Abstract
Objective
Saposhnikoviae Radix (Fangfeng in Chinese), the roots of Saposhnikovia divaricata, lacks commodity specification and grade standardization in the current market. This study investigated the existing specifications and grades of Saposhnikoviae Radix to provide a standardized scientific reference for its market use.
Methods
Based on a textual research of Chinese herbal medicine from the Han Dynasty to the present, medicinal materials of different specifications and grades obtained from Saposhnikoviae Radix in the main producing areas of China were collected and the markets for these materials were investigated. Field investigations were performed in the major producing areas such as Northeast China, Hebei Province, and Inner Mongolia. Four major Chinese herbal medicine markets in China were investigated. Sensory indices were used to categorize the two specifications (wild and cultivated) according to the shape, color, texture, and cross-section. High-performance liquid chromatography was performed to determine the active components. Vernier calipers and measuring tape were used to measure the diameter and length, respectively, of 41 samples. Using Excel and the R Language software, cluster analysis and descriptive statistical analysis were performed to assist in the application of new specifications and grades based on physical characteristics, pharmacological activity, and chemical composition.
Results
The two specifications (wild and cultivated) of Saposhnikoviae Radix were divided into three grades each based on the length and diameter. Prim-O-glucosylcimifugin, 5-O-methylvisamminoside, and the length of Saposhnikoviae Radix can be used as a basis for classifying the commodity specifications and grades. The specifications and grade standards of Saposhnikoviae Radix were established based on the following eight aspects: shape, surface characteristics, texture, cross section, taste, prim-O-glucosylcimifugin content, 5-O-methylvisamminoside content and length.
Conclusion
The formulation of this standard stipulates the commodity specification level of Saposhnikoviae Radix. It is also suitable for the evaluation of commodity specifications in the process of production, circulation and use of Saposhnikoviae Radix.
Keywords: classification, commodity specification and grade, market, Saposhnikovia divaricata (Turcz.) Schischk, Saposhnikoviae Radix, production area
1. Introduction
Saposhnikoviae Radix (SR) is the root portion of Saposhnikovia divaricata (Turcz.) Schischk. (a perennial herb belonging to the Umbelliferae family) and is a commonly used traditional Chinese medicine (Fig. 1) (Peng, 2013). It has been used in China for several years for the treatment of the common cold, headache, rheumatic diseases, arthralgia, rubella, pruritus, and tetanus (National Pharmacopoeia Committee, 2015). In ancient books such as the Shennong’s Classic of Materia Medica, the use of SR is generally prescribed to dispel wind to release the exterior pattern, to dispel dampness, to relieve pain, and to arrest convulsions, which roughly corresponds to its modern applications. Presently, owing to its efficacy, SR is a popular product worldwide, especially in Japan, Korea, Mongolia, and Russia (Magsar, 2006).
Fig. 1.
Image of S. divaricata (A) and Saposhnikoviae Radix (B).
Although it is one of the most important Chinese medicinal components available in the market, SR has not been classified and assigned with commodity specifications and grades. SR, with thick and large roots, has been previously reported as an excellent product. However, most roots are now sold as a unified, ungraded product at the production site. Presently, SR’s grade in the market is based on the diameter of the root. However, no uniform standard or criterion exists. Therefore, it is important to formulate a new standard for the specification and grade of the commodity. This study investigated the historical evolution of SR through a research literature review and summarized the previous findings on the reported specifications and grades, so as to provide a basis for the current study. In this study, field surveys and sampling were conducted in the main production areas and the major herbal medicine markets in China. A detailed summary of the processing and grading of origin and market division methods was also conducted. Based on the literature and field survey data, we formulated specifications and grades of SR to promote its rational application in the market and safe medicinal use.
2. Materials and methods
2.1. Materials
Forty-one samples of SR were collected from the main production areas and four major herbal medicine markets in China, and all samples were identified as the dried roots of S. divaricata by Professor Minhui Li at the Baotou Medical College, Inner Mongolia.
2.2. Literature survey
To lay a foundation for field and market investigations and the formulation of standards, we reviewed and categorized the herbal textual records of SR from the Han Dynasty to the present and systematically summarized the quality evaluation records in the ancient Chinese books, current printed research literature, electronic resources (such as Baidu Scholar, Google Scholar, Web of Science, PubMed, and CNKI), research articles, Ph.D. and M.Sc. dissertations, and existing local standards.
2.3. Field and market investigation
Based on the previous literature survey conducted, the members of the investigation team visited the major herbal medicine markets and main production areas in China to investigate the distribution of SR in the market. Field investigations were conducted in the main production areas, including northeastern China, Hebei, and Inner Mongolia. Four S. divaricata farms located in Tailai in Heilongjiang, Hulunbuir and Chifeng in Inner Mongolia, and Anguo in Hebei were visited and investigated to understand the areas of cultivation, planting systems, and growing conditions of cultivated S. divaricata. For the wild S. divaricata, a field survey was conducted by designating a sample area (10 m × 10 m) in northeastern China and Inner Mongolia. One kilogram of S. divaricata was collected as a sample from each study area.
Market investigation was conducted in Anguo, Bozhou, Hehuachi, and Yulin herbal medicine markets (four major herbal medicine markets in China), located in Hebei, Anhui, Sichuan, and Guangxi, respectively. Six stores involved in the sale of S. divaricata were randomly selected to investigate its demand and market price according to the sales records. One kilogram of S. divaricata, without reference to its specification and grade, was purchased as a sample from each store.
2.4. Observation of appearance characteristics of SR samples
Based on traditional experience, combined with herbal research findings, market research findings, and the 2015 Chinese Pharmacopoeia’s description of the morphological characteristics, we selected the diameter and length of SR as quantitative indicators. For each sample collected, we randomly selected 10 SR samples for the measurement of diameter using a Vernier caliper and length using a measuring tape. The measurements were accurate to 0.01 mm and 0.1 cm, respectively. The color was observed and recorded.
2.5. Determination of active components of SR samples
2.5.1. High-performance liquid chromatography
A Waters C18 column (5 μm, 4.6 mm × 250 mm) was used for performing high-performance liquid chromatography (HPLC) (Thermo Fisher Scientific, Waltham, MA, USA). The mobile phase acetonitrile (A)-aqueous solution (B) was eluted by conducting gradient elution. The elution procedure was as follows: 0–20 min, 15%–25% A; 20–30 min, 25%–50% A; 30–40 min, 50%–50% A, and the flow rate was 1 mL/min. The detection wavelength was 254 nm, column temperature 30 °C, injection volume 10 L, and the number of theoretical trays calculated according to glucosinolates were not <2000.
