Abstract
Surgery can cure or significantly improve both the frequency and intensity of seizures in patients with medication-refractory epilepsy. The set of diagnostic and therapeutic interventions involved in the path from initial consultation to definitive surgery is complex and includes a multidisciplinary team of neurologists, neurosurgeons, neuroradiologists, and neuropsychologists, supported by a very large epilepsy-dedicated clinical architecture. In recent years, new practices and technologies have emerged that dramatically expand the scope of interventions performed: stereoelectroencephalography has become widely adopted for seizure localization; stereotactic laser ablation has enabled more focal, less-invasive, destructive interventions; and new brain stimulation devices have unlocked treatment of eloquent foci and multifocal-onset etiologies. This article articulates and illustrates the full framework for how epilepsy patients are considered for surgical intervention, with particular attention given to stereotactic approaches.
Most patients with epilepsy can achieve good seizure control with antiseizure medications. Approximately 30% of people with epilepsy do not achieve seizure remission despite two or more medication trials1–3. These individuals would be classified as having refractory or intractable epilepsy4. In individuals with refractory epilepsy, surgical evaluation should be considered early5. This is especially true in those with focal epilepsies localized to the temporal lobe in whom surgery may result in a high likelihood of seizure freedom6. In patients with temporal lobe epilepsy and concordance between EEG localization and a structural abnormality on imaging, they can have up to 80% chance of seizure freedom7.
There is often a delay before referral for epilepsy surgery. In adult patients, there can be up to a 20-year delay between epilepsy onset and time of surgery8, 9. In children, this delay is less but on average 9 years and 7 to 8 anti-seizure medication trials later10. Reasons for delay can include delay in epilepsy diagnosis, delay in referral to an epilepsy center, waiting for epilepsy to be “outgrown,” continued medication trials with low likelihood of remission, lack of findings on imaging, and external patient factors (lower socioeconomic status, cultural beliefs, reluctance to undergo surgery)11, 12. However, the evidence for the benefit of surgical intervention is clear: In the only randomized controlled trial of epilepsy surgery vs medical management, surgery outperformed medical management (58% vs 8% free of impaired-awareness seizures, and 38% vs 3% free of all seizures at one year)13. In a trial of early epilepsy surgery vs medical therapy for mesial temporal lobe epilepsy, 11/15 in the surgery group compared to 0/23 in the medical group were seizure free at 2-year follow up14.
The concept of epilepsy surgery goes back millennia to Hippocrates15; however, our understanding of epilepsy and its mechanisms have evolved significantly from the simple skull trephinations performed in antiquity. Modern epilepsy surgery is a relatively recent development, starting with Sir Victor Horsley in the late 19th century, who anatomically localized patients’ epileptic onset based upon their symptoms during seizures and cured them by resection16, 17. Following advances in stereotactic targeting18, 19, Bickford & Cairns20–23 and later Talairach24 pioneered diagnosis of seizure onset from electrical activity measured with penetrating depth electrodes. Talairach’s frame, enabling pure lateral trajectories guided by angiogram to avoid vasculature, is the foundation of modern stereotactic EEG25, 26. This pioneering work allowed for chronic intracranial EEG recording which had previously not been possible. In the decades since, magnetic resonance imaging and intraoperative neuronavigation have enabled millimeter-scale precision for targeting with direct knowledge of the cortical anatomy beneath. Today, the stereotactic EEG approach consists of customized trajectories that optimize sampling with skewed trajectories that specifically target imaging-identified pathologies and suspected seizure-onset zones, while maintaining distance from dangerous structures. In the last decade, stereotactic placement of clear cannulas combined with the ability to measure temperature in real time with MRI (MR thermometry) has seen the emergence of laser heating to heat and destroy the seizure focus, sparing the patient a craniotomy with damage to unrelated brain tissue27. As this shift towards minimally invasive epilepsy surgery continues to evolve in the modern era, the process from diagnostic workup to definitive intervention has become more nuanced. This manuscript aims to highlight the potential uses of stereotaxy in medically intractable epilepsy and to provide a resource to navigate the decision-making process in epilepsy surgery (Figure 1).
Figure 1:
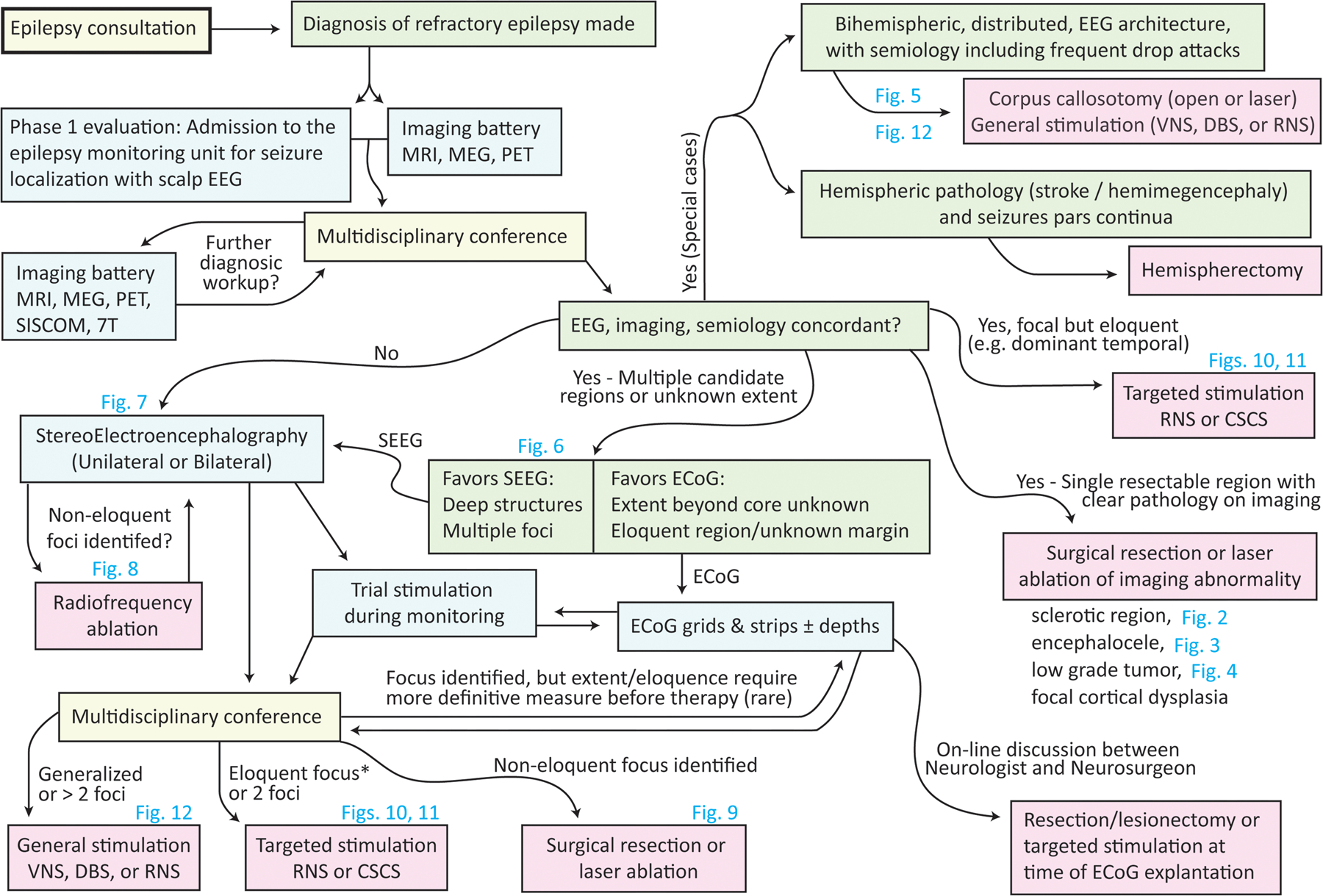
Overview of the decision-making process in stereotactic epilepsy surgery. The process begins in the top left, with a consultation to the epileptologist. Steps illustrated in this manuscript are noted by corresponding figure number.
From referral to multidisciplinary conference
Once a diagnosis of refractory epilepsy is made, the next step should be consideration of epilepsy surgery. In all patients with surgically remediable epilepsy, early referral is better. In children, earlier referral can be associated with improved developmental outcomes28. Reasons for referral to a epilepsy center include: age less than 2 years old29; epilepsy is not controlled within two years of onset or after trials of two or more medications; intolerable side effects are experienced; disabling seizures; imaging demonstrating a focal unilateral lesion consistent with seizure semiology; an epileptic encephalopathy, with lack of expected developmental progression, plateauing or regression coincident with seizure onset or increase in frequency; and an etiology which requires special dietary or medical management – i.e. glucose transporter deficiency, Dravet syndrome, etc.29.
Comprehensive presurgical evaluation at an epilepsy center typically begins with attempts at seizure classification and anatomic localization of the seizure onset zone (SOZ). This starts with a thorough seizure history and evaluation by an epileptologist (Figure 1). Continuous video EEG monitoring is necessary to record symptoms before, during, and after the seizure (ictal semiology) and correlate these with electroencephalographic findings. In order to determine whether there is an identifiable anatomic abnormality (i.e. whether this represents lesional focal epilepsy), high-fidelity imaging including a brain MRI, ideally on a 3 Tesla MRI scanner when possible, including thin cuts through the hippocampi and sequences which can aid in the identification of abnormalities, such as double-inversion recovery (DIR) which can highlight gray matter abnormalities such as focal cortical dysplasia or migrational abnormalities30.
When a patient is non-lesional or “MRI-negative” additional studies should be performed. Imaging modalities such as PET coregistered to CT or MRI can identify areas of focal hypometabolism suggestive of a seizure onset zone in the interictal period or hypermetabolism, which can be seen following a recent seizure31, 32. Other modalities which can aid in localization can include studies such as magnetoencephalography, which can be an adjunct to EEG to identify a cluster of electrical dipoles that localize the SOZ. During a monitoring unit evaluation, performing ictal SPECT (single photon emission computed tomography) can help identify areas of cerebral hypermetabolism which may indicate the SOZ. This can further be enhanced by co-registration to MRI as is done with SISCOM (subtraction ictal-SPECT coregistered to MRI)31, 33 and with statistical parametric mapping (SPM)34.
Following completion of phase 1 (admission to the epilepsy monitoring unit (EMU) for seizure localization with scalp EEG) of the presurgical evaluation, many centers convene a multidisciplinary epilepsy surgery conference that involves epileptologists, neurosurgeons, neuropsychologists, and neuroradiologists. Neuropsychologists evaluate for cognitive deficits that can help in assessing the patient-specific morbidity of particular surgical interventions, particularly in function related to memory, naming, or speech35. One of the key benefits of this step in the process is the potential to re-review previous imaging or electrophysiological studies for evaluation of subtle findings that may have been overlooked on initial radiologic interpretation36. Following this meeting, the committee may recommend (with the weight and gravity of a consensus): further diagnostic studies; medical management without surgical intervention; a definitive procedure, such as neurostimulator implantation, resection, or disconnection; or phase 2 evaluation with intracranial EEG may be the next step.
A question of concordance
When evaluating candidacy for epilepsy surgery, one of the primary goals is to evaluate for concordance (agreement) in the hypothesized SOZ between seizure semiology, diagnostic electrophysiology (EEG), and abnormalities on imaging37–39. Having concordance increases the likelihood that the suspected epileptogenic zone has been correctly identified and increases the likelihood of a seizure-free outcome: When there is an identifiable lesion, the odds of seizure freedom can be 2.5 times higher compared to those without a structural abnormality, therefore, it can be important to pursue multiple modalities when assessing for concordance40.
Magnetic resonance imaging (MRI) is the cornerstone of lesion identification, and most patients undergoing presurgical evaluation will already have updated neuroimaging to assess whether they may have lesional focal epilepsy. In patients with MRI-negative epilepsy who have not undergone recent imaging (within the past 6–12 months), it may be reasonable to repeat imaging as part of the phase 1 evaluation. This is particularly true if technology has changed or improved or if, for example, the patient previously had MRI imaging on a 1.5 tesla coil MRI and now a 3 tesla MRI scanner is available41. While not routinely clinically available, though often available on a research basis, 7 tesla MRI can identify subtle malformations of cortical development that were initially not identified (or were inconclusive) on 3 tesla MRI42, 43. Having a standardized epilepsy imaging protocol as well as staff trained to interpret the sequences is critical in the evaluation44–47. Utilizing specific sequences, such as double inversion recovery (DIR) can be helpful in identifying a lesional abnormality30. Image post-processing, such as morphometric analysis, can be particularly valuable in the workup of non-lesional focal epilepsy48–51.
In addition to MRI, a number of ancillary studies can be performed to further refine SOZ localization and help to create a surgical plan. Ictal SPECT is an imaging study where a radiotracer is injected during a seizure shortly after clinical or electrographic seizure onset52–54. Once the radiotracer is injected it is selectively concentrated in areas of hyperperfusion and hypermetabolism within the brain, which, during the early phase of seizures, identifies the SOZ. With late injections, these may be non-localizing as electrical hyperactivity may have diffused or spread due to generalization of epileptiform activity. Interictal SPECT can also be helpful, where the injection is repeated during a period of time without seizure, and areas of hypoperfusion/metabolism are of interest. Subtraction ictal SPECT coregistered to MRI (SISCOM) subtracts ictal and interictal SPECT studies and overlays them on MRI, which can sometimes identify a more nuanced area of interest for the SOZ, or where a subtle malformation may have missed on initial inspection52, 55–57.
Statistical parametric mapping (SPM) is an image processing tool that analyzes cerebral blood flow changes comparing voxels and can be helpful in identification of seizure onset zone hyperperfusion58, 59. This information can also be coregistered to MRI, i.e. StatisCOM, to again help point out subtle abnormalities not initially identified on imaging34.
Positron emission tomography (PET) is a nuclear medicine study which evaluates energy utilization, commonly in the form of 18 F-fluorodeoxyglucose (FDG) assessing areas of hypometabolism for identification of the epileptogenic region60. This can be particularly helpful in the identification of focal cortical dysplasia (FCD) overlooked on initial inspection of the MRI. Coregistration of PET onto MRI or CT can identify areas of relative hypo- and hyper-metabolism, which can indicate the SOZ in its ictal or interictal states. Emerging techniques coregister PET with ictal SPECT61.
Digital analysis of raw EEG data and synthesis with imaging can be helpful for understanding seizure initiation and propagation in an anatomically grounded framework, and aid in planning if the patient is a surgical candidate62–67. Similarly, magnetoencephalography (MEG) is a complementary electrophysiology technique that records magnetic fields generated by cellular activity68. Information from MEG is combined with the structural MRI in order to localize the source of the generated signal (magnetic source imaging - MSI)69. MEG can be particularly helpful for confirming a lesion or the area surrounding a lesion is the SOZ, with anatomic colocalization of epileptic spike source to pathology on imaging70, 71. In non-lesional (MRI-negative) epilepsy cases, source localization of interictal dipole clusters can help identify a region of suspicion that could be further evaluated with sEEG electrodes during subsequent intracranial monitoring. In those with restricted zones of dipole clusters, seizure free outcomes may still be achieved with normal or non-lesional MRI72.
In some patients with clear concordance, there is limited benefit from additional evaluation and a definitive procedure is recommended as the next step. These situations may be because there is a clear lesion that can be implicated as causative for the patient’s epilepsy (i.e. encephalocele, tumor, etc.) or a procedure with known benefits (corpus callosotomy for atonic seizures). If the abnormality is far from eloquent cortex or felt to be fairly “low risk, high reward” such as in non-dominant temporal lobe with concordant seizure semiology and EEG findings, then many may go directly to surgery. If the seizure semiology does not match with the expected imaging findings, then further evaluation with sEEG would be reasonable to try to find the suspected seizure onset zone.
