Abstract
Background:
Idiopathic Parkinson’s disease (iPD) is associated with two distinct brain networks, PDRP and PDCP, which correlate respectively with motor and cognitive symptoms. The relationship between the two networks in individual patients is unclear.
Objective:
To determine whether a consistent relationship exists between these networks, we measured the difference between PDRP and PDCP expression, termed delta, on an individual basis in independent populations of patients with iPD (n=356), idiopathic REM sleep behavioral disorder (iRBD) (n=21), genotypic Parkinson’s disease patients carrying GBA1 variants (n=12) or the LRRK2-G2019S mutation (n=14), atypical parkinsonian syndromes (APS, n=238), and healthy control subjects (n=95) from the US, Slovenia, India, and South Korea.
Methods:
We used [18F]-fluorodeoxyglucose PET and resting-state fMRI to quantify delta and to compare the measure across samples; changes in delta over time were likewise assessed in longitudinal patient samples. Lastly, we evaluated delta in prodromal individuals with iRBD and in genotypic Parkinson’s disease.
Results:
Delta was abnormally elevated in each of the four iPD samples (p<0.05) as well as in the at risk iRBD group (p<0.05), with increasing values over time (p<0.001). PDRP predominance was also present in genotypic Parkinson’s disease, with higher values in patients with GBA1 variants compared to the less aggressive LRRK2-G2019S mutation (p=0.005). This trend was not observed in APS patients, who were accurately discriminated from iPD based on PDRP expression and delta (AUC=0.85, p<0.0001).
Conclusions:
PDRP predominance, quantified by delta, assays the spread of dysfunction from motor to cognitive networks in Parkinson’s disease patients. Delta may therefore aid in differential diagnosis and in tracking disease progression in individual patients.
Keywords: Parkinson’s disease, network analysis, FDG-PET, rs-fMRI, disease progression
Introduction
While symptom manifestation and disease progression vary across individual patients with idiopathic Parkinson’s disease (iPD), the underlying histopathological changes and the time sequence of the regions involved are relatively stereotyped.1–4 iPD is associated with two distinct metabolic networks, the PD-related pattern (PDRP) and the PD-related cognitive pattern (PDCP) that relate respectively to the motor and cognitive manifestations of the disorder.5–7 Longitudinal imaging studies with [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) have revealed significant PDCP elevations beginning 4–6 years after motor onset.8–10 Thus, the PDRP is expected to have dominant expression compared to PDCP when measured in the same patients. In this vein, PDRP predominance can be understood in terms of the regional sequence of histopathological changes in iPD as originally described by Braak.1,4,7 Abnormalities in disease-related network may even precede the onset of motor symptoms, as evident from the presence of PDRP elevations in patients with idiopathic REM sleep behavioral disorder (iRBD), a prodromal form of the disorder.11–15 By contrast, PDRP predominance is unlikely in atypical parkinsonian syndromes (APS) such as multiple system atrophy (MSA) and progressive supranuclear palsy (PSP), in which Braak’s sequence does not apply.16–18
In the current study, we evaluated PDRP dominance in multiple samples of iPD patients of varying disease duration who were scanned with FDG-PET at four different sites. For each patient, we measured PDRP and PDCP expression levels, and computed the difference in these values, which was termed delta. We examined the relationship of delta to disease duration in cross-sectional iPD samples and over time. Likewise, delta in iPD patients was compared to corresponding values in subjects with iRBD,19 and in patients with PD genotypes associated with the LRRK2-G2019S mutation and GBA1 variants associated with slow and more rapid disease progression. The resulting data were compared with corresponding measures obtained in APS patients. Lastly, as proof-of-principle, the potential for broader applications of delta was demonstrated by similar results obtained non-invasively using resting-state fMRI (rs-fMRI).
Methods
Human Subjects
We studied independent groups of iPD patients and matched healthy control (HC) subjects. In each group, patients were diagnosed clinically with iPD according to UK PD Society Brain Bank criteria,20 without prior history of neuroleptic exposure, structural brain abnormalities or encephalitis. Ethical permission for these studies was obtained from the Institutional Review Board of Northwell Health, Manhasset, NY, National Medical Ethics Committee of the Republic of Slovenia, ethical committee at Institute of Nuclear Medicine and Allied Sciences, New Delhi, India and Institutional Review Board at Asan Medical Center, Seoul, Republic of Korea. Written consent was obtained from each subject following detailed explanation of the procedures.
Feinstein Institutes
Feinstein A (cross-sectional analysis; Table S1):
To explore the hypothesis of PDRP dominance in iPD, we computed PDRP and PDCP expression values and measured delta in FDG-PET scans from a sample composed of 172 iPD patients and 20 age-matched HC subjects from the Feinstein Institutes, Manhasset, NY, USA. Patients in this sample were subdivided by symptom duration into the following groups: early iPD (< 4 years), intermediate iPD (4–8 years), and late iPD (> 8 years). These cut-off values were chosen based on the natural history of iPD.21,22 The Feinstein A sample also included a prodromal group composed of 16 individuals with polysomnography-confirmed iRBD diagnosed according to established criteria.23 Demographic and clinical data are presented in Table S1. Limited PET data from Feinstein A subjects have been reported previously.11,24,25 To determine whether delta measurements are stable in single subjects, we explored an additional group of 14 iPD subjects who underwent repeat FDG-PET over an eight week period as part of a test-retest study26; within-subject reliability for the measure was assessed by computing the intraclass correlation coefficient (ICC) as described elsewhere.27,28
Feinstein B (longitudinal analysis; Table S2):
The Feinstein B sample was composed of two longitudinal iPD cohorts. The first cohort (Feinstein B1) consisted of 11 early (symptom duration of two years) iPD patients who were scanned with FDG-PET at baseline, 24 months and 48 months at the Feinstein Institutes. The second cohort (Feinstein B2) consisted of 22 late iPD patients who were randomized to sham surgery as part of a double-blind gene therapy trial and scanned with FDG-PET at baseline and 12 months after the procedure.9,26
Feinstein C (diagnostic study; Table S1):
To compare delta in APS with corresponding values in iPD, we analyzed FDG-PET scans from 71 parkinsonian subjects in whom the clinical diagnosis was uncertain at the time of imaging. These patients were followed by blinded movement disorders experts until a final clinical diagnosis was made.25,29 These patients were subsequently diagnosed as MSA (n=41) or PSP (n=30) on clinical grounds.25
Feinstein D (genotypic Parkinson’s disease (gPD); Table S3):
We computed delta in FDG-PET scans from gPD patients carrying either the LRRK2-G2019S mutation (PD-LRRK2, n=14) or GBA1 variants (PD-GBA, n=12), associated respectively with slow and more rapid clinical progression.30,31 Values in this genotypic sample were compared to data from matched samples of sporadic iPD (sPD, n=14) and HC subjects (n=14).32
Other Datasets (iPD and atypical parkinsonian syndromes; Table S1)
For further validation, we measured delta in FDG-PET data from patients with clinical parkinsonism (iRBD, iPD, MSA, or PSP) and HC scanned at other sites: Slovenia, India and South Korea. As in the Feinstein C sample, values at the following sites were computed in patients with parkinsonism who were diagnosed with iPD or APS on strictly clinical grounds, blind to image analysis. Patients from Slovenia and India were recruited regardless of cognitive function while dementia was an exclusion criterion for patients from South Korea. These cohorts are described in detail in Supplementary Materials. Limited PET data from these samples have been reported previously.33–35
Imaging
FDG-PET
Following an overnight fast, patients and control subjects at each site were scanned with FDG-PET on their respective imaging site. Feinstein A-D samples were scanned with FDG-PET using the GE Advance tomograph (General Electric, Milwaukee, WI) at the Feinstein Institutes (Manhasset, NY, USA). The scanning protocol at this site has been detailed elsewhere.24,25,32 Scanning at other sites was conducted on local imaging platforms.33–35 The Feinstein A-D samples and the India sample were scanned in the off-state, whereas those in the Slovenia and South Korea samples were scanned on their usual medications.
