Abstract
Background:
While men and women with the LRRK2 G2019S variant appear to be equally likely to have Parkinson disease (PD), the sex-distribution among GBA variant carriers with PD, including limited to specific variant severities of GBA, is not well understood. Further, the sex-specific genetic contribution to PD without a known genetic variant is controversial.
Objectives:
To better understand sex differences in genetic contribution to PD, especially sex-specific frequencies among GBA variant carriers with PD (GBA PD) and LRRK2-G2019S variant carriers with PD (LRRK2 PD).
Methods:
We assess differences in the sex-specific frequency in GBA PD, including in subsets of GBA variant severity, LRRK2 PD, and idiopathic PD in an Ashkenazi Jewish cohort with PD. Further, we expand prior work evaluating differences in family history of Parkinsonism.
Results:
Both idiopathic PD (267/420 men, 63.6%) (p<0.001) and GBA PD overall (64/107, 59.8%) (p=0.042) were more likely to be men, while no difference was seen in LRRK2 PD (50/99, 50.5%) and LRRK2/GBA PD (5/10, 50%). However, among GBA PD probands, severe variant carriers were more likely to be women (15/19 women, 79.0%) (p=0.005), while mild variant carriers (44/70 men, 62.9%) (p=0.039) and risk-variant carriers (15/17 men, 88.2%) (p=0.001) were more likely to be men.
Conclusions:
Our study demonstrates that the male-sex predominance present in GBA PD overall was not consistent across GBA variant severities, and a female-sex predominance was present among severe GBA variant carriers. Thus, research and trial designs for PD should consider sex-specific differences, including across GBA variant severities.
Introduction
There is a consistent sex discrepancy in the prevalence of Parkinson disease (PD)1–4, with disease occurring approximately 1.4 times more frequently in men.5 Differences in sex-related frequencies are not well understood, nor are the differential contributions of protective and deleterious factors between men and women.6,7,8–15,16,17 These include longer exposure to potentially protective endogenous or exogenous sex hormones in women.11–15 It has been postulated that men have a higher relative contribution of non-genetic risk factors than women 7, including greater exposure to occupational environmental toxins.8 Differences may also be attributable to sex-specific genetic factors. 18,19 However, evaluation of GWAS data did not identify disparate heritability estimates of autosomal dominant loci between men and women with PD20, and data are conflicting regarding whether family history is increased in women with PD.7,21,22
Differences in sex-related frequency of disease occur in some, but not all genetic forms of PD, and this may depend not only on the gene but also the particular variant.23 However, the evidence is not consistent across studies, including across separate meta-analyses. For example, the male/female ratio in PD associated with the LRRK2 G2019S variant (LRRK2 PD) consistently approximates 50:5021,24–27,15. A landmark meta-analysis evaluating men and women carrying LRRK2 variants overall, and separately by G2019 and G2385 variants, demonstrated that both sexes shared similar odds for developing PD, lending support to the hypothesis that the genetic load in LRRK2 PD outweighs sex-specific protective or deleterious factors. 28 A follow-up meta-analysis studying sex differences in LRRK2 PD demonstrated a higher prevalence of PD in women carrying LRRK2 variants that was attributed to the G2019S variant but not the G2385 variant 29. The distribution of sex in PD associated with GBA variants (GBA PD), the leading genetic contributor to PD, is controversial. While most have shown a male predominance, or more equal sex distribution, a subsequent meta-analysis demonstrated a higher prevalence of women among GBA PD in North America and Europe, but not in Asia or Oceana. 30–32,33 It has separately been suggested that the sex distribution in GBA PD may also vary depending on the specific GBA variant under study.23
To better understand sex differences in genetic contribution to PD and among genetic subgroups, we assess the sex distribution among carriers of GBA variants and/or LRRK2 G2019S, as well as potential gene associated sex differences in family history in a cohort comprising GBA PD, LRRK2 PD, and PD without genetic variants.
Methods
Subjects:
Consecutive probands with PD who reported Ashkenazi Jewish ancestry were invited to a study of genetics of Parkinson research at Mount Sinai Beth Israel/Mount Sinai (New York, USA), including a subset previously reported.7 Participants provided written informed consent, and the study was approved by the Institutional Review Board.
All participants were genotyped for LRRK2 G2019S and the 11 most common GBA variants in the Ashkenazi Jewish population (N370S, L444P, 84GG, IVS2+1, V394L, D409H, A456P, R496H, RecNciI, E326K, T369M,) using the Tag-It™ Mutation Detection Kit (Luminex Molecular Diagnostics, Toronto, ON, Canada) according to the manufacturer’s instructions. Multiplex polymerase chain reaction was used to amplify the regions around the target genes. These regions were subjected to allele-specific primer extension, hybridized to specific Luminex® beads via Universal Tags, and sorted on a Luminex® 100 IS platform (Luminex Corporation, Austin, TX, USA). Genotyping was then completed using the Tag-It™Data Analysis Software (Luminex Molecular Diagnostics), and as previously described.34,35,36
Family history of parkinsonism in first-degree relatives of probands was determined by self-report through family history screen (standardized family history questionnaire or clinical research screen), or by pedigree review.7
Data Sharing:
Data requests from qualified investigators for purposes of replicating procedures and results can be made to the corresponding author for a subset of de-identified data, for which consent for sharing was obtained.
Analysis:
Differences in sex distribution, in the cohort overall, and in probands based on their LRRK2 G2019S and GBA variant status, were determined using one-sample tests of equality of proportions. Odds-ratios were estimated using logistic regression for the association between reported sex and genetic status. GBA variants were subcategorized into three groups based on the variant “severity” and the association between the GBA variant and Gaucher disease (GD), as severe (when biallelic causing neuronopathic GD [84GG, IVS2+1, L444P, RecNcil, V394L]), mild (associated with GD type 1 when biallelic mild or together with a severe variant [N370S, R496H]), and risk-variant (not causing Gaucher disease when biallelic, [E326K, T369M]).37 Screening did not detect any carriers of GBA D409H, and GBA A456P was only present among RecNcil carriers. To determine whether family history of parkinsonism in first-degree relatives differed by sex overall, and separately based on LRRK2 and GBA variant status, sex-specific history of parkinsonism in a first-degree relative was analyzed using unadjusted logistic regression to estimate odds-ratios and standard errors, as well as adjusted for proband age at time of family history collection and age at onset of PD. A sensitivity analysis limited to the history of parkinsonism in a parent was also performed. All analyses were performed using Stata statistical software version 16 (StataCorp, TX).
Results:
Demographic, clinical and family history in first-degree relatives was available for 636 probands with PD, including: 420 idiopathic PD, 99 LRRK2 PD, 107 GBA PD, and 10 LRRK2/GBA PD. Among the 636 PD probands, 250 were women (39.3%) compared with 386 men (60.7%). Family history was obtained by family history screen (n=454) and pedigree review (n=182). 609 of the probands had complete information for the adjusted analysis.
