Abstract
Aims
An increasing body of evidence suggests that drug-coated balloon (DCB) angioplasty represents a valuable option for revascularization in selected patients with coronary bifurcation disease. However, there remains a paucity of real-world observational evidence on the efficacy of DCB in left main (LM) true bifurcation lesion. We compared clinical and angiographic outcomes of hybrid [DCB + drug-eluting stent (DES)] versus DES-only strategy (provisional stenting or two-stent strategies) in de novo LM true bifurcated lesions.
Methods
The primary endpoint was the 2-year composite rate of target lesion failure (TLF): cardiac death, target vessel myocardial infarction (TVMI), or clinically driven target lesion revascularization (CD-TLR). A routine 1-year angiographic follow-up was scheduled. Propensity-score matching was utilized to assemble a cohort of patients with similar baseline characteristics.
Results
Among 1077 eligible patients, 199 who received DCB treatment and 398 who were assigned to DES therapy had similar propensity scores and were included in the analysis. TLF within 2 years occurred in 13 patients (7.56%) assigned to DCB group, and 52 patients (14.36%) assigned to DES group (odds ratio: 0.487; 95% confidence interval: 0.258–0.922; P = 0.025; Log-rank P = 0.024). Compared with the DES group, the DCB group resulted in a lower rate of CD-TLR (2.91% vs. 9.42%; P = 0.007). Cardiac death, TVMI, all-cause mortality, and stent thrombosis were comparable between both groups. Patients treated with DES-only were associated with a higher late lumen loss (0.42 ± 0.62 mm vs. 0.13 ± 0.42 mm, P < 0.001) compared with the DCB group at 1 year. In sensitivity analysis, the DCB group also presented a lower incidence of TLF, CD-TLR and stent thrombosis both compared to the two-stent strategy and compared to provisional stenting (Ps < 0.05).
Conclusion
The 2-year results of PCI utilizing DCB for LM true bifurcation lesions are superior to employing DES alone in terms of safety and effectiveness.
Keywords: drug-coated balloon, drug-eluting stent, left main, true bifurcation, de novo
Graphical Abstract
DCB, drug-coated balloon; LM, left main; PSM, propensity score matching; DES, drug-eluting stent; TLF, target lesion failure; CD-TLR, clinically driven target lesion revascularization. 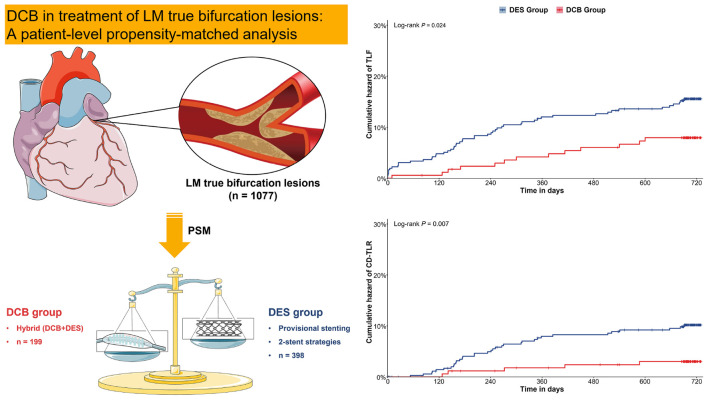
Introduction
Left main (LM) bifurcation is consistently distinct from other bifurcations (1), and the side branch (SB) is often the circumflex (LCx), which is frequently angular and has a large reference diameter, making it challenging to treat. Oftentimes, LCx acute occlusion causes significant ischemia. It has been reported that the T-shaped bifurcation angle of the LM may also alter the implantation procedure, and a steeply angled LCx take-off may affect the prognosis following LM stenting. According to recent myocardial revascularization guidelines, patients with significant LM disease with a low or moderate SYNTAX score have a class I indication for percutaneous coronary intervention (PCI) (2). However, bifurcation stenting is frequently associated with greater rates of restenosis and thrombosis, especially in complex procedures (3). Provisional side branch intervention is preferred for most bifurcation lesions (3, 4). Nonetheless, provisional stenting may not always be appropriate for LM bifurcation lesions. Accordingly, further studies are essential to developing new technologies to reduce restenosis in bifurcation lesions.
Nowadays, drug-coated balloons (DCBs) can be used to deliver antiproliferative drugs without the need for implanting permanent prostheses. DCB has well-established efficacy in treating coronary in-stent restenosis (ISR) (5, 6) and de novo lesions (7–9). Notably, the past decade has witnessed significant inroads achieved in DCB technology, lesion preparation, and clinical experience for bifurcation PCI (10–12).
To the best of our knowledge, no study has hitherto explored the utilization of DCB in LM true bifurcation lesions. Our multicenter study retrospectively evaluates the effect of hybrid [DCB + drug-eluting stent (DES)] and DES-only techniques on 2-year outcomes in LM true bifurcation diseases.
Materials and methods
Study subjects
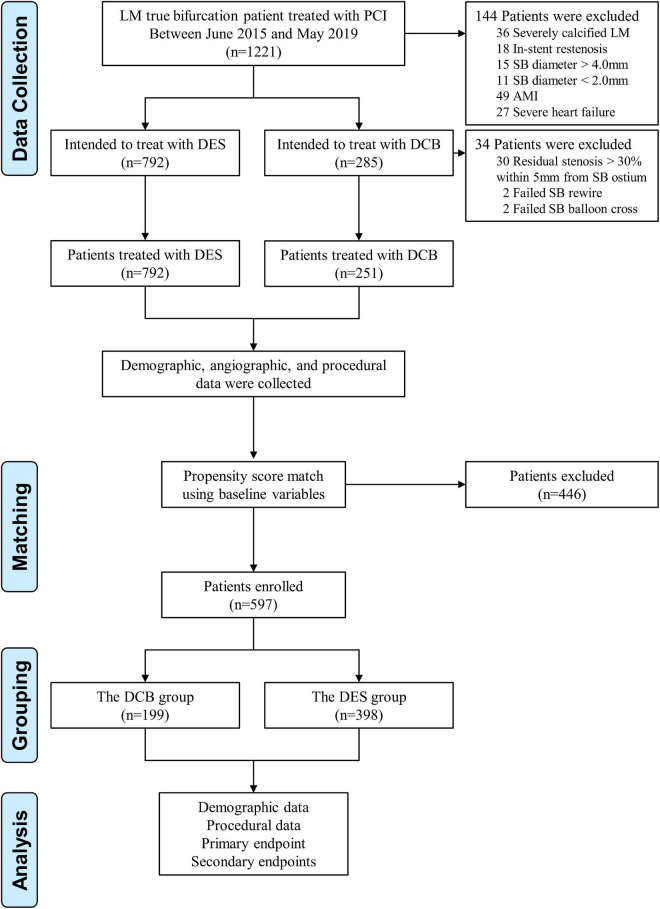
Patients were enrolled consecutively from June 2015 to May 2019 at three Chinese centers (including the First Affiliated Hospital of Zhengzhou University, Jincheng People’s Hospital, and the Fifth Affiliated Hospital of Zhengzhou University). All patients submitted written informed permission, which was authorized by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. Patients that exhibited stable coronary disease or unstable angina pectoris and underwent PCI for de novo coronary lesions (diameter stenosis > 50%) at the LM bifurcation (Medina 1,0,1, 0,1,1 or 1,1,1) (13), with an SB diameter ≥ 2.0 mm, were included in the present study. Patients with the following criteria were excluded: (1) angiographic evidence of severely calcified LM lesions requiring atherectomy; (2) ISR; (3) Inherence to the procedural steps of optimization defined below as extracted from angiographic images and reports reviews; (4) acute myocardial infarction (MI); (5) cardiogenic shock or unstable hemodynamics (Figure 1).
FIGURE 1.
Study population. LM, left main; PCI, percutaneous coronary intervention; SB, side branch; AMI, acute myocardial infarction; DCB, drug-coated balloon; DES, drug-eluting stent.
Patients received aspirin (300 mg loading dose) or long-term aspirin medication prior to the intervention. A loading dosage of 600 mg of clopidogrel or 180 mg of ticagrelor was given. Dual antiplatelet treatment (DAPT) was provided for the duration recommended by guidelines (2, 14). Women of reproductive potential and those with a life expectancy less than one year, as well as patients with contraindications to DAPT, bivalirudin, heparin, paclitaxel, or –limus, were excluded from the study. A standard computerized case report form was used to collect the data.
Study procedures
In the DES group, LM true bifurcation lesions were treated with any solution using DES alone, in accordance with the recommendations of the European Bifurcation Club (EBC) (1), including provisional SB interventional approach or any two-stent strategies.
