Abstract
Background
Non‐Hispanic Black individuals may be less likely to receive a diagnosis of dementia compared to non‐Hispanic White individuals. These findings raise important questions regarding which factors may explain this observed association and any differences in the time to which disparities emerge following dementia onset.
Methods
We conducted a retrospective cohort study using survey data from the 1995 to 2016 Health and Retirement Study linked with Medicare fee‐for‐service claims. Using the Hurd algorithm (a regression‐based approach), we identified dementia onset among older adult respondents (age ≥65 years) from the Telephone Interview for Cognitive Status and proxy respondents. We determined date from dementia onset to diagnosis using Medicare data up to 3 years following onset using a list of established diagnosis codes. Cox Proportional Hazards modeling was used to examine the association between an individual's reported race and likelihood of diagnosis after accounting for sociodemographic characteristics, income, education, functional status, and healthcare use.
Results
We identified 3435 older adults who experienced a new onset of dementia. Among them, 30.1% received a diagnosis within 36 months of onset. In unadjusted analyses, the difference in cumulative proportion diagnosed by race continued to increase across time following onset, p‐value <0.001. 23.8% of non‐Hispanic Black versus 31.4% of non‐Hispanic White participants were diagnosed within 36 months of dementia onset, Hazard Ratio = 0.73 (95% CI: 0.61, 0.88). The association persisted after adjustment for functional status and healthcare use; however, these factors had less of an impact on the strength of the association than income and level of education.
Conclusion
Lower diagnosis rates of dementia among non‐Hispanic Black individuals persists after adjustment for sociodemographic characteristics, functional status, and healthcare use. Further understanding of barriers to diagnosis that may be related to social determinants of health is needed to improve dementia‐related outcomes among non‐Hispanic Black Americans.
Keywords: dementia, diagnosis, disparities, older adults, race
Key points
Non‐Hispanic Black older adults, compared to non‐Hispanic White older adults, are less likely to receive a diagnosis for dementia.
This difference in the likelihood of a dementia diagnosis persists for up to 3 years following dementia onset after accounting for differences in sociodemographic characteristics, functional status, and healthcare use.
Differences in income and level of education are the largest factors that influence the association.
Why does this paper matter?
Among older adults with cognitive decline, non‐Hispanic Black persons are less likely to receive a diagnosis. The association persists after accounting for differences in sociodemographic characteristics, functional status, and use of healthcare. These results support the growing body of evidence that race influences dementia diagnosis.
INTRODUCTION
Due to an increase in population age and longevity, the number of Americans with Alzheimer's disease and related dementias (ADRD) is expected to increase from the current estimate of 5 million to over 13 million by 2050. 1 A major goal of the National Plan to Address Alzheimer's Disease is to enhance care quality and efficiency by ensuring timely and accurate clinical diagnosis. 2 Timely clinical diagnosis provides opportunity for early intervening in care coordination, advanced care planning, and mitigation of risky behaviors. 3 Yet more than 60% of Americans with ADRD experience a delay or completely missed diagnosis. 4 Clinical diagnosis of ADRD requires a comprehensive medical history, cognitive assessment, physical exam conducted by a healthcare provider with dementia expertise, laboratory testing, and neuroimaging to identify potential structural changes. 5 Barriers to high‐value care exist particularly among disadvantaged groups that could impact an individual's ability to receive a prompt and accurate diagnosis.
Prior research has identified evidence of potential differences in the likelihood of receiving an ADRD clinical diagnosis by race and ethnicity. 6 , 7 , 8 , 9 , 10 , 11 For instance, several studies compared the identification of dementia using cognitive assessment to that using administrative claims (as a measure of clinical diagnosis). Chen et al. 6 found that 85% of non‐Hispanic White older adults have cognitive assessment scores consistent with dementia and have a clinical diagnosis, compared to only 74% of non‐Hispanic Black older adults. Similarly, comparing dementia prevalence based on cognitive assessment to diagnoses in administrative data, large differences exist by race and ethnicity—while 39% of non‐Hispanic Black beneficiaries had dementia based on the cognitive assessment, only 17% were clinically diagnosed (whereas among non‐Hispanic White beneficiaries, rates of dementia based on cognitive assessment and administrative claims were equivalent). 7 The few recent studies to examine the likelihood of clinical diagnosis (using administrative claims or self‐report) among older adults with known ADRD suggest that the odds of receiving a clinical diagnosis for non‐Hispanic Black individuals is approximately 20%–26% lower than that for non‐Hispanic White. 8 , 9 Tsoy et al. 11 found that non‐Hispanic Black beneficiaries (compared to non‐Hispanic White) were 27% less likely to receive a mild cognitive impairment versus dementia diagnosis (suggesting a delay in identification of cognitive decline); however, there was no statistically significant difference in the number of appropriate ADRD diagnostic services between the groups.