2.5.2. Preparation of reference solution
The appropriate amount of the reference material was accurately weighed and dissolved in methanol to prepare a solution of cimicillin glycoside, cimicillin (ampicillin), 5-O-methylvisammiol glycoside, and hesperidin.
2.5.3. Preparation of test solution
SR was ground and subjected to filtration through a 40-mesh sieve. The powder was dried to a constant weight, and 0.25 g sample was placed in a conical flask, to which 10 mL of methanol was added. The flask was stoppered and weighed. The mixture was refluxed for 2 h in a water bath, cooled, and the weight loss was compensated with the addition of methanol. It was thoroughly shaken and filtered through a 0.22 μm filter membrane to obtain the filtrate.
2.6. Statistical analysis and establishment of commodity specifications and grade standards
Data analysis was performed using R language (The R Foundation for Statistical Computing, Vienna, Austria) and Microsoft Office Excel Professional Plus 2016 (Microsoft Corporation, Redmond, WA, USA). R language was used to perform cluster analysis for the quantitative indicators of each SR sample to obtain the categories of all measured samples. Then, the measurement data in each category was graded by using Excel and descriptive statistical analysis with a 95 % confidence interval. The results of the analysis were used to establish the specifications and grades of SR.
3. Results
3.1. Literature survey
3.1.1. Herbal textual research
SR is referred to as Fangfeng, Tongyun, Huicao, Baizhi, and Pingfeng in ancient Chinese books. It has a long history of medicinal application, and its medical application was first reported in Shennong’s Classic of Materia Medica, the oldest pharmaceutical monograph of traditional Chinese medicine written during the reign of the Qin and Han Dynasties. In this monograph, SR usage has been prescribed for treating dizziness, blindness, dysphoria, apoplexia, joint pain, and for providing relaxation to the body when used for a long term (Liu & Zhao, 2017). In addition to the records of its therapeutic effects, the production areas of SR have been recorded in detail in different dynasties, and include Shaanxi, Heilongjiang, Shandong, Henan, Jiangsu, Zhejiang, and northern Hubei (Table 1). There have been significant changes in these areas from ancient to modern times. Table 1 showed that the northward movement of the production areas of S. divaricata was primarily related to the destruction of the wild resources caused by the expansion of cultivated land associated with population growth (Wang & Lou, 1989).
Table 1.
Herbal textual research of SR.
| Dynasty | Monographs | Medicinal material names | Original plants | Traditional uses | Producing areas |
|---|---|---|---|---|---|
| Han Dynasty (A.D. 220–450) |
Mingyi Bielu | Huicao, Baizhi, Pingfeng, etc | S. divaricata | Dispel wind and relieve pain | Shayuan (The Weinan region of Shaanxi), Chuanze, Handan (the southern of Hebei Province), Langya (now in the Shandong Province) and Shangcai (now in the Henan Province) |
| Northern and Southern Dynasties (A.D. 480–498) | Bencao Jing Jizhu | Tongyun, Huicao, Baizhi, Pingfeng, etc | Peucedanum wawrii (Wolff) Su | Dispel wind and relieve pain | Pengcheng (now the Xuzhou in Jiangsu), Lanling (now in the Shandong Province), Yuzhou (The Lianyungang region of Jiangsu); Xiangyang (now in the Hubei Province), Yiyang (now the Xinyang of Henan) |
| Tang Dynasty (A.D. 657–659) |
Xinxiu Bencao | Tongyun, Huicao, Baizhi, Pingfeng, etc | P. ledebourielloides K. T. Fu | Dispel wind, relieve pain and relieve internal heat or fever | Longshan in Qizhou (now the southwest of Shandong), Zizhou (now the Zibo of Shandong), Yanzhou (now in the Guizhou Province), Qingzhou (now in the Weifang of Shandong) and Shayuan (The Weinan region of Shaanxi) |
| Song Dynasty (CE 1020–1101) | Bencao Tujing | Fangfeng | S. divaricata |
Dispel wind and relieve pain |
Shayuan (The Weinan region of Shaanxi), Chuanze, Handan (the southern of Hebei province) and Shangcai (now in the Henan Province); Jingdong (now the Kaifeng of Henan), Huai and Zhe areas (now the regions of Jiangsu and Zhejiang |
| Shifangfeng | P. ledebourielloides | Hezhongfu (now in the Yongji of Shanxi) | |||
| Song Dynasty (CE 1082) | Zhenglei Bencao | Haizhou Fangfeng | S. divaricata | Dispel wind, relieve pain, tonifying spleen and relieve uneasiness of mind and body tranquilization | The same as Mingyi Bielu, Bencao Jing Jizhu, Xinxiu Bencao, Bencao Tujing recorded |
| Hezhongfu Fangfeng | unknown | ||||
| Qizhou Fangfeng | P. wawrii | ||||
| Tongzhou Fangfeng | P. ledebourielloides | ||||
| MingDynast (CE 1406) | Materia Medica for Famine Relief | Tongyun, Huicao, Baizhi, Pingfeng, etc | S. divaricata | Relieve internal heat or fever | Shayuan (The Weinan region of Shaanxi), Chuanze, Handan (the southern of Hebei province), Langya (now in the Shandong Province) and Shangcai (now in the Henan Province); Shaanxi, Shandong and Zhongmu (now the area of Hebi Mountain in Henan Province) |
| Ming Dynasty (CE 1436) | Materia Medica of South Yunnan | Zhuye Fangfeng | Seseli mairei Wolff | Dispel wind, dehumidification and relieve pain | Yunnan Province |
| Xinye Fangfeng | Pimpinella candolleana Wight et Arn. | Warm the spleen and stomach for dispelling cold | Yunnan Province | ||
| Xiuqiu Fangfeng | Leucas caliata Benth | Remove the carbuncle | Yunnan Province | ||
| Ming Dynasty (CE 1590) | Compendium of Materia Medica | Tongyun, Huiyun, Huicao, Pingfen, etc | S. divaricata | Dispel wind, relieve pain, relieve internal heat or fever and hidroschesis | The same as Mingyi Bielu, Bencao Jing jizhu, Xinxiu Bencao, Bencao Tujing recorded |
| Shifangfeng | Glehnia littoralis F. Schmidt ex Miq. | Dispel wind and relieve pain | Jianghuai Region (now the plain of the middle and lower reaches of the Changjiang River, the north of the Changjiang River and the south of the Huaihe River) | ||
| Qing Dynasty (1848) |
Zhiwu Mingshi Tukao | Shifangfeng (China), Bou-hu (Japan), Bangpung (Korea) | G. littoralis | Dispel wind and relieve pain | Huaiyuan, Tongcheng, Taihe (now in the bengbu, anqing, fuyang of Anhui Province, respectively) |
| Republic of China period | Yaowu Chuchan Bian | Fangfeng | S. divaricata | Dispel wind and relieve pain | Taonan (now in Heilongjiang Province) |
Moreover, according to the ancient illustrations discovered in China, other umbelliferous plants such as Peucedanum ledebourielloides K. T. Fu (Huashan, eastern Shaanxi Province) and P. wawrii (Wolff) Su (Lianyungang, Northern Jiangsu Province), which are now produced in the Weinan area of Shaanxi Province, may provide further evidence for the original plant species of Fangfeng used in ancient times (Fig. 2). Additionally, Glehnia littoralis Fr. Schmidt ex Miq. may be the original plant known as Shifangfeng listed under the Fangfeng record in the Compendium of Materia Medica of the Ming Dynasty (Bencao Gangmu, Ming Dynasty, CE 1590), the root of which is now used as Glehniae Radix and as SR in North Korea and Japan.
