Abstract
Early hypotheses of claustrum function were fueled by neuroanatomical data and yielded suggestions that the claustrum is involved in processes ranging from salience detection to multisensory integration for perceptual binding. While these hypotheses spurred useful investigations, incompatibilities inherent in these views must be reconciled to further conceptualize claustrum function amid a wealth of new data. Here, we review the varied models of claustrum function and synthesize them with developments in the field to produce a novel functional model: Network Instantiation in Cognitive Control (NICC). This model proposes that frontal cortices direct the claustrum to flexibly instantiate cortical networks to subserve cognitive control. We present literature support for this model and provide testable predictions arising from this conceptual framework.
Keywords: cognition, attention, working memory, cognitive network, cortex, cortical network
A PRIMER ON THE CLAUSTRUM
The claustrum is a thin, elongated sub-cortical telencephalic nucleus (Figure 1). It possesses bidirectional connections with many areas of the cerebral cortex and receives input from select subcortical regions [1–13] (Figure 2; see Supplementary Table S1). The claustrum is positioned between the insula and putamen and, in primates, is bounded by the external and extreme capsules (Figure 1). The claustrum appears in all therian mammals, at least some monotremes [14–16], and a potential homologue was recently discovered in reptiles [17,18], and possibly birds [19]. The claustrum is composed of spiny glutamatergic projection neurons that exit the claustrum to predominantly target cortex. In addition, multiple subpopulations of GABAergic, aspiny interneurons reside in the claustrum. They are differentiated by their expression of calcium-binding proteins or neuropeptides, including parvalbumin [20–22], somatostatin, and vasoactive intestinal peptide [20].
Figure 1. The location of the claustrum in mouse, monkey, and human.

The claustrum (red, CL) in mouse lies lateral to the striatum (Str) and external capsule of white matter and is positioned within the deep layers of insular cortex (IC) [145]. The claustrum in primates is located between two white matter (WM) structures, the external and extreme capsules. Lateral to the primate extreme capsule of white matter lies the insular cortex (IC), and medial to the external capsule of white matter are the striatal caudate (Cd) and putamen (Pu). The claustrum is likely present in all mammals [14–16], in at least some reptiles [17], and an analogous structure is identified in avian species [19]. As such, the claustrum is speculated to have formed early in the evolution of amniotes [146,147]. This figure was created using BioRender.
Figure 2. Anatomical connections of the claustrum in rodents, cats, and monkeys.

A survey of known inputs to the claustrum (left) and outputs (right) from the claustrum. Cortical (top) and subcortical (bottom) connections are depicted. Studies used to generate this figure are reviewed in Table S1. Abbreviations: A1, primary auditory cortex; ACC, anterior cingulate cortex; AP, anterior pole; IPL, inferior parietal lobule; M1, primary motor cortex; PtA, parietal association cortex; S1, primary somatosensory cortex; V1/V2, primary and secondary visual cortex.
The elongated shape of the claustrum and its proximity to the dorsolateral striatum (putamen in primates), the insular cortex, and white matter structures have historically limited the ability to specifically manipulate the claustrum. As a result, most early hypotheses of claustrum function are disproportionately based on structural data obtained from neuronal tract-tracing. However, the advent of neural circuit-specific approaches and whole-brain functional imaging enables a more direct assessment of claustrum function through the recording of claustrum activity in the context of behavior and cognition. Considering the resulting expanded functional dataset, the diverse field of claustrum hypotheses may now be revisited and refined.
Here we first describe previous hypotheses of claustrum function before advancing a new theory that unifies earlier concepts: specifically, that the claustrum receives frontal cortical signals for coordinating the engagement of downstream cortical areas to meet cognitive demands. We term this model network instantiation in cognitive control (NICC). We describe evidence for the NICC model, examine the model’s similarities and differences with other theories of claustrum function, and articulate predictions of the NICC model that can be tested in future research.
EXISTING FUNCTIONAL HYPOTHESES
Models of claustrum function fall into three “families” of hypotheses that relate to: integration of cortical sensory information, salience and attentional processing, and cortical network dynamics. These hypotheses will be described in roughly the order they have been proposed.
Integration
Guided by an early claustrum lesion study in dogs in which auditory conditioning was impaired [23], along with observations of non-somatotopic multimodal responses in the claustrum, Spector et al. [24] hypothesized that the claustrum associates sensory stimuli across modalities. The involvement of the claustrum in sensory processing was supported in an earlier study in curarized cat in which claustrum neuronal firing was observed in response to somatic nerve stimulation [25].
In an influential proposal, Ettlinger and Wilson [26] postulated that all areas of cortex have access to information from other (sensory) cortices through the claustrum, passing information through a series of cortico-claustro-cortical channels. In this way, the claustrum would function as a cross-modal transfer locus to support sensory integration. Crick and Koch [27] expanded upon the Ettlinger and Wilson [26] sensory integration model by proposing that the claustrum participates in perceptual binding by coordinating cortical processing. Perceptual binding refers to the process by which prior experience and ongoing sensory information gathered across multiple modalities are combined into a singular, cohesively perceived experience.
Following tract-tracing studies demonstrating widespread bi-directional claustrum connectivity with the cerebral cortex, Alloway et al. [28] proposed a somewhat divergent function compared to Crick and Koch. They proposed a sensorimotor integration function that, at least in rodents, would serve to bilaterally coordinate whisker movement. This proposal was a landmark in proposing motor control as a possible outcome of claustrum-mediated sensory integration. Later, an investigation by Smith et al. [29] established that the claustrum receives input from the whisker representation in motor cortex (but not somatosensory cortex). This led to an updated proposal that the claustrum cannot participate in cross-modal integration of whisker representations, though it may still participate in coordination of the two cortices. More recently, Chevee et al. [30] detected few to no responses to sensory stimuli in mouse anterior claustrum, but instead observed abundant motor planning responses during a cross-modal sensory task.
In an elaboration upon the “binding hypothesis” of Crick and Koch, Smythies et al. [31] proposed that the claustrum synchronizes oscillations from different cortical regions to give rise to binding. This hypothesis was further refined by Smythies et al. [32], which, similar to Crick and Koch [27], speculated about the existence of intra-claustral interactions and the possibility of dynamic cortico-claustro-cortical circuits that would serve as a mechanism to regulate cortical synchrony for perceptual binding.
These iterations of the “binding hypothesis” rely upon claustrum neurons being responsive to multimodal sensory inputs. While a function for the claustrum in multimodal sensory processing is supported by early work in curarized cat [24,25] and during multimodal tasks in human positron emission tomography [33], it is challenged by later observations of unimodal responses to naturalistic stimuli in awake monkeys [34] and an observed lack of sensory responses in anterior claustrum of mouse in response to visual or tactile stimuli [30]. The “binding hypothesis” also suggests that the claustrum is integral in producing states of consciousness. However, in a study of five epilepsy patients, bilateral electrical stimulation of the claustrum did not result in loss or alteration of subjective awareness [35], and the claustrum remains relatively quiescent during a conscious resting state [24,25,34,36]. Despite a lack of strong evidence for the claustrum as a conductor of consciousness, these series of hypotheses generated core concepts that guided the emergence of a new generation of salience and attentional models of claustrum function.
Salience Detection and/or Attention
The salience detection/attention model of claustrum function was prompted by anatomical data showing that the claustrum is strongly connected with anterior cingulate cortex (ACC), which is implicated in monitoring conflicts in decision making processing [37,38] and is a component of the salience network (SN) [39,40]. In this model, as proposed by Remedios et al. [41], the claustrum identifies changes in the sensory scene to alert other brain areas, a process that was proposed to contribute to sensory awareness. Observations that responses in primate claustrum are primarily unimodal [34], similar regardless of the type of auditory stimuli (naturalistic or conspecific vocalization), only active at stimulus onset, and scaled with signal-to-noise ratio [41] support this hypothesis.
In an updated view, Goll et al. [42] proposed that the claustrum mediates the allocation of cortical processing for selective attention. In this model, claustro-cortical projections suppress cortical activity to reduce processing of unattended information. Thus, the claustrum is proposed as an “attentional searchlight”, a term Crick [43] coined when discussing a similar function for the thalamic reticular nucleus. This hypothesis was recently tested in mouse, where it was found that claustrum neurons in rodent appear to primarily encode signals related to motor planning, rather than the attended sensory modality [30].
Later, in response to functional magnetic resonance imaging (fMRI) observations of functional connectivity between the claustrum, insula, medial prefrontal cortex, and cingulate cortex in rodent, Smith et al. [44] proposed that the claustrum integrates sensory and limbic information to facilitate both bottom-up and top-down attentional processes. Specifically, the claustrum was proposed to integrate input from the basolateral amygdala, mediodorsal thalamus, and the medial prefrontal cortex to then distribute that information to cortical areas of the relevant modality, such as the frontal eye field, ultimately resulting in attentional and behavioral orientation to a stimulus; a function not unlike that proposed for the superior colliculus [45]. Smith et al. [44] proposed this model as a circuit-based elaboration of a previous hypothesis, forwarded by Reser et al. [6], who suggested that due to the large voxel size in fMRI, some activity historically attributed to the anterior insula may be a product of claustrum activity. Thus, it would follow that some roles attributed to anterior insula (such as participation in the SN and salience processing) may in fact be claustrum functions. Jackson et al. [46], in a recent review of claustrum literature, reinforced a focus on claustrum as a contributor to attentional processes by distributing limbic information to cortex to inform actions and perception via inhibitory control of cortex.
Cortical Networks and Cognitive Control
The claustrum was recently proposed to play a role in cortical network switching [6,7]. In this section we will first give a brief overview of cortical networks and cognitive control, then more specifically describe how the claustrum fulfills a key mechanism of network interaction facilitating cognitive control. This lays the groundwork for the presentation of our new model of claustrum as a key hub modulating networks of cognitive control, presented in the next section.
Cognitive control is the ability to initiate, maintain, and monitor relevant information during goal-directed action, as opposed to habitual stimulus-response behavior [47,48]. Cognitive control, therefore, enables adaptive behavior in response to environmental challenges. This is distinct from other cognitive sub-processes such as selective attention, which is the ability to increase signal-to-noise detection of particular sensory modalities over others or to increase signal-to-noise detection of features within a sensory modality [49]. As an umbrella cognitive faculty, cognitive control is the ability to muster, for instance, working memory and selective attention processes for task performance.
The degree to which cognitive control is implemented, known as cognitive demand, is a function of task difficulty [50]. Task difficulty increases with task variable and rule number, as well as rule complexity. In an animal laboratory setting, an example of a “cognitively non-demanding” task would be a requirement to nose-poke a lone port each time it illuminates for a sugar reward (e.g., the one-choice serial response time task). This contrasts with a “high cognitive demand” rodent task wherein five ports may each, randomly, illuminate with each trial (e.g., the five-choice serial response time task), requiring the animal to monitor five different ports to successfully poke the one that illuminates. Despite the same physical requirement between the two tasks (correct nose poke required for a reward), the five-choice task is more difficult to learn and, once learned, error rates are higher compared to the one-choice task version. Thus, the higher cognitive demand of the five-choice task requires greater mobilization of cognitive control to perform.
