Abstract
Intravascular schistosomes may control immune and hemostatic responses by regulating the nature and amount of selected host purinergic signaling molecules (such as ATP, ADP and NAD) surrounding them. Such metabolites are collectively known as the worm’s “purinergic halo”. Host-interactive, membrane-bound, tegumental ectonucleotidases, notably SmATPDase1, SmNPP5, SmAP and SmNACE, can degrade proinflammatory, pro-thrombotic and immunomodulatory purinergic metabolites like those listed. A common catabolic product is the anti-inflammatory metabolite adenosine that can additionally be taken in by the worms as food. We envision the tegumental ectonucleotidases as having a twofold role at the worm surface: first, they degrade potentially harmful host signaling molecules and second, they generate vital nutrients around the worms from where these can be conveniently imported.
Keywords: schistosome, immune modulation, thromboregulation, ATP, adenosine, NAD
Schistosomes and host purinergic signaling
Schistosomiasis is a neglected tropical disease (NTD) affecting > 200 million people globally and causing over 200,000 deaths per year [1]. The disease is caused by parasitic trematodes called schistosomes. Three main species cause the disease in humans: Schistosoma mansoni, S. haematobium and S. japonicum [2]. Eggs produced by mature adult worms are shed into the environment where they hatch, liberating larval forms called miracidia. These infect freshwater snails where they replicate asexually to generate a second larval life stage called the cercaria. People become infected when they come into contact with fresh water containing cercariae that have emerged from infected snails. A cercaria can penetrate human skin where it transforms into a juvenile called a schistosomulum. Next, schistosomula invade a blood vessel and migrate to the liver and then move either to the blood vessels of the intestine (S. mansoni, S. japonicum), or the urinary tract (S. haematobium) [3]. Mature females lay eggs, many of which exit the body with feces or urine to complete the life cycle. However, some eggs do not leave the body but lodge in host tissues where they become surrounded by immune cells forming structures called granulomas (see Glossary). These destroy the eggs and neutralize otherwise pathogenic egg antigens, but also result in fibrinogenesis that is the cause of much of the pathology of infection [4]. Symptoms of schistosomiasis include abdominal pain, diarrhea and blood in the urine or stool. Chronic infection can lead to liver and kidney damage, infertility, or bladder cancer.
While schistosome eggs provoke immunological scrutiny, resulting in the formation of granulomas, the egg producers (i.e., the adult worms themselves) provoke no overt inflammation in the vasculature [5]. Unlike eggs, no equivalent collections of immune cells are detected around adult male or female worms lying exposed in the vasculature of experimentally infected animals [5]. Furthermore, the worms do not appear to trigger host coagulation cascades – no blood clots or assemblages of platelets are seen around the worms in vivo [5]. This is striking since adult schistosomes are relatively large (~10mm × 1mm) and would be predicted to obstruct blood flow, especially in smaller blood vessels – a circumstance that ordinarily provokes thrombus formation. We argue that one major mechanism used by schistosome parasites to regulate host immune and hemostatic responses is their ability to modulate the type and amount of host purinergic signaling molecules, such as adenosine triphosphate (ATP) and adenosine diphosphate (ADP), around the worms in the vasculature [6]. Schistosome achieve this regulation using several membrane-bound ectonucleotidases. In this review we describe how the worms shape this collection of host molecules - collectively known as the “purinergic halo” - surrounding them [7].
Extracellular ATP, ADP and Adenosine
While nucleotides, like ATP, are largely intracellular, they are released into the extracellular space following cell damage or death. The release of these normally intracellular molecules constitutes a “danger signal” for the body and such molecules are known as alarmins or damage-associated molecular patterns (DAMPs) [8,9]. Even if cells do not die, DAMPs can also be released in response to stressors such as mechanical stimulation [10] and hypoxia [11]. Schistosomes utilize spine-laden suckers to migrate throughout the blood stream and we hypothesize that these mechanically stress the endothelium. Further, we propose that the large worms can impinge on blood flow causing (at least transient) hypoxia. These circumstances should lead to the release of DAMPs like ATP.
Extracellular ATP is profoundly proinflammatory; it can bind to excitatory ATP receptors, comprising inotropic P2XR and metabotropic P2YR subtypes, that can (1) promote inflammatory cell migration, (2) redirect T-helper (Th) cell differentiation, (3) activate the NLRP3 inflammasome, (4) promote cytokine and chemokine release, (5) induce oxygen and nitrogen radical formation and can (6) be directly cytotoxic [12–14].
To avoid prolonged ATP-induced inflammation and any resulting collateral damage, ATP signaling must be terminated in an efficient manner. This is achieved by the dephosphorylation of ATP usually through ADP, to adenosine monophosphate (AMP) and then to adenosine by the action of a series of ectoenzymes. Adenosine has remarkable anti-inflammatory properties, as compared with ATP. Adenosine can e.g., inhibit neutrophil adhesion to endothelial cells and reduce the production of superoxide anions as well as lower the release of pro-inflammatory cytokines [15,16]. Adenosine can facilitate the release of the anti-inflammatory cytokine IL-10, from monocytes [17]. The relationship between extracellular ATP and adenosine has been called the Yin and Yang in immune responses since adenosine acts as a negative feed-back signal that suppresses and reverts ATP-driven immune activation [18].