To ensure compatibility across the sites, we used the same protocols for image preprocessing and network computations. The scans from each subject were realigned separately, spatially normalized to a standard Montreal Neurological Institute (MNI)-based PET brain template and smoothed with an isotropic Gaussian kernel (10 mm) in all directions to improve the signal-to-noise ratio. Image processing was performed using SPM5 (Wellcome Department of Cognitive Neurology, University College, London)36 implemented in Matlab 7.0.1 (MathWorks, Sherborn, MA).
Resting-state fMRI
To show the potential generalizability of the FDG-PET findings and to prove the feasibility of non-invasive measurements of delta using rs-fMRI, we computed these values in 20 iPD (14M/6F, age 59.8± 8.5 [mean ± SD] years) and 20 HC subjects (12M/8F, age 52.6 ± 9.3 years).6 They underwent rs-fMRI on a General Electric 3.0 Tesla Signa HDxt scanner (General Electric, Milwaukee, WI) at North Shore University Hospital. The iPD subjects were scanned in a medication-free (off) state, approximately 12 h after the cessation of antiparkinsonian medication. The details of scan acquisition, network identification, and subject score measurements are provided elsewhere.6 As with FDG-PET, standardized expression values for the rs-fMRI-based PDRP and PDCP topographies were computed in each of the subjects; the resulting measures were used to compute delta as described below. For the rs-fMRI delta measure, test-retest reliability was assessed within-session as described previously.6,27,28
Network Computations
For FDG-PET, expression values for the PDRP and PDCP topographies were computed on a prospective case basis using ScAnVp software (available at http://feinsteinneuroscience.org/). 37 To ensure comparable measures across centers, subject scores for both patterns were z-scored with respect to corresponding local HC values,38 and the resulting values were used to compute delta for each scan. Analogous computations were performed on the rs-fMRI scans as detailed elsewhere.6,39 Delta values were then computed as the difference in PDRP and PDCP z-scores for the subjects in each of the samples.
Statistics
For each sample, we evaluated within-subject differences in PDRP and PDCP expression using paired t-tests. In the cross-sectional samples, group differences in delta were evaluated using one-way ANOVA, incorporating the post-hoc Bonferroni correction for multiple comparisons. A similar approach was used to assess differences in delta for the iPD and APS groups at the four sites. In the longitudinal samples, changes in delta over time were evaluated using RMANOVA in the early iPD (3-time point) data and the paired t-test in the late iPD (2-time point) data. Longitudinal changes in delta for the combined sample were analyzed using a mixed model, with disease duration as a fixed effect and cohort (early/late) as random effects.
To capture trends in delta associated with disease duration, and to facilitate interpretation of measure in individual subjects, we additionally considered delta as an ordinal variable. To this end, individual values were categorized according to sign as being either PDRP predominant (delta≥+0.5), neutral (+0.5>delta>−0.5), or PDCP predominant (delta≤−0.5). The cut-off value ± 0.5 was chosen according to the delta distribution in HC. Differences in the proportion of cases in the various delta categories were evaluated by Chi-square tests. In the data from each site, trends across subgroups ordered by disease duration were evaluated using Jonckheere-Terpstra (J-T) tests.
Delta values from iPD and APS patients at each site were compared using the Kruskal-Wallis test. For iPD and APS samples pooled across sites, the delta distributions were compared using the Kolmogorov-Smirnov test. Finally, the role of delta in differential diagnosis was assessed in the pooled data using logistic regression. We first used PDRP expression and imaging site as predictors and then added delta as an additional predictor. Models were constructed using leave-one-out cross-validation to reduce subject-dependent bias.40 Receiver-operating characteristic (ROC) curves were plotted for both models and the respective areas-under-the-curve (AUC) were compared.41
Statistical tests were performed using JMP 14 (SAS Institute Inc., Cary, NC) and SPSS 28 (IBM Corp., Armonk, NY) and GraphPad Prism v8 (GraphPad Software, San Diego, CA). Results were considered significant for p<0.05 (two tailed).
Data Availability
The data that support the findings of this study are available upon request.
Results
PDRP Predominance in iPD and iRBD
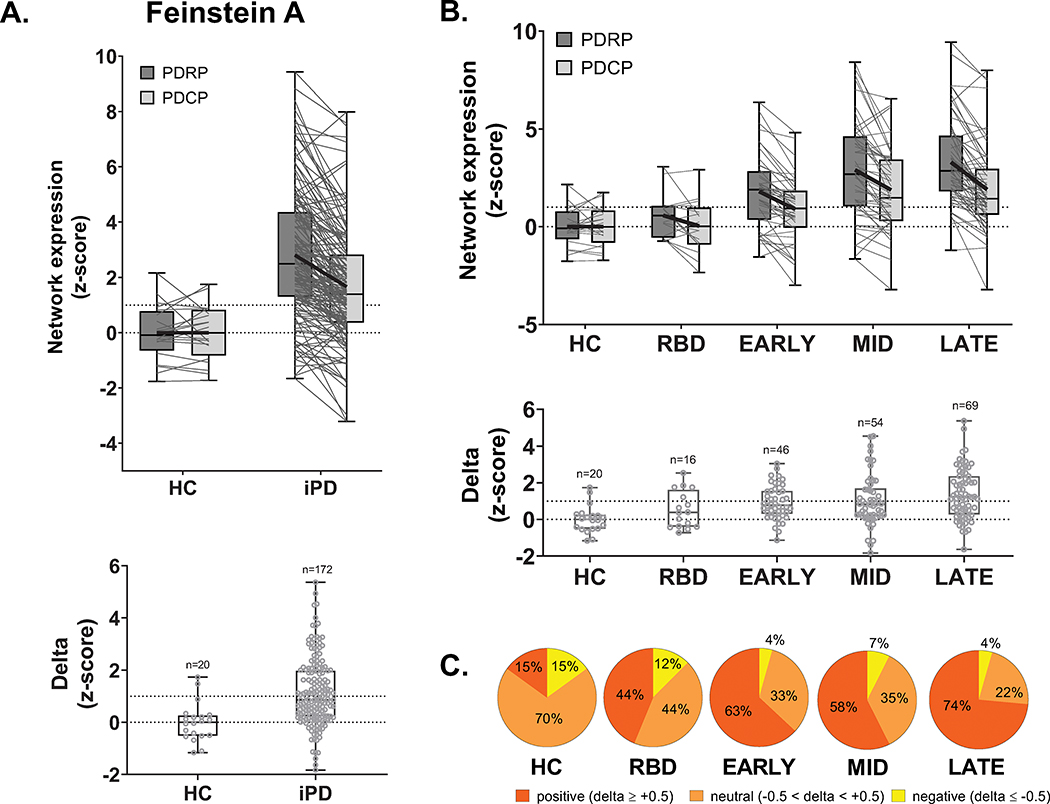
PDRP expression in Feinstein A iPD patients was elevated relative to PDCP values measured in the same subjects (p<0.0001, paired t-test; Fig. 1A, top). Accordingly, delta was positive in these patients, with higher values compared to HC subjects (p=0.0002, Student’s t-test; Fig. 1A, bottom). Delta values displayed excellent test-retest reliability (ICC=0.91, 95% confidence interval (CI) [0.74, 0.97], p<0.0005) (see Methods). When the Feinstein A iPD patients were stratified by duration (Fig. 1B, top), significant increases were observed in the expression of both PDRP (F4,200=14.8, p<0.0001) and PDCP (F4,200=7.7, p<0.0001; one-way ANOVA; Table S4), with higher delta in patients with longer duration (F4,200=5.7, p=0.0002; Fig. 1B, bottom). A similar trend was seen when delta was treated as an ordinal variable (Fig. 1C). Whereas only 15% of HC subjects had delta values greater than +0.5, corresponding values in iPD ranged from 63% in early patients to 74% at late stage (p<0.0005, J-T test).
