Abstract
Iron deficiency and/or iron deficiency anemia complicate nearly 50% of pregnancies globally, negatively impacting both maternal and fetal outcomes. Iron deficiency can cause a range of symptoms that range from aggravating to debilitating including fatigue, poor quality of life, pagophagia and restless leg syndrome. Iron deficiency and iron deficiency anemia are also associated with maternal complications including preterm labor, increased rates of cesarean delivery, postpartum hemorrhage and maternal death. Fetal complications include increased rates of low birth weight and small for gestational age newborns. Prenatal maternal anemia has also been associated with autism spectrum disorders in the neonate, although causation is not established. Deficiency in the newborn is associated with compromised memory, processing, and bonding, with some of these deficits persisting into adulthood. Despite the prevalence and consequences associated with iron deficiency in pregnancy, data show that it is routinely undertreated. Due to the physiologic changes of pregnancy, all pregnant individuals should receive oral iron supplementation. However, the bioavailability of oral iron is poor and it is often ineffective at preventing and treating iron deficiency. Likewise, it frequently causes gastrointestinal symptoms that can worsen quality of life in pregnancy. Intravenous iron formulations administered in a single or multiple dose series are now available. There is increasing data suggesting that newer intravenous formulations are safe and effective in the second and third trimesters and should be strongly considered in pregnant individuals without optimal response to oral iron repletion.
Keywords: iron deficiency, oral iron, anemia, blood loss, pregnancy
Introduction:
Iron deficiency is the most common micronutrient deficiency in the world1 and consequently anemia of pregnancy is most commonly caused by iron deficiency.2 In 2020, the worldwide maternal mortality ratio was estimated at 152 deaths per 100,000 live births, although the true number may be higher.3 The World Health Organization (WHO) has previously estimated the global burden of deaths attributed to anemia in women of reproductive age, with an estimated incidence of 16,800 to 28,000 deaths annually.4 Though the proportion of death during childbirth due to iron deficiency anemia (IDA) is unknown, IDA complicates approximately 30–60% of pregnancies globally.5–7 Due to an increase in iron deficiency throughout gestation, nearly 75% of individuals have iron deficiency or IDA by the third trimester.8 Individuals of color and or low socioeconomic status disproportionately experience IDA during pregnancy.9,10 Despite the high prevalence, health inequalities, and negative impact to both mother and fetus,11 iron deficiency remains underdiagnosed, understudied and undertreated.
The diagnosis and treatment of iron deficiency anemia in pregnancy, while relatively straightforward, is often overlooked and not optimally managed by healthcare providers.12 This is largely because many practitioners lack adequate knowledge on iron repletion and often approach treatment using an excessive use of oral iron, which offers marginal to no benefit with increasing doses, but can cause significant gastrointestinal toxicity.13,14 In addition, older formulations of intravenous iron that require multiple small doses (e.g., iron sucrose) and ferric gluconate are still being administered as opposed to newer formulations which are more convenient, require a single dose, and have similar safety profiles.
In the following review we outline the impact of iron deficiency on pregnancy, from the global impact to fetal and maternal complications. We focus on advances in our understanding of iron absorption and oral iron replacement and subsequently discuss recent and ongoing studies of intravenous iron repletion in pregnancy. Finally, we suggest an evidence-based workflow for the diagnosis and treatment of iron deficiency in pregnancy to guide healthcare providers in the management of this common, but treatable complication of pregnancy.
The Incidence of iron deficiency in pregnancy:
Iron deficiency in reproductive age women is common, affecting more than 40% of first trimester pregnancies in the US using standard cutoffs of percent transferrin saturation and/or serum ferritin (<50 μg/L).15–17 Pregnant individuals who are teenagers, have short interpregnancy intervals, are from socially disadvantages populations, or who are recent immigrants from developing nations are particularly affected.18 The incidence of iron deficiency without anemia has not been well described, but appears to increase throughout gestation, with 30–50% of pregnant individuals affected by low serum ferritin by the end of pregnancy.7,19–22 As red blood cell mass expands throughout gestation, over a quarter of individuals develop resultant anemia by the third trimester.19–21 A significant challenge in interpreting the literature is a lack of consensus on the laboratory diagnosis of iron deficiency in pregnancy as existing studies use inconsistent ferritin cutoffs for determining iron deficiency. Iron deficiency anemia in pregnancy is variably defined as serum ferritin <15–30 μg/L with a hemoglobin <11 g/dL and hematocrit <33%.1,23 Recently published World Health Organization (WHO) guidelines have recommended using a cutoff of ferritin <15 μg/L.24 Using a ferritin <30 μg/L improves the specificity and sensitivity for diagnosing iron deficiency;7,25,26 however, research on pregnancy-specific cut-offs are lacking. At least two clinical trials16,17 have suggested using a ferritin cut off of 50 μg/L reduces symptoms in females, and some authors have advocated for the use of this cutoff in clinic practice.27,28
Racial and ethnic disparities in iron deficiency and iron deficiency anemia
The prevalence of iron deficiency highlights racial and ethnic disparities in the United States, but the underlying etiology of these observations are not well understood. A recent study found that amongst individuals with isolated iron deficiency, prevalence was highest in Mexican American (20.5%) and non-Hispanic Black women (19.9%) and lower in other Hispanic (13.5%) and non-Hispanic, non-Black women (7.7%).10 Similarly, the large, cross-sectional Hemochromatosis and Iron Overload Screening Study (HEIRS) conducted at five centers in the United States and Canada found that a higher proportion of Hispanic and black women of reproductive age were iron deficient as compared to similarly aged white and Asian women.9
Historically, different thresholds have been used for the diagnosis of anemia in Black women in the United Sates. Until recently, the recommendation was to lower hemoglobin (Hgb) cutoffs by 0.8 g/dL, making a Hgb less than 10.2 g/dL consistent with anemia in Black women.29 Using these cutoffs, it has been reported that the prevalence of anemia in pregnant individuals is two times higher in Black females (35.38/1,000; RR=2) than in white (18.02/1,000) in the United States.30 This is alarming given the racial disparities that exist for pregnancy-related mortality rates (PRMR) in Black females versus their white counterparts. According to a report published by the CDC in 2019, the PRMR of Black women in the United States is 40.8/100,000 births compared to 13/100,000 birth for white females. Specifically, Black females constitute 9.7% of deaths attributed to hemorrhage, compared to only 9.1% of white females despite the racial demographics of the country.31 A recent secondary analysis of a prospective cohort study demonstrated use of race-based definitions of antepartum anemia are associated with increased odds of anemia upon presentation to labor and delivery in Black women.32 As a result, The American College of Obstetricians and Gynecologists (ACOG) no longer recommends race-based definitions of anemia.33
The pathophysiology of iron deficiency and iron deficiency anemia in pregnancy:
The majority of iron deficiency occurs in menstruating individuals, and as such, many women are iron deficient at the time of conception. In a sentinel study published in 1967, Scott and Pritchard reported that 58% of healthy 18 year old, non-pregnant females has absent hemosiderin on marrow aspiration, suggesting low iron stores.34 Pregnancy further increases physiologic iron demands as iron is preferentially directed to the developing fetus over the pregnant individual.35 Physiologically, red blood cell mass expands approximately 25% with a singleton gestation, while blood volume expands by 50%.36–38 The greater expansion in plasma volume is typically reflected by a decrease in Hgb. Anemia in pregnancy can be attributed to an expected dilutional effect;33 however, given the incidence of iron deficiency in pregnancy there is risk of iron-deficient erythropoiesis further worsening anemia. Furthermore, there is additional total iron loss associated with pregnancy and lactation (approximately 1 gram for an average weight of 55 kg).39–43 This includes approximately 350 mg associated with fetal and placental development, 500mg with expansion in red cell mass, and 250mg associated with blood loss at delivery.39–43 On average, a typical diet in high resource areas contains 12–18 mg of elemental iron per day.44 To replace the demands of pregnancy on iron stores, the recommended daily intake of ferrous iron during pregnancy is 27 mg (present in most prenatal vitamins) and 10 mg during lactation in individuals without ovulatory suppression. Although in individuals who have menstrual suppression in the setting of lactation, the net loss is marginal.41,44 Data from both primate and human studies have demonstrated that despite the biologic processes that aim to divert iron to the fetus, infants born to iron deficient mothers often have iron deficiency in infancy.18,21,45,46 This is because fetal iron is derived from maternal iron stores. Iron is preferentially transported across the placenta to the fetus, even if this causes iron deficiency in the mother.47 However, when maternal iron sources are exhausted and there is maternal anemia, fetal iron stores are at risk for being inadequate.48 Some data suggest that teenage pregnancies are also at higher risk for iron deficiency presumed due to increased maternal nutritional requirements for iron.49
Symptoms of iron deficiency and anemia:
Iron deficiency can be asymptomatic, but for other patients it can cause distressing symptoms. Symptoms of iron deficiency include fatigue, irritability, dyspnea, headache, hair loss, poor concentration, pica, restless legs syndrome, and reduced physical performance. These symptoms are often dismissed as normal during pregnancy as they can also be attributed to the physiologic changes in pregnancy. Pagophagia, a specific form of pica entailing eating of ice, has a 95% specificity for iron deficiency in women.50,51 The presence of anemia, if severe, may amplify many of these symptoms.
