Abstract
Background:
Patients with indeterminate liver nodules, classified as LR-3 and 4 observations per Liver Imaging Reporting and Data System (LI-RADS), are at risk of developing hepatocellular carcinoma (HCC), but risk estimates remain imprecise.
Methods:
We conducted a systematic review of Ovid MEDLINE, EMBASE, and Cochrane databases from inception to December 2021 to identify cohort studies examining HCC incidence among patients with LR-3 or LR-4 observations on CT or MRI. Predictors of HCC were abstracted from each study, as available.
Results:
Of 13 total studies, 9 conducted LR-3 observation-level analyses, with the proportions of incident HCC ranging from 1.2%-12.5% at 12 months and 4.2%-44.4% during longer study follow-up. Among 3 studies with patient-level analyses, 8%-22.2% of patients with LR-3 lesions developed LR-4 observations and 11.1%-24.5% developed HCC. Among 9 studies conducting LR-4 observation-level analyses, incident HCC ranged from 30.8%-44.0% at 12 months and 30.9%-71.0% during study follow-up; conversely, 6%-42% of observations were downgraded to LR-3 or lower. Patient-level factors associated with HCC included older age, male sex, higher AFP levels, viral etiology, and prior history of HCC; observation-level factors included maximum diameter, threshold growth, T2 hyperintensity, and visibility on ultrasound. Studies were limited by small sample sizes, inclusion of patients with prior HCC, short follow-up duration, and failure to account for clustering of observations within patients or competing risks of transplantation and death.
Conclusion:
LR-3 and 4 observations have elevated but variable risk of HCC. Higher quality studies are necessary to identify high-risk patients who warrant close CT or MRI-based follow-up.
Keywords: liver cancer, LI-RADS, surveillance, hepatocellular carcinoma
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide and one of the fastest increasing causes of cancer-related death in the United States.1 2 Patients with early-stage HCC are eligible to curative therapies and have a median survival exceeding 10 years, whereas those with more advanced tumor burden have a median survival of only 2-3 years.3,4 This marked survival disparity underlies the rationale of HCC surveillance among at-risk patients, including those with cirrhosis.5
Professional society guidelines from the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) recommend surveillance using semi-annual ultrasound with or without alpha-fetoprotein (AFP), and those with positive surveillance results should undergo diagnostic evaluation with multi-phase CT or contrast-enhanced MRI.6,7 In 2011, the American College of Radiology developed a standardized Liver Imaging Reporting and Data System (LI-RADS) to describe liver observations on diagnostic CT or MRI in at-risk patients (i.e., cirrhosis and chronic HBV), with LI-RADS classifications ranging from LR-1 (definitely benign) to LR-5 (definitely HCC) based on several imaging features: liver nodule size, arterial phase hyperenhancement, and venous or delayed phase washout.8,9 LR-3 (intermediate probability for HCC) and LR-4 (suspicious for HCC) observations are commonly encountered in patients undergoing diagnostic imaging after positive surveillance tests and have an intermediate risk of developing HCC.10,11 In clinical practice, many patients with indeterminate nodules undergo surveillance every 3-6 months, rather than immediate biopsy, given potential sampling error in smaller lesions and risk of complications (e.g., bleeding, tumor seeding).12,13
A prior systematic review demonstrated that a substantial portion of LR-3 and LR-4 observations are in fact HCC at time of initial detection, but there is a need to better understand the natural history of these observations, particularly the risk of developing HCC during follow-up.11 The natural history of patients with intermediate- and high-risk observations has important diagnostic and therapeutic implications, including determining optimal follow-up strategies for these patients.13 There have been an increasing number of studies examining HCC risk over time for LR-3 and LR-4 observations, although small sizes of individual study cohorts have generated imprecise estimates. The objectives of our study were to: 1) characterize the natural history of LR-3 and LR-4 observations, including incidence of HCC and mean time to HCC diagnosis, and 2) describe predictors of progression to HCC, through a systematic review.
METHODS
Search Strategy
We conducted a computer-assisted search of Ovid MEDLINE, EMBASE, and Cochrane Central databases to identify relevant articles published from database inception through December 31, 2021 using the following keywords: (Liver imaging reporting and data system) or LIRADS or LI-RADS or LI-RAD$. We performed manual searches of reference lists to identify citations that may have been missed by the computer-assisted search. Finally, we consulted with expert hepatologists and radiologists to identify additional references or unpublished data. This study was conducted in accordance with Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines and registered with PROSPERO (International Prospective Register of Systematic Reviews) under ID CRD42022303833.
Study Selection
One investigator (MK) reviewed all citations retrieved from the search strategy to generate a list of potentially relevant articles. If the applicability of a study could not be determined by title or abstract alone, the full text was reviewed. Full texts were independently checked for possible inclusion by a second investigator (AGS) and disagreements were resolved through discussion.
Studies were included if they included patients with LR-3 or LR-4 observations and followed them longitudinally using CT or MRI for development of clinical outcomes of interest. Our primary outcome was development of HCC (LR-5); secondary outcomes included progression of LR-3 observations to LR-4, downgrading of LR-3 observations to LR-2 or lower, and downgrading of LR-4 observations to LR-3 or lower. Additional exclusion criteria included studies that relied on contrast-enhanced ultrasound, studies with non-human data, lack of primary data, non-English studies, and incomplete data for incident HCC during follow-up. If duplicate publications used the same cohort of patients, the study with more complete data was included.
