Abstract
Introduction
Guidelines have improved the management of prosthetic joint infections (PJI). However, it is necessary to reassess the incidence and risk factors for treatment failure (TF) of Staphylococcus aureus PJI (SA-PJI) including functional loss, which has so far been neglected as an outcome.
Methods
A retrospective cohort study of SA-PJI was performed in 19 European hospitals between 2014 and 2016. The outcome variable was TF, including related mortality, clinical failure and functional loss both after the initial surgical procedure and after all procedures at 18 months. Predictors of TF were identified by logistic regression. Landmark analysis was used to avoid immortal time bias with rifampicin when debridement, antibiotics and implant retention (DAIR) was performed.
Results
One hundred twenty cases of SA-PJI were included. TF rates after the first and all surgical procedures performed were 32.8% and 24.2%, respectively. After all procedures, functional loss was 6.0% for DAIR and 17.2% for prosthesis removal. Variables independently associated with TF for the first procedure were Charlson ≥ 2, haemoglobin < 10 g/dL, bacteraemia, polymicrobial infection and additional debridement(s). For DAIR, TF was also associated with a body mass index (BMI) > 30 kg/m2 and delay of DAIR, while rifampicin use was protective. For all procedures, the variables associated with TF were haemoglobin < 10 g/dL, hip fracture and additional joint surgery not related to persistent infection.
Conclusions
TF remains common in SA-PJI. Functional loss accounted for a substantial proportion of treatment failures, particularly after prosthesis removal. Use of rifampicin after DAIR was associated with a protective effect. Among the risk factors identified, anaemia and obesity have not frequently been reported in previous studies.
Trial registration
This study is registered at clinicaltrials.gov, registration no. NCT03826108.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00701-0.
Keywords: Prosthetic joint infection, Staphylococcus aureus, Outcome, Clinical failure, Functional failure
Plain Language Summary
Staphylococcus aureus is one of the most virulent bacteria and frequently causes prosthetic joint infections.
Knowledge of the treatment of this type of infection has advanced in recent years, and treatment guidelines have led to improved management. Typically, the successful treatment of these infections has been determined by clinical cure, that is, the symptoms of infection have disappeared, but has not taken into account loss of function (such as significant difficulties walking), which is critical for the patient’s quality of life. Our aim in this study was to evaluate the success of current management strategies for S. aureus prosthetic joint infection, including recovery of functionality, and the factors that predict why some of these infections are not cured, to identify areas for improvement.
In a multinational cohort of 128 patients with S. aureus prosthetic joint infection, rates of treatment failure were found to be high, with significant rates of loss of function, especially when the prosthesis needed to be removed. Loss of function was less frequent when the infection was initially treated with surgical cleaning without removal of the prosthesis, even when this procedure failed at first. We found that anaemia and obesity were associated with lower treatment success, and that the probability of treatment success increased when surgical cleaning without prosthesis removal was performed early, and when the antibiotic rifampicin was used in combination with another antibiotic.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00701-0.
Key Summary Points
| Why carry out this study? |
| Staphylococcus aureus has been associated with a higher rate of prosthetic joint infection (PJI) and treatment failure compared with other pathogens. |
| Changes in the management of S. aureus PJI (SA-PJI) in recent decades make it necessary to reassess the incidence and risk factors for treatment failure, including functional loss which has previously been neglected as an outcome. |
| What was learned from the study? |
| A considerable proportion of SA-PJIs failed after initial surgical treatments, although a substantial part of them can be rescued by additional procedures. |
| Significant functional loss must be considered in addition to clinical failure, mainly in patients in whom the prosthesis was removed, with no possibility of recovery. |
| In patients managed with debridement, antibiotic and prosthesis retention (DAIR), the loss of function was lower even if this procedure fails. |
| Anaemia and obesity were risk factors for treatment failure that are rarely reported. |
| The importance of some risk factors for treatment failure was reinforced, including the protective role of rifampicin-based treatment in DAIR. |
Digital Features
This article is published with digital features, including [list digital features available e.g. a video abstract and slide deck], to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.21163246.
Introduction
Staphylococcus aureus is widely recognized as a key microorganism causing prosthetic joint infection (PJI), and has been associated with higher rates of treatment failure (TF) compared with other aetiologies [1]. However, studies evaluating TF have traditionally focused on outcomes related primarily with control of the infection [2–8], while functional outcomes have been given little consideration. A definition of TF that integrates clinical and functional aspects could provide a more realistic measurement of the consequences of S. aureus-PJI (SA-PJI).
TF rates may vary according to patient characteristics, infection type (early, delayed or late) and surgical management. Implementation of the recommendations included in the guidelines for the management of PJI [9, 10] may have positively influenced changes in the rates and predictors of TF in general, and particularly in patients managed with debridement, antibiotics and implant retention (DAIR). For SA-PJI, however, there is little published data on the outcomes associated with different therapeutic strategies and their predictors, and the timing and role of rifampicin in combination with other antibiotics, particularly in patients undergoing DAIR, remain controversial.
Assessments of TF tend to be analysed for the first surgical procedure performed to treat the infection, which is important to evaluate the outcomes associated for the decision about which procedure must be performed. However, additional procedures are usually performed if that first procedure fails to cure the infection, which may rescue some patients but could also affect the functional outcome. Evaluations of TF considering all the procedures performed are not usually made. Such an evaluation would provide a more global view of the final consequences of SA-PJI.
The objectives of this study were to provide updated rates of TF in SA-PJI, taking into account clinical and functional aspects, and to investigate the predictors of TF according to different management strategies. The assessment of both objectives will be carried out for both the first surgical procedure and all additional procedures performed on patients in whom the first one failed.
Methods
Study Design, Sites and Period
This study is part of the ARTHR-IS project (registered at clinicaltrials.gov: NCT03826108) and was designed to evaluate the incidence, risk factors and predictors of SA-PJI TF after primary hip and knee arthroplasty. A retrospective cohort study was conducted in 19 European hospitals to identify patients older than 18 years who received a primary arthroplasty between 1 January 2014 and 31 December 2016 and developed post-surgical knee or hip PJI due to S. aureus within the first year after the procedure. The participating sites, located in Spain, Italy, France, Germany, UK and the Netherlands, were selected using the CLIN-NET network (https://www.combacte.com/about/clin-net/), based on their research experience and data collection capability.
The STROBE recommendations were followed for the reporting of the study (Supplementary Table S1).
Participants
Patients with post-surgical hip or knee PJI due to S. aureus diagnosed within the first year after primary arthroplasty were included. The criteria used to define SA-PJI were as follows: presence of at least one sign or symptom of PJI, including joint pain and/or swelling, or a sinus tract communicating with the prosthesis; and the isolation of S. aureus from (a) ≥ one joint aspirate culture, (b) ≥ two periprosthetic tissue samples and (c) blood cultures with no other obvious source of infection. The patients were identified by reviewing medical records from microbiological laboratory databases, local PJI surveillance databases and discharge reports.
Variables and Definitions
The primary outcome variable was TF until month 18 after the first surgical procedure performed, and was analysed separately both for the first surgical procedure performed (mimicking an intention-to-treat analysis for that procedure) and for all procedures performed (including those performed after failure of the first one). TF was defined as a composite variable including SA-PJI-related mortality, clinical failure and functional failure. Clinical failure was defined as persistence or recurrence of signs or symptoms of infection. For the analysis of the first procedure, this also included the need for an additional course of antibiotics beyond the initial one, the need to use long-term suppressive antibiotic therapy and need of prosthesis removal if not performed as the initial surgical procedure. Functional failure was defined as impeded or significantly impaired walking due to prosthetic loosening or the need to perform a Girdlestone procedure or arthrodesis. Finally, TF was also analysed in the subgroup of patients who underwent DAIR as the first procedure.
Potential predictors of TF were selected according to previous studies [2–8] and additional hypotheses developed by the project team, and are included in Tables 1 and 2.
Table 1.
