Abstract
Background.
Intravenous immune checkpoint inhibition is an effective anti-cancer strategy for BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) but may be associated with greater systemic toxicity compared to localized therapies.
Objective:
We assessed the safety and antitumor activity of intravesical pembrolizumab combined with BCG.
Design, Setting and Participants:
A 3+3 phase I trial of pembrolizumab + BCG was conducted in patients with BCG-unresponsive NMIBC (NCT02808143).
Intervention:
Pembrolizumab was given intravesically (1–5 mg/kg for 2 hours) beginning 2 weeks prior to BCG induction until recurrence. Urine profiling during treatment and spatial transcriptomic profiling of pre- and post- treatment tumors was conducted to identify biomarkers that correlated with response.
Outcomes and Statistical Analysis:
Safety, tolerability with Kaplan-Meier survival analysis.
Results and Limitations:
Nine patients completed therapy. Median follow-up was 35 months for 5 patients still alive at the end of the trial. The trial was closed due to the Covid19 pandemic. Grade 1–2 urinary symptoms were common. The maximum tolerated dose was not reached, however one dose-limiting toxicity was reported (grade 2 diarrhea) in the only patient who reached 52-weeks without recurrence. One death occurred from myasthenia gravis that was deemed potentially related to treatment. The 6-month and 1-year recurrence-free rates were 67% (95% CI: 42–100%) and 22% (95% CI: 6.5–75%) respectively. Pembrolizumab was detected in the urine and not in blood. CD4+ T cells were significantly increased in the urine post-treatment, and transcriptomic analysis identified decreased expression of T-cell exhaustion markers in late recurrences.
Conclusion:
We demonstrate that intravesical pembrolizumab is safe, feasible and capable of eliciting strong immune responses in a clinical setting and should be investigated further
Patient Summary:
Direct application of pembrolizumab to the bladder is a promising alternative for BCG-unresponsive NMIBC and should be investigated further.
Keywords: Clinical trial, BCG, digital spatial profiling, bladder cancer, intravesical pembrolizumab
Introduction
Treatment for non-muscle invasive bladder cancer (NMIBC) is endoscopic resection followed by intravesical adjuvant chemotherapy or BCG (Bacillus Calmette–Guérin)[1]. Non-responders to BCG have limited options. In 2021, the first intravenous anti-PD1 checkpoint immunotherapy (CPI) was approved for patients with BCG-unresponsive NMIBC with carcinoma in situ[2]. Although the 3-month response was 41% (39/96), the median duration of complete response (CR) was 16 months (95% CI: 7–36) with 19% (18/96) CR at 12 months. While pembrolizumab was well-tolerated, 66% of patients reported an adverse-event (AE) with a total of 11 serious AEs in 8 patients. Hence, even though some patients responded to pembrolizumab, more than 80% did not respond and 2/3 reported AEs.
We hypothesized that changing the route of delivery could limit toxicity. For more than 40 years, urologists have administered medication into the bladder to treat bladder cancer[3, 4]. Recently several trials have focused on intravesical administration of immune-modulating agents to generate a localized immune response [5]. To date, activity and safety profile of CPIs administered as an intravesical therapy has not been investigated. Recent pre-clinical studies in mice suggest an increase in lymphocytes in the bladder after intravesical CPI antibodies[6]. Herein, we report safety, toxicity and clinical response from the first-in-human clinical trial of intravesical administration of an immune checkpoint inhibitor.
Methods
Study Design and participants:
NCT02808143 was a Phase I dose-escalation trial with a 3+3 design with increasing doses of intravesical pembrolizumab with BCG in patients unresponsive to BCG. All patients were recruited from one institution. The trial was approved by Northwestern University IRB (STU00202754) and the Robert H. Lurie Comprehensive Cancer Center Scientific Review Committee and Data Safety and Monitoring Committee. Patients had a history of high-grade bladder cancer (Ta, T1 or Tis) and were treated with at least 7 doses of BCG (5 during induction and 2 during maintenance). Patients were also eligible if they were BCG unresponsive and had been treated with at least 3 doses of a salvage regimen (such as gemcitabine and/or docetaxel). Any patient with an invasion (T1) must have had imaging within 60 days, and all participants had to have a ECOG performance of 0 or 1. Inclusion and exclusion criteria are listed in the protocol available on request.
Study procedures and dose-escalation scheme:
After enrollment, patients received a single intravesical dose of pembrolizumab at the specified dose-level (1 or 2 mg/kg) at week -2 prior to induction (Figure 1B). Participants were then treated with BCG (TICE, 50 mg) (weeks 0–5), intravesical pembrolizumab (weeks 0, 2, 4). After induction, participants received only intravesical pembrolizumab during maintenance, first every 2 weeks until week 17 and then every 4 weeks for the remainder of the year. DLTs were defined as a serious adverse event from week -2 through week 5 and defined by CTCAE v 4.03 criteria. Additional details can be found in the Supplementary methods.
Figure 1: Clinical trial design.
