SUMMARY
Gut-microbiota membership is associated with diverse neuropsychological outcomes, including substance use disorders (SUDs). Here, we use mice colonized with Citrobacter rodentium or the human γ-Proteobacteria commensal Escherichia coli HS as a model to examine the mechanistic interactions between gut microbes and host responses to cocaine. We find that cocaine exposure increases intestinal norepinephrine levels that is sensed through the bacterial adrenergic receptor QseC to promote intestinal colonization of γ-Proteobacteria. Colonized mice showed enhanced host cocaine-induced behaviors. The neuroactive metabolite glycine, a bacterial nitrogen source is depleted in the gut and cerebrospinal fluid of colonized mice. Systemic glycine repletion reversed, and γ-Proteobacteria mutated for glycine uptake did not alter the host response to cocaine. γ-Proteobacteria modulated glycine levels are linked to cocaine-induced transcriptional plasticity in the nucleus accumbens through glutamatergic transmission. The mechanism outline here could potentially be exploited to modulate reward-related brain circuits that contribute to SUDs.
Keywords: Cocaine, Norepinephrine, QseC, Proteobacteria, Microbiota, host-microbe interactions, substance abuse disorders (SUDs), Citrobacter rodentium, Glycine
Graphical Abstract

eTOC blurb
Cuesta et al. demonstrate in mice that cocaine exposure increases norepinephrine in the gut facilitating Proteobacteria colonization. This gut microbiota shift toward proteobacteria leads to a depletion of glycine from the host, which, in turn, facilitates cocaine-induced addiction-like behaviors in mice.
INTRODUCTION
The human gut microbiota contains different bacterial phyla including Bacteroidetes, Firmicutes and Proteobacteria (Eckburg et al., 2005; Frank et al., 2007; Human Microbiome Project, 2012; Ley et al., 2006). Distinct microbiota compositions have been shown to profoundly impact host homeostasis, physiology, metabolic profile, and vulnerability to disease (Holmes et al., 2012; Li et al., 2008; Nicholson et al., 2012). Reciprocally, host genetics and environmental factors, such as diet, lifestyle, pathogenic infections, and antibiotics usage shape the establishment of unique microbiota enterotypes (Arumugam et al., 2011; Human Microbiome Project, 2012; Wu et al., 2011), revealing a strong bidirectional crosstalk between the host and its microbiome.
Interkingdom communication has been exploited by different bacteria, such as invading enteric pathogens, which have evolved sensing mechanisms to identify host signals to maximize their colonization and establishment in the gut (Baumler and Sperandio, 2016). γ-Proteobacteria, including the human enteric pathogen enterohemorrhagic Escherichia coli (EHEC) and its murine counterpart Citrobacter rodentium, sense the host neurotransmitter norepinephrine (NE) through the QseC bacterial adrenergic receptor to regulate their virulence repertoire and colonize the gut (Clarke et al., 2006; Moreira et al., 2016), leading to a γ-Proteobacteria bloom within the intestine (Lopez et al., 2016; Lupp et al., 2007). Unlike mammalian adrenergic receptors that belong to the G-coupled protein receptor (GPCR) family, QseC is a membrane bound histidine sensor kinase (HK). This receptor directly binds and responds to NE to initiate a complex regulatory cascade that activates the transcription of key virulence and gut colonization genes in γ-Proteobacteria (Clarke et al., 2006; Moreira et al., 2016). C. rodentium murine colonization is promoted by host NE within the intestine in a QseC-dependent manner (Moreira et al., 2016) and, consistently, dopamine-β-hydroxylase knockout mice (DBH−/−), which do not produce NE, depict less colonization and virulence expression by C. rodentium (Moreira et al., 2016).
Recently, many studies have shown that gut microbiota composition, as well as certain gut microbes, can strongly modulate different host social, communicative, stress-related, and cognitive behaviors (Vuong et al., 2017). Furthermore, an altered gut microbiota has been associated with diverse neurological disorders in humans and animal models (Cenit et al., 2017; Lucerne and Kiraly, 2021; Meckel and Kiraly, 2019; Needham et al., 2020; Vuong et al., 2017). However, the studies reporting these findings to date present challenges in determining whether the changes observed in the microbiota are causative, promote and/or enhance disease, or instead are a consequence of an otherwise unrelated pathology. A similar scenario is observed regarding the role of the gut microbiota on addiction and substance use disorders (SUDs). While it is known that psychostimulants such as cocaine can lead to changes in gut microbiota composition (Lee et al., 2018; Scorza et al., 2019; Volpe et al., 2014; Xu et al., 2017), and that microbiota depletion affects cocaine responses (Kiraly et al., 2016; Lee et al., 2018), the molecular mechanisms behind these interactions are poorly understood.
Psychostimulants such as cocaine, act systemically blocking the catecholamines transporters, augmenting their signaling (Zimmerman, 2012). This suggests that these recreational drugs could modulate the function of QseC-expressing bacteria in the intestine, potentially impacting host homeostasis and their vulnerability to develop SUDs. Hence, here, we used C. rodentium colonization of mice to explore whether and how cocaine-induced increase in catecholamine levels affect intestinal colonization. Then, we evaluated the consequences of γ-Proteobacteria intestinal colonization in cocaine-induced behavioral and neuronal plasticity. We show that cocaine exposure increases intestinal levels of NE, which is sensed through the bacterial-adrenergic receptor QseC to increase intestinal colonization of γ-Proteobacteria. Metabolomics and genetic experiments revealed that because γ-Proteobacteria in the gut use glycine as a nitrogen source, this neuroactive metabolite is depleted in in the intestine and also the cerebrospinal fluid (CSF), enhancing host cocaine-induced behaviors. Both systemic repletion of glycine and intestinal colonization with γ-Proteobacteria unable to uptake glycine reversed this response. Transcriptomic studies suggest a role of microbiota-modulated glycine levels in cocaine-induced changes in the nucleus accumbens transcription profile through the glutamatergic transmission, which has been linked to addiction behaviors.
RESULTS
Cocaine Exposure Enhances Murine Susceptibility to Enteric Pathogens
To establish whether cocaine exposure can modulate gut bacterial function, mice were infected with the murine pathogen C. rodentium (used extensively as a surrogate model for the human pathogen EHEC (Borenshtein et al., 2008)) and treated with cocaine (15mg/kg, i.p.) or saline (vehicle, 1ml/kg, i.p.) (Figure 1A). Cocaine exposure increased pathogen burden in cecum tissues compared to saline-treated infected animals (Figure 1C). Moreover, cocaine-treated mice showed increased markers of intestinal disease in response to C. rodentium infection as revealed by increased cecal expression of the proinflammatory cytokines Nos2, Mip and Cxcl1b (Figure 1F - H) and by gross pathology in the cecal tissues (Figure S2A). Of note, under these conditions, cocaine treatment in non-infected animals (PBS) did not alter proinflammatory cytokine levels or cecal pathology (Figures 1F – H and S2A). Tissue colonization of C. rodentium is strictly dependent on the locus of enterocyte effacement (LEE)-encoded type three secretion system (T3SS) (McDaniel et al., 1995). T3SSs are molecular syringes employed by pathogens to translocate effector proteins into host-cells that highjack their function, promoting pathogen colonization (Galan and Wolf-Watz, 2006). Transcript levels of the LEE-encoded genes espA and tir in attached bacteria to the cecum from cocaine-treated infected mice were significantly higher compared with infected animals treated with saline (Figure 1D and E). These data demonstrate that cocaine exposure by increasing C. rodentium virulence expression leads to more severe intestinal disease.
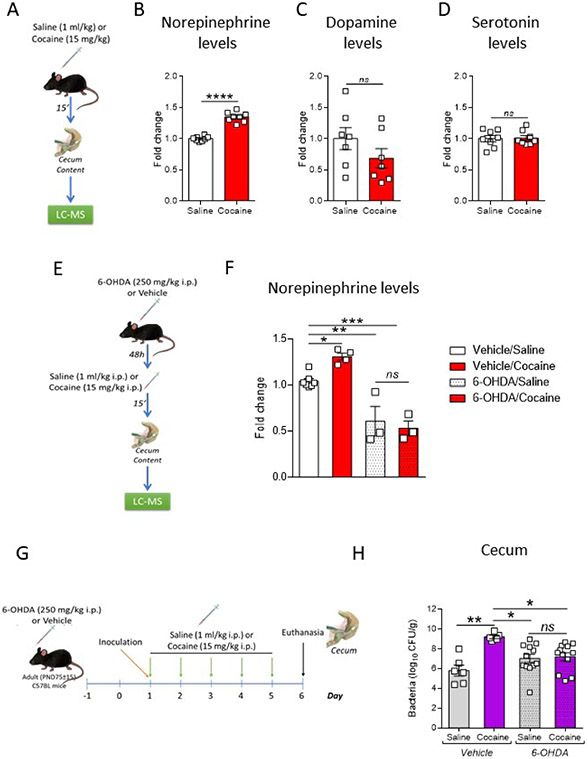
Figure 1. Repeated cocaine exposure increases C. rodentium colonization and infectious disease by means of the bacterial norepinephrine sensing QseC receptor.
(A) Timeline of treatment and experimental procedures.
(B) QseC signaling receptor senses norepinephrine (NE) to regulate virulence.
(C) C. rodentium WT and ΔqseC burdens in cecal tissues harvested from saline- or cocaine-treated mice. (n = 6 – 9/2 cohorts per group; two-way ANOVA, with Bonferroni post hoc analysis, ns: non-significant; *p<0.05)
(D - E) Top: Relative expression of C. rodentium WT and ΔqseC LEE-encoded espA (D) and tir (E) in cecal tissue-attached bacteria recovered from saline- or cocaine-treated mice. (n = 3 – 9/2 cohorts per group; two-way ANOVA, with Bonferroni post hoc analysis, *p<0.05; **p<0.01). Bottom: Schematic depicting the genetic organization of the locus of enterocyte effacement (LEE) pathogenicity island.
(F – H) Relative cecal transcript levels of Nos2 (F), of Mip (G), and Cxcl1b (H) in PBS-treated or C. rodentium WT or ΔqseC -infected saline- or cocaine-exposed mice. (n = 6 – 9/2 cohorts per group; two-way ANOVA, with Bonferroni post hoc analysis, ns: non-significant; **p<0.01; ***p<0.001; ****p<0.0001).
Data presented as average ± SEM.
Cocaine acts by blocking the catecholamines transporter, increasing the levels of these neurotransmitters, and consequently their signaling (Zimmerman, 2012). After administration, cocaine is widely distributed in the body (Giroud et al., 2004; Organization, 2020; Som et al., 1994), and both the NE transporter (NET) and the dopamine (DA) transporter (DAT) are expressed in cecum tissue (Li et al., 2010; Li et al., 2006) (Figure S1A). Therefore, to dissect the mechanisms underlying the cocaine-induced increase in virulence, we first assessed whether cocaine itself or DA could directly affect C. rodentium growth or virulence. No changes were found in growth or virulence expression (expression of LEE- ler, tir, espA and espB genes or proteins) after in vitro exposure to cocaine (Figure S1C - E) or to DA (Figure S1F - H). C. rodentium senses NE through the bacterial adrenergic receptor QseC, which leads to activation of LEE-encoded genes promoting pathogen colonization of the gut (Clarke et al., 2006; Moreira et al., 2016) (Figure 1B). Our data suggest a NE-mediated effect of cocaine on C. rodentium increased virulence in mice. Congruently, metabolomic determinations revealed that cocaine increased NE levels in the cecum (Figure 2A - B) without altering DA (Figure 2C). Furthermore, no changes were observed in the levels of serotonin (Figure 2D), another neurotransmitter whose uptake can be affected by cocaine and that modulates C. rodentium infection (Kumar et al., 2020).
Figure 2. Repeated cocaine exposure increases the levels of norepinephrine in the intestine modulating C. rodentium colonization.
(A) Timeline of treatment and experimental procedures.