Collectively previous work suggests a lower clinical likelihood in diagnosis among non‐Hispanic Black individuals may be explained by any number of factors including differences in demographic factors, income, level of education, functional status, and/or healthcare access. However, these prior studies differ in several, important features including methodology (e.g., identify ADRD using different data sources and algorithms, some of which are known to have poorer accuracy for racial/ethnic groups), comprehensive inclusion of exploratory factors, and examination of differences in diagnosis across time. Therefore, we used a nationally representative sample to examine the time from dementia onset to clinical diagnosis among older US adults. We examine differences in the duration of time to diagnosis (and likelihood of receiving a diagnosis at all) by race after accounting for important, potentially explanatory factors.
METHODS
We conducted a retrospective cohort study using data from the Health and Retirement Study (HRS) linked with Medicare data to investigate, by race, time from dementia onset to ADRD clinical diagnosis as indicated by first diagnosis in claims. Older adult (ages ≥65 years) non‐Hispanic White (hereafter, “White”) and non‐Hispanic Black (hereafter, “Black”) respondents to the HRS who had evidence of a new onset of dementia from 1998 to 2013 were included in our study. We were interested in observing whether the average time to diagnosis varies by race and which factors potentially explain the association between race and clinical diagnosis. We conceptualized the categories of factors potentially explaining differences in clinical diagnosis by race as demographic characteristics (age, sex, and marital status), income, level of education, functional status, and healthcare use. We examined the association after progressively accounting for these factors in our analyses.
Study population (older adults identified with dementia)
The HRS is a nationally representative, longitudinal study of adults 51 years and older with an oversampling of African American and Latino Americans. Since the start of the study in 1992, more than 37 thousand respondents have been surveyed during the two‐year waves on such measures as their sociodemographic characteristics, health, employment and earnings, and family structure. The HRS re‐interview response rate ranges from 68.8% to 92.3% with a mean of 73.0%. Approximately 80% of HRS respondents give permission for their survey data to be linked to Medicare claims data. 12 The linked Medicare data include inpatient (hospital and skilled nursing), outpatient, physician, and home health claims.
Among the health data collected with HRS is a cognitive assessment that includes the Telephone Interview for Cognitive Status (TICS), administered by telephone or in‐person at every survey wave (approximately every 2 years). 13 For an HRS respondent who is unable or unwilling to complete the survey, a proxy respondent (e.g., spouse) is identified to answer survey questions and is administered the Jorm Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE). 14
Different methods exist to identify cognitive impairment and dementia using data from the TICS and/or IQCODE, with varying levels of accuracy. 15 We used the Hurd method, a regression‐based approach, for the identification of dementia onset because it is least affected by inherent racial biases. More specifically, the Hurd method has been shown to have the following attributes: (1) comparable sensitivity and specificity across racial/ethnic groups, (2) achieves higher than 80% accuracy across racial/ethnic groups, and (3) overall has higher than a 75% sensitivity and 90% specificity. 16 In addition to using cognitive assessment scores (or, for individuals with proxy respondents, IQCODE data) the Hurd method accounts for the change in cognitive function from the previous wave and adjusts for sociodemographic characteristics and functional status that are highly correlated with dementia severity 17 —therefore the algorithm is well suited to detect dementia across racial and ethnic groups with its incorporation of functional decline (versus other approaches that use only function at a single point in time).
From 1998–1999 (wave 4) to 2012–2013 (wave 11) we identified 5783 HRS older adult study participants who were classified with dementia based on the Hurd method (Figure 1). Because the TICS is administered approximately every 2 years, for those newly identified as having cognitive decline the true onset of dementia likely occurred at some point before the date of the HRS interview of the respondent (or proxy respondent). To account for this, we used the midpoint between the date of the survey (TICS) first identified and the prior survey. 18 Among those identified with dementia, we restricted to participants whose race was reported as either White or Black and who were continuously enrolled in Medicare fee‐for‐service and not enrolled in managed care in the year before dementia onset as well as the 3 years after onset. We removed 372 older adults who received a dementia clinical diagnosis before the onset identified in HRS. After restricting to White and Black individuals with complete data, our final sample consisted of 3435 HRS respondents.
FIGURE 1.
