Abstract
Introduction
People living with HIV (PLWH) who engaged in chemsex are at risk of potential drug–drug interactions (pDDIs) with recreational drugs. This study aimed to characterize pDDIs between antiretroviral treatment (ART) and chemsex drugs and evaluate their association with unscheduled relevant hospital consultations.
Methods
We conducted a single-center, retrospective, observational study in a series of gay, bisexual, and other men who have sex with men (gbMSM) living with HIV who engaged in chemsex and who attended a tertiary hospital in Barcelona, Spain, from February 2018 through August 2019. Associations between all recorded pDDIs and relevant unscheduled consultations were estimated using the incidence rate (IR) per 100 person-years of those events compared between patients with no pDDI (green flag) or moderate severity pDDI (orange flag) with patients with high severity pDDI (red flag) using the incidence rate ratio (IRR).
Results
Among 172 PLWH engaged in chemsex, 249 ART regimens were prescribed: 44% based on integrase inhibitors, 30% on boosted ART, and 26% based on non-nucleoside reverse transcriptase inhibitors. The substances and recreational drugs most frequently used were erectile dysfunction agents (83%), methamphetamine (79%), GHB (77%), and alkyl nitrites (71%). Polydrug use was reported in 52%. We observed 2048 pDDIs. Of these, 23% were orange flag pDDIs; 88% related to boosted ARTs. The IR of the 285 unscheduled relevant episodes in patients with orange flag pDDIs was 64.67 (95% CI 40.07–89.28). The IRR of green flag pDDIs was 1.05 (95% CI 0.60–1.8; p = 0.876).
Conclusion
One in four pDDIs were of moderate severity but no significant increase in the incidence of unscheduled relevant consultations was observed. A high number of unscheduled consultations, predominantly for psychiatric events and intoxication, were observed. Beyond using non-boosted ART to minimize pDDIs, other factors related to the practice of chemsex must be addressed, in order to offer a better approach.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00694-w.
Keywords: Chemsex, Drug–drug interactions, HIV, Antiretroviral therapy
Key Summary Points
| People living with HIV (PLWH) prescribed with ART are at risk of potential drug–drug interactions (pDDIs) with recreational drugs. |
| This article describes the pDDIs between ART and chemsex drugs in a cohort of PLWH engaged in chemsex from a tertiary Hospital in Barcelona, Spain, and evaluate their association with unscheduled relevant hospital consultations. |
| Among 172 participants, we observed 2048 pDDIs. Of these, 23% were orange flag pDDIs and mainly related to boosted ARTs. The IR of the 285 unscheduled relevant episodes in patients with orange flag pDDIs was 64.67 (95% CI 40.07–89.28). The IRR of green flag pDDIs was 1.05 (95% CI 0.60–1.8; p = 0.876). |
| Despite one in four pDDIs being of moderate severity, no significant increase in the incidence of unscheduled relevant consultations was found. Beyond using non-boosted ART to minimize pDDIs, other factors related to the practice of chemsex must be addressed, to offer a better approach. |
Introduction
HIV infection has evolved from a deadly disease to a chronic condition as a result of many improvements in antiretroviral treatment (ART) alongside the provision of specialized follow-up of people living with HIV (PLWH) [1]. Life-long treatment with ART for PLWH confers a risk of potential drug–drug interactions (pDDIs) due to co-administration of other drugs, potentially leading to toxicity or decreased efficacy of ART or non-ART treatment [2–5].
Previous studies of pDDIs between ART and other medications have been conducted in specific populations including elderly PLWH [2], people who require opioid therapy [6], and patients with a high burden of comorbidities [7]. Severe manifestations of pDDIs have been reported with evidence for the involvement of several metabolic pathways [8, 9]. Other studies have described polypharmacy in PLWH and its implications on adherence to ART, risk of pDDIs, and related adverse effects [10].
Interactions between ART and illicit recreational drugs represent a substantial clinical concern. Drugs use patterns and trends have changed in recent years; chemsex is an example of a new phenomenon related to drug use and sex. Chemsex is defined as the intentional use of recreational drugs, before or during sex, among gay, bisexual, and other men who have sex with men (gbMSM) to facilitate, enhance, and prolong sexual intercourse [11]. Chemsex has an impact on sexual health and increases the risk of transmission of HIV, hepatitis C virus (HCV), and other sexually transmitted diseases (STDs) [12]. Data from our hospital corroborate the aforementioned concerns and describe a heterogeneous cohort of chemsex users with health, social, and psychological implications [13].
The most commonly used drugs for chemsex are γ-hydroxybutyric acid (GHB) and analogues, mephedrone, and methamphetamine [14]. Other frequently used substances include cocaine, ecstasy, ketamine, erectile dysfunction agents, and alkyl nitrites (poppers) [15].
The complexity of drug use during chemsex increases the potential risk of pDDIs between drugs and prescribed medications—specifically ART [16]. To date, only one clinical trial of pDDIs between ART and chemsex drugs has been reported in non drug users [17]. The existence of pDDIs between ART and drugs has been hypothesized on the basis of the results of in vitro assays and pharmacokinetic and pharmacodynamic studies [18–20]. Further, clinical cases of pDDIs between ART and recreational drugs have been reported [21–25].
Cytochrome P450-CYP3A4 and CYP2D6 inhibitors, such as ritonavir or cobicistat, and CYP450 inductors, such as non-nucleoside reverse transcriptase inhibitors (NNRTI), confer an increased risk of interactions with other cytochrome-metabolized drugs [11]. Specific chemsex drugs are also metabolized through CYP3A4 or CYP2D6 which can lead to increased or decreased plasma levels of the drugs involved. Nucleoside reverse transcriptase inhibitors (NRTI), unboosted integrase inhibitors (INSTI), and maraviroc appear to be associated with less potent pDDIs [19].