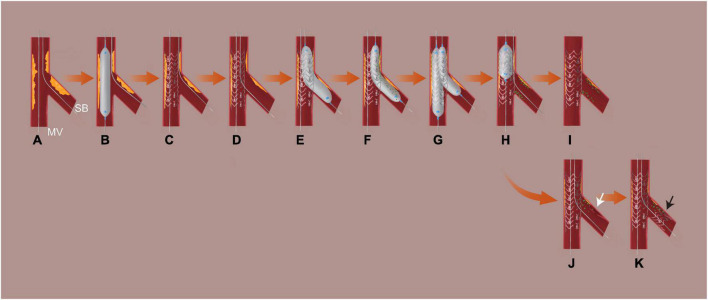
In the DCB group, appropriate lesion preparation was emphasized prior to DCB treatment. Both branches were wired, and the main vessel (MV) was pre-dilated. Subsequently, the stent was implanted in the MV and fully expanded. Then, the SB was rewired from the distal strut mesh, and SB lesion preparation was conducted (plaque modification and expand the orifice of SB). Dilatation was required using a plain or non-compliant balloon with a balloon-to-vessel ratio of 0.8–1.0. In the absence of a major, flow-limiting dissection [<Type C according to the NHLBI classification (15)] and if residual stenosis was ≤ 30% based on at least two perpendicular angiographic views, DCB angioplasty was conducted. For residual stenosis ≤ 30% only in the proximal 5 mm following lesion preparation, irrelevant of other segments, a DCB + DES approach was initially selected in SB. To achieve full coverage of the dissection and severe elastic recoil segment, DCB angioplasty was performed first, followed by stent placement. The distance between the stent’s proximal end and the SB ostium should be more than 3 mm. Patients with residual stenosis > 30% within 5 mm of the SB ostium after SB lesion preparation were eliminated (switching to a 2-stent strategy or SB palliative DCB angioplasty). Finally, kissing balloon inflation and the proximal optimizing technique (POT) were conducted. In this investigation, the DCB used was coated with a matrix of paclitaxel and iopromide (SeQuent™ Please, B. Braun, Melsungen, Germany). To avoid a geographic mismatch, the DCB catheter was extended 4–5 mm into the MV and 2–3 mm beyond the pre-dilated area. Using a balloon-to-vessel ratio of 0.8–1.0, the DCB diameters were matched to the reference vessel diameters. At a pressure of > 7 bars, the suggested inflating time was at least 30 s. If the outcome of DCB treatment was unsatisfactory owing to significant residual stenosis or dissections, a new-generation DES was implanted (Figure 2).
FIGURE 2.
Procedural steps in DCB group. (A) Wiring both branches; (B) MV lesion preparation; (C) MV stent implantation; (D) rewire SB from the distal strut; (E) SB lesion preparation: plaque modification and expand the orifice of SB; (F) SB DCB angioplasty; (G) final kissing inflation; (H) POT if necessary; (I) final result I; (J) dissection (≥type C or flow-limited, white arrow); (K) bailout stent deployment (black arrow, the distance between the proximal end of the stent and the ostium of the vessel ≥ 3 mm) and final result II. MV, denotes main vessel; NC, non-compliant; SB, side branch; DCB, drug-coated balloon; POT, proximal optimizing technique. If there was a residual stenosis ≤ 30% only in proximal 5 mm regardless of other segment after SB lesion preparation, a DCB + DES strategy was initially selected. DCB angioplasty was done first, followed by stent deployment to ensure complete coverage of the dissection and severe elastic recoil segment (residual stenosis > 30%). The distance between the proximal end of the stent and the ostium of SB ≥ 3 mm.
Non-target lesions were first treated if present.
Follow-up
For 2 years, clinical follow-up was done through office visits or telephone calls every 3 months. Following evaluating the main clinical endpoint, coronary angiography was planned for all patients 12 months after the index procedure, unless clinical indications warranted an earlier angiographic evaluation.
Endpoint and definitions
Target lesion failure (TLF), composed of cardiac death, target vessel myocardial infarction (TVMI), or clinically driven target lesion revascularization (CD-TLR) after 2 years of follow-up, was the primary endpoint. When the cause of death was undetermined or unknown, cardiogenic causes were assumed. Periprocedural myocardial infarction (≤48 h) was defined as cardiac troponin values at least five times the 99th percentile upper reference limit (URL) of the assay plus either: (1) new ischemic ECG changes or development of new pathological Q waves; (2) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality; or (3) angiographically documented graft or coronary artery occlusion or new severe stenos. Spontaneous myocardial infarction (after 48 h) was defined as a clinical symptom consistent with MI with cardiac troponin values > 1 × URL and new ST-segment elevation or depression or other abnormalities as described above (16). All MIs were deemed TVMIs unless it could be proven that they were caused by non-target vessels (17). Angina or ischemia due to the target lesion necessitating repeat PCI or coronary artery bypass graft (CABG) surgery because of restenosis or thrombosis of the target lesion, which encompassed the proximal and distal edge segments of both branches, was defined as CD-TLR. Secondary outcomes, including all-cause mortality, cardiac death, TVMI, CD-TLR, and stent thrombosis (ST), were also assessed. ST was defined in line with the definition provided by the Academic Research Consortium (17).
Quantitative coronary angiographic assessment
For quantitative coronary angiographic (QCA) analysis, edge detection methods and a bifurcation algorithm (QAngio XA version 7.3, Medis Medical Imaging, Leiden, Netherlands) were used (18). The guiding catheter was employed as the reference for calibration. Measurements were performed at baseline, after the procedure and during follow-up angiography. The MV was determined based on the anatomical nature of the lesion. The QCA variables analyzed included: (a) lesion length; (b) reference vessel diameter (RVD); (c) minimal lumen diameter (MLD); (d) percent diameter stenosis; (e) acute luminal gain (MLD immediately after the procedure minus the MLD before the procedure); and (f) late lumen loss (LLL) (MLD immediately after the procedure minus the MLD at follow up).
Statistical analysis
All findings were analyzed using R version 3.6.1 (The R Foundation, Vienna, Austria1) and EmpowerStats (R) (X&Y Solutions Inc., Boston, MA, USA2). Categorical data were reported as frequencies (percentages), whereas continuous variables were presented as means ± standard deviation. Using Fisher’s exact test for categorical variables and the Mann–Whitney U test (Wilcoxon rank sum test) for continuous variables, the DCB and DES groups were compared.
Due to disparities in baseline characteristics between eligible participants in the two groups of the observational trial, 1:2 propensity score matching (PSM) was utilized to select patients with similar baseline data. After evaluation of covariates associated clinically and/or statistically with the treatment group and removal of repeatedly defined or collinear variables, including baseline characteristics, risk factors, clinical conditions at admission and treatment during operation, 13 variables (including age, sex, diabetes mellitus, hypertension, hyperlipidemia, renal insufficiency, clinical presentation, family history of coronary artery disease, smoking history, prior PCI history, previous MI history, previous CABG history, and left ventricular ejection fraction) were included in the PSM model using greedy (nearest neighbor) matching without replacement and a caliper of 0.02 (Supplementary Figure 1). In both the whole population and the propensity-matched cohort, the primary and secondary outcomes (DCB vs. DES) were evaluated. Using a log-rank test, the outcomes were compared and shown as Kaplan–Meier (KM) curves.
According to age (<60 years, or ≥60 years), gender (male or female), diabetes status (yes or no), and SYNTAX score (0–22, 23–32, or >32), subgroup analyses were conducted. To ensure a baseline balance between the DCB and DES groups throughout subgroup analysis, only matched pairings within a subgroup were selected. For instance, for diabetic patients, only matched pairs of diabetic patients in the DCB and DES groups were included in the study. To evaluate heterogeneity of treatment impact across subgroups, stratified analyses were conducted.
Separate propensity-matched cohorts of patients who received PCI with DCB as opposed to those who had the 2-stent technique and those who underwent PCI with DCB as compared to those who underwent provisional stenting were also assessed for the 2-year primary and secondary outcomes.
For all reported analyses, a two-sided P-value < 0.05 was statistically significant.
Results
Baseline clinical, angiographic, and procedural characteristics
A total of 1,077 LM true bifurcation patients treated with PCI met our inclusion and exclusion criteria. Of these, 792 patients were intended to treat with DES, and the remaining 285 patients were intended to treat with DCB. Finally, 34 patients with residual stenosis > 30% within 5 mm from SB ostium (n = 30); failed SB rewiring (n = 2) and failed SB balloon cross (n = 2) were excluded in the DCB group (Figure 1). Baseline characteristics are shown in Table 1. After matching, 597 patients (199 in the DCB group and 398 in the DES group) were selected. Statistical differences between the groups in age, renal insufficiency, and left ventricular ejection fraction were reduced upon patient matching.
TABLE 1.
