Summary
Analysis of tumor infiltrating lymphocyte (TIL) functional states, particularly tumor-reactive PD-1T TILs, within specific spatial context, can server as a biologically informed predictive marker of immunotherapy that may be superior to standard clinical biomarkers. High-plex quantitative immune cell phenotyping within their spatial context has tremendous potential in immuno-oncology.
In this issue of Clinical Cancer Research, Hummelink and colleagues report on PD-1T TILs, a tumor-reactive tumor infiltrating T lymphocyte (TIL) pool, as a predictive biomarker for immunotherapy in non-small cell lung cancer (NSCLC) (1). PD-1T TILs represent an intratumoral CD8+ T cell population with high PD-1 expression, distinct transcriptional profiles and increased tumor recognition capacity (2). This subset of PD-1+ tumor infiltrating T cells is preferentially recruited in tertiary lymphoid structures (TLS) and can be identified by bright PD-1 expression that can be digitally quantified and distinguished from other PD-1+ cells (1, 2). Hummelink and colleagues report their findings on the predictive accuracy of PD-1T TILs in the context of immune checkpoint inhibitor (ICI) therapy for patients with non-small cell lung cancer (NSCLC) receiving nivolumab or pembrolizumab. Following a digital workflow for PD-1T TIL quantification in formalin fixed paraffin embedded tissue, the authors evaluated the association of PD-1T TIL density with clinical outcomes, focusing on disease control at 6 months (a surrogate endpoint also known as durable clinical benefit (3)) as their primary endpoint. The predictive accuracy of PD-1T TIL density (AUC ROC 0.72-0.79) was superior to that of PD-L1 TPS score, commonly used in NSCLC to identify tumors more likely to regress with immune checkpoint inhibitors-ICI (AUC ROC 0.58). Notably, the predictive nature of PD-1T TILs may be enhanced for determining long-term clinical outcome and sustained clinical response past 6 months (ROC AUC 0.79-0.89 for prediction of disease control at 12 months). As PD-1T TILs were predominantly found in tertiary lymphoid structures (1, 2) and the role of mature TLS in anti-tumor immune responses in the context of immune checkpoint blockade (4), the authors investigated the incremental value of assessing PD-1T TILs over the number of TLS within the analyzed tumors; these analyses showed that the predictive value of PD-1T TILs was not driven by TLS density alone (ROC AUC for the latter 0.62). Taken together, these findings build on the previously reported role of this functionally distinct subset of CD8+ intratumoral T cells (2) and support PD-1T TILs as a putative determinant of response to immune checkpoint blockade and suggest that prospective validation in larger cohorts should be prioritized.
The study of Hummelink et al., emphasizes that a nuanced spatially-informed quantitative analysis, that captures T cell populations with unique functional properties and tumor recognition capacities, may more accurately identify individuals more likely to respond to immune checkpoint blockade compared to conventionally used biomarkers. Currently established predictive biomarkers of ICI response include microsatellite instability (MSI) (5), which is detected in <5% of human cancers, as well as PD-L1 expression and tumor mutation burden (TMB), that both suffer from technical and biological limitations. The clinical utility of PD-L1 testing varies based on the cancer type evaluated and the ICI therapy considered (6), with several phase III trials failing to reproduce the association between PD-L1 expression and ICI response (7, 8). Similarly, with the exception of MSI-high tumors, the predictive value of TMB is cancer-lineage dependent (9) and not consistently predictive of ICI response (10, 11). In contrast to PD-L1 expression or TMB that serve as surrogates of an anti-tumor immune response, PD-1T TILs are an indicator that an effective tumor-specific T cell response has occurred and can therefore serve as a biologically relevant measure of clinical outcomes. Furthermore, as PD-1T TIL density was largely independent from PD-L1 TPS in the study by Hummelink et al.; it is conceivable that PD-1T TIL density may be informative for PD-L1 negative tumors as well as tumors with PD-L1 TPS in the gray zone of 1-50% (Figure 1). Conceptually, PD-1T TILs can be used as a footprint for active tumor-specific adaptive immune responses and therefore might enable patient selection for immune checkpoint inhibitors in cancers with marginal anti-PD1 response rates, for example ovarian and breast cancer.
Figure 1. Impact and roles for spatially resolved high-plex assays in immunotherapy treatment and biomarker discovery.