Figure 1. PDRP predominance in idiopathic Parkinson’s disease (iPD): Cross-sectional analysis.
(A) Top: PDRP and PDCP expression values measured in the cross-sectional Feinstein A sample (see text). The gray lines in the box plots connect PDRP and PDCP values measured in each of the subjects. Bold black lines connect mean PDRP and PDCP values for each group. Bottom: The difference between PDRP and PDCP expression values, termed delta, was computed for each subject. (B) Delta values from iPD patients (n=172) were stratified by symptom duration into early (< 4 years; n=46), intermediate (4–8 years; n=54) and late (> 8 years; n=69) subgroups and compared to at risk idiopathic REM sleep behavior disorder (iRBD) (n=16) and age- and gender-matched healthy control (HC) subjects (n=20). Stepwise increases in this measure were seen with longer duration (F4, 200=14.8, p<0.0001; one-way ANOVA). (C) PDRP predominance, measured in each group by the percentage of delta values ≥0.5 (red), likewise increased with advancing disease (z=4.3, p<0.0005, Jonckheere-Terpstra test of trends; see text).
An analogous effect was present in the longitudinal Feinstein B sample (Fig. S1). PDRP and PDCP expression values rose over time in both early and late stage patients (Feinstein B1: PDRP: p<0.0001, PDCP: p=0.01, RMANOVA; Feinstein B2: PDRP: p=0.002, PDCP: p=0.03, paired t-tests), with increasing delta in both cohorts. To assess this effect in the combined longitudinal sample, we used a mixed regression model, which revealed a highly significant increase in delta over time (t=3.56, p=0.0007), consistent with the cross-sectional data.
Validation in Independent Patient Samples
For validation, we measured PDRP/PDCP expression values and delta in iPD and HC subjects from the Slovenia, India, and South Korea sites (Fig. 2). Mean delta was positive and abnormally elevated in iPD patients compared to corresponding HC subjects (p≤0.03). In these samples, the proportion of PDRP predominant cases ranged from 0–15% in HC, increasing to 28–65% in early and 70–89% in late stage iPD (Slovenia: p<0.0005, India: p=0.047, South Korea: p<0.005; J-T tests).
Figure 2. PDRP predominance in three independent Parkinson’s disease validation samples.
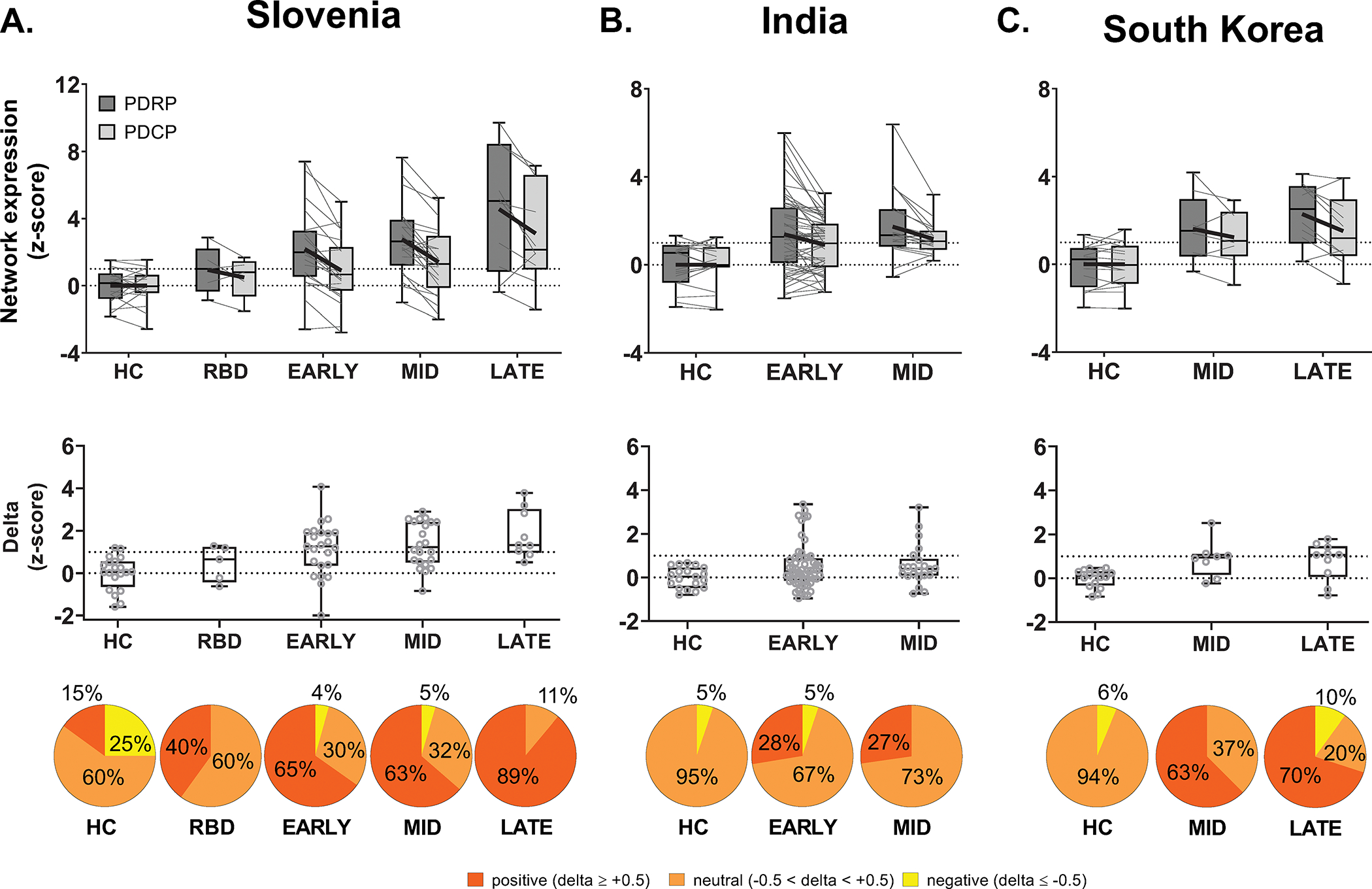
Bar graphs of PDRP and PDCP expression (top) and delta (middle) (mean ± SE) measured in idiopathic Parkinson’s disease (iPD) and healthy control (HC) validation samples from (A) Slovenia, (B) India, and (C) South Korea (see Table S1). For each sample, the gray lines in the box plots (top) connect PDRP and PDCP values measured in each of the subjects. Bold black lines connect mean PDRP and PDCP values for each group. As in Feinstein A, mean delta was positive in the validation samples, denoting greater expression in PDRP relative to PDCP in iPD, with increasing values in patients with longer symptom duration. Also as in Feinstein A, iRBD subjects in the Slovenia sample had delta that was between mean values for HC and early iPD. Bottom: The distribution of delta also differed across the patients in different disease stage (Slovenia: p<0.0005, India: p=0.047, South Korea: p<0.005; Jonckheere-Terpstra tests of trends; see text).