Mapping techniques to identify brain function
Non-invasive functional mapping is essential for understanding the epileptic-eloquent interface and counseling patients and their families about surgical risk or neuromodulatory side effects. These mapping studies are performed to localize eloquent brain areas involved with speech/language, sensorimotor function, vision, and memory. Functional MRI (fMRI) does this by measuring MR correlates of blood oxygenation while patients perform tasks in the scanner73, 74. The subtlety of mapping scales with patient participation and can be performed in young children for motor localization75, 76. Active functional mapping can be performed non-invasively with transcranial magnetic stimulation (TMS) – a stimulation method that applies changing magnetic fields outside the head to induce electrical currents within the brain to map language and motor function77, 78. TMS is effective in adult and pediatric epilepsy patients with a minimal side effect profile, including headaches and scalp discomfort, and a small (<1%) risk of seizure79–83. As an interesting aside, TMS can also be used as an intermittent neuromodulatory therapy for refractory focal epilepsy84–87.
Targeted brain tissue ablation with laser interstitial thermal therapy (LITT)
Laser interstitial thermal therapy (LITT, Figure 2) is a minimally invasive, stereotactic technique that has been adopted within the last decade for the treatment of refractory epilepsy in both adults and children88–91. LITT burns brain tissue by laser heating with a fiber optic filament advanced through an irrigating cooling catheter that is stereotactically placed in the SOZ, while monitoring heat distribution within the brain volume using continuous MRI (modified T2* sequence). By virtue of the real-time temperature maps from MRI (MR Thermography - Figure 3), LITT is safer than classical radiofrequency (RF) ablation when lesioning sensitive brain areas. Laser ablation often follows sEEG-based identification of the SOZ and can be helpful for deeper and difficult to reach structures92. Real-time thermal mapping is used to show the area of burn surrounding the area of interest (Figures 2, 3, 4, 5, & 9). The temperature limits are set to protect injury to sensitive structures, where low-temperature triggers are placed on imaging, and automatically turn off the laser before the tissue is burned. This can be repeated multiple times, and along multiple trajectories to destroy the SOZ.
Figure 2: Dual filament LITT ablation for mesial temporal sclerosis.
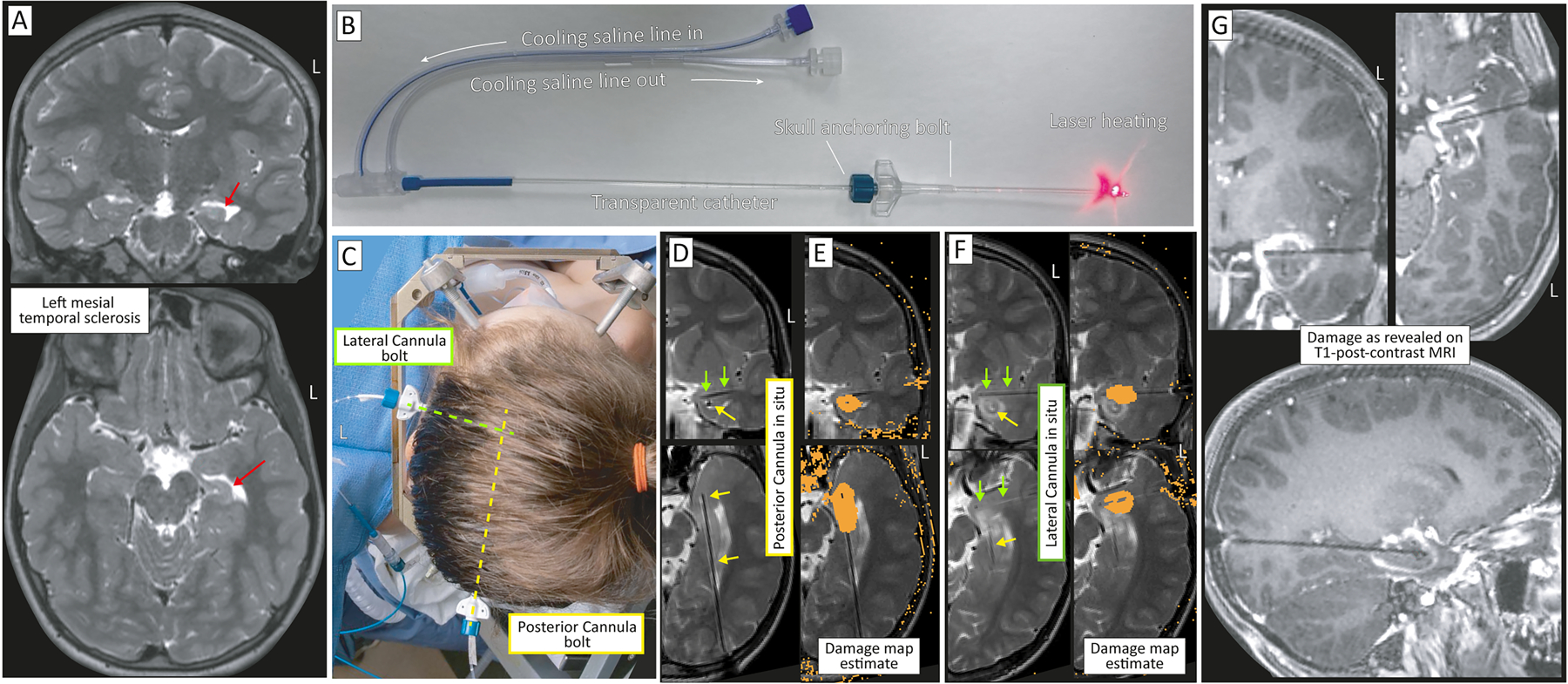
(A) Coronal (top) and axial (bottom) T2 MRI showing left hippocampal mesial temporal sclerosis. Sclerotic hippocampus indicated by red arrow. (B) The transparent laser cannula is continually cooled with cycled saline and held in place with a skull alignment and anchoring bolt that can be entirely plastic (as seen here), or metal & plastic (seen in C). (C) The mesial temporal structures are targeted with two cannulas. One is from a posterior approach (yellow-dashed), targeting the body and lateral head of the hippocampus, and the other is from a lateral approach, targeting the amygdala and the superior-medial aspect of the hippocampal head. (D) The posterior-approach laser cannula seen by air artifact on in-plane pseudo-coronal (top) and pseudo-axial (bottom) T2 MRI, indicated by yellow arrows. Green arrows show the lateral approach cannula, which can also be seen on the coronal image. (E) Thermal damage map from the posterior cannula in pseudo-coronal (top) and axial (bottom) sections. (F) As in D&E, but for the lateral approach cannula, indicated in green arrows. Note the ablation from the posterior approach (yellow arrow) that can be seen on the coronal image. (G) Post-ablation damage revealed on T1 post-contrast (gadolinium) MRI, in coronal (left), axial (right), and sagittal (bottom) sections.
Figure 3: Illustration of laser interstitial thermal therapy (LITT) for a left temporal encephalocele.
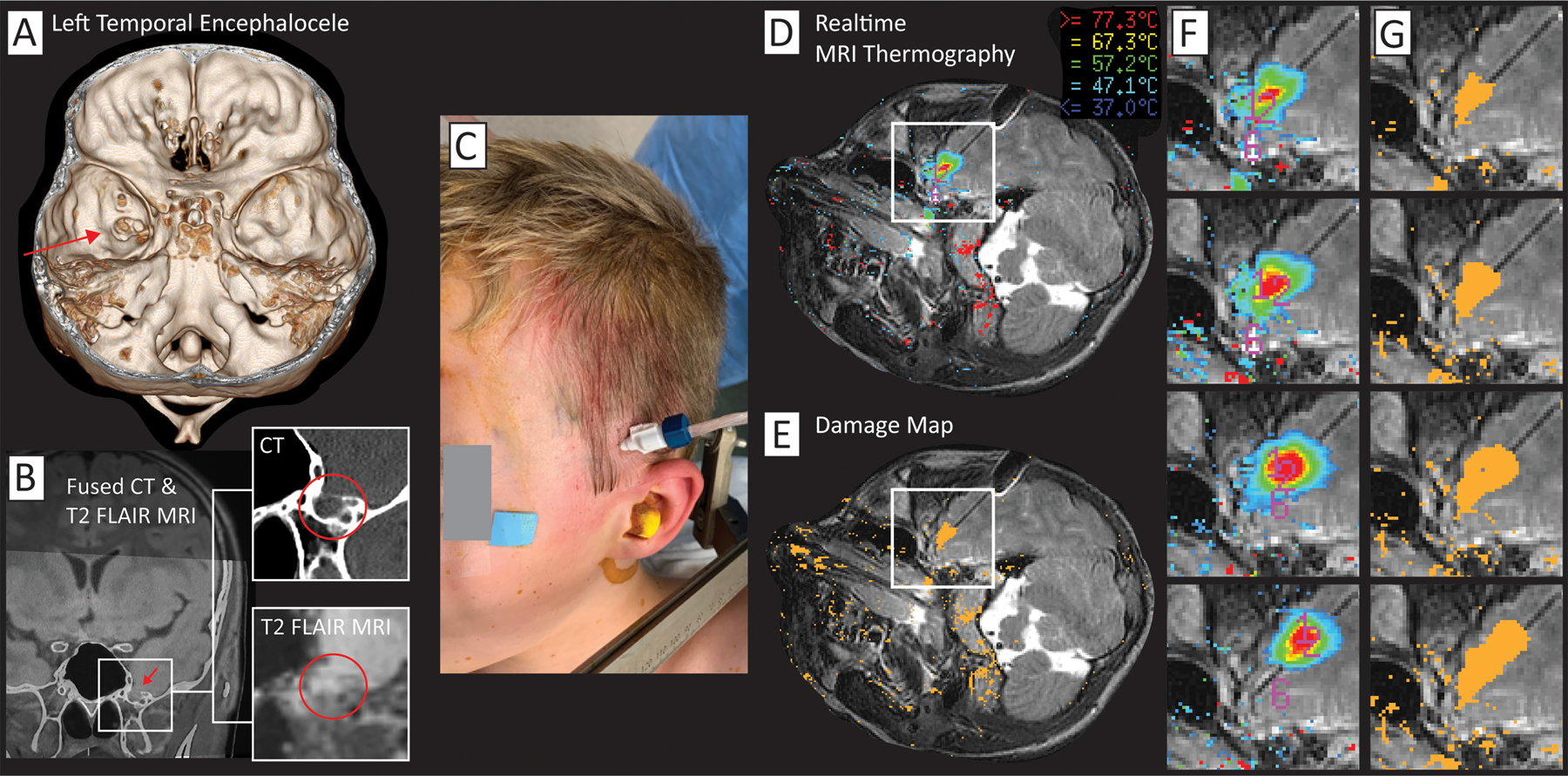
(A) A left anteromedial temporal skull base defect can is seen on 3D rendering (red arrow). (B) The skull defect and herniating brain tissue is seen in coronal section. (C) Operative photograph showing site of cannula insertion through skull bolt. (D) Realtime image of temperature map from MR thermography (modified T2* sequence). (E) Realtime cumulative damage map for estimated permanent burn using the Arrhenius equation172. (F) Sequential ablations - realtime MRI thermography (from white box in (D). (G) Sequential cumulative damage map (from white box in (E).
Figure 4: Coordinated biopsy and LITT of an epileptogenic tumor.
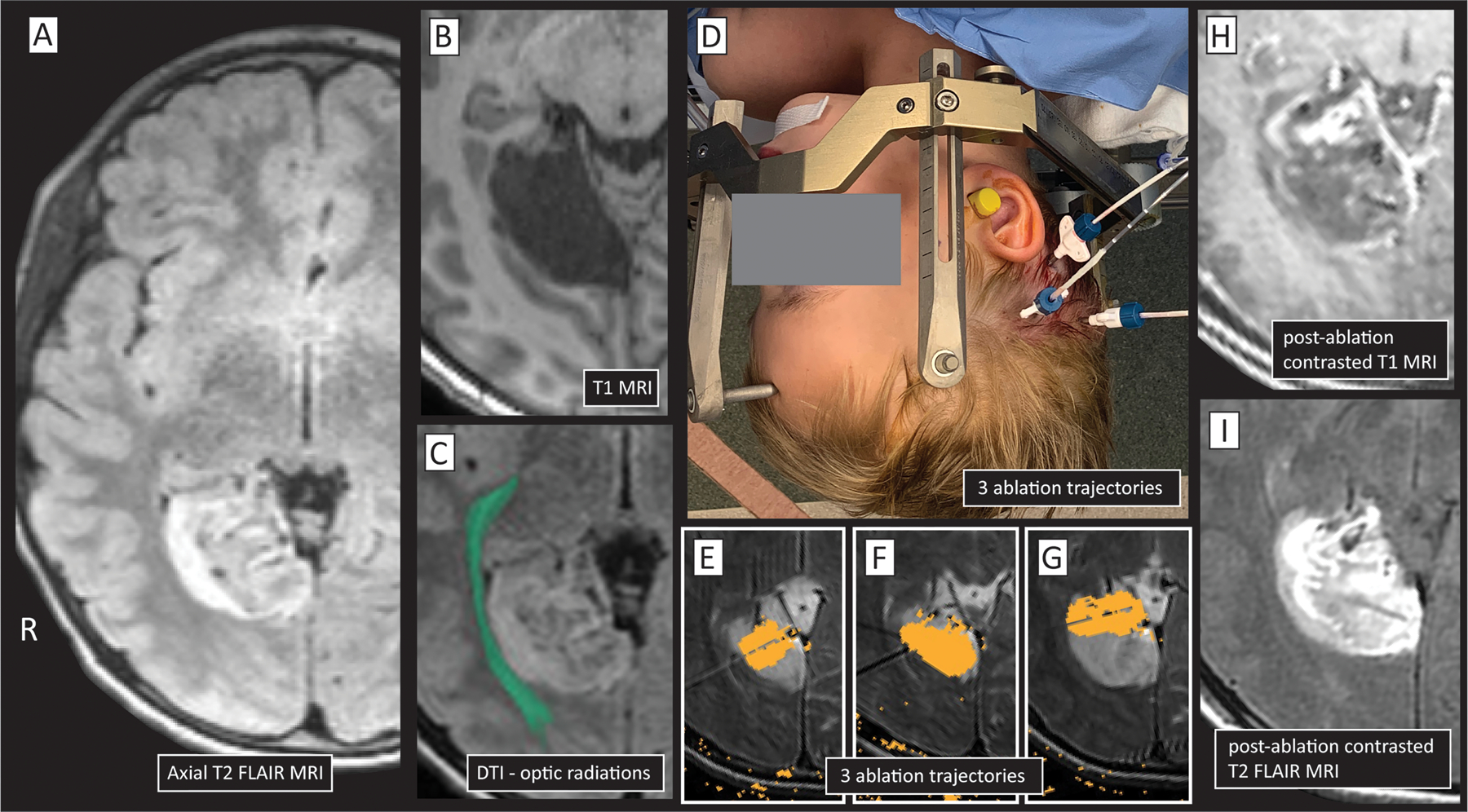
(A&B) A child with intractable seizures was found to have a right-sided lingual gyrus lesion, seen here in T1&T2 axial sections. (C) Diffusion tractography imaging (DTI) showed close proximity of the lesion to the optic radiations. (D) Three laser cannulas were placed stereotactically. A needle biopsy was performed through the posterior trajectory prior to cannula placement, and the lesion was found to be a dysembryoplastic neuroepithelial tumor (DNET). (E-G) Damage maps for 3 laser trajectories. (H&I) Post-ablation damage revealed on contrasted (gadolinium) T1 (H) and T2 (I) MRIs.
Figure 5: Complete corpus callosotomy performed by four-cannula LITT approach.