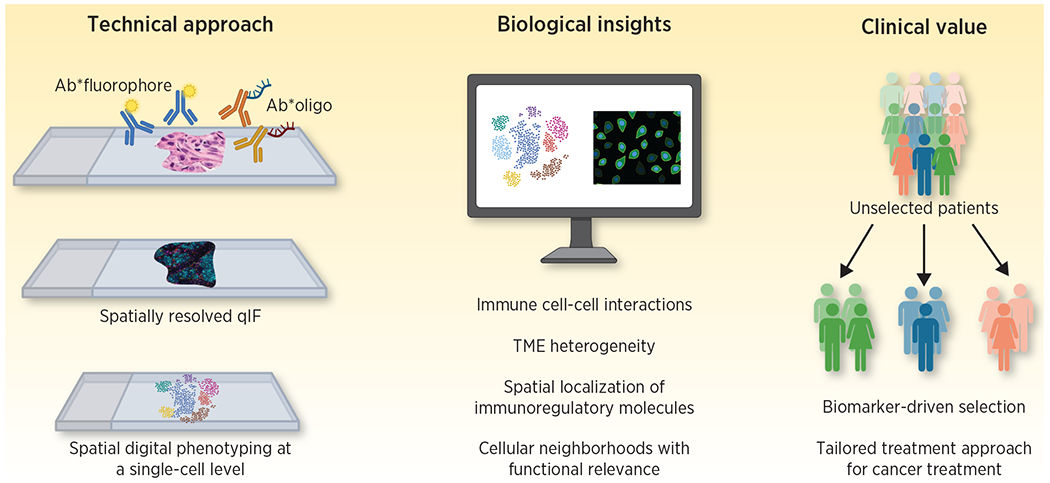
Spatially resolved high-plex methods including quantitative immunofluorescence and digital spatial profiling are high throughput approaches that allow for simultaneous identification of multiple biomarkers in their spatial context. These methods have the unique potential to provide insights in the phenotype and spatial localization of immune cell subsets and thus serve as biology-informed biomarkers reflecting the quality and architecture of anti-tumor immune responses. As such, they can be incorporated in patient selection strategies for cancer immunotherapy as well as used as a platform for novel biomarker discovery. Adapted from an image created with BioRender.com.
The value of TILs in reflecting adaptive anti-tumor immune responses and ultimately clinical responses with ICI therapy has been previously demonstrated (12), with emerging studies supporting the additive benefit of considering TIL functional profiles and their spatial localization within the tumor microenvironment (TME). To this end, spatially resolved multiplex immunofluorescence analyses have uniquely enabled spatial mapping of immune cells and assessment of their heterogeneity in the TME (13–15), revealing relationships among TIL subpopulations that are linked with differential ICI clinical outcomes (16). Furthermore, evaluation of PD-1/PD-L1 proximity rather than PD-L1 expression alone may more optimally distinguish tumors more likely to regress with ICI therapy (17). In addition to evaluation of the PD-1/PD-L1 axis, spatially resolved quantitative immunofluorescence approaches have the potential to interrogate interactions and localization of immunoregulatory molecules such as IDO-1, LAG-3, TIGIT, TIM-3, and VISTA, providing a unique opportunity to understand mechanisms of response and resistance to novel checkpoint inhibitors currently tested in clinical trials. Overall, these approaches have been shown to more accurately predict ICI response compared to PD-L1 expression and tumor mutation burden (18).
Similarly, spatial phenotyping by reconstruction of cellular neighborhoods has pointed towards local enrichment in immune cell subpopulations and differential organization of the TME that is reflective of distinct anti-tumor immunity states (19). Implementation of photo-cleavable oligonucleotide tags attached to antibodies or RNA probes has further increased the multiplexing capacity, dynamic range and level of detection of digital spatial profiling approaches. Spatial transcriptomics represent another avenue of interrogation of immune cell spatial heterogeneity, with neoantigen-reactive T cell clones shown to harbor unique transcriptomic profiles that are further differentiated in the TME of ICI responsive tumors (20). While spatially resolved and high-plex assays may uniquely assess the immune contexture of tumors at a single-cell resolution, further standardization is required to generate analytical platforms that allow for measurement of complex spatial associations. Notably, these approaches are more likely to succeed when representative of spatial and functional interactions, following the paradigm of the study by Hummelink and colleagues that relied on interrogation of a TIL subset previously functionally characterized and found to be tumor-reactive (2).
Collectively, high-plex quantitative evaluation of immune cell subpopulation phenotypes, in their spatial context, holds unique promise as a near-term improved biomarker of treatment response and has tremendous potential for ICI biomarker discovery, especially for the subset of tumors with low PD-L1 expression and/or low tumor mutation burden.
Funding:
This work was supported in part by the US National Institutes of Health grants CA121113 (to VA) and UM1CA186690-06, P50CA254865-01A1, P30CA047904-32, and 1R01DE031729-01A1 (to JL), and the Department of Defense Congressionally Directed Medical Research Programs grant CA190755 (VA).
Conflict of Interest
VA: V.A. receives research funding to Johns Hopkins University from Astra Zeneca and Delfi Diagnostics and has received research funding to Johns Hopkins University from Bristol-Myers Squibb in the past 5 years. V.A is an inventor on patent applications (63/276,525, 17/779,936, 16/312,152, 16/341,862, 17/047,006 and 17/598,690) submitted by Johns Hopkins University related to cancer genomic analyses, ctDNA therapeutic response monitoring and immunogenomic features of response to immunotherapy that have been licensed to one or more entities. Under the terms of these license agreements, the University and inventors are entitled to fees and royalty distributions.
