Abstract
Alkannin/shikonin (A/S) and their derivatives are naturally occurring naphthoquinones majorly found in Boraginaceae family plants. They are integral constituents of traditional Chinese medicine Zicao (roots of Lithospermum erythrorhizon). In last two decades significant increase in pharmacological investigations on alkannin/shikonin and their derivatives has been reported that resulted in discovery of their novel mechanisms in various diseases and disorders. This review throws light on recently conducted pharmacological investigations on alkannin/shikonin and their derivatives and their outputs. Various analytical aspects are also discussed and brief summary of patent applications on inventions containing alkannin/shikonin and its derivatives is also provided.
Keywords: alkannin, naphthoquinones, patents, shikonin
1. Introduction
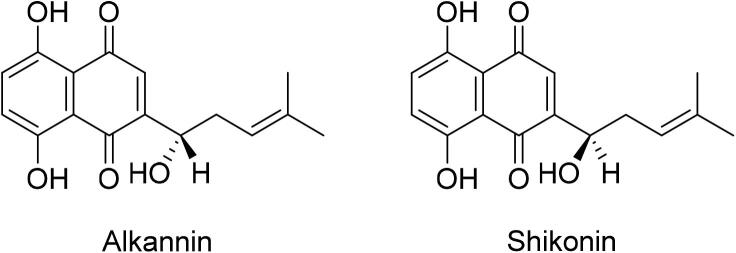
Alkannin and shikonin (A/S) are enantiomeric pair and naphthoquinone pigments (Boulos, Rahama, Hegazy, & Efferth, 2019) which are well known for their therapeutic, cosmetic and coloring applications (Fig. 1). Plants containing these bioactive pigments are traditionally used for curing various ailments since centuries. Alkannin was initially reported as a principle component of the root bark of with records of traditional utilization for 4th century BCE for various ailments, principally for ulcers (Papageorgiou et al., 1999, Weigle, 1974). On the other hand, Alkanna tinctoria Tausch. Plant of European Origin belonging to Boraginaceae family shikonin was isolated from the root bark of Chinese medicinal plant Lithospermum erythrorhizon Sieb. et Zucc (Boraginaceae) which is well known in China by various traditional names i.e. tzuts’ao, tzu-ken, hung-tzu ken, etc (Huu Tung, Du, Wang, Yuan, & Shoyama, 2013). It is an integral component of traditional Chinese medicine Zicao (roots of L. erythrorhizon) which has successful history in treatment of various inflammatory and infectious diseases (Andújar, Ríos, Giner, & Recio, 2013, Papageorgiou et al., 1999, Winter, 1984a, Winter, 1984b, Khan and Abourashed, 2010).
Fig. 1.
Chemical structures of alkannin and shikonin.
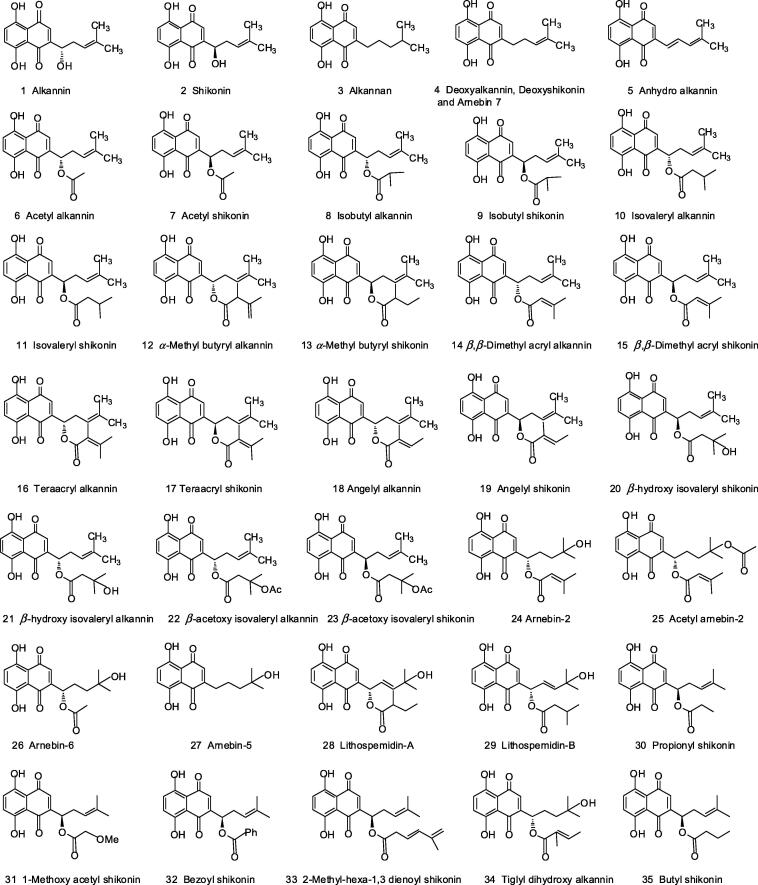
Apart from these plants, a wide range of plants belonging to Boraginaceae family are known to possess these enantiomers and their derivatives. In fact acetylshikonin was first isolated from L. erythrorhizon by Kuroda and Wada in 1922, later shikonin and its other derivatives were also identified (Kuroda & Wada, 1936). It took almost 14 years to identify accurate structure of shikonin (reported as 5,8-dihydroxy-2-[(1R)-1-hydroxy-4-methyl-3-pentenyl]-1,4 naphthoquinone in 1936 by Brockmann); Subsequently, it’s another enantiomer alkannin was identified by the same group (Albreht et al., 2009, Brockmann, 1936). Approximately, 35 derivatives (Fig. 2) of alkannin and shikonin have been isolated from various plants of Boraginaceae family and extensively investigated for wide range of biological activities including wound healing, antimicrobial (Aburjai, Al-Janabi, Al-Mamoori, & Azzam, 2019), anti-acne (Fang & Shoukang, 1998), antiulcer (Singh & Sharma, 2012), anti-inflammatory (Lee et al., 2016), anticancer (Sun, Zhang, Liu, & Guan, 2019) activities, etc.
Fig. 2.
Structural representation of alkannin, shikonin and their derivatives.
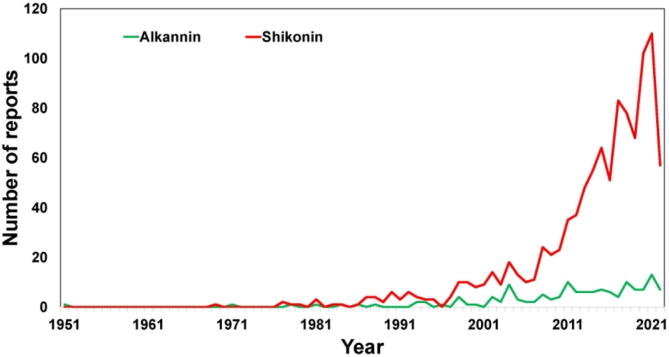
In the time frame of 1969 to 2021, a total of 634 full text reports are available in PubMed database and out of these, and 606 reports are published after 2000, showing the increased interest of research groups in A/S and their derivatives. Trend analysis suggests that researchers are more focused on shikonin than alkannin (Fig. 3). An exhaustive review of A/S and their derivatives was first published by Papageorgiou group in 1999 (Papageorgiou, Assimopoulou, Couladouros, Hepworth, & Nicolaou, 1999). A decade later, another update was published with prime focus on wound healing and associated bioactivities (Papageorgiou, Assimopoulou, & Ballis, 2008). In 2013, Andujar group published a compilation containing pharmacological investigations on A/S and their derivatives for the period of 2002 to 2013 (Andújar, Ríos, Giner, & Recio, 2013). Subsequently, various review reports were published by different research groups with a focus on either individual bioactivity or on individual derivative. A/S and their derivatives possess enantiomeric properties that make their analysis quite complex. Surface-enhanced Raman Spectroscopy (SERS) and chiral HPLC have been successfully utilized for differentiating A/S and their derivatives (Cañamares, et al., 2022; Azuma et al., 2016). Literature analysis suggests that after 2008 (Papageorgiou, Assimopoulou, & Ballis, 2008), any review update regarding analytical aspects of A/S and their derivatives is not available. Thus, there is a dire need of an updated compilation containing all pharmacological, analytical and miscellaneous investigations on A/S and their derivatives. Forecasting the marketable potential of A/S and their derivatives, wide range of patents have been filed by various research groups around the globe for various applications to safeguard their usage. This review is primarily focused on providing update on various investigations on A/S and their derivatives from year 2008 to 2021 along with thorough insight on the patent applications filed.
Fig. 3.
Trend analysis of reports indexed in “PubMed” database with keywords “Alkannin” and “Shikonin” from 1951 to 2021.
2. Pharmacological activities
2.1. Wound healing activity
Dried roots of Arnebia guttata Bung, Arnebia euchroma (Royle) Johnston, and Lithospermum erythrorhizon Sieb. Et Zucc loaded oil based ointment (Zicao) has been widely used for treatment of wounds (Chak, Hsiao, & Chen, 2013, Hsiao, Tsai, & Chak, 2012, Lu et al., 2008, Zeng & Zhu, 2014). The major active components of Zicao include shikonin and its derivatives such as deoxyshikonin, acetylshikonin and β,β’-dimethylacrylshikonin. Furthermore, to overcome the demerits of this oil based ointments such as discomfort, irritation and difficulty in cleaning, soluble water based topical preparation such as Zicao-HP-β-CD complex was formulated using 2-hydroxypropyl-β-cyclodextrin to form water-soluble complex which resulted in its enhanced bioavailability and stability. The active ingredients of Zicao enhance collagen synthesis in granuloma tissues and promote inactivation of tumor necrosis factor-α gene expression (Chen, Yu, Hsu, Tsai, & Tsai, 2018). On the other hand, Jawoongo, a Korean traditional medicine has been found highly effective in removing necrotic tissue caused by burn wounds. Jawoongo consists of Lithospermi Radix, Angelicae Gigantis Radix, Ronicerae Flos, Glycyrrhizae Radix, Coptidis Rhizome and Scutellariae Radix. The major active ingredient is Lithospermi Radix which mainly comprises of deoxyshikonin. It significantly increases the phosphorylation of p38 and ERK1/2 in a concentration dependent manner. Additionally, it activates Mitogen-activated protein kinase (MAPK) signaling which promotes cellular migration and angiogenesis. It was observed that deoxyshikonin induced migration and proliferation in HaCaT cells mediated through activation of p38 and ERK respectively. Thus, the study demonstrated that deoxyshikonin possesses strong ability for proliferation, migration and tube formation of HaCaT and HUVEC cells, which in turn promotes angiogenesis (Kim, Lee, & Yook, 2013, Park et al., 2017).
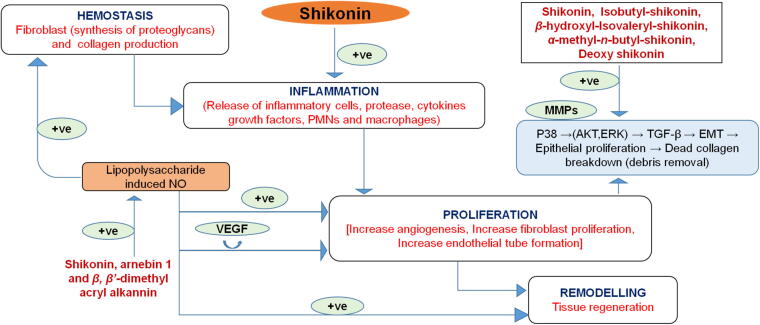
Recently, an increased attention is focused on the herbal medicines attributing to their quality, safety and efficacy. Since ancient times, people have used plant based preparations to promote wound healing process (Fronza, Heinzmann, Hamburger, Laufer, & Merfort, 2009). Various plants especially belonging to Boraginaceae family have been reported to possess excellent therapeutic potential in wound management. The main active metabolites of this family are naphthoquinones which possess anti-inflammatory, anti-microbial, anti-oxidant activities contributing to wound healing (Lee et al., 2016). Meanwhile, additional studies demonstrated that therapeutic benefits of roots of Boraginaceae family plants are wider than its aerial parts. The most active components found in roots are shikonin, alkannin, deoxyshikonin and acetylshikonin. Traditionally, the root extract of Onosma dichroantha Boiss. has been used in Iran for healing burn wounds. Furthermore, the cyclohexane fraction has been found to be most potent inhibitor of lipopolysaccharide induced nitrogen oxide production which accelerates fibroblast proliferation, tissue regeneration and angiogenesis. Active components present in the cyclohexane fraction were found to be shikonin, arnebin-1 and β,β’-dimethyl acrylalkannin. Among all of these components, arnebin-1 has pro-angiogenic and synergistic effects with vascular endothelial growth factor (VEGF) which further augments the wound healing process (Safavi et al., 2019). Similarly, several other phytoconstituents isolated from n-hexane-dichloromethane extract of Onosma argentatum Hub.-Mor. roots i.e. deoxyshikonin, acetylshikonin, 3-hydroxyisovalerylshikonin and 5–8-O-dimethylacetylshikonin were found to be effective in treatment of burns wounds. In another study, the efficacy of mixture of olive oil, beeswax and root extract of Alkanna tinctoria Tausch. was examined on burn wounds which showed rapid epithelization and angiogenesis (Gümüş & Özlü, 2017). Moreover, this extract has been established to increase fibroblasts production which amplifies tissue regeneration and provides better perfusion to wound area resulting in granulation tissue formation (Yazdinezhad, Monsef-Esfahani, & Ghahremani, 2013) (Fig. 4). The healing effects of ointment loaded with Arnebia euchroma extract were also compared with standard silver sulfadiazine on second degree burns and the extract demonstrated higher efficacy. Fibroblast proliferation, cell migration and collagen synthesis were observed to be the major mechanisms in its healing process (Nasiri et al., 2016).
Fig. 4.
Impact of shikonin and derivatives on different phases of wound healing.
Furthermore, the active constituents of L. erythrorhizon such as shikonin, isobutyl-shikonin, β-hydroxyl-isovaleryl-shikonin and α-methyl-n-butyl-shikonin were loaded in chitosan/gelatin-based scaffolds and examined for their wound healing potential. The results demonstrated the mechanism of healing via regulation of epithelial-mesenchymal transition (EMT) through TGF-β expression (Table 1) (Hsiao, Tsai, & Chak, 2012, Wang, Kravchuk, & Kimble, 2010, Yao, Chen, Chen, Li, & Huang, 2019).
Table 1.
