Abstract
Caveolae, specialized plasma membrane invaginations present in most cell types, play important roles in multiple cellular processes including cell signaling, lipid uptake and metabolism, endocytosis and mechanotransduction. They are found in almost all cell types but most abundant in endothelial cells, adipocytes and fibroblasts. Caveolin-1 (Cav1), the signature structural protein of caveolae was the first protein associated with caveolae, and in association with Cavin1/PTRF is required for caveolae formation. Genetic ablation of either Cav1 or Cavin1/PTRF downregulates expression of the other resulting in loss of caveolae. Studies using Cav1-deficient mouse models have implicated caveolae with human diseases such as cardiomyopathies, lipodystrophies, diabetes and muscular dystrophies. While caveolins and caveolae are extensively studied in extra-ocular settings, their contributions to ocular function and disease pathogenesis are just beginning to be appreciated. Several putative caveolin/caveolae functions are relevant to the eye and Cav1 is highly expressed in retinal vascular and choroidal endothelium, Müller glia, the retinal pigment epithelium (RPE), and the Schlemm’s canal endothelium and trabecular meshwork cells. Variants at the CAV1/2 gene locus are associated with risk of primary open angle glaucoma and the high risk HTRA1 variant for age-related macular degeneration is thought to exert its effect through regulation of Cav1 expression. Caveolins also play important roles in modulating retinal neuroinflammation and blood retinal barrier permeability. In this article, we describe the current state of caveolin/caveolae research in the context of ocular function and pathophysiology. Finally, we discuss new evidence showing that retinal Cav1 exists and functions outside caveolae.
Keywords: Caveolae, Caveolin, Cavin1/PTRF, Inflammation, Retinal vasculature, Glaucoma, Non-caveolar Cav1, Müller glia, Retina
1. Introduction
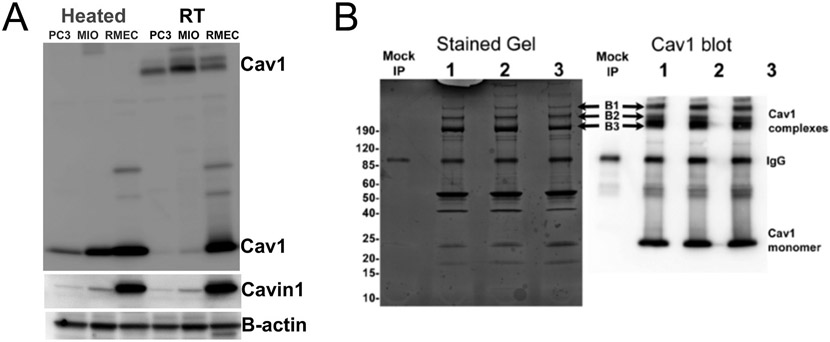
In 1953, in a brief statement on the ultrastructure of capillaries in the Journal of Applied Physics, George Palade was the first to describe “… a large number of vesicles concentrated immediately under the cell membranes facing both the capillary lumen and pericapillary spaces” (Palade, 1953). Palade later published the detailed analysis of these vesicles where he proposed their involvement in transendothelial transport (Palade, 1961). In 1955, these vesicle-like structures were identified in the gall bladder epithelium of mice and renamed “caveolae intracellulare” (Yamada, 1955). The discovery of caveolin-1 (Cav1) almost forty years later as the essential structural protein required for caveolae biogenesis (Glenney and Soppet, 1992; Rothberg et al., 1992) enabled caveolar scientists to study the functions of caveolae using molecular techniques, further enriching our understanding of these subcellular structures. In the years that followed, another protein called Cavin1 or PTRF (Polymerase I and Transcript Release Factor) was also found to be necessary for caveolae biogenesis, as it is required to sequester Cav1 into caveolae (Briand et al., 2011; Liu et al., 2008; Liu and Pilch, 2008). Both Cav1 and Cavin1/PTRF are co-expressed in multiple tissues, whereby deletion of one downregulates expression of the other, resulting in loss of caveolae (Hill et al., 2008; Liu et al., 2008). Currently, functions of Cav1 are studied mainly in the context of caveolae formation. However, we know that Cav1 can exist and function independent of caveolae (Head and Insel, 2007; Lajoie and Nabi, 2010; Pol et al., 2020). For example, the PC3 prostate cancer cell line expresses Cav1 predominantly outside caveolae (also known as non-caveolar Cav1), as they abundantly express Cav1 without Cavin1/PTRF (Gould et al., 2010; Moon et al., 2014; Nassar et al., 2015). This clearly calls for further investigations to define the functions of Cav1 in regulating cellular processes both in caveolar and non-caveolar domains. In this article, we highlight the central role of Cav1 and Cavin1/PTRF in the biogenesis, structure and function of caveolae with a particular focus on the molecular mechanisms by which caveolins, inside and outside of caveolae, regulate ocular functions. Particular emphasis is placed on the putative roles of caveolins in retinal inflammatory responses, retinal vascular structure and function, and on aqueous humor drainage and intraocular pressure control.
1.1. Caveolae: specialized plasma membrane invaginations
Caveolae are 50–100 nm diameter plasma membrane vesicular invaginations first described in endothelial cells (Palade, 1961). Their unique flask-shaped appearance (Fig. 1A), resembling the Greek letter omega (Ω) makes them morphologically distinct from other membrane structures in an electron micrograph (Palade, 1961; Richter et al., 2008; Yamada, 1955). Electron microscopic analysis revealed that they are present most cell types, with greatest abundance in endothelial cells, smooth muscle cells, fibroblast and adipocytes (Hansen et al., 2009; Hansen et al., 2013; Parton and del Pozo, 2013; Scherer et al., 1997). While similar to clathrin-coated pits, caveolae possess unique features that significantly distinguish them. Unlike clathrin-coated pits, there is significant evidence of variation in caveolae density from cell to cell. For example, adipocytes possess up to one million caveolae, which increases their surface area by about 50% (Thorn et al., 2003). However, caveolae are not observed in other cell types such as lymphocytes (Fra et al., 1994, 1995), kidney proximal tubule cells (Zhuang et al., 2011), and neurons (Trushina et al., 2006b). In addition, variation in caveolae density also occurs within the same cells, which is in stark contrast to the relatively constant density of clathrin-coated pits. For example, Parat et al., evaluated differential polarization of caveolae during migration of aortic endothelial cells and found a higher density of caveolae in the basolateral and front ends of the cells (Parat et al., 2003).
Fig. 1.
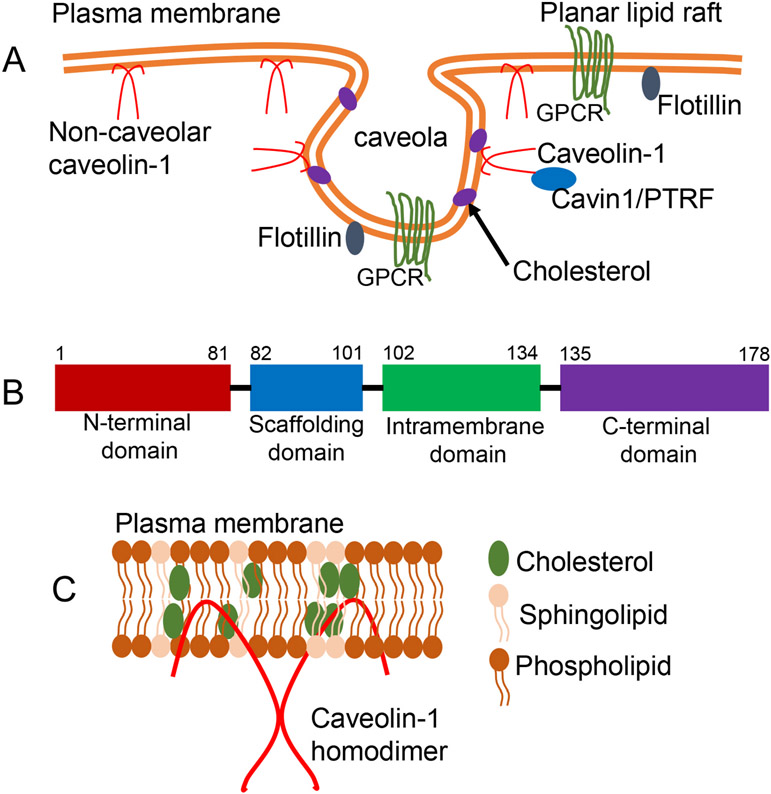
Caveolae are flask-shaped plasma membrane invaginations. A) Schematic representation of planar and caveolar membranes. The plasma membranes of many cells consist of planar (flat) or non-planar (invaginated or flasked-shaped) lipid rafts. In cells expressing both Cav1 and Cavin1/PTRF, Cav1 resides and functions predominantly in caveolae domains. On the other hand, when Cav1 is expressed without Cavin1/PTRF such as in prostate cancer (PC3) cells, Cav1 resides outside caveolae in non-caveolar Cav1 scaffolds. B) Schematic diagram of Cav1 domains. All three caveolins possess an N-terminal domain, a scaffolding domain, an intramembrane domain and a C-terminal domain. C) Diagram showing Cav1 membrane topology and homooligomer of Cav1. Cav1 assumes an unusual membrane topology with both its N- and C- terminals facing the cytoplasm.
In addition to their striking morphological features, caveolae are defined based on their protein composition. Cav1 and Cavin1/PTRF are proteins associated with caveolae domains, and are essential for caveolae biogenesis (Hansen et al., 2009, 2013; Hill et al., 2008; Rothberg et al., 1992). The lipid composition and biophysical characteristics of caveolae indicates that they represent a subdomain of plasma membrane lipid rafts, enriched in cholesterol and sphingolipids (Lajoie and Nabi, 2010). However, Cav1 expression is one of the key features that distinguishes caveolae from other lipid rafts (Glenney and Soppet, 1992; Rothberg et al., 1992).
1.2. Cav1, the core structural protein of caveolae
Our understanding of caveolae was further enhanced nearly four decades after their discovery when caveolin protein was identified and found essential for caveolae biogenesis (Parton and Collins, 2016; Parton et al., 2006). Caveolin was discovered in an antibody screen, and originally identified as a 22 kDa tyrosine-phosphorylated substrate of v-Src in Rous sarcoma virus-transformed chicken fibroblasts (Glenney, 1989). Monoclonal antibodies against caveolin were shown by immuno-electron microscopy to decorate the cytoplasmic protein coat of caveolae (Glenney and Soppet, 1992). This was the first clear evidence that the protein was a crucial component of caveolae (Glenney and Soppet, 1992; Rothberg et al., 1992). Caveolin was later renamed Caveolin-1 (Cav1) due to the discovery of other isoforms of the protein (Scherer et al., 1996). Three classes of caveolins- Cav1, Cav2 and Cav3 exist in mammalian cells, of which only Cav1 and Cav3 are responsible for caveolae formation (Cohen et al., 2004; Williams and Lisanti, 2004). Cav1 is ubiquitous, and expressed in almost all cells types including endothelial cells, adipocytes and fibroblast (non-striated muscle cells) (Drab et al., 2001; Razani et al., 2001; Razani and Lisanti, 2001; Rothberg et al., 1992); Cav2 is co-expressed with Cav1, and depends on hetero-oligomerization with Cav1 for membrane localization and function (Scherer et al., 1996, 1997). Cav3 is exclusively expressed in striated and some smooth muscle cells where it is the main caveolin responsible for caveolae formation (Gazzerro et al., 2010; Tang et al., 1996; Way and Parton, 1995). Ectopic expression of Cav1 or Cav3 in caveolin-deficient cells results in significant increases in the number of morphologically identifiable caveolae, while deletion inhibits caveolae formation (Jung et al., 2018; Parton et al., 2006).
All three caveolins possess four main domains: an amino (N)-terminal domain, a scaffolding domain, an intramembrane domain and a carboxyl (C)-terminal domain (Fiala and Minguet, 2018; Parton, 2018; Root et al., 2015) (Fig. 1B). Caveolins possess a signature structural motif ‘FEDVIAEP’ within their hydrophobic N-terminal domain that is conserved across species (Head and Insel, 2007; Razani et al., 2002c; Williams and Lisanti, 2004). Caveolins share significant homology: Cav3 is 85% similar and 65% identical to Cav1, while Cav2 is 58% similar and 38% identical to Cav1 (Cohen et al., 2004). There are two main isoforms of Cav1: α and β. Cav1α is the best studied and has 178 amino acids (residues 1–178), with an estimated molecular weight of 24 kDa Cav1β (21 kDa, residues 32–178) is a truncated version of Cav1α, with 31 fewer amino acids that are translated from an alternative, internal start site (Scherer et al., 1995). Cav1 localizes to the plasma membrane such that the N-terminal and C-terminal domains both face the cytoplasm forming a hairpin loop (Fig. 1C) (Parton, 2018; Root et al., 2015). This unusual topology requires that cells be permeabilized to detect Cav1 proteins by immunohistochemistry. The C-terminal domain of Cav1 has palmitoylation sites (Dietzen et al., 1995; Parat and Fox, 2001), while the N-terminal domain possesses the tyrosine (Tyr14) phosphorylation site originally identified by Glenney and colleagues (Glenney, 1989; Glenney and Soppet, 1992; Rothberg et al., 1992).
The Cav1 scaffolding domain (CSD) is responsible for membrane binding, oligomerization, protein interaction and cholesterol recognition. Cav1 interacts with several signaling molecules including G-protein subunits, receptor and non-receptor tyrosine kinases, endothelial nitric oxide synthase (eNOS), and small GTPases using its scaffolding domain (Hoop et al., 2012; Schlegel et al., 1999). The CSD interacts with signaling molecules through a highly conserved caveolin-binding motif which is rich in aromatic residues (Couet et al., 1997; Hoop et al., 2012). The CSD contains residues required for oligomerization of Cav1 monomers into oligomers. Cav1 forms homo-oligomeric complexes of 14–16 Cav1 monomers, which further assemble in the Golgi into higher molecular weight aggregates of 130–150 Cav1 molecules. An estimated 150 Cav1 molecules is required per caveola formed (Fiala and Minguet, 2018; Hayer et al., 2010; Parton and del Pozo, 2013). Cav1 also forms hetero-oligomers with Cav2, which is required for membrane localization and function of the Cav2 protein (Scherer et al., 1996, 1997).
1.3. Cavin1/PTRF is required to sequester Cav1 into caveolae
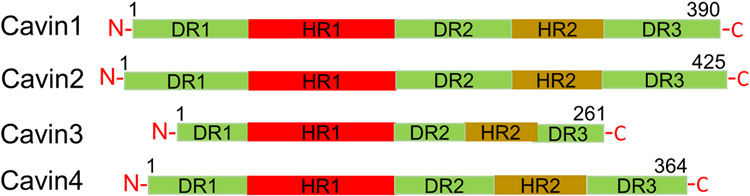
The previously accepted paradigm that Cav1 was the only structural protein required for caveolae biogenesis became obsolete with the discovery of Cavin1 as an essential caveolae-forming protein. Cavin1 was originally called as “PTRF” (or Polymerase I and Transcript Release Factor), a protein necessary to re-initiate paused transcription complexes catalyzed by RNA Polymerase I (Hasegawa et al., 2000; Jansa et al., 1998, 2001). PTRF was later identified as a key structural component of caveolae and found to be essential for caveolae biogenesis (Aboulaich et al., 2004; Vinten et al., 2001, 2005), hence, it was renamed “Cavin1”. The Cavin family of proteins is made up of four members: Cavin1/PTRF, which is required for caveolae formation (Jansa et al., 1998); Cavin2 or Serum Deprivation Protein Response (SDPR) which does not affect caveolae number but regulates caveolar morphology or curvature (Hansen et al., 2009); Cavin3 which regulates caveolae budding or vesicle movement (McMahon et al., 2009); and Cavin4 or MURC (Muscle restricted coiled-coiled Protein) which is a muscle-specific Cavin responsible for caveolae formation (Bastiani et al., 2009; Tagawa et al., 2008). All Cavins are cytosolic proteins, share sequence homology and have been isolated together with caveolae preparations (Bastiani et al., 2009; Hill et al., 2008; Kovtun et al., 2015). Cavins associate with one another to form higher molecular weight homo- and heteromeric complexes, which may or may not be associated with caveolae (Bastiani et al., 2009). A striking feature of Cavins is the presence of a PEST motif (sequences rich in proline, glutamic acid, serine and threonine) thought to play a role in proteolytic sensitivity (Aboulaich et al., 2004). Cavin proteins share characteristic domain architecture made up of two α-helical regions called HR1 and HR2, rich in basic residues, which are highly conserved. These regions are linked together by three disordered regions (DR1, DR2 and DR3) enriched in acidic residues (Tillu et al., 2021) (Fig. 2). The disordered domains in Cavin1 are thought to be responsible for electrostatic interactions with caveolar membrane and Cav1 protein (Tillu et al., 2021). They also share leucine zipper domains, which play a role in protein-protein interactions, and phosphatidylserine sites indicating their tendency to be phosphorylated (Aboulaich et al., 2004; Briand et al., 2011; Kovtun et al., 2015).
Fig. 2.
Schematic representation depicting the main features of Cavin family of proteins. All Cavins possess two regions of α-helices called HR1 and HR2, which are rich in basic residues, and linked by disordered acidic sequences called DR1, DR2 and DR3.
In most cells and tissues, Cavin1/PTRF and Cav1 proteins are co-expressed and morphologically-identifiable caveolae are observable. Deletion of Cavin1/PTRF results in a significant downregulation of Cav1 protein expression (Hill et al., 2008; Liu et al., 2008). Liu et al., demonstrated that global Cavin1/PTRF KO mice exhibited significantly diminished Cav1 protein levels and were devoid of morphologically identifiable caveolae, while simultaneously increasing their Cav1 mRNA transcript levels (Liu et al., 2008). Furthermore, deletion of Cav1 results in concomitant loss of Cavin family protein expression (Hansen et al., 2013). Overexpression of Cavin1/PTRF in Cavin1/PTRF1-deficient cells results in a concomitant increase in Cav1 protein expression and stabilization, and significantly increases the number of morphologically identifiable caveolae. Proteomic analysis to identify protein-protein interactions identified Cav1 and Cavin1/PTRF to be co-regulated, with loss of Cavin1/PTRF associated with loss of Cav1 (Davalos et al., 2010; Pan et al., 2017). Cavin1/PTRF stabilizes Cav1 in pancreatic cancer cells by inhibiting its internalization and lysosomal degradation (Liu et al., 2014; Meng et al., 2015). It is estimated that Cavin1/PTRF exists in an equivalent or 1:1 ratio to Cav1 in caveolae (Hill et al., 2008). These results underscore the critical role of Cavin1/PTRF in regulating Cav1 expression and caveolae formation. Additionally, Cavin1/PTRF promotes efficient ribosomal RNA (rRNA) transcription in mature adipocytes in response to metabolic stress (Liu and Pilch, 2008) and plays a role in multiple physiological processes including muscle physiology, metabolic regulation, lipid metabolism (Liu et al., 2008), and senescence (Volonte and Galbiati, 2011). Genetic mutation in Cavin1/PTRF is associated with diseases such as lipodystrophy (Liu et al., 2008) and muscular dystrophy (Ding et al., 2017; Taniguchi et al., 2016), which might be associated with downregulation of Cav1 and inhibition of caveolae formation.
While Cavin1/PTRF is required to sequester Cav1 into caveolae, studies have shown that Cav1 can be stably expressed in cells such as PC3 prostate cancer cells without Cavin1/PTRF. In these cells, Cav1 exists in planar or flat membranes which have been described as non-caveolar Cav1 scaffolds (Fig. 1A) (Hill et al., 2008; Khater et al., 2019a, 2019b; Moon et al., 2014).
1.4. Caveolae biogenesis
Caveolae formation starts with the synthesis of Cav1 in the endoplasmic reticulum (ER) in a signal recognition-dependent manner. Cav1 is synthesized as an integral membrane protein, and assumes an unusual topology, with both its N- and C-terminals facing the cytoplasm (Fig. 1C) (Monier et al., 1995). After synthesis, Cav1 forms homo-oligomer of 12–16 molecules, which are transported to the Golgi through a process dependent on coat protein complex II (Hayer et al., 2010). In the Golgi, Cav1 oligomers assemble into higher order oligomers (Hayer et al., 2010; Sargiacomo et al., 1995), associate with lipid rafts, and adopt detergent-resistant properties, which are similar to Cav1 complexes at the plasma membrane. A pool of newly formed Cav1 remains in the Golgi in many cell types and is not associated with detergent-resistant membranes. The difference in properties of Golgi and plasma membrane pools of Cav1 suggests a change in the characteristics of Cav1 prior to exit from the Golgi (Pol et al., 2005). There seems to be a direct connection between Cav1 oligomerization and exit from Golgi to the plasma membrane. Cav1 mutants that fail to oligomerize are trapped in the Golgi and degraded (Kirkham et al., 2008; Luetterforst et al., 1999; Machleidt et al., 2000). The exact mechanism of Cav1 exit from the Golgi is not fully understood but cholesterol and glycosphingolipids are important regulators of Cav1 budding. Addition of cholesterol to Cav1 oligomers in the Golgi is the rate-limiting step in Cav1 exit and trafficking to the plasma membrane is enhanced by addition of cholesterol and inhibited by cholesterol depletion (Pol et al., 2005). Studies using live-cell imaging of newly synthesized green fluorescent protein (GFP)-tagged Cav1 (Cav1-GFP) suggest that exocytic caveolar proteins directly transport Cav1 oligomer from the Golgi to the plasma membrane (Tagawa et al., 2005). The exocytic caveolar proteins carry a defined number of Cav1 molecules similar to the number of Cav1 molecules in mature caveolae. Caveolar carrier proteins fuse directly with the plasma membrane in a process regulated by the SNARE protein syntaxin-6 (Choudhury et al., 2006; Jung et al., 2012).
The final step in caveolae biogenesis is the recruitment and association of Cavin proteins with Cav1-enriched membrane domains. Cavin proteins form hetero-oligomeric complexes of approximately 60–80 Cavin molecules, which are recruited to the plasma membrane in a Cavin1/PTRF-dependent manner. Cavin proteins associate with the plasma membrane Cav1 but not the Golgi pool to form surface caveolae (Bastiani and Parton, 2010; Hayer et al., 2010). Other proteins like EH domain-containing protein 2 (EHD2) and PACSIN2 may be recruited to the plasma membrane prior to caveolae maturation (Hansen et al., 2011; Moren et al., 2012). The lipid composition of the plasma membrane plays a role in recruitment of proteins to and stabilization of mature caveolae. Cholesterol depletion disrupts the interaction between Cav1 and Cavin1/PTRF, which in turn disrupts caveolar architecture (Fielding and Fielding, 2000; Rothberg et al., 1992; Sohn et al., 2018). The Cavin proteins interact with phosphatidylserine (PS) and phosphatidylinositol (4,5) biphosphate (Burgener et al., 1990) on the plasma membrane ensuring that caveolae only form on the plasma membrane (Hirama et al., 2017).
1.5. Functions of caveolae
Since their discovery, the physiological significance of caveolae in regulating cellular processes has been a major topic of study. The identification of first Cav1 and later Cavin1/PTRF as important structural components required for caveolae biogenesis enabled cell biological and biochemical investigations into the role of caveolae. Using Cav1 and Cavin1/PTRF KO models, multiple studies have implicated caveolae in regulation of cell signaling (Lisanti et al., 1995), lipid metabolism (Pilch et al., 2011; Razani et al., 2002a), mechanotransduction (Sinha et al., 2011) and endocytosis (Sun et al., 2010).
1.5.1. Caveolae/Cav1 in cell signaling
Caveolae are specialized lipid rafts, which play important roles in cell signaling by concentrating or segregating receptors and signaling intermediates. This provides a favorable microenvironment for kinases and phosphatases to modify downstream signaling targets (Chidlow and Sessa, 2010; Simons and Toomre, 2000). Cav1 acts as a scaffold and interacts with molecules such as G-proteins, epidermal growth factor receptor (EGFR) and platelet-derived growth factor through the Cav1 scaffolding domain (Hoop et al., 2012; Schlegel et al., 1999). While there is some controversy about Cav1 interactions with other molecules, there is substantial evidence of Cav1 interaction with endothelial nitric oxide synthase (eNOS). Cav1 is a negative regulator of eNOS. In endothelial cells, the interaction of Cav1 with eNOS results in inhibition of eNOS activity. The Cav1/eNOS interaction is an important physiological mechanism for control of vascular reactivity, which might play a role in disease progression through regulation of the inflammatory response (Garcia-Cardena et al., 1996, 1997). Interestingly, the inhibitory effects of Cav1 on eNOS are only observed when Cav1 is present in caveolae, while Cav1 present in non-caveolar domains is unable to inhibit eNOS. In 2001, Sowa et al., demonstrated that expression of Cav1 in Cav1-deficient cells resulted in caveolae biogenesis, which in turn promoted the interaction between Cav1 and eNOS, resulting in inhibition of nitric oxide (NO) release (Sowa et al., 2001). However, in cells where Cav1 was present in non-caveolar domains, they reported significantly less interaction between Cav1 and eNOS resulting in less inhibition of eNOS function and increased NO release. Thus, they postulated that when Cav1 is present in caveolae, it is closer in proximity to eNOS, facilitating protein-protein interaction that may induce its negative regulation of eNOS function (Sowa et al., 2001).