Sex distribution
Sex distribution within groups:
Both idiopathic PD (267/420 men, 63.6% men, p<0.001) and GBA PD overall (64/107 men, 59.8%, p=0.042) were more likely to be men. In contrast, there was no difference in sex-specific distribution in LRRK2 PD (50/99 men, 50.5%) (p=0.920) and LRRK2/GBA PD (5/10 men, 50%) (p=1).
However, among GBA PD probands, severe GBA variant carriers were more likely to be women (15/19 women, 79.0% women, p=0.005), while it was the reverse association with mild GBA variant carriers (44/70 men, 62.9% men, p=0.039), and variant GBA carriers (15/17 men, 88.2% men, p=0.001), who were both more likely to be men.
Sex distribution between groups:
A greater proportion of LRRK2 were women compared with idiopathic PD (LRRK2 PD vs. idiopathic PD 49.5% vs. 36.4, p=0.017), and GBA PD (LRRK2 vs GBA 49.5% vs 40.2%, p=0.18), though the latter comparison was not statistically significant (Table 1, Figure 1).
Table 1:
Summary of sex distribution among each major PD group, and separately among GBA-variant severities
| Among PD groups | ||||
|---|---|---|---|---|
|
| ||||
| Cohort | N | Women (N, % cohort) | Men (N, % cohort) | p-value |
|
| ||||
| Idiopathic PD | 420 | 153 (36.4%) | 267 (63.6%) | <0.001 |
| LRRK2 PD | 99 | 49 (49.5%) | 50 (50.5%) | 0.920 |
| LRRK2/GBA PD | 10 | 5 (50.0%) | 5 (50.0%) | 1.00 |
| GBA PD | 107 | 43 (40.2%) | 64 (59.8%) | 0.042 |
|
| ||||
| Among GBA-variant severities | ||||
|
| ||||
| Severe GBA PD | 19 | 15 (79.0%) | 4 (21.1%) | 0.005 |
| Mild GBA PD | 70 | 26 (37.1%) | 44 (62.9%) | 0.377 |
| Risk-variant GBA PD | 17 | 2 (11.8%) | 15 (88.2%) | 0.002 |
Within group summary of sex distribution in PD overall and in the different genetic groups. Although idiopathic PD and GBA PD are more likely to be men, LRRK2 PD and LRRK2/GBA PD were not. Further, carriers of a severe GBA variant were more likely to be women, unlike carriers of a mild GBA variant, who were more likely to be men. One GBA PD among men was screened as part of an outside study and they were excluded from the variant-specific analysis. Verification of GBA variant status for this participant using our cohort’s methods is ongoing.
Figure 1: Sex Distribution of PD overall and in IPD, LRRK2 and GBA, including by GBA variant. 1A. Comparison of the frequency of LRRK2 and GBA variants (overall) among men and women.
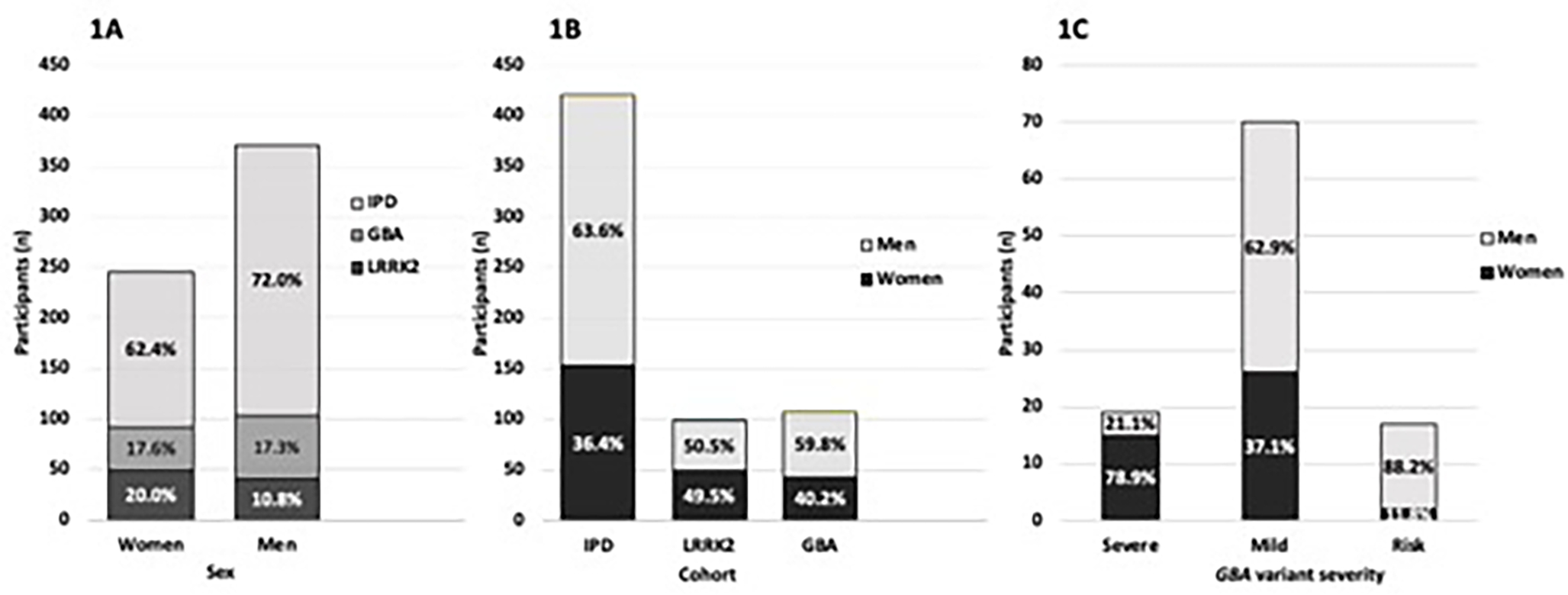
Note the greater proportion of women vs. men with LRRK2 PD (20% vs. 10.8%), similar proportion of GBA PD in both (17.6% vs. 17.3%), and subsequent smaller proportion of idiopathic PD comprising women vs men (62.4% vs. 72.0%) (p=0.057). 1B. Distribution of sex by PD groups. Note the overall greater percentage of men with GBA PD vs. women with GBA-PD (p=0.042), unlike LRRK2 PD (p=0.920), and similar to IPD (p<0.001) 1C. Sex frequency in GBA-PD separated by variant severity, as defined by severe, mild or risk-variant.37 GBA variants include the major variants (both severe and mild variants), which, when bi-allelic decrease activity of the glucocerebrosidase enzyme and cause Gaucher disease (GD), but when monoallelic increase the risk of synucleinopathies including PD, PD with dementia (PD-D), and dementia with Lewy bodies (DLB); and risk-variants, which when biallelic, do not cause Gaucher disease, but are associated with increased risk of PD and DLB.37,56,73 Similar to 1B where GBA variants overall are associated with greater risk in men, there is significant increase in men with mild variants and risk-variants. In stark contrast, however, there is greater frequency of severe variants in women (p=0.001).