Maternal complications of iron deficiency and iron deficiency anemia:
Isolated iron deficiency (iron deficiency without anemia, also referred to as latent iron deficiency) and iron deficiency anemia in pregnant individuals is known to increase maternal morbidity and mortality (Figure 1).52,53 In pregnancy, untreated isolated iron deficiency is associated with an increased risk of iron deficiency anemia, placental hypertrophy, and maternal hypothyroidism.52,54,55 Maternal consequences of iron deficiency anemia include abnormal thyroid function, preterm labor, preterm birth (OR 1.54; 95%CI, 1.36–1.76), placental abruption, preeclampsia, eclampsia, and cesarean delivery (OR 1.30; 95%CI, 1.13–1.49).35,52,55–58 In addition, maternal iron deficiency anemia is associated with an increased risk of postpartum depression, decreased quality of life, severe, severe postpartum hemorrhage, maternal shock, increased admission to maternal intensive care unit, hysterectomy (aOR 7.66; 95%CI 4.57–12.85),59 antenatal and postnatal maternal sepsis, need for blood transfusion (OR 5.48; 95%CI, 4.57–6.58), poor wound healing, cardiac failure, and even maternal death (OR 2.36; 95% CI 1.60–3.48).7,33,52,55–58
Figure 1.
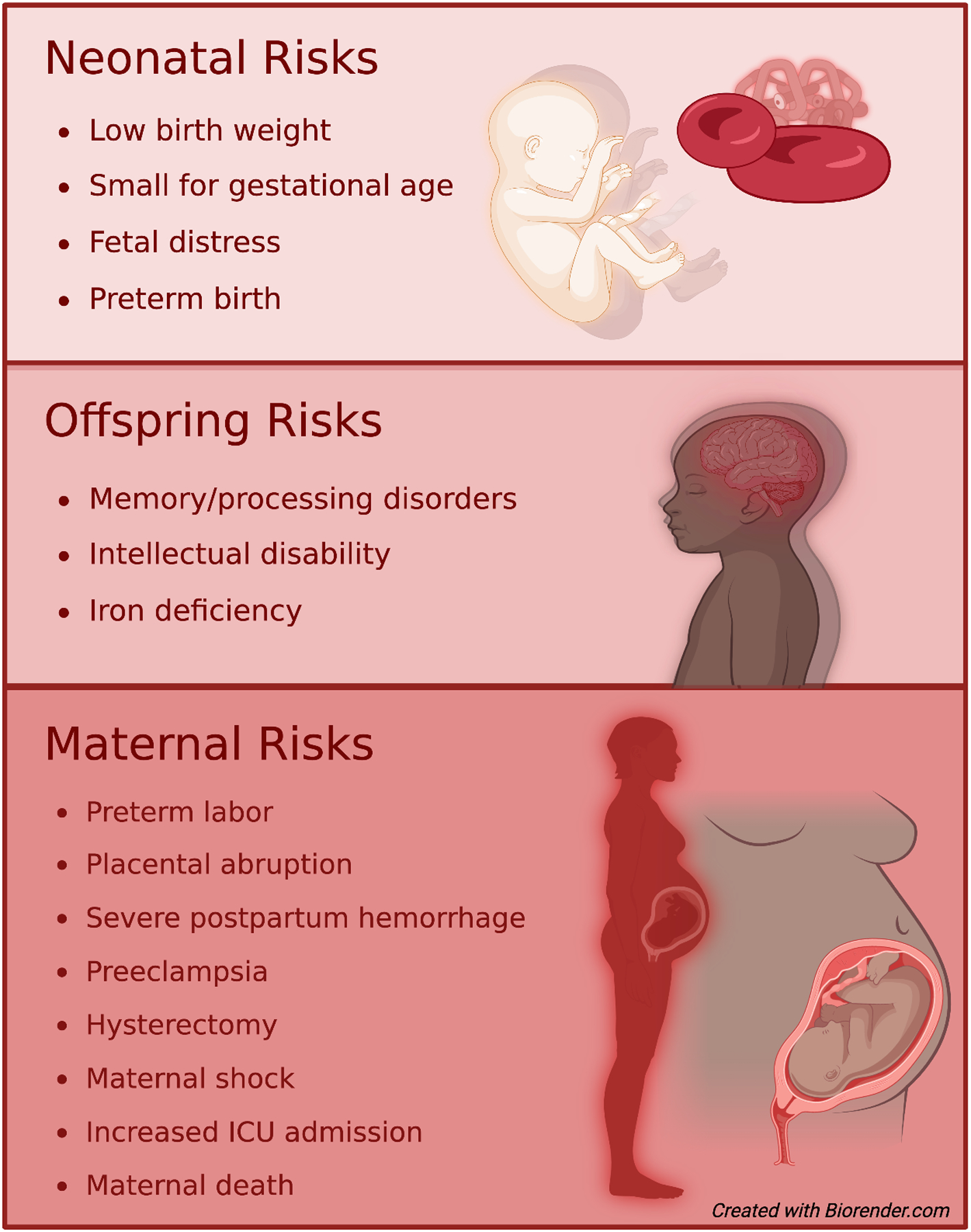
Maternal and offspring complications imparted by iron deficiency anemia
Fetal complications of maternal iron deficiency and iron deficiency anemia:
Severe iron deficiency in pregnancy has been shown to increase fetal morbidity (Figure 1). Findings from animal models suggest that iron deficiency during critical periods of fetal development results in altered brain metabolism, neurotransmission, epigenetics and myelination in offspring that persist into later in life.60,61 Clinical studies have suggested these findings occur in humans as well.62,63 Iron deficiency has also been shown to impact the fetal cardiovascular system including reduced circulation, slowed cardiovascular development, and enlarged hearts.39 Additionally, offspring born to iron deficient mothers are at higher risk for hypertension, obesity, and changes in lipid metabolism, although these results may be confounded by the impact of maternal obesity on fetal iron stores.55,64
Maternal iron deficiency is associated with an increased risk for adverse consequences to their offspring including low birth weight, small size for gestational age, and higher rates of fetal distress.65 Additionally, offspring are at an increased risk of neurological disorders such as autism spectrum disorder, attention deficit disorder, and other intellectual disabilities.18,61 In 2019, Wiergersma et al. published a cohort study of over 500,000 infants and nearly 300,000 mothers that found a significant association between diagnosis of anemia prior to 30 weeks and autism spectrum disorder (OR 1.44; 95% CI 1.13–1.84).66 Maternal iron deficiency with or without associated anemia, especially with a ferritin level of <12 μg/L, can adversely affect the fetal iron status.22 There is a lack of data to validate a ferritin threshold used to diagnose iron deficiency in pregnancy; Shao et al. found a significant association between maternal ferritin <13.6 μg/L and fetal iron deficiencies.67 Iron deficiency can negatively impact growth and function in multiple major organ systems, including the heart, skeletal muscle, brain, and gastrointestinal tract.68–72 Fetal iron deficiency is also associated with an increased predisposition to early onset postnatal iron deficiency, approximately 14% of term infants born to iron-deficient mothers have a serum ferritin concentration <30 μg/L at birth.19 Infants born to mothers with mild to moderate iron deficiency anemia who appear to be iron sufficient at birth still carry a risk for iron deficiency through the first year of life.21,46
