidneys on each slide; data from eight fields was averaged for each sample. The glomeruli, tubular casts, and large vessels were excluded from the analysis. For each kidney, 100 randomly selected glomeruli were blindly scored on a 0-4 scale. A score of 0 equates to healthy, undamaged glomeruli, with the score increasing based on the amount of glomerulosclerosis, mesangial expansion, capillary health, and hypertrophy/atrophy, up to a score of 4, indicating glomeruli that are severely damaged and barely functioning.
Aldosterone and Catecholamine ELISA.
Plasma aldosterone levels were evaluated by ELISA (ADI-900-173, Enzo, Farmingdale, NY) as described(12). Urinary adrenaline and noradrenaline were measured by ELISA (IB89154R, Immuno-Biological Laboratories, Inc. (IBL-America), Minneapolis, MN).
Statistical analysis.
SigmaPlot 12.5 software (Systat Software, San Jose, CA) was used to perform statistical tests. Data was compared using the Student’s t-test, and the Shapiro-Wilk test was used to test for normality. In the case of experiments with two variables and the use of repeated measures, two-way ANOVA was performed using Graph Pad Prism 9.3 software (GraphPad Software, La Jolla, California) with Holm-Sidak for multiple comparisons. Summarized data are reported as mean±SEM, and statistical significance was determined at P<0.05.
PCR, sequencing, and Western blot methods are described in the supplement.
Results
Per1 knockout exacerbates hypertension in Dahl SS rats on a high salt diet.
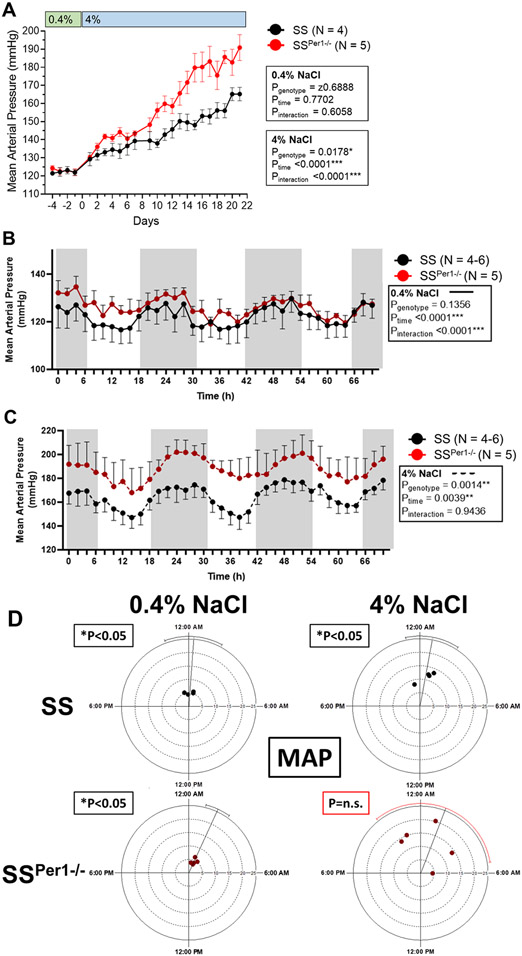
Previous work has found that PER1 plays an important role in renal sodium handling and in blood pressure. In this study, we aim to examine the effects of PER1 knockout in a salt-sensitive rat model. Mean arterial pressure (MAP) was not different between groups when the animals were on the normal 0.4% NaCl diet. Chronic blood pressure recordings show an exacerbation in the development of hypertension in SSPer1−/− compared to SS animals during three weeks on a 4% NaCl diet (Figure 1A). Diurnal blood pressure values were averaged for every 2 hr on the last three days on 0.4% and 4% NaCl diets. Both SSPer1−/− and SS rats exhibit dipping blood pressure during inactive periods on 0.4% NaCl (Figure 1B) and NaCl (Figure 1C) diets. Two-way repeated measures (RM) ANOVA demonstrated a significant genotype effect only during the high salt diet (Figure 1C).
Figure 1. Knockout of PER1 in Dahl SS rats exacerbates salt-sensitive hypertension.