While the iRBD sample was comparatively small, the data suggest that the trend toward PDRP predominance was present before the onset of motor symptoms. Given the similarity of the Feinstein A and Slovenia iRBD data, with comparable elevations in mean delta in both samples (Figs. 1B, 2A), values from the two sites were combined for further analysis. A significant increase was noted compared to HC (p<0.04, Student’s t-test). Indeed, consistent with the trend toward increasing delta with disease progression, 38% of iRBD subjects were PDRP predominant compared to only 15% of HC (p=0.03, Chi-square test).
PDRP Predominance: Effect of Parkinson’s Disease Genotype
To assess the influence of the common PD susceptibility genes on delta, we measured these values (Feinstein D) in PD patients carrying the LRRK2-G2019S mutation (PD-LRRK2) or GBA1 variants (PD-GBA) and compared the measures to matched groups of sporadic PD (sPD) and HC subjects in this sample. Differences in PDRP and PDCP expression have been reported for matched groups of PD-LRRK2, PD-GBA, and sporadic iPD patients (Table S3). Delta likewise differed across the groups (p=0.0003; one-way ANOVA; Fig. 3A, top), with greater values in PD-GBA compared to PD-LRRK2 (p=0.005) but not sPD (p=0.15, post-hoc tests). Genotypic differences in delta were reflected in the proportion of PDRP predominant cases in each group (Fig. 3A, bottom). 57% of sPD patients in the sporadic group had delta values of +0.5 or greater, which was similar to the corresponding percentages in iPD subgroups of equivalent duration (Table S5). By contrast, 83% of PD-GBA were PDRP predominant but only 43% of PD-LRRK2.
Figure 3. Effect of genotype on PDRP predominance.
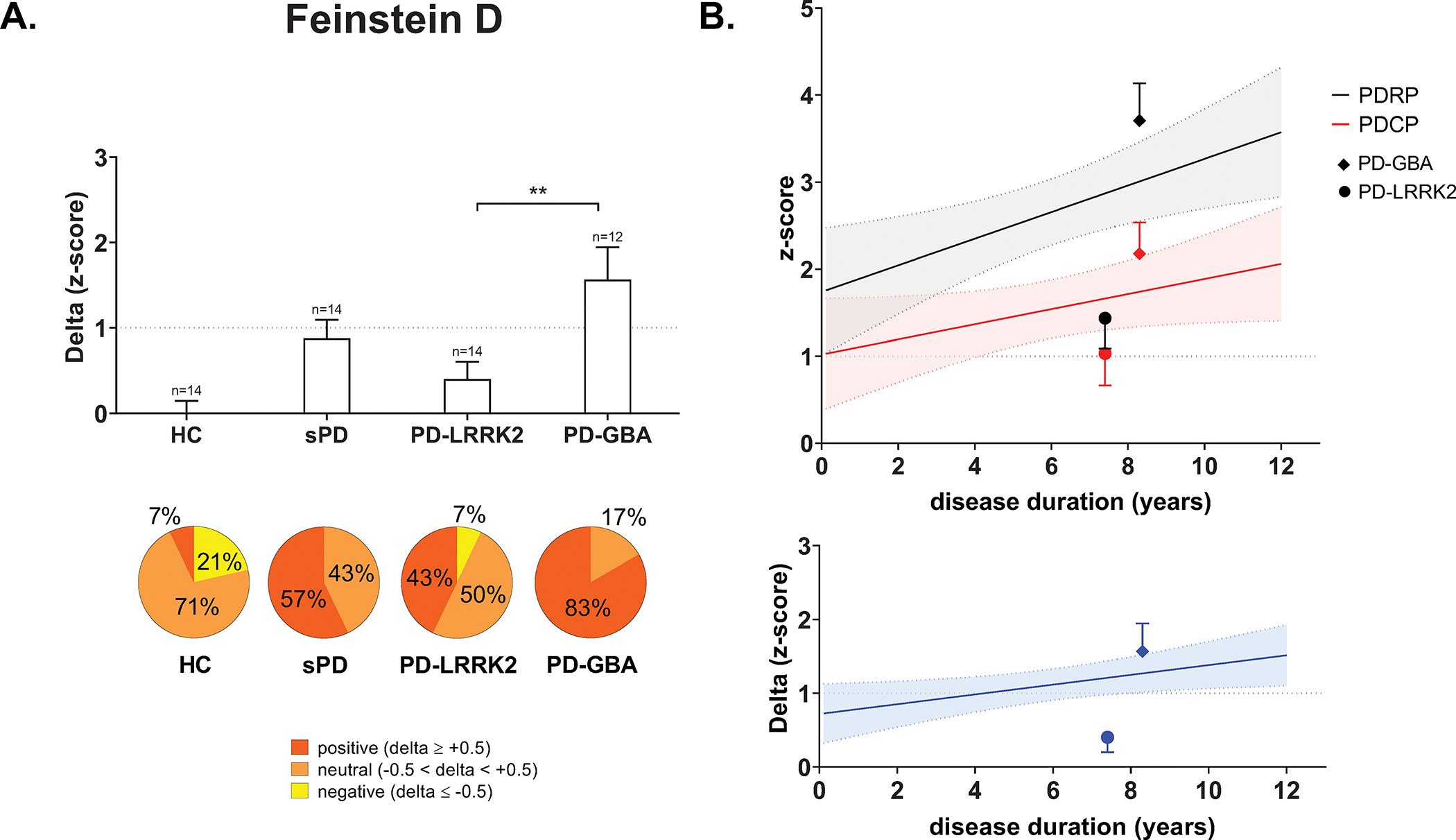
(A) Top: Bar graphs of delta (mean ± SE) computed in genotypic Parkinson’s disease (gPD) patients with the LRRK2-G2019S mutation (PD-LRRK2), GBA1 variants (PD-GBA), and a matched group with sporadic disease (sPD) as well as healthy control subjects (HC). Significant differences in the measure were seen across groups (F3, 50=7.5, p=0.0003; one-way ANOVA), with greater values in PD-GBA compared to PD-LRRK2 (**p<0.01; post-hoc Bonferroni test). Bottom: The distribution of delta values also differed across the patients with pronounced PDRP dominance (red) in PD-GBA compared to the other groups (χ23=16.6, p=0.0009; Kruskal-Wallis test). (B) Top: Mean pattern expression for the PD-LRRK2 and PD-GBA groups (see text) plotted with respect to regression lines of pattern expression against duration determined based on the Feinstein A sample (PDRP, black line; PDCP, red line). Bottom: Mean delta for the PD-LRRK2 and PD-GBA groups plotted with respect to the corresponding regression line based on the Feinstein A sample (blue line). Despite increased expression of both patterns in PD-GBA, delta for this genotype was at the upper bound of the 95% confidence interval for the non-genotypic iPD reference sample. In PD-LRRK2, by contrast, mean delta fell below the regression line, outside the 95% confidence interval of the reference sample.