Odds ratios comparing GBA PD to idiopathic PD and LRRK2 PD, and separately comparing categories of GBA variants to each other, idiopathic PD and LRRK2 PD are shown in Table 2.
Table 2:
Regression analysis of sex distribution among each major PD group and separately among GBA-variant severities
| Model comparing odds of being a woman among PD groups | ||
|
| ||
| Cohort | OR (95% CI) | p-value |
|
| ||
| LRRK2 PD vs Idiopathic PD | 1.71 (1.10–2.66) | 0.017 |
| LRRK2 PD vs LRRK2/GBA PD | 0.98 (0.27–3.60) | 0.976 |
| LRRK2/GBA PD vs Idiopathic PD | 1.75 (0.50–6.12) | 0.385 |
| GBA PD vs Idiopathic PD | 1.17 (0.76–1.81) | 0.473 |
| GBA PD vs LRRK2 PD | 0.69 (0.39–1.19) | 0.180 |
| GBA PD vs LRRK2/GBA PD | 0.67 (0.18–2.46) | 0.548 |
|
| ||
| Model comparing odds of being a woman including GBA-variant severities | ||
|
| ||
| Cohort | OR (95% CI) | p-value |
|
| ||
| Severe GBA PD vs Idiopathic PD | 6.54 (2.13–20.07) | 0.001 |
| Severe GBA PD vs LRRK2 PD | 3.83 (1.19–12.34) | 0.025 |
| Severe GBA PD vs LRRK2/GBA PD | 3.75 (0.71–19.71) | 0.118 |
| Severe GBA PD vs Mild GBA PD | 6.35 (1.90–21.17) | 0.003 |
| Severe GBA PD vs Risk-variant GBA PD | 28.12 (4.46–177.46) | <0.001 |
| Mild GBA PD vs Idiopathic PD | 1.03 (0.61–1.74) | 0.909 |
| Mild GBA PD vs LRRK2 PD | 1.66 (0.88–3.10) | 0.112 |
| Mild GBA PD vs LRRK2/GBA PD | 0.59 (0.16–2.24) | 0.439 |
| Mild GBA PD vs Risk-variant GBA PD | 4.43 (20.94–0.94) | 0.060 |
| Risk-variant GBA PD vs Idiopathic PD | 0.23 (0.05–1.03) | 0.055 |
| Risk-variant GBA PD vs LRRK2 PD | 0.14 (0.03–0.92) | 0.010 |
| Risk-variant GBA PD vs LRRK2/GBA PD | 0.13 (0.02–0.92) | 0.040 |
Two models estimating odds ratios, comparing odds of being a woman between groups. As was expected, LRRK2 PD were more likely to be women than idiopathic PD (OR, SE: 1.71, 1.10–2.66; p=0.017). Although the odds of being a woman were not different between GBA PD and idiopathic PD (OR, SE: 1.17, 0.76–1.81; p=0.473) or GBA PD and LRRK2 PD (OR, SE: 0.69, 0.39–1.19; p=0.180), severe GBA PD were more likely to be women than both idiopathic PD (6.54, 2.13–20.07; p=0.001), LRRK2 PD (3.83, 1.19–12.34; p=0.025), and even mild GBA PD (6.35, 1.90–21.17; p=0.003).
Of note, severe GBA variant carriers were more likely to be women than idiopathic PD (OR (95% CI) = 6.54 (2.13–20.07), p=0.001), LRRK2 PD (3.83 (1.19–12.34), p=0.025), as well as mild GBA variant carriers (6.35 (1.90–21.17), p=0.003) (Table 2).
Family history of parkinsonism
Among idiopathic PD, female probands were more likely to report a family history of parkinsonism in a first-degree relative compared to male probands (22.5% of women vs 15.4% of men) (OR (SE) =1.70 (0.43), p=0.038) (Figure 2). In the genetic forms, there was no difference in the likelihood to report a family history of parkinsonism in a first-degree relative between men and women: among LRRK2 PD (40.8% of women vs. 30.6% of men, OR (SE)=1.23 (0.51), p=0.622), GBA PD (18.6% of women vs. 18.8% of men, OR (SE)=0.99 (0.50), p=0.985), or LRRK2/GBA PD (20.0% of women vs. 40.0% of men, OR (SE)=0.38 (0.54), p=0.497). There was also no difference when separately subdividing GBA PD by the severity of variants. All results were maintained when adjusting for proband age at time of family history collection and age at onset of PD.
Figure 2. Proportion of participants, overall and by PD group, with a family history of PD, stratified by sex.