(A) Mean arterial pressure (MAP) was determined by averaging daily pressure recordings from 9:00 am to 12:00 pm in SS and SSPer1−/− rats before and after diet change of 0.4% to 4% NaCl. SS have an N=4 and SSPer1−/− N=5. *P<0.05 was considered significant. (B) Diurnal blood pressure recordings over the last three days on 0.4% NaCl diet; each point is a 2-hour average. (C) Diurnal blood pressure recordings over the last three days on 4% NaCl diet; each point is a 2-hour average. Shaded regions in B and C indicate the active period when lights are off in the animal facility. Data in B and C were evaluated using two-way RM ANOVA; for data in Panels B, and C, N=4 and 5 for SS and SSPer1−/− rats, respectively. (D) Rayleigh plots represent a 24-hour clock, with midnight (the midpoint of the active phase for rats) at the top and noon (midpoint of the rest phase) at the bottom. The placement of each dot relative to time on these 24-hour clocks represents the acrophase, or time of peak MAP. The distance of each dot relative to the center represents the amplitude. Error bars represent the 95% confidence interval, with red bars indicating statistically significant variance in acrophase. P-values indicate results from Rayleigh test statistic. For Rayleigh plots, N=4 SS and N=5 SSPer1−/−.
Cosinor analysis was performed to test the effect of PER1 knockout on the circadian characteristics of the MAP data. Mesor (midline estimating statistic of rhythm, or time-averaged mean), acrophase (time of peak), amplitude (distance from MESOR to acrophase), and robustness (strength of fit to a cosine function) were calculated and compared using two-way ANOVA to test for effects of genotype and high salt diet (Table 1). The only significant genotype-dependent effect on MAP was observed in the mesor values, demonstrating the magnitude of the effect of PER1 knockout to increase MAP in response to a high salt diet (~40 mmHg increase in SS vs. ~60 mmHg increase in SSPer1−/−). The effect of diet to increase the mesor and the interaction of genotype and diet were also significant for the MAP mesor. Consistent with previous findings in rats (19, 20), the high salt diet increased the amplitude of MAP, and this effect was independent of genotype.
Table 1.
Cosinor Analysis of Mean Arterial Pressure
| Treatment Group |
Mesor | Amplitude | Robustness | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Average | SEM | Two-Way ANOVA |
Average | SEM | Two- Way ANOVA |
Average | SEM | Two- Way ANOVA |
|
| SS Normal salt | 125.2 | 1.5 |
Pgenotype 0.0001
Pdiet <0.0001 Pinteraction 0.0003 |
5.1 | 0.3 | Pgenotype 0.6225 Pdiet 0.0002 Pinteraction 0.3969 |
59.0 | 4.3 | Pgenotype 0.1601 Pdiet 0.7252 Pinteraction 0.4187 |
| SS High salt | 164.1 | 4.4 | 11.3 | 1.3 | 69.8 | 3.1 | |||
| SSPer1−/− Normal salt | 126.3 | 1.1 | 4.5 | 0.6 | 53.2 | 11.6 | |||
| SSPer1−/− High salt | 187.9 | 2.9 | 13.5 | 2.8 | 48.9 | 15.6 | |||
Data were evaluated by two-way ANOVA to compare effects of diet and genotype. N=4 SS, N=5 SSPer1−/−
Acrophase values were evaluated by Rayleigh plot (21) (Figure 1D). The presence of the red error bar for SSPer1−/− MAP on the high salt diet indicates loss of synchrony among the SSPer1−/− rats in response to the high salt diet. The Rayleigh test statistic corresponds to the uniformity of data in a circular, in this case, clock-based, plot. The Rayleigh test determines if the time of an event (like the peak, or acrophase, of MAP) occurs in a synchronized manner (P<0.05). Perfect synchrony amongst the rats would have all peaks clustered together, whereas circadian desynchrony would have P>0.05 due to random distribution of peak times. The Dahl SS control rats exhibited significance (P<0.05) for MAP, regardless of diet. In contrast, SSPer1−/− rats exhibited significance (P<0.05) only on normal salt diet, indicating desynchrony amongst the animals in response to the high salt diet.
Heart rate rhythms were assessed in SS and SSPer1−/− rats on the normal salt diet and after 21 days of the high salt diet (Figure S3A-B). Genotype effects on heart rate were not apparent (P>0.5 by two-way (RM) ANOVA). Cosinor analysis, however, revealed that SSPer1−/− rats exhibited significantly lower amplitude of heart rate and also decreased robustness in response to the high salt diet (Table S1). Rayleigh plots for heart rate acrophase demonstrated synchrony in SS control and SSPer1−/− rats, regardless of diet (Figure S3C). Genotype effects on activity were not apparent on the normal salt diet (Figure S4A). In contrast, two-way RM ANOVA demonstrated a significant genotype effect during the high salt diet (Figure S4B). Cosinor analysis demonstrated that circadian activity patterns were also altered by loss of PER1, with the SSPer1−/− rats exhibiting decreased mesor and amplitude in response to the high salt diet (Table S2). Significant Rayleigh test p-values were observed for the SS and SSPer1−/− rats on both normal salt and high salt diets indicating that synchrony of activity amongst the animals was not altered by genotype or diet (Figure S4C).