Data Extraction and Quality Assessment
Two investigators (MK and AGS) independently extracted required information from eligible studies using standardized forms, with discrepancies resolved via discussion. The data extraction form included the following: study characteristics including study period and location, inclusion and exclusion criteria, characteristics and size of the cohort, LI-RADS version, imaging characteristics of liver observations, and duration of follow-up. We also recorded the following data: number of incident HCC during follow-up, imaging modality, median time to HCC development, predictors for development of HCC, and number of observations that progressed from LR-3 to LR-4. Patient-level data were collected when available, although most studies only reported results as observation-level data. Two investigators (MK and AGS) assessed study quality by a modified checklist based upon the National Institute of Health (NIH) study quality assessment tool for observational cohort studies.14
Statistical Analysis
For each study, we abstracted the proportion of patients who developed HCC or observations that progressed to HCC during follow-up. When available, we abstracted HCC incidence rates, although many studies reported proportions who developed HCC at specific timepoints from LR-3/LR-4 diagnosis during follow-up. Pooled proportions of HCC at set time points, e.g., one year, were calculated using the metaprop command, with random effects. Subset analyses were planned for the following predefined subsets of studies: 1) LR-3 vs. LR-4, 2) LI-RADS version, and 3) duration of follow-up. Data analysis were performed using Stata 11 (StataCorp, College Station, TX).
RESULTS
Study Characteristics
We identified 13 eligible studies, including 11 full texts and 2 abstracts.15–27 Four studies exclusively evaluated LR-3 observations, one evaluated LR-4 observations, and the remaining eight evaluated both LR-3 and LR-4 observations (Figure 1). Characteristics of studies are detailed in Table 1. All studies were retrospective in design, with median duration of follow-up ranging from 5 months to 33 months. Most studies were conducted in the United States, whereas two were from Canada, one Europe, and two from Asia. Most studies were conducted as observation-level analyses, with only three studies having conducted patient-level analyses. Seven studies exclusively evaluated patients with LR-3 or LR-4 observations on MRI at baseline, whereas 6 studies included patients with either MRI or CT. Only four studies included histologic confirmation as a component for HCC diagnosis, whereas the remaining studies relied on radiologic diagnosis.
Figure 1.
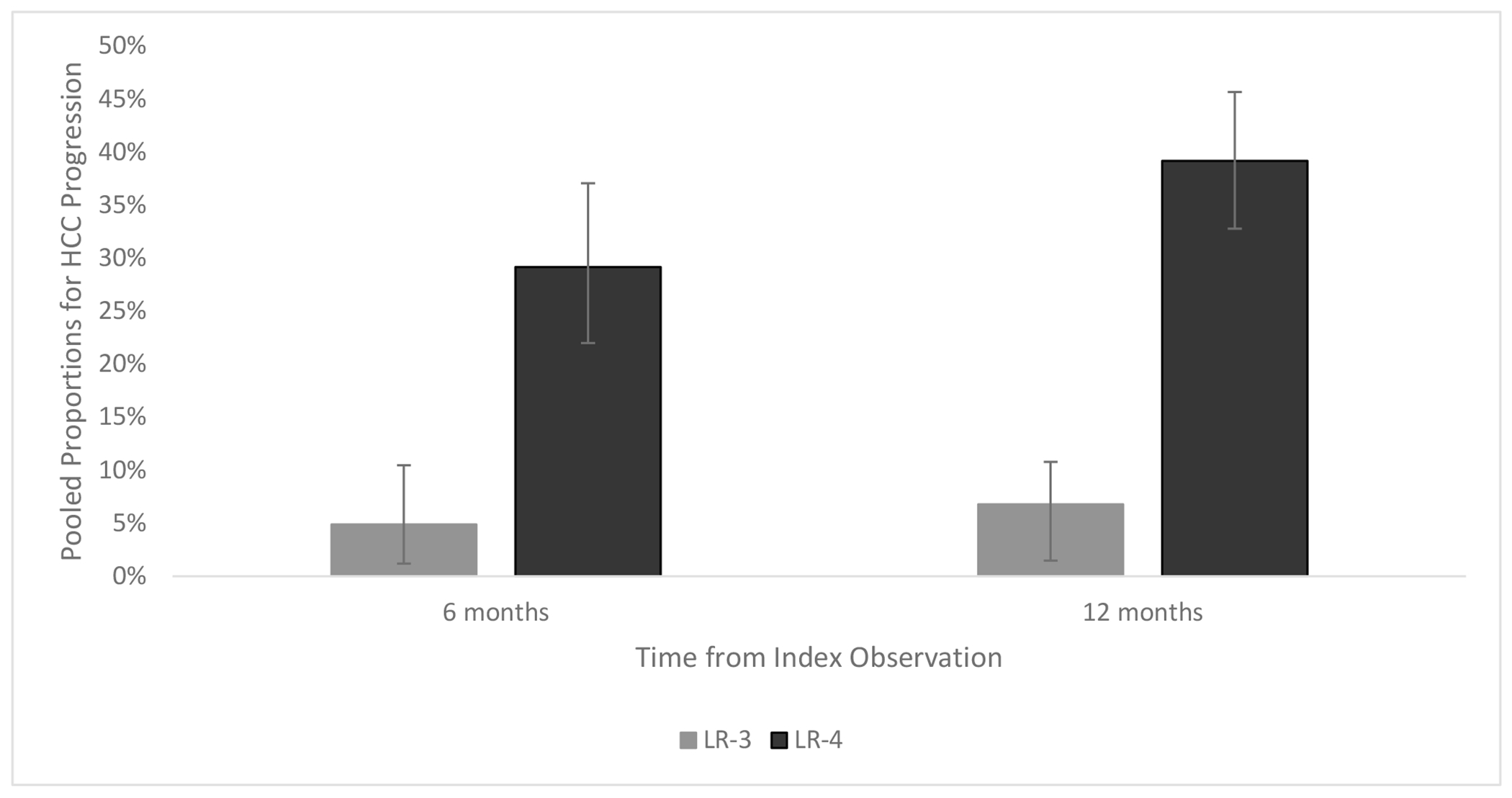
Pooled Proportions for LR-3 and LR-4 Progression to HCC at 6 months and 12 months
Table 1:
Characteristics of Included Studies
| Author Year | Study Location | Study Years | LIRADS version | % Cirrhosis | Initial Imaging Modality | Level of Analysis | LR Category | Number patients | Number lesions | Initial Observation Size | Duration follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Choi 2013 | USA | 2008-2011 | 2011 | 100% | MRI | lesion | 3 | 52 | 69 | 0.8 cm (mean) | 11.2 (mean) |