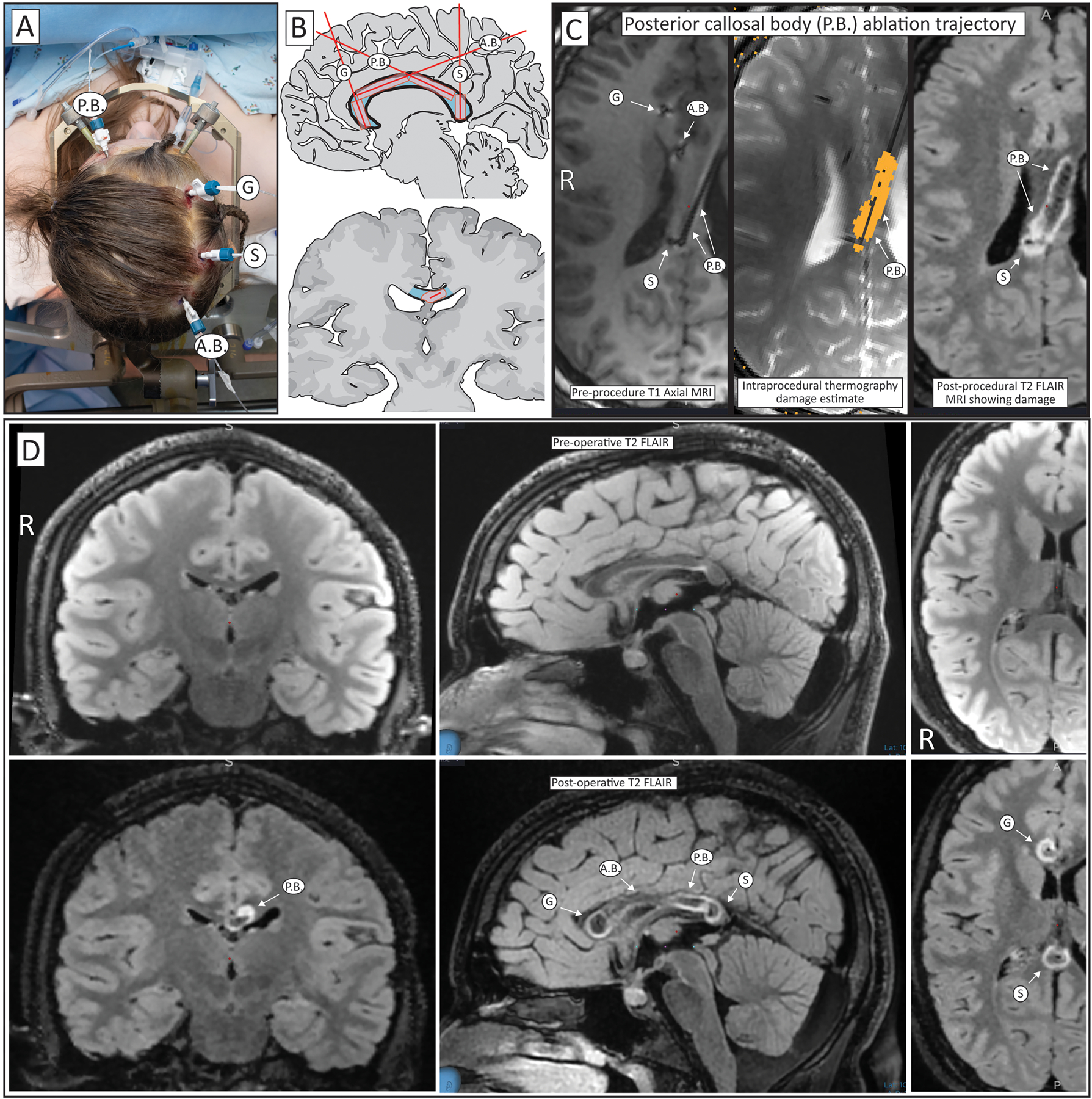
(A) Four skull bolts are placed with an anterior-posterior posterior callosal body (P.B.) trajectory, transverse trajectories through the genu (G) and splenium (S), and a posterior-anterior anterior callosal body (A.B.) trajectory. (B) Segments of the corpus callosum traversed by laser cannulas in cartoonized sagittal (upper) and coronal (lower) images. (C) Illustration using axial FLAIR MRI imaging of an ablation trajectory in post-placement, pre-treatment imaging where artifact from air shows the cannulas (left), during the treatment where the estimated damage can be seen in orange (middle), and post-treatment, where the ablated region can be seen with hyperintensity. (D) Pre-operative (top) and post-treatment (bottom) FLAIR imaging in coronal (left), sagittal (middle), and axial (right) sections, showing the ablation extent.
Fig 9. Peri-insular seizure onset zone intervention.
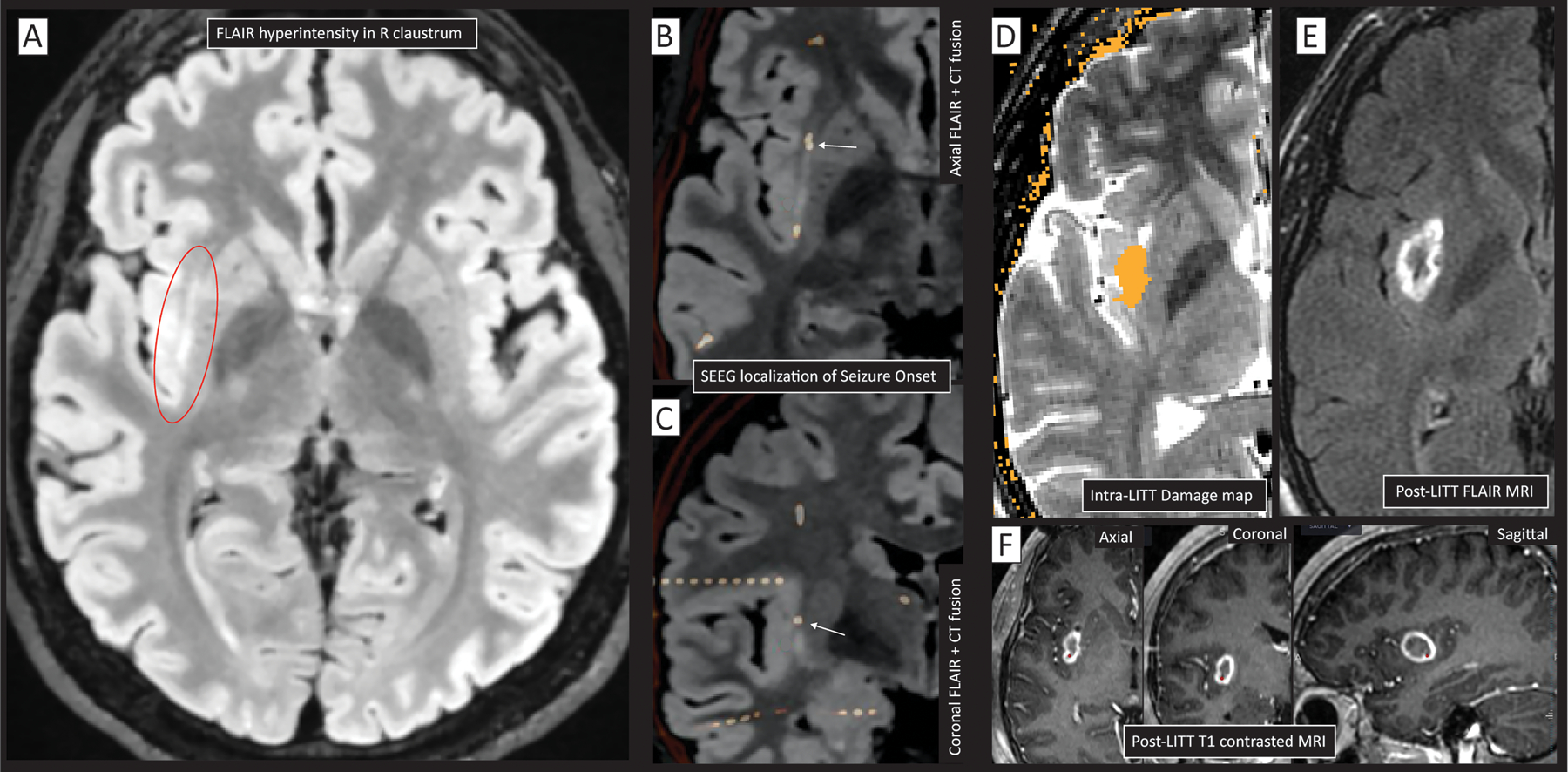
(A) A hyperintensity is seen on MR FLAIR sequence in the right claustrum (circled in red), but scalp EEG non-focal within the right hemisphere. (B&C) Implanted SEEG electrodes (white-orange) fused to the FLAIR MRI localized seizure initiation (white arrow) to the claustrum/insula (B-axial, C-coronal). RF ablation changed seizure semiology, though did not eliminate seizures altogether. (D) Based upon causal suggestion from RF, LITT was performed, with intraoperative damage estimate (orange) overlaid on axial T2 MRI. (E) Post-LITT axial FLAIR MRI. (F) Post-LITT gadolinium-contrasted T1 MRI seen in axial, coronal, and sagittal sections. Note that the peri-insular seizures were also captured in electrodes out of plane in addition to that noted by white arrow in panels C&D.
The most classic, and most successful, target for surgical intervention in epilepsy is mesial temporal sclerosis (MTS, also called hippocampal sclerosis), a pathology characterized by atrophy and scarring down/sclerosing of the hippocampus with loss of normal cytoarchitecture93, 94. Stereotactic laser ablation can be used as an alternative to an open anterior temporal lobectomy with amygdalohippocampectomy (Figure 2)95. Studies comparing stereotactic laser ablation to standard anterior temporal lobectomy (ATL) for mesial temporal sclerosis have found that LITT fully preserves naming compared with >75% deficit rate with ATL96, 97. While there are still some memory deficits seen with dominant mesial temporal LITT, these are less severe and far less frequent than dominant ATL96, 98. As currently performed, LITT provides a slightly lower probability of seizure freedom versus standard temporal lobectomy for MTS95, 99–102. However, LITT does not preclude subsequent resection, so ATL may follow if improvement is only partial or non-sustained. This can still be an appealing surgical option given the minimally invasive nature compared to open resection, and, anecdotally, many patients who decline an open resection do choose to undergo LITT.
Encephaloceles are herniations of brain tissue into a skull defect, which can be either acquired or congenital. Some acquired causes are previous head trauma or prior neurosurgical intervention 103. These can be found in any location, but more commonly identified in the anterior and middle cranial fossae, where they are epileptogenic and resection or ablation is generally recommended103–105. For some encephaloceles, ablation via stereotactic laser thermal ablation is emerging as an approach to minimize morbidity while stopping seizure activity from spreading to other brain regions, although it does not directly repair the cranial defect (Figure 3)106, 107.
In patients with epileptogenic tissue aberrancies, such as focal cortical dysplasia, gray matter heterotopia, cortical tubers, etc., consideration can be made for LITT in place of open resection. This can follow in cases of strong initial EEG concordance or from an sEEG evaluation if confirmation of SOZ is needed. Laser ablation can also be performed initially as part of a staged procedure, to be followed by open resection in the case of incomplete seizure control or as part of a planned staged approach for larger lesions108. In some pathologies, LITT may be the clearly preferred approach for surgery, as in the case of hypothalamic hamartoma, where endoscopic or open resection may be associated with increased risk of complications and lower rates of seizure freedom109–112.
Stereotactic innovation has enabled new avenues for therapy for epileptogenic brain tumors. Using stereotaxy, tumors can be biopsied to obtain diagnostic tissue, followed by laser ablation of the tumor, which can be particularly useful for deep or difficult to access tumors113, 114. For a number of tumor types, the diagnostic yield of stereotactic versus open biopsy can be similar115, while the frequency of epilepsy associated with brain tumors is variable depending on the tumor pathology. A number of brain tumors are known to be highly epileptogenic, such as neuroglial tumors and gangliogliomas, where rates of epilepsy could approach 100%, though the natural history of the lesions may be otherwise relatively benign116. Neuroglial tumors, such as dysembryoplastic neuroepithelial tumor (DNET) and polymorphous low-grade neuroepithelial tumor of the young (PLNTY), while rare, are among the most common causes of intractable focal epilepsy117. The prototypic scenario where laser ablation may be recommended would be in the case of epilepsy associated with hypothalamic hamartoma118. For patients with imaging and history consistent with these tumors or previous subtotal resection, ablation can be considered over open resection, particularly for hard to reach or deep tumors119, 120 (Figure 4).
Stereotactic disconnection surgery – laser corpus callosotomy
In the case of intractable atonic seizures or drop attacks, there may be no discrete regions of seizure onset. The sequelae of these seizures may be dramatic and, cumulatively, life threatening with frequent injury. As drop attacks result from interhemispheric sustainment of seizure activity with synchronized loss of function, a palliative corpus callosotomy to prevent spread between the hemispheres may be the next recommended step. While atonic seizures can often be seen in individuals with Lennox-Gastaut syndrome, they may also be seen in other childhood onset epilepsies and epileptic encephalopathies. These and other refractory epilepsies may respond to callosotomy 121–123. Corpus callosotomy involves dissection of the fibers of the corpus callosum as an effort to prevent rapid propagation of epileptic activity between the hemispheres. While callosotomies have traditionally been performed via an open approach, laser ablation has recently been adopted as an alternative by placement of multiple thermal ablation catheters throughout part or all of the corpus callosum (Figure 5). LITT is significantly less invasive than the open approach, which involves craniotomy, dissection, brain retraction, potential injury to the pericallosal arteries, and blood loss124. The most common complication of complete corpus callosotomy, which can be seen with both open and laser approaches, would be a disconnection syndrome, characterized by ataxia, aphasia/mutism, apraxia, anomia, and alien hand syndrome, which often is transient. Recovery time and length of hospital stay are dramatically reduced following laser callosotomy compared with an open approach. LITT can also be performed to complete a prior partial callosotomy or any residual connections from a prior open resection125–127.
Decision point: should this patient undergo stereo-EEG?
Stereoelectroencephalography (sEEG) records electrical signals within the brain by using electrodes that are implanted using a minimally invasive procedure where wires are passed through 2 mm burr holes in the skull. These electrodes help locate the seizure source when scalp EEG is unclear or imaging is negative, but seizure activity is still suspected or observed (Table 1). sEEG may also be used to map function in areas of the brain to be removed via resection or thermal ablation or to assess whether patients would benefit from implanted stimulation therapy.
Table 1.
The role of stereoelectroencephalography (sEEG) in epilepsy treatment.
|
Refining the hypothesis
• SOZ hypothesis may be based on limited evidence • There can be discordance between EEG, imaging, and/or ancillary studies • Scalp EEG may be poorly localizing • Clinical semiology may not be specific to a particular region |
|
Sampling a deeper focus • sEEG electrodes can sample deep structures, while studies like ECoG cannot • Can sample multiple targets with a single lead • Ability to sample white matter tracts • Ability to sample networks beyond cortex, examining spread into the thalamus and other deep structures. |
|
Seizure localization
• Scalp EEG may show secondary ictal propagation and be falsely localizing • Can sample areas of interest widely with electrodes • Can home in on the SOZ (not just seizure propagation to the surface) and minimize/refine potential area of resection/ablation |
|
Defining the borders of resection • sEEG can help with mapping the borders of resection • Multiple electrodes can be implanted surrounding a lesion/abnormality • Contacts within the SOZ and other active contacts of secondary propagation can be identified • Can be done extraoperatively with seizure localization and intraoperatively with electrical stimulation of active contacts |
|
Mapping eloquent cortex/tracts • sEEG can be utilized for mapping of eloquent cortex and white matter tracts. • Can be useful to sample around and within a malformation as dysplastic, epileptogenic, cortex can be intermingled with eloquent cortex |
|
Assessing candidacy for RF ablation • RF ablation can be performed through sEEG electrodes • SOZ in noneloquent cortex (determined by stimulation) can be considered for RF ablation • Response to RF ablation may predict future response to laser ablation or resection • Well tolerated – low risk, performed at bedside |
|
Assessing candidacy for neuromodulation with DBS or RNS using trial stimulation • Stimulation montages targeting the SOZ(s) or thalamus independently or in tandem can be assessed. • Response to different programming and parameters can be assessed • Implanted thalamic electrode stimulation can be used to test response to DBS or RNS |
Until recently, most patients who currently undergo sEEG in the United States would instead have been monitored with grid, strip, and depth electrodes using traditional electrocorticography (Figure 6), which records from larger areas of the brain surface following craniotomy. In contrast, sEEG can be performed via small holes drilled in the skull, anchors placed over these holes, and then placement of the intracranial sEEG electrodes through these holes guided by stereotactic navigation to pre-planned locations. Between 10 to 15 electrode leads are typically placed during sEEG implantation (with more than 200 total electrode contacts) depending on the set of hypotheses for location(s) of the seizure onset zone (Figure 7). Many centers now have robotic-assisted sEEG implantation, which can speed up implantation time up to 2.5 times faster than frame-based approaches128. Additionally, robotic-assisted implantation has been associated with improved target accuracy (reduced deviations from the intended trajectory) and reduced rates of catastrophic complications129. Recently, head-mounted 3-D printed customized stereotactic fixtures, with all trajectories pre-aligned, have allowed for rapid sEEG placement with high accuracy and fewer free parameters than other approaches130–132.
