Highlights
-
•
A microcosm test for chemical fate analysis in planted filters was developed.
-
•
Mass balance of the model 13C,15N-pesticide glyphosate fate was established.
-
•
Glyphosate was mainly adsorbed on gravel in the microcosms.
-
•
Plants promoted glyphosate mineralization and microbial assimilation of 13C and 15N.
-
•
Microcosm test is simple & feasible for fate analysis of non-volatile chemicals.
Keywords: Planted filter, Fate, Mass balance, Pesticide, Microbial degradation, Adsorption
Abstract
Planted filters are often used to remove pesticides from runoff water. However, the detailed fate of pesticides in the planted filters still remains elusive. This hampers an accurate assessment of environmental risks of the pesticides related to their fate and thereby development of proper mitigation strategies. In addition, a test system for the chemical fate analysis including plants and in particular for planted filters is not well established yet. Therefore, we developed a microcosm test to simulate the fate of pesticide in planted filters, and applied 2-13C,15N-glyphosate as a model pesticide. The fate of 2-13C,15N-glyphosate in the planted microcosms over 31 day-incubation period was balanced and compared with that in the unplanted microcosms. The mass balance of 2-13C,15N-glyphosate turnover included 13C mineralization, degradation products, and the 13C and 15N incorporation into the rhizosphere microbial biomass and plants. We observed high removal of glyphosate (> 88%) from the water mainly due to adsorption on gravel in both microcosms. More glyphosate was degraded in the planted microcosms with 4.1% of 13C being mineralized, 1.5% of 13C and 3.8% of 15N being incorporated into microbial biomass. In the unplanted microcosms, 1.1% of 13C from 2-13C,15N-glyphosate was mineralized, and only 0.2% of 13C and 0.1% of 15N were assimilated into microbial biomass. The total recovery of 13C and 15N was 81% and 85% in planted microcosms, and 91% and 93% in unplanted counterparts, respectively. The microcosm test was thus proven to be feasible for mass balance assessments of the fate of non-volatile chemicals in planted filters. The results of such studies could help better manage and design planted filters for pesticide removal.
Graphical abstract
1. Introduction
Pesticides in agricultural and urban areas are often carried by runoff water to surface waters which are the habitats of aquatic biota and drinking water supplies for humans. Pesticides can be toxic to aquatic biota (Morrissey et al., 2015; Stenstrom et al., 2021) and human beings (Mostafalou and Abdollahi, 2017). In order to protect aquatic ecosystems and drinking water resources, planted filters like constructed wetlands, vegetated ditches, and riparian buffer strips are often used to remove pesticides from runoff water (Syversen and Bechmann, 2004; Vymazal and Bfezinova, 2015). Pesticides can be removed by planted filters via sorption on filter matrices, (bio)degradation, and plant uptake and phytotransformation. Pesticides sorbed on matrices can still be released to waters, thus posing delayed environmental and toxicological risks (Maillard and Imfeld, 2014). Pesticides may also be completely removed from water through (bio)degradation processes by mineralization and incorporation of elements into microbial biomass, resulting in detoxification of the pesticides (Kästner et al., 2014). Nevertheless, during the degradation of pesticides, toxic and persistent degradation products may also be formed. Moreover, an enhanced uptake of pesticides by plants can improve the removal efficiency of pesticides from water. However, the comprehensive fate of pesticides in planted filters is elusive, which prevents predicting environmental risks associated with the pesticide fate and developing planted filters optimized for pesticide removal.
Laboratory microcosm experiments are usually conducted to simulate the fate of chemicals in the environment (OECD, 2002a, 2002b, 2004). Although pesticide fate has been widely determined in different environmental matrices such as soil and water-sediment with stable or radioactive isotope-labeled pesticide (Nowak et al., 2018; Shrestha et al., 2016; Wang et al., 2016), fate analyses in the presence of plants are rare and there is no generally accepted test which includes plants. Previous studies of chemical fate with plants usually applied complex air flow-through setup and could not elucidate the detailed microbial degradation processes of the applied 14C-labeled chemicals (Hand et al., 2020; Sun et al., 2014). Plants can influence the fate of pesticide in the environmental matrices via uptake and the formed rhizosphere conditions. Plants diffuse oxygen into the matrices changing the redox conditions; they consume nutrients (such as NO3−) in the matrices and also release root exudates which provide carbon sources for microorganisms (Jones et al., 2009). We thus hypothesized that the degradation rate and the microbial metabolism of pesticide as a carbon and nitrogen source are different in planted matrices compared to that in the unplanted counterparts.
Therefore, the overall aim of this study was to develop a microcosm test for detailed fate assessment of isotope-labeled pesticides in planted filters. Reed canary grass (Phalaris arundinacea) was planted due to its frequent application in planted filters (Vymazal, 2013) and the prominent development of roots (Vymazal and Kröpfelová, 2008) which might favor degradation of pesticides in the filter. Gravel was employed as the substrate for the microcosms because of its common use. 13C and 15N co-labeled glyphosate (N-(phosphonomethyl) glycine) was selected as a model pesticide because of the following three reasons. Firstly, glyphosate is one of the most applied pesticide worldwide and can be carried away from the application site by runoff water (Lupi et al., 2019; Maggi et al., 2020), which is one important reason for its frequent detection in surface waters worldwide (Carles et al., 2019; Geng et al., 2021; Okada et al., 2020). Secondly, the degradation pattern of glyphosate is well-known (Zhan et al., 2018), which allows to track the detailed fate of the pesticide and thus facilitates studying the influence of rhizosphere. Thirdly, although high removal of glyphosate by planted filters was reported, the detailed fate processes contributing to the removal are still unexplored (Imfeld et al., 2013; López-Chávez et al., 2021).
The specific objective of the study was to establish the comprehensive mass balance of 2-13C,15N-glyphosate fate, which included quantifying the isotope labels in (i) mineralization, (ii) degradation products, (iii) incorporation of 13C and 15N into microbial biomass (amino acids) in the rhizosphere, and (iv) plant biomass (roots and shoots). In addition, we also investigated the label distribution in different compartments (pore water, gravel, plants, and microbial pellets from rhizosphere) of the microcosms. The obtained results in planted microcosms were compared with that in unplanted microcosms.
2. Materials and methods
2.1. Chemicals
2-13C,15N-glyphosate (99 atom% 13C, 98 atom% 15N) and unlabeled glyphosate (> 99% purity) were purchased from Sigma-Aldrich (St. Louis, USA) and used for the incubation experiment. AMPA and sarcosine (purity > 98%) were both obtained from Sigma-Aldrich, and glycine (J.T.Baker™) was obtained from Avantor (Deventer, Netherland). All other chemicals were of analytical grade and purchased from Sigma-Aldrich. MilliQ water (18 MΩ) was produced from a Milli-Q machine (Merck, Darmstadt, Germany). Chemicals used for LC-MS analysis were listed in Text S1, S 1.1.