Cortical networks underlie cognitive control.
Cognitive control demands flexible engagement of cognitive processes from moment to moment. Meeting these demands is the flexible engagement of networks of brain regions. Networks are defined as collections of functionally correlated regions (as assessed during whole-brain neuroimaging) identified during task-based or task-free (i.e., resting state) periods of measurement [51–53], and largely include cortical regions [54–56] across species [54,57–64] (Box 1). Several groups attempted to map the human brain’s network organization [65–69]. Such parcellations are not identical, but they consistently include local sensory and somatomotor networks, as well as reproducible, geographically distributed networks of association cortices. A widely cited seven-network parcellation [65] of the human brain includes: visual and somatomotor networks; a default mode network (DMN) notable for increased metabolic activity at rest and decreased BOLD signal during externally focused tasks [53,70]; a frontoparietal network (FPN) active during tasks; dorsal (DAN) and ventral (VAN) attention networks active during tasks; and a limbic network. More granular parcellations reveal additional networks, such as sub-components of the DMN [54], as well as the SN and a cingulo-opercular network (CON) overlapping with the VAN [52]. More broadly, a commonly used gross taxonomy divides the brain into two anti-correlated networks: a task-positive network active during tasks and a task-negative network (the latter largely overlaps with the DMN) [71].
Box 1. From Cortical Synchrony to Network Emergence.
Synchronous oscillations between different cortical areas are theorized to be vital for cross-regional communication and necessary for cortical network performance [148]. This is because input from other cortical areas, synchronized with local oscillations, would be more likely to elicit action potentials than off-phase input [149,150], thereby decreasing cortical noise and amplifying signal [151]. As such, transmission of neural signals between non-synchronized regions is depressed, while transmission between synchronous regions is enhanced. This process allows for selective gating of information flow through the brain, as directly demonstrated by the ability, in humans, to direct top-down signals from prefrontal cortex to different posterior visual areas depending on the type of task being performed [152].
Evidence indicates that increases in cortical synchrony are associated with increased network integrity. Electroencephalography (EEG) detected cortical oscillations correlate with functional connectivity and network strength across a variety of task-states [153–155]. Causally, synchronous transcranial alternating current stimulation (tACS) of left and right somatosensory cortex increases functional connectivity between those areas [156]. During a working memory task, power-synchronous tACS stimulation of the middle frontal gyrus and inferior parietal lobule nodes (right FPN associated areas) enhances FPN activation in humans [157]. Synchronous network processing is required for optimal cognition and task performance. Induction of synchrony using tACS stimulation improves human performance on a working memory task [157], while optogenetically driving fast-spiking interneurons in mouse barrel cortex induces theta synchrony and improves somatosensory discrimination [158]. Additionally, transcranial magnetic stimulation enabled disruption of frontal and parietal cortices engaged in networked processing disrupts task goal updating [159]. These findings support the view that synchronized oscillatory activity supports cross-regional communication, cognitive network integrity, and performance on tasks that require multiple cognitive faculties. Thus, the ability to induce cortical synchrony for enhanced network integrity may support a cognitive control function.
Several cortical networks composed of prefrontal cortical regions are associated with cognitive control [72]. The FPN [62] comprises regions associated with functions such as decision-making, maintaining task sets, and working memory. The DAN [73,74] is “involved in preparing and applying goal-directed (top-down) selection for stimuli and responses” [74], while the VAN directs orientation toward salient stimuli in a bottom-up fashion [74]. As mentioned above, the SN and CON largely overlap with the VAN. The SN – as the name implies – activates in the presence of salient stimuli or thoughts [75], and the CON shows sustained activity related to maintaining and updating rules in a task [76]. While the CON and SN are distinct networks, they possess similar topography and the labels are sometimes used interchangeably in the literature [55,66].
Several models have been proposed for how these cortical networks interact to dynamically support cognitive states. They include the VAN updating the DAN in response to potentially important environmental sensory stimuli [74], the SN or CON performing the “switch” between task-negative and task-positive networks [39,77,78], and a dual-network model wherein a rapid-adaptive FPN and a set-maintenance CON act in parallel. That is, the FPN quickly adapts to initiate and adjust cognitive control during a task while the CON, on a longer timescale, supports task set maintenance [79].
With this high degree of complexity, the field has gravitated to graph theory analyses of whole brain imaging data to understand network interactions. Conceptualizing the brain as a collection of nodes (brain regions) connected by edges (structural/functional connectivity) allows analysis of the networks in terms of communities of nodes varying in the number (nodal degree) and diversity (participation coefficient) of their connections. For example, diffusion tensor imaging data across 21 human participants was used to identify a collection of regions that possess a high number of connections (large nodal degree) throughout the brain [80]. A largely distinct but overlapping set of hubs, labeled the “diverse club”, which connect multiple network communities, is identified [81]. These hub regions exhibit substantial overlap with cognitive control-associated networks such as the FPN, DAN, and CON, and hub activation modulates task-based cortical network functional connectivity [81,82]. As such, dynamic cognitive responding to changing task demands requires hub activation to orchestrate the substrate of cognitive control: cortical networks. Therefore, investigation of high participation coefficient regions (i.e., network hub regions) may yield insight into the neural circuit basis of cortical network emergence.
The claustrum as a locus for cognitive control.
The claustrum possesses hallmark characteristics of a network hub region. The claustrum has the highest connectivity per unit volume in the brain [10]. Similar to diverse club hubs [80,81], the effects of removing claustral connections from network graphs suggest they efficiently link multiple brain networks in health [10]. In many brain disorders, dysfunction is more likely to be found in highly connected regions, such as diverse club regions, than elsewhere [82]. Accordingly, claustrum abnormalities are associated with a variety of neuropsychiatric symptoms [10,83], including difficulties in cognitive control [84].
The claustrum is structurally connected with cognitive control regions. In humans, the claustrum shares its densest structural connectivity with frontal cortices [10], which anchor cognitive control networks [72]. In monkey, the claustrum projects to areas 9, 10, and 12, which include the frontal pole and dorsal and ventral rostral prefrontal cortices [7]. The claustrum also receives strong input from frontal cortical regions including the dorsolateral prefrontal cortex in monkey [85–88] and the ACC in monkey and rodents [6,11,12,89]. Taking just one of these areas as a representative to describe what type of signals the claustrum may receive from frontal regions that orchestrate cognitive control, the ACC is a key node in the SN and CON, which has been placed within a “core system” for cognitive control [77,90]. Dorsal ACC is proposed to be responsible for determining the expected value of control: defined as an evaluation of the potential benefits of exerting cognitive control against the potential costs [90]. Thus, the claustrum is situated to receive these and other types of cognitive control signals to direct dynamic interplay of cortical networks to optimize cognitive control. In the next section, we describe a model for claustrum function that expands on this general concept.
THE NICC MODEL
We propose that the claustrum serves a cognitive control function by instantiating a cortical network with appropriate network integrity for optimal cognitive performance. To accomplish this, the NICC model proposes that the claustrum: 1) receives a signal from frontal cortices to prompt the emergence of a specific network (a network initiation signal); 2) transforms and amplifies this signal and; 3) broadcasts the transformed signal through return projections to frontal cortices and projections to posterior sensory and association cortices to disrupt prior processing and bring targeted cortical network nodes into phase (Figure 3). This allows other inputs (such as from thalamus) to set oscillatory frequency and continue maintenance of synchronization. We propose this process is integral to the successful performance of tasks that require cognitive control.
Figure 3. The Network Instantiation in Cognitive Control (NICC) model.
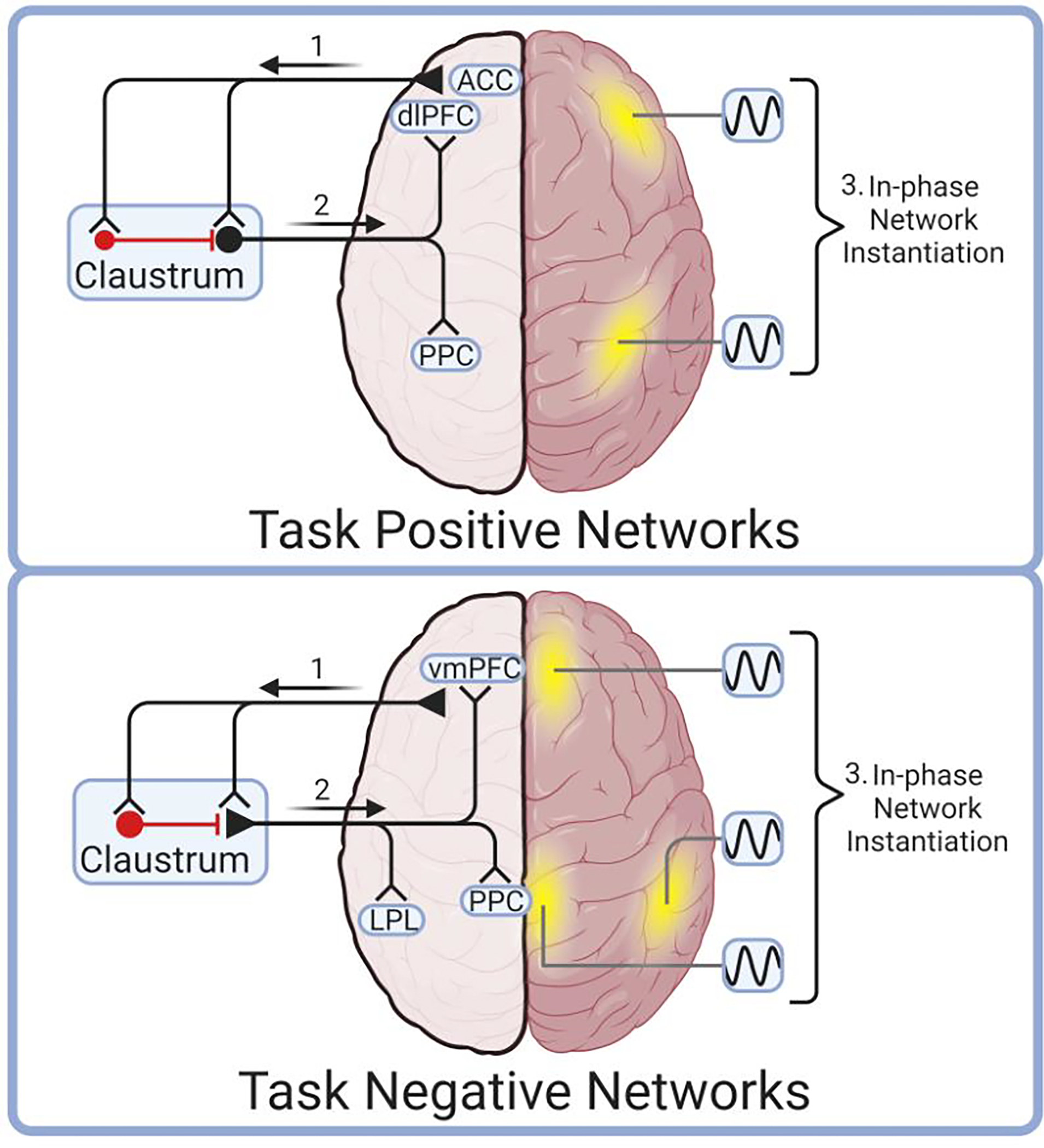
The NICC model proposes that frontal regions send cognitive control information to claustrum (1), which then transforms, amplifies, and broadcasts this information to cortical network nodes (2). These cortical nodes are brought into phase by claustro-cortical input to instantiate task-positive (top) or task-negative (bottom) cortical networks (3). In this model, unique frontal cortical inputs project to unique claustrum projection neurons that selectively target, and therefore instantiate, a given network composed of cortical nodes (yellow). For instance, the anterior cingulate cortex (ACC) is proposed to activate claustrum neurons that selectively synchronize components of the task positive network, such as the dorsolateral prefrontal cortex (dlPFC) and posterior parietal cortex (PPC) (top). Other networks may be instantiated in a similar fashion by frontal cortical activation of claustrum, which synchronizes, for instance, task negative network nodes including the ventromedial prefrontal cortex (vmPFC), posterior cingulate (PCC) and the lateral parietal lobe (LPL). This figure was created using BioRender.