Fig. 2.
Images of herbal textual research. A–D: Song Dynasty (CE1082) Haizhoufangfeng, Tongzhoufangfeng, Hezhongfufangfeng, Qizhoufangfeng from Zhenglei Bencao; E: Ming Dynasty (CE1406) Tongyun (also called Huicao, Baizhi, Pingfeng, etc.) from Jiuhuang Bencao; F: Qing Dynasty (CE1848) Shifangfeng from Zhiwu Mingshi Tukao.
In erstwhile dynasties, the production area was an important aspect of assessing the quality of SR. For example, Xinxiu Bencao (Tang Dynasty, A.D. 657–659) recorded that the quality of SR produced in Shandong was appreciable. SR was also produced in the Weinan area of Shaanxi Province. However, its quality was lower than that produced in Shandong owing to its loose texture. Furthermore, the ancient records on the quality of SR also emphasized its morphological characteristics, such as texture, color, and smell. For example, Dissemination of Materia Medica (Bencao Jiyao, Ming Dynasty, CE 1496) recorded that high-quality SR should be dense in texture, rich in oil, with a hard root-head. The quality of SR after subjection to bolting was considered to be low, and it was not used in medicine (Sun et al. 2010). Another well-known book of the Ming Dynasty, Collected Essentials of Species of Materia Medica (Bencao Pinhui Jingyao), recorded the characteristics of SR, in the same manner as Bencao Jiyao. The Compendium of Materia Medica reports that high-quality SR is yellow in color, while low-quality SR is white with a loose texture and is not used in medicine. Moreover, Reverence for the Origin of Materia Medica (Bencao Chongyuan, Qing Dynasty, CE 1674) recorded the characteristics of high-quality SR as follows: yellow, sweet, and fragrant.
In modern times, the Empirical Identification of 500 Chinese Medicinal Materials in Common Use (Lu, 1999) states that the quality of SR is evaluated by the number of lateral roots, the diameter of the upper portion of the root, and the length of the root. The Chinese Pharmacopoeia (2015) also states the minimum quality required for SR based on its basic characteristics, including diameter, length, color, texture, and smell.
3.2. Field and market investigation
3.2.1. Field investigation
The results of the field investigation showed that the planting area of each major production region increased with the increase in market demand. During the last three years, the planting area amounted to 660–830, 250–330, and 830 acres in Inner Mongolia, Anguo in Hebei, and the three provinces of the Northeast, respectively. When the dried roots produced per acre were computed at 250 kg, the total output amounted to 2875–3250 t.
As the largest production area of S. divaricata, northeast China primarily produces wild S. divaricata. However, the wild resources of S. divaricata in these areas have been significantly reduced owing to overexploitation and wasteland reclamation. Therefore, in the 1980s, a few main production areas (such as Heilongjiang) have been established as nature reserves for the protection of the wild resources of S. divaricata. Presently, SR is produced mainly as a semi-wild product in northeastern China.
In Inner Mongolia, the second-largest producer of SR, the wild resources of S. divaricata are rich, and cultivation mainly imitates the wild growth patterns; As a result, it is generally planted on hillside wastelands (Fig. 3). Detailed information on the status of cultivation in Inner Mongolia revealed the following four main points. First, although a substantial number of farmers continue to harvest wild S. divaricata, the number has decreased. Second, because the seed vigor of cultivated S. divaricata is low, many growers use wild seeds for planting. Third, S. divaricata cannot be easily re-moisturized after complete dehydration. Hence, the fresh herbs in these areas should only be spread out for 3 to 7 d without the occurrence of drying. Fourth, currently, medicinal material cultivation companies are adopting a primarily “order-oriented” production system and utilize specific technical cultivation procedures, and such approaches are gradually being realized for standardization because of which more traceable systems can be established. Both the wild and cultivated S. divaricata types are commonly used in the current market.
Fig. 3.
Images of cultivated S. divaricata in Inner Mongolia, China.
As wild resources are rare, Hebei Province primarily produces cultivated S. divaricata. According to the survey results, we found that the cultivated S. divaricata in Hebei Province is planted by conducting direct seeding and transplanting. The production of cultivated S. divaricata in Hebei is the highest amongst those reported in various regions; however, often many lateral roots are observed, and such a product is considered as an inferior product of low quality.
3.2.2. Market investigation
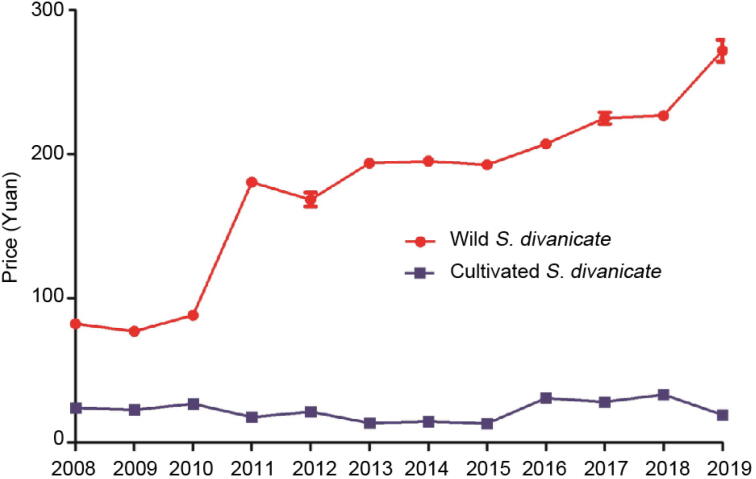
The results of the market investigation indicated that the demand and market price of S. divaricata exhibit the same trend. Guan Fangfeng (SR produced mainly in northeast China), which is of good quality and sufficient efficacy, is considered as the leading herbal medicine in markets across the country, and there is a tendency for the market price to exhibit yearly increase along with an increased demand. According to the research data, the output of wild Guan Fangfeng produced in Inner Mongolia and northeastern China was about 20,000 t in the early 21st century but decreased significantly to approximately 2000 t in 2008. In 2012, it was reduced to approximately 1300 t, the reduction being 15 times less than that reported at the beginning of the century (Ding, 2012). Market investigations showed that the demand for SR increased by 1300–1500 t and 7000–8000 t during 2000–2001 and 2012–2014, respectively, which indicated a 5-fold increase over the past 14 years and an increase in growth has been projected in the future. Guan Fangfeng was temporarily unavailable during the SARS period, causing the price to increase significantly to 120 Yuan/kg, which was unprecedented throughout the medicinal material market. Overall, the price has increased owing to a decrease in wild resources over the past decade. Presently, the price has increased beyond 200 Yuan/kg and continuous increase in the price has been witnessed.
