Abstract
Zedoary tumeric (Curcumae Rhizoma, Ezhu in Chinese) has a long history of application and has great potential in the treatment of liver cancer. The antiliver cancer effect of zedoary tumeric depends on the combined action of multiple pharmacodynamic substances. In order to clarify the specific mechanism of zedoary tumeric against liver cancer, this paper first analyzes the mechanism of its single pharmacodynamic substance against liver cancer, and then verifies the joint anti liver cancer mechanism of its “pharmacodynamic group”. By searching the research on the antihepatoma effect of active components of zedoary tumeric in recent years, we found that pharmacodynamic substances, including curcumol, zedoarondiol, curcumenol, curzerenone, curdione, curcumin, germacrone, β-elemene, can act on multi-target and multi-channel to play an antihepatoma role. For example, curcumin can regulate miR, GLO1, CD133, VEGF, YAP, LIN28B, GPR81, HCAR-1, P53 and PI3K/Akt/mTOR, HSP70/TLR4 and NF-κB. Wnt/TGF/EMT, Nrf2/Keap1, JAK/STAT and other pathways play an antihepatoma role. Network pharmacological analysis showed that the core targets of the “pharmacodynamic group” for anti-life cancer are AKT1, EGFR, MAPK8, etc, and the core pathways are neuroactive live receiver interaction, nitrogen metabolism, HIF-1 signaling pathway, etc. At the same time, by comparing and analyzing the relationship between the specific mechanisms of pharmacodynamic substance and “pharmacodynamic group”, it is found that they have great reference significance in target, pathway, biological function, determination of core pharmacodynamic components, formation of core target protein interaction, in-depth research of single pharmacodynamic substance, increasing curative effect and so on. By analyzing the internal mechanism of zedoary tumeric pharmacodynamic substance and “pharmacodynamic group” in the treatment of liver cancer, this paper intends to provide some ideas and references for the deeper pharmacological research of zedoary tumeric and the relationship between pharmacodynamic substance and “pharmacodynamic group”.
Keywords: action mechanism, Curcumae Rhizoma, liver cancer, pharmacodynamic groups, pharmacodynamic substances, zedoary turmeric
1. Introduction
Liver cancer is the sixth most common invasive cancer in the world and the third leading cause of cancer death. Asia is one of the regions with the highest number of liver cancer patients in the world. Among them, China accounts for more than 50% of the number of liver cancer patients in the world, and the 5-year survival rate of liver cancer patients in China is only 12.1% (Li et al., 2020, Watanabe et al., 2021, Zeng et al., 2018). There are many factors inducing liver cancer, including hepatitis virus infection, alcoholic liver injury, nonalcoholic fatty liver disease, liver fibrosis, obesity, diabetes, and so on. Liver cancer tumors are mostly found in the middle and late stages. They lose the chance of liver transplantation or radical surgery. Radiotherapy and chemotherapy usually fail to achieve satisfactory results. The incidence rate and mortality rate are close to 1, which has caused serious medical and social burden worldwide (Scaglione et al., 2020, GBD, 2013). Nowadays, the treatment methods of liver cancer mainly include surgical resection, liver transplantation, radiotherapy, chemotherapy, targeted therapy, and so on. However, the above treatments have some problems in the clinic, such as large toxic and side effects, high requirements for organ function, easy recurrence, easy drug resistance and so on. Therefore, it is very important to continue to explore and develop more effective treatment measures for liver cancer. At present, traditional Chinese medicine can significantly reduce the toxic and side effects of treatment methods, prolong the survival time of patients, improve the treatment effect, and improve the quality of life of patients (Yuan et al., 2017, Li, 2016). Traditional Chinese medicine has the outstanding curative effects in anti-tumor. A variety of traditional Chinese medicine components have been used in the cancer clinics. New drugs with the anti-tumor effect of traditional Chinese medicine have great research and development value.