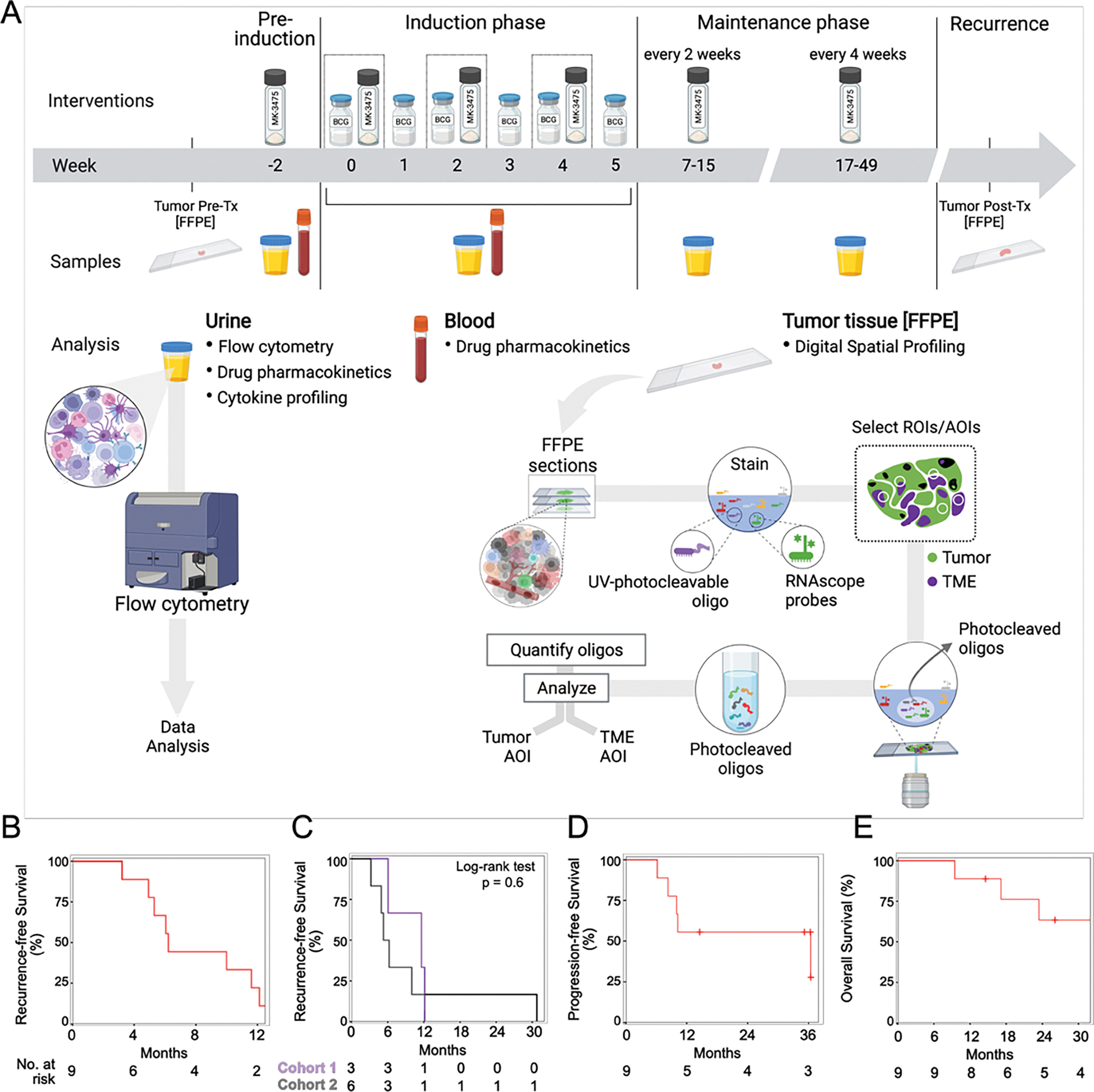
A) Treatment schema with dosing schedule of pembrolizumab (MK-3475) and BCG at each stage of the clinical trial. B-E) Kaplan-Meier curves showing B) Recurrence-free survival for the entire cohort (n=9) C) Recurrence-free survival by dose cohort (Cohort 1 at 1 mg/kg, and Cohort 2 at 2 mg/kg) D) Progression-free survival and E) Overall survival for the entire cohort.
Outcome measures:
At each visit, patients underwent an evaluation and physical exam to identify any adverse events with endoscopic evaluation at week 17 and every 8 weeks until the completion of the trial at week 52 (last cystoscopy on week 49). Urine was analyzed for cytopathology and any concerning lesion was biopsied. Any high-grade recurrence, whether in the bladder or throughout the urinary tract, was considered a recurrence and treatment was halted. After the last dose of trial therapy, patients were followed clinically for disease recurrence and survival every 3 months (± 30 days) during years 1 and 2.
Statistics:
This is a Phase I dose-escalation study using the 3+3 design with 4 doses. All analyses were conducted using R version-4.0.3 or GraphPad Prism version-9.3.1. Wilcoxon signed rank test was used for paired sample comparisons (e.g.: pre- to post comparison). For comparing independent groups, Kruskal-Wallis test was used to generate p-values. For repeated measures across different timepoints, mixed effects model was used to generate p-values. Recurrence-free (RFS), progression-free (PFS) and overall survival (OS) were defined as time from start of pre-induction until the corresponding event. Patients were censored for PFS and OS on the date of last cystoscopy or imaging without observed progression or date last known alive. Survival estimates were obtained using the method of Kaplan-Meier and groups were compared using the log-rank test.
Detailed methods for urine pK, urine flow cytometry, serum pK, urine cytokine analysis, GeoMx digital spatial profiling and PDL1 immunohistochemistry are included in the Supplementary methods.
Results
Clinical trial design
Eleven patients were screened, and 9 patients were enrolled between June 2016 and May 2020 when the trial was closed due to the COVID-19 pandemic (Supp Figure 1A). Patients were followed up through May 2021. Patient demographics are described in Table 1. All patients received a pre-induction dose at week -2 (Figure 1A) followed by BCG on weeks 0–5 along with intravesical pembrolizumab on weeks 0, 2, and 4. After induction, participants received intravesical pembrolizumab every two-weeks until week-17 (first cystoscopy), and then every four-weeks for the remainder of the year.