| Tang 2019 | Canada | 2009-2015 | 2011 | 100% | CT/MRI | lesion | 3 | NR | 53 | 1.9 cm (mean) | 6.5 (median) |
| 4 | 37 | ||||||||||
| Ojeda 2021 | USA | 2013-2017 | 2011 - 2014 | 100% | CT/MRI | patient | 3 | 212 | NR | NR | 9.0 (median) |
| Sofue 2017 | USA | 2010-2013 | 2014 | 100% | MRI | lesion | 4 | 139 | 181 | 1.6 cm | 5.1 (median) |
| Tanabe 2016 | USA | 2011-2015 | 2014 | 98.70% | CT/MRI | lesion | 3 | NR | 166 | 1.0 cm (mean) | 17.7 (mean) |
| 4 | 152 | ||||||||||
| Hong 2019 | USA | 2014-2017 | 2014 | NR | CT/MRI | lesion | 3 | NR | 186 | NR | 7.5 (median) |
| 4 | 133 | ||||||||||
| Arvind 2022 | USA | 2015-2018 | 2014 | 100% | CT/MRI | patient | 3 | 458 | 648 | 1 cm (median) | 17.9 (median) |
| Agnello 2020 | Italy | 2013-2018 | 2018 | 100% | MRI | lesion | 3 | 34 | 55 | 35% <1 cm | 33.6 (mean) |
| 4 | 15 | 19 | 53% <1 cm | ||||||||
| Kim 2020 | S. Korea | 2014-2015 | 2018 | 85% | MRI | lesion | 3 | NR | 38 | 45% <1 cm | 13.5 (median) |
| 4 | 68 | 43% <1 cm | |||||||||
| Smereka 2020 | USA | 2008-2018 | 2018 | NR | MRI | lesion | 3 | NR | 257 | 1.2 cm | 13.8 (mean) |
| 4 | 83 | ||||||||||
| Ranathunga 2021 | Canada | 2018-2020 | 2018 | 85.7% | MRI | patient | 3 | 36 | 36 | 1.3 cm (mean) | 17.8 (mean) |
| Tang 2017 | USA | NR | NR | 100% | CT/MRI | lesion | 3 | NR | 57 | NR | NR |
| 4 | 38 | ||||||||||
| Xing 2018 | China | 2012-2017 | NR | 100% | MRI | lesion | 3 | NR | 71 | NR | 16.3 (mean) |
| 4 | 32 | 12.4 (mean) |
NR = not reported
Clinical Outcomes of Patients with LR-3 Observations
Of the 12 studies evaluating clinical outcomes in patients with LR-3 observations, 9 were observation-level (n=952 total observations) and 3 were patient-level (n=706 total patients) analyses. (Table 2) Among studies with observation-level analyses, the proportion of LR-3 observations that progressed to HCC ranged from 0% to 30.2% (pooled proportion 4.9%, 95%CI 1.2 – 10.5%; I2=89%), at 6 months, 1.2% to 12.5% (pooled proportion 6.8%, 95%CI 2.8 – 12.1%; I2=84%) at 12 months, and 4.2% to 44.4% over the study periods, which ranged from 1 to 10 years. An additional 1.4% to 17.7% of observations were upgraded from LR-3 to LR-4, whereas 16% to 68% were downgraded to LR-2 or lower. Few studies reported details of why lesions were upgraded to LR-4 (e.g., growth, ancillary features) or downgraded (e.g., stability, change in appearance, no longer seen), although two studies reported ancillary features as a common reason for change in category. The proportion of patients who developed HCC at 6 and 12 months (7% and 11%, respectively) was consistent in the one observation-level study that relied on reports instead of independent review of imaging. Among the four studies using LI-RADS v2018, 11.1% to 44.4% of LR-3 observations developed HCC, 5.5% to 22.2% of observations were upgraded to LR-4, and 25% to 52% were downgraded to LR2 or lower during study follow-up.