Despite reported pDDIs between chemsex drugs and ART, the increased risk of clinical events associated to pDDIs between ART and chemsex drugs has yet to be determined. Accordingly, the main objective of the present study was to evaluate pDDIs between ARTs and chemsex drugs in a series of gbMSM living with HIV who engaged in chemsex and who attended our hospital. We further aimed to evaluate the association between pDDIs and unscheduled clinical visits.
Methods
Study Design
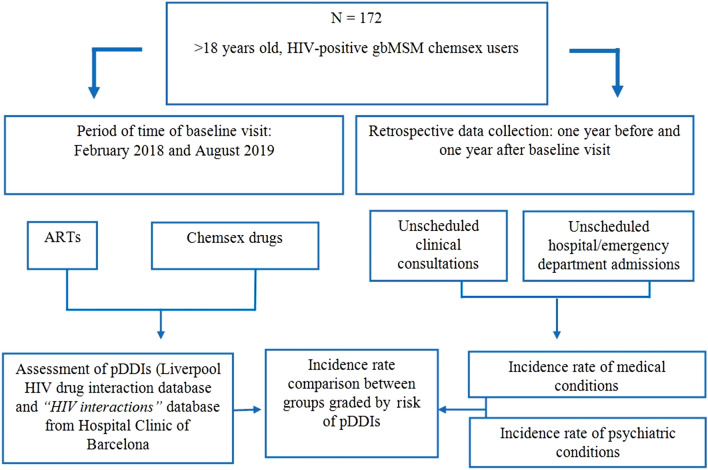
We conducted a retrospective, observational, single-center study on pDDIs between ART and recreational drugs in a series of PLWH gbMSM who engaged in chemsex and who attended the Hospital Clinic de Barcelona (HCB). Methods are summarized in Fig. 1.
Fig. 1.
Flow diagram
Study Period, Setting, and Population
HCB is a referral hospital for the treatment of HIV infection located in an area with a large gay population in Barcelona. The subjects included retrospectively in this study belong to an ongoing prospective study, named the Care_ChemS_CliniC Study (CSC Study), funded by an international grant from ViiV Healthcare through its Positive Pathways program. The CSC study aims to provide facilities for the diagnosis, treatment, and follow-up of HIV, HCV, and other STDs to chemsex users. We performed a comprehensive analysis of relevant pDDIs between ARTs and chemsex drugs among participants included in the CSC Study between February 2018 and August 2019. Participants met the following eligibility criteria: age greater than 18 years; gbMSM on ART; history of intentional drug use in a sexualized context (at least once a month in the previous 6 months or more than 10 times during the previous year); and provision of signed informed consent.
Study Variables and Data Collection
Data on the following variables were collected: age; place of birth; type of drug; polydrug use (defined as use of at least three drugs); route and frequency of drug use; ART regimen 1 year after and 1 year before baseline visit; viral load (VL); and CD4+ lymphocyte count at the time of inclusion. pDDIs were interpreted according to ART and chemsex drugs used by each patient.
To evaluate the potential association between pDDIs and clinical events, we recorded unscheduled medical visits of study participants, defined as non-appointed medical consultations and/or hospital/emergency department admissions due to medical or psychiatric conditions in an interval of 1 year before and 1 year after their baseline visit. Diagnoses made during unscheduled visits were codified according to the ICD-10-CM [26]. We defined relevant unscheduled visits as those that may have been associated with drug effects (adverse and side effects or intoxication).
Sources of Information
Data were collected in the electronic medical record system of the HIV Unit and in specific electronic case report form (eCRF) implemented in REDCap hosted at HCB.
Potential DDIs were checked using two freely available online software tools: the Liverpool HIV drug interaction database website [26] and “HIV Interactions” from HCB [27]. The Liverpool HIV drug interactions database categorized pDDI into four groups, illustrated by colored flags, as follows: green for “no expected interactions”; yellow for “potential weak interaction”; orange for “potential interaction”; and red for “interaction contraindicating its co-administration.” The allocation of each flag is based on available evidence, whose level of quality ranged according to GRADE criteria [28]. The HCB database, “HIV interactions,” analyses pDDIs between ART and other drugs (including recreational drugs and herbal medicine). The HCB database color-codes potential interactions into three categories: green when there is no clinically significant interaction; orange when there is a pDDI that may justify a dose adjustment; and red when co-administration is contraindicated. To simplify results, we coded interactions using colors described in the Liverpool database and have provided descriptions of differences between the Liverpool and HCB databases.
Statistical Analysis
Descriptive statistics of qualitative variables were based on frequencies and percentages. Quantitative variables are described as the mean and standard deviation (SD) or median and interquartile range (IQR). The incidence rate (IR) of unscheduled consultations was estimated as the number of new events per 100 person-years using the negative binomial regression model and was compared between groups using the incidence rate ratio (IRR). IR and IRR were reported along with their 95% confidence intervals (CI). Statistical analyses were performed using Stata (Release 17. StataCorp, College Station, TX).
Ethical Considerations
The present study adhered to the ethical principles as set forth in the Declaration of Helsinki and followed all principles of good clinical practice. Ethics approval was previously obtained from the local research ethics committee for the CSC Study (HCB/2017/0909).
Results
Study Population
A total of 172 PLWH gbMSM who engaged in chemsex were included. Demographic, clinical, and HIV-related characteristics are summarized in Table 1. The median CD4+ T cell count was 677 (IQR 523–854) cells/mm3. Detectable VL (at least 50 copies/ml) was observed in 24 (14%) patients, two of whom were treatment-naive. Among the 249 ART regimens registered during the study period, INSTI-based regimens were the most frequently prescribed (44%). Boosted ART (INSTI boosted with cobicistat or protease inhibitors boosted with cobicistat or ritonavir) was prescribed in 30% of participants, and NNRTI in 26%.
Table 1.