We additionally examined the relationship of delta in PD-LRRK2 and PD-GBA with corresponding values in non-genotypic iPD patients as a function of symptom duration. In PD-GBA, mean PDRP expression exceeded the 95% CI of the regression line against duration calculated based on the non-genotypic Feinstein A iPD sample; mean PDCP was on the upper bound of the corresponding regression line (Fig. 3B, top). In PD-LRRK2, by contrast, mean PDRP and PDCP expression levels were both below the lower bounds of the corresponding confidence intervals. Applying the same approach to delta (Fig. 3B, bottom), we found that the mean value for PD-GBA was at the upper bound of the 95% CI of the Feinstein A regression line, while that for PD-LRRK2 was below the corresponding lower bound. In aggregate, the findings suggest that PDRP predominance in PD-LRRK2 is less than would be predicted for patients with non-genotypic disease of comparable duration.
Delta in Atypical Parkinsonian Syndromes
In contrast to iPD, in which delta was frequently positive in all samples, this effect was not observed in APS patients scanned at the same sites (Fig. S2). Thus, delta differed significantly for iPD, MSA, and PSP patients in each of the samples (p≤0.0002; one-way ANOVA; Table S6). For MSA, the measure was reduced compared to iPD for patients in the Feinstein, Slovenia, and India sites, but not in the South Korea sample. That said, at all four sites, PSP patients had lower delta values compared to iPD and MSA.
We next compared the distribution of delta values for iPD and APS patients over the entire study population. To this end, individual subjects were categorized by their ordinal values, and positive, neutral, and negative cases were pooled separately across sites (Table S7). We found that the distribution of delta values (Fig. 4A) differed significantly for the pooled iPD and APS samples (d=0.36, p<0.0001; Kolmogorov-Smirnov test). 93% of the iPD population (Fig. 4B, top) had delta values that were either positive or neutral; negative values were observed in only 7% of cases. By contrast, in the APS population (Fig. 4B, bottom), the proportions of positive, neutral, and negative cases were similar, with 30–40% in each category.
Figure 4. Frequency distribution of individual delta values from pooled idiopathic Parkinson’s disease and atypical parkinsonian syndrome.
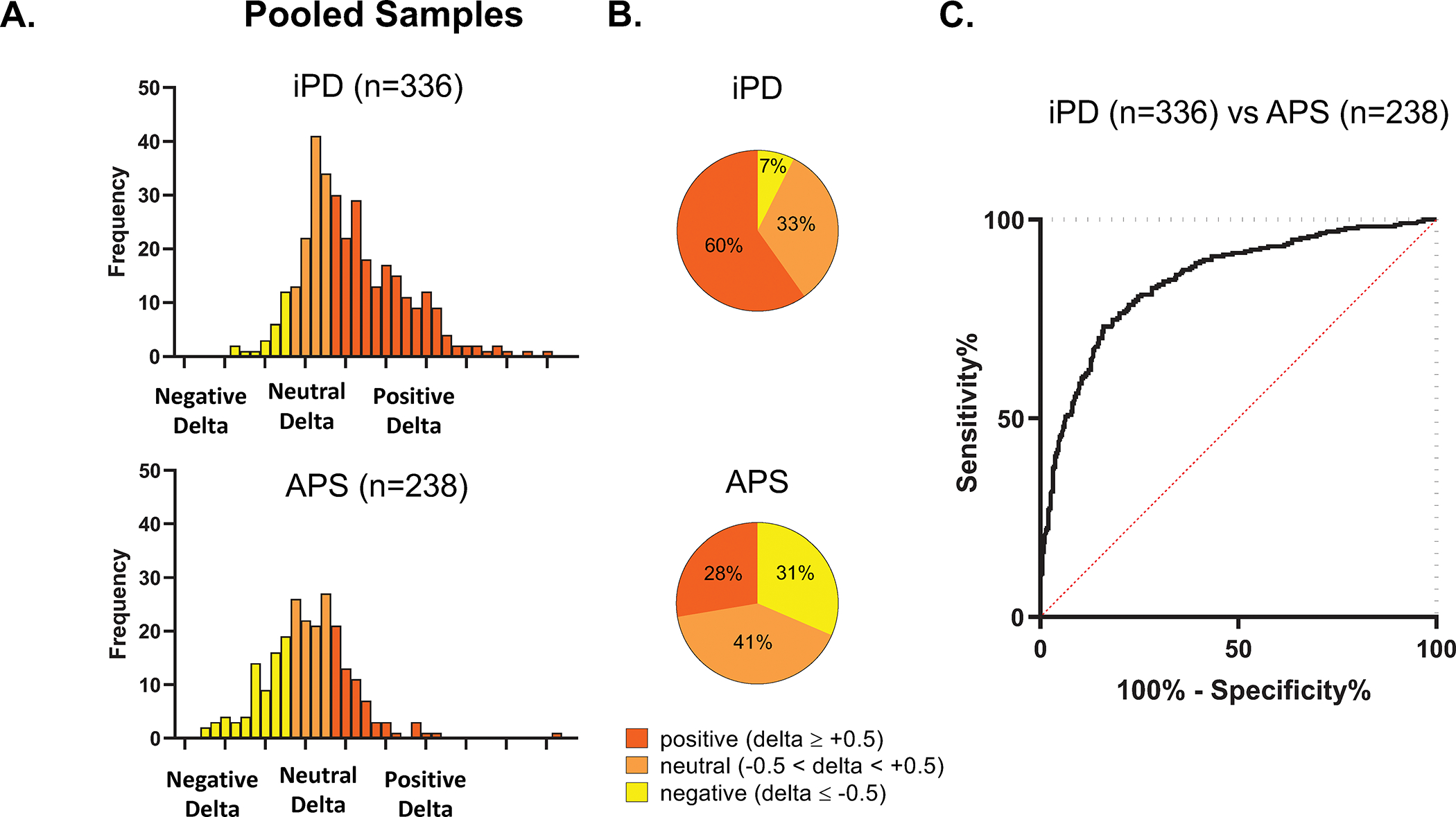
(A) Frequency histograms for delta values from the pooled samples of iPD (n=336) and APS (n=238) patients from the four sites (see Fig. S2). Delta values in each diagnostic category were divided into groups based on the sign and magnitude of the individual measurements (see text). The distribution of the individual delta values differed significantly for the two populations (d=0.36, p<0.0001; Kolmogorov-Smirnov test). (B) Displays of delta values in the pooled samples revealed a significant difference in the proportion of PDRP predominant cases (red), with 60% in iPD compared to 28% in APS. The proportion of PDCP dominant cases (yellow) also differed, with 7% in iPD compared to 31% in APS. (C) Receiver-operating characteristic (ROC) curve showing accurate discrimination of iPD and APS patients across samples based on PDRP expression and delta (AUC=0.85, p<0.0001; see text).