Table 1:
Baseline demographics
| Treatment group | |||
|---|---|---|---|
| Cohort 1 (N = 3) | Cohort 2 (N = 6) | ||
| Age | |||
| Median (yr) | 76 | 82 | |
| Sex - no. | |||
| Male | 3 | 5 | |
| ECOG performance status score | |||
| 0 | 3 | 6 | |
| Tumor stage - no. | |||
| Tis | 1 | 2 | |
| TaHG | 0 | 2 | |
| TaHG + CIS | 1 | 1 | |
| T1 | 1 | 0 | |
| T1HG | 0 | 1 | |
| PD-L1 status | |||
| Combined positive score ≤ 10 | 2 | 4 | |
| Adverse events attributed to BCG | |||
| Maximum Event Grade | |||
| Grade 1–2 | 3 | 6 | |
| Adverse events attributed to Pembrolizumab | |||
| Maximum Event Grade | |||
| Grade 1–2 | 3 | 4 | |
| Grade 5 | 0 | 1 | |
| Number of doses of BCG | Overall (N = 9) | ||
| Median (range) | 12 (6–27) | ||
| Other BCG unresponsive therapies - no. | |||
| Docetaxel | 3 | ||
| Gemcitabine | 1 | ||
| Nadofaragene Firadenovec | 1 | ||
Toxicity
There were 21 Grade 1–2 adverse events related to BCG and/or pembrolizumab with one Grade 5 adverse event related to pembrolizumab (Table 2). Nearly all BCG-related adverse events were bladder-related with the most common being gross hematuria (5) (Table 2). Adverse events are described in Table 2. The trial was closed early due to COVID-19 outbreak. Three patients were treated at a starting dose of pembrolizumab 1 mg/kg (cohort 1), and six patients were treated at 2 mg/kg (cohort 2). One DLT of grade 2, diarrhea lasting 21 days, was observed during treatment in cohort 2. One patient died due to treatment-related myasthenia gravis, an autoimmune disorder. The patient presented with vision changes and an elevated anti-ACHR antibody level. This was originally reported 117 days after the last dose of pembrolizumab. The patient was treated with pyridostigmine, prednisone, and IVIG and expired 10 months after symptom onset. All patients in both dose cohorts experienced grade 1–2 events (3/3 and 6/6 patients) related to BCG.
Table 2:
Summary of Adverse events
| Event - no. (%) | Grade 1 or 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
|
| ||||
| Adverse Events attributed to BCG | ||||
| Any | 21 (100) | 0 | 0 | 0 |
| Hematuria | 5 (55.6) | 0 | 0 | 0 |
| Diarrhea | 2 (22.2) | 0 | 0 | 0 |
| Fatigue | 2 (22.2) | 0 | 0 | 0 |
| Renal and urinary disorders - other, specify | 2 (22.2) | 0 | 0 | 0 |
| Urinary tract infection | 2 (22.2) | 0 | 0 | 0 |
| Abdominal pain | 1 (11.1) | 0 | 0 | 0 |
| Anemia | 1 (11.1) | 0 | 0 | 0 |
| Cystitis noninfective | 1 (11.1) | 0 | 0 | 0 |
| Flatulence | 1 (11.1) | 0 | 0 | 0 |
| Nausea | 1(11.1) | 0 | 0 | 0 |
| Pain | 1(11.1) | 0 | 0 | 0 |
| Urinary frequency | 1 (11–1) | 0 | 0 | 0 |
| Urinary urgency | 1(11.1) | 0 | 0 | 0 |
| Adverse Events attributed to Pembrolizumab | ||||
| Any | 21 (88.9) | 0 | 0 | 1 (11.1) |
| Hematuria | 4 (44.4) | 0 | 0 | 0 |
| Diarrhea | 2 (22.2) | 0 | 0 | 0 |
| Fatigue | 2 (22.2) | 0 | 0 | 0 |
| Renal and urinary disorders - other, specify | 2 (22.2) | 0 | 0 | 0 |
| Urinary frequency | 2 (22.2) | 0 | 0 | 0 |
| Abdominal pain | 1 (11–1) | 0 | 0 | 0 |
| Anemia | 1(11.1) | 0 | 0 | 0 |
| Autoimmune disorder | 0 | 0 | 0 | 1 (11.1) |
| Cystitis noninfective | 1 (11–1) | 0 | 0 | 0 |
| Flatulence | 1 (11.1) | 0 | 0 | 0 |
| Nausea | 1 (11.1) | 0 | 0 | 0 |
| Pain | 1 (11.1) | 0 | 0 | 0 |
| Pruritus | 1(11.1) | 0 | 0 | 0 |
| Urinary frequency | 1 (11–1) | 0 | 0 | 0 |
| Urinary urgency | 1(11.1) | 0 | 0 | 0 |
Clinical Response Data
Response to pembrolizumab and BCG was evaluated by cystoscopy, urine cytology and bladder biopsy when indicated. Median follow-up time among 5 patients who were alive at the end of the study was 35 months (IQR: 26–36). All patients recurred with recurrence free survival (RFS) rate at 3, 6 and 12 months of 100%, 67% (95% CI: 42–100%) and 22% (95%CI: 7–75%) respectively. (Figure 1B, Supp Fig 1B). Median RFS was 6.2 months (95% CI: 5-NA). No differences in RFS rates was observed between the 2 cohorts (Log-rank test, p-value = 0.6) (Figure 1C). The patient with the longest time to recurrence of 31 months, was also the only participant who experienced a DLT.