Screening recommendations:
There is not a clear consensus on screening protocols, or even the routine use of screening for iron deficiency in pregnancy. Few organizations support screening for isolated iron deficiency given the paucity of studies demonstrating risk amelioration with treatment. However, recently the United Kingdom officially recommended risk-based screening for iron deficiency in individuals with history of anemia, parity greater than or equal to three, multiple gestation, interpregnancy interval <1 year, poor dietary habits, vegetarian or vegan diet, pregnant teenagers, those at high risk of bleeding during birth, or in individuals who decline blood products.73 They recommend the diagnosis of iron deficiency be made using a serum ferritin of less than or equal to 30 μg/L until quality data suggests the use of an alternative cut-off.73 The United States Preventative Services Taskforce (USPSTF) has concluded that the current body of evidence is insufficient to assess the balance of benefits and harms of screening for iron deficiency anemia in pregnant individuals to prevent adverse maternal health and birth outcomes.74 Counter to this, the American College of Obstetricians and Gynecologists (ACOG) recommends screening for anemia in pregnant individuals and implementing universal iron supplementation to meet the iron requirements of pregnancy.33 They only recommend assessment of iron stores in the setting of previously diagnosed anemia. Serum ferritin has the highest sensitivity and specificity for diagnosing iron deficiency in pregnancy, but there is not a clear consensus on the ideal diagnostic threshold. Ferritin levels of less than 30 μg/L have been endorsed by ACOG as diagnostic for iron deficiency,33 while the WHO has suggested a cutoff of ferritin levels less than 15 μg/L24 and others have suggested thresholds as high as 50 micrograms/L.75
Treatment:
Oral Iron in pregnancy:
Multiple national societal guidelines including ACOG33 and the Centers for Disease Control and Prevention (CDC)23 recommend that all pregnant individuals receive oral iron supplementation. At our institution, our practice is to offer a single dose of oral iron daily (or every other day in the event of toxicity) (Figure 2).23 However, there have been a number of high-quality studies that demonstrate increased absorption of oral iron with every other day dosing.76–78 A workshop by the National Academy of Sciences concluded that the recommended daily intake of iron during pregnancy is 27 mg of elemental iron and in lactation is 9 mg.79 As oral iron is well known to result in gastrointestinal side effects in a large proportion of pregnant individuals, we assess for any intolerance at follow up visits. The frequency and severity of gastrointestinal side effects associated with oral iron supplementation can vary and include constipation, abdominal pain, flatulence, nausea, vomiting and diarrhea (Table 1).80 A prior meta-analysis of 20 trials demonstrated an increased incidence of gastrointestinal side effects with oral ferrous sulfate compared to placebo (OR=2.32, 95% CI 1.74–3.08, p<0.001) and as a result, reduced compliance with therapy in 30–70% of symptomatic cases.80 Currently, oral iron represents the only safe option for repletion during the first trimester of pregnancy, given the lack of safety data during this crucial window of fetal development and organogenesis. As such, we reserve intravenous (IV) iron for the second and third trimester (Figure 2).
Figure 2.
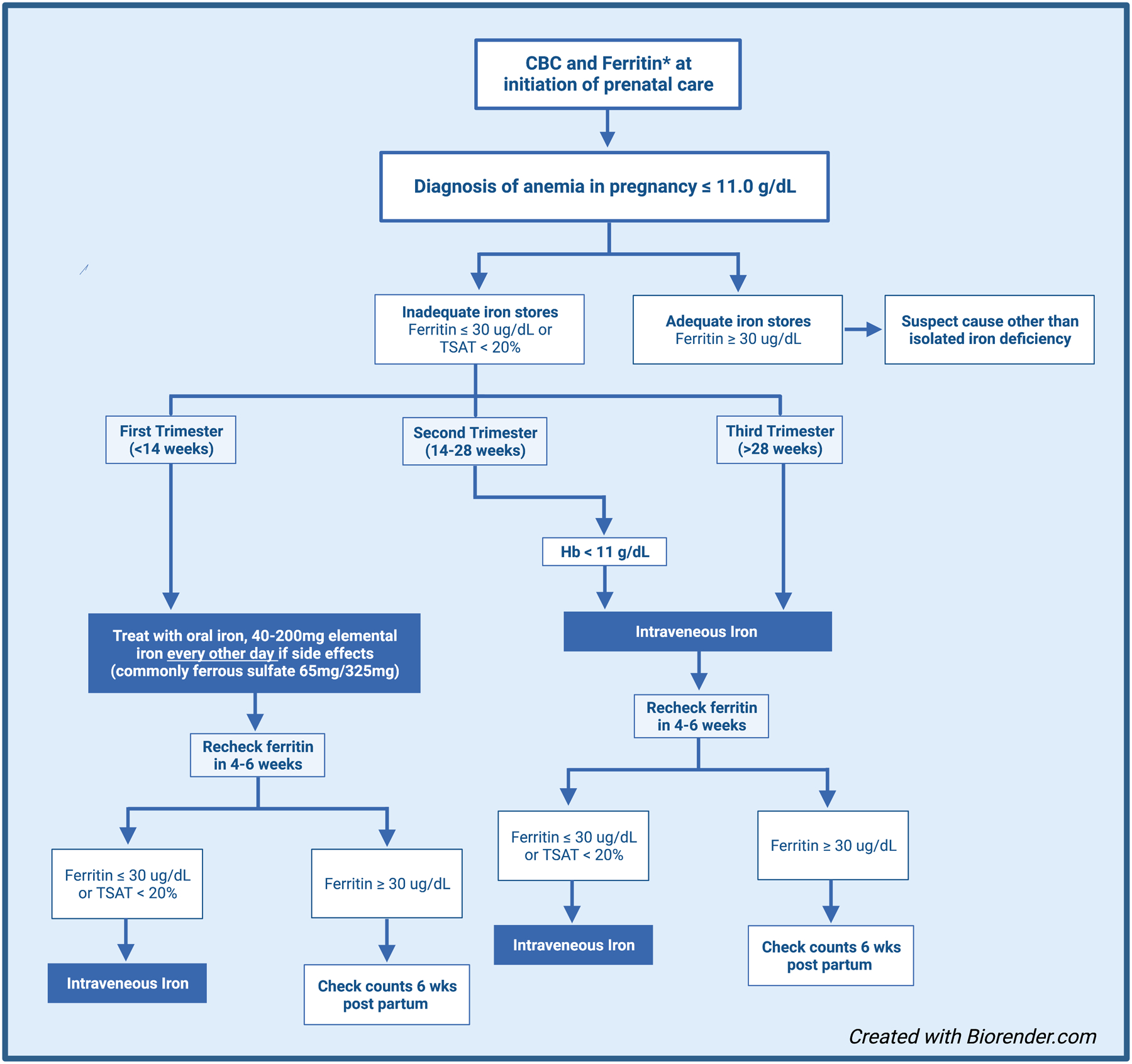
Proposed algorithm for diagnosing and treating iron deficiency anemia in pregnancy.