Combining the results of our investigations conducted in each market with the data presented in the literatures and export records derived from the herbal medicine market, we found that between 2008 and 2019, the price of wild SR showed an upward trend, while that of cultivated one remained relatively stable (Fig. 4). However, the survey showed that there was a marked increase in the price of wild SR, which might be attributable to the exhaustion of the inventory 2011 when the avian flu virus spread globally. As the main component of Fangfeng Tongsheng Powder, SR plays an important role in mounting an immune response against the avian influenza virus. Therefore, the demand for medicinal materials markedly increased in 2011, resulting in a corresponding price increase. The data were derived from different markets based on the analysis of five random samples acquired at different times each year.
Fig. 4.
Price trend of SR from 2008 to 2019.
3.3. Morphological characteristics and active component content of S. divaricate
3.3.1. Morphological characteristics of S. divaricate
The diameter and the length of the S. divaricate samples collected from the major herbal medicine markets and main production areas were detailed in Table 2, along with their mean and standard deviation values.
Table 2.
Detail information of S. divaricate samples and their diameter and length measurements
| No. | Market specifications (main type) | Herbal medicine markets or producing areas | Collection time | Diameter [min, max] (mm) | Length [min, max] (cm) | Color |
|---|---|---|---|---|---|---|
| 1 | Wild S. divaricate | Sunrise Oriental Pharmaceutical Company in Ulanhot, Inner Mongolia | September 11, 2017 | 7.85 ± 1.59 [5.54, 11] | 13.26 ± 1.38 [11.1, 15.2] | Gray-brown |
| 2 | Tsining District in Wulanchabu, Inner Mongolia | September 5, 2017 | 5.51 ± 0.99 [4.46, 7.6] | 12.18 ± 1.21 [10.2, 14.3] | ||
| 3 | Zhalantun in Hulunbuir, Inner Mongolia | September 8, 2017 | 4.87 ± 0.48 [4.02, 5.58] | 18.37 ± 1.94 [15.8, 22.2] | ||
| 4 | Horch Village of Arun Banner in Hulunbuir, Inner Mongolia | September 11, 2017 | 7.85 ± 1.14 [5.53, 9.13] | 14.6 ± 1.12 [14.4, 16.9] | ||
| 5 | Hailar District in Hulunbuir, Inner Mongolia | September 9, 2017 | 7.38 ± 1.38 [6.05, 10.9] | 14.01 ± 1.4 [12, 15.5] | ||
| 6 | Anguo herbal medicine market | September 12, 2017 | 12.37 ± 2.96 [9.6, 16.7] | 13.91 ± 2.18 [11.4, 17.9] | ||
| 7 | Rongxing Hall Pharmaceutical Company in Chifeng, Inner Mongolia | September 17, 2017 | 6.13 ± 1.55 [4.77, 10.1] | 12.01 ± 2.84 [4.4, 14.5] | ||
| 8 | Prairie Chenbarhu Banner in Hulunbuir, Inner Mongolia | September 8, 2017 | 7.74 ± 1.45 [5.58, 10.4] | 16.23 ± 3.27 [7.4, 18.8] | ||
| 9 | Hailar District in Hulunbuir, Inner Mongolia | September 9, 2017 | 6.38 ± 0.98 [4.45, 7.34] | 14.87 ± 1.8 [12.2, 17.2] | ||
| 10 | Xin Barag Left Banner in Hulunbuir, Inner Mongolia | September 13, 2017 | 6.65 ± 1.31 [4.75, 8.43] | 18.48 ± 1.69 [16, 21.5] | ||
| 11 | Bozhou herbal medicine market | September 15, 2017 | 8.92 ± 0.91 [7.12, 10.2] | 13.85 ± 1.11 [12, 15.4] | ||
| 12 | Hehuachi herbal medicine market | September 20, 2017 | 6.74 ± 1.5 [5.12, 9.28] | 11.45 ± 1.19 [10.3, 13.5] | ||
| 13 | Xinghe County in Wulanchabu, Inner Mongolia | August 9, 2018 | 8.68 ± 1.2 [6.93, 10.2] | 15.76 ± 1.57 [13.4, 18] | ||
| 14 | Yulin herbal medicine market | September 14, 2017 | 5.46 ± 0.78 [4.29, 6.66] | 11.19 ± 0.8 [10.2, 12.2] | ||
| 15 | Siziwang Banner in Wulanchabu, Inner Mongolia | August 11, 2018 | 8.86 ± 1.97 [6, 12.8] | 13.12 ± 0.87 [11.8,14.6] | ||