1.5.2. Caveolae/Cav1 in lipid metabolism
Multiple studies have shown caveolae to play a role in lipid uptake and metabolism (Del Pozo et al., 2021; Pilch and Liu, 2011). Cav1 not only interacts with cholesterol (Murata et al., 1995), but also binds to fatty acids (Trigatti et al., 1999). Caveolae are thought to regulate trafficking into and accumulation of lipid in adipocytes, as Cav1 localizes to lipid droplets both in vivo and in vitro (Martin and Parton, 2005; Parton and del Pozo, 2013). In cultured cells, addition of cholesterol has been shown to increase Cav1 trafficking from plasma membrane to lipid droplets (Pol et al., 2005). Inducing expression of Cav1 in Cav1-deficient cells enhances fatty acids uptake into cells and increases levels of free cholesterol (Fielding and Fielding, 2001; Fu et al., 2004). In hepatocytes, Cav1 regulates several lipid metabolic processes including lipid storage and fatty acid oxidation and Cav1 deletion impairs lipid storage and reduces the level of total tryglycerides (Fernandez-Rojo et al., 2012, 2013; Fernandez-Rojo and Ramm, 2016). Further, mice deficient in Cav1 present with lipodystrophy, characterized by reduced fat mass, smaller adipocytes, are resistant to diet induced obesity and show hypertriglyceridemia (Razani et al., 2002a).
1.5.3. Caveolae/Cav1 in mechanotransduction
Caveolae play a role in mechanosensation in response to different mechanical stimuli such as stretching. Cell stretching disrupts the association between Cav1 and Cavin1/PTRF, resulting in caveolae flattening (Parton and del Pozo, 2013; Sinha et al., 2011). Caveolae flatten in response to increased transmural pressure in capillaries causing the caveolar membrane to be incorporated into the plasma membrane (Lee and Schmid-Schonbein, 1995). In this way, caveolae are thought to act as membrane reserves regulating volume-sensitive ion channels in response to cellular swelling (Kozera et al., 2009). Sinha et al. (2011) employed different types of controlled stresses to evaluate the role of caveolae in mechanical stress responses. They reported an immediate disappearance of caveolae induced by cell osmotic swelling or uniaxial stretching, which was associated with reduced Cav1/Cavin1/PTRF interaction and an increase in free Cav1. They also showed that release of the mechanical stress resulted in reassembly of caveolae (Sinha et al., 2011). Caveolae disassembly provides a mechanism to buffer membrane tension, thereby protecting cells from rupture (Cheng et al., 2015; Dulhunty and Franzini-Armstrong, 1975; Elliott et al., 2016). In addition to sensing swelling and stretch, caveolae can also mediate responses to hydrodynamic forces. In particular, flow-mediated activation of eNOS is associated with dissociation of eNOS from Cav1 (Rizzo et al., 1998) and Cav1 null mice are deficient in shear stress-mediated nitric oxide production (Chai et al., 2013). These results suggests that caveolae can sense, transduce, and buffer changing biomechanical perturbations. The disruption of Cav1-Cavin1/PTRF interaction during caveolae flattening may also be necessary to allow these Cavins to mediate other signaling pathways in response to mechanical stimuli (Bastiani and Parton, 2010).
1.5.4. Caveolae/Cav1 in endocytosis
Caveolae have been implicated in endocytosis of diverse molecules including glycosphingolipids and integrins (Anderson et al., 1992; Benlimame et al., 1998; Schnitzer et al., 1994), although there is controversy about whether caveolae facilitate or reduce endocytosis. For example, while multiple studies have shown Cav1 expression to significantly increase caveolae-mediated endocytosis (Gonzalez et al., 2007; Schnitzer et al., 1994; Sun et al., 2010), others have shown Cav1 over-expression to suppress caveolae-medicated endocytosis (Le et al., 2002). In addition, the internalization of glycophosphatidylinositol (GPI)-anchored proteins through caveolar pathways is not altered with or without Cav1 expression (Bhagatji et al., 2009). This obvious inconsistency is thought to result from cell-specific differences in levels of Cav1 expression and lipids involved in caveolae-mediated endocytosis. The main challenge to fully understanding the role of Cav1 in endocytosis stems from the fact that the same molecules can be endocytosed by different pathways. For example, the Simian-Virus 40 (SV40) can be internalized in both caveolar and non-caveolar vesicles (Pelkmans et al., 2001). Caveolae-mediated endocytosis involves interaction of Cav1 with actin cytoskeleton which determines whether caveolae remain at the surface or travel to internal sites. This process is mediated by tyrosine phosphorylation of Cav1 (Parton et al., 1994; Pelkmans et al., 2001).
2. Cav1 and Cavin1/PTRF expression in the retina
2.1. Cav1 expression in the retina
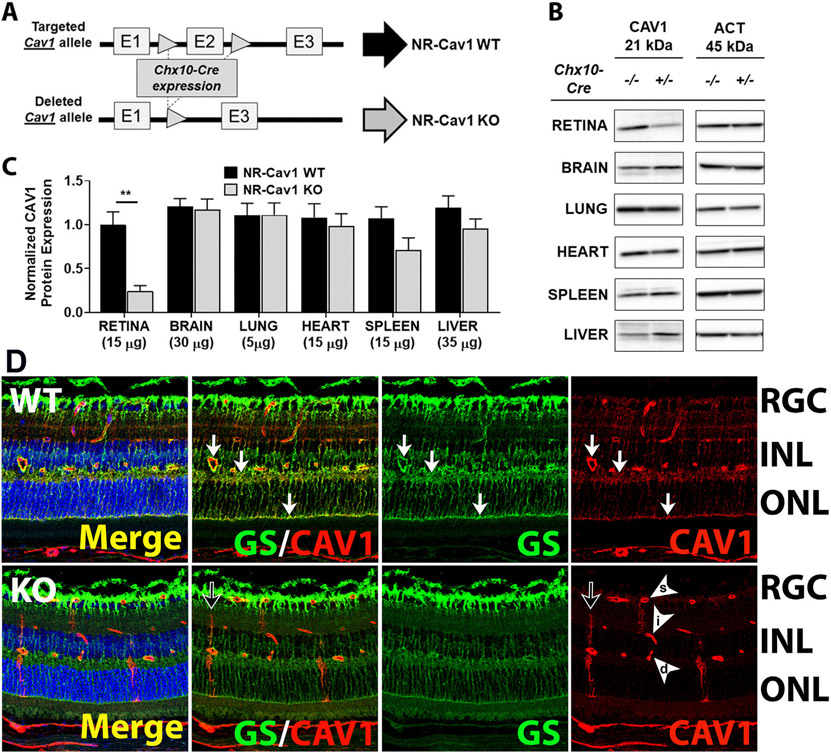
The expression of Cav1 in the retina has been extensively reviewed (Gu et al., 2017). Cav1 is highly expressed in Müller glia, retinal pigment epithelium (RPE), and in choroidal and retinal blood vessels (Gu et al., 2014b; Gurley et al., 2020; Li et al., 2012). Cav1 is also expressed in photoreceptors (Elliott et al., 2003; Kachi et al., 2001) and retinal ganglion cells (Zhang et al., 2017) albeit at much lower levels than in other cell types. Early in retinal development, Cav1 localizes predominantly to RPE, choroidal and retinal vasculature from post-natal day P0, and remains prominent in the vasculature throughout development. However, Cav1 expression is weak or absent in the developing P0 neuroretina (made up of Müller glia and neurons) and only becomes detectable between P7 and P10 in cells with Müller glia morphology. Between P7 and P10, Cav1 expression is high in the neuroretina and co-localizes with glutamine synthetase, a marker for mature Müller glia (Gu et al., 2014b). Interestingly, this corresponds with the time of Müller glia differentiation, indicating that Cav1 may be a differentiation marker (Nelson et al., 2011). The developmental expression of Cav1 also suggests that most of Cav1 expression in the neuroretina is from Müller glia and not neurons, as neuronal development begins much earlier (Fan et al., 2016) than the onset of Cav1 expression in the neuroretina. More evidence for this hypothesis comes from a recently published study using our neuroretina-specific Cav1 KO mouse model (Chx10-cre Cav1 floxed mice). We used Cre/Lox recombination technology to knockout Cav1 specifically from the neuroretina of mice, with no effect on endothelial Cav1 expression. Using our Chx10-cre, Cav1 is deleted from neurons and Müller glia cells of the neuroretina, but not from other cells like astrocytes, RPE, or retinal vascular endothelial cells. As seen in Fig. 3B and C, whole retinal tissue lysates from neuroretina-specific Cav1 KO mice show a 76% reduction in Cav1 protein level when compared to WT, suggesting that most of the Cav1 expression in the retina is from the neuroretinal compartment (Gurley et al., 2020). To confirm that Cav1 in this model targets the neuroretina specifically, we performed immunohistochemistry analysis on WT and neuroretina-specific Cav1 KO retina sections. Neuroretina-specific deletion of Cav1 was shown to primarily target Müller glia (Fig. 3D), suggesting that Cav1 expression in the neuroretina is mostly from Müller glia and not the neurons (Fig. 3) (Gurley et al., 2020). Given the colocalization of neuroretinal Cav1 with glutamine synthetase and the distinct Müller glial morphology of Cav1 immunoreactive cells, we hypothesize that the majority of Cav1 expression comes from Müller glia. These results are in agreement with previously published single cell gene expression data showing Cav1 mRNA transcripts to be more highly enriched in Müller glia than in other cells types of the neuroretina (Hoang et al., 2020; Macosko et al., 2015; Roesch et al., 2008). Taken together, these data confirm that Müller glia Cav1 expression accounts for over 70% of all Cav1 expression in the retina.
Fig. 3.
Most Cav1 expression in the retina is from the neuroretina. (A) Schematic representation of neuroretinal Cav1 knockout strategy using Cre-lox recombination technology (Chx10-cre/Cav1-floxed mouse model). (B) Representative western blots of whole cell lysate from different tissues. Neuroretinal deletion of Cav1 produced more than 70% reduction in Cav1 expression in whole cell lysate. (C) Densitometry analysis of western blots showing significant reduction in Cav1 protein expression in retina-specific Cav1 KO whole cell lysate. (D) Immunohistochemistry staining of WT and neuroretina-specific Cav1 KO retina sections, stained with anti-Cav1 antibodies and Müller glia-specific marker, anti-glutamine synthetase (GS) showing high expression of Cav1 in Müller glia, which co-localizes with GS. Neuroretinal deletion of Cav1 significantly downregulates Müller glia Cav1 expression, with no effect on Cav1 expression in choroidal and retinal vasculature. Adapted from (Gurley et al., 2020).
2.2. Cavin1/PTRF expression in the retina
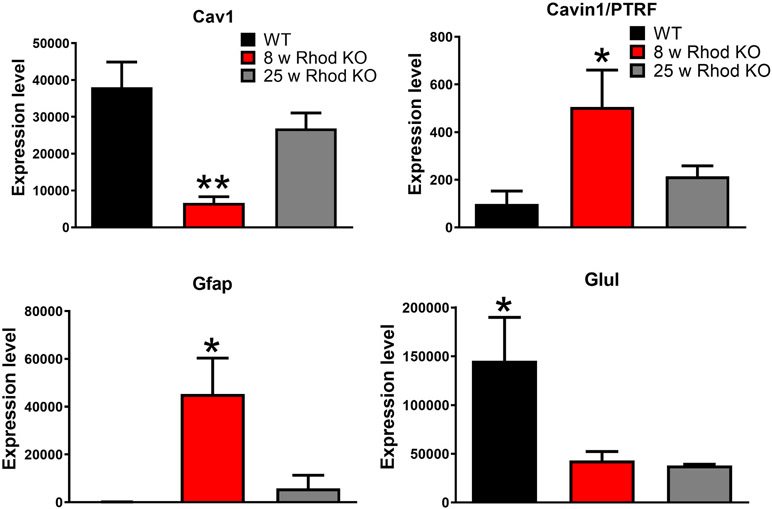
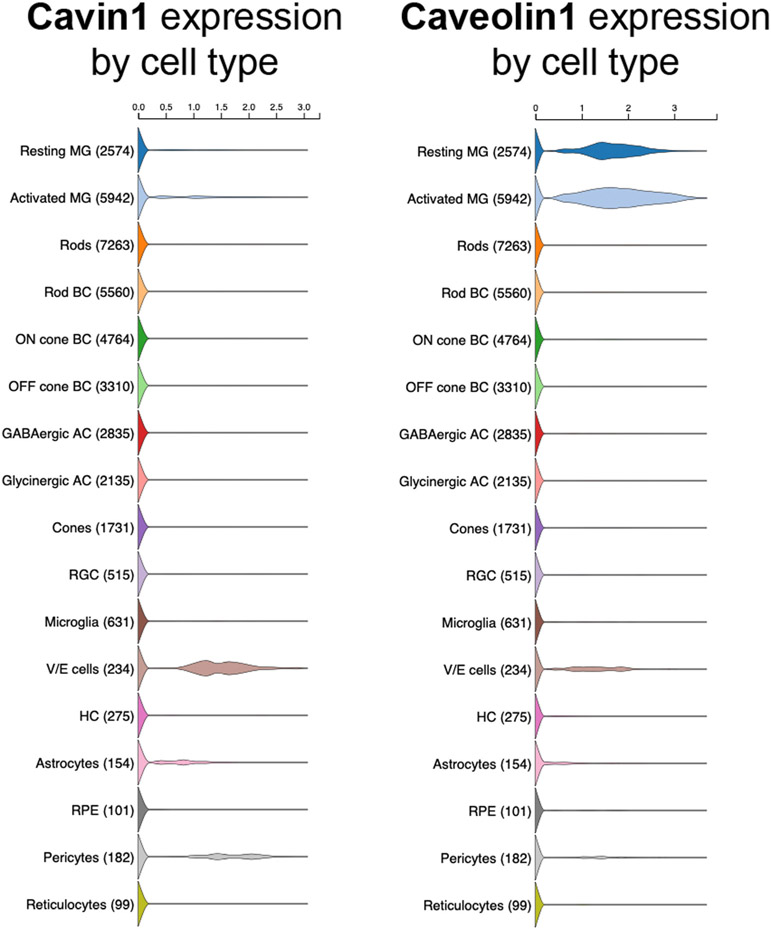
While the expression and cellular localization of Cav1 in the retina are well established, the expression of Cavin1/PTRF in the retina has not received attention. Although the universally accepted dogma that Cav1 expression generally reflects Cavin1/PTRF expression, analysis of recently published data indicates that the expression patterns of Cav1 and Cavin1/PTRF in the retina might be different. For example, single cell RNAseq data published by Macosko et al., showed Cav1 to be highly enriched in Müller glia, while Cavin1/PTRF was highly enriched in endothelial cells and pericytes (Macosko et al., 2015). Analysis of transcriptional data from isolated Müller glia in the rhodopsin knockout model of retinitis pigmentosa originally published by Roesch et al., (2012) showed a decrease in Cav1 mRNA transcripts in Müller glia at the peak of rod degeneration (8 weeks of age), which returned to control levels by the peak of cone degeneration (25 weeks of age) (Fig. 4). The decrease in Cav1 mRNA coincided with a decrease in glutamine synthetase and an increase in glial fibrillary acidic protein (GFAP) mRNAs, indicating Müller glial dedifferentiation and gliosis (Roesch et al., 2012). Interestingly, our analysis also revealed that Cavin1/PTRF mRNA transcript levels were very low in healthy Müller glia and increased modestly at the onset of rod degeneration (Fig. 4). Cavin1/PTRF mRNA levels returned to baseline expression at the onset of cone degeneration (Roesch et al., 2012). Therefore, in the rhodopsin KO model, Cav1 expression was actually opposite to Cavin1/PTRF expression: as Cav1 expression decreased, Cavin1/PTRF expression increased. Further, we analyzed a recently published single-cell RNAseq dataset by Hoang et al., (2020) and found that Cav1 is abundantly expressed in Müller glia and vascular endothelium, while Cavin1/PTRF was mainly expressed in vascular endothelium and pericytes (Fig. 5). Intriguingly, although resting Müller glial do not express Cavin1/PTRF, when subjected to stress, they are capable of upregulating Cavin1/PTRF expression (Fig. 5) (Hoang et al., 2020). Taken together, these results indicate that Cavin1/PTRF expression in the retina is mainly from the endothelium and not the neuroretina. Additionally, our results suggest that Müller glia abundantly express Cav1 without Cavin1/PTRF and, thus, are likely devoid of morphologically identifiable caveolae.
Fig. 4.
Transcriptional data replotted from microarray data from individual Müller glia from control and Rhodopsin knockout (Rhod KO) mice originally published by (Roesch et al., 2012). In this model rod death peaks at eight weeks (8 w) of age and cone death at 25 weeks (25 w). Cav1 expression is reduced in Müller glia at the peak of rod degeneration but has recovered by the peak of cone degeneration where as Cavin1/PTRF expression shows an opposing expression pattern. Gfap and Glul are indicators for gliosis and Müller glial differentiation status, respectively.
Fig. 5.
Violin plots of cell type-specific expression of Cavin1/PTRF and Cav1 in retinal single cell RNAseq data plotted from Hoang et al. (2020) (Hoang et al., 2020). As shown, Cav1 expression is high in resting and activated (by toxic NMDA insult) Müller glia (MG), vascular endothelium (V/E) and pericytes. Cavin1/PTRF is virtually undetectable in resting MG with a small number of cells increasing in expression upon activation. Cavin1/PTRF expression is high in V/E and pericytes. Available interactively at: https://proteinpaint.stjude.org/F/2019.retina.scRNA.html.
3. Caveolae and ocular diseases
Caveolae/Cav1 play important roles in modulating a variety of ocular functions that are relevant to ocular pathophysiology (Gu et al., 2017). Perhaps not surprisingly, caveolins have also been directly and indirectly associated with ocular diseases including glaucoma and age-related macular degeneration (Janssen et al., 2013). Here, we will focus on recent studies that enlighten our understanding of the role of caveolae/Cav1 in ocular inflammatory processes, glaucoma, and blood-retinal barrier and retinal vascular functions.
3.1. Caveolae/Cav1 in regulation of retinal inflammation
3.1.1. Cav1 plays paradoxical, cell-intrinsic roles in retinal innate immune responses
A robust literature describes important roles for Cav1 and caveolae in regulation of innate immune responses outside of the eye (Chidlow and Sessa, 2010; de Almeida, 2017; Shihata et al., 2016). Caveolae are also important sites for pathogen entry into cells. Several species of bacteria, including Escherichia coli, Pseudomonas aeruginosa and Mycobacteria species are known to hijack the caveolar endocytic pathway to gain intracellular access (Sowa, 2012; Zaas et al., 2005, 2009). Simian virus 40 (SV40) also enters the cell by caveolae-mediated endocytosis and is delivered to the endoplasmic reticulum through an intermediate organelle called the ‘caveosome’ (Pelkmans et al., 2001). In the cornea, caveolae provide a pathway for adenoviral entry (Yousuf et al., 2013). In addition to pathogen entry, caveolae provide a favorable microenvironment to concentrates receptors such as toll-like receptors (TLRs) and signaling molecules, like eNOS, that are involved in inflammatory responses (Chidlow and Sessa, 2010; Okamoto et al., 1998) and Cav1 is an important regulator of pro-inflammatory cytokines (Codrici et al., 2018; Guo et al., 2012; Takamura et al., 2019; Weiss et al., 2015). Codrici et al. (2018) used a Cav1 KO mouse model to assess inflammatory status by quantifying pro- and anti-inflammatory cytokines and reported a low-grade pro-inflammatory status in Cav1 KO mice, characterized by moderate increases in pro-inflammatory cytokines IL-6 and TNF-α. Similarly, Cav1 deficient mice also exhibited significantly higher levels of IL-6 and TNF-α upon induction of sepsis (Feng et al., 2010). Surprisingly, Cav1 deficiency has also been shown to blunt NF-ƙB activation in response to systemic endotoxemia with LPS-challenged Cav1--deficient mice exhibiting reduced vascular leakage, immune cell infiltration, and edema (Garrean et al., 2006). However, when Cav1 is ablated specifically from the endothelium, an enhanced inflammatory response to LPS is observed in the lung (Oliveira et al., 2017). These results imply an important but complex role in innate immune regulation outside of the eye. We hypothesize that these paradoxical responses result from different Cav1-dependent modulatory actions in different cell types.
At present there is not consensus about whether Cav1 promotes (Jiao et al., 2013; Lv et al., 2010; Mirza et al., 2010) or suppresses (Wang et al., 2009; Zhang et al., 2013) innate responses such as those mediated by TLR4 activation. Two mechanisms have been proposed to explain how Cav1 regulates TLR4: 1) by direct interaction with the receptor, resulting in either stimulation or inhibition of signaling (Wang et al., 2006), and 2) by an indirect mechanism involving over-activation of endothelial nitric oxide synthase (eNOS), resulting in NF-ƙB activation (Garrean et al., 2006). In macrophages, Cav1 binds to and inhibits TLR4 signaling resulting in suppression of pro-inflammatory cytokine secretion, while enhancing production of anti-inflammatory cytokines (Wang et al., 2006). TLR4 activation by LPS recruits the myeloid differentiation primary response (MyD88) adaptor protein through its TIR (Toll/Il-1 receptor) domain, which in turn initiates MyD88-dependent and independent pathways, resulting in activation of NF-ƙB. NF-ƙB activation mediates production of cytokines, chemokines and other molecules involved in the inflammatory process including IL-6, IL-1β, TNF-α (Medzhitov, 2001). In 2014, Li et al. published additional paradoxical data on the role of Cav1 in regulating innate immune response in the eye (Li et al., 2014). In response to intraocular LPS challenge, they observed a simultaneous decrease in pro-inflammatory cytokine secretion and an increase in immune cell influx into retinas of global Cav1 KO mice (Li et al., 2014). One explanation for the confusing disconnect between pro-inflammatory cytokine levels and immune cell recruitment resulting from global Cav1 deficiency is that the inflammatory response is suppressed in one cell type (e.g., Müller glia) while being simultaneously enhanced in another (e.g., vascular endothelium). In other words, Cav1’s influence over inflammatory responses are cell-intrinsic.
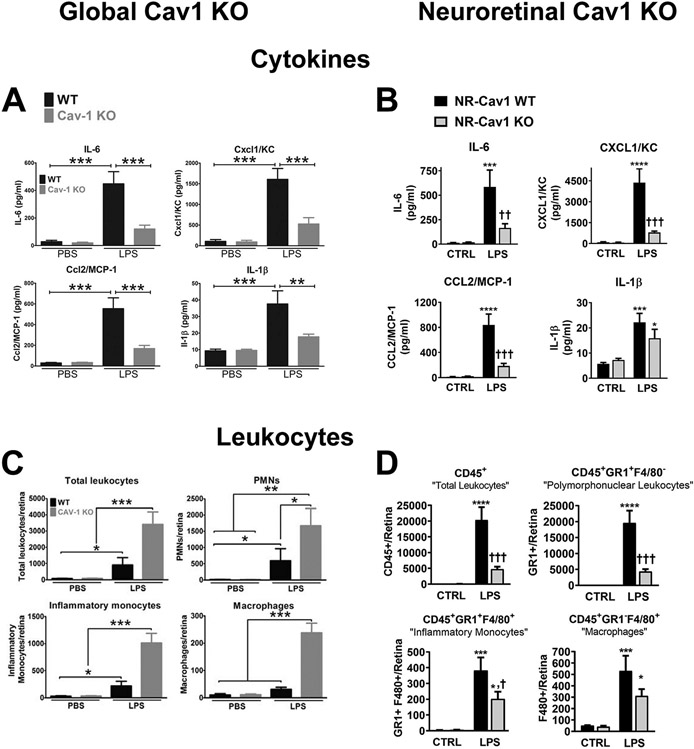
3.1.2. Müller glia Cav1 is the main driver of retinal innate immune response
To begin to address the hypothesis that Cav1 differentially modulates immune responses in different cellular contexts, we took advantage of novel Cav1 floxed mice to conditionally delete Cav1 in the neuroretina (ret-Cav1-KO) while leaving other tissues/cell types, Cav1-competent (Gurley et al., 2020). In this model, Cav1 is deleted from neuroretinal progenitors which give rise to both Müller glia and neurons (Gurley et al., 2020; Reagan et al., 2016). To determine whether neuroretinal deletion of Cav1 affects proteins involved in innate immune responses, Gurley et al., (2020) performed quantitative proteomic analysis of membrane proteins from ret-Cav1-KO mice and found 23 downregulated and 46 upregulated proteins associated with six functionally relevant immune or stress pathways (Gurley et al., 2020). To confirm whether neuroretinal deletion of Cav1 affects innate immune responses, they used multiplex cytokine panels and flow cytometry to measure the levels of proinflammatory cytokines and immune cell influx, respectively, into ret-Cav1-KO mice in response to TLR4 activation. Consistent with previous findings in global Cav1 KO (Fig. 6A) (Li et al., 2014), a signficant reduction in pro-inflammatory cytokine secretion into the retina of ret-Cav1-KO mice was observed (Fig 6B). In contrast to the paradoxical results observed in global Cav1 KO mice where cytokine response and immune cell infiltration were disconnected (Fig 6C), ret-Cav1-KO mice exhibited suppression of TLR4-mediated immune cells influx into the retina (Fig. 6D), consistent with the suppressed cytokines (Gurley et al., 2020). These findings suggest that neuroretinal Cav1 promotes a pro-inflammatory response to TLR4 activation and deletion of Cav1 from the neuroretina is sufficient to inhibit both cytokine secretion and immune cell influx into the retina. To further assess the cell-specific roles of Cav1 in modulating inflammatory responses, Gurley et al., (2020) measured cytokine levels and immune cell influx into the retina of endothelium-specific Cav1 KO (endo-Cav1-KO) mice challenged with intraocular LPS. Deletion of Cav1 in the vascular endothelium, did not blunt the inflammatory response to LPS (Gurley et al., 2020), further confirming that neuroretinal Cav1 is the key driver of the innate immune response in the retina. Since Müller glia Cav1 expression account for greater than 70% of all retinal Cav1 expression (Gurley et al., 2020), these results support the hypothesis that Cav1 expression in Müller glia is the main driver of the inflammatory response observed in the neuroretinal Cav1 KO model.
Fig. 6.