*Our institutional practice is to treat isolated iron deficiency with IV iron in the 2nd and 3rd trimester
Table 1.
Characteristics of different oral iron formulations.
| Formulations | Benefits | Complications |
|---|---|---|
| Ferrous Iron Salts * | Inexpensive, widely available | Tendency to cause gastrointestinal side effects |
| Polysaccharide-Iron Complex | Potentially lower risk of gastrointestinal intolerance, better taste profile | Less effective than ferrous salts in at least one randomized clinical trial of children. |
| Heme Iron | Higher absorption rate | Expensive. Not be a viable option for vegetarian or vegan individuals. |
| Carbonyl Iron | Inexpensive | No clear efficacy or side effect benefit as compared |
| Iron Protein Succinylate | Some data to suggest there may be better tolerability and efficacy as compared to ferrous salts. | Unsuitable for individuals with hypersensitivity to milk protein |
| Iron Amino Acid Chelates ** | Less susceptible to dietary interactions. | May be more expensive than ferrous salts |
Ferrous sulfate, Ferrous fumarate, Ferrous gluconate
Ferrous Bisglycinate, Ferric Trisglycinate
Intravenous iron in Pregnancy:
Intravenous (IV) Iron has been demonstrated to be safe and effective in multiple studies of pregnant individuals (Table 2).81–83 As a general rule, and reiterated throughout this text, the use of IV iron is restricted to the second and third trimester due to a paucity of safety data in early gestation. Doses are similar to those used in nonpregnant individuals and the drugs appear equally well-tolerated in pregnancy (Table 2). Our practice has shifted to the use of single dose infusions due to the relative ease and patient satisfaction associated with these formulations as compared to older formulations that required multiple visits.84 Individual clinical data on the available formulations are outlined below:
Table 2.
Characteristics of different IV iron formulations.
| Drug | Trade name | Maximum approved dose (mg) | # of doses | Duration (min) | Benefit | Side Effect |
|---|---|---|---|---|---|---|
| Low Molecular Weight Iron Dextran | INFeD | 1000 | 1 | 60 | Total dose infusion; cost effective | Although initially there were concerns for a slightly higher risk of anaphylaxis, more recent data has shown a similar safety profile to other common products. |
| Ferric gluconate | Ferrlecit | 125 | 8 | 60 | Safe in history of dextran induced anaphylaxis | Hypotension, flushing, headache |
| Iron sucrose | Venofer | 200–300 per dose, 1000 mg total | 3–5 | 15 | Safe in history of dextran induced anaphylaxis | Diarrhea, headache, nausea, dizziness, hypotension |
| Ferumoxytol | Feraheme | 510 | 2 | 15 | Total dose in 2 infusions | interferes with certain MRIs. Diarrhea, headache, nausea, dizziness, hypotension |
| Iron isolamtoside | Monoferric | 1000 | 1 | 15 | Total dose infusion; no test dose required | Appears similar to other commonly used products |
| Ferric carboxymaltose | Ferinject or Injectafer | 750 | 2 | 15 | Multiple clinical trials in pregnancy | May have higher rates of hypophosphatemia then other products. |
Hypersensitivity reactions are common with all intravenous formulation and are often mild and transient. Severe reactions are generally a response to the carbohydrate shell, and most individuals will be able to tolerate a different formulation with a different carbohydrate molecule.
Iron Sucrose:
A recent large phase III trial comparing iron sucrose to oral iron enrolled 2,018 pregnant individuals with iron deficiency anemia, 999 of whom received iron sucrose.81 The trial did not demonstrate any difference between intravenous and oral iron therapy in reducing adverse maternal outcomes. While the total dose has been criticized as subtherapeutic, the protocol involved dosing similar to that administered in the United States in pregnancy. This study highlighted the safety of iron sucrose pregnancy however, which was not dissimilar to oral iron. Minor side effects were more common in individuals receiving oral iron (21% vs 16%). While safe, iron sucrose is generally administered at lower doses (generally 200mg per session) requiring multiple visits. For this reason, we tend to favor the higher-dose infusions outlined in the following sections.
Low Molecular Weight Iron Dextran (LMWID):
LMWID can be offered in a single infusion (1000 mg IV over 1 hour) suggesting it to be a more time and cost-effective option. The safety of LMWID has been demonstrated in several clinical trials of pregnant individuals.
The first prospective study in the United States examined 74 oral iron-intolerant pregnant individuals receiving 1,000mg of LMWID.57 Of those, 60 had paired pre- and posttreatment data. The average pre- and post-hemoglobin concentrations were 9.7 and 10.8 g/dL (p<0.00001), transferrin saturations of 11.7% and 22.6% (p=0.0003) and ferritin levels of 14.5 and 126.3 ng/mL (p<0.000001) respectively. Six participants had minor infusion reactions that resolved, and no infants were diagnosed with iron deficiency anemia.
One trial treated 100 pregnant individuals with iron deficiency anemia with LMWID, comparing their outcomes to 50 matched controls receiving oral iron supplementation (ferrous sulfate 200 mg three times a day).85 The mean increase of hemoglobin in the intervention group was 2.43 gm/dl (95% CI 2.4 – 3.8) and for the control group it was 0.7 gm/dl (95% CI 0.6–2.3). Flushing and palpitations were observed in 4% of the intervention group and none in the control group. No significant adverse reactions were observed in either group.
Another study evaluated 189 consecutives, unselected second and third trimester gravidas refractory to oral iron.82 All received a test dose of 25 mg LMWID and were monitored for adverse events during the 60-min infusion. No serious adverse events occurred, but approximately 2% of participants experienced transient infusion reactions. Hemoglobin improved by 1–1.9 g/dL in 82% and ≥2 g/dL in 24%. Second trimester treatment was not associated with a greater hemoglobin improvement than third trimester treatment.