2.2. Gravel
Gravel (Quartz, 6 – 8 mm; WECO GmbH & Co. KG, Leer, Germany) for ornamental use was purchased from a local market (Leipzig, Germany). The gravel was sieved to remove the fine particles (< 4 mm). The iron and aluminum from amorphous metal oxides in gravel were extracted with ammonium oxalate solution according to the method of Schwertmann (1964) and determined with inductively coupled plasma optical emission spectrometry (ICP-OES) (ARCOS, Spectro GmbH; Kleve, Germany). The amorphous iron content was 150 mg/kg, and the amorphous aluminum content was 26 mg/kg. The organic matter content measured by loss on ignition (at 450°C) was 0.2% (w/w). The pH was 4.5 (H2O) and 4.3 (0.01 M CaCl2).
2.3. Setup of the microcosms and incubation with glyphosate
Five different treatments were prepared: (1) planted microcosm with 2-13C,15N-glyphosate (planted, labeled test), (2) planted microcosm with unlabeled glyphosate (unlabeled control), (3) planted microcosm without glyphosate (blank control), (4) unplanted microcosm with 2-13C,15N-glyphosate (unplanted control), (5) unplanted microcosm with unlabeled glyphosate (unplanted, unlabeled control). The amount of 13C and 15N (calculated based on total C or N concentration and the ratio of the heavy isotope to the light one as at%) in the labeled treatments (1 and 4) was corrected for the natural abundance of these isotopes by subtracting the amount of 13C or 15N in the corresponding treatments with unlabeled glyphosate (2 and 5). The blank control (3) provided the information about the potential inhibitive effect of glyphosate on plant growth. All treatments consisted of three replicates.
The microcosms were set up in 1 L glass jars. The jar was sealed with a glass lid with a rubber sealing ring. 1000 g of gravel was placed in each microcosm. The setup of planted microcosms is illustrated in Fig. 1A. The lid was perforated with six holes of 1 cm diameter, allowing plants to grow through as well as watering and sampling (Fig. 1B). Three to four seedlings of reed canary grass (stem height around 20 cm) were planted in the microcosms and grew with 2 g/L fertilizer (Hakaphos® Soft Spezial) which was replenished every week for two months before the application of glyphosate. One week before glyphosate application, the rhizosphere part was sealed for plant adaption by closing the holes on the lid. The holes for plants were first filled with soft clay and finally sealed with silicone rubber sealant (TFC silicone rubber Type 1; Troll Factory, Riede, Germany). The other holes were closed by rubber stoppers. For trapping 13CO2 and 12CO2, a 25 mL glass vessel for NaOH solution was placed in the rhizosphere part; the vessel was held in position by a stainless steel wire mounted to a rubber stopper. Sampling and replacement of NaOH solution could be conducted by a syringe through the cannula installed in the rubber stopper. When not in use, the cannula was closed with a luer lock (B. Braun, Melsungen, Germany). For the unplanted microcosms, the lids of the jars did not contain any holes. The unplanted microcosms were fertilized with 2 g/L Hakaphos® Soft Spezial twice (1st: directly after preparation of the microcosms, 2nd: one week before glyphosate application). The glass vessels for NaOH solution were mounted inside the microcosms. The lids were opened during sampling and replacement of NaOH solution for the short time needed for the procedure. The outer walls of all the microcosm jars were covered with aluminum foil to minimize algae growth. All the microcosms were placed in a greenhouse with controlled conditions and located at Helmholtz-Centre for Environmental Research (UFZ, Leipzig, Germany). The temperature was kept at 22°C from 6 am to 9 pm, and at 16°C at night, thus providing standard conditions allowing for optimal plant growth. Light produced from Master SON-PIA lamps (400 W, Phillips, Belgium) was supplied when the illuminance by natural light fell below 60 klx.
Fig. 1.
Diagrams of setup (A) and lid (B) of the planted microcosms. The lid holes 1 – 3 allowed the plants to grow through the lid; they were sealed with clay and silicone rubber sealant. The hole 4 was for supplementing water, and hole 5 was for sampling pore water, and both were closed with rubber stoppers. Hole 6 was for sampling and replacement of NaOH solution performed through an installed cannula in the rubber stopper.
After around two months, when the stem height of the plants reached around 30 – 40 cm, water was removed from all the microcosms by evapotranspiration (for the planted microcosms) or by decanting (for the unplanted microcosms). Thereafter, 200 mL of 20 mg/L glyphosate solution (unlabeled or 2-13C,15N co-labeled) in tap water or only tap water (blank control) was loaded into the microcosms. The applied concentration of glyphosate was much higher than that found in the runoff water or surface waters (ranged from several to hundreds of μg/L (Geng et al., 2021; Lefrancq et al., 2017)). However, such high amount of glyphosate was needed to ensure isotopic enrichment in the samples not being masked by the natural abundances of 13C and 15N. Previous study by Wang et al. (2016) showed that the mineralization rate of glyphosate at high concentration (50 mg/L) in water-sediment was a bit slower than that at low concentration of 3 mg/L, but the ultimate cumulative mineralization at the two concentrations was the same (about 60% of the initially added 13C) at the end (80 days).
The resulting water level in each microcosm was around 1 cm below the top of the gravel. NaOH solution (2 M, 10 mL) was added to the glass vessels. The last supply of nutrients to all microcosms was one week before dosing glyphosate. During the incubation period, the planted microcosms were supplemented only with tap water every two days to compensate for water loss by plant transpiration and to maintain the water level. Water was not supplemented for the unplanted microcosms due to insignificant loss. All microcosms were incubated for 31 days.
2.4. Sampling and analysis
2.4.1. Sampling procedure
During the incubation period, NaOH solution with CO2/13CO2 trapped was sampled and replaced every four days for the unplanted microcosms, and every two days for the planted microcosms due to the high respiration of rhizosphere. Three water samples of around 300 µL were taken from the microcosms to determine the concentrations of glyphosate and its degradation products (AMPA, sarcosine and glycine) on days 0, 5, 13, 22, and 31.