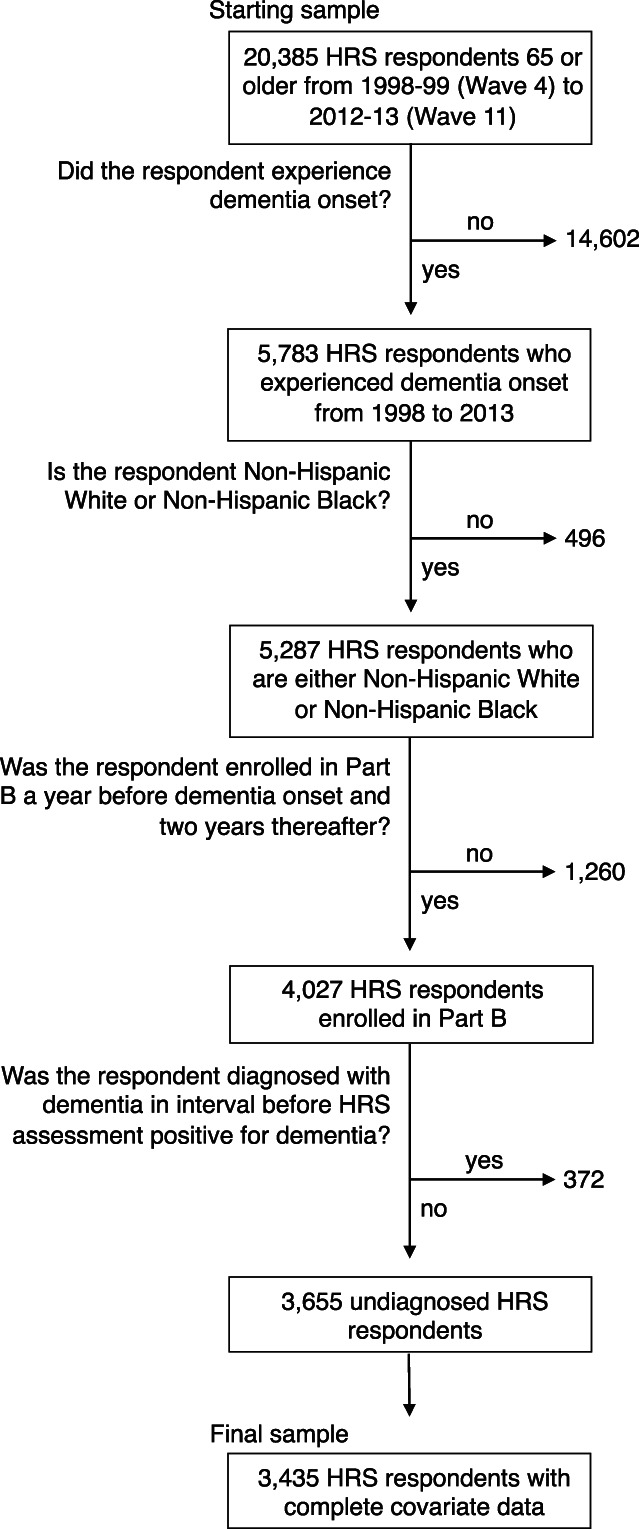
Identification of study cohort. HRS, health and retirement study.
ADRD clinical diagnosis identified using medicare claims
The primary dependent variable was a clinical diagnosis of ADRD identified using Medicare claims. Medicare switched from using International Classification of Diseases (ICD) version 9 to version 10 in October 2015; therefore, we used a combination of ICD‐9 and ICD‐10 codes to identify ADRD clinical diagnosis in our cohort of older adults. Using a list of established ICD‐9 and ICD‐10 19 , 20 codes that have been shown to capture ADRD, we identified a first ADRD diagnosis from the study participant's healthcare claims. Anticipating that more disadvantaged groups may be less likely to receive an ADRD clinical diagnosis, we used an inclusive definition of a diagnosis, defined as the first evidence of an ADRD diagnostic code appearing in any diagnostic position on the claim for any ambulatory or inpatient health service. Previous reports have shown 3 years is sufficient to identify an ADRD clinical diagnosis, hence, we searched for diagnosis up to 36 months after onset. 21 , 22
For each study participant who received an ADRD clinical diagnosis identified by the occurrence of a diagnosis appearing on a claim, we calculated the number of days that elapsed from the estimated dementia onset (identified based on midpoint date between HRS interview dates) and date of the healthcare claim.
Covariates
Explanatory factors included demographic measures (age at the time of the survey, sex, marital status, 23 income, and level of education), functional limitations, and healthcare use (number of ambulatory visits, hospitalizations, as well as self‐reported diagnoses from health encounters). Median household income was separated into quartiles and level of education was separated into the following ordinal categories: High School or less, Some college or Associate's degree, Bachelor's degree, and Graduate or advanced degree. We identified study participants with self‐reported (or proxy‐reported) health conditions including hypertension, a mental health disorder (i.e., depression, anxiety, or other psychiatric disorder), diabetes, respiratory illness, cardiovascular disease (i.e., heart failure and/or history of myocardial infarction), stroke, and cancer. Limitations in activities of daily living (ADLs) and instrumental ADLs (IADLs) were identified by self‐ or proxy‐report. Last, we determined the amount of informal caregiving received; collapsed into none, moderate (1–13 h), and high (14 or more hours per week), and the number of ambulatory healthcare visits for evaluation and management 24 in the year before dementia onset (from Medicare claims), which can represent access to care (and therefore opportunities for diagnosis).