Structural and Functional Connectivity
The model presented above is built upon existing data relating to the connectivity of the claustrum with network nodes and the known functional interactions between network nodes. That is, during task engagement, frontal cortical activation precedes activation of posterior cortex in macaques [91,92], and frontal cortical activity precedes network emergence in humans [93,94]. This suggests the claustrum is directed by frontal cortical input to synchronize these frontal areas with posterior cortical areas. This requires synaptic connectivity between these cortical areas through claustrum. Supporting this, rodent frontal cortices strongly project to the claustrum, whereas posterior sensory and sensory association cortices do so weakly [11,13,95,96]. Of these frontal inputs, only ACC input to claustrum has been extensively studied, though observations of characteristics of claustrum responses to ACC input may be representative of other frontal cortical inputs. For instance, optogenetic stimulation of ACC inputs readily drives action potentials in claustrum projection neurons in mouse acute brain slices, while stimulation of inputs from various combinations of posterior sensory cortical inputs does not [95]. Providing a synaptic link between frontal and distant posterior cortices, claustrum neurons projecting to posterior parietal association and visual cortices are among those that fire in response to ACC afferent stimulation [97]. The rodent claustrum also provides dense innervation to frontal areas, including the ACC and the prelimbic prefrontal cortex [11,12]. With the synaptic link between frontal and posterior cortices through the claustrum established, it is now important to note that multiple input-specific, synaptic pathways of information exist through the claustrum [98]. This provides a synaptic basis for multiple networks to be supported through the claustrum as directed by various frontal cortices. Thus, there exists a neural circuit mechanism by which given frontal cortices communicate, through the claustrum, to specific downstream cortical nodes composing a network (Figure 3).
The connectivity necessary for the claustrum to support cortical network nodes is observed in monkey as well. The claustrum projects to dorsolateral prefrontal cortex in capuchin monkey [4,7] and posterior parietal cortex in capuchin and macaque monkeys [99]. These regions are key nodes of the task-positive FPN [74,94,100,101]. Regarding task-negative networks, the posterior cingulate cortex, inferior parietal lobule, entorhinal cortex, ventromedial prefrontal cortex (which includes frontal pole), and dorsomedial prefrontal cortex are established nodes of the DMN [102–104]. The claustrum bidirectionally connects with posterior cingulate cortex in both the Crab-eating and rhesus macaque [105], projects to frontal pole in capuchin monkey [7], receives frontal pole projections in macaque [86], receives projections from the inferior parietal lobule in stump-tailed monkey [106], and projects to entorhinal cortex in macaque [107,108]. Unlike human and macaque DMN, the marmoset frontal pole [109] and dorsolateral prefrontal cortex (specifically area 8aD) [110] are identified as the prefrontal nodes of DMN. In this species, the claustrum provides up to 50% of subcortical input to the frontal pole [111]. These structural and functional data gathered from rodent and primate are reflected in human functional connectivity data [112,113], further indicating a role for claustrum in the regulation of cortical networks. However, there are important caveats regarding claustrum signal detection using functional neuroimaging.
Usage of fMRI to study the claustrum is challenged by the large voxel size of fMRI compared to the claustrum, which results in contamination of claustrum signal by nearby putamen and insula. As such, special analysis techniques such as Small Region Confound Correction are required to obtain a meaningful claustrum signal [112]. Using this approach, signals from structures neighboring the claustrum are regressed from claustrum signal, resulting in a claustrum signal that bears little statistical resemblance to adjacent structures. Small Region Confound Correction, when applied to resting state fMRI reveals that the human claustrum is functionally connected to task-positive networks, task-negative networks, and several others [112,113] (Figure 4).
Figure 4. The claustrum is functionally connected with many cortical networks at rest.
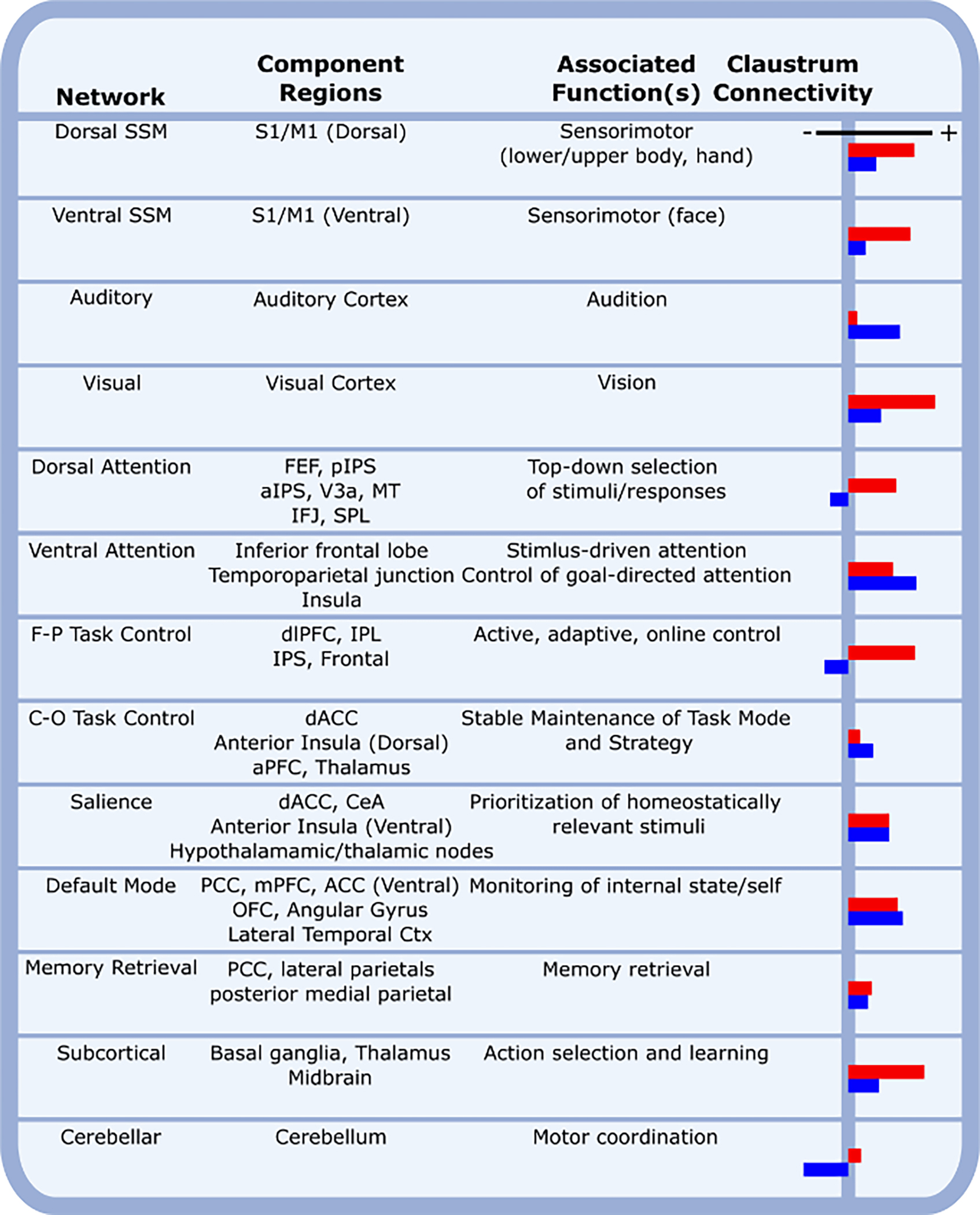
Human cortical networks are listed, as defined by the Power Atlas [66], along with network component regions, the associated functions of each network, and the functional connectivity of the claustrum with each network as previously determined [113]. Red and blue bars represent left and right claustrum functional connectivity, respectively. Abbreviations: aPFC, anterior prefrontal cortex; aIPS, anterior intraparietal sulcus; CeA, central amygdala; C-O, cingulo-opercular; Ctx, cortex; dACC, dorsal anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; FEF, frontal eye field; F-P, frontoparietal; IFJ, inferior frontal junction; IPL, inferior parietal lobe; IPS, intraparietal sulcus; M1, primary motor cortex; mPFC, medial prefrontal cortex; MT, middle temporal visual area; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; pIPS, posterior intraparietal sulcus; S1, primary somatosensory cortex; SPL, superior parietal lobule; SSM, sensory/somatomotor; V3a; visual cortex (V3 accessory cortex);.
There is high expression of serotonin 2A receptor in the claustrum [114–120], which is the primary target for the psychedelic drug psilocybin. Testing the hypothesis that administration of psilocybin affects claustrum activity and its functional connectivity with cortical networks, Barrett et al. [113] applied Small Region Confound Correction to find that psilocybin depresses claustrum activity and disrupts its functional connectivity with several cortical networks. Specifically, functional connectivity of the right claustrum with DMN decreases and functional connectivity with FPN increases, while the integrity of both DMN and FPN networks is associated with functional connectivity with the right claustrum [113]. This provides a degree of causal evidence in humans that suggests the claustrum may participate in cortical network dynamics.
Data from these structural and functional connectivity studies position the claustrum to support networks comprised of both frontal and posterior cortical areas and sets the stage for synchronization of these spatially distant cortical areas by the claustrum in response to a top-down frontal cortical signal. Next, we will present mechanistic explanations based on extant data for how the claustrum responds to top-down frontal cortical signals and how the claustrum may, in turn, participate in bringing cortices into phase to facilitate synchronization for network instantiation [121].