Characteristics of 128 patients with Staphylococcus aureus prosthetic joint infections (SA-PJI)
| Variables | No. of patients (percentage), except where specified |
|---|---|
| Spain | 52 (40.6) |
| France | 15 (11.7) |
| Germany | 7 (5.5) |
| United Kingdom | 16 (12.5) |
| Italy | 17 (13.3) |
| The Netherlands | 21 (16.4) |
| Male sex | 65 (50.8) |
| Born abroad | 2 (1.5) |
| Age in years; median (IQR) | 73 (59.3–80.8) |
| Body mass index units; median (IQR) | 31.2 (25.6–35.1) |
| ASA 3-4 assessment for primary arthroplasty | 64 (50%) |
| Charlson comorbidity index; median (IQR) | 1 (1–2) |
| Comorbidities | |
| Chronic heart failure | 17 (13.3) |
| Chronic pulmonary disease | 30 (23.4) |
| Diabetes mellitus | 29 (22.7) |
| Chronic renal insufficiency | 5 (3.9) |
| Reason for arthroplasty | |
| Osteoarthritis | 83 (64.8) |
| Hip fracture | 34 (26.6) |
| Osteonecrosis | 5 (3.9) |
| Others | 6 (4.6) |
| Type of arthroplasty | |
| Total hip arthroplasty | 50 (39.1) |
| Partial hip arthroplasty | 27 (21.1) |
| Total knee arthroplasty | 47 (36.7) |
| Partial knee arthroplasty | 4 (3.1) |
| Methicillin-resistant Staphylococcus aureus | 28 (21.9) |
| Polymicrobial infection | 36 (28.1) |
| Bacteraemia | 25 (19.5) |
| Days from arthroplasty to onset of SA-PJI symptoms; median (IQR) | 24 (15–36) |
| Symptoms and signs of SA-PJI | |
| Fever | 32 (25.0) |
| Joint pain | 70 (54.7) |
| Suppuration | 89 (69.5) |
| Cellulitis | 38 (29.7) |
| Wound dehiscence | 56 (43.8) |
| Articular swelling | 18 (14.1) |
| Sinus tract | 8 (6.3) |
| Laboratory data at diagnosis of SA-PJI; median (IQR) | |
| Haemoglobin (g/dL) | 10.5 (9.5–11.4) |
| Blood leucocytes (cells/μL) | 9600 (7550–12,950) |
| C-reactive protein (mg/L) | 84.5 (23.2–224.2) |
| Erythrocyte sedimentation rate (mm/h) | 68.5 (39.8–92.3) |
| Type of initial surgical procedure performed to treat SA-PJI | |
| Debridement and prosthesis retention | 99 (77.3) |
| Partial removal and reimplantation | 6 (4.7) |
| One-stage replacement and reimplantation | 4 (3.1) |
| Two-stage replacement and reimplantation | 13 (10.2) |
| Girdlestone procedure (hip resection arthroplasty) | 6 (4.7) |
| Days from onset of SA-PJI symptoms to first surgical procedure performed; median (IQR) | 4 (1–11) |
| Days of antibiotic treatment to treat PJI; median (IQR) | |
| Empirical intravenous | 2 (1–4) |
| Target intravenous | 16 (11–34) |
| Target oral | 50 (33–80) |
| Total | 73 (56–96) |
| Target oral antibiotics | |
| Rifampicin | 103 (80.5) |
| Fluoroquinolones | 73 (63.5) |
| Clindamycin | 17 (14.8) |
| Trimethoprim–sulfamethoxazole | 15 (13.0) |
| Tetracyclines | 3 (2.6) |
| Linezolid | 4 (3.5) |
| Others | 3 (2.6) |
Table 2.
Bivariate analysis of potential predictors of treatment failure among patients with SA-PJI: after the first surgical procedure, after DAIR, and after all surgical procedures performed
| Failure after the initial surgical procedure (n = 128; failures = 42) | Failure after DAIR as first surgical procedure (n = 99; failures = 31) | Failure at 18 months after all procedures performed (n = 128; failures = 31) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | No. failure (%) | Relative risk (95% CI) | p-Value | No. failure (%) | Relative risk (95% CI) | p-Value | No. failure (%) | Relative risk (95% CI) | p-Value |
| Age | |||||||||
| ≥ 80 years | 13 (40.6) | 1.6 (0.7–3.6) | 0.277 | 8 (38.1) | 1.5 (0.5–4.1) | 0.450 | 12 (37.5) | 2.4 (1.1–5.8) | 0.043 |
| < 80 years | 29 (30.2) | 23 (29.5) | 19 (19.8) | ||||||
| Sex | |||||||||
| Male | 22 (33.8) | 1.1 (0.5–2.3) | 0.800 | 15 (30.6) | 1.07 (0.4–2.5) | 0.880 | 17 (26.2) | 1.2 (0.5–2.8) | 0.378 |
| Female | 20 (31.7) | 16 (32.0) | 14 (22.2) | ||||||
| Charlson index ≥ 2 | |||||||||
| Yes | 21 (47.7) | 2.7 (1.3–5.9) | 0.009 | 16 (44.4) | 2.6 (1.1–6.1) | 0.033 | 15 (34.1) | 2.2 (0.9–5.0) | 0.059 |
| No | 21 (25.0) | 15 (23.8) | 16 (19.0) | ||||||
| Haemoglobin < 10 mg/dl | |||||||||
| Yes | 23 (48.9) | 3.1 (1.4–6.7) | 0.003 | 18 (50.0) | 3.8 (1.6–9.4) | 0.002 | 18 (38.3) | 3.2 (1.4–7.5) | 0.005 |
| No | 19 (23.5) | 13 (20.6) | 13 (16.0) | ||||||
| Leukocytes ≥ 7500/μL | |||||||||
| Yes | 29 (31.9) | 0.9 (0.4–2.2) | 0.882 | 19 (27.9) | 1.9 (0.5–7.6) | 0.383 | 24 (26.4) | 2.3 (0.7–7.4) | 0.211 |
| No | 10 (33.3) | 9 (37.5) | 4 (13.3) | ||||||
| C-reactive protein ≥ 150 mg/La | |||||||||
| Yes | 18 (36.7) | 1.3 (0.6–2.8) | 0.478 | 10 (31.3) | 1.0 (0.4–2.5) | 0.992 | 15 (30.6) | 1.7 (0.7–3.8) | 0.221 |
| No | 22 (30.6) | 19 (31.1) | 15 (20.8) | ||||||
| Erythrocyte sedimentation rate ≥ 60 mm/hb | |||||||||
| Yes | 9 (42.9) | 2.1 (0.5–8.6) | 0.261 | 3 (37.5) | 1.3 (0.2–8.6) | 0.557 | 8 (38.1) | 8.6 (0.9–78.7) | 0.051 |
| No | 4 (26.7) | 4 (30.8) | 1 (6.7) | ||||||
| Body mass index > 30 kg/m2 | |||||||||
| Yes | 24 (34.8) | 1.2 (0.6–2.5) | 0.608 | 21 (36.8) | 1.9 (0.8–4.5) | 0.122 | 15 (21.8) | 0.7 (0.3–1.7) | 0.479 |
| No | 18 (30.5) | 10 (23.8) | 16 (27.1) | ||||||
| Hip fracture as the reason for arthroplasty | |||||||||
| Yes | 17 (50.0) | 2.7 (1.2–6.2) | 0.010 | 12 (48.0) | 2.7 (1.04–6.8) | 0.035 | 17 (50.0) | 5.7 (2.3–13.7) | 0.001 |
| No | 25 (26.6) | 19 (25.7) | 14 (14.9) | ||||||
| Type of arthroplasty | |||||||||
| Total knee arthroplasty | 12 (25.5) | Ref | 10 (27.0) | Ref | 7 (14.9) | Ref | |||
| Total hip arthroplasty | 14 (28.0) | 1.1 (0.5–2.8) | 0.784 | 11 (26.8) | 0.9 (0.4–2.7) | 0.984 | 8 (16.0) | 1.1 (0.4–3.3) | 0.880 |
| Partial hip arthroplasty | 14 (51.9) | 3.1 (1.1–8.5) | 0.025 | 9 (50.0) | 2.7 (0.8–8.7) | 0.097 | 14 (51.9) | 6.1 (2.0–18.5) | 0.001 |
| Partial knee arthroplasty | 2 (50.0) | 2.9 (0.4–23.0) | 0.310 | 1 (33) | 1.3 (0.1–16.6) | 0.815 | 2 (50.0) | 5.7 (0.7–47.5) | 0.107 |
| Radiological signs of infectionc | |||||||||
| Yes | 6 (42.8) | 4.6 (0.8–26.0) | 0.080 | 5 (44.0) | 1.76 (0.2–7.7) | 0.866 | 2 (20.0) | 0.56 (0.1–2.8) | 0.479 |
| No | 20 (35.1) | 14 (32.6) | 19 (31.1) | ||||||
| Fever > 38 °C: | |||||||||
| Yes | 13 (40.6) | 1.6 (0.7–3.6) | 0.190 | 9 (40.9) | 1.7 (0.6–4.6) | 0.199 | 10 (31.3) | 1.6 (0.7–3.9) | 0.200 |
| No | 29 (30.2) | 22 (28.6) | 21 (21.9) | ||||||
| Sinus tract at diagnosis: | |||||||||
| Yes | 6 (75.0) | 7 (1.3–36.0) | 0.015 | 2 (100) | – | 0.096 | 6 (75.0) | 11.4 (2.2–59.9) | 0.003 |
| No | 36 (30.0) | 29 (29.9) | 25 (20.8) | ||||||
| Methicillin-resistant S. aureus | |||||||||
| Yes | 13 (46.4) | 2.1 (0.9–5.0) | 0.068 | 8 (40.0) | 1.6 (0.6–4.5) | 0.249 | 11 (39.3) | 2.6 (1.0–6.4) | 0.035 |
| No | 29 (29.0) | 23 (29.1) | 20 (20.0) | ||||||
| Polymicrobial infection | |||||||||
| Yes | 15 (41.7) | 1.7 (0.8–3.8) | 0.130 | 13 (40.6) | 1.8 (0.7–4.5) | 0.160 | 11 (30.6) | 1.6 (0.6–3.7) | 0.205 |