Neuroretinal Cav1 deletion suppresses endotoxin-induced immune response. (A) Deletion of Cav1 globally suppresses inflammatory cytokine secretion into the retina in response to TLR4 activation. (B) Neuroretinal deletion of Cav1 suppresses proinflammatory cytokine secretion into the retina in response to TLR4 activation. In both global and neuroretinal Cav1 KO models, there is a suppression of proinflammatory cytokine secretion into the retina. Proinflammatory cytokines were measured using a multiplex cytokine panel after intraocular injection of LPS. (C) Global Cav1 deletion enhances immune cell influx into the retina. (D) Neuroretina deletion of Cav1 suppresses immune cell influx into the retina. Leukocyte infiltration into the retina was measured by flow cytometry. A and C adapted from (Li et al., 2014); B and D adapted from (Gurley et al., 2020).
Intriguingly, the inflammatory response to TLR4 activation in our neuroretinal Cav1 KO model is opposite to that reported for endothelium-specific Cav1 deletion outside the eye. When endo-Cav1-KO mice were challenged with LPS in the lungs, both immune cell infiltrate and pro-inflammatory cytokines were significantly increased (Oliveira et al., 2017). This highlights important questions about why the neuroretinal/Müller glial pool of Cav1 behaves differently than the vascular endothelial pool. Because Cavin1 expression and morphological caveolae are found in lung endothelium (Hansen et al., 2013; Liu et al., 2008; Schubert et al., 2001), but not in Müller glia, we speculate that the localization of Cav1 within or outside of caveolae in these different cell types may be responsible for differential inflammatory control.
3.2. Caveolae/Cav1 in retinal vasculature and regulation of blood-retinal barrier function
3.2.1. Ocular vessel anatomy and structure
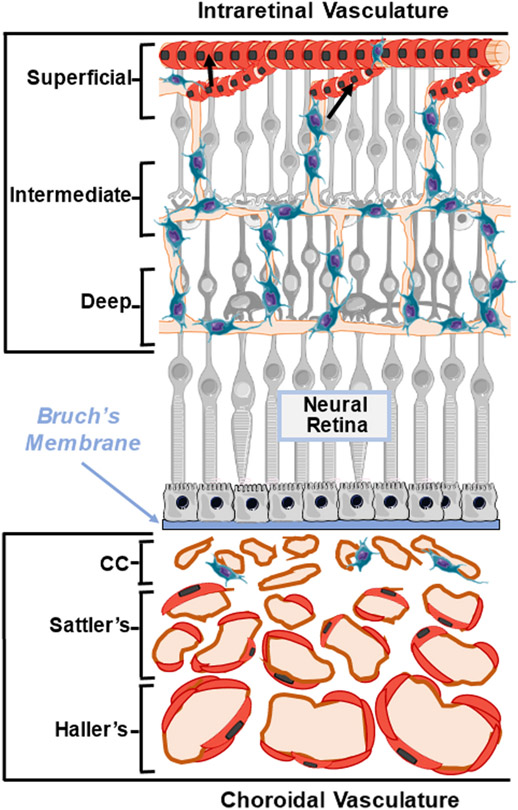
Like other organs, the eye utilizes a well-developed vascular system to deliver nutrients and oxygen, dispose of tissue waste, and provide a mechanism for immune cell recruitment following infection and/or injury. The vascular beds most intimately associated with the inner structures of the developed eye include the posterior choroidal and intraretinal vessels, as well as the specialized blood-lymphatic structures of the anterior aqueous humor outflow pathway (discussed in Section 3.3). The choroidal and intraretinal vessels ultimately support retinal tissue for phototransduction and synaptic processing of visual stimuli. The choroid is located posterior to the retina and behind the RPE. The choroid is generally separated into three vascular layers. During development, the ophthalmic artery branches and diverging posterior ciliary arteries give rise to the choroid while an alternate central retinal artery promotes development of the intraretinal vasculature (Fig. 7). The largest arteries and veins that branch from the posterior ciliary vessels form the outermost Haller’s layer. Anterior to Haller’s layer is Sattler’s layer, which consists of small and medium sized arterioles and venules. Finally, the choriocapillaris (CC), located immediately posterior to the RPE of the retina, is comprised of capillary-caliber endothelial vessels derived from hemangioblast precursor cells (Hasegawa et al., 2007). While choroidal layers containing the arteries of Haller’s and Sattler’s layers contain smooth muscle cells (SMCs), the smaller vessels of the CC are supported by mural pericytes (PCs). The choroidal vasculature supplies the outer one third of the retina, which houses photoreceptor cells. For a detailed review of choroidal structure and physiology, please refer to Nickla and Wallman (2010) (Nickla and Wallman, 2010).
Fig. 7.
Schematic diagram representing choroidal and intraretinal vascular beds. Diagram illustrates the composition of intraretinal vasculature that supports the inner neural retina (top) and choroidal vasculature that supports the outer neural retina (bottom). The intraretinal vessels form three distinct interconnected (superficial, intermediate, and deep) layers, which are associated with mural SMCs and or PCs. The choroidal vessels and supporting mural SMCs and PCs are located posterior to the RPE and Bruch’s membrane and are composed of the choriocapillaris (CC), Sattler’s layer, and Haller’s layer. Black arrows indicated SMCs on retinal arterioles.
The intraretinal vessels arise from the central retinal artery and comprise three distinct, yet interconnected layers within the inner two thirds of retinal tissue (Fig. 7). The largest vessels in the intraretinal vascular bed are small superficial arterioles and venules, which are located in the anterior retinal neural fiber layer (RNFL). These larger vessels are connected to anastomosed intermediate- and deep-capillary vascular plexi located in the inner and outer plexiform layers (IPL and OPL), respectively. While mural PCs are associated with all three layers of the intraretinal vasculature, SMCs are restricted to the larger branches of the superficial arterioles. Whereas the choroid is fenestrated and supports the photoreceptors of the outer retina via the RPE, the intraretinal vessels support retinal neurons located in the inner nuclear and retinal ganglion cell (INL and RGC) layers. This unique arrangement of ocular blood vessels maintains the high metabolic delivery requirements of the retina while also permitting sufficient passage of light for visual phototransduction.
3.2.2. Cav1/caveolae in retinal barrier function and transport
Cav1 is highly expressed in both choroidal and intraretinal vascular endothelial cells (ECs), and to a lesser extent, SMCs and PCs (Gu et al., 2014a; Li et al., 2012; Voigt et al., 2019). Cav1 plays an important role in maintenance of intraretinal barrier function, which allows for highly regulated transport of molecules into retinal tissue. Whether caveolae participate in transendothelial transport in the intraretinal vasculature has been a matter of some debate (Gardiner and Archer, 1986; Raviola and Butler, 1983). However, suppression of Cav1-mediated transcytosis is an important contributor to the development of blood-retinal barrier properties (Chow and Gu, 2017) and caveolar transcytosis is contributes to pathological permeability in the retina and brain (Knowland et al., 2014; Li et al., 2014; Wang et al., 2020b). In the choroidal vasculature, the CC harbors fenestrated vascular endothelia which allows for passage of small molecules through the vessel wall (Raviola, 1977). These specialized choroidal fenestrae openings are covered by a small diaphragm formed by bundles of PLVAP (plasmalemma vesicle-associated protein) which further enhances selective passage of small molecules and solutes (Guo et al., 2016). Interestingly, PLVAP localizes with CAV1 and forms stomatal diaphragms that cover caveolae invaginations. However, Cav1 depletion does not affect vascular fenestrae formation (Warren et al., 2010) and fenestrae appear normal in the CC of Cav1 null mice (Nakanishi et al., 2016). Likewise, deletion of Plvap is not required for caveolae formation as subcutaneous capillaries of Plvap−/− mouse embryos retain caveolar structures (Herrnberger et al., 2012b). While the choroid is semi-permeable, the outer retinal barrier (ORB) function is maintained structurally by tight junctions between RPE cells. While choroidal vessels exhibit high Cav1 expression, the fully-developed RPE exhibits very low Cav1 expression. Unfortunately, investigations of choroidal functions, including other possible roles for Cav1, are hampered by the technical difficulty of visualizing the choroidal vascular bed which is masked by the pigmented RPE. At present, we have not observed any effect of global or RPE-specific Cav1 depletion on outer retinal barrier integrity.
While Cav1 is not likely required for RPE barrier function, transcellular transport of select large molecules across the choroidal and intraretinal vessel requires Cav1/caveolae. A key study from Dr. Jerry Lutty’s laboratory showed that gp60 receptor-mediated albumin transport across choroidal ECs required Cav1 as albumin was retained in the CC vascular lumen of global-Cav1 KO mice (Nakanishi et al., 2016). These findings may have important implications for age-related macular degeneration as caveolae area of the CC is increased in geographic at-rophy while fenestrations are concomitantly reduced (Grebe et al., 2019). Caveolar-mediated endocytosis also plays a role in cellular membrane lipid trafficking and cholesterol distribution (Chaudhary et al., 2014; Shvets et al., 2015). Additionally, Cav1 over-expression/caveolae generation regulates clathrin-independent endocytosis (Chaudhary et al., 2014; Shvets et al., 2015).
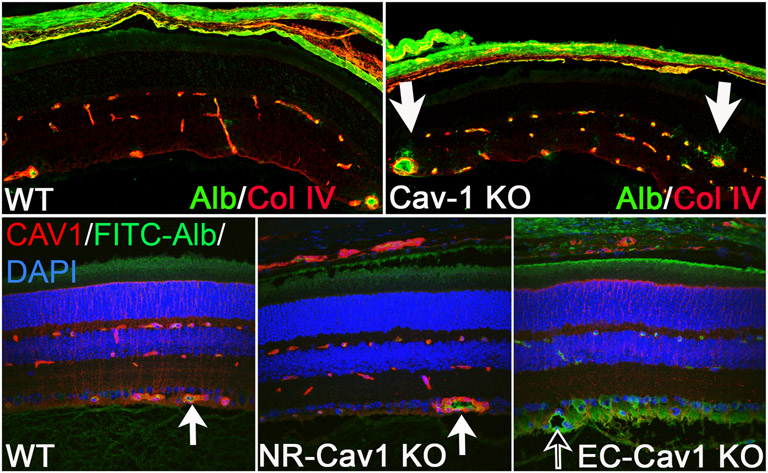
In contrast to the ORB, the intraretinal blood-retinal-barrier (IRB; blood-retinal-barrier, BRB) is compromised in Cav1−/− mice as BRB breakdown and fluorescein leakage (i.e., FITC-dextran, 4 kDa) from superficial vessels was previously observed (Gu et al., 2014a) (Fig. 8). Interestingly, Song et al. also found greater cerebral albumin leakage in Cav1−/− mice, suggesting that depletion of Cav1 also contributes to blood-brain-barrier (BBB) breakdown (Song et al., 2007). Further, we have recently shown that EC-specific Cav1 depletion was sufficient to induce BRB permeability as extravascular albumin localization was observed in retinal tissue sections from endothelium-specific Cav1 KO mice, but not neural retinal-Cav1 KO mice (Gurley et al., 2020) (Fig. 8). Intriguingly, BRB permeability induced by ocular inflammation is actually reduced by global Cav1 depletion (Li et al., 2014) suggesting that the mechanism of basal versus pathological permeability may be different. However, as we previously showed that Cav1 depletion does not affect retinal junctional protein expression (Gu et al., 2014a), the precise mechanisms involved in Cav1-mediated BRB integrity are under further investigation. Together, this supports that EC-Cav1, specifically, plays a crucial in maintenance of BRB function and regulates retinal vascular permeability.
Fig. 8.
Endogenous albumin detection in Global-, NR-, and Endo-Cav1 KO retinal tissue. Immunohistochemical staining of mouse retinal sections from global-Cav1 KO (top; adapted from (Gu et al., 2014a)) compared to NR- and Endo-Cav1 KO animals (bottom; (Gurley et al., 2020). Enhanced endogenous albumin detection was observed in global--Cav1 KO retinas and Endo(Tie2)-Cav1 KO animals compared to WT and NR (Chx10)-Cav1 KO retinas. White arrows in the top panel from the Gu et al., 2014a study highlight areas of albumin leakage in global-Cav1 KO superficial vessels. Black arrow in the bottom panel from the Gurley et al. (2020) study indicates albumin extravasation into the RNFL in Endo-Cav1 KO retinas, whereas albumin is contained within vascular lumens of WT and NR-Cav1 KO retinal vessels (bottom white arrows). WT, wild-type; “Cav1-KO” = global-Cav1 KO; NR, neural retinal; Endo, endothelial; Alb/FITC-Alb, fluorescein isothiocyanate-conjugated albumin; Col IV, collagen IV; CAV1, caveolin-1; DAPI, 4′,6-diamidino-2-pheylindole.
For an extensive review on BRB permeability and the role of Cav1 in BRB transport, please see Klaassen et al. (Klaassen et al., 2013). We have also previously discussed the role of nonvascular cells (i.e., Müller glia) in formation of the BRB (Gu et al., 2017). As previously mentioned, both Müller glial-derived Cav1 and EC-Cav1 play distinct, but important roles in modulating retinal permeability during inflammation (see Section 3.1). Additionally, Cav1 transcript and protein expression is upregulated in whole retina in response to oxygen-induced retinopathy and thought to contribute to disruption of the BRB as silencing Cav1 expression reduced retinal vascular albumin leakage in retinal tissue (Tian et al., 2012). However, whether Müller-or EC-derived Cav1 are responsible for this effect is unclear.
3.2.3. Cav1/caveolae in retinal function and retinal vascular structure
Cav1 depletion is known to affect retinal function. We originally reported reduced scotopic retinal responses Cav1−/− animals (Li et al., 2012), a finding recently independently replicated in a study suggesting that this defect reflects energy deficiencies in the Cav1 null retina (Tang et al., 2021). Cav1 KO mice exhibit alterations in RPE subretinal ion homeostasis which was attributed to enhanced RPE Na+/K+ ATPase activity (Li et al., 2012). While Cav1 and Na+/K+ATPase expression was found to be colocalized near the RPE, it is interesting to speculate whether the high expression of Cav1 in the neighboring choroid plays a role in maintaining the RPE subretinal microenvironment. The loss of Cav1 in the RPE is partially responsible for the ERG defect as RPE-specific Cav1 null mice display a modest but significant ERG deficit (Sethna et al., 2016). In addition to these ERG deficits, two recent reports independently observed significantly reduced positive scotopic threshold responses (pSTR) which are thought to be an indicator of reduced ganglion cell function (Abbasi et al., 2020; Loo et al., 2021). Whether the significant intraocular pressure elevations observed in Cav1 null mice (discussed in section 3.3) is responsible for the reduced pSTR remains to be determined.
The mural cells of the choroid receive innervation via neural varicosities (as opposed to the neuromuscular junctions found in skeletal muscle tissue). No direct innervation of intraretinal vasculature is present; however, autoregulatory mechanisms exist that regulate SMC-mediated myogenic tone in response to change in neural physiology. In SMCs, caveolae are located on the cell surface and sarcolemma and mediate Ca2+ uptake via voltage-gated channels. Thus, both choroidal and intraretinal vessels have the capacity to respond to vasoactive mediators. For a detailed review on choroidal and intraretinal blood flow regulation responses, please refer to Kur et al. (Kur et al., 2012). Whether PCs harbor contractile activity is controversial and hinges on a debate as to whether PCs express contractile proteins (i.e., α-SMA) necessary to mediate contraction (Alarcon-Martinez et al., 2018; Hill et al., 2015).
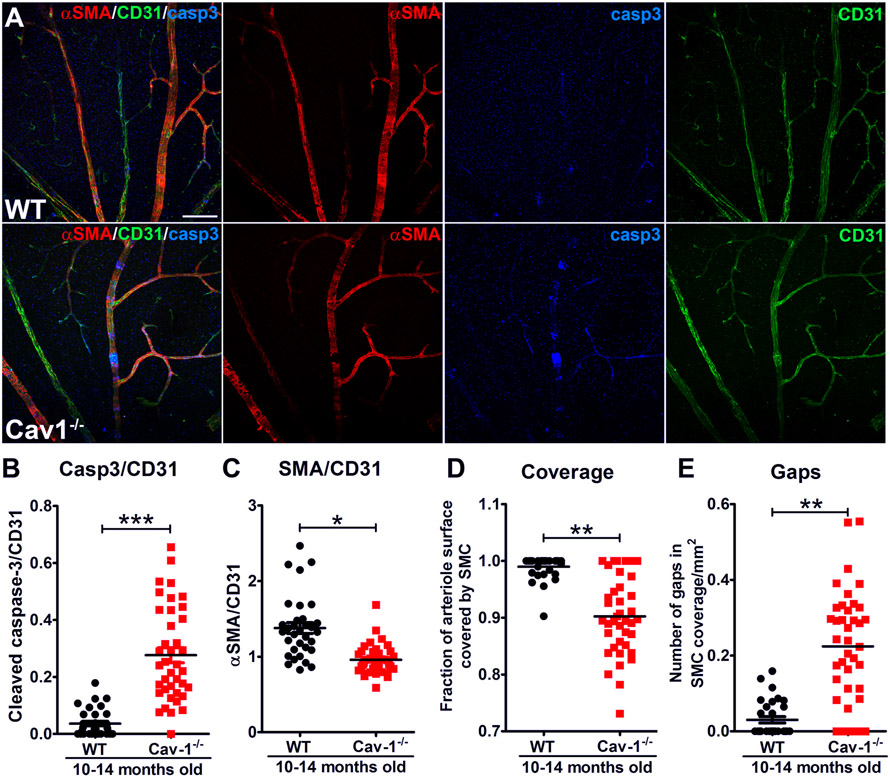
We previously showed that Cav1 ablation results in intraretinal BRB breakdown in retinal venules (Gu et al., 2014a) (Fig. 8). Interestingly, we also showed that Cav1−/− mice also exhibit areas of retinal arteriolar SMC loss as defined by loss of α-smooth muscle actin expression in surrounding mural cells (Reagan et al., 2018). Some of these “SMC gap” regions were shown to retain NG2 and/or apoptotic marker, cleaved caspase3, suggesting that heterogenous mural cell remodeling and cell death occurs with Cav1 depletion (Fig. 9 from (Reagan et al., 2018)). It is interesting to speculate that this mural remodeling could result in stasis of fluid and eventual retinal vascular breakdown, which could contribute to the previously-observed reduced retinal function and venule leakage in Cav1−/− animals (Gu et al., 2014a; Li et al., 2012; Reagan et al., 2018). The mechanism for retinal arteriolar SMC loss in Cav1−/− mice is unclear. However, given that, in some instances, non-smooth muscle actin-expressing mural cells remain in “gap” regions, it is possible that SMCs may undergo dedifferentiation with Cav1 depletion. Interestingly, the Cerebral Autosomal Dominant Arteriopathy with Sub-cortical Infarcts and Leukoencephalopathy (CADASIL) mouse model, which harbors a defective Notch3 receptor, exhibits a remarkably similar phenotype of retinal arteriolar SMC loss akin to that observed in our Cav1 KO animals (Henshall et al., 2015; Liu et al., 2010; Machuca-Parra et al., 2017). This suggests that Cav1 may be involved in SMC-EC Notch signaling and required for sustained SMC mural maintenance in adult retinal vasculature.
Fig. 9.
Cav1 deficiency results in segmental loss of alpha smooth muscle actin (αSMA) in retinal arterioles (A,D,E). The segmental gaps in αSMA staining (red) retain immunoreactivity for the endothelial marker CD31 (green) indicating that the vessel is still intact. Regions deficient in αSMA staining show increased immunoreactivity for cleaved caspase-3 (Casp3) indicating localized cell death at these gaps that are deficient in contractile smooth muscle (A,B,C). Reproduced from (Reagan et al., 2018).
3.3. Caveolae/Cav1 and glaucoma, intraocular pressure, and aqueous humor drainage
Glaucoma is a heterogeneous group of optic neuropathies characterized by progressive degeneration of RGCs resulting from damage at the optic disc. The primary and modifiable risk factor for glaucoma is intraocular pressure (IOP). Primary open angle glaucoma (POAG), the most common form of glaucoma, is frequently associated with elevated IOP, with the magnitude of IOP elevation corresponding to severity of RGC death (Jonas et al., 2017; Nickells et al., 2012; Weinreb et al., 2014; Weinreb and Khaw, 2004). Intraocular pressure is a function of a balance between secretion of aqueous humor at the ciliary body and its drainage via conventional and unconventional outflow pathways. Regulation at the level of the conventional outflow pathway sets IOP and patients with POAG exhibit increased resistance to aqueous humor drainage through the conventional outflow pathway (Grant, 1951, 1963). Subsequent elevation of IOP causes mechanical stress at the optic disk and deforms the lamina cribrosa, damaging the RGC axons and optic nerve head vasculature (Aghsaei Fard and Ritch, 2020; Jonas et al., 2017; Stamer and Acott, 2012).
IOP is dynamically regulated by controlling the rate of aqueous humor drainage through the conventional outflow pathway under conditions of fluctuating mechanical loads (Safa et al., 2022; Xin et al., 2018). The precise site of resistance to aqueous drainage is at the interface of Schlemm’s canal (SC) inner wall and the immediately adjacent trabecular meshwork (TM), the so-called juxtacanalicular tissue (JCT) (Grant, 1963; Vahabikashi et al., 2019). The rate of aqueous drainage through the conventional outflow pathway is dependent on IOP and the JCT responds to pressure fluctuations by modulating the resistance to outflow. Aqueous humor exiting the eye first passes through the inner layers of the TM, and then to the JCT and SC inner wall. The fluid must then pass through part of the blood-aqueous barrier, the inner wall of SC wall to enter its lumen. Once in the SC lumen, the aqueous humor exits the SC via collector channels that connect to the distal, episcleral venous system. The pressure within this distal vasculature, the so-called episcleral venous pressure, also provides resistance to outflow and contributes to IOP (McDonnell et al., 2018). The dynamic responsiveness of the TM, SC, and distal vasculature requires mechanical sensors to facilitate rapid and homeostatic modulation of outflow resistance to maintain IOP within a normal range. We hypothesize that caveolae expressed in the conventional outflow pathway are critical components of the mechanosensory system that transduces mechanical information (IOP fluctuations) to changes in outflow resistance, a premise initially supported by genetic evidence associating polymorphisms in genes encoding caveolins with both POAG risk and elevated IOP.
3.3.1. Variants in CAV1/2 are associated with POAG risk and IOP
For adult-onset forms of glaucoma, such as POAG, most genes associated with risk have been identified by genome wide association studies (GWAS). The CAV1 and CAV2 genes encode caveolin-1 (Cav1) and caveolin-2 (Cav2) scaffolding proteins, respectively, with Cav1, but not Cav2, required for the biosynthesis of caveolae (Drab et al., 2001; Le Lay and Kurzchalia, 2005; Razani et al., 2001, 2002b). In 2010, Thorleifsson and colleagues conducted a GWAS of subjects from Iceland and found a common genetic variant, rs4236601, on chromosome 7q31 in an intergenic region between the CAV1 and CAV2 loci that was replicated in independent cohorts from Sweden, the United Kingdom and Australia (Thorleifsson et al., 2010). The CAV1/2 POAG risk variants have also been found in populations from the United States (Loomis et al., 2014; Wiggs et al., 2011), Pakistan (Micheal et al., 2014), China (Rong et al., 2016), and Korea (Kim et al., 2015) but not from African and Afro-Caribbean populations (Cao et al., 2012; Liu et al., 2013; Williams et al., 2015) suggesting that determinants of POAG risk are associated with ethnicity (Rong et al., 2016; Zukerman et al., 2020). Importantly, CAV1/2 variants have also been associated with the major risk factor for POAG, elevated IOP (Chen et al., 2014; Hysi et al., 2014; Khawaja et al., 2018; Kim et al., 2015; MacGregor et al., 2018; Ozel et al., 2014) suggesting that the POAG risk is related to dysregulated IOP homeostasis. Although variants near the CAV1/2 genes are strongly associated with both IOP regulation and POAG risk, CAV1/2 single-nucleotide polymorphisms (SNPS) have also been associated with normal tension glaucoma in Asian populations (Kato et al., 2013; Lu et al., 2020). However, because IOP values were not stratified in these studies, we cannot exclude an CAV1/2 variants on outflow/IOP in these studies.