Claustrum Responsivity to Top-Down Cortical Input
If claustrum projection neurons initiate synchronization of constellations of cortical areas to give rise to task-relevant networks, then the activity of claustrum neurons would be expected to activate: 1) at the initiation of a task and, therefore, at network instantiation; 2) in a manner that briefly amplifies incoming signals; 3) faithfully in response to frontal input and otherwise remain quiescent.
Claustrum activity at task-initiation.
Mouse ACC input activity and the activity of the claustrum itself increases prior to the presentation of a cue in the cognitively demanding five choice serial response time task [95,122]. Photoinhibition of either ACC afferents to the claustrum, or the claustrum itself, diminishes task performance in mouse [95,122]. Inhibition of claustrum, or ACC afferents, during performance of the less cognitively demanding one choice serial response time task does not impair performance [95,122]. Adding to this in mouse, blanket claustrum inhibition decreases performance in both pup-retrieval and two-alternative forced-choice tasks, but only in the presence of an auditory distractor [123]. In humans, claustrum activity is significantly greater than baseline only at the onset of difficult blocks of trials in the multi-source interference task [112]. This activity peak occurs when task-positive networks emerge and the DMN disengages [112], i.e., during the switch from the DMN to the task-positive network that occurs to allow optimal performance on the difficult task. These data support the notion that the claustrum activates at the initiation of task-positive networks and is necessary for optimal performance in tasks requiring those networks.
Claustrum amplification of input signals.
An ideal network instantiation signal must be both powerful, to interrupt any previous cortical processing, and transient, to prevent interference with subsequent task-relevant processing. The claustrum possesses internal microcircuitry that allows the shaping of cortical inputs into transiently amplified claustro-cortical outputs. To produce amplification of frontal input signals, roughly half of claustrum projection neurons respond to input non-linearly; these neurons burst fire disproportionately to cortical input strength [20,22]. On average across all claustrum projection neuron types, optogenetic activation of ACC inputs at 1–10 Hz input frequencies produces 50–80 Hz claustrum projection neuron firing frequencies [95]. This amplified output firing is transient, lasting a few seconds before exponentially decaying to below input frequencies [22]. Additionally, cortical inputs to the claustrum innervate both claustrum projection neurons and parvalbumin-positive interneurons, the latter of which provides a classical feedforward microcircuit inhibition onto claustrum projection neurons that constrain their firing [95,124]. This corresponds with observations of claustrum neuron firing transience: 1) in monkey (~0.2 seconds) in vivo [41]; 2) in mouse acute brain slice where a less than one second firing period in claustrum neurons is observed in response to a five second 20 Hz optogenetic stimulation of ACC afferents [95,97]; and 3) in disinhibited bat claustrum, where neither spontaneous nor evoked firing events display repetitive firing or after-discharge firing [125].
Faithful claustrum responsivity to frontal cortical input over quiescent background activity.
In acute brain slices, claustrum projection neurons readily fire in response to excitatory input from frontal cortices, but these neurons do not fire in response to sensorimotor cortical inputs in mouse [22]. Generally, claustrum firing in the absence of a stimulus is sparse, as commonly noted across in vivo electrophysiological recordings in cat [24,25], rabbit [36], and monkey [34]. In bat, disinhibited claustrum readily engages in spontaneous or electrically evoked single spike population firing events but does not produce repetitive or after-discharge firing [125]. Providing a possible mechanism for this quiescence, local parvalbumin-positive interneurons possess high electrical interconnectivity [124] and strongly innervate claustrum projection neurons [95,124] (Figure 5).
Figure 5. Cortico-claustro-cortical processing.

Claustrum projection neurons receive strong input from cortex, specifically frontal regions [11,95,97] (A), and are strongly inhibited by an interconnected local parvalbumin-positive interneuron population (red). Claustrum projection neurons course predominantly to cortex with weaker innervation of neighboring claustrum interneurons [124]; a subset of claustrum projection neurons burst fire in response to activation (Type II), while others exhibit only single action potential responses (Type I) [22]. Together, this configuration of claustrum microcircuitry allows for sufficient cortical input (that which exceeds the inhibitory “threshold” imposed by local interneurons) to be transformed and amplified for claustro-cortical broadcast (B). This figure was created using BioRender.
Claustrum Synchronization of Cortex
The NICC model proposes that the claustrum brings cortical activity into phase to instantiate a distributed network. This proposal is supported by existing evidence: the claustrum coordinates synchronous cortical slow-wave activity in awake mice by producing a sustained cortical down-state [126], which is similar to the sustained cortical suppression observed in awake head-fixed mice [127]. Furthermore, the claustrum generates synchronous sharp waves in slow-wave sleep in reptiles [17]. These studies establish the potential ability of the claustrum to synchronize cortex for network initiation in waking states (Box 2).
Box 2. The Claustrum Across Contexts.
The NICC model is proposed as a common, cross-species network function of the claustrum. Given that task-positive and task-negative cortical networks emerge across a variety of internally- and externally-directed tasks, claustrum activity is expected to be associated with an equally wide variety of functions ascribed to these tasks. For instance, attention tasks [160–162], working memory tasks [157,163], and experimental pain [164,165] all induce task-positive network emergence, and contextual and cued fear conditioning are associated with DMN emergence [166]. Thus, the claustrum is predicted to activate with each, despite not playing a unique role in, for instance, pain processing per se. In rabbit, optimal acquisition of classically conditioned eyeblink responses, but not expression of learned responses, requires the claustrum [36]. In this case, the claustrum is seemingly involved in stimulus-response behavior acquisition, but not expression. Additionally, the claustro-entorhinal circuit is required for optimal contextual fear conditioning [167]. In isolation, this appears to indicate that claustrum directly enables affective learning, but may truly reflect claustrum-directed recruitment of DMN. These wide-ranging claustrum responses, coupled with the wide-ranging anatomical connections of the claustrum, may yield disparate claustrum functional hypotheses if data are not collectively considered.
An important consideration is that the NICC model could be just one, specific example of a broader functional capacity for the claustrum in enabling distant inter-areal cortical synchronization. Thus, the claustrum may interact with and organize networks beyond prefrontal cortex anchored networks, such as sensorimotor networks. Additionally, the claustrum may engage in inter-areal coordination that may not be recognized as a “cortical network”. The claustrum may also, in states such as sleep, enable broad, cortex-wide synchronization. The claustrum is required for slow-wave activity coordination in sleeping rodent [126], and the putative reptile claustrum homologue is similarly required for sharp-wave events in sleeping Pogona vitticeps [17]. Considering that species vary greatly by sensory, motor, and cognitive specialization, the claustrum may have evolved to support inter-areal cortical synchrony across a variety of functions based on the unique strategies by which an organism exploits its environment. Ultimately, it will be critical to understand why cross-cortical synchronization occurs in brains to provide deeper insight into claustrum function. In this endeavor, it is important to realize the limitations of experimental tasks and the language that we use to define brain functionality and resultant behavior – limitations that may represent our largest challenge in understanding the claustrum across contexts.
To achieve cross-cortical synchrony, the NICC model proposes that the claustrum activates cortical inhibitory microcircuits to achieve cross-cortical down states, from which synchronous activation emerges. In awake mice, optogenetic activation of claustrum input to medial prefrontal cortex transiently inhibits net cortical pyramidal neuron activity by activating cortical parvalbumin positive and neuropeptide Y positive interneurons [127]. Supporting the notion that claustral inhibition of cortex may override ongoing cortical activity, optogenetic activation of claustro-cortical neurons results in greater inhibition of mouse medial prefrontal cortical neuron firing in vivo than all other major subcortical inputs [127]. This cortical inhibition is followed by a resumption of excitation, transiently driving pyramidal neuron activity above baseline levels [46,127]. The NICC model proposes that when this occurs simultaneously across disparate areas of cortex, it allows network instantiation by inducing a temporally phase-locked cortical up-state (Figure 6). From this up-state, other inputs to cortex may sustain cortical oscillations underlying network activity.
Figure 6. Claustrum innervation and modulation of cortex.
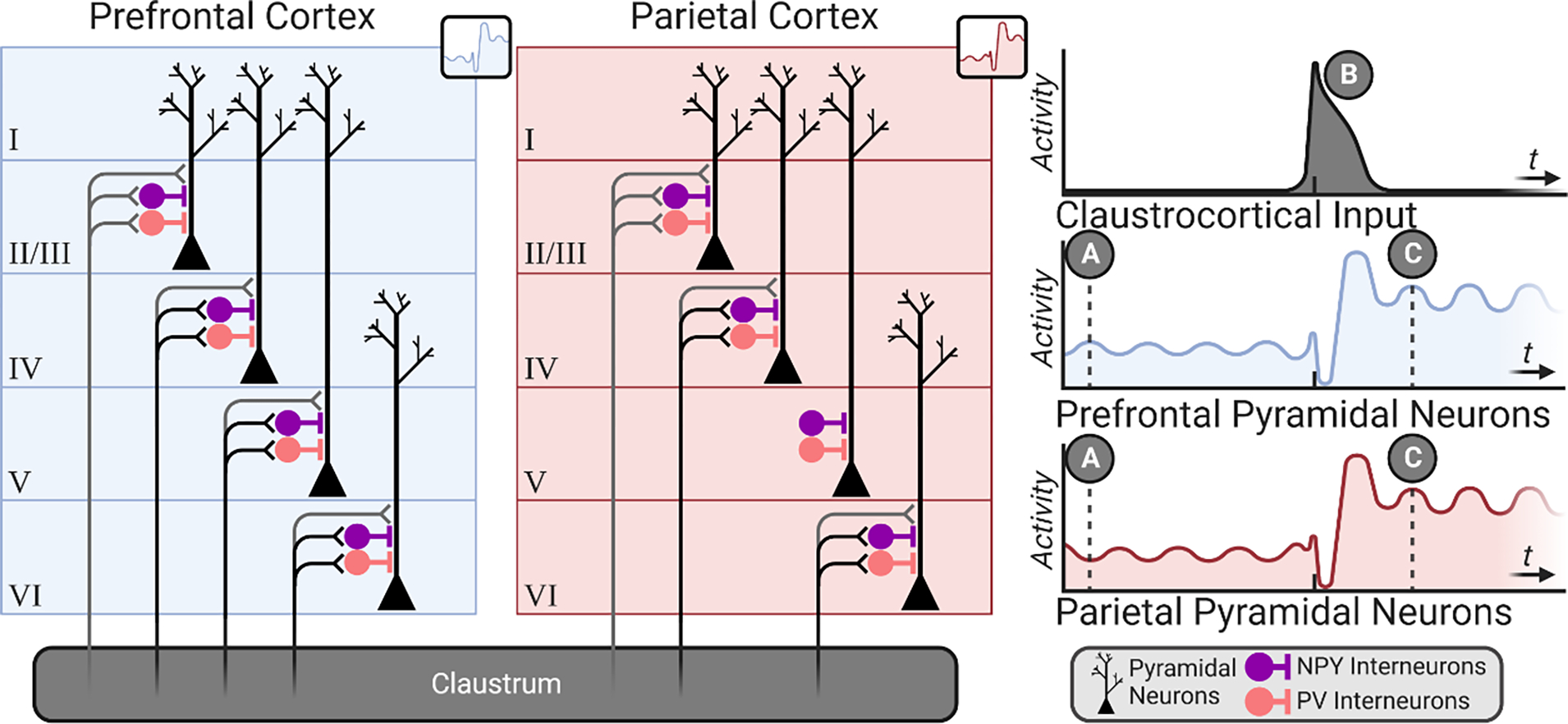
Claustrum afferents are biased toward deeper layers of cortex and to neuropeptide Y (NPY) and parvalbumin (PV)-expressing interneurons. The exception to this is a lack of claustro-cortical input to layer V parietal cortex in mouse [97], which likely reflects a lack of claustrum control of layer V neurons projecting to dorsolateral striatum/putamen. Task-positive cortical network components (e.g., prefrontal cortex and parietal cortex) at cognitive rest display relatively desynchronized oscillations (A). Claustrum firing initially elicits strong inhibition of pyramidal neurons via activation of PV-expressing and NPY-expressing interneurons, followed by resumption of pyramidal neuron excitation mediated, in part, by direct claustro-cortical input (B). This induces a phase-locking of activity across cortical regions, from which subsequent oscillatory frequencies may be governed by cortico-cortical and thalamocortical circuits. With disparate cortical regions now participating in phase-locked activity, a synchronized network is instantiated (C). This figure was created using BioRender.