Zedoary tumeric (Curcumae Rhizoma, Ezhu in Chinese) is the dry rhizome of Curcuma phaeocaulis Val., Curcuma kwangsiensis S. G. Lee et C. F. Liang or Curcuma wenyujin Y. H. Chen et C. Ling, and sesquiterpenoids are its main active components and pharmacodynamic substance. Modern studies show that zedoary tumeric has the effects of antithrombotic, antitumor, antibacterial, anti-inflammatory, and anti-virus activities. Zedoary tumeric is good at breaking blood and Qi, soothing the liver and relieving depression, protecting the liver and anti-tumor. At present, there are many studies, and the outstanding curative effect of zedoary tumeric in liver cancer has also been confirmed. Some active components, including curcumol, zedoarondiol, curcumenol, curdione, curcumin, germacrone β-elemene, have been proved to exist in a large number in zedoary tumeric (Gao et al., 2017, Chen et al., 2008, Xu et al., 2015, Matsuda et al., 1998, Lou et al., 2009), and it has also been reported that these components have significant anticancer activity (Mao et al., 2013, Pan et al., 2018, Ahmed Hamdi et al., 2014, Zeng et al., 2012, Feng et al., 2021), and these components have been identified as quality markers of liver protection and anticancer of zedoary tumeric (Li, Cao, & Hao, 2021). The research idea of this paper is shown in Fig. 1. At present, the pharmacodynamic substance of zedoary tumeric in the treatment of liver cancer has been determined and verified, but there is still a lack of in-depth and systematic comprehensive analysis of these substances. Therefore, based on the above research basis, by integrating the validated pharmacodynamic substances of zedoary tumeric anti-liver cancer, the internal mechanism of these pharmacodynamic substances play the role of anti liver cancer in zedoary tumeric as a whole, and the relationship between these pharmacodynamic substances and “pharmacodynamic group” anti liver cancer is analyzed. It provides certain reference value and ideas and methods for the follow-up development and application of zedoary tumeric and the research of traditional Chinese medicine pharmacodynamic substance and “pharmacodynamic group”.
Fig. 1.
Research strategy of internal mechanism of zedoary turmeric in treatment of liver cancer.
2. Mechanism of zedoary tumeric pharmacodynamic substances in treatment of liver cancer
2.1. Curcumin
Curcumin, a diphenylmethane compound, is a hydrophobic polyphenol. It shows a good application prospect in the treatment of liver diseases. It has the ability to regulate a variety of cancer signal pathways controlling differentiation, growth, and malignant transformation, and plays an anti liver cancer role through a variety of molecular mechanisms (Yong & Peng, 2014). Curcumin can induce the production of reactive oxygen species in human cancer cells and arrest the cell cycle in G0/G1 phase (Wang et al., 2018). Because curcumin has strong anticancer activity and can inhibit a variety of cancers, scholars have studied it widely. The specific mechanisms of curcumin in treating liver cancer are as follows.
2.1.1. Regulation of gene expression
MicroRNA (MIR) family genes affect the insulin-like growth factor-1 (IGF-1) / IGF-1 receptor signal axis and regulate the development and metastasis of cancer (Shastri et al, 2020). Curcumin can play an anticancer role by bidirectional regulating the expression of MIR. Curcumin may regulate the biological process of liver cancer cells by up-regulating the expression of mir-29 and reducing the expression of vascular endothelial growth factor (VEGF) (Chen, Xu, & Zhao, 2020). Curcumin can also down-regulate the expression of miR-21, up-regulate the expression of TIMP3 and inhibit the transforming growth factor-β 1(TGF-β1)/recombinant human mothers against decapentaplegic homolog 3 (Smad3) signal pathway and can inhibit the proliferation of liver cancer HepG2 and HCCLM3 cells (Li et al., 2020).
Glo1 is widely distributed in the cytoplasm and participates in the detoxification of methylglyoxal. It is overexpressed in a variety of cancers. Its overexpression is related to multidrug resistance (MDR) in cancer chemotherapy (Wang et al., 2012). Curcumin can inhibit the proliferation of liver cancer HepG2 cells by inhibiting the activity of Glo1, resulting in the increase of methylglyoxal content (He, Tang, & Huang, 2020).
CD133 can recognize tumor-initiating cells and cancer stem cells. CD133 can be used as a biomarker for detecting liver cancer (Jun et al., 2019). Curcumin can inhibit HepG2 proliferation of liver cancer cells by reducing the expression of HepG2 stem cell marker CD133 and reducing the expression of Oct4 (Wen, Jiang, & Fan, 2019).