Knockout of Per1 results in lower animal body weight.
Upon completion of the dietary protocol, animals were weighed, euthanized, and organs were removed, weighed, and collected. SSPer1−/− animals displayed similar body weights to SS rats when fed a normal salt (0.4% NaCl) diet (Figure S5A), but significantly lower body weights than SS rats at the end of the 3-week challenge with high salt diet (Figure S5B). Furthermore, SSPer1−/− rats showed trends for increased kidney (Figure S5C) and heart (Figure S5D) weights relative to their body weight following three weeks on high salt diet.
Evaluation of renal function and damage.
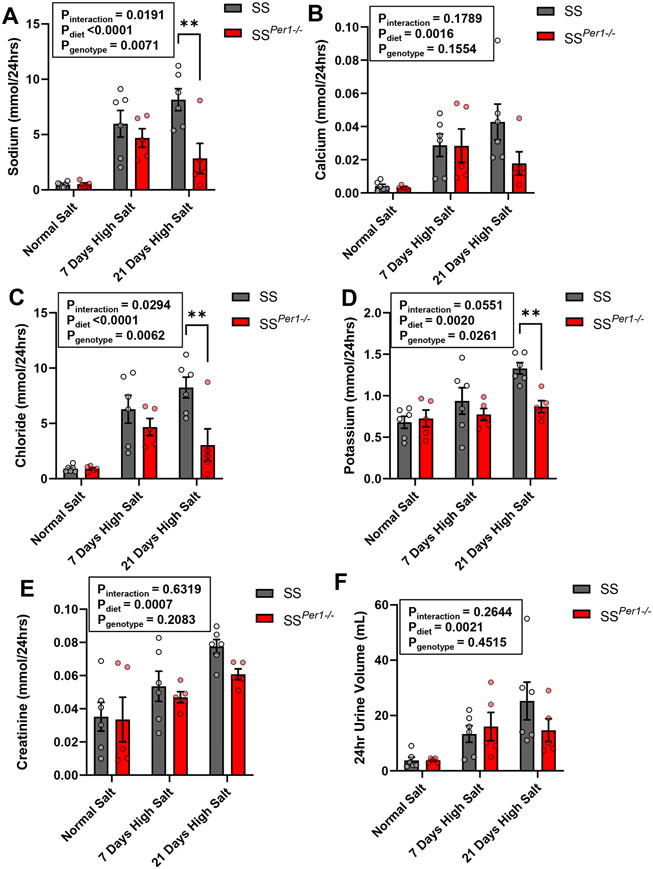
Twenty-four hr urine collections were made during different timepoints on the normal and high salt diets, and plasma was collected during animal euthanasia. SSPer1−/− displayed altered electrolyte handling, evidenced by significantly decreased plasma potassium and calcium (Table S3). Importantly, SSPer1−/− rats exhibited decreased urinary sodium excretion on day 21 of the 4% NaCl diet (Figure 2A), and this effect was similar for chloride and potassium (Figure 2C-D). Calcium levels were not different between groups (Figure 2B). Genotype-dependent effects on creatinine excretion were not apparent (Figure 2E). SSPer1−/− rats appeared to produce less urine by day 21 of the high salt diet (Figure 2F).
Figure 2. SSPer1−/− rats exhibit altered urinary electrolytes compared to SS rats.
(A-D) Values for Na+, Ca2+, Cl−, and K+ normalized to creatinine in SS and SSPer1−/− rats fed a normal 0.4% NaCl diet and 4% NaCl diet (7 and 21 days, respectively). (E) and (F) Creatinine and urine volume values for the same groups. Error bars are SEM, **P < 0.01, ***P < 0.001, and ****P < 0.0001, respectively as determined by two-way ANOVA with Sidak’s post-hoc analysis, N=5 for SS and SSPer1−/− rats.