Table 2:
Clinical outcomes, stratified by LR-3 vs. LR-4 category
| 8h | Number of Observations | HCC at 6 months (%) | HCC at 12 months (%) | Total Proportion with HCC | Total Proportion with LR4 | Median time to HCC (months) | % Downgraded |
|---|---|---|---|---|---|---|---|
| LR-3 Observations | |||||||
| Choi 2013 | 69 | 1.4% | 4.3% | 4.3% | 1.4% | 16.0% | |
| Tang 2019 | 53 | 30.2% | 32.1% | 6.1 (IQR 4.6 – 13.1) | |||
| Ojeda 2021 | 20.4% | 24.5% | 8.0% | 6.4 | |||
| Tanabe 2016 | 166 | 0% | 1.2% | 4.2% | 4.8% | 68.0% | |
| Hong 2019 | 186 | 7.0% | 11.0% | 8.6% | 17.7% | 16.0% | |
| Arvind 2022 | 458 | 5.1% | 12.5% | 24.1% | |||
| Agnello 2020 | 55 | 1.8% | 9.1% | 30.9% | 5.5% | 27.2 (range 5.3-56.1) | 52.0% |
| Kim 2020 | 38 | 5.3% | 4.4% | 15.8% | |||
| Smereka 2020 | 257 | 44.4% | |||||
| Ranathunga 2022 | 36 | 11.1% | 22.2% | 25.0 – 30.6% | |||
| Tang 2017 | 57 | 36.5% | 45.9 | ||||
| Xing 2018 | 71 | 18.3% | 8.5% | 25.4% | |||
| LR-4 Observations | |||||||
| Tang 2019 | 37 | 56.8% | N/A | 4.7 (IQR 2.5 – 14.5) | |||
| Sofue 2017 | 181 | 30.9% | N/A | 5.1 | 42.0% | ||
| Tanabe 2016 | 52 | 28.8% | 30.8% | 36.5% | N/A | 17.0% | |
| Hong 2019 | 133 | 32.0% | 44.0% | 33.1% | N/A | 20.0% | |
| Agnello 2020 | 19 | 10.5% | 31.6% | 42.1% | N/A | 19.4 (range 3.2 - 65.5) | 37.0% |
| Kim 2020 | 68 | 35.3% | 39.7% | 54.4% | N/A | ||
| Smereka 2020 | 83 | 69.9% | N/A | ||||
| Tang 2017 | 38 | 71.0% | N/A | 10.9 | |||
| Xing 2018 | 32 | 46.9% | N/A | 6.3% | |||
Among the 3 studies that conducted patient-level analyses, Arvind and colleagues reported 5.1% and 12.5% of patients with LR-3 observations developed HCC at 6 and 12 months, respectively, with an HCC incidence rate of 84 per 1000 person-years.16 Similarly, Ojeda et al. reported 20.4% of patients developed HCC within one year of LR-3 observation diagnosis.20 Two of the patient-level studies relied on imaging reports, with Ranathunga et al the only to independently review imaging studies. The study by Ranathunga and colleagues was also the only study to perform patient-level analysis using LI-RADS v2018 and reported 11.1% of 36 patients developed HCC over the mean follow-up 17.8-month study period.21 Across all three studies, the proportion of patients who developed HCC ranged from 11.1% to 24.5%, and an additional 8% to 22.2% developed an LR-4 observation during median follow up of 9 to 17.9 months .
Clinical Outcomes of Patients with LR-4 Observations
The 9 studies evaluating outcomes in patients with LR-4 observations were all observation-level (n=743 total observations) analyses (Table 2). The proportion of LR-4 observations that progressed to HCC ranged from 10.5% to 35.3% (pooled proportion 29.2%; 95%CI 22.0 – 37.1%; I2=38%) at 6 months, 30.8% to 44.0% at 12 months (pooled proportion 39.2%, 95%CI 32.9 – 45.7%; I2=10%), and 30.9% to 71.0% over the entire study periods of 1 to 10 years, respectively. However, 6.0% to 42.0% of LR-4 observations were downgraded to LR-3 or lower. The proportion of patients who developed HCC at 6 and 12 months (32% and 44%, respectively) was consistent in the one observation-level study that relied on reports instead of independent review of imaging. Three studies assessed outcomes using LI-RADS v2018, with 10.5% to 35.3% of observations developing HCC within 6 months and 42.1% to 69.9% developing HCC during the study period.
Predictors of HCC Development
Seven studies reported patient- or observation-level factors associated with development of HCC in LR-3 or LR-4 observations (Table 3). Patient-level factors associated with HCC included older age, male sex, higher AFP levels, viral liver disease etiology, and history of HCC, although these were not consistently reported across all studies. Observation-level risk factors included maximum diameter of the LR-3 or LR-4 observation >1cm, threshold growth, T2 hyperintensity, and visibility on ultrasound; however, several studies failed to find any significant associations. No studies evaluated the association between liver dysfunction and incident HCC among LR-3 or LR-4 observations.
Table 3:
NIH quality assessment of studies
| Author Year | Study Population Representative | Sample Size Justified | Sufficient time frame* | Exposure valid and reliable** | Outcome valid and reliable | Loss to follow-up ≤20% | Assessed Confounders |
|---|---|---|---|---|---|---|---|
| Choi 2013 | Yes | No | No | Yes | Yes | Yes | No |
| Tang 2019 | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Ojeda 2021 | Yes | No | Yes | No | Yes | Yes | Yes |
| Sofue 2017 | Yes | No | No | Yes | Yes | Yes | Yes |
| Tanabe 2016 | Yes | No | Yes | Yes | Yes | Yes | No |
| Hong 2019 | Yes | No | Yes | No | Yes | Yes | Yes |
| Arvind 2022 | Yes | No | Yes | No | Yes | No | Yes |
| Agnello 2020 | Yes | No | Yes | Yes | Yes | Yes | No |
| Kim 2020 | Yes | No | Yes | Yes | Yes | Yes | No |
| Smereka 2020 | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Ranathunga 2022 | Yes | No | Yes | Yes | Yes | Yes | No |
| Tang 2017 | Yes | No | Yes | Yes | Yes | NR | Yes |
| Xing 2018 | Yes | No | Yes | Yes | Yes | Yes | No |
Sufficient timeframe >12 months
Independent Review conducted on imaging for LIRADS categorization
All studies defined the research objective, defined the study population, measured exposure prior to outcome ascertainment.
Questions regarding differential exposure, assessing exposure more than once over time, and at least 50% participation were not applicable.
Quality Assessment
Details of the quality assessment are reported in Table 4. All studies defined the research objective, defined the study population, and measured the exposure prior to outcome ascertainment. All but three studies used independent review of imaging to assess LR-3 or LR-4 diagnosis, increasing rigor of exposure assessment; however, most did not require multiple readers and three studies relied on radiology reports rather than blinded re-interpretation. Six studies allowed use of CT or MR imaging for index imaging to define LR-3 or LR-4 category, although the two modalities differ in test performance, particularly when considering ancillary features that can be used to upgrade or downgrade LI-RADS categorization. Further, at least three studies included patients with a history of HCC, with some (e.g., Agnello et al) having over 50% with a history of HCC, whereas others did not specifically remark on inclusion or exclusion of these patients. Inclusion of these patients may affect reported HCC risk and limit generalizability to patients without a history of HCC. Lack of individual patient-level data or subgroup analyses among studies precluded analyses among those without a history of HCC.