After 31 days, when the incubation was terminated, the microcosms were opened. Plants were retrieved by carefully separating the roots from gravel. They were then divided into shoots and roots, and the fresh weights were recorded. The roots and shoots were thereafter lyophilized and ground to powder with a mixer mill (MM2000; Retsch, Haan, Germany) prior to further analysis. The pore water was withdrawn after manually shaking the gravel and pore water for a few minutes. The suspended microbial biomass in the pore water was collected by centrifugation (5000 g, 20 min, 4°C) and retrieving the pellets. Biofilms attached to plant roots and gravel were extracted with 200 mL and 300 mL of 10 mM phosphate buffered saline (PBS) solution (pH 7), respectively (Weber and Legge, 2010) by shaking at 300 rpm (Unimax 1010, Heidolph; Schwabach, Germany) for 1 h at 25°C. The extraction was conducted twice consecutively. The obtained extracts were then centrifuged and the pellets were collected after removing the supernatants. All harvested microbial biomass pellets were lyophilized for further analysis, and the dry weights were recorded.
2.4.2. Mineralization analysis
13CO2 from the mineralization of 13C in 2-13C,15N-glyphosate was quantified based on the amount of total inorganic carbon (TIC) trapped into the NaOH solution and the 13C/12C-isotope ratio (at% 13C/12C) of CO2 released by addition of phosphoric acid (85%) in an airtight headspace vial (Girardi et al., 2013). The TIC was measured with a Total Organic Carbon analyzer (Shimadzu, Duisburg, Germany); the at% 13C/12C of the CO2 was analyzed with a gas chromatography-combustion-isotope ratio mass spectrometer (GC-C-irMS) which consisted of an Agilent 6890N Network GC system coupled to a Finnigan MAT 253 irMS (Thermo, Bremen, Germany) via a ConFlo IV (Thermo, Bremen, Germany). The GC column used for CO2 separation was a PoraplotQ column (25 m × 0.32 mm × 1 μm; Chrompack, Middleburg, USA). For the detailed instrumental setup, please refer to Text S2.
2.4.3. Extraction and analysis of glyphosate and its degradation products
Pore water. Glyphosate and its degradation products (AMPA, sarcosine, and glycine) in the pore water (for the sampling procedure, see Section 2.4.1) were determined with LC-MS/MS (see below) after centrifugation (5000 g, 20 min, 4°C).
Adsorption on gravel. Glyphosate and AMPA adsorbed on gravel were extracted with 400 mL of 0.3 M NaOH solution by shaking (300 rpm, 1 h, 25°C) after removing the biofilm on gravel by PBS solution. The extraction was conducted three times consecutively. The extracts were combined and diluted with MilliQ H2O in an appropriate amount to have concentrations of glyphosate and AMPA in the calibration range of 0.05 – 50 µg/L for quantification.
Plants. To extract glyphosate and AMPA from the plant material, 80 mg of shoot or 25 mg of root was mixed with 10 mL of H2O/DCM (1/1, v/v). Before the extraction, the plant material was spiked with 50 µL of 10 mg/L 13C315N-glyphosate and 13C15N-AMPA in MilliQ water as internal standards to correct for the extraction efficiency and matrix effect. The extraction was conducted by vortexing for 1 h and subsequently sonicating (35 kHz; Bandelin Sonorex Tk 52, Berlin, Germany) for 20 min. The mixtures were then centrifuged (10 000 g, 20 min, 4°C). The supernatant from the upper water phase (500 µL) was withdrawn and diluted to a final volume of 1 mL with MilliQ H2O prior to the quantification of glyphosate and AMPA.
The analyses of glyphosate, AMPA, sarcosine, and glycine were described in detail in Text S1. Briefly, they were analyzed by LC-MS/MS after derivatization with fluorenylmethyloxycarbonyl chloride (FMOC-Cl) (Muskus et al., 2019; Sun et al., 2019). The LC-MS/MS comprised a 1260 Infinity II LC system (Agilent, Santa Clara, USA) coupled to a QTRAP 6500+ MS (AB Sciex, Darmstadt, Germany) with an electrospray ionization (ESI) source. A ZORBAX Extend-C18 column (2.1 × 100 mm, 3.5 µm particle size; Narrow Bore RR, Agilent, US) was used to separate the analytes. Glyphosate and AMPA were separated with a gradient of 5 mM ammonium acetate (pH 9) and methanol as mobile phases and detected in negative ion mode. Sarcosine and glycine were separated with a gradient of 5 mM acetic acid/ammonium acetate and acetonitrile as mobile phases and detected in positive ion mode. The limits of quantification (LOQ) were 0.04 µg/L for glyphosate and 0.12 µg/L for AMPA, 0.64 µg/L for sarcosine and 0.69 µg/L for glycine.
2.4.4. Analysis of amino acids (AAs)
AAs were hydrolyzed from the proteins of microbial biomass pellets collected from the endpoint (pore water, PBS extracts of gravel and root biofilm) with 6 M HCl at 110˚C for 22 h according to the method of Nowak et al. (2011). The obtained hydrolysates were then passed through a glass microfiber filter (0.7 µm, Puradisc 25 GF/F; Whatman, Buckinghamshire, UK) and dried under N2 prior to derivatization. The details of derivatization and subsequent purification were described previously (Nowak et al., 2011). Finally, the samples were dissolved in hexane with 20% ethyl acetate for further analysis.
The amounts of 13C and 15N incorporated into the AAs were calculated based on the amount of individual AA measured by GC-MS and their corresponding isotopic composition (13C/12C and 15N/14N) determined by GC-C-irMS. The detailed instrumental methods are listed in Text S3.
2.4.5. Analysis by elemental analyzer
The total amounts of C and N, and the corresponding isotope ratios of C (13C/12C) and N (15N/14N) in the microbial pellets, plants, and the endpoint pore water were analyzed with elemental analyzer (EA)-irMS (EA Flash 2000, Thermo; ConFlo IV, Thermo; Delta V Advantage IRMS, Thermo, Bremen, Germany). All calibration curves for C and N concentrations exhibited R2 > 0.99.
2.5. Mass balance and data analysis
The amounts of 13C and 15N were determined in different compartments of the microcosms: pore water, gravel, plants, NaOH-trapping solution, and pellets collected from the endpoint pore water, gravel biofilm and root biofilm. The recovered amounts of 13C and 15N in each compartment are presented as the percentages of the initially applied amount of 2-13C,15N-glyphosate. The mass balance of 13C and 15N was calculated by the sum of the percentages. The measured amounts of 13C and 15N in the AAs were used to represent the labeled microbial proteins. Since protein accounts for around 55% of the dry weight of microbial cells (Madigan, 2015), a conversion factor of 1.8 was used to estimate the total amounts of 13C and 15N from 2-13C,15N-glyphosate incorporated into the microbial biomass.
All data are presented as average value (± standard deviation) of three replicates. The difference between the planted microcosms and the unplanted microcosms was compared by using unpaired t-test, and was considered significant if p < 0.05.