SIMILARITIES AND DIFFERENCES WITH PREVIOUS MODELS
The NICC model integrates the current literature on claustrum function, builds upon previous models, and provides testable hypotheses (Box 3.). Advancing the proposals by Crick and Koch [27] and, later, Smythies et al. [32] that the claustrum coordinates widespread cortical areas, the NICC model attributes this cortical coordination to a cognitive control function rather than generating conscious percepts. Although, other functions beyond cognitive control are possible assuming that the claustrum is a general cortical network organizer (Box 2.). Building on the model proposed by Vidyasagar and Levichkina [128], in which claustrum enhances cortical synchrony by passing low frequency “cross frequency coupling” signals between cortical areas, the NICC model posits that the claustrum may perform such a function for the purpose of network instantiation; the maintenance of the instantiated network may be thereafter supported by other inter-cortical connections or thalamocortical connections [128–134].
Box 3. Testable Predictions of the NICC Model.
The NICC model yields specific structural and functional predictions to guide further investigation of the claustrum. A selection of these predictions is listed below. Some of these predictions are partially supported by available data, while others will serve as essential future tests of the model’s veracity.
- Connectivity
- Individual claustrum neurons should preferentially project to different groups of cortical areas depending on the frontal inputs they receive. These targets should map onto areas identified as nodes of specific task-positive (e.g. FPN) and task-negative (e.g. DMN) cortical networks.
- Support: FPN node-associated cortico-claustro-cortical circuit [97].
- Functional Circuits and Cortical Networks
- Claustrum neuron activity should not encode sensory information but should encode aspects of task demand.
- Support: rodent claustrum signals motor planning related signals over sensory signals [30].
- Claustrum projection neurons should fire at the volitional initiation of internal reflection or another activity requiring DMN activation.
- Inputs capable of eliciting claustrum firing in physiological conditions should be from cortices associated with cognitive control (frontal regions).
- Disruption of claustrum function at the onset of a task should impair the initiation, segregation and/or integrity of cortical networks.
- Degree of claustrum activation may scale with the integrity of the produced cortical network to meet task demands.
- Cognition and Behavior
- Selective suppression of different claustrum sub-circuits should elucidate the role of specific nodes within a network to behavior and network performance.
- Initiating cognitive control in humans should correlate with claustrum activation.
- Support: Claustrum activation is detected at the onset of a difficult task set, but not at initiation of an easy task set in humans [112].
- The claustrum should activate while switching between tasks that require different cortical network configurations and significant cognitive load demands.
Goll et al. [42] and later Jackson et al. [127] proposed that the claustrum supresses activity in cortical areas encoding unattended modalities to subserve selective attention (i.e. an attentional “spotlight” role). The NICC model submits that the claustrum is involved in an umbrella cognitive control function that will be recruited by a variety of tasks, and that the claustrum inhibits cortex to then allow cortical re-activation for synchronization of cortical nodes participating in a network. This “synchronized rebound” is not in conflict with the proposal by Jackson et al. [46] that argues that claustrum feed-forward inhibition of cortex, combined with the bifurcation of claustro-cortical axons to innervate different cortical layers and regions, could serve to synchronize spike timing and facilitate communication between different cortical areas.
Jackson et al. [46] and Smith et al. [44] propose the claustrum encodes salient stimuli. The NICC model adopts a more nuanced view on salience by predicting that task-relevant stimuli may activate claustrum, downstream of frontal cortical activation, to prompt network recruitment. In this way, the claustrum would appear to activate with a salient stimulus but is instead encoding cognitive control signals for network recruitment. A corollary prediction of this is that the claustrum would not activate with salient stimuli that are not task relevant (i.e. stimuli that do not elicit networks). Further distinguishing the NICC model from the Smith et al. [44] model, the NICC model identifies frontal cortices as the primary drivers of claustrum activation rather than the claustrum interfacing sensorimotor and limbic information (Box 4).
Box 4. Subcortical Inputs to Claustrum.
Corticoclaustral and claustrocortical pathways predominate the connectivity profile of the claustrum and the NICC model proposes that these connections are central to orchestrating cortical networks. However, the claustrum also receives subcortical projections. These subcortical regions include dorsal raphe nucleus, nucleus basalis, basolateral amygdala, and midline intralaminar thalamic nuclei. While the functional roles of these inputs are more unresolved than the function of the claustrum itself, function may be speculatively inferred based on the NICC model. Cholinergic inputs from the nucleus basalis may contribute vigilance state information for optimized network instantiation. Similarly, emotional valence state information may be supplied to the claustrum from the basolateral amygdala to muster cortical networks required for threat avoidance and fear learning; for instance, by recruiting the DMN, which is involved in affective learning [166]. The DMN contains entorhinal cortex [102–104], which requires claustral input for acquisition of contextual fear conditioning [167]. In the same vein, the rostral intralaminar thalamic nuclei, which are thought to integrate ascending reticular arousal system information with top-down information from ACC and orbitofrontal cortices, may modulate claustrum activity for network instantiation dependent upon behavioral engagement demands [168]. Given that dorsal raphe serotonin neurons begin firing at 1–5 Hz with wakefulness [169,170], serotonin signaling in the claustrum may allow for discrete network states in wakefulness or cortex-wide synchronization in sleep [17,126]. This notion suggests a powerful role for serotonin modulation of claustrum activity [171]. Supporting this, administration of the pan-serotonin receptor agonist psilocybin decreases claustrum activity and disrupts cortical networks [113]. These emerging findings suggest a powerful influence of subcortical projections to claustrum and, as such, these connections are poised to deepen our understanding of claustrum function despite their minority status.
The NICC model of claustrum function is synergistic with that of other possible circuit-based mechanisms of cortical network emergence, including cortico-thalamo-cortical and cortico-cortical processing. Thalamic nuclei, such as the mediodorsal nucleus, the pulvinar nucleus, and the thalamic reticular nucleus, each uniquely regulate cortical processing. In turn, the NICC model proposes an equally unique regulatory role for the claustrum over cortical processing. Indeed, in contrast to the claustrum, the thalamic mediodorsal nucleus input to prefrontal cortex continues throughout a task [134] and may sustain attention on the task by amplifying local prefrontal cortex connectivity [133]. The pulvinar nucleus mediates synchrony between neighboring (non-distant) cortical regions [130] and is implicated in task-switching within the visual domain [135]. Unlike the claustrum, and similarly to the mediodorsal nucleus, the pulvinar is highly active, consistently exchanging signals with cortex [135]. The thalamic reticular nucleus functions as a “neuronal oscillator” [132] that determines the frequency of cortical rhythms [131] and is implicated in selective attention similar to the “searchlight” hypotheses of selective attention proposed by Crick and Koch [43,136,137]. Ultimately, both the claustrum and thalamic nuclei must bear separate but mutually dependent roles in cortical processing for cognition. Finally, cortico-cortical circuits are proposed to support network architecture. For example, the mouse DMN, as defined by fMRI analysis, is composed of cortical regions that are preferentially connected through layer 2/3 cortico-cortical connections [129]. This finding provides a potential circuit mechanism for within-network maintenance. Future investigation should therefore test predictions of the NICC model within the context of the distributed network of extended cortical systems (Box 3).
Behaviorally, the NICC model proposes a function for the claustrum in cognitive control that is distinct from bottom-up salience detection, which activates the SN. Anterior insula activation predicts FPN activation and DMN deactivation [138] and anterior insula dysfunction is associated with impaired network switching [139]. These findings led to the proposal that the SN mediates network switching [39]. The NICC model is not incompatible with the SN-directed network switching model and may be situated to test its predictions. For example, the NICC model may provide one possible neural circuit explanation for how the SN achieves network switching: SN signaling to the claustrum may instantiate a task-positive network (e.g., FPN) for optimal task performance.
Finally, the NICC model provides testable hypotheses to experimentally disentangle the respective functions of the anterior insula and the claustrum. It is proposed that some portion of claustrum signal in fMRI is misattributed to the neighboring anterior insula [6,7,44]. This is used as support for the notion that the claustrum is involved in salience detection, perhaps rather than the anterior insula. To aid in disambiguating this issue, the NICC model predicts that SN node lesions, for instance, would disallow claustrum activation in experimental contexts where SN activation precedes task-positive network emergence. In turn, claustrum lesions would not affect SN activation, but would disallow SN-directed task-positive network emergence. Thus, the NICC model functions as a unique conceptual framework from which to derive circuit-level, testable predictions of network activity in cognitive control (Box 3).
CONCLUDING REMARKS
The NICC model posits that the claustrum instantiates cortical networks through synchronization of cortical network nodes in a manner commensurate with the cognitive demand of a given task. This function is achieved in three parts: the claustrum receives frontal cortical task demand information, transforms and amplifies this signal, and finally synchronizes targeted cortical areas to instantiate a cognitive network. This model relies on the current status of data from the claustrum field, which greatly requires further expansion. Several avenues of investigation are ripe for exploration (see Outstanding Questions). A great deal of further work characterizing claustrum macro- and microcircuitry is required across species. This will lay the groundwork for understanding how neuromodulation within the claustrum may be supporting flexible cortical network support. An understanding of neuromodulatory mechanisms will reveal critical insight into how disease pathology and exposure to drugs of abuse may be hijacking claustrum function and, therefore cognitive control. Indeed, cognitive control is subject to dysfunction in a range of neuropsychiatric disorders [140–144]. Determination of the claustrum’s role in these, and other, illnesses represents a nascent field. If further supported by these new lines of investigation, the NICC model provides a useful conceptual framework with which to understand how the claustrum supports cognition and cortical network emergence in health and disease.