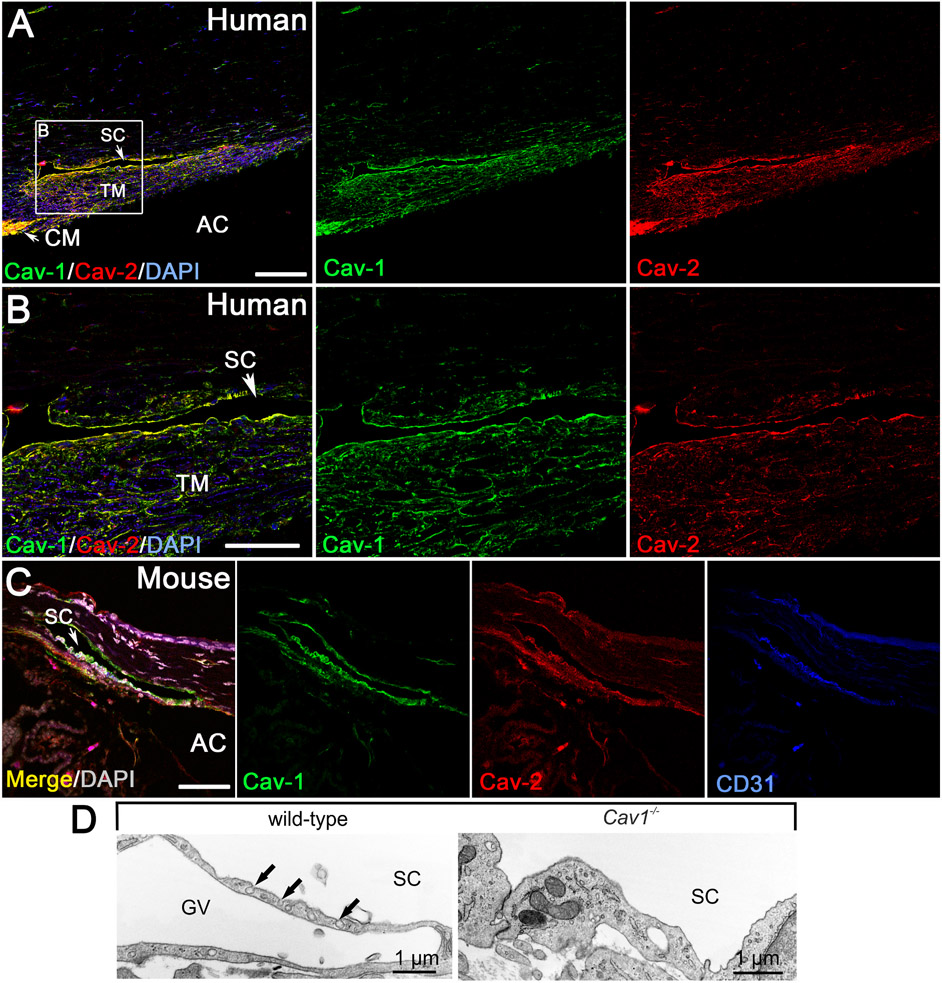
Given the association of CAV1/2 gene variants with elevated IOP and POAG, Elliott et al. (2016) evaluated the localization of caveolae-associated gene products in the conventional outflow pathway (Elliott et al., 2016). The SC endothelium and TM cells of the human conventional outflow tract are strongly immunoreactive for both Cav1 and Cav2 (Fig. 10A and B). Similar immunolocalization is also found in the mouse SC and TM (Fig. 10C), highlighting the utility of mouse models in which the Cav1 gene has been disrupted to examine outflow pathway function/dysfunction. The SC and TM also robustly express Cavin1/PTRF (Elliott et al., 2016), a protein necessary for caveolae formation in concert with Cav1 (Hill et al., 2008; Liu et al., 2008; Liu and Pilch, 2008). Not surprisingly, morphologically identifiable caveolae are abundant in both the TM and SC by transmission electron microscopy (Herrnberger et al., 2012a; Tamm, 2009), which are effectively ablated when the Cav1 gene is globally deleted in mice ((Elliott et al., 2016) and Fig. 10D).
Fig. 10.
Localization of caveolins to the human (A,B) and mouse (C) conventional outflow tract. Caveolae are abundant features of the Schlemm’s canal (SC) endothelium (D) and trabecular meshwork and are absent from global Cav1−/− mice. Adapted from (Elliott et al., 2016).
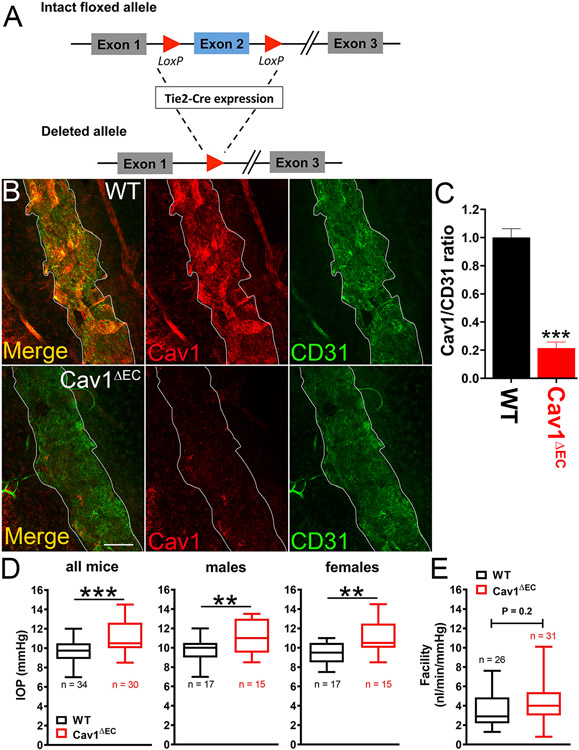
3.3.2. Global Cav1 deficiency results in elevated IOP and reduced conventional outflow facility
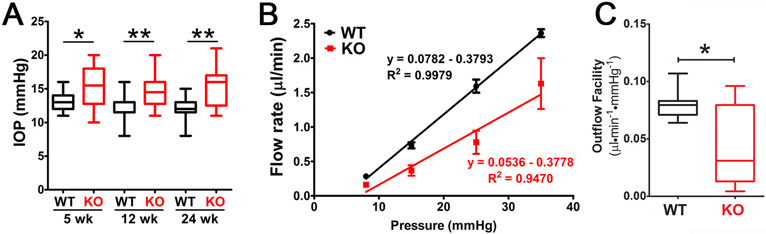
The influence of caveolae on IOP homeostasis was evaluated by Elliott et al., (2016) using mice in which the Cav1 gene was globally deleted. A significant and sustained elevation in IOP was found as early as 5 weeks of age in Cav1 KO mice (Fig. 11A), which was confirmed in subsequent independent studies (Lei et al., 2016; Loo et al., 2021; Song et al., 2017). As shown in Fig. 11B and C, the elevation in IOP in Cav1 knockout mice corresponded to a significant increase in conventional outflow resistance as measured in enucleated mouse eyes (Elliott et al., 2016). Increased outflow resistance in Cav1 KO mice was also independently observed in other studies (Kizhatil et al., 2016; Lei et al., 2016). Although the outflow pathway is dysfunctional in Cav1-deficient mice, the anterior chamber iridocorneal angle is open and the outflow pathway morphology and ultrastructure is unremarkable (Elliott et al., 2016). As such, the global Cav1 KO mouse meets several criteria defining it as a mouse model of ocular hypertension (McDowell et al., 2022), but the mechanism by which IOP is elevated and outflow facility reduced is a matter of ongoing investigation.
Fig. 11.
Global Cav1 deficiency results in increased IOP as measured by rebound tonometry (A) and reduced outflow facility (B,C) as measured by ex vivo perfusion. Adapted from (Elliott et al., 2016).
One explanation that has been proposed for the effects of Cav1 deficiency on IOP and aqueous humor drainage is that loss of Cav1 results in hyperactivity of endothelial nitric oxide synthase (eNOS) and subsequent nitrative stress in the outflow tract (Lei et al., 2016; Song et al., 2017). As discussed earlier, Cav1 is a well-established negative regulator of eNOS activity (Garcia-Cardena et al., 1996; Sowa et al., 2001) and Cav1 deletion enhances eNOS activity in the cardiovascular system (Murata et al., 2007). In iridocorneal angle tissue, endothelial-specific Cav1 deletion results in a basal increase in phosphorylation of an activating site on eNOS, supporting the idea that Cav1 is a negative regulator of eNOS in the outflow pathway (De Ieso et al., 2020). Furthermore, outflow facility (inverse of outflow resistance) is more dramatically reduced by eNOS inhibition in Cav1-deficient mice than in controls. As eNOS is a pressure-dependent regulator of IOP (Stamer et al., 2011) and nitric oxide is a crucial and clinically important player in lowering IOP (Reina-Torres et al., 2021), it seems paradoxical that increased eNOS activity as a consequence of Cav1 deletion would result in increased IOP. However, the chronic dysregulation of eNOS could result in outflow pathway dysfunction. In fact, transient suppression of Cav1 in the outflow tract actually decreases outflow resistance in perfused human donor eyes (Aga et al., 2014), while chronic Cav1 deletion impairs it (Elliott et al., 2016; Kizhatil et al., 2016; Lei et al., 2016). Thus, the idea that chronic, unregulated production of NO leads to outflow pathway tissue damage and dysfunction is intriguing but the target(s) of nitrative damage have yet to be identified. An interesting candidate is the stretch-activated transient receptor potential vanilloid isoform 4 (TRPV4) channel which is active in the TM and is regulated by cholesterol (Lakk et al., 2021). It was recently shown that TRPV4 activity is impaired by caveolar peroxynitrite in the pulmonary vasculature (Daneva et al., 2021). Thus it is conceivable that eNOS hyperactivity secondary to Cav1 depletion results in indirect nitrative modifications of other putative mechanosensors.
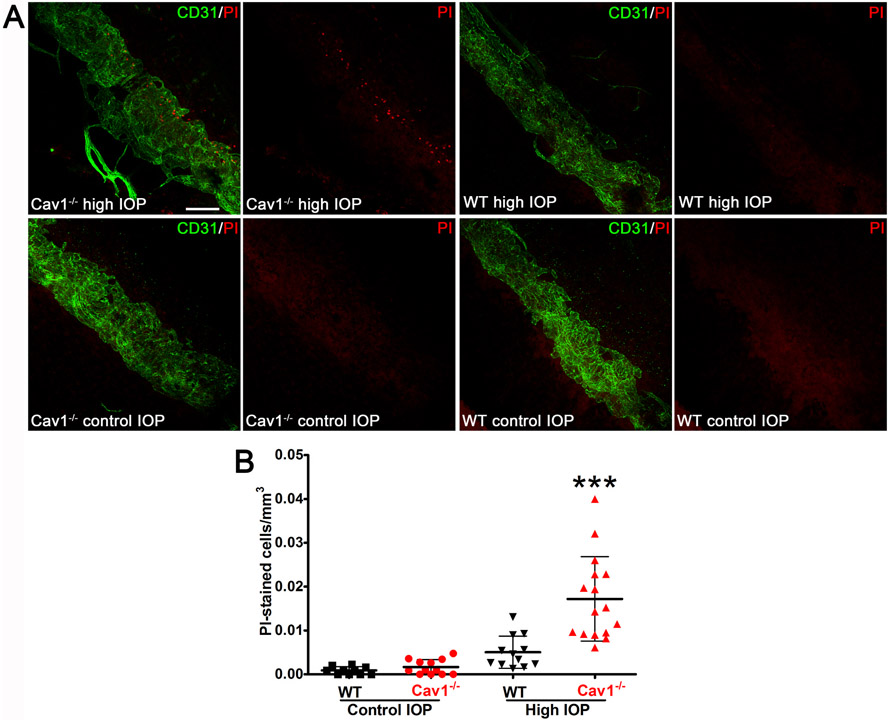
Another possible mechanism by which Cav1 deficiency could result in outflow pathway damage/dysfunction is via loss of protection from mechanical stress. Caveolae were originally hypothesized as reservoirs of membrane to protect against stretch in skeletal muscle in the 1970’s (Dulhunty and Franzini-Armstrong, 1975). This concept was revisited and supported experimentally nearly 40 years later in elegant studies both in muscle (Lo et al., 2015) and in vascular endothelium (Cheng et al., 2015). As the conventional outflow pathway is subjected to a variety of biomechanical stresses (Safa et al., 2022), we hypothesized that caveolae serves to provide protection against fluctuating, IOP-induced mechanical loads (Elliott et al., 2016). To test this, we experimentally elevated the IOP in eyes from caveolae-deficient, global Cav1 KO and caveolae-competent control mice in the presence of the membrane impermeable dye, propidium iodide with the idea that if IOP elevation induced cellular damage, the dye would have intracellular access. As shown in Fig. 12A and B incorporation of propidium iodide into cells of the outflow tissue was significantly increased in Cav1-deficient eyes that were subjected to IOP challenge compared with control providing support for a role of caveolae in outflow tract mechanoprotection.
Fig. 12.
Caveolae protect conventional outflow pathway tissue against rupture from experimentally-induced IOP elevations. Eyes were pressurized to 50 mmHg for 30 min and tissue was stained with propidium iodide (PI). Cell rupture allows PI to enter cells and label nuclei. As shown in representative panels (A) and in the quantitative analysis (B), caveolae ablation results in enhanced IOP-induced cell rupture. Reproduced from (Elliott et al., 2016).
3.3.3. Impact of Cav1/caveolae individual cell type of the outflow pathway
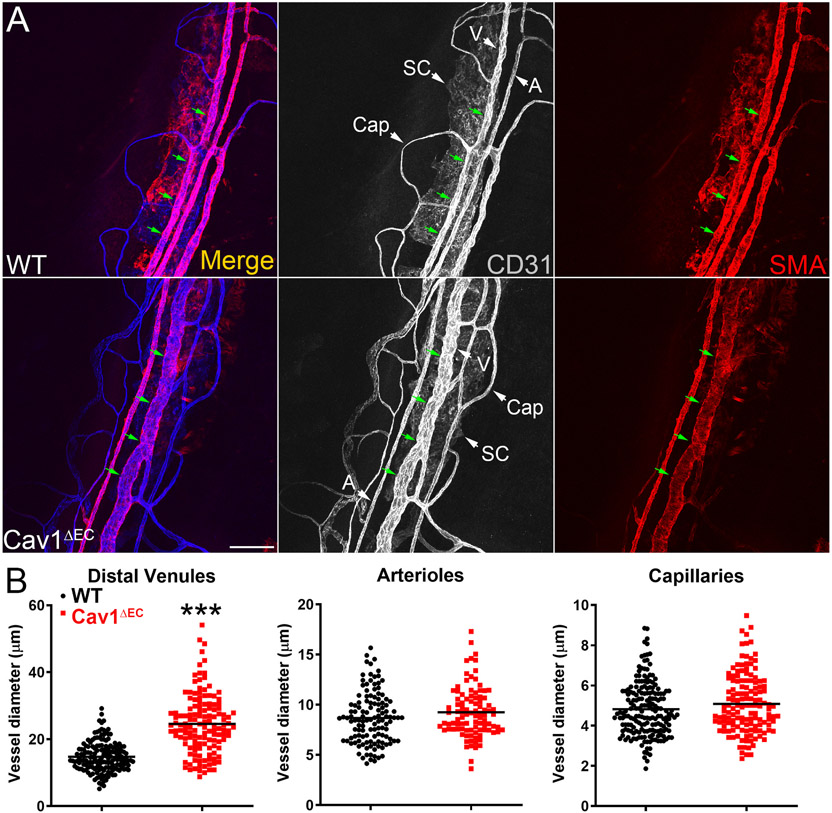
As indicated in Fig. 10, caveolins and caveolae are abundant features of both the TM, and SC. While a connection between global Cav1 expression, IOP, and conventional outflow pathway function has been established, the cell type-specific effects of caveolae are only beginning to be elucidated. We were the first to generate cell type-specific Cav1 null mice using cre/lox technology with a variety of cre drivers (Gurley et al., 2020; Oliveira et al., 2017; Reagan et al., 2016; Sethna et al., 2016). To examine the role of endothelium-derived Cav1 on IOP control, they generated an endothelium-specific Cav1 KO (Cav1ΔEC) mouse model using Tie2-driven cre expression (Fig. 13A and (De Ieso et al., 2020)). Tie2 is expressed by the SC endothelium but not the TM, and is important for SC development and function (Kim et al., 2017; Kizhatil et al., 2014; Li et al., 2020a; Thomson et al., 2014). They showed that Tie2-driven cre, which is extremely efficient at recombination in blood vessel endothelium (Oliveira et al., 2017), can also efficiently delete the Cav1 floxed allele from the SC (Fig. 13B and C) and (De Ieso et al., 2020)). Endothelium-specific deletion of Cav1 resulted in a modest but significant IOP elevation compared to control mice (Fig. 13D and (De Ieso et al., 2020)). However, unlike global Cav1 KO mice, outflow facility in Cav1ΔEC mice was not significantly different from controls (Fig. 13E and (De Ieso et al., 2020)). They did find, however, a significant and dramatic enlargement of venules in the distal outflow pathway of Cav1ΔEC mice (Fig. 14A and B and (De Ieso et al., 2020)). A similar distal outflow pathway vessel enlargement is observed in lysyl oxidase-like-1 (LOXL1) null mice, which also display elevated IOP without reduced outflow facility (Li et al., 2020b). Intriguingly, global deletion of Cav1 also results in venule enlargement in the retinal venous drainage system (Gu et al., 2014a). While at present only speculative, the venule enlargement both in distal outflow and in the retina may reflect stasis of venous flow. If this is the case, it is possible that the local venous pressure is actually higher in Cav1 null eyes resulting in IOP elevation. As outflow facility measurements were made on enucleated eyes, where venous pressure is effectively zero, the enlarged vessels might result in enhanced outflow facility, ex vivo, masking an SC-specific phenotype. While this hypothesis remains to be tested experimentally, it provides a possible explanation for the disconnect between IOP and outflow facility in Cav1ΔEC eyes. Deleting Cav1 in the SC, using a more specific cre driver that does not target the systemic vasculature, would better elucidate the contribution of SC-specific caveolae on aqueous humor outflow.
Fig. 13.
Impact of endothelial Cav1 on outflow pathway function. Endothelium-specific Cav1 null mice (Cav1ΔEC) were generated by Cre/lox technology using Cav1 floxed and Tie2-Cre mice (A) resulting in efficient deletion of Cav1 from the SC endothelium (B,C). Cav1ΔEC mice display modest but significant IOP elevation (D) without a concomitant reduction in outflow facility (E). Adapted from (De Ieso et al., 2020).
Fig. 14.
Limbal venules (V) downstream of the SC (the distal outflow vessels) are specifically enlarged in Cav1ΔEC mice. Representative confocal images (A) of the limbal region of mouse anterior segment wholemounts stained with Pecam1 (CD31) and alpha smooth muscle actin (SMA). Distal limbal venules (V) but not limbal arterioles (A) or capillaries (Cap) are significantly enlarged in Cav1ΔEC eyes (B). Reproduced from (De Ieso et al., 2020).
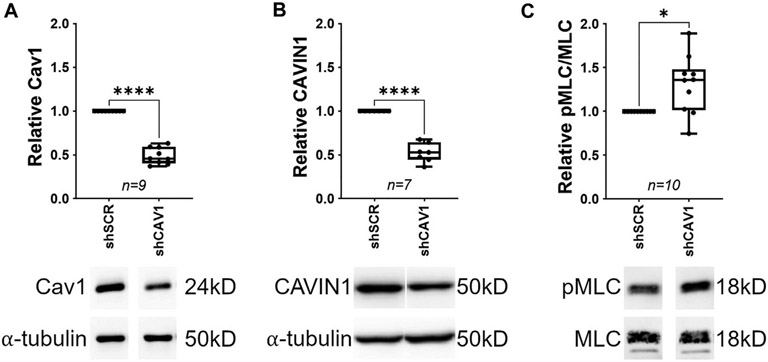
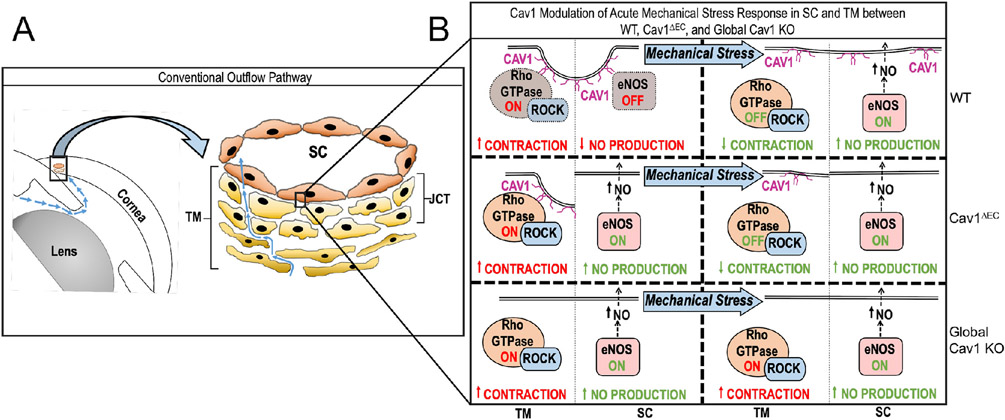
Another explanation that remains to be tested is that the TM, which is rich in caveolae and remains Cav1-competent in the Cav1ΔEC model, compensates to normalize outflow facility. It is clear that caveolins/caveolae play important roles in TM function. Expression of caveolar components including Cav1 and Cavin1 are induced by dexamethasone in TM cells (Clark et al., 2013; Surgucheva and Surguchov, 2011; Yemanyi et al., 2020). Silencing caveolins in TM cells results in increased expression of matrix remodeling enzymes suggesting that the composition/structure of extracellular matrix is regulated by caveolae (Aga et al., 2014). Recently, De Ieso et al. (2022) have shown that silencing Cav1 in TM cells results in increased myosin light chain phosphorylation suggesting increased contractility of TM upon Cav1 depletion (Fig. 15 and (De Ieso et al., 2022)). Cav1 regulation of myosin light chain phosphorylation occurs via RhoA activation and is dependent on the substrate stiffness on which TM cells are plated (De Ieso et al., 2022). Collectively, these published studies suggest that caveolae directly modulate contractility and matrix remodeling in the TM. It is also clear that caveolae indirectly modulate TM contractility via eNOS regulation and NO production by the SC endothelium, which physically interacts with the underlying TM (Chang et al., 2015; Dismuke et al., 2014; Reina-Torres et al., 2021; Stamer et al., 2011). Our current understanding of how caveolins/caveolae in the outflow pathway regulate TM contractility via direct and indirect mechanisms is summarized in Fig. 16 taken from De Ieso et al., (2020). Upon mechanical perturbation of outflow pathway cells, caveolae disassemble to release Rho-GTPase and eNOS from TM and SC caveolae, respectively. This results in a suppression of Rho/Rock signaling in the TM and an increase NO production in the SC.
Fig. 15.
Silencing Cav1 (A) in human trabecular meshwork (TM) cells results in downregulation of Cavin1 (B) and increased phosphorylation of myosin light chain (C), a surrogate marker for enhanced TM cell contractility. Reproduced from (De Ieso et al., 2022).
Fig. 16.
Schematic of how caveolae may modulate the physiologic response to mechanical stress in the SC and TM of the conventional outflow pathway function. Cav1 modulates IOP induced eNOS activation in the SC and, potentially, Rho/ROCK signaling in the TM, independently. (WT, wild type; Cav1ΔEC, endothelium-specific Cav1 KO mouse) Reproduced from (De Ieso et al., 2020).
4. Cav1 exists and functions outside of caveolae
As Cav1 is essential for the formation of caveolae, most of its functions have been attributed to roles within these domains. However, there is substantial evidence that Cav1 can exist and function in some cells outside of caveolae (Pol et al., 2020). Cells like neurons, lymphocytes, hepatocytes and organisms such as Caenorhabditis elegans express Cav1 with no morphologically identifiable caveolae, indicating a caveolae-independent role for Cav1 (Head and Insel, 2007; Pol et al., 2020). A pool of Cav1 is also found in the Golgi complex of many cells and can only be detected using specific antibodies, further confirming the presence of Cav1 in cell compartments other than plasma membrane caveolae (Bush et al., 2006; Luetterforst et al., 1999). It is possible that even within cells that possess numerous caveolae, caveolar and non-caveolar Cav1 pools co-exist in a dynamic relationship whereby membrane caveolae assemble and disassemble in response to different stimuli, and release Cav1 onto planar membranes. This concept was alluded to in the discussion of caveolae mechano-transduction/mechanoprotection in the conventional outflow tract. During periods of stretching or swelling, caveolae disassemble, disrupting Cav1-Cavin1/PTRF interactions and increase the amount of non-caveolar Cav1 on the plasma membrane (Sinha et al., 2011). However, the exact role of and mechanisms by which non-caveolar Cav1 regulates cellular processes is still unclear. Non-caveolar Cav1 is implicated in cancer progression and metastasis (Gould et al., 2010), innate immune responses (Moon et al., 2014), cell migration (Aung et al., 2011), and neuronal plasticity (Egawa et al., 2018). In this section, we will discuss the role of non-caveolar Cav1 in different cell types including neurons, lymphocytes and focus on the role of non-caveolar Cav1 in progression and metastasis of prostate cancer (PC3) cells. Finally, we will highlight data from our laboratory providing the first evidence that non-caveolar Cav1 in Müller glia plays a role in retinal innate immune responses.
4.1. Non-caveolar Cav1 in neurons
Several studies report expression of all three caveolin isoforms in neurons (Boulware et al., 2007; Galbiati et al., 1998; Zschocke et al., 2002) with no morphologically identifiable caveolae detected, suggesting major roles for non-caveolar caveolins in neuronal processes. Cav1 plays a role in synaptic transmission and neural plasticity. A recently published study by Koh and colleagues monitored synaptic vesicle dynamics in the absence of Cav1 and found impaired synaptic vesicle exocytosis and slower synaptic transmission in Cav1-deficient neuron (Koh et al., 2021). In primary rodent neurons and in human neurons derived from pluripotent stem cells, neuron-targeted over-expression of Cav1 enhanced lipid raft formation, promoted growth of dendrites and increased molecular markers of synaptic plasticity such as synaptobrevin, syntaxin and synaptophysin (Head et al., 2011). Egawa and colleagues demonstrated that in vivo Cav1 delivery into the hippocampus improved synaptic plasticity as evidenced by increase in number of synapses and increased myelination (Egawa et al., 2018). Improved synaptic plasticity improves learning and memory in adult and aged mice (Mandyam et al., 2017). In neurons, Cav1 serves as scaffolds for binding or interacting with signaling complexes required for synaptic transmission and plasticity. For example, via the CSD, Cav1 binds to and regulates glutamate and AMPA receptors by its activity on phospholipase A2 (PLA2) (Gaudreault et al., 2004a; Head and Insel, 2007). Cav1 expression in hippocampal neurons increases upon treatment with glutamate and AMPA (Bu et al., 2003), while Cav1 deficiency attenuates metabotropic glutamate receptor activation (Takayasu et al., 2010). Taken together, these results suggest that non-caveolar Cav1 may be involved in the regulation of both ionotropic and metabotropic receptors in neurons.