3. Results and discussion
3.1. Glyphosate and its degradation products
3.1.1. Concentrations in the pore water
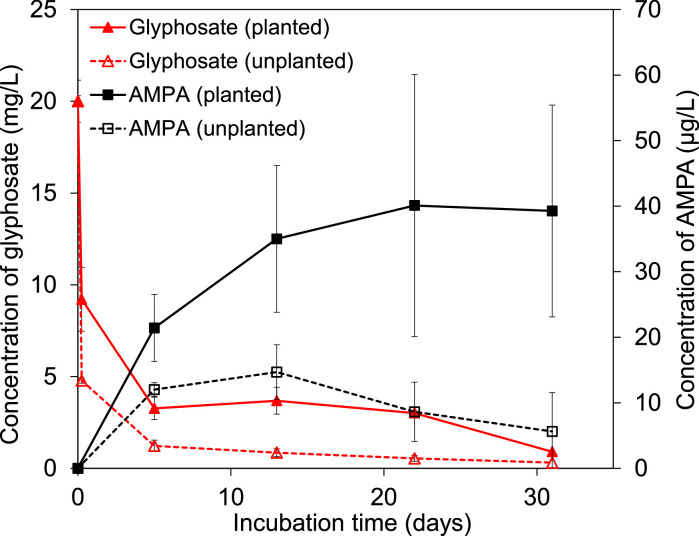
The concentrations of glyphosate, AMPA, glycine, and sarcosine in the pore water were monitored over the incubation period to check the dissipation of glyphosate and the detection of degradation products. The concentrations of glyphosate in the pore water dropped rapidly within several hours to 9.2 ± 1.2 mg/L in the planted and to 4.8 ± 0.3 mg/L in the unplanted microcosms after glyphosate application (Fig. 2). Such a rapid dissipation of glyphosate from the pore water implied that sorption occurred in the microcosms. The glyphosate concentration then decreased slowly from day 5 onwards. AMPA was already detected on day 5 at 21 ± 5 μg/L in the planted and at 12 ± 1 μg/L in the unplanted microcosms. Then the concentration of AMPA increased to a maximum of 40 ± 20 μg/L on day 22 in the planted microcosms and remained almost stable thereafter. The concentration of AMPA in the unplanted microcosms reached a maximum of 15 ± 4 μg/L on day 13 and afterwards decreased slightly until the end. We did not detect glycine and sarcosine on any sampling date. Both sarcosine and glycine produced from glyphosate degradation in the microcosms may have been utilized very fast by the microorganisms.
Fig. 2.
Concentrations of glyphosate and AMPA in the pore water of planted and unplanted microcosms during the 31-day incubation period. Results are presented as mean values of three replicates with error bars indicating standard deviations.
Noteworthy is that the concentrations of AMPA were two orders of magnitude lower than those of glyphosate. Comparing with the initially applied concentration of glyphosate (20 mg/L), relatively low concentrations of glyphosate (< 1 mg/L) and AMPA (maximum 40 µg/L) were detected in the pore water after 31 days, indicating high removal of glyphosate from the water in both microcosms.
The concentrations of glyphosate in the unplanted were always lower (p < 0.05) at each sampling date than in the planted microcosms. This thus suggests that the unplanted microcosms exhibited higher removal of glyphosate from the water than the planted microcosms. However, higher (p < 0.05) concentrations of AMPA were detected on day 31 in the planted (39 ± 16 μg/L) than in the unplanted microcosms (6 ± 6 μg/L). After terminating the incubation, 10.9 ± 2.2% of the initial amount of glyphosate was found in the planted pore water, and AMPA accounted for 0.4 ± 0.2% of the initial amount of glyphosate. In the unplanted microcosms, glyphosate accounted for 1.8 ± 1.1% and AMPA for 0.1 ± 0.03%. High removal of glyphosate from water was also observed by López-Chávez et al. (2021). In their study, more than 98% of the initially applied glyphosate was dissipated during water passage through both planted and unplanted wetlands after 47 days. However, the removal of glyphosate from the planted pore water in this study (around 89%) was lower than that in their study. The high removal in their unplanted wetlands was mainly due to sorption of the glyphosate on the gravel, while that in their planted wetlands was attributed to both sorption and degradation of the glyphosate.
3.1.2. Adsorption on gravel
The adsorption capacity of glyphosate, AMPA, glycine, and sarcosine on the gravel was studied in an independent batch experiment (details in Text S4), and the result was presented in Fig. S1. It showed that more than 85% of the initially applied amount of glyphosate and AMPA was adsorbed on the gravel within 2 h, and the adsorption increased to 98% after 24 h. The adsorption of glycine and sarcosine on the gravel was less than 20%.
After terminating the incubation of the microcosms, the glyphosate and AMPA adsorbed on gravel were released by both PBS solution and NaOH solution (Table S3). Glyphosate and AMPA were desorbed into the PBS solution due to the competition of phosphate in PBS with the phosphonate group of glyphosate and AMPA for sorption sites on gravel (López-Chávez et al., 2021; Pereira et al., 2019). In total, the glyphosate adsorbed on gravel accounted for 59.5 ± 6.5% of the initially added glyphosate in the planted microcosms, and for 86.4 ± 1.2% in the unplanted microcosms. Thus, the adsorption of glyphosate on gravel was the main fate of glyphosate in the microcosms. In addition, the adsorption was lower (p < 0.05) in the planted microcosms than in the unplanted counterparts. Therefore, higher removal of glyphosate from the pore water was observed in the unplanted microcosms in Section 3.1.1.
The high adsorption of glyphosate on gravel was also observed in other studies (López-Chávez et al., 2021; Strange-Hansen et al., 2004). The adsorption of glyphosate was associated with the contents of iron and aluminum oxides of the solid matrices (Piccolo et al., 1994). The iron and aluminum oxides are prone to form inner sphere complexes with the phosphonate group of glyphosate, as the distance of the OH-groups of the phosphonate group matches well with the lattice geometry of the oxides (Sheals et al., 2002). In addition, iron-and aluminum-oxides have high point of zero charge (pHPZC) (above 7.0) (Pereira et al., 2019). At pH < pHPZC, they are positively charged and thus can sorb the negatively charged glyphosate present at common environmental pH conditions. Hence, the adsorption of glyphosate on gravel is also associated with pH. Although the amorphous iron and aluminum contents in the gravel (150 mg Fe/kg and 26 mg Al /kg) of this study were lower than those of the gravel (202 mg Fe/kg and 49 mg Al /kg) studied by Albers et al. (2020), the adsorption of glyphosate on the gravel in this study was higher. This divergence might be due to the lower pH of the gravel in this study (4.5) than those in the study of Albers (8.4 and 9.0). At low pH, the iron-and aluminum-oxides are more positively charged and thus can sorb the negatively charged glyphosate stronger and with a higher capacity.