| No | 27 (29.3) | 18 (26.9) | 20 (21.7) | ||||||
| Presence of bacteraemia | |||||||||
| Yes | 13 (52.0) | 2.7 (1.1–6.7) | 0.023 | 9 (52.9) | 3.1 (1.05–8.9) | 0.037 | 10 (40.0) | 2.6 (1.0–6.6) | 0.040 |
| No | 29 (28.2) | 22 (26.8) | 21 (20.4) | ||||||
| Type of first surgical therapy | |||||||||
| Debridement or partial replacement | 32 (30.5) | 0.5 (0.2–1.4) | 0.229 | – | – | – | 22 (21.0) | 0.4 (0.2–1.1) | 0.061 |
| Total prosthesis replacement | 10 (43.5) | 9 (39.1) | |||||||
| Polyethylene/mobile component replacementd | |||||||||
| Yes | – | – | – | 14 (24.1) | 0.4 (0.2–1.1) | 0.067 | – | – | – |
| No | 17 (41.5) | ||||||||
| Days from symptom onset to surgery > 21 | |||||||||
| Yes | – | – | – | 6 (54.5) | 3.3 (0.8–11.1) | 0.081 | – | – | |
| No | 5 (45.5) | ||||||||
| Appropriate indication for DAIRe | |||||||||
| Yes | – | – | – | 0 (20.8) | 0.38 (0.15–0.92) | 0.029 | – | – | – |
| No | 21 (51.0) | ||||||||
| Need for additional surgery not due to persistent infectionf | |||||||||
| Yes | 16 (55.2) | 3.45 (1.5–8.1) | 0.004 | 11 (55.0) | 3.6 (1.3–9.9) | 0.013 | 13 (44.8) | 3.6 (1.5–8.9) | 0.005 |
| No | 26 (26.3) | 9 (45.0) | 18 (18.2) | ||||||
| Additional procedures due to failure of first procedure | |||||||||
| 0 | – | – | – | – | – | 12 (14.0) | Ref | ||
| 1 | – | 3 (21.4) | 1.68 (0.4–6.9) | 0.471 | |||||
| 2 | 8 (50.0) | 6.17 (1.9–19.5) | 0.002 | ||||||
| 3 | 3 (60.0) | 9.25 (1.4–61.2) | 0.021 | ||||||
| ≥ 4 | 5 (71.4) | 12.3 (1.0–146.8) | 0.047 | ||||||
| Inadequate empirical antimicrobial therapyg | |||||||||
| Yes | 5 (55.6) | 3.5 (0.9–14.3) | 0.077 | 5 (55.6) | 4.8 (1.1–20.5) | 0.038 | 4 (44.4) | 3.4 (0.8–14.1) | 0.096 |
| No | 22 (26.2) | 13 (20.6) | 16 (19.0) | ||||||
| Rifampicin used in antibiotic regimen | |||||||||
| Yes | 31 (30.1) | 0.5 (0.2–1.3) | 0.138 | 22 (26.2) | 0.2 (0.1–0.7) | 0.009 | 22 (21.4) | 0.5 (0.2–1.2) | 0.104 |
| No | 11 (44.0) | 9 (60.0) | 9 (36.0) | ||||||
| Fluoroquinolone used in antibiotic regimen | |||||||||
| Yes | 20 (27.4) | 0.6 (0.3–1.2) | 0.133 | 16 (26.7) | 0.4 (0.2–1.4) | 0.216 | 12 (16.4) | 0.4 (0.2–0.8) | 0.018 |
| No | 22 (40.0) | 15 (38.5) | 19 (34.5) | ||||||
| Rifampicin plus quinolones used in regimen | |||||||||
| Yes | 18 (27.7) | 0.6 (0.3–1.4) | 0.210 | 14 (25.5) | 0.6 (0.4–1.2) | 0.160 | 11 (16.9) | 0.4 (0.2–0.9) | 0.040 |
| No | 24 (38.1) | 17 (38.6) | 20 (31.7) | ||||||
DAIR debridement and implant retention
aData available for 121 patients
bData available for 36 patients
cData available for 71 patients. Radiological signs of infection is defined as the presence of periprosthetic lucency or signs of loosening of prosthetic components
dApplies only in case of DAIR
ePerformed < 21 days from symptom onset to surgery, absence of sinus tract and replacement of polyethylene or mobile components
fAdditional interventions caused by bleeding, haematoma or devitalized tissues
gData for 93 patients, defined as inactive antibiotics in vitro used before the susceptibility results were available
DAIR as primary treatment procedure was considered appropriate if it was performed < 21 days from onset of PJI symptoms, there was no sinus tract communicating with the joint prosthesis and replacement of polyethylene or mobile components was performed according to IDSA guidelines [9]. The definitions for other variables are included in the Supplementary Table S2.
Data Collection and Ethical Aspects
Data collection was supervised locally by staff with relevant expertise in the field. Data were entered into an anonymized electronic case report form and checked for missing values and inconsistencies. The study was approved by the Ethics Committees at each site (Supplementary Table S5). The need to obtain written informed consent was waived owing to the retrospective nature of the study and anonymized data, except in the case of the French hospitals where a letter of non-opposition was sent to eligible patients. All patients included in these centres therefore gave their authorization to participate.
Statistical Analysis
For bivariate analysis of the association of exposure variables with TF, relative risks with 95% confidence interval (CI) were calculated; p values were calculated by Chi-square or Fisher’s exact test, as appropriate. Continuous variables were categorized after analysing for stratified associations with TF. Multivariable analyses were performed by logistic regression: the effect of study site was controlled for using a generalized linear mixed model in which study sites were considered random effects. Variables with p value < 0.15 in bivariate analysis, and those considered as potentially relevant from clinical judgement, were entered into the models and selected using a manual stepwise backward procedure. Variables with p value < 0.1 were kept in the models. Collinearity and modification effects between variables were studied when clinically sound. The predictive ability of each model was examined by calculating their areas under the receiver operating characteristic (AUROC) curves with 95% CIs. For the effect of rifampicin in the subgroup of patients who underwent DAIR as first surgical treatment, a propensity score (PS) was calculated using a non-parsimonious multivariate logistic regression model, in which the outcome variable was combination therapy with rifampicin. As further sensitivity analyses for the impact of rifampicin therapy, parsimonious multivariate logistic regression models were performed, in which one or two other variables were removed. In addition, to avoid immortal time bias, landmark analysis was used, excluding patients who died or failed treatment in the first 21 days after debridement.
Results
Patient Characteristics and Treatment Failure Rates
A total of 130 cases of SA-PJI were detected, and 128 were included (relevant follow-up data were missing for the other two). The median number of cases per hospital was 7 (interquartile range [IQR] 5–9). The median age of patients was 73 years (IQR 59–81 years); 65 (50.8%) were males; 77 (60.2%) had hip arthroplasty (50 total and 27 partial) and 51 (39.8%) knee arthroplasty (47 total and 4 partial). Patient characteristics are presented in Table 1. Infection-related symptoms started a median of 24 (IQR 15–36) days after the primary arthroplasty (Supplementary Fig. S1), while the first surgical procedure for treatment of infection was performed a median of 4 days (IQR 1–11) after symptom onset. Bacteraemia occurred in 25 cases (19.5%). Overall, out of 128 PJI cases, 28 (21.9%) were due to methicillin-resistant S. aureus (MRSA) strains.