VEGF and its receptor VEGFR2 play an important role in angiogenesis. The expression level of VEGF also reflects the growth level of the tumor. VEGF can promote tumor growth by promoting new capillaries (Di et al., 2017, Bai et al., 2020). In today's new target research of tumor treatment, looking for drugs to inhibit tumor angiogenesis has become a research trend. Because such drugs do not produce drug resistance, they have become an ideal drug for tumor treatment and have high research and development value. The level of VEGF in patients with liver cancer is significantly increased. Studies have shown that curcumin can inhibit the proliferation of hepatocellular carcinoma (HCC) cells in vivo and in vitro by reducing the expression of VEGF (Pan et al., 2018).
Overexpression of the Yap gene is easy to cause liver enlargement and tumorigenesis. Therefore, regulating Yap expression is very important to maintain liver homeostasis. Studies have confirmed that curcumin can promote oxidative stress and induce apoptosis of hepg2 liver cancer cell line by downregulating Yap expression (Yu, Zhang, & Gu, 2020).
LIN28B can participate in the process of tumorigenesis and development. Its expression level is significantly downregulated during cell differentiation, and plays the role of Oncogene by promoting malignant transformation. It is overexpressed in human liver cancer cells. LIN28B is involved in liver cancer by downregulating the expression of a variety of tumor-related Mir (Ma, Zhao, Chen, & Zhang, 2018). Curcumin can reduce the chemoresistance of liver cancer Hep3B and HepG2 cells by downregulating the expression of LIN28B (Tian et al., 2019).
Lactic acid regulates the DNA repair mechanism of the MDR family by stimulating its G protein-coupled receptor 81 (GPR81) / hydroxycarboxylic acid receptor-1 (hcar-1) (Wagner, Kania, Blauz, & Ciszewski, 2017). MDR-1 can induce cancer cell apoptosis through hcar-1 signal. The expression of MDR-1 was also correlated with the expression of hypoxia-inducible factor-1 (HIF-1). Curcumin can down-regulate the expression of hcar-1 / GPR81 and promote the apoptosis of liver cancer HepG2 cells (Wagner, Kania, Blauz, & Ciszewski, 2017).
As an important tumor suppressor gene in the course of cancer, p53 can prevent cancer cell proliferation and induce apoptosis. Studies (Fu et al., 2018, Li et al., 2015) have shown that curcumin can up-regulate the expression of p53 and activate the corresponding tumor suppressor pathway to promote the apoptosis of cancer cells.
2.1.2. Regulation of signal pathway
2.1.2.1. PI3K / protein kinase B (Akt) / mTOR signaling pathway
Phosphotidylinoside-3-kinases (PI3K) and its downstream threonine-protein kinase (Akt) and mammalian target of rapamycin (mTOR) constitute the PI3K/Akt/mTOR signal pathway. Studies have confirmed that this signal pathway is related to the occurrence and development of cancer and plays an important role in the growth, proliferation, diffusion, metastasis, and apoptosis of cancer cells (Hassan, Akcakanat, Holder, & Meric-Bernstam, 2013). The anti-liver cancer effect of curcumin depends on this pathway. Curcumin can also inhibit the activation of the PI3K/Akt signaling pathway, down-regulate the expression of PI3K/Akt protein, and reduce the tolerance of cells to chemotherapeutic drug adriamycin (Wang et al., 2018, Wei and Liu, 2018).
2.1.2.2. External heat shock protein 70 (HSP70) / toll like receptor 4 (TLR4) signaling pathway
HSP70 can stimulate the expression of TLR4 on the surface of immune cells and tumor cells, and the combination of them activates nuclear transcription factor-κB (NF-κB). NF promotes the transcription of cytokines, chemokines and growth factors-κB pathway and affects the process of tumorigenesis and development. Studies have shown that curcumin can inhibit NF-κB transcription (Lim et al., 2016). Curumin can also inhibit HSP70/TLR4 signal transduction, inhibit tumor cells by reducing the level of inducible heat shock protein 70 (ehsp70), and thus inhibit NF induced by TLR4 Signal transduction-κB pathway (Ren et al., 2018).