(B – D) Quantification of norepinephrine (B), dopamine (C) and serotonin (D) levels in cecal contents harvested from mice 15 minutes after an acute saline (1ml/kg, i.p.) or cocaine (15mg/kg, i.p.) injection using liquid chromatography with tandem mass spectrometry (LC-MS) apparatus. (n = 7 – 8/2 cohorts per group; Student’s t test, ns: non-significant; ****p<0.0001).
(E) Timeline of treatment and experimental procedures.
(F) Quantification of norepinephrine levels in cecal contents harvested from 6-hydroxydopamine (6-OHDA)- or vehicle-treated animals 15 minutes after an acute saline (1ml/kg, i.p.) or cocaine (15mg/kg, i.p.) injection. (n = 3 – 7/1 – 2 cohorts per group; two-way ANOVA with Bonferroni post hoc analysis, ns: non-significant; *p<0.05; **p<0.01; ***p<0.001).
(G) Timeline of treatment and experimental procedures.
(H) C. rodentium burdens in cecal tissues harvested from 6-OHDA)- or vehicle-treated animals repeatedly exposed to saline (1ml/kg, i.p.) or cocaine (15mg/kg, i.p.). (n = 5 – 8/2 cohorts per group, two-way ANOVA with Bonferroni post hoc analysis, ns: non-significant; *p<0.05; **p<0.01).
Data presented as average ± SEM.
To determine whether cocaine-induced increase in C. rodentium infection was mediated by NE signaling, we assessed whether preventing the pathogen to sense NE by genetically deleting the QseC receptor (ΔqseC) would abrogate this phenotype. C. rodentium ΔqseC is attenuated for infection (Figures 1 and S1B), and the absence of this receptor prevented cocaine from enhancing bacterial colonization, LEE-encoded gene expression and proinflammatory cytokine expression compared to mice treated with saline (Figure 1A - H). Furthermore, to functionally confirm the role of NE in the cocaine-induced increase in C. rodentium colonization, we used chemical denervation or sympathectomy. Animals were systemically treated with 6-Hydroxydopamine hydrobromide (6-OHDA) to impair noradrenergic nerve terminals in the peripheral nervous system (Kruszewska et al., 1995). As expected, by using this intervention we were able to block the cocaine-mediated increase in NE levels in the cecum (Figure 2E - F). Congruently, 6-OHDA treatment also prevented cocaine-induced increase in C. rodentium burdens in colonic tissues (Figure 2G and H), supporting a role for a drug increase in NE levels as a promotor of virulence. Collectively, these data propose a mechanistic model where cocaine exposure acts as a pro-virulence mediator for C. rodentium by increasing NE levels in the cecal tissues, which activates QseC signaling promoting the subsequent expression of the LEE and pathogen colonization.
Enhanced Behavioral Response to Cocaine is Driven by γ-Proteobacteria Colonization and not Inflammation
Gut dysbiosis has been corelated with the pathogenesis of SUDs (Lucerne and Kiraly, 2021; Meckel and Kiraly, 2019; Salavrakos et al., 2021). C. rodentium infection is known to alter the microbiota composition, promoting an expansion of γ-Proteobacteria (Lopez et al., 2016; Lupp et al., 2007).Therefore, we investigated whether the infection with C. rodentium can alter behavioral plastic changes that are observed after repeated exposure to cocaine. We found that C. rodentium infected mice showed a significant increase in the locomotor response to repeated exposure to cocaine in comparison to their PBS-mock-infected counterparts (Figure 3A), an effect that was not observed in infected mice after a saline injection.
Figure 3. γ-Proteobacteria composition modulates the host behavioral response to repeated cocaine exposure.
(A) Top: Timeline of treatment and experimental procedures. Bottom: Behavioral activity recordings after cocaine or saline injections on days 1, 3 and 5 in PBS-mock- or C. rodentium-infected mice. Activity tracing for cocaine-treated mice in day 5 is also shown. (n = 13 – 14/2 cohorts per group, two-way repeated measures ANOVA, with Bonferroni post hoc analysis vs. PBS/cocaine, **p<0.01).
(B) Top: Timeline of treatment and experimental procedures. Bottom: Behavioral activity recordings after cocaine or saline injections on days 1, 3 and 5 in mice exposed to 3% dextran sodium sulfate (DSS) or vehicle throughout drinking water. Activity tracing for cocaine-treated mice in day 5 is also shown. (n = 6 – 8/2 cohorts per group).
(C) Microbiota composition at the phylum level in C. rodentium infected and non-infected mice treated with cocaine. (n = 7/3 cohorts per group, two-way ANOVA, with Bonferroni post hoc analysis vs. same phylum in PBS/cocaine, **p<0.01).
(D) Top: Timeline of treatment and experimental procedures. Bottom: Behavioral activity recordings after cocaine or saline injections on days 1, 3 and 5 in mice antibiotic (ABX)-treated reconstituted with a full microbiota (fecal microbiota transplant, FMT) or with the avirulent C. rodentium escN mutant (ΔescN) or with the human E. coli HS. Activity tracing for cocaine-treated mice in day 5 is also shown. (n = 5 – 9/2 cohorts per group, two-way repeated measures ANOVA, with Bonferroni post hoc analysis vs. ABX/FMT/cocaine, *p<0.05, **p<0.01).
Data presented as average ± SEM.
High levels of gut inflammation were detected in mice inoculated with C. rodentium and treated with cocaine (Figure 1F - H and S2A). Therefore, to untangle whether this increased response to cocaine is due to the pathogen itself, to the inflammation they cause, or a combination of both, we used a model of chemically-induced colitis by treatment with dextran sodium sulfate (DSS). DSS directly damages the epithelium and induces severe colitis in the absence of any bacterial pathogen (Winter et al., 2013) (Figure S2A). No differences were found in the behavioral responses elicited by cocaine in animals exposed to DSS regarding their vehicle counterparts (Figure 3B). These data indicate that gut inflammation and colitis are not mediating the increase in cocaine behavioral responses observed with C. rodentium infection.
C. rodentium itself is a γ-Proteobacteria, moreover its infection promotes expansion of Proteobacteria (specifically, γ-Proteobacteria) within the intestine (Lopez et al., 2016; Lupp et al., 2007). Using metagenomic analysis, we found that overall, in comparison with mock-infected mice, animals infected with C. rodentium showed a significant change in microbiota composition (Figures 3C and S2B). We found that while the non-infected mice were γ-Proteobacteria-free (our animals are purchased from Jackson’s laboratory, which are free of γ--Proteobacteria), C. rodentium-infected animals had a striking increase in Proteobacteria (Figure 3C and S2B). As expected, this increase was mostly due (~93%) to the presence of C. rodentium. Therefore, we reasoned that it was the bloom in γ-Proteobacteria driven by C. rodentium colonization, rather than the inflammatory process induced by the pathogen in the gut, that was responsible for the increased cocaine behavioral responses. To confirm this hypothesis, we employed a genetically engineered avirulent C. rodentium strain, ΔescN. EscN is LEE-encoded and is a structural ATPase that is essential for virulence-associated T3SS activity (Zarivach et al., 2007) and colonization of the mouse gut. It has been demonstrated, that in the absence of commensal microbiota, ΔescN can colonize mice without inducing inflammation (Lopez et al., 2016) (Figure S3 and S6C). Therefore, we depleted the murine microbiota by treating mice with antibiotics (ABXs) (Curtis et al., 2014; Kuss et al., 2011) and then reconstituted them with C. rodentium ΔescN or with a normal murine microbiota (fecal microbiota transplant, FMT). The behavioral evaluation revealed a significant increase in cocaine responses in C. rodentium ΔescN reconstituted mice when compared with their FMT-reconstituted counterparts (Figure 3D). Furthermore, consistent with this hypothesis of a γ-Proteobacteria-rich intestinal microbial environment mediating cocaine-increased behavioral responses, we found that animals reconstituted with the human γ-Proteobacteria commensal Escherichia coli HS (E. coli HS (Rasko et al., 2008b)) also recapitulated the increase in the cocaine-induced locomotion phenotype (Figure 3D). Altogether, these data indicate that a change in the microbial gut composition, through a bloom in γ-Proteobacteria, mediates alterations in the behavioral plasticity induced by cocaine.
Gut Metabolic Changes induced by γ-Proteobacteria Colonization Affect Cocaine Induced Behaviors and Signaling Pathways in the Brain
Because modifications in the gut-microbial composition impact the host metabolic profile (Holmes et al., 2012; Li et al., 2008; Nicholson et al., 2012), we next investigated whether the changes in the behavioral responses to cocaine were associated with metabolite alterations in the C. rodentium ΔescN and E. coli HS colonized mice. We used high-resolution liquid chromatography and mass spectrometry (LC–MS) to perform untargeted comparative metabolomics of cerebrospinal fluid (CSF) obtained from the ABX-treated mice that were reconstituted with FMT, C. rodentium ΔescN or E. coli HS and treated with cocaine. Integrated pathway analyses of the metabolites of the CSF were performed using MetaboAnalyst 5.0 (Pang et al., 2021). No significant pathways differences were observed between C. rodentium ΔescN and E. coli HS (Figure S4A). Therefore, we performed an integrated pathway analysis comparing the metabolites of the CSF of FMT-reconstituted mice with the metabolites of the CSF of γ-Proteobacteria-reconstituted mice (C. rodentium ΔescN and E. coli HS combined).
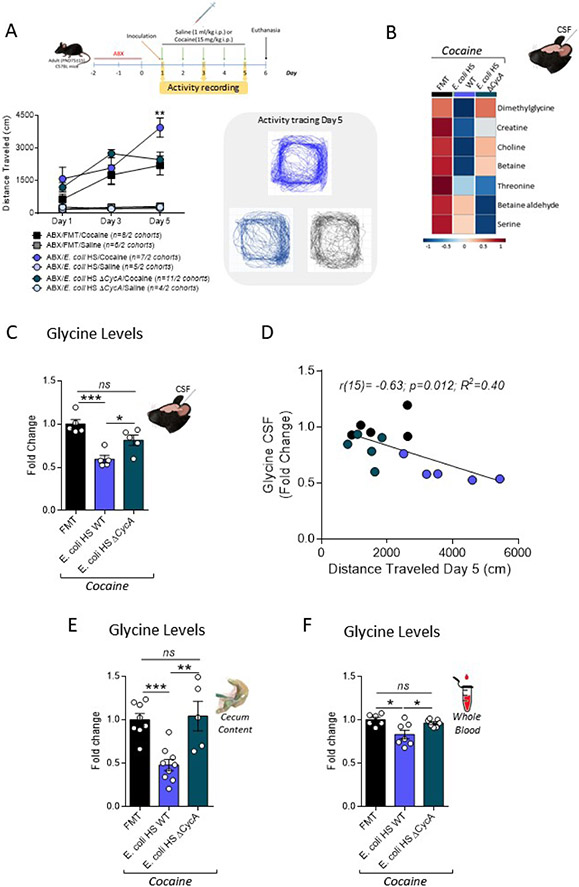
This analysis revealed several significantly affected metabolic pathways (Figure 4A). In particular, the glycine, serine and threonine metabolism pathway was one of most impacted and significantly altered. Notably, of the 11 hits detected by the pathway analysis (Figure S4B), 7 were precursors of the amino acid neurotransmitter and N-methyl-D-aspartate (NMDA) receptor agonist, glycine (Figures 4B and S4C), and all of them were downregulated in γ-Proteobacteria-reconstituted mice (Figure 4C). Glycine has been associated with neuropsychiatric disorders such as schizophrenia and drug addiction (Harvey and Yee, 2013; Marques et al., 2020). Furthermore, glycine is consumed at high rates and favors the growth of γ-Proteobacteria (Goldfarb et al., 2011; Han et al., 2002; Zampieri et al., 2019), which can use it as a nitrogen source (Figure S4D). Since glycine was not detected by the LC-MS analysis (not included within the panel of tested metabolites), we directly measured it in CSF samples. A significant reduction in the levels of glycine was observed in the CSF of mice treated with cocaine that were reconstituted with C. rodentium ΔescN or E. coli HS compared to animals reconstituted with FMT (Figure 4D). Moreover, glycine levels in the CSF negatively correlated with the behavioral murine response elicited by cocaine (Figure 4E). In line with the hypothesis that a decrease in host CSF levels of glycine stems from the reduction of this amino acid in the intestine of reconstituted with C. rodentium ΔescN or E. coli HS mice, glycine levels in the cecum were also reduced in mice reconstituted with C. rodentium ΔescN or E. coli HS compared to FMT (Figure 4F).