Mechanisms involved and pharmacological outcomes from various investigation of alkannin/shikonin containing plant extracts, alkannin/shikonin and its derivatives on different wound models.
| Test compounds/extract | Cell cultures/In vitro/In vivo assays | Mechanism involved/ Pharmacological outcomes |
Wound types | References |
|---|---|---|---|---|
| Shikonin, isobutylshikonin, β-hydroxylisovalerylshikonin, α-methyl-n-butyl-shikonin | Cytotoxicity assay using L929 mouse fibroblasts; In vivo wound healing assay |
Proliferation of fibroblasts; Synergistic effect of gelatin and chitosan promote granulation tissue formation |
Skin wounds | Yao, Chen, Chen, Li, & Huang, 2019 |
| Shikonin | Hypertropic scar derived fibroblasts (HSF) and normal fibroblasts (nHSF) cell lines | Activation of Erk1/2 and p38α/β pathway; Induction of hypertropic scar derived fibroblasts apoptosis |
Hpertropic or keloid scars | Xie et al., 2015 |
| Shikonin, β,β’-dimethylacryl shikonin β,β’-dimethylacryl alkannin |
Murine macrophages (RAW264.7), normal human skin fibroblasts (Hs27), human microvascular endothelial cells (HMEC-1), zebrafish line TG (fli1: EGFP) | Inhibition of LPS-induced NO production thereby promoting tissue regeneration and angiogenesis | Burn wounds | Safavi et al., 2019 |
| Alkannin, shikonin, juglone, α-napthoquinone, lapachol, deoxyshikonin, β,β’-dimethylacrylshikonin, acetylshikonin. | Human dermal scar-derived fibroblasts (HSF) and Human ‘normal’ dermal fibroblasts (nsHSF) | Inhibition of TGF-β1 induced collagen deposition and cell mediated contraction; Phosphorylation of P-Erk and NF-κβ |
Dermal scars | Fan et al., 2019 |
| Alkanna strigose extract | Excision and incision wound models | Increase in wound contraction rate and promoting granulation tissue formation. | Suppurative wounds | Aburjai, Al-Janabi, Al-Mamoori, & Azzam, 2019 |
| 2-methyl-n-butrylshikonin, acetylshikonin, isovalerylshikonin, deoxyshikonin | Anti-oxidant activity using DPPH assay and wound healing activity using Linear incision wound model | Accelerative effect on proliferation and migration thereby promoting re-epitheliazation | Incision wounds | Eruygur, Yılmaz, Kutsal, Yücel, & Üstün, 2016 |
| 2-bromo-1,4-naphthoquinone, 2-N-isonicotinoyl-hydrazide-1,4-naphthoquinone, 1-N-Isonicotinoyl-hydrazone-[2hydroxy-3-(3-methyl-2-butenyl)]-1,4-naphthoquinone |
Mouse fibroblast cell lines 3T3, MTT assay, Scratch assay, Excision wound model | Inhibition of lysophosphatidic acid signaling pathway and MAPK signaling pathway | Diabetic wounds | Cardoso et al., 2018 |
| Shikonin, acetylshikonin, β,β’-dimethylacrylshikonin | Excision wound model | Increase in collagen fibre levels in granuloma tissue via expression of TNF-α | Excision wounds | Chen, Yu, Hsu, Tsai, & Tsai, 2018 |
| Deoxyshikonin | Human umbilical vein vascular endothelial cells (HUVECs), immortalized human kerationocytes (HaCaT) | Stimulation of phosphorylation of p38 and extracellular signal regulated kinase. | Full-thickness dermal wounds | Park et al., 2017 |
| Arnebia euchroma roots | Randomized, single blind clinical trials | Promote angiogenesis via increased expression of matrix mucopolysaccharide deposition, collagen synthesis and fibroblasts proliferation. | Second degree burns wounds | Nasiri et al., 2016 |
| Echium arenarium extract | Murine 218 macrophagic cells (Raw264.7). Bacillus cereus, Listeria monocytogenes, Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus (MRSA), Enterrococcus faecalis, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Leishmania major (GLC94) and Leishmania infantum (LV05) |
Anti-oxidant, anti-bacterial activity and anti-leishmanial activity | Cutaneous leishamaniatic wounds | Kefi, et al., 2018 |
| Alkanna tinctoria extract | Experimental study on patients with second degree burns. | Increased fibroblastic activity and accelerated granulation. | Full-thickness burn wounds | Gümüş & Özlü, 2017 |
2.2. Antimicrobial activity
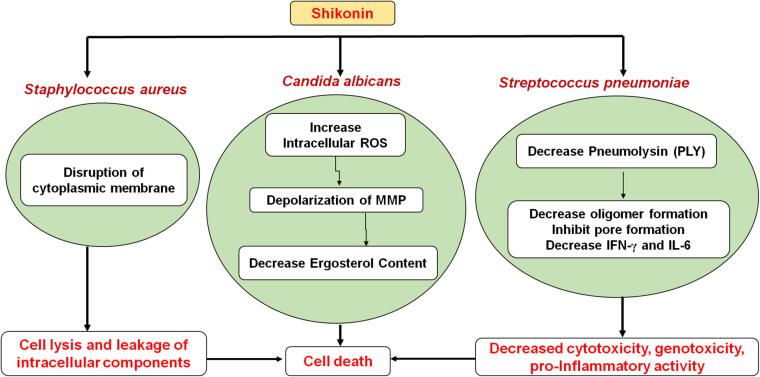
Traditional Chinese herb L. erythrorhizon has been widely used in treatment of a wide range of infections (Yan, Tan, Miao, Wang, & Cao, 2019). Candida albicans is the major opportunistic pathogen and major cause of fungal infections in humans. Shikonin showed significant inhibitory effect on the growth of C. albicans through multiple mechanisms. It markedly increases the intracellular ROS (reactive oxygen species) and causes depolarization of mitochondrial membrane potential. It was observed to reduce the ergosterol content also. Further, it could lead to the upregulation of thioredoxin reductase-related gene (TRR1), NADPH oxidoreductase-related gene (EBP1) and mitochondrial respiratory electron transport chain-related gene (MRF1) (Fig. 5) (Miao et al., 2012). Moreover, shifting mitochondrial aerobic respiration and promoting endogenous reactive oxygen species augmentation contributes to DNA damage (Liao et al., 2016). On the other hand, Staphylococcus aureus is one of the most common and predominant causes of persistent infections in chronic wounds; It contributes the nosocomial infections and hence, proved to be biggest pathogenic burden. Being an adaptable pathogen, it has ability to gain resistance against broad spectrum of antibiotics. Resistance development occurs as a result of horizontal gene transfer (HGT) via transduction, conjugation, or transformation (Craft, Nguyen, Berg, & Townsend, 2019). However, resistance to methicillin and other β-lactam antibiotics is acquired due to mecA gene transfer situated on a mobile genomic element, the Staphylococcal chromosome cassette mec (SCCmec) (Deurenberg & Stobberingh, 2009). In order to combat multidrug resistance, shikonin is of utmost importance. The TEM images of shikonin treated Methicillin-resistant Staphylococcus aureus (MRSA) shows disruption of cytoplasmic membrane and cell lysis with subsequent leakage of intracellular components. In addition, shikonin directly binds to peptidoglycan (PGN) which is main component of Gram-positive bacterial cell wall (Lee et al., 2015).
Fig. 5.
Mechanistic action of shikonin and its derivatives on various microbial strains viz. Staphylococcus aureus, Candida albicans and Streptococcus pneumoniae where ROS is reactive oxygen species and MMP is mitochondrial membrane potential.
Streptococcus pneumoniae is another pathogen causing severe infections in humans. Pneumolysin (PLY) is vital virulence trait of S. pneumoniae which possesses cytotoxicity, genotoxicity and pro-inflammatory activity. The treatments for this microbe were limited due to its ubiquitous antibiotic resistance. Shikonin has been found as therapeutically effective for S. pneumoniae based pneumonia as it antagonizes the hemolytic activity of PLY thereby reducing the cytotoxicity of PLY. It also inhibits oligomers formation and block pore formation on the cell membrane which leads to decreased production of IFN-γ and IL-6 (Zhao et al., 2017). Moreover, shikonin was also found effective for periodontal diseases as it has ability to inhibit Porphyromonas gingivalis, Fusobacterium nucleatum, Streptococcus mutans and Lactobacillus acidophilus which are most susceptible bacterial strains involved in dental caries (Table 2) (Li, Xu, Zhu, & Wang, 2012). In the latest studies, shikonin and its derivatives including shikonin glucoside, 4-chlorophenylacetyl shikonin, lithospermidin B and Angelyl shikonin were assessed for protein binding with Main protease (Mpro) of SARS CoV-2 revealed shikonin and some derivatives as potential antiviral agent of Covid (Woo & Das, 2022).
Table 2.
Mechanisms involved and pharmacological outcomes from various antimicrobial investigations on alkannin/shikonin and its derivatives.
| Shikonin and its derivatives | Cells/Targeted strains | Mechanism involved | References |
|---|---|---|---|
| Shikonin | Human lung epithelial cells (A549) Murine model of endonasal pulmonary infection; Streptococcus pneumoniae strain D39 serotype 2 (NCTC 7466) |
Antagonistic effect on haemolytic activity of pneumolysin (PLY); Reduce the cytotoxicity of PLY by inhibiting oligomers formation and blocking pore formation on the cell membrane; Decreased production of IFN-γ and IL-6. |
Zhao et al., 2017 |
| Aspergillus terreus (NCCPF860035) | Upregulation of Mpkc, spm1, protein kinase (Pkc-c), protein kinase (dsk1) serine/threonine-protein kinase and small GTPase ras-1 proteins; Moderate increase in cAMP. |
Shishodia & Shankar, 2020 | |
| SC5314, SN250 strains | Shifting mitochondrial aerobic respiration and promoting endogenous reactive oxygen species augmentation thereby causing DNA damage. | Liao et al., 2016 | |
| Agrobacterium rhizogenes 15834 | − | Boehm, Sommer, Li, & Heide, 2000 | |
| S. aureus ATCC 33591 (MRSA) and S. aureus ATCC 25923 (MSSA) | − | Lee et al., 2015 | |
| Iranian Arnebia euchroma extract |
Trichophyton mentagrophytes (PTCC5054), Microsporum canis (PTCC5069), Trichophyton rubrum (PTCC5143), Candida albicans (PTCC5027), Aspergillus fumigatus (PTCC5009) and Penicillium chrysogenum (PTCC5076) | − | Sabokbar, Tabaraie, Karimi, & Talebi, 2017 |
| Lithospermum erythrorhizon seeds | Bacillus subtilis 613R, Clavibacter michigenensis subsp. nebraskensis CN74-1, Agrobacterium radiobacter K84, Agrobacterium tumefaciens C58, Escherichia coli ESS, Erwinia carotovora ATCC 15713, Pseudomonas aureofaciens, Pseudomonas fluorescens, Pseudomonas syringae B, Ralstonia solanacearum, and Serratia marsecens | − | Brigham, Michaels, & Flores, 1999 |
| Acetylshikoninshikonin, β-sitosterol, β,β-dimethylacryl shikonin and deoxyshikonin from L. erythrorhizon |
Porphyromonas gingivalis (ATCC 33277), Streptococcus mutans (UA 159), Fusobacterium nucleatum (ATCC 25586) and Lactobacillus acidophilus (ATCC 4356) | − | Li, Xu, Zhu, & Wang, 2012 |
| β,β-dimethyl acrylshikonin, isovaleryl shikonin, β-hydroxyiso valerylshikonin and shikonin isovalerate from Arnebia hispidissima (Lehm.) DC. | Escherichia coli (ATCC-5922), Klebsiella pneumonie (ATCC-59008) Eterobacter cloacae (ATCC25924), Bacillus subtilis (ATCC-10031), Staphylococcus aureus (ATCC-25923), Streptococcus pneumoniae (ATCC-10032), Aspergillus niger, Rhizoctonia phaseoli, Aspergillus flavus, Penicillium chrysogenum and Candida albicans | − | Singh & Sharma, 2012 |
| Shikonin | Candida albicans (SC5314) | Increased intracellular ROS and depolarization of mitochondrial membrane potential | Miao et al., 2012 |
| Deoxyalkannin, alkannin, acetylalkannin, Isobutyryl alkannin, β-hydroxyiso valerylalkannin, 2′'-(S)-α-methylbutyryl alkannin, Propionyl alkannin, methyl jasmonate and Teracrylalkannin. | Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), Staphylococcus epidermidis (ATCC 12228), Klebsiella pneumoniae (ATCC 13883), Enterobacter cloacae (ATCC 13047), Pseudomonas aeruginosa (ATCC 227853) Candida albicans (ATCC 10231), Candida tropicalis (ATCC 13801) and Candida glabrata (ATCC 28838). | − | Damianakos et al., 2012 |
| Alkannin, shikonin, acetyl alkannin, acetyl shikonin, β,β-dimethyl acryloyl alkannin isovaleryl alkannin, and R-methylbutyryl alkannin, Cinnamoyl alkannin, 3,4-(methylenedioxy)cinnamoyl alkannin, isobutyryl alkannin from Arnebia euchroma | S. aureus, E. faecalis and MRSA. | − | Shen et al., 2002 |
2.3. Anticancer activity
Cancer is one of the most fatal diseases and one of the primary causes of deaths globally. The incidence of cancer in India has been expanding in the last two decades as in other developing nations. Not only the incidence but pattern has also changed to a great extent (Ferlay et al., 2010, Jha, 2009, Guddati, 2012, Rocconi et al., 2012). Anticancer drug resistance is another major obstacle in the effective cancer treatment. It is known that conventional anticancer drugs are likely to cause apoptosis. Due to sensitivity to neoplastic cells to apoptosis, they significantly become resistant via antiapoptotic progression and dysregulation of apoptotic machinery (Han et al., 2007). In addition, antiapoptotic progression in neoplastic cells involves overexpression of antiapoptotic proteins (Bcl-2, Bcl-x1, Mcl-1, c-FLIP), proapoptotic proteins mutations (p53, Apaf-1, Bax, FAS) and loss of caspases (Caspase-3 and Caspase-8) which significantly contributes to drug resistance (Bonora et al., 2015). Therefore, defects in the apoptotic signaling and upregulation of apoptotic inducers enormously limit the effectiveness of chemotherapy. Presently, overcoming the drug transporter-mediated resistance is possible as it works on fewer targets whereas apoptosis mediated drug resistance is highly difficult because of multiple potential targets (Han et al., 2007).