Outstanding Questions.
How do claustrum projection neuron subtypes uniquely contribute to the control of cortical networks and cognition?
How do unique claustrum interneuron populations contribute to claustrum function and cognitive control?
How do subcortical projections (e.g., from the supramammillary nucleus) to the claustrum modulate claustrum function and cognitive control?
What are the mechanisms underlying synaptic plasticity in the claustrum?
How do synaptic plasticity mechanisms support state- and learning-dependent control of cortical networks?
How do claustrum connectivity profiles differ across species with unique cognitive capabilities (e.g., rodent, feline, bat, cetacea, non-human primate, and human)?
How does claustrum instantiation of cortical networks extend to distantly related species (e.g., reptiles, and birds)?
How does the claustrum differ in its ability to synchronize cortex in sleep and wake states from that of other structures (e.g., thalamic nuclei)?
What is the role of the claustrum in age-related cognitive decline?
Does the claustrum contribute to cognitive dysfunction in neuropsychiatric illness (e.g., schizophrenia)?
Is claustrum dysfunction associated with sleep disorders?
Given the high degree of connectivity with cortex, how does claustrum activation in response to non-invasive brain stimulation of cortex contribute to therapeutic effects?
Does the claustrum contribute to the long-lasting pro-cognitive effects of psychedelic drug therapy?
Supplementary Material
Highlights.
Modern neuroscience approaches have expanded investigation into the functional role of the claustrum, one of the most highly connected regions of the brain.
Emerging data across rodent studies demonstrate that the claustrum is required for optimal cognitive performance and synchronizes distant cortical areas.
Human whole brain functional imaging data demonstrate the claustrum activates during difficult versions of a cognitive task and with the emergence of task-positive cortical networks.
We propose a functional role for the claustrum in cortical network instantiation underlying cognitive control.
FUNDING
This work was supported by the US National Institute on Alcohol Abuse and Alcoholism grant R01AA028070 (B.N.M.).
Footnotes
COMPETING INTERESTS
FSB is on the scientific advisory board for Wavepaths, Ltd. and a scientific advisor for Mindstate Design Labs, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Macchi G et al. (1981) The organization of the claustroneocortical projections in the cat studied by means of the HRP retrograde axonal transport. J. Comp. Neurol. 195, 681–695. 10.1002/cne.901950411 [DOI] [PubMed] [Google Scholar]
- 2.Baizer JS et al. (1997) Projections from the claustrum to the prelunate gyrus in the monkey. Exp. Brain Res. 113, 564–568. 10.1007/pl00005607 [DOI] [PubMed] [Google Scholar]
- 3.Sadowski M et al. (1997) Rat’s claustrum shows two main cortico-related zones. Brain Res. 756, 147–152 [DOI] [PubMed] [Google Scholar]
- 4.Tanne-Gariepy J et al. (2002) Projections of the claustrum to the primary motor, premotor, and prefrontal cortices in the macaque monkey. J. Comp. Neurol. 454, 140–157. 10.1002/cne.10425 [DOI] [PubMed] [Google Scholar]
- 5.Hur EE and Zaborszky L (2005) Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study [corrected]. J. Comp. Neurol. 483, 351–373. 10.1002/cne.20444 [DOI] [PubMed] [Google Scholar]
- 6.Reser DH et al. (2017) Topography of claustrum and insula projections to medial prefrontal and anterior cingulate cortices of the common marmoset (Callithrix jacchus). J. Comp. Neurol. 525, 1421–1441. 10.1002/cne.24009 [DOI] [PubMed] [Google Scholar]
- 7.Reser DH et al. (2014) Claustrum projections to prefrontal cortex in the capuchin monkey (Cebus apella). Frontiers in systems neuroscience 8, 123. 10.3389/fnsys.2014.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milardi D et al. (2015) Cortical and subcortical connections of the human claustrum revealed in vivo by constrained spherical deconvolution tractography. Cereb. Cortex 25, 406–414. 10.1093/cercor/bht231 [DOI] [PubMed] [Google Scholar]
- 9.Arrigo A et al. (2017) Inter-hemispheric Claustral Connections in Human Brain: A Constrained Spherical Deconvolution-Based Study. Clinical neuroradiology 27, 275–281. 10.1007/s00062-015-0492-x [DOI] [PubMed] [Google Scholar]
- 10.Torgerson CM et al. (2015) The DTI connectivity of the human claustrum. Hum. Brain Mapp. 36, 827–838. 10.1002/hbm.22667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q et al. (2017) Organization of the connections between claustrum and cortex in the mouse. J. Comp. Neurol. 525, 1317–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White MG et al. (2017) Cortical hierarchy governs rat claustrocortical circuit organization. J. Comp. Neurol. 525, 1347–1362. 10.1002/cne.23970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zingg B et al. (2018) Input-output organization of the mouse claustrum. J. Comp. Neurol. 526, 2428–2443. 10.1002/cne.24502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler AB et al. (2002) Apparent absence of claustrum in monotremes: implications for forebrain evolution in amniotes. Brain Behav. Evol. 60, 230–240. 10.1159/000066698 [DOI] [PubMed] [Google Scholar]
- 15.Ashwell KW et al. (2004) The claustrum is not missing from all monotreme brains. Brain Behav. Evol. 64, 223–241 [DOI] [PubMed] [Google Scholar]
- 16.Suarez R et al. (2018) A pan-mammalian map of interhemispheric brain connections predates the evolution of the corpus callosum. Proceedings of the National Academy of Sciences 115, 9622–9627. 10.1073/pnas.1808262115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norimoto H et al. (2020) A claustrum in reptiles and its role in slow-wave sleep. Nature 578, 413–418. 10.1038/s41586-020-1993-6 [DOI] [PubMed] [Google Scholar]
- 18.Puelles L (2022) Current Status of the Hypothesis of a Claustro-Insular Homolog in Sauropsids. Brain Behav. Evol. 96, 212–241. 10.1159/000520742 [DOI] [PubMed] [Google Scholar]
- 19.Puelles L et al. (2016) Selective early expression of the orphan nuclear receptor Nr4a2 identifies the claustrum homolog in the avian mesopallium: Impact on sauropsidian/mammalian pallium comparisons. J. Comp. Neurol. 524, 665–703. 10.1002/cne.23902 [DOI] [PubMed] [Google Scholar]
- 20.Graf M et al. (2020) Identification of Mouse Claustral Neuron Types Based on Their Intrinsic Electrical Properties. eNeuro 7. 10.1523/ENEURO.0216-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown SP et al. (2017) New Breakthroughs in Understanding the Role of Functional Interactions between the Neocortex and the Claustrum. J. Neurosci. 37, 10877–10881. 10.1523/JNEUROSCI.1837-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White MG and Mathur BN (2018) Claustrum circuit components for top-down input processing and cortical broadcast. Brain Structure and Function 223, 3945–3958. 10.1007/s00429-018-1731-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chorazyna H et al. (1965) Changes in auditory conditioning in dogs after lesions of the claustrum. Proc XXIII Int Cong Physiol Sci Tokyo 13, 456–457 [Google Scholar]
- 24.Spector I et al. (1974) Sensory properties of single neurons of cat’s claustrum. Brain Res. 66, 39–65 [Google Scholar]
- 25.Segundo JP and Machne X (1956) Unitary responses to afferent volleys in lenticular nucleus and claustrum. J. Neurophysiol. 19, 325–339. 10.1152/jn.1956.19.4.325 [DOI] [PubMed] [Google Scholar]
- 26.Ettlinger G and Wilson WA (1990) Cross-modal performance: behavioural processes, phylogenetic considerations and neural mechanisms. Behav. Brain Res. 40, 169–192. 10.1016/0166-4328(90)90075-p [DOI] [PubMed] [Google Scholar]
- 27.Crick FC and Koch C (2005) What is the function of the claustrum? Philosophical Transactions of the Royal Society B: Biological Sciences 360, 1271–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alloway KD et al. (2009) Bilateral projections from rat MI whisker cortex to the neostriatum, thalamus, and claustrum: forebrain circuits for modulating whisking behavior. J. Comp. Neurol. 515, 548–564. 10.1002/cne.22073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JB et al. (2012) Rat claustrum coordinates but does not integrate somatosensory and motor cortical information. J. Neurosci. 32, 8583–8588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevee M et al. (2022) Neural activity in the mouse claustrum in a cross-modal sensory selection task. Neuron 110, 486–501 e487. 10.1016/j.neuron.2021.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smythies J et al. (2012) The Functional Anatomy of the Claustrum: The Net That Binds. WMC Neuroscience, [Google Scholar]
- 32.Smythies JR et al. (2014) Hypotheses relating to the function of the claustrum. In The Claustrum, pp. 299–352, Elsevier [Google Scholar]
- 33.Hadjikhani N and Roland PE (1998) Cross-modal transfer of information between the tactile and the visual representations in the human brain: a positron emission tomographic study. J. Neurosci. 18, 1072–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remedios R et al. (2010) Unimodal responses prevail within the multisensory claustrum. J. Neurosci. 30, 12902–12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bickel S and Parvizi J (2019) Electrical stimulation of the human claustrum. Epilepsy & Behavior 97, 296–303. 10.1016/j.yebeh.2019.03.051 [DOI] [PubMed] [Google Scholar]
- 36.Reus-García M et al. (2020) The Claustrum is Involved in Cognitive Processes Related to the Classical Conditioning of Eyelid Responses in Behaving Rabbits. Cereb. Cortex 31, 281–300. 10.1093/cercor/bhaa225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Botvinick MM (2007) Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cognitive, Affective, & Behavioral Neuroscience 7, 356–366. 10.3758/cabn.7.4.356 [DOI] [PubMed] [Google Scholar]
- 38.Carter CS and van Veen V (2007) Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective, & Behavioral Neuroscience 7, 367–379. 10.3758/cabn.7.4.367 [DOI] [PubMed] [Google Scholar]
- 39.Menon V and Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function 214, 655–667. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathur BN (2014) The claustrum in review. Frontiers in systems neuroscience 8, 48. 10.3389/fnsys.2014.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remedios R et al. (2014) A role of the claustrum in auditory scene analysis by reflecting sensory change. Frontiers in systems neuroscience 8, 44. 10.3389/fnsys.2014.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goll Y et al. (2015) Attention: the claustrum. Trends Neurosci. 38, 486–495. 10.1016/j.tins.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 43.Crick F (1984) Function of the thalamic reticular complex: the searchlight hypothesis. Proceedings of the National Academy of Sciences 81, 4586–4590. 10.1073/pnas.81.14.4586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith JB et al. (2019) A Role for the Claustrum in Salience Processing? Frontiers in neuroanatomy 13, 64. 10.3389/fnana.2019.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basso MA et al. (2021) Unraveling circuits of visual perception and cognition through the superior colliculus. Neuron, 918–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson J et al. (2020) The Anatomy and Physiology of Claustrum-Cortex Interactions. Annu. Rev. Neurosci. 43, 231–247. 10.1146/annurev-neuro-092519-101637 [DOI] [PubMed] [Google Scholar]
- 47.Gratton G et al. (2018) Dynamics of cognitive control: Theoretical bases, paradigms, and a view for the future. Psychophysiology 55. 10.1111/psyp.13016 [DOI] [PubMed] [Google Scholar]
- 48.Botvinick MM et al. (2001) Conflict monitoring and cognitive control. Psychological review 108, 624–652. 10.1037/0033-295x.108.3.624 [DOI] [PubMed] [Google Scholar]