The lower adsorption of glyphosate on the planted gravel than on the unplanted gravel can be attributed to the following four explanations. (1) The dense biofilms developed on the planted gravel sheltered the adsorption sites of glyphosate. The extracted biomass from the gravel biofilm was more than 10-fold higher in the planted microcosms (31.5 ± 4.8 mg) than that in the unplanted counterparts (2.5 ± 0.4 mg). (2) The higher amount of phosphorus in the planted microcosms occupied some sorption sites for glyphosate (Table S4). Phosphate was found to compete for sorption sites of glyphosate in soils or minerals (Borggaard and Gimsing, 2008; Gimsing et al., 2004). The sorption capacity for glyphosate thus can be reduced by prior application of phosphate (Munira et al., 2018). In this study, prior to glyphosate application, nutrient containing phosphate was supplied more often to the planted microcosms for supporting plant growth than to the unplanted microcosms (Section 2.3). Although the plants utilized substantial part of the added phosphate, there could be still some phosphate adsorbed on gravel hampering the adsorption of glyphosate. (3) Root exudates or their mediated microbial activity liberated the adsorbed glyphosate (Li et al., 2021). Root exudates were not analyzed in this study. Instead, the DOC concentration in the pore water was measured, which was higher in the planted microcosms (14.2 ± 2.3 mg/L) than in the unplanted microcosms (7.9 ± 0.8 mg/L) suggesting root exudates were present. Besides, the higher amount of microbial biomass collected from the planted microcosms also indicated the contribution of root exudates. (4) More metal-oxides in gravel were presumably reduced in the planted microcosms than in the unplanted counterparts; therefore, the sorption of glyphosate in the planted microcosms decreased. A black color presumably caused by precipitation of metal sulfides under anoxic conditions (Janssen et al., 1997) was observed at the greater depth of the planted gravel (Fig. S2), but not in the unplanted microcosms, which suggests the planted microcosms were more anoxic. In summary, both the rhizosphere condition and nutrient application for plant development may have influenced the sorption of glyphosate on gravel in the planted microcosms, but we cannot decide which factor contributed most in this study.
3.2. Mineralization of glyphosate
The release of 13CO2 from 2-13C,15N-glyphosate was measured over the 31-day incubation period. The detected 13CO2 in the microcosms increased slowly from day 0 without a lag phase (Fig. 3). The ultimate cumulative mineralization of 13C from 2-13C,15N-glyphosate accounted for 4.1% of the initially added 13C in the planted microcosms, and 1.1% in the unplanted microcosms. The higher mineralization of 2-13C,15N-glyphosate in the planted microcosms than in the unplanted microcosms could be attributed to the higher amount of DOC in the planted microcosms (Table S4) which could have promoted the activity of microbial degraders.
Fig. 3.
Cumulative mineralization of 13C from 2-13C,15N-glyphosate in the planted and unplanted microcosms during the 31-day incubation period. Results are presented as mean values of three replicates with error bars indicating standard deviations.
The mineralization of glyphosate was relatively low in the microcosms compared with that in soil and water-sediment reported by others (Muskus et al., 2019; Sun et al., 2019; Wang et al., 2016). Mineralization of glyphosate was found to be negatively correlated to its adsorption by soil (Zablotowicz et al., 2009). However, higher adsorption of glyphosate on soil than on gravel was reported (Albers et al., 2020; Strange-Hansen et al., 2004). Moreover, although the adsorption capacity of gravel in this study is lower than that reported by Strange-Hansen et al. (2004), the mineralization of glyphosate in this study was much lower than that in Strange-Hansen’ study with comparable concentration of glyphosate (17 mg/L in their study) applied. Therefore, the low mineralization of glyphosate in this study cannot be attributed only to its high adsorption on the gravel. The microbial activities and microcosm conditions may also have influenced the mineralization of glyphosate (Stenrod et al., 2006; von Wirén‐Lehr et al., 1997). Several studies have shown that glyphosate is less degraded in oxygen-limited soil microcosms (Kanissery et al., 2015; la Cecilia and Maggi, 2018). As the microcosms in this study were water-saturated, we assume that the oxygen level was low, at least at greater depths. Although plants can transport oxygen into the microcosms, the planted microcosms are still believed to be oxygen deficient. The evidence for this is the black color at the greater depth of the gravel (Fig. S2) as discussed in Section 3.1.2.
3.3. Incorporation of elements into microbial biomass
The 13C and 15N derived from 2-13C,15N-glyphosate in AAs were used to quantify the label incorporation into the microbial biomass. 1.5 ± 0.9% of the initially applied 13C and 3.8 ± 1.7% of the initially applied 15N (considering the conversion factor 1.8, see Section 2.5) were incorporated into the microbial biomass collected from the planted microcosms. However, the isotope assimilation into the biomass was much lower in the unplanted microcosms (0.2 ± 0.08% of the initially applied 13C and 0.1 ± 0.01% of the initially applied 15N). Although these percentage values are very low, the 13C and 15N isotope enrichments of AAs were significant as compared to the unlabeled control (natural abundance).
The incorporation of 13C and 15N into the microbial biomass indicated that 2-13C,15N-glyphosate degraded through sarcosine/glycine pathways apart from AMPA pathways (Fig. 4). Glycine was most abundant in 13C and 15N according to the labeling patterns of individual AA (Fig. 5). The 13C15N-glycine could be either the direct product of glycine pathway or yielded from the degradation of 13C15N-sarcosine; both pathways are energetically favorable (Brock et al., 2019). The produced glycine can be directly incorporated into the microbial proteins as a monomeric building block, which also saves energy for microorganisms during anabolism (Nelson et al., 2017).
Fig. 4.
Potential degradation pathways of 2-13C,15N-glyphosate in the microcosms, adapted from Wang et al. (2016). The 13C label is marked with red dots, while the 15N label is marked with blue dots. TCC indicates the tricarboxylic acid cycle.
Fig. 5.
Labeling patterns of 13C-amino acids (AAs) and 15N-AAs derived from the microbial biomass on day 31 in the planted microcosms (A, B, respectively) and unplanted microcosms (C, D, respectively). Results are shown as mean values of three replicates with error bars indicating standard deviations.