Ferric Carboxymaltose (FCM):
FCM is typically administered as two doses of 750 mg at least 7 days apart. While effective, it carries a higher rate of hypophosphatemia then other iron formulations, however this effect seems to be short-lived in pregnant individuals if it occurs.86,87 Multiple clinical trials have found FCM to be safe in the second and third trimester of pregnancy.88–90
A prospective observational study analyzed data from 863 pregnant individuals with iron deficiency anemia treated with FCM.91 The authors found significantly increased hemoglobin in individuals with mild, moderate, and severe iron deficiency anemia and individuals with isolated iron deficiency at 3- and 6-week post-infusion (p < 0.01 for all). No serious adverse events were recorded, with minor temporary side effects (including local skin irritation, nausea, and headache) occurring in 11% of individuals. No adverse fetal or neonatal outcomes were observed.
Ferumoxytol:
Ferumoxytol can be given as two 510 mg doses approximately a week apart. Several studies also report the use of a single 1,020 mg dose.92–95 One study recently reported the outcomes of 131 iron-deficient second- and third-trimester pregnant individuals who received either 510 mg of IV ferumoxytol twice or 1,020 mg once.96 Among all infusions of 1,020 mg or 510 mg, minor infusion reactions were observed in 19 (14%), of which 11 occurred with the 510 mg infusion, and 8 with the 1,020 mg infusion. All reactions were self-limited with resolution within minutes, and all but one (patient had refused) received the full intended dose. There were no serious adverse events, episodes of hypotension or hospitalizations.
Ferric derisomaltose:
Although recently approved in the United States to be given as a single 1,000 mg dose over 15–20 minutes, pregnancy specific data has yet to be published. Recently, a single-center, open-label, randomized controlled trial demonstrated superiority of IV ferric derisomaltose over oral iron for the treatment of persistent iron deficiency anemia (ferritin < 30μg/L) in early second trimester (14–21 weeks) resulting in treatment of anemia in addition to improved fatigue and quality of life.97 A study outlining the safety of ferric derisomaltose in pregnancy as compared to iron sucrose is currently ongoing (NCT05251493).
Postpartum iron deficiency:
Postpartum iron deficiency anemia is largely caused by blood loss or inadequate dietary iron intake/uptake. This can often result in symptoms such as fatigue, dyspnea and light-headedness. A recent meta-analysis evaluated use of intravenous iron as compared to oral iron for the treatment of IDA in the postpartum period.98 The study included 15 trials including more than 2000 postpartum individuals. Use of IV iron resulted in hemoglobin concentrations that were higher at 6 weeks postpartum with fewer GI side effects. Overall the safety profile was reassuring with an anaphylaxis rate of 0.6%.98 The use of single dose IV iron is also associated with a significant reduction in physical fatigue within 12 weeks following postpartum hemorrhage when compared to oral iron supplementation.99 Use of IV iron for postpartum anemia is also associated with improved physical performance and quality of life.100 Historically the robustness of the existing literature was deemed insufficient to determine the utility of iron replacement therapy for treating postpartum isolated iron deficiency.101 However, the contemporary data above suggest that there may be a role for IV iron in women with postpartum anemia who fail to tolerate or respond to oral iron.
Isolated iron deficiency has also been demonstrated to significantly increase the risk of postpartum depression.102,103 A prospective, randomized, controlled, intervention trial was conducted to examine the relationship between iron deficiency anemia and postpartum depression in 81 mothers. The study included three treatment groups: nonanemic controls and anemic mothers receiving either placebo or 125mg of daily iron.103 The study found that iron replacement resulted in a 25% improvement (p<0.05) in the previously iron-deficient mother’s depression and stress scales at 10 weeks and 9 months postpartum. Multivariate analysis demonstrated a strong association between markers of iron status (e.g. hemoglobin, mean corpuscular volume, transferrin saturation) and markers of behavior (e.g. anxiety, stress, depression). The study concluded that there is a strong association between maternal iron status and depression, stress and cognitive function in the postpartum period.
Although the data are limited, iron deficiency anemia has also been associated with insufficient milk supply in lactating individuals. A longitudinal study of 630 lactating individuals documented a greater percentage of individuals with anemia having insufficient milk supply compared to non-anemic individuals (19.7% vs. 11.4%, p <0.01).104 The study also reported primary reasons cited for weaning. Of those weaning primarily for an indication of insufficient milk supply, 29.6% were anemic and 20.8% were non-anemic. Further studies are needed to elucidate if this relationship is causative and whether treatment of anemia impacts duration of breastfeeding.
Conclusion:
Isolated iron deficiency remains underdiagnosed and undertreated in pregnancy, even as its high prevalence and morbidity have profound and potentially addressable effects on public and global health. Most pregnant individuals do not have sufficient iron stores to meet prenatal requirements and this deficit is exacerbated by the physiologic changes of pregnancy. The data show a consistent association between maternal isolated iron deficiency and poor neurodevelopmental outcomes from infancy through adolescence. Currently, the primary intervention for isolated iron deficiency is oral iron supplementation. However, oral iron is poorly tolerated and there is increasing data to support the increased efficacy and reduced side effects offered by intravenous iron formulations. Compared to oral iron, intravenous iron is better tolerated and serious side effects are rare. Isolated iron deficiency, in the absence of anemia, has both negative maternal and neonatal implications; however, there are a lack of standardized definitions for iron deficiency and screening or treatment recommendations in pregnant individuals contribute to underdiagnosis. In addition, there is accumulating evidence suggesting that circulating and dietary iron biomarkers in pregnant individuals are associated with gestational diabetes, but existing studies have high heterogeneity in their analyses. Long-term, prospective studies are needed to elucidate associations between the treatment of iron deficiency with or without anemia and maternal and neonatal outcomes. In addition, as newer intravenous iron formulations with improved side effect profiles and shorter durations of administration become available, randomized controlled trials in pregnancy are critical to guide prenatal providers on iron supplementation, and improve the reproductive health and quality of life of millions of pregnant individuals worldwide.
What is the new aspect of your work?
We focus on advances in iron absorption and oral iron replacement, along with recent and ongoing studies of intravenous iron repletion in pregnancy.
What is the central finding of your work?
We outline the impact of iron deficiency on pregnancy, from the global impact to fetal and maternal complications.
What is (or could be) the specific clinical relevance of your work?
We suggest an evidence-based workflow for the diagnosis and treatment of iron deficiency in pregnancy to guide healthcare providers in the management of this common, but treatable complication of pregnancy.
Financial Support:
JJ. Shatzel is supported by the National Heart, Lung, and Blood Institute/National Institutes of Health (R01HL151367). J. Lo is supported by the National Institutes of Health (P51-OD-011092) and the Silver Family Innovation Award.
Footnotes
Disclosures: JJ. Shatzel reports receiving consulting fees from Aronora Inc. The remaining authors have nothing to disclose.
Data Availability:
The data used to support the findings of this study are included within the article.
References
- 1.WHO/UNICEF/UNU. Iron deficiency anaemia: assessment, prevention and control, a guide for programme managers. World Health Organization; Geneva Switzerland. 2001. WHO/NHD/01.3. In. [Google Scholar]
- 2.Lee AI, Okam MM. Anemia in pregnancy. Hematol Oncol Clin North Am. 2011;25(2):241–259, vii. [DOI] [PubMed] [Google Scholar]
- 3.Bill & Melinda Gates Foundation. Maternal Mortality. 2022. Available from https://www.gatesfoundation.org/goalkeepers/report/2021-report/progress-indicators/maternal-mortality/. Last accessed September 19, 2022.