To determine whether delta can be used to enhance diagnostic accuracy, we constructed a logistic regression model to discriminate iPD from APS based on PDRP expression and delta values. Indeed, this two predictor model (Fig. 4C) was accurate (AUC=0.85, 95% CI [0.82, 0.88]; ROC analysis) and provided better discrimination than one based on PDRP alone (AUC=0.80, 95% CI [0.77, 0.84], p<0.0005; paired difference).
Non-invasive Measurements of Delta using Resting-state fMRI
Lastly, we note that delta can also be computed using PDRP and PDCP expression values obtained non-invasively using rs-fMRI. As with FDG-PET, expression values for PDRP were greater than PDCP (Fig. 5A) in rs-fMRI scans from iPD patients (p<0.002; paired t-test) but not HC (p>0.99). Delta values computed in the rs-fMRI data exhibited good test-retest reliability (ICC=0.75, 95% CI [0.65, 0.82], p=0.003). Mean delta was positive in the iPD group (Fig. 5B), with significantly elevated values compared to HC (p=0.004, Student’s t-test). Likewise, the distribution of delta values also differed across groups (Fig. 5C), with PDRP predominance in 75% of iPD but only 30% of control subjects (p=0.002, Mann-Whitney U test).
Figure 5. Increased delta in idiopathic Parkinson’s disease: Non-invasive measurement with resting-state fMRI.
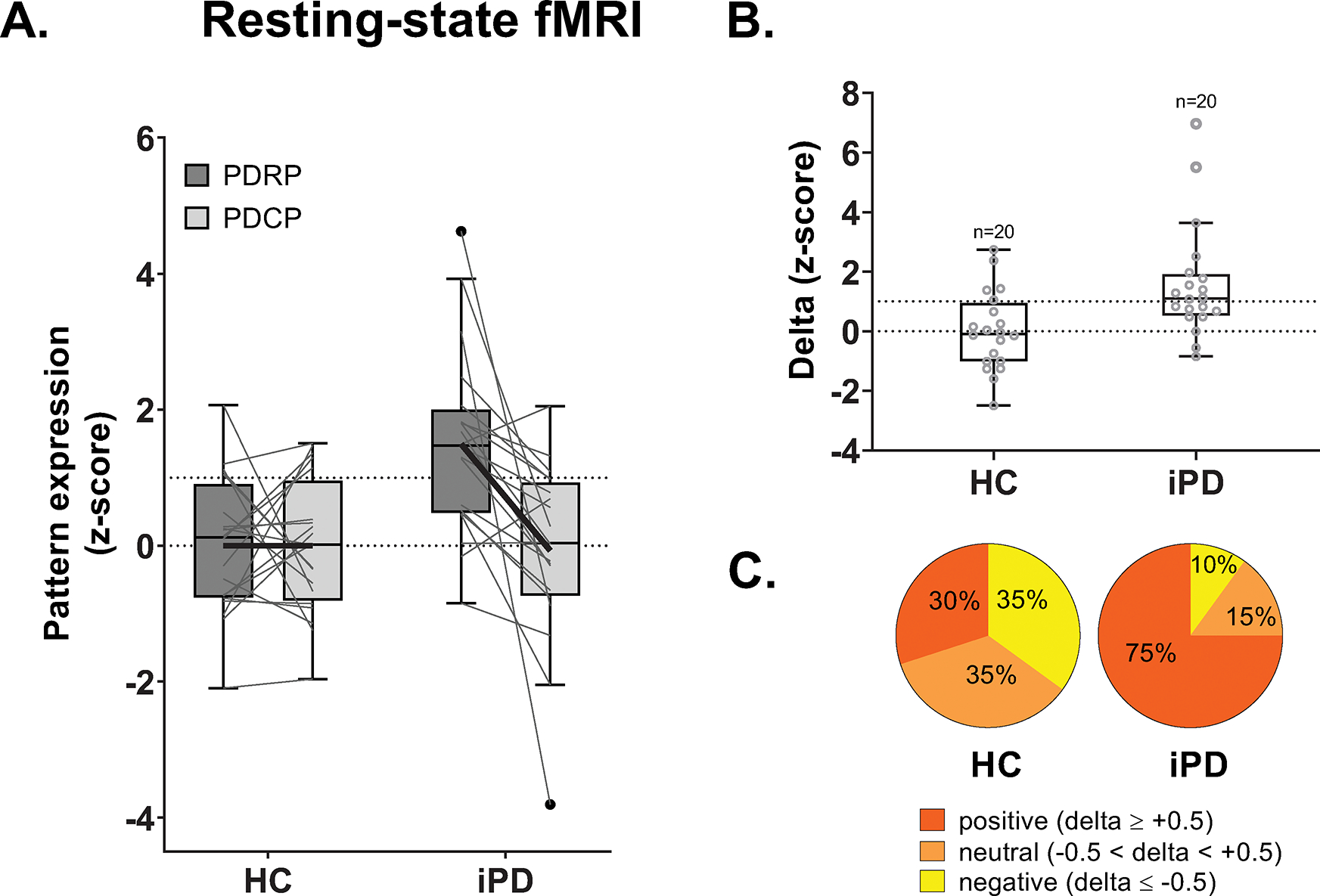
(A) PDRP and PDCP expression values measured in rs-fMRI scans from 20 idiopathic Parkinson’s disease (iPD) and 20 healthy control (HC) subjects (see Methods). The gray lines in the box plots connect PDRP and PDCP values measured in each of the subjects. Bold black lines connect mean PDRP and PDCP values for each group. (B) Delta was computed for each subject. Values were significantly increased in iPD subjects compared to HC subjects (p=0.004, Student’s t-test), even after excluding two outliers with very high delta values (p=0.01). (C) As with FDG-PET, the proportion of PDRP predominant cases was greater in iPD compared to HC (p=0.002; Mann-Whitney U test), with 75% in patients and 30% in control subjects. [The whiskers were plotted by the Tukey method.]