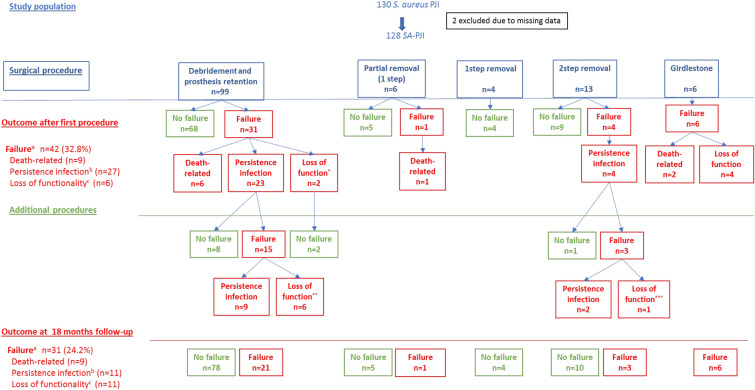
Figure 1 shows patient outcomes according to the first and additional surgical procedures performed. The rate of TF after the first procedure was 32.8% (42 patients; 95% CI 25.2–41.3%). TF was due to clinical failure in 27 cases (21.1%), related deaths in 9 (7%) and loss of function in 6 (4.7%). Median days until failure was 126 (IQR 34–335). Deaths occurred at a median of 21 (IQR 13–48) days after the first surgical procedure performed.
Fig. 1.
Outcome of patients with SA-PJI according to type of procedure performed to treat the infection. This figure shows the outcome of patients with SA-PJI according to the first type of procedure performed to treat the infection. For boxes on the left, 99 patients receiving initial debridement, antibiotic and implant retention (DAIR) were included: treatment was successful in 68 patients and 31 failed, including 6 deaths, 23 clinical failures and 2 loss of function. The 25 surviving patients with failure after the first procedure underwent subsequent procedures. After 18 months and considering additional procedures, 15 were still failing (9 clinical failures and 6 loss of function). Similar explanations apply to other surgical procedures. aFailure: a composite variable that included related mortality, clinical failure and functional loss (see Methods section). bIncludes: persistence or relapse of infection, need for extra courses of antibiotics after initial scheduled treatment and need for suppressive antibiotic therapy. cIncludes severe impairment of limb function that impedes or makes walking very difficult, including Girdlestone or arthrodesis. *Two aseptic loosening. **Two arthrodesis (one hip, one knee), four prosthesis removal waiting for a new prosthesis. ***One arthrodesis
After further surgical interventions, 11 patients who failed the first procedure (9 with persistent infection, 2 with functional failure due to prosthesis loosening after DAIR) were rescued. After 18 months of follow-up, TF was 24.2% (95% CI 17.5–32.3%). The reasons for failure were 9 related deaths (7.0%), 11 cases of clinical failure and 11 cases of functional loss (8.5%, respectively). Excluding functional loss, the failure rate was 15.5%.
Of the 99 patients who received DAIR as a first intervention to treat the SA-PJI (Fig. 1), 31 (31.3%) failed treatment due to death (n = 6), clinical failure (n = 23) or loss of function (n = 2). Despite further interventions, 15 were still failing at the end of the 18-month follow-up.
Of the 29 patients who received other types of first interventions to treat SA-PJI, 11 (37%) failed treatment, broken down as follows: related death (n = 3), clinical failure (n = 4) and loss of function (n = 4). Notwithstanding further interventions, 7 were still failing at the end of the 18-months follow-up.
A summary of the rates and reasons for treatment failure is provided in Supplementary Table S3, including the rate of TF for prosthesis removal as first procedure.
Analysis of Variables Associated with Treatment Failure
Table 2 presents the bivariate analysis of risk factors for TF after the initial surgery. Variables with p-value < 0.15 were Charlson index ≥ 2, haemoglobin < 10 g/dL, hip fracture as the reason for arthroplasty, type of arthroplasty, radiological signs of infection, sinus tract, MRSA, polymicrobial infection, bacteraemia, need for additional procedures not due to persistent infection, inadequate empirical antimicrobial treatment, and treatment with rifampicin and fluoroquinolones. After controlling for hospital and type of initial surgery performed, multivariable analysis retained Charlson index ≥ 2 [adjusted OR 2.5 (95% CI 1.1–5.9)], haemoglobin < 10 g/dL [aOR 3.6 (95% CI 1.6–8.4)], bacteraemia [aOR 2.7 (95% CI 1.0–7.6)], polymicrobial infection [aOR 3.1 (95% CI 1.2–8.2)] and need for additional procedures not due to persistent infection [aOR 2.7 (95% CI 1.1–6.6)] as variables independently associated with TF following the first surgical procedure (Table 3). The AUROC of the model for the observed data was 0.82 (95% CI 0.74–0.89).
Table 3.
Multivariate analysis of associations of potential predictors of treatment failure among patients with SA-PJI after the first surgical procedure, after DAIR, and after all surgical procedures performed
| Failure after the initial surgical procedure performed (n = 128; failures = 42)a | Failure after debridement and prosthesis retention as first surgery procedure performed (n = 99; failures = 31)b | Failure at 18 months after the initial and additional surgical procedures performed (n = 128; failures = 31)c | ||||
|---|---|---|---|---|---|---|
| Variable | Adjusted OR (95%IC) | p-Value | Adjusted OR (95%IC) | p-Value | Adjusted OR (95%IC) | P-Value |
| Charlson index ≥ 2 | 2.5 (1.1–5.9) | 0.030 | ||||
| Body mass index > 30 kg/m2 | – | – | 3.9 (1.2–12.6) | 0.021 | – | |
| Hip fracture | – | – | – | 4.6 (1.6–12.9) | 0.005 | |
| Haemoglobin < 10 g/dL | 3.6 (1.6–8.4) | 0.003 | 5.2 (1.9–14.5) | 0.002 | 2.5 (1.0–6.6) | 0.059 |
| Bacteraemia | 2.7 (1.0–7.6) | 0.058 | 6.4 (1.7–24.2) | 0.007 | – | – |
| Days from symptom onset to surgery | – | – | 1.03 (1.01–1.06) | 0.003 | – | – |
| Polymicrobial infection | 3.1 (1.2–8.2) | 0.021 | – | – | ||
| Additional surgery not due to persistent infection | 2.7 (1.1–6.6) | 0.033 | 4.4 (1.4–13.9) | 0.044 | 3.2 (1.1–8.9) | 0.028 |
| Rifampicin therapy | – | – | 0.2 (0.1–0.7) | 0.015 | – | – |
BMI body mass index
aThe AUROC curve of the model was 0.82 (95% CI 0.74–0.89)
bThe AUROC curve of the model was 0.84 (95%CI 0.76–0.93). The model prediction was similar when rifampicin was introduced at more than 21 or 28 days
cThe AUROC curve of the model was 0.80 (95% CI 0.71–0.90)
In the specific analysis performed to investigate the factors associated with TF of SA-PJI after DAIR as the first surgical procedure, the variables with p-values < 0.15 identified in bivariate analysis were the same as for the whole cohort, except for radiological signs of infection and MRSA, and with the addition of body mass index (BMI) > 30 kg/m2, interval > 21 days from symptom onset to surgery, polyethylene/mobile component replacement and appropriate indication for DAIR (Table 2). No statistically significant differences were found between patients treated with and without combination rifampicin therapy (Supplementary Table S4). As we were unable to obtain a PS sufficiently predictive for the probability of receiving rifampicin (AUROC curves < 0.5), a PS was not useful to provide additional control for confounding for the indication of rifampicin. On multivariable analysis (Table 3), variables independently associated with TF of DAIR were: BMI > 30 kg/m2 [aOR 3.9 (95% CI 1.2–12.6)], haemoglobin < 10 g/dL [aOR 5.2 (95% CI 1.9–14.5)], bacteraemia [aOR 6.4 (95% CI 1.7–24.2)], days from symptom onset to surgery [aOR 1.03 (95% CI 1.01–1.06)], and need for additional debridements after the first procedure not due to persistent infection [aOR 4.4 (95% IC 1.4–13.9)]; use of rifampicin was a protective factor [aOR 0.2 (95% CI 0.1–0.7)]. The AUROC of the model for the observed data was 0.84 (95% CI 0.76–0.93). The model prediction was similar when rifampicin was considered to be used for more than 21 or 28 days. For the different models made in which one or two variables other than rifampicin were removed, the estimates of the OR for rifampicin ranked from 0.15 (95% CI 0.04–0.5) to 0.2 (95% CI 0.1–0.7). In the landmark analysis excluding patients who died or failed in the first 21 days after debridement, use of rifampicin remained protective for TF [adjusted ORs (95%CI), 0.22 (0.06–0.80); AUROC curve of the model, 0.82 (95% CI 0.72–0.91)].