Noteworthy is that the amount of 15N-labeled glutamate was much lower than 15N-glycine in the unplanted microcosms (Fig. 5D), while the 15N-labeled glutamate had similar amount to 15N-glycine in the planted microcosms (Fig. 5B). This indicates deamination of 15N-glutamate could have happened in the unplanted microcosms. The higher amount of inorganic N in the unplanted microcosms than that in planted microcosms could have led to deamination of AAs to excrete the overloaded 15N from sarcosine/glycine degradation pathway. The possible inorganic 15N is unlikely to derive from 15N-AMPA degradation, because the degradation of AMPA can be inhibited by the low C/N ratios in the microcosms (DOC/TN ratio in the endpoint pore water was 8.27 ± 2.83 in the planted microcosms and was 0.04 ± 0.003 in the unplanted counterparts, see Table S4) (Brock et al., 2019).
3.4. Uptake by plants
Glyphosate did not influence the biomass of plants which was comparable in microcosms with and without glyphosate (Table S5). A small portion of 13C (1.3% of the initially added 2-13C,15N-glyphosate) was only found in the roots (Table S6) with EA analysis, while 15N was found both in shoots and roots and accounted for 2.2% in total. However, 2-13C,15N-glyphosate was detected also in shoots with LC-MS/MS analysis. It suggests that a small amount of 13C was also incorporated into the shoots, but it was not detectable due to the high carbon content of shoots. The glyphosate in plants (shoots + roots) accounted for 0.4 ± 0.3% of the initially added glyphosate, whereas AMPA for 0.02 ± 0.01%. The amounts of 2-13C,15N-glyphosate and 15N-AMPA in plants were three to five times lower than the total amounts of 13C and 15N in plants. The possible reasons can be that: i) glyphosate and AMPA in plants were mostly transformed, ii) more degradation products of glyphosate were transported into plants than glyphosate itself, and iii) glyphosate or AMPA formed phase-II-conjugates or underwent phase-III-compartmentation in plants, which masked their presence and detection in plants (Van Eerd et al., 2003).
3.5. Mass balance of 13C and 15N from 2-13C,15N-glyphosate in the microcosms
The 13C and 15N mass balances from 2-13C,15N-glyphosate in the microcosms are shown in Fig. 6A–D. The recovery of 13C and 15N was 80.5 ± 8.5% and 84.8 ± 4.5% in the planted microcosms, and 90.7 ± 0.5% and 93.1 ± 0.4% in the unplanted microcosms. The recovery of 13C and 15N was thus lower in the planted microcosms than that in the unplanted counterparts. The lower recovery of 13C presumably resulted from the loss of 13CO2 by plant aerenchyma (Grundmann et al., 2008). The lost 13CO2 could result from the degradation of 2-13C,15N-glyphosate or its degradation products in plants. The lower recovery of 15N could be ascribed to the loss of 15N2 which could result from inorganic 15N reduction under anaerobic conditions in the planted microcosms.
Fig. 6.
Distribution of 13C and 15N label equivalents from 2-13C,15N-glyphosate in different compartments of the planted (A, C) and unplanted (B, D) microcosms (% of the initially applied isotope label), and composition of 15N in pore water and adsorbed on gravel of the planted (E) and unplanted (F) microcosms. Mean values of three replicates are shown.
In both microcosms, 13C and 15N was dominantly adsorbed on gravel as 2-13C,15N-glyphosate and in particular in the unplanted microcosms (> 86% of the initially added isotope). In the planted pore water, 15N was attributed to 2-13C,15N-glyphosate and 15N-AMPA (Fig. 6E). In the unplanted pore water, around 12.3% of 15N could not be identified (Fig. 6F) and it was most likely inorganic 15N (15NH4+ or 15NO3−) derived from the deamination of the 15N-AAs (Section 3.3).
4. Conclusions and outlook
With the microcosm test, we established the mass balance of the model pesticide – glyphosate fate in planted and unplanted water-gravel microcosms and achieved good recovery of the pesticide. The comprehensive fate of the pesticide in the rhizosphere was elucidated by including sorption on substrate, mineralization, degradation products, and incorporation of elements into microbial biomass and compared with that in unplanted microcosms. Plants influenced the microbial utilization of the pesticide as a carbon and nitrogen source as revealed by 13C,15N-colabeling approach. Although the degradation of the pesticide was promoted in the planted microcosms, the adsorption on the gravel as the dominant fate was lower than that in unplanted microcosms, leading to higher amount of the pesticide detected in the planted pore water. In this case, strategies, such as increasing the hydraulic retention time of water, are needed to increase degradation of the pesticide in planted filters for meeting the requirement of water quality for effluent.
The fate of chemicals under the influence of plants is largely unknown especially in planted filters. This microcosm set-up could serve as a reference for such studies, but establishing mass balance of the chemical fate is only possible for non-volatile compounds. The advantage of this microcosm set-up is the simplicity and that it can be used for other substrates to be applied in wetlands or for agricultural soils. Furthermore, the influence of plants on chemical fate applying microcosm set-up under controlled laboratory conditions is not considered yet in regulatory testing of chemicals (e.g. REACH regulation). Therefore, this microcosm test also could be developed as a future standardized OECD test for regulatory testing of chemicals.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This collaborative research has been partly supported by the EU WaterJPI – 2015 AWARE project (PCIN – 2017 – 067) and the Research Council of Norway (RCN 272309/E50). We also thank NIBIO and UFZ for financial support, and the China Scholarship Council (CSC) for funding Yuying Jing. The research was also financed by the ELECTRA project that received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 826244. In addition, we thank Steffen Kümmel (UFZ, Isotope Biogeochemistry) for his help with isotopic analysis.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.watres.2022.119211.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Albers C.N., Jacobsen O.S., Bester K., Jacobsen C.S., Carvalho P.N. Leaching of herbicidal residues from gravel surfaces–a lysimeter-based study comparing gravels with agricultural topsoil. Environ. Pollut. 2020;266 doi: 10.1016/j.envpol.2020.115225. [DOI] [PubMed] [Google Scholar]