Baseline demographics and HIV-related characteristics
| Demographic characteristics | |
| Mean (SD) | |
| Age (years) | 39 (9) |
| n (%) | |
| Region of origin (n = 172) | |
| Spain | 56 (33%) |
| Europe (w/o Spain) | 29 (17%) |
| Latin America | 82 (48%) |
| Australia/Oceania | 1 (1%) |
| Asia | 1 (1%) |
| Africa | 2 (1%) |
| USA | 1 (1%) |
| Clinical characteristics | |
| Median (IQR) | |
| CD4 (cells/mm3) (n = 171) | 677 (523; 854) |
| CD8 (cells/mm3) (n = 170) | 811 (617; 1010) |
| CD4/CD8 ratio (n = 170) | 0.8 (0.6; 1.1) |
| n (%) | |
| Plasma HIV RNA-VL (copies/ml) | |
| Detectable | 24 (14%) |
| Undetectablea | 147 (86%) |
| Median (IQR) | |
| Plasma HIV RNA-VL (copies/ml) (n = 24) | 4950 (109; 88,750) |
| ART characteristics | |
| ART (n = 249)b | n (%) |
| InSTI/b or PI/b | 75 (30%) |
| InSTI | 109 (44%) |
| NNRTI | 65 (26%) |
SD standard deviation, IQR interquartile range, VL viral load, InSTI/b integrase strand transfer inhibitors boosted with cobicistat/ritonavir, PI/b protease inhibitors boosted with cobicistat/ritonavir, InSTI integrase strand transfer inhibitors, NNRTI non-nucleoside reverse transcriptase inhibitors.
aUndetectable VL: < 50 copies/ml
b249 treatment regimens (in 172 patients), 1 year before and 1 year after the baseline visit
The most frequently used drugs were erectile dysfunction (ED) agents (143/172; 83%), methamphetamine (136/172; 79%), GHB (132/172; 77%), poppers (122/172; 71%), and cocaine (96/172; 56%). Polydrug use was reported in 88/169 individuals (52%) and alcohol consumption during chemsex in 33/143 (23%). Drug use characterization is displayed in Table 2.
Table 2.
Drug-use characterization
| n (%) | |
|---|---|
| Chemsex drugs | |
| Cocaine (n = 172) | 96 (56%) |
| Ketamine (n = 172) | 74 (43%) |
| GHB/GBL (n = 172) | 132 (77%) |
| Methamphetamine (n = 172) | 136 (79%) |
| Mephedrone (n = 172) | 85 (49%) |
| Speed (n = 172) | 60 (35%) |
| Ecstasy (n = 172) | 76 (44%) |
| MDMA (n = 172) | 62 (36%) |
| Alkyl nitrites (poppers) (n = 172) | 122 (71%) |
| Erectile dysfunction agents (n = 172) | 143 (83%) |
| Cannabis (n = 172) | 51 (30%) |
| Number of drugs used (before or during chemsex) | |
| 1 drug (n = 167) | 16 (9%) |
| 2 drugs (n = 167) | 65 (38%) |
| Polydrug usea (n = 167) | 88 (52%) |
| Route of drug administration | |
| Oral (n = 170) | 128 (75%) |
| Inhaled (n = 171) | 135 (79%) |
| Sniffed (n = 172) | 113 (66%) |
| Sublingual (n = 171) | 18 (11%) |
| Rectal (n = 172) | 29 (17%) |
| Intravenous (slamming) (n = 170) | 26 (15%) |
| Frequency of use | |
| Every day (n = 167) | 11 (7%) |
| Every week (n = 169) | 76 (45%) |
| Every month (n = 168) | 65 (39%) |
| < 1 time per month (n = 165) | 29 (18%) |
Ecstasy includes “designer drugs”, MDMA 3,4-methylenedioxymethamphetamine, GHB γ-hydroxybutyric acid, GBL γ-butyrolactone
Erectile dysfunction agents include sildenafil, tadalafil, vardenafil
aActive use of three or more drugs before or during chemsex
pDDIs Among PLWH Chemsex Users
By identifying all combinations of chemsex drugs and ARTs taken by each patient we obtained 2048 pDDIs. According to the Liverpool interaction checker, the proportions of each pDDI category were 76% green flags (n = 1565); 1% yellow flags (n = 21); 23% orange flags (n = 462); and no red flag interactions, as illustrated in Fig. 2.
Fig. 2.
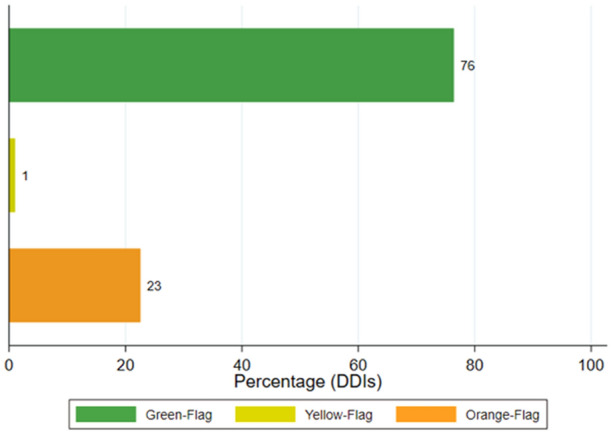
Proportions of potential DDIs (ART–chemsex drug interactions) according to severity. Potential DDIs between ART and recreational drugs are represented as different colors according to the severity of potential DDIs: orange flag (potential interaction), yellow flag (potential weak interaction), and green flag (no expected interactions). No red flags were reported
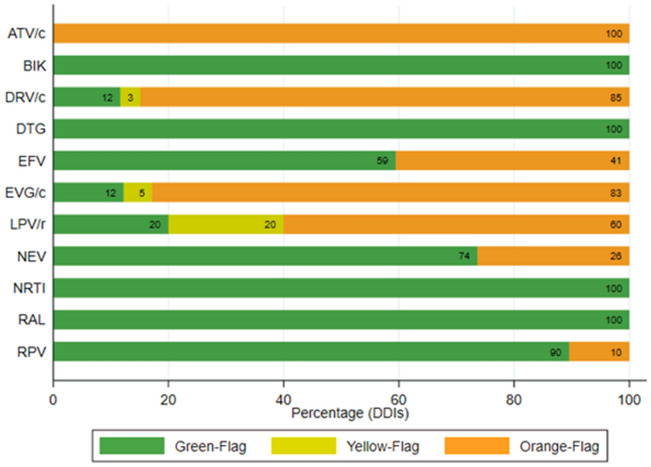
According to the Liverpool interaction checker, 88% of orange flags involved boosted ARTs whereas the remaining 8% were due to NNRTI (Fig. 3).