| 16 | Tumd Left Banner in Wulanchabu, Inner Mongolia | August 23, 2018 | 6.83 ± 1.41 [4.48, 8.88] | 15.59 ± 1.73 [12.3, 18.4] | ||
| 17 | Horinger County in Huhhot, Inner Mongolia | August 9, 2018 | 5.79 ± 1.02 [4.03, 7.04] | 10.44 ± 1.08 [8.4, 11.9] | ||
| 18 | Qinglongshan town in Tongliao, Inner Mongolia | September 16, 2018 | 6.27 ± 1.17 [4.09, 7.56] | 13.95 ± 2.04 [11.3, 17.1] | ||
| 19 | Xin Barag Right Banner in Hulunbuir, Inner Mongolia | July 19, 2018 | 8.91 ± 2.47 [5.57, 13.4] | 13.25 ± 1.57 [10.8, 15.7] | ||
| 20 | Yakeshi in Hulunbuir, Inner Mongolia | September 10, 2018 | 8.63 ± 1.15 [7.02, 10.9] | 17.94 ± 3.15 [13, 22.2] | ||
| 21 | Daur Autonomous Banner in Hulunbuir, Inner Mongolia | July 17, 2018 | 10.18 ± 3.6 [7.15, 17.6] | 15.67 ± 2 [13.2, 18.2] | ||
| 22 | Bairin Left Banner in Chifeng, Inner Mongolia | August 14, 2018 | 6.89 ± 0.83 [5.21, 7.91] | 13.65 ± 1.32 [11.5, 15.4] | ||
| 23 | West Ujimqin banner in Xilingol League, Inner Mongolia | June 21, 2018 | 5.42 ± 1.12 [3.57, 6.96] | 11.55 ± 1.88 [9.6, 14.8] | ||
| 24 | Cultivated S. divaricate | Sunrise Oriental Pharmaceutical Company in Ulanhot, Inner Mongolia | September 11, 2017 | 4.35 ± 0.55 [3.56, 5.2] | 17.25 ± 0.84 [15.8, 18.4] | Brownish yellow or light brown |
| 25 | Hehuachi herbal medicine market | September 20, 2017 | 4.9 ± 0.75 [4.18, 6.59] | 13.41 ± 0.95 [12.1, 15.2] | ||
| 26 | Rongxing Hall Pharmaceutical Company in Chifeng, Inner Mongolia | September 11, 2017 | 4.94 ± 0.96 [3.2, 6.79] | 20.64 ± 1.1 [19.2,22] | ||
| 27 | Yulin herbal medicine market | September 14, 2017 | 4.77 ± 1.1 [3.24, 7] | 16.28 ± 1.72 [12.7, 18.4] | ||
| 28 | Fengzhen in Wulanchabu, Inner Mongolia | April 6, 2017 | 4.72 ± 0.98 [3.58, 6.59] | 10.29 ± 1.62 [8.3, 12.5] | ||
| 29 | Guyuan County in Zhangjiakou, Inner Mongolia | September 11, 2017 | 4.62 ± 0.5 [3.67, 5.45] | 10.07 ± 1.6 [8.5, 14.1] | ||
| 30 | Bozhou herbal medicine market | September 15, 2017 | 6.57 ± 0.64 [5.63, 8] | 16.59 ± 3.68 [8.7, 20.1] | ||
| 31 | Bozhou herbal medicine market | September 15, 2017 | 15 ± 3.31 [9.56, 19.4] | 10.08 ± 2.4 [5.5, 13] | ||
| 32 | Bozhou herbal medicine market | September 15, 2017 | 6.47 ± 1.51 [4.04, 8.95] | 17.85 ± 3.93 [10.8, 24.8] | ||
| 33 | Bozhou herbal medicine market | September 15, 2017 | 6.4 ± 1.54 [4.37, 8.66] | 12.81 ± 1.77 [10.6, 17.1] | ||
| 34 | Bozhou herbal medicine market | September 15, 2017 | 4.41 ± 0.81 [3.16, 5.5] | 19.02 ± 1.77 [16, 22] | ||
| 35 | Anguo herbal medicine market | September 12, 2017 | 4.62 ± 1.02 [3.06, 6.26] | 22.49 ± 2.9 [18.5, 28] | ||
| 36 | Anguo herbal medicine market | September 12, 2017 | 3.72 ± 0.83 [2.66, 5.39] | 26.51 ± 2.36 [22.5, 29] | ||
| 37 | Anguo herbal medicine market | September 12, 2017 | 3.23 ± 0.85 [1.81, 4.41] | 21.59 ± 1.57 [18.8, 23.8] | ||
| 38 | Anguo herbal medicine market | September 12, 2017 | 12.32 ± 3.79 [6.72, 17.1] | 19.71 ± 3.87 [10.5, 22.8] | ||
| 39 | Anguo herbal medicine market | September 12, 2017 | 12.19 ± 1.91 [9.23, 14.8] | 9.49 ± 0.88 [8.3, 10.5] | ||
| 40 | Anguo herbal medicine market | September 12, 2017 | 12.61 ± 2.4 [8.88, 16.1] | 16.41 ± 3.18 [12, 23] | ||
| 41 | Yulin herbal medicine market | September 14, 2017 | 6.01 ± 1.12 [4.31, 7.38] | 17.7 ± 2.27 [13.1, 20] |
3.3.2. Determination of content of active components of S. divaricate
Forty-one batches of sample solutions were prepared according to the methods described in Section 2.5.3, and the contents of glucosinol, 5-O-methylvisaminoside, and hypericin were determined using the chromatographic procedures described in Section 2.5.1. The results were shown in Table 3.
Table 3.
Contents of four components in 41 batches of S. divaricate.