Statistical analyses
To compare sociodemographic characteristics, functional status, and healthcare use (as well as informal caregiving) according to race we used bivariate statistical tests. Logit models were used to calculate the association between race and a clinical diagnosis after adjustment for differences. We used time‐to‐event (i.e., survival analysis) for all other analyses that allowed us to account for the time to clinical diagnosis up to 3 years after dementia identification in HRS. For these analyses, the duration under observation was the number of days between the estimated date of dementia onset and the date of the claim where the first ADRD clinical diagnosis was documented. Therefore, older adults who did not receive an ADRD clinical diagnosis while under observation were considered right censored. We used the Kaplan–Meier method to estimate the cumulative incidence of clinical diagnosis separately by race. The Log‐rank test was used to compare the cumulative incidence of ADRD clinical diagnosis by race. Cox Proportional Hazards regression was used to estimate associations between independent variables and the likelihood of diagnosis and coefficients were exponentiated to express associations as hazards ratios (HR). We did not include informal caregiving in our statistical models due to collinearity with functional status and because caregiving is only assessed among participants with a functional limitation. A sequential modeling approach, with five separate Cox Proportional Hazard models, was used to identify factors contributing to differences in the probability of diagnosis by race. This included a naïve “unadjusted” model with only the dependent variable (clinical diagnosis) and the independent variable of interest (race) that identified population‐level differences in probability of diagnosis. Subsequent models incrementally introduced sets of additional measures representing potential facilitators or barriers to health care use that could potentially explain differences in diagnosis probability: demographic factors, income, level of education, functional limitations, and health care status and utilization. For all analyses, the statistical significance level was set at a critical alpha level of 0.05, 2‐sided. Analyses were performed using a combination of Stata version 16.0 (StataCorp, College Station, TX, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Among the 3435 older adults who experienced a new onset of dementia, 30.1% (1033 of 3435) received an ADRD diagnosis within 3 years of onset. Among all respondents, sociodemographic characteristics among White versus Black study participants differed—Black respondents were younger than White participants (mean age 80.2 vs. 83.1 years, p‐value <0.001) and more likely to have lower education and income (Table 1). 46.0% of Black participants were poor/near‐poor versus only 19.9% among White participants and 66.7% of Black participants had a High School or less education level compared to only 34.8% among White participants, p‐value <0.001 for both. Black participants were less likely to be married (30.6% vs. 42.2%) and more likely to have self‐reported comorbidities including hypertension and diabetes. The mean number of ADLs and IADLs were higher among Black than White participants. Black participants were more likely to report receiving high levels of informal caregiving yet had fewer ambulatory healthcare visits compared to White participants.
TABLE 1.
Characteristics of study participants
| Characteristic | By Race | ||
|---|---|---|---|
| Non‐hispanic white | Non‐hispanic black | p‐value a | |
| Sample, No. | 2846 | 589 | |
| Sociodemographic characteristics | |||
| Mean age in years (SD) | 83.1 (6.6) | 80.2 (7.3) | <0.001 |
| Age category, No. (%) | <0.001 | ||
| 65–74 years | 278 (9.8) | 131 (22.2) | |
| 75–84 years | 1314 (46.2) | 278 (47.2) | |
| 85 or older | 1254 (44.0) | 180 (30.6) | |
| Sex, No. (%) | 0.2 | ||
| Male | 1085 (38.1) | 208 (35.3) | |
| Female | 1761 (61.9) | 381 (64.7) | |
| Received an ADRD diagnosis, No. (%) | 893 (31.4) | 140 (23.8) | <0.001 |
| Income category, No. (%) | <0.001 | ||
| Poor/near poor (quartile 1) | 566 (19.9) | 269 (46.0) | |
| Low (quartile 2) | 698 (24.5) | 164 (27.8) | |
| Middle (quartile 3) | 766 (26.9) | 92 (15.6) | |
| High (quartile 4) | 816 (28.7) | 64 (10.9) | |
| Level of education, No. (%) | <0.001 | ||
| High School or less | 989 (34.8) | 393 (66.7) | |
| Some college or Associate's degree | 1046 (36.8) | 124 (21.1) | |
| Bachelor's degree | 485 (17.0) | 36 (6.1) | |
| Graduate or advanced degree | 326 (11.5) | 36 (6.1) | |
| Marital status, No. (%) | <0.001 | ||
| Never married | 67 (2.4) | 21 (3.6) | |
| Widowed/Divorced/Separated | 1579 (55.5) | 388 (65.9) | |
| Married | 1200 (42.2) | 180 (30.6) | |
| Health status | |||
| No. with hypertension diagnosis (%) | 1790 (62.9) | 452 (76.7) | <0.001 |
| No. with mental health diagnosis (%) | 590 (20.7) | 109 (18.5) | 0.2 |
| No. with diabetes diagnosis (%) | 558 (19.6) | 186 (31.6) | <0.001 |
| No. with respiratory illness diagnosis (%) | 424 (14.9) | 47 (8.0) | <0.001 |
| No. with cardiovascular diagnosis b (%) | 1220 (42.9) | 208 (35.3) | <0.01 |
| No. with stroke diagnosis (%) | 604 (21.2) | 114 (19.4) | 0.3 |
| No. with cancer diagnosis (%) | 572 (20.1) | 82 (13.9) | <0.01 |
| Functional status | |||
| Mean No. of ADLs (SD) | 1.2 (1.6) | 1.3 (1.7) | 0.2 |
| Mean No. of IADLs (SD) | 1.4 (1.7) | 1.5 (1.7) | 0.5 |
| Heath service use | |||
| Mean No. of ambulatory EM visits in year before onset (SD) | 9.3 (9.4) | 7.2 (8.7) | <0.001 |
| Mean No. of hospitalizations in 1 year before onset (SD) | 0.3 (0.8) | 0.4 (0.8) | 0.7 |
| Caregiving hours, No. (%) | 0.06 | ||
| None | 1577 (55.4) | 314 (55.3) | |
| Moderate (1–13 h per week) | 613 (21.5) | 113 (19.2) | |
| High (14 or more hours per week) | 656 (23.1) | 162 (27.5) | |
Abbreviations: ADL, activities of daily living; EM, evaluation and management; IADL, instrumental activities of daily living.
t‐test used to compare means, Chi‐squared test used to compare proportions.