There is evidence linking Cav1 expression to neuronal maturation independent of caveolae. Shikanai et al. (2018), showed high Cav1 expression in immature neurons of the developing cerebral cortex, which promoted neurite pruning, process elongation and neuronal migration. Cav1 knockdown in immature neurons impaired clathrin-independent endocytosis of cell adhesion molecules such as N-cadherin and neural cell adhesion molecule L1, which are required for early neuronal maturation in the developing cerebral cortex. In this model, Cav1 was shown to be essential for the early steps of neuronal development in vivo by mediating caveolae-independent trafficking of N-cadherin and L1 (Shikanai et al., 2018). D’Orlando and colleagues demonstrated that Cav1 expression in some neurons is developmentally regulated, since migrating immature GN11 neuronal cells expressed Cav1, which was lost in fully differentiated cells. Cav1 expression in mature GN11 neuronal cells increased upon activation of estrogen receptor β (D’Orlando et al., 2008), suggesting that neurons can upregulate Cav1 expression in response to different activating stimuli. Further, Cav1 supports axonal growth of motor neurons and affect neuromuscular development in Xenopus (Breuer et al., 2020). Dysregulation of Cav1 has been implicated in neurodegenerative diseases including Huntington’s disease, Alzheimer and schizophrenia (Allen et al., 2011; Gaudreault et al., 2004b; Kassan et al., 2017; Trushina et al., 2006a), most of which are caused by immaturity of neurons in the caudate nucleus, prefrontal cortex and temporal cortex.
The expression of Cav1 in retinal neurons is less clear as the bulk of Cav1 immunoreactivity is associated with Müller glia and retinal vasculature (Gurley et al., 2020; Li et al., 2012). However, Cav1 is expressed in photoreceptors (Corley and Albert, 2011; Elliott et al., 2003, 2008; Vladimirov et al., 2018), where rare morphological caveolae have also been observed (Kachi et al., 2001). Expression of Cav1 has also been reported in RGCs (Gupta et al., 2012) where it may be involved in neuroprotective signaling (Abbasi et al., 2020, 2021; Zhang et al., 2017) but whether the impact of Cav1 on neuroprotection is via its actions directly within RGCs has not been firmly established.
4.2. Non-Caveolar Cav1 in immune cells
Cav1 is expressed in immune cells (Fra et al., 1995; Kiss et al., 2000; Medina et al., 2006b) and has established roles in formation of immunological synapses in chronic lymphocytic leukemia (CLL) (Gilling et al., 2012). Gilling et al. (2012) found Cav1 expression to correlate with clinical outcomes in CLL, with Cav1 expression seven-fold higher in CLL lymph node cells than controls. They also reported decreased migration and proliferation in Cav1-deficient CLL cells, and inability of B and T cells to form immune synapses upon Cav1 deletion suggesting that Cav1 deficiency leads to immunosuppression (Gilling et al., 2012). Cav1 also plays a role in B-cell, T-cell and macrophage signaling. Cav1 deficiency impaired macrophage phagocytosis, which is thought to alter innate immune responses, immune regulation and increase susceptibility to autoimmune diseases (Li et al., 2005). Caveolae have been observed in macrophages (Kiss and Kittel, 1995) and Cav1 is crucial for macrophage differentiation (Fu et al., 2012). Cav1-deficient macrophages display increased inflammatory responses and increased nitric oxide production in response to LPS, indicating an anti-inflammatory role for Cav1 in macrophages (Medina et al., 2006a). These data are consistent with studies showing an anti-inflammatory role for Cav1 in cells with morphologically identifiable caveolae (Wang et al., 2006). Cav1 regulates lymphocyte trafficking into the central nervous system in experimental autoimmune encephalomyelitis (EAE) by modulating expression of cell adhesion molecules ICAM-1 and VCAM-1. An important feature of most neuroinflammatory diseases is massive influx of T lymphocytes into the CNS. In EAE, Cav1 enhances T-cell infiltration into the CNS, highlighting its pathological involvement in neuroinflammation (Wu et al., 2016). Cav1 expression in human and murine T cells increases after allogeneic hematopoietic cell transplantation (Schonle et al., 2016). In murine B-lymphocytes, Cav1 mRNA and protein levels increase in response to LPS, while Cav1 deletion suppresses IgG3 secretion in response to LPS (Medina et al., 2006b). Furthermore, Cav1 in resting B cells is important for clustering and signaling of B cell antigen receptors and Cav1 deficiency leads to spontaneous B cell activation and impaired removal of autoreactive B cells (Minguet et al., 2017). These results suggest a highly regulated role for Cav1 in B- and T lymphocytes. In addition to the complex role of Cav1 within immune cells, the important impact of Cav1 in blood-CNS barrier transcytosis (Knowland et al., 2014) and integrity (Song et al., 2007) likely plays a crucial role in control of immune cell infiltration (Engel et al., 2011; Lutz et al., 2017). In fact, Cav1 may play a role in transcellular migration of immune cells through the RPE (Omri et al., 2011). However, we previously speculated that the paradoxical enhancement of immune cell infiltration (in the context of suppressed cytokine production) into the retina following LPS challenge could result from the impact of Cav1 deficiency on the blood-retinal barrier integrity (Li et al., 2014). The complex functions of Cav1 both within immune cells and within barrier-forming endothelia requires further study in models in which Cav1 can be manipulated in a cell-specific context.
Cav1 and Cav3 are expressed in brain microglia which change isoform expression dependent on activation state (Niesman et al., 2013). In cultured microglia, phosphorylation of Cav1 is important for internalization of the plasma membrane sodium-vitamin C cotransporter, a protein critical for microglial activation (Portugal et al., 2017). However, in the retina, we have not observed expression of Cav1 in microglia a finding supported by single cell RNAseq studies of retina (see https://proteinpaint.stjude.org/F/2019.retina.scRNA.html with data from (Hoang et al., 2020)). Furthermore, deletion of Cav1 from the neuroretina/Müller glia and not microglia is sufficient to suppress TLR4-mediated innate immune responses (Gurley et al., 2020). Although the jury is still out as to whether caveolins are expressed in retinal microglia, the important role that caveolins play in neuroinflammation/neuroprotection merits a more rigorous assessment.
4.3. Non-Caveolar Cav1 in prostate cancer (PC3) cells promote cancer progression
The generally accepted paradigm for many years was that Cav1 expression paralleled Cavin1/PTRF expression and that deletion of one downregulates expression of the other resulting in loss of caveolae (Hill et al., 2008; Liu et al., 2008; Liu and Pilch, 2008). However, Hill et al., (2008) showed that the PC3 prostate cancer cell line abundantly expresses Cav1 without Cavin1/PTRF, and is devoid of morphologically identifiable caveolae. Ectopic expression of Cavin1/PTRF in PC3 cells dramatically increased caveolae density, further confirming the role of Cavin1/PTRF as essential to caveolae biogenesis (Hill et al., 2008). These results were independently confirmed by Gould and colleagues who showed that loss of Cavin1/PTRF expression and membrane caveolae correlated with prostate cancer progression (Gould et al., 2010). Moon et al., (2014) published additional evidence for the non-caveolar localization of Cav1 in PC3 cells, by comparing expression of Cav1 and Cavin1/PTRF in HeLa, LNCaP, 22Rv1 and PC3 cell lines. They reported abundant expression of Cav1 and Cavin1/PTRF in HeLa cells, with very low or no expression in LNCaP and 22Rv1 cell lines. However, PC3 cells abundantly expressed Cav1 with no Cavin1/PTRF expression, indicating that Cav1 in PC3 resides predominantly in non-caveolar domains (Moon et al., 2014). These observations raised important questions about the role of non-caveolar Cav1 in PC3 cells.
Multiple studies have shown Cav1 to play an important role in cancer progression, since Cav1 is overexpressed or mutated in many solid tumors (Campos et al., 2019; Diaz et al., 2020; Nunez-Wehinger et al., 2014). However, there is controversy about the role of Cav1 in cancer development. For example, Cav1 suppresses tumor growth by inhibiting cell proliferation and anchorage-independent growth (Engelman et al., 1997; Galbiati et al., 1998; Glenney, 1989). Mutations in the Cav1 gene accelerate onset of breast cancers and lung metastasis in mice (Williams et al., 2004). Capozza and colleagues also demonstrated that Cav1-deficient mice were more susceptible to carcinogen-induced tumorigenesis, with a quicker onset of skin tumors than WT, suggesting a tumor suppressor role for Cav1 (Capozza et al., 2003). On the other hand, Cav1 overexpression has been shown to promote cancer cell survival, growth and metastasis (Ketteler and Klein, 2018), which are major hallmarks of cancer progression. Taken together, these results suggest that Cav1 can either suppress or promote tumorigenesis in a tumor-specific and stage-specific context. Cav1 is highly expressed in PC3 cells and is implicated in the life-threatening characteristics of prostate cancer including metastasis and angiogenesis. In the early stages of prostate cancer development, loss of Cav1 enhances cells proliferation and survival, while upregulation of Cav1 in later stages promotes invasiveness, metastasis and drug resistance (Aung et al., 2011; Moon et al., 2014; Nassar et al., 2013, 2015). In addition to regulating cancer progression, Moon et al., also found that non-caveolar Cav1 in PC3 cell significantly enhanced IL-6 expression, which was attenuated by co-expression with Cavin1/PTRF (Moon et al., 2014). These results suggest that in addition to promoting cancer progression, non-caveolar Cav1 in PC3 also regulates the tumor immune microenvironment. Expression of Cavin1/PTRF in PC3 seems to neutralize non-caveolar domains, thereby inhibiting its cancer-promoting actions (Moon et al., 2014).
4.4. Non caveolar Cav1 in Müller glia modulates retinal innate immune responses
We have previously established that neuroretinal Cav1 expression accounts for over 70% of all Cav1 protein expression in the retina, and showed that the majority of Cav1 in the neuroretina localizes to Müller glia (Gurley et al., 2020). Further, we demonstrated that Cavin1/PTRF localizes to retinal and choroidal blood vessels but not the neuroretina, suggesting that retinal Müller glia abundantly express Cav1 without Cavin1/PTRF, and are devoid of morphologically identifiable caveolae (Enyong et al., 2020). Thus, unlike most cells and tissues where Cav1 and Cavin1/PTRF are co-expressed and build caveolae (Hill et al., 2008; Liu et al., 2008), Müller glia more closely resemble PC3 prostate cancer cells that express Cav1 independently of Cavin1/PTRF. Although less well-studied than its functions within caveolae, several roles have been proposed for non-caveolar Cav1 including lipid trafficking, endocytosis, and regulation of immune responses (Pol et al., 2020). We hypothesize that this non-caveolar Cav1 is crucial to Müller glia function and is an area of active study.
4.4.1. Non-caveolar Cav1 in MIO-M1 Müller glia exist as heat-resistant, high molecular weight complexes
While Müller glia abundantly express Cav1, we have recently established that they do not co-express significant levels of Cavin1/PTRF (see Figs. 5 and 17 and (Enyong et al., 2022; Enyong et al., 2020)). Interestingly, we found that nearly the entire pool of Cav1 protein in MIO-M1 Müller glia exists in high molecular weight complexes that are resistant to denaturation unless heated at 98 °C in reducing SDS-PAGE buffer (Fig. 17A) (Enyong et al., 2022). The majority of Cav1 in PC3 prostate cancer cells (which also do not express Cavin1/PTRF) is also found in high molecular weight aggregates. However, in retinal microvascular endothelial cells (RMEC), which robustly express Cavin1/PTRF and make abundant caveolae, most of the Cav1 is dissociated to monomer even without heating in reducing SDS-PAGE buffer (Fig. 17A). As Cavin1/PTRF is thought to sequester and stabilize Cav1 within caveolae (Inder et al., 2012), the mechanism by which Cav1 is stabilized in the absence of Cavin1/PTRF is not known. To identify proteins that might associate with Cav1 in the absence of Cavin1/PTRF, we evaluated the protein interactome of these non-caveolar Cav1 high molecular weight complexes by immunoprecipitation and mass spectrometry. As shown in Fig. 17B, high molecular weight Cav1 complexes able to be recovered after Cav1 immunoprecipitation and visualized on SDS-PAGE gels (Fig. 17B). Mass spectrometric analysis of high molecular weight Cav1 complexes identified 33 proteins that interact with non-caveolar Cav1. Interestingly, many of these interacting proteins including β-actin, myosins, filamins, vimentin, nestin, plectin, microtubule-associated proteins are members of the three major components of the cytoskeleton (Fletcher and Mullins, 2010) (Fig. 18 and (Enyong et al., 2022)). It is currently unclear why Cav1 exist in this aggregated form in MIO-M1 Müller glia and PC3 cells, as opposed to the monomeric form in Cavin1/PTRF-expressing RMEC. We speculate that these Cavin1/PTRF-deficient Cav1 oligomers may represent “Cav1 scaffolds” previously described by Nabi and colleagues (Khater et al., 2019a, 2019b; Zheng et al., 2011) and may indicate a strong interaction between non-caveolar Cav1 scaffolds and the cytoskeleton. Ectopic expression of Cavin1/PTRF in MIO-M1 Müller glia may provides a mechanism to biochemically resolve non-caveolar Cav1 scaffold domains.
Fig. 17.
Cav1 in MIO-M1 cells exists in denaturation-resistant, high molecular weight complexes. (A) Representative Western blots showing expression of Cav1 and Cavin1 in PC3 prostate cancer cells, MIO-M1 Müller glia and retinal microvascular endothelial cells (RMEC). MIO-M1 Müller glia, like endogenous Müller glia abundantly express Cav1. In these cells and PC3 cells, nearly all of Cav1 is found in high molecular weight complexes that are only dissociated upon heating in reducing SDS-PAGE buffer. On the contrary, Cav1 in RMEC cells is predominantly monomeric both without and with heating in reducing SDS-PAGE sample buffer. (B) Stained gel and representative Western blot showing that the high molecular Cav1 complexes are recoverable after immunoprecipitation. High molecular weight complex bands from the stained gel (B1, B2 and B3 indicated by arrows) were excised and analyzed by mass spectrometry. Adapted from Enyong et al., 2022) (Enyong et al., 2022).
Fig. 18.
Proteins that interact with high molecular weight Cav1 complexes are associated with the cell cytoskeleton. Cav1 protein-protein interaction by STRING analysis. Cav1 complexes were analyzed by mass spectrometry and STRING open-source database was used to identify protein-protein interactions. A total of 33 proteins were found to interact with Cav1 complexes, most of which play a role in the cytoskeletal architecture.
4.4.2. Non-caveolar Cav1 in Müller glia enhance TLR4-mediated immune response
Previously, we have shown that neuroretinal-derived Cav1 enhances innate immune response to TLR4 activation by promoting pro-inflammatory cytokine secretion and immune cell influx into the retina (Gurley et al., 2020). Intriguingly, non-caveolar Cav1 in prostate cancer cells promotes IL-6 secretion which can be suppressed by Cavin1/PTRF expression (Inder et al., 2012; Moon et al., 2014). Since Müller glia Cav1 accounts for over 70% of all retinal Cav1 expression, and having established that Cav1 is highly expressed in Müller glia independent of Cavin1/PTRF, we speculated that non-caveolar Cav1 in Müller glia is the key driver of the inflammatory response observed in our neuroretinal-specific Cav1 KO model. Similarly, we have shown that non-caveolar Cav1 in MIO-M1 Müller glia enhances TLR4-mediated IL-6 response, in vitro, which can be blunted by silencing Cav1 (Enyong et al., 2020, 2021). These findings are consistent with and support data from our neuroretinal Cav1 KO model, in vivo (Gurley et al., 2020), suggesting that non-caveolar Cav1 in Müller glia plays an important role in regulation of innate immune response in the retina. Intriguingly, the IL-6 response to TLR4 activation in our neuroretinal-specific Cav1 KO model was exactly opposite to what has been observed in endothelium-specific Cav1 KO. Oliveira et al. (2017) showed enhanced inflammatory response in the lungs of endothelium-specific Cav1 KO mice (Oliveira et al., 2017). These paradoxical results raise an important question about why the same treatment (i.e., silencing Cav1) produces different results in different cells. We speculate that the localization of Cav1 within or outside caveolae differentially modulates innate immune responses. Thus, we hypothesize that when Cav1 is present in caveolae like in endothelial cells, TLR4 signaling is suppressed but when Cav1 is present in non-caveolar domains, like in Müller glia, TLR4 signaling is enhanced.
The mechanism by which the differential localization of Cav1 modulates TLR4-mediated responses remains to be elucidated but one possibility is that the membrane composition is different when Cav1 is non-caveolar versus when sequestered in caveolae. During caveolae biogenesis, Cav1 forms oligomers of 12–15 Cav1 molecules, which associate with lipid rafts in the Golgi and adopt detergent-resistant properties similar to Cav1 on the membrane. These Cav1 oligomers assemble into higher order oligomers containing approximately 150 Cav1 molecules, which are transported to the plasma membrane where they interact with Cavin1/PTRF to form caveolae (Hayer et al., 2010; Sargiacomo et al., 1995). However, Khater and colleagues used super-resolution microscopy to demonstrate that non-caveolar Cav1 in PC3 cells can be delivered to the plasma membrane in smaller complexes of 5–8 Cav1 molecules in the absence of Cavin1/PTRF (Khater et al., 2019b), the so-called non-caveolar “Cav1 scaffolds”. These scaffolds can reassemble into higher order domains on the plasma membrane in the absence of Cavin1/PTRF (Khater et al., 2019b; Pol et al., 2020). Therefore, in the absence of Cavin1/PTRF and membrane caveolae, Cav1 on the plasma membrane exist mainly as small oligomeric complexes instead of approximately 150 Cav1 molecules present in caveolae. Interestingly, these non-caveolar Cav1 scaffolds are relatively unstable and more susceptible to internalization and degradation than caveolar Cav1 (Hill et al., 2008; Pol et al., 2020). Since MIO-M1 Müller glia express Cav1 in non-caveolar domains, it is interesting to speculate that they share similar non-caveolar Cav1 oligomeric structures with PC3 cells. It is likely that the instability of non-caveolar Cav1 scaffolds in MIO-M1 Müller glia renders them more readily accessible to immune mediators including immune ligands and receptors, which in turn facilitates ligand-receptor interactions and thus enhances the inflammatory response. We further speculate that sequestration of non-caveolar Cav1 scaffolds into caveolae by expression of Cavin1/PTRF provides a mechanism to stabilize these domains, thereby reducing accessibility to immune mediators, resulting in blunting of the inflammatory response. Also, expression of Cavin1/PTRF in MIO-M1 Müller glia (like in PC3 cells) likely affects the mechanism by which non-caveolar Cav1 scaffolds are trafficked to the plasma membrane, which may have implications for further downstream immune signaling.
5. Future directions and conclusions
The discovery of Cav1 (Glenney and Soppet, 1992; Rothberg et al., 1992) and Cavin1/PTRF (Hill et al., 2008) as core structural components of caveolae facilitated cell biological, biochemical and immunological investigations to understand the role of caveolae in cellular processes. In non-ocular systems, caveolae play a role in cell signaling, lipid metabolism, mechanotransduction, and endocytosis (Lisanti et al., 1995; Pilch and Liu, 2011; Razani et al., 2002a; Sinha et al., 2011; Sun et al., 2010). Loss of caveolae due to deletion of either Cav1 or Cavin1/PTRF genes results in diseases such as lipodystrophies (Liu et al., 2008) and cardiomyopathies (Taniguchi et al., 2016). However, the role of caveolae as key regulators of ocular disease pathogenesis has only recently been appreciated. Caveolae are implicated in primary open angle glaucoma (Thorleifsson et al., 2010), neuroprotection (Reagan et al., 2016) and maintenance of blood-retinal barrier function (Gu et al., 2014a). Since Cav1 and Cavin1/PTRF are highly expressed in multiple ocular cells (Macosko et al., 2015), and given both proteins co-regulate each other in most tissues (Hill et al., 2008; Liu et al., 2008), it is important to understand how their interaction contributes to ocular diseases. As more in-depth knowledge about caveolae comes to light, more questions arise about the role of Cav1 outside caveolae. While the functions of Cav1 are studied mainly in the context of caveolae, we have identified a pool of Cav1 that resides preferentially outside caveolae (also called non-caveolar Cav1). Our data show that Cav1 is stably expressed in Müller glia of the retina without Cavin1/PTRF, and resides predominantly outside caveolae. As Müller glia lack morphological caveolae it is unlikely that this non-caveolar Cav1 participates in the well-established mechanotransducive/mechanoprotecdtive function of caveolae. Because Müller glia have well-established and critical functions in the retina, they represent an ideal system to evaluate the functions of non-caveolar Cav1, in vivo.
A strategy to target Cav1 function that has not received attention in the retina, is to ectopically express Cavin1/PTRF to drive non-caveolar Cav1 into caveolae. Sequestering non-caveolar Cav1 by expressing Cavin1/PTRF in PC3 cells reduces cancer growth, migration and metastasis, partly by suppressing IL-6 secretion (Aung et al., 2011; Moon et al., 2014). We have also found that non-caveolar Cav1 in the retina promotes IL-6 production and secretion and ablating Cav1 can suppress inflammation-induced IL-6 production (Gurley et al., 2020). We hypothesize that ectopic expression of Cavin1/PTRF in Müller glia, in vivo, will sequester Cav1 into caveolae, and also blunt the inflammatory response. Studies have shown that most retinal neurodegenerative diseases trigger an inflammatory response, which may affect disease outcome. Sequestration of Müller glia Cav1 into caveolae, in vivo, by ectopic expression of Cavin1/PTRF may act to control local inflammation in the retina. Thus, future studies can take advantage of Cavin1/PTRF expression in Muller glia in vivo to shed more light on various roles of non-caveolar Cav1.
In addition to the ways in which the unique non-caveolar localization of Cav1 in Müller glia can impact ocular physiology and pathophysiology, there are also important contributions that traditional caveolae functions make in the eye. As discussed earlier, in the outflow tract, it is clear from global Cav1 KO mice, that caveolae contribute to protection of outflow pathway cells from mechanical perturbation (Elliott et al., 2016). This suggests that caveolae disassemble in response to mechanical stimulation and it is also clear that this disassembly is important in mechanically-induced activation of eNOS in the SC (De Ieso et al., 2020; Lei et al., 2016) and, potentially, Rho/ROCK signaling in the TM (De Ieso et al., 2022). Whether other signaling molecules that are mechanically disengaged from caveolae during disassembly (e.g., Cavin1/PTRF (Elliott et al., 2016);) contribute to IOP-induced homeostatic responses in the outflow pathway remains to be determined. Furthermore, the relative importance of caveolae function in the SC versus the TM is still not completely understood, in vivo, as the TM-specific genetic manipulation has not been reported and the SC targeting, to date, has impacted the entire vasculature. Given that endothelium-specific Cav1 ablation resulted in increased IOP elevation without reduced outflow facility as well as a dramatic enlargement of distal vasculature, developing a model that more specifically targets the SC without impacting the systemic vasculature is needed. The hypothesis that caveolins are involved in mechanotransduction and mechanoprotection may also be relevant for biomechanical stress that occurs at the lamina cribrosa in the optic nerve head. We have observed caveolin expression in this region of murine eyes but it remains to be determined if loss of caveolins makes the laminar region more sensitive to mechanical perturbations. Finally, there is currently a significant gap in our understanding of the contribution of the gene variants with POAG risk. Most of the POAG- and IOP-associated gene variants are found in an intergenic region between CAV1 and CAV2 that contains a number of transcription factor binding sites (Loomis et al., 2014; Wiggs et al., 2011). In fact, several CAV1/2 risk alleles (rs17518872, rs4236601, and rs10262524) are associated with reduced expression of CAV1 and/or CAV2 in non-ocular tissues (Hysi et al., 2014; Kim et al., 2015). Thus, a careful assessment of CAV1/2 variants with expression in ocular tissues is needed.
Traditional caveolae function is also relevant to the function of the retinal and choroidal vasculature. There is clear evidence that the establishment of the blood-retinal barrier in the inner retinal vasculature is in part via downregulation of caveolae-mediated transcellular transport (Chow and Gu, 2017). Furthermore, caveolae-mediated permeability increases in the retina and brain during neuroinflammation/injury (Knowland et al., 2014; Li et al., 2014; Wang et al., 2020b; Yang et al., 2020). In addition to direct contribution of caveolae to transendothelial transport, they may also play important roles in vascular smooth muscle cell contractility. We have found a significant downregulation of αSMA expression and loss of mural cells in Cav1-deficient mice (Reagan et al., 2018). However, a mechanism for this mural cell phenotypic change is still not elucidated but may involve caveolar regulation of Notch signaling (Geles et al., 2021; Qin et al., 2016; Wang et al., 2020a). Thus, a reassessment of the contribution of caveolins/caveolae to vascular homeostasis in barrier-forming vascular beds (e.g., in the eye and brain) will be a valuable contribution.
Acknowledgements
This work was supported in part by NIH Grants R01EY019494 (MHE), R01EY028608 (MHE, WDS), NEI Core Grants P30EY021725, and P30EY005722, the BrightFocus Foundation grant G2013092, and by unrestricted grants to the OUHSC Department of Ophthalmology and Duke Eye Center from Research to Prevent Blindness, Inc. Mr. Eric Enyong is a Presbyterian Health Foundation Research Scholar and Dr. Jami Gurley is the recipient of American Heart Association Career Development Award 935236.