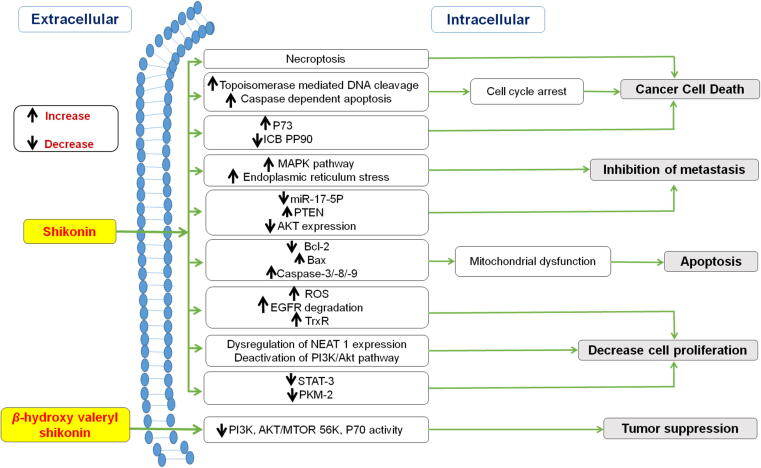
Owing to its strong and broad spectrum anti-cancer activity, shikonin and its derivatives are gaining popularity. A study by a research group revealed the necroptotic mechanism of shikonin to promote non-apoptotic cell death (Degterev et al., 2005). Moreover, shikonin could circumvent cancer drug resistance through induction of necroptosis. Necroptosis is a programmed cell death characterized by necrotic cell morphology and activation of autophagy (Han et al., 2007). Also, shikonin promotes topoisomerase mediated DNA cleavage, caspase–dependent apoptosis and cell cycle arrest via activation of tumor suppressor gene p73 and downregulation of ICBP90 (Fig. 6). Additionally, p73 is responsible for transcription of various p53 target genes such as p16INK4A, PIUMA (p53-upregulated modulator of cell death) and p21 (Jang, Hong, Jeong, & Kim, 2015).
Fig. 6.
Mechanistic anticancer pathways of shikonin and β-hydroxy valeryl shikonin.
A recent report indicates that ICBP90 is overexpressed in patients with cervical cancer. Cervical cancer is second most malignant tumor in women after breast cancer. Annually, the global rate of cervical cancers is about 60 million cases with 25 million deaths (Kaarthigeyan, 2012). Also, high risk human papillomavirus infection (HPV) long term infection of HPV is the leading cause of cervical intraepithelial neoplasia, precancerous lesions and cervical carcinoma (Cook et al., 2017). Previous studies demonstrated that β-hydroxyisovaleryl shikonin (β-HIVS), a shikonin derivative, possesses inhibitory effect on HeLa cells through apoptosis and prevent tumor cell proliferation. β-HIVS retards PI3K activity and downregulates AKT/mTOR signaling along with reduced P7056K expression levels which ultimately leads to tumor suppression (Lu et al., 2015).
On the other side, breast cancer is most prevalent malignancy in women. Recently, triple-negative breast cancer (TNBC) accounts for about 20% of all new cases of breast cancer accompanied with higher grade and distinct metastatic potential. Therefore, suppression of metastasis might be a promising therapy for TNBC patients (Lambert, Pattabiraman, & Weinberg, 2017, Temian, Pop, Irimie, & Berindan-Neagoe, 2018). Essentially, epithelial-to-mesenchymal transition (EMT) plays a pivotal role in regulating metastasis process. EMT involves loss of epithelial phenotypes and the gain of mesenchymal features. It is characterized by downregulation of epithelial cell-surface markers such as occludin, E-cadherin and zonula occludens-1 whereas upregulation of mesenchymal markers such as N-cadherin and vimentin. Of particular interest, shikonin has been established as an effective strategy with good therapeutic potential for TNBC patients. It significantly reduces the expression of miR-17-5p which leads to activation of tumor suppressor gene (PTEN). However, overexpression of PTEN downregulates the Akt expression thereby inhibiting metastasis (Bao et al., 2020).
In recent years, the incidence of colon cancer is considerably increasing in western countries attributing to unhealthy lifestyles. The safety and efficacy of shikonin was determined against colon cancer. Studies demonstrated that shikonin promotes cell death via mitochondrial dysfunction which is induced by downregulation of Bcl-2 and upregulation of Bax, Caspase-3 and Caspase-9. In addition, activation of MAPK pathway and increased endoplasmic reticulum stress triggers apoptosis (Han et al., 2019, Liang et al., 2017). Specifically, anti-cancer activity of shikonin against gefitinib–resistant non-small cell lung cancer (NSCLC) was investigated. Shikonin showed strong cytotoxicity against NSCLC cell lines. Also, it effectively generates ROS and stimulates EGFR degradation resulting in inhibition of TrxR thereby inducing apoptosis (Li et al., 2017). Another study on paclitaxel-resistant non-small cell lung cancer, shikonin induces dysregulation of NEAT1 expression which leads to deactivation of PI3K/Akt pathway hence, inhibiting cell proliferation. Simultaneously, shikonin considerably increases expression of PARP and caspase-3 and caspase 9 cleavages (Zang, Rao, Zhu, Wu, & Jiang, 2020). Researchers reported that activation of STAT3 and PKM2 regulates cell proliferation (Cao et al., 2020, Hoshino et al., 2007). Therefore, STAT3 and PKM2 can be considered as key targets for tumor suppression. Recent studies indicated that shikonin markedly reduced the expression of STAT3dimer and PKM2 gene thereby inhibiting inhibits melanoma cell growth (Cao et al., 2020, Liu et al., 2020). Furthermore, the deactivation of NFƙB also contributes in inhibiting cancer-inducing inflammation by decreasing release of inflammatory cytokines such as COX-2, iNOS and IL-6 (Table 3). In the recent studies shikonin was tested against Acute Myeloid Leukemia. Shikonin impairs the mitochondrial activity and electron transport chain complex-II to selectively target leukemia cells (Roma et al., 2022). Moreover, inhibitory potential of shikonin was reported on Sunitinib-Resistant renal carcinoma cells. It acts by necrosome complex formation and downregulation of AKT/mTOR signaling pathway (Markowitsch et al., 2022). Lately, shikonin was tested and found effectively active against Mutant-non small lung cancer cells. It induces necrosis and apoptosis of cancer cells via thioredoxin reductase 1 inhibition following SecTRAPs generation and oxygen-coupled redox cycling pathway (Zhang et al., 2022). One of the study demonstrated anticancer effect of shikonin against colon cancer cells. It triggers the apoptosis of cancer cells by checking the cancer cell growth in S phase of cell cycle (Chen et al., 2021). Shikonin is found to be a potential inhibitor in pancreatic cancer as it mediates PD-L1 degradation which in turn suppresses immune evasion in pancreatic cancer cells via NF-κB/STAT3 and NF-κB/CSN5 signaling pathway (Ruan et al., 2021). The anticancer potential of shikonin co delivered with siTGF-β against triple negative breast cancer cells was investigated by Li et al and this co-delivery approach was found to be magnificently efficacious for the same (Li et al., 2022). In a nutshell, shikonin/alkannin and their derivatives are promising candidates for anticancer activity which act by various signaling pathways.
Table 3.
Mechanism involved and pharmacological outcomes from various anticancer investigations on shikonin and its derivatives.
| Compounds | Cell lines/In vitro/In vivo assay | Mechanism involved | Types of cancer | References |
|---|---|---|---|---|
| Shikonin | Human normal lung fibroblast cell line CCD19 and human NSCLC cell lines (HCC827, H1650 and H1975) | Induces EGFR degradation causes deactivation of Tyr1173 and Tyr1068 of EGFR; Inhibits TrxR1 to activate ROS-mediated apoptosis |
Gefitinib-resistant non-small cell lung cancer | Li et al., 2017 |
| Human epithelial colorectal adenocarcinoma Caco-2 cells. AOM/DSS model. |
Inhibition of COX-2, iNOS and IL-6 via deactivation of NFƙB; Inhibits Bcl-2 and activates Caspase-3 |
Colon cancer | Andújar, Ríos, Giner, & Recio, 2013 | |
| Normal human colon epithelial cell line (NCM460), well-differentiated colon carcinoma cell lines (HT29 and HCT116), poorly differentiated colon carcinoma cell line (SW480). Nude mouse tumor xenograft model |
Overexpression of SIRT2; Inhibits the viability of SW480 cells and arrests the cell cycle at the G2/M stage; Inhibition of ERK1/2 phosphorylation |
Colorectal cancer | Zhang et al., 2017 | |
| MCF-7 and SK-BR-3 cells | Downregulation of ERα, GPER, EGFR and p-ERK expressions; Inhibits the proliferation in MCF-7 and SK-BR-3 cells; Arrest cell cycle at G0/G1 phase in MCF-7 and induce apoptosis in SK-BR-3 cells |
Breast cancer | Yang et al., 2019 | |
| Human lung cancer cells (A549) Nude mouse tumor xenograft model |
Significant increase in RIP1 levels leading to necroptosis | Non-small cell lung cancer | Kim et al., 2017 | |
| Human colon cancer cell lines HCT116, SW480 and human normal colon mucosal epithelial cell line NCM460. | Decreased Bcl-2 and Bcl-xl expression; Increased caspase 3 and 9 activities. Depolarization of mitochrondrial membrane potential |
Colon cancer | Liang et al., 2017 | |
| Myelogenous leukaemia cell line (K562cells), breast cancer cell line (MCF-7cells) and cervical cancer cells (HeLa cells) Xenograph tumour model | Irreversible inhibition of human recombinant CDC25 phosphatases; Inhibit dephosphorylation of CDK1 thereby inducing cell cycle arrest at G2/M phase |
Cancer | Zhang et al., 2019 | |
| Human lung cancer cells (A549) Nude mouse tumor xenograft model |
Suppression of NEAT1 and Akt signaling | Paclitaxel-resistant non-small cell lung cancer | Zang, Rao, Zhu, Wu, & Jiang, 2020 | |
| Breast cancer cell line (MCF-7 cells) | Downregulation of Bax expression and reduced exosomal secretion leading to suppress proliferation | Breast cancer | Wei et al., 2016 | |
| Human colon cancer cell line (SNU-407)Terminal deoxynucleotidyl transferase-mediated digoxigenin dUTP nick-end labeling (TUNEL) assay. |
Induces the mitochondrial dysfunction via downregulation of Bcl-2 and upregulation of Bax, Caspase-3 and Caspase-9; Activation of MAPK Pathway. Increased Ca2+ levels leading to ER stress |
Colon cancer | Han et al., 2019 | |
| Murine mammary cancer (4 T1) and human breast cancer cells (MDA-MB-231) Orthotopic model of murine mammary cancer cells. |
Activation of p38 and JNK signaling pathways; Increase caspase3/7 activity; Inhibits proliferation, migration and invasion ability of cells |
Breast cancer | Xu et al., 2019 | |
| Human breast cancer cells (MDA-MB-231) | Reduced expression of miR-17-5p and upregulation of PTEN expression with decreased levels of Akt and p-Akt leads to EMT suppression | Triple negative breast cancer | Bao et al., 2020 | |
| Human ovarian cancer cell (SKOV3) | Downregulation of Bcl-2, AKT and PI3K whereas upregulation of Bax, Caspase-3 and Caspase-9 | Ovarian cancer | Zhang et al., 2020 | |
| Human melanoma (A375) and normal human liver-derived cells (MIHA) Zebrafish Tumor Model |
Reduced expression of STAT3 dimer. Decreased levels of Bcl-2, Mcl-1, MMP-9 and MMP-2 |
Malignant melanoma | Cao et al., 2020 | |
| Human colorectal adenocarcinoma (SW620 and HCT116 cell lines) Subcutaneous tumor mouse model |
Induce autophagosome formation via LC3 cleavage; Upregulate expression and promote galectin-1 dimerization |
Colorectal carcinoma | Zhang et al., 2020 | |
| Hepatocellular carcinoma cells lines (LM3, SMMC-7721, Huh-7, and HepG2) and a normal liver cell line (LO2) | Downregulates the expression of PKM2; Increased expression of Bax, cyto C, cleaved Caspase-9, and cleaved Caspase-3, and decreased expression of Bcl-2 |
Hepatocellular Carcinoma | Liu et al., 2020 | |
| Hepatocellular carcinoma cell lines (Huh-7 and HepG2) | Modulation of the SMAD7/TGF‐β signaling pathway through regulation of miR‐106b | Hepatocellular carcinoma | Li & Zeng, 2020 | |
| Shikonin, acetyl shikonin, and β,β-dimethyl acryl shikonin | Human breast cancer cells (MDA-MB-231) and Murine mammary cancer (4T1). | Shikonin was found most potent against TNBC cell lines; Upregulates E-catherin levels whereas downregulates N-cadherin and vimentin levels; Stimulate β-catenin degradation through enhanced GSK-3β levels |
Triple-negative breast cancer | Chen et al., 2019 |
| Shikonin, Deoxyshikoninand β,β-Dimethyl acrylshikonin | Human amelanotic malignant melanoma cell line A375 (CRL1619), mouse metastatic melanoma cell line B16-F10 (CRL-6475) and mouse nonmetastatic melanoma cell line B16-F0 (CRL6322), | Induces p53-mediated cell cycle arrest;Stimulates ROS -mediated Endoplasmic Reticulum (ER) stress. Activation of p-ERK, p-p38, Caspase-3 and Caspase-9 |
Skin cancer | Ng, Upton, Leavesley, & Fan, 2020 |
2.4. Miscellaneous activities
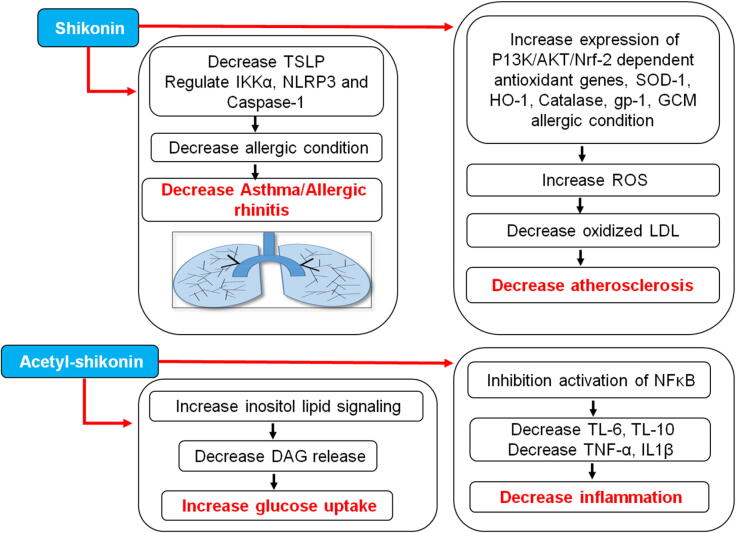
Apart from pharmacological activities discussed above alkannin/shikonin and their derivatives also possess therapeutic potential against phytogenotoxicity, bronchial asthma, peptic ulcer, spasmogenicity, atherosclerosis, inflammatory diseases, ischemic heart diseases, cataract, hepatotoxicity and impotency (Fig. 7) (Yildirim, 2020). Onosma, the biggest genus of Boraginaceae family, is being used as traditional medicine since centuries (Davis, 1970). Shikonin and its derivatives have also been reported to inhibit oxidized low-density lipoprotein (LDL) induced monocyte adhesion by deactivation of NFƙβ and hence used in treatment of atherosclerosis. It is well known that oxidized LDL plays a key role in thrombosis, endothelium apoptosis and vascular smooth muscle proliferation. In addition, it also stimulates release of inflammatory mediators such as cytokines and reactive oxygen species. Moreover, activation of NFƙβ further upregulates the expression of intracellular adhesion molecule (ICAM-1), E-selectin, vascular cell adhesion molecule and monocyte chemotactic protein-1. Hence, the accumulation of oxidized low-density lipoprotein (oxLDL) and inflammatory cells lead to atherosclerosis. Shikonin has also been found effective in retarding oxLDL mediated ROS production through induction of expression of PI3K/Akt/Nrf 2- dependent antioxidant genes such as SOD-1, HO-1, Catalase, GPx-1, GCLM, and GSR (Huang et al., 2015).