Includes heart failure and myocardial infarction.
Association between race and likelihood of ADRD diagnosis
In a naïve analysis unadjusted for any covariates, the cumulative proportion who received an ADRD clinical diagnosis differed by race initially after the estimated dementia onset and the difference increased across 36 months, Log‐rank p‐value <0.001 (Figure 2). Up to 3 years after dementia onset, Black respondents were less likely to receive an ADRD clinical diagnosis compared to White respondents; 23.8% versus 31.4%, respectively, were diagnosed, with an unadjusted Hazard Ratio = 0.73 (0.61, 0.88) (Figure 3 and Table 2). Progressively adjusting for explanatory factors overall attenuated but did not remove the association between race and ADRD diagnosis—most notably, inclusion of demographics, income, and level of education reduced the association from HR = 0.73 to 0.79 (Table S1). However, additional adjustment for functional status (ADLs and IADL limitations) and healthcare use did not affect the association in an appreciable way.
FIGURE 2.
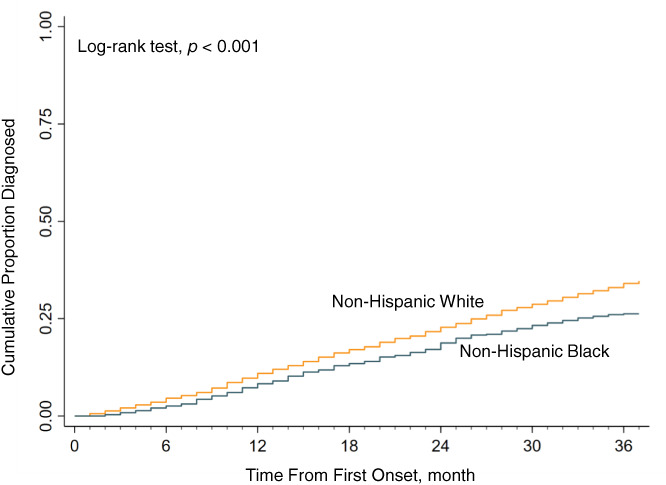
Unadjusted cumulative proportion diagnosed since ADRD identified in the health and retirement study by race. ADRD, Alzheimer's disease and other related dementias.
FIGURE 3.
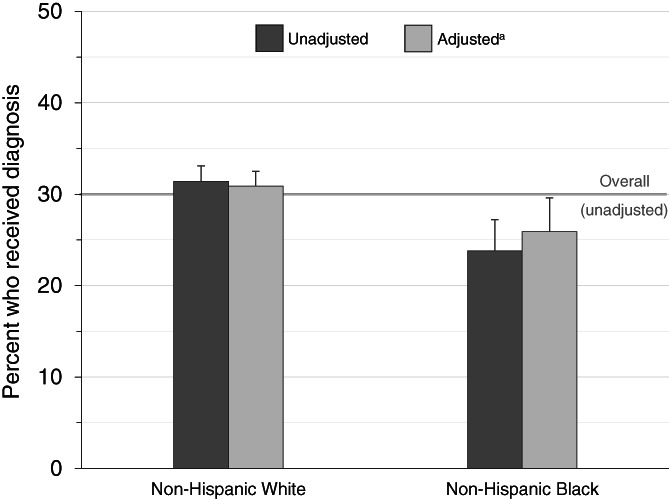
Percent of older adults with ADRD who received a clinical diagnosis within three years after dementia identified in the health and retirement study by race. ADRD, Alzheimer's disease and related dementias. (A) Adjusted for: age (continuous), sex, marital status, income, level of education, No. of ADLs, No. of IADLs, hypertension diagnosis, mental health illness diagnosis, diabetes diagnosis, respiratory illness diagnosis, cardiovascular diagnosis, stroke diagnosis, cancer diagnosis, within the year before onset No. of ambulatory EM visits and No. of hospitalizations
TABLE 2.
Hazard ratios for the association between non‐hispanic black race (vs. non‐hispanic white, reference) and ADRD clinical diagnosis
| Hazard ratio (95% CI) | |
|---|---|
| Unadjusted | 0.73 (0.61, 0.88) |
| Adjusted | |
| Demographics a | 0.74 (0.62, 0.89) |
| Income category b | 0.77 (0.64, 0.92) |
| Level of education c | 0.79 (0.66, 0.96) |
| Functional status d | 0.81 (0.67, 0.98) |
| Healthcare use e | 0.81 (0.67, 0.99) |
Abbreviation: CI, confidence interval.