Abbreviations
- BRB
Blood-retina barrier
- Cav1
Caveolin-1
- CC
choriocapillaris
- CSD
Caveolin scaffolding domain
- EC
endothelial cell
- EAE
experimental autoimmune encephalomyelitis
- Endo-Cav1-KO
Endothelial-specific Cav1 knockout
- eNOS
Endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- GFAP
Glial fibrillary acid protein
- GFP
Green fluorescence protein
- GS
Glutamine synthetase
- GWAS
genome wide assosciation study
- HREC
Human retinal endothelial cells
- IgG
Immunoglobulins
- IHC
Immunohistochemistry
- IL-6
Interleukin-6
- IOP
Intraocular pressure
- INL
inner nuclear layer
- JCT
juxtacanalicular tissue
- KO
Knockout
- LPS
Lipopolysaccharide
- mRNA
Messenger ribonucleic acid
- MyD88
myeloid differentiation primary response protein
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
nitric oxide
- OPL
outer plexiform layer
- PLVAP
plasmalemma vesicle associated protein
- PC
pericyte
- POAG
Primary open angle glaucoma
- pSTR
Positive scotopic threshold response
- PTRF
Polymerase I and Transcript Release Factor
- Ret-Cav1-KO
Neuroretina-specific Cav1 knockout
- RGC
Retinal ganglion cell
- RMEC
retinal microvascular endothelial cells
- RPE
Retinal pigment epithelium
- RNFL
retinal nerve fiber layer
- SC
Schlemm’s canal
- shRNA
Short hairpin RNA
- SNPs
single nucleotide polymorphisms
- SMC
smooth muscle cell
- STAT
Signal transduction and activator of transcription
- TIR
Toll/IL-1 receptor domain
- TLR
Toll-like receptor
- TM
trabecular meshwork
- TNF-α
Tumor necrosis factor alpha
- IPL
inner plexiform layer
Footnotes
CRediT authorship contribution statement
Eric N. Enyong: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Jami M. Gurley: Conceptualization, Methodology, Writing – original draft. Michael L. De Ieso: Conceptualization, Methodology, Writing – review & editing. W. Daniel Stanier: Conceptualization, Methodology, Writing – review & editing, Supervision, Project administration, Funding acquisition. Michael H. Elliott: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Data availability
Data will be made available on request.
References
- Abbasi M, Gupta VK, Chitranshi N, Gupta V, Ranjbaran R, Rajput R, Pushpitha K, Kb D, You Y, Salekdeh GH, Parton RG, Mirzaei M, Graham SL, 2021. Inner retinal injury in experimental glaucoma is prevented upon AAV mediated Shp2 silencing in a caveolin dependent manner. Theranostics 11, 6154–6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbasi M, Gupta VK, Chitranshi N, Gupta VB, Mirzaei M, Dheer Y, Garthwaite L, Zaw T, Parton RG, You Y, Graham SL, 2020. Caveolin-1 ablation imparts partial protection against inner retinal injury in experimental glaucoma and reduces apoptotic activation. Mol. Neurobiol 57, 3759–3784. [DOI] [PubMed] [Google Scholar]
- Aboulaich N, Vainonen JP, Stralfors P, Vener AV, 2004. Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase I and transcript release factor (PTRF) at the surface of caveolae in human adipocytes. Biochem. J 383, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aga M, Bradley JM, Wanchu R, Yang YF, Acott TS, Keller KE, 2014. Differential effects of caveolin-1 and -2 knockdown on aqueous outflow and altered extracellular matrix turnover in caveolin-silenced trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci 55, 5497–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghsaei Fard M, Ritch R, 2020. Optical coherence tomography angiography in glaucoma. Ann. Transl. Med 8, 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon-Martinez L, Yilmaz-Ozcan S, Yemisci M, Schallek J, Kilic K, Can A, Di Polo A, Dalkara T, 2018. Capillary pericytes express alpha-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA, Yadav PN, Setola V, Farrell M, Roth BL, 2011. Schizophrenia risk gene CAV1 is both pro-psychotic and required for atypical antipsychotic drug actions in vivo. Transl. Psychiatry 1, e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RG, Kamen BA, Rothberg KG, Lacey SW, 1992. Potocytosis: sequestration and transport of small molecules by caveolae. Science 255, 410–411. [DOI] [PubMed] [Google Scholar]
- Aung CS, Hill MM, Bastiani M, Parton RG, Parat MO, 2011. PTRF-cavin-1 expression decreases the migration of PC3 prostate cancer cells: role of matrix metalloprotease 9. Eur. J. Cell. Biol 90, 136–142. [DOI] [PubMed] [Google Scholar]
- Bastiani M, Liu L, Hill MM, Jedrychowski MP, Nixon SJ, Lo HP, Abankwa D, Luetterforst R, Fernandez-Rojo M, Breen MR, Gygi SP, Vinten J, Walser PJ, North KN, Hancock JF, Pilch PF, Parton RG, 2009. MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J. Cell. Biol 185, 1259–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiani M, Parton RG, 2010. Caveolae at a glance. J. Cell Sci 123, 3831–3836. [DOI] [PubMed] [Google Scholar]
- Benlimame N, Le PU, Nabi IR, 1998. Localization of autocrine motility factor receptor to caveolae and clathrin-independent internalization of its ligand to smooth endoplasmic reticulum. Mol. Biol. Cell 9, 1773–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagatji P, Leventis R, Comeau J, Refaei M, Silvius JR, 2009. Steric and not structure-specific factors dictate the endocytic mechanism of glycosylphosphatidylinositol-anchored proteins. J. Cell. Biol 186, 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG, 2007. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J. Neurosci.: Off. J. Soc. Neurosci 27, 9941–9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer M, Berger H, Borchers A, 2020. Caveolin 1 is required for axonal outgrowth of motor neurons and affects Xenopus neuromuscular development. Sci. Rep 10, 16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand N, Dugail I, Le Lay S, 2011. Cavin proteins: new players in the caveolae field. Biochimie 93, 71–77. [DOI] [PubMed] [Google Scholar]
- Bu J, Bruckner SR, Sengoku T, Geddes JW, Estus S, 2003. Glutamate regulates caveolin expression in rat hippocampal neurons. J. Neurosci. Res 72, 185–190. [DOI] [PubMed] [Google Scholar]
- Burgener R, Wolf M, Ganz T, Baggiolini M, 1990. Purification and characterization of a major phosphatidylserine-binding phosphoprotein from human platelets. Biochem. J 269, 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush WS, Ihrke G, Robinson JM, Kenworthy AK, 2006. Antibody-specific detection of caveolin-1 in subapical compartments of MDCK cells. Histochem. Cell Biol 126, 27–34. [DOI] [PubMed] [Google Scholar]
- Campos A, Burgos-Ravanal R, Gonzalez MF, Huilcaman R, Lobos Gonzalez L, Quest AFG, 2019. Cell intrinsic and extrinsic mechanisms of caveolin-1-enhanced metastasis. Biomolecules 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Jiao X, Liu X, Hennis A, Leske MC, Nemesure B, Hejtmancik JF, 2012. CDKN2B polymorphism is associated with primary open-angle glaucoma (POAG) in the Afro-Caribbean population of Barbados, West Indies. PLoS One 7, e39278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozza F, Williams TM, Schubert W, McClain S, Bouzahzah B, Sotgia F, Lisanti MP, 2003. Absence of caveolin-1 sensitizes mouse skin to carcinogen-induced epidermal hyperplasia and tumor formation. Am. J. Pathol 162, 2029–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Q, Wang XL, Zeldin DC, Lee HC, 2013. Role of caveolae in shear stress-mediated endothelium-dependent dilation in coronary arteries. Cardiovasc. Res 100, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Stamer WD, Bertrand J, Read AT, Marando CM, Ethier CR, Overby DR, 2015. Role of nitric oxide in murine conventional outflow physiology. Am. J. Physiol. Cell Physiol 309, C205–C214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N, Gomez GA, Howes MT, Lo HP, McMahon KA, Rae JA, Schieber NL, Hill MM, Gaus K, Yap AS, Parton RG, 2014. Endocytic crosstalk: cavins, caveolins, and caveolae regulate clathrin-independent endocytosis. PLoS Biol. 12, e1001832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Klein AP, Klein BE, Lee KE, Truitt B, Klein R, Iyengar SK, Duggal P, 2014. Exome array analysis identifies CAV1/CAV2 as a susceptibility locus for intraocular pressure. Invest. Ophthalmol. Vis. Sci 56, 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JP, Mendoza-Topaz C, Howard G, Chadwick J, Shvets E, Cowburn AS, Dunmore BJ, Crosby A, Morrell NW, Nichols BJ, 2015. Caveolae protect endothelial cells from membrane rupture during increased cardiac output. J. Cell. Biol 211, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow JH Jr., Sessa WC, 2010. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc. Res 86, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A, Marks DL, Proctor KM, Gould GW, Pagano RE, 2006. Regulation of caveolar endocytosis by syntaxin 6-dependent delivery of membrane components to the cell surface. Nat. Cell Biol 8, 317–328. [DOI] [PubMed] [Google Scholar]
- Chow BW, Gu C, 2017. Gradual suppression of transcytosis governs functional blood-retinal barrier formation. Neuron 93, 1325–1333 e1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, Nosie A, Walker T, Faralli JA, Filla MS, Barrett-Wilt G, Peters DM, 2013. Comparative genomic and proteomic analysis of cytoskeletal changes in dexamethasone-treated trabecular meshwork cells. Mol. Cell. Proteomics : MCP 12, 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codrici E, Albulescu L, Popescu ID, Mihai S, Enciu AM, Albulescu R, Tanase C, Hinescu ME, 2018. Caveolin-1-Knockout mouse as a model of inflammatory diseases. J. Immunol. Res, 2498576, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, Lisanti MP, 2004. Role of caveolae and caveolins in health and disease. Physiol. Rev 84, 1341–1379. [DOI] [PubMed] [Google Scholar]
- Corley SC, Albert AD, 2011. Assessment of bovine rod outer segment disk membrane heterogeneity utilizing flow cytometry. Exp. Eye Res 92, 20–27. [DOI] [PubMed] [Google Scholar]
- Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP, 1997. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem 272, 6525–6533. [DOI] [PubMed] [Google Scholar]
- D’Orlando C, Guzzi F, Gravati M, Biella G, Toselli M, Meneveri R, Barisani D, Parenti M, 2008. Retinoic acid- and phorbol ester-induced neuronal differentiation down-regulates caveolin expression in GnRH neurons. J. Neurochem 104, 1577–1587. [DOI] [PubMed] [Google Scholar]
- Daneva Z, Marziano C, Ottolini M, Chen YL, Baker TM, Kuppusamy M, Zhang A, Ta HQ, Reagan CE, Mihalek AD, Kasetti RB, Shen Y, Isakson BE, Minshall RD, Zode GS, Goncharova EA, Laubach VE, Sonkusare SK, 2021. Caveolar peroxynitrite formation impairs endothelial TRPV4 channels and elevates pulmonary arterial pressure in pulmonary hypertension. Proc. Natl. Acad. Sci. U. S. A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos A, Fernandez-Hernando C, Sowa G, Derakhshan B, Lin MI, Lee JY, Zhao H, Luo R, Colangelo C, Sessa WC, 2010. Quantitative proteomics of caveolin-1-regulated proteins: characterization of polymerase i and transcript release factor/CAVIN-1 IN endothelial cells. Mol. Cell. Proteomics : MCP 9, 2109–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida CJG, 2017. Caveolin-1 and caveolin-2 can Be antagonistic partners in inflammation and beyond. Front. Immunol 8, 1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ieso ML, Gurley JM, McClellan ME, Gu X, Navarro I, Li G, Gomez-Caraballo M, Enyong E, Stamer WD, Elliott MH, 2020. Physiologic consequences of caveolin-1 ablation in conventional outflow endothelia. Invest. Ophthalmol. Vis. Sci 61, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ieso ML, Kuhn M, Bernatchez P, Elliott MH, Stamer WD, 2022. A role of caveolae in trabecular meshwork mechanosensing and contractile tone. Front. Cell Dev. Biol 10, 855097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo MA, Lolo FN, Echarri A, 2021. Caveolae: mechanosensing and mechanotransduction devices linking membrane trafficking to mechanoadaptation. Curr. Opin. Cell Biol 68, 113–123. [DOI] [PubMed] [Google Scholar]
- Diaz MI, Diaz P, Bennett JC, Urra H, Ortiz R, Orellana PC, Hetz C, Quest AFG, 2020. Caveolin-1 suppresses tumor formation through the inhibition of the unfolded protein response. Cell Death Dis. 11, 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzen DJ, Hastings WR, Lublin DM, 1995. Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. J. Biol. Chem 270, 6838–6842. [DOI] [PubMed] [Google Scholar]
- Ding SY, Liu L, Pilch PF, 2017. Muscular dystrophy in PTFR/cavin-1 null mice. JCI Insight. 2, e91023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismuke WM, Liang J, Overby DR, Stamer WD, 2014. Concentration-related effects of nitric oxide and endothelin-1 on human trabecular meshwork cell contractility. Exp. Eye Res 120, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV, 2001. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293, 2449–2452. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Franzini-Armstrong C, 1975. The relative contributions of the folds and caveolae to the surface membrane of frog skeletal muscle fibres at different sarcomere lengths. J. Physiol 250, 513–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa J, Zemljic-Harpf A, Mandyam CD, Niesman IR, Lysenko LV, Kleschevnikov AM, Roth DM, Patel HH, Patel PM, Head BP, 2018. Neuron-targeted caveolin-1 promotes ultrastructural and functional hippocampal synaptic plasticity. Cerebr. Cortex 28, 3255–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MH, Ashpole NE, Gu X, Herrnberger L, McClellan ME, Griffith GL, Reagan AM, Boyce TM, Tanito M, Tamm ER, Stamer WD, 2016. Caveolin-1 modulates intraocular pressure: implications for caveolae mechanoprotection in glaucoma. Sci. Rep 6, 37127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MH, Fliesler SJ, Ghalayini AJ, 2003. Cholesterol-dependent association of caveolin-1 with the transducin alpha subunit in bovine photoreceptor rod outer segments: disruption by cyclodextrin and guanosine 5’-O-(3-thiotriphosphate). Biochemistry 42, 7892–7903. [DOI] [PubMed] [Google Scholar]
- Elliott MH, Nash ZA, Takemori N, Fliesler SJ, McClellan ME, Naash MI, 2008. Differential distribution of proteins and lipids in detergent-resistant and detergent-soluble domains in rod outer segment plasma membranes and disks. J. Neurochem 104, 336–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D, Beckers L, Wijnands E, Seijkens T, Lievens D, Drechsler M, Gerdes N, Soehnlein O, Daemen MJ, Stan RV, Biessen EA, Lutgens E, 2011. Caveolin-1 deficiency decreases atherosclerosis by hampering leukocyte influx into the arterial wall and generating a regulatory T-cell response. Faseb. J. : Off. Publ. Feder. Am. Soc. Exp. Biol 25, 3838–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Wykoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP, 1997. Recombinant expression of caveolin-1 in oncogenieally transformed cells abrogates anchorage-independent growth. J. Biol. Chem 272, 16374–16381. [DOI] [PubMed] [Google Scholar]
- Enyong EN, Gurley J, Gyening Y, Agbaga MPG, Elliott MH, 2021. Compositional analysis of non-caveolar Caveolin-1-containing domains in Muller glia. Invest. Ophthalmol. Vis. Sci 62. [Google Scholar]
- Enyong EN, Gurley J, Sjoelund V, Elliott MH, 2022. Caveolin-1 in Müller glia exist as heat-resistant, high molecular weight complexes. Adv. Exp. Med. Biol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyong EN, Gyening YK, Agbaga MPG, Ding SY, Pilch P, Liu LB, Elliott MH, 2020. The role of non-caveolar caveolin-1 in Muller glia. Invest. Ophthalmol. Vis. Sci 61. [Google Scholar]
- Fan WJ, Li X, Yao HL, Deng JX, Liu HL, Cui ZJ, Wang Q, Wu P, Deng JB, 2016. Neural differentiation and synaptogenesis in retinal development. Neural. Regen. Res 11, 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Guo L, Song Z, Gao H, Wang D, Fu W, Han J, Li Z, Huang B, Li XA, 2010. Caveolin-1 protects against sepsis by modulating inflammatory response, alleviating bacterial burden, and suppressing thymocyte apoptosis. J. Biol. Chem 285, 25154–25160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Rojo MA, Gongora M, Fitzsimmons RL, Martel N, Martin SD, Nixon SJ, Brooks AJ, Ikonomopoulou MP, Martin S, Lo HP, Myers SA, Restall C, Ferguson C, Pilch PF, McGee SL, Anderson RL, Waters MJ, Hancock JF, Grimmond SM, Muscat GE, Parton RG, 2013. Caveolin-1 is necessary for hepatic oxidative lipid metabolism: evidence for crosstalk between caveolin-1 and bile acid signaling. Cell Rep. 4, 238–247. [DOI] [PubMed] [Google Scholar]
- Fernandez-Rojo MA, Ramm GA, 2016. Caveolin-1 function in liver physiology and disease. Trends Mol. Med 22, 889–904. [DOI] [PubMed] [Google Scholar]
- Fernandez-Rojo MA, Restall C, Ferguson C, Martel N, Martin S, Bosch M, Kassan A, Leong GM, Martin SD, McGee SL, Muscat GE, Anderson RL, Enrich C, Pol A, Parton RG, 2012. Caveolin-1 orchestrates the balance between glucose and lipid-dependent energy metabolism: implications for liver regeneration. Hepatology 55, 1574–1584. [DOI] [PubMed] [Google Scholar]
- Fiala GJ, Minguet S, 2018. Caveolin-1: the unnoticed player in TCR and BCR signaling. Adv. Immunol 137, 83–133. [DOI] [PubMed] [Google Scholar]
- Fielding CJ, Fielding PE, 2000. Cholesterol and caveolae: structural and functional relationships. Biochim. Biophys. Acta 1529, 210–222. [DOI] [PubMed] [Google Scholar]
- Fielding CJ, Fielding PE, 2001. Caveolae and intracellular trafficking of cholesterol. Adv. Drug Deliv. Rev 49, 251–264. [DOI] [PubMed] [Google Scholar]
- Fletcher DA, Mullins RD, 2010. Cell mechanics and the cytoskeleton. Nature 463, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fra AM, Williamson E, Simons K, Parton RG, 1994. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J. Biol. Chem 269, 30745–30748. [PubMed] [Google Scholar]
- Fra AM, Williamson E, Simons K, Parton RG, 1995. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc. Natl. Acad. Sci. U. S. A 92, 8655–8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Hoang A, Escher G, Parton RG, Krozowski Z, Sviridov D, 2004. Expression of caveolin-1 enhances cholesterol efflux in hepatic cells. J. Biol. Chem 279, 14140–14146. [DOI] [PubMed] [Google Scholar]
- Fu Y, Moore XL, Lee MK, Fernandez-Rojo MA, Parat MO, Parton RG, Meikle PJ, Sviridov D, Chin-Dusting JP, 2012. Caveolin-1 plays a critical role in the differentiation of monocytes into macrophages. Arterioseler. Thromb. Vase. Biol 32, e117–125. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Gil O, Zanazzi G, Salzer JL, Sargiacomo M, Scherer PE, Engelman JA, Schlegel A, Parenti M, Okamoto T, Lisanti MP, 1998. Expression of caveolin-1 and -2 in differentiating PC12 cells and dorsal root ganglion neurons: caveolin-2 is up-regulated in response to cell injury. Proc. Natl. Acad. Sci. U. S. A 95, 10257–10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Fan R, Stern DF, Liu J, Sessa WC, 1996. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J. Biol. Chem 271, 27237–27240. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC, 1997. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J. Biol. Chem 272, 25437–25440. [DOI] [PubMed] [Google Scholar]
- Gardiner TA, Archer DB, 1986. Does unidirectional vesicular transport occur in retinal vessels? Br. J. Ophthalmol 70, 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB, 2006. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J. Immunol 177, 4853–4860. [DOI] [PubMed] [Google Scholar]
- Gaudreault SB, Chabot C, Gratton JP, Poirier J, 2004a. The caveolin scaffolding domain modifies 2-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor binding properties by inhibiting phospholipase A2 activity. J. Biol. Chem 279, 356–362. [DOI] [PubMed] [Google Scholar]
- Gaudreault SB, Dea D, Poirier J, 2004b. Increased caveolin-1 expression in Alzheimer’s disease brain. Neurobiol. Aging 25, 753–759. [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Sotgia F, Bruno C, Lisanti MP, Minetti C, 2010. Caveolinopathies: from the biology of caveolin-3 to human diseases. Eur. J. Hum. Genet. : EJHG (Eur. J. Hum. Genet.) 18, 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geles KG, Gao Y, Giannakou A, Sridharan L, Yamin TT, Zhang J, Karim R, Bard J, Piche-Nicholas N, Charati M, Maderna A, Lucas J, Golas J, Guffiroy M, Pirie-Shepherd S, Roy M, Qian J, Franks T, Zhong W, O’Donnell CJ, Tchistiakova L, Gerber HP, Sapra P, 2021. NOTCH3-targeted antibody drug conjugates regress tumors by inducing apoptosis in receptor cells and through transendocytosis into ligand cells. Cell Rep. Med 2, 100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilling CE, Mittal AK, Chaturvedi NK, Iqbal J, Aoun P, Bierman PJ, Bociek RG, Weisenburger DD, Joshi SS, 2012. Lymph node-induced immune tolerance in chronic lymphocytic leukaemia: a role for caveolin-1. Br. J. Haematol 158, 216–231. [DOI] [PubMed] [Google Scholar]
- Glenney JR Jr., 1989. Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J. Biol. Chem 264, 20163–20166. [PubMed] [Google Scholar]
- Glenney JR Jr., Soppet D, 1992. Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc. Natl. Acad. Sci. U. S. A 89, 10517–10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MI, Krizman-Genda E, Robinson MB, 2007. Caveolin-1 regulates the delivery and endocytosis of the glutamate transporter, excitatory amino acid carrier 1. J. Biol. Chem 282, 29855–29865. [DOI] [PubMed] [Google Scholar]
- Gould ML, Williams G, Nicholson HD, 2010. Changes in caveolae, caveolin, and polymerase 1 and transcript release factor (PTRF) expression in prostate cancer progression. Prostate 70, 1609–1621. [DOI] [PubMed] [Google Scholar]
- Grant WM, 1951. Clinical tonography. Trans. Am. Acad. Ophthalmol. Otolaryngol 55, 774–781. [PubMed] [Google Scholar]
- Grant WM, 1963. Experimental aqueous perfusion in enucleated human eyes. Arch. Ophthalmol 69, 783–801. [DOI] [PubMed] [Google Scholar]
- Grebe R, Mughal I, Bryden W, McLeod S, Edwards M, Hageman GS, Lutty G, 2019. Ultrastructural analysis of submacular choriocapillaris and its transport systems in AMD and aged control eyes. Exp. Eye Res 181, 252–262. [DOI] [PubMed] [Google Scholar]
- Gu X, Fliesler SJ, Zhao YY, Stallcup WB, Cohen AW, Elliott MH, 2014a. Loss of caveolin-1 causes blood-retinal barrier breakdown, venous enlargement, and mural cell alteration. Am. J. Pathol 184, 541–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Reagan A, Yen A, Bhatti F, Cohen AW, Elliott MH, 2014b. Spatial and temporal localization of caveolin-1 protein in the developing retina. Adv. Exp. Med. Biol 801, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Reagan AM, McClellan ME, Elliott MH, 2017. Caveolins and caveolae in ocular physiology and pathophysiology. Prog. Retin. Eye Res 56, 84–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhang H, Hou Y, Wei T, Liu J, 2016. Plasmalemma vesicle-associated protein: a crucial component of vascular homeostasis. Exp. Ther. Med 12, 1639–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Shen N, Yuan K, Li J, Wu H, Zeng Y, Fox J 3rd, Bansal AK, Singh BB, Gao H, Wu M, 2012. Caveolin-1 plays a critical role in host immunity against Klebsiella pneumoniae by regulating STAT5 and Akt activity. Eur. J. Immunol 42, 1500–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta VK, You Y, Klistorner A, Graham SL, 2012. Shp-2 regulates the TrkB receptor activity in the retinal ganglion cells under glaucomatous stress. Biochim. Biophys. Acta 1822, 1643–1649. [DOI] [PubMed] [Google Scholar]
- Gurley JM, Gmyrek GB, McClellan ME, Hargis EA, Hauck SM, Dozmorov MG, Wren JD, Carr DJJ, Elliott MH, 2020. Neuroretinal-derived caveolin-1 promotes endotoxin-induced inflammation in the murine retina. Invest. Ophthalmol. Vis. Sci 61, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CG, Bright NA, Howard G, Nichols BJ, 2009. SDPR induces membrane curvature and functions in the formation of caveolae. Nat. Cell Biol 11, 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CG, Howard G, Nichols BJ, 2011. Pacsin 2 is recruited to caveolae and functions in caveolar biogenesis. J. Cell Sci 124, 2777–2785. [DOI] [PubMed] [Google Scholar]
- Hansen CG, Shvets E, Howard G, Riento K, Nichols BJ, 2013. Deletion of cavin genes reveals tissue-specific mechanisms for morphogenesis of endothelial caveolae. Nat. Commun 4, 1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, McLeod DS, Bhutto IA, Prow T, Merges CA, Grebe R, Lutty GA, 2007. The embryonic human choriocapillaris develops by hemo-vasculogenesis. Dev. Dynam 236, 2089–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Takeuchi A, Miyaishi O, Xiao H, Mao J, Isobe K, 2000. PTRF (polymerase I and transcript-release factor) is tissue-specific and interacts with the BFCOL1 (binding factor of a type-I collagen promoter) zinc-finger transcription factor which binds to the two mouse type-I collagen gene promoters. Biochem. J 347 (Pt 1), 55–59. [PMC free article] [PubMed] [Google Scholar]