Figure 4. Local and systemic metabolic changes driven by γ-Proteobacteria gut colonization affect cocaine-induced behaviors.
(A) Metabolic pathway analysis of cerebrospinal fluid (CSF) samples collected 24hs after the last cocaine treatment injection in mice antibiotic (ABX)-treated reconstituted with a full microbiota (fecal microbiota transplant, FMT) in comparison with mice reconstituted with C. rodentium escN deficient mutant (ΔescN) or the human commensal E. coli HS (evaluated as a single, combined group). (n = 3 – 4/2 cohorts per group).
(B-C) Glycine synthesis pathway (adapted from the Kyoto Encyclopedia of Genes and Genomes, KEGG). Blue boxes indicate downregulated precursors (blue filled boxes: statistically significantly downregulated; blue empty boxes: non-statistically significantly downregulated) that were identified as hits during the partway analysis performed in (A) and which average levels are depicted in (C) (measured using liquid chromatography with tandem mass spectrometry). (n = 3 – 4/2 cohorts per group, Student’s t test, ABX/FMT/Cocaine vs ABX/Proteobacteria/Cocaine).
(D) Glycine levels in CSF collected 24h after the last cocaine treatment injection in mice ABX-treated reconstituted with FMT, ΔescN or E. coli HS. (n = 4 – 5/2 cohorts per group, one-way ANOVA with Bonferroni post hoc analysis, ***p<0.001; ****p<0.0001).
(E) Negative correlation between glycine levels in CSF and the behavioral response observed in cocaine-treated mice at day 5. The black circles represent FMT-reconstituted, the green ones, ΔescN-reconstituted and the blue, E. coli HS-reconstituted animals.
(F) Glycine levels in cecal contents collected 24hs after the last cocaine treatment injection in mice ABX-treated reconstituted with FMT, ΔescN or E. coli HS. (n = 5/2 cohorts per group, one-way ANOVA with Bonferroni post hoc analysis, **p<0.01).
(G) Volcano plot of differential expression of genes in the Nucleus accumbens in cocaine-exposed mice ABX-treated reconstituted with FMT in comparison with mice reconstituted with ΔescN or E. coli HS (evaluated as a single, combined group). Mice were euthanized 24hs after the last cocaine treatment injection. Differentially expressed genes (DEGs) between Proteobacteria-reconstituted versus FMT-reconstituted mice are shown as red dots (upregulation) or blue dots (downregulation). (n= 3/2 cohorts per group, DESeq2 Wald test, false discovery rate (FDR) < 0.1).
(H) Heat maps showing the top 50 most significantly upregulated or downregulated DEGs.
(I) Significantly enriched KEGG pathways analysis based on all the DEGs.
Data presented as average ± SEM.
To further evaluate the hypothesis that an alteration in glycine levels is mediating the changes observed in the behavioral responses to cocaine in C. rodentium ΔescN and E. coli HS reconstituted mice, we assessed modifications at neuroanatomical levels. Genome-wide transcriptional profiling was performed in the nucleus accumbens (NAcc), an area of the brain highly related to cocaine-induced synaptic plasticity. NAcc tissue dissected from C. rodentium ΔescN or E. coli HS reconstituted mice treated with cocaine, exhibited comparable transcriptomes with no significant differential expressed genes (DEGs) (Figure S4E). Therefore, as performed with the metabolomic data, we compared the transcriptomes of the NAcc of FMT mice to the γ-Proteobacteria-reconstituted mice (C. rodentium ΔescN and E. coli HS combined) after cocaine exposure. Several significant DEGs were observed between FMT and γ-Proteobacteria-reconstituted animals (Figure 4G and H). Consistent with the involvement of glycine, pathway enrichment analysis for DEGs using the Kyoto Encyclopedia of Genes and Genomes (KEGG) showed strong alterations in pathways involved in the glutamatergic synapse, as well as in pathways that regulate the dopaminergic synaptic function (Figure 4I) between FMT and γ-Proteobacteria-reconstituted mice. These findings strongly suggest a role of altered glycine levels in cocaine-induced transcriptional plasticity in the NAcc of γ-Proteobacteria-reconstituted mice.
Glycine Levels Modulate Host Susceptibility to Cocaine-Induced Behaviors
To functionally validate the reduction of glycine as a mediator of the increase in cocaine behavioral responses, we directly treated C. rodentium ΔescN- or E. coli HS-reconstituted mice with i.p. glycine before each cocaine injection (Figure 5A and S5). Remarkably, glycine pretreatment prevented the exacerbated behavioral response observed after cocaine in these mice compared to FMT-reconstituted animals. Furthermore, similar results were observed when using the glycine reuptake inhibitor and NMDA partial co-agonist, sarcosine (Zhang et al., 2009a; Zhang et al., 2009b) (Figure 5B and S5B). Together, these observations indicate a role for glycine levels in the intestine and the brain, in determining vulnerability to behavioral plastic changes elicited by repeated cocaine exposure.
Figure 5. Glycine and Sarcosine pretreatment prevents changes in cocaine behavioral responses associated with γ-Proteobacteria gut colonization.
(A) Top: Timeline of treatment and experimental procedures. Bottom: Behavioral activity recordings after cocaine or saline injections on days 1, 3 and 5 of mice antibiotic (ABX)-treated reconstituted with a full microbiota (fecal microbiota transplant, FMT) or with the avirulent C. rodentium escN mutant (ΔescN) or with the human commensal E. coli HS and pretreated with glycine or vehicle. (n = 4 – 11/2 – 3 cohorts per group, two-way repeated measures ANOVA, with Bonferroni post hoc analysis vs. ABX/FMT/PBS/cocaine, *p<0.05; **p<0.01).
(B) Top: Timeline of treatment and experimental procedures. Bottom: Behavioral activity recordings after cocaine or saline injections on days 1, 3 and 5 of mice antibiotic (ABX)-treated reconstituted with a full microbiota (fecal microbiota transplant, FMT) or with the avirulent C. rodentium escN mutant (ΔescN) or with the human commensal E. coli HS and, pretreated with sarcosine or vehicle. (n = 5 – 11/2 – 3 cohorts per group, two-way repeated measures ANOVA, with Bonferroni post hoc analysis vs. ABX/FMT/PBS/cocaine, *p<0.05; **p<0.01).
Data presented as average ± SEM.
Proteobacteria-mediated Glycine Depletion Modulate Host Behavioral Responses to Cocaine
The cycA gene encodes a permease that mediates glycine transport uptake in different Enterobacteriaceae (Cosloy, 1973; Ghrist and Stauffer, 1995; Robbins and Oxender, 1973; Wargel et al., 1971; Wargel et al., 1970), including E. coli HS and C. rodentium. Therefore, we next tested whether a cycA deletion would reduce bacterial-induced host glycine depletion, reversing the increased vulnerability to cocaine-induced behavioral responses using ABX-treated mice reconstituted with a E. coli HS ΔcycA mutant (Figure S6A-C). Remarkably, the increase in the cocaine-induced locomotor behavioral response was no longer observed in animals reconstituted with E. coli HS ΔcycA, when compared with FMT reconstituted mice (Figure 6A). Importantly, most of the precursors of the glycine pathway (Figure 6B), as well as total glycine levels (Figure 6C), were restored to FMT-reconstituted mice levels in CSF samples collected from E. coli HS ΔcycA. Once again, we observed a significant negative correlation between glycine levels in the CSF and the behavioral response to cocaine on day 5 of the different groups (Figure 6D). As predicted, there were no differences in glycine levels in the cecum of mice reconstituted with E. coli HS ΔcycA compared to FMT-reconstituted animals (Figure 6E). Finally, we also observed a decrease in glycine in whole blood levels in mice reconstituted with E. coli HS WT in comparison with FMT animals, that was not observed when the animals were reconstituted using E. coli HS ΔcycA (Figure 6F). These data are consistent with the hypothesis that γ-Proteobacteria colonization drives changes in the intestinal metabolome that affects the systemic and CSF metabolic landscape regulating host glycine levels, and behavioral responses to cocaine.
Figure 6. γ-Proteobacteria gut colonization modulates host metabolic glycine levels altering cocaine-induced behavioral plasticity.
(A) Top: Timeline of treatment and experimental procedures. Bottom: Behavioral activity recordings after cocaine or saline injections on days 1, 3 and 5 in mice antibiotic (ABX)-treated reconstituted with a full microbiota (fecal microbiota transplant, FMT) or with the human commensal E. coli HS WT or with E. coli HS lacking the glycine transporter (E. coli HS ΔcycA). Activity tracing for cocaine-treated mice in day 5 is also shown. (n = 4 – 11/2 cohorts per group, two-way repeated measures ANOVA, with Bonferroni post hoc analysis vs. ABX/FMT/cocaine, **p<0.01).
(B) Glycine synthesis pathway precursors average levels in cerebrospinal fluid (CSF) samples measured by liquid chromatography with tandem mass spectrometry apparatus. (n = 3-4/2 cohorts per group).
(C) Glycine levels in CSF collected 24hs after the last cocaine treatment injection in mice ABX-treated reconstituted with FMT, E. coli HS WT or ΔcycA. (n = 5/2 cohorts per group, one-way ANOVA with Bonferroni post hoc analysis, ns: non-significant; *p<0.05; ***p<0.001).
(D) Negative correlation between glycine levels in CSF and the behavioral response observed in cocaine-treated mice at day 5. The black circles represent FMT-reconstituted, the teal ones, E. coli HS ΔcycA-reconstituted and the blue, E. coli HS WT-reconstituted mice.
(E) Glycine levels in cecal contents collected 24hs after the last cocaine treatment injection in mice ABX-treated reconstituted with FMT, E. coli HS WT or ΔcycA. (n = 5 – 9/2 – 3 cohorts per group, one-way ANOVA with Bonferroni post hoc analysis, ns: non-significant; **p<0.01; ***p<0.001).
(F) Glycine levels in whole blood collected 24hs after the last cocaine treatment injection in mice ABX-treated reconstituted with FMT, E. coli HS WT or ΔcycA. (n = 6 – 7/2 cohorts per group, one-way ANOVA with Bonferroni post hoc analysis, ns: non-significant; *p<0.05).
Data presented as average ± SEM.
Proteobacteria-mediated Glycine Depletion Modulate Host Rewarding Effects of Cocaine
To further evaluate the effects of γ-Proteobacteria composition in the rewarding properties of cocaine we used conditioned place preference (CPP). CPP is a standard preclinical behavioral model largely used to study the rewarding effects of drugs that mimics the association of environmental cues with the drug experience that have been shown to play a critical role in acquiring and maintaining drug-taking behaviors in humans (Bardo and Bevins, 2000; McKendrick and Graziane, 2020; Prus et al., 2009). We found that ABX-treated animals reconstituted with a normal fecal microbiota (FMT) do not demonstrate significant drug seeking behaviors to 5 mg/kg i.p. dose of cocaine (Figure 7). However, significant cocaine-seeking behavior was observed in animals colonized with C. rodentium ΔescN or with the commensal E. coli HS (Figure 7). Furthermore, similar to FMT, mice reconstituted whit the E. coli HS ΔcycA, that cannot uptake glycine, did not develop significant cocaine seeking behaviors (Figure 7). These data further support the hypothesis that γ-Proteobacteria driven glycine depletion can modulate drug-seeking and rewarding responses associated to cocaine exposure.
Figure 7. γ-Proteobacteria gut colonization modulates the development of cocaine-induce place preference.
(A) Timeline of treatment and experimental procedures for Conditioned place preference (CPP).