Base model covariates include age (continuous, years), sex (male, female), marital status (never married, widowed/divorced/separated, married).
Further adjusted for income (lowest quartile 1 to highest quartile 4).
Further adjusted for level of education (high school or less, some college or Associate's degree, Bachelor's degree, Graduate or advanced degree).
Further adjusted for No. of ADLs, No of IADLs, hypertension diagnosis, mental health diagnosis, diabetes diagnosis, respiratory illness diagnosis, cardiovascular diagnosis (includes heart failure and myocardial infarction), stroke diagnosis, cancer diagnosis.
Further adjusted for within the year before onset No. of ambulatory EM visits and No. of hospitalizations.
Predictors of receiving an ADRD clinical diagnosis
In fully adjusted analyses (including all covariates), female sex (compared to male) was associated with a higher likelihood of ADRD diagnosis (HR = 1.20, 95% CI: 1.03, 1.39). A dose–response relationship of diagnostic likelihood was observed across level of education. Specifically, a High School or less education was associated with a 35% lower likelihood of diagnosis (HR = 0.65, 95% CI: 0.52, 0.81) compared to Graduate or advanced degree attainment. Having an additional IADL limitation was associated with a higher likelihood of diagnosis.
DISCUSSION
In this retrospective cohort study among a large sample of older adults with a new onset of dementia in the HRS, Black participants were less likely to receive an ADRD clinical diagnosis up to 3 years after onset. Differences by race in the likelihood of clinical diagnosis were only partially explained by factors including income and education measures that were strongly independently associated with diagnosis. Together the findings identify differences by race in ADRD diagnosis even after accounting for commonly observed facilitators or barriers to health care use and quality of care, potentially suggesting embedded disparities in care systems.
Prior studies using similar data sources reveal point‐in‐time, cross‐sectional differences in dementia diagnosis by race. Leveraging self‐reports of whether a healthcare professional provided a diagnosis of dementia, Lin et al. 8 estimated 26% lower odds of diagnosis for Black compared to White individuals who were screened as having dementia. Lower odds for Black versus White individuals were also observed when using administrative data to assess whether a clinical diagnosis was offered for those later screened as having dementia. 9 Our longitudinal analyses add to this literature, in identifying a race‐associated diagnosis gap immediately present following estimated dementia onset and that continues to grow up to 36 months after onset. Together this work suggests substantial, persistent, and growing race‐based gaps in dementia diagnosis that may indicate detection occurring at more advanced stages of the disease; for Black patients and family members, this could impede care choices including obtaining long‐term services and supports and may translate to costly, emergent care for unmet needs related to the underlying condition. 25
Given the strong relationship between higher educational levels and likelihood of an undetected dementia diagnosis, 4 these gaps could be attributable to differences by race in education‐related factors such as health care literacy, health communication, ability to navigate the healthcare system, or quality of care. Likelihood of a clinical diagnosis may also reflect structural or physiological differences in the clinical evidence of cognitive decline (e.g., increased healthcare encounters translating to more opportunities to observe impairment, or more rapid cognitive decline translating to clearer symptomatology of underlying disease). However, diagnosis differences persisted after adjustment for education, healthcare encounters, and biological sex, which is associated with cognitive decline patterns and encounters with the healthcare system, 26 , 27 plus functional limitations that are indicators of cognitive decline.
Limitations
This study has limitations that must be acknowledged. First, we were interested in estimating the time elapsed between dementia onset (time from identification in HRS) and clinical diagnosis— thus, older adults were removed from our study if the ADRD clinical diagnosis occurred before identification by HRS. However, our final sample consisted of a reasonable proportion of non‐Hispanic Black individuals (17.1%) and race did not differ statistically between those included versus excluded from our study (p‐value = 0.23). Second, we relied on the use of administrative data to identify an ADRD diagnosis. Claims are imperfect measures of treatment received and there is the potential that an individual received a diagnosis without an appropriate billing ICD code—thus, there is potential for misclassification bias. We do not suspect though that misclassification would differ by race, given the strong incentive to bill for any clinical time, including obtaining diagnoses; if anything, reimbursement for clinical time and therefore likelihood of an appropriate billing code should be greater for Black individuals who are more likely to present for ADRD‐related treatment at a time of crisis. 28 Further, we deemed an individual diagnosed if any claim whatsoever appeared with an ADRD diagnosis biasing our analyses towards identification of diagnosed individuals.
These limitations notwithstanding, our findings raise clinical concerns. Although earlier detection does not likely help to impede disease progression, given that dementia is a cure‐less disease, those with delayed diagnoses may have less developed care plans, which could affect their function and safety. For instance, while fall risks are 30% greater for older adults with versus without cognitive impairment, 29 , 30 they can be mitigated through the presence of formal and informal caregivers 31 who offer assistance with daily activities and support for management of behavioral symptoms and co‐occurring chronic conditions. Earlier clinical detection for Black patients (whether through improved patient‐provider communication, or uncovering implicit bias) could therefore improve patient supports and outcomes, while also potentially alleviating patient and family member anxieties about behavior changes related to cognitive decline.