Progression occurred in 5 patients with a median PFS of 36 months (95%CI: 10-NR). PFS rate at 6- and 12-months were 100% and 56% (95%CI: 31–100%) (Figure 1D, Supp Fig 1B). There were no significant differences in recurrence and progression rates when the cohort was stratified by CIS (Supp Figure 1B–1C). Progression to locally advanced cancer (T2+Nany, TxN+, or M+) occurred in 4/9 patients, with recurrence in 6/9 outside of the treated bladder to UTUC (2), lung (1), prostate (2) and pelvis (1) (Supplementary Table 5). Death occurred in 4/9 patients (Figure 1E, Supp Fig 1D). Major cancer surgeries after enrollment included radical cystectomy (2), nephroureterectomy (1), and a distal ureterectomy with partial cystectomy (1).
Response During Therapy: Urine analytes
To determine if pembrolizumab was confined to the bladder, we evaluated plasma and urine levels of pembrolizumab for Cohort 1, and serum levels in the patient with DLT and grade 5 SAE. We found no detectable levels of pembrolizumab in the blood (serum or plasma) in any patient. We were able to detect pembrolizumab in the urine samples collected at week -2 and week 4. No detectable levels of pembrolizumab were found in the urine sample collected prior to treatment at week 4 (Supp Figure 2A).
To understand local immune changes that occurred after intravesical pembrolizumab administration, we prospectively evaluated urine of patients collected during the trial (Figure 1A) [7]. To identify potential mechanisms of response, we compared patients with early recurrence (n=5, median months to recurrence: 5.3, 95% CI: 4.9-NA) to late recurrence (n=4, median: 12 months, 95% CI: 10-NA).
At baseline, the patient that developed myasthenia gravis had the highest level of CD45+ immune cells with an increase of 8.7-fold over the median of other patients at baseline, (pre-treatment, week -2) (Figure 2A).
Figure 2: Progressive remodeling of local immune contents over the course of treatment by urine analysis of immune cells and cytokines.
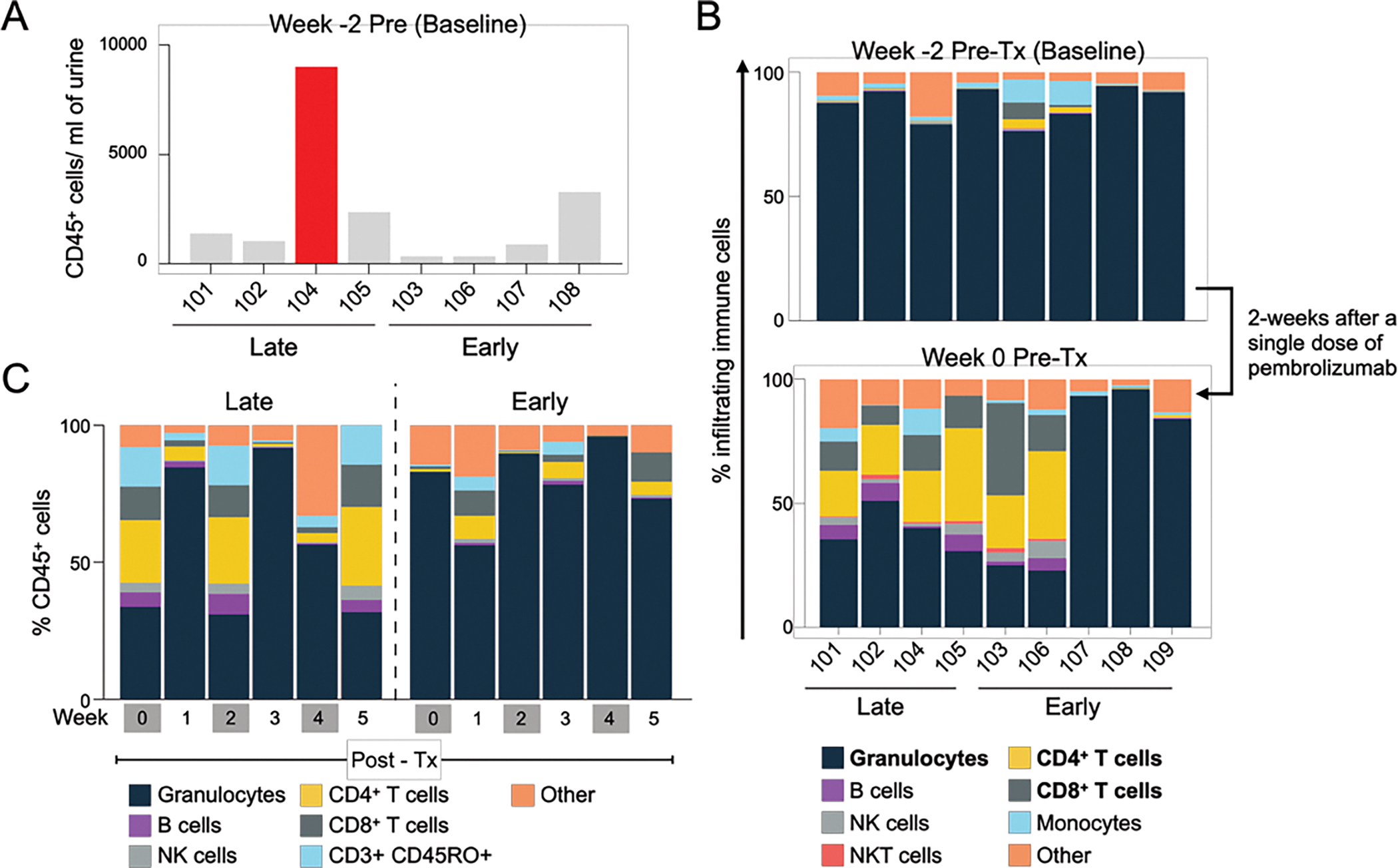
A) CD45+ cell number in the urine at baseline prior to start of treatment. B) Composition of immune cells in the urine at baseline and 2 weeks after administration of single dose of pembrolizumab. C) Changes in immune cell population in the urine during the induction period (Week 0–5). Each bar represents mean of indicated immune cells for each group. (n=4 for Late responders, n=5 for Early responders).