- Borggaard O.K., Gimsing A.L. Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manag. Sci. 2008;64(4):441–456. doi: 10.1002/ps.1512. [DOI] [PubMed] [Google Scholar]
- Brock A.L., Rein A., Polesel F., Nowak K.M., Kastner M., Trapp S. Microbial turnover of glyphosate to biomass: utilization as nutrient source and formation of AMPA and biogenic NER in an OECD 308 test. Environ. Sci. Technol. 2019;53(10):5838–5847. doi: 10.1021/acs.est.9b01259. [DOI] [PubMed] [Google Scholar]
- Carles L., Gardon H., Joseph L., Sanchis J., Farre M., Artigas J. Meta-analysis of glyphosate contamination in surface waters and dissipation by biofilms. Environ. Int. 2019;124:284–293. doi: 10.1016/j.envint.2018.12.064. [DOI] [PubMed] [Google Scholar]
- Geng Y., Jiang L., Zhang D., Liu B., Zhang J., Cheng H., Wang L., Peng Y., Wang Y., Zhao Y. Glyphosate, aminomethylphosphonic acid, and glufosinate ammonium in agricultural groundwater and surface water in China from 2017 to 2018: Occurrence, main drivers, and environmental risk assessment. Sci. Total Environ. 2021;769 doi: 10.1016/j.scitotenv.2020.144396. [DOI] [PubMed] [Google Scholar]
- Gimsing A.L., Borggaard O.K., Sestoft P. Modeling the kinetics of the competitive adsorption and desorption of glyphosate and phosphate on goethite and gibbsite and in soils. Environ. Sci. Technol. 2004;38(6):1718–1722. doi: 10.1021/es030572u. [DOI] [PubMed] [Google Scholar]
- Girardi C., Nowak K.M., Carranza-Diaz O., Lewkow B., Miltner A., Gehre M., Schaffer A., Kastner M. Microbial degradation of the pharmaceutical ibuprofen and the herbicide 2,4-D in water and soil - use and limits of data obtained from aqueous systems for predicting their fate in soil. Sci. Total Environ. 2013;444:32–42. doi: 10.1016/j.scitotenv.2012.11.051. [DOI] [PubMed] [Google Scholar]
- Grundmann S., Dörfler U., Ruth B., Loos C., Wagner T., Karl H., Munch J.C., Schroll R. Mineralization and transfer processes of 14 C-labeled pesticides in outdoor lysimeters. Water Air Soil Pollut. Focus. 2008;8(2):177–185. [Google Scholar]
- Hand L.H., Gougoulias C., Bramke I., Thomas K.A., Oliver R.G. Evaluation of the rhizosphere contribution to the environmental fate of the herbicide prometryn. Environ. Toxicol. Chem. 2020;39(2):450–457. doi: 10.1002/etc.4604. [DOI] [PubMed] [Google Scholar]
- Imfeld G., Lefrancq M., Maillard E., Payraudeau S. Transport and attenuation of dissolved glyphosate and AMPA in a stormwater wetland. Chemosphere. 2013;90(4):1333–1339. doi: 10.1016/j.chemosphere.2012.04.054. [DOI] [PubMed] [Google Scholar]
- Jones D.L., Nguyen C., Finlay R.D. Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant Soil. 2009;321(1-2):5–33. [Google Scholar]
- Kanissery R.G., Welsh A., Sims G.K. Effect of soil aeration and phosphate addition on the microbial bioavailability of carbon-14-glyphosate. J. Environ. Qual. 2015;44(1):137–144. doi: 10.2134/jeq2014.08.0331. [DOI] [PubMed] [Google Scholar]
- Kästner M., Nowak K.M., Miltner A., Trapp S., Schäffer A. Classification and modelling of Nonextractable Residue (NER) formation of xenobiotics in soil - a synthesis. Crit. Rev. Environ. Sci. Technol. 2014;44(19):2107–2171. [Google Scholar]
- la Cecilia D., Maggi F. Analysis of glyphosate degradation in a soil microcosm. Environ. Pollut. 2018;233:201–207. doi: 10.1016/j.envpol.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Lefrancq M., Jadas-Hécart A., La Jeunesse I., Landry D., Payraudeau S. High frequency monitoring of pesticides in runoff water to improve understanding of their transport and environmental impacts. Sci. Total Environ. 2017;587-588:75–86. doi: 10.1016/j.scitotenv.2017.02.022. [DOI] [PubMed] [Google Scholar]
- Li H., Bolscher T., Winnick M., Tfaily M.M., Cardon Z.G., Keiluweit M. Simple plant and microbial exudates destabilize mineral-associated organic matter via multiple pathways. Environ. Sci. Technol. 2021;55(5):3389–3398. doi: 10.1021/acs.est.0c04592. [DOI] [PubMed] [Google Scholar]
- López-Chávez M.Y., Alvarez-Legorreta T., Infante-Mata D., Dunn M.F., Guillén-Navarro K. Glyphosate-remediation potential of selected plant species in artificial wetlands. Sci. Total Environ. 2021;781 [Google Scholar]
- Lupi L., Bedmar F., Puricelli M., Marino D., Aparicio V.C., Wunderlin D., Miglioranza K.S. Glyphosate runoff and its occurrence in rainwater and subsurface soil in the nearby area of agricultural fields in Argentina. Chemosphere. 2019;225:906–914. [Google Scholar]
- Madigan M., Martinko J.M., Bender K.S., Buckley D.H., Stahl D.A. Pearson Inc.; Boston, USA: 2015. Brock Biology of Microorganisms. [Google Scholar]
- Maggi F., la Cecilia D., Tang F.H., McBratney A. The global environmental hazard of glyphosate use. Sci. Total Environ. 2020;717 doi: 10.1016/j.scitotenv.2020.137167. [DOI] [PubMed] [Google Scholar]
- Maillard E., Imfeld G. Pesticide mass budget in a stormwater wetland. Environ. Sci. Technol. 2014;48(15):8603–8611. doi: 10.1021/es500586x. [DOI] [PubMed] [Google Scholar]
- Morrissey C.A., Mineau P., Devries J.H., Sanchez-Bayo F., Liess M., Cavallaro M.C., Liber K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ. Int. 2015;74:291–303. doi: 10.1016/j.envint.2014.10.024. [DOI] [PubMed] [Google Scholar]
- Mostafalou S., Abdollahi M. Pesticides: an update of human exposure and toxicity. Arch. Toxicol. 2017;91(2):549–599. doi: 10.1007/s00204-016-1849-x. [DOI] [PubMed] [Google Scholar]
- Munira S., Farenhorst A., Akinremi W. Phosphate and glyphosate sorption in soils following long-term phosphate applications. Geoderma. 2018;313:146–153. [Google Scholar]