Fig. 3.
pDDI severity according to ART. Potential DDIs between ART and chems are represented as different colors according to the severity of potential DDIs: orange flag (potential clinical relevance), yellow flag (weak clinical relevance), and green flag (absence of potential DDIs). ATV/c atazanavir/cobicistat, BIK tenofovir-alafenamide/emtricitabine/bictegravir, DRV/c darunavir/cobicistat, DTG dolutegravir, EFV efavirenz, EVG/c elvitegravir/cobicistat, LPV/r lopinavir/ritonavir, NEV nevirapine, NRTI nucleosides reverse transcriptase inhibitors, RAL raltegravir, RPV rilpivirine
The HCB “HIV Interactions” webpage had some differences compared to the Liverpool database: red flags were described for atazanavir–methamphetamine (less than 1%), elvitegravir/cobicistat–3,4-methylenedioxy-N-methamphetamine (MDMA) (1%), and darunavir/cobicistat–MDMA (1%) interactions; and orange flags (potential DDI) were found for efavirenz–MDMA (less than 1%), efavirenz–methamphetamine (less than 1%), and efavirenz–mephedrone (less than 1%) interactions.
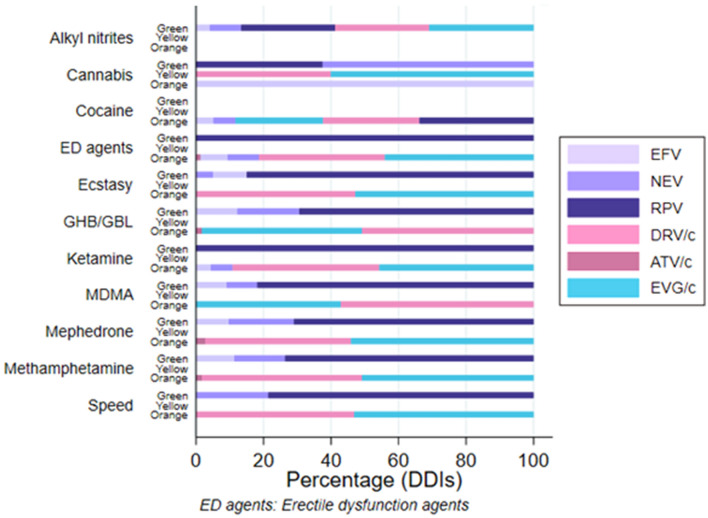
We calculated the proportions of ART–chemsex drug interactions for each severity category. Figure 4 illustrates the proportions of green, yellow, and orange flags for each chemsex drug in our cohort according to the Liverpool interaction checker. Only the seven ARTs associated with yellow and/or orange flags are shown. Cocaine was associated with the most orange flags (n = 77), with 55% due to interactions with boosted ARTs. ED agents were associated with 75 orange flags, with 82% due to interactions with boosted ARTs and 18% due to interactions with NNTRI. Methamphetamine was associated with 59 orange flags, all due to interactions with boosted ART. GHB was associated with 59 orange flags, all due to interactions with boosted ART. Other drugs are described in Appendix 1 in the supplementary material.
Fig. 4.
Distribution of ARTs, chems, and DDIs. Chemsex drugs are displayed on the ordinate axis and are subdivided according to green, yellow, and orange flags. Bars are illustrated with colors representing the proportion of ART corresponding to each flag. On every bar appearing from left to right, the proportion of flags is presented from lowest to highest. Lilac represents NNRTI, rose represents boosted PI, and turquoise represents boosted InSTI. EFV efavirenz, NEV nevirapine, RPV rilpivirine, DRV/c darunavir/cobicistat, ATV/c atazanavir/cobicistat, EVG/c elvitegravir/cobicistat
Unscheduled Clinical Visits
A total of 85% (146/172) of patients attended for unscheduled clinical visits. We identified 603 clinical attendances, according to the ICD-10-CM, of 437 (72%) corresponded to medical reasons and 166 (28%) to psychiatric reasons. The IR (95%CI) of unscheduled visits was 175.29 (151.6–202.69) per 100 person-years.
Of these 603 unscheduled clinical visits, 218 were selected as relevant episodes (36%) and these involved 73 patients. The IR (95% CI) of relevant unscheduled visits was 63.37 (47.95–83.76) per 100 person-years. Of these episodes, 24% (n = 52) were due to medical reasons and 76% (n = 166) to psychiatric reasons. The IR (95% CI) for relevant unscheduled visits due to medical reasons was 15.12 (9–21.23) per 100 person-years and the IRR for unscheduled visits due to psychiatric reasons was 3.19 (1.89–5.4; p < 0.0001).
The most frequent relevant diagnoses were related to anxiety disorders 62/218 (28%), intoxication 49/218 (22%), and psychotic disorders 29/218 (13%) (Table 3).
Table 3.