Fig. 7.
Mechanistic activity of shikonin and acetyl shikonin for treatment of asthma, arthrosclerosis, diabetes and inflammation. LDL, low density lipoprotein; DAG, diacylglycerol; ROS, reactive oxygen species; TSLP, thymic stromal lymphopoietin; IL, interleukins; GCM, global compact on migration.
Furthermore, the oxidative stress is the major cause of various other medical conditions such as ageing, diabetes, stroke, neurodegenerative disorders, cancer etc. Oxidative stress is often accompanied with higher blood sugar levels. The skeletal muscle cells are rich in insulin-sensitive glucose transporters named as glucose transporter 4 (GLUT4). Their main function is translocation of glucose from cytoplasm to cell membrane aiding in glucose uptake. Therefore, it plays imperative role in regulation of homeostasis of glucose. However, the contraction-induced release of reactive oxygen species (ROS) and activation of AMP activated protein kinase (AMPK) may also lead to increased glucose uptake in skeletal muscle cells (Mao, Yu, Li, & Li, 2008, Su et al., 2016). Subsequently, acetylshikonin-induced glucose uptake was significantly inhibited by reduction of PLC-β3 in L6 myotubes, which makes it evident that acetylshikonin-induced glucose uptake may be triggered by activation of inositol lipid signaling and increased DAG release (Huang et al., 2019). On the other hand, ageing is also considered as biggest cause of Alzheimer’s disease. Various studies have shown that oxidative stress, neuronal apoptosis and neuroinflammation plays critical role in pathogenesis of Alzheimer’s disease (Heneka, 2015). SIRT1 is essentially involved in cognitive functions and shows protective effect against aging-related neuronal degeneration. Thus, SIRT1 can be the most promising therapeutic target for Alzheimer’s disease. Multiple studies reported that chronic inflammation associated with raised levels of pro-inflammatory mediators such as IL-6, IL-10, TNF-α and IL-1β. Notably, acetylshikonin reduced the levels of these mediators via inhibiting the activation of NFƙβ and thereby reducing inflammation. Simultaneously, it also inhibits the activation of p21/p53 signaling pathway (Chang et al., 2015). Furthermore, overexpression of thymic stromal lymphopoietin (TSLP) is a major factor contributing to allergic diseases such as asthma, allergic rhinitis etc. Epithelial cell-derived TSLPs control the allergic condition via regulating the activation of T-cells, mast cells, and dendritic cells. The findings of the study elucidated that shikonin as well as L. erythrorhizon aqueous extract was able to downregulate TSLP production as well as markedly attenuated the levels of IKKα, NLRP3 and Caspase-1 (Yen et al., 2017). Besides having multiple pharmacological effects, naphthoquinones are also considered as potent allelochemicals as they hold good potential to defend against predators. Previous studies demonstrated that juglone, 1,4-naphthoquinone, plumbagin and 2-methoxy-1,4-naphthoquinone showed anti-feedant activity against the cabbage looper Trichoplusia ni (Akhtar, Isman, Niehaus, Lee, & Lee, 2012). Napthoquinones were also found effective against the dry bean pests Epilachna varivestis and Acanthoscelides obtectus (Cespedes et al., 2016). Moreover, the extreme toxicity of juglone against Myzocallis walshii and plumbagin against Tetrany chusurticae, Myzus persicae and Illinoia liriodendri were also investigated. These studies substantiate that juglone and plumbagin are effective insecticidal and acaricidal agents. The inhibitory and toxicity potential of Onosma visianii roots against Spodoptera littoralis was also investigated. The main active constituents of O. visianii roots include isovalerylshikonin and isobutyrylshikonin. Being highly lipophilic in nature, these active moieties easily enter the insect exoskeleton and hinder the physiological processes. Moreover, the ester groups of these moieties increases cuticle penetration via linkage with hydroxyl groups and significantly inhibits acetylcholinesterase (AChE) enzymes. Additionally, it leads to inhibition of mitochondrial respiration thereby controlling larval growth (Table 4) (Akhtar, Isman, Lee, Lee, & Lee, 2012, Pavela, 2013).
Table 4.
Mechanism involved and pharmacological outcomes from miscellaneous investigations on alkannin/shikonin containing plant extracts, alkannin/shikonin and its derivatives.
| Test compounds | Cell lines/In vitro/In vivo assay | Mechanism involved | Disease targeted | References |
|---|---|---|---|---|
| Lithospermum erythrorhizon and Angelica sinensis extract | Human Bronchial Epithelial cell line (BEAS-2B) | Anti-inflammatory effect in Der-p2-stimulated BEAS-2 β cells; Inhibition of TSLP production and suppression of IKKα, caspase-1 and NLRP3 |
Allergic diseases such as asthma, atopic dermatitis and allergic rhinitis | Yen et al., 2017 |
| Onosma tauricum extract | Anti-oxidant assays (DPPH, CUPRAC, ferrous ion chelating, FRAP, polumolybednum, ABTS) and Enzyme inhibitory assays (AChE, α-amylase, BChE, tyrosinase, α-glucosidase) | Anti-oxidant and enzyme inhibitory activity | Evaluation of antioxidant and enzyme inhibitory potential | Kirkan et al., 2018 |
| Onosma sieheana and Onosma stenoloba extracts | Total phenolic assay, total flavonoid assay, anti-oxidant assay, tyrosinase assay, α-amaylase assay. | Anti-tyrosinase activity. Increased expression of p-Erk1/2 and reduced extression of tyrosinase related protein 1 and 2 |
Diabetes | Sarikurkcu, Sahinler, Ceylan, & Tepe, 2020 |
| Lithospermum radix aqueous extract | Sub-acute oral toxicity | Suppression of spinal inflammation | Chemotherapy induced neuropathy. | Kim et al., 2019 |
| Shikonin | PAI-1 activity assay, Clot lysis assay, mouse arterial thrombosis model, Mouse liver fibrosis model. | Inhibition of plasminogen activator inhibitor-1 activity; Anti-thrombotic and anti-fibrotic effect |
Fibrinolysis | Han et al., 2016 |
| Human endothelial cell line derived from human lung carcinoma cells and human umbilical vein endothelial cells | Induction of expression of PI3K/ Akt/Nrf 2- dependent antioxidant genes such as SOD-1, HO-1, Catalase, GPx-1, GCLM and GSR; Inhibition of oxLDL-induced intracellular ROS accumulation via NF-ƙβ adhesion |
Atherosclerosis | Huang et al., 2015 | |
| Human umbilical vein endothelial cells (HUVEC), human fibroblast-like synoviocyte (HFLS).Collagen-induced arthritis model. In vivo chick chorioallantoic membrane (CAM) assay. Ex vivo rat aortic ring assay |
Inhibition of pro-angiogenic mediators such as TNF-α, IL-12, TGF-β, IL-6, IL-8, VEGF, PDGF,IL-17A and MMPs; Increase expression of Treg/Th17 by deactivation of TLR4/MyD88 pathway |
Rheumatoid arthritis | Liu et al., 2020 | |
| Isovaleryl shikonin and isobutyryl shikonin | Acute toxicity, chronic toxicity, growth inhibition, antifeedant activity, AChE inhibitory activity and antioxidant assay. | Inhibition of AChE enzymes; Inhibition of mitochondrial respiration thereby inhibiting larval growth |
Inhibition of larval growth of Tobacco cutworm Spodoptera littoralis. | Sut et al., 2017 |
| Acetylshikonin | Behavioral testing (Morris Water Maze test) |
Inhibition of activation of p53/p21 signaling pathway; Upregulation of SIRTI in hippocampus; Anti-apoptotic activity in neuronal cells and attenuated H2O2 induced oxidative stress |
Alzheimer’s disease | Li, Zeng, Su, He, & Zhu, 2018 |
| L6 rat skeletal muscle cells. Alloxan-induced type I diabetic models. | Activation of p2C-β3/PKCδ cascades via activation of inositol lipid signaling and increase in DAG release | Diabetes. | Huang et al., 2019 | |
| Acetylshikonin and isobutyryl shikonin | Anti-genotoxic properties (Umu-test) and cytotoxicity assay (Lung fibroblast cell line (V79) | Inhibition of p450 enzymes, Free radical scavenging activity and anti-genotoxic activity | Carcinogenesis | Skrzypczak et al., 2015 |
3. Isolation and analytical aspects of A/S and their derivatives
A/S and their derivatives have been reported to be isolated from various Boraginaceae family plants (Table 5) amongst which L. erythrorhizon (Lee et al., 2016, Rajasekar et al., 2012, Han et al., 2007, Azuma et al., 2016) and Alkanna tinctoria Tausch. (Mohammed, 2016, Rashan et al., 2018, Jaradat et al., 2018) yield high content of shikonin and alkannin derivatives, respectively. Adding on, the petroleum ether and chloroform fraction of dried roots of L. erythorrhizon elute β,β-dimethylacrylshikonin, isobutyl shikonin, shikonin, 5,8-dihydroxy-2- (-1-methoxy-4-methyl-3-pentenyl) – I, 4 naphthalene dione and – sitosterol, mixture of caffeic acid esters when subjected to column chromatography (Han et al., 2007). On the other hand, column chromatography of powdered roots of A. tinctoria led to elute alkannin, angenylalkannin, 5-methoxy angenylalkannin, alkanfuranol, alkandiol, acetylalkannin whereas dimethylacrylshikonin was obtained using reverse phase column chromatography (Tung, Du, Yuan, Shoyama, & Wang, 2013). On similar lines, Lithospermum euchroma when subjected to column chromatography resulted in the production of acetyl shikonin and β,β’-dimethylacylshikonin (Cheng et al., 2008). Moreover, chiral column in HPLC was used to separate out the enantiomeric excess from the mixture of A/S obtained from dried (under reduce pressure with aid of P2O5) chloroform residue of L. erythrorhizon (Azuma et al., 2016). Furthermore, percolation and soxhlet extraction was used sequentially followed by thin layer chromatography to obtain arnebin-1, arnebin-2, arnebin-3, arnebin-4, arnebin-5, arnebin-6 arnebin-7, tiglic acid, arnebinone, alkannin, arnebinol, cycloarnebin-7, β,β-dimethylacryl shikonin, isovaleryl shikonin, β-hydroxyisovaleryl shikonin and shikonin isovalerate from the powdered roots of Alkanna hispidissima (Singh & Sharma, 2012, Yusufoglu et al., 2018). Percolation technique was also used to obtain acetyl alkannin and β,β-dimethylacryl alkannin from the viscous red residue of Arnebia nobilis Reichb. f. (Mohapatra et al., 2016). In another report, solid liquid extraction and HPLC-VIS technique was used to elute angelylshikonin, 2-methyl-n-butyrylshikonin, and isovaleryl shikonin from the dried roots of Echium etalicum (Albreht, Vovk, Simonovska, & Srbinoska, 2009). In addition to the conventional methods of extraction and isolation, a novel method called supercritical CO2 method was used to isolate A/S from the powdered roots of A. tinctoria. Supercritical CO2 functions as non-polar, lipophilic solvent with alkannin/ shikonin. It was reported that highest yield was obtained at higher temperature and lower flow rates (Akgun, Erkucuk, Pilavtepe, & Yesil-Celiktas, 2011). Subsequently, the ultrasonication technique was also exploited to primarily obtain deoxyshikonin and other naphthoqimone derivatives from the residual extract of Lomandra hastilis (Park et al., 2017, Utkina & Pokhilo, 2017). On the similar lines, L. erythrorhizon extract was subjected to sonication to obtain acetylshikonin, shikonin, deoxyshikonin, β-sitosterol and β,β-dimethylacrylshikonin (Li, Xu, Zhu, & Wang, 2012). Furthermore, Microwave assisted extraction/isolation followed by reverse phase chromatography was used to obtain shikonin and its derivatives from the dried roots of L. erythorrhizon. Moreover, the Sephadex column has also been used in one of the reports where L. erythrorhizon was taken to elute isobutyrylshikonin (Park, Woo, Kim, Choi, & Park, 2020, Yen et al., 2017). Lately, the higher yield of acetylshikonin in Echium plantagineum has been reported by overexpression of cloned EpGHQH1 (geranylhydroquinone 3″-hydroxylase candidate gene) (Fu et al., 2021). Also, the production of alkannin and shikonin was found to be increased in hairy roots of A. tinctoria when introduced with bacteria belonging to Chitinophaga sp., Allorhizobium sp., Duganella sp., and Micromonospora sp. (Rat et al., 2021).
Table 5.