(B) CPP score for a cocaine dose of 5 mg/kg in mice antibiotic (ABX)-treated reconstituted with a full microbiota (fecal microbiota transplant, FMT) or with the avirulent C. rodentium escN mutant (ΔescN) or with the human commensal E. coli HS WT or with E. coli HS lacking the glycine transporter (E. coli HS ΔcycA). (n = 6 – 9/2 – 3 cohorts per group, two-way repeated measures ANOVA, with Bonferroni post hoc analysis, ns: non-significant: *p<0.05; **p<0.01; ****p<0.0001).
Data presented as average ± SEM.
DISCUSSION
Growing evidence suggests a bidirectional interaction between the gut microbiome and drug abuse (Meckel and Kiraly, 2019). However, the mechanisms by which abused substances modulate and/or alter microbial composition and the consequences of these changes for cocaine-induced neuroplastic changes remain scarce. Our findings establish that an alteration in the levels of host catecholamines induced by recreational drugs such as cocaine can be sensed by γ-Proteobacteria pathogens. Furthermore, we found that this type of compositional microbial change towards γ-Protobacteria can lead to a depletion of host neuroactive metabolites from the gut and the cerebrospinal fluid, affecting cocaine-induced NAcc plasticity and addiction-like behaviors.
Our results support a model of cocaine-induced increase of NE signaling to intestinal pathogens through the activation of the bacterial adrenergic receptor QseC that results in more severe intestinal disease and colonization. Importantly, QseC is also encoded in the core genomes of several other pathogens (Moreira and Sperandio, 2012; Moreira et al., 2010), as well as nonpathogenic γ--Proteobacteria (Rasko et al., 2008a; Rooks et al., 2017). Therefore, it is possible that psychostimulants modulate many additional aspects of bacterial function in both pathogenic and commensal bacteria, increasing their ability to colonize the gut. Indeed, exposure to cocaine and amphetamines has been also reported to increase commensal Protobacteria in mice (Angoa-Pérez et al., 2020; Chivero et al., 2019; Ning et al., 2017), which further support a role of host catecholamine levels as modulators of commensal bacteria dynamics.
Changes in microbiota composition impact host homeostasis, physiology, metabolic profile, and vulnerability to disease (Holmes et al., 2012; Li et al., 2008; Nicholson et al., 2012). Specifically, we found that a γ-Proteobacteria expansion (driven by an enteric infection) increases cocaine-induced responses. It is known that microbiota composition can be altered through multiple genetic and environmental cues, including diet, antibiotic usage and pathogenic insults among others. Notably, interventions such as high fat diets and oral antibiotic treatment that promote γ-Proteobacteria expansion (Jeong et al., 2019; Kim et al., 2017; Litvak et al., 2017; Rizzatti et al., 2017; Shin et al., 2015), have also be correlated to increased behaviors associated to cocaine exposure (Baladi et al., 2012; Blanco-Gandia et al., 2017a; Blanco-Gandia et al., 2017b; Blanco-Gandia et al., 2018; Blanco-Gandia and Rodriguez-Arias, 2017; Clasen et al., 2020a; Clasen et al., 2020b; Kiraly et al., 2016; Serafine et al., 2015; Serafine et al., 2016). It is therefore tempting to speculate a potential synergistic contribution of a bloom in γ-Proteobacteria in the gut to these cocaine responses.
Regarding the molecular mechanisms, our data reveal that γ-Proteobacteria colonization cause a significant reduction in glycine levels in the gut, in the blood and the CSF leading to an exacerbated response to cocaine. This type of bacterial impact on the host metabolome is not unexpected. Amino acid levels in the gastrointestinal tract are highly affected by the microbiota (Claus et al., 2008; Lahiri et al., 2019; Mardinoglu et al., 2015; Whitt and Demoss, 1975). Glycine, in particular, is considered a conditionally essential amino acid and approximately 50% of its bioavailability depends on dietary uptake in the intestine, (Alves et al., 2019; Li and Wu, 2018) where it can be also highly catabolized by different microbial members. This means, that a decrease in glycine bioavailability in the intestine will lead to lower levels of this amino acid to be absorbed by the host, which will reduce its concentration systemically. This modulatory effect of the gut microbiota has been largely observed for many metabolites (e.g. glucose and indole [which is exclusively produced by gut microbes]) and amino acids in many different tissues, including the brain and the CSF (Chu et al., 2019; Kawase et al., 2017; Lahiri et al., 2019; Manca et al., 2020; Mardinoglu et al., 2015; Matsumoto et al., 2013; Velagapudi et al., 2010; Wikoff et al., 2009). Moreover, recently Lai et al. (2021) published metabolomic signatures owing to gut microbiota, yielding hundreds of identified metabolites including 533 altered for feces, 231 for sera, and 58 for brain with numerous significantly enriched pathways involving aromatic amino acids and neurotransmitters. Both, C. rodentium and E coli HS can use glycine as a N source, and indeed, Proteobacteria consume it at high rates during growth (Goldfarb et al., 2011; Han et al., 2002; Zampieri et al., 2019) reducing its availability for the host. Furthermore, reduced circulating glycine levels is consistently reported in patients and murine models of nonalcoholic fatty liver disease (NAFLD), and in line with our data, both, humans and animal models of this disease show consistent increased levels of Proteobacteria in their gut (Kapil et al., 2016; Michail et al., 2015; Rom et al., 2020; Zhu et al., 2013).
Once in the brain, glycine works as the obligatory co-agonist of the NMDA receptor, modulating calcium-dependent neuronal excitation and plasticity affecting glutamatergic and dopaminergic synapses (Molander and Soderpalm, 2005; Zafra et al., 2017). Congurent with these modulatory effects of glycine, our data show that γ-Proteobacteria-monocolonized mice present transcriptional alterations in both, glutamatergic and dopaminergic synapsis in the NAcc after cocaine exposure. Furthermore, we demonstrated that this γ-Proteobacteria-mediated glycine reduction is sufficient to modulate cocaine locomotor and rewarding properties. Similar results were previously reported using other glycine agonists, such as D-serine and sarcosine, were the administration of these compounds systemically or intra-NAcc prevented the development of behavioral sensitization as well as the expression of cocaine-induced CPP (Curcio et al., 2013; Liu et al., 2016; Yang et al., 2013). Increases in glycine levels, by using a glycine transporter-1 inhibitor, has also been shown to facilitate cocaine-cue extinction and attenuate reacquisition of cocaine-seeking behaviors (Nic Dhonnchadha et al., 2012). Notably, selectively bred high-responder rats present lower glycine levels in the NAcc after a cocaine injection when compared to their low-responder counterparts (Mabrouk et al., 2018). Combined with our data, these reports support glycine as potent regulator of cocaine responses. While the specific molecular mechanisms at the level of the NAcc remain to be established, glycine reduction could lead to a hypofunction of the NMDA receptors that could, in turn, alter cocaine-induced plasticity (Curcio et al., 2013; D'Ascenzo et al., 2014; Liu et al., 2016). The RNAseq data revealed the Grin2b as one of the most differentially upregulated genes in the cocaine-treated γ-Proteobacteria-reconstituted group in comparison with animals recolonized with a full microbiota. GluN2B-containing NMDARs have been previously reported to be transiently upregulated in NAcc medium spiny neurons (MSNs). This increase seems to be related to the generation of new silent synapses, a key cellular event that would mediate cocaine-induced neurobehavioral plasticity (Brown et al., 2011; Huang et al., 2009; Neumann et al., 2016). Future studies will be aimed at determining the molecular basis for how microbiota-mediated glycine depletion affect cellular activity in the NAcc.
Drug use and SUDs affect more than 27 million people in the United States, leading to more than $740 billion annually in costs related to crime, lost work productivity and health care (Birnbaum et al., 2011; Florence et al., 2016; Xu et al., 2015). Unfortunately, despite many years of research, no successful therapeutic targets or evidence-based treatments have been developed. Innovative ideas and more integrative approaches are sorely needed. Microbial composition can strongly modulate the host’s metabolic profile and vulnerability to disease. Here we show a defined mechanisms by which a specific microbial compositional shift in the gut modulated behavioral, rewarding and neuroplastic changes induced by repeated exposure to psychostimulants. Moreover, we show that, by manipulating specific bacterial processes, microbial-induced host metabolic alterations can be reversed and, thus, the increased responses to cocaine. More broadly, our findings demonstrate that intestinal bacteria can serve as a potentially engineered signaling node within the enteric system that impacts behavioral and molecular targets affected during the course of psychiatric diseases, advancing our understanding of these mental conditions, and shifting the current brain-centered paradigm to a more systemic and holistic view in neuropsychiatric research and treatment.
LIMITATIONS OF STUDY
Here, we show that different behavioral responses associated with cocaine exposure in mice (behavioral sensitization and drug seeking behaviors) can be affected by changes in the gut microbial composition. We acknowledge that there are limitations in our studies. The animal colonization experiments with C. rodentium or commensal E. coli HS were performed in mice whose microbiota was depleted through antibiotic treatment. One would considered also performing these experiments in germ-free animals. However, it is technically unfeasible to run the behavioral experiments using germ-free mice. The animals are transferred one by one to the behavioral boxes for these tests and there is no way to have these two different sets of boxes (locomotion and CPP), which are also hooked to a computer for analyses, within germ-free bubbles. Moreover, additional work will be necessary to confirm the translational value of these results to interventions in human SUDs. A major challenge is whether microbiota changes in addicted individuals are the result of cocaine modulating the norepinephrine levels in the gut, or if the often-poor nutrition of these individuals is driving these changes. Finally, it would be valuable to establish a more precise mechanism by which this γ-Proteobacteria-driven glycine depletion impacts the nucleus accumbens and the reward circuitry function.
STARS METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and request for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Vanessa Sperandio (vsperandio@wisc.edu).
Materials Availability
All unique reagents generated in this study are available by request made to the lead contact.
Data and Code Availability
The raw data of RNA sequencing was deposited at GEO database. The access number is listed in the key resources table.
This study did not report the original code.