Fig 6. Electrocorticography (ECoG) and stereoelectroencephalography (SEEG).
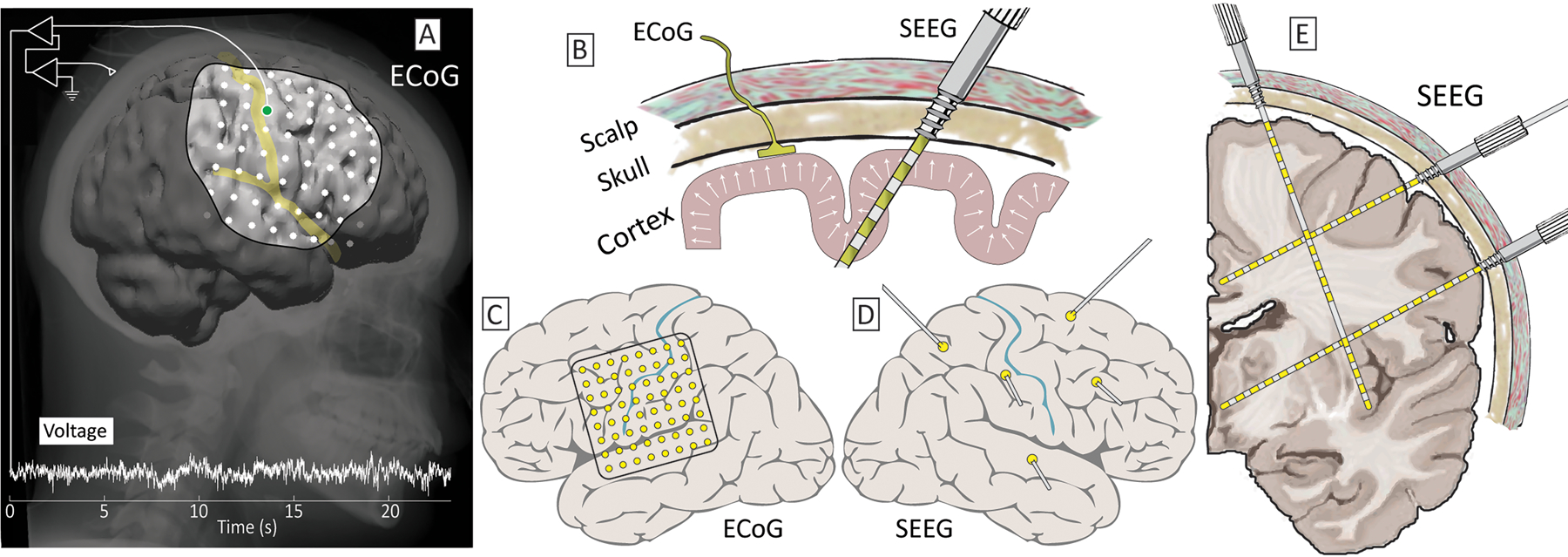
(A) Grids of brain surface ECoG electrodes are placed through large openings in the skull (craniotomies). (B) Recently, there has been increasing use of stereotactically-placed depth electrodes – SEEG, placed through bolts embedded in the skull. (C) ECoG electrodes sample the exposed, convexity, brain surface at regular intervals. (D) SEEG samples this convexity irregularly and sparsely, though can be targeted precisely. (E) SEEG is used to precisely sample surface & deep gray matter as well as subcortical nuclei to identify seizure onset zones and potential therapeutic loci.
Fig 7. Stereoelectroencephalography implantation.
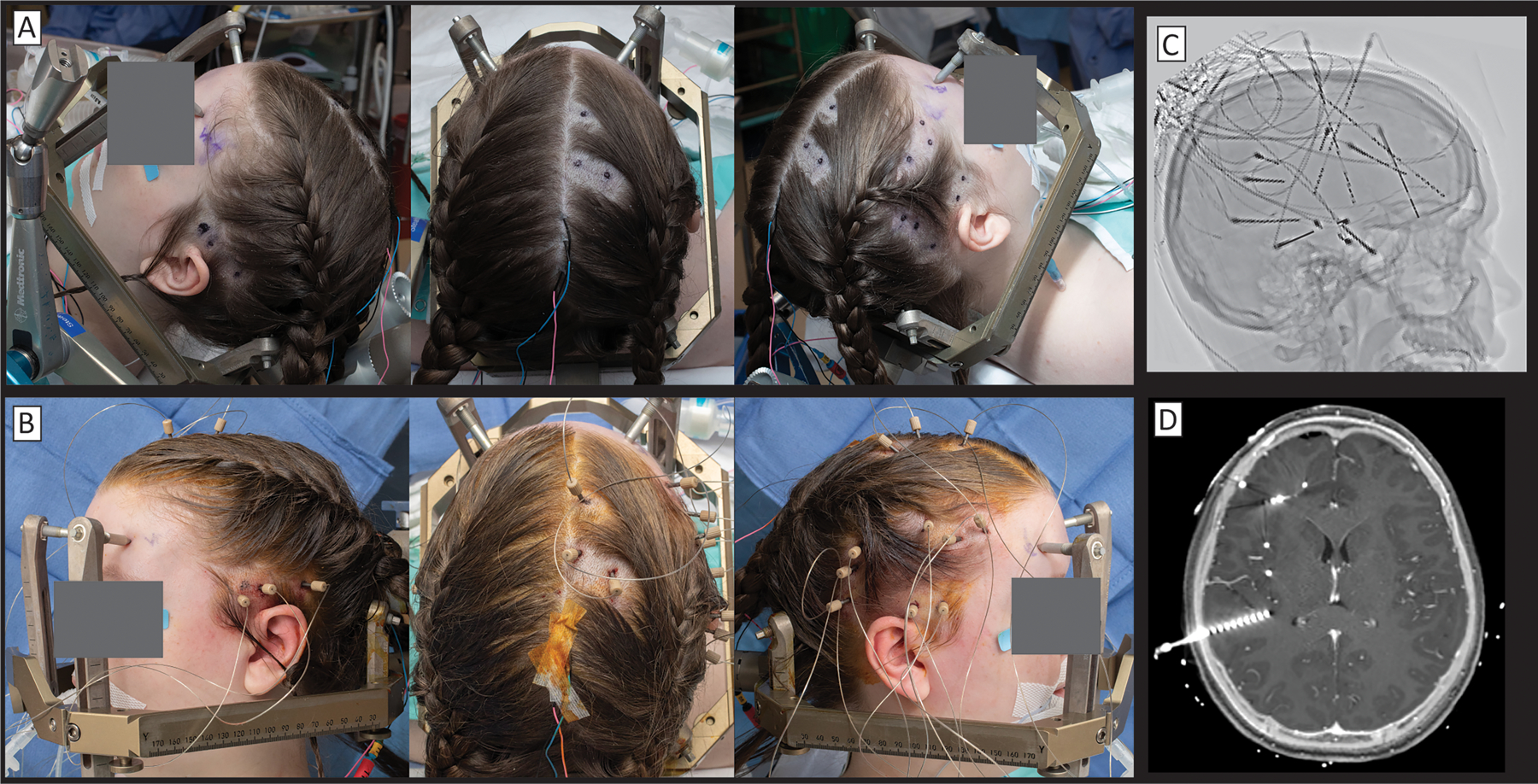
(A) Preoperatively, insertion sites are marked and small shaves are made. (B) SEEG bolts are placed stereotactically and SEEG leads are advanced in-line through them. (C) An inverted x-ray shows a variety of trajectories. Note that many surgeons are increasingly placing “skew” trajectories that follow gyral anatomy rather than pure lateral trajectories that dominated in Europe in previous generations. (D) Fusion of CT to post-gadolinium enhancement T1 MRI shows the precise relationship of each electrode to underlying brain anatomy.
sEEG in lesional epilepsy:
sEEG can serve an important role in the diagnosis and treatment of lesional epilepsy. In the case of multiple evident lesions on brain imaging, with inconclusive scalp EEG to delineate between them, implanted sEEG electrodes can isolate which is the SOZ. In the lesional case where the SOZ is coarsely known, sEEG can to help define the extent of resection – defining the epileptic margin – while also enabling extraoperative cortical stimulation mapping, defining the functional margin (eloquent boundary). The two margins can then be weighed against one another to determine what intervention will maximize reduction in seizures while minimizing the risk of significant deficit. This approach is particularly useful with malformations of cortical development, where eloquent cortex can be intermixed with dysplastic cortex and there is markedly increased risk of deficit if function is not characterized prior to resection133. At the brain surface, stimulation mapping with ECoG has been shown to improve seizure-free outcomes while minimizing post-operative deficits134, 135. Mapping with sEEG electrodes has the benefit of sampling brain structures throughout the brain volume, which cannot be done with brain-surface ECoG grid & strip electrodes. However, the extent of contiguous coverage with sEEG is limited by sparse sampling, while ECoG can provide a regular sampling of the brain surface surrounding the superficial lesion margin of lesion for direct epileptic and functional mapping136, 137. Despite this, electrical stimulation mapping with sEEG can still be helpful with mapping motor and language function, but more likely underrepresents the extent of eloquent cortex138, 139. One benefit of deeper sampling with sEEG mapping is the ability to assess propagation and spread of activity from stimulated contacts in gray matter through white matter which can assist with mapping the epileptic network140. In select patients, awake resection with intraoperative ECoG can be considered to further delineate epileptic and eloquent cortex if needed141, even in patients who may have undergone prior sEEG monitoring.
sEEG in non-lesional epilepsy
While seizure-free outcomes for patients without obvious imaging abnormalities (“imaging negative”, non-lesional) are typically considered lower compared to lesional epilepsy, sEEG can significantly improve the prospect of seizure reduction or freedom129, 142, 143. In non-lesional epilepsy, the set of regions targeted by sEEG is primarily driven by the scalp EEG findings and the seizure semiology, with a canonical set of trajectories for each candidate brain region. For example, epigastric rising sensation and olfactory auras suggest the mesial temporal lobe144, prompting subsequent sEEG lead placement in the hippocampal body & head and the amygdala. Scalp EEG with diffuse lateral interictal epileptiform discharges over frontal, temporal, and central leads would prompt placement of sEEG leads into the ipsilateral insula. Ancillary metabolic imaging studies like PET and SPECT may also provide candidate SOZs for sEEG lead placement. MEG, when available, can also guide sEEG targeting as an electrophysiological adjunct to EEG, with ictal dipoles interpolated on a co-registered MRI145.
Radiofrequency ablation through stereoelectroencephalography (SEEG) electrodes
As previously discussed, sEEG can also be performed as the first part of a staged intervention, such as radiofrequency ablation (RF) or LITT. RF ablation is performed by delivering high levels of electrical current through the implanted sEEG leads to burn adjacent epileptogenic tissue (principally in Europe, and now being increasingly adopted in the United states)146–149. The procedure is performed after a period of prolonged inpatient monitoring, where the seizure focus (or foci) has been localized, and clinical stimulation mapping has been performed through the sEEG leads to rule out post-procedural deficit. It may be performed at the bedside, with typically a neurosurgeon attaching RF cables to the sEEG leads and delivering high power (typically ~5 watts) current sequentially through a set of pre-determined contacts using a clinical radiofrequency generator. A neurologist reviews the EEG traces from the adjacent leads during the ablation and clinically monitors the patient for seizures or behavioral changes. While the RF ablation volumes are smaller than other techniques like LITT (~3.5mm versus >12mm diameter149), RF ablation may serve several important purposes: 1) RF ablation can be performed to “mark” seizure onset zone as (Figure 8); 2) Some patients experience a sustained reduction of seizures following RF ablation alone146, 148; 3) In those with even a transient reduction in seizure frequency or a change in seizure character, RF helps to identify regions for future LITT, resection, or neurostimulation to produce a more lasting effect; 4) The implanted electrophysiology may continue to be monitored after the RF ablation, providing the epilepsy team with novel understanding of the patient’s seizure network to devise permanent treatment strategies (this is particularly relevant in multifocal epilepsy, where only a subset of foci may be ablated due to eloquence).
Fig 8. Radiofrequency ablation (RF) through stereoelectroencephalography electrodes.
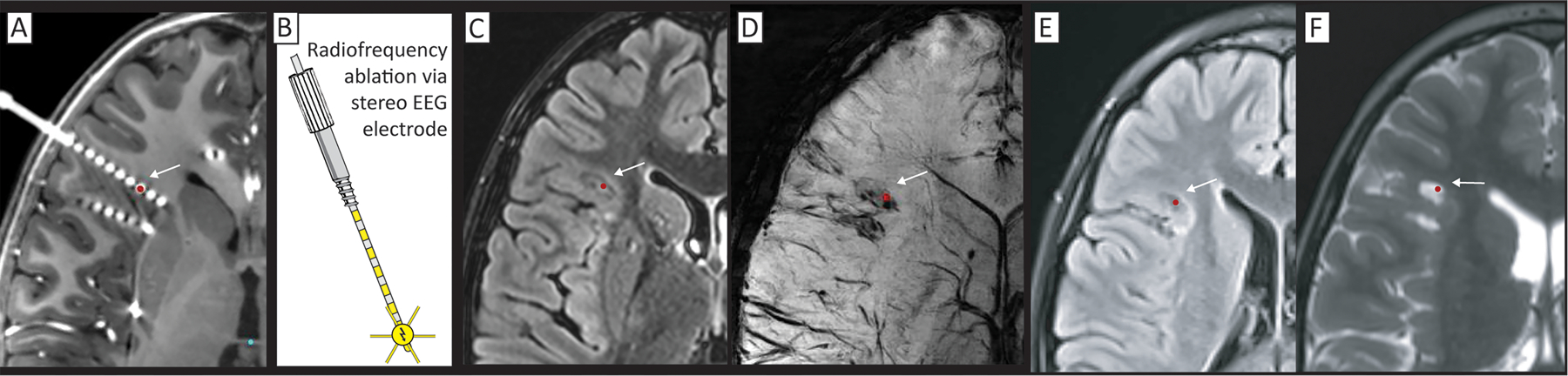
(A) Seizure onset was identified by SEEG in several of the deepest contacts, centered at the red dot, and indicated by an arrow (on CT fused to gadolinium-contrasted T1). (B) RF ablation is performed by passing current directly through the SEEG electrodes, using a grounding pad on the leg. (C-D) Immediate post-procedure MR imaging on T2 FLAIR (C), and susceptibility-weighting (D). (E&F) 16-month post-procedure T2 FLAIR (E) and standard T2 (F) showing persistent lesion effect.