Next, we investigated whether a single dose of pembrolizumab could alter local immune environment by comparing urine collected at baseline (pre-treatment, Week -2) to that collected 2-weeks after first dose of intravesical pembrolizumab (pre-treatment, Week 0). Although most pre-treatment urine collected at baseline had a large concentration of granulocytes, we observed a significant decrease in granulocytes (Median: 41%, 95% CI: 0–60%, Wilcoxon signed rank test, p=0.023), and a significant increase in CD4+ (Median: 18%, 95% CI: 0.2–33%, p=0.023) and CD8+ T cells (Median: 12%, 95% CI: 0–14.2, p=0.015) after a single dose of pembrolizumab (Figure 2B). Since this measurement was performed two weeks later, alterations in immune cell populations reflect at-least transient changes to the microenvironment after a single intravesical dose of pembrolizumab.
To identify immune changes that occurred with BCG and pembrolizumab, we compared urinary immune profiles between early and late-recurrences at each week during the six weeks of induction. Most striking differences in immune populations were observed for time-points when the patients received BCG and pembrolizumab (weeks 0, 2 and 4) relative to BCG alone (weeks 1, 3 and 5). Relative to early recurrences, late recurring populations had significantly lower fractions of granulocytes and a higher lymphocyte fraction in particular higher CD4+ and CD8+ T cells at weeks 0 and 2 (Figure 2C and Supp Table 6).
By urine cytokine profiling, we detect increased levels of BCG-regulated inflammatory cytokines (TNF-alpha and IL-8) [8, 9], chemokines such as MIP-1 beta and monocyte chemoattractant (MCP-1) increased from week -2 to 5 during induction treatment (Supp Figure 2B).
Collectively, multi-omic urine analysis identified immune recruitment to the bladder following exposure to intravesical BCG and pembrolizumab.
Spatial Transcriptomic Analysis
We sought to understand how intravesical pembrolizumab and BCG affected bladder tumors and investigate mechanisms of resistance. Given the technical limitations in profiling CIS by bulk RNA-Seq or macro-dissection, we performed transcriptomic analysis of pre- and post-treatment (Tx) tumors using Nanostring’s digital spatial profiling (DSP) technology (Figure 1A)[10]. Using DNA, pan-cytokeratin, CD68 and CD3 as protein markers for the tumor and tumor micro-environment (TME), we evaluated 151 areas of interest (AOIs) collectively from 8 patients, 86 from post-treatment and 65 from pre-treatment tumors. Principal component analysis (PCA) confirmed discrete clustering of PanCK+ (tumor) and PanCK− (TME) AOIs (Figure 3A). We first evaluated bladder cancer subtypes in PanCK+ AOIs. While one tumor had features of a basal/squamous tumor, the rest expressed only luminal markers [11]. We compared AOIs from multiple regions, and found little intra-tumoral spatial heterogeneity in tumor subtype and found no changes in subtype after treatment. (Figure 3B)
Figure 3: Changes in the tumor microenvironment as a consequence of therapy.
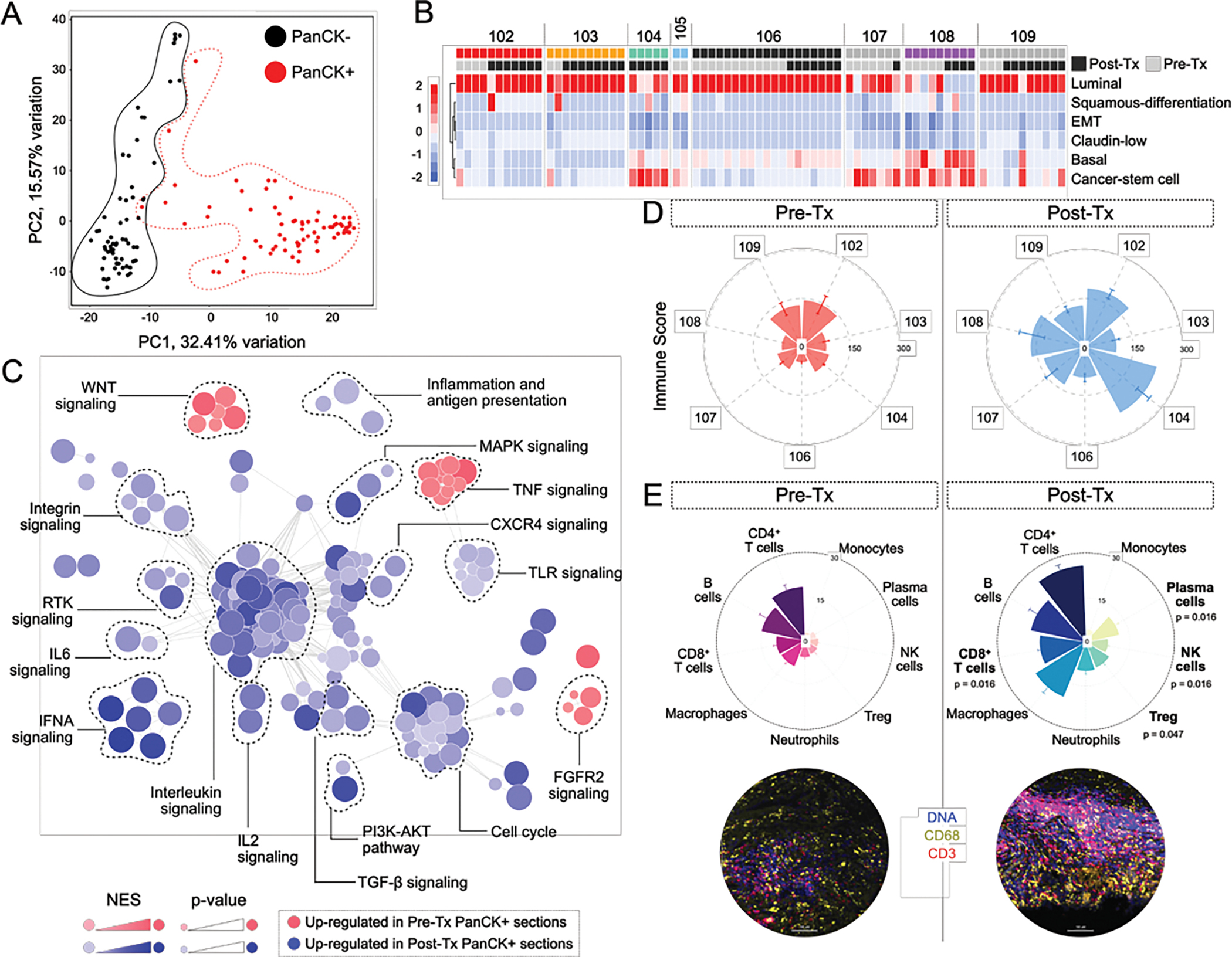