Demographic characteristics before and after propensity-score matching*.
| Variable | All patients | Propensity-matched sample | ||||
|
|
|
|||||
| DCB group (n = 251) |
DES group (n = 792) |
P-value | DCB group (n = 199) |
DES group (n = 398) |
P-value | |
| Age (years) | 61.28 ± 11.14 | 63.91 ± 7.76 | 0.001 | 63.75 ± 8.13 | 64.48 ± 7.75 | 0.261 |
| Sex (Male) | 188 (74.90%) | 586 (73.99%) | 0.774 | 145 (72.86%) | 299 (75.13%) | 0.551 |
| Comorbidity | ||||||
| Diabetes mellitus | 96 (38.25%) | 299 (37.75%) | 0.888 | 77 (38.69%) | 165 (41.46%) | 0.517 |
| Hypertension | 125 (49.80%) | 399 (50.38%) | 0.873 | 107 (53.77%) | 205 (51.51%) | 0.602 |
| Hyperlipidemia | 63 (25.10%) | 241 (30.43%) | 0.105 | 54 (27.14%) | 119 (29.90%) | 0.483 |
| History of smoking | 89 (35.46%) | 295 (37.25%) | 0.609 | 72 (36.18%) | 138 (34.67%) | 0.716 |
| Renal insufficiency | 9 (3.59%) | 61 (7.70%) | 0.023 | 7 (3.52%) | 24 (6.03%) | 0.192 |
| Clinical presentation | 0.433 | 0.478 | ||||
| Stable angina | 91 (36.25%) | 309 (39.02%) | 75 (37.69%) | 162 (40.70%) | ||
| Unstable angina | 160 (63.75%) | 483 (60.98%) | 124 (62.31%) | 236 (59.30%) | ||
| Previous MI history | 15 (5.98%) | 56 (7.07%) | 0.549 | 11 (5.53%) | 26 (6.53%) | 0.631 |
| Previous PCI history | 42 (16.73%) | 138 (17.42%) | 0.801 | 31 (15.58%) | 74 (18.59%) | 0.362 |
| Previous CABG history | 4 (1.59%) | 10 (1.26%) | 0.691 | 3 (1.51%) | 7 (1.76%) | 0.822 |
| Family history of CAD | 52 (20.72%) | 188 (23.74%) | 0.322 | 43 (21.61%) | 90 (22.61%) | 0.781 |
| LVEF | 59.59 ± 5.78 | 58.27 ± 6.15 | 0.005 | 59.64 ± 5.21 | 59.38 ± 5.60 | 0.941 |
*Plus–minus values are means ± SD. DCB, drug-coated balloon; DES, drug-eluting stent; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; DES, drug-eluting stent.
Procedural and angiographic baseline features are shown in Tables 2, 3. There were 597 lesions after matching, of which 199 (33.33%) were treated with DCB (hybrid strategy) and 398 (66.67%) with DES-only strategy (DK crush 113; Culotte 90; T stent 18; kissing stent 4; and provisional stenting 173). The mean SYNTAX score of the matched cohort was 32.23 (±7.08), with multivessel disease present in 87.60% of cases. The distal LM bifurcation lesion was classified as Medina 1,1,1; 0,1,1; and 1,0,1 in 80.90%, 10.22%, and 8.88% of cases, respectively and 18.93% were trifurcations lesions. The lesion characteristics of the two groups were similar before and after matching, including RVD, stenosis (%), and lesion length (Ps > 0.05).
TABLE 2.
Lesion characteristics before and after propensity-score matching*.
| Variable | All patients | Propensity-matched sample | ||||
|
|
|
|||||
| DCB group (n = 251) |
DES group (n = 792) |
P-value | DCB group (n = 199) |
DES group (n = 398) |
P-value | |
| Classifications | 0.120 | 0.376 | ||||
| Medina 0,1,1 | 19 (7.57%) | 87 (10.98%) | 16 (8.04%) | 45 (11.31%) | ||
| Medina 1,1,1 | 214 (85.26%) | 629 (79.42%) | 167 (83.92%) | 316 (79.40%) | ||
| Medina 1,0,1 | 18 (7.17%) | 76 (9.60%) | 16 (8.04%) | 37 (9.30%) | ||
| Trifurcation | 52 (20.72%) | 141 (17.80%) | 0.300 | 44 (22.11%) | 69 (17.34%) | 0.160 |
| MV | ||||||
| Total occlusion | 9 (3.59%) | 29 (3.66%) | 0.955 | 7 (3.52%) | 19 (4.77%) | 0.478 |
| Intracoronary thrombus | 2 (0.80%) | 2 (0.25%) | 0.246 | 2 (1.01%) | 2 (0.50%) | 0.604 |
| Calcified lesions | 76 (30.28%) | 214 (27.02%) | 0.315 | 60 (30.15%) | 112 (28.14%) | 0.609 |
| Reference diameter (mm) | 3.30 ± 0.22 | 3.32 ± 0.23 | 0.246 | 3.29 ± 0.22 | 3.31 ± 0.22 | 0.254 |
| MLD (mm) | 0.93 ± 0.34 | 0.96 ± 0.35 | 0.148 | 0.91 ± 0.34 | 0.95 ± 0.37 | 0.186 |
| Diameter stenosis (QCA) | 0.72 ± 0.10 | 0.71 ± 0.10 | 0.210 | 0.72 ± 0.10 | 0.71 ± 0.11 | 0.240 |
| Area stenosis (QCA) | 0.91 ± 0.05 | 0.91 ± 0.06 | 0.210 | 0.91 ± 0.05 | 0.91 ± 0.06 | 0.240 |
| Lesion length (mm) | 31.73 ± 14.66 | 30.07 ± 12.54 | 0.500 | 32.63 ± 15.05 | 30.38 ± 13.05 | 0.189 |
| SB | ||||||
| Total occlusion | 8 (3.19%) | 25 (3.16%) | 0.981 | 6 (3.02%) | 10 (2.51%) | 0.720 |
| Intracoronary thrombus | 2 (0.80%) | 7 (0.88%) | 1.000 | 1 (0.50%) | 4 (1.01%) | 0.669 |
| Calcified lesions | 23 (9.16%) | 82 (10.35%) | 0.585 | 20 (10.05%) | 45 (11.31%) | 0.642 |
| Severe tortuous | 63 (25.10%) | 226 (28.54%) | 0.289 | 53 (26.63%) | 108 (27.14%) | 0.896 |
| Reference diameter (mm) | 3.01 ± 0.49 | 2.97 ± 0.32 | 0.264 | 3.00 ± 0.49 | 2.98 ± 0.32 | 0.584 |
| MLD (mm) | 0.97 ± 0.40 | 0.99 ± 0.36 | 0.786 | 0.95 ± 0.40 | 1.02 ± 0.35 | 0.069 |
| Diameter stenosis (QCA) | 0.67 ± 0.12 | 0.67 ± 0.12 | 0.857 | 0.67 ± 0.12 | 0.66 ± 0.11 | 0.225 |
| Area stenosis (QCA) | 0.88 ± 0.07 | 0.88 ± 0.07 | 0.833 | 0.88 ± 0.07 | 0.87 ± 0.07 | 0.317 |
| Lesion length (mm) | 15.25 ± 6.96 | 15.02 ± 6.94 | 0.636 | 15.51 ± 7.07 | 15.11 ± 6.80 | 0.490 |
| Bifurcation angle (Degrees) | 78.89 ± 23.16 | 78.78 ± 23.11 | 0.963 | 78.47 ± 22.82 | 76.84 ± 23.02 | 0.427 |
| Multivessel disease | 223 (88.84%) | 701 (88.51%) | 0.885 | 175 (87.94%) | 348 (87.44%) | 0.861 |
| SYNTAX score | 32.51 ± 7.13 | 31.93 ± 6.88 | 0.146 | 32.47 ± 7.24 | 32.11 ± 7.01 | 0.440 |
| 0–22 | 28 (11.16%) | 91 (11.49%) | 0.884 | 24 (12.06%) | 50 (12.56%) | 0.978 |
| 23–32 | 87 (34.66%) | 286 (36.11%) | 67 (33.67%) | 135 (33.92%) | ||
| >32 | 136 (54.18%) | 415 (52.40%) | 108 (54.27%) | 213 (53.52%) | ||
*Plus–minus values are means ± SD. DCB, drug-coated balloon; DES, drug-eluting stent; MV, main vessel; SB, side branch; MLD, minimal lumen diameter; QCA, quantitative coronary angiographic; SYNTAX, synergy between percutaneous coronary intervention with taxus and cardiac surgery.
TABLE 3.