The connection between sEEG and brain stimulation to treat epilepsy
During phase 2 monitoring, it may be discovered that the patient is not a focal resection candidate, most commonly because the patient has multifocal epilepsy or the identified SOZ is in eloquent cortex. In cases of multifocal or eloquent SOZ, sEEG can be used in the evaluation for candidacy for neuromodulation. In light of this, the preoperative sEEG planning should answer the question, “Could this patient benefit from neuromodulation, and will this monitoring plan determine the best neuromodulation strategy?” In the case of lesional epilepsy, where the extent of SOZ extension into eloquent cortex is unknown, adequate electrode coverage of the lesion borders can be used for trial stimulation and to see if the patient would benefit from focal stimulation (i.e. responsive neurostimulation (RNS) or chronic subthreshold cortical stimulation(CSCS)). For patients with multifocal, diffuse, or generalized epileptic networks, a more generalized stimulation modality (deep brain stimulation (DBS) or RNS into thalamic targets) may be more helpful, and sEEG implants into the thalamus may help determine optimal permanent stimulating electrode placement150–152. Trial stimulation through sEEG electrodes delivers current to the same locations that would be implanted with RNS or DBS, while continuing to observe the patient in the epilepsy monitoring unit and observing changes in electrographic interictal spikes, electrographic seizures, and clinical seizures153.
Brain stimulation for epilepsy with chronically implanted electronic devices
Implanted brain stimulation for epilepsy currently falls into two general paradigms. The first is to target electrode contacts to the identified SOZ specific to the patient being treated. The second paradigm is to target electrode contacts to a part of the brain that receives a confluence of inputs from distributed circuitry, typically in the thalamus. Neurostimulation strategies in both paradigms may be implemented using a “responsive” (RNS) approach with current delivery explicitly triggered by events identified from the measured voltage trace (Figure 10)151, 154, 155. Alternately, stimulation may be delivered according to a prescheduled pattern, independent of underlying brain activity. When prescheduled stimulation is delivered to the SOZ it is called chronic subthreshold cortical stimulation (CSCS), where ‘subthreshold’ refers to the calibration of parameters so that stimulation does not induce a perception by the patient (Figure 11)156, 157. Prescheduled stimulation of the thalamus is called deep brain stimulation (DBS, Figure 12).
Fig 10. Responsive neurostimulation (RNS), illustrated for bitemporal epilepsy with for the Neuropace system.
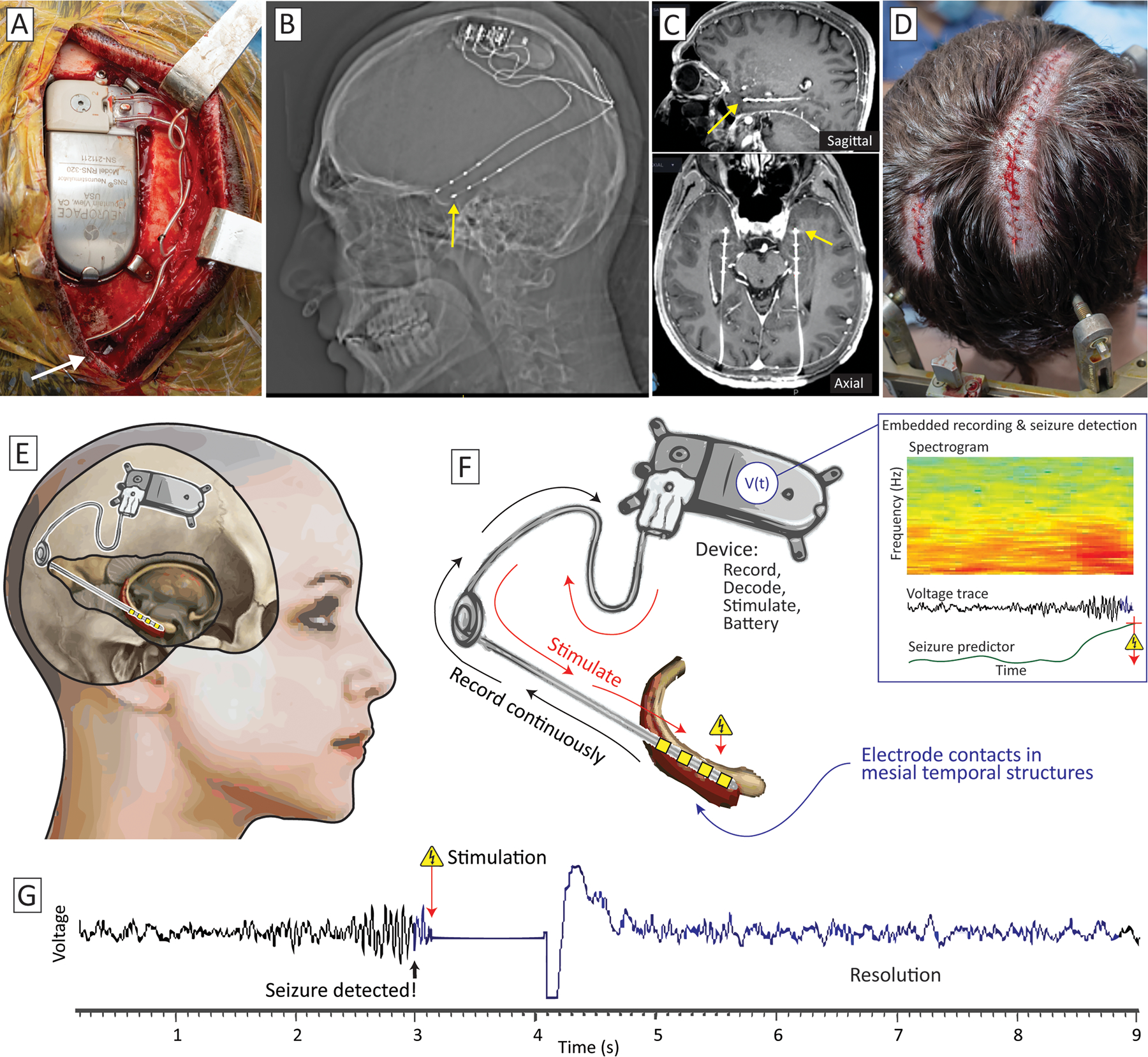
(A) The sense & stimulate device is embedded in a tray in the right parietal boss of the skull, and leads are placed stereotactically (insertion site the right lead shown with a white arrow). (B) Lateral x-ray shows leads bilaterally. (C) Fused post-implant CT to gadolinium-contrasted T1 MRI in sagittal (upper panel) and axial (lower panel) planes, showing the leads traversing the hippocampus and extending into the anterior-inferior portion of the amygdala. (D) Incisions for device implantation, with the left incision for insertion and anchoring of left lead, and the closed right incision from (A). (E) Schematic of implantation from a right-sided view showing a common trajectory (here terminating in the hippocampus). (F) Data are recorded continuously from the implanted structure and processed in the RNS device. When the custom-parameterized predictor exceeds a threshold (indicating seizure detection), electrical stimulation pulses are sent back into the lead. (G) Example voltage trace from an implanted patient, showing emergence of a seizure, stimulation, and resolution of the seizure.
Fig 11. Constant subthreshold cortical stimulation (CSCS) of two spatially-separated SOZs.
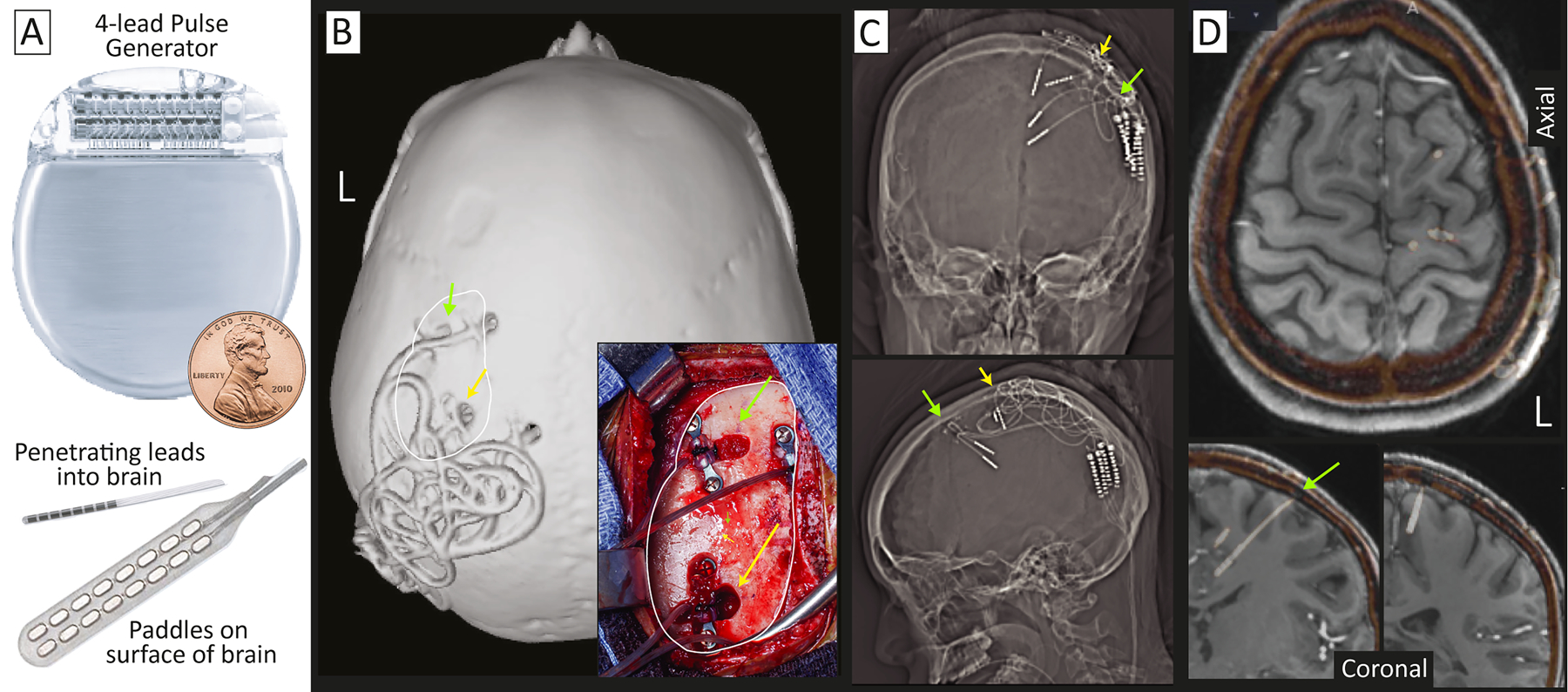
(A) Co-opted pulse generators typically used for DBS are used to deliver electrical current into the seizure network using paddle or penetrating lead electrodes. Target sites within the seizure network are initially identified with ECoG or SEEG. (B) An example implant with penetrating leads, seen on a skull surface rendering and intraoperative photograph (inset, with corresponding skull surface indicated by white trace). (C) AP and lateral x-rays showing penetrating leads. (D) Co-registered Implanted SEEG electrodes (white-orange) fused to the gadolinium-contrasted T1 MRI show implantation in the primary and pre-motor areas. Yellow and green indicate corresponding sites in (B-D).
Fig 12. Deep brain stimulation to treat epilepsy.
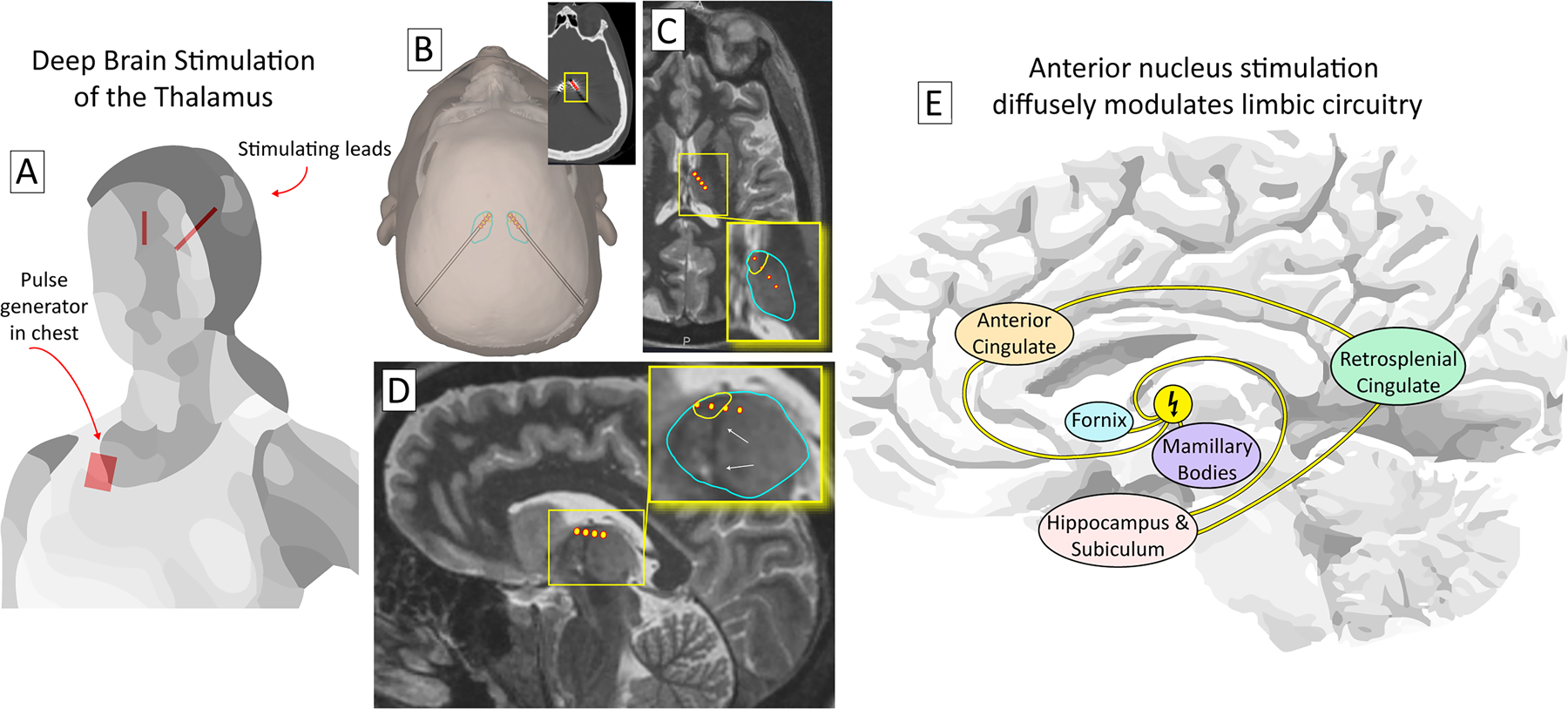
(A) A deep brain stimulation system consists of stimulating electrodes that target central brain structures connected to a pulse generator (placed in the chest). (B) The thalamus may be approached from a posterior-to-anterior trajectory173, as illustrated, or a superior-to-inferior trajectory through the ventricle. Inset shows electrode positions on post-implant CT. (C) The anterior nucleus of thalamus (ANT - yellow encircled region within blue encircled thalamus in inset) is the most common target for deep brain stimulation. A trajectory is shown with 4 contacts in the thalamus, 2 of which lie within the ANT, in axial section. (D) The same trajectory as (C), but in sagittal section. The ANT lies immediately superior to the termination of the mammillothalamic tract (white arrows). (E) Because of its diffuse projections, DBS of the ANT is thought to suppress seizures throughout the limbic network, despite not targeting the sites of seizure onset directly.