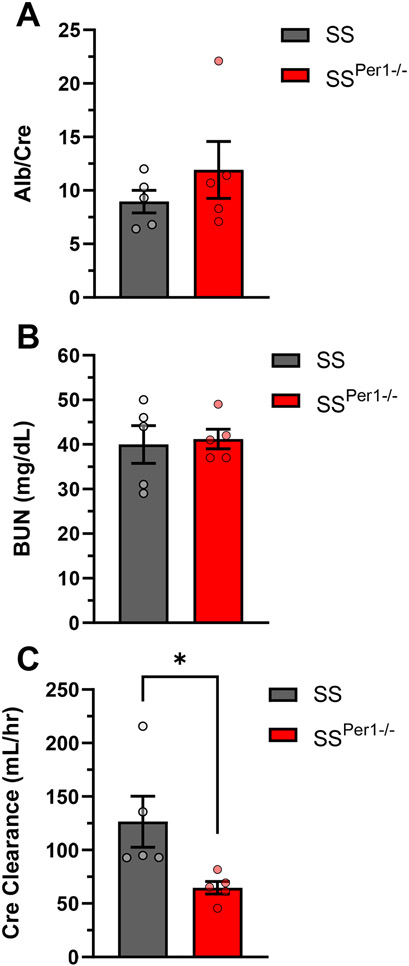
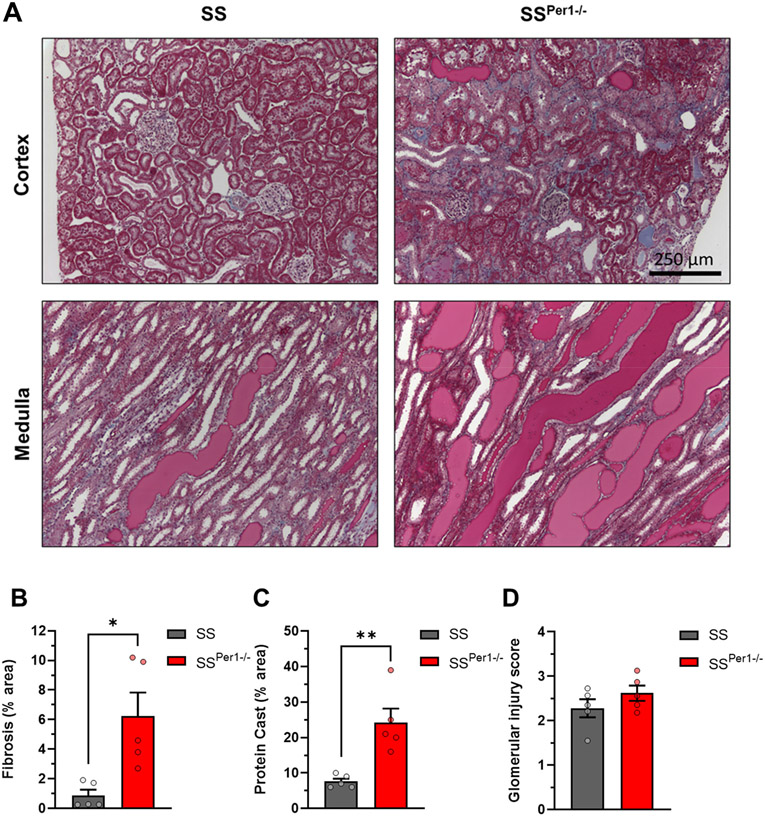
Parameters of renal function were analyzed, such as the plasma albumin normalized to plasma creatinine (Figure 3A), and blood urea nitrogen (BUN) (Figure 3B), both of which showed no differences between groups. Interestingly, creatinine clearance (Figure 3C) was significantly decreased in SSPer1−/− animals, which likely caused the significant increase in plasma creatinine in the SSPer1−/− rats (Table S3), suggesting that these animals have an impaired glomerular filtration rate. At the endpoint, kidneys were collected from animals, fixed in formalin, and kidney tissue was stained with Masson’s trichrome. Representative cortical and medullary images in SSPer1−/− and SS animals are shown in Figure 4A. SSPer1−/− rats experienced increased cortical and medullary damage, as evidenced by significantly increased fibrosis (Figure 4B) and protein cast (Figure 4C) percentages, respectively. Despite showing signs of increased damage of kidney tissue, there was no difference in glomerular injury (Figure 4D) between SS and SSPer1−/− rats.
Figure 3. SSPer1−/− rats exhibit decreased renal function compared to SS rats.
Assessment of renal function and histology in SS and SSPer1−/− rats following 3 weeks on high salt diet. (A) Ratio of plasma albumin normalized to plasma creatinine in SS and SSPer1−/− rats. (B) Plasma blood urea nitrogen (BUN). (C) Calculated creatinine clearance using 24-hour urine excretion. Error bars are SEM, *P < 0.05, N=5 rats in both groups.
Figure 4. Kidney injury is exacerbated in SSPer1−/− rats following three weeks of high salt diet.
(A) Representative images of cortex and medulla from SS and SSPer1−/− rats. (B) Calculated area percentage of fibrosis in cortical tissue. (C) Calculated area percentage of protein casts in medullary tissue. (D) Glomerular injury score, 0-4 scale, each point contains an average of 100 randomly scored glomeruli per animal. Error bars are SEM, *P < 0.05, **P < 0.01, N=5 for SS and SSPer1−/− rats.