2.1.2.3. NF-κB signal path
Modern research found that the NF-κB signaling pathway is frequently up-regulated in the development of liver disease and liver cancer, and this pathway can protect cancer cells from stress induction, so as to play a protective role against cancer cells. In addition, activation of the NF-κB signaling pathway is often associated with HCC progression, while disruption of the NF-κB signaling pathway has been shown to induce the development of tumor promoter cells (TICs) in liver cancer (Pai and Sukumar, 2020, He and Karin, 2011). Studies have shown that curcumin specifically destroys NF-κB signaling pathway, which is a potential treatment for hepatocellular carcinoma with poor prognosis (Marquardt et al., 2015).
2.1.2.4. Wnt/ β-catenin and transforming growth factor (TGF) / epithelial mesenchymal transition (EMT) pathway
Unc119 can regulate the expression of cyclin D1 (CCND1) and cyclin E1 (CCNE1) and promote cell proliferation, while Wnt/β-abnormal activation of the catenin signaling pathway is associated with many tumors, including HCC (Lei et al., 2015, Qiu et al., 2018). Studies have shown that curcumin activates Wnt by inhibiting unc119 expression/ β-catenin signaling pathway and TGF / EMT pathway play a role in inhibiting liver cancer (Zhao, Malhotra, & Seng, 2019).
2.1.2.5. Nrf2 / Keap1 signal path
Nuclear transcription factor E2 related factor 2 (Nrf2) is a cytoplasmic transcription factor that can regulate antioxidant and stress-related enzymes. When combined with Nrf2, kelch-like epichlorohydrin-associated protein 1 (Keap1) can accelerate Nrf2 ubiquitination and proteasome-dependent degradation (Mishra, Zhong, & Kowluru, 2014). Nrf2 / Keap1 pathway plays an important role in tumor development and application of tumor drug resistance. Inhibition of Nrf2 activation can be used as an important strategy to inhibit tumor growth and overcome chemoresistance (Jeddi, Soozangar, Sadeghi, Somi, & Samadi, 2017). Curcumin can reduce the expression of Keap1 protein and mRNA, increase the expression of Nrf2 protein and mRNA, and activate the signal of downstream antioxidant response elements, so as to reduce liver injury in liver cancer mice (Mu, Jin, & Chen, 2020). Some studies have also confirmed that curcumin can inhibit the expression of connective tissue growth factors by upregulating Nrf2 and achieving the protective effect on HCC (Shao et al., 2019).
2.1.2.6. JAK / STAT signal path
Janus kinase/signal transducers and activators of the transcription (JAK/STAT) signal pathway can regulate cytokine-mediated immune response. Stat molecular pathway is also involved in cell proliferation and apoptosis. The imbalance of this pathway is considered to be an important inducement for the formation of various cancers. Abnormal activation of signal transducers and activators of the transcription 3 (STAT3) signal pathway promotes the occurrence and progression of tumors. JAK/STAT signal is the target of cancer treatment and drug research and development. Studies have shown that curcumin can not only inhibit JAK/STAT3 phosphorylation, but also inhibit the expression of downstream targets of STAT3, such as cyclin B1, BCL XL, VEGF, intercellular adhesion molecule-1 (ICAM-1) (Xu and Zhu, 2017). Curcumin can also up-regulate endoplasmic reticulum stress marker protein glucose regulatory protein 78 (GRP78), phosphorylated eukaryotic initiation factor 2 by inhibiting STAT3 expression α (p-elF2 α), phosphorylated c-Jun amino-terminal enzyme (p-JNK), CCAAT enhancer-binding protein homologous protein (CHOP), cysteine containing aspartate proteolytic enzyme-4 (Caspase-4) expression, down-regulate the expression of p-STAT3, and then induce apoptosis of human liver cancer BEL-7404 cells (Zhang, Zhang, & Shen, 2020). Other studies have shown that blocking its anti-apoptotic function by blocking the phosphorylation of stat3ser727 site or the nuclear localization of pser727 STAT3 protein is a new strategy for the treatment of chemotherapy resistance induced by human liver cancer sodium glycine deoxycholate (gcda) (Wang et al., 2020).