Stimulating the SOZ
Electrical stimulation at the site of seizure initiation can arrest seizure progression acutely158, 159, stop seizures from initiating to reduce their frequency over time160, and induces plasticity in the seizure circuits161. Both RNS (closed-loop sensing and stimulation) and CSCS (open loop stimulation) of the SOZ have been shown to be effective (with 75–90% reduction in seizures) and are particularly useful for eloquent cortex SOZs162–165 (Figure 11). Stimulating electrodes may be placed on the exposed brain surface with paddle-style electrodes or depth electrodes. Permanent stimulator implantation of the SOZ will follow a period of implanted monitoring, except in the case of clearly concordant lesional epilepsy.
Stimulating the seizure circuit with electrode contacts in the thalamus
In contrast with the patient-idiosyncratic targeting of the SOZ, it is also possible to target central brain nodes where propagating seizure activity converges. For epilepsy, these targets have been in the thalamus. The stimulation presumably works by arresting generalization of seizures (i.e. disrupting ictal activity spreading via the thalamus), by disentraining hyperconnectivity in seizure circuits, and by modulating SOZs interictally in such a way that they are less epileptogenic. Anterior nucleus of the thalamus (ANT) has been assessed and approved through an FDA premarket approval clinical trial, finding a 56% and 69% seizure reduction at 2 and 5 years, respectively166–168. However, the ANT is a component of limbic circuitry and is not a universal node in all seizure networks. Most epilepsies come from discrete networks, so the nucleus selected for thalamic stimulation should be determined by the putative network involved. In the emerging framework for patient-specific thalamic stimulation, the centromedian nucleus is suggested for basal-ganglial, motoric, and generalized epilepsies due to its unique widespread connectivities152, 169. The pulvinar has been suggested as a common target for occipital-onset seizures (especially those with occipital horn periventricular nodular heterotopias), and those with eye movement semiologies170. The central lateral (intralaminar) nucleus is being trialed for non-lesional, extratemporal epilepsies of impaired awareness171. Further targets will emerge based upon evolving neuroscientific understanding of how the hemispheres interact with the thalamus, and what circuit dysfunctions underly different seizure types.
We believe that stereotactic approaches are a natural extension of the personalized approach that is essential for the idiosyncratic nature of epilepsy. Moving forward, optimized therapies will move beyond a “node-based” philosophy, toward a “network-based” philosophy, where patient-specific SEEG findings will guide stimulation and/or ablation of multiple nodes within and across networks specific to that patient. Treatment of SOZs in the hemispheres with ablation or stimulation may be paired with thalamic stimulation for a more comprehensive seizure suppression strategy. For tandem SOZ+thalamic stimulation multi-lead stimulation strategy can be trialed over several days in the epilepsy monitoring unit through implanted SEEG arrays after the diagnostic portion of the SEEG monitoring period is complete.
Conclusion
Recent advancements in stereotactic neurosurgery are facilitating less-invasive, more sophisticated interventions for epilepsy. This manuscript illustrates the decision-making process that guides each patient from their initial presentation to definitive therapy, highlighting the roles of SEEG, LITT, and brain stimulation. These techniques are the core of a network-based paradigm for epilepsy therapy, which is a concept that has been talked about for a long time but is only recently being realized in clinical practice.
Article Highlights:
New advances in MR-guided laser ablation, depth electrode measurement, and brain stimulation have dramatically expanded and complicated the range of neurosurgical interventions to treat patients with epilepsy.
Decision making in neurosurgical treatment of epilepsy is highly complex and requires multiple stages of coordinated discussion between neurologists, neurosurgeons, neuroradiologists, and neuropsychologists.
Stereotactic interventions access deep areas of the brain through penetrating electrode leads and laser cannulas advanced via several square millimeter holes drilled through the skull.
Acknowledgments
We would like to thank our colleagues in Epilepsy Neurology, Neurosurgery, Neuropsychology, and Neuroradiology at Mayo Clinic for the development of the practice that we describe here. Drs. Gregory Cascino, Elaine Wirrell, Jeffrey Britton, and Gregory Worrell provided helpful reading of this manuscript with helpful suggestions to improve the text. KJM was supported by the Brain & Behavior Research Foundation with a NARSAD Young Investigator Grant, by the Brain Research Foundation with a Fay/Frank Seed Grant, and by NIH-NCATS CTSA KL2 TR002379.
Abbreviations and Acronyms:
- CSCS
chronic subthreshold cortical stimulation
- CT
computed tomography
- DBS
deep brain stimulation
- ECoG
electrocorticograph
- EEG
electroencephalography
- EMU
epilepsy monitoring unit
- LITT
laser interstitial thermal therapy
- MEG
magnetoencephalography
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- RF
radiofrequency ablation
- RNS
responsive neurostimulation
- sEEG
stereoelectroencephalography
- SOZ
seizure onset zone
- SPECT
single photon emission computed tomography
- VNS
vagal nerve stimulation
Footnotes
Disclosures
Neither of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Kwan P, Brodie MJ. Early identification of refractory epilepsy N Engl J Med 2000. Feb 3;342:314–319. [DOI] [PubMed] [Google Scholar]
- 2.Callaghan BC, Anand K, Hesdorffer D, Hauser WA, French JA. Likelihood of seizure remission in an adult population with refractory epilepsy Ann Neurol 2007. Oct;62:382–389. [DOI] [PubMed] [Google Scholar]
- 3.Luciano AL, Shorvon SD. Results of treatment changes in patients with apparently drug-resistant chronic epilepsy Ann Neurol 2007. Oct;62:375–381. [DOI] [PubMed] [Google Scholar]
- 4.Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy N Engl J Med 2011. Sep 8;365:919–926. [DOI] [PubMed] [Google Scholar]
- 5.Jobst BC, Cascino GD. Resective epilepsy surgery for drug-resistant focal epilepsy: a review JAMA 2015. Jan 20;313:285–293. [DOI] [PubMed] [Google Scholar]
- 6.Engel J Jr., Wiebe S, French J, Sperling M, Williamson P, Spencer D, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons Neurology 2003. Feb 25;60:538–547. [DOI] [PubMed] [Google Scholar]
- 7.Cascino GD. Surgical treatment for epilepsy Epilepsy Res 2004. Jul-Aug;60:179–186. [DOI] [PubMed] [Google Scholar]
- 8.Cascino GD. Functional imaging demonstrates source of epileptiform discharges in generalized epilepsy Epilepsy Curr 2004. Nov-Dec;4:223–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg AT. Understanding the delay before epilepsy surgery: who develops intractable focal epilepsy and when? CNS Spectr 2004. Feb;9:136–144. [DOI] [PubMed] [Google Scholar]
- 10.Sperling MR, O’Connor MJ, Saykin AJ, Plummer C. Temporal lobectomy for refractory epilepsy JAMA 1996. Aug 14;276:470–475. [PubMed] [Google Scholar]
- 11.Dlugos DJ. The early identification of candidates for epilepsy surgery Arch Neurol 2001. Oct;58:1543–1546. [DOI] [PubMed] [Google Scholar]
- 12.Steinbrenner M, Kowski AB, Holtkamp M. Referral to evaluation for epilepsy surgery: Reluctance by epileptologists and patients Epilepsia 2019. Feb;60:211–219. [DOI] [PubMed] [Google Scholar]
- 13.Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness, Efficiency of Surgery for Temporal Lobe Epilepsy Study G. A randomized, controlled trial of surgery for temporal-lobe epilepsy N Engl J Med 2001. Aug 2;345:311–318. [DOI] [PubMed] [Google Scholar]
- 14.Engel J Jr., McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial JAMA 2012. Mar 7;307:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsoucalas G, Spengos K, Panayiotakopoulos G, Papaioannou T, Karamanou M. Epilepsy, theories and treatment inside corpus Hippocraticum Current pharmaceutical design 2017;23:6369–6372. [DOI] [PubMed] [Google Scholar]
- 16.Meador KJ, Loring DW, Flanigin HF. History of Epilepsy Surgery J Epilepsy 1989;2:21–25. [Google Scholar]
- 17.Taylor DC. One hundred years of epilepsy surgery: Sir Victor Horsley’s contribution Journal of neurology, neurosurgery, and psychiatry 1986;49:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiegel EA, Wycis HT, Marks M, Lee AJ. Stereotaxic apparatus for operations on the human brain Science 1947;106:349–350. [DOI] [PubMed] [Google Scholar]
- 19.Blomstedt P, Olivecrona M, Sailer A, Hariz MI. Dittmarand The History Of Stereotaxy; Orrats, Rabbits, And References Neurosurgery 2007;60:198–202. [DOI] [PubMed] [Google Scholar]
- 20.Bickford RG, Petersen MC, Dodge HW Jr., Sem-Jacobsen CW. Observations on depth stimulation of the human brain through implanted electrographic leads Proc Staff Meet Mayo Clin 1953. Mar 25;28:181–187. [PubMed] [Google Scholar]
- 21.Dodge HW Jr., Bailey AA, Bickford RG, Petersen MC, Sem-Jacobsen CW, Miller RH. Neurosurgical and neurologic application of depth electrography Proc Staff Meet Mayo Clin 1953. Mar 25;28:188–191. [PubMed] [Google Scholar]
- 22.Dodge HW Jr., Holman CB, Sem-Jacobsen CW, Bickford RG, Petersen MC. Technic of depth electrography Proc Staff Meet Mayo Clin 1953. Mar 25;28:147–155. [PubMed] [Google Scholar]
- 23.Woltman HW. Symposium on intracerebral electrography; introduction Proc Staff Meet Mayo Clin 1953. Mar 25;28:145–147. [PubMed] [Google Scholar]
- 24.Reif PS, Strzelczyk A, Rosenow F. The history of invasive EEG evaluation in epilepsy patients Seizure 2016. Oct;41:191–195. [DOI] [PubMed] [Google Scholar]
- 25.Harary M, Cosgrove GR. Jean Talairach: a cerebral cartographer Neurosurg Focus 2019. Sep 1;47:E12. [DOI] [PubMed] [Google Scholar]
- 26.Kelly PJ. Stereotactic navigation, Jean Talairach, and I Neurosurgery 2004. Feb;54:454–463; discussion 463–454. [DOI] [PubMed] [Google Scholar]
- 27.Willie JT, Laxpati NG, Drane DL, Gowda A, Appin C, Hao C, et al. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy Neurosurgery 2014;74:569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer FB, Marsh WR, Laws ER Jr., Sharbrough FW. Temporal lobectomy in children with epilepsy J Neurosurg 1986. Mar;64:371–376. [DOI] [PubMed] [Google Scholar]
- 29.Cross JH, Jayakar P, Nordli D, Delalande O, Duchowny M, Wieser HG, et al. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery Epilepsia 2006. Jun;47:952–959. [DOI] [PubMed] [Google Scholar]
- 30.Wong-Kisiel LC, Britton JW, Witte RJ, Kelly-Williams KM, Kotsenas AL, Krecke KN, et al. Double Inversion Recovery Magnetic Resonance Imaging in Identifying Focal Cortical Dysplasia Pediatr Neurol 2016. Aug;61:87–93. [DOI] [PubMed] [Google Scholar]
- 31.So EL, Lee RW. Epilepsy surgery in MRI-negative epilepsies Curr Opin Neurol 2014. Apr;27:206–212. [DOI] [PubMed] [Google Scholar]
- 32.Hammers A. PET in MRI-negative refractory focal epilepsy MRI-negative epilepsy: evaluation and surgical management 2015:28. [Google Scholar]
- 33.O’Brien TJ, So EL, Cascino GD, Hauser MF, Marsh WR, Meyer FB, et al. Subtraction SPECT coregistered to MRI in focal malformations of cortical development: localization of the epileptogenic zone in epilepsy surgery candidates Epilepsia 2004. Apr;45:367–376. [DOI] [PubMed] [Google Scholar]
- 34.Sulc V, Stykel S, Hanson DP, Brinkmann BH, Jones DT, Holmes DR 3rd, et al. Statistical SPECT processing in MRI-negative epilepsy surgery Neurology 2014. Mar 18;82:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherman EM, Wiebe S, Fay‐McClymont TB, Tellez‐Zenteno J, Metcalfe A, Hernandez‐Ronquillo L, et al. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates Epilepsia 2011;52:857–869. [DOI] [PubMed] [Google Scholar]
- 36.Kenney DL, Kelly-Williams KM, Krecke KN, Witte RJ, Watson RE Jr., Kotsenas AL, et al. Usefulness of repeat review of head magnetic resonance images during presurgical epilepsy conferences Epilepsy Res 2016. Oct;126:106–108. [DOI] [PubMed] [Google Scholar]
- 37.Uijl SG, Leijten FS, Arends JB, Parra J, van Huffelen AC, Moons KG. Decision-making in temporal lobe epilepsy surgery: the contribution of basic non-invasive tests Seizure 2008;17:364–373. [DOI] [PubMed] [Google Scholar]
- 38.So EL. Value and limitations of seizure semiology in localizing seizure onset Journal of clinical neurophysiology 2006;23:353–357. [DOI] [PubMed] [Google Scholar]
- 39.Elwan S, Alexopoulos A, Silveira DC, Kotagal P. Lateralizing and localizing value of seizure semiology: Comparison with scalp EEG, MRI and PET in patients successfully treated with resective epilepsy surgery Seizure 2018;61:203–208. [DOI] [PubMed] [Google Scholar]
- 40.Tellez-Zenteno JF, Hernandez Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis Epilepsy Res 2010. May;89:310–318. [DOI] [PubMed] [Google Scholar]
- 41.Craven I, Griffiths PD, Hoggard N. Magnetic resonance imaging of epilepsy at 3 Tesla Clin Radiol 2011. Mar;66:278–286. [DOI] [PubMed] [Google Scholar]
- 42.Burkett BJ, Fagan AJ, Felmlee JP, Black DF, Lane JI, Port JD, et al. Clinical 7-T MRI for neuroradiology: strengths, weaknesses, and ongoing challenges Neuroradiology 2021. Feb;63:167–177. [DOI] [PubMed] [Google Scholar]
- 43.Guye M, Bartolomei F, Ranjeva JP. Malformations of cortical development: The role of 7-Tesla magnetic resonance imaging in diagnosis Rev Neurol (Paris) 2019. Mar;175:157–162. [DOI] [PubMed] [Google Scholar]
- 44.Von Oertzen J, Urbach H, Jungbluth S, Kurthen M, Reuber M, Fernandez G, et al. Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy J Neurol Neurosurg Psychiatry 2002. Dec;73:643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernasconi A, Bernasconi N, Bernhardt BC, Schrader D. Advances in MRI for ‘cryptogenic’ epilepsies Nat Rev Neurol 2011. Feb;7:99–108. [DOI] [PubMed] [Google Scholar]
- 46.Bronen RA, Fulbright RK, Kim JH, Spencer SS, Spencer DD. A systematic approach for interpreting MR images of the seizure patient AJR Am J Roentgenol 1997. Jul;169:241–247. [DOI] [PubMed] [Google Scholar]