Table 4:
Factors associated with development of HCC in patients with LR-3 or LR-4 observations.
| Author Year | Patient-level factors | Observation-level factors |
|---|---|---|
| Tang 2019 | Age, per 10-year increase (OR 1.76, 95%CI 1.00–3.07) | Diameter >2cm (OR 21.69, 95% CI 5.36–87.74) Delayed washout (OR 5.34, 95% CI 1.90–15.03) |
| Ojeda 2021 | Male sex (HR: 4.27, 95% CI 1.75-9.27) Age, per 10-year increase (HR: 1.49, 95% CI 1.03-1.91) |
|
| Sofue 2017 | Hepatitis C etiology (HR 1.69, 95%CI 1.07–2.75) | Threshold growth (HR 3.71, 95% CI 1.51–8.75) Mild-to-moderate T2 hyperintensity (HR 1.84, 95% CI 1.22–2.76) |
| Hong 2019 | Follow-up duration, per year (OR = 1.77, 95%CI 1.06-2.95) | |
| Arvind 2022 | Age >60 years (HR 1.82, 95% CI 1.06-3.13) Male sex (HR 1.83, 95% CI 1.02-3.30) AFP >10 ng/mL (HR 3.11, 95% CI 1.80-5.36) |
LR-3 diameter ≥1.0 cm (HR 1.86; 95%CI 1.05-3.31) |
| Agnello 2020 | LR3: diameter ≥ 1 cm (OR 6.07; 95%CI 0.12-60.28) LR4: diameter ≥ 1 cm (OR 8.95, 95%CI 0.73 – 111.8) |
|
| Smereka 2020 | Viral liver disease etiology (p = 0.01) Presence of HCC (p = 0.02) LI-RADS v2017 vs. 2018 (p=0.04) |
Mild-to-moderate T2 hyperintensity (p<0.001) |
| Tang 2017 | Male sex Liver disease etiology |
LI-RADS major features (arterial phase hyperenhancement, delayed washout, presence of pseudocapsule) Interval growth Lesions visible on US |
All studies were retrospective with convenience samples, without justification of sample size or power calculations. Many studies were small, with less than 100 patients, creating imprecise estimates for each individual study and precluding identification of risk factors for progression. Five studies had a median follow-up time shorter than 12 months and one study did not report length of follow-up, limiting the ability to determine incident HCC risk in LR-3 or LR-4 observations in these studies. This is particularly noteworthy as Hong et al. reported follow-up duration was associated with risk of incident HCC. However, most studies required at least one follow-up imaging study for study inclusion with Arvind and colleagues being the only study to include all-comers with LR-3 observations, including one-third of patients failing to have any follow-up imaging. All studies had valid assessment of HCC status, although there was a risk of ascertainment bias given biopsy was not routinely performed in observations that failed to meet imaging criteria for HCC diagnosis. Further, most studies examined risk of progression to HCC within LR-3 or LR-4 observations, with only three conducting patient-level analyses and accounting for HCC development elsewhere in the liver. Most studies failed to report the proportion of patients who underwent liver transplantation or died during follow-up and did not account for these competing risks when estimating HCC incidence rates. Further, the LI-RADS criteria and performance have evolved over time; LI-RADS v2011 and v2014 classified observations between 1-2 cm with arterial phase hyperenhancement and only delayed washout (i.e. without capsule, threshold growth, or antecedent ultrasound visibility) as LR-4 despite meeting AASLD criteria for HCC.28 Finally, observation-level analyses in most studies failed to account for clustering within patients, which is necessary given patient-level factors such as older age, male sex and elevated AFP were commonly reported risk factors for development of HCC.
DISCUSSION
LR-3 and LR-4 observations are commonly detected in patients undergoing HCC surveillance. Understanding their risk of developing HCC is of critical importance to guide decision-making for management and follow-up. The 12 studies evaluating LR-3 observations found a wide range in observation-level HCC risk, from 15.8% to 44.4%; patient-level risk ranged from 11.1% to 24.5% across studies. Similarly, the 9 studies evaluating LR-4 observations found a wide range in observation-level HCC risk, from 30.9% to 71.0%. Studies have identified several factors associated with HCC risk including older age, male sex, viral liver disease etiology, history of HCC, and maximum diameter of the observation.