Procedural characteristics before and after propensity-score matching*.
| Variable | All patients | Propensity-matched sample | ||||
|
|
|
|||||
| DCB group (n = 251) |
DES group (n = 792) |
P-value | DCB group (n = 199) |
DES group (n = 398) |
P-value | |
| Transradial approach | 239 (95.22%) | 711 (89.77%) | 0.008 | 191 (95.98%) | 361 (90.70%) | 0.021 |
| 6F guiding catheter used | 174 (69.32%) | 425 (53.66%) | <0.001 | 133 (66.83%) | 223 (56.03%) | 0.011 |
| MV lesion preparation (pre-dilation) | 251 (100.00%) | 792 (100.00%) | - | 199 (100.00%) | 398 (100.00%) | - |
| MV stent | ||||||
| Diameter (mm) | 3.19 ± 0.39 | 3.19 ± 0.40 | 0.992 | 3.16 ± 0.39 | 3.17 ± 0.39 | 0.959 |
| Total length (mm) | 37.84 ± 14.60 | 36.20 ± 12.70 | 0.458 | 38.69 ± 15.05 | 36.49 ± 13.19 | 0.248 |
| Covered ostial LM | 148 (58.96%) | 478 (60.35%) | 0.695 | 116 (58.29%) | 234 (58.79%) | 0.906 |
| SB lesion preparation | ||||||
| Semi-compliant balloon | 251 (100.00%) | 792 (100.00%) | - | 199 (100.00%) | 398 (100.00%) | - |
| Non-compliant balloon | 205 (81.67%) | 316 (39.90%) | <0.001 | 164 (82.41%) | 161 (40.45%) | <0.001 |
| Maximum pre-dilation balloon diameter (mm) | 2.86 ± 0.52 | 2.30 ± 0.33 | <0.001 | 2.84 ± 0.51 | 2.31 ± 0.34 | <0.001 |
| Maximum pre-dilation balloon diameter/RD ratio | 0.93 ± 0.08 | 0.78 ± 0.07 | <0.001 | 0.93 ± 0.08 | 0.77 ± 0.07 | <0.001 |
| SB stent (2-stent strategies) | ||||||
| Number of DESs used (per lesion) | – | 1.12 ± 0.329 | – | – | 1.13 ± 0.34 | – |
| Diameter (mm) | – | 2.82 ± 0.31 | – | – | 2.81 ± 0.30 | – |
| Total length (mm) | – | 22.07 ± 7.46 | – | – | 22.37 ± 7.54 | – |
| DCB/DES use (hybrid strategy) | ||||||
| Number of DCBs used (per lesion) | 1.01 ± 0.11 | – | 1.02 ± 0.12 | – | ||
| DCB diameter (mm) | 2.89 ± 0.39 | – | 2.88 ± 0.39 | – | ||
| DCB/RD ratio | 0.95 ± 0.09 | – | 0.95 ± 0.09 | – | ||
| Length of DCB balloon (mm) | 18.51 ± 4.93 | – | 18.84 ± 5.22 | – | ||
| Inflation pressure (bar) | 8.31 ± 1.10 | – | 8.25 ± 1.06 | – | ||
| DCB + DES (planned)† | 99 (39.44%) | – | 69 (34.67%) | – | ||
| SB dissection after DCB intervention (%) | - | |||||
| None | 184 (73.31%) | – | 141 (70.85%) | – | ||
| Type A | 39 (15.54%) | – | 34 (17.09%) | – | ||
| Type B | 19 (7.57%) | – | 17 (8.54%) | – | ||
| Type C | 5 (1.99%) | – | 5 (2.51%) | – | ||
| Type D | 3 (1.20%) | – | 1 (0.50%) | – | ||
| Type E | 1 (0.40%) | – | 1 (0.50%) | – | ||
| SB bailout stenting | 9 (3.59%) | – | – | 7 (3.52%) | – | – |
| Final strategy | – | – | ||||
| Hybrid strategy (DES + DCB) | 251 (100.00%) | – | 199 (100.00%) | |||
| DK crush | – | 239 (30.18%) | – | 113 (28.39%) | ||
| Culotte | – | 173 (21.84%) | – | 90 (22.61%) | ||
| T stent | – | 33 (4.17%) | – | 18 (4.52%) | ||
| Kissing stent | – | 9 (1.13%) | – | 4 (1.01%) | ||
| 1-stent strategy | – | 338 (42.68%) | – | 173 (43.47%) | ||
| Final kissing inflation | 246 (98.01%) | 721 (91.04%) | <0.001 | 197 (98.99%) | 360 (90.45%) | <0.001 |
| POT performed | 246 (98.01%) | 721 (91.04%) | <0.001 | 197 (98.99%) | 360 (90.45%) | <0.001 |
| Staged PCI | 97 (38.65%) | 314 (39.65%) | 0.777 | 84 (42.21%) | 166 (41.71%) | 0.907 |
| Complete revascularization | 204 (81.27%) | 639 (80.68%) | 0.835 | 161 (80.90%) | 315 (79.15%) | 0.614 |
| Procedural IVUS use | 108 (43.03%) | 345 (43.56%) | 0.882 | 87 (43.72%) | 173 (43.47%) | 0.953 |
| Procedural time (min) | 66.49 ± 33.46 | 75.83 ± 30.92 | <0.001 | 65.50 ± 32.34 | 77.49 ± 30.72 | <0.001 |
| Glycoprotein IIb/IIIa inhibitor used | 128 (51.00%) | 392 (49.49%) | 0.679 | 104 (52.26%) | 198 (49.75%) | 0.563 |
*Plus–minus values are means ± SD. DCB, drug-coated balloon; DES, drug-eluting stent; RD, reference diameter; MV, main vessel; SB, side branch; LM, left main; DK, double kissing; POT, proximal optimization technique; PCI, percutaneous coronary intervention; IVUS, intravascular ultrasound.
†For diffuse lesion in SB, DCB + DES implantation was initially selected after SB lesion preparation. DCB angioplasty was done first, followed by stent deployment to ensure complete coverage of the dissection and severe elastic recoil segment (residual stenosis > 30%). The distance between the proximal end of the stent and the ostium of SB ≥ 3 mm.
Lesion preparation of the MV and SB was done for all lesions. The proportion of non-compliant balloons for SB preparation in the DCB group was significantly higher than in the DES group (82.41% vs. 40.45%, P < 0.001), which is consistent with clinical practice. More subjects underwent kissing inflation and POT in the DCB group compared with the DES group. In the DCB group, a DCB + DES strategy in SB was initially selected for 69 (34.67%) patients due to diffuse SB lesions. The bailout stenting rate was low in the DCB group (3.59%) due to appropriate lesion preparation. The rates of staged PCI and complete revascularization were similar in the 2 groups, although procedural times were longer in the DES group than in the DCB group. Furthermore, 260 (43.55%) patients underwent intravascular ultrasound (IVUS) guidance during the procedure.
Clinical outcomes
At 30-day follow-up, TLF occurred more frequently in the DES group than in the DCB group (3.52% vs. 0.50%; P = 0.026). There were no statistical differences regarding the incidence of cardiac death, TVMI, CD-TLR, and ST (Ps > 0.05).
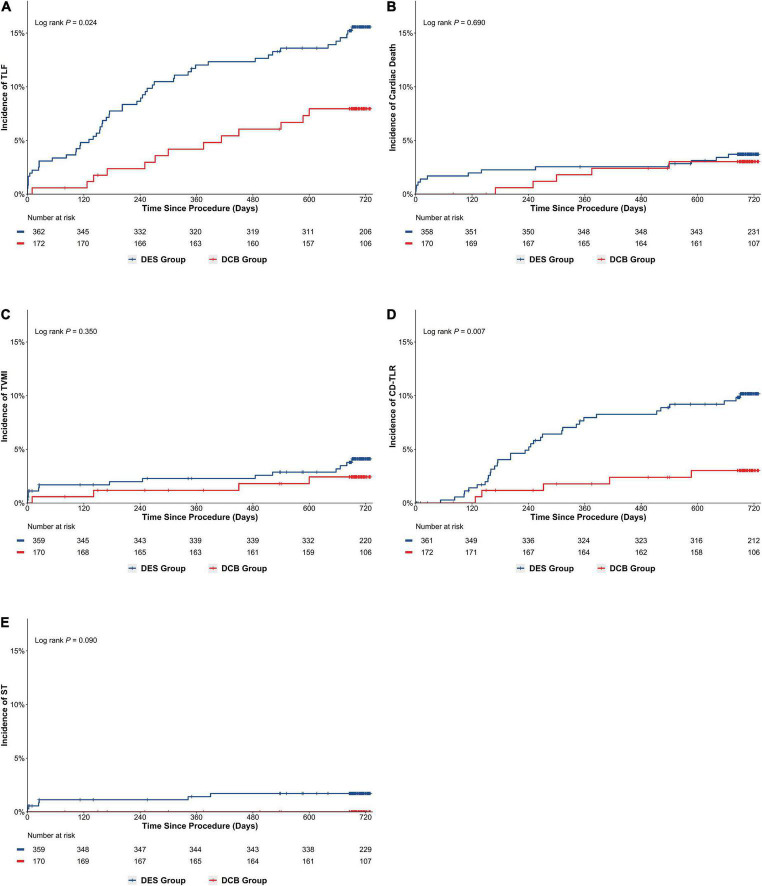
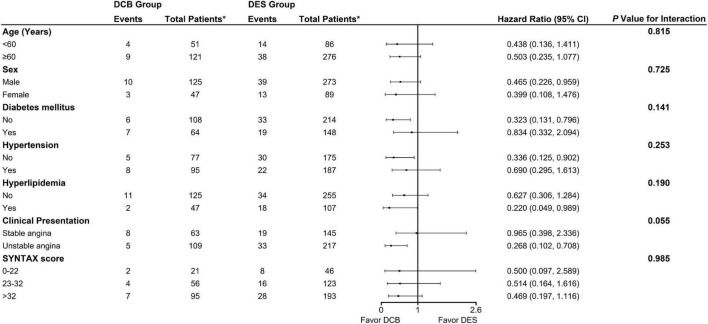
Following up on 89.36% of patients for a median of 729 days revealed TLF incidence rates of 8.64 and 14.64% in DCB and DES groups, respectively [Odds ratio (OR), 0.551; 95% Confidence interval (CI), 0.330–0.921; P = 0.021]. After PSM, the DCB group exhibited lower TLF incidence than the DES group (7.56% vs. 14.36%; OR, 0.487; 95% CI, 0.258–0.922; P = 0.025; Log-rank P = 0.024) (Table 4 and Figure 3A). The results were largely similar in the subgroup analyses based on selected characteristics. Moreover, there were no significant interactions between subgroups for the 2-year rate of TLF (Figure 4).