2.1.2.7. MAPK signal path
Mitogen-activated protein kinase (MAPK) is a group of serine-threonine protein kinases composed of p38 MAPK, c-Jun N-terminal kinases (JNK), and extracellular signal-regulated kinases (ERK). As an important transmitter from the cell surface to the nucleus, this signal cascade controls a variety of biological processes, including cell proliferation, immune response, and carcinogenesis. Studies have shown that curcumin can activate p38 MAPK and JNK pathways, and the inhibitors of p38 MAPK and JNK can inhibit curcumin-induced apoptosis protease cysteine protease-9/-3 (Caspases-9/-3). Curcumin can also inhibit p38MAPK phosphorylation, then inhibit the proliferation of liver cancer-resistant cell HepG2/ADM, and reverse its adriamycin resistance (Yu et al., 2016, Cao et al., 2020).
2.2. Curcumol
Curcumol is a guaiacane sesquiterpene natural compound. As a characteristic active component of zedoary tumeric, curcumol has strong anticancer activity. Curcumol can inhibit the cell proliferation by blocking cell cycle in G0/G1 and G2/M phases (Huang, Wang, & Lu, 2013). Because of its obvious curative effect and broad development prospect, it has become a research hotspot of scholars. Its specific anticancer mechanism is as follows.
2.2.1. Regulation of gene expression
The latest research shows that curcumol can inhibit the expression of COX-2, VEGF (Tang & Li, 2007), p-pi3k, p-Akt (Wu, Li, & Peng, 2019), cyclin A1 (Huang et al., 2013), up-regulate the expression of Caspase-3 (Wu et al., 2019), p21 WAF1, p27kip1 and Cdk8 (Huang et al., 2013), so as to promote the proliferation and apoptosis of life cancerhepg2.
Curcumol can also regulate the expression of a variety of tumor suppressor genes and proto-oncogenes. EZH2 protein has histone methyltransferase activity and can regulate the development of cancer. Abnormal activation of EZH2 can inhibit the normal expression of tumor suppressor genes. Curcumol can inhibit cancer genes by inhibiting the expression of EZH2. Isocitrate dehydrogenase (IDH) is a key enzyme in the tricarboxylic acid cycle. IDH gene mutation will cause tumors (Zhou et al., 2018). IDH1 is the expression product of IDH, which can promote NADPH and α-formation of ketoglutarate (Liu & Ling, 2015). Curcumol can downregulate oncogene IDH1 and up-regulate reactive oxygen species (ROS) in tumor cells, so as to achieve the effect of tumor inhibition (Zang, 2017). PCNA and p27 are important tumor suppressor genes regulating the cell cycle. Curcumol can down-regulate the expression of the PCNA gene and upregulate the expression of the tumor suppressor gene p27 (Ma, 2015). P53 and p73, as characteristic tumor suppressor genes, can induce cell cycle arrest and apoptosis (Sun, Han, & Wang, 2001). Curcumol can up-regulate the expression of oncogene p73 and activate the expression of tumor suppressor gene p53 (Huang et al., 2017).
2.2.2. Regulation of signal pathway
Modern studies have shown that curcumol inhibits PI3K/Akt (Wu et al., 2019), Raf/MEK/ERK (Guo, 2018), microRNA-30a-5p and hippo (Yu et al., 2021) signaling pathways, and activates PRB (Huang et al., 2013, Huang et al., 2018) pathways, so as to play an anti liver cancer role.
2.2.3. Reducing liver injury
Liver diseases such as liver injury, liver fibrosis, and hepatitis are the great inducements of liver cancer. Curcumol can reduce chronic liver injury by reducing the expression of Kr ü ppel like factor 5 (KLF5). It can also reduce the production of reactive oxygen species in mitochondria of sinusoidal endothelial cells (LSEC) and improve the morphology of LSEC mitochondria. In addition, curcumol can inhibit KLF5 mediated LSEC angiogenesis by inhibiting reactive oxygen species / ERK signal transduction (Gao et al., 2021). Recent studies have also found that curcumol may protect the liver from the phenotypic changes of early and late fibrosis through the urokinase plasminogen activator/urokinase type plasminogen activator receptor pathway (Li et al., 2020).