A) PCA plot showing individual region of interest (ROI) profiled from pre- and post-Tx tumor sections. B) Heatmap showing transcriptomic subtypes identified for each PanCK+ ROI within the cohort. C) Gene expression programs identified in PanCK+ segments pre- and post-treatment (Tx). D) Immune scores calculated from PanCK− segments for each analyzed sample. Bars represent Mean ± SEM. E) Immune cell counts within the tumor microenvironment derived by deconvolution of PanCK− RNA expression profiles of pre- and post-Tx ROIs (Top). Bars represent Mean ± SEM. Representative images shown for pre- and post-Tx groups (Bottom).
Next, we compared transcriptomic profile of pre- and post-treatment tumor epithelium (PanCK+) using gene-set enrichment analysis and found a significant enrichment of inflammatory pathways in PanCK+ post-treatment tumor AOIs (Figure 3C). Pre-treatment tumor AOIs expressed minimal inflammatory gene expression programs, with the exception of pro-inflammatory TNF signaling, suggestive of prior BCG treatment. Post-treatment tumors depicted an inflamed phenotype with enrichment of pathways involved in immune regulation (inflammation and antigen presentation, TGF-β signaling) and cytokine signaling (IL6, IL2, IFNA, CXCR4, TLR and Interleukin signaling).
Next, we evaluated changes in the TME (PanCK−), comparing AOIs of the same patient before and after therapy. We found a significant increase in ImmuneScore (median increase: 48, 95% CI: 12–104) (Wilcoxon signed rank test, p = 0.031) (Figure 3D) in post-treatment TMEs. In particular we found a significant increase in plasma (median difference: 6.5, 95% CI: 4.3–9.2), NK, (median difference: 3, 95% CI: 1.4–4.2), CD8+ T (median difference: 6.9, 95% CI: 4.5–9.9) and Treg cells (median difference: 7.0, 95% CI: 2.2–8.1) in post-treatment samples relative to pre-treatment (Figure 3E). This increase was most pronounced in the patient that developed myasthenia gravis (Patient 104) (Supp Figure 3A, 3B).
To identify mechanisms of resistance or response to intravesical pembrolizumab and BCG, we compared spatial profiling of pre-treatment tumor and TME from early and late recurrences. Tumor epithelium AOIs from early recurrences showed an inflamed phenotype with upregulation of TNF-mediated inflammatory signaling, MHC class II signaling. In contrast, late recurrences were enriched for pathways involved in cell cycle regulation, DNA damage response, and innate immune pathways (Figure 4A).
Figure 4: Differences in transcriptional programs in tumor and TME from early and late responders.
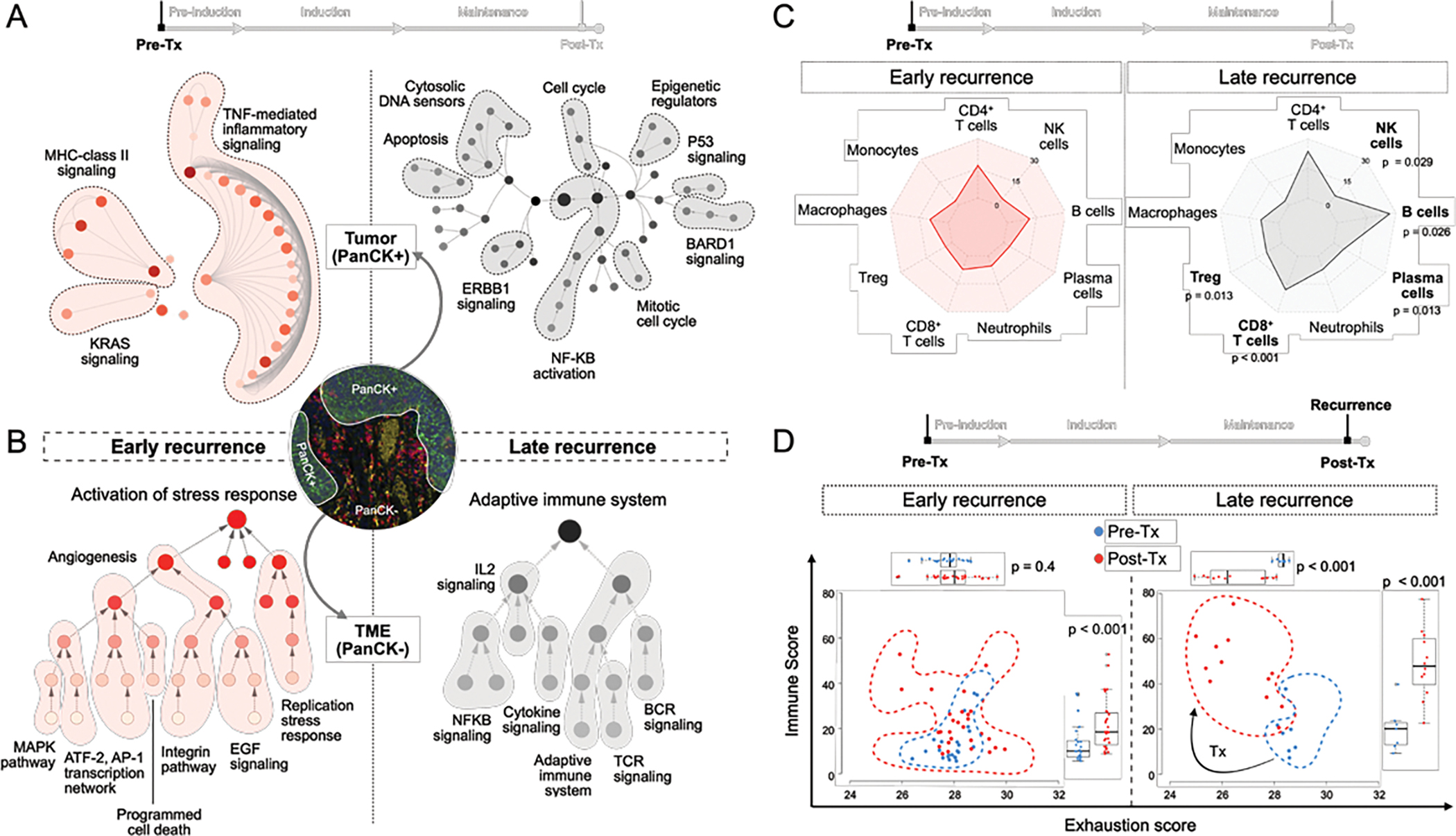
A) Gene expression programs identified in pre-treatment PanCK+ ROIs from early and late recurrences. B) Gene expression programs identified in pre-treatment PanCK− ROIs from early and late recurrences. C) Radar plot of Immune cell distribution in PanCK− pre-Tx ROIs from early and late responding tumors. Each point represents mean values. (p-values from comparing immune cell populations in early vs late recurrences were generated using Kruskal-Wallis test) D) Changes in Immune score and Exhaustion score for each PanCK− ROI pre-to-post treatment segregated by early and late responders.