In animals, a series of ectonucleotidases, grouped into four families, drive the ATP to adenosine catabolic pathway. These are: ectonucleoside triphosphate diphosphohydrolases (ENTPDases or apyrases), ectonucleotide pyrophosphatase/phosphodiesterases (ENPPs), alkaline phosphatases (APs) and ecto-5′-nucleotidases (5′-NTs) [12]. Examples of these enzymes include the ENTPDase CD39 and the ENPP CD203 both of which can cleave ATP and ADP to generate AMP, and the 5’-NT CD73 and APs that dephosphorylate AMP to generate adenosine. Early biochemical experiments, and more recent proteomic analyses, has demonstrated that intravascular schistosomes express on their tegumental surfaces homologs of three of these four enzyme groups [19–21]. An ENTPDase, an ENPP and an AP enzyme have all been described [22]. No 5’-NT homolog has been identified in the schistosome tegumental proteome.
Schistosome nucleotide metabolizing ectoenzymes
The S. mansoni tegumental ENTPDase is the 544 amino acid ATP diphosphohydrolase designated SmATPDase1 [23,24]. The protein is predicted to possess two transmembrane domains (one towards the amino terminus and one towards the carboxyl terminus) that are required for enzyme functionality [23]. In recombinant form, SmATPDase1 degrades ATP and ADP in a cation dependent manner with optimal activity seen at alkaline pH. The Km of rSmATPDase1 for ATP is ~400 μM and for ADP, ~250 μM [23]. Live schistosomes whose SmATPDase1 gene has been suppressed using RNA interference (RNAi) are significantly impaired in their ability to degrade these nucleotides compared to control worms [23]. Suppression of a second S. mansoni ATPDase (SmATPDase2) has no impact on the ability of worms to cleave exogenous nucleotides from which we conclude that this second enzyme is not host interactive but is instead expressed on the parasite’s internal tissues [24].
The S. mansoni tegumental ENPP is the 458 amino acid, GPI-linked pyrophosphatase/phosphodiesterase SmNPP5 [25,26]. Recombinant SmNPP5 cleaves ATP with a Km of ~220 μM [27] and ADP with a Km of ~250 μM [28]. Optimal activity is seen at pH 8–8.5. Note that while SmATPDase1 and SmNPP5 both cleave ATP, they do so in a distinct manner: SmATPDase1 cleaves ATP’s terminal (gamma) phosphate to generate ADP whereas SmNPP5 cleaves ATP to generate AMP plus pyrophosphate (PPi). SmNPP5 has been shown to be essential for schistosome survival since parasites whose SmNPP5 gene is suppressed using RNAi are greatly compromised in their ability to establish infection in experimental animals [25].
A third ectonucleotidease enzyme is a S. mansoni 536 amino acid, GPI-linked tegumental alkaline phosphatase designated SmAP [29,30]. The purified, recombinant enzyme dephosphorylates AMP with a Km of ~650 μM and at a pH optimum of 9 [30]. The ability of living parasites to cleave exogenous AMP and generate adenosine is very largely abolished when SmAP gene expression is suppressed following RNAi treatment targeting the gene [30].
These three proteins have all been detected in the S. mansoni tegument in multiple proteomics analyses [22] and all have been independently immunolocalized in the schistosome tegument [23–26,29,30]. All three are therefore host-interactive and all are highly expressed in the parasite’s intravascular life stages [23–26,29,30]. Working in unison, SmATPDase1, SmNPP5 and SmAP could efficiently convert the proinflammatory DAMP, extracellular ATP into its anti-inflammatory product, adenosine, thereby creating a more immunologically benign environment for the worms within the host vasculature. Figure 1 (right; reactions 1 to 4) illustrates the ATP to adenosine catabolic pathway and, in colored boxes alongside the arrows, denotes the principal schistosome (on top), and equivalent human (below), enzymes that drive the process.
Figure 1. Enzymatic reactions that dictate the nature of the intravascular schistosome purinergic halo.
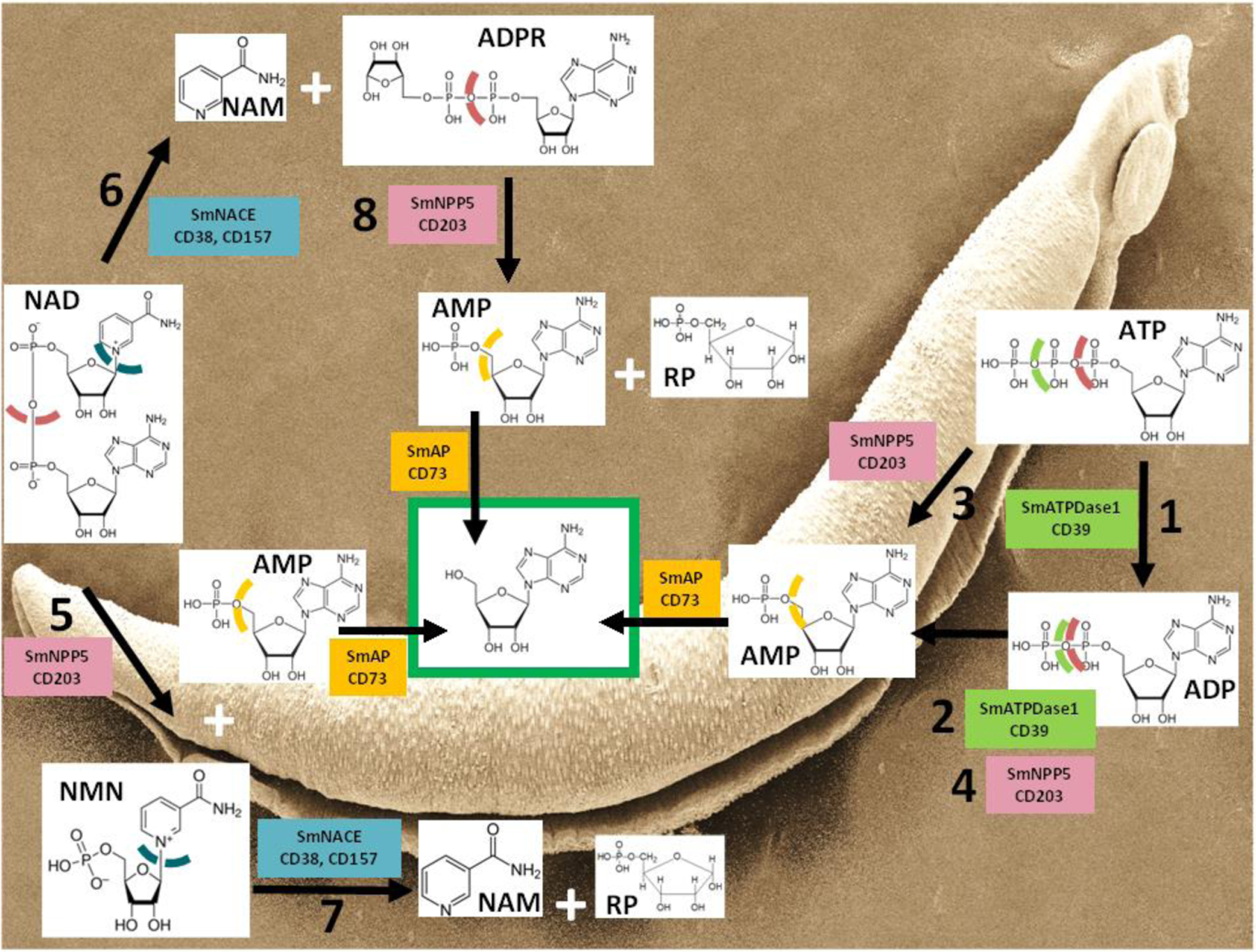