Various investigations carried out for analysis of alkannin/shikonin and its derivatives.
| Plant | Extraction process | Solvent systems | Methods | Constituents | References |
|---|---|---|---|---|---|
| Lithospermum erythrorhizon | Sonication | Gradient elution: Petroleum ether-ethyl acetate, petroleum ether - dichloromethane, petroleum ether - acetone, and petroleum ether - ethyl acetate and acetone | Silica gel column chromatography | Acetylshikonin, shikonin, deoxyshikonin, β-sitosterol and β,β-dimethylacrylshikonin | Li, Xu, Zhu, & Wang, 2012 |
| Solid liquid extraction | – | Open column of silica gel chromatography | Shikonin, acetylshikonin,5,8-dihydroxy-1.4-naphthoquinone (DH), 1,4naphthoquinone (NAP) and β,β’-dimethylacylshikonin. |
Cheng et al., 2008 | |
| Solid-liquid extraction | n-hexane/2- propanol (90:10, volume percentage) | Chiral HPLC | Shikonin (an improved method) | Azuma et al., 2016 | |
| Maceration | 50% hexane in CH2Cl2, CH2Cl2, 5% and 33% acetone in CH2Cl2, and 5% and 33% methanol in CH2Cl2 | Silica gel column chromatography and Sephadex column with methanol. | Isobutyrylshikonin | Park, Woo, Kim, Choi, & Park, 2020 | |
| Maceration | 0.085% H3PO4 buffer and acetonitrile: 10%–25% for 20 min; 25%–70% for 30 min; 70%–90% for 40 min; 80%–90% for 60 min; and 100% for 65 min. | Reverse phase column chromatography | Shikonin, bhydroxyisovalerylshikonin, acetylshikonin, β-acetoxyisovalerylshikonin, deoxyshikonin, isobutyrylshikonin, β,β-dimethylacrylshikonin, and methyl-n-butyrylshikonin |
Yen et al., 2017 | |
| Ultrasonic extraction. | Methanol and water with 0.5% acetic acid. | Reverse phase column chromatography | Deoxyshikonin | Park et al. (2017) | |
| Onosma visianii | Soxhlet extraction | methanol and water (0.1% formic acid) (90:10) | Semipreparative HPLC | Isovalerylshikonin, isobutyrylshikonin,acetylshikonin, hydroxyisovalerylshikonin, shikonin-β,β-dimethylacrylate, propionylshikonin, 5,8 dimethoxy acetylshikonin, 1-(5,8-dimethoxy-1,4-dioxo-1,4-dihydronaphthalen-2-yl)- 4-methylpent-3-en-1-yl 2-methylbutanoate, 5,8 -dimethoxy isobutyrylshikonin, 5,8-O-dimethyldeoxyshikonin, 2-(4-hydroxy-4-methylpent-2-en-1-yl)-5,8-dimethoxynaphthalene-1,4-dione. | Sut et al., 2017 |
| Alkanna strigosa | Soxhlet extraction | (CHCl3: MeOH: H2O) (5:4:1) | Preparative tlc | Alkannin and shikonin | Aburjai, Al-Janabi, Al-Mamoori, & Azzam, 2019 |
| Echium italicum | Maceration | Hexane-Etilacetae | silica gel column chromatography | 2-Methyl-n-butyrylshikonin, isovalerylshikonin, acetylshikonin and deoxyshikonin | Eruygur, Yılmaz, Kutsal, Yücel, & Üstün, 2016 |
| Lomandra hastilis | Sonication | n-hexane–acetone (3:1) | Preparative TLC | 5,8-Dihydroxy-2-ethyl-3,6,7-trimethoxy-1,4- naphthoquinone, lomazarin, 2-(1′- acetoxyethyl)-5,8-dihydroxy-3,6,7-trimethoxy-1,4-naphthoquinone, 5,8-dihydroxy-3,6,7-trimethoxy-2-(1′-methoxyethyl)-1,4- naphthoquinone, isonorlomazarin, 5,8- dihydroxy-2-(1′-hydroxyethyl)-1,4-naphthoquinone, 2-(1′- acetoxyethyl)-5,8-dihydroxy-1,4-naphthoquinone, 5,6,8- trihydroxy-2-ethyl-3,7-dimethoxy-1,4-naphthoquinone and ethylmompain dimethyl ether. | Utkina & Pokhilo, 2017 |
| Alkanna tinctoria | Extraction with 95% EtOH |
|
|
Angelylalkannin, 5-methoxy angenyalkannin, alkanfuranol, alkandiol, acetylalkannin and dimethylacrylshikonin. | Tung, Du, Yuan, Shoyama, & Wang, 2013 |
| supercritical CO2 extraction | 0.025% aqueous TFA and acetonitrile | HPLC-PDA analysis | Alkannin/shikonin | Akgun, Erkucuk, Pilavtepe, & Yesil-Celiktas, 2011 | |
| Echium italicum | Solid–liquid extraction | MeOH:HCOOH (20:1, volume percentage) and THF:MeCN:H2O:HCOOH (30:20:50:0.5, volume percentage) |
Chiral thin-layer chromatography and semi-preparative HPLC | Angelylshikonin, 2-methyl-n-butyrylshikonin, and isovaleryl shikonin | Albreht, Vovk, Simonovska, & Srbinoska, 2009 |
| Arnebia nobilis Reichb.f. | Percolation | – | Silica gel chromatography | Acetyl alkannin, acetoxyisovaleryl alkannin and β,β dimethylacryl alkannin | Mohapatra et al., 2016 |
| Alkanna hispidissima | Percolation | Hexane - acetone - acetic acid | Thin layer chromatography | Arnebin-1, arnebin-2, arnebin-3, arnebin-4, arnebin-5, arnebin-6 arnebin-7, tiglicacid, arnebinone, alkannin, arnebinol, and cycloarnebin-7. | Yusufoglu et al., 2018 |
4. Patent applications
Forecasting the market potential, numerous patent applications on inventions containing alkannin/shikonin and its derivatives have been filed by various research groups across the world. Brief details of these applications are divided into two categories viz. therapeutic and analytical and are summarized in Table 6, Table 7.
Table 6.
Therapeutic patents of alkannin/shikonin and their derivatives.
| Titles | Targeted diseases | Mechanism of action | References |
|---|---|---|---|
| Acylatedalkannin or shikonninderivs.- useful as dermatological, bactericidal and fungicidal medicaments | Treatment of skin lesions: ulcers, burns, wounds, scruf, skin cancers | Antibacterial and anti-inflammatory effect | Papageorgiou, 1980 |
| Process for preparing arnebia root medicine with broad-spectrum medical functions | Measles, rashes, ulcer sores, eczema, burns | Proliferation of fibroblasts | Song, 2004 |
| Alkannin derivatives as immune inhibitors and metal complexes thereof | Arthritis, scleredema, lupus erythematosus, HIV infection and malignant tumor | Immunological suppression of chemokines amd HIV-type 1 | Li & Hu, 2004 |
| Use of alkannin in preparing medicine for treating tumor disease | Treatment of tumor, effective on eh tumour, effective on medicine resisting tumor cells | Killing tumor cells with p-glycoprotein | Hu & Fang, 2005 |
| Application of shikonnin in preparing medicine for inducing apoptosis | Treatment of tumor | Shikonin induces ROS production and cytochrome c release in cancer cells. | Hu & Han, 2007 |
| Application of Xinjiang radix macrotomiae for treating flatwart, common wart and fig wart | Treatment of verrucous disease, Flat wart, common wart, fig wart | Diminishing the inflammation of hurt on an afflicted part, healing of hurt without leaving scar | Li & Chen, 2009 |
| Method of treatment of virus infections using shikonnin compounds | Virus infections, mycoplasma infections, malignant tumor | Promoting idiosyncratic cell mediated immunity and improves immune response of T-lymphocytes | Wang, 2008 |
| Antineoplastic sulphur-comtaining alkannin and naphthoquinone derivatives | Antineoplastic | Inhibition of tumor cell growth | Li, Zhao, Xei, He, & Guo, 2008 |
| Antineoplastic alkannatinctoria ketoximes derivatives | Antineoplastic | Retard tumor cell growth | Li & Zhao, 2010 |
| Application of alkannin in preparation of pyuruvate kinase inhibitor | Psoriasis, herpes simplex keratitis | Inhibition of PMK2 activity | Hu, 2011 |
| Medical application of radix arnebiaeseulithospermi naphthoquinone compounds | Crohn’s disease | Inhibition of NF-kβ and STAT-3 | Liu & Fan, 2014a |
| Medical application of gromwell naphthoquinone compounds | Ulcerative colitis | Inhibition of COX-2 and cytokines (INF-γ and IL-6) | Liu & Fan, 2014b |
| Medical application of lithospermumnahthoquinone compounds | Chronic obstructive pulmonary disease (COPD) | Inhibition of PDE-4 | Liu & Fan, 2014c |
| Pharmaceutical composition for treating flatwart and verruca vulgaris and prepation method for pharmaceutical composition | Flat wart, verruca vulgaris | Resisting inflammation, killing viruses and realizing quick apoptosis of skin vegetation cells. | Yuan & Wang, 2016 |
| Compound traditional Chinese medicine for preventing and treating stigmatosis of freashwater fish | Stigmatosis | Inhibition of influenza virus, gram positive and gram negative bacteria | Liu, Zhao & Wang, 2017 |
| Compositions for metabolic disorders comprising alkannin as an active ingredient | Obesity, hyperlipidemia, fatty liver | Activation of AMPK (AMP activated protein kinase) | Yoon, Lee, Jang, & Jeong, 2017 |
| Application of alkannin in preparation of medicine for treating upper and lower respiratory tract allergic disease | Allergic rhinitis and allergic asthma | Activation and differentiation of TH cells and cytokine secretion | Liu & Yu, 2017 |
| Hydroxynaphthoquinone compounds for treatment of non-small cell lung cancer | Non– small cell lung cancer | Inhibition of EGFR kinase activity and induction of apoptosis in cancer cells | Liu, Leung, Li, & Fan, 2018 |
| Herqueiazole-containing medicine for controlling inflammation | Inflammation | Synergistic effect of shikonin and herqueiazole | Zhuang & Zhang, 2017 |
| External biological preparation for feminine vagina prophylaxis and health-care as well as treatment of gynaecological genital tract inflammation, and preparation method | Cervical erosion, vaginitis, pelvic inflammation | Eliminating vaginal bacteria and maintaining vaginal flora and acid base balance | Wang & Chen, 2017 |
| Omeprazole enteric-coated capsules capable of inhibiting gastric acid secretion | Gastric and duodenal ulcer | Inhibition of H+, K+ ATPase enzyme activity | Li, 2018 |
| Composition for treating burns and scalds | Treating burns and scalds. | Antibacterial and antiinflammatorey effect | Liu, Wei, Zhong, Zuo, Yi, & Yang, 2017 |
| Shikonin and derivant thereof are as the application of gene therapy sensitizer | Cancer | Inhibition of TNF-ᾳ | Ling, Wang, Wang, & Zhang, 2017 |
| Externally-applied anti-inflammatory agent containing radix lithospermii extract | Inflammation | Inhibition of STAT 3 (Signal transducers and activators of transcription) pathway | Chu, 2018a, Chu, 2018b |
Table 7.
Analytical/Biosynthetic patents of alkannin/shikonin and their derivatives.
| Patent No./ Filling date | Plants | Titles | Conditions | Methods of extraction | Compounds | References |
|---|---|---|---|---|---|---|
| CN1079239C (12-04-1995) | Comfrey roots | Gromwell prepn. With wide medical effect and its prepn. Process | Refined oil | Decoction | Shikonin | Song, 2002 |
| CN1117525A (13-06-1995) | Alkanna tinctoria shoots | Arnebia euchroma (Royle) Johnst. Cell cultivation and prodn. Process by solid two step method | AG-7 growth culture medium and AP-5 germ culture medium | Cell suspension culture | Deoxyshikonin, acetylshikonin, β,β- dimethyl acrylamide shikonin, dimethylpenteneshikonin | Ye, Li, Song, & Chen, 1996 |
| CN1253972A (29-11-1999) | Lithospermum officinale roots | Alkannia and its extraction method | Liquid CO₂ | Super critical CO₂ extraction | Shikonin, acetylshikonin, dimethyl acrylamide shikonin, β hydroxyl isovalerylshikonin, 2,3 dimethyl pentene shikonin. | Xu, Wang, & Huang, 2000 |
| CN1384149A (17-05-2002) | Comfrey roots | Gromwell haematochrome extracting process | Liquid CO₂ | Super critical CO₂ extraction | Alkannin | Wang, 2002 |
| CN1633841A (26-12-2003) | Arnebia euchroma | Method for promoting Xinjiang alkannatinctoria callus growth using rare earth element | N₆ solid medium | Callus growth culture | Shikonin | Wang, Fang, & Wang, 2005 |
| CN1546450A (08-01-2004) | Dried Arnebia roots | Preparation method of high purity alkannaphthaquinone | Supercritical CO₂ | Super critical CO₂ extraction | Alkannin | Li & Hu, 2004 |
| CN101434530A (12-12-2008) | Comfrey dried purple roots | Method for extracting alkannin from alkanet | Ethanol | Solid liquid extraction | Shikonin | Zu et al., 2009 |
| CN101942212A (15-07-2010) | Comfrey powder | Method of extracting alkannin naphthoquinone pigment | 1,1,1,2- Tetrafluoro ethane | Molecular distillation | Dimethyl acrylamide shikonin, isovalerylshikonin | Liu & Liu, 2011 |
| CN101906028B (26-08-2010) | Comfrey roots powder | Method for extracting benzoquinone compound in lithospermum | n-Hexane or petroleum ether | Multiple reflux extraction | Alkannin | Yan, Xu, Yu, & Lei, 2013 |
| CN102228499A (20-06-2011) | Arnebia roots | Method for separating naphthoquinone active ingredients from sinkiangarnebia root | Petroleum ether, ethylacetate | Ultrasonic extraction | Deoxyshikonin, acetyl shikonin, shikonin, β,β’-metho acryloyloxyshikonin, isobutyrylshikonin, β hydroxyl isovalerylshikonin | Yuan & Yuan, 2011 |
| CN103373913A (15-04-2012) | Comfrey purple grass powder | Extraction method of alkannin | Cyclohexane | Maceration | Shikonin | Pan, Wang, Tang, Li, Wang, & Zhou, 2013 |
| CN103664566A (02-12-2013) | Comfrey purple grass | Alkannin extraction device | Petroleum ether | Ultrasonic crusher extraction | Shikonin | Tang, Wang, & Zhou, 2014 |
| CN105949045A (28-07-2014) | Arnebia roots | Method for extracting alkannin from arnebia roots | Supercritical CO₂ | Super critical fluid extraction | Shikonin | Guo, Zhang, & Xu, 2016 |
| CN105348065A (04-12-2015) | Lithospermum erythrorhizon | Preparation method for high-purity alkannin from lithospermumerythrorhizon | Petroleum ether | Percolation | Shikonin | Yang & Yang, 2016 |
| CN104774151A (30-01-2015) | Lithospermum mongolia | Preparation technology of mount taishan Radix Lithospermi naphthoquinone active monomers | Petroleum ether, hexanoicacid Capro lactone Hexylalcohol-water. | High performance counter current chromatography | Isopentyl shikonin, hexylshikonnin, isobutyl shikoin | Lei, Haiwei, Jiang, Zhai, Yi, & Jiang, 2015 |
| CN107151203A (03-03-2016) | Arnebia euchroma | Method for separating and preparing natural naphthoquinone compounds | n-Hexane, ethylacetate, acetonitrile, water | High speed counter current chromato Graphy | Deoxyshikonin, propionyl shikonin, β,β dimethylacryl shikonnin, isovalerylshikonin. | He, Qing, & Zhang, 2017 |
| CN108409570A (06-03-2018) | Arnebia euchroma | Fast and efficient purification method comfrey acetyl shikonnin | Ethylacetate/petroleum ether | Reverse phase silica gel chromatography | Acetylshikonin | Jiang, Lin, & Zhai, 2018 |
5. Conclusion
Alkannin/shikonin and its derivatives possess a wide variety of pharmacological activities. These constituents are majorly investigated for their wound healing, antimicrobial and anticancer potential. In the last decade, various mechanisms of alkannin/shikonin and their derivatives are explored implicated in wide variety of diseases. The present study suggests the higher applicability of alkannin/shikonin and its derivatives are in the development of potent and safer wound healing and anticancer agents. Various analytical investigations are also discussed that will help the analysts for more efficient analysis of alkannin/shikonin and its derivatives from different sources. Brief patent summary is provided to highlight the future marketable potential of alkannin/shikonin and its derivatives. The appropriate knowledge of the pharmacological aspects of A/S and their derivatives will not only benefit the natural product researchers but also the pharmaceutical/formulation scientists in their future course of action. Further, the advanced and novel drug delivery systems could be used to mask the limitations of these derivatives including their low solubility and photo degradation. Despite having magnificent pharmacological potential, there is a dire need to collect remarkable data related to their toxicological and safety profile which can establish the clinical usage of these components.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are thankful to all the scientists for carrying out in-depth study on alkannin/shikonin and their derivatives that was helpful in framing this article.The authors duly acknowledge RUSA 2.0 Scheme (Component-4) of Ministry of Education, Government of India to facilitate the current work.