- Hayer A, Stoeber M, Bissig C, Helenius A, 2010. Biogenesis of caveolae: stepwise assembly of large caveolin and cavin complexes. Traffic 11, 361–382. [DOI] [PubMed] [Google Scholar]
- Head BP, Hu Y, Finley JC, Saldana MD, Bonds JA, Miyanohara A, Niesman IR, Ali SS, Murray F, Insel PA, Roth DM, Patel HH, Patel PM, 2011. Neuron-targeted caveolin-1 protein enhances signaling and promotes arborization of primary neurons. J. Biol. Chem 286, 33310–33321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head BP, Insel PA, 2007. Do caveolins regulate cells by actions outside of caveolae? Trends Cell Biol. 17, 51–57. [DOI] [PubMed] [Google Scholar]
- Henshall TL, Keller A, He L, Johansson BR, Wallgard E, Raschperger E, Mae MA, Jin S, Betsholtz C, Lendahl U, 2015. Notch3 is necessary for blood vessel integrity in the central nervous system. Arterioscler. Thromb. Vase. Biol 35, 409–420. [DOI] [PubMed] [Google Scholar]
- Herrnberger L, Ebner K, Junglas B, Tamm ER, 2012a. The role of plasmalemma vesicle-associated protein (PLVAP) in endothelial cells of Schlemm’s canal and ocular capillaries. Exp. Eye Res 105, 27–33. [DOI] [PubMed] [Google Scholar]
- Herrnberger L, Seitz R, Kuespert S, Bosl MR, Fuchshofer R, Tamm ER, 2012b. Lack of endothelial diaphragms in fenestrae and caveolae of mutant Plvap-deficient mice. Histochem. Cell Biol 138, 709–724. [DOI] [PubMed] [Google Scholar]
- Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, Hancock JF, Parton RG, 2008. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 132, 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J, 2015. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 87, 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirama T, Das R, Yang Y, Ferguson C, Won A, Yip CM, Kay JG, Grinstein S, Parton RG, Fairn GD, 2017. Phosphatidylserine dictates the assembly and dynamics of caveolae in the plasma membrane. J. Biol. Chem 292, 14292–14307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T, Wang J, Boyd P, Wang F, Santiago C, Jiang L, Yoo S, Lahne M, Todd LJ, Jia M, Saez C, Keuthan C, Palazzo I, Squires N, Campbell WA, Rajaii F, Parayil T, Trinh V, Kim DW, Wang G, Campbell LJ, Ash J, Fischer AJ, Hyde DR, Qian J, Blackshaw S, 2020. Gene regulatory networks controlling vertebrate retinal regeneration. Science 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoop CL, Sivanandam VN, Kodali R, Srnec MN, van der Wei PC, 2012. Structural characterization of the caveolin scaffolding domain in association with cholesterol-rich membranes. Biochemistry 51, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysi PG, Cheng CY, Springelkamp H, Macgregor S, Bailey JNC, Wojciechowski R, Vitart V, Nag A, Hewitt AW, Hohn R, Venturini C, Mirshahi A, Ramdas WD, Thorleifsson G, Vithana E, Khor CC, Stefansson AB, Liao J, Haines JL, Amin N, Wang YX, Wild PS, Ozel AB, Li JZ, Fleck BW, Zeller T, Staffieri SE, Teo YY, Cuellar-Partida G, Luo X, Allingham RR, Richards JE, Senft A, Karssen LC, Zheng Y, Bellenguez C, Xu L, Iglesias AI, Wilson JF, Kang JH, van Leeuwen EM, Jonsson V, Thorsteinsdottir U, Despriet DDG, Ennis S, Moroi SE, Martin NG, Jansonius NM, Yazar S, Tai ES, Amouyel P, Kirwan J, van Koolwijk LME, Hauser MA, Jonasson F, Leo P, Loomis SJ, Fogarty R, Rivadeneira F, Kearns L, Lackner KJ, de Jong P, Simpson CL, Pennell CE, Oostra BA, Uitterlinden AG, Saw SM, Lotery AJ, Bailey-Wilson JE, Hoffnan A, Vingerling JR, Maubaret C, Pfeiffer N, Wolfs RCW, Lemij HG, Young TL, Pasquale LR, Delcourt C, Spector TD, Klaver CCW, Small KS, Burdon KP, Stefansson K, Wong TY, Group, B.G., Consortium, N., Wellcome Trust Case Control, C., Viswanathan A, Mackey DA, Craig JE, Wiggs JL, van Duijn CM, Hammond CJ, Aung T, 2014. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat. Genet 46, 1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder KL, Zheng YZ, Davis MJ, Moon H, Loo D, Nguyen H, Clements JA, Parton RG, Foster LJ, Hill MM, 2012. Expression of PTRF in PC-3 Cells modulates cholesterol dynamics and the actin cytoskeleton impacting secretion pathways. Mol. Cell. Proteomics : MCP 11. M111 012245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansa P, Burek C, Sander EE, Grummt I, 2001. The transcript release factor PTRF augments ribosomal gene transcription by facilitating reinitiation of RNA polymerase I. Nucleic Acids Res. 29, 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansa P, Mason SW, Hoffmann-Rohrer U, Grummt I, 1998. Cloning and functional characterization of PTRF, a novel protein which induces dissociation of paused ternary transcription complexes. EMBO J. 17, 2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen SF, Gorgels TG, Ramdas WD, Klaver CC, van Duijn CM, Jansonius NM, Bergen AA, 2013. The vast complexity of primary open angle glaucoma: disease genes, risks, molecular mechanisms and pathobiology. Prog. Retin. Eye Res 37, 31–67. [DOI] [PubMed] [Google Scholar]
- Jiao H, Zhang Y, Yan Z, Wang ZG, Liu G, Minshall RD, Malik AB, Hu G, 2013. Caveolin-1 Tyr14 phosphorylation induces interaction with TLR4 in endothelial cells and mediates MyD88-dependent signaling and sepsis-induced lung inflammation. J. Immunol 191, 6191–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S, 2017. Glaucoma. Lancet 390, 2183–2193. [DOI] [PubMed] [Google Scholar]
- Jung JJ, Inamdar SM, Tiwari A, Choudhury A, 2012. Regulation of intracellular membrane trafficking and cell dynamics by syntaxin-6. Biosci. Rep 32, 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung W, Sierecki E, Bastiani M, O’Carroll A, Alexandrov K, Rae J, Johnston W, Hunter DJB, Ferguson C, Gambin Y, Ariotti N, Parton RG, 2018. Cell-free formation and interactome analysis of caveolae. J. Cell. Biol 217, 2141–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachi S, Yamazaki A, Usukura J, 2001. Localization of caveolin-1 in photoreceptor synaptic ribbons. Invest. Ophthalmol. Vis. Sci 42, 850–852. [PubMed] [Google Scholar]
- Kassan A, Egawa J, Zhang Z, Almenar-Queralt A, Nguyen QM, Lajevardi Y, Kim K, Posadas E, Jeste DV, Roth DM, Patel PM, Patel HH, Head BP, 2017. Caveolin-1 regulation of disrupted-in-schizophrenia-1 as a potential therapeutic target for schizophrenia. J. Neurophysiol 117, 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Meguro A, Nomura E, Uemoto R, Nomura N, Ota M, Kashiwagi K, Mabuchi F, Iijima H, Kawase K, Yamamoto T, Nakamura M, Negi A, Sagara T, Nishida T, Inatani M, Tanihara H, Aihara M, Araie M, Fukuchi T, Abe H, Higashide T, Sugiyama K, Kanamoto T, Kiuchi Y, Iwase A, Chin S, Ohno S, Inoko H, Mizuki N, 2013. Association study of genetic variants on chromosome 7q31 with susceptibility to normal tension glaucoma in a Japanese population. Eye 27, 979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketteler J, Klein D, 2018. Caveolin-1, cancer and therapy resistance. Int. J. Cancer 143, 2092–2104. [DOI] [PubMed] [Google Scholar]
- Khater IM, Aroca-Ouellette ST, Meng F, Nabi IR, Hamarneh G, 2019a. Caveolae and scaffold detection from single molecule localization microscopy data using deep learning. PLoS One 14, e0211659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khater IM, Liu Q, Chou KC, Hamarneh G, Nabi IR, 2019b. Super-resolution modularity analysis shows polyhedral caveolin-1 oligomers combine to form scaffolds and caveolae. Sci. Rep 9, 9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja AP, Cooke Bailey JN, Wareham NJ, Scott RA, Simcoe M, Igo RP Jr., Song YE, Wojciechowski R, Cheng CY, Khaw PT, Pasquale LR, Haines JL, Foster PJ, Wiggs JL, Hammond CJ, Hysi PG, Eye, U.K.B., Vision, C., Consortium, N., 2018. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat. Genet 50, 778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Park DY, Bae H, Park DY, Kim D, Lee CK, Song S, Chung TY, Lim DH, Kubota Y, Hong YK, He Y, Augustin HG, Oliver G, Koh GY, 2017. Impaired angiopoietin/Tie2 signaling compromises Schlemm’s canal integrity and induces glaucoma. J. Clin. Invest 127, 3877–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim K, Heo DW, Kim JS, Park CK, Kim CS, Kang C, 2015. Expression-associated polymorphisms of CAV1-CAV2 affect intraocular pressure and high-tension glaucoma risk. Mol. Vis 21, 548–554. [PMC free article] [PubMed] [Google Scholar]
- Kirkham M, Nixon SJ, Howes MT, Abi-Rached L, Wakeham DE, Hanzal-Bayer M, Ferguson C, Hill MM, Fernandez-Rojo M, Brown DA, Hancock JF, Brodsky FM, Parton RG, 2008. Evolutionary analysis and molecular dissection of caveola biogenesis. J. Cell Sci 121, 2075–2086. [DOI] [PubMed] [Google Scholar]
- Kiss AL, Kittel A, 1995. Early endocytotic steps in elicited macrophages: omega-shaped plasma membrane vesicles at their cell surface. Cell Biol. Int 19, 527–538. [DOI] [PubMed] [Google Scholar]
- Kiss AL, Turi A, Mullner N, Timar J, 2000. Caveolin isoforms in resident and elicited rat peritoneal macrophages. Eur. J. Cell. Biol 79, 343–349. [DOI] [PubMed] [Google Scholar]
- Kizhatil K, Chlebowski A, Tolman NG, Freeburg NF, Ryan MM, Shaw NN, Kokini AD, Marchant JK, John SW, 2016. An in vitro perfusion system to enhance outflow studies in mouse eyes. Invest. Ophthalmol. Vis. Sci 57, 5207–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizhatil K, Ryan M, Marchant JK, Henrich S, John SW, 2014. Schlemm’s canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol. 12, e1001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen I, Van Noorden CJ, Schlingemann RO, 2013. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res 34, 19–48. [DOI] [PubMed] [Google Scholar]
- Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, Steinberg GK, Barres BA, Nimmerjahn A, Agalliu D, 2014. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 82, 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S, Lee W, Park SM, Kim SH, 2021. Caveolin-1 deficiency impairs synaptic transmission in hippocampal neurons. Mol. Brain 14, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun O, Tillu VA, Ariotti N, Parton RG, Collins BM, 2015. Cavin family proteins and the assembly of caveolae. J. Cell Sci 128, 1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozera L, White E, Calaghan S, 2009. Caveolae act as membrane reserves which limit mechanosensitive I(Cl,swell) channel activation during swelling in the rat ventricular myocyte. PLoS One 4, e8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kur J, Newman EA, Chan-Ling T, 2012. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res 31, 377–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie P, Nabi IR, 2010. Lipid rafts, caveolae, and their endocytosis. Int. Rev. Cell Molecular. Biol 282, 135–163. [DOI] [PubMed] [Google Scholar]
- Lakk M, Hoffmann GF, Gorusupudi A, Enyong E, Lin A, Bernstein PS, Toft-Bertelsen T, MacAulay N, Elliott MH, Krizaj D, 2021. Membrane cholesterol regulates TRPV4 function, cytoskeletal expression, and the cellular response to tension. J. Lipid Res 62, 100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Lay S, Kurzchalia TV, 2005. Getting rid of caveolins: phenotypes of caveolin-deficient animals. Biochim. Biophys. Acta 1746, 322–333. [DOI] [PubMed] [Google Scholar]
- Le PU, Guay G, Altschuler Y, Nabi IR, 2002. Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J. Biol. Chem 277, 3371–3379. [DOI] [PubMed] [Google Scholar]
- Lee J, Schmid-Schonbein GW, 1995. Biomechanics of skeletal muscle capillaries: hemodynamic resistance, endothelial distensibility, and pseudopod formation. Ann. Biomed. Eng 23, 226–246. [DOI] [PubMed] [Google Scholar]
- Lei Y, Song M, Wu J, Xing C, Sun X, 2016. eNOS activity in CAV1 knockout mouse eyes. Invest. Ophthalmol. Vis. Sci 57, 2805–2813. [DOI] [PubMed] [Google Scholar]
- Li G, Nottebaum AF, Brigell M, Navarro ID, Ipe U, Mishra S, Gomez-Caraballo M, Schmitt H, Soldo B, Pakola S, Withers B, Peters KG, Vestweber D, Stamer WD, 2020a. A small molecule inhibitor of VE-PTP activates Tie2 in Schlemm’s canal increasing outflow facility and reducing intraocular pressure. Invest. Ophthalmol. Vis. Sci 61, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Schmitt H, Johnson WM, Lee C, Navarro I, Cui J, Fleming T, Gomez-Caraballo M, Elliott MH, Sherwood JM, Hauser MA, Farsiu S, Ethier CR, Stamer WD, 2020b. Integral role for lysyl oxidase-like-1 in conventional outflow tissue function and behavior. Faseb. J. : Off. Publ. Feder. Am. Soc. Exp. Biol 34, 10762–10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Scherl A, Medina F, Frank PG, Kitsis RN, Tanowitz HB, Sotgia F, Lisanti MP, 2005. Impaired phagocytosis in caveolin-1 deficient macrophages. Cell Cycle 4, 1599–1607. [DOI] [PubMed] [Google Scholar]
- Li X, Gu X, Boyce TM, Zheng M, Reagan AM, Qi H, Mandal N, Cohen AW, Callegan MC, Carr DJ, Elliott MH, 2014. Caveolin-1 increases proinflammatory chemoattractants and blood-retinal barrier breakdown but decreases leukocyte recruitment in inflammation. Invest. Ophthalmol. Vis. Sci 55, 6224–6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, McClellan ME, Tanito M, Garteiser P, Towner R, Bissig D, Berkowitz BA, Fliesler SJ, Woodruff ML, Fain GL, Birch DG, Khan MS, Ash JD, Elliott MH, 2012. Loss of caveolin-1 impairs retinal function due to disturbance of subretinal microenvironment. J. Biol. Chem 287, 16424–16434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti MP, Tang Z, Scherer PE, Kubler E, Koleske AJ, Sargiacomo M, 1995. Caveolae, transmembrane signalling and cellular transformation. Mol. Membr. Biol 12, 121–124. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang W, Kennard S, Caldwell RB, Lilly B, 2010. Notch3 is critical for proper angiogenesis and mural cell investment. Circ. Res 107, 860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, Ravid K, Pilch PF, 2008. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metabol. 8, 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Pilch PF, 2008. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J. Biol. Chem 283, 4314–4322. [DOI] [PubMed] [Google Scholar]
- Liu L, Xu HX, Wang WQ, Wu CT, Chen T, Qin Y, Liu C, Xu J, Long J, Zhang B, Xu YF, Ni QX, Li M, Yu XJ, 2014. Cavin-1 is essential for the tumor-promoting effect of caveolin-1 and enhances its prognostic potency in pancreatic cancer. Oncogene 33, 2728–2736. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hauser MA, Akafo SK, Qin X, Miura S, Gibson JR, Wheeler J, Gaasterland DE, Challa P, Herndon LW, International Consortium of African Ancestry, R.i.G., Ritch R, Moroi SE, Pasquale LR, Girkin CA, Budenz DL, Wiggs JL, Richards JE, Ashley-Koch AE, Allingham RR, 2013. Investigation of known genetic risk factors for primary open angle glaucoma in two populations of African ancestry. Invest. Ophthalmol. Vis. Sci 54, 6248–6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HP, Nixon SJ, Hall TE, Cowling BS, Ferguson C, Morgan GP, Schieber NL, Fernandez-Rojo MA, Bastiani M, Floetenmeyer M, Martel N, Laporte J, Pilch PF, Parton RG, 2015. The caveolin-cavin system plays a conserved and critical role in mechanoprotection of skeletal muscle. J. Cell. Biol 210, 833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo JH, Lee YS, Woon CY, Yong VHK, Tan B, Schmetterer L, Chong RS, 2021. Loss of caveolin-1 impairs light flicker-induced neurovascular coupling at the optic nerve head. Front. Neurosci 15, 764898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis SJ, Kang JH, Weinreb RN, Yaspan BL, Cooke Bailey JN, Gaasterland D, Gaasterland T, Lee RK, Lichter PR, Budenz DL, Liu Y, Realini T, Friedman DS, McCarty CA, Moroi SE, Olson L, Schuman JS, Singh K, Vollrath D, Wollstein G, Zack DJ, Brilliant M, Sit AJ, Christen WG, Fingert J, Kraft P, Zhang K, Allingham RR, Pericak-Vanee MA, Richards JE, Hauser MA, Haines JL, Pasquale LR, Wiggs JL, 2014. Association of CAV1/CAV2 genomic variants with primary open-angle glaucoma overall and by gender and pattern of visual field loss. Ophthalmology 121, 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SY, Rong SS, Wu Z, Huang C, Matsushita K, Ng TK, Leung CKS, Kawashima R, Usui S, Tam POS, Tsujikawa M, Young AL, Zhang M, Wiggs JL, Nishida K, Tham CC, Pang CP, Chen LJ, 2020. Association of the CAV1-CAV2 locus with normal-tension glaucoma in Chinese and Japanese. Clin. Exp. Ophthalmol 48, 658–665. [DOI] [PubMed] [Google Scholar]
- Luetterforst R, Stang E, Zorzi N, Carozzi A, Way M, Parton RG, 1999. Molecular characterization of caveolin association with the Golgi complex: identification of a cis-Golgi targeting domain in the caveolin molecule. J. Cell. Biol 145, 1443–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz SE, Smith JR, Kim DH, Olson CVL, Ellefsen K, Bates JM, Gandhi SP, Agalliu D, 2017. Caveolin1 is required for Th1 cell infiltration, but not tight junction remodeling, at the blood-brain barrier in autoimmune neuroinflammation. Cell Rep. 21, 2104–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv XJ, Li YY, Zhang YJ, Mao M, Qian GS, 2010. Over-expression of caveolin-1 aggravate LPS-induced inflammatory response in AT-1 cells via up-regulation of cPLA2/p38 MAPK. Inflammation research. Off. J. Eur. Histamine Res. Soc 59, 531–541 [et al. [DOI] [PubMed] [Google Scholar]
- MacGregor S, Ong JS, An J, Han X, Zhou T, Siggs OM, Law MH, Souzeau E, Sharma S, Lynn DJ, Beesley J, Sheldrick B, Mills RA, Landers J, Ruddle JB, Graham SL, Healey PR, White AJR, Casson RJ, Best S, Grigg JR, Goldberg I, Powell JE, Whiteman DC, Radford-Smith GL, Martin NG, Montgomery GW, Burdon KP., Mackey DA, Gharahkhani P, Craig JE, Hewitt AW, 2018. Genome-wide association study of intraocular pressure uncovers new pathways to glaucoma. Nat. Genet 50, 1067–1071. [DOI] [PubMed] [Google Scholar]
- Machleidt T, Li WP, Liu P, Anderson RG, 2000. Multiple domains in caveolin-1 control its intracellular traffic. J. Cell. Biol 148, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machuca-Parra AI, Bigger-Alien AA, Sanchez AV, Boutabla A, Cardona-Velez J, Amarnani D, Saint-Geniez M, Siebel CW, Kim LA, D’Amore PA, Arboleda-Velasquez JF, 2017. Therapeutic antibody targeting of Notch3 signaling prevents mural cell loss in CADASIL. J. Exp. Med 214, 2271–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA, 2015. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Schilling JM, Cui W, Egawa J, Niesman IR, Kellerhals SE, Staples MC, Busija AR, Risbrough VB, Posadas E, Grogman GC, Chang JW, Roth DM, Patel PM, Patel HH, Head BP, 2017. Neuron-targeted caveolin-1 improves molecular signaling, plasticity, and behavior dependent on the Hippocampus in adult and aged mice. Biol. Psychiatr 81, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Parton RG, 2005. Caveolin, cholesterol, and lipid bodies. Semin. Cell Dev. Biol 16, 163–174. [DOI] [PubMed] [Google Scholar]
- McDonnell F, Dismuke WM, Overby DR, Stamer WD, 2018. Pharmacological regulation of outflow resistance distal to Schlemm’s canal. Am. J. Physiol. Cell Physiol 315, C44–C51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell CM, Kizhatil K, Elliott MH, Overby DR, van Batenburg-Sherwood J, Millar JC, Kuehn MH, Zode G, Acott TS, Anderson MG, Bhattacharya SK, Bertrand JA, Borras T, Bovenkamp DE, Cheng L, Danias J, De Ieso ML, Du Y, Faralli JA, Fuchshofer R, Ganapathy PS, Gong H, Herberg S, Hernandez H, Humphries P, John SWM, Kaufman PL, Keller KE., Kelley MJ, Kelly RA, Krizaj D, Kumar A, Leonard BC, Lieberman RL, Liton P, Liu Y, Liu KC, Lopez NN, Mao W, Mavlyutov T, McDonnell F, McLellan GJ, Mzyk P, Nartey A, Pasquale LR, Patel GC, Pattabiraman PP, Peters DM, Raghunathan V, Rao PV, Rayana N, Raychaudhuri U, Reina-Torres E, Ren R, Rhee D, Chowdhury UR, Samples JR, Samples EG, Sharif N, Schuman JS, Sheffield VC, Stevenson CH, Soundararajan A, Subramanian P, Sugali CK, Sun Y, Toris CB, Torrejon KY, Vahabikashi A, Vranka JA, Wang T, Willoughby CE, Xin C, Yun H, Zhang HF, Fautsch MP, Tamm ER, Clark AF, Ethier CR, Stamer WD, 2022. Consensus recommendation for mouse models of ocular hypertension to study aqueous humor outflow and its mechanisms. Invest. Ophthalmol. Vis. Sci 63, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon KA, Zajicek H, Li WP, Peyton MJ, Minna JD, Hernandez VJ, Luby-Phelps K, Anderson RG, 2009. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J. 28, 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina FA, de Almeida CJ, Dew E, Li J, Bonuccelli G, Williams TM, Cohen AW, Pestell RG, Frank PG, Tanowitz HB, Lisanti MP, 2006a. Caveolin-1-deficient mice show defects in innate immunity and inflammatory immune response during Salmonella enterica serovar Typhimurium infection. Infect. Immun 74, 6665–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina FA, Williams TM, Sotgia F, Tanowitz HB, Lisanti MP, 2006b. A novel role for caveolin-1 in B lymphocyte function and the development of thymus-independent immune responses. Cell Cycle 5, 1865–1871. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol 1, 135–145. [DOI] [PubMed] [Google Scholar]
- Meng F, Joshi B, Nabi IR, 2015. Galectin-3 overrides PTRF/Cavin-1 reduction of PC3 prostate cancer cell migration. PLoS One 10, e0126056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheal S, Ayub H, Khan MI, Bakker B, Schoenmaker-Koller FE, Ali M, Akhtar F, Khan WA, Qamar R, den Hollander AI, 2014. Association of known common genetic variants with primary open angle, primary angle closure, and pseudoexfoliation glaucoma in Pakistani cohorts. Mol. Vis 20, 1471–1479. [PMC free article] [PubMed] [Google Scholar]
- Minguet S, Klasener K, Schaffer AM, Fiala GJ, Osteso-Ibanez T, Raute K, Navarro-Lerida I, Haiti FA, Seidl M, Reth M, Del Pozo MA, 2017. Caveolin-1-dependent nanoscale organization of the BCR regulates B cell tolerance. Nat. Immunol 18, 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza MK, Yuan J, Gao XP, Garrean S, Brovkovych V, Malik AB, Tiruppathi C, Zhao YY, 2010. Caveolin-1 deficiency dampens Toll-like receptor 4 signaling through eNOS activation. Am. J. Pathol 176, 2344–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV, 1995. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol. Biol. Cell 6, 911–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H, Lee CS, Inder KL, Sharma S, Choi E, Black DM, Le Cao KA, Winterford C, Coward JI, Ling MT, Australian Prostate Cancer B, Craik DJ, Parton RG, Russell PJ, Hill MM, 2014. PTRF/cavin-1 neutralizes non-caveolar caveolin-1 microdomains in prostate cancer. Oncogene vol. 33, 3561–3570. [DOI] [PubMed] [Google Scholar]
- Moren B, Shah C, Howes MT, Schieber NL, McMahon HT, Parton RG, Daumke O, Lundmark R, 2012. EHD2 regulates caveolar dynamics via ATP-driven targeting and oligomerization. Mol. Biol. Cell 23, 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K, 1995. VIP21/caveolin is a cholesterol-binding protein. Proc. Natl. Acad. Sci. U. S. A 92, 10339–10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC, 2007. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J. Exp. Med 204, 2373–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M, Grebe R, Bhutto IA, Edwards M, McLeod DS, Lutty GA, 2016. Albumen transport to Bruch’s membrane and RPE by choriocapillaris caveolae. Invest. Ophthalmol. Vis. Sci 57, 2213–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar ZD, Hill MM, Parton RG, Francois M, Parat MO, 2015. Non-caveolar caveolin-1 expression in prostate cancer cells promotes lymphangiogenesis. Oncoscience 2, 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar ZD, Hill MM, Parton RG, Parat MO, 2013. Caveola-forming proteins caveolin-1 and PTRF in prostate cancer. Nat. Rev. Urol 10, 529–536. [DOI] [PubMed] [Google Scholar]
- Nelson BR, Ueki Y, Reardon S, Karl MO, Georgi S, Hartman BH, Lamba DA, Reh TA, 2011. Genome-wide analysis of Muller glial differentiation reveals a requirement for Notch signaling in postmitotic cells to maintain the glial fate. PLoS One 6, e22817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickells RW, Howell GR, Soto I, John SW, 2012. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu. Rev. Neurosci 35, 153–179. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wallman J, 2010. The multifunctional choroid. Prog. Retin. Eye Res 29, 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesman IR, Zemke N, Fridolfsson HN, Haushalter KJ, Levy K, Grove A, Schnoor R, Finley JC, Patel PM, Roth DM, Head BP, Patel HH, 2013. Caveolin isoform switching as a molecular, structural, and metabolic regulator of microglia. Mol. Cell. Neurosci 56, 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Wehinger S, Ortiz RJ, Diaz N, Diaz J, Lobos-Gonzalez L, Quest AF, 2014. Caveolin-1 in cell migration and metastasis. Curr. Mol. Med 14, 255–274. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP, 1998. Caveolins, a family of scaffolding proteins for organizing "preassembled signaling complexes" at the plasma membrane. J. Biol. Chem 273, 5419–5422. [DOI] [PubMed] [Google Scholar]