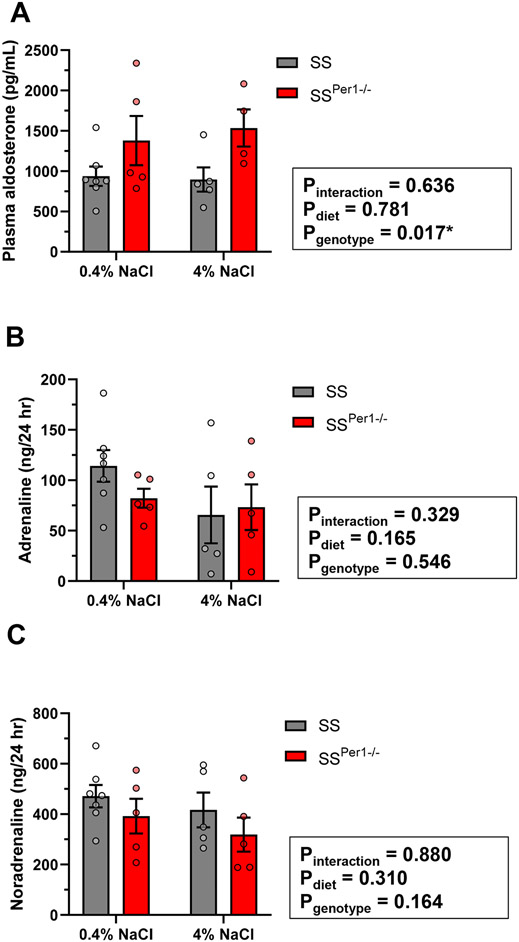
Finally, plasma aldosterone levels were determined, showing that SSPer1−/− rats exhibit higher plasma aldosterone levels compared to SS rats (Figure 5A). To test a putative role for sympathetic activation in the PER1-dependent phenotype, urine catecholamines were measured. Neither genotype nor diet effects were observed in adrenaline or noradrenaline (Figures 5B-C).
Figure 5. SSPer1−/− rats exhibit increased plasma aldosterone compared to SS rats.
(A). Plasma aldosterone levels were determined by ELISA in SS and SSPer1−/− rats fed a normal 0.4% NaCl diet or 4% NaCl diet for 21 days. Urine adrenaline (B) and noradrenaline (C) were measured by ELISA in samples collected from SS and SSPer1−/− rats fed a normal 0.4% NaCl diet or 4% NaCl diet for 21 days. Data are expressed as mean +/− SEM and were analyzed by two-way ANOVA for diet and genotype effects.
Discussion
In this study, we aimed to examine the effects of PER1 knockout in a well-established model of salt-induced hypertension, the Dahl SS rat (22-24). SSPer1−/− rats experience a greater increase in MAP in response to a high salt diet and this effect is associated with decreased sodium excretion. Furthermore, SSPer1−/− rats were smaller in size and showed signs of increased kidney damage and reduced kidney function after high salt diet. These animals are the first rat model with global knockout of a circadian gene on a salt-sensitive background; the data obtained from these studies clearly demonstrate an important role for PER1 in the regulation of salt-sensitive blood pressure and sodium handling.
An interesting outcome of this study is the unexpected finding that SSPer1−/− rats exhibited desynchrony in MAP rhythms, as indicated by loss of synchrony in acrophase following a high salt diet. This finding was unexpected since the rats were housed in a normal 12:12 light:dark cycle. We suspected that circadian rhythm changes such as the desynchrony in MAP observed in SSPer1−/− rats on a high salt diet would be unlikely unless animals are subjected to light cycle manipulations (25). Desynchrony may be considered a form of circadian disruption (26); additional studies are required to determine whether the desynchrony in SSPer1−/− rats is internal (misalignment between the peripheral clocks and the central clock) or external (misalignment between behavior and the external or light/dark cycle). Since activity rhythms were preserved, future studies will test whether internal misalignment occurs in this rat model. Although knockout of the core clock gene BMAL1 in Sprague Dawley rats lowered blood pressure, it had no apparent effects on circadian rhythms in blood pressure (14). Our data suggest that PER1 is required for maintaining circadian synchrony in blood pressure, which is critical for homeostasis, but this must be tested under constant conditions such as total darkness.
Another interesting aspect from the cosinor analysis of MAP rhythms was that the amplitude was increased in both SS and SSPer1−/− rats in response to the high salt diet, a finding that has been observed in rats in previous studies (19, 27). Becker et al. postulated that sympathetic tone may play a role in this effect which is one reason why we investigated urine catecholamines in our model. We did not detect any diet- or genotype-dependent differences in urine adrenaline or noradrenaline, but additional studies looking at earlier time points are necessary to further investigate this mechanism.