- 47.Wehner T, Luders H. Role of neuroimaging in the presurgical evaluation of epilepsy J Clin Neurol 2008. Mar;4:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner J, Weber B, Urbach H, Elger CE, Huppertz HJ. Morphometric MRI analysis improves detection of focal cortical dysplasia type II Brain 2011. Oct;134:2844–2854. [DOI] [PubMed] [Google Scholar]
- 49.Wong-Kisiel LC, Tovar Quiroga DF, Kenney-Jung DL, Witte RJ, Santana-Almansa A, Worrell GA, et al. Morphometric analysis on T1-weighted MRI complements visual MRI review in focal cortical dysplasia Epilepsy Res 2018. Feb;140:184–191. [DOI] [PubMed] [Google Scholar]
- 50.House PM, Holst B, Lindenau M, Voges B, Kohl B, Martens T, et al. Morphometric MRI analysis enhances visualization of cortical tubers in tuberous sclerosis Epilepsy Res 2015. Nov;117:29–34. [DOI] [PubMed] [Google Scholar]
- 51.House PM, Lanz M, Holst B, Martens T, Stodieck S, Huppertz HJ. Comparison of morphometric analysis based on T1- and T2-weighted MRI data for visualization of focal cortical dysplasia Epilepsy Res 2013. Oct;106:403–409. [DOI] [PubMed] [Google Scholar]
- 52.O’Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Bohnen NI, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus Neurology 1998. Feb;50:445–454. [DOI] [PubMed] [Google Scholar]
- 53.O’Brien TJ, Zupanc ML, Mullan BP, O’Connor MK, Brinkmann BH, Cicora KM, et al. The practical utility of performing peri-ictal SPECT in the evaluation of children with partial epilepsy Pediatr Neurol 1998. Jul;19:15–22. [DOI] [PubMed] [Google Scholar]
- 54.So EL, O’Brien TJ, Brinkmann BH, Mullan BP. The EEG evaluation of single photon emission computed tomography abnormalities in epilepsy J Clin Neurophysiol 2000. Jan;17:10–28. [DOI] [PubMed] [Google Scholar]
- 55.von Oertzen TJ, Mormann F, Urbach H, Reichmann K, Koenig R, Clusmann H, et al. Prospective use of subtraction ictal SPECT coregistered to MRI (SISCOM) in presurgical evaluation of epilepsy Epilepsia 2011. Dec;52:2239–2248. [DOI] [PubMed] [Google Scholar]
- 56.Brinkmann BH, O’Brien TJ, Mullan BP, O’Connor MK, Robb RA, So EL. Subtraction ictal SPECT coregistered to MRI for seizure focus localization in partial epilepsy Mayo Clin Proc 2000. Jun;75:615–624. [DOI] [PubMed] [Google Scholar]
- 57.Ahnlide JA, Rosen I, Linden-Mickelsson Tech P, Kallen K. Does SISCOM contribute to favorable seizure outcome after epilepsy surgery? Epilepsia 2007. Mar;48:579–588. [DOI] [PubMed] [Google Scholar]
- 58.Chang DJ, Zubal IG, Gottschalk C, Necochea A, Stokking R, Studholme C, et al. Comparison of statistical parametric mapping and SPECT difference imaging in patients with temporal lobe epilepsy Epilepsia 2002. Jan;43:68–74. [DOI] [PubMed] [Google Scholar]
- 59.McNally KA, Paige AL, Varghese G, Zhang H, Novotny EJ Jr., Spencer SS, et al. Localizing value of ictal-interictal SPECT analyzed by SPM (ISAS) Epilepsia 2005. Sep;46:1450–1464. [DOI] [PubMed] [Google Scholar]
- 60.la Fougere C, Rominger A, Forster S, Geisler J, Bartenstein P. PET and SPECT in epilepsy: a critical review Epilepsy Behav 2009. May;15:50–55. [DOI] [PubMed] [Google Scholar]
- 61.Perissinotti A, Ninerola-Baizan A, Rubi S, Carreno M, Marti-Fuster B, Aparicio J, et al. PISCOM: a new procedure for epilepsy combining ictal SPECT and interictal PET Eur J Nucl Med Mol Imaging 2018. Dec;45:2358–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma P, Scherg M, Pinborg LH, Fabricius M, Rubboli G, Pedersen B, et al. Ictal and interictal electric source imaging in pre-surgical evaluation: a prospective study Eur J Neurol 2018. Sep;25:1154–1160. [DOI] [PubMed] [Google Scholar]
- 63.Michel CM, He B. EEG source localization Handb Clin Neurol 2019;160:85–101. [DOI] [PubMed] [Google Scholar]
- 64.Michel CM, Grave de Peralta R, Lantz G, Gonzalez Andino S, Spinelli L, Blanke O, et al. Spatiotemporal EEG analysis and distributed source estimation in presurgical epilepsy evaluation J Clin Neurophysiol 1999. May;16:239–266. [DOI] [PubMed] [Google Scholar]
- 65.Rose S, Ebersole JS. Advances in spike localization with EEG dipole modeling Clin EEG Neurosci 2009. Oct;40:281–287. [DOI] [PubMed] [Google Scholar]
- 66.Ebersole JS. Noninvasive localization of epileptogenic foci by EEG source modeling Epilepsia 2000;41 Suppl 3:S24–33. [DOI] [PubMed] [Google Scholar]
- 67.Sperli F, Spinelli L, Seeck M, Kurian M, Michel CM, Lantz G. EEG source imaging in pediatric epilepsy surgery: a new perspective in presurgical workup Epilepsia 2006. Jun;47:981–990. [DOI] [PubMed] [Google Scholar]
- 68.Bagic A, Funke ME, Ebersole J, Committee APS. American Clinical MEG Society (ACMEGS) position statement: the value of magnetoencephalography (MEG)/magnetic source imaging (MSI) in noninvasive presurgical evaluation of patients with medically intractable localization-related epilepsy J Clin Neurophysiol 2009. Aug;26:290–293. [DOI] [PubMed] [Google Scholar]
- 69.Knowlton RC, Shih J. Magnetoencephalography in epilepsy Epilepsia 2004;45 Suppl 4:61–71. [DOI] [PubMed] [Google Scholar]
- 70.Otsubo H, Ochi A, Elliott I, Chuang SH, Rutka JT, Jay V, et al. MEG predicts epileptic zone in lesional extrahippocampal epilepsy: 12 pediatric surgery cases Epilepsia 2001. Dec;42:1523–1530. [DOI] [PubMed] [Google Scholar]
- 71.Morioka T, Nishio S, Ishibashi H, Muraishi M, Hisada K, Shigeto H, et al. Intrinsic epileptogenicity of focal cortical dysplasia as revealed by magnetoencephalography and electrocorticography Epilepsy Res 1999. Feb;33:177–187. [DOI] [PubMed] [Google Scholar]
- 72.RamachandranNair R, Otsubo H, Shroff MM, Ochi A, Weiss SK, Rutka JT, et al. MEG predicts outcome following surgery for intractable epilepsy in children with normal or nonfocal MRI findings Epilepsia 2007. Jan;48:149–157. [DOI] [PubMed] [Google Scholar]
- 73.Glover GH. Overview of functional magnetic resonance imaging Neurosurg Clin N Am 2011. Apr;22:133–139, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tyndall AJ, Reinhardt J, Tronnier V, Mariani L, Stippich C. Presurgical motor, somatosensory and language fMRI: Technical feasibility and limitations in 491 patients over 13 years Eur Radiol 2017. Jan;27:267–278. [DOI] [PubMed] [Google Scholar]
- 75.Choudhri AF, Patel RM, Siddiqui A, Whitehead MT, Wheless JW. Cortical Activation Through Passive-Motion Functional MRI AJNR Am J Neuroradiol 2015. Sep;36:1675–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shurtleff H, Warner M, Poliakov A, Bournival B, Shaw DW, Ishak G, et al. Functional magnetic resonance imaging for presurgical evaluation of very young pediatric patients with epilepsy J Neurosurg Pediatr 2010. May;5:500–506. [DOI] [PubMed] [Google Scholar]
- 77.Starnes K, Miller K, Wong-Kisiel L, Lundstrom BN. A Review of Neurostimulation for Epilepsy in Pediatrics Brain Sci 2019. Oct 18;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen R, Spencer DC, Weston J, Nolan SJ. Transcranial magnetic stimulation for the treatment of epilepsy Cochrane Database Syst Rev 2016. Aug 11:CD011025. [DOI] [PubMed] [Google Scholar]
- 79.Allen CH, Kluger BM, Buard I. Safety of Transcranial Magnetic Stimulation in Children: A Systematic Review of the Literature Pediatr Neurol 2017. Mar;68:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMSCG. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research Clin Neurophysiol 2009. Dec;120:2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tarapore PE, Picht T, Bulubas L, Shin Y, Kulchytska N, Meyer B, et al. Safety and tolerability of navigated TMS for preoperative mapping in neurosurgical patients Clin Neurophysiol 2016. Mar;127:1895–1900. [DOI] [PubMed] [Google Scholar]
- 82.Pereira LS, Muller VT, da Mota Gomes M, Rotenberg A, Fregni F. Safety of repetitive transcranial magnetic stimulation in patients with epilepsy: A systematic review Epilepsy Behav 2016. Apr;57:167–176. [DOI] [PubMed] [Google Scholar]
- 83.Stultz DJ, Osburn S, Burns T, Pawlowska-Wajswol S, Walton R. Transcranial Magnetic Stimulation (TMS) Safety with Respect to Seizures: A Literature Review Neuropsychiatr Dis Treat 2020;16:2989–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rotenberg A. Prospects for clinical applications of transcranial magnetic stimulation and real-time EEG in epilepsy Brain Topogr 2010. Jan;22:257–266. [DOI] [PubMed] [Google Scholar]
- 85.Theodore WH. Transcranial Magnetic Stimulation in Epilepsy Epilepsy Curr 2003. Nov;3:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cantello R, Rossi S, Varrasi C, Ulivelli M, Civardi C, Bartalini S, et al. Slow repetitive TMS for drug-resistant epilepsy: clinical and EEG findings of a placebo-controlled trial Epilepsia 2007. Feb;48:366–374. [DOI] [PubMed] [Google Scholar]
- 87.Sun W, Mao W, Meng X, Wang D, Qiao L, Tao W, et al. Low-frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy: a controlled clinical study Epilepsia 2012. Oct;53:1782–1789. [DOI] [PubMed] [Google Scholar]
- 88.Wicks RT, Jermakowicz WJ, Jagid JR, Couture DE, Willie JT, Laxton AW, et al. Laser Interstitial Thermal Therapy for Mesial Temporal Lobe Epilepsy Neurosurgery 2016. Dec;79 Suppl 1:S83–S91. [DOI] [PubMed] [Google Scholar]
- 89.Fayed I, Sacino MF, Gaillard WD, Keating RF, Oluigbo CO. MR-Guided Laser Interstitial Thermal Therapy for Medically Refractory Lesional Epilepsy in Pediatric Patients: Experience and Outcomes Pediatr Neurosurg 2018;53:322–329. [DOI] [PubMed] [Google Scholar]
- 90.Curry DJ, Gowda A, McNichols RJ, Wilfong AA. MR-guided stereotactic laser ablation of epileptogenic foci in children Epilepsy Behav 2012. Aug;24:408–414. [DOI] [PubMed] [Google Scholar]
- 91.Kang JY, Wu C, Tracy J, Lorenzo M, Evans J, Nei M, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy Epilepsia 2016. Feb;57:325–334. [DOI] [PubMed] [Google Scholar]
- 92.Cobourn K, Fayed I, Keating RF, Oluigbo CO. Early outcomes of stereoelectroencephalography followed by MR-guided laser interstitial thermal therapy: a paradigm for minimally invasive epilepsy surgery Neurosurg Focus 2018. Sep;45:E8. [DOI] [PubMed] [Google Scholar]
- 93.Le S, Ho AL, Fisher RS, Miller KJ, Henderson JM, Grant GA, et al. Laser interstitial thermal therapy (LITT): Seizure outcomes for refractory mesial temporal lobe epilepsy Epilepsy Behav 2018. Dec;89:37–41. [DOI] [PubMed] [Google Scholar]
- 94.Gross RE, Willie JT, Drane DL. The Role of Stereotactic Laser Amygdalohippocampotomy in Mesial Temporal Lobe Epilepsy Neurosurg Clin N Am 2016. Jan;27:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kerezoudis P, Parisi V, Marsh WR, Kaufman TJ, Lehman VT, Worrell GA, et al. Surgical Outcomes of Laser Interstitial Thermal Therapy for Temporal Lobe Epilepsy: Systematic Review and Meta-analysis World Neurosurg 2020. Nov;143:527–536 e523. [DOI] [PubMed] [Google Scholar]
- 96.Donos C, Breier J, Friedman E, Rollo P, Johnson J, Moss L, et al. Laser ablation for mesial temporal lobe epilepsy: surgical and cognitive outcomes with and without mesial temporal sclerosis Epilepsia 2018;59:1421–1432. [DOI] [PubMed] [Google Scholar]
- 97.Drane DL, Loring DW, Voets NL, Price M, Ojemann JG, Willie JT, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy Epilepsia 2015. Jan;56:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bermudez CI, Jermakowicz WJ, Kolcun JPG, Sur S, Cajigas I, Millan C, et al. Cognitive outcomes following laser interstitial therapy for mesiotemporal epilepsies Neurology: Clinical Practice 2020;10:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Willie JT, Laxpati NG, Drane DL, Gowda A, Appin C, Hao C, et al. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy Neurosurgery 2014. Jun;74:569–584; discussion 584–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Youngerman BE, Oh JY, Anbarasan D, Billakota S, Casadei CH, Corrigan EK, et al. Laser ablation is effective for temporal lobe epilepsy with and without mesial temporal sclerosis if hippocampal seizure onsets are localized by stereoelectroencephalography Epilepsia 2018. Mar;59:595–606. [DOI] [PubMed] [Google Scholar]
- 101.Waseem H, Vivas AC, Vale FL. MRI-guided laser interstitial thermal therapy for treatment of medically refractory non-lesional mesial temporal lobe epilepsy: Outcomes, complications, and current limitations: A review J Clin Neurosci 2017. Apr;38:1–7. [DOI] [PubMed] [Google Scholar]
- 102.Waseem H, Osborn KE, Schoenberg MR, Kelley V, Bozorg A, Cabello D, et al. Laser ablation therapy: An alternative treatment for medically resistant mesial temporal lobe epilepsy after age 50 Epilepsy Behav 2015. Oct;51:152–157. [DOI] [PubMed] [Google Scholar]
- 103.Wind JJ, Caputy AJ, Roberti F. Spontaneous encephaloceles of the temporal lobe Neurosurg Focus 2008;25:E11. [DOI] [PubMed] [Google Scholar]
- 104.Faulkner HJ, Sandeman DR, Love S, Likeman MJ, Nunez DA, Lhatoo SD. Epilepsy surgery for refractory epilepsy due to encephalocele: a case report and review of the literature Epileptic Disord 2010. Jun;12:160–166. [DOI] [PubMed] [Google Scholar]
- 105.Saavalainen T, Jutila L, Mervaala E, Kalviainen R, Vanninen R, Immonen A. Temporal anteroinferior encephalocele: An underrecognized etiology of temporal lobe epilepsy? Neurology 2015. Oct 27;85:1467–1474. [DOI] [PubMed] [Google Scholar]
- 106.Ranjan M, Wilfong AA, Boerwinkle V, Jarrar R, David Adelson P. Temporal encephalocele: a novel indication for magnetic resonance-guided laser interstitial thermal therapy for medically intractable epilepsy Epileptic Disord 2019. Jun 1;21:265–270. [DOI] [PubMed] [Google Scholar]
- 107.Youngerman BE, Save AV, McKhann GM. Magnetic Resonance Imaging-Guided Laser Interstitial Thermal Therapy for Epilepsy: Systematic Review of Technique, Indications, and Outcomes Neurosurgery 2020. Apr 1;86:E366–E382. [DOI] [PubMed] [Google Scholar]
- 108.Ellis JA, Mejia Munne JC, Wang SH, McBrian DK, Akman CI, Feldstein NA, et al. Staged laser interstitial thermal therapy and topectomy for complete obliteration of complex focal cortical dysplasias J Clin Neurosci 2016. Sep;31:224–228. [DOI] [PubMed] [Google Scholar]
- 109.Ng YT, Rekate HL, Prenger EC, Wang NC, Chung SS, Feiz-Erfan I, et al. Endoscopic resection of hypothalamic hamartomas for refractory symptomatic epilepsy Neurology 2008. Apr 22;70:1543–1548. [DOI] [PubMed] [Google Scholar]