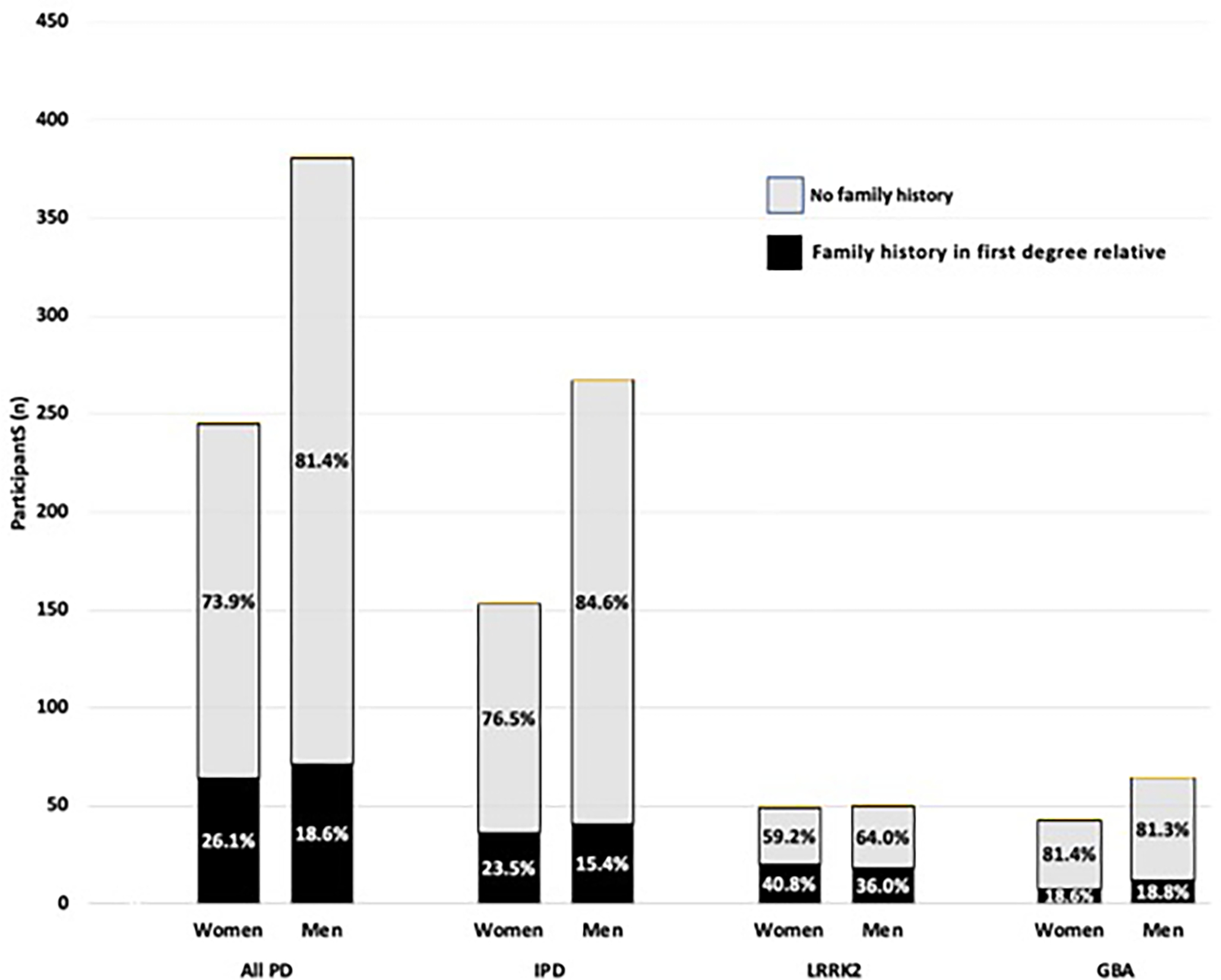
As the black signifies individuals with parkinsonism in a first-degree relative, the grey area nonetheless includes a subset that likely have a major genetic contribution that is yet to be determined. As anticipated, because of lower penetrance in GBA PD compared to LRRK2 PD, the proportion with first degree family member with parkinsonism is greater in the LRRK2 PD (38.4%) than GBA PD (18.7%) (p=0.002). As a smaller proportion of men have a first-degree family history of parkinsonism, there is a larger contribution of other factors including additional genetic, epigenetic, environmental, hormonal and structurally different factors.19,68,69 We cannot exclude that there is also an undetermined “protective factor” portion that is also responsible for the smaller percentage of non-first-degree family member cases in the women. We postulate that the differential lipid response to inflammation that may be responsible for sex differences in GBA variant PD may play a role in idiopathic PD as well. 57–61
Because the penetrance of PD associated variants increases with age30, and siblings are younger and less likely to have reached older ages than the parents, we performed a sensitivity analysis limiting the history of parkinsonism to the parents of the probands. Results from the overall model were maintained such that among idiopathic PD, women were more likely to report a family history of parkinsonism in a parent compared to men (19.6% vs. 11.6%, OR (SE)=1.86 (0.52), p=0.027). There was no difference found between men and women in the likelihood of parkinsonism in a parent among LRRK2 PD (28.6% vs. 32.0%, OR (SE)=0.85 (0.37), p=0.711), GBA PD (11.6% vs. 14.1%, OR (SE)=0.80 (0.48), p=0.715), or LRRK2/GBA PD (20.0% vs. 40.0%, OR (SE)=0.38 (0.54), p=0.497). Lastly, while idiopathic PD were more likely to report a history of parkinsonism in a father (43/61, 70.5%) compared to a mother (19/61, 31.2%) (p=0.002), there was no difference in maternal or paternal risk of parkinsonism among LRRK2 PD or GBA PD (Table 3).
Table 3:
Differences in reported history of paternal and maternal parkinsonism between PD groups
| Parkinsonism in parent N (%) | Parkinsonism in mother N (%) | Parkinsonism in father N (%) | p | |
|---|---|---|---|---|
| Idiopathic PD | 61/420 (14.5%) | 19/61 (31.2%) | 43/61 (70.5%) | 0.002 |
| LRRK2 PD | 30/99 (30.3%) | 16/30 (53.3%) | 16/30 (53.3%) | 1 |
| GBA PD | 14/107 (13.1%) | 5/14 (35.7%) | 9/14 (64.3%) | 0.268 |
| LRRK2 GBA PD | 3/10 (30.3%) | 2/3 (66.7%) | 1/3 (33.3%) | 0.531 |
While idiopathic PD were more likely to report a history of parkinsonism in a father (43/61, 70.5%) compared to a mother (19/61, 31.2%) (p=0.002), there was no difference in maternal or paternal risk of parkinsonism among LRRK2 PD or GBA PD
No difference in age at onset was found between men and women in any PD group. Similarly, proband age at time of family history collection was not different between men and women (p=0.443).
Discussion:
Our study demonstrates that although male-sex predominance was present in GBA PD overall, it was not consistent across GBA variant types and was even reversed for severe variants. This supports that sex differences in GBA PD may be variant dependent.23 Most surprising was our finding that severe-GBA variant carriers were most likely to be women (15/19), while mild-GBA variant carriers and GBA risk-variant carriers (comprised of E326K and T369M carriers) were more likely to be men (15/17). We also replicated most previous reports demonstrating a greater frequency of men in GBA PD overall when considering all GBA variants together33,38, although some have also shown that GBA PD may have similar sex ratios between men and women,30–32 or even greater frequency of women among certain risk variants.39
In order to better address the impact of sex on PD prevalence in genetic subtypes, meta-analyses, including in both Ashkenazi Jewish and non-Ashkenazi Jewish populations, as well as a diversity of mutation types, are necessary. Our study may be divergent from other studies for several reasons. Heterogeneity in cohorts and the frequency of genotypic variants across different ethnic groups40,41, as well as differences in GBA screening methods, may have contributed to the diverse reporting of sex distribution in GBA PD overall.28 European cohorts are more likely to have a greater proportion of severe variant carriers than, for example, Ashkenazi Jewish cohorts in whom the mild variants are most frequent.42,43 GBA screening methodologies, notoriously difficult because of a pseudogene, have changed over time, as has our knowledge of variants considered pathogenic.44 Case ascertainment decisions, specifically whether variant cases (i.e. GBA E326K and GBA T369M) are included as GBA PD, or excluded as GBA DLB, for example, and clinical characteristics, such as whether subjects with primarily dementia versus PD are screened, may lead to significant cohort differences including sex-related differences. Although we did not have systematic data on dementia in family members, our finding that the male sex predominance varied across GBA variant types suggests that the population studied and GBA variants screened likely contribute to some of the phenotypic heterogeneity. A recent meta-analysis in GBA carriers suggests men are more likely to develop DLB compared to women (OR, [95% CI]: 1.60 [0.93, 2.74]), though the increase was not statistically significant (p=0.09).45 To better discern risk-specific sex differences in these sub-groups, inclusion of participants with PD dementia and DLB in future study is warranted. Alternatively, our sex difference may be due to chance and sample size.