- 49.Desimone R and Duncan J (1995) Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222. 10.1146/annurev.ne.18.030195.001205 [DOI] [PubMed] [Google Scholar]
- 50.Tsaparlis G (2014) Cognitive Demand. In Encyclopedia of Science Education (Gunstone R, ed), pp. 1–4, Springer; Netherlands [Google Scholar]
- 51.Biswal B et al. (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541. 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- 52.Power JD et al. (2014) Studying brain organization via spontaneous fMRI signal. Neuron 84, 681–696. 10.1016/j.neuron.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greicius MD et al. (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences 100, 253–258. 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buckner RL and DiNicola LM (2019) The brain’s default network: updated anatomy, physiology and evolving insights. Nature Reviews Neuroscience 20, 593–608. 10.1038/s41583-019-0212-7 [DOI] [PubMed] [Google Scholar]
- 55.Gratton C et al. (2018) Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron 98, 439–452. 10.1016/j.neuron.2018.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bressler SL and Menon V (2010) Large-scale brain networks in cognition: emerging methods and principles. Trends in cognitive sciences 14, 277–290. 10.1016/j.tics.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 57.Chuang KH and Nasrallah FA (2017) Functional networks and network perturbations in rodents. NeuroImage 163, 419–436. 10.1016/j.neuroimage.2017.09.038 [DOI] [PubMed] [Google Scholar]
- 58.Lu H et al. (2012) Rat brains also have a default mode network. Proceedings of the National Academy of Sciences 109, 3979–3984. 10.1073/pnas.1200506109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gozzi A and Schwarz AJ (2016) Large-scale functional connectivity networks in the rodent brain. NeuroImage 127, 496–509. 10.1016/j.neuroimage.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 60.Arsenault JT et al. (2018) Attention Shifts Recruit the Monkey Default Mode Network. J. Neurosci. 38, 1202–1217. 10.1523/JNEUROSCI.1111-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vincent JL et al. (2007) Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447, 83–86. 10.1038/nature05758 [DOI] [PubMed] [Google Scholar]
- 62.Vincent JL et al. (2008) Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 100, 3328–3342. 10.1152/jn.90355.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bressler SL (1995) Large-scale cortical networks and cognition. Brain Res. Rev. 20, 288–304. 10.1016/0165-0173(94)00016-i [DOI] [PubMed] [Google Scholar]
- 64.Mantini D et al. (2011) Default mode of brain function in monkeys. J. Neurosci. 31, 12954–12962. 10.1523/JNEUROSCI.2318-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas Yeo B et al. (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Power JD et al. (2011) Functional network organization of the human brain. Neuron 72, 665–678. 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cole MW et al. (2014) Intrinsic and task-evoked network architectures of the human brain. Neuron 83, 238–251. 10.1016/j.neuron.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glasser MF et al. (2016) A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gordon EM et al. (2017) Precision Functional Mapping of Individual Human Brains. Neuron 95, 791–807 e797. 10.1016/j.neuron.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spreng RN et al. (2010) Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage 53, 303–317. 10.1016/j.neuroimage.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fox MD et al. (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences 102, 9673–9678. 10.1073/pnas.050413610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menon V and D’Esposito M (2022) The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology 47, 90–103. 10.1038/s41386-021-01152-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szczepanski SM et al. (2013) Functional and structural architecture of the human dorsal frontoparietal attention network. Proceedings of the National Academy of Sciences 110, 15806–15811. 10.1073/pnas.1313903110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corbetta M and Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nature reviews neuroscience 3, 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- 75.Seeley WW et al. (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dosenbach NU et al. (2007) Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences 104, 11073–11078. 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dosenbach NU et al. (2006) A core system for the implementation of task sets. Neuron 50, 799–812. 10.1016/j.neuron.2006.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu T et al. (2019) Anterior insular cortex is a bottleneck of cognitive control. NeuroImage 195, 490–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dosenbach NU et al. (2008) A dual-networks architecture of top-down control. Trends in cognitive sciences 12, 99–105. 10.1016/j.tics.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van den Heuvel MP and Sporns O (2011) Rich-club organization of the human connectome. J. Neurosci. 31, 15775–15786. 10.1523/JNEUROSCI.3539-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bertolero MA et al. (2017) The diverse club. Nature communications 8, 1–11. 10.1038/s41467-017-01189-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crossley NA et al. (2014) The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 137, 2382–2395. 10.1093/brain/awu132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zuhorn F et al. (2021) Parainfectious encephalitis in COVID-19:“the claustrum sign”. Journal of neurology 268, 2031–2034. 10.1007/s00415-020-10185-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sener R (1993) The claustrum on MRI: normal anatomy, and the bright claustrum as a new sign in Wilson’s disease. Pediatric radiology 23, 594–596. 10.1007/BF02014975 [DOI] [PubMed] [Google Scholar]
- 85.Selemon LD and Goldman-Rakic PS (1988) Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J. Neurosci. 8, 4049–4068. 10.1523/JNEUROSCI.08-11-04049.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Petrides M and Pandya DN (2007) Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J. Neurosci. 27, 11573–11586. 10.1523/JNEUROSCI.2419-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stepniewska I et al. (2018) Frontal eye field in prosimian galagos: Intracortical microstimulation and tracing studies. J. Comp. Neurol. 526, 626–652. 10.1002/cne.24355 [DOI] [PubMed] [Google Scholar]
- 88.Pearson R et al. (1982) The organization of the connections between the cortex and the claustrum in the monkey. Brain Res. 234, 435–441. 10.1016/0006-8993(82)90883-6 [DOI] [PubMed] [Google Scholar]
- 89.Vogt BA et al. (1979) Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science 204, 205–207. 10.1126/science.107587 [DOI] [PubMed] [Google Scholar]
- 90.Shenhav A et al. (2013) The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79, 217–240. 10.1016/j.neuron.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buschman TJ and Miller EK (2007) Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315, 1860–1862. 10.1126/science.1138071 [DOI] [PubMed] [Google Scholar]
- 92.Gregoriou GG et al. (2009) High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324, 1207–1210. 10.1126/science.1171402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grent T and Woldorff MG (2007) Timing and sequence of brain activity in top-down control of visual-spatial attention. PLoS Biol. 5, e12. 10.1371/journal.pbio.0050012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li L et al. (2010) Role of frontal and parietal cortices in the control of bottom-up and top-down attention in humans. Brain Res. 1344, 173–184. 10.1016/j.brainres.2010.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.White MG et al. (2018) Anterior Cingulate Cortex Input to the Claustrum Is Required for Top-Down Action Control. Cell reports 22, 84–95. 10.1016/j.celrep.2017.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith JB and Alloway KD (2010) Functional specificity of claustrum connections in the rat: interhemispheric communication between specific parts of motor cortex. J. Neurosci. 30, 16832–16844. 10.1523/JNEUROSCI.4438-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.White MG and Mathur BN (2018) Frontal cortical control of posterior sensory and association cortices through the claustrum. Brain Structure and Function 223, 2999–3006. 10.1007/s00429-018-1661-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chia Z et al. (2020) Synaptic Connectivity between the Cortex and Claustrum Is Organized into Functional Modules. Curr. Biol. 30, 2777–2790. 10.1016/j.cub.2020.05.031 [DOI] [PubMed] [Google Scholar]
- 99.Leichnetz GR (2001) Connections of the medial posterior parietal cortex (area 7m) in the monkey. The Anatomical Record: An Official Publication of the American Association of Anatomists 263, 215–236. 10.1002/ar.1082 [DOI] [PubMed] [Google Scholar]
- 100.Kastner S and Ungerleider LG (2000) Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 23, 315–341. 10.1146/annurev.neuro.23.1.315 [DOI] [PubMed] [Google Scholar]
- 101.Fan J et al. (2005) The activation of attentional networks. NeuroImage 26, 471–479. 10.1016/j.neuroimage.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 102.Sheline YI et al. (2009) The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences 106, 1942–1947. 10.1073/pnas.0812686106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buckner RL et al. (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- 104.Raichle ME et al. (2001) A default mode of brain function. Proceedings of the National Academy of Sciences 98, 676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baleydier C and Mauguiere F (1980) The duality of the cingulate gyrus in monkey. Neuroanatomical study and functional hypothesis. Brain 103, 525–554. 10.1093/brain/103.3.525 [DOI] [PubMed] [Google Scholar]
- 106.Weber JT and Yin TC (1984) Subcortical projections of the inferior parietal cortex (area 7) in the stump-tailed monkey. J. Comp. Neurol. 224, 206–230. 10.1002/cne.902240204 [DOI] [PubMed] [Google Scholar]
- 107.Insausti R et al. (1987) The entorhinal cortex of the monkey: III. Subcortical afferents. J. Comp. Neurol. 264, 396–408. 10.1002/cne.902640307 [DOI] [PubMed] [Google Scholar]
- 108.Mufson EJ and Mesulam MM (1982) Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J. Comp. Neurol. 212, 23–37. 10.1002/cne.902120103 [DOI] [PubMed] [Google Scholar]
- 109.Buckner RL and Margulies DS (2019) Macroscale cortical organization and a default-like apex transmodal network in the marmoset monkey. Nature communications 10, 1976. 10.1038/s41467-019-09812-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu C et al. (2019) Anatomical and functional investigation of the marmoset default mode network. Nature communications 10, 1–8. 10.1038/s41467-019-09813-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burman KJ et al. (2011) Subcortical projections to the frontal pole in the marmoset monkey. Eur. J. Neurosci. 34, 303–319. 10.1111/j.1460-9568.2011.07744.x [DOI] [PubMed] [Google Scholar]
- 112.Krimmel SR et al. (2019) Resting state functional connectivity and cognitive task-related activation of the human claustrum. NeuroImage 196, 59–67. 10.1016/j.neuroimage.2019.03.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barrett FS et al. (2020) Psilocybin acutely alters the functional connectivity of the claustrum with brain networks that support perception, memory, and attention. NeuroImage 218, 116980. 10.1016/j.neuroimage.2020.116980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pasqualetti M et al. (1996) Comparative anatomical distribution of serotonin 1A, 1Dα and 2A receptor mRNAs in human brain postmorten. Mol. Brain Res. 39, 223–233. 10.1016/0169-328x(96)00026-5 [DOI] [PubMed] [Google Scholar]