Any additional information required to reanalyze the data reported in this paper is available upon request of the lead contact.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-EspA-polyclonal, rabbit | UT Southwestern, Vanessa Sperandio | N/A |
| Anti-EspB-polyclonal, rabbit | UT Southwestern, Vanessa Sperandio | N/A |
| Anti-rabbit-HRP, goat | BioRad | Cat# 1706515 |
| Bacterial and Virus Strains | ||
| E. coli HS | Center for Vaccine Development at the University of Maryland School of Medicine | N/A |
| C. rodentium DBS100 | Tufts University, John Leong | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Dulbecco eagle medium, low glucose | Invitrogen | Cat# 11885-084 |
| Cocaine Hydrochloride | Sigma-Aldrich | Cat#C5776 |
| LB agar, Miller | Fisher Scientific | Cat# BP1425 |
| LB broth, Miller | Fisher Scientific | Cat# BP9723 |
| Ampicillin powder | Sigma-Aldrich | Cat# A9518 |
| Naladixic acid power | Fisher Scientific | Cat# BP908 |
| L-(+)-Arabinose | Sigma-Aldrich | Cat# A3256 |
| Dextran sulfate sodium salt | MP Biomedicals | Cat#021601109 |
| Phusion High fidelity PCR master mix | Thermo Fisher | Cat# F-531L |
| Random primers | Invitrogen | Cat# 48190-011 |
| Super script II reverse transcriptase | Invitrogen | Cat# 18064-014 |
| Sybr green master mix | Thermo Fisher | Cat# 4309155 |
| Sarcosine | Sigma-Aldrich | Cat#131776 |
| Glycine Hydrochloride | Sigma-Aldrich | Cat# G2879 |
| Trizol reagent | Thermo Fisher | Cat# 1559618 |
| Chloroform | IBM Scientific | Cat# 67-66-3 |
| dNTP mix 10mM | Invitrogen | Cat# 18-427-088 |
| Bovine Serum Albumin | Sigma-Aldrich | Cat# A2153 |
| Dopamine Hydrochloride | Sigma-Aldrich | Cat# H8502 |
| 6-hydroxydopamine Hydrochloride | Sigma-Aldrich | Cat# H4381 |
| RNAlater-ICE | Fisher Scientific | Cat# 44-275-75 |
| Protease inhibitor cocktail | Sigma-Aldrich | Cat# P8849-5 |
| Dithiothreitol (DTT) | Fisher Scientific | Cat# BP172-25 |
| Formaldehyde | Fisher Scientific | Cat# BP539 |
| Brain heart infusion agar | Becton Dickinson | Cat# 2418102 |
| Dextran sulfate sodium salt | Fisher Scientific | Cat# 02-160-1109 |
| Metronidazole | MP biomedicals | Cat# 0215571025 |
| Neomycin trisulfate salt hydrate | Sigma-Aldrich | Cat# N5285 |
| Vancomycin hydrochloride | Research Products International | Cat# V06500 |
| Critical Commercial Assays | ||
| RNeasy Power Microbiome | Qiagen | Cat# 26000-50 |
| RNeasy Plus Mini Kit | Qiagen | Cat# 74134 |
| RiboPure RNA isolation Kit | Ambion | Cat# 1405072 |
| GenElute Plasmid Miniprep | Sigma | Cat# PLN350-1KT |
| QIAquick PCR purification kit | Qiagen | Cat# 28906 |
| Glycine Assay Kit | Sigma-Aldrich | Cat# MAK261 |
| Deposited Data | ||
| RNA sequencing data | https://www.ncbi.nlm.nih.gov/geo/ | GSE212421 |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J mice | The Jackson laboratory | Cat# 000664 |
| Oligonucleotides | ||
| Primers for qRT-PCR used in this study are listed in Table S1 | This paper | N/A |
| Software and Algorithms | ||
| Prism 6 | GraphPad | N/A |
| QuantStudio real-time PCR software v1.3 | Thermo Fisher | N/A |
| Image Lab 5.2.1 | BioRad | N/A |
| iDEP.91 | http://bioinformatics.sdstate.edu/idep/ | N/A |
| CLC Bio microbial genomics module | https://www.qiagenbioinformatics.com/plugins/clc-microbial-genomics-module/ | N/A |
| MetaboAnalyst 5.0 | https://www.metaboanalyst.ca/ | N/A |
| Fiji Image J | https://fiji.sc/ | N/A |
| Fusion 6.5v | Omnitech Electronics Incorporated | N/A |
| MultiQuant 3.0.3. Software | https://sciex.com/products/software/multiquant-software | N/A |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
C57BL/6 adult (postnatal day, PND, 75±15) male mice were obtained from Jackson Laboratory (arrival weight range: 20±3 g). All mice were housed in specific pathogen free conditions at UT Southwestern, and acclimatized for one week before being used for experiments. All mice were maintained under specific pathogen-free conditions, housed in individual cages with no more than 5 mice per cage, with controlled room temperature (22-25°C) and humidity (45-75%), on a 12-h light-dark cycle, and with autoclaved standard chow and water. All animal protocols were approved by the UT Southwestern Medical Center Institutional Animal Care and Use Committee.
Bacterial Strains
Citrobacter rodentium strain DBS100, and Escherichia coli HS were grown overnight in Luria bertani (LB) broth at 250 rpm and 37°C prior to subculture for in vitro experiments or mouse infections as described below. All bacteria at stationary phase was mixed with sterile glycerol (25% final concentration) and kept as stocks at −80°C.
Bacterial strains and growth conditions
All the bacterial strains used in this study are listed in Table S1. Unless otherwise indicated, all bacterial strains were grown overnight aerobically in LB prior to subculture for in vitro experiments or mouse infections. For in vitro studies, C. rodentium strains were subcultured in DMEM with 0.1% glucose (Gibco) and grown in standing cultures (microaerobic) at 37°C (LEE-inducing conditions) or subcultured in minimal medium (MM) and grown microaerobically at 37°C. E coli HS strains were subcultured in MM and grown microaerobically at 37°C. Where indicated, bacteria were grown in the presence of dopamine (Sigma) or its vehicle (saline solution + 0.01M HCl), cocaine (Sigma) or its vehicle (saline solution) or Glycine (Sigma).
Isogenic mutants were generated using the lamda red recombinase method as previously described (Datsenko and Wanner, 2000). For growth measurements, bacteria were grown under microaerobic conditions using 96-well microplates.
METHOD DETAILS
Western blot assays for secreted proteins
Secreted proteins were isolated as previously described (Ellermann et al., 2020). Briefly, bovine serum albumin (BSA) was used as a loading control and added to secreted protein samples. Proteins were separated using a gradient 4-15% SDS-PAGE gel, transferred to a polyvinylidene fluoride membrane and blocked with 5% milk in phosphate buffered saline (PBS) with 0.05% Tween. Membranes were probed using anti-EspA or anti-EspB primary antibodies, followed by incubation with secondary antibodies conjugated to streptavidin-horseradish peroxidase. Membranes were exposed with the Bio-Rad ChemiDoc Touch Imaging System with Image Lab 5.2.1 software for image analysis.
C. rodentium infections
Mice were orally infected with 1 x 109 CFU of C. rodentium DBS100 WT or with QseC deficient mutant, ΔqseC or mock-treated with PBS. Fecal pellets were collected throughout the course of infection to enumerate fecal bacteria loads by quantitative culture using selective media for DBS100 (nalidixic acid). At necropsy, cecal tissues were harvested to quantify C. rodentium burden, for pathology analysis and for RNA isolation from tissues.
Cocaine treatment
Three hours after infection and for five consecutive days, mice were injected i.p. once a day with either cocaine (15 mg/kg) (Sigma) or saline (1 ml/kg).
RNA isolation and quantitative real-time PCR
For host and bacterial gene expression, RNA was isolated from cecal tissues snap frozen in liquid nitrogen using the TriZol method (ThermoFisher Scientific) according to the manufacturer’s instructions. For bacterial expression of LEE genes in vitro RNA was extracted using the RiboPure bacterial isolation kit (Ambion) per the manufacturer’s instructions. cDNA was synthesized using Superscript II reverse transcriptase (ThermoFisher Scientific). qPCR was performed in a QuantStudio 6 Flex Instrument (Life Technologies) with Power SYBR Green (Applied Biosystems) using the following PCR conditions: a single hold at 50°C for 2 minutes and at 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. Each PCR was performed in 10 μL reactions and contained the following: 1x SYBR green mix and 0.25 μM of each primer. Melting curves were assessed to ensure specificity of the PCR products. The relative abundance of mammalian mRNA transcripts was calculated using the delta delta CT method and normalized to Gapdh levels. The relative abundance of bacterial mRNA transcripts was calculated using the delta delta CT method and normalized to rpoA levels. Table S2 lists qRT-PCR primers to amplify the different transcripts.
Gross pathology and histopathology
Cecal tip was fixed in 10% neutral buffered formalin. Histological inflammation scores (0-20) of hematoxylin and eosin (H&E) stained sections were blindly assessed as previously described (Gibson et al., 2008). Briefly, inflammation was assessed based on the following histopathological features: epithelial hyperplasia (0-4), submucosal edema (0-4), goblet cell depletion (0-4), epithelial integrity (0-4) and polymononuclear (PMN) cell and inflammatory monocyte infiltration (0-4). Data are expressed as the sum of these individual scores (0-20). Histological inflammation scores and representative images corresponding to all the non-antibiotic treated animals were assigned parallel during the same experimental evaluation. The same criterion was applied to the antibiotic-treated groups.
Neurotransmitter measurements in cecal contents
Cecal content samples were collected and frozen immediately at −80°C and then processed as described (Wojnicz et al., 2016). Briefly, the samples were homogenized for protein precipitation in an ice-cold solution containing 75% acetonitrile, 1% formic acid and 0.2 μM of the internal heavy standards (IS) in a 4:1 proportion (solvent:cecum content). The samples were then centrifugated at high speed (~17000g) for 40 min at 4°C. 200 μL of the supernatant were collected, evaporated to dryness at 45°C, resuspended in 100 μL of mobile phase (95:5, 0.2%formic acid:acetonitrile) and submitted to the UT Southwestern Medical Center Metabolomic Core to be evaluated using a Liquid Chromatography with tandem mass spectrometry (LC-MS) apparatus. All the peaks were validated using the pure compound as a control. Peaks representing targeted masses and LC retention times were confirmed manually and the data analyzed using relative quantifications.
16S rRNA sequencing and analysis
For 16S rRNA microbiota profiling, genomic DNA was isolated from feces that were collected at euthanasia, snap frozen and maintained at −80°C and processed as in (Ellermann et al., 2020). Briefly, the hypervariable region V3 & V4 of bacterial 16S rRNA gene were captured using the Illumina Nextera protocol and a single amplicon of about 460 bp was amplified as described in the Illumina protocol. The PCR product were cleaned using Agencourt AmpureXP beads from Beckman Counter Genomics. Illumina adaptor and barcode sequences were ligated to the amplicons in order to attach them to MiSeqDx flow cell and for multiplexing. Quality and quantity of each sequencing library was assessed using Bioanlyzer and picogreen measurements, respectively. About 6 pM of pooled libraries was loaded onto a MiSeqDX flow cell and sequenced using PE300 (Paired end 300 bp) v3 kit. Raw fastq files were demultiplexed based on unique barcodes and assessed for quality. Samples with more than 50 K QC pass sequencing reads were used for downstream 16S operational taxonomic units (OTU) analysis. Taxonomic classification and OTU abundance analysis were done using CLC Bio microbial genomics module (https://digitalinsights.qiagen.com/plugins/clc-microbial-genomics-module/). Individual sample reads were annotated with the Greengenes database and taxonomic features were determined.
Chemical sympathectomy
6-Hydroxydopamine hydrobromide (6-OHDA, Sigma Chemical Co., St. Louis, MO) was dissolved in sterile saline containing 0.01% of the antioxidant ascorbate and injected i.p. at concentration of 250 mg/kg (Kruszewska et al., 1995). Control animals received the respective vehicle.
Cocaine-induced locomotor activity recordings and analyses
Mice were injected i.p. once a day, for 5 consecutive days with cocaine (15 mg/kg) or saline (1 ml/kg). After the first, the third and the last injection of the treatment, their locomotor activity was measured during 20 min (Delint-Ramirez et al., 2018; Kourrich et al., 2013; Smith et al., 2016) in a SuperFlex open field equipped with infrared photobeams located around the perimeter of the arenas interfaced to a computer running Fusion 6.5v software (Omnitech Electronics Incorporated, OH). Injections in day 2 and 4 were administered in the mice home cages.
Conditioned Place Preference (CPP)
Mice were evaluated for cocaine place preference using a SuperFlex 3 chambered CPP equipped with infrared photobeams located around the perimeter of the arenas interfaced to a computer running Fusion 6.5v software (Omnitech Electronics Incorporated, OH) using an unbiased design. The two end chambers have distinct visual (vertical or horizontal striped walls) and tactile (small holes vs. grid flooring) cues to allow differentiation. During the pretest day mice were allowed to freely explore all three chambers for 20 minutes. Mice that showed significant avoidance for one of the two chambers (<20% of the total time in the chamber) were excluded from further analysis (<10% of animals tested). Groups were then balanced and adjusted to balance out any pre-existing chamber bias. A total of four conditioning sessions were carried out by pairing an injection of saline with one chamber, and one of cocaine (5 mg/kg, i.p.) with the other chamber during four consecutive days. CPP posttest was carried out 24h after the las conditioning session during which mice were again allowed to explore all chambers freely. Place preference scores were taken during the pretest and the posttest as time on the cocaine paired side – time on the saline paired side.
Chemically-induced colitis
Mice were orally administered 3% dextran sulfate sodium (DSS) in their drinking water and allowed to consume ad libitum to damages the epithelium and induces severe colitis (Winter et al., 2013).
Antibiotic treatment and microbiota reconstitution
Mice were orally treated with a four-antibiotic (ABX) cocktail (ampicillin, vancomycin, metronidazole and neomycin) for 3 days to deplete their natural gut microbiota, as previously described (Curtis et al., 2014; Kuss et al., 2011). 24 hrs after the last antibiotic dose, mice were reconstituted with 1 x 109 CFU of C. rodentium DBS770 ΔescN, or with 1 x 1010 CFU E. coli HS WT or with 1 x 1010 CFU E. coli HS CycA deficient mutant, ΔCycA, or reconstituted with a full microbiota (fecal microbiota transplant, FMT). Cecal contents were collected at euthanasia to enumerate bacteria loads by quantitative culture using selective media in aerobic conditions (LB with chloramphenicol for C. rodentium DBS770 ΔescN, MacConkey for E. coli HS, BHI-Blood for FMT and LB with Kanamycin for E. coli HS ΔCycA).