References
- Aburjai T., Al-Janabi R., Al-Mamoori F., Azzam H. In vivo wound healing and antimicrobial activity of Alkanna strigose. Wound Medicine. 2019;25(1):100–152. [Google Scholar]
- Akgun I.H., Erkucuk A., Pilavtepe M., Yesil-Celiktas O. Optimization of total alkannin yields of Alkanna tinctoria by using sub- and supercritical carbon dioxide extraction. Journal of Supercritical Fluid. 2011;57:31–37. [Google Scholar]
- Akhtar Y., Isman M.B., Lee C.H., Lee S.G., Lee H.S. Toxicity of quinones against two-spotted spider mite and three species of aphids in laboratory and greenhouse conditions. Industrial Crops and Products. 2012;37(1):536–541. [Google Scholar]
- Akhtar Y., Isman M.B., Niehaus L.A., Lee C.H., Lee H.S. Antifeedant and toxic effects of naturally occurring and synthetic quinones to the cabbage looper, Trichoplusiani. Crop Protection. 2012;31(1):8–14. [Google Scholar]
- Albreht A., Vovk I., Simonovska B., Srbinoska M. Identification of shikonin and its ester derivatives from the roots of Echium italicum L. Journal of Chromatography A. 2009;1216(3):156–162. doi: 10.1016/j.chroma.2009.01.098. [DOI] [PubMed] [Google Scholar]
- Andújar I., Ríos J.L., Giner R.M., Recio M.C. Pharmacological properties of shikonin - a review of literature since 2002. Planta Medica. 2013;79(18):1685–1697. doi: 10.1055/s-0033-1350934. [DOI] [PubMed] [Google Scholar]
- Azuma H., Li J., Youda R., Suzuki T., Miyamoto K., Taniguchi T., Nagasaki T. Improved isolation procedure for shikonin from the root of the Chinese medicinal plant Lithospermum erythrorhizon and its solubilization with cyclodextrins. Journal of Applied Research on Medicinal and Aromatic Plants. 2016;3:58–63. [Google Scholar]
- Bao C., Liu T., Qian L., Xiao C., Zhou X., Ai H.…Pan J. Shikonin inhibits migration and invasion of triple-negative breast cancer cells by suppressing epithelial-mesenchymal transition via miR-17-5p/PTEN/Akt pathway. Journal of Cancer. 2020;1(12):76–88. doi: 10.7150/jca.47553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm R., Sommer S., Li S.M., Heide L. Genetic engineering on shikonin biosynthesis: Expression of the bacterial ubiA gene in Lithospermum erythrorhizon. Plant and Cell Physiology. 2000;41(8):911–919. doi: 10.1093/pcp/pcd013. [DOI] [PubMed] [Google Scholar]
- Bonora M., Wieckowski M.R., Chinopoulos C., Kepp O., Kroemer G., Galluzzi L., Pinton P. Molecular mechanisms of cell death: Central implication of ATP synthase in mitochondrial permeability transition. Oncogene. 2015;34(12):1475–1486. doi: 10.1038/onc.2014.96. [DOI] [PubMed] [Google Scholar]
- Boulos J.C., Rahama M., Hegazy M.E.F., Efferth T. Shikonin derivatives for cancer prevention and therapy. Cancer Letters. 2019;10(459):248–267. doi: 10.1016/j.canlet.2019.04.033. [DOI] [PubMed] [Google Scholar]
- Brigham L.A., Michaels P.J., Flores H.E. Cell-specific production and antimicrobial activity of naphthoquinones in roots of Lithospermum erythrorhizon. Plant Physiology. 1999;119(2):417–428. doi: 10.1104/pp.119.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmann H. The constitutions of alkannin, shikonin and alkannan. Liebigs Annalen der Chemie. 1936;521:1–47. [Google Scholar]
- Cañamares M.V., Mieites-Alonso M.G., Leona M. Raman, SERS and DFT analysis of the natural red dyes of Japanese origin alkannin and shikonin. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2022;265:1–8. doi: 10.1016/j.saa.2021.120382. [DOI] [PubMed] [Google Scholar]
- Cao H.H., Liu D.Y., Lai Y.C., Chen Y.Y., Yu L.Z., Shao M., Liu J.S. Inhibition of the STAT3 signaling pathway contributes to the anti-melanoma activities of shikonin. Frontiers in Pharmacology. 2020;27(11):1–12. doi: 10.3389/fphar.2020.00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso S.H., de Oliveira C.R., Guimarães A.S., Nascimento J., dos Santos Carmo J.D., de Souza Ferro J.N., Barreto E. Synthesis of newly functionalized 1,4-naphthoquinone derivatives and their effects on wound healing in alloxan-induced diabetic mice. Chemico-Biological Interactions. 2018;291:55–64. doi: 10.1016/j.cbi.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Cespedes C.L., Lina-Garcia L., Kubo I., Salazar J.R., Ariza-Castolo A., Alarcon J., Seigler D.S. Calceolaria integri folias complex, reduces feeding and growth of Acanthoscelides obtectus, and Epilachna varivestis. A new source of bioactive compounds against dry bean pests. Industrial Crops and Products. 2016;30(89):257–267. [Google Scholar]
- Chak K.F., Hsiao C.Y., Chen T.Y. A study of the effect of shiunko, a traditional Chinese herbal medicine, on fibroblasts and its implication on wound healing processes. Advances in Wound Care. 2013;2(8):448–455. doi: 10.1089/wound.2012.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Su H., Zhang D., Wang Y., Shen Q., Liu B., Shen H.M. AMPK-dependent phosphorylation of GAPDH triggers Sirt1 activation and is necessary for autophagy upon glucose starvation. Molecular Cell. 2015;60(6):930–940. doi: 10.1016/j.molcel.2015.10.037. [DOI] [PubMed] [Google Scholar]
- Chen T.C., Yu S.C., Hsu C.M., Tsai F.J., Tsai Y. A water-based topical Chinese traditional medicine (Zicao) for wound healing developed using 2-hydroxypropyl-β-cyclodextrin. Colloids and Surfaces B: Biointerfaces. 2018;165:67–73. doi: 10.1016/j.colsurfb.2018.02.013. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen Z.Y., Chen L., Zhang J.Y., Fu L.Y., Zhang Y., Shen X.C. Shikonin inhibits triple-negative breast cancer-cell metastasis by reversing the epithelial-to-mesenchymal transition via glycogen synthase kinase 3β-regulated suppression of β-catenin signaling. Biochemical Pharmacology. 2019;166:33–45. doi: 10.1016/j.bcp.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Chen Y., Si L., Zhang J., Yu H., Liu X., Chen Y., Wu Y. Uncovering the antitumor effects and mechanisms of shikonin against colon cancer on comprehensive analysis. Phytomedicine. 2021;82 doi: 10.1016/j.phymed.2021.153460. [DOI] [PubMed] [Google Scholar]
- Cheng Y.W., Chang C.Y., Lin K.L., Hu C.M., Lin C.H., Kang J.J. Shikonin derivatives inhibited LPS-induced NOS in RAW 264.7 cells via downregulation of MAPK/NF-κB signaling. Journal of Ethnopharmacology. 2008;120:264–271. doi: 10.1016/j.jep.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Chu, H. (2018). A kind of extracting method of alkannin. Patent No. CN108558631A.
- Chu, H. (2018). A kind of externally applied anti-inflammation agent containing Radix Arnebiae extract. Patent No. CN108143830A.
- Cook D.A., Smith L.W., Law J., Mei W., van Niekerk D.J., Ceballos K., Krajden M. Aptima HPV Assay versus Hybrid Capture® 2 HPV test for primary cervical cancer screening in the HPV FOCAL trial. Journal of Clinical Virology. 2017;87:23–29. doi: 10.1016/j.jcv.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Craft K.M., Nguyen J.M., Berg L.J., Townsend S.D. Methicillin-resistant Staphylococcus aureus (MRSA): Antibiotic-resistance and the biofilm phenotype. Medicinal Chemistry Communications. 2019;10(8):1231–1241. doi: 10.1039/c9md00044e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damianakos H., Kretschmer N., Sykłowska-Baranek K., Pietrosiuk A., Bauer R., Chinou I. Antimicrobial and cytotoxic isohexenyl naphthazarins from Arnebia euchroma (Royle) Jonst. (Boraginaceae) callus and cell suspension culture. Molecules. 2012;17(12):14310–14322. doi: 10.3390/molecules171214310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P.H. Flora of Turkey and the East Aegean Islands. Flora of Turkey and the East Aegean Islands. 1970;3:645. [Google Scholar]
- Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N.…Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature Chemical Biology. 2005;2:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Deurenberg R.H., Stobberingh E.E. The molecular evolution of hospital-and community-associated methicillin-resistant Staphylococcus aureus. Current Molecular Medicine. 2009;9(2):100–115. doi: 10.2174/156652409787581637. [DOI] [PubMed] [Google Scholar]
- Eruygur N., Yılmaz G., Kutsal O., Yücel G., Üstün O. Bioassay-guided isolation of wound healing active compounds from Echium species growing in Turkey. Journal of Ethnopharmacology. 2016;185:370–376. doi: 10.1016/j.jep.2016.02.045. [DOI] [PubMed] [Google Scholar]
- Fan C., Lim L.K., Loh S.Q., Lim K.Y., Upton Z., Leavesley D. Application of “macromolecular crowding” in vitro to investigate the naphthoquinones shikonin, naphthazarin and related analogues for the treatment of dermal scars. Chemico-Biological Interactions. 2019;310:1–14. doi: 10.1016/j.cbi.2019.108747. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Fronza M., Heinzmann B., Hamburger M., Laufer S., Merfort I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. Journal of Ethnopharmacology. 2009;126(3):463–467. doi: 10.1016/j.jep.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Fu J., Lu G., Yang M., Zhao H., Jie W., Fazal A.…Yang Y. Cloning and functional analysis of EpGHQH1 in shikonin production of Echium plantagineum. Plant Cell, Tissue and Organ Culture. 2021;144:533–543. [Google Scholar]
- Guddati A.K. Ovarian cancer stem cells: Elusive targets for chemotherapy. Medical Oncology. 2012;29(5):3400–3408. doi: 10.1007/s12032-012-0252-6. [DOI] [PubMed] [Google Scholar]
- Gümüş K., Özlü Z.K. The effect of a beeswax, olive oil and Alkanna tinctoria (L.) Tausch mixture on burn injuries: An experimental study with a control group. Complementary Therapies in Medicine. 2017;34:66–73. doi: 10.1016/j.ctim.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Guo Y., Zhang M., Xu B. Method for extracting alkannin from arnebia roots. Patent No. 2016 CN105949045A. [Google Scholar]
- Han T., Zhang G., Yan D., Yang H., Ma T., Ye Z. Modulation of plasminogen activator inhibitor-1 (PAI-1) by the naphthoquinone shikonin. Fitoterapia. 2016;113:117–122. doi: 10.1016/j.fitote.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Han W., Li L., Qiu S., Lu Q., Pan Q., Gu Y.…Hu X. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Molecular Cancer Therapeutics. 2007;6(5):1641–1649. doi: 10.1158/1535-7163.MCT-06-0511. [DOI] [PubMed] [Google Scholar]
- Han X., Kang K.A., Piao M.J., Zhen A.X., Hyun Y.J., Kim H.M.…Hyun J.W. Shikonin exerts cytotoxic effects in human colon cancers by inducing apoptotic cell death via the endoplasmic reticulum and mitochondria-mediated pathways. Biomolecules & Therapeutics. 2019;27(1):41–47. doi: 10.4062/biomolther.2018.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, J., Qing, M., & Zhang, S. (2017). Separate the method for preparing natural naphthoquinone compound. Patent No. CN107151203A.
- Heneka M.T. Neuroinflammation in Alzheimer's disease. The Lancet Neurology. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A., Hirst J.A., Fujii H. Regulation of cell proliferation by interleukin-3-induced nuclear translocation of pyruvate kinase. Journal of Biological Chemistry. 2007;282(24):17706–17711. doi: 10.1074/jbc.M700094200. [DOI] [PubMed] [Google Scholar]
- Hu, X. (2011). Application of alkannin in preparation of pyruvate kinase inhibitor. Patent No. CN102068421A.
- Hu, X., & Fang, J. (2005). Use of alkannin in preparing medicine for treating tumor disease. Patent No. CN1579378A.
- Hu, X., & Han, W. (2007). Application of shikonin in preparing medicine for inducing apoptosis. Patent No. CN1931152A.