2.2.4. Others
The anticancer effect of curcumol also depends on its effect on the invasion, migration, and metabolism of tumor cells. The migration and invasion of malignant tumors are the main causes of poor prognosis, clinical treatment failure, and death. The occurrence, invasion, and metastasis of tumors is a complex and multi-step process of multi-factor and multi-gene coordination. The inactivation of the tumor suppressor gene is closely related to tumor metastasis and invasion. Curcumol can induce TGF by regulating E-cadherin and N-cadherin-β 1 mediated epithelial-mesenchymal transformation, which affects the migration of cancer cells. The process of cell proliferation is accompanied by nucleic acid metabolism. If nucleic acid metabolism is inhibited, the process of cell proliferation will be affected accordingly. Curcumol also has a direct antitumor effect, which will affect the synthesis of nucleic acid, and the mechanism may be related to its inhibition of cellular RNA synthesis (Yan et al., 2018).
2.3. β-Elemene
β-Elemene is a national class II non-cytotoxic anti-tumor drug. β-Elemene Emulsion Injection with elemene as the main component has been approved as a class II new anticancer drug by the state in 1995. β-Elemene can inhibit tumor proliferation by inducing apoptosis, blocking tumor cells from G0 and G1 to S phase and from S phase to G2 and M phase, so as to reduce the ability of cell division and proliferation (Li, Mo, & Zhao, 2018).
β-Elemene not only exerts an anticancer effect, but also enhances immune function. In the clinical application of liver cancer, β-elemene can significantly reduce the symptoms of patients with liver cancer and delay the course of the disease. It has great development and promotion value. Its specific mechanisms are as follows.
2.3.1. Effect on gene expression
The latest research found that it can down-regulate the expression of survivin mRNA, survivin (Li, Mo, & Zhao, 2018) and Bcl-2 (Tong, 2013) protein in tumor tissues and upregulate the expression of Caspase-3 mRNA, Caspase-3 (Tong, 2013) and p53 (Di et al., 2017) protein, so as to promote cancer cell apoptosis and play an anti-tumor role. β-Elemene can effectively inhibit the invasion and metastasis of human liver cancer cells by reducing the expression of matrix metalloproteinase-2 (MMP-2) and MMP-9 protein (Li & Zhuang, 2012).
2.3.2. Impact on signal pathway
Current research shows that β-lemene can adjust ERK, Akt, Wnt and glial matching factor β (GMF β)-anti mitogen-activated protein kinase 3/6 (mkk3/6) signaling pathway and induces apoptosis (Que, 2015). β-Elemene can block the Notch signaling pathway during the differentiation of endothelial progenitor cells (EPC), thus inhibiting EPC's participation in tumor angiogenesis (Meng, 2010).
2.3.3. Others
β-Elemene can also enhance the immune function and play an anti-tumor role by inducing a cellular immune response, mediator fluid immune response, regulating cytokines (interleukin (IL), interferon (IFN) and tumor necrosis factor (TNF)), and enhancing the immunogenicity of tumor cells (Shen & Ni, 2014).
2.4. Germacrone
Germacrone is a monocyclic sesquiterpene compound with strong antitumor activity. It can inhibit the proliferation and promote apoptosis of liver cancer cells. Its antitumor mechanism is mainly to induce cancer cell apoptosis and block G2 / M cycle (Liu et al., 2013, Wang, 2013, Fang, 2013, Liu, 2013). Some studies (Wang, 2013, Fang, 2013, Liu, 2013) also found that germacrone selectively inhibited the proliferation of liver cancer cells. The concentration of germacrone is 80–240 μmol/L can promote the apoptosis of liver cancer cells, and it is expected to make compound preparations alone or with other substances for the prevention or treatment of liver cancer.
2.4.1. Effect on gene expression
Germacrone can inhibit the proliferation of liver cancer cells and promote the apoptosis of liver cancer cells by upregulating p53 and Bax (Liu et al., 2013, Sadia et al., 2014), downregulating Bcl-2 (Liu et al., 2013, Sadia et al., 2014) and cyclinB1 (Wang, 2013, Fang, 2013, Liu, 2013).