Fecal microbiota transplantation (FMT)
Fecal pellets from age matching male controls were collected immediately upon defecation under sterile conditions and resuspended in PBS containing 0.05% of L-cysteine as a reducing agent (~5 pellets/1 ml of buffer), vortexed, briefly spined and transplanted into recipient mice by gavage with 200 μl of the supernatant.
Cerebrospinal fluid collection
Cerebrospinal fluid (CSF) was collected after euthanasia using the modified cisternal puncture method (Sakic, 2019). Samples were snap frozen and maintained at −80°C until processing.
Cerebrospinal fluid untargeted metabolomic
For metabolites extraction, 2 μl of sample were homogenized in an ice-cold solution containing 40% ethanol, 80% acetonitrile, 0.1% formic acid and 0.2 μM of the internal heavy standards (IS) in a 4:1 proportion (solvent:CSF) and maintained at −20°C for 1 hour. The samples were then centrifugated at high speed (~17000g) for 40 min at 4°C, the supernatants were collected, evaporated to dryness at 45°C, resuspended in 75 μL of mobile phase (80:205, acetonitrile:water) and submitted to the UT Southwestern Medical Center Metabolomic Core to be evaluated using a Liquid Chromatography with tandem mass spectrometry (LC-MS) apparatus. MultiQuant software (AB Sciex, Version 2.1) was used for peak analysis of ~110 targeted metabolites, with standards run consecutively. Peaks representing targeted masses and LC retention times were confirmed manually. To perform pathway analysis and create heatmaps, data was analyzed using MetaboAnalyst version 5.0 (Pang et al., 2021).
Glycine levels determination
Glycine levels in cerebrospinal fluid, in cecal contents and in trunk whole blood were determined using the Glycine Assay Kit (MAK261, Sigma-Aldrich, USA) as per manufacturer’s protocol.
Glycine and sarcosine pretreatment
Glycine hydrochloride (Sigma) was dissolved in PBS, its pH adjusted to 7.4 using NaOH. A dose of 400 mg/kg was administered i.p. 30min before each cocaine or saline injection (D'Souza et al., 2000; Kanahara et al., 2008; Olsson et al., 2021). Sarcosine (Sigma) was dissolved in PBS and 300 mg/kg were administered i.p. 30min before each cocaine or saline injection (Chan et al., 2012; Fone et al., 2020; Pei et al., 2019). In both cases, PBS injections were used as a vehicle control.
NAcc dissection, RNAseq library preparation, sequencing and analysis
After euthanasia, mice brains were rapidly removed, snap frozen and stored at −80°C. Before dissection, mice brains were transferred to RNAlater-ICE® (Ambion) and maintained at −20°C for at least 48hs as per manufacturer’s protocol. For NAcc tissue dissection, mice brains were placed in a metal brain mice matrix on ice, razor blades were placed at 1-mm separation, and two bilateral punches per animal were taken containing the NAcc (predominantly the core subregion). Total RNA was immediately isolated using the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s instructions.
A total of 1 μg from each DNase-treated RNA sample were processed for sequencing by the UT Southwestern Medical Center Next Generation Sequencing Core as described in (Martins et al., 2020). Read counts were generated using feature Counts (Liao et al., 2014) and analyzed and visualized using iDEP.91 (Ge et al., 2018). Briefly, after ID conversion, low and non-expressed genes (less than 0.5 counts per million reads in more than 70% of all the samples) were omitted from the analysis and data was transformed using the regularized log (rlog) transformation. Differentially expressed genes (DEGs) were selected using the DESeq2 package, with a false discovery rate (FDR) < 0.1. DEGs were then subjected to enrichment analysis based on the hypergeometric distribution using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analysis
All values are presented as means ± SEM. Statistical significance of differences between groups was determined using two-tailed unpaired Student’s t test when 2 experimental groups were compared. Correlations were calculated using the Pearson correlation coefficient with two-tailed analysis on the fold-change data. Statistical differences between more than two groups were analyzed with one-way, two-way, or two-way repeated-measures ANOVAs as indicated, followed by Bonferroni multiple comparison post hoc tests. All enumeration of bacteria by serial dilution and plating was log transformed to normalize the data.
The individual statistic for the Cocaine-induced locomotor activity analysis can be find in the Document S1.
Supplementary Material
Table S1. Bacterial strains and Oligonucleotide primers used in this study. Related to bacterial strains and growth conditions in STAR methods section.
Highlights.
Cocaine raises gut norepinephrine levels facilitating Proteobacteria colonization
Proteobacteria colonization depletes glycine in gut, blood, and CSF in mice
Glycine depletion alters cocaine-induced neuroplasticity and drug responses
Systemic or bacteria-mediated glycine replenishment restores cocaine responses
ACKNOWLEDGEMENTS
We thank Fernando Martins and Melissa Ellermman for their help with experimental design and data analysis. We thank the Cell Imaging Core for assistance with microscopy, the Histology Core for aiding in pathology sample preparation, the High Throughput Sequencing Core for their aid with RNAseq. This study was supported by the NIH grants: AI053067, AI154597, and AI155398
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AND MATERIALS AVAILABILITY
All data, materials and strains published in this article are available upon request
DECLARATION OF INTERESTS
The authors declare no competing interests
REFERENCES
- Alves A, Bassot A, Bulteau AL, Pirola L, and Morio B (2019). Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Pérez M, Zagorac B, Winters AD, Greenberg JM, Ahmad M, Theis KR, and Kuhn DM (2020). Differential effects of synthetic psychoactive cathinones and amphetamine stimulants on the gut microbiome in mice. PLOS ONE 15, e0227774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al. (2011). Enterotypes of the human gut microbiome. Nature 473, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Koek W, Aumann M, Velasco F, and France CP (2012). Eating high fat chow enhances the locomotor-stimulating effects of cocaine in adolescent and adult female rats. Psychopharmacology (Berl) 222, 447–457. [DOI] [PubMed] [Google Scholar]
- Bardo MT, and Bevins RA (2000). Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 153, 31–43. [DOI] [PubMed] [Google Scholar]
- Barthold SW, Coleman GL, Bhatt PN, Osbaldiston GW, and Jonas AM (1976). The etiology of transmissible murine colonic hyperplasia. Lab Anim Sci 26, 889–894. [PubMed] [Google Scholar]
- Baumler AJ, and Sperandio V (2016). Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, and Roland CL (2011). Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain medicine 12, 657–667. [DOI] [PubMed] [Google Scholar]
- Blanco-Gandia MC, Aracil-Fernandez A, Montagud-Romero S, Aguilar MA, Manzanares J, Minarro J, and Rodriguez-Arias M (2017a). Changes in gene expression and sensitivity of cocaine reward produced by a continuous fat diet. Psychopharmacology (Berl) 234, 2337–2352. [DOI] [PubMed] [Google Scholar]
- Blanco-Gandia MC, Cantacorps L, Aracil-Fernandez A, Montagud-Romero S, Aguilar MA, Manzanares J, Valverde O, Minarro J, and Rodriguez-Arias M (2017b). Effects of bingeing on fat during adolescence on the reinforcing effects of cocaine in adult male mice. Neuropharmacology 113, 31–44. [DOI] [PubMed] [Google Scholar]
- Blanco-Gandia MC, Montagud-Romero S, Aguilar MA, Minarro J, and Rodriguez-Arias M (2018). Housing conditions modulate the reinforcing properties of cocaine in adolescent mice that binge on fat. Physiol Behav 183, 18–26. [DOI] [PubMed] [Google Scholar]
- Blanco-Gandia MC, and Rodriguez-Arias M (2017). Bingeing on fat increases cocaine reward. Oncotarget 8, 16105–16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenshtein D, McBee ME, and Schauer DB (2008). Utility of the Citrobacter rodentium infection model in laboratory mice. Current opinion in gastroenterology 24, 32–37. [DOI] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, et al. (2011). A Silent Synapse-Based Mechanism for Cocaine-Induced Locomotor Sensitization. J Neurosci 31, 8163–8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenit MC, Sanz Y, and Codoner-Franch P (2017). Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol 23, 5486–5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MH, Chung SS, Stoker AK, Markou A, and Chen HH (2012). Sarcosine attenuates toluene-induced motor incoordination, memory impairment, and hypothermia but not brain stimulation reward enhancement in mice. Toxicol Appl Pharmacol 265, 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivero ET, Ahmad R, Thangaraj A, Periyasamy P, Kumar B, Kroeger E, Feng D, Guo ML, Roy S, Dhawan P, et al. (2019). Cocaine Induces Inflammatory Gut Milieu by Compromising the Mucosal Barrier Integrity and Altering the Gut Microbiota Colonization. Scientific reports 9, 12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Murdock MH, Jing D, Won TH, Chung H, Kressel AM, Tsaava T, Addorisio ME, Putzel GG, Zhou L, et al. (2019). The microbiota regulate neuronal function and fear extinction learning. Nature 574, 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MB, Hughes DT, Zhu C, Boedeker EC, and Sperandio V (2006). The QseC sensor kinase: a bacterial adrenergic receptor. Proceedings of the National Academy of Sciences of the United States of America 103, 10420–10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen MM, Riley AL, and Davidson TL (2020a). Hippocampal-Dependent Inhibitory Learning and Memory Processes in the Control of Eating and Drug Taking. Curr Pharm Des 26, 2334–2352. [DOI] [PubMed] [Google Scholar]
- Clasen MM, Sanon TV, Hempel BJ, Nelson KH, Kearns DN, Davidson TL, and Riley AL (2020b). Ad-libitum high fat diet consumption during adolescence and adulthood impacts the intravenous self-administration of cocaine in male Sprague-Dawley rats. Experimental and clinical psychopharmacology 28, 32–43. [DOI] [PubMed] [Google Scholar]
- Claus SP, Tsang TM, Wang Y, Cloarec O, Skordi E, Martin FP, Rezzi S, Ross A, Kochhar S, Holmes E, et al. (2008). Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol Syst Biol 4, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosloy SD (1973). D-serine transport system in Escherichia coli K-12. Journal of bacteriology 114, 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio L, Podda MV, Leone L, Piacentini R, Mastrodonato A, Cappelletti P, Sacchi S, Pollegioni L, Grassi C, and D'Ascenzo M (2013). Reduced D-serine levels in the nucleus accumbens of cocaine-treated rats hinder the induction of NMDA receptor-dependent synaptic plasticity. Brain 136, 1216–1230. [DOI] [PubMed] [Google Scholar]
- Curtis MM, Hu Z, Klimko C, Narayanan S, Deberardinis R, and Sperandio V (2014). The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell host & microbe 16, 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ascenzo M, Podda MV, and Grassi C (2014). The role of D-serine as co-agonist of NMDA receptors in the nucleus accumbens: relevance to cocaine addiction. Front Synaptic Neurosci 6, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Gil R, Cassello K, Morrissey K, Abi-Saab D, White J, Sturwold R, Bennett A, Karper LP, Zuzarte E, et al. (2000). IV glycine and oral D-cycloserine effects on plasma and CSF amino acids in healthy humans. Biol Psychiatry 47, 450–462. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, and Wanner BL (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97, 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delint-Ramirez I, Garcia-Oscos F, Segev A, and Kourrich S (2018). Cocaine engages a non-canonical, dopamine-independent, mechanism that controls neuronal excitability in the nucleus accumbens. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, and Relman DA (2005). Diversity of the human intestinal microbial flora. Science 308, 1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermann M, Pacheco AR, Jimenez AG, Russell RM, Cuesta S, Kumar A, Zhu W, Vale G, Martin SA, Raj P, et al. (2020). Endocannabinoids Inhibit the Induction of Virulence in Enteric Pathogens. Cell 183, 650–665 e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence CS, Zhou C, Luo F, and Xu L (2016). The Economic Burden of Prescription Opioid Overdose, Abuse, and Dependence in the United States, 2013. Medical care 54, 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KCF, Watson DJG, Billiras RI, Sicard DI, Dekeyne A, Rivet JM, Gobert A, and Millan MJ (2020). Comparative Pro-cognitive and Neurochemical Profiles of Glycine Modulatory Site Agonists and Glycine Reuptake Inhibitors in the Rat: Potential Relevance to Cognitive Dysfunction and Its Management. Mol Neurobiol 57, 2144–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, and Pace NR (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 104, 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JE, and Wolf-Watz H (2006). Protein delivery into eukaryotic cells by type III secretion machines. Nature 444, 567–573. [DOI] [PubMed] [Google Scholar]