- Hsiao C.Y., Tsai T.H., Chak K.F. The molecular basis of wound healing processes induced by Lithospermi Radix: A proteomics and biochemical analysis. Evidence-Based Complementary and Alternative Medicine. 2012:1–15. doi: 10.1155/2012/508972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.S., Lin A.H., Yang T.C., Liu K.L., Chen H.W., Li C.K. Shikonin inhibits oxidized LDL-induced monocyte adhesion by suppressing NFκB activation via up-regulation of PI3K/Akt/Nrf2-dependent antioxidation in EA. hy926 endothelial cells. Biochemical Pharmacology. 2015;93(3):352–361. doi: 10.1016/j.bcp.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Huang W., Zeng J., Liu Z., Su M., Li Q., Zhu B. Acetyl shikonin stimulates glucose uptake in L6 myotubes via a PLC-β3/PKCδ-dependent pathway. Biomedicine & Pharmacotherapy. 2019;112:1–9. doi: 10.1016/j.biopha.2019.01.049. [DOI] [PubMed] [Google Scholar]
- Huu Tung N., Du G.J., Wang C.Z., Yuan C.S., Shoyama Y. Naphthoquinone components from Alkanna tinctoria (L.) Tausch show significant antiproliferative effects on human colorectal cancer cells. Phytotherapy Research. 2013;27(1):66–70. doi: 10.1002/ptr.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S.Y., Hong D., Jeong S.Y., Kim J.H. Shikonin causes apoptosis by up-regulating p73 and down-regulating ICBP90 in human cancer cells. Biochemical and Biophysical Research Communications. 2015;465:71–76. doi: 10.1016/j.bbrc.2015.07.131. [DOI] [PubMed] [Google Scholar]
- Jaradat N.A., Zaid A.N., Hussen F., Issa L., Altamimi M., Fuqaha B., Assadi M. Phytoconstituents, antioxidant, sun protection and skin anti-wrinkle effects using four solvents fractions of the root bark of the traditional plant Alkanna tinctoria (L.) European Journal of Integrative Medicine. 2018;21:88–93. [Google Scholar]
- Jha P. Avoidable global cancer deaths and total deaths from smoking. Nature Reviews Cancer. 2009;9(9):655–664. doi: 10.1038/nrc2703. [DOI] [PubMed] [Google Scholar]
- Jiang, H., Lin, Z., & Zhai, J. (2018). Acetylshikonin rapidly and efficiently purification process in Asian puccoon. Patent No. CN108409570A.
- Kaarthigeyan K. Cervical cancer in India and HPV vaccination. Indian Journal of Medical and Paediatric Oncology. 2012;33(1):7–12. doi: 10.4103/0971-5851.96961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefi S., Essid R., Mkadmini K., Kefi A., Haddada F.M., Tabbene O., Limam F. Phytochemical investigation and biological activities of Echium arenarium (Guss) extracts. Microbial Pathogenesis. 2018;118:202–210. doi: 10.1016/j.micpath.2018.02.050. [DOI] [PubMed] [Google Scholar]
- Khan, I., & Abourashed, E. (2010). Encyclopedia of common natural ingredients. http://www.dankunlimited.com/uno_pound_sharing/Know_files/-leung-s-encyclopedia-of-common-natural-ingredients-2010.
- Kim H.J., Hwang K.E., Park D.S., Oh S.H., Jun H.Y., Yoon K.H., Kim Y.S. Shikonin-induced necroptosis is enhanced by the inhibition of autophagy in non-small cell lung cancer cells. Journal of Translational Medicine. 2017;15(1):1–12. doi: 10.1186/s12967-017-1223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.C., Lee C.H., Yook T.H. Effects of anti-inflammatory and Rehmanniae Radix pharmacopuncture on atopic dermatitis in NC/Nga mice. Journal of Acupuncture and Meridian Studies. 2013;6(2):98–109. doi: 10.1016/j.jams.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Kim N.S., Shin S., Park H.S., Kwon H.J., Son H.Y., Bang O.S. Sub-chronic oral toxicity of the aqueous extract of Lithospermi Radix in Fischer 344 rats. Journal of Ethnopharmacology. 2019;235:406–414. doi: 10.1016/j.jep.2019.01.034. [DOI] [PubMed] [Google Scholar]
- Kirkan B., Sarikurkcu C., Ozer M.S., Cengiz M., Atılgan N., Ceylan O., Tepe B. Phenolic profile, antioxidant and enzyme inhibitory potential of Onosmatauricum var. tauricum. Industrial Crops and Products. 2018;125:549–555. [Google Scholar]
- Kuroda C., Wada M. Studies on the constitution of shikonin syntheses of iso-hexyl-naphthazarin and the related compounds. Proceedings of the Imperial Academy. 1936;12(8):239–241. [Google Scholar]
- Lambert A.W., Pattabiraman D.R., Weinberg R.A. Emerging biological principles of metastasis. Cell. 2017;168(4):670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.Y., Choi S.I., Han S.H., Lee Y.J., Choi J.G., Lee Y.S.…Kim G.S. Potential of pseudoshikonin I isolated from Lithospermi Radix as inhibitors of MMPs in IL-1β-induced SW1353 cells. International Journal of Molecular Sciences. 2016;17(8):1–9. doi: 10.3390/ijms17081350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.S., Lee D.Y., Kim Y.B., Lee S.W., Cha S.W., Park H.W., Han S.H. The mechanism underlying the antibacterial activity of shikonin against methicillin-resistant Staphylococcus aureus. Evidence-Based Complementary and Alternative Medicine. 2015:1–10. doi: 10.1155/2015/520578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, F., Haiwei, J., Jiang, H., Zhai, J., Yi, S., & Jiang, H. (2015). Preparation technology of Mount Taishan Radix Lithospermi naphthoquinone active monomers. Patent no. CN104774151A.
- Li J., Zhao M., Liang W., Wu S., Wang Z., Wang D. Codelivery of Shikonin and siTGF-β for enhanced triple negative breast cancer chemo-immunotherapy. Journal of Controlled Release. 2022;342:308–320. doi: 10.1016/j.jconrel.2022.01.015. [DOI] [PubMed] [Google Scholar]
- Li M.Y., Xu Z.T., Zhu C.L., Wang J. Effect of different derivatives of shikonin from Lithospermum erythrorhizon against the pathogenic dental bacteria. Current Pharmaceutical Analysis. 2012;8(3):255–260. [Google Scholar]
- Li Q., Zeng J., Su M., He Y., Zhu B. Acetylshikonin from Zicao attenuates cognitive impairment and hippocampus senescence in D-galactose-induced aging mouse model via upregulating the expression of SIRT1. Brain Research Bulletin. 2018;137:311–318. doi: 10.1016/j.brainresbull.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Li, S., & Hu, K. (2004). Alkannin derivatives as immune inhibitors and metal complexes thereof. Patent No. CN1546450A.
- Li, S., & Zhao, L. (2010). Antineoplastic Alkanna tinctoria ketoximes derivatives. Patent No. CN101265212B.
- Li, S., & Chen, F. (2009). Application of Xinjiang Radix macrotomiae for treating flat wart, common wart and fig wart. Patent No. CN101342170A.
- Li, S., Zhao, L., Xei, T., He, Y., & Guo, X. (2008). Antineoplastic sulfur-containing alkannin and naphthoquinones derivatives. Patent No. CN101239936A.
- Li X., Zeng X. Shikonin suppresses progression and epithelial–mesenchymal transition in hepatocellular carcinoma (HCC) cells by modulating miR-106b/SMAD7/TGF-β signaling pathway. Cell Biology International. 2020;44(2):467–476. doi: 10.1002/cbin.11247. [DOI] [PubMed] [Google Scholar]
- Li X., Fan X.X., Jiang Z.B., Loo W.T., Yao X.J., Leung E.L., Liu L. Shikonin inhibits gefitinib-resistant non-small cell lung cancer by inhibiting TrxR and activating the EGFR proteasomal degradation pathway. Pharmacological Research. 2017;115:45–55. doi: 10.1016/j.phrs.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Li, Y. (2018). Omeprazole enteric-coated capsules capable of inhibiting gastric acid secretion. Patent No. CN107595845A.
- Liang W., Cui J., Zhang K., Xi H., Cai A., Li J.…Wang N. Shikonin induces ROS-based mitochondria-mediated apoptosis in colon cancer. Oncotarget. 2017;8(65):109094–109106. doi: 10.18632/oncotarget.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z., Yan Y., Dong H., Zhu Z., Jiang Y., Cao Y. Endogenous nitric oxide accumulation is involved in the antifungal activity of shikonin against Candida albicans. Emerging Microbes & Infections. 2016;5(1):1–6. doi: 10.1038/emi.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, C., Wang, Y., Wang, L., & Zhang, Y. (2017). Shikonin and derivant thereof are as the application of gene therapy sensitizer. Patent No. CN104127885B.
- Liu, D., Zhao, J., & Wang, L. (2017). Compound traditional Chinese medicine for preventing and treating stigmatosis of freshwater fish. Patent No. CN106692630A.
- Liu, J., & Yu, J. (2017). Application of alkannin in preparation of medicine for treating upper and lower respiratory tract allergic disease. Patent No. CN106389397A.
- Liu, K., & Fan, H. (2014). Medical application of gromwell naphthaquinone compounds. Patent No. CN103919756A.
- Liu, K., & Fan, H. (2014). Medical application of Lithospermum naphthoquinone compounds. Patent No. CN103690518A.
- Liu, K., & Fan, H. (2014). Medical application of Radix Arnebiae seu lithospermi naphthoquinone compounds. Patent No. CN103735538A.
- Liu, L., Leung, L. H., Li, X., & Fan, X. (2018). Hydroxynaphthoquinone compounds for treatment of non-small cell lung cancer. Patent No. US9913811B2.
- Liu T., Li S., Wu L., Yu Q., Li J., Feng J.…Guo C. Experimental study of hepatocellular carcinoma treatment by shikonin through regulating PKM2. Journal of Hepatocellular Carcinoma. 2020;7:19–31. doi: 10.2147/JHC.S237614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., & Liu, K. (2011). Method for extracting alkannin naphthoquinone pigment. Patent No. CN101942212A.
- Liu, Z., Wei, Y., Zhang, H., Zuo, J., Yi, M., & Yang, Y. (2017). Composition for treating burns and scalds. Patent No. CN107149641A.
- Lu P.J., Yang C., Lin C.N., Li C.F., Chu C.C., Wang J.J., Chen J.Y. Shiunko and acetylshikonin promote reepithelialization, angiogenesis, and granulation tissue formation in wounded skin. The American Journal of Chinese Medicine. 2008;36(01):115–123. doi: 10.1142/S0192415X08005631. [DOI] [PubMed] [Google Scholar]
- Lu D., Qian J., Li W., Feng Q., Pan S., Zhang S. β-hydroxyisovaleryl shikonin induces human cervical cancer cell apoptosis via PI3K/AKT/mTOR signaling. Oncology Letters. 2015;10(6):3434–3442. doi: 10.3892/ol.2015.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., Yu C.R., Li W.H., Li W.X. Induction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Research. 2008;18(8):879–888. doi: 10.1038/cr.2008.86. [DOI] [PubMed] [Google Scholar]
- Markowitsch S.D., Vakhrusheva O., Schupp P., Akele Y., Kitanovic J., Slade K.S., Juengel E. Shikonin inhibits cell growth of sunitinib-resistant renal cell carcinoma by activating the necrosome complex and inhibiting the AKT/mTOR signaling pathway. Cancers. 2022;14(5):11–14. doi: 10.3390/cancers14051114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Zhao L., Li C., Shang Q., Lu H., Fu Z., Cao Y. Inhibitory effect of shikonin on Candida albicans growth. Biological and Pharmaceutical Bulletin. 2012;35(11):1956–1963. doi: 10.1248/bpb.b12-00338. [DOI] [PubMed] [Google Scholar]
- Mohammed M. Evaluation of antipyretic, antinocieptive and sedative effects of Tribulus terrestris, Mimosa pigra and Alkanna tinctoria methanolic extracts. Journal of Phytopharmacology. 2016;5:105–107. [Google Scholar]
- Mohapatra S., Varma R.S., Kumar L.M.S., Thiyagarajan O.S., Vijaykumar M., Dilmel K., Patki P.S. Anti-skin ageing activity of napthoquinones from Arnebia nobilis Reichb.f. Natural Products Research. 2016;30:574–577. doi: 10.1080/14786419.2015.1025232. [DOI] [PubMed] [Google Scholar]
- Nasiri E., Hosseinimehr S.J., Hosseinzadeh A.Z., Azadbakht M., Akbari J., Azadbakht M. The effects of Arnebia euchroma ointment on second-degree burn wounds: A randomized clinical trial. Journal of Ethnopharmacology. 2016;189:107–116. doi: 10.1016/j.jep.2016.05.029. [DOI] [PubMed] [Google Scholar]
- Pan, X., Wang, F., Tang, X., Li, S., Wang, C., & Zhou, J. (2013). Extraction method of alkannin. Patent No. CN103373913A.
- Papageorgiou, V. P. (1980). Acylated alkannin or shikonin derivs. - Useful as dermatological, bactericidal and fungicidal medicaments. Patent No. DE2831786A1.