Unscheduled clinical visits and hospital admissions
| Diagnosis | ICD-10 code | Prevalence of consultations and admissions by flag color | Prevalence of consultations and admissions | |
|---|---|---|---|---|
| Green | Orange | |||
| Adjustment disorders | F43.2 | 1 (1%) | 2 (2%) | 3 (1%) |
| Anxiety disorder, unspecified | F41.9 | 32 (32%) | 30 (25%) | 62 (28%) |
| Chest pain, unspecified | R07.9 | 2 (2%) | 4 (3%) | 6 (3%) |
| Dehydration | E86.0 | 0 (0%) | 1 (1%) | 1 (0%) |
| Insomnia and other sleep disorders | G47.0 | 5 (5%) | 3 (3%) | 8 (4%) |
| Ischemic heart disease | I20.9 | 1 (1%) | 0 (0%) | 1 (0%) |
| Other stimulant abuse with stimulant-induced mood disorder | F15.14 | 7 (7%) | 10 (8%) | 17 (8%) |
| Other stimulant abuse with stimulant-induced psychotic disorder | F15.15 | 9 (9%) | 20 (17%) | 29 (13%) |
| Other stimulant dependence or abuse | F15.2 | 11 (11%) | 15 (13%) | 26 (12%) |
| Stimulant abuse with intoxication | F15.12 | 23 (23%) | 26 (22%) | 49 (22%) |
| Suicide attempt and suicidal ideations | T14.91 | 7 (7%) | 5 (4%) | 12 (6%) |
| Syncope and collapse | R55 | 1 (1%) | 2 (2%) | 3 (1%) |
| Toxic liver disease with acute hepatitis | K71.2 | 0 (0%) | 1 (1%) | 1 (0%) |
| Total | 99 (100%) | 119 (100%) | 218 (100%) | |
ICD-10 10th revision of the International Statistical Classification of Diseases and Related Health Problems
To analyze clinical and psychiatric unscheduled visits, a single flag was assigned to each patient. In patients taking multiple drugs at risk of causing DDIs (i.e., polydrug users), the flag with the highest severity was assigned. Of these, 47% of patients (n = 80) were assigned a green flag and reported 99 unscheduled visits, with an IR (95% CI) of 61.97 (36.5–87.25) per 100 person-years. Orange flags were assigned to 53% of participants (n = 92) accounting for 119 unscheduled visits with an IR (95% CI) of 64.67 (40.07–89.28) per 100 person-years. The IRR for green flag DDIs was 1.05 (95% CI 0.60–1.8; p = 0.876).
There were 75 unscheduled visits identified as being due to intoxication and stimulant abuse, corresponding to 38 patients. Of these, 18 patients (47%) were labelled with green flags and 20 (53%) with orange flags. The IR of patients with orange flag DDIs was 22.28 (95% CI 10.78–33.79), and the IRR for green flag DDIs was 1.05 (95% CI 0.49–2.24; p = 0.902).
Discussion
To our knowledge, this is the first study to assess pDDIs between chemsex drugs and ART in a sample comprised exclusively of PLWH who engaged in chemsex. A recent study described intoxication caused by potential chemsex-related drugs; however, the results were not evaluated on the basis of pDDIs as in the present study [27].
Unboosted INSTI-based regimens were the most widely prescribed in our series, yet 30% of individuals were receiving boosted ART, predominantly with cobicistat; this was a lower percentage than reported by previous studies that evaluated ART interactions in chemsex users [28].
We found that most combinations of ART and chemsex drugs did not result in significant drug interactions, as demonstrated by 76% of pDDIs being green-flagged in the present study. Only 1% of pDDIs were yellow-flagged and 23% orange-flagged. Although no red-flagged pDDIs were observed according to the Liverpool interaction checker, we observed red-flagged pDDIs described on the HCB “HIV Interactions” webpage [29]. The differences were minimal between the two databases and most theoretical pDDIs were based on low to very low levels of evidence due to a lack of reported data regarding pDDIs between chemsex drugs and ART [19]. We recommend confirming pDDIs using a range of databases when there is scare evidence.
As expected, boosted ARTs were associated with the highest proportions of yellow- and orange-flagged pDDIs, followed by NNRTI (efavirenz and nevirapine). Boosted ARTs inhibit CYP3A4 and present a higher risk of pDDI when co-administered with other drugs as they may increase plasma levels of drugs that are metabolized by the same pathway [19]. As almost all NNRTIs are inducers of CYP3A4, co-administration of NNRTIs may decrease the efficacy of the other substances leading to increased drug dosages and intoxication [8].
Among the substances used in this series of chemsex users, cocaine was associated the highest number of orange-flagged DDIs, followed by ED agents, methamphetamine, and GHB, respectively. Despite this, cocaine pDDIs were weaker compared to other drugs [19]. Only a small proportion of cocaine is metabolized by CYP3A4 which leads to the formation of norcocaine, a toxic metabolite associated with liver toxicity [19].
ED agents are predominantly metabolized via CYP3A. Co-administration with cobicistat or ritonavir may result in increased plasma concentrations of ED agents and lead to adverse reactions [24, 25].
GHB and GBL are central nervous system depressants (CNS) used recreationally to increase relaxation and euphoria. The metabolism of GHB and its precursors is mediated by dehydrogenases; the role of CYP450 to GHB metabolism remains unclear [19]. A recent study reported that neither pharmacokinetic nor pharmacodynamic pDDIs were found between GHB and cobicistat; however, this study had a small sample size of 10 participants [17]. Increases in deaths associated with GHB overdose have been reported in recent years, including in PLWH [23, 28, 30–32]; however, other pharmacokinetic or pharmacodynamic factors may contribute. For example, the narrow therapeutic index of GHB (doses greater than 3 ml can be lethal) and co-administration with ethanol or ketamine may increase the risk of CNS depression [19]. Methamphetamine, the second most frequent drug used in our cohort, is predominantly metabolized by CYP2D6 [19]. A case of severe toxicity has been reported as a result of co-administration of methamphetamine and ritonavir [33].