Discussion
Idiopathic PD is characterized by PDRP predominance that is present consistently in multiple independent patient samples, both cross-sectional and longitudinal. The relationship between PDRP and PDCP was remarkably constant across cross-sectional cohorts regardless of minor local differences in patient recruitment and imaging protocols. Namely, in the two cohorts that underwent FDG-PET on levodopa treatment (Slovenia and South Korea), medication may have caused lower PDRP and consequently delta values.8 However, despite this effect, we were still able to capture significant PDRP predominance that increased with disease duration. The PDRP comprises metabolic changes in the brainstem, basal ganglia, and limbic regions, the functional counterparts of Braak stage III-IV pathology.7,42,43 The PDCP on the other hand involves changes in the ventral default mode network (DMN), as well as other fronto-temporal regional components44,45 characterizing functional abnormalities that involve neocortical regions which are associated with Braak stage V.44,45 Given that network progression in iPD is linear and the rise in PDRP expression begins earlier than PDCP,5,8–11 delta positivity can be viewed as representing the stereotyped caudo-rostral sequence of pathological involvement that characterizes this disorder.1,4 Accordingly, Parkinson’s disease patients with clinically aggressive genotypes such as PD-GBA have greater degree of PDRP predominance than their non-genotypic counterparts, consistent with faster progression in this genotype.31 While the less aggressive PD-LRRK2 mutation exhibits PDRP predominance, delta values were lower than in sporadic iPD patients of comparable duration and disability, consistent with slower disease progression in this genotype.30 That said, longitudinal imaging studies are needed to document changes in delta that take place over time in each genotype. Similarly, potential differences in delta across different iPD phenotypes46 may be indicated by comparing the measure in iPD patients from different sites. While no specific criteria were used to recruit iPD patients in Feinstein A, Slovenia and India, those in the South Korea cohort were limited to patients with intermediate or late disease duration without dementia. We hypothesize that majority of these patients had benign mild-motor or intermediate iPD given that dementia appears early in the more severe phenotype.46 Consistent with the genotype analysis, the specific recruitment of mild or intermediate patients would explain the relatively low PDRP, PDCP and delta values seen in this population. Similarly, the difference in recruitment among sites may also explain the plateau, which was present in Feinstein A in late-stage patients (>8 years). The Feinstein A subjects underwent FDG-PET imaging on an outpatient basis, which may have limited participation of severely affected patients with dementia. However, an analogous plateau was not observed in the Slovenia cohort, in which a large percentage of patients were hospitalized with advanced disease at the time of imaging and also had significant cognitive loss.
Despite a significant trend of delta progression over time in all the cross-sectional (Table S5) and longitudinal cohorts, we did not find significant differences between subgroups classified by disease duration in the cross-sectional cohorts (Table S4). This may have been due to relatively small number of participants in each of the subgroups.
In addition to increasing steadily with disease progression, PDRP predominance is likely to have begun before the onset of motor manifestations. Thus, individuals with iRBD studied at two independent sites had increased frequencies of positive delta compared to matched groups of healthy control subjects. That said, larger iRBD samples will be needed to substantiate this finding.
The stereotyped sequence of pathological changes inherent to iPD is not present in APS such as MSA and PSP.16–18 Moreover, the metabolic patterns associated with these disorders are distinct from the PDRP, and individual patients may express more than a single network in a given scan.25,34,35 That said, delta measurements from the four sites revealed PDRP predominance in over 60% of iPD patients compared to only 28% of APS. It is noteworthy that while iPD and APS patients were separable based on PDRP expression (AUC=0.80), diagnostic accuracy was significantly improved when delta was included in the logistic regression model. Superior accuracy (AUC>0.90) was achievable, however, using a 2-level logistic regression procedure based on PDRP, MSA-related pattern (MSARP), and PSP-related pattern (PSPRP) expression values.25,33,34,47 Even so, the simpler model based on PDRP and delta has a number of advantages. Unlike PDRP and PDCP, MSARP and PSPRP have not been extensively validated across centers and imaging platforms.33,34,48–50 This is particularly relevant in the rs-fMRI domain in which PDRP and PDCP networks have recently been identified and validated,6,39,44 whereas MSA- and PSP-related networks analogous to those identified using FDG-PET have yet to be characterized using this modality. Our preliminary results suggest that the stereotyped relationship between PDRP and PDCP can also be discerned using non-invasive rs-fMRI techniques, which are better suited for large-scale clinical trials and potential diagnostic use.
In summary, the data suggest that delta, as the difference between PDRP and PDCP expression in a given patients, provides unique information regarding the underlying disease process that is not conveyed by either measure alone. Indeed, by incorporating information regarding the spread of metabolic pathology rostrally beyond the PDRP space, delta can be used to determine whether the observed changes are consistent with an iPD reference sample of equivalent duration. In this regard, increased delta would denote greater progression than predicted based on the reference population, whereas reduced delta suggests potentially less aggressive disease.
Supplementary Material
Acknowledgments
The authors thank Drs. Madhavi Tripathi and Chong Sik Lee for sharing FDG-PET data from their subjects and Dr. Luka Ležaić for FDG-PET data processing from UMC Ljubljana.
Funding Sources:
Aspects of this work were supported by grants to D.E. from the National Institute of Neurological Disorders and Stroke (P50 NS 071675 [Morris K. Udall Center of Excellence for Parkinson’s Disease Research at The Feinstein Institute for Medical Research]) and The Michael J. Fox Foundation for Parkinson’s Research and to M.T. from the Slovenian Research Agency (grant P1-0389, research projects J7-2600 and J7-3150). T.R. is a recipient of the Fulbright Foreign Student Program sponsored by the U.S. Department of State’s Bureau of Educational and Cultural Affairs. K.A.S. is the recipient of a Research Fellowship for Clinical Neurologists (PF-FBS-1929) from the Parkinson’s Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the other funding agencies.
Footnotes
Conflict of Interest: D.E. serves on the scientific advisory boards of and has received fees from The Michael J. Fox Foundation for Parkinson’s Research and Ovid Therapeutics (unrelated to manuscript); receives consulting fees from MeiraGTx (unrelated to manuscript); has received grants from NIH (NINDS, NIAID) (unrelated to manuscript); and is the coinventor of patents re: Markers for use in screening patients for nervous system dysfunction and a method and apparatus for using same, without financial gain. M.T. has received honoraria from CARSOpharm d.o.o. and AbbVie Biofarmacevtska druzba d.o.o. for lectures and consultancies. All other authors disclose no relevant conflict of interest.