The Schistosoma mansoni (Sm) enzymes listed are expressed at the worm’s external surface. Chemical structures of key purinergic metabolites are depicted along with, in colored boxes, the names of the following key schistosome tegumental ectoenzymes (and functional host homologs): S. mansoni ATP diphosphohydrolase1 - SmATPDase1 (CD39), S. mansoni nucleotide pyrophosphatase/phosphodiesterase5 – SmNPP5 (CD203), S. mansoni alkaline phosphatase – SmAP (CD73), S. mansoni NAD-catabolizing enzyme- SmNACE (CD38, CD157). Color-coded, dashed lines indicate sites of cleavage by the various enzymes. The green box encloses adenosine (Ado), a terminal product of several pathways. A scanning electron micrograph image of an adult S. mansoni male is shown in the background. Ado: Adenosine, ADP: Adenosine diphosphate, ADPR: Adenosine diphosphate ribose, AMP: Adenosine monophosphate, ATP: Adenosine triphosphate, NAD: Nicotinamide adenine dinucleotide, NAM: Nicotinamide, NMN: Nicotinamide mononucleotide, RP: Ribose phosphate
Note that this reaction series also efficiently removes the potently pro-thrombotic molecule ADP. ADP promotes platelet activation and aggregation, so an ability by schistosome SmATPDase1 and SmNPP5 to cleave extracellular ADP could block this and any resulting thrombus formation in the vicinity of the worms [31]. This would allow the parasites greater freedom of movement in the bloodstream. We have shown that SmNPP5 can inhibit ADP-mediated platelet aggregation in a dose-dependent manner, as measured by multiple electrode aggregometry (MEA) using whole blood [28]. Inhibition is lost following heat treatment of SmNPP5. ADP can also contribute to inflammation, e.g., by triggering the P2Y12 receptor to activate NF-κB and the NLRP3 inflammasome [32]. Thus, the efficient hydrolysis of extracellular ADP by the schistosome ectoenzymes would complement the effects of ATP degradation, thus further minimizing host pro-inflammatory signaling pathways around the worms.
It has already been noted that suppressing SmATPDase1 expression in intravascular schistosomes significantly diminishes their ability to cleave exogenous ATP and ADP [23]. However similar suppression of SmNPP5 – an enzyme that unambiguously also cleaves ATP and ADP – has no significant effect on the worms’ abilities to cleave these metabolites [25,27]. A likely explanation for this conundrum stems from the several fold greater relative amount of SmATPDase1 at the schistosome surface compared to SmNPP5 [21]. Thus, the abundance of SmATPDase1 masks any impact of SmNPP5 suppression on the worm’s ability to cleave ATP and ADP. This suggests that SmATPDase1 is the key ectoenzyme employed by worms within the vasculature to hydrolyze extracellular ATP and ADP, and that reaction 1 and 2 (Figure 1) predominate in vivo. This conclusion then leaves unclear the primary function of SmNPP5 which we argue here is to cleave a different biomolecule that is also of relevance regarding the schistosome’s purinergic halo - nicotinamide adenine dinucleotide (NAD).
Extracellular NAD
Like ATP, NAD is found inside all living cells and is central to cellular metabolism. In cells, NAD acts e.g., as a coenzyme in redox reactions and as a donor of ADP-ribose moieties in ADP-ribosylation reactions. In addition, and again like ATP, NAD can be released from cells where it can have profound impact [33]. For instance, extracellular NAD can influence the fate of T lymphocyte subsets; it has been shown to induce murine T regulatory cells (T regs) to undergo apoptosis [34]. In addition to its ability to cleave ATP, SmNPP5 cleaves NAD with a Km of ~200 μM to generate NMN and AMP (Figure 1, reaction 5). Interestingly, a second tegumental ectoenzyme can additionally cleave exogenous NAD, but does so in a different manner compared to SmNPP5. This second enzyme is SmNACE – a 303 amino acid, GPI-linked ectoenzyme [35] that, like the other schistosome enzymes described here, was also identified in much tegumental proteomics work and was immunolocalized to the tegument of intravascular stage schistosomes [19–21]. While SmNACE exhibits sequence similarity with members of the ADP-ribosyl cyclase family, such as human CD38 and CD157, unlike these enzymes it does not demonstrate significant cyclase activity i.e., it does not cleave NAD to generate cADPR [36]. Instead SmNACE cleaves NAD (with a Km of ~50 μM) to generate nicotinamide and ADP-ribose (ADPR, Figure 1, reaction 6). The two schistosome NAD-cleaving ectoenzymes, SmNACE and SmNPP5, can additionally process one of the other’s NAD cleavage reaction products. Thus, SmNACE has the ability to cleave NMN into nicotinamide and ribose phosphate (reaction 7 in Figure 1) and SmNPP5 can cleave ADPR to generate AMP and ribose phosphate (reaction 8 in Figure 1) [37].