| No. | Market specifications (main type) | Herbal medicine markets or producing areas | Collection time | Weight (g) | Contents of four components (%) |
|||
|---|---|---|---|---|---|---|---|---|
| Prim-O-glucosylcimifugin |
Cimifugin |
5-O-methylvisamminoside |
Sec-Oglucosylhamaudol |
|||||
| 1 | Wild S. divaricate | Sunrise Oriental Pharmaceutical Company in Ulanhot, Inner Mongolia | September 11, 2017 | 0.2507 | 0.9130 | 0.0864 | 0.2996 | 0.0540 |
| 2 | Tsining District in Wulanchabu, Inner Mongolia | September 5, 2017 | 0.2504 | 1.1720 | 0.0338 | 1.1995 | 0.0463 | |
| 3 | Zhalantun in Hulunbuir, Inner Mongolia | September 8, 2017 | 0.2503 | 1.5866 | 0.0359 | 1.1986 | 0.0664 | |
| 4 | Horch Village of Arun Banner in Hulunbuir, Inner Mongolia | September 11, 2017 | 0.2503 | 0.6201 | 0.0727 | 0.1919 | 0.0340 | |
| 5 | Hailar District in Hulunbuir, Inner Mongolia | September 9, 2017 | 0.2503 | 0.6054 | 0.0641 | 0.5701 | 0.0351 | |
| 6 | Anguo herbal medicine market | September 12, 2017 | 0.2504 | 1.4624 | 0.0432 | 0.5781 | 0.0400 | |
| 7 | Rongxing Hall Pharmaceutical Company in Chifeng, Inner Mongolia | September 17, 2017 | 0.2505 | 0.7797 | 0.0281 | 0.2068 | 0.0326 | |
| 8 | Prairie Chenbarhu Banner in Hulunbuir, Inner Mongolia | September 8, 2017 | 0.2501 | 0.8913 | 0.1323 | 0.3167 | 0.0572 | |
| 9 | Hailar District in Hulunbuir, Inner Mongolia | September 9, 2017 | 0.2507 | 0.5988 | 0.0282 | 0.1001 | 0.0571 | |
| 10 | Xin Barag Left Banner in Hulunbuir, Inner Mongolia | September 13, 2017 | 0.2507 | 0.6055 | 0.0725 | 0.1300 | 0.0339 | |
| 11 | Bozhou herbal medicine market | September 15, 2017 | 0.2504 | 0.5774 | 0.1118 | 0.2114 | 0.0543 | |
| 12 | Hehuachi herbal medicine market | September 20, 2017 | 0.2504 | 0.5770 | 0.1102 | 0.2084 | 0.0540 | |
| 13 | Xinghe County in Wulanchabu, Inner Mongolia | August 9, 2018 | 0.2504 | 0.7620 | 0.0865 | 0.3647 | 0.0522 | |
| 14 | Yulin herbal medicine market | September 14, 2017 | 0.2506 | 0.7183 | 0.0890 | 0.3587 | 0.0491 | |
| 15 | Siziwang Banner in Wulanchabu, Inner Mongolia | August 11, 2018 | 0.2504 | 0.6911 | 0.0345 | 0.3551 | 0.0660 | |
| 16 | Tumd Left Banner in Wulanchabu, Inner Mongolia | August 23, 2018 | 0.2503 | 0.6533 | 0.0422 | 0.3296 | 0.0485 | |
| 17 | Horinger County in Huhhot, Inner Mongolia | August 9, 2018 | 0.2504 | 0.7655 | 0.0699 | 0.3963 | 0.0656 | |
| 18 | Qinglongshan town in Tongliao, Inner Mongolia | September 16, 2018 | 0.2506 | 1.4089 | 0.1329 | 1.0280 | 0.0588 | |
| 19 | Xin Barag Right Banner in Hulunbuir, Inner Mongolia | July 19, 2018 | 0.2504 | 0.5917 | 0.0203 | 0.3028 | 0.0326 | |
| 20 | Yakeshi in Hulunbuir, Inner Mongolia | September 10, 2018 | 0.2503 | 0.6950 | 0.0748 | 0.6512 | 0.0838 | |
| 21 | Daur Autonomous Banner in Hulunbuir, Inner Mongolia | July 17, 2018 | 0.2504 | 0.5345 | 0.0617 | 0.3751 | 0.0459 | |
| 22 | Bairin Left Banner in Chifeng, Inner Mongolia | August 14, 2018 | 0.2506 | 0.9429 | 0.0583 | 0.3633 | 0.0547 | |
| 23 | West Ujimqin banner in Xilingol League, Inner Mongolia | June 21, 2018 | 0.2504 | 1.5412 | 0.0147 | 0.5191 | 0.0948 | |
| 24 | Cultivated S. divaricate | Sunrise Oriental Pharmaceutical Company in Ulanhot, Inner Mongolia | September 11, 2017 | 0.2506 | 0.4544 | 0.0231 | 0.6538 | 0.0328 |
| 25 | Hehuachi herbal medicine market | September 20, 2017 | 0.2500 | 1.1037 | 0.0577 | 0.9913 | 0.0573 | |
| 26 | Rongxing Hall Pharmaceutical Company in Chifeng, Inner Mongolia | September 11, 2017 | 0.2509 | 0.5232 | 0.0418 | 0.3508 | 0.0851 | |
| 27 | Yulin herbal medicine market | September 14, 2017 | 0.2507 | 0.4189 | 0.0431 | 0.4633 | 0.0222 | |
| 28 | Fengzhen in Wulanchabu, Inner Mongolia | April 6, 2017 | 0.2504 | 0.7197 | 0.1962 | 0.6777 | 0.0752 | |
| 29 | Guyuan County in Zhangjiakou, Inner Mongolia | September 11, 2017 | 0.2509 | 0.8350 | 0.0081 | 0.6386 | 0.0269 | |
| 30 | Bozhou herbal medicine market | September 15, 2017 | 0.2507 | 0.3655 | 0.0154 | 0.3549 | 0.0316 | |
| 31 | Bozhou herbal medicine market | September 15, 2017 | 0.2504 | 0.6932 | 0.0295 | 0.5329 | 0.0592 | |
| 32 | Bozhou herbal medicine market | September 15, 2017 | 0.2509 | 0.7285 | 0.0058 | 0.5160 | 0.0619 | |
| 33 | Bozhou herbal medicine market | September 15, 2017 | 0.2501 | 0.5730 | 0.0186 | 0.1827 | 0.0012 | |
| 34 | Bozhou herbal medicine market | September 15, 2017 | 0.2509 | 0.2581 | 0.0031 | 0.3155 | 0.0147 | |
| 35 | Anguo herbal medicine market | September 12, 2017 | 0.2509 | 0.0147 | 0.0542 | 0.0056 | 0.0391 | |
| 36 | Anguo herbal medicine market | September 12, 2017 | 0.2503 | 0.0180 | 0.0489 | 0.0036 | 0.0356 | |
| 37 | Anguo herbal medicine market | September 12, 2017 | 0.2508 | 0.0190 | 0.0558 | 0.0042 | 0.0789 | |
| 38 | Anguo herbal medicine market | September 12, 2017 | 0.2504 | 1.2216 | 0.0320 | 0.6528 | 0.0461 | |
| 39 | Anguo herbal medicine market | September 12, 2017 | 0.2503 | 0.3375 | 0.2349 | 0.7949 | 0.0812 | |
| 40 | Anguo herbal medicine market | September 12, 2017 | 0.2503 | 0.5776 | 0.1118 | 0.2115 | 0.0543 | |
| 41 | Yulin herbal medicine market | September 14, 2017 | 0.2506 | 0.4544 | 0.0231 | 0.6538 | 0.0328 | |
3.4. Specification and grade of SR samples
3.4.1. Analysis of correlation between morphological characteristics and internal components
The results showed no significant correlation between the external indices of SR (P < 0.01). However, there were significant positive correlations between cimicifugin glycoside and 5-O-methylvisamminoside (P < 0.01), between cimicifugin and helonoside (P < 0.05), and between cimicifugin glycoside and the length (P < 0.05). The results of the correlation analysis shown in Fig. 5 indicated that cimicifugin glycoside, 5-O-methylvisamminoside, and the length of SR can be used as a basis for classifying the commodity specifications of SR.
Fig. 5.
Correlation between appearance index and internal index.