- 4.Murray C, Lopez AD. Global and regional cause-of-death patterns in 1990. Bulletin of the World Health Organization. 1994;72(3):447. [PMC free article] [PubMed] [Google Scholar]
- 5.Osungbade KO, Oladunjoye AO. Preventive treatments of iron deficiency anaemia in pregnancy: a review of their effectiveness and implications for health system strengthening. J Pregnancy. 2012;2012:454601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamani AR, Farajzadegan Z, Ghahiri A, KHADEMLOU M, Golshiri P. Effectiveness of twice weekly iron supplementation compared with daily regimen in reducing anemia and iron deficiency during pregnancy: a randomized trial in Iran. 2008.
- 7.Daru J, Allotey J, Peña-Rosas JP, Khan KS. Serum ferritin thresholds for the diagnosis of iron deficiency in pregnancy: a systematic review. Transfus Med. 2017;27(3):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang G, Lausman A, Abdulrehman J, et al. Prevalence of iron deficiency and iron deficiency anemia during pregnancy: a single centre Canadian study. Blood. 2019;134:3389. [Google Scholar]
- 9.Barton JC, Wiener HH, Acton RT, et al. Prevalence of iron deficiency in 62,685 women of seven race/ethnicity groups: The HEIRS Study. PLoS One. 2020;15(4):e0232125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell R, Wang H, Ahmed R. Risk Factors Contributing to Racial/Ethnic Disparities in Iron Deficiency in US Women. Current Developments in Nutrition. 2021;5(Supplement_2):725–725. [Google Scholar]
- 11.Ren A, Wang J, Ye RW, Li S, Liu JM, Li Z. Low first-trimester hemoglobin and low birth weight, preterm birth and small for gestational age newborns. Int J Gynaecol Obstet. 2007;98(2):124–128. [DOI] [PubMed] [Google Scholar]
- 12.Read AJ, Waljee AK, Sussman JB, et al. Testing Practices, Interpretation, and Diagnostic Evaluation of Iron Deficiency Anemia by US Primary Care Physicians. JAMA Network Open. 2021;4(10):e2127827–e2127827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewkowitz AK, Gupta A, Simon L, et al. Intravenous compared with oral iron for the treatment of iron-deficiency anemia in pregnancy: a systematic review and meta-analysis. J Perinatol. 2019;39(4):519–532. [DOI] [PubMed] [Google Scholar]
- 14.Lewkowitz AK, Stout MJ, Cooke E, et al. Intravenous versus Oral Iron for Iron-Deficiency Anemia in Pregnancy (IVIDA): A Randomized Controlled Trial. Am J Perinatol. 2022;39(8):808–815. [DOI] [PubMed] [Google Scholar]
- 15.Auerbach M, Abernathy J, Juul S, Short V, Derman R. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med. 2021;34(6):1002–1005. [DOI] [PubMed] [Google Scholar]
- 16.Verdon F, Burnand B, Stubi CL, et al. Iron supplementation for unexplained fatigue in non-anaemic women: double blind randomised placebo controlled trial. Bmj. 2003;326(7399):1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaucher P, Druais PL, Waldvogel S, Favrat B. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. Cmaj. 2012;184(11):1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaime-Perez JC, Herrera-Garza JL, Gomez-Almaguer D. Sub-optimal fetal iron acquisition under a maternal environment. Arch Med Res. 2005;36(5):598–602. [DOI] [PubMed] [Google Scholar]
- 20.Scholl TO. Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr. 2005;81(5):1218s–1222s. [DOI] [PubMed] [Google Scholar]
- 21.Kilbride J, Baker TG, Parapia LA, Khoury SA, Shuqaidef SW, Jerwood D. Anaemia during pregnancy as a risk factor for iron-deficiency anaemia in infancy: a case-control study in Jordan. Int J Epidemiol. 1999;28(3):461–468. [DOI] [PubMed] [Google Scholar]
- 22.Kelly AM, MacDonald DJ, McDougall AN. Observations on maternal and fetal ferritin concentrations at term. Br J Obstet Gynaecol. 1978;85(5):338–343. [DOI] [PubMed] [Google Scholar]
- 23.Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47(Rr-3):1–29. [PubMed] [Google Scholar]
- 24.Organization WH. Anaemia in Women and Children: WHO Global Anaemia Estimates, 2021 Edition. World Health Organization: Geneva, Switzerland. 2021. [Google Scholar]
- 25.Seid MH, Derman RJ, Baker JB, Banach W, Goldberg C, Rogers R. Ferric carboxymaltose injection in the treatment of postpartum iron deficiency anemia: a randomized controlled clinical trial. Am J Obstet Gynecol. 2008;199(4):435.e431–437. [DOI] [PubMed] [Google Scholar]
- 26.van den Broek NR, Letsky EA, White SA, Shenkin A. Iron status in pregnant women: which measurements are valid? Br J Haematol. 1998;103(3):817–824. [DOI] [PubMed] [Google Scholar]
- 27.DeLoughery TG. Microcytic anemia. N Engl J Med. 2014;371(14):1324–1331. [DOI] [PubMed] [Google Scholar]
- 28.Elstrott B, Khan L, Olson S, Raghunathan V, DeLoughery T, Shatzel JJ. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol. 2020;104(3):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woteki CE, Earl R. Iron deficiency anemia: recommended guidelines for the prevention, detection, and management among US children and women of childbearing age. 1994. [PubMed]
- 30.Adebisi OY, Strayhorn G. Anemia in pregnancy and race in the United States: blacks at risk. Fam Med. 2005;37(9):655–662. [PubMed] [Google Scholar]
- 31.Petersen EE, Davis NL, Goodman D, et al. Racial/ethnic disparities in pregnancy-related deaths—United States, 2007–2016. Morbidity and Mortality Weekly Report. 2019;68(35):762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamm RF, Wang EY, Levine LD, Srinivas SK. Association Between Race and Hemoglobin at Delivery or Need for Transfusion When Using Race-Based Definitions for Treatment of Antepartum Anemia. Obstet Gynecol. 2021;138(1):108–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anemia in Pregnancy: ACOG Practice Bulletin, Number 233. Obstet Gynecol. 2021;138(2):e55–e64. [DOI] [PubMed] [Google Scholar]
- 34.Scott DE, Pritchard JA. Iron deficiency in healthy young college women. JAMA. 1967;199(12):897–900. [PubMed] [Google Scholar]
- 35.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1 Suppl):257s–264s. [DOI] [PubMed] [Google Scholar]
- 36.de Haas S, Ghossein-Doha C, van Kuijk SM, van Drongelen J, Spaanderman ME. Physiological adaptation of maternal plasma volume during pregnancy: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;49(2):177–187. [DOI] [PubMed] [Google Scholar]
- 37.Chesley LC. Plasma and red cell volumes during pregnancy. Am J Obstet Gynecol. 1972;112(3):440–450. [DOI] [PubMed] [Google Scholar]
- 38.Pritchard JA. CHANGES IN THE BLOOD VOLUME DURING PREGNANCY AND DELIVERY. Anesthesiology. 1965;26:393–399. [DOI] [PubMed] [Google Scholar]
- 39.Bothwell TH. Overview and mechanisms of iron regulation. Nutr Rev. 1995;53(9):237–245. [DOI] [PubMed] [Google Scholar]
- 40.Bothwell TH, Charlton R, Cook J, Finch CA. Iron metabolism in man. Blackwell Scientific Publications.; 1979. [Google Scholar]
- 41.Fransson GB, Lönnerdal B. Iron in human milk. J Pediatr. 1980;96(3 Pt 1):380–384. [DOI] [PubMed] [Google Scholar]