An ability of SmNPP5 and SmNACE to cleave extracellular NAD could have a bearing on T cell homeostasis during schistosome infection. The process of NAD-induced T cell death (NICD), mentioned earlier, occurs through the action of a T cell surface mono-ADP ribosyltransferase (ART2) that catalyzes the transfer of the ADPR group from NAD to the cell surface ATP receptor P2X7R to induce apoptosis [38]. Murine Tregs (CD4+CD25+FoxP3+ cells that act to limit excessive immune responses) are particularly sensitive to NICD because they highly express the P2X7 receptor [39]. We hypothesize that an ability of schistosomes to cleave NAD could limit NICD and preserve Treg numbers. This could benefit the worms by suppressing potentially deleterious anti-parasitic activities of Th cells in rodents. We have shown that SmNPP5 can block NAD-induced T cell death in vitro [40]. It is notable that schistosome infection in experimental animals does lead to the expansion of Tregs [41,42] and the action of the schistosome NAD cleaving ectoenzymes could bear some responsibility for this. That said, it has been shown that suppressing the SmNPP5 gene, but not the SmNACE gene, significantly diminishes the ability of live schistosomes to cleave exogenous NAD [40]. This leads to the hypothesis that it is SmNPP5 that predominantly cleaves NAD in the vasculature and that reaction 5 (Figure 1) predominates in vivo. Note that since ATP can additionally drive T cell apoptosis, schistosome mediated cleavage of this molecule could help to prevent this, the result being further protection for Tregs. It has been shown that live worms (as well as rSmNPP5) can block ATP-driven T cell killing [27].
Since extracellular NAD can exert proinflammatory effects, e.g., stimulating human granulocytes and potentially recruiting them to sites of inflammation [43], schistosome-mediated NAD cleavage could additionally mitigate this outcome. Another way that cleaving extracellular NAD could benefit intravascular schistosomes stems from the finding that NAD pools are rapidly depleted in inflammatory macrophages. Thus, NAD salvage from the environment is essential for such macrophages to maintain a pro-inflammatory state [44]. Schistosome-mediated cleavage of serum NAD would lower the pool of extracellular NAD to help starve local macrophages and thus create a more immunologically benign environment for the worms.
Since SmNPP5 is the principal NAD cleaving ectoenzyme of intravascular worms, this leaves the primary physiological function of SmNACE unclear. We hypothesize that the main role of SmNACE is to cleave NMN [37]. This liberates the essential metabolite nicotinamide (vitamin B3) in the local vicinity of the worms from where it could be easily imported and used in central metabolism [45]. It has been reported that schistosomes lack the enzymes required for de novo NAD biosynthesis [45], making salvage of e.g., nicotinamide (generated by the action of SmNACE), vital for worm survival. Indeed, it has been demonstrated that inhibiting NAD salvage metabolism in schistosomes impedes worm development, reproduction, and survival both in vitro and in vivo [45]. Finally, since extracellular nicotinamide can impinge on monocyte differentiation, resulting in strongly reduced pro-inflammatory features [46], any nicotinamide generated by the action of schistosome ectoenzymes that is not taken in by the worms could act in this manner as an immunomodulator.
Blocking host purinergic signaling while generating simple purines as nutrients
Figure 1 illustrates the many reactions driven by schistosome ectoenzymes to control their purinergic halo. Note the central position of adenosine (green box) that is generated following SmAP-mediated cleavage of AMP (with AMP being a product of several of the reaction pathways depicted). Likely the convergence on adenosine generation reflects this molecule’s already discussed beneficial (for the worms) anti-inflammatory and immunosuppressive properties [47,48]. In the same way, adenosine is reported to help tumor cells evade immune-mediated killing; the activity on tumor cells of homologs of the schistosome ectoenzymes discussed here - CD39 (SmATPDase), CD203 (SmNPP5), and CD73 (SmAP), have been found to favor tumor growth and dissemination, largely by working in the same sequential manner to cleave NAD and ATP into anti-inflammatory adenosine [49–51]. This set of reactions creates a more immunotolerant environment for tumors, as it does for schistosomes.
Also of note is that schistosomes are incapable of de novo purine synthesis; they are dependent on salvage of purines (like adenosine) from the external environment for their core metabolism [52]. Thus, generating abundant free adenosine using the pathways depicted in Figure 1 may not only have an immunosuppressive impact but may also generate an essential food in the local environment of the worms from where it can be conveniently imported. The known ability of SmAP to dephosphorylate other common nucleotides like CMP, GMP and TMP to generate cytidine, guanosine, and thymidine respectively should also facilitate the easy uptake of these nucleosides by the worms as nutrients [53].