Table 2 also presents the bivariate analysis of risk factors for patients who failed after all procedures performed. In addition to the variables identified for the first procedure, TF was also associated with age > 80 years, prosthesis removal as first procedure, need to perform additional joint surgery not due to persistent infection and non-use of rifampicin and fluoroquinolones in combination. On multivariate analysis, hip fracture [aOR 4.6 (95% CI 1.6–12.9)], haemoglobin level < 10 g/dL [aOR 2.5 (95% CI 1.0–6.6)] and need to perform additional joint surgery not due to persistent infection [aOR 3.2 (95% IC 1.1–8.9)] were independently associated with TF at 18 months (Table 3). The AUROC of the model for the observed data was 0.80 (95% CI 0.71–0.90).
Discussion
In this study, we found that nearly a third of initial surgical procedures resulted in TF. The TF rate decreased when further surgical procedures were performed. Importantly, we estimated the impact of significant functional loss. When DAIR was used as the first procedure, even though it is a less aggressive strategy, additional procedures rescued a significant proportion of initial failures without increasing loss of function. In addition, the predictors of TF for SA-PJIs were identified, and the role of rifampicin in patients undergoing DAIR was confirmed.
A review of the literature on SA-PJI studies focusing on treatment outcomes highlighted the difficulties of comparing different study results owing to heterogeneity in study design, case definitions adopted, length of follow-up and types of analyses used (Table 4). Overall, previously reported TF rates ranged from 0% to 16.6% for prosthesis removal [4, 5, 8, 11] and 13.6% to 63% for DAIR [2, 4–8, 12–15]. It should also be noted that, to the best of the authors’ knowledge, functional outcome was not considered at all in previous studies, despite it being critical to the quality of life of patients. Functional loss is significantly influenced by the surgical procedures performed and this information is therefore relevant to the decision-making process.
Table 4.
Summary of published studies on the outcome and management of PJI including ≥ 20 cases focusing on S. aureus
| References | Years of diagnosis and design | Types of infection and number of patients | Number of patients and management | Cure or failure definition | Outcome | Factors associated with outcome |
|---|---|---|---|---|---|---|
| Brandt et al. (1997) [2] |
1980–1991 Retrospective cohort, unicentric |
Post-surgical knee and hip SA-PJI N = 33 (3 MRSA) |
DAIR (n = 33) Median 28 days intravenous (IV) antibiotics (beta-lactams, 91%; vancomycin, 9%) |
Failure: persistence or recurrence of clinical signs of PJI |
Failure: 59% at 1 year 63% at 2 years |
Multivariable (HR): DAIR performed > 2 days after symptom onset |
| Salgado et al. (2007) [3] |
1998–2004 Retrospective cohort, unicentric |
Hip and knee SA-PJI N = 45 (12 MRSA) |
DAIR (n = 20) Partial/total 1 T (n = 4) 2 T (n = 15) Resection arthroplasty (n = 6) Median 42 days IV antibiotics (beta-lactams or vancomycin, 51% with rifampicin). In four DAIR, oral rifampicin-quinolone |
Failure: relapse, reinfection, death-related, other signs of clinical failure |
Failure: 38%, 32% in MSSA, 50% in MRSA Follow-up: median 190 days (range 4–2279 daus) |
Multivariable: MRSA, TKA, retention of prosthesis |
| Vilchez et al. (2010) [14] |
2000–2007 Retrospective cohort, unicentric |
Early (< 2 months, < 15 days of symptoms) SA-PJI, total and partial hip and knee prosthesis N = 53 (4 MRSA) |
DAIR (n = 53) Mean 10.6 days iv antibiotics (cloxacillin if MSSA, vancomycin if MRSA) and 88 days oral (levofloxacin plus rifampicin) |
Cure: Absence of infection symptoms, aseptic loosening that required exchange prosthesis |
Failure: 24.5% Follow-up: 2 years |
Multivariable (HR): CRP > 22 mg/dl Need for 2nd DAIR Prosthesis age > 25 days |
| Joulie et al. (2011) [4] |
2001–2006 Prospective cohort, unicentric (reference) |
Any total and partial hip and knee SA-PJI N = 95 evaluable (25% MRSA) |
DAIR (n = 30), 1 T (n = 15), 2 T (n = 25), resection (n = 10) Mean 7 days IV antibiotics (not specified, adapted to susceptibility) and 122.3 days oral (not specified, adapted to susceptibility, 64% combined with rifampicin) |
Cure: ESR and/or CRP normal, non-inflammatory scar with no fistula, no antibiotics since discharge and no reintervention needed |
Cure: DAIR, 57%; 1 T, 94%; 2 T, 86.2%; Resection, 34% Follow-up: minimum of 12 months |
Multivariable for cure: Monomicrobial Prosthesis removal |
| Senneville et al. (2011) [5] |
2000–2006 Retrospective cohort, unicentric |
Total hip and knee SA-PJI N = 98 (17.3% MRSA) |
DAIR (n = 41) (performed if symptoms < 4 weeks) 1 T (n = 14) 2 T (n = 26) Resection arthroplasty (n = 9) Arthrodesis (n = 8) Mean 7 days IV antibiotics (not specified, adapted to susceptibility) and 3–6 months oral (rifampicin, n = 68; rifampicin-fluorquinolone, n = 39). Six DAIR with suppressive treatment |
Cure: no local or systemic signs of infection, no need for reintervention or new antibiotic therapy, no infection-related deaths |
Cure: 78.6%; DAIR, 78.0%; 1 T, 100%; 2 T, 84.6%; Resection, 44.4%; Arthrodesis, 62.5% Follow-up: minimum of 2 years |
Multivariable for cure: ASA score ≤ 2, use of rifampicin-fluoroquinolone |
| Lora-Tamayo et al. (2013) [6] |
2003–2010 Retrospective cohort, multicentre |
Early and haematogenous hip (total and partial) and knee SA-PJI N = 345 (23% MRSA) |
DAIR (n = 345) Median days of antibiotics: 94 in MRSA and 91 in MSSA. Rifampicin combinations in 88%: beta-lactams (13%) or quinolones (75%, mainly levofloxacin) in MSSA, and glycopeptides (18%, namely vancomycin), cotrimoxazole (46%), linezolid (24%) or clindamycin (10%) in MRSA |
Failure: infection-related death, prosthesis replacement, need for further debridements |
Failure: 45% (infection-related death, 7%) – Early failure (30 days), 29% ; Failure during antibiotic therapy, 32%; – Failure after antibiotic therapy, 39%; Follow-up: 2 years |
Multivariable (HR) for early failure: male, rheumatoid arthritis, bacteraemia, polymicrobial, CRP > 100 mg/L Failure during therapy: higher age, immunosuppressive drugs, MRSA, sinus tract, abnormal radiography, need ≥ 2 debridements, no use of rifampicin Failure after therapy: haematogenous infection, debridement delay, need for ≥ 2 debridements |
| Gómez-Junyent et al. (2021) [11] |
2003–2010 Retrospective cohort, multicentre |
Hip and knee SA-PJI N = 249 (early, n = 141; haematogenous, n = 26; chronic, n = 82) |
Implant removal (161 initial therapy, 88 salvage) 1 T, n = 17 (6.8%) 2 T, n = 188 (75.5%) Hip resection, n = 44 (17.7%) |
Failure: local failure and/or all-cause mortality within 60 days |
Failure: 15.6% Local failure: 9.3% Mortality: 12.8% Follow-up: median 781 days, interquartile range [IQR] 355–1375 days |
Multivariable for failure: ≥ 2 comorbidities |
| Betz et al. (2015) [15] |
1996–2012 Retrospective cohort, unicentric |
Hip (total and partial) PJI N = 29 (12 MSSA, 17 MRSA, 9 streptococci) |
DAIR (n = 38) All exchange of mobile parts. Antibiotic adapted to susceptibility, with median duration of 12 weeks (IV for a median of 14 days). Rifampicin use: 23 (60.5%) |
Persistence or recurrence of PJI |
Failure: 18.4% MSSA, 9.5% MRSA, 29.4% Streptococci, 0% Follow-up: minimum of 2 years |
Univariate analysis: no factors associated with failure |
| Bouaziz et al. (2018) [13] |
2000–2010 Retrospective cohort, two centres |
Hip and knee MSSA-PJI N = 85 (early, 33; delayed, 11; late, 45) |
DAIR (n = 62) Antibiotic not specified, except for rifampicin Rifampicin use: early 20 (62%), delayed 7 (63%), late 26 (58%) |
Failure: need for further surgery to control PJI (or to treat superinfection), amputation or infection-related death |
Failure: 42% Early, 10 (30%) Delayed, 4 (36%) Late, 23 (51%) Follow-up: 2.8 ± 2.2 years |
Multivariable (HR) for failure: non-compliance with surgical IDSA guidelines (namely DAIR performed if duration of symptoms < 3 weeks or joint age < 30 days, and stable implant without sinus tract) |
| Lesens et al. (2018) [12] |
2010–2014 Retrospective cohort, multicentre |
Hip, knee, shoulder, elbow SA-PJI N = 137 (early acute, n = 63; early chronic, n = 26; late acute, n = 35; late chronic, n = 13) MRSA 27 (19.7%) |