During the study period, 85% of participants attended for unscheduled clinical visits. These patients are likely to require extra care and higher health budgets. Other studies evaluating unscheduled care in PLWH have concluded that a large number of emergency room visits and hospital admissions could be prevented by early, low-cost interventions and primary care [34]. The implementation of such measures should be further evaluated in the chemsex population.
Seventy-three patients attended as a result of adverse drug effects, 23% for medical reasons, and the rest as a result of psychiatric reasons. A high prevalence of psychiatric comorbidity has been reported among chemsex users [35]. In a study of methamphetamine users, 72% were found to have psychiatric comorbidity [36]. In our study, the IRR of relevant unscheduled medical consultations for psychiatric versus medical reasons was statistically significant.
However, a causal association between moderate pDDIs and a higher incidence of unscheduled clinical consultations could not be demonstrated. We also evaluated pDDIs in the subgroup of patients who attended for intoxication, with no differences observed in IRR between patients classified with green-flagged pDDIs and orange-flagged pDDIs.
Despite these results we consider it is necessary to individualize ART regimens in every patient who uses drugs and provide education regarding pDDIs, particularly when initiating or changing treatments. Clinicians should be aware of other factors that may increase the risk of intoxication and events related to drug consumption and abuse, such as polydrug use [37], frequency and routes of drug use that may increase bioavailability, such as rectal and intravenous routes [8, 38].
In our study population, 14% of participants had detectable VL, a rate comparable to a recent study on chemsex use and its impacts across European countries [39]. Detectable VL in PLWH who engaged in chemsex may be related to suboptimal ART adherence, for which clinical assessment is of particular importance. Methamphetamine, GHB, cocaine, and mephedrone were the most frequently used drugs in our study, corroborating previous reports [11, 40]. Half of participants self-reported polydrug use, which is known to be associated with harmful physical and psychiatric effects such as dependence, overdose, psychiatric disorders, and death [37].
Our study has limitations related to its observational and retrospective nature. We analyzed participants included in the CSC Study, which probably are not all the people who practice chemsex that we are following in our HIV Unit. We were unable to assess pDDIs between recreational or chemsex drugs and non-ART medications. Further, the number of unscheduled visits may have been underestimated as a result of a number of factors: potentially incomplete information on admissions to other hospitals; pDDIs may have led to toxicity not serious enough to prompt participants to seek medical advice; and our classification of relevant diagnoses may not have included other drug-related events. Finally, we have not differentiated between patients with or without pre-existing medical and psychiatric comorbidities, and their potential impact on the unscheduled visits.
Conclusion
One in four pDDIs between drugs and ART were of moderate severity predominantly with boosted ARTs. A very high rate of unscheduled clinical consultations was observed in PLWH who practice chemsex, predominantly related to psychiatric events and intoxication.
We observed no evidence of an increased incidence of unscheduled relevant medical and psychiatric consultations related to orange-flagged pDDI. Other factors such as polydrug use, high-risk routes of administration, toxic doses, and duration of exposure to substance use should be considered. However, we think that the recommended ART for these users should be based on unboosted regimens, as one more strategy, but not the only one, in the holistic and multidisciplinary management of PLWH engaged in chemsex.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The Final Degree Project of the student Maria José Nebot, from the Faculty of Medicine of the University of Barcelona, has been part of this work. We would like to thank all the study participants for their involvement in the study.
Funding
This work was supported by an international grant from ViiV Healthcare within its Positive Pathways program, supporting the CSC Study, including the Rapid Service Fee of this specific publication.
Author Contributions
L.D.M. and M.M.R contributed equally to the study. J.M. and J.L.B contributed equally to the study. L.D.M., M.J.N and M.M.R designed the study. D.S. collaborated in the study design as a ViiV representative. L.D.M, M.L, M.J.N, A.U, B. T., A.G.C., A.I., J.A, I.C., E.M., J.L.B., J.B., L.M., J.M. and M.M.R recruited patients for the study. M.T. and E.S. contributed to pharmacological and toxicological analyses. L.D.M., E.D.L., J.M. and M.M.R analyzed and interpreted data. L.D.M., M.J.N, M.T., M.L., J.L.B., J.M and M.M.R were involved in drafting the manuscript. All authors were involved in reviewing the manuscript and approved the final version.
Prior Presentation
This manuscript is based on work presented as e-poster, number EPB190, in the 24th International AIDS Conference, Montreal, Canada, 29 July–2 August, 2022.
Disclosures
L.D.M. has received fees to give lectures from Gilead, MSD, ViiV, AbbVie and Janssen-Cilag. M.J.N has nothing to disclose. M.M.R. has received fees to give lectures from Gilead, MSD, ViiV, AbbVie and Janssen-Cilag. E.D.L. has nothing to disclose. M.T. has received clinical research grants from Janssen, Gilead Sciences and ViiV Healthcare and has received financial compensation for speaking engagements from Gilead Sciences, Janssen, MerckSharp & Dohme, ViiV Healthcare and Theratechnologies. M.L. has received fees to give lectures from Gilead, MSD, ViiV, AbbVie and Janssen-Cilag. JA has participated in advisory boards and received consulting honoraria, research grants, or both, from Gilead Sciences, Janssen Pharmaceuticals, and ViiV Healthcare, all outside of this work. E.M. has received honoraria for lectures or advisory boards from Gilead and Janssen and his institution has received research grants from MSD and ViiV. L.M. has received honoraria for lectures from Lundbeck, Gilead and Neuraxpharm. J.B. has received honoraria for lectures or advisory boards from Ferrer Internacional, Gilead, Janssen, MSD and ViiV. A.U. has received fees to give lectures from Gilead, ViiV, and Janssen-Cilag. B.T. has received fees to give lectures from Gilead, MSD, ViiV, AbbVie and Janssen-Cilag. A.G.C. has received fees to give lectures and participate in advisory boards from Gilead, MSD, ViiV, AbbVie and Janssen-Cilag. A.I. has received educational grants from MSD and Gilead. J.L.B. has received honoraria for lectures or advisory boards from Gilead, Janssen, MSD. I.C. has received fees to give lectures from Gilead, MSD, ViiV, AbbVie and Janssen-Cilag. D.S. is an employee of ViiV Healthcare and shareholder of GlaxoSmithKline. J.M. has received honoraria, speakers’ fees, consultant fees or funds for research from MSD, Roche, Boehringer-Ingelheim, ViiV, Gilead, Janssen, BMS, AbbVie. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Compliance with Ethics Guidelines
The present study adhered to the ethical principles as set forth in the Declaration of Helsinki and followed all principles of good clinical practice. Ethics approval was previously obtained from the local research ethics committee for the CSC Study (HCB/2017/0909).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lorena De La Mora and Maria Martinez-Rebollar contributed equally to the study as first and co-corresponding authors.