- Oliveira SDS, Castellon M, Chen J, Bonini MG, Gu X, Elliott MH, Machado RF, Minshall RD, 2017. Inflammation-induced caveolin-1 and BMPRII depletion promotes endothelial dysfunction and TGF-beta-driven pulmonary vascular remodeling. Am. J. Physiol. Lung Cell Mol. Physiol 312, L760–L771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omri S, Behar-Cohen F, de Kozak Y, Sennlaub F, Verissimo LM, Jonet L, Savoldelli M, Omri B, Crisanti P, 2011. Microglia/macrophages migrate through retinal epithelium barrier by a transcellular route in diabetic retinopathy: role of PKCzeta in the Goto Kakizaki rat model. Am. J. Pathol 179, 942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozel AB, Moroi SE, Reed DM, Nika M, Schmidt CM, Akbari S, Scott K, Rozsa F, Pawar H, Musch DC, Lichter PR, Gaasterland D, Branham K, Gilbert J, Garnai SJ, Chen W, Othman M, Heckenlively J, Swaroop A, Abecasis G, Friedman DS, Zack D, Ashley-Koch A, Ulmer M, Kang JH, Consortium, N., Liu Y, Yaspan BL, Haines J, Allingham RR, Hauser MA, Pasquale L, Wiggs J, Richards JE, Li JZ, 2014. Genome-wide association study and meta-analysis of intraocular pressure. Hum. Genet 133, 41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade GE, 1953. Fine structure of blood capillaries. J. Appl. Phys 24, 1424, 1424. [Google Scholar]
- Palade GE, 1961. Blood capillaries of the heart and other organs. Circulation 24, 368–388. [DOI] [PubMed] [Google Scholar]
- Pan D, Gao J, Zeng X, Ma G, Li N, Huang X, Du X, Miao Q, Lian J, Xu L, Zhou H, Chen S, 2017. Quantitative proteomic Analysis Reveals up-regulation of caveolin-1 in FOXP3-overexpressed human gastric cancer cells. Sci. Rep 7, 14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parat MO, Anand-Apte B, Fox PL, 2003. Differential caveolin-1 polarization in endothelial cells during migration in two and three dimensions. Mol. Biol. Cell 14, 3156–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parat MO, Fox PL, 2001. Palmitoylation of caveolin-1 in endothelial cells is post-translational but irreversible. J. Biol. Chem 276, 15776–15782. [DOI] [PubMed] [Google Scholar]
- Parton RG, 2018. Caveolae: structure, function, and relationship to disease. Annu. Rev. Cell Dev. Biol 34, 111–136. [DOI] [PubMed] [Google Scholar]
- Parton RG, Collins BM, 2016. Unraveling the architecture of caveolae. Proc. Natl. Acad. Sci. U. S. A 113, 14170–14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, del Pozo MA, 2013. Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol 14, 98–112. [DOI] [PubMed] [Google Scholar]
- Parton RG, Hanzal-Bayer M, Hancock JF, 2006. Biogenesis of caveolae: a structural model for caveolin-induced domain formation. J. Cell Sci 119, 787–796. [DOI] [PubMed] [Google Scholar]
- Parton RG, Joggerst B, Simons K, 1994. Regulated internalization of caveolae. J. Cell. Biol 127, 1199–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L, Kartenbeck J, Helenius A, 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol 3, 473–483. [DOI] [PubMed] [Google Scholar]
- Pilch PF, Liu L, 2011. Fat caves: caveolae, lipid trafficking and lipid metabolism in adipocytes. Trends Endocrinol. Metabol.: TEM (Trends Endocrinol. Metab.) 22, 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch PF, Meshulam T, Ding S, Liu L, 2011. Caveolae and lipid trafficking in adipocytes. Clin. Lipidol 6, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol A, Martin S, Fernandez MA, Ingelmo-Torres M, Ferguson C, Enrich C, Parton RG, 2005. Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies. Mol. Biol. Cell 16, 2091–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol A, Morales-Paytuvi F, Bosch M, Parton RG, 2020. Non-caveolar caveolins - duties outside the caves. J. Cell Sci 133. [DOI] [PubMed] [Google Scholar]
- Portugal CC, Socodato R, Canedo T, Silva CM, Martins T, Coreixas VS, Loiola EC, Gess B, Rohr D, Santiago AR, Young P, Minshall RD, Paes-de-Carvalho R, Ambrosio AF, Relvas JB, 2017. Caveolin-1-mediated internalization of the vitamin C transporter SVCT2 in microglia triggers an inflammatory phenotype. Sci. Signal 10. [DOI] [PubMed] [Google Scholar]
- Qin WD, Zhang F, Qin XJ, Wang J, Meng X, Wang H, Guo HP, Wu QZ, Wu DW, Zhang MX, 2016. Notch1 inhibition reduces low shear stress-induced plaque formation. Int. J. Biochem. Cell Biol 72, 63–72. [DOI] [PubMed] [Google Scholar]
- Raviola G, 1977. The structural basis of the blood-ocular barriers. Exp. Eye Res 25 (Suppl. 1), 27–63. [DOI] [PubMed] [Google Scholar]
- Raviola G, Butler JM, 1983. Unidirectional vesicular transport mechanism in retinal vessels. Invest. Ophthalmol. Vis. Sci 24, 1465–1474. [PubMed] [Google Scholar]
- Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE, Lisanti MP, 2002a. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem 277, 8635–8647. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H Jr., Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP, 2001. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem 276, 38121–38138. [DOI] [PubMed] [Google Scholar]
- Razani B, Lisanti MP, 2001. Two distinct caveolin-1 domains mediate the functional interaction of caveolin-1 with protein kinase A. Am. J. Physiol. Cell Physiol 281, C1241–C1250. [DOI] [PubMed] [Google Scholar]
- Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H Jr., Christ GJ, Edelmann W, Lisanti MP, 2002b. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol. Cell. Biol 22, 2329–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Woodman SE, Lisanti MP, 2002c. Caveolae: from cell biology to animal physiology. Pharmacol. Rev 54, 431–467. [DOI] [PubMed] [Google Scholar]
- Reagan A, Gu X, Hauck SM, Ash JD, Cao G, Thompson TC, Elliott MH, 2016. Retinal caveolin-1 modulates neuroprotective signaling. Adv. Exp. Med. Biol 854, 411–418. [DOI] [PubMed] [Google Scholar]
- Reagan AM, Gu X, Paudel S, Ashpole NM, Zalles M, Sonntag WE, Ungvari Z, Csiszar A, Otalora L, Freeman WM, Stout MB, Elliott MH, 2018. Age-related focal loss of contractile vascular smooth muscle cells in retinal arterioles is accelerated by caveolin-1 deficiency. Neurobiol. Aging 71, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina-Torres E, De Ieso ML, Pasquale LR, Madekurozwa M, van Batenburg-Sherwood J, Overby DR, Stamer WD, 2021. The vital role for nitric oxide in intraocular pressure homeostasis. Prog. Retin. Eye Res 83, 100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter T, Floetenmeyer M, Ferguson C, Galea J, Goh J, Lindsay MR, Morgan GP, Marsh BJ, Parton RG, 2008. High-resolution 3D quantitative analysis of caveolar ultrastructure and caveola-cytoskeleton interactions. Traffic 9, 893–909. [DOI] [PubMed] [Google Scholar]
- Rizzo V, McIntosh DP, Oh P, Schnitzer JE, 1998. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. J. Biol. Chem 273, 34724–34729. [DOI] [PubMed] [Google Scholar]
- Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL, 2008. The transcriptome of retinal Muller glial cells. J. Comp. Neurol 509, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch K, Stadler MB, Cepko CL, 2012. Gene expression changes within Muller glial cells in retinitis pigmentosa. Mol. Vis 18, 1197–1214. [PMC free article] [PubMed] [Google Scholar]
- Rong SS, Chen LJ, Leung CK, Matsushita K, Jia L, Miki A, Chiang SW, Tam PO, Hashida N, Young AL, Tsujikawa M, Zhang M, Wang N, Nishida K, Pang CP, 2016. Ethnic specific association of the CAV1/CAV2 locus with primary open-angle glaucoma. Sci. Rep 6, 27837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root KT, Plucinsky SM, Glover KJ, 2015. Recent progress in the topology, structure, and oligomerization of caveolin: a building block of caveolae. Curr. Top. Membr 75, 305–336. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG, 1992. Caveolin, a protein component of caveolae membrane coats. Cell 68, 673–682. [DOI] [PubMed] [Google Scholar]
- Safa BN, Wong CA, Ha J, Ethier CR, 2022. Glaucoma and biomechanics. Curr. Opin. Ophthalmol 33, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M, Scherer PE, Tang Z, Kubler E, Song KS, Sanders MC, Lisanti MP, 1995. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc. Natl. Acad. Sci. U. S. A 92, 9407–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP, 1997. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J. Biol. Chem 272, 29337–29346. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP, 1996. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc. Natl. Acad. Sci. U. S. A 93, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Tang Z, Chun M, Sargiacomo M, Lodish HF, Lisanti MP, 1995. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. J. Biol. Chem 270, 16395–16401. [DOI] [PubMed] [Google Scholar]
- Schlegel A, Schwab RB, Scherer PE, Lisanti MP, 1999. A role for the caveolin scaffolding domain in mediating the membrane attachment of caveolin-1. The caveolin scaffolding domain is both necessary and sufficient for membrane binding in vitro. J. Biol. Chem 274, 22660–22667. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, Allard J, 1994. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell. Biol 127, 1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonle A, Hard FA, Mentzel J, Noltner T, Rauch KS, Prestipino A, Wohlfeil SA, Apostolova P, Hechinger AK, Melchinger W, Fehrenbach K, Guadamillas MC, Folio M, Prinz G, Ruess AK, Pfeifer D, del Pozo MA, Schmitt-Graeff A, Duyster J, Hippen KI, Blazar BR, Schachtrup K, Minguet S, Zeiser R, 2016. Caveolin-1 regulates TCR signal strength and regulatory T-cell differentiation into alloreactive T cells. Blood 127, 1930–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert W, Frank PG, Razani B, Park DS, Chow CW, Lisanti MP, 2001. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J. Biol. Chem 276, 48619–48622. [DOI] [PubMed] [Google Scholar]
- Sethna S, Chamakkala T, Gu X, Thompson TC, Cao G, Elliott MH, Finnemann SC, 2016. Regulation of phagolysosomal digestion by caveolin-1 of the retinal pigment epithelium is essential for vision. J. Biol. Chem 291, 6494–6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihata WA, Michell DL, Andrews KL, Chin-Dusting JP, 2016. Caveolae: a role in endothelial inflammation and mechanotransduction? Front. Physiol 7, 628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai M, Nishimura YV, Sakurai M, Nabeshima YI, Yuzaki M, Kawauchi T, 2018. Caveolin-1 promotes early neuronal maturation via caveolae-independent trafficking of N-cadherin and L1. iScience 7, 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvets E, Bitsikas V, Howard G, Hansen CG, Nichols BJ, 2015. Dynamic caveolae exclude bulk membrane proteins and are required for sorting of excess glycosphingolipids. Nat. Commun 6, 6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Toomre D, 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol 1, 31–39. [DOI] [PubMed] [Google Scholar]
- Sinha B, Roster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P, 2011. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144, 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J, Lin H, Fritch MR, Tuan RS, 2018. Influence of cholesterol/caveolin-1/caveolae homeostasis on membrane properties and substrate adhesion characteristics of adult human mesenchymal stem cells. Stem Cell Res. Ther 9, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Ge S, Pachter JS, 2007. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood 109, 1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Wu J, Lei Y, Sun X, 2017. Genetic deletion of the NOS3 gene in CAV1−/− mice restores aqueous humor outflow function. Invest. Ophthalmol. Vis. Sci 58, 4976–4987. [DOI] [PubMed] [Google Scholar]
- Sowa G, 2012. Role of Caveolin Proteins in Sepsis. Pediatrics & therapeutics current research 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa G, Pypaert M, Sessa WC, 2001. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc. Natl. Acad. Sci. U. S. A 98, 14072–14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Acott TS, 2012. Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol 23, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR, 2011. eNOS, a pressure-dependent regulator of intraocular pressure. Invest. Ophthalmol. Vis. Sci 52, 9438–9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SW, Zu XY, Tuo QH, Chen LX, Lei XY, Li K, Tang CK, Liao DF, 2010. Caveolae and caveolin-1 mediate endocytosis and transcytosis of oxidized low density lipoprotein in endothelial cells. Acta Pharmacol. Sin 31, 1336–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgucheva I, Surguchov A, 2011. Expression of caveolin in trabecular meshwork cells and its possible implication in pathogenesis of primary open angle glaucoma. Mol. Vis 17, 2878–2888. [PMC free article] [PubMed] [Google Scholar]
- Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A, 2005. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J. Cell. Biol 170, 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa M, Ueyama T, Ogata T, Takehara N, Nakajima N, Isodono K, Asada S, Takahashi T, Matsubara H, Oh H, 2008. MURC, a muscle-restricted coiled-coil protein, is involved in the regulation of skeletal myogenesis. Am. J. Physiol. Cell Physiol 295, C490–C498. [DOI] [PubMed] [Google Scholar]
- Takamura N, Yamaguchi Y, Watanabe Y, Asami M, Komitsu N, Aihara M, 2019. Downregulated Caveolin-1 expression in circulating monocytes may contribute to the pathogenesis of psoriasis. Sci. Rep 9, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayasu Y, Takeuchi K, Kumari R, Bennett MV, Zukin RS, Francesconi A, 2010. Caveolin-1 knockout mice exhibit impaired induction of mGluR-dependent long-term depression at CA3-CA1 synapses. Proc. Natl. Acad. Sci. U. S. A 107, 21778–21783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm ER, 2009. The trabecular meshwork outflow pathways: structural and functional aspects. Exp. Eye Res 88, 648–655. [DOI] [PubMed] [Google Scholar]
- Tang Y, Fang W, Xiao Z, Song M, Zhuang D, Han B, Wu J, Sun X, 2021. Nicotinamide ameliorates energy deficiency and improves retinal function in Cav-1 (−/−) mice. J. Neurochem 157, 550–560. [DOI] [PubMed] [Google Scholar]
- Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP, 1996. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem 271, 2255–2261. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Maruyama N, Ogata T, Kasahara T, Nakanishi N, Miyagawa K, Naito D, Hamaoka T, Nishi M, Matoba S, Ueyama T, 2016. PTRF/Cavin-1 deficiency causes cardiac dysfunction accompanied by cardiomyocyte hypertrophy and cardiac fibrosis. PLoS One 11, e0162513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson BR, Heinen S, Jeansson M, Ghosh AK, Fatima A, Sung HK, Onay T, Chen H, Yamaguchi S, Economides AN, Flenniken A, Gale NW, Hong YK, Fawzi A, Liu X, Kume T, Quaggin SE, 2014. A lymphatic defect causes ocular hypertension and glaucoma in mice. J. Clin. Invest 124, 4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, DeWan A, Sigurdsson A, Jonasdottir A, Gudjonsson SA, Magnusson KP, Stefansson H, Lam DS, Tam PO, Gudmundsdottir GJ, Southgate L, Burdon KP, Gottfredsdottir MS, Aldred MA, Mitchell P, St Clair D, Collier DA, Tang N, Sveinsson O, Macgregor S, Martin NG, Cree AJ, Gibson J, Macleod A, Jacob A, Ennis S, Young TL, Chan JC, Karwatowski WS, Hammond CJ, Thordarson K, Zhang M, Wadelius C, Lotery AJ, Trembath RC, Pang CP, Hoh J, Craig JE, Kong A, Mackey DA, Jonasson F, Thorsteinsdottir U, Stefansson K, 2010. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat. Genet 42, 906–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn H, Stenkula KG, Karlsson M, Ortegren U, Nystrom FH, Gustavsson J, Stralfors P, 2003. Cell surface orifices of caveolae and localization of caveolin to the necks of caveolae in adipocytes. Mol. Biol. Cell 14, 3967–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian XF, Xia XB, Xu HZ, Xiong SQ, Jiang J, 2012. Caveolin-1 expression regulates blood-retinal barrier permeability and retinal neovascularization in oxygen-induced retinopathy. Clin. Exp. Ophthalmol 40, e58–66. [DOI] [PubMed] [Google Scholar]
- Tillu VA, Rae J, Gao Y, Ariotti N, Floetenmeyer M, Kovtun O, McMahon KA, Chaudhary N, Parton RG, Collins BM, 2021. Cavin1 intrinsically disordered domains are essential for fuzzy electrostatic interactions and caveola formation. Nat. Commun 12, 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigatti BL, Anderson RG, Gerber GE, 1999. Identification of caveolin-1 as a fatty acid binding protein. Biochem. Biophys. Res. Commun 255, 34–39. [DOI] [PubMed] [Google Scholar]
- Trushina E, Du Charme J, Parisi J, McMurray CT, 2006a. Neurological abnormalities in caveolin-1 knock out mice. Behav. Brain Res. 172, 24–32. [DOI] [PubMed] [Google Scholar]
- Trushina E, Singh RD, Dyer RB, Cao S, Shah VH, Parton RG, Pagano RE, McMurray CT, 2006b. Mutant huntingtin inhibits clathrin-independent endocytosis and causes accumulation of cholesterol in vitro and in vivo. Hum. Mol. Genet 15, 3578–3591. [DOI] [PubMed] [Google Scholar]
- Vahabikashi A, Gelman A, Dong B, Gong L, Cha EDK, Schimmel M, Tamm ER, Perkumas K, Stamer WD, Sun C, Zhang HF, Gong H, Johnson M, 2019. Increased Stiffness and Flow Resistance of the Inner Wall of Schlemm’s Canal in Glaucomatous Human Eyes. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinten J, Johnsen AH, Roepstorff P, Harpoth J, Tranum-Jensen J, 2005. Identification of a major protein on the cytosolic face of caveolae. Biochim. Biophys. Acta 1717, 34–40. [DOI] [PubMed] [Google Scholar]
- Vinten J, Voldstedlund M, Clausen H, Christiansen K, Carlsen J, Tranum-Jensen J, 2001. A 60-kDa protein abundant in adipocyte caveolae. Cell Tissue Res. 305, 99–106. [DOI] [PubMed] [Google Scholar]
- Vladimirov VI, Zernii EY, Baksheeva VE, Wimberg H, Kazakov AS, Tikhomirova NK, Nemashkalova EL, Mitkevich VA, Zamyatnin AA Jr., Lipkin VM, Philippov PP, Permyakov SE, Senin II, Koch KW, Zinchenko DV, 2018. Photoreceptor calcium sensor proteins in detergent-resistant membrane rafts are regulated via binding to caveolin-1. Cell Calcium 73, 55–69. [DOI] [PubMed] [Google Scholar]
- Voigt AP, Mulfaul K, Mullin NK, Flamme-Wiese MJ, Giacalone JC, Stone EM, Tucker BA, Scheetz TE, Mullins RF, 2019. Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration. Proc. Natl. Acad. Sci. U. S. A 116, 24100–24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonte D, Galbiati F, 2011. Polymerase I and transcript release factor (PTRF)/cavin-1 is a novel regulator of stress-induced premature senescence. J. Biol. Chem 286, 28657–28661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Lao M, Xu Z, Ding M, Guo S, Li L, 2020a. Caveolin1 modulates hypertensive vascular remodeling via regulation of the Notch pathway. Mol. Med. Rep 22, 4320–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM, 2009. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J. Immunol 182, 3809–3818. [DOI] [PubMed] [Google Scholar]
- Wang XM, Kim HP, Song R, Choi AM, 2006. Caveolin-1 confers antiinflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am. J. Respir. Cell Mol. Biol 34, 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu CH, Huang S, Fu Z, Tomita Y, Britton WR, Cho SS, Chen CT, Sun Y, Ma JX, He X, Chen J, 2020b. Wnt signaling activates MFSD2A to suppress vascular endothelial transcytosis and maintain blood-retinal barrier. Sci. Adv 6, eaba7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren A, Cogger VC, Arias IM, McCuskey RS, Le Couteur DG, 2010. Liver sinusoidal endothelial fenestrations in caveolin-1 knockout mice. Microcirculation 17, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M, Parton RG, 1995. M-caveolin, a muscle-specific caveolin-related protein. FEBS Lett. 376, 108–112. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Aung T, Medeiros FA, 2014. The pathophysiology and treatment of glaucoma: a review. JAMA 311, 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Khaw PT, 2004. Primary open-angle glaucoma. Lancet 363, 1711–1720. [DOI] [PubMed] [Google Scholar]
- Weiss CR, Guan Q, Ma Y, Qing G, Bernstein CN, Warrington RJ, Peng Z, 2015. The potential protective role of caveolin-1 in intestinal inflammation in TNBS-induced murine colitis. PLoS One 10, e0119004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Kang JH, Yaspan BL, Mirel DB, Laurie C, Crenshaw A, Brodeur W, Gogarten S, Olson LM, Abdrabou W, DelBono E, Loomis S, Haines JL, Pasquale LR, Consortium, G., 2011. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum. Mol. Genet 20, 4707–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Carmichael TR, Allingham RR, Hauser M, Ramsay M, 2015. The genetics of POAG in black South Africans: a candidate gene association study. Sci. Rep 5, 8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP, 2004. The caveolin proteins. Genome Biol. 5, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG, Lisanti MP, 2004. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J. Biol. Chem 279, 51630–51646. [DOI] [PubMed] [Google Scholar]
- Wu H, Deng R, Chen X, Wong WC, Chen H, Gao L, Nie Y, Wu W, Shen J, 2016. Caveolin-1 is critical for lymphocyte trafficking into central nervous system during experimental autoimmune encephalomyelitis. J. Neurosci. : Off. J. Soc. Neurosci 36, 5193–5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin C, Song S, Johnstone M, Wang N, Wang RK, 2018. Quantification of pulse-dependent trabecular meshwork motion in normal humans using phase-sensitive OCT. Invest. Ophthalmol. Vis. Sci 59, 3675–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada E, 1955. The fine structure of the gall bladder epithelium of the mouse. J. Biophys. Biochem. Cytol 1, 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JM, Park CS, Kim SH, Noh TW, Kim JH, Park S, Lee J, Park JR, Yoo D, Jung HH, Takase H, Shima DT, Schwaninger M, Lee S, Kim IK, Lee J, Ji YS, Jon S, Oh WY, Kim P, Uemura A, Ju YS, Kim I, 2020. Dll4 suppresses transcytosis for arterial blood-retinal barrier homeostasis. Circ. Res 126, 767–783. [DOI] [PubMed] [Google Scholar]
- Yemanyi F, Baidouri H, Burns AR, Raghunathan V, 2020. Dexamethasone and glucocorticoid-induced matrix temporally modulate key integrins, caveolins, contractility, and stiffness in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci 61, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf MA, Zhou X, Mukherjee S, Chintakuntlawar AV, Lee JY, Ramke M, Chodosh J, Rajaiya J, 2013. Caveolin-1 associated adenovirus entry into human corneal cells. PLoS One 8, e77462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaas DW, Duncan M, Rae Wright J, Abraham SN, 2005. The role of lipid rafts in the pathogenesis of bacterial infections. Biochim. Biophys. Acta 1746, 305–313. [DOI] [PubMed] [Google Scholar]
- Zaas DW, Swan ZD, Brown BJ, Li G, Randell SH, Degan S, Sunday ME, Wright JR, Abraham SN, 2009. Counteracting signaling activities in lipid rafts associated with the invasion of lung epithelial cells by Pseudomonas aeruginosa. J. Biol. Chem 284, 9955–9964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Xu J, Liu R, Chen W, Chen Q, Hu W, Zhou L, Zhang R, Xu H, Lin D, Li X, Tang Z, 2017. Caveolin-1 protects retinal ganglion cells against acute ocular hypertension injury via modulating microglial phenotypes and distribution and activating AKT pathway. Sci. Rep 7, 10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PX, Murray TS, Villella VR, Ferrari E, Esposito S, D’Souza A, Raia V, Maiuri L, Krause DS, Egan ME, Bruscia EM, 2013. Reduced caveolin-1 promotes hyperinflammation due to abnormal heme oxygenase-1 localization in lipopolysaccharide-challenged macrophages with dysfunctional cystic fibrosis transmembrane conductance regulator. J. Immunol 190, 5196–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YZ, Boscher C, Inder KL, Fairbank M, Loo D, Hill MM, Nabi IR, Foster LJ, 2011. Differential impact of caveolae and caveolin-1 scaffolds on the membrane raft proteome. Mol. Cell. Proteomics : MCP 10. M110 007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZJ, Marshansky V, Breton S, Brown D, 2011. Is caveolin involved in normal proximal tubule function? Presence in model PT systems but absence in situ. Am J. Physiol. Renal 300, F199–F206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschocke J, Manthey D, Bayatti N, van der Burg B, Goodenough S, Behl C, 2002. Estrogen receptor alpha-mediated silencing of caveolin gene expression in neuronal cells. J. Biol. Chem 277, 38772–38780. [DOI] [PubMed] [Google Scholar]
- Zukerman R, Harris A, Vercellin AV, Siesky B, Pasquale LR, Ciulla TA, 2020. Molecular genetics of glaucoma: subtype and ethnicity considerations. Genes 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.