From a policy perspective, our findings may implicate broader issues within the healthcare system. The findings fit a broader pattern of race‐associated gaps in healthcare, such as the diagnosis and treatment of prostate and breast cancers. 32 , 33 Treatment differences by race have also been observed for individuals with ADRD, with Black individuals less likely than White individuals to access antidementia medications, but more likely to receive care only at a time of crisis, 28 and to report unmet dementia‐related needs and have worse health care outcomes. 34 More broadly, care for Black and Hispanic patients is highly concentrated among US hospitals 35 , 36 that have been found to have relatively poor quality of care, plus lower revenue and nurse staffing levels. 35 , 36 Further study is warranted to understand the potential causes of dementia detection differences and whether Medicare might consider the use of reimbursement‐related incentives (e.g., bonus or penalty additions to traditional diagnosis‐related payments) to address these race‐associated gaps.
CONCLUSIONS AND IMPLICATIONS
This study offers additional insight into the association between race and ADRD diagnosis among older U.S. adults by finding the association persists after accounting for differences in functional status and healthcare use. In sum, these findings suggest that race is influential in diagnosis likelihood because of structural and systematic factors including level of education and interactions within the healthcare system; however, future studies are needed to fully explain factors that underpin dementia diagnosis. Careful evaluation and assessment should be conducted with older adults given dementia is difficult to diagnose and early interventions with healthcare support are important for promoting optimal health in this population.
AUTHOR CONTRIBUTIONS
Study concept and design: Davis, Bynum, Langa, and Hoffman. Acquisition of subjects and/or data: Hoffman and Kang. Analysis and interpretation of data: Davis, Hoffman, and Kang. Preparation of manuscript: All authors.
FUNDING INFORMATION
The Health and Retirement Study is funded by the National Institute on Aging (U01 AG009740) and the Social Security Administration, and performed at the Institute for Social Research, University of Michigan, Ann Arbor. Drs Davis and Bynum were supported by grant P01 AG019783 from the National Institute on Aging. Dr Langa was supported by grants P30 AG024824, P30 AG053760, and R01 AG053972 from the National Institute on Aging. Dr Hoffman was supported by the grant P30 AG066582 from the National Institute on Aging.
CONFLICT OF INTEREST
Drs Davis and Bynum were supported by grant P01 AG019783 from the National Institute on Aging. Dr Langa was supported by grants P30 AG024824, P30 AG053760, and R01 AG053972 from the National Institute on Aging. Dr Hoffman was supported by the grant P30 AG066582 from the National Institute on Aging. Dr Davis received financial report from Regional Anesthesia & Pain Medicine for consulting statistical review. Dr Harris, Dr Kang, and Ms Lee have no conflicts to report.
SPONSOR'S ROLE
The funders had no role in the study design, data collection, management, and analysis, nor any participation in the preparation, review, and approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Supporting information
Table S1. Hazard ratios for the association between race and ADRD clinical diagnosis
ACKNOWLEDGMENTS
Dr Davis affirms that everyone who contributed significantly to the work are listed as a co‐author.
Davis MA, Lee KA, Harris M, et al. Time to dementia diagnosis by race: A retrospective cohort study. J Am Geriatr Soc. 2022;70(11):3250‐3259. doi: 10.1111/jgs.18078
Funding information Institute for Social Research, University of Michigan, Ann Arbor, MI; National Institute on Aging, Grant/Award Numbers: P01 AG019783, P30 AG024824, P30 AG053760, P30 AG066582, R01 AG053972, U01 AG009740; U.S. Social Security Administration
REFERENCES
- 1. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1786‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. US Department of Health and Human Services National Plan to Address Alzheimer's disease. Accessed April 15, 2021. https://aspe.hhs.gov/report/national-plan-address-alzheimers-disease-2020-update. Published 2020 update.