TABLE 4.
Risk of clinical outcomes at 30-day and long-term follow-up*.
| Endpoint | All patients | Propensity-matched sample | ||||||
|
|
|
|||||||
| DCB group (n = 251) |
DES group (n = 792) |
Odds ratio (95% CI) | P-value | DCB group (n = 199) |
DES group (n = 398) |
Odds ratio (95% CI) | P-value | |
| 30-day follow up, n% | 251 (100.00%) | 792 (100.00%) | – | – | 199 (100.00%) | 398 (100.00%) | – | – |
| TLF† | 1 (0.40%) | 26 (3.28%) | 0.118 (0.016–0.873) | 0.012 | 1 (0.50%) | 14 (3.52%) | 0.139 (0.018–1.061) | 0.026 |
| Cardiac death | 0 (0.00%) | 9 (1.14%) | – | 0.124 | 0 (0.00%) | 6 (1.51%) | – | 0.186 |
| TVMI | 1 (0.40%) | 15 (1.89%) | 0.207 (0.057–1.576) | 0.138 | 1 (0.50%) | 6 (1.51%) | 0.330 (0.039–2.760) | 0.434 |
| Periprocedural | 0 (0.00%) | 10 (1.26%) | – | 0.130 | 0 (0.00%) | 4 (1.01%) | – | 0.307 |
| Non-periprocedural | 1 (0.40%) | 15 (0.63%) | 0.630 (0.073–5.414) | 1.000 | 1 (0.50%) | 2 (0.50%) | 1.000 (0.090–11.095) | 1.000 |
| Clinically driven TLR | 0 (0.00%) | 2 (0.25%) | – | 1.000 | 0 (0.00%) | 0 (0.00%) | – | – |
| Stent thrombosis | 0 (0.00%) | 10 (1.26%) | – | 0.130 | 0 (0.00%) | 4 (1.01%) | – | 0.307 |
| Definite | 0 (0.00%) | 2 (0.25%) | – | 1.000 | 0 (0.00%) | 0 (0.00%) | – | – |
| Probable | 0 (0.00%) | 8 (1.01%) | – | 0.210 | 0 (0.00%) | 4 (1.01%) | – | 0.307 |
| 1-year follow up, n% | 236 (94.02%) | 762 (96.21%) | – | 0.137 | 187 (93.97%) | 385 (96.73%) | – | 0.112 |
| TLF† | 10 (4.24%) | 75 (9.82%) | 0.406 (0.207–0.800) | 0.007 | 6 (3.21%) | 42 (10.91%) | 0.271 (0.113–0.649) | 0.002 |
| Cardiac death | 2 (0.85%) | 16 (2.10%) | 0.399 (0.091–1.746) | 0.271 | 2 (1.07%) | 9 (2.34%) | 0.452 (0.097–2.112) | 0.517 |
| TVMI | 3 (1.27%) | 19 (2.49%) | 0.504 (0.148–1.717) | 0.264 | 2 (1.07%) | 8 (2.08%) | 0.509 (0.107–2.423) | 0.511 |
| Clinically driven TLR | 7 (2.97%) | 49 (6.41%) | 0.446 (0.199–0.998) | 0.044 | 3 (1.60%) | 27 (7.01%) | 0.216 (0.065–0.722) | 0.006 |
| Stent thrombosis | 0 (0.00%) | 13 (1.71%) | – | 0.047 | 0 (0.00%) | 5 (1.30%) | – | 0.178 |
| Definite | 0 (0.00%) | 4 (0.52%) | – | 0.578 | 0 (0.00%) | 1 (0.26%) | – | 1.000 |
| Probable | 0 (0.00%) | 9 (1.18%) | – | 0.126 | 0 (0.00%) | 4 (1.04%) | – | 0.309 |
| All-cause death | 5 (2.12%) | 21 (2.76%) | 0.764 (0.285–2.048) | 0.591 | 5 (2.67%) | 10 (2.60%) | 1.030 (0.347–3.058) | 1.000 |
| 2-year follow up, n% | 218 (86.85%) | 714 (90.15%) | – | 0.140 | 170 (85.43%) | 358 (89.95%) | – | 0.103 |
| TLF† | 19 (8.64%) | 106 (14.64%) | 0.551 (0.330–0.921) | 0.021 | 13 (7.56%) | 52 (14.36%) | 0.487 (0.258–0.922) | 0.025 |
| Cardiac death | 7 (3.21%) | 30 (4.20%) | 0.756 (0.328-1.747) | 0.512 | 5 (2.94%) | 13 (3.63%) | 0.804 (0.282–2.293) | 0.683 |
| TVMI | 5 (2.29%) | 29 (4.05%) | 0.556 (0.213–1.454) | 0.225 | 4 (2.35%) | 14 (3.90%) | 0.594 (0.192–1.832) | 0.359 |
| Clinically driven TLR | 9 (4.09%) | 70 (9.68%) | 0.398 (0.195–0.810) | 0.009 | 5 (2.91%) | 34 (9.42%) | 0.288 (0.111–0.750) | 0.007 |
| Stent thrombosis | 0 (0.00%) | 17 (2.38%) | – | 0.018 | 0 (0.00%) | 6 (1.67%) | – | 0.184 |
| Definite | 0 (0.00%) | 6 (0.84%) | – | 0.345 | 0 (0.00%) | 1 (0.28%) | – | 1.000 |
| Probable | 0 (0.00%) | 11 (1.54%) | – | 0.077 | 0 (0.00%) | 5 (1.39%) | – | 0.182 |
| All-cause death | 12 (5.50%) | 41 (5.74%) | 0.956 (0.493–1.854) | 0.894 | 10 (5.88%) | 19 (5.31%) | 1.115 (0.507–2.453) | 0.786 |
*DCB, drug-coated balloon; DES, drug-coated balloon; CI, confidence interval; TLF, target lesion failure; TVMI, target vessel myocardial infarction; TLR, target lesion revascularization; MI, myocardial infraction.
†TLF defined as the composite outcome of cardiac death, target vessel myocardial infarction, and clinical driven target lesion revascularization.
FIGURE 3.
Cumulative risks of the study outcomes in the matched cohort (Kaplan–Meier time-to-first event curves). (A) TLF; (B) cardiac death; (C) TVMI; (D) CD-TLR; (E) ST. TLF, target vessel failure; TVMI, target vessel myocardial infraction; CD-TLR, clinically driven target lesion revascularization; ST, stent thrombosis.
FIGURE 4.
Subgroup analysis: Risk of primary outcome among propensity-score matched cohort. “*Total patients” means numbers of patients at follow up. DCB, drug-coated balloon; DES, drug-eluting stent; SYNTAX, synergy between percutaneous coronary intervention with taxus and cardiac surgery.
After matching, KM analysis (Table 4 and Figure 3) revealed that the cumulative rate of CD-TLR (2.91% vs. 9.42%; OR, 0.288; 95% CI, 0.111–0.750; P = 0.007; Log-rank P = 0.007) was significantly lower in the DCB group than in the DES group at 2 years. However, no significant difference was found between the DCB and DES groups in the incidence of cardiac death (2.94% vs. 3.63%; P = 0.683), TVMI (2.35% vs. 3.90%; P = 0.359), ST (0.00% vs. 1.67%; P = 0.184) or all-cause death (5.88% vs. 5.31%; P = 0.786).