References
- 1.Braak H, Tredici K Del, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 2.Beach TG, Adler CH, Lue LF, et al. Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117(6):613–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler CH, Beach TG, Zhang N, et al. Unified staging system for Lewy body disorders: Clinicopathologic correlations and comparison to Braak staging. J Neuropathol Exp Neurol. 2019;78(10):891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson’s disease. Park Relat Disord. 2010;16(2):79–84. [DOI] [PubMed] [Google Scholar]
- 5.Eidelberg D Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009;32(10):548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vo A, Sako W, Fujita K, et al. Parkinson’s disease-related network topographies characterized with resting state functional MRI. Hum Brain Mapp. 2017;38(2):617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schindlbeck KA, Eidelberg D. Network imaging biomarkers: insights and clinical applications in Parkinson’s disease. Lancet Neurol. 2018;17(7):629–640. [DOI] [PubMed] [Google Scholar]
- 8.Niethammer M, Eidelberg D. Metabolic brain networks in translational neurology: Concepts and applications. Ann Neurol. 2012;72(5):635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Tang C, Feigin A, et al. Changes in network activity with the progression of Parkinson’s disease. Brain. 2007;130(7):1834–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang CC, Poston KL, Dhawan V, Eidelberg D. Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson’s disease. J Neurosci. 2010;30(3):1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holtbernd F, Gagnon J-F, Postuma RB, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology. 2014;82(7):620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu P, Yu H, Peng S, et al. Consistent abnormalities in metabolic network activity in idiopathic rapid eye movement sleep behaviour disorder. Brain. 2014;137(12):3122–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meles SK, Renken RJ, Janzen AHO, et al. The metabolic pattern of idiopathic REM sleep behavior disorder reflects early-stage Parkinson’s disease. J Nucl Med. 2018;59(9):1437–1444. [DOI] [PubMed] [Google Scholar]
- 14.Shin JH, Lee JY, Kim YK, et al. Parkinson Disease-Related Brain Metabolic Patterns and Neurodegeneration in Isolated REM Sleep Behavior Disorder. Neurology. 2021;97(4):e378–e388. [DOI] [PubMed] [Google Scholar]
- 15.Han X, Wu P, Alberts I, et al. Characterizing the heterogeneous metabolic progression in idiopathic REM sleep behavior disorder. NeuroImage Clin. 2020;27:102294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovacs GG, Lukic MJ, Irwin DJ, et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 2020;140(2):99–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briggs M, Allinson KSJ, Malpetti M, Spillantini MG, Rowe JB, Kaalund SS. Validation of the new pathology staging system for progressive supranuclear palsy. Acta Neuropathol. 2021;141(5):787–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brettschneider J, Irwin DJ, Boluda S, et al. Progression of alpha-synuclein pathology in multiple system atrophy of the cerebellar type. Neuropathol Appl Neurobiol. 2017;43(4):315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain. 2019;142(3):744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284–2303. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong MJ, Okun MS. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA - J Am Med Assoc. 2020;323(6):548–560. [DOI] [PubMed] [Google Scholar]
- 23.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72(15):1296–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattis PJ, Niethammer M, Sako W, et al. Distinct brain networks underlie cognitive dysfunction in Parkinson and Alzheimer diseases. Neurology. 2016;87(18):1925–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang CC, Poston KL, Eckert T, et al. Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol. 2010;9(2):149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko JH, Feigin A, Mattis PJ, et al. Network modulation following sham surgery in Parkinson’s disease. J Clin Invest. 2014;124(8):3656–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallgren KA. Computing Inter-Rater Reliability for Observational Data: An Overview and Tutorial. Tutor Quant Methods Psychol. 2012;8(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cicchetti DV. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instruments in Psychology. Psychol Assess. 1994;6(4):284–290. [Google Scholar]
- 29.Schindlbeck KA, Gupta DK, Tang CC, et al. Neuropathological correlation supports automated image-based differential diagnosis in parkinsonism. Eur J Nucl Med Mol Imaging. 2021;48(11):3522–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saunders-Pullman R, Mirelman A, Alcalay RN, et al. Progression in the LRRK2-Asssociated Parkinson Disease Population. JAMA Neurol. 2018;75(3):312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis MY, Johnson CO, Leverenz JB, et al. Association of GBA mutations and the E326K polymorphism with motor and cognitive progression in parkinson disease. JAMA Neurol. 2016;73(10):1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schindlbeck KA, Vo A, Nguyen N, et al. LRRK2 and GBA Variants Exert Distinct Influences on Parkinson’s Disease-Specific Metabolic Networks. Cereb Cortex. 2020;30(5):2867–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripathi M, Tang CC, Feigin A, et al. Automated Differential Diagnosis of Early Parkinsonism Using Metabolic Brain Networks: A Validation Study. J Nucl Med. 2016;57(1):60–66. [DOI] [PubMed] [Google Scholar]
- 34.Rus T, Tomše P, Jensterle L, et al. Differential diagnosis of parkinsonian syndromes: a comparison of clinical and automated - metabolic brain patterns’ based approach. Eur J Nucl Med Mol Imaging. 2020;47(12):2901–2910. [DOI] [PubMed] [Google Scholar]
- 35.Ko JH, Lee CS, Eidelberg D. Metabolic network expression in parkinsonism: Clinical and dopaminergic correlations. J Cereb Blood Flow Metab. 2017;37(2):683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friston K, Ashburner J, Kiebel S, Nichols T, Penny W, eds. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London: Elsevier; 2007. [Google Scholar]
- 37.Spetsieris PG, Eidelberg D. Scaled subprofile modeling of resting state imaging data in Parkinson’s disease: Methodological issues. Neuroimage. 2011;54(4):2899–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomše P, Jensterle L, Rep S, et al. The effect of 18F-FDG-PET image reconstruction algorithms on the expression of characteristic metabolic brain network in Parkinson’s disease. Phys Medica. 2017;41:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rommal A, Vo A, Schindlbeck KA, et al. Parkinson’s disease-related pattern (PDRP) identified using resting-state functional MRI: Validation study. Neuroimage: Reports. 2021;1(3):100026. [Google Scholar]
- 40.Demšar J, Curk T, Erjavec A, et al. Orange: Data mining toolbox in python. J Mach Learn Res. 2013;14:2349–2353. [Google Scholar]
- 41.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 42.Ko JH, Spetsieris PG, Eidelberg D. Network structure and function in Parkinson’s disease. Cereb Cortex. 2018;28(12):4121–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spetsieris PG, Eidelberg D. Spectral guided sparse inverse covariance estimation of metabolic networks in Parkinson’s disease. Neuroimage. 2021;226:117568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schindlbeck KA, Vo A, Mattis PJ, et al. Cognition-Related Functional Topographies in Parkinson’s Disease: Localized Loss of the Ventral Default Mode Network. Cereb Cortex. 2021;31(11):5139–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spetsieris PG, Ko JH, Tang CC, et al. Metabolic resting-state brain networks in health and disease. Proc Natl Acad Sci U S A. 2015;112(8):2563–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Pablo-Fernández E, Lees AJ, Holton JL, Warner TT. Prognosis and Neuropathologic Correlation of Clinical Subtypes of Parkinson Disease. JAMA Neurol. 2019;76(4):470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papathoma P-E, Markaki I, Tang CC, et al. A replication study, systematic review and meta-analysis of automated image-based diagnosis in parkinsonism. Sci Rep. 2022;12(1):2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eckert T, Tang C, Ma Y, et al. Abnormal metabolic networks in atypical parkinsonism. Mov Disord. 2008;23(5):727–733. [DOI] [PubMed] [Google Scholar]
- 49.Martí-Andrés G, van Bommel L, Meles SK, et al. Multicenter Validation of Metabolic Abnormalities Related to PSP According to the MDS-PSP Criteria. Mov Disord. 2020;35(11):2009–2018. [DOI] [PubMed] [Google Scholar]
- 50.Teune LK, Bartels AL, De Jong BM, et al. Typical cerebral metabolic patterns in neurodegenerative brain diseases. Mov Disord. 2010;25(14):2395–2404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon request.