- 3. Dubois B, Padovani A, Scheltens P, Rossi A, Dell'Agnello G. Timely diagnosis for Alzheimer's disease: a literature review on benefits and challenges. J Alzheimers Dis. 2015;49(3):617‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lang L, Clifford A, Wei L, et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta‐analysis. BMJ Open. 2017;7(2):e011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review. JAMA. 2019;322(16):1589‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Y, Tysinger B, Crimmins E, Zissimopoulos JM. Analysis of dementia in the US population using Medicare claims: insights from linked survey and administrative claims data. Alzheimers Dement. 2019;5:197‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu Y, Chen Y, Crimmins E, Zissimopoulos JM. Sex, race, and age differences in prevalence of dementia in medicare claims and survey data. J Gerontol Series B. 2021;76(3):596‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin PJ, Daly A, Olchanski N, et al. Dementia diagnosis disparities by race and ethnicity. Alzheimers Dement. 2020;16(S10): e043183. [Google Scholar]
- 9. Lin PJ, Emerson J, Faul JD, et al. Racial and ethnic differences in knowledge about One's dementia status. J Am Geriatr Soc. 2020;68(8):1763‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of dementia: an observational study of patterns in diagnosis and awareness in US older adults. J Gen Intern Med. 2018;33(7):1131‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsoy E, Kiekhofer RE, Guterman EL, et al. Assessment of racial/ethnic disparities in timeliness and comprehensiveness of dementia diagnosis in California. JAMA Neurol. 2021;78:657‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lima JC, Ogarek J, Mor V. Untapped potential: using the HRS‐medicare‐linked files to study the changing nursing home population. Med Care. 2018;56(3):216‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brandt J, Spencer M, Folsetein M. The telephone interview for cognitive status. Neuropsychiat Neuropsychol Behav Neurol. 1988;1:111‐117. [Google Scholar]
- 14. Jorm AF. A short form of the informant questionnaire on cognitive decline in the elderly (IQCODE): development and cross‐validation. Psychol Med. 1994;24(1):145‐153. [DOI] [PubMed] [Google Scholar]
- 15. Gianattasio KZ, Wu Q, Glymour MM, Power MC. Comparison of methods for algorithmic classification of dementia status in the health and retirement study. Epidemiology. 2019;30(2):291‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gianattasio KZ, Ciarleglio A, Power MC. Development of algorithmic dementia ascertainment for racial/ethnic disparities research in the US health and retirement study. Epidemiology. 2020;31(1):126‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnes LL, Bennett DA. Alzheimer's disease in African Americans: risk factors and challenges for the future. Health Aff. 2014;33(4):580‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Law CG, Brookmeyer R. Effects of mid‐point imputation on the analysis of doubly censored data. Stat Med. 1992;11(12):1569‐1578. [DOI] [PubMed] [Google Scholar]
- 19. Taylor DH, Østbye T, Langa KM, Weir D, Plassman BL. The accuracy of medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17(4):807‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maust DT, Strominger J, Kim HM, et al. Prevalence of central nervous system–active polypharmacy among older adults with dementia in the US. JAMA. 2021;325(10):952‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor DH, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer's disease. J Clin Epidemiol. 2002;55(9):929‐937. [DOI] [PubMed] [Google Scholar]
- 22. Gianattasio KZ, Prather C, Glymour MM, Ciarleglio A, Power MC. Racial disparities and temporal trends in dementia misdiagnosis risk in the United States. Alzheimers Dement. 2019;5:891‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kotagal V, Langa KM, Plassman BL, et al. Factors associated with cognitive evaluations in the United States. Neurology. 2015;84(1):64‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Centers for Medicare & Medicaid Sevices . Berenson‐Eggers Type of Service (BETOS) Codes. Accessed February 15, 2021. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareFeeforSvcPartsAB/Downloads/BETOSDescCodes.pdf.
- 25. Hoffman GJ, Maust DT, Harris M, Ha J, Davis MA. Medicare spending associated with a dementia diagnosis among older adults. J Am Geriatr Soc. 2022;70(9):2592‐2601. doi: 10.1111/jgs.17835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levine DA, Gross AL, Briceno EM, et al. Sex differences in cognitive decline among US adults. JAMA Netw Open. 2021;4(2):e210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cylus J, Hartman M, Washington B, Andrews K, Catlin A. Pronounced gender and age differences are evident in personal health care spending per person. Health Aff (Project Hope). 2011;30(1):153‐160. [DOI] [PubMed] [Google Scholar]
- 28. Cooper C, Tandy AR, Balamurali TBS, Livingston G. A systematic review and meta‐analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry. 2010;18(3):193‐203. [DOI] [PubMed] [Google Scholar]
- 29. Ganz DA, Latham NK. Prevention of falls in community‐dwelling older adults. New Engl J Med. 2020;382(8):734‐743. [DOI] [PubMed] [Google Scholar]
- 30. Montero‐Odasso M, Speechley M. Falls in cognitively impaired older adults: implications for risk assessment and prevention. J Am Geriatr Soc. 2018;66(2):367‐375. [DOI] [PubMed] [Google Scholar]
- 31. Hoffman GJ, Hays RD, Wallace SP, Shapiro MF, Yakusheva O, Ettner SL. Receipt of caregiving and fall risk in U.S. community‐dwelling older adults. Med Care. 2017;55(4):371‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pietro GD, Chornokur G, Kumar NB, Davis C, Park JY. Racial differences in the diagnosis and treatment of prostate cancer. Int Neurourol J. 2016;20(Suppl 2):S112‐S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer‐specific survival by race and ethnicity in the United States. JAMA. 2015;313(2):165‐173. [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Yu F, Cai X, Caprio TV, Li Y. Functional outcome in home health: do racial and ethnic minority patients with dementia fare worse? PLoS One. 2020;15(5):e0233650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jha AK, Orav EJ, Li Z, Epstein AM. Concentration and quality of hospitals that care for elderly black patients. Arch Intern Med. 2007;167(11):1177‐1182. [DOI] [PubMed] [Google Scholar]
- 36. Jha AK, Orav EJ, Zheng J, Epstein AM. The characteristics and performance of hospitals that care for elderly Hispanic Americans. Health Aff. 2008;27(2):528‐537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Hazard ratios for the association between race and ADRD clinical diagnosis