We perceive the action of schistosome ectoenzymes on the host biomolecules discussed as being twofold. First, cleaving the target molecule negates its potentially harmful impact on schistosomes. Thus, as outlined in this review, proinflammatory ATP and prothrombotic ADP signaling events are tempered; NAD-driven Treg apoptosis in mice, and sustained macrophage inflammatory responses, are curtailed. A second important outcome of target molecule cleavage is the generation of valuable biochemical products that are essential for the worms. The central role of adenosine (as an anti-inflammatory agent and as a food source) generated following ATP, ADP or NAD catabolism, was emphasized earlier. The same rationale applies to other targeted molecules; cleavage of NMN, as noted above, generates vital nicotinamide (vitamin B3). In addition, since NMN can itself be proinflammatory, (e.g., stimulating NF-κB activity and triggering iNOS induction in human vascular smooth muscle [54]) lowering the level of this molecule via SmNACE-mediated cleavage, may, by itself, be selectively advantageous for schistosomes. Similarly, ADPR, acting through transient receptor potential (TRP) channel TRPM2, is reportedly capable of controlling immune cell chemotaxis and oxidative stress signal transduction [55]. So, cleaving any ADPR that is generated e.g., by SmNACE-mediated NAD hydrolysis, (Figure 1, reaction 6), would help terminate these processes, in addition to generating product (AMP) from which useful adenosine can be conveniently derived. However, the physiological significance of these pathways under conditions of natural schistosome infection has not been elucidated [56].
Comparative properties of the schistosome nucleotide metabolizing ectoenzymes
The biochemical characteristics of the four S. mansoni tegumental ectonucleotidases SmATPDase1, SmNPP5, SmAP and SmNACE are summarized in Table 1. All four are most highly expressed in the adult schistosome life stages. Three (SmNPP5, SmAP and SmNACE) are GPI-linked at the surface. SmATPDase1 has carboxyl and amino terminal transmembrane domains, and these are vital for functional enzymatic activity [23]. Three of the four (SmATPDase1, SmNPP5 and SmAP) require divalent cations for function and, unlike SmNACE, their activities are shut down in the presence of the chelating agent EDTA. All four maintain substantial catalytic activity under alkaline conditions; for two (SmNPP5 and SmAP) the pH optimum is ≥ 8. The relevance of the sustained activity under alkaline conditions is unclear since the worms live in highly buffered, more pH-neutral blood. Known substrates for each enzyme and Km values (where available) are listed. Note the inclusion of additional substrates for some enzymes that are not discussed in depth here. For instance, SmAP can dephosphorylate the lipid biomolecule sphingosine-1-phosphate (S1P) and the linear polymer polyphosphate (polyP) – which may have consequences for immune and hemostatic responses [53,57]. Finally, SmAP also cleaves pyridoxal 5’phosphate (PLP, vitamin B6), likely in order to generate free pyridoxal for easy uptake by the worms as food [58].
Table 1.
Comparative biochemical characteristics of the four tegumental ectoenzymes that are known to control the S. mansoni purinergic halo.
| SmATPDase1 | SmNPP5 | SmNACE | SmAP | |
|---|---|---|---|---|
| Size (AA#) | 544 | 458 | 303 | 536 |
| Mol Mass (Da) | 61,353 | 52,563 | 34,758 | 59,375 |
| pI | 9.11 | 6.28 | 7.13 | 5.92 |
| GPI-linked | − | + | + | + |
| Glycoprotein | ? | + | + | + |
| pH optimum (range) | ATP: 8.5–10 ADP: 7.5–10 |
ATP: 8–8.5 ADP: 7–10 |
ATP: 7–8 ADP: 7–10 |
ATP: 9 ADP: 8–10 |
| Cation requirement | Ca≥Mg | Mg=Ca=Zn | None | Mg >>Ca>Zn>Cu |
| Substrates (K m , μM±SD) | ATP (400 ±20) ADP (252 ±20) |
NAD (200 ±0.6) ATP (217 ±26) ADP (246 ±34) ADPR |
NAD (49 ±3.5) NADP (14 ±2.9) NDG (23 ±1.1) NMN (1084 ±164) |
AMP (650 ±22) CMP (630 ±20) GMP (640 ±11) TMP (595 ±33) pNPP (288 ±12) polyP (6900 ±1000) S1P, PLP |
| Accession# | AY323529 | EU769293 | AAX35328 | EU040139 |
| References | [23, 24] | [25, 26–28, 40] | [35, 40, 45] | [29, 30, 53, 56, 57] |
Km values reported here were measured at each enzyme’s optimal pH. Whether SmATPDase1 is glycosylated is unknown, indicated by “?”. GenBank accession numbers for each enzyme are listed. AA#: amino acid number, Mol Mass (Da): Molecular Mass (Daltons), pI: isoelectric point. The full designations of most listed substrates are given in Figure 1. NADP, nicotinamide adenine dinucleotide phosphate; NGD, nicotinamide guanine dinucleotide; pNPP p-nitrophenyl phosphate; polyP, polyphosphate; S1P, sphingosine-1-phosphate; PLP, pyridoxal phosphate.
Concluding Remarks
Here we have described the ability of schistosome parasites to cleave a collection of host cell signaling molecules such as ATP, ADP, and NAD. However, the full range of substrates acted upon by the ectonucleotidases discussed has not been elucidated. Further, the broader relevance of purinergic halos to other schistosome life stages (e.g., eggs in host tissues or sporocysts within the intermediate snail host) is unresolved (see Outstanding Questions). Since all of the enzymes discussed in this review are known to be expressed at the surface of intravascular schistosomes and interact with extracellular host metabolites, this suggests that they may be especially accessible to the action of immune effectors or chemical inhibitors. Efforts to identify drugs that block SmNACE function are on-going [59,60]. We argue that an ability to control the worm’s purinergic halo, as outlined in this report, is essential for schistosome development and survival. Therefore, blocking the action of one or more of the four enzymes discussed here, by immunological or chemical means, offers one pathway to potentially debilitate the worms and terminate schistosome infection.