Prior work has examined the effects of PER1 knockout on renal electrolyte handling and blood pressure in different strains of mice. Salt-sensitive 129/sv mice with global knockout of PER1 had lower blood pressure compared to WT mice at baseline and after high salt and deoxycorticosterone pivalate (DOCP, an aldosterone analog) treatment (9). It is important to note that a key difference between the salt-sensitive 129/sv mice and Dahl S rats is that the 129/sv mice have an extra copy of the renin gene whereas the Dahl S rat is a model of low renin (28, 29). Our finding that SSPer1−/− rats exhibit elevated plasma aldosterone compared to SS controls suggests a connection between PER1 and the renin angiotensin aldosterone system. These findings are in line with our previous work in C57BL/6 PER1 knockout mice, where deletion of PER1 exacerbated the hypertensive response to a high salt diet plus mineralocorticoid (DOCP), a model of low renin, high aldosterone blood pressure changes (10). SSPer1−/− rats excreted significantly less sodium and potassium than SS control rats and had lower plasma potassium. These differences in electrolyte handling may contribute to the worsened hypertension in the SSPer1−/− animals. The Gumz lab has consistently observed a role for PER1 in the regulation of the expression of ENaC as well as other genes in the kidney (9-11, 30-33). We recently showed that kidney-specific PER1 knockout mice on a C57BL/6 background exhibit increased sodium retention in response to a high salt diet plus DOCP (12). It is clear that the action of PER1 likely depends on a variety of factors, such as variation in genetics.
Chronic hypertension often causes kidney damage which can ultimately lead to kidney failure. SSPer1−/− rats developed a significantly higher MAP than control SS rats, which likely caused increased renal damage, evidenced by higher fibrosis and protein casts. Glomeruli, however, appeared to be of similar health in both groups, suggesting the kidney damage was tubular and interstitial. Furthermore, only creatinine clearance was significantly lower in SSPer1−/− rats while Albumin/Creatinine and BUN were not. This indicates some degree of renal damage and reduction in GFR, however due to the absence of differences in glomerular damage, it is likely that GFR was not drastically affected. Importantly, blood pressure of SSPer1−/− and SS rats was not different when animals were fed a normal salt diet, which indicates the key role of PER1 in the development of salt-induced hypertension. Interestingly, the SSPer1−/− group weighed significantly less following 21 days of high salt, despite having the same body weight at the time of telemeter surgery, so it is unlikely this difference is developmental. The difference in weight is potentially due to altered feeding habits, but unfortunately this can only be speculated as food consumption was not measured. Ultimately, it is evident that disrupting or altering these circadian mechanisms can have many deleterious effects, which is seen in shift workers, who are at greater risk of developing harmful health conditions (3). The tie between circadian rhythm and eating patterns may have influenced the food intake of the animals, which could explain the weight differences between SSPer1−/− and control rats (34).
One of the limitations of this study is the use of only male animals. Other studies have found differences between male and female PER1 knockout mice (11). Kidney-specific cadherin BMAL1 knockout (KS-BMAL1 KO) mice also exhibit sex-specific control of blood pressure (35). Zhang et al. showed similar results to knockout BMAL1 using Aquaporin2-Cre (31). Furthermore, as it was recently reported by Johnston et al., in BMAL1 global knockout rats generated on the Sprague Dawley rat background, Bmal1−/− rats have a clear sex dependent dissociation between circadian blood pressure and control of sodium excretion (14). Thus, it would be beneficial to examine possible sex differences in this animal model. Another limitation was the use of 24-hour urine collections which did not allow for determination of possible differences in night/day patterns of renal excretory function. Future studies will employ increased time points for sampling in order to determine if SS and SSPer1−/− rats exhibit loss of normal rhythms in renal function.
Perspectives. Overall, we have created a novel model for studying the role of the circadian clock protein PER1 in salt-sensitive hypertension. This is the first rat model with global knockout of PER1 that does not require mineralocorticoid treatment to induce hypertension. Our studies indicate that PER1 is indeed important in the development of salt-sensitive hypertension and kidney injury in Dahl salt-sensitive rats. The finding that loss of PER1 is associated with increased plasma aldosterone levels as well as worsened hypertension and increased kidney injury suggests that PER1 is an important link between two homeostatic mechanisms: the circadian clock and the renin angiotensin aldosterone system. Future studies are necessary to determine the mechanisms by which PER1 contributes to homeostatic regulation of blood pressure and kidney function.
Supplementary Material
Pathophysiological Novelty and Relevance
What is new?
First knockout of a circadian clock gene in Dahl SS rats
Loss of PER1 exacerbates the hypertensive and renal damage response to a high salt diet
SSPer1−/− rats exhibit signs of circadian desynchrony in blood pressure
What is relevant?