- 42.Milman N. Prepartum anaemia: prevention and treatment. Ann Hematol. 2008;87(12):949–959. [DOI] [PubMed] [Google Scholar]
- 43.Milman N, Bergholt T, Byg KE, Eriksen L, Graudal N. Iron status and iron balance during pregnancy. A critical reappraisal of iron supplementation. Acta Obstet Gynecol Scand. 1999;78(9):749–757. [PubMed] [Google Scholar]
- 44.Russell R, Beard JL, Cousins RJ, et al. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. A report of the panel on micronutrients, subcommittees on upper reference levels of nutrients and of interpretation and uses of dietary reference intakes, and the standing committee on the scientific evaluation of dietary reference intakes food and nutrition board Institute of medicine. 2001:797. [Google Scholar]
- 45.Golub MS, Hogrefe CE, Tarantal AF, et al. Diet-induced iron deficiency anemia and pregnancy outcome in rhesus monkeys. Am J Clin Nutr. 2006;83(3):647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colomer J, Colomer C, Gutierrez D, et al. Anaemia during pregnancy as a risk factor for infant iron deficiency: report from the Valencia Infant Anaemia Cohort (VIAC) study. Paediatr Perinat Epidemiol. 1990;4(2):196–204. [DOI] [PubMed] [Google Scholar]
- 47.Gambling L, Danzeisen R, Gair S, et al. Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem J. 2001;356(Pt 3):883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fall C, Group INAC. Iron requirements and iron status during infancy. Paper presented at: International Nutritional Anemia Consultative Group. Report of the 2004 symposium. Iron deficiency in early life: challenges and progress. Lima: 2004. [Google Scholar]
- 49.Soares NN, Mattar R, Camano L, Torloni MR. Iron deficiency anemia and iron stores in adult and adolescent women in pregnancy. Acta Obstet Gynecol Scand. 2010;89(3):343–349. [DOI] [PubMed] [Google Scholar]
- 50.Chansky MC, King MR, Bialkowski W, et al. Qualitative assessment of pica experienced by frequent blood donors. Transfusion. 2017;57(4):946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bryant BJ, Yau YY, Arceo SM, Hopkins JA, Leitman SF. Ascertainment of iron deficiency and depletion in blood donors through screening questions for pica and restless legs syndrome. Transfusion. 2013;53(8):1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juul SE, Derman RJ, Auerbach M. Perinatal iron deficiency: implications for mothers and infants. Neonatology. 2019;115(3):269–274. [DOI] [PubMed] [Google Scholar]
- 53.Leonard AJ, Chalmers KA, Collins CE, Patterson AJ. Comparison of two doses of elemental iron in the treatment of latent iron deficiency: efficacy, side effects and blinding capabilities. Nutrients. 2014;6(4):1394–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu X, Shan Z, Li C, et al. Iron deficiency, an independent risk factor for isolated hypothyroxinemia in pregnant and nonpregnant women of childbearing age in China. The Journal of Clinical Endocrinology & Metabolism. 2015;100(4):1594–1601. [DOI] [PubMed] [Google Scholar]
- 55.Govindappagari S, Newman R, Burwick R. Iron-deficiency anemia in pregnancy and the role of intravenous iron. Contemporary OB/GYN Journal. 2021;66(07). [Google Scholar]
- 56.Drukker L, Hants Y, Farkash R, Ruchlemer R, Samueloff A, Grisaru-Granovsky S. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55(12):2799–2806. [DOI] [PubMed] [Google Scholar]
- 57.Auerbach M, James SE, Nicoletti M, et al. Results of the First American Prospective Study of Intravenous Iron in Oral Iron-Intolerant Iron-Deficient Gravidas. Am J Med. 2017;130(12):1402–1407. [DOI] [PubMed] [Google Scholar]
- 58.Zimmermann MB, Burgi H, Hurrell RF. Iron deficiency predicts poor maternal thyroid status during pregnancy. J Clin Endocrinol Metab. 2007;92(9):3436–3440. [DOI] [PubMed] [Google Scholar]
- 59.Harrison RK, Lauhon SR, Colvin ZA, McIntosh JJ. Maternal anemia and severe maternal morbidity in a US cohort. Am J Obstet Gynecol MFM. 2021;3(5):100395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pisansky MT, Wickham RJ, Su J, et al. Iron deficiency with or without anemia impairs prepulse inhibition of the startle reflex. Hippocampus. 2013;23(10):952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tran PV, Fretham SJ, Carlson ES, Georgieff MK. Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr Res. 2009;65(5):493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tamura T, Goldenberg RL, Hou J, et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140(2):165–170. [DOI] [PubMed] [Google Scholar]
- 63.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64(5 Pt 2):S34–43; discussion S72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bahr TM, Benson AE, Kling PJ, Ohls RK, Ward DM, Christensen RD. Maternal obesity and impaired offspring neurodevelopment: could fetal iron deficiency be a pathogenic link? J Perinatol. 2021;41(5):1199–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McArdle HJ, Gambling L, Kennedy C. Iron deficiency during pregnancy: the consequences for placental function and fetal outcome. Proceedings of the Nutrition Society. 2014;73(1):9–15. [DOI] [PubMed] [Google Scholar]
- 66.Wiegersma AM, Dalman C, Lee BK, Karlsson H, Gardner RM. Association of prenatal maternal anemia with neurodevelopmental disorders. JAMA psychiatry. 2019;76(12):1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shao J, Lou J, Rao R, et al. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142(11):2004–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aggett PJ. Trace elements of the micropremie. Clin Perinatol. 2000;27(1):119–129, vi. [DOI] [PubMed] [Google Scholar]
- 69.Blayney L, Bailey-Wood R, Jacobs A, Henderson A, Muir J. The effects of iron deficiency on the respiratory function and cytochrome content of rat heart mitochondria. Circ Res. 1976;39(5):744–748. [DOI] [PubMed] [Google Scholar]
- 70.Berant M, Khourie M, Menzies IS. Effect of iron deficiency on small intestinal permeability in infants and young children. J Pediatr Gastroenterol Nutr. 1992;14(1):17–20. [DOI] [PubMed] [Google Scholar]
- 71.Guiang SF 3rd, Merchant JR, Eaton MA, Fandel KB, Georgieff MK. Intracardiac iron distribution in newborn guinea pigs following isolated and combined fetal hypoxemia and fetal iron deficiency. Can J Physiol Pharmacol. 1998;76(9):930–936. [DOI] [PubMed] [Google Scholar]
- 72.Mackler B, Grace R, Finch CA. Iron deficiency in the rat: effects on oxidative metabolism in distinct types of skeletal muscle. Pediatr Res. 1984;18(6):499–500. [DOI] [PubMed] [Google Scholar]
- 73.Pavord S, Daru J, Prasannan N, Robinson S, Stanworth S, Girling J. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2020;188(6):819–830. [DOI] [PubMed] [Google Scholar]