DAIR (n = 137) Mean duration antibiotic: 12.6 weeks Rifampicin use: 85 (65%) Rifampicin plus fluorquinolone: 63 (47.4%) Suppressive treatment: 14 (10.2%) |
Failure: prosthesis removal, death, additional debridement or course of antibiotics, clinical and microbiological signs of infection |
Failure: 25% Mortality: 8.8% Follow-up: 2 years |
Multivariable (HR) for failure: longer AB duration (protective), rifampicin regimen (protective), smoking, early infection (protective) |
| Wouthuyzen-Bakker et al. (2018) [7] |
2005–2015 Retrospective cohort, two centres |
Early hip and knee MSSA-PJI treated with rifampicin plus levofloxacin (n = 40) or moxifloxacin (n = 19) N = 58 |
DAIR (n = 58) Duration antibiotic therapy about 90 days. IV cloxacillin or flucloxacillin 7–14 days followed rifampicin plus fluorquinolone |
Failure: need for revision surgery and/or suppressive antimicrobial therapy because of persistent infection, death-related infection, reinfection or relapse with S. aureus |
Cure: 86.4% (87.5% in levofloxacin group, 84.2% in moxifloxacin group) Follow-up: minimum 2 years |
Not performed |
| Muñoz-Gallego et al. (2020) [8] | 2016–2017 Prospective cohort, multicentre |
Hip (total and partial) and knee SA-PJI N = 85 (19 MRSA) Early (< 90 days): 45 Chronic (> 90 days): 21 Haematogenous: 19 |
DAIR (n = 55) Prosthesis removal (n = 30) Duration and type of antibiotic not specified |
Failure: all-cause death within 90 days, persistent or relapsing signs of staphylococcal infection, need for salvage therapy except for extra debridements in the first 30 days, suppressive antimicrobial treatment |
Overall failure: 36.4% (DAIR, 47.2%; prosthesis removal, 16.6%) Follow-up: at least 1 year |
Univariate analysis: Global failure associated with DAIR DAIR: failure associated with delay of debridement and no use of rifampicin |
| ARTHR-IS cohort |
2014–2016 Retrospective cohort, multicentre |
Hip (total and partial) and knee postsurgical SA-PJI N = 128 (28 MRSA) |
DAIR (n = 99) 1 T (n = 10) 2 T (n = 13) Resection (n = 6) Median days of antibiotics: 16 IV and 50 oral. Rifampicin combinations in 103 (80.5%); with quinolones in 42 (65.8%) |
Clinical and functional failure after the first procedure and at 18 months Clinical failure: all-cause deaths in first 2 months, SA-PJI-related death, persistence or recurrence of signs or symptoms of infection; need for additional course of antibiotics including suppressive therapy, removal of the prosthesis if DAIR. Functional failure: significantly impaired walking due to prosthesis loosening, Girdlestone or arthrodesis |
Overall clinical and functional failure after first surgical procedure: 32.8% (DAIR, 33.3%; prosthesis removal, 37.1%) Considering only clinical failure: 28.1% Failure at 18 months: 24.2% (DAIR: 21.2%; prosthesis removal, 34.4%) Considering only clinical failure: 15.5% (DAIR: 15.5%; prosthesis removal: 17.2%) Follow-up: 18 months |
Multivariable analysis: Failure after first surgical procedure: Charlson ≥ 2, haemoglobin level < 10 mg/dL, bacteraemia, polymicrobial infection and need to perform additional debridement In DAIR: those above and BMI > 30, and time between symptom onset and DAIR; rifampicin use was protective At 18 months: haemoglobin level < 10 mg/dL, hip fracture and need for additional joint, surgical procedures not related to infection control |
1 T one-stage replacement, 2 T two-stage replacement, CRP C-reactive protein, DAIR debridement, antibiotics, and implant retention, ESR erythrocyte sedimentation rate, HR hazard ratio, IV intravenous, MRSA methicillin-resistant S. aureus., MSSA methicillin-susceptible S. aureus, PJI prosthetic joint infection, SA-PJI prosthetic joint infection produced by S. aureus, TKA total knee arthroplasty
When DAIR was analysed as the initial procedure, the TF rate was 31.3% (29.2% without considering functional loss), which is slightly higher than reported in more recent observations [7, 12] but lower than in older publications [2–4, 6, 13] (Table 4). However, the TF rate decreased to 21.2% after additional procedures, and to 15% if functional loss was not taken into account (which is more consistent with definitions in previous reports). The lower TF rates for DAIR reported by the latest studies (and by this one) could be attributed to better patient selection for this treatment strategy and to the involvement of multidisciplinary teams in the management of PJI. Indeed, Bouaziz et al. [13] found an overall TF rate of 42%, which decreased to 30% when DAIR was performed according to the latest guidelines [9]. Our data further suggest that appropriate patient selection favours more positive outcomes, and that an initial TF can be rescued without significant functional loss in a considerable number of patients.
The overall TF rates, including clinical and functional updates, for prosthesis removal in our cohort (34.4%) may seem relatively high when compared with other studies, but when only clinical cure was considered: the rate of TF for SA-PJI was 17.2%, which is similar to that found in other series [4, 5, 11]. The high proportion of TF after prosthesis removal related to functional loss is noteworthy and reinforces the importance of early diagnosis of SA-PJI to increase the likelihood of being treated with DAIR.
Since the decision to perform DAIR or remove the prosthesis as first procedure is strongly influenced by patient and infection characteristics, we did not try to compare the outcomes of the two procedures as they are not comparable. Instead, we focused our analysis on identifying potential predictors for TF. Regarding the variables identified, the Charlson index is a predictor of survival and also of prognosis of many infections; similarly, other studies have used the ASA index [5] or ≥ 2 comorbidities for similar reasons [11]. Anaemia was previously identified as a predictor in one study [16], but was not assessed at all in most of the others. Anaemia is a potential marker of nutritional status that can increase tissue hypoxia or even indicate a systemic inflammatory process. As in our study, bacteraemia, polymicrobial infection and the need for additional debridement were also predictors of TF in other studies [6, 14].
Since DAIR is the most frequent initial procedure, we also analysed predictors of TF in this subgroup. Apart from the above variables, obesity and delay in performing DAIR were also identified as risk factors, while the use of rifampicin had a protective effect. Obesity is a risk factor for PJI and was also found to be associated with TF in hip PJI undergoing two-stage replacement [17], but was not even considered in most studies. Obesity could be associated with wound complications, additional debridement, impaired innate immune response and changes in the pharmacokinetics of some antimicrobial drugs [18]. Delayed DAIR following onset of PJI symptoms is a known factor for TF regardless of the microorganism involved, but is particularly pertinent in the case of SA-PJI [2, 6, 12–14]. Establishing a threshold is complex. The 21-day threshold for performing DAIR recommended by the guidelines [9] was based on one small-scale study experience [19], and a delay of > 2 days in patients with SA-PJI treated with beta-lactams was associated with increased TF in another study [2] but not when a rifampicin-fluoroquinolone combination was used [6–14, 16]. In our cohort, we found an increased risk for each day of delay, supporting the recommendation that debridement should be performed as early as possible.
Rifampicin in combination with other antibiotics (mainly fluoroquinolones) was reported to increase cure rates in a small randomized trial [19] and in observational studies [5, 6, 12, 20–22]. However, two recent meta-analyses found controversial results on the role of rifampicin: one found no benefit in staphylococcal infections [23] and the other only a limited impact [24]. The studies included in the meta-analysis had a considerable risk of selection and immortal time bias. In our study, on the other hand, we found that rifampicin combinations were associated with a protective effect, even after performing sensitivity and landmark analyses.