Josep Mallolas and José L. Blanco contributed equally to the study as co-senior authors.
References
- 1.Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016;11:492–500. doi: 10.1097/COH.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Courlet P, Livio F, Guidi M, et al. Polypharmacy, drug–drug interactions, and inappropriate drugs: new challenges in the aging population with HIV. Open Forum Infect Dis. 2019;6:ofz531. doi: 10.1093/ofid/ofz531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Núñez-Núñez M. Interacciones potenciales en una cohorte de pacientes VIH positivos de edad avanzada. Farm Hosp. 2018;04:163–167. doi: 10.7399/fh.10921. [DOI] [PubMed] [Google Scholar]
- 4.Livio F, Marzolini C. Prescribing issues in older adults living with HIV: thinking beyond drug–drug interactions with antiretroviral drugs. Ther Adv Drug Saf. 2019;10:2042098619880122. doi: 10.1177/2042098619880122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marzolini C, Livio F. Prescribing issues in elderly individuals living with HIV. Expert Rev Clin Pharmacol. 2019;12:643–659. doi: 10.1080/17512433.2019.1627200. [DOI] [PubMed] [Google Scholar]
- 6.Meemken L, Hanhoff N, Tseng A, Christensen S, Gillessen A. Drug–drug interactions with antiviral agents in people who inject drugs requiring substitution therapy. Ann Pharmacother. 2015;49:796–807. doi: 10.1177/1060028015581848. [DOI] [PubMed] [Google Scholar]
- 7.Fantoni M, Del Borgo C, Autore C. Evaluation and management of metabolic and coagulative disorders in HIV-infected patients receiving highly active antiretroviral therapy. AIDS. 2003:S162–S169. [DOI] [PubMed]
- 8.Manzardo C, Tuset M, Miró JM, Gatell JM. Interacciones graves o potencialmente letales entre antirretrovirales y otros medicamentos. Enferm Infecc Microbiol Clin. 2015;33:e15–e30. doi: 10.1016/j.eimc.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Marzolini C, Elzi L, Gibbons S, et al. Prevalence of comedications and effect of potential drug–drug interactions in the Swiss HIV Cohort Study. Antivir Ther. 2010;15:413–423. doi: 10.3851/IMP1540. [DOI] [PubMed] [Google Scholar]
- 10.López-Centeno B, Badenes-Olmedo C, Mataix-Sanjuan Á, et al. Polypharmacy and drug–drug interactions in people living with human immunodeficiency virus in the region of Madrid, Spain: a population-based study. Clin Infect Dis. 2020;71:353–362. doi: 10.1093/cid/ciz811. [DOI] [PubMed] [Google Scholar]
- 11.Bourne A, Reid D, Hickson F, Torres-Rueda S, Steinberg P, Weatherburn P. ‘Chemsex’ and harm reduction need among gay men in South London. Int J Drug Policy. 2015;26:1171–1176. doi: 10.1016/j.drugpo.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Sewell J, Miltz A, Lampe FC, et al. Poly drug use, chemsex drug use, and associations with sexual risk behaviour in HIV-negative men who have sex with men attending sexual health clinics. Int J Drug Policy. 2017;43:33–43. doi: 10.1016/j.drugpo.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 13.De La Mora L, Laguno M, De Lazzari E, et al. Vulnerability conditions in a cohort of men who have sex with men who engage in chemsex in Barcelona city: a cross-sectional study. Sex Res Soc Policy. 2022. 10.1007/s13178-022-00702-1.