3.4.2. Descriptive statistical analysis
The results of the descriptive statistical analysis of the morphology and component indices shown in Table 4 indicated that the coefficient of variation of 5-O-methylvisamminoside in wild and cultivated SR was significant with 68.48% and 67.12%, respectively; The variation coefficient of diameter and hematoxylin was not significant; the coefficient of variation of the length index was the lowest and was not significant. Moreover, the content of 5-O-methylvisamminoside in SR varies considerably, and this finding can be used as a basis for the classification of the product specifications and grades of SR.
Table 4.
Descriptive statistical analysis of wild SR and cultivated SR.
| wild SR |
cultivated SR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Minimum (cm) | Maximum (cm) | Mean value (cm) | Standard deviation | Coefficient of variation | Minimum (cm) | Maximum (cm) | Mean value (cm) | Standard deviation | Coefficient of variation | |
| Prim-O-glucosylcimifugin | 0.45 | 1.59 | 0.84 | 0.34 | 40.47 | 0.00 | 0.31 | 0.13 | 0.09 | 66.93 |
| Cimifugin | 0.01 | 0.13 | 0.07 | 0.03 | 51.64 | 0.00 | 0.23 | 0.06 | 0.07 | 113.23 |
| 5-O-methylvisamminoside | 0.03 | 0.30 | 0.11 | 0.08 | 68.48 | 0.00 | 0.25 | 0.11 | 0.07 | 67.12 |
| Sec-O-glucosylhamaudol | 0.03 | 0.09 | 0.05 | 0.02 | 29.33 | 0.00 | 0.09 | 0.05 | 0.02 | 52.26 |
| Diameter | 4.87 | 12.61 | 7.64 | 2.02 | 26.38 | 3.23 | 15.00 | 6.43 | 3.39 | 52.82 |
| Length | 10.44 | 18.48 | 14.24 | 2.25 | 15.77 | 9.49 | 26.51 | 16.58 | 4.96 | 29.90 |
3.4.3. Principal component analysis
The results of the principal component analysis were shown in Table 5. The first principal component characteristic root was 2.0154 and the contribution rate was 33.5898%, the second principal component characteristic root was 1.3031 and the contribution rate was 21.7185%, the third principal component characteristic root was 1.1002 and the contribution rate was 18.3370%, and the cumulative contribution rate was 73.6453%. The first principal component characteristic root was 1.5602 and the contribution rate was 44.9946%, the second principal component characteristic root was 1.5602 and the contribution rate was 26.0033%, the third principal component characteristic root was 1.1225 and the contribution rate was 18.7078%, and the cumulative contribution rate was 89.7057 %. The first principal component and the second principal component can well reflect the quality of SR. Additionally, the size of the eigenvector represents the contribution of each index to the principal component. According to the results of the analysis conducted herein, the index of SR classification with a larger eigenvector in the first principal component was cimicillin glycoside, and the index with a larger eigenvector in the second principal component was 5-O-methylvisamminoside.
Table 5.
Principal component analysis of wild SR and cultivated SR.
| Items | Principal component of wild SR |
Principal component of cultivated SR |
||||
|---|---|---|---|---|---|---|
| First principal component | Second principal component | Third principal component | First principal component | Second principal component | Third principal component | |
| Prim-O-glucosylcimifugin | 0.6183 | 0.2457 | –0.5593 | 0.7509 | –0.5931 | –0.0391 |
| Cimifugin | –0.3678 | 0.5227 | 0.4498 | 0.3582 | 0.5836 | 0.6783 |
| 5-O-Methylvisamminoside | 0.7609 | 0.4035 | 0.0280 | 0.7401 | –0.4196 | 0.4204 |
| Sec-O-glucosylhamaudol | 0.4775 | –0.0061 | 0.7444 | 0.6636 | 0.6729 | 0.0306 |
| Diameter | –0.8215 | 0.1860 | –0.1503 | 0.4957 | 0.4657 | –0.6466 |
| Length | –0.1263 | 0.8787 | –0.0870 | –0.8795 | 0.1484 | 0.2552 |
| Characteristic root | 2.0154 | 1.3031 | 1.1002 | 2.6997 | 1.5602 | 1.1225 |
| Contribution rate | 33.5898 | 21.7185 | 18.3370 | 44.9946 | 26.0033 | 18.7078 |
| Cumulative contribution rate | 33.5898 | 55.3083 | 73.6453 | 44.9946 | 70.9979 | 89.7057 |
3.4.4. Specification and grade of SR samples
Based on the conduction of a review of ancient herbal books, modern monographs, national, trade, and local standards, and international pharmacopeia (European Pharmacopoeia European Pharmacopoeia Commission, 2013, Japanese Pharmacopoeia Editorial Committee, 2011, The Korea Food and Drug Administration, 2012), a complete and standard specification and grade of SR was established through the combination of traditional and modern analytical methods. More specifically, in the present study, cimicillin glycoside, 5-O-methylvisamminoside, and length were used as indices for clustering. Wild and cultivated SR were preliminarily clustered into three groups. The k-cluster center values of wild SR and those of cultivated SR were shown in Table 6. Considering the final clustering center value of the k-cluster as the reference value, combined with the actual operation and the market classification of the specifications and grade of SR commodity, along with the consideration of expert suggestions, the standard of product specifications and grades for SR was established as shown in Table 7. Furthermore, the two specifications were divided into three grades each (Fig. 6A and B) and included descriptions based on their diameter and length. This current standard can be used to define the specifications and grades of SR and can be adopted for its production, marketing, sales, and utilization.
Table 6.
Final cluster centers of K-means cluster analysis of wild SR and cultivated SR.
| Items | Cluster center of wild SR |
Cluster center of cultivated SR |
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Prim-O-glucosylcimifugin | 0.81 | 0.82 | 1.00 | 0.02 | 0.11 | 0.21 |
| 5-O-Methylvisamminoside | 0.09 | 0.12 | 0.18 | 0.03 | 0.10 | 0.16 |
| Length (cm) | 14.54 | 11.47 | 18.26 | 23.53 | 18.13 | 11.03 |
Table 7.