- Muskus A.M., Krauss M., Miltner A., Hamer U., Nowak K.M. Effect of temperature, pH and total organic carbon variations on microbial turnover of 13C315N-glyphosate in agricultural soil. Sci. Total Environ. 2019;658:697–707. doi: 10.1016/j.scitotenv.2018.12.195. [DOI] [PubMed] [Google Scholar]
- Nelson D.L., Lehninger A.L., Cox M.M. Macmillan; 2017. Lehninger Principles of Biochemistry. [Google Scholar]
- Nowak K.M., Miltner A., Gehre M., Schaffer A., Kastner M. Formation and fate of bound residues from microbial biomass during 2,4-D degradation in soil. Environ. Sci. Technol. 2011;45(3):999–1006. doi: 10.1021/es103097f. [DOI] [PubMed] [Google Scholar]
- Nowak K.M., Telscher M., Seidel E., Miltner A. Unraveling microbial turnover and non-extractable residues of bromoxynil in soil microcosms with 13C-isotope probing. Environ. Pollut. 2018;242:769–777. doi: 10.1016/j.envpol.2018.07.049. [DOI] [PubMed] [Google Scholar]
- OECD . OECD; Paris: 2002. Test No. 307: Aerobic and Anaerobic Transformation in Soil. [Google Scholar]
- OECD . OECD; Paris: 2002. Test No. 308: Aerobic and Anaerobic Transformation in Aquatic Sediment Systems. [Google Scholar]
- OECD . OECD; Paris: 2004. OECD Guidelines for the Testing of Chemicals. [Google Scholar]
- Okada E., Allinson M., Barral M.P., Clarke B., Allinson G. Glyphosate and aminomethylphosphonic acid (AMPA) are commonly found in urban streams and wetlands of Melbourne. Water Res. 2020;168 doi: 10.1016/j.watres.2019.115139. [DOI] [PubMed] [Google Scholar]
- Pereira R.C., Anizelli P.R., Di Mauro E., Valezi D.F., da Costa A.C.S., Zaia C.T.B., Zaia D.A. The effect of pH and ionic strength on the adsorption of glyphosate onto ferrihydrite. Geochem. Trans. 2019;20(1):1–14. doi: 10.1186/s12932-019-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo A., Celano G., Arienzo M., Mirabella A. Adsorption and desorption of glyphosate in some European soils. J. Environ. Sci. Health Part B. 1994;29(6):1105–1115. [Google Scholar]
- Schwertmann U. Differenzierung der eisenoxide des bodens durch extraktion mit ammoniumoxalat-Lösung. Z. Pflanzenernähr. Düng. Bodenkd. 1964;105(3):194–202. [Google Scholar]
- Sheals J., Sjoberg S., Persson P. Adsorption of glyphosate on goethite: molecular characterization of surface complexes. Environ. Sci. Technol. 2002;36(14):3090–3095. doi: 10.1021/es010295w. [DOI] [PubMed] [Google Scholar]
- Shrestha P., Junker T., Fenner K., Hahn S., Honti M., Bakkour R., Diaz C., Hennecke D. Simulation studies to explore biodegradation in water–sediment systems: from OECD 308 to OECD 309. Environ. Sci. Technol. 2016;50(13):6856–6864. doi: 10.1021/acs.est.6b01095. [DOI] [PubMed] [Google Scholar]
- Stenrod M., Charnay M.P., Benoit P., Eklo O.M. Spatial variability of glyphosate mineralization and soil microbial characteristics in two Norwegian sandy loam soils as affected by surface topographical features. Soil Biol. Biochem. 2006;38(5):962–971. [Google Scholar]
- Stenstrom J.R., Kreuger J., Goedkoop W. Pesticide mixture toxicity to algae in agricultural streams ? Field observations and laboratory studies with in situ samples and reconstituted water. Ecotoxicol. Environ. Saf. 2021;215 doi: 10.1016/j.ecoenv.2021.112153. [DOI] [PubMed] [Google Scholar]
- Strange-Hansen R., Holm P.E., Jacobsen O.S., Jacobsen C.S. Sorption, mineralization and mobility of N-(phosphonomethyl)glycine (glyphosate) in five different types of gravel. Pest Manag. Sci. 2004;60(6):570–578. doi: 10.1002/ps.842. [DOI] [PubMed] [Google Scholar]
- Sun F.F., Kolvenbach B.A., Nastold P., Jiang B.Q., Ji R., Corvini P.F.X. Degradation and metabolism of tetrabromobisphenol A (TBBPA) in submerged soil and soil-plant systems. Environ. Sci. Technol. 2014;48(24):14291–14299. doi: 10.1021/es503383h. [DOI] [PubMed] [Google Scholar]
- Sun M.J., Li H., Jaisi D.P. Degradation of glyphosate and bioavailability of phosphorus derived from glyphosate in a soil-water system. Water Res. 2019;163 doi: 10.1016/j.watres.2019.07.007. [DOI] [PubMed] [Google Scholar]
- Syversen N., Bechmann M. Vegetative buffer zones as pesticide filters for simulated surface runoff. Ecol. Eng. 2004;22(3):175–184. [Google Scholar]
- Van Eerd L.L., Hoagland R.E., Zablotowicz R.M., Hall J.C. Pesticide metabolism in plants and microorganisms. Weed Sci. 2003;51(4):472–495. [Google Scholar]
- von Wirén-Lehr S., Komoßa D., Gläßgen W.E., Sandermann Jr, H., Scheunert I. Mineralization of [14C] glyphosate and its plant-associated residues in arable soils originating from different farming systems. Pestic. Sci. 1997;51(4):436–442. [Google Scholar]
- Vymazal J. Emergent plants used in free water surface constructed wetlands: a review. Ecol. Eng. 2013;61:582–592. [Google Scholar]
- Vymazal J., Bfezinova T. The use of constructed wetlands for removal of pesticides from agricultural runoff and drainage: a review. Environ. Int. 2015;75:11–20. doi: 10.1016/j.envint.2014.10.026. [DOI] [PubMed] [Google Scholar]
- Vymazal J., Kröpfelová L. Springer Science & Business Media; 2008. Wastewater Treatment in Constructed Wetlands with Horizontal Sub-Surface Flow. [Google Scholar]
- Wang S., Seiwert B., Kastner M., Miltner A., Schaffer A., Reemtsma T., Yang Q., Nowak K.M. Bio)degradation of glyphosate in water-sediment microcosms - a stable isotope co-labeling approach. Water Res. 2016;99:91–100. doi: 10.1016/j.watres.2016.04.041. [DOI] [PubMed] [Google Scholar]
- Weber K.P., Legge R.L. Method for the detachment of culturable bacteria from wetland gravel. J. Microbiol. Methods. 2010;80(3):242–250. doi: 10.1016/j.mimet.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Zablotowicz R.M., Accinelli C., Krutz L.J., Reddy K.N. Soil depth and tillage effects on glyphosate degradation. J. Agric. Food Chem. 2009;57(11):4867–4871. doi: 10.1021/jf900272w. [DOI] [PubMed] [Google Scholar]
- Zhan H., Feng Y.M., Fan X.H., Chen S.H. Recent advances in glyphosate biodegradation. Appl. Microbiol. Biotechnol. 2018;102(12):5033–5043. doi: 10.1007/s00253-018-9035-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.