Available data highlight several critical points that directly inform surveillance recommendations. First, LR-3 and LR-4 observations are both associated with a high risk of HCC, particularly within the first six months after detection, suggesting prevalent HCC at time of LR-3 or LR-4 detection. These data suggest that biopsy should be considered for patients in whom an HCC diagnosis would immediately impact clinical management. One such example would be a patient with a liver observation exceeding 2 cm in diameter, in whom an HCC diagnosis could warrant liver transplantation with MELD exception points. Second, we found that risk of HCC extends beyond the first six months, suggesting these patients remain at higher risk of HCC than cirrhosis patients without LR-3 or LR-4 observations. These data underscore the importance of continued close follow-up for these patients, with some of these patients potentially warranting CT or MR imaging.29 Third, although some of these observations have a high risk of HCC, several others were downgraded during follow-up. The variation in natural history likely parallels the heterogeneity in what these observations represent. A single-center study examining histopathologic correlates of radiographic LR-3 and LR-4 observations ranged from perfusion abnormalities or regenerative nodules to dysplastic nodules or HCC.30 Finally, traditional risk factors such as older age, male sex, viral liver disease, and larger observation size are inconsistently associated with HCC and imperfect to accurately risk stratify patients and inform surveillance strategies. While AFP has been shown to be an early detection and prognostic marker for HCC31, most studies did not include AFP in their multivariable model. There is a clear need for future studies to examine blood- and imaging-based biomarkers that could further identify which patients are at highest risk of HCC and warrant more intensive monitoring versus those who are lower risk and can be safely monitored by ultrasound-based surveillance. Radiomics has been a field of growing interest for tumor detection and prognostication, and algorithms incorporating features such as T2 hyperintensity and restricted diffusion on MRI may be able to identify observations with higher HCC risk.32 Similarly, blood-based biomarkers are undergoing validation for risk stratification among patients with cirrhosis and should be tested in patients with LR-3 or LR-4 observations.33
Despite these important clinical implications, we identified notable limitations of the current literature, highlighting a need for more robust data in this area (Table 5). Understanding these limitations can inform recommendations for future studies on this topic. First, most studies were retrospective in nature with variable surveillance strategies, including differences in imaging tests, surveillance intervals, and use of biopsy. Indeed, Arvind and colleagues highlighted that many patients with LR-3 observations failed to undergo surveillance CT or MR imaging and few underwent biopsy.16 Lack of standardized evaluation, compounded by short study intervals and competing risks in this population, creates a high risk of ascertainment bias, so HCC risk estimates are likely underestimated. There is a need for future prospective data among patients with LR-3 or LR-4 observations using a standardized protocol or CT or MR imaging and biopsy as needed to better characterize natural history. Second, study populations were heterogeneous, and several studies did not explicitly exclude patients with a history of HCC. The histopathologic character of the LR-3 or LR-4 observation, and corresponding HCC risk, may differ based on presence of absence of HCC elsewhere in the liver. LR-3 and LR-4 lesions are more likely to be HCC in patients with existing LR-5 lesions.34 Further, it would be difficult to differentiate progression of an LR-3/LR-4 observation from HCC recurrence, given the HCC may occur within or outside the index observation. Indeed, prior history of HCC was one of the factors associated with future HCC risk across studies.22 Therefore, future studies should either exclude patients with a history of HCC or reporting findings stratified by HCC history status. Third, few studies, even those using independent review of imaging, required multiple readers to determine LR-3 or LR-4 status, which is concerning given the suboptimal inter-rater reliability of LI-RADS assessment.35 This was also compounded by inclusion of both CT and MR imaging, which differ in their ability to assess ancillary findings and distinguish between LR-3 or LR-4 observations.36 Further, changes in LI-RADS criteria over time due to version updates (e.g., observations 1-2 cm with arterial phase hyperenhancement and delayed washout being classified as LR-4 vs. LR-5) can complicate interpretation of these data. Future studies should use independent review of imaging by multiple readers and stratify results by CT vs. MR imaging and imaging features, as possible. Finally, most studies to date have been observation-level analyses, which fail to adjust for clustering of the observations within patients. Further, patient-level analyses more closely parallel management strategies where cost effectiveness of surveillance strategies would be informed by overall patient risk rather than each individual observation. Patient-level analyses are also more accurate given HCC may develop outside of the LR-3/LR-4 observation, occurring in nearly one-third of patients in prior studies.16
Table 5:
Recommendations for future studies evaluating LR-3 or LR-4 natural history
| Study Quality Limitations | Recommendations for Future Studies |
|---|---|
| Lack of independent review of imaging and reliance on pre-existing imaging reports | Independently review imaging using multiple readers to account for inter-reader variability |
| Variation in initial and follow-up imaging modality (CT vs MRI) | Use the same imaging modality during follow-up in prospective cohorts or report results stratified by CT vs MRI |
| Heterogenous patient population, including some patients with a history of HCC may affect reported HCC risk | Exclude patients with prior risk of HCC, or report results stratified by HCC history status |
| Short and variable follow-up | Consistent follow-up time points and longer follow-up of at least one year after index LR-3 or LR-4 observation |
| Ascertainment bias based on variable follow-up imaging | Prospective studies with standardized surveillance strategies for patients with LR-3 and LR-4 lesions |
| Inconsistent outcomes, precluding pooling of result | Consistently report outcomes of interest including proportion of incident HCC and downgrading at set time points (e.g., 6 and 12 months) as well as annual HCC incidence rates |
| Observation-level analysis fails to adjust for clustering of lesions within patients | Perform patient-level analysis to account for HCC development in separate locations in the liver |
| Failure to account for liver transplantation or death | Conduct competing risk analysis with liver transplantation and death as competing outcomes |
| Lack of data on predictors for HCC or downgrading, with inconsistent use of multivariable models | Consistently report risk factors for HCC development and risk factors for downgrading |
While our study has several strengths, there are several inherent limitations, primarily due to the limitations of the included studies. First, clinical heterogeneity in LI-RADS assessments, surveillance strategies, and duration of follow-up precluded rigorous pooled results. In addition to addressing specific study-level limitations as discussed above, future studies should consistently report desired outcomes including proportion of HCC at set time points (e.g., 6 and 12 months), annual HCC incidence rates, and proportion of LR-3 and LR-4 observations that were downgraded to more benign LI-RADS classifications during follow-up. The reason for downgrade of LR-3 and LR-4 lesions, such as poor arterial phase contrast timing, was not mentioned in most studies, and should also be further explored. Second, we performed a study-level instead of individual patient-level meta-analysis, so we could not perform some subgroup analyses of interest, such as HCC risk in patients without a history of prior HCC. Additionally, variation between MRI and CT imaging modality was present, raising the concern that LR-3 or 4 on CT does not necessarily correlate with LR-3 or 4 on MRI.37 Third, included studies focused on progression to HCC as the primary outcome and did not report on other clinical outcomes (e.g., tumor stage or mortality). Despite these limitations, we believe our systematic review provides valuable insights into currently available literature on risk of incident HCC in LR-3 or LR-4 observations, given prior meta-analyses have largely focused on risk of prevalent HCC at time of LR-3 or LR-4 detection. In that regard, our systematic review and meta-analysis did not have any overlapping studies from the prior systematic review by Van der Pol and colleagues.11 Further, our study critically evaluated the existing literature and provided recommendations for future research.