Specification and grade of SR.
| Items | Grades |
|||
|---|---|---|---|---|
| First grade | Second grade | Third grade | ||
| Wild SR | Shape | Long cylindrical or conical | ||
| Surface character | The surface is rough, light brown or brown. The upper part of root reveals dense crosswise wrinkles like ring nodes, and sometimes reveals brown and hair-like remains of leaf sheath. The root reveals many longitudinal wrinkles and scars of rootlets. | |||
| Texture | Soft, easily broken | |||
| Cross section | Cross section is uneven, cortex is grayish brown and reveals many lacunae and xylem is yellow. | |||
| Taste | Characteristic odor and slightly sweet. | |||
| Content of Prim-O-glucosylcimifugin | ≥1.00 | ≥0.82 | ≥0.81 | |
| Content of 5-O-methylvisamminoside | ≥0.18 | ≥0.12 | ≥0.09 | |
| Length (cm) | ≥18.26 | ≥11.47 | ≥14.54 | |
| Cultivated SR | Shape | Long cylindrical or conical, occasionally branched | ||
| Surface character | The surface is smooth, light brown or faint yellow. The upper part of root reveals few or no crosswise wrinkles. The root reveals many longitudinal wrinkles and scars of rootlets. | |||
| Texture | Hard, easily broken | |||
| Cross section | Cross section is even, cortex is yellowish-brown and reveals few lacunae and xylem is pale yellow. | |||
| Taste | Faint odor, slightly sweet. | |||
| Content of Prim-O-glucosylcimifugin | ≥0.21 | ≥0.11 | ≥0.02 | |
| Content of 5-O-methylvisamminoside | ≥0.16 | ≥0.10 | ≥0.03 | |
| Length (cm) | ≥11.03 | ≥18.13 | ≥23.53 | |
Note: The characters of wild-cultivated SR are close to the wild, and can be divided according to the standard of the wild SR.
Fig. 6.
Images of SR and its specification and grade (A: different grades of cultivated SR; B: different grades of wild SR).
4. Discussion
In recent years, with the increasing scarcity of wild sources of SR, the market demand in and outside China continues to witness an increase (Chen et al., 2014). Owing to the differences in planting region, climate, and cultivation techniques, the quality of commercial medicinal materials circulated in the market is markedly different, and a substantial number of local substitutes and counterfeits of SR derived from different sources are available in the market. Therefore, it is imperative to establish the standard of SR and to standardize the planting, collection, and processing of SR.
First, this study reviewed and categorized literatures on SR dating back to the Han Dynasty, and on this basis, the members of the investigation team visited the main Chinese herbal medicine market and main producing areas in China and investigated the distribution of SR in the market. The results of field and market investigation showed that with the increase in the market demand of SR, the planting area of each main producing area also increased, and this led to the observation of an upward trend for the market price of SR. Combined with modern analysis methods, it can be concluded that cimicifugin glycoside, 5-O-methylvisamminoside, and the length of SR can be used as a basis for classifying the commodity specifications of SR. Wild and cultivated SR were preliminarily clustered into three groups. Additionally, the commodity specifications and grade standards of SR were established based on the following eight aspects: shape, surface characteristics, texture, cross section, taste, prim-O-glucosylcimifugin content, 5-O-methylvisamminoside content, and length. This provides a scientific basis for the rational use of SR in the market.
Presently, SR is widely used in the market; however, if consumers decide to use only wild products, the supply of wild SR will decrease compared to the demand, and the wild resources of traditional Chinese medicine may be subjected to considerable damage. Therefore, it is difficult for consumers to perform a selection between wild and cultivated SR. Notably, because the characteristics of wild-cultivated SR are similar to wild one, consumers can consider using the wild-cultivated. Additionally, according to the regulations stipulated by the Chinese Pharmacopoeia (2020 edition), the total prim-O-glucosylcimifugin and 5-O-methylvisamminoside contents in dried SR should not be <0.24%. In this study, the contents of 18 batches of SR cultivated products were determined, of which only three batches of medicinal materials did not meet the requirements prescribed by the Chinese Pharmacopoeia. It can be inferred that the cultivation of SR holds considerable potential. Therefore, it is suggested that consumers should buy cultivated varieties of SR that meet this standard as the effect is similar to that exhibited by the wild one, and such a selection and consumption would also be conducive to the protection of traditional Chinese medicine resources.
5. Conclusion
Although SR has been used in China, Japan, South Korea, and other countries and regions, the quality requirements and standards of SR are not the same in each country. Therefore, the establishment of internationally recognized quality standards of SR medicinal materials is of significance for protecting and promoting the healthy development of SR planting, sales, trade, and other related industries in and outside China. This study provides insights into the establishment of a new standard for the specification and grade of SR, promotes the standardization of the traditional Chinese medicine industry, and lays a foundation for the subsequent development.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by 2017 Inner Mongolia Autonomous Region Health Commission Planning Research Project for “Study on specification and grade of Saposhnikoviae Radix” (No. 201703058), “the Fourth Survey on Chinese Materia Medica Resource” [No. Finance Society [2019] 39], China Agriculture Research System (No. CARS-21), Transformation Program of Scientifific and Technological Achievements of Inner Mongolia (No. CGZH2018174).
Contributor Information
Chunhong Zhang, Email: zchlhh@126.com.
Minhui Li, Email: prof_liminhui@yeah.net.
References
- Chen S., Pang X.H., Song J.Y., Shi L.C., Yao H., Han J.P., Leon C. A renaissance in herbal medicine identification: From morphology to DNA. Biotechnology Advances. 2014;32(7):1237–1244. doi: 10.1016/j.biotechadv.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Ding L.W. Analysis on the trend of production and marketing of Guan Fangfeng. Modern Chinese Medicine. 2012;14(3):53–56. [Google Scholar]
- European Pharmacopoeia Commission (2013). European Pharmacopoeia. 8th ed. Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM): Strasbourg.
- Japanese Pharmacopoeia Editorial Committee (2011). Japanese Pharmacopoeia. 16th ed. The Ministry of Health, Labour and Welfare: Tokyo.
- Liu Z.Y., Zhao Y.R. Herbal textual research on the Saposhnikovia divaricata. Medical Science. 2017;2017(04):226. [Google Scholar]
- Lu G.P. China Press of Traditional Chinese Medicine; Beijing: 1999. The Empirical Identification of 500 Chinese Medicinal Materials in Common use. [Google Scholar]
- Magsar U. Description of species Saposhnikovia divaricata Schischkin (Umbelliferae Juss.) in Mongolia. Proceedings of Institute of Botany. 2006;16:51–55. [Google Scholar]
- National Pharmacopoeia Committee . China Medical Science Press; Beijing: 2015. Chinese Pharmacopoeia of 2015th. [Google Scholar]
- Peng C. China Press of Traditional Chinese Medicine; Beijing: 2013. Chinese authentic medicinal materials. [Google Scholar]
- The Korea Food and Drug Administration (2012). The Korean Pharmacopoeia. 10th ed. The Korea Food and Drug Administration Press: Seoul.
- Wang J.H., Lou Z.C. Herbalogical studies of the Chinese drug Fangfeng. China Journal of Chinese Materia Medica. 1989;14(10):3–5. [PubMed] [Google Scholar]