Quantitative coronary angiographic measurement
Angiographic follow-up was completed in 65.96% (688/1043) of patients. Results from the QCA data analysis are shown in Tables 2, 5. Baseline characteristics as assessed by QCA were comparable between the two groups. MLD post-intervention and acute lumen gain were similar in both groups, including MV and SB. During follow-up angiography, the MLD of SB in the DES group was smaller than the DCB group (2.05 ± 0.72 mm vs. 2.32 ± 0.71 mm, P < 0.001) while LLL was larger (0.42 ± 0.62 mm vs. 0.13 ± 0.42 mm, P < 0.001). Results were comparable before and after PSM.
TABLE 5.
Quantitative coronary angiographic (QCA) results before and after propensity-score matching*.
| Variable | All patients | Propensity-matched sample | ||||
|
|
|
|||||
| DCB group (n = 251) |
DES group (n = 792) |
P-value | DCB group (n = 199) |
DES group (n = 398) |
P-value | |
| Lesions performed angiographic follow up | 176 (70.12%) | 512 (64.65%) | 0.111 | 136 (68.34%) | 258 (64.82%) | 0.392 |
| Main vessel | ||||||
| Post-intervention MLD (mm) | 3.12 ± 0.22 | 3.14 ± 0.21 | 0.084 | 3.14 ± 0.22 | 3.10 ± 0.22 | 0.016 |
| Acute lumen gain (mm) | 2.19 ± 0.35 | 2.18 ± 0.37 | 0.318 | 2.20 ± 0.39 | 2.19 ± 0.36 | 0.728 |
| Follow up MLD (mm) | 2.89 ± 0.53 | 2.91 ± 0.48 | 0.606 | 2.85 ± 0.54 | 2.93 ± 0.49 | 0.170 |
| Late lumen loss (mm) | 0.24 ± 0.45 | 0.23 ± 0.42 | 0.817 | 0.25 ± 0.48 | 0.22 ± 0.42 | 0.523 |
| Side branch | ||||||
| Post-intervention MLD (mm) | 2.50 ± 0.55 | 2.45 ± 0.40 | 0.550 | 2.47 ± 0.40 | 2.48 ± 0.52 | 0.708 |
| Acute lumen gain (mm) | 1.53 ± 0.58 | 1.46 ± 0.48 | 0.132 | 1.46 ± 0.47 | 1.53 ± 0.57 | 0.132 |
| Follow up MLD (mm) | 2.34 ± 0.71 | 2.04 ± 0.70 | <0.001 | 2.32 ± 0.71 | 2.05 ± 0.72 | <0.001 |
| Late lumen loss (mm) | 0.12 ± 0.40 | 0.42 ± 0.59 | <0.001 | 0.13 ± 0.42 | 0.42 ± 0.62 | <0.001 |
*Plus–minus values are means ± SD. DCB, drug-coated balloon; DES, drug-coated balloon; MLD, minimal lumen diameter.
Sensitivity analysis
During analysis of PCI with DCB versus the 2-stent technique (225 matched pairs), the DCB group was associated with lower risks of TLF (9.60% vs. 17.14%, P = 0.026), CD-TLR (4.55% vs. 11.90%, P = 0.007) and ST (0.00% vs. 2.87%, P = 0.031) while the risks of other outcomes were similar (Supplementary Table 1). During analysis of PCI with DCB versus provisional stenting (202 matched pairs), DCB was associated with significantly lower rates of TLF (7.39 and 14.20%, respectively; P = 0.039), CD-TLR (3.41 and 8.57%, respectively; P = 0.041), and ST (0.00 and 2.89%, respectively; P = 0.030) but similar risks of other outcomes (Supplementary Table 2).
Discussion
This is the first multicenter research to investigate the viability of a hybrid technique employing DES in the main vessel and DCB in the side branch for the treatment of patients with LM true bifurcation lesions, as far as we are aware. Importantly, we found that compared to the DES group, patients treated with DCB had decreased rates of TLF and CD-TLR at 2 years. The incidence rates of cardiac death, TVMI, ST, and all-cause mortality were comparable between the two groups, indicating that DCB (hybrid approach) was both safe and effective for treating de novo LM true bifurcation lesions. Therefore, DCB may be an alternative to stenting or perhaps the first-choice therapy for individuals who are qualified. For DCB/DES-treated SB, we discovered that the LLL at follow-up was greater in the DES group than in the DCB group. Due to the fact that our results are based on PSM, it is unlikely that they are the result of negative confounding. In addition, the validity of these data was validated using subgroup analysis and sensitivity analysis techniques.
Given the EBC’s KISSS (keep it simple, swift, and safe) recommendation, provisional SB stenting should be considered the standard therapeutic approach (19). However, the double-kissing crush approach offers the most favorable outcome data for LM true bifurcation lesions (20, 21). During clinical practice, deciding whether to execute SB dilation following crossover stenting in the LM can be challenging. In accordance with the “provisional” method, SB intervention is advised if the SB outcome is deemed sub-optimal. However, defining a “sub-optimal” result for the LCx ostium is challenging and undefined. Even in the absence of a sub-optimal SB result, the need of eliminating stent struts from the SB ostium in order to improve access to the LCx remains contentious. In fact, the presence of “floating” struts across the ostium may aid in the development of “fenestrated” LCx ostial restenosis. In contrast, a thorough registry of patients treated with crossover stenting from LM to the left anterior descending (LAD) branch indicated that the 5-year cumulative incidence of TLR was not significantly different between the kissing balloon and non-kissing balloon groups (22). Therefore, the appropriate management of coronary bifurcation lesions remains debatable, especially with LM true bifurcations (23).
Drug-coated balloon are emerging devices with established efficacy in ISR, de novo, and small vessels that may provide potential advantages in bifurcation PCI and circumvent the restrictions encountered with this group of lesions (24, 25). In the last several years, many clinical trials and registries have studied the efficacy of DCB in treating bifurcation lesions. In bifurcation lesions, the postulated function of DCB is to retain the simplicity of provisional stenting while reducing SB restenosis. However, it has been observed that the combination of pre-dilatation with a DCB and MV stenting with a bare metal stent is inferior to DES plus uncoated balloon due to unsatisfactory outcomes in the MV and equivalent outcomes in the side branch (11, 26). Thus, there are presently two DCB therapy options for bifurcation lesions: (1) DCBs in both MV and SB, and (2) DCB in SB and DES in MV.
Schulz et al. (27) investigated a DCB-only strategy initially, reporting a low incidence of SB and MV restenosis (3.3 and 6.6%, respectively) using second-generation DCB. Furthermore, a reduced prevalence of MACE (7.7%) was also observed, with 33% of the treated lesions located at the LM bifurcation. In the PEPCAD-BIF trial (28), after SB and MV predilatation, patients were randomly allocated to undergo either a DCB therapy of the MV and SB or plain balloon angioplasty. As potential benefits, the authors indicated the low incidence of TLR (31% vs. 9.4%), the increased physiological blood flow achieved, and the lack of carina shift. Bruch et al. (29) compared a DCB-only approach with a DCB + DES strategy. In the absence of thrombotic events, they identified a 9-month TLR of 4.6% and major adverse cardiovascular events (MACE) of 6.2% in patients treated with a DCB-only strategy. For specific bifurcation lesions, they found that therapy with DCB alone was both safe and effective.
Fifty patients in the DEBSIDE study (30) were treated with DES in the MV and the DANUBIO balloon (a new type of DCB) in the SB. After 12 months, the incidence of TLR was 10% in the MV and 2% in the SB. In contrast, the BIOLUX-I study (31) assessed the feasibility of provisional stenting with an everolimus-eluting stent (EES) in the MV and a paclitaxel-coated balloon in the SB. Following a 9-month follow-up, the MACE, TLR, and LLL were all low, and 11 of the 35 lesions were identified by core lab analysis to be true bifurcation lesions. The SARPEDON study (10) evaluated the effectiveness of treating the SB ostium with DCB following DES implantation in MV, followed by the kissing balloon technique. At 1-year of follow-up, the MACE rate was 19.0%, with three target vessel revascularization and two all-cause death. The rates of restenosis for MV and SB were 4 and 6%, respectively, with all SB restenosis occurring at the ostium.
The German Consensus Group initially suggested using DCB to treat bifurcation lesions (32). The administration of DCB maintains the natural morphology of the bifurcation, which is especially important in the carina area, and enables the uniform distribution of a high dose of the antiproliferative drug throughout the whole vessel surface. The use of DCB in the SB reduces the risk of incomplete coverage of the bifurcation region, scaffolding of the SB ostium, stent distortions in the MV by SB access, and overlapping and crushing of multiple metal layers and polymers with uncontrolled drug release compared to the other 2-stent techniques. The use of DCB provides a theoretical advantage over the plain balloon, with favorable vessel remodeling and plaque stabilization, as well as improved late angiographic findings, even with the simplest provisional approach.