The intriguing finding of female predominance among severe GBA carriers will be most important if replicated in further work, but merits discussion as the sex differences in PD are poorly understood. The finding raises questions about possible pathophysiologic differences in expression of the variants.46,47 Severe variants confer the greatest genetic risk of GBA related PD as they have higher penetrance, as well as earlier age of onset.37,48 As such, one might postulate there would be fewer male/female differences. Indeed this is the case for LRRK2 PD, which has a higher penetrance than mild variant or risk-variant GBA, approximating 25% by the age of 80, and no sex-related differences in penetrance.49 We speculate that the sex ratio reversal we observed for severe-GBA variants could be a consequence of a difference in disease expression, but one that encompasses not only PD, but DLB. The spectrum of GBA related synucleinopathy may be thought of as ranging from DLB with particularly early cortical Lewy body deposition, to Parkinson Disease with dementia (PD-D), with cortical Lewy bodies later in the clinical course, to the mildest, Parkinson Disease, with a lower cortical Lewy body burden.23,31,50–54 We propose that men with severe variants may be more likely to develop DLB, with more widespread cortical pathology, while those with mild and risk variants, will more often develop PD-D and PD. In contrast, the women with severe GBA variants may be more likely to develop PD-D and PD, while women with mild and risk-variant variants may be slightly less likely to develop PD than men.
Support for this sex-related differential expression hypothesis includes the observations that GBA variants are also the leading genetic risk factor for DLB, that sex-related differences in GBA variants have been observed both in GBA PD and GBA related DLB23, and that there is a propensity for dementia and more widespread synuclein pathology in men.55 There is also greater risk for dementia in male GBA carriers with parkinsonism, including carriers of the severe GBA variant, L444P.23 Further, in one pathologic and clinical study of DLB, GBA variants were present in 36% of pure DLB cases, and the great majority, 90%, of GBA variant carriers were men.56 It is thus possible that our findings point not to the lack of expression of severe GBA variants in men, but to a varied phenotype of DLB rather than PD. Hence the “missing” severe GBA carriers may not be controls, but rather may have DLB, and the sex-differences described in our study may reflect the preferential sampling of parkinsonism patients (PD and PD-D) from our movement disorders clinic. It is likely that DLB and severe PD-D would instead have presented to the cognitive/behavioral clinic. Had we also evaluated DLB patients, we may have found that the male severe GBA variant carriers clustered in the DLB cohort. However, we did not include DLB in our cohort, and in order to test this hypothesis, future studies are needed that systematically recruit both DLB and PD.
Further, others have reported sex differences that depend on the particular variant, but in a different direction than ours.23 Straniero et al, found that the GBA T369M carriers in their DLB cohort were more likely to be women, whereas in our sample, probands carrying a GBA T369M variant were more likely to be men (7/7 GBA PD, 100%). This highlights that in order to determine the validity of our hypothesis it will be important not only to look at variant severity, but at the sex distribution among GBA PD with severe, mild, and risk-variant GBA variants.
The etiology of sex differences in GBA PD, and idiopathic PD as well, might be attributable in part to sexual dimorphism in sphingolipid metabolism, inflammation, and microgliosis, as well as the more widely studied differences in distribution of environmental and hormonal factors.57–61 These factors may play a role in idiopathic PD without GBA variants, or may be exaggerated in those harboring GBA variants. As GBA-related effects may be mediated, at least in part by substrate accumulation of sphingolipids,62 we postulate that women may be less vulnerable to the additional sphingolipid accumulation than men.
Further, as glucocerebrosidase activity may be decreased in idiopathic PD63, a group that also has a greater predominance of men, our study raises the question as to whether sex-related differences in sphingolipids and lipid metabolism might play a role not only in GBA related parkinsonism, but in idiopathic PD as well. Estrogen may also modulate lipid rafts and preserve neuronal membrane lipids.64 Further, sex has an effect on monocyte gene expression in PD, with greater inflammatory response to lipopolysaccharide in women with PD,57 and overall enhanced expression of interleukin 6, TNF-alpha, and interleukin 1 beta in astrocytes in response to lipopolysaccaride stimulation.65 Several lines of evidence support that sex-hormones may also mediate the microglia response, and the differential immune response could thus mediate sex-dependent effects of inflammation61,66,67. Thus, the degree to which lipid changes are associated with specific GBA variants and present in non-GBA variant PD warrants further examination.
As predicted, our study confirms that the male predominance of PD is lost in LRRK2 G2019S carriers compared with idiopathic PD.21,24,25,27 This is in line with most other studies of major LRRK2 variants, and further supports that the genetic contributions in LRRK2 G2019S carriers outweighs other sex-related factors associated with development of disease.
Finally, in this larger follow-up analysis we continue to demonstrate that a greater proportion of women with idiopathic PD report family history of PD, leading us to postulate that the relative contribution of “extra-genetic” factors is greater among men than women (Figure 2). This is consistent with some,7,21 but not all prior studies. These “extra-genetic” factors may include epigenetic influences, as well as environmental, hormonal and other contributors.10,19,68,69
Our cohort has several limitations. Our sample size was smaller than others, and we did not have complete GBA sequencing. However, because we limited our cohort to Ashkenazi Jews, and were able to ascertain the major GBA variants in this population, it is likely that only a few additional GBA cases were missed.70 Additionally, because we limited our analysis to the LRRK2 G2019S variant, we cannot evaluate the sex-related effect that might be seen with other LRRK2 variants. Further, for our investigation of family history in first-degree relatives, multiple sources of information (pedigree vs. family history screen) were utilized in order to determine the proband’s family history of parkinsonism. However, in sensitivity analyses adjusting for information source, all significant findings remained. We were also not able to clinically confirm the reported diagnosis of parkinsonism in many first-degree relatives. We also cannot exclude that the greater family history seen in female probands compared to male probands is explained by a sex-specific, differential recall bias of PD in a family member,71 though whether a sex-specific differential recall of family history of PD exists has not been consistently shown,72 and our study supports previous reports of a greater family history in women compared to men.7,21 Lastly, it is a limitation that our results may not be generalizable to other populations, particularly those with different founder effects.