- 115.Pompeiano M et al. (1994) Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Mol. Brain Res. 23, 163–178. 10.1016/0169-328x(94)90223-2 [DOI] [PubMed] [Google Scholar]
- 116.Hamada S et al. (1998) Localization of 5-HT2A receptor in rat cerebral cortex and olfactory system revealed by immunohistochemistry using two antibodies raised in rabbit and chicken. Mol. Brain Res. 54, 199–211. 10.1016/s0169-328x(97)00322-7 [DOI] [PubMed] [Google Scholar]
- 117.Lopez-Gimenez JF et al. (1997) Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn-Schmiedeberg’s archives of pharmacology 356, 446–454. 10.1007/pl00005075 [DOI] [PubMed] [Google Scholar]
- 118.López-Giménez JF et al. (2001) Mapping of 5-HT2A receptors and their mRNA in monkey brain:[3H] MDL100, 907 autoradiography and in situ hybridization studies. J. Comp. Neurol. 429, 571–589. [DOI] [PubMed] [Google Scholar]
- 119.Zea-Ponce Y et al. (2002) Pharmacokinetics and brain distribution in non human primate of R (−)[{sup 123} I] DOI, A 5HT {sub 2A/2C} serotonin agonist. Nuclear medicine and biology 29, 575–583. 10.1016/s0969-8051(02)00306-2 [DOI] [PubMed] [Google Scholar]
- 120.Hall H et al. (2000) Autoradiographic localization of 5-HT2A receptors in the human brain using [3H] M100907 and [11C] M100907. Synapse 38, 421–431. [DOI] [PubMed] [Google Scholar]
- 121.Raut RV et al. (2021) Global waves synchronize the brain’s functional systems with fluctuating arousal. Science advances 7. 10.1126/sciadv.abf2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.White MG et al. (2020) The Mouse Claustrum Is Required for Optimal Behavioral Performance Under High Cognitive Demand. Biological psychiatry 88, 719–726. 10.1016/j.biopsych.2020.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Atlan G et al. (2018) The Claustrum Supports Resilience to Distraction. Curr. Biol. 28, 2752–2762 e2757. 10.1016/j.cub.2018.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim J et al. (2016) Synaptic Organization of the Neuronal Circuits of the Claustrum. J. Neurosci. 36, 773–784. 10.1523/JNEUROSCI.3643-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Orman R et al. (2017) Claustrum of the short-tailed fruit bat, Carollia perspicillata: Alignment of cellular orientation and functional connectivity. J. Comp. Neurol. 525, 1459–1474. 10.1002/cne.24036 [DOI] [PubMed] [Google Scholar]
- 126.Narikiyo K et al. (2020) The claustrum coordinates cortical slow-wave activity. Nat. Neurosci. 23, 741–753. 10.1038/s41593-020-0625-7 [DOI] [PubMed] [Google Scholar]
- 127.Jackson J et al. (2018) Inhibitory Control of Prefrontal Cortex by the Claustrum. Neuron 99, 1029–1039 e1024. 10.1016/j.neuron.2018.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vidyasagar TR and Levichkina E (2019) An Integrated Neuronal Model of Claustral Function in Timing the Synchrony Between Cortical Areas. Frontiers in neural circuits 13, 3. 10.3389/fncir.2019.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Whitesell JD et al. (2021) Regional, Layer, and Cell-Type-Specific Connectivity of the Mouse Default Mode Network. Neuron 109, 545–559 e548. 10.1016/j.neuron.2020.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saalmann YB et al. (2012) The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337, 753–756. 10.1126/science.1223082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Clemente-Perez A et al. (2017) Distinct Thalamic Reticular Cell Types Differentially Modulate Normal and Pathological Cortical Rhythms. Cell reports 19, 2130–2142. 10.1016/j.celrep.2017.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Steriade M and Deschenes M (1984) The thalamus as a neuronal oscillator. Brain Res. Rev. 8, 1–63. 10.1016/0165-0173(84)90017-1 [DOI] [PubMed] [Google Scholar]
- 133.Schmitt LI et al. (2017) Thalamic amplification of cortical connectivity sustains attentional control. Nature 545, 219–223. 10.1038/nature22073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bolkan SS et al. (2017) Thalamic projections sustain prefrontal activity during working memory maintenance. Nat. Neurosci. 20, 987–996. 10.1038/nn.4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yantis S et al. (2002) Transient neural activity in human parietal cortex during spatial attention shifts. Nat. Neurosci. 5, 995–1002. 10.1038/nn921 [DOI] [PubMed] [Google Scholar]
- 136.Wimmer RD et al. (2015) Thalamic control of sensory selection in divided attention. Nature 526, 705–709. 10.1038/nature15398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McAlonan K et al. (2006) Attentional modulation of thalamic reticular neurons. J. Neurosci. 26, 4444–4450. 10.1523/JNEUROSCI.5602-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sridharan D et al. (2008) A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences 105, 12569–12574. 10.1073/pnas.0800005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang Z et al. (2021) Anterior insula regulates brain network transitions that gate conscious access. Cell Reports 35, 109081. 10.1016/j.celrep.2021.109081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lesh TA et al. (2011) Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology 36, 316–338. 10.1038/npp.2010.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wilcox CE et al. (2014) Cognitive control in alcohol use disorder: deficits and clinical relevance. Reviews in the Neurosciences 25, 1–24. 10.1515/revneuro-2013-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Douglas VI (1999) Cognitive control processes in attention deficit/hyperactivity disorder. In Handbook of disruptive behavior disorders, pp. 105–138, Springer [Google Scholar]
- 143.Harvey P-O et al. (2005) Cognitive control and brain resources in major depression: an fMRI study using the n-back task. NeuroImage 26, 860–869. 10.1016/j.neuroimage.2005.02.048 [DOI] [PubMed] [Google Scholar]
- 144.Zeier JD et al. (2012) Cognitive control deficits associated with antisocial personality disorder and psychopathy. Personality Disorders: Theory, Research, and Treatment 3, 283–293. 10.1037/a0023137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mathur BN et al. (2009) Proteomic analysis illuminates a novel structural definition of the claustrum and insula. Cereb. Cortex 19, 2372–2379. 10.1093/cercor/bhn253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Puelles L (2014) Deve lopment and evolution of the claustrum. In The Claustrum, pp. 119–176, Elsevier [Google Scholar]
- 147.Bruguier H et al. (2020) In search of common developmental and evolutionary origin of the claustrum and subplate. J. Comp. Neurol. 528, 2956–2977. 10.1002/cne.24922 [DOI] [PubMed] [Google Scholar]
- 148.Buzsaki G and Watson BO (2012) Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues in clinical neuroscience 14, 345–367. 10.31887/DCNS.2012.14.4/gbuzsaki [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fries P (2005) A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in cognitive sciences 9, 474–480. 10.1016/j.tics.2005.08.011 [DOI] [PubMed] [Google Scholar]
- 150.Salinas E and Sejnowski TJ (2000) Impact of correlated synaptic input on output firing rate and variability in simple neuronal models. J. Neurosci. 20, 6193–6209. 10.1523/JNEUROSCI.20-16-06193.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sohal VS et al. (2009) Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702. 10.1038/nature07991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morishima Y et al. (2009) Task-specific signal transmission from prefrontal cortex in visual selective attention. Nat. Neurosci. 12, 85–91. 10.1038/nn.2237 [DOI] [PubMed] [Google Scholar]
- 153.Herweg NA et al. (2016) Theta-Alpha Oscillations Bind the Hippocampus, Prefrontal Cortex, and Striatum during Recollection: Evidence from Simultaneous EEG-fMRI. J. Neurosci. 36, 3579–3587. 10.1523/JNEUROSCI.3629-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mizuhara H et al. (2004) A long-range cortical network emerging with theta oscillation in a mental task. Neuroreport 15, 1233–1238. 10.1097/01.wnr.0000126755.09715.b3 [DOI] [PubMed] [Google Scholar]
- 155.Mizuhara H et al. (2005) Long-range EEG phase synchronization during an arithmetic task indexes a coherent cortical network simultaneously measured by fMRI. NeuroImage 27, 553–563. 10.1016/j.neuroimage.2005.04.030 [DOI] [PubMed] [Google Scholar]
- 156.Bachinger M et al. (2017) Concurrent tACS-fMRI Reveals Causal Influence of Power Synchronized Neural Activity on Resting State fMRI Connectivity. J. Neurosci. 37, 4766–4777. 10.1523/JNEUROSCI.1756-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Violante IR et al. (2017) Externally induced frontoparietal synchronization modulates network dynamics and enhances working memory performance. Elife 6. 10.7554/eLife.22001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cardin JA et al. (2009) Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667. 10.1038/nature08002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Muhle-Karbe PS et al. (2014) Transcranial magnetic stimulation dissociates prefrontal and parietal contributions to task preparation. J. Neurosci. 34, 12481–12489. 10.1523/JNEUROSCI.4931-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Berry AS et al. (2017) Distinct Frontoparietal Networks Underlying Attentional Effort and Cognitive Control. J. Cognit. Neurosci. 29, 1212–1225. 10.1162/jocn_a_01112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Coull JT et al. (1996) A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia 34, 1085–1095. 10.1016/0028-3932(96)00029-2 [DOI] [PubMed] [Google Scholar]
- 162.Dodds CM et al. (2011) Dissociating inhibition, attention, and response control in the frontoparietal network using functional magnetic resonance imaging. Cereb. Cortex 21, 1155–1165. 10.1093/cercor/bhq187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Murray JD et al. (2017) Working Memory and Decision-Making in a Frontoparietal Circuit Model. J. Neurosci. 37, 12167–12186. 10.1523/JNEUROSCI.0343-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kong J et al. (2013) Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain 154, 459–467. 10.1016/j.pain.2012.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Seminowicz DA and Davis KD (2007) Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J. Neurophysiol. 97, 3651–3659. 10.1152/jn.01210.2006 [DOI] [PubMed] [Google Scholar]
- 166.Zidda F et al. (2018) Default mode network connectivity of fear- and anxiety-related cue and context conditioning. NeuroImage 165, 190–199. 10.1016/j.neuroimage.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 167.Kitanishi T and Matsuo N (2017) Organization of the Claustrum-to-Entorhinal Cortical Connection in Mice. J. Neurosci. 37, 269–280. 10.1523/JNEUROSCI.1360-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cover KK and Mathur BN (2021) Rostral Intralaminar Thalamus Engagement in Cognition and Behavior. Frontiers in Behavioral Neuroscience 15, 652764. 10.3389/fnbeh.2021.652764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Trulson ME and Jacobs BL (1979) Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 163, 135–150. 10.1016/0006-8993(79)90157-4 [DOI] [PubMed] [Google Scholar]
- 170.Wu MF et al. (2004) Activity of dorsal raphe cells across the sleep-waking cycle and during cataplexy in narcoleptic dogs. The Journal of physiology 554, 202–215. 10.1113/jphysiol.2003.052134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wong KLL et al. (2021) Changing the Cortical Conductor’s Tempo: Neuromodulation of the Claustrum. Frontiers in neural circuits 15, 658228. 10.3389/fncir.2021.658228 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