Overall, currently available data are inconclusive and leave many concerns unsolved. The primary one is that the sequence of SB with DCB treatment remains unknown. In this research, a stent was first implanted in the MV. It should be borne in mind that DCB angioplasty of the SB first may complicate SB rewiring after MV stenting because the SB may be dissected during the DCB intervention. The SB ostium may have substantial residual stenosis and compromise the eventual therapeutic effect if the SB orifice is not dilated sufficiently after MV stenting. To prevent difficulties in rewiring the SB following MV stenting, the SB ostium may be protected by a jailed balloon or initially inflated with a small balloon. Moreover, SB rewiring via the distal struts of the stent is advised because this offers better scaffolding of the SB ostium and prevents stent distortion.
In addition, there are several difficulties, including SB lesion preparation and procedure (with or without final kissing ballooning or repeat POT). Appropriate lesion preparation improves acute gain, remodeling and prevents flow-limiting dissection. The SB ostium has greater calcium and fibrous tissue composition and a greater elastic recoil. In trying to attain the aim of ≤30% residual stenosis within 5 mm of the SB ostium, the average maximum balloon-vessel diameter ratio reached 0.93, which can significantly prolong the balloon expansion period in clinical practice. In this research, 30 (10.53%) patients were excluded owing to residual stenosis > 30% within 5 mm from the SB ostium. For bifurcation lesion preparation, non-compliant and plain semi-compliant balloons were used more often than scoring balloons since after implanting the MV stent, the scoring balloon may get trapped in the struts and cannot be withdrawn, or this type of balloon may cause damage to the DES polymer. Of these 30 patients, 25 still underwent palliative side branch DCB angioplasty (residual stenosis was 30–50%). The area of the side branch supply was not particularly large in some cases, with 30–50% residual stenosis, which was sufficient to provide blood supply to the corresponding myocardium; and balloon underexpansion was observed when lesion preparation at the side branch ostium. If SB stent implantation is undertaken on these individuals, it will surely lead to underexpansion or malposition of the SB stent. It is widely recognized that underexpansion or malposition of a stent is a significant cause of stent failure. In the remaining five cases, 2-stent was instead performed. During the 2-year follow-up, there were no clinical events among these patients.
Side branch dilation alone reduces MV stent volume and distorts MV stent symmetry; accordingly, a kissing balloon should be used to remedy stent distortion (33). The MV balloon should be inflated first, followed by simultaneous deflation of both balloons (34, 35). Importantly, the fractal geometry of bifurcations necessitates controlled modification of the 3D meshwork of the stent for optimal coronary bifurcation treatment (36). POT should be regarded as a routine procedure in bifurcation treatment since it permits reconstruction of the basic physiologic anatomy and facilitates wire interchange (37).
The mean SB lesion length in the DCB group was 15.51 (±7.07) mm, indicating that the DCB-only technique is not always practicable. After SB lesion preparation, a DCB + DES approach was initially adopted for 69 (34.67%) patients. This finding offers us sufficient confidence to perform enough pre-dilation without the misgivings of severe dissection.
Given that stent thrombosis at LM bifurcation lesions is critical, it is essential to minimize the risk of stent thrombosis to the greatest extent feasible. During the 2-year follow-up period, no thrombotic events occurred in the DCB group in this study. Although there is no statistical difference between the two groups, the value of 0% has a significant value.
Notably, some patients in the DCB group had a negative LLL of SB. Nonetheless, the fundamental cause could not be elucidated, although it is widely thought to result from vascular remodeling. Moreover, even if a severe dissection occurs during DCB angioplasty, switching to a 2-stent technique is not needed. The method of bailout stenting used in this study differs from earlier studies. It is well-established that the bailout stent should always be located at least 3 mm from the ostium of SB. Moreover, a 3–5 mm distance prevents acute occlusion of the SB in the event of severe dissection or hemorrhage. Importantly, the stent covered only the segment with dissection > type B and/or residual stenosis > 30% following DCB angioplasty, which forms the basis of our innovative technique for achieving high success rates and great clinical results without the risk of acute SB occlusion. Simultaneously, this method eliminates the metal struts covering the carina by the 2-stent technique and drastically minimizes the operation duration, procedure complexity, and learning curve.
Study limitations
Due to the non-randomized nature of this observational research, it is susceptible to selection and ascertainment bias despite our rigorous PSM. Due to the exploratory character of this work, an “a priori” sample size calculation was not performed. In addition, over 90% of the patients had multivessel disease, and most had undergone complete revascularization. Moreover, the complexity of lesions (median SYNTAX score was 33 [17–42]) may increase the incidence of clinical events. Based on the KM curves of TLF, TVMI, and CD-TLR, the incidence gap between the two groups grew substantially over time. Therefore, there is reason to assume that the clinical results of the DCB group will become more robust as the length of the follow-up increases. Lastly, the DCB-only method in patients with LM true bifurcation is unquestionably interesting; nonetheless, no statistical analysis could be conducted due to the lack of available data, emphasizing the need for further studies.
Conclusion
In patients with LM true bifurcation disease undergoing PCI, the DCB group was associated with a lower risk of prespecified clinical outcomes at 2 years. In terms of both safety and efficacy, PCI with DCB was superior to other DES-only strategies. Our findings warrant further investigation in large, randomized clinical trials with long-term follow-up to define optimal treatment strategies for this special coronary lesion set.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
CQ, WL, ZH, JD, and LP contributed to conception and design of the study. LP, WL, SP, XW, YS, MP, XQ, GS, and PZ organized the database. LP performed the statistical analysis. LP and WL wrote the first draft of the manuscript. LP, WL, and ZH wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
We thank the physicians who helped this research (especially Guanghui Liu, Luosha Zhao, Youyou Du, Zhenwen Huang, Feifei Zhang, Xule Wang, and Xiaolin Zheng) and colleagues who participated in data collection (including Peng Qin, Sen Guo, Yanjun Zhou, Wencai Zhang, Shuai Zhou, Ran Li, and Qiangwei Shi).