Knockout of the circadian clock protein PER1 increases salt-sensitive hypertension
The SSPer1−/− rats exhibit changes in rhythms of mean arterial pressure, raising the possibility that this model may be relevant to hypertension and kidney disease in humans who suffer from circadian disruption
Pathophysiological Implications?
This study is the first to demonstrate that knockout of the circadian clock gene Per1 in male Dahl SS rats results in altered circadian rhythms of blood pressure, increased salt-sensitivity, worsened hypertension, and augmented renal injury compared to control Dahl SS rats. The magnitude and direction of the effect of PER1 on blood pressure depend on the rodent strain and the dietary salt content, highlighting the importance of using diverse animal models in order to better understand hypertension and salt-sensitivity and increase the translational potential of basic research studies in these areas.
Acknowledgments
The authors would like to thank Dr. Oleg Palygin (Medical University of South Carolina) for helpful discussions and Mr. Kit-Yan Cheng (University of Florida) for technical support.
Sources of Funding
This research was supported by the National Institute of Health R35 HL135749 (to A.S.), R01 DK109570 (to M.L.G. and A.S.), R56 DK128271 (to M.L.G.), R24 HL114474 (to A.M.G.), and the Department of Veteran Affairs I01 BX004024 (to A.S.).
Nonstandard acronyms:
- SS
Dahl S rat
- KO
knockout
- MAP
mean arterial pressure
Footnotes
Disclosures
The authors declare no conflict of interest.
References
- 1.Buijs FN, León-Mercado L, Guzmán-Ruiz M, Guerrero-Vargas NN, Romo-Nava F, and Buijs RM. The Circadian System: A Regulatory Feedback Network of Periphery and Brain. Physiology 31: 170–181, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Richards J, and Gumz ML. Advances in understanding the peripheral circadian clocks. FASEB J 26: 3602–3613, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douma LG, and Gumz ML. Circadian clock-mediated regulation of blood pressure. Free Rad Biol & medicine 119: 108–114, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crislip GR, Masten SH, and Gumz ML. Recent advances in understanding the circadian clock in renal physiology. Curr Opin Physiol 5: 38–44, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crane BR, and Young MW. Interactive features of proteins composing eukaryotic circadian clocks. Ann Rev Biochem 83: 191–219, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Johnston JG, and Pollock DM. Circadian regulation of renal function. Free Radic Biol Med 119: 93–107, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solocinski K, and Gumz ML. The Circadian Clock in the Regulation of Renal Rhythms. J Biol Rhythms 30: 470–486, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stow LR, Richards J, Cheng KY, Lynch IJ, Jeffers LA, Greenlee MM, Cain BD, Wingo CS, and Gumz ML. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension 59: 1151–1156, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alli A, Yu L, Holzworth M, Richards J, Cheng KY, Lynch IJ, Wingo CS, and Gumz ML. Direct and indirect inhibition of the circadian clock protein Per1: effects on ENaC and blood pressure. Am J Physiol Renal Physiol 316: F807–f813, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solocinski K, Holzworth M, Wen X, Cheng KY, Lynch IJ, Cain BD, Wingo CS, and Gumz ML. Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice. Acta Physiol 220: 72–82, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douma LG, Solocinski K, Holzworth MR, Crislip GR, Masten SH, Miller AH, Cheng KY, Lynch IJ, Cain BD, Wingo CS, and Gumz ML. Female C57BL/6J mice lacking the circadian clock protein PER1 are protected from nondipping hypertension. Am J Physiol Regu Integr Comp Physiol 316: R50–r58, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douma LG, Costello HM, Crislip GR, Cheng KY, Lynch IJ, Juffre A, Barral D, Masten SH, Roig E, Beguiristain K, Li W, Bratanatawira P, Wingo CS, and Gumz ML. Kidney-Specific KO of the Circadian Clock Protein PER1 Alters Renal Sodium Handling, Aldosterone Levels, and Kidney/Adrenal Gene Expression. Am J Physiol Renal Physiol 322: F449–F459, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speed JS, Hyndman KA, Roth K, Heimlich JB, Kasztan M, Fox BM, Johnston JG, Becker BK, Jin C, Gamble KL, Young ME, Pollock JS, and Pollock DM. High dietary sodium causes dyssynchrony of the renal molecular clock in rats. Am J Physiol Renal Physiol 314: F89–f98, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston JG, Speed JS, Becker BK, Kasztan M, Soliman RH, Rhoads MK, Tao B, Jin C, Geurts AM, Hyndman KA, Pollock JS, and Pollock DM. Diurnal Control of Blood Pressure Is Uncoupled From Sodium Excretion. Hypertension 75: 1624–1634, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, and Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A 104: 