JJL: DSMB: Abbvie, Immutep, Evaxion; Scientific Advisory Board: (no stock) 7 Hills, Bright Peak, Exo, Fstar, Inzen, RefleXion, Xilio (stock) Actym, Alphamab Oncology, Arch Oncology, Kanaph, Mavu, NeoTx, Onc.AI, OncoNano, Pyxis, STipe, Tempest; Consultancy with compensation: Abbvie, Bayer, Bristol-Myers Squibb, Castle, CHECKMATE, Codiak, Crown, Day One, Duke St, EMD Serono, Endeavor, Flame, Genentech, Gilead, Glenmark, HotSpot, Kadmon, Janssen, Ikena, Immunocore, Incyte, IO Biotech, Macrogenics, Merck, Nektar, Novartis, Partner, Pfizer, Regeneron, Roivant, Servier, STINGthera, Synlogic, Synthekine; Research Support: (all to institution for clinical trials) AbbVie, Astellas, Astrazeneca, Bristol-Myers Squibb, Corvus, Day One, EMD Serono, Fstar, Genmab, Ikena, Immatics, Incyte, Kadmon, KAHR, Macrogenics, Merck, Moderna, Nektar, Next Cure, Numab, Palleon, Pfizer, Replimmune, Rubius, Servier, Scholar Rock, Synlogic, Takeda, Trishula, Tizona, Xencor; Patents: (both provisional) Serial #15/612,657 (Cancer Immunotherapy), PCT/US18/36052 (Microbiome Biomarkers for Anti-PD-1/PD-L1 Responsiveness: Diagnostic, Prognostic and Therapeutic Uses Thereof)
References
- 1.Hummelink K, van der Noort V, Muller M, Schouten RD, Lalezari F, Peters D, et al. PD-1T TILs as a predictive biomarker for clinical benefit to PD-1 blockade in patients with advanced NSCLC. Clin Cancer Res. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nature medicine. 2018;24(7):994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anagnostou V, Yarchoan M, Hansen AR, Wang H, Verde F, Sharon E, et al. Immuno-oncology Trial Endpoints: Capturing Clinically Meaningful Activity. Clin Cancer Res. 2017;23(17):4959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fridman WH, Meylan M, Petitprez F, Sun CM, Italiano A, Sautes-Fridman C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nature reviews Clinical oncology. 2022;19(7):441–57. [DOI] [PubMed] [Google Scholar]
- 5.Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022;386(25):2363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nature reviews Clinical oncology. 2021;18(6):345–62. [DOI] [PubMed] [Google Scholar]
- 7.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litchfield K, Reading JL, Puttick C, Thakkar K, Abbosh C, Bentham R, et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell. 2021;184(3):596–614.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGrail DJ, Pilie PG, Rashid NU, Voorwerk L, Slagter M, Kok M, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32(5):661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anagnostou V, Bardelli A, Chan TA, Turajlic S. The status of tumor mutational burden and immunotherapy. Nature cancer. 2022;3(6):652–6. [DOI] [PubMed] [Google Scholar]
- 12.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry S, Giraldo NA, Green BF, Cottrell TR, Stein JE, Engle EL, et al. Analysis of multispectral imaging with the AstroPath platform informs efficacy of PD-1 blockade. Science. 2021;372(6547). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carstens JL, Correa de Sampaio P, Yang D, Barua S, Wang H, Rao A, et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun. 2017;8:15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giraldo NA, Berry S, Becht E, Ates D, Schenk KM, Engle EL, et al. Spatial UMAP and Image Cytometry for Topographic Immuno-oncology Biomarker Discovery. Cancer immunology research. 2021;9(11):1262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez de Rodas M, Nagineni V, Ravi A, Datar IJ, Mino-Kenudson M, Corredor G, et al. Role of tumor infiltrating lymphocytes and spatial immune heterogeneity in sensitivity to PD-1 axis blockers in non-small cell lung cancer. Journal for immunotherapy of cancer. 2022;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavrielatou N, Liu Y, Vathiotis I, Zugazagoitia J, Aung TN, Shafi S, et al. Association of PD-1/PD-L1 Co-location with Immunotherapy Outcomes in Non-Small Cell Lung Cancer. Clin Cancer Res. 2022;28(2):360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu S, Stein JE, Rimm DL, Wang DW, Bell JM, Johnson DB, et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA Oncol. 2019;5(8):1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schurch CM, Bhate SS, Barlow GL, Phillips DJ, Noti L, Zlobec I, et al. Coordinated Cellular Neighborhoods Orchestrate Antitumoral Immunity at the Colorectal Cancer Invasive Front. Cell. 2020;183(3):838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caushi JX, Zhang J, Ji Z, Vaghasia A, Zhang B, Hsiue EH, et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature. 2021;596(7870):126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