- Ge SX, Son EW, and Yao R (2018). iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinformatics 19, 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghrist AC, and Stauffer GV (1995). The Escherichia coli glycine transport system and its role in the regulation of the glycine cleavage enzyme system. Microbiology (Reading) 141 (Pt 1), 133–140. [DOI] [PubMed] [Google Scholar]
- Gibson DL, Ma C, Rosenberger CM, Bergstrom KS, Valdez Y, Huang JT, Khan MA, and Vallance BA (2008). Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cellular microbiology 10, 388–403. [DOI] [PubMed] [Google Scholar]
- Giroud C, Michaud K, Sporkert F, Eap C, Augsburger M, Cardinal P, and Mangin P (2004). A fatal overdose of cocaine associated with coingestion of marijuana, buprenorphine, and fluoxetine. Body fluid and tissue distribution of cocaine and its metabolites determined by hydrophilic interaction chromatography-mass spectrometry(HILIC-MS). J Anal Toxicol 28, 464–474. [DOI] [PubMed] [Google Scholar]
- Goldfarb KC, Karaoz U, Hanson CA, Santee CA, Bradford MA, Treseder KK, Wallenstein MD, and Brodie EL (2011). Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Frontiers in microbiology 2, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Doverskog M, Enfors SO, and Haggstrom L (2002). Effect of glycine on the cell yield and growth rate of Escherichia coli: evidence for cell-density-dependent glycine degradation as determined by (13)C NMR spectroscopy. J Biotechnol 92, 237–249. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, and Yee BK (2013). Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence and pain. Nature reviews Drug discovery 12, 866–885. [DOI] [PubMed] [Google Scholar]
- Holmes E, Li JV, Marchesi JR, and Nicholson JK (2012). Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab 16, 559–564. [DOI] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, et al. (2009). In vivo cocaine experience generates silent synapses. Neuron 63, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project C (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong MY, Jang HM, and Kim DH (2019). High-fat diet causes psychiatric disorders in mice by increasing Proteobacteria population. Neurosci Lett 698, 51–57. [DOI] [PubMed] [Google Scholar]
- Kanahara N, Shimizu E, Ohgake S, Fujita Y, Kohno M, Hashimoto T, Matsuzawa D, Shirayama Y, Hashimoto K, and Iyo M (2008). Glycine and D: -serine, but not D: - cycloserine, attenuate prepulse inhibition deficits induced by NMDA receptor antagonist MK-801. Psychopharmacology (Berl) 198, 363–374. [DOI] [PubMed] [Google Scholar]
- Kapil S, Duseja A, Sharma BK, Singla B, Chakraborti A, Das A, Ray P, Dhiman RK, and Chawla Y (2016). Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol 31, 213–221. [DOI] [PubMed] [Google Scholar]
- Kawase T, Nagasawa M, Ikeda H, Yasuo S, Koga Y, and Furuse M (2017). Gut microbiota of mice putatively modifies amino acid metabolism in the host brain. Br J Nutr 117, 775–783. [DOI] [PubMed] [Google Scholar]
- Kim S, Covington A, and Pamer EG (2017). The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol Rev 279, 90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Walker DM, Calipari ES, Labonte B, Issler O, Pena CJ, Ribeiro EA, Russo SJ, and Nestler EJ (2016). Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Scientific reports 6, 35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su TP, and Bonci A (2013). Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell 152, 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszewska B, Felten SY, and Moynihan JA (1995). Alterations in cytokine and antibody production following chemical sympathectomy in two strains of mice. J Immunol 155, 4613–4620. [PubMed] [Google Scholar]
- Kumar A, Russell RM, Pifer R, Menezes-Garcia Z, Cuesta S, Narayanan S, MacMillan JB, and Sperandio V (2020). The Serotonin Neurotransmitter Modulates Virulence of Enteric Pathogens. Cell host & microbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, and Pfeiffer JK (2011). Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 334, 249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S, Kim H, Garcia-Perez I, Reza MM, Martin KA, Kundu P, Cox LM, Selkrig J, Posma JM, Zhang H, et al. (2019). The gut microbiota influences skeletal muscle mass and function in mice. Science translational medicine 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Liu CW, Yang Y, Hsiao YC, Ru H, and Lu K (2021). High-coverage metabolomics uncovers microbiota-driven biochemical landscape of interorgan transport and gut-brain communication in mice. Nature communications 12, 6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Vuong HE, Nusbaum DJ, Hsiao EY, Evans CJ, and Taylor AMW (2018). The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology 43, 2606–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Peterson DA, and Gordon JI (2006). Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124, 837–848. [DOI] [PubMed] [Google Scholar]
- Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, Zhang Y, Shen J, Pang X, Zhang M, et al. (2008). Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A 105, 2117–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, and Wu G (2018). Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 50, 29–38. [DOI] [PubMed] [Google Scholar]
- Li Z, Caron MG, Blakely RD, Margolis KG, and Gershon MD (2010). Dependence of serotonergic and other nonadrenergic enteric neurons on norepinephrine transporter expression. J Neurosci 30, 16730–16740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZS, Schmauss C, Cuenca A, Ratcliffe E, and Gershon MD (2006). Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knockout mice. J Neurosci 26, 2798–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, and Shi W (2014). featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. [DOI] [PubMed] [Google Scholar]
- Litvak Y, Byndloss MX, Tsolis RM, and Baumler AJ (2017). Dysbiotic Proteobacteria expansion: a microbial signature of epithelial dysfunction. Current opinion in microbiology 39, 1–6. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, Gu XH, Yang YJ, Yin XP, Xu LJ, and Wang W (2016). D-Serine in the nucleus accumbens region modulates behavioral sensitization and extinction of conditioned place preference. Pharmacol Biochem Behav 143, 44–56. [DOI] [PubMed] [Google Scholar]
- Lopez CA, Miller BM, Rivera-Chavez F, Velazquez EM, Byndloss MX, Chavez-Arroyo A, Lokken KL, Tsolis RM, Winter SE, and Baumler AJ (2016). Virulence factors enhance Citrobacter rodentium expansion through aerobic respiration. Science 353, 1249–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucerne KE, and Kiraly DD (2021). The role of gut-immune-brain signaling in substance use disorders. Int Rev Neurobiol 157, 311–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, and Finlay BB (2007). Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell host & microbe 2, 204. [DOI] [PubMed] [Google Scholar]
- Mabrouk OS, Han JL, Wong JT, Akil H, Kennedy RT, and Flagel SB (2018). The in Vivo Neurochemical Profile of Selectively Bred High-Responder and Low-Responder Rats Reveals Baseline, Cocaine-Evoked, and Novelty-Evoked Differences in Monoaminergic Systems. ACS Chem Neurosci 9, 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manca C, Shen M, Boubertakh B, Martin C, Flamand N, Silvestri C, and Di Marzo V (2020). Alterations of brain endocannabinoidome signaling in germ-free mice. Biochim Biophys Acta Mol Cell Biol Lipids 1865, 158786. [DOI] [PubMed] [Google Scholar]
- Mardinoglu A, Shoaie S, Bergentall M, Ghaffari P, Zhang C, Larsson E, Backhed F, and Nielsen J (2015). The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol 11, 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques BL, Oliveira-Lima OC, Carvalho GA, de Almeida Chiarelli R, Ribeiro RI, Parreira RC, da Madeira Freitas EM, Resende RR, Klempin F, Ulrich H, et al. (2020). Neurobiology of glycine transporters: From molecules to behavior. Neurosci Biobehav Rev 118, 97–110. [DOI] [PubMed] [Google Scholar]
- Martins FH, Kumar A, Abe CM, Carvalho E, Nishiyama-Jr M, Xing C, Sperandio V, and Elias WP (2020). EspFu-Mediated Actin Assembly Enhances Enteropathogenic Escherichia coli Adherence and Activates Host Cell Inflammatory Signaling Pathways. mBio 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Kibe R, Ooga T, Aiba Y, Sawaki E, Koga Y, and Benno Y (2013). Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front Syst Neurosci 7, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel TK, Jarvis KG, Donnenberg MS, and Kaper JB (1995). A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci U S A 92, 1664–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendrick G, and Graziane NM (2020). Drug-Induced Conditioned Place Preference and Its Practical Use in Substance Use Disorder Research. Frontiers in behavioral neuroscience 14, 582147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckel KR, and Kiraly DD (2019). A potential role for the gut microbiome in substance use disorders. Psychopharmacology (Berl) 236, 1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michail S, Lin M, Frey MR, Fanter R, Paliy O, Hilbush B, and Reo NV (2015). Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol 91, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander A, and Soderpalm B (2005). Glycine receptors regulate dopamine release in the rat nucleus accumbens. Alcoholism, clinical and experimental research 29, 17–26. [DOI] [PubMed] [Google Scholar]
- Moreira CG, Russell R, Mishra AA, Narayanan S, Ritchie JM, Waldor MK, Curtis MM, Winter SE, Weinshenker D, and Sperandio V (2016). Bacterial Adrenergic Sensors Regulate Virulence of Enteric Pathogens in the Gut. MBio 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira CG, and Sperandio V (2012). Interplay between the QseC and QseE bacterial adrenergic sensor kinases in Salmonella enterica serovar Typhimurium pathogenesis. Infection and immunity 80, 4344–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira CG, Weinshenker D, and Sperandio V (2010). QseC mediates Salmonella enterica serovar typhimurium virulence in vitro and in vivo. Infection and immunity 78, 914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham BD, Kaddurah-Daouk R, and Mazmanian SK (2020). Gut microbial molecules in behavioural and neurodegenerative conditions. Nat Rev Neurosci 21, 717–731. [DOI] [PubMed] [Google Scholar]
- Neumann PA, Wang Y, Yan Y, Wang Y, Ishikawa M, Cui R, Huang YH, Sesack SR, Schluter OM, and Dong Y (2016). Cocaine-Induced Synaptic Alterations in Thalamus to Nucleus Accumbens Projection. Neuropsychopharmacology 41, 2399–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Pinard E, Alberati D, Wettstein JG, Spealman RD, and Kantak KM (2012). Inhibiting glycine transporter-1 facilitates cocaine-cue extinction and attenuates reacquisition of cocaine-seeking behavior. Drug and alcohol dependence 122, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, and Pettersson S (2012). Host-gut microbiota metabolic interactions. Science 336, 1262–1267. [DOI] [PubMed] [Google Scholar]
- Ning T, Gong X, Xie L, and Ma B (2017). Gut Microbiota Analysis in Rats with Methamphetamine-Induced Conditioned Place Preference. Frontiers in microbiology 8, 1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson Y, Hoifodt Lido H, Danielsson K, Ericson M, and Soderpalm B (2021). Effects of systemic glycine on accumbal glycine and dopamine levels and ethanol intake in male Wistar rats. J Neural Transm (Vienna) 128, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W.H. (2020). Cocaine.