- Papageorgiou V.P., Assimopoulou A.N., Couladouros E.A., Hepworth D., Nicolaou K.C. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angewandte Chemie. 1999;38(3):270–301. doi: 10.1002/(SICI)1521-3773(19990201)38:3<270::AID-ANIE270>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ng J.J., Upton Z., Leavesley D., Fan C. Investigating the effects of shikonin, deoxyshikonin, and (β, β-Dimethylacryl) shikonin on melanoma cell lines. Natural Product Communications. 2020;15(4):1–12. [Google Scholar]
- Papageorgiou V., Assimopoulou A., Ballis A. Alkannins and shikonins: A new class of wound healing agents. Current Medicinal Chemistry. 2008;15:3248–3267. doi: 10.2174/092986708786848532. [DOI] [PubMed] [Google Scholar]
- Park D.G., Woo B.H., Kim H.J., Choi Y.W., Park H.R. Isobutyrylshikonin has a potentially stronger cytotoxic effect in oral cancer cells than its analogue shikonin in vitro. Archives of Oral Biology. 2020:1–34. doi: 10.1016/j.archoralbio.2020.104774. [DOI] [PubMed] [Google Scholar]
- Park J.Y., Kwak J.H., Kang K.S., Jung E.B., Lee D.S., Lee S., Kim S.N. Wound healing effects of deoxyshikonin isolated from Jawoongo: In vitro and in vivo studies. Journal of Ethnopharmacology. 2017;199:128–137. doi: 10.1016/j.jep.2016.10.031. [DOI] [PubMed] [Google Scholar]
- Pavela R. Efficacy of naphthoquinones as insecticides against the house fly, Musca domestica L. Industrial Crops and Products. 2013;43:745–750. [Google Scholar]
- Rajasekar S., Park D.J., Park C., Park S., Park Y.H., Kim S.T., Choi Y.W. In vitro and in vivo anticancer effects of Lithospermum erythrorhizon extract on B16F10 murine melanoma. Journal of Ethnopharmacology. 2012;144:335–345. doi: 10.1016/j.jep.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Rashan L., Hakkim F.L., Fiebig H.H., Kelter G., Merfort I., Al-Buloshi M., Hasson S.S. In vitro anti-proliferative activity of the Rubia tinctorum and Alkanna tinctoria root extracts in panel of human tumor cell lines hasson. Jordan Journal of Biological Sciences. 2018;11(5):489–494. [Google Scholar]
- Rat A., Naranjo H.D., Krigas N., Grigoriadou K., Maloupa E., Alonso A.V., Willems A. Endophytic bacteria from the roots of the medicinal plant Alkanna tinctoria tausch (Boraginaceae): Exploration of plant growth promoting properties and potential role in the production of plant secondary metabolites. Frontiers in Microbiology. 2021;12:1–14. doi: 10.3389/fmicb.2021.633488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocconi R.P., Sullivan P., Long B., Blaize M., Brown J., Arbuckle J., Finan M.A. Treatment of chemotherapy-induced anemia in ovarian cancer patients: Does the use of erythropoiesis-stimulating agents worsen survival? International Journal of Gynecologic Cancer. 2012;22(5):786–791. doi: 10.1097/IGC.0b013e31825104f4. [DOI] [PubMed] [Google Scholar]
- Roma A., Tcheng M., Ahmed N., Walker S., Jayanth P., Minden M.D., Spagnuolo P.A. Shikonin impairs mitochondrial activity to selectively target leukemia cells. Phytomedicine Plus. 2022;100300 [Google Scholar]
- Ruan Z., Liang M., Shang L., Lai M., Deng X., Su X. Shikonin-mediated PD-L1 degradation suppresses immune evasion in pancreatic cancer by inhibiting NF-κB/STAT3 and NF-κB/CSN5 signaling pathways. Pancreatology. 2021;21(3):630–641. doi: 10.1016/j.pan.2021.01.023. [DOI] [PubMed] [Google Scholar]
- Sabokbar A., Tabaraie B., Karimi M.Z., Talebi S. Study of the antifungal activity of shikonin and alcoholic–oily extracts of Iranian Arnebia euchroma L. International Journal of Basic Science in Medicine. 2017;2(2):106–110. [Google Scholar]
- Safavi F., Farimani M.M., Golalipour M., Leung P.C., Lau K.M., Kwok H.F., Lau C.S. Investigations on the wound healing properties of Onosma dichroantha Boiss root extracts. South African Journal of Botany. 2019;125:344–352. [Google Scholar]
- Sarikurkcu C., Sahinler S.S., Ceylan O., Tepe B. Onosmapulchra: Phytochemical composition, antioxidant, skin-whitening and anti-diabetic activity. Industrial Crops and Products. 2020;154:1126–1132. [Google Scholar]
- Shen C.C., Syu W.J., Li S.Y., Lin C.H., Lee G.H., Sun C.M. Antimicrobial activities of naphthazarins from Arnebia euchroma. Journal of Natural Products. 2002;65(12):1857–1862. doi: 10.1021/np010599w. [DOI] [PubMed] [Google Scholar]
- Shishodia S.K., Shankar J. Proteomic analysis revealed ROS-mediated growth inhibition of Aspergillus terreus by shikonin. Journal of Proteomics. 2020;30:1038–1049. doi: 10.1016/j.jprot.2020.103849. [DOI] [PubMed] [Google Scholar]
- Singh B., Sharma R.A. Anti-inflammatory and antimicrobial activity of shikonin derivatives from Arnebia hispidissima (Lehm.) DC. Phytopharmacology. 2012;3(1):68–81. doi: 10.1078/0944-7113-00262. [DOI] [PubMed] [Google Scholar]
- Skrzypczak A., Przystupa N., Zgadzaj A., Parzonko A., Sykłowska-Baranek K., Paradowska K., Nałęcz-Jawecki G. Antigenotoxic, anti-photogenotoxic and antioxidant activities of natural naphthoquinoneshikonin and acetylshikonin and Arnebiae uchroma callus extracts evaluated by the umu-test and EPR method. Toxicology in Vitro. 2015;30(1):364–372. doi: 10.1016/j.tiv.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Song, A. (2002). Gromwell prepn. with wide medical effect and its prepn. Process. Patent No. CN1079239C.
- Song, A. (2004). Process for preparing Arnebia root medicine with broad-spectrum medical functions. Patent no. CN1178678C.
- Su M., Huang W., Zhu B. Acetylshikonin from Zicao prevents obesity in rats on a high-fat diet by inhibiting lipid accumulation and inducing lipolysis. PLoS ONE. 2016;11(1):1–12. doi: 10.1371/journal.pone.0146884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Zhang J., Liu M., Guan L. Alkannin inhibits proliferation, migration and invasion of hepatocellular carcinoma cells via regulation of miR-92a. Biomedicine and Pharmacotheraphy. 2019;114:1–9. doi: 10.1016/j.biopha.2019.108782. [DOI] [PubMed] [Google Scholar]
- Sut S., Pavela R., Kolarčik V., Lupidi G., Maggi F., Dall’Acqua S., Benelli G. Isobutyrylshikonin and isovalerylshikonin from the roots of Onosma visianii inhibit larval growth of the tobacco cutworm Spodoptera littoralis. Industrial Crops and Products. 2017;109:266–273. [Google Scholar]
- Tang, H., Wang, X., & Zhou, T. (2014). Alkannin extraction device. Patent No. CN103664566A.
- Temian D.C., Pop L.A., Irimie A.I., Berindan-Neagoe I. The epigenetics of triple-negative and basal-like breast cancer: Current knowledge. Journal of Breast Cancer. 2018;21(3):233–243. doi: 10.4048/jbc.2018.21.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung N.H., Du G.J., Yuan C.S., Shoyama Y., Wang C.Z. Isolation and chemopreventive evaluation of novel naphthoquinone compounds from Alkanna tinctoria. Anti-Cancer Drugs. 2013;24:1058–1068. doi: 10.1097/CAD.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F. (2008). Method of treatment of virus infections using shikonin compounds. Patent No. US20080182900A1.
- Wang, H., & Chen, Z. (2017). A kind of external application biological agent and preparation method for vagina prevention, health care and treatment gynaecology genital inflammation. Patent No. CN107456509A.
- Wang, S. (2002). Gromwell haematochrome extracting process. Patent No. CN1384149A.
- Utkina N.K., Pokhilo N.D. Antioxidant activity of 1’-hydroxyethylnaphthazarins and their derivatives. Natural Product Communications. 2017;12(12):1885–1888. [Google Scholar]
- Wang, Y., Fang, G., & Wang, X. (2005). Method for promoting Xinjiang Alkanna tinctoria callus growth using rare-earth element. Patent No. CN1633841A.
- Wang X.Q., Kravchuk O., Kimble R.M. A retrospective review of burn dressings on a porcine burn model. Burns. 2010;36(5):680–687. doi: 10.1016/j.burns.2009.06.200. [DOI] [PubMed] [Google Scholar]
- Wei Y., Li M., Cui S., Wang D., Zhang C.Y., Zen K., Li L. Shikonin inhibits the proliferation of human breast cancer cells by reducing tumor-derived exosomes. Molecules. 2016;21(6):777–787. doi: 10.3390/molecules21060777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle P. Watson-Guptil Publications; 1974. Ancient Dyes for Modern Weavers; p. 50. [Google Scholar]
- Winter R. A consumers dictionary of cosmetic ingredients. Crown. 1984;23 [Google Scholar]
- Winter R. A consumers dictionary of food additives. Crown. 1984;25 [Google Scholar]
- Woo J., Das R. Binding mechanisms of shikonin derivatives targeting SARS-CoV-2 main protease. The FASEB Journal. 2022;36 [Google Scholar]
- Xie Y., Fan C., Dong Y., Lynam E., Leavesley D.I., Li K., Upton Z. Functional and mechanistic investigation of shikonin in scarring. Chemico-Biological Interactions. 2015;228:18–27. doi: 10.1016/j.cbi.2014.12.037. [DOI] [PubMed] [Google Scholar]
- Xu J., Koizumi K., Liu M., Mizuno Y., Suzaki M., Iitsuka H., Shimada Y. Shikonin induces an anti-tumor effect on murine mammary cancer via p38-dependent apoptosis. Oncology Reports. 2019;41(3):2020–2026. doi: 10.3892/or.2019.6966. [DOI] [PubMed] [Google Scholar]
- Xu, S., Wang, S., & Huang, M. (2000). Alkannia and its extraction method. Patent No. CN1253972A.
- Yan, X., Xu, X., Yu, B., & Lei, F. (2013). Method for extracting benzoquinone compound in lithospermum. Patent No. CN101906028B.
- Yan Y., Tan F., Miao H., Wang H., Cao Y. Effect of shikonin against Candida albicans biofilms. Frontiers in Microbiology. 2019;10:1–11. doi: 10.3389/fmicb.2019.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., & Yang, Y. (2016). Preparation method for high-purity alkannin from Lithospermum erythrorhizon. Patent No. CN105348065A.
- Yang Y., Gao W., Tao S., Wang Y., Niu J., Zhao P., Yang L. ER-mediated anti-tumor effects of shikonin on breast cancer. European Journal of Pharmacology. 2019;863:1–9. doi: 10.1016/j.ejphar.2019.172667. [DOI] [PubMed] [Google Scholar]
- Yao C.H., Chen K.Y., Chen Y.S., Li S.J., Huang C.H. Lithospermi Radix extract-containing bilayer nanofiber scaffold for promoting wound healing in a rat model. Materials Science and Engineering: C. 2019;96:850–858. doi: 10.1016/j.msec.2018.11.053. [DOI] [PubMed] [Google Scholar]
- Ye, H., Li, G., Song, H., & Chen, J. (1996). Arnebia euchroma (Royle) Johnst. Cell cultivation and prodn. Process by solid two step method. Patent No. CN1117525A.
- Yazdinezhad A., Monsef-Esfahani H., Ghahremani M.H. Effect of Alkanna frigida extracts on 3T3 fibroblast cell proliferation. International Journal of Pharmacy and Biological Sciences. 2013;3:212–215. [Google Scholar]
- Yen C.Y., Chiang W.D., Liu S.Y., Wang K.T., Liao E.C., Hsieh C.L. Lithospermum erythrorhizon extract inhibits Der p2-induced inflammatory response through alleviation of thymic stromal lymphopoietin, nuclear factor Kappa B, and inflammasome expression in human bronchial epithelial cells. Journal of Ethnopharmacology. 2017;201:1–8. doi: 10.1016/j.jep.2017.02.038. [DOI] [PubMed] [Google Scholar]
- Yildirim A.B. Ultraviolet-B-induced changes on phenolic compounds, antioxidant capacity and HPLC profile of in vitro-grown plant materials in Echium orientale L. Industrial Crops and Products. 2020;153:1–7. [Google Scholar]
- Yoon, Y., Lee, D., Jang, J., & Jeong, Y. (2017). Compositions for metabolic disorders comprising alkannin as an active ingredient. Patent No. KR20170142223A.
- Yuan, H., & Wang, X. (2016). Pharmaceutical composition for treating flat wart and verruca vulgaris and preparation method for pharmaceutical composition. Patent No. CN105456315A.
- Yuan, X., & Yuan, P. (2011). Method for separating naphthoquinone active ingredients from Sinkiang arnebia root. Patent No. CN102228499A.
- Yusufoglu H.S., Soliman G.A., Foudah A.I., Abdulkader M.S., Alqarni M.H., Alam A., Salkini M.A. Standardization and antioxidant studies of Arnebia hispidissima. International Journal of Pharmacology. 2018;14:428–436. [Google Scholar]
- Zang F., Rao Y., Zhu X., Wu Z., Jiang H. Shikonin suppresses NEAT1 and Akt signaling in treating paclitaxel-resistant non-small cell of lung cancer. Molecular Medicine. 2020;26:1–7. doi: 10.1186/s10020-020-00152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Zhu B.H. Arnebin-1 promotes the angiogenesis of human umbilical vein endothelial cells and accelerates the wound healing process in diabetic rats. Journal of Ethnopharmacology. 2014;154(3):653–662. doi: 10.1016/j.jep.2014.04.038. [DOI] [PubMed] [Google Scholar]
- Zhang L.L., Zhan L., Jin Y.D., Min Z.L., Wei C., Wang Q., Yuan Q. SIRT2 mediated antitumor effects of shikonin on metastatic colorectal cancer. European Journal of Pharmacology. 2017;797:1–8. doi: 10.1016/j.ejphar.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Zhang N., Peng F., Wang Y., Yang L., Wu F., Wang X.…He G. Shikonin induces colorectal carcinoma cells apoptosis and autophagy by targeting galectin-1/JNK signaling axis. International Journal of Biological Sciences. 2020;16(1):147–161. doi: 10.7150/ijbs.36955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Gao Q., Li W., Zhu L., Shang Q., Feng S., Su Z. Shikonin inhibits cancer cell cycling by targeting Cdc25s. BMC Cancer. 2019;19(1):1–9. doi: 10.1186/s12885-018-5220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sun S., Xu W., Yang R., Yang Y., Guo J., Xu J. Thioredoxin reductase 1 inhibitor shikonin promotes cell necroptosis via SecTRAPs generation and oxygen-coupled redox cycling. Free Radical Biology and Medicine. 2022;180:52–62. doi: 10.1016/j.freeradbiomed.2021.12.314. [DOI] [PubMed] [Google Scholar]
- Zhao X., Zhou Y., Wang L., Li M., Shi D., Li D., Wang J. Shikonin alleviates the biotoxicity produced by pneumococcal pneumolysin. Life Sciences. 2017;177:1–7. doi: 10.1016/j.lfs.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Zhuang, H., & Zhang, C. (2017). Herqueiazole-containing medicine for controlling inflammation. Patent No. CN106562959A.
- Zu, Y., Yang, L., Zhang, L., Liu, T., Wang, H., Shi, Z., Zhao, C., Yang, F., & Sun, Z. (2009). Method for extracting alkannin from alkanet. Patent No. CN101434530A.