Conclusion
Our study highlights sex-related differences in PD, suggesting a proportionally greater non-genetic risk in men that appears to explain much of the sex difference, as well as a postulated sex-related expression difference in phenotype relative to GBA variants. As variants in GBA constitute the leading genetic etiology of PD and DLB, and clinical trials addressing GBA have begun, it will be essential to consider sex related differences in trial design, including allocation schema to treatment and placebo arms. Further study, including meta-analysis in Ashkenazi Jewish and non-Ashkenazi Jewish cohorts continues to be warranted.
Acknowledgements:
The authors are grateful to the study participants who graciously donated their time and energy for this study.
Funding/Sponsor:
Main funding for this proposal was provided by: NIH-NINDS U01 NS107016, NIH-NINDS U01 NS094148, Bigglesworth Family Foundation, Empire Clinical Research Investigator Program, Bachman-Strauss Chair, Bonnie and Tom Strauss Center for Movement Disorders and an anonymous donor.
Footnotes
Conflict of interest statement: None of the authors reports any conflict of interest.
Financial Disclosure: R Saunders-Pullman received funding for this project from the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS) (U01NS107016-01A1 and U01NS094148-01), the Michael J Fox Foundation for Parkinson’s Disease and the Bigglesworth Family Foundation. SB Bressman also received funding from the Michael J Fox Foundation for Parkinson’s Disease. The following authors have no disclosures relevant to this manuscript: RA Ortega, C Wang, L Ozelius, D Raymond, K Leaver, N Urval, V Katsnelson, M Swan, V Shanker.
REFERENCES
- 1.Baldereschi M, Di Carlo A, Rocca WA, et al. Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology. 2000;55(9):1358–1363. doi: 10.1212/WNL.55.9.1358 [DOI] [PubMed] [Google Scholar]
- 2.Morioka S, Sakata K, Yoshida S, et al. Incidence of Parkinson disease in Wakayama, Japan. J Epidemiol. 2002;12(6):403–407. doi: 10.2188/JEA.12.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Lau LML, Giesbergen PCLM, De Rijk MC, Hofman A, Koudstaal PJ, Breteler MMB. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63(7):1240–1244. doi: 10.1212/01.WNL.0000140706.52798.BE [DOI] [PubMed] [Google Scholar]
- 4.Taylor KSM, Cook JA, Counsell CE. Heterogeneity in male to female risk for Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(8):905. doi: 10.1136/JNNP.2006.104695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray Dorsey E, Elbaz A, Nichols E, et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939–953. doi: 10.1016/S1474-4422(18)30295-3/ATTACHMENT/30B76483-5DB3-41F7-993B-E817505F2129/MMC1.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marras C, Saunders-Pullman R. The complexities of hormonal influences and risk of Parkinson’s disease. Mov Disord. 2014;29(7):845–848. doi: 10.1002/MDS.25891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders-Pullman R, Stanley K, Luciano MS, et al. Gender differences in the risk of familial parkinsonism: beyond LRRK2? Neurosci Lett. 2011;496(2):125–128. doi: 10.1016/J.NEULET.2011.03.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frigerio R, Sanft KR, Grossardt BR, et al. Chemical exposures and Parkinson’s disease: A population-based case–control study. Mov Disord. 2006;21(10):1688–1692. doi: 10.1002/MDS.21009 [DOI] [PubMed] [Google Scholar]
- 9.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Risk factors for Parkinson’s disease may differ in men and women: An exploratory study. Horm Behav. 2013;63(2):308. doi: 10.1016/J.YHBEH.2012.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ascherio A, Chen H, Schwarzschild MA, Zhang SM, Colditz GA, Speizer FE. Caffeine, postmenopausal estrogen, and risk of Parkinson’s disease. Neurology. 2003;60(5):790–795. doi: 10.1212/01.WNL.0000046523.05125.87 [DOI] [PubMed] [Google Scholar]
- 11.Popat RA, Van Den Eeden SK, Tanner CM, et al. Effect of reproductive factors and postmenopausal hormone use on the risk of Parkinson disease. Neurology. 2005;65(3):383–390. doi: 10.1212/01.WNL.0000171344.87802.94 [DOI] [PubMed] [Google Scholar]
- 12.Yoo JE, Shin DW, Jang W, et al. Female reproductive factors and the risk of Parkinson’s disease: a nationwide cohort study. Eur J Epidemiol. 2020;35(9):871–878. doi: 10.1007/S10654-020-00672-X [DOI] [PubMed] [Google Scholar]
- 13.Gatto NM, Deapen D, Stoyanoff S, et al. Lifetime exposure to estrogens and Parkinson’s disease in California teachers. Parkinsonism Relat Disord. 2014;20(11):1149–1156. doi: 10.1016/J.PARKRELDIS.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 14.Nicoletti A, Nicoletti G, Arabia G, et al. Reproductive factors and Parkinson’s disease: a multicenter case-control study. Mov Disord. 2011;26(14):2563–2566. doi: 10.1002/MDS.23951 [DOI] [PubMed] [Google Scholar]
- 15.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70(3):200–209. doi: 10.1212/01.WNL.0000280573.30975.6A [DOI] [PubMed] [Google Scholar]
- 16.Agin-Liebes J, Cortes E, Vonsattel JP, Marder K, Alcalay RN. Movement disorders rounds: A case of missing pathology in a patient with LRRK2 Parkinson’s disease. Park Relat Disord. 2020;74:76–77. doi: 10.1016/j.parkreldis.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortese M, Riise T, Engeland A, Ascherio A, Bjørnevik K. Urate and the risk of Parkinson’s disease in men and women. Published online 2018. doi: 10.1016/j.parkreldis.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 18.Canonico M, Pesce G, Bonaventure A, et al. Increased Risk of Parkinson’s Disease in Women after Bilateral Oophorectomy. Mov Disord. 2021;36(7):1696–1700. doi: 10.1002/MDS.28563 [DOI] [PubMed] [Google Scholar]
- 19.Elbaz A, Clavel J, Rathouz PJ, et al. Professional exposure to pesticides and Parkinson disease. Ann Neurol. 2009;66(4):494–504. doi: 10.1002/ANA.21717 [DOI] [PubMed] [Google Scholar]