In summary, we found that some patients with LR-3 and LR-4 observations have a high risk of developing HCC, whereas others are downgraded to more benign categories during follow-up. This variability in natural history underscores a need for risk stratification tools to better identify patients in need of more intensive surveillance strategies. Given limitations in study quality for current studies, there is a critical need for robust prospective studies on this topic.
Supplementary Material
Financial Source:
This study was conducted with support from NIH U01 CA230694, U01 CA23099, and R01 CA212008. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding agencies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript.
Conflict of Interest:
Neehar Parikh has served as a consultant or on advisory boards for Bayer, Eisai, Bristol-Myers Squibb, Exelixis, Eli Lilly, Wako Diagnostics, Exact Sciences, and Freenome. He has received research funding from Exact Sciences, Glycotest, Bayer, and Target Pharmasolutions.
Jorge Marrero has served as a consultant for Glycotest.
Takeshi Yokoo receives research support from Siemens Healthineers, Guerbet, Bracco, Bayer, and GE.
None of the other authors have any relevant conflicts of interest.
Nicole Rich has served as a consultant for AstraZeneca.
Amit Singal has served as a consultant or on advisory boards for Bayer, Wako Diagnostics, Exact Sciences, Roche, Glycotest, and GRAIL.
Data Sharing:
Data collection forms, extracted data, and other materials are available upon request to corresponding author.
REFERENCES
- 1.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. January 21 2021;7(1):6. doi: 10.1038/s41572-020-00240-3 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. Jan 2022;72(1):7–33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 3.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. Apr 2014;11(4):e1001624. doi: 10.1371/journal.pmed.1001624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris PS, Hansen RM, Gray ME, Massoud OI, McGuire BM, Shoreibah MG. Hepatocellular carcinoma surveillance: An evidence-based approach. World J Gastroenterol. Apr 07 2019;25(13):1550–1559. doi: 10.3748/wjg.v25.i13.1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singal AG, Zhang E, Narasimman M, et al. HCC Surveillance Improves Early Detection, Curative Treatment Receipt, and Survival in Patients with Cirrhosis: A Systematic Review and Meta-Analysis. J Hepatol. Feb 06 2022;doi: 10.1016/j.jhep.2022.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.easloffice@easloffice.eu EAftSotLEa, Liver EAftSot. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. July 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 7.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. January 2018;67(1):358–380. doi: 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 8.Elsayes KM, Kielar AZ, Chernyak V, et al. LI-RADS: A conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. Review. Journal of Hepatocellular Carcinoma. 2019;6:49–69. doi: 10.2147/JHC.S186239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunha GM, Sirlin CB, Fowler KJ. Imaging diagnosis of hepatocellular carcinoma: LI-RADS. Review. Chinese Clinical Oncology. 2020;9(3)doi: 10.21037/cco-20-107 [DOI] [PubMed] [Google Scholar]
- 10.Tang A, Singal AG, Mitchell DG, et al. Introduction to the Liver Imaging Reporting and Data System for Hepatocellular Carcinoma. Review. Clinical Gastroenterology and Hepatology. June 2019;17(7):1228–1238. doi: 10.1016/j.cgh.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 11.van der Pol CB, Lim CS, Sirlin CB, et al. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy-A Systematic Review. Gastroenterology. March 2019;156(4):976–986. doi: 10.1053/j.gastro.2018.11.020 [DOI] [PubMed] [Google Scholar]
- 12.Dahdaleh FS, Naffouje SA, Sherman SK, Kamarajah SK, Salti GI. Tissue Diagnosis Is Associated With Worse Survival in Hepatocellular Carcinoma: A National Cancer Database Analysis. Am Surg. Apr 08 2021:3134821991983. doi: 10.1177/0003134821991983 [DOI] [PubMed] [Google Scholar]
- 13.Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. February 2020;72(2):250–261. doi: 10.1016/j.jhep.2019.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NIH. Study Quality Assessment Tools. 2022. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 15.Agnello F, Albano D, Sparacia G, et al. Outcome of LR-3 and LR-4 observations without arterial phase hyperenhancement at Gd-EOB-DTPA-enhanced MRI follow-up. Clinical Imaging. December 2020;68:169–174. doi: 10.1016/j.clinimag.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 16.Arvind A, Joshi S, Zaki T, et al. Risk of Hepatocellular Carcinoma in Patients with Indeterminate (LI-RADS 3) Liver Observations. Clinical Gastroenterology & Hepatology. Dec 10 2021;10:10. doi: 10.1016/j.cgh.2021.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JY, Cho HC, Sun M, Kim HC, Sirlin CB. Indeterminate observations (Liver Imaging Reporting and Data System category 3) on MRI in the cirrhotic liver: Fate and clinical implications. American Journal of Roentgenology. November 2013;201(5):993–1001. doi: 10.2214/AJR.12.10007 [DOI] [PubMed] [Google Scholar]
- 18.Hong CW, Park CC, Mamidipalli A, et al. Longitudinal evolution of CT and MRI LI-RADS v2014 category 1, 2, 3, and 4 observations. European Radiology. 01 Sep 2019;29(9):5073–5081. doi: 10.1007/s00330-019-06058-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YY, Choi JY, Kim SU, et al. MRI Ancillary Features for LI-RADS Category 3 and 4 Observations: Improved Categorization to Indicate the Risk of Hepatic Malignancy. American Journal of Roentgenology. December 2020;215(6):1354–1362. doi: 10.2214/AJR.20.22802 [DOI] [PubMed] [Google Scholar]