Next, we evaluated the pre-treatment TME of early and late recurrent tumors. Early recurrent TME was enriched in stress response pathways, stromal signatures and angiogenesis with a higher Stromal score and significantly elevated levels of PDGFRB (Figure 4B, Supp Figure 3C). In contrast, late recurring TME had an enrichment of pathways associated with adaptive immune system processes (TCR, BCR signaling), cytokine signaling (IL2, NF-KB pathways) (Figure 4B) along with a significantly higher Immune score (median difference: 26, 95% CI: 12–95) (Supp Figure 3D).
In particular, we find elevated CD8+ T (median difference: 12, 95% CI:6–19), Treg (median difference: 9.2, 95% CI:2.3–11), B (median difference: 9.9, 95% CI:3.5–36), plasma (median difference: 4.8, 95% CI:1–5.5) and NK cells (median difference: 2.2, 95% CI:0.18–5.2) in late recurring tumors relative to early recurrences (pre-Tx) (Figure 4C). To characterize the activation state of immune response, we compared immune cell counts and exhaustion score. Late recurrent tumors had a large significant increase in immune cells in the TME, and a significant reduction in exhaustion markers pre-to-post treatment. (Figure 4D).
Overall, this is the first direct comparison of the heterogeneity of tumor and TME in NMIBC and BCG unresponsive NMIBC by spatial profiling. Our data indicates that differences in clinical endpoints of BCG unresponsive tumors may be secondary to distinct pathways of the tumor/TME, with responsive tumors showing greater T-cell infiltration with less exhaustion while resistant tumors have increased neutrophils and a stroma-rich microenvironment (Supp Figure 3E).
Discussion
Although checkpoint immunotherapy has revolutionized the treatment of solid tumors, toxicity of CPI and their efficacy in early stage NMIBC require further investigation for broader use. In this trial, we attempted to mitigate the toxicity of CPI by direct application of pembrolizumab into the bladder, potentially avoiding systemic toxicity. We combined pembrolizumab with BCG with the intent to both increase immune recruitment but also decrease exhaustion. We had initially hoped to deliver higher doses of pembrolizumab, however our trial was discontinued at the start of the COVID-19 pandemic.
We did not detect pembrolizumab in the serum or plasma in all five patients tested, however, two patients experienced toxicity during treatment, and both adverse events occurred at the higher dose of pembrolizumab tested of 2 mg/kg. While we cannot conclude that lower dosing of pembrolizumab is safe, higher doses may not be needed to modulate the immune response. We observed increased lymphocyte recruitment, suggestive of immune modulation two weeks after a single dose of intravesical pembrolizumab.
Like other immunotherapies, we find a correlation between inflammatory response and durability of clinical response. The patient that had the longest response to therapy, greater than 12 months without high grade recurrence, was also the patient that experienced a DLT during induction. Except for two patients, intravesical pembrolizumab was well tolerated with only grade 1–2 adverse events that were mostly urinary-related. Thus, intravesical delivery of CPI is a viable strategy, for patients with BCG-unresponsive NMIBC, meriting further investigation of dose, delivery, and patient selection.