- 14.Bourne A, Reid D, Hickson F, Torres Rueda S, Weatherburn P. The Chemsex Study: drug use in sexual settings among gay and bisexual men in Lambeth. Southwark: Sigma Research, London School of Hygiene & Tropical Medicine; 2014. [Google Scholar]
- 15.Schmidt AJ, Bourne A, Weatherburn P, et al. Illicit drug use among gay and bisexual men in 44 cities: findings from the European MSM Internet Survey (EMIS) Int J Drug Policy. 2016;38:4–12. doi: 10.1016/j.drugpo.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Daskalopoulou M, Rodger A, Phillips AN, et al. Recreational drug use, polydrug use, and sexual behaviour in HIV-diagnosed men who have sex with men in the UK: results from the cross-sectional Astra study. Lancet HIV. 2014;1:e22–e31. doi: 10.1016/S2352-3018(14)70001-3. [DOI] [PubMed] [Google Scholar]
- 17.Moltó J, Bailón L, Pérez-Mañá C, et al. Absence of drug–drug interactions between γ-hydroxybutyric acid (GHB) and cobicistat. J Antimicrob Chemother. 2021;77:181–184. doi: 10.1093/jac/dkab359. [DOI] [PubMed] [Google Scholar]
- 18.Antoniou T, Tseng AL-I. Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother. 2002;36:1598–1613. doi: 10.1345/aph.1A447. [DOI] [PubMed] [Google Scholar]
- 19.Bracchi M, Stuart D, Castles R, Khoo S, Back D, Boffito M. Increasing use of “party drugs” in people living with HIV on antiretrovirals: a concern for patient safety. AIDS. 2015;29:1585–1592. doi: 10.1097/QAD.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 20.Colfax G, Guzman R. Club drugs and HIV infection: a review. Clin Infect Dis. 2006;42:1463–1469. doi: 10.1086/503259. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Rao PS, Earla R, Kumar A. Drug–drug interactions between anti-retroviral therapies and drugs of abuse in HIV systems. Expert Opin Drug Metab Toxicol. 2015;11:343–355. doi: 10.1517/17425255.2015.996546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry JA, Hill IR. Fatal interaction between ritonavir and MDMA. Lancet. 1998;352:1751–1752. doi: 10.1016/S0140-6736(05)79824-X. [DOI] [PubMed] [Google Scholar]
- 23.Harrington RD, Woodward JA, Hooton TM, Horn JR. Life-threatening interactions between HIV-1 protease inhibitors and the illicit drugs MDMA and gamma-hydroxybutyrate. Arch Intern Med. 1999;159:2221–2224. doi: 10.1001/archinte.159.18.2221. [DOI] [PubMed] [Google Scholar]
- 24.Muirhead GJ, Wulff MB, Fielding A, Kleinermans D, Buss N. Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. Br J Clin Pharmacol. 2000;50:99–107. doi: 10.1046/j.1365-2125.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loulergue P, Gaillard R, Mir O. Interaction involving tadalafil and CYP3A4 inhibition by ritonavir. Scand J Infect Dis. 2011;43:239–240. doi: 10.3109/00365548.2010.526139. [DOI] [PubMed] [Google Scholar]
- 26.ICD. ICD-10-CM—International Classification of Diseases. ICD-10-CM/PCS transition. https://www.cdc.gov/nchs/icd/icd10cm_pcs_background.htm. Accessed April 4, 2022.
- 27.Vallecillo G, Losada A, Inciarte A, et al. Increasing emergency department admissions for chemsex-related intoxications in Barcelona, Spain, among people living with HIV: an observational study from 2018 to 2020. BMC Public Health. 2022;22:346. doi: 10.1186/s12889-022-12763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adler Z, Fitzpatrick C, Broadwell N, Churchill D, Richardson D. Chemsex and antiretroviral prescribing in an HIV cohort in Brighton, UK. HIV Med. 2022 doi: 10.1111/hiv.13239. [DOI] [PubMed] [Google Scholar]
- 29.Interacciones VIH. http://www.interaccionesvih.com/. Accessed June 25, 2021.
- 30.Caldicott DGE, Chow FY, Burns BJ, Felgate PD, Byard RW. Fatalities associated with the use of gamma-hydroxybutyrate and its analogues in Australasia. Med J Aust. 2004;181:310–313. doi: 10.5694/j.1326-5377.2004.tb06295.x. [DOI] [PubMed] [Google Scholar]
- 31.Hockenhull J, Murphy KG, Paterson S. An observed rise in γ-hydroxybutyrate-associated deaths in London: evidence to suggest a possible link with concomitant rise in chemsex. Forensic Sci Int. 2017;270:93–97. doi: 10.1016/j.forsciint.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 32.Corkery JM, Loi B, Claridge H, et al. Gamma hydroxybutyrate (GHB), gamma butyrolactone (GBL) and 1,4-butanediol (1,4-BD; BDO): a literature review with a focus on UK fatalities related to non-medical use. Neurosci Biobehav Rev. 2015;53:52–78. doi: 10.1016/j.neubiorev.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Hales G, Roth N, Smith D. Possible fatal interaction between protease inhibitors and methamphetamine. Antivir Ther. 2000;5:19. doi: 10.1177/135965350000500108. [DOI] [PubMed] [Google Scholar]
- 34.Grant C, Bergin C, O’Connell S, Cotter J, Ní CC. High-cost, high-need users of acute unscheduled HIV care: a cross-sectional study. Open Forum Infect Dis. 2020;7:ofaa037. doi: 10.1093/ofid/ofaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohn A, Sander D, Köhler T, et al. Chemsex and mental health of men who have sex with men in Germany. Front Psychiatry. 2020;11:542301. doi: 10.3389/fpsyt.2020.542301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gavín P, Arbelo N, Monràs M, et al. Methamphetamine use in chemsex and its consequences on mental health: a descriptive study. Rev Esp Salud Publica. 2021;95:e202108108. [PubMed] [Google Scholar]
- 37.Grov C, Kelly BC, Parsons JT. Polydrug use among club-going young adults recruited through time-space sampling. Subst Use Misuse. 2009;44:848–864. doi: 10.1080/10826080802484702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolengevich-Segal H, Gonzalez-Baeza A, Valencia J, et al. Drug-related and psychopathological symptoms in HIV-positive men who have sex with men who inject drugs during sex (Slamsex): data from the U-SEX GESIDA 9416 Study. PLoS ONE. 2019;14:e0220272. doi: 10.1371/journal.pone.0220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitlock GG, Protopapas K, Bernardino JI, et al. Chems4EU: chemsex use and its impacts across four European countries in HIV-positive men who have sex with men attending HIV services. HIV Med. 2021;22:944–957. doi: 10.1111/hiv.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González-Baeza A, Dolengevich-Segal H, Pérez-Valero I, et al. Sexualized drug use (chemsex) is associated with high-risk sexual behaviors and sexually transmitted infections in HIV-positive men who have sex with men: data from the U-SEX GESIDA 9416 study. AIDS Patient Care STDs. 2018;32:112–118. doi: 10.1089/apc.2017.0263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.