- 20.Blauwendraat C, Iwaki H, Makarious MB, et al. Investigation of Autosomal Genetic Sex Differences in Parkinson’s Disease. Ann Neurol. 2021;90(1):35. doi: 10.1002/ANA.26090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cilia R, Siri C, Rusconi D, et al. LRRK2 mutations in Parkinson’s disease: confirmation of a gender effect in the Italian population. Parkinsonism Relat Disord. 2014;20(8):911–914. doi: 10.1016/J.PARKRELDIS.2014.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rybicki BA, Johnson CC, Peterson EL, Kortshab GX, Gorell JM. A family history of Parkinson’s disease and its effect on other PD risk factors. Neuroepidemiology. 1999;18(5):270–278. doi: 10.1159/000026222 [DOI] [PubMed] [Google Scholar]
- 23.Straniero L, Asselta R, Bonvegna S, et al. The SPID-GBA study: Sex distribution, Penetrance, Incidence, and Dementia in GBA-PD. Neurol Genet. 2020;6(6). doi: 10.1212/NXG.0000000000000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark LN, Wang Y, Karlins E, et al. Frequency of LRRK2 mutations in early- and late-onset Parkinson disease. Neurology. 2006;67(10):1786–1791. doi: 10.1212/01.WNL.0000244345.49809.36 [DOI] [PubMed] [Google Scholar]
- 25.Orr-Urtreger A, Shifrin C, Rozovski U, et al. The LRRK2 G2019S mutation in Ashkenazi Jews with Parkinson disease: is there a gender effect? Neurology. 2007;69(16):1595–1602. doi: 10.1212/01.WNL.0000277637.33328.D8 [DOI] [PubMed] [Google Scholar]
- 26.San Luciano M, Wang C, Ortega RA, et al. Sex differences in LRRK2 G2019S and idiopathic Parkinson’s Disease. Ann Clin Transl Neurol. 2017;4(11):801–810. doi: 10.1002/acn3.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alcalay RN, Mirelman A, Saunders-Pullman R, et al. Parkinson disease phenotype in Ashkenazi jews with and without LRRK2 G2019S mutations. Mov Disord. 2013;28(14):1966–1971. doi: 10.1002/mds.25647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gan-Or Z, Leblond CS, Mallett V, Orr-Urtreger A, Dion PA, Rouleau GA. LRRK2 mutations in Parkinson disease; a sex effect or lack thereof? A meta-analysis. Parkinsonism Relat Disord. 2015;21(7):778–782. doi: 10.1016/J.PARKRELDIS.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Yan X, Lv H, Liu Y, He Z, Luo X. Gender differences in prevalence of LRRK2-associated Parkinson disease: A meta-analysis of observational studies. Neurosci Lett. 2020;715:134609. doi: 10.1016/J.NEULET.2019.134609 [DOI] [PubMed] [Google Scholar]
- 30.Simuni T, Brumm MC, Uribe L, et al. Clinical and Dopamine Transporter Imaging Characteristics of Leucine- Rich Repeat Kinase 2 (LRRK2) and Glucosylceramidase Beta (GBA) Parkinson’s Disease Participants in the Parkinson’s Progression Markers Initiative: A Cross-Sectional Study. Mov Disord. 2020;35(5):833. doi: 10.1002/MDS.27989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Setó-Salvia N, Pagonabarraga J, Houlden H, et al. Glucocerebrosidase mutations confer a greater risk of dementia during Parkinson’s disease course. Mov Disord. 2012;27(3):393–399. doi: 10.1002/MDS.24045 [DOI] [PubMed] [Google Scholar]
- 32.Li Q, Jing Y, Lun P, Liu X, Sun P. Association of gender and age at onset with glucocerebrosidase associated Parkinson’s disease: a systematic review and meta-analysis. Neurol Sci. 2021;42(6):2261–2271. doi: 10.1007/S10072-021-05230-1 [DOI] [PubMed] [Google Scholar]
- 33.Malek N, Weil RS, Bresner C, et al. Research paper: Features of GBA-associated Parkinson’s disease at presentation in the UK Tracking Parkinson’s study. J Neurol Neurosurg Psychiatry. 2018;89(7):702. doi: 10.1136/JNNP-2017-317348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozelius LJ, Senthil G, Saunders-Pullman R, et al. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2006;354(4):424–425. doi: 10.1056/NEJMC055509 [DOI] [PubMed] [Google Scholar]
- 35.Nichols WC, Pankratz N, Marek DK, et al. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology. 2009;72(4):310. doi: 10.1212/01.WNL.0000327823.81237.D1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran EE, Bressman SB, Ortega RA, et al. Cognitive Functioning of Glucocerebrosidase ( GBA) Non-manifesting Carriers. Front Neurol. 2021;12. doi: 10.3389/FNEUR.2021.635958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gan-Or Z, Amshalom I, Kilarski LL, et al. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology. 2015;84(9):880–887. doi: 10.1212/WNL.0000000000001315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gan-Or Z, Bar-Shira A, Mirelman A, et al. LRRK2 and GBA mutations differentially affect the initial presentation of Parkinson disease. Neurogenetics. 2010;11(1):121–125. doi: 10.1007/S10048-009-0198-9/TABLES/2 [DOI] [PubMed] [Google Scholar]
- 39.Tan EK, Tong J, Fook-Chong S, et al. Glucocerebrosidase Mutations and Risk of Parkinson Disease in Chinese Patients. Arch Neurol. 2007;64(7):1056–1058. doi: 10.1001/ARCHNEUR.64.7.1056 [DOI] [PubMed] [Google Scholar]
- 40.Horowitz M, Pasmanik-Chor M, Borochowitz Z, et al. Prevalence of glucocerebrosidase mutations in the Israeli Ashkenazi Jewish population. Hum Mutat. 1998;12(4):240–244. doi: [DOI] [PubMed] [Google Scholar]
- 41.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2004;351(19):1972–1977. doi: 10.1056/NEJMOA033277 [DOI] [PubMed] [Google Scholar]
- 42.Lesage S, Anheim M, Condroyer C, et al. Large-scale screening of the Gaucher’s disease-related glucocerebrosidase gene in Europeans with Parkinson’s disease. Hum Mol Genet. 2011;20(1):202–210. doi: 10.1093/HMG/DDQ454 [DOI] [PubMed] [Google Scholar]
- 43.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter Analysis of Glucocerebrosidase Mutations in Parkinson’s Disease. N Engl J Med. 2009;361(17):1651–1661. doi: 10.1056/nejmoa0901281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horowitz M, Wilder S, Horowitz Z, Reiner O, Gelbart T, Beutler E. The human glucocerebrosidase gene and pseudogene: Structure and evolution. Genomics. 1989;4(1):87–96. doi: 10.1016/0888-7543(89)90319-4 [DOI] [PubMed] [Google Scholar]
- 45.Liu L, Li J, Quan W, et al. Effect of GBA gene variants on clinical characteristics of dementia with Lewy bodies: a review and meta-analyses. Neurol Sci. 2022;43(6):3541–3550. doi: 10.1007/S10072-022-06031-W/FIGURES/5 [DOI] [PubMed] [Google Scholar]