3450–3455, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudemiller NP, and Mattson DL. Candidate genes for hypertension: insights from the Dahl S rat. Am J Physiol Renal Physiol 309: F993–995, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Pochynyuk OM, Jacob HJ, Geurts AM, Hodges MR, and Staruschenko A. Essential role of Kir5.1 channels in renal salt handling and blood pressure control. JCI insight 2:e92331, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spires D, Ilatovskaya DV, Levchenko V, North PE, Geurts AM, Palygin O, and Staruschenko A. Protective role of Trpc6 knockout in the progression of diabetic kidney disease. Am J Physiol Renal Physiol 315: F1091–f1097, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker BK, Feagans AC, Chen D, Kasztan M, Jin C, Speed JS, Pollock JS, and Pollock DM. Renal denervation attenuates hypertension but not salt sensitivity in ET(B) receptor-deficient rats. Am J Physiol Regu Integr Comp Physiol 313: R425–r437, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Speed JS, Hyndman KA, Kasztan M, Johnston JG, Roth KJ, Titze JM, and Pollock DM. Diurnal pattern in skin Na(+) and water content is associated with salt-sensitive hypertension in ET(B) receptor-deficient rats. Am J Physiol Regu Integr Comp Physiol 314: R544–r551, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Refinetti R, Lissen GC, and Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res 38: 275–325, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polina I, Domondon M, Fox R, Sudarikova AV, Troncoso M, Vasileva VY, Kashyrina Y, Gooz MB, Schibalski RS, DeLeon-Pennell KY, Fitzgibbon WR, and Ilatovskaya DV. Differential effects of low-dose sacubitril and/or valsartan on renal disease in salt-sensitive hypertension. Am J Physiol Renal Physiol 319: F63–f75, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lerman LO, Kurtz TW, Touyz RM, Ellison DH, Chade AR, Crowley SD, Mattson DL, Mullins JJ, Osborn J, Eirin A, Reckelhoff JF, Iadecola C, and Coffman TM. Animal Models of Hypertension: A Scientific Statement From the American Heart Association. Hypertension 73: e87–e120, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fehrenbach DJ, Abais-Battad JM, Dasinger JH, Lund H, and Mattson DL. Salt-sensitive increase in macrophages in the kidneys of Dahl SS rats. Am J Physiol Renal Physiol 317: F361–f374, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, and Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30: 525–536, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Vetter C. Circadian disruption: What do we actually mean? Eur J Neurosci 51: 531–550, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock DM, and Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281: F144–150, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Hartner A, Cordasic N, Klanke B, Veelken R, and Hilgers KF. Strain differences in the development of hypertension and glomerular lesions induced by deoxycorticosterone acetate salt in mice. Nephrol Dial Transplant 18: 1999–2004, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Jiang J, Stec DE, Drummond H, Simon JS, Koike G, Jacob HJ, and Roman RJ. Transfer of a salt-resistant renin allele raises blood pressure in Dahl salt-sensitive rats. Hypertension 29: 619–627, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Richards J, Greenlee MM, Jeffers LA, Cheng KY, Guo L, Eaton DC, and Gumz ML. Inhibition of αENaC expression and ENaC activity following blockade of the circadian clock-regulatory kinases CK1δ/ε. Am J Physiol Renal Physiol 303: F918–927, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gumz ML, Cheng KY, Lynch IJ, Stow LR, Greenlee MM, Cain BD, and Wingo CS. Regulation of αENaC expression by the circadian clock protein Period 1 in mpkCCD(c14) cells. Bioch Biophy Acta 1799: 622–629, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards J, Jeffers LA, All SC, Cheng KY, and Gumz ML. Role of Per1 and the mineralocorticoid receptor in the coordinate regulation of αENaC in renal cortical collecting duct cells. Front Physiol 4: 253, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douma LG, Holzworth MR, Solocinski K, Masten SH, Miller AH, Cheng KY, Lynch IJ, Cain BD, Wingo CS, and Gumz ML. Renal Na-handling defect associated with PER1-dependent nondipping hypertension in male mice. Am J Physiol Renal Physiol 314: F1138–f1144, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendoza J. Food intake and addictive-like eating behaviors: Time to think about the circadian clock(s). Neurosci Biobehav Rev 106: 122–132, 2019. [DOI] [PubMed] [Google Scholar]
- 35.Crislip GR, Douma LG, Masten SH, Cheng KY, Lynch IJ, Johnston JG, Barral D, Glasford KB, Holzworth MR, Verlander JW, Wingo CS, and Gumz ML. Differences in renal BMAL1 contribution to Na(+) homeostasis and blood pressure control in male and female mice. Am J Physiol Renal Physiol 318: F1463–f1477, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.