Although previous studies have found higher TF rates in PJIs caused by MRSA compared with susceptible strains [3, 15], our data did not demonstrate this association, which is in line with more recent observations [5, 6]. Whether this is due to the use of anti-MRSA drugs with good bioavailability and anti-biofilm activity, such as linezolid, would require further studies.
Finally, we also analysed the predictors of TF after all procedures had been performed. Apart from other factors, hip fracture increased the risk of TF, probably reflecting the frailty of the patients affected. In these patients, the first surgical approach was crucial, since initial TF was followed by functional failure in all cases (data not shown). In a previous multicentre cohort study of patients with hip PJIs, fracture was also associated with clinical failure and worse functional prognosis [25]. The risk of TF also increased when additional joint surgery not due to persistent infection was performed; this may have been due to tissue damage, delayed healing or facilitation of bacterial superinfections.
This study has some limitations that should be considered when interpreting the results. The sample size was too small to investigate predictors of other initial surgical procedures and may have been insufficient to detect additional predictors of TF; its retrospective design limited the available variables; residual confounding is also possible; we did not collect data about ethnicity of the patients; finally, some changes in management may have occurred during the study period. Some strengths include that it is a multinational study, the definitions of TF including clinical and functional aspects and the good predictive ability of the models developed.
Conclusions
In conclusion, we observed that a considerable proportion of SA-PJIs failed after initial surgical treatments, although a substantial part of them were recovered with further procedures. Significant functional loss should be considered alongside clinical failure, and the importance of certain risk factors for TF was confirmed, including the protective role of rifampicin-based treatment in DAIR.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all study participants for their collaboration, without which this manuscript would have been impossible.
Other members of the ARTHR-IS group are: Nienke Cuperus and Giuseppe Manfré (Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands) collaborated in the selection process of the participating centers. Ana Isabel Suárez-Barrenechea and Alvaro Pascual-Hernandez (Department of microbiology, Hospital Universitario Virgen Macarena), Alba Rivera (Deparment of Microbiology, Hospital de la Santa Creu i Sant Pau), Xavier Crusi and Marcos Jordán (Department of traumatology, Hospital de la Santa Creu i Sant Pau), Nicolò Rossi (Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy), Tessa van de Kerkhof (Department of Orthopaedic Surgery & Trauma, Máxima MC, Eindhoven, the Netherlands; Department of Orthopaedic Surgery & Trauma, Catharina Hospital, Eindhoven, the Netherlands), Juan P. Horcajada, Joan Gómez-Junyent and Albert Alier (Hospital del Mar, Institut Hospital del Mar d'Investigacions Mèdiques, Universitat Pompeu Fabra, Barcelona, Spain), Miranda van Rijen and Jannie Romme (Amphia Hospital, Breda, Netherlands), Juliane Ankert (Jena University Hospital—Jena, Germany), Celia Whitehouse and Adrian Jones (Norfolk and Norwich University Hospital—Norwich, UK), Javier Cobo and Javier Moreno (Hospital Universitario Ramón y Cajal—Madrid, Spain), Anne Meheut (Centre Hospitalier Universitaire de Rennes—Rennes, France), Claire Gledel (Orthopedic surgery department, Croix Rousse Hospital—Lyon, France), Pauline Perreau (Centre Hospitalier Universitaire de Bordeaux—Bordeaux, France), Remco J.A. van Wensen (Department of Orthopaedic Surgery & Trauma, Catharina Hospital, Eindhoven, the Netherland), Gabriella Lindergard (North Manchester General Hospital—Manchester, UK) collaborated in the data collection.
Funding
This work was supported by the Innovative Medicines Initiative Joint Undertaking (grant agreement No. 115523), COMBACTE-NET consortium (European Union FP7/2007–2013 and GlaxoSmithKline Biologicals SA, as EFPIA partner). R.E, L.S, O. M, R. E-S, J. L–T, J. P, J. R-B and MD. del T are members of the Spanish Network for Research in Infectious Diseases (REIPI), supported by Plan Nacional de I + D + i 2013‐2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia, Innovación y Universidades, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0001; 0002; 0005; 0009; 0011; 0015), co‐ financed by European Development Regional Fund “A way to achieve Europe”, Operative Program Intelligence Growth 2014‐2020. The study sponsor is also funding the journal's Rapid Service Fee. Role of funding source: The funders had no influence on the analysis and decision to published; GlaxoSmithKline Biologicals SA was provided the opportunity to review a version of this manuscript for factual accuracy; authors are solely responsible for final content and interpretation.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Espindola R, Vella V, Rodríguez-Baño J, Del Toro MD contributed in the conception and design of the study, analysis and interpretation of data and drafting the article. Benito N, Mur I, Tedeschi S, Zamparini E, Hendriks J, Sorlí L, Murillo O, Soldevila L, Scarborough M, Scarborough C, Kluytmans J, Ferrari MC, Pletz M, Mcnamara I, Escudero-Sanchez R, Arvieux C, Batailler C, Dauchy F-A, Liu W-Y, Lora-Tamayo J, Praena J, Ustianowski J contributed in the acquisition of data and revising the article. Cinconze E, Pellegrini M and Bagnoli F participated in the analysis and interpretation of data and revision the article. All authors gave their final approval of the version to be submitted.
Prior Presentation
This study was previously presented as a poster at the 32nd European Congress of Clinical Microbiology & Infectious Diseases (ECCMIC), held in Lisbon, Portugal, April 23–26.
Disclosures
Venanzio Vella, Elisa Cinconze, Michele Pellegrini and Fabio Bagnoli are employees of the GSK group of companies. Venanzio Vella, Michele Pellegrini and Fabio Bagnoli hold shares in the GSK group of companies. Fabio Bagnoli holds patents pending and issued patents on Staphylococcus aureus vaccine formulations. Venanzio Vella, Elisa Cinconze, Michele Pellegrini and Fabio Bagnoli declare no other financial or non-financial relationships and activities. Reinaldo Espindola, Natividad Benito, Isabel Mur, Sara Tedeschi, Eleonora Zamparini, Johannes G.E. Hendriks, Luisa Sorlí, Oscar Murillo, Laura Soldevila, Mathew Scarborough, Claire Scarborough, Jan Kluytmans, Mateo Carlo Ferrari, Mathias W. Pletz, Iain Mcnamara, Rosa Escudero-Sanchez, Cedric Arvieux, Cecile Batailler, Frédéric-Antoine Dauchy, Wai-Yan Liu, Jaime Lora-Tamayo, Julia Praena, Andrew Ustianowski, Jesús Rodríguez-Baño and Maria Dolores Del Toro have no conflicts to declare. At present, the affiliation of Reinaldo Espindola is Infectious Diseases and Microbiology Clinical Unit, Hospital Universitario Virgen de Valme, Seville, Spain.
Compliance with Ethics Guidelines
The study was approved by the Ethics Committees at each site. The need to obtain written informed consent was waived due to the retrospective nature of the study and anonymized data, except in the case of the French hospitals. The approval of the ethics committee of all the participating centers, with the names and references, are reflected in the table S5 of the supplementary material.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Members of The ARTHR-IS Group are listed in Acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jesús Rodríguez-Baño and Maria Dolores del Toro contributed equally as senior authors of the study.
Contributor Information
Maria Dolores del Toro, Email: mdeltoro@us.es.