- 110.Ng YT, Rekate HL, Prenger EC, Chung SS, Feiz-Erfan I, Wang NC, et al. Transcallosal resection of hypothalamic hamartoma for intractable epilepsy Epilepsia 2006. Jul;47:1192–1202. [DOI] [PubMed] [Google Scholar]
- 111.Du VX, Gandhi SV, Rekate HL, Mehta AD. Laser interstitial thermal therapy: A first line treatment for seizures due to hypothalamic hamartoma? Epilepsia 2017. Jun;58 Suppl 2:77–84. [DOI] [PubMed] [Google Scholar]
- 112.Curry DJ, Raskin J, Ali I, Wilfong AA. MR-guided laser ablation for the treatment of hypothalamic hamartomas Epilepsy Res 2018. May;142:131–134. [DOI] [PubMed] [Google Scholar]
- 113.Smith JS, Quinones-Hinojosa A, Barbaro NM, McDermott MW. Frame-based stereotactic biopsy remains an important diagnostic tool with distinct advantages over frameless stereotactic biopsy J Neurooncol 2005. Jun;73:173–179. [DOI] [PubMed] [Google Scholar]
- 114.Tilgner J, Herr M, Ostertag C, Volk B. Validation of intraoperative diagnoses using smear preparations from stereotactic brain biopsies: intraoperative versus final diagnosis--influence of clinical factors Neurosurgery 2005. Feb;56:257–265; discussion 257–265. [DOI] [PubMed] [Google Scholar]
- 115.Woodworth G, McGirt MJ, Samdani A, Garonzik I, Olivi A, Weingart JD. Accuracy of frameless and frame-based image-guided stereotactic brain biopsy in the diagnosis of glioma: comparison of biopsy and open resection specimen Neurol Res 2005. Jun;27:358–362. [DOI] [PubMed] [Google Scholar]
- 116.van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management Lancet Neurol 2007. May;6:421–430. [DOI] [PubMed] [Google Scholar]
- 117.Englot DJ, Chang EF. Rates and predictors of seizure freedom in resective epilepsy surgery: an update Neurosurg Rev 2014. Jul;37:389–404; discussion 404–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harvey AS, Freeman JL. Epilepsy in hypothalamic hamartoma: clinical and EEG features Semin Pediatr Neurol 2007. Jun;14:60–64. [DOI] [PubMed] [Google Scholar]
- 119.Buckley R, Estronza-Ojeda S, Ojemann JG. Laser Ablation in Pediatric Epilepsy Neurosurg Clin N Am 2016. Jan;27:69–78. [DOI] [PubMed] [Google Scholar]
- 120.Buckley RT, Wang AC, Miller JW, Novotny EJ, Ojemann JG. Stereotactic laser ablation for hypothalamic and deep intraventricular lesions Neurosurg Focus 2016. Oct;41:E10. [DOI] [PubMed] [Google Scholar]
- 121.Graham D, Tisdall MM, Gill D. Corpus callosotomy outcomes in pediatric patients: A systematic review Epilepsia 2016. Jul;57:1053–1068. [DOI] [PubMed] [Google Scholar]
- 122.Bower RS, Wirrell E, Nwojo M, Wetjen NM, Marsh WR, Meyer FB. Seizure outcomes after corpus callosotomy for drop attacks Neurosurgery 2013. Dec;73:993–1000. [DOI] [PubMed] [Google Scholar]
- 123.Moseley BD, Nickels K, Wirrell EC. Surgical outcomes for intractable epilepsy in children with epileptic spasms J Child Neurol 2012. Jun;27:713–720. [DOI] [PubMed] [Google Scholar]
- 124.Caruso JP, Janjua MB, Dolce A, Price AV. Retrospective analysis of open surgical versus laser interstitial thermal therapy callosotomy in pediatric patients with refractory epilepsy J Neurosurg Pediatr 2021. Jan 22:1–9. [DOI] [PubMed] [Google Scholar]
- 125.Roland JL, Akbari SHA, Salehi A, Smyth MD. Corpus callosotomy performed with laser interstitial thermal therapy J Neurosurg 2019. Dec 13:1–9. [DOI] [PubMed] [Google Scholar]
- 126.Badger CA, Lopez AJ, Heuer G, Kennedy BC. Systematic review of corpus callosotomy utilizing MRI guided laser interstitial thermal therapy J Clin Neurosci 2020. Jun;76:67–73. [DOI] [PubMed] [Google Scholar]
- 127.Ho AL, Miller KJ, Cartmell S, Inoyama K, Fisher RS, Halpern CH. Stereotactic laser ablation of the splenium for intractable epilepsy Epilepsy Behav Case Rep 2016;5:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vakharia VN, Sparks R, O’Keeffe AG, Rodionov R, Miserocchi A, McEvoy A, et al. Accuracy of intracranial electrode placement for stereoencephalography: A systematic review and meta-analysis Epilepsia 2017. Jun;58:921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cardinale F, Rizzi M, Vignati E, Cossu M, Castana L, d’Orio P, et al. Stereoelectroencephalography: retrospective analysis of 742 procedures in a single centre Brain 2019. Sep 1;142:2688–2704. [DOI] [PubMed] [Google Scholar]
- 130.Balanescu B, Franklin R, Ciurea J, Mindruta I, Rasina A, Bobulescu RC, et al. A personalized stereotactic fixture for implantation of depth electrodes in stereoelectroencephalography Stereotactic and functional neurosurgery 2014;92:117–125. [DOI] [PubMed] [Google Scholar]
- 131.Pistol C, Daneasa A, Ciurea J, Rasina A, Barborica A, Oane I, et al. Accuracy and Safety of Customized Stereotactic Fixtures for Stereoelectroencephalography in Pediatric Patients Stereotactic and Functional Neurosurgery 2021;99:17–24. [DOI] [PubMed] [Google Scholar]
- 132.Yu H, Pistol C, Franklin R, Barborica A. Clinical accuracy of customized stereotactic fixtures for stereoelectroencephalography World neurosurgery 2018;109:82–88. [DOI] [PubMed] [Google Scholar]
- 133.Marusic P, Najm IM, Ying Z, Prayson R, Rona S, Nair D, et al. Focal cortical dysplasias in eloquent cortex: functional characteristics and correlation with MRI and histopathologic changes Epilepsia 2002. Jan;43:27–32. [DOI] [PubMed] [Google Scholar]
- 134.Gelinas JN, Battison AW, Smith S, Connolly MB, Steinbok P. Electrocorticography and seizure outcomes in children with lesional epilepsy Childs Nerv Syst 2011. Mar;27:381–390. [DOI] [PubMed] [Google Scholar]
- 135.Asano E, Juhasz C, Shah A, Sood S, Chugani HT. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery Brain 2009. Apr;132:1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Britton JW. Electrical Stimulation Mapping With Stereo-EEG Electrodes J Clin Neurophysiol 2018. Mar;35:110–114. [DOI] [PubMed] [Google Scholar]
- 137.Jayakar P, Gotman J, Harvey AS, Palmini A, Tassi L, Schomer D, et al. Diagnostic utility of invasive EEG for epilepsy surgery: Indications, modalities, and techniques Epilepsia 2016. Nov;57:1735–1747. [DOI] [PubMed] [Google Scholar]
- 138.Arya R, Ervin B, Dudley J, Buroker J, Rozhkov L, Scholle C, et al. Electrical stimulation mapping of language with stereo-EEG Epilepsy Behav 2019. Oct;99:106395. [DOI] [PubMed] [Google Scholar]
- 139.Arya R, Ervin B, Holloway T, Dudley J, Horn PS, Buroker J, et al. Electrical stimulation sensorimotor mapping with stereo-EEG Clin Neurophysiol 2020. Aug;131:1691–1701. [DOI] [PubMed] [Google Scholar]
- 140.Grande KM, Ihnen SKZ, Arya R. Electrical Stimulation Mapping of Brain Function: A Comparison of Subdural Electrodes and Stereo-EEG Front Hum Neurosci 2020;14:611291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maesawa S, Nakatsubo D, Fujii M, Iijima K, Kato S, Ishizaki T, et al. Application of Awake Surgery for Epilepsy in Clinical Practice Neurol Med Chir (Tokyo) 2018. Oct 15;58:442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mariani V, Revay M, D’Orio P, Rizzi M, Pelliccia V, Nichelatti M, et al. Prognostic factors of postoperative seizure outcome in patients with temporal lobe epilepsy and normal magnetic resonance imaging J Neurol 2019. Sep;266:2144–2156. [DOI] [PubMed] [Google Scholar]
- 143.McGonigal A, Bartolomei F, Regis J, Guye M, Gavaret M, Trebuchon-Da Fonseca A, et al. Stereoelectroencephalography in presurgical assessment of MRI-negative epilepsy Brain 2007. Dec;130:3169–3183. [DOI] [PubMed] [Google Scholar]
- 144.Blair RD. Temporal lobe epilepsy semiology Epilepsy research and treatment 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stefan H, Trinka E. Magnetoencephalography (MEG): past, current and future perspectives for improved differentiation and treatment of epilepsies Seizure 2017;44:121–124. [DOI] [PubMed] [Google Scholar]
- 146.Cossu M, Fuschillo D, Casaceli G, Pelliccia V, Castana L, Mai R, et al. Stereoelectroencephalography-guided radiofrequency thermocoagulation in the epileptogenic zone: a retrospective study on 89 cases J Neurosurg 2015. Dec;123:1358–1367. [DOI] [PubMed] [Google Scholar]
- 147.Bourdillon P, Devaux B, Job-Chapron AS, Isnard J. SEEG-guided radiofrequency thermocoagulation Neurophysiol Clin 2018. Feb;48:59–64. [DOI] [PubMed] [Google Scholar]
- 148.Bourdillon P, Isnard J, Catenoix H, Montavont A, Rheims S, Ryvlin P, et al. Stereo electroencephalography-guided radiofrequency thermocoagulation (SEEG-guided RF-TC) in drug-resistant focal epilepsy: Results from a 10-year experience Epilepsia 2017. Jan;58:85–93. [DOI] [PubMed] [Google Scholar]
- 149.Cossu M, Cardinale F, Casaceli G, Castana L, Consales A, D’Orio P, et al. Stereo-EEG-guided radiofrequency thermocoagulations Epilepsia 2017. Apr;58 Suppl 1:66–72. [DOI] [PubMed] [Google Scholar]
- 150.Wong JK, Bergman H, Muthuraman M, Groppa S, Sheth SA, Bronte-Stewart HM, et al. Proceedings of the Ninth Annual Deep Brain Stimulation Think Tank: Advances in Cutting Edge Technologies, Artificial Intelligence, Neuromodulation, Neuroethics, Pain, Interventional Psychiatry, Epilepsy and Traumatic Brain Injury Frontiers in Human Neuroscience 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Elder C, Friedman D, Devinsky O, Doyle W, Dugan P. Responsive neurostimulation targeting the anterior nucleus of the thalamus in 3 patients with treatment-resistant multifocal epilepsy Epilepsia Open 2019. Mar;4:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kwon CS, Schupper AJ, Fields MC, Marcuse LV, La Vega‐Talbott M, Panov F, et al. Centromedian thalamic responsive neurostimulation for Lennox‐Gastaut epilepsy and autism Annals of Clinical and Translational Neurology 2020;7:2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lundstrom BN, Meisel C, Van Gompel J, Stead M, Worrell G. Comparing spiking and slow wave activity from invasive electroencephalography in patients with and without seizures Clinical Neurophysiology 2018;129:909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures Neurology 2015. Feb 24;84:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Geller EB. Responsive neurostimulation: Review of clinical trials and insights into focal epilepsy Epilepsy Behav 2018. Nov;88S:11–20. [DOI] [PubMed] [Google Scholar]
- 156.Child ND, Stead M, Wirrell EC, Nickels KC, Wetjen NM, Lee KH, et al. Chronic subthreshold subdural cortical stimulation for the treatment of focal epilepsy originating from eloquent cortex Epilepsia 2014;55:e18–e21. [DOI] [PubMed] [Google Scholar]
- 157.Lundstrom BN, Van Gompel J, Britton J, Nickels K, Wetjen N, Worrell G, et al. Chronic Subthreshold Cortical Stimulation to Treat Focal Epilepsy JAMA Neurol 2016. Nov 1;73:1370–1372. [DOI] [PubMed] [Google Scholar]
- 158.Lesser R, Kim S, Beyderman L, Miglioretti DL, Webber W, Bare M, et al. Brief bursts of pulse stimulation terminate afterdischarges caused by cortical stimulation Neurology 1999;53:2073–2073. [DOI] [PubMed] [Google Scholar]
- 159.Osorio I, Frei MG, Manly BF, Sunderam S, Bhavaraju NC, Wilkinson SB. An introduction to contingent (closed-loop) brain electrical stimulation for seizure blockage, to ultra-short-term clinical trials, and to multidimensional statistical analysis of therapeutic efficacy Journal of Clinical Neurophysiology 2001;18:533–544. [DOI] [PubMed] [Google Scholar]
- 160.Heck CN, King‐Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, et al. Two‐year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial Epilepsia 2014;55:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Khambhati AN, Shafi A, Rao VR, Chang EF. Long-term brain network reorganization predicts responsive neurostimulation outcomes for focal epilepsy Science Translational Medicine 2021;13:eabf6588. [DOI] [PubMed] [Google Scholar]
- 162.Skarpaas TL, Jarosiewicz B, Morrell MJ. Brain-responsive neurostimulation for epilepsy (RNS® System) Epilepsy research 2019;153:68–70. [DOI] [PubMed] [Google Scholar]
- 163.Child ND, Stead M, Wirrell EC, Nickels KC, Wetjen NM, Lee KH, et al. Chronic subthreshold subdural cortical stimulation for the treatment of focal epilepsy originating from eloquent cortex Epilepsia 2014. Mar;55:e18–21. [DOI] [PubMed] [Google Scholar]
- 164.Jobst BC, Kapur R, Barkley GL, Bazil CW, Berg MJ, Bergey GK, et al. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas Epilepsia 2017. Jun;58:1005–1014. [DOI] [PubMed] [Google Scholar]
- 165.Lundstrom BN, Gompel JV, Khadjevand F, Worrell G, Stead M. Chronic subthreshold cortical stimulation and stimulation-related EEG biomarkers for focal epilepsy Brain Communications 2019;1:fcz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy Neurology 2015. Mar 10;84:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy Epilepsia 2010;51:899–908. [DOI] [PubMed] [Google Scholar]
- 168.Salanova V, Sperling MR, Gross RE, Irwin CP, Vollhaber JA, Giftakis JE, et al. The SANTÉ study at 10 years of follow‐up: Effectiveness, safety, and sudden unexpected death in epilepsy Epilepsia 2021. [DOI] [PubMed] [Google Scholar]
- 169.Warren AE, Dalic LJ, Thevathasan W, Roten A, Bulluss KJ, Archer J. Targeting the centromedian thalamic nucleus for deep brain stimulation Journal of Neurology, Neurosurgery & Psychiatry 2020;91:339–349. [DOI] [PubMed] [Google Scholar]
- 170.Burdette D, Mirro EA, Lawrence M, Patra SE. Brain‐responsive Corticothalamic Stimulation in the Pulvinar Nucleus for the Treatment of Regional Neocortical Epilepsy: A case series Epilepsia Open 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gummadavelli A, Motelow JE, Smith N, Zhan Q, Schiff ND, Blumenfeld H. Thalamic stimulation to improve level of consciousness after seizures: evaluation of electrophysiology and behavior Epilepsia 2015;56:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McNichols RJ, Gowda A, Kangasniemi M, Bankson JA, Price RE, Hazle JD. MR thermometry-based feedback control of laser interstitial thermal therapy at 980 nm Lasers Surg Med 2004;34:48–55. [DOI] [PubMed] [Google Scholar]
- 173.Wang Y-C, Grewal SS, Middlebrooks EH, Worrell GA, Stead M, Lundstrom BN, et al. Targeting analysis of a novel parietal approach for deep brain stimulation of the anterior nucleus of the thalamus for epilepsy Epilepsy research 2019;153:1–6. [DOI] [PubMed] [Google Scholar]