- 46.Cantuti-Castelvetri I, Keller-McGandy C, Bouzou B, et al. Effects of Gender on Nigral Gene Expression and Parkinson Disease. Neurobiol Dis. 2007;26(3):606. doi: 10.1016/J.NBD.2007.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simunovic F, Yi M, Wang Y, Stephens R, Sonntag KC. Evidence for gender-specific transcriptional profiles of nigral dopamine neurons in Parkinson disease. PLoS One. 2010;5(1). doi: 10.1371/JOURNAL.PONE.0008856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gan-Or Z, Giladi N, Rozovski U, et al. Genotype-phenotype correlations between gba mutations and parkinson disease risk and onsetsymbol. Neurology. 2008;70(24):2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29 [DOI] [PubMed] [Google Scholar]
- 49.Marder K, Wang Y, Alcalay RN, et al. Age-specific penetrance of LRRK2 G2019S in the Michael J. Fox Ashkenazi Jewish LRRK2 Consortium. Neurology. 2015;85(1):89–95. doi: 10.1212/WNL.0000000000001708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goker-Alpan O, Giasson BI, Eblan MJ, et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67(5):908–910. doi: 10.1212/01.WNL.0000230215.41296.18 [DOI] [PubMed] [Google Scholar]
- 51.Clark LN, Kartsaklis LA, Gilbert RW, et al. Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol. 2009;66(5):578–583. doi: 10.1001/ARCHNEUROL.2009.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winder-Rhodes SE, Evans JR, Ban M, et al. Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain. 2013;136(2):392–399. doi: 10.1093/brain/aws318 [DOI] [PubMed] [Google Scholar]
- 53.Brockmann K, Srulijes K, Hauser AK, et al. GBA-associated PD presents with nonmotor characteristics. Neurology. 2011;77(3):276–280. doi: 10.1212/WNL.0B013E318225AB77 [DOI] [PubMed] [Google Scholar]
- 54.Neumann J, Bras J, Deas E, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132(7):1783. doi: 10.1093/BRAIN/AWP044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cholerton B, Johnson CO, Fish B, et al. Sex differences in progression to mild cognitive impairment and dementia in Parkinson’s disease. Parkinsonism Relat Disord. 2018;50:29–36. doi: 10.1016/J.PARKRELDIS.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gámez-Valero A, Prada-Dacasa P, Santos C, et al. GBA Mutations Are Associated With Earlier Onset and Male Sex in Dementia With Lewy Bodies. Mov Disord. 2016;31(7):1066–1070. doi: 10.1002/MDS.26593 [DOI] [PubMed] [Google Scholar]
- 57.Carlisle SM, Qin H, Hendrickson RC, et al. Sex-based differences in the activation of peripheral blood monocytes in early Parkinson disease. NPJ Park Dis. 2021;7(1). doi: 10.1038/S41531-021-00180-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mielke MM, Bandaru VVR, Han D, et al. Demographic and clinical variables affecting mid- to late-life trajectories of plasma ceramide and dihydroceramide species. Aging Cell. 2015;14(6):1014–1023. doi: 10.1111/ACEL.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flores-Cuadrado A, Saiz-Sanchez D, Mohedano-Moriano A, et al. Astrogliosis and sexually dimorphic neurodegeneration and microgliosis in the olfactory bulb in Parkinson’s disease. npj Park Dis 2021 71. 2021;7(1):1–13. doi: 10.1038/s41531-020-00154-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vegeto E, Bonincontro C, Pollio G, et al. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 2001;21(6):1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brockmann K, Apel A, Schulte C, et al. Inflammatory profile in LRRK2-associated prodromal and clinical PD. J Neuroinflammation. 2016;13(1):1–13. doi: 10.1186/S12974-016-0588-5/FIGURES/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alecu I, Bennett SAL. Dysregulated Lipid Metabolism and Its Role in α-Synucleinopathy in Parkinson’s Disease. Front Neurosci. 2019;13(APR). doi: 10.3389/FNINS.2019.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alcalay RN, Levy OA, Waters CC, et al. Glucocerebrosidase activity in Parkinson’s disease with and without GBA mutations. Brain. 2015;138(9):2648–2658. doi: 10.1093/brain/awv179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marin R, Diaz M. Estrogen Interactions With Lipid Rafts Related to Neuroprotection. Impact of Brain Ageing and Menopause. Front Neurosci. 2018;12(MAR). doi: 10.3389/FNINS.2018.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cerri S, Mus L, Blandini F. Parkinson’s Disease in Women and Men: What’s the Difference? J Parkinsons Dis. 2019;9(3):501. doi: 10.3233/JPD-191683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vegeto E, Pollio G, Pellicciari C, Maggi A. Estrogen and progesterone induction of survival of monoblastoid cells undergoing TNF-alpha-induced apoptosis. FASEB J. 1999;13(8):793–803. doi: 10.1096/FASEBJ.13.8.793 [DOI] [PubMed] [Google Scholar]
- 67.Liu X, Fan XL, Zhao Y, et al. Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-alpha and estrogen receptor-beta in microglia. J Neurosci Res. 2005;81(5):653–665. doi: 10.1002/JNR.20583 [DOI] [PubMed] [Google Scholar]
- 68.Mariani E, Lombardini L, Facchin F, et al. Sex-Specific Transcriptome Differences in Substantia Nigra Tissue: A Meta-Analysis of Parkinson’s Disease Data. Genes (Basel). 2018;9(6). doi: 10.3390/GENES9060275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saunders-Pullman R, Gordon-Elliott J, Parides M, Fahn S, Saunders HR, Bressman S. The effect of estrogen replacement on early Parkinson’s disease. Neurology. 1999;52(7):1417–1421. doi: 10.1212/WNL.52.7.1417 [DOI] [PubMed] [Google Scholar]
- 70.Ruskey JA, Greenbaum L, Roncière L, et al. Increased yield of full GBA sequencing in Ashkenazi Jews with Parkinson’s disease. Eur J Med Genet. 2019;62(1):65. doi: 10.1016/J.EJMG.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elbaz A, McDonnell SK, Maraganore DM, et al. Validity of family history data on PD: evidence for a family information bias. Neurology. 2003;61(1):11–17. doi: 10.1212/01.WNL.0000068007.58423.C2 [DOI] [PubMed] [Google Scholar]
- 72.Marder K, Levy G, Louis ED, et al. Accuracy of family history data on Parkinson’s disease. Neurology. 2003;61(1):18–23. doi: 10.1212/01.WNL.0000074784.35961.C0 [DOI] [PubMed] [Google Scholar]
- 73.Tsuang D, Leverenz JB, Lopez OL, et al. GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology. 2012;79(19):1944. doi: 10.1212/WNL.0B013E3182735E9A [DOI] [PMC free article] [PubMed] [Google Scholar]