- Pang Z, Zhou G, Chong J, and Xia J (2021). Comprehensive Meta-Analysis of COVID-19 Global Metabolomics Datasets. Metabolites 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei JC, Hung WL, Lin BX, Shih MH, Lu LY, Luo DZ, Tai HC, Studer V, Min MY, and Lai WS (2019). Therapeutic potential and underlying mechanism of sarcosine (N-methylglycine) in N-methyl-D-aspartate (NMDA) receptor hypofunction models of schizophrenia. J Psychopharmacol 33, 1288–1302. [DOI] [PubMed] [Google Scholar]
- Prus AJ, James JR, and Rosecrans JA (2009). Conditioned Place Preference. In Methods of Behavior Analysis in Neuroscience, nd, and Buccafusco JJ, eds. (Boca Raton (FL)). [Google Scholar]
- Rasko DA, Moreira CG, Li de R, Reading NC, Ritchie JM, Waldor MK, Williams N, Taussig R, Wei S, Roth M, et al. (2008a). Targeting QseC signaling and virulence for antibiotic development. Science 321, 1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasko DA, Rosovitz MJ, Myers GS, Mongodin EF, Fricke WF, Gajer P, Crabtree J, Sebaihia M, Thomson NR, Chaudhuri R, et al. (2008b). The pangenome structure of Escherichia coli: comparative genomic analysis of E. coli commensal and pathogenic isolates. Journal of bacteriology 190, 6881–6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzatti G, Lopetuso LR, Gibiino G, Binda C, and Gasbarrini A (2017). Proteobacteria: A Common Factor in Human Diseases. Biomed Res Int 2017, 9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JC, and Oxender DL (1973). Transport systems for alanine, serine, and glycine in Escherichia coli K-12. Journal of bacteriology 116, 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom O, Liu Y, Liu Z, Zhao Y, Wu J, Ghrayeb A, Villacorta L, Fan Y, Chang L, Wang L, et al. (2020). Glycine-based treatment ameliorates NAFLD by modulating fatty acid oxidation, glutathione synthesis, and the gut microbiome. Science translational medicine 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks MG, Veiga P, Reeves AZ, Lavoie S, Yasuda K, Asano Y, Yoshihara K, Michaud M, Wardwell-Scott L, Gallini CA, et al. (2017). QseC inhibition as an antivirulence approach for colitis-associated bacteria. Proc Natl Acad Sci U S A 114, 142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakic B (2019). Cerebrospinal fluid collection in laboratory mice: Literature review and modified cisternal puncture method. J Neurosci Methods 311, 402–407. [DOI] [PubMed] [Google Scholar]
- Salavrakos M, Leclercq S, De Timary P, and Dom G (2021). Microbiome and substances of abuse. Prog Neuropsychopharmacol Biol Psychiatry 105, 110113. [DOI] [PubMed] [Google Scholar]
- Scorza C, Piccini C, Martinez Busi M, Abin Carriquiry JA, and Zunino P (2019). Alterations in the Gut Microbiota of Rats Chronically Exposed to Volatilized Cocaine and Its Active Adulterants Caffeine and Phenacetin. Neurotox Res 35, 111–121. [DOI] [PubMed] [Google Scholar]
- Serafine KM, Bentley TA, Koek W, and France CP (2015). Eating high fat chow, but not drinking sucrose or saccharin, enhances the development of sensitization to the locomotor effects of cocaine in adolescent female rats. Behav Pharmacol 26, 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafine KM, Labay C, and France CP (2016). Dietary supplementation with fish oil prevents high fat diet-induced enhancement of sensitivity to the locomotor stimulating effects of cocaine in adolescent female rats. Drug and alcohol dependence 165, 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin NR, Whon TW, and Bae JW (2015). Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33, 496–503. [DOI] [PubMed] [Google Scholar]
- Smith LN, Penrod RD, Taniguchi M, and Cowan CW (2016). Assessment of Cocaine-induced Behavioral Sensitization and Conditioned Place Preference in Mice. Journal of visualized experiments : JoVE, 53107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Som P, Oster ZH, Wang GJ, Volkow ND, and Sacker DF (1994). Spatial and temporal distribution of cocaine and effects of pharmacological interventions: wholebody autoradiographic microimaging studies. Life Sci 55, 1375–1382. [DOI] [PubMed] [Google Scholar]
- Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, Felin J, Perkins R, Boren J, Oresic M, et al. (2010). The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res 51, 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe GE, Ward H, Mwamburi M, Dinh D, Bhalchandra S, Wanke C, and Kane AV (2014). Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. Journal of studies on alcohol and drugs 75, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HE, Yano JM, Fung TC, and Hsiao EY (2017). The Microbiome and Host Behavior. Annual review of neuroscience 40, 21–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargel RJ, Hadur CA, and Neuhaus FC (1971). Mechanism of D-cycloserine action: transport mutants for D-alanine, D-cycloserine, and glycine. Journal of bacteriology 105, 1028–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargel RJ, Shadur CA, and Neuhaus FC (1970). Mechanism of D-cycloserine action: transport systems for D-alanine, D-cycloserine, L-alanine, and glycine. Journal of bacteriology 103, 778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt DD, and Demoss RD (1975). Effect of microflora on the free amino acid distribution in various regions of the mouse gastrointestinal tract. Appl Microbiol 30, 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, and Siuzdak G (2009). Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A 106, 3698–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, et al. (2013). Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339, 708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicz A, Ortiz JA, Casas AI, Freitas AE, Lopez MG, and Ruiz-Nuno A (2016). Data supporting the rat brain sample preparation and validation assays for simultaneous determination of 8 neurotransmitters and their metabolites using liquid chromatography-tandem mass spectrometry. Data Brief 7, 714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. (2011). Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Bishop EE, Kennedy SM, Simpson SA, and Pechacek TF (2015). Annual healthcare spending attributable to cigarette smoking: an update. American journal of preventive medicine 48, 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Xie Z, Wang H, Shen Z, Guo Y, Gao Y, Chen X, Wu Q, Li X, and Wang K (2017). Bacterial Diversity of Intestinal Microbiota in Patients with Substance Use Disorders Revealed by 16S rRNA Gene Deep Sequencing. Scientific reports 7, 3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FY, Lee YS, Cherng CG, Cheng LY, Chang WT, Chuang JY, Kao GS, and Yu L (2013). D-cycloserine, sarcosine and D-serine diminish the expression of cocaine-induced conditioned place preference. J Psychopharmacol 27, 550–558. [DOI] [PubMed] [Google Scholar]
- Zafra F, Ibanez I, Bartolome-Martin D, Piniella D, Arribas-Blazquez M, and Gimenez C (2017). Glycine Transporters and Its Coupling with NMDA Receptors. Adv Neurobiol 16, 55–83. [DOI] [PubMed] [Google Scholar]
- Zampieri M, Horl M, Hotz F, Muller NF, and Sauer U (2019). Regulatory mechanisms underlying coordination of amino acid and glucose catabolism in Escherichia coli. Nature communications 10, 3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarivach R, Vuckovic M, Deng W, Finlay BB, and Strynadka NC (2007). Structural analysis of a prototypical ATPase from the type III secretion system. Nat Struct Mol Biol 14, 131–137. [DOI] [PubMed] [Google Scholar]
- Zhang HX, Hyrc K, and Thio LL (2009a). The glycine transport inhibitor sarcosine is an NMDA receptor co-agonist that differs from glycine. The Journal of physiology 587, 3207–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HX, Lyons-Warren A, and Thio LL (2009b). The glycine transport inhibitor sarcosine is an inhibitory glycine receptor agonist. Neuropharmacology 57, 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, and Gill SR (2013). Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 57, 601–609. [DOI] [PubMed] [Google Scholar]
- Zimmerman JL (2012). Cocaine intoxication. Critical care clinics 28, 517–526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Bacterial strains and Oligonucleotide primers used in this study. Related to bacterial strains and growth conditions in STAR methods section.
Data Availability Statement
The raw data of RNA sequencing was deposited at GEO database. The access number is listed in the key resources table.
This study did not report the original code.
Any additional information required to reanalyze the data reported in this paper is available upon request of the lead contact.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-EspA-polyclonal, rabbit | UT Southwestern, Vanessa Sperandio | N/A |
| Anti-EspB-polyclonal, rabbit | UT Southwestern, Vanessa Sperandio | N/A |
| Anti-rabbit-HRP, goat | BioRad | Cat# 1706515 |
| Bacterial and Virus Strains | ||
| E. coli HS | Center for Vaccine Development at the University of Maryland School of Medicine | N/A |
| C. rodentium DBS100 | Tufts University, John Leong | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Dulbecco eagle medium, low glucose | Invitrogen | Cat# 11885-084 |
| Cocaine Hydrochloride | Sigma-Aldrich | Cat#C5776 |
| LB agar, Miller | Fisher Scientific | Cat# BP1425 |
| LB broth, Miller | Fisher Scientific | Cat# BP9723 |
| Ampicillin powder | Sigma-Aldrich | Cat# A9518 |
| Naladixic acid power | Fisher Scientific | Cat# BP908 |
| L-(+)-Arabinose | Sigma-Aldrich | Cat# A3256 |
| Dextran sulfate sodium salt | MP Biomedicals | Cat#021601109 |
| Phusion High fidelity PCR master mix | Thermo Fisher | Cat# F-531L |
| Random primers | Invitrogen | Cat# 48190-011 |
| Super script II reverse transcriptase | Invitrogen | Cat# 18064-014 |
| Sybr green master mix | Thermo Fisher | Cat# 4309155 |
| Sarcosine | Sigma-Aldrich | Cat#131776 |
| Glycine Hydrochloride | Sigma-Aldrich | Cat# G2879 |
| Trizol reagent | Thermo Fisher | Cat# 1559618 |
| Chloroform | IBM Scientific | Cat# 67-66-3 |
| dNTP mix 10mM | Invitrogen | Cat# 18-427-088 |
| Bovine Serum Albumin | Sigma-Aldrich | Cat# A2153 |
| Dopamine Hydrochloride | Sigma-Aldrich | Cat# H8502 |
| 6-hydroxydopamine Hydrochloride | Sigma-Aldrich | Cat# H4381 |
| RNAlater-ICE | Fisher Scientific | Cat# 44-275-75 |
| Protease inhibitor cocktail | Sigma-Aldrich | Cat# P8849-5 |
| Dithiothreitol (DTT) | Fisher Scientific | Cat# BP172-25 |
| Formaldehyde | Fisher Scientific | Cat# BP539 |
| Brain heart infusion agar | Becton Dickinson | Cat# 2418102 |
| Dextran sulfate sodium salt | Fisher Scientific | Cat# 02-160-1109 |
| Metronidazole | MP biomedicals | Cat# 0215571025 |
| Neomycin trisulfate salt hydrate | Sigma-Aldrich | Cat# N5285 |
| Vancomycin hydrochloride | Research Products International | Cat# V06500 |
| Critical Commercial Assays | ||
| RNeasy Power Microbiome | Qiagen | Cat# 26000-50 |
| RNeasy Plus Mini Kit | Qiagen | Cat# 74134 |
| RiboPure RNA isolation Kit | Ambion | Cat# 1405072 |
| GenElute Plasmid Miniprep | Sigma | Cat# PLN350-1KT |
| QIAquick PCR purification kit | Qiagen | Cat# 28906 |
| Glycine Assay Kit | Sigma-Aldrich | Cat# MAK261 |
| Deposited Data | ||
| RNA sequencing data | https://www.ncbi.nlm.nih.gov/geo/ | GSE212421 |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J mice | The Jackson laboratory | Cat# 000664 |
| Oligonucleotides | ||
| Primers for qRT-PCR used in this study are listed in Table S1 | This paper | N/A |
| Software and Algorithms | ||
| Prism 6 | GraphPad | N/A |
| QuantStudio real-time PCR software v1.3 | Thermo Fisher | N/A |
| Image Lab 5.2.1 | BioRad | N/A |
| iDEP.91 | http://bioinformatics.sdstate.edu/idep/ | N/A |
| CLC Bio microbial genomics module | https://www.qiagenbioinformatics.com/plugins/clc-microbial-genomics-module/ | N/A |
| MetaboAnalyst 5.0 | https://www.metaboanalyst.ca/ | N/A |
| Fiji Image J | https://fiji.sc/ | N/A |
| Fusion 6.5v | Omnitech Electronics Incorporated | N/A |
| MultiQuant 3.0.3. Software | https://sciex.com/products/software/multiquant-software | N/A |