Outstanding Questions.
What is the full range of substrates of each of the schistosome tegumental ectonucleotidases?
How does ectonucleotidase-mediated substrate cleavage impact parasite survival and growth?
What is the relative density of each ectonucleotidase in the tegument, how does this change during development and how are the ectonucleotidases organized relative to one another at the parasite surface?
Regarding the action of the ectonucleotidases on their currently known substrates, which is more important for the worms: cleavage of signaling molecules to impede host biochemistry or generating smaller purinergic products for direct uptake as food?
Do other schistosome species express homologous ectonucleotidases and in a similar arrangement compared to S. mansoni?
Why do the ectonucleotidases display sustained high activity at alkaline pH, given the more neutral pH environment of their intravascular habitat?
How do the ectonucleotidases, and other tegumental proteins, traffic to the schistosome surface?
How relevant is control of the purinergic halo to other schistosome life cycle stages, e.g., to sporocysts in the intermediate snail host or to eggs in the definitive host?
Could chemicals that inhibit one or more of the ectonucleotidases form the basis of new anti-schistosome therapies?
How widespread is the distribution of ectonucleotidases among different parasite groups and might this be related to the habitat of the parasites (blood v gut, for example)?
Supplementary Material
Highlights.
Blood stage Schistosoma mansoni express a series of membrane-bound, tegumental ectonucleotidases at the host parasite interface that are designated SmATPDase1, SmNPP5, SmAP and SmNACE.
These enzymes can degrade exogenous, host purinergic signaling molecules such as proinflammatory ATP, pro-thrombotic ADP, and immunomodulatory NAD, to control what is collectively known as the worm’s “purinergic halo”.
A common product of purinergic catabolism is the anti-inflammatory metabolite adenosine. Since schistosomes cannot synthesize purines de novo, adenosine can additionally be taken in by the worms as food.
In this manner, schistosome tegumental ectonucleotidases not only degrade potentially harmful host signaling molecules but additionally generate vital nutrients in the vicinity of the worms from where these can be conveniently taken in.
Acknowledgements
This work was funded with support from the National Institutes of Health - National Institute of Allergy and Infectious Disease, grant AI056273.
Glossary
- Alkaline phosphatase (AP)
an enzyme that catalyzes the hydrolysis of organic phosphate esters present in the extracellular space, converting e.g., AMP to adenosine and phosphate.
- Damage-associated molecular pattern (DAMP)
endogenous danger molecules (such as ATP) released from damaged or stressed cells that can bind to cellular receptors to activate the innate immune system.
- Ecto-5′-nucleotidase (5′-NT)
a GPI-anchored extracellular enzyme that hydrolyses AMP to adenosine and phosphate.
- Ectonucleotide pyrophosphatase/phosphodiesterase (ENPP)
an extracellular enzyme that can hydrolyze nucleotides e.g., ATP and ADP and (for some members of this protein family) lysophospholipids.
- Ectonucleoside triphosphate diphosphohydrolase (ENTPDase or apyrase)
an extracellular enzyme that catalyzes the hydrolysis of ATP and ADP to AMP and phosphate.
- Granuloma
an organized aggregation of immune cells that surround schistosome eggs in host tissues.
- Inducible nitric oxide synthase (iNOS)
an inducible enzyme that catalyzes the production of the signaling molecule nitric oxide (NO) from L-arginine.
- Michaelis constant (Km)
the concentration of substrate at which an enzyme’s reaction velocity is half maximal; a measure of the affinity of an enzyme for its substrate.
- Multiple electrode aggregometry (MEA)
a test of platelet function (aggregation) in whole blood.
- NAD-induced T cell death (NICD)
nicotinamide adenine dinucleotide (NAD) released during cell damage or inflammation can trigger T lymphocyte apoptosis.
- NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3)
a molecular sensor that mediates inflammatory cytokine secretion in response to infection and cell damage.
- Nuclear factor-κB (NF-κB)
a transcription factor that regulates inflammatory responses.
- P2XR
cell surface ATP receptor, membrane ion channel stimulated by the binding of extracellular ATP.
- P2YR
cell surface G protein-coupled receptor, activated by nucleotides such as ATP.
- Purinergic halo
the collection of purine-containing molecules (notably ADP, ATP, and adenosine) surrounding a cell, tissue, or organism.
- Purinergic signaling
a form of extracellular signaling mediated by purine-containing molecules (notably ADP, ATP, and adenosine) that can activate a cell’s purinergic receptors and regulate cell function.