- 74.Cantor AG, Bougatsos C, Dana T, Blazina I, McDonagh M. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: a systematic review for the US Preventive Services Task Force. Annals of internal medicine. 2015;162(8):566–576. [DOI] [PubMed] [Google Scholar]
- 75.Koulaouzidis A, Cottier R, Bhat S, Said E, Linaker BD, Saeed AA. A ferritin level >50 microg/L is frequently consistent with iron deficiency. Eur J Intern Med. 2009;20(2):168–170. [DOI] [PubMed] [Google Scholar]
- 76.Uyoga MA, Mikulic N, Paganini D, et al. The effect of iron dosing schedules on plasma hepcidin and iron absorption in Kenyan infants. Am J Clin Nutr. 2020;112(4):1132–1141. [DOI] [PubMed] [Google Scholar]
- 77.Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981–1989. [DOI] [PubMed] [Google Scholar]
- 78.Stoffel NU, von Siebenthal HK, Moretti D, Zimmermann MB. Oral iron supplementation in iron-deficient women: How much and how often? Molecular Aspects of Medicine. 2020;75:100865. [DOI] [PubMed] [Google Scholar]
- 79.National Academies of Sciences, Engineering, Medicine: The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. 2017. Last accessed, July 20, 2022. [PubMed]
- 80.Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PloS one. 2015;10(2):e0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neogi SB, Devasenapathy N, Singh R, et al. Safety and effectiveness of intravenous iron sucrose versus standard oral iron therapy in pregnant women with moderate-to-severe anaemia in India: a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Glob Health. 2019;7(12):e1706–e1716. [DOI] [PubMed] [Google Scholar]
- 82.Wong L, Smith S, Gilstrop M, et al. Safety and efficacy of rapid (1,000 mg in 1 hr) intravenous iron dextran for treatment of maternal iron deficient anemia of pregnancy. Am J Hematol. 2016;91(6):590–593. [DOI] [PubMed] [Google Scholar]
- 83.Varde KN. TREATMENT OF 300 CASES OF IRON DEFICIENCY OF PREGNANCY BY TOTAL DOSE INFUSION OF IRON-DEXTRAN COMPLEX. J Obstet Gynaecol Br Commonw. 1964;71:919–922. [DOI] [PubMed] [Google Scholar]
- 84.Arastu AH, Elstrott BK, Martens KL, et al. Analysis of Adverse Events and Intravenous Iron Infusion Formulations in Adults With and Without Prior Infusion Reactions. JAMA Network Open. 2022;5(3):e224488–e224488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ayub R, Tariq N, Adil MM, Iqbal M, Junaid A, Jaferry T. Efficacy and safety of total dose infusion of low molecular weight iron dextran in the treatment of iron deficiency anemia during pregnancy. J Coll Physicians Surg Pak. 2008;18(7):424–427. [PubMed] [Google Scholar]
- 86.Glaspy JA, Lim-Watson MZ, Libre MA, et al. Hypophosphatemia Associated with Intravenous Iron Therapies for Iron Deficiency Anemia: A Systematic Literature Review. Ther Clin Risk Manag. 2020;16:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wolf M, Rubin J, Achebe M, et al. Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia: Two Randomized Clinical Trials. JAMA. 2020;323(5):432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Froessler B, Collingwood J, Hodyl NA, Dekker G. Intravenous ferric carboxymaltose for anaemia in pregnancy. BMC Pregnancy and Childbirth. 2014;14(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jose A, Mahey R, Sharma JB, et al. Comparison of ferric Carboxymaltose and iron sucrose complex for treatment of iron deficiency anemia in pregnancy- randomised controlled trial. BMC Pregnancy and Childbirth. 2019;19(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kant S, Haldar P, Malhotra S, Kaur R, Rath R, Jacob OM. Intravenous ferric carboxymaltose rapidly increases haemoglobin and serum ferritin among pregnant females with moderate-to-severe anaemia: A single-arm, open-label trial. Natl Med J India. 2020;33(6):324–328. [DOI] [PubMed] [Google Scholar]
- 91.Froessler B, Gajic T, Dekker G, Hodyl NA. Treatment of iron deficiency and iron deficiency anemia with intravenous ferric carboxymaltose in pregnancy. Arch Gynecol Obstet. 2018;298(1):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karki NR, Auerbach M. Single total dose infusion of ferumoxytol (1020 mg in 30 minutes) is an improved method of administration of intravenous iron. Am J Hematol. 2019;94(9):E229–e231. [DOI] [PubMed] [Google Scholar]
- 93.Khan H, May P, Kuo E, et al. Safety and efficacy of a single total dose infusion (1020 mg) of ferumoxytol. Ther Adv Hematol. 2021;12:20406207211006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsin J, Au L, Lustik M, et al. Implementing total-dose ferumoxytol in lieu of split-dose ferumoxytol. J Am Pharm Assoc (2003). 2021;61(6):e99–e104. [DOI] [PubMed] [Google Scholar]
- 95.Cohen J, Khudanyan A, Lu J, et al. A multicenter study evaluating the effectiveness and safety of single-dose low molecular weight iron dextran vs single-dose ferumoxytol for the treatment of iron deficiency. Am J Hematol. 2020;95(12):1572–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gerb J, Strauss W, Derman R, et al. Ferumoxytol for the treatment of iron deficiency and iron-deficiency anemia of pregnancy. Ther Adv Hematol. 2021;12:20406207211018042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hansen R, Sommer VM, Pinborg A, et al. Intravenous ferric derisomaltose versus oral iron for persistent iron deficient pregnant women: a randomised controlled trial. Arch Gynecol Obstet. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sultan P, Bampoe S, Shah R, et al. Oral vs intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2019;221(1):19–29.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Holm C, Thomsen LL, Norgaard A, Langhoff-Roos J. Single-dose intravenous iron infusion or oral iron for treatment of fatigue after postpartum haemorrhage: a randomized controlled trial. Vox Sang. 2017;112(3):219–228. [DOI] [PubMed] [Google Scholar]
- 100.Daniilidis A, Panteleris N, Vlachaki E, Breymann C, Assimakopoulos E. Safety and efficacy of intravenous iron administration for uterine bleeding or postpartum anaemia: a narrative review. J Obstet Gynaecol. 2018;38(4):443–447. [DOI] [PubMed] [Google Scholar]
- 101.Markova V, Norgaard A, Jørgensen KJ, Langhoff-Roos J. Treatment for women with postpartum iron deficiency anaemia. Cochrane Database Syst Rev. 2015;2015(8):Cd010861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Azami M, Badfar G, Khalighi Z, et al. The association between anemia and postpartum depression: A systematic review and meta-analysis. Caspian J Intern Med. 2019;10(2):115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beard JL, Hendricks MK, Perez EM, et al. Maternal Iron Deficiency Anemia Affects Postpartum Emotions and Cognition. The Journal of Nutrition. 2005;135(2):267–272. [DOI] [PubMed] [Google Scholar]
- 104.Henly SJ, Anderson CM, Avery MD, Hills-Bonczyk SG, Potter S, Duckett LJ. Anemia and insufficient milk in first-time mothers. Birth. 1995;22(2):86–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