The ARTHR-IS Group:
Nienke Cuperus, Giuseppe Manfré, Ana Isabel Suárez-Barrenechea, Alvaro Pascual-Hernandez, Alba Rivera, Xavier Crusi, Marcos Jordán, Nicolò Rossi, Tessa vande Kerkhof, Juan P. Horcajada, Joan Gómez-Junyent, Albert Alier, Miranda van Rijen, Jannie Romme, Juliane Ankert, Celia Whitehouse, Adrian Jones, Javier Cobo, Javier Moreno, Anne Meheut, Claire Gledel, Pauline Perreau, Remco J. A. van Wensen, and Gabriella Lindergard
References
- 1.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27:302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandt CM, Sistrunk WW, Duffy MC, et al. Staphylococcus aureus prosthetic joint infection treated with debridement and prosthesis retention. Clin Infect Dis 1997;24:914–919. https://pubmed.ncbi.nlm.nih.gov/9142792/. Accessed 4 June 2021. 10.1093/CLINIDS/24.5.914. [DOI] [PubMed]
- 3.Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Related Res. 2007:48–53. https://pubmed.ncbi.nlm.nih.gov/17534195/. Accessed 3 June 2021. 10.1097/BLO.0b013e3181123d4e. [DOI] [PubMed]
- 4.Joulie D, Girard J, Mares O, et al. Factors governing the healing of Staphylococcus aureus infections following hip and knee prosthesis implantation: a retrospective study of 95 patients. Orthop Traumatol Surg Res 2011;97:685–692. https://pubmed.ncbi.nlm.nih.gov/22000281/. Accessed 20 Mar 2022. 10.1016/J.OTSR.2011.05.013. [DOI] [PubMed]
- 5.Senneville E, Joulie D, Legout L, et al. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to staphylococcus aureus. Clin Infect Dis 2011;53:334–340. https://pubmed.ncbi.nlm.nih.gov/21810745/. Accessed 3 June 2021. 10.1093/CID/CIR402. [DOI] [PMC free article] [PubMed]
- 6.Lora-Tamayo J, Murillo O, Iribarren JA, et al. A large multicenter study of methicillin-susceptible and methicillin-resistant staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis 2013;56:182–194. https://pubmed.ncbi.nlm.nih.gov/22942204/. Accessed 3 June 2021. 10.1093/cid/cis746. [DOI] [PubMed]
- 7.Wouthuyzen-Bakker M, Tornero E, Morata L, et al. Moxifloxacin plus rifampin as an alternative for levofloxacin plus rifampin in the treatment of a prosthetic joint infection with Staphylococcus aureus. Int J Antimicrob Agents 2018;51:38–42. https://pubmed.ncbi.nlm.nih.gov/28668678/. Accessed 20 Mar 2022. 10.1016/J.IJANTIMICAG.2017.04.011. [DOI] [PubMed]
- 8.Muñoz-Gallego I, Viedma E, Esteban J, et al. Genotypic and phenotypic characteristics of staphylococcus aureus prosthetic joint infections: insight on the pathogenesis and prognosis of a multicenter prospective cohort. Open forum Infect Dis 2020;7. https://pubmed.ncbi.nlm.nih.gov/33005695/. Accessed 20 Mar 2022. 10.1093/OFID/OFAA344. [DOI] [PMC free article] [PubMed]
- 9.Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the infectious diseases Society of America. Clin Infect Dis 2013;56. https://pubmed.ncbi.nlm.nih.gov/23223583/. Accessed 13 June 2021. 10.1093/CID/CIS803. [DOI] [PubMed]
- 10.Ariza J, Cobo J, Baraia-Etxaburu J, et al. Executive summary of management of prosthetic joint infections. Clinical practice guidelines by the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). Enferm Infecc Microbiol Clin 2017;35:189–195. https://linkinghub.elsevier.com/retrieve/pii/S0213005X16302816. Accessed 1 Apr 2019. 10.1016/j.eimc.2016.08.012. [DOI] [PubMed]
- 11.Gómez-Junyent J, Lora-Tamayo J, Baraia-Etxaburu J, et al. Implant removal in the management of prosthetic joint infection by staphylococcus aureus: outcome and predictors of failure in a large retrospective multicenter study. Antibiotics 2021;10:1–13. https://pubmed.ncbi.nlm.nih.gov/33530523/. Accessed 3 June 2021. 10.3390/antibiotics10020118. [DOI] [PMC free article] [PubMed]
- 12.Lesens O, Ferry T, Forestier E, et al. Should we expand the indications for the DAIR (debridement, antibiotic therapy, and implant retention) procedure for Staphylococcus aureus prosthetic joint infections? A multicenter retrospective study. Eur J Clin Microbiol Infect Dis 2018;37:1949–1956. https://pubmed.ncbi.nlm.nih.gov/30083889/. Accessed 20 Mar 2022. 10.1007/S10096-018-3330-7. [DOI] [PubMed]
- 13.Bouaziz A, Uçkay I, Lustig S, et al. Non-compliance with IDSA guidelines for patients presenting with methicillin-susceptible Staphylococcus aureus prosthetic joint infection is a risk factor for treatment failure. Med Mal Infect 2018;48:207–211. https://pubmed.ncbi.nlm.nih.gov/29122410/. Accessed 3 June 2021. 10.1016/J.MEDMAL.2017.09.016. [DOI] [PubMed]
- 14.Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect 2011;17:439–444. https://pubmed.ncbi.nlm.nih.gov/20412187/. Accessed 3 June 2021. 10.1111/J.1469-0691.2010.03244.X. [DOI] [PubMed]
- 15.Betz M, Abrassart S, Vaudaux P, et al. Increased risk of joint failure in hip prostheses infected with Staphylococcus aureus treated with debridement, antibiotics and implant retention compared to Streptococcus. Int Orthop 2015;39:397–401. https://pubmed.ncbi.nlm.nih.gov/25183296/. Accessed 20 Mar 2022. 10.1007/S00264-014-2510-Z. [DOI] [PubMed]
- 16.Swenson RD, Butterfield JA, Irwin TJ, Zurlo JJ, Davis CM. Preoperative anemia is associated with failure of open debridement polyethylene exchange in acute and acute hematogenous prosthetic joint infection. J Arthroplasty 2018;33:1855–1860. https://pubmed.ncbi.nlm.nih.gov/29555498/. Accessed 4 June 2021. 10.1016/J.ARTH.2018.01.042. [DOI] [PubMed]
- 17.Ahmad SS, Orlik L, Ahmad SJS, Albers CE, Siebenrock KA, Klenke FM. Obesity and smoking predict the results of two-stage exchange in septic revision hip arthroplasty: a cohort study. Orthop Traumatol Surg Res 2019;105:467–471. https://pubmed.ncbi.nlm.nih.gov/30922806/. Accessed 4 June 2021. 10.1016/j.otsr.2019.01.006. [DOI] [PubMed]
- 18.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis 2006;6:438–446. https://pubmed.ncbi.nlm.nih.gov/16790384/. Accessed 20 Mar 2022. 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed]
- 19.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. J Am Med Assoc 1998;279:1537–1541. https://pubmed.ncbi.nlm.nih.gov/9605897/. Accessed 4 June 2021. 10.1001/jama.279.19.1537. [DOI] [PubMed]
- 20.El Helou OC, Berbari EF, Lahr BD, et al. Efficacy and safety of rifampin containing regimen for staphylococcal prosthetic joint infections treated with debridement and retention. Eur J Clin Microbiol Infect Dis 2010;29:961–967. https://pubmed.ncbi.nlm.nih.gov/20505968/. Accessed 4 June 2021. 10.1007/s10096-010-0952-9. [DOI] [PubMed]
- 21.Widmer AF, Gaechter A, Ochsner PE, Zimmerli W. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin Infect Dis 1992;14:1251–1253. https://pubmed.ncbi.nlm.nih.gov/1623081/. Accessed 4 June 2021. 10.1093/clinids/14.6.1251. [DOI] [PubMed]
- 22.Beldman M, Löwik C, Soriano A, et al. If, when, and how to use rifampin in acute staphylococcal periprosthetic joint infections, a multicentre observational study. Clin Infect Dis 2021. https://pubmed.ncbi.nlm.nih.gov/33970214/. Accessed 4 June 2021. 10.1093/cid/ciab426. [DOI] [PMC free article] [PubMed]
- 23.Aydın O, Ergen P, Ozturan B, Ozkan K, Arslan F, Vahaboglu H. Rifampin-accompanied antibiotic regimens in the treatment of prosthetic joint infections: a frequentist and Bayesian meta-analysis of current evidence. Eur J Clin Microbiol Infect Dis 2021;40:665–671. https://pubmed.ncbi.nlm.nih.gov/33125602/. Accessed 20 Mar 2022. 10.1007/S10096-020-04083-4. [DOI] [PubMed]
- 24.Scheper H, Gerritsen LM, Pijls BG, Van Asten SA, Visser LG, De Boer MGJ. Outcome of debridement, antibiotics, and implant retention for staphylococcal hip and knee prosthetic joint infections, focused on rifampicin use: a systematic review and meta-analysis. Open forum Infect Dis 2021;8. https://pubmed.ncbi.nlm.nih.gov/34258321/. Accessed 20 Mar 2022. 10.1093/OFID/OFAB298. [DOI] [PMC free article] [PubMed]
- 25.Del Toro MD, Nieto I, Guerrero F, et al. Are hip hemiarthroplasty and total hip arthroplasty infections different entities? The importance of hip fractures. Eur J Clin Microbiol Infect Dis 2014;33:1439–1448. https://pubmed.ncbi.nlm.nih.gov/24671411/. Accessed 4 June 2021. 10.1007/s10096-014-2091-1. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.