- T regulatory cells (T regs)
a population of T lymphocytes that can suppress immune responses to help maintain homeostasis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Colley DG et al. (2014) Human schistosomiasis. Lancet 383, 2253–2264. 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McManus DP et al. (2018) Schistosomiasis. Nat Rev Dis Primers 4, 13. 10.1038/s41572-018-0013-8 [DOI] [PubMed] [Google Scholar]
- 3.Nation CS et al. (2020) Schistosome migration in the definitive host. PLoS Negl Trop Dis 14, e0007951. 10.1371/journal.pntd.0007951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stavitsky AB (2004) Regulation of granulomatous inflammation in experimental models of schistosomiasis. Infect Immun 72, 1–12. 10.1128/IAI.72.1.1-12.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keating JH et al. (2006) No overt cellular inflammation around intravascular schistosomes in vivo. The Journal of parasitology 92, 1365–1369. 10.1645/GE-864R.1 [DOI] [PubMed] [Google Scholar]
- 6.Bhardwaj R and Skelly PJ (2009) Purinergic signaling and immune modulation at the schistosome surface? Trends Parasitol 25, 256–260. 10.1016/j.pt.2009.03.004 [DOI] [PubMed] [Google Scholar]
- 7.Antonioli L et al. (2013) CD39 and CD73 in immunity and inflammation. Trends Mol Med 19, 355–367. 10.1016/j.molmed.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Idzko M et al. (2007) Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 13, 913–919. 10.1038/nm1617 [DOI] [PubMed] [Google Scholar]
- 9.Di Virgilio F et al. (2009) Extracellular nucleotides as negative modulators of immunity. Curr Opin Pharmacol 9, 507–513. 10.1016/j.coph.2009.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Homolya L et al. (2000) Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol 150, 1349–1360. 10.1083/jcb.150.6.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNamara N et al. (2001) ATP transduces signals from ASGM1, a glycolipid that functions as a bacterial receptor. Proc Natl Acad Sci U S A 98, 9086–9091. 10.1073/pnas.161290898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Virgilio F et al. (2020) Purinergic signaling, DAMPs, and inflammation. Am J Physiol Cell Physiol 318, C832–C835. 10.1152/ajpcell.00053.2020 [DOI] [PubMed] [Google Scholar]
- 13.Junger WG (2011) Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 11, 201–212. 10.1038/nri2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asgari E et al. (2013) C3a modulates IL-1beta secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood 122, 3473–3481. 10.1182/blood-2013-05-502229 [DOI] [PubMed] [Google Scholar]
- 15.Barletta KE et al. (2012) Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol 32, 856–864. 10.1161/ATVBAHA.111.226845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Virgilio F and Vuerich M (2015) Purinergic signaling in the immune system. Auton Neurosci 191, 117–123. 10.1016/j.autneu.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 17.Hasko G and Pacher P (2012) Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol 32, 865–869. 10.1161/ATVBAHA.111.226852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faas MM et al. (2017) Extracellular ATP and adenosine: The Yin and Yang in immune responses? Mol Aspects Med 55, 9–19. 10.1016/j.mam.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 19.Braschi S et al. (2006) Proteomic analysis of the schistosome tegument and its surface membranes. Mem Inst Oswaldo Cruz 101 Suppl 1, 205–212. S0074-02762006000900032 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Braschi S and Wilson RA (2006) Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol Cell Proteomics 5, 347–356. M500287-MCP200 [pii] 10.1074/mcp.M500287-MCP200 [DOI] [PubMed] [Google Scholar]
- 21.Castro-Borges W et al. (2011) Enzymatic shaving of the tegument surface of live schistosomes for proteomic analysis: a rational approach to select vaccine candidates. PLoS Negl Trop Dis 5, e993. 10.1371/journal.pntd.0000993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skelly P and Wilson R (2006) Making Sense of the Schistosome Surface. Advances in parasitology 63, 185–284 [DOI] [PubMed] [Google Scholar]
- 23.Da’dara AA et al. (2014) Schistosome tegumental ecto-apyrase (SmATPDase1) degrades exogenous pro-inflammatory and pro-thrombotic nucleotides. PeerJ 2, e316. 10.7717/peerj.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Da’dara AA et al. (2014) Schistosome apyrase SmATPDase1, but not SmATPDase2, hydrolyses exogenous ATP and ADP. Purinergic Signal 10, 573–580. 10.1007/s11302-014-9416-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhardwaj R et al. (2011) Tegumental Phosphodiesterase SmNPP-5 Is a Virulence Factor for Schistosomes. Infect Immun 79, 4276–4284. 10.1128/IAI.05431-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rofatto HK et al. (2009) Characterization of phosphodiesterase-5 as a surface protein in the tegument of Schistosoma mansoni. Mol Biochem Parasitol 166, 32–41. S0166-6851(09)00068-1 [pii] 10.1016/j.molbiopara.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 27.Nation CS et al. (2022) Schistosomes Impede ATP-Induced T Cell Apoptosis In Vitro: The Role of Ectoenzyme SmNPP5. Pathogens 11, 155 doi: 10.3390/pathogens11020155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elzoheiry M et al. (2018) The Essential Ectoenzyme SmNPP5 from the Human Intravascular Parasite Schistosoma mansoni is an ADPase and a Potent Inhibitor of Platelet Aggregation. Thromb Haemost. 10.1055/s-0038-1641715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araujo-Montoya BO et al. (2011) Schistosoma mansoni: molecular characterization of Alkaline Phosphatase and expression patterns across life cycle stages. Exp Parasitol 129, 284–291. 10.1016/j.exppara.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 30.Bhardwaj R and Skelly PJ (2011) Characterization of schistosome tegumental alkaline phosphatase (SmAP). PLoS Negl Trop Dis 5, e1011. 10.1371/journal.pntd.0001011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Da’dara AA and Skelly PJ (2014) Schistosomes versus platelets. Thrombosis research 134, 1176–1181. 10.1016/j.thromres.2014.09.032 [DOI] [PubMed] [Google Scholar]