Footnotes
Funding
This study was funded by the Medical Science and Technique Research Plan of Henan Province (Provincial and Ministerial Co-construction Project) (Grant No. SB201901027).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1028007/full#supplementary-material
References
- 1.Burzotta F, Lassen JF, Banning AP, Lefevre T, Hildick-Smith D, Chieffo A, et al. Percutaneous coronary intervention in left main coronary artery disease: the 13th consensus document from the European Bifurcation Club. EuroIntervention. (2018) 14:112–20. 10.4244/EIJ-D-18-00357 [DOI] [PubMed] [Google Scholar]
- 2.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Chen S, Huang L, Hildick-Smith D, Ferenc M, Jabbour RJ, et al. Definite stent thrombosis after drug-eluting stent implantation in coronary bifurcation lesions: a meta-analysis of 3,107 patients from 14 randomized trials. Catheter Cardiovasc Interv. (2018) 92:680–91. 10.1002/ccd.27443 [DOI] [PubMed] [Google Scholar]
- 4.Zimarino M, Corazzini A, Ricci F, Di Nicola M, De Caterina R. Late thrombosis after double versus single drug-eluting stent in the treatment of coronary bifurcations: a meta-analysis of randomized and observational Studies. JACC Cardiovasc Interv. (2013) 6:687–95. 10.1016/j.jcin.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 5.Lu W, Zhu Y, Han Z, Wang X, Wang X, Qiu C. Drug-coated balloon in combination with bare metal stent strategy for de novo coronary artery disease: a PRISMA-compliant meta-analysis of randomized clinical trials. Medicine. (2017) 96:e6397. 10.1097/MD.0000000000006397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Lu W, Wang X, Pan L, Fu W, Liu Q, et al. Drug-coated balloon angioplasty: predicting outcomes based on different patterns of drug-eluting stent restenosis. Int J Cardiovasc Imaging. (2020) 36:171–8. 10.1007/s10554-019-01681-y [DOI] [PubMed] [Google Scholar]
- 7.Lu W, Zhu Y, Han Z, Sun G, Qin X, Wang Z, et al. Short-term outcomes from drug-coated balloon for coronary de novo lesions in large vessels. J Cardiol. (2019) 73:151–5. 10.1016/j.jjcc.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 8.Jeger RV, Farah A, Ohlow MA, Mangner N, Mobius-Winkler S, Weilenmann D, et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet. (2020) 396:1504–10. 10.1016/S0140-6736(20)32173-5 [DOI] [PubMed] [Google Scholar]
- 9.Ali RM, Degenhardt R, Zambahari R, Tresukosol D, Ahmad WA, Kamar H, et al. Paclitaxel-eluting balloon angioplasty and cobalt-chromium stents versus conventional angioplasty and paclitaxel-eluting stents in the treatment of native coronary artery stenoses in patients with diabetes mellitus. EuroIntervention. (2011) 7(Suppl K):K83–92. 10.4244/EIJV7SKA15 [DOI] [PubMed] [Google Scholar]
- 10.Jim MH, Lee MK, Fung RC, Chan AK, Chan KT, Yiu KH. Six month angiographic result of supplementary paclitaxel-eluting balloon deployment to treat side branch ostium narrowing (SARPEDON). Int J Cardiol. (2015) 187:594–7. 10.1016/j.ijcard.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 11.Lopez Minguez JR, Nogales Asensio JM, Doncel Vecino LJ, Sandoval J, Romany S, Martinez Romero P, et al. A prospective randomised study of the paclitaxel-coated balloon catheter in bifurcated coronary lesions (BABILON trial): 24-month clinical and angiographic results. EuroIntervention. (2014) 10:50–7. 10.4244/EIJV10I1A10 [DOI] [PubMed] [Google Scholar]
- 12.Megaly M, Rofael M, Saad M, Shishehbor M, Brilakis ES. Outcomes with drug-coated balloons for treating the side branch of coronary bifurcation lesions. J Invasive Cardiol. (2018) 30:393–9. [PubMed] [Google Scholar]
- 13.Medina A, Suarez de Lezo J, Pan M. [A new classification of coronary bifurcation lesions]. Rev Esp Cardiol. (2006) 59:183. [PubMed] [Google Scholar]
- 14.Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2018) 39:213–60. 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 15.Huber MS, Mooney JF, Madison J, Mooney MR. Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am J Cardiol. (1991) 68:467–71. 10.1016/0002-9149(91)90780-o [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72:2231–64. 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 17.Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. (2007) 356:1020–9. 10.1056/NEJMoa067731 [DOI] [PubMed] [Google Scholar]
- 18.Collet C, Onuma Y, Cavalcante R, Grundeken M, Genereux P, Popma J, et al. Quantitative angiography methods for bifurcation lesions: a consensus statement update from the European Bifurcation Club. EuroIntervention. (2017) 13:115–23. 10.4244/EIJ-D-16-00932 [DOI] [PubMed] [Google Scholar]
- 19.Burzotta F, Lassen JF, Lefevre T, Banning AP, Chatzizisis YS, Johnson TW, et al. Percutaneous coronary intervention for bifurcation coronary lesions: the 15(th) consensus document from the European Bifurcation Club. EuroIntervention. (2021) 16:1307–17. 10.4244/EIJ-D-20-00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen SL, Zhang JJ, Han Y, Kan J, Chen L, Qiu C, et al. Double kissing crush versus provisional stenting for left main distal bifurcation lesions: DKCRUSH-V randomized trial. J Am Coll Cardiol. (2017) 70:2605–17. 10.1016/j.jacc.2017.09.1066 [DOI] [PubMed] [Google Scholar]
- 21.Chen SL, Xu B, Han YL, Sheiban I, Zhang JJ, Ye F, et al. Comparison of double kissing crush versus Culotte stenting for unprotected distal left main bifurcation lesions: results from a multicenter, randomized, prospective DKCRUSH-III study. J Am Coll Cardiol. (2013) 61:1482–8. 10.1016/j.jacc.2013.01.023 [DOI] [PubMed] [Google Scholar]
- 22.Nishida K, Toyofuku M, Morimoto T, Ohya M, Fuku Y, Higami H, et al. Prognostic impact of final kissing balloon technique after crossover stenting for the left main coronary artery: from the AOI-LMCA registry. Cardiovasc Interv Ther. (2019) 34:197–206. 10.1007/s12928-018-0522-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elwany M, Palma GD, Cortese B. Treatment of coronary bifurcation lesions: current knowledge and future perspectives. Future Cardiol. (2018) 14:165–79. 10.2217/fca-2017-0068 [DOI] [PubMed] [Google Scholar]
- 24.Pan L, Lu W, Han Z, Pan S, Wang X, Shan Y, et al. Clinical outcomes of drug-coated balloon in coronary lesions: a real-world, all-comers study. Clin Res Cardiol. (2022) 111:732–41. 10.1007/s00392-021-01895-y [DOI] [PubMed] [Google Scholar]
- 25.Yerasi C, Case BC, Forrestal BJ, Torguson R, Weintraub WS, Garcia-Garcia HM, et al. Drug-coated balloon for De Novo coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 75:1061–73. 10.1016/j.jacc.2019.12.046 [DOI] [PubMed] [Google Scholar]
- 26.Stella PR, Belkacemi A, Dubois C, Nathoe H, Dens J, Naber C, et al. A multicenter randomized comparison of drug-eluting balloon plus bare-metal stent versus bare-metal stent versus drug-eluting stent in bifurcation lesions treated with a single-stenting technique: six-month angiographic and 12-month clinical results of the drug-eluting balloon in bifurcations trial. Catheter Cardiovasc Interv. (2012) 80:1138–46. 10.1002/ccd.23499 [DOI] [PubMed] [Google Scholar]
- 27.Schulz A, Hauschild T, Kleber FX. Treatment of coronary de novo bifurcation lesions with DCB only strategy. Clin Res Cardiol. (2014) 103:451–6. 10.1007/s00392-014-0671-9 [DOI] [PubMed] [Google Scholar]
- 28.Kleber FX, Rittger H, Ludwig J, Schulz A, Mathey DG, Boxberger M, et al. Drug eluting balloons as stand alone procedure for coronary bifurcational lesions: results of the randomized multicenter PEPCAD-BIF trial. Clin Res Cardiol. (2016) 105:613–21. 10.1007/s00392-015-0957-6 [DOI] [PubMed] [Google Scholar]
- 29.Bruch L, Zadura M, Waliszewski M, Platonic Z, Eranen J, Scheller B, et al. Results from the international drug coated balloon registry for the treatment of bifurcations. can a bifurcation be treated without stents? J Interv Cardiol. (2016) 29:348–56. 10.1111/joic.12301 [DOI] [PubMed] [Google Scholar]
- 30.Berland J, Lefevre T, Brenot P, Fajadet J, Motreff P, Guerin P, et al. DANUBIO - a new drug-eluting balloon for the treatment of side branches in bifurcation lesions: six-month angiographic follow-up results of the DEBSIDE trial. EuroIntervention. (2015) 11:868–76. 10.4244/EIJV11I8A177 [DOI] [PubMed] [Google Scholar]
- 31.Worthley S, Hendriks R, Worthley M, Whelan A, Walters DL, Whitbourn R, et al. Paclitaxel-eluting balloon and everolimus-eluting stent for provisional stenting of coronary bifurcations: 12-month results of the multicenter BIOLUX-I study. Cardiovasc Revasc Med. (2015) 16:413–7. 10.1016/j.carrev.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 32.Kleber FX, Mathey DG, Rittger H, Scheller B. German Drug-eluting Balloon Consensus G. How to use the drug-eluting balloon: recommendations by the German consensus group. EuroIntervention. (2011) 7(Suppl K):K125–8. 10.4244/EIJV7SKA21 [DOI] [PubMed] [Google Scholar]
- 33.Rahman S, Leesar T, Cilingiroglu M, Effat M, Arif I, Helmy T, et al. Impact of kissing balloon inflation on the main vessel stent volume, area, and symmetry after side-branch dilation in patients with coronary bifurcation lesions: a serial volumetric intravascular ultrasound study. JACC Cardiovasc Interv. (2013) 6:923–31. 10.1016/j.jcin.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 34.Sawaya FJ, Lefevre T, Chevalier B, Garot P, Hovasse T, Morice MC, et al. Contemporary approach to coronary bifurcation lesion treatment. JACC Cardiovasc Interv. (2016) 9:1861–78. 10.1016/j.jcin.2016.06.056 [DOI] [PubMed] [Google Scholar]
- 35.Murasato Y, Finet G, Foin N. Final kissing balloon inflation: the whole story. EuroIntervention. (2015) 11(Suppl V):V81–5. 10.4244/EIJV11SVA18 [DOI] [PubMed] [Google Scholar]
- 36.Finet G, Gilard M, Perrenot B, Rioufol G, Motreff P, Gavit L, et al. Fractal geometry of arterial coronary bifurcations: a quantitative coronary angiography and intravascular ultrasound analysis. EuroIntervention. (2008) 3:490–8. 10.4244/eijv3i4a87 [DOI] [PubMed] [Google Scholar]
- 37.Burzotta F, Trani C. Technical aspects of provisional stenting in percutaneous treatment of complex bifurcation lesions. Interv Cardiol. (2013) 8:96–9. 10.15420/icr.2013.8.2.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.