- 20.Ojeda PI, Hannan LM, Mieloszyk RJ, et al. Is There a Difference Between LI-RADS 3 to LI-RADS 5 Progression Assessment Using CT Versus MR? A Retrospective, Single-Center, Longitudinal Study of Patients Who Underwent 5082 Radiologic Examinations for Surveillance of Hepatocellular Carcinoma Over a 43-Month Period. Current Problems in Diagnostic Radiology. 2021;doi: 10.1067/j.cpradiol.2021.03.016 [DOI] [PubMed] [Google Scholar]
- 21.Ranathunga D, Osman H, Islam N, et al. Progression Rates of LR-2 and LR-3 Observations on MRI to Higher LI-RADS Categories in Patients at High Risk for Hepatocellular Carcinoma: A Retrospective Study. AJR American journal of roentgenology. 2021;13doi: 10.2214/AJR.21.26376 [DOI] [PubMed] [Google Scholar]
- 22.Smereka P, Doshi AM, Lavelle LP, Shanbhogue K. New arterial phase enhancing nodules on MRI of cirrhotic liver: Risk of progression to hepatocellular carcinoma and implications for LI-RADS classification. American Journal of Roentgenology. August 2020;215(2):382–389. doi: 10.2214/AJR.19.22033 [DOI] [PubMed] [Google Scholar]
- 23.Tanabe M, Kanki A, Wolfson T, et al. Imaging outcomes of liver imaging reporting and data system version 2014 category 2, 3, and 4 observations detected at CT and MR imaging. Radiology. October 2016;281(1):129–139. doi: 10.1148/radiol.2016152173 [DOI] [PubMed] [Google Scholar]
- 24.Tang E, Hall G, Yu D, Hopman W, Menard A, Nanji S. Retrospective analysis of lirads observations: Correlation with clinical and pathological outcomes. Conference Abstract. Hpb 2017;19(Supplement 1):S141. [Google Scholar]
- 25.Tang EST, Hall G, Yu D, Menard A, Hopman W, Nanji S. Predictors and Cumulative Frequency of Hepatocellular Carcinoma in High and Intermediate LI-RADS Lesions: A Cohort Study from a Canadian Academic Institution. Annals of Surgical Oncology. 15 Aug 2019;26(8):2560–2567. doi: 10.1245/s10434-019-07386-9 [DOI] [PubMed] [Google Scholar]
- 26.Xing F, Lu J, Zhang T, Miao X, Zhang X, Jiang J. Category modifications and prognosis of cirrhotic nodules depending on MRI imaging report and data system of LR-2, LR-3 and LR-4. [Chinese]. Mrilr-2, lr-3, lr-4. Chinese Journal of Radiology (China). 10 Apr 2018;52(4):272–276. doi: 10.3760/cma.j.issn.1005-1201.2018.04.007 [DOI] [Google Scholar]
- 27.Sofue K, Burke LMB, Nilmini V, et al. Liver imaging reporting and data system category 4 observations in MRI: Risk factors predicting upgrade to category 5. Journal of Magnetic Resonance Imaging. September 2017;46(3):783–792. doi: 10.1002/jmri.25627 [DOI] [PubMed] [Google Scholar]
- 28.Kierans AS, Song C, Gavlin A, et al. Diagnostic Performance of LI-RADS Version 2018, LI-RADS Version 2017, and OPTN Criteria for Hepatocellular Carcinoma. AJR Am J Roentgenol. November 2020;215(5):1085–1092. doi: 10.2214/AJR.20.22772 [DOI] [PubMed] [Google Scholar]
- 29.Tzartzeva K, Obi J, Rich NE, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. May 2018;154(6):1706–1718.e1. doi: 10.1053/j.gastro.2018.01.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn C, Lin B, Rich NE, Patel MS, Gopal P, Singal A. Correlation of LI-RADS 3 or 4 Observations with Histopathologic Diagnosis in Patients with Cirrhosis. Clin Gastroenterol Hepatol. 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parikh ND, Mehta AS, Singal AG, Block T, Marrero JA, Lok AS. Biomarkers for the Early Detection of Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev. December 2020;29(12):2495–2503. doi: 10.1158/1055-9965.EPI-20-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harding-Theobald E, Louissaint J, Maraj B, et al. Systematic review: radiomics for the diagnosis and prognosis of hepatocellular carcinoma. Aliment Pharmacol Ther. October 2021;54(7):890–901. doi: 10.1111/apt.16563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujiwara N, Kobayashi M, Fobar AJ, et al. A blood-based prognostic liver secretome signature and long-term hepatocellular carcinoma risk in advanced liver fibrosis. Med (N Y). Jul 09 2021;2(7):836–850.e10. doi: 10.1016/j.medj.2021.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee TH, Hirshman N, Cardona DM, et al. LR-3 and LR-4 Lesions Are More Likely to Be Hepatocellular Carcinoma in Transplant Patients with LR-5 or LR-TR Lesions. Dig Dis Sci. Mar 07 2022;doi: 10.1007/s10620-022-07428-5 [DOI] [PubMed] [Google Scholar]
- 35.Fowler KJ, Tang A, Santillan C, et al. Interreader Reliability of LI-RADS Version 2014 Algorithm and Imaging Features for Diagnosis of Hepatocellular Carcinoma: A Large International Multireader Study. Radiology. January 2018;286(1):173–185. doi: 10.1148/radiol.2017170376 [DOI] [PubMed] [Google Scholar]
- 36.Basha MAA, Alazzazy MZ, Ahmed AF, et al. Does a combined ct and mri protocol enhance the diagnostic efficacy of li-rads in the categorization of hepatic observations? A prospective comparative study. European Radiology. 24 Jan 2018;28(6):2592–2603. doi: 10.1007/s00330-017-5232-y [DOI] [PubMed] [Google Scholar]
- 37.Kim BR, Lee JM, Lee DH, et al. Diagnostic performance of gadoxetic acid-enhanced liver MR imaging versus multidetector CT in the detection of dysplastic nodules and early hepatocellular carcinoma. Radiology. October 2017;285(1):134–146. doi: 10.1148/radiol.2017162080 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collection forms, extracted data, and other materials are available upon request to corresponding author.