- 32.Suzuki T et al. (2020) Extracellular ADP augments microglial inflammasome and NF-kappaB activation via the P2Y12 receptor. Eur J Immunol 50, 205–219. 10.1002/eji.201848013 [DOI] [PubMed] [Google Scholar]
- 33.Billington RA et al. (2006) Emerging functions of extracellular pyridine nucleotides. Mol Med 12, 324–327. 10.2119/2006-00075.Billington [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adriouch S et al. (2012) Extracellular NAD(+): a danger signal hindering regulatory T cells. Microbes Infect 14, 1284–1292. 10.1016/j.micinf.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 35.Goodrich SP et al. (2005) Production of calcium-mobilizing metabolites by a novel member of the ADP-ribosyl cyclase family expressed in Schistosoma mansoni. Biochemistry 44, 11082–11097. 10.1021/bi050704r [DOI] [PubMed] [Google Scholar]
- 36.Kuhn I et al. (2013) Schistosoma mansoni NAD(+) catabolizing enzyme: identification of key residues in catalysis. Biochim Biophys Acta 1834, 2520–2527. 10.1016/j.bbapap.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 37.Nation CS et al. (2022) NAD-catabolizing ectoenzymes of Schistosoma mansoni. Biochem J 479, 1165–1180. 10.1042/BCJ20210784 [DOI] [PubMed] [Google Scholar]
- 38.Adriouch S et al. (2007) NAD+ released during inflammation participates in T cell homeostasis by inducing ART2-mediated death of naive T cells in vivo. J Immunol 179, 186–194. 10.4049/jimmunol.179.1.186 [DOI] [PubMed] [Google Scholar]
- 39.Hubert S et al. (2010) Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J Exp Med 207, 2561–2568. 10.1084/jem.20091154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nation CS et al. (2020) The essential schistosome tegumental ectoenzyme SmNPP5 can block NAD-induced T cell apoptosis. Virulence 11, 568–579. 10.1080/21505594.2020.1770481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang CL et al. (2019) Role of regulatory T cells in Schistosoma-mediated protection against type 1 diabetes. Mol Cell Endocrinol 491, 110434. 10.1016/j.mce.2019.04.014 [DOI] [PubMed] [Google Scholar]
- 42.Tang CL et al. (2011) Effect of CD4+ CD25+ regulatory T cells on the immune evasion of Schistosoma japonicum. Parasitol Res 108, 477–480. 10.1007/s00436-010-2089-2 [DOI] [PubMed] [Google Scholar]
- 43.Bruzzone S et al. (2006) Extracellular NAD+ regulates intracellular calcium levels and induces activation of human granulocytes. Biochem J 393, 697–704. 10.1042/BJ20051302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cameron AM et al. (2019) Inflammatory macrophage dependence on NAD(+) salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat Immunol 20, 420–432. 10.1038/s41590-019-0336-y [DOI] [PubMed] [Google Scholar]
- 45.Schultz MD et al. (2020) Inhibition of the NAD salvage pathway in schistosomes impairs metabolism, reproduction, and parasite survival. PLoS Pathog 16, e1008539. 10.1371/journal.ppat.1008539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss R et al. (2015) Nicotinamide: a vitamin able to shift macrophage differentiation toward macrophages with restricted inflammatory features. Innate Immun 21, 813–826. 10.1177/1753425915602545 [DOI] [PubMed] [Google Scholar]
- 47.Hasko G and Cronstein BN (2004) Adenosine: an endogenous regulator of innate immunity. Trends Immunol 25, 33–39 [DOI] [PubMed] [Google Scholar]
- 48.Sitkovsky MV and Ohta A (2005) The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol 26, 299–304 [DOI] [PubMed] [Google Scholar]
- 49.Antonioli L et al. (2013) Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 13, 842–857. 10.1038/nrc3613 [DOI] [PubMed] [Google Scholar]
- 50.Morandi F et al. (2018) Microvesicles released from multiple myeloma cells are equipped with ectoenzymes belonging to canonical and non-canonical adenosinergic pathways and produce adenosine from ATP and NAD(). Oncoimmunology 7, e1458809. 10.1080/2162402X.2018.1458809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horenstein AL et al. (2019) Functional insights into nucleotide-metabolizing ectoenzymes expressed by bone marrow-resident cells in patients with multiple myeloma. Immunol Lett 205, 40–50. 10.1016/j.imlet.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 52.Senft AW et al. (1972) Purine metabolism in Schistosoma mansoni. Int J Parasitol 2, 249–260. 10.1016/0020-7519(72)90013-6 [DOI] [PubMed] [Google Scholar]
- 53.Elzoheiry M et al. (2018) Intravascular Schistosoma mansoni Cleave the Host Immune and Hemostatic Signaling Molecule Sphingosine-1-Phosphate via Tegumental Alkaline Phosphatase. Front Immunol 9, 1746. 10.3389/fimmu.2018.01746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romacho T et al. (2009) Extracellular PBEF/NAMPT/visfatin activates pro-inflammatory signalling in human vascular smooth muscle cells through nicotinamide phosphoribosyltransferase activity. Diabetologia 52, 2455–2463. 10.1007/s00125-009-1509-2 [DOI] [PubMed] [Google Scholar]
- 55.Guse AH (2015) Calcium mobilizing second messengers derived from NAD. Biochim Biophys Acta 1854, 1132–1137. 10.1016/j.bbapap.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 56.Silva CLM (2016) Purinergic signaling in schistosomal infection. Biomed J 39, 316–325. 10.1016/j.bj.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elzoheiry M et al. (2019) Schistosomes can hydrolyze proinflammatory and prothrombotic polyphosphate (polyP) via tegumental alkaline phosphatase, SmAP. Mol Biochem Parasitol, 111190. 10.1016/j.molbiopara.2019.111190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Da’dara AA et al. (2020) Vitamin B6 Acquisition and Metabolism in Schistosoma mansoni. Front Immunol 11, 622162. 10.3389/fimmu.2020.622162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacques SA et al. (2015) Discovery of Potent Inhibitors of Schistosoma mansoni NAD(+) Catabolizing Enzyme. J Med Chem 58, 3582–3592. 10.1021/acs.jmedchem.5b00203 [DOI] [PubMed] [Google Scholar]
- 60.Muller-Steffner H et al. (2017) Efficient Inhibition of SmNACE by Coordination Complexes Is Abolished by S. mansoni Sequestration of Metal. ACS Chem Biol 12, 1787–1795. 10.1021/acschembio.7b00186 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