Evaluation of tissue and blood from the one patient with a grade 5 autoimmune adverse event identified possible mechanisms of systemic immune activation from intravesical therapy. This patient had the highest number of CD45+ cells in the urine prior to treatment initiation and this number increased further during the course of treatment. Although this resulted in a longer duration in delay to recurrence, the enhanced immune activation from intravesical therapy may have hastened progression of myasthenia gravis. While BCG, and advanced age have been associated with myasthenia gravis, only a few case reports have described myasthenia gravis associated with IV checkpoint therapy[12, 13].
There are several limitations of our study. Our sample size was limited to nine patients due to premature closure of the trial. Although we attempt to describe our findings for pre- and post-treatment therapy samples, validation in larger cohorts is necessary. The rate of extra-vesical recurrence were greater than expected, and may reflect the limited number of treatment options available for patients recruited in this study.
Conclusions
Here, we demonstrate that intravesical pembrolizumab is a safe and feasible alternative in patients with BCG unresponsive NMIBC. We attempted to decrease toxicity of anti-PD-1 therapy by intravesical delivery, however 2/9 patients had a DLT or a SAE.
We are encouraged by the broad-range of immune responses observed in urine collected during trial and in the tumor tissue at the end of the trial. Changes in the immune niche are detectable in the urine as early as after one dose of pembrolizumab. Nevertheless, as evident by analysis of recurrences observed in our trial, duration of anti-tumor immunity is dependent on pre-treatment tumor composition and future trials should explore combination therapies and careful patient selection to improve efficacy.
Overall, intravesical administration of pembrolizumab is a promising therapeutic modality, capable of generating long-lasting anti-tumor immunity and should be investigated further.
Supplementary Material
Take Home Message:
We conducted a phase I clinical trial of intravesical pembrolizumab and BCG. The trial found both local and systemic toxicity and identified a subset of patients that had a longer response with decreased immune exhaustion.
Acknowledgments
We thank Arighno Das for technical assistance.
Financial Disclosures:
RSS: is PI of S1602, which utilizes TICE BCG provided by Merck
TK: Serves on DSMC for Merck trials, but no COI
JJM: received funding from Merck for the described clinical trial through the MISP, funding from Lurie Cancer Center, and participated in advisory boards for Merck
Funding Support/Role of the Sponsor.
This study was supported by funding from Merck Investigator Studies Program (MISP) and the Robert H. Lurie Cancer Center. JM is supported by grants from the VHA BX005599 and BX003692. This work was supported by the Northwestern University RHLCCC Flow Cytometry Facility and the Quantitative Data Sciences Core (MK and SM) under the Cancer Center Support Grant (NCI CA060553).
References
- [1].Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. 2016;196:1021–9. [DOI] [PubMed] [Google Scholar]
- [2].Balar AV, Kamat AM, Kulkarni GS, Uchio EM, Boormans JL, Roumiguié M, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021;22:919–30. [DOI] [PubMed] [Google Scholar]
- [3].Chade DC, Shariat SF, Dalbagni G. Intravesical therapy for urothelial carcinoma of the urinary bladder: a critical review. Int Braz J Urol. 2009;35:640–50; discussion 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Herr HW, Morales A. History of bacillus Calmette-Guerin and bladder cancer: an immunotherapy success story. J Urol. 2008;179:53–6. [DOI] [PubMed] [Google Scholar]
- [5].Giannarini G, Agarwal N, Apolo AB, Briganti A, Grivas P, Gupta S, et al. Urologists, You’ll Never Walk Alone! How Novel Immunotherapy and Modern Imaging May Change the Management of Non-muscle-invasive Bladder Cancer. Eur Urol Oncol. 2021. [DOI] [PubMed] [Google Scholar]
- [6].Kirschner AN, Wang J, Rajkumar-Calkins A, Neuzil KE, Chang SS. Intravesical Anti-PD-1 Immune Checkpoint Inhibition Treats Urothelial Bladder Cancer in a Mouse Model. J Urol. 2021;205:1336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wong YNS, Joshi K, Khetrapal P, Ismail M, Reading JL, Sunderland MW, et al. Urine-derived lymphocytes as a non-invasive measure of the bladder tumor immune microenvironment. J Exp Med. 2018;215:2748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].de Boer EC, Somogyi L, de Ruiter GJ, de Reijke TM, Kurth KH, Schamhart DH. Role of interleukin-8 in onset of the immune response in intravesical BCG therapy for superficial bladder cancer. Urol Res. 1997;25:31–4. [DOI] [PubMed] [Google Scholar]
- [9].Kamat AM, Briggman J, Urbauer DL, Svatek R, Nogueras González GM, Anderson R, et al. Cytokine Panel for Response to Intravesical Therapy (CyPRIT): Nomogram of Changes in Urinary Cytokine Levels Predicts Patient Response to Bacillus Calmette-Guérin. Eur Urol. 2016;69:197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pelka K, Hofree M, Chen JH, Sarkizova S, Pirl JD, Jorgji V, et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell. 2021;184:4734–52.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fong MHY, Feng M, McConkey DJ, Choi W. Update on bladder cancer molecular subtypes. Transl Androl Urol. 2020;9:2881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Takizawa T, Kojima M, Suzuki S, Osada T, Kitagawa S, Nakahara J, et al. New onset of myasthenia gravis after intravesical Bacillus Calmette-Guerin: A case report and literature review. Medicine (Baltimore). 2017;96:e8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Becquart O, Lacotte J, Malissart P, Nadal J, Lesage C, Guillot B, et al. Myasthenia Gravis Induced by Immune Checkpoint Inhibitors. J Immunother. 2019;42:309–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