2.4.2. Impact on signal pathway
Studies have confirmed that germacrone can induce apoptosis of human liver cancer cells by inhibiting JAK2/STAT3 signaling pathway (Liu et al., 2013).
2.5. Curdione
Curdione, as one of the main active components of zedoary tumeric, can inhibit tumor cell proliferation and invasion, promote tumor cell apoptosis, and block cell cycle (Jiang and Wang, 2021, Zhang et al., 2021). Curdione can regulate MAPK and Akt signaling pathways, downregulate the phosphorylation levels of ERK, JNK, and Akt proteins, and reduce the expression of MMP2 and MMP-9 (Sun, Jahnke, & Lv, 2019).
2.6. Curzerenone, zedoarondiol and curcumenol
It is reported that curzerenone, zedoarondiol and curcumenol also have strong antitumor effects. They also have protective effects on acute liver injury. They can induce the production of nitric oxide by macrophages in vivo, inhibit the cytotoxic activity of galactosamine and galactosamine induced liver injury, and protect tumor necrosis factor-α. It also has an obvious inhibitory effect (Zeng et al., 2012, Feng et al., 2021). However, there are few studies on these active ingredients and a lack of specific mechanism research. They have great research and development prospects in anti-tumor.
The above analysis shows that curcumol, zedoarondiol, curcumenol, curzerenone, curdione, curcumin, germacrone, β-elemene are the pharmacodynamic substance of zedoary tumeric with high research significance and development value. These components can play a role in the treatment of liver cancer by acting on multi-target and multi-channel, which shows a synergistic effect in the treatment of liver cancer. Among these compounds, curcumin and zedoary tumeric alcohols are widely studied. Curcumin has a wide range of pharmacological activities and has become one of the research hotspots. It shows good therapeutic potential in malignant tumors, rheumatic diseases, neuroinflammatory diseases, infectious diseases, atherosclerosis, and myocardial infarction. zedoary tumeric alcohol, as a characteristic component of zedoary tumeric, has strong anti-inflammatory and antiviral activities. However, the bioavailability of curcumin and zedoary tumeric alcohol is low. Therefore, at present, more studies have formed new derivatives with higher bioavailability by modifying their structures, which is of great significance to their subsequent research and development.
3. Mechanism of zedoary turmeric pharmacodynamic group in treatment of liver cancer
3.1. Research methods
3.1.1. Prediction of potential targets of zedoary turmeric pharmacodynamic group
Obtain the SMILES number of active components in the Pubchem database, import the compound SMILES number into Swiss targetprediction database for target prediction, and obtain the target information corresponding to each active compound by Tcmsp database. The SwissTargetPrediction database and TCMSP database were integrated to obtain the target information related to the chemical composition of zedoary tumeric, so as to obtain the potential target library of active compounds.
3.1.2. Collection of liver cancer disease targets and screening of common targets of pharmacodynamic group action targets and disease targets
The keyword “liver cancer” was retrieved from the Genecards database to obtain relevant disease targets and gene libraries. The active ingredients and liver cancer-related genes were used to obtain the common targets for the treatment of liver cancer by using the online Wayne analysis tool.
3.1.3. Construction of protein protein interaction (PPI) network and analysis of key targets
Import the integrated target information into the String 10 database, limit the species to “Homo sapiens”, set the minimum interaction threshold to medium “medium confidence” (0.4), obtain PPI information, import the file into Cytoscape 3.7.2 software, draw PPI network, analyze network topology parameters and screen out key protein targets.
3.1.4. Go biological function annotation and KEGG pathway enrichment analysis
Metascape database was used for go gene enrichment analysis and signal pathway KEGG enrichment analysis of zedoary tumeric anti-liver cancer target, and bioinformatics platform medium and high-level bubble diagram was used for visual analysis of biological function and pathway.
3.1.5. Construction of compound target pathway molecular pharmacological network
The target contained in the first 20 pathways and the compounds corresponding to the target is used to construct the compound target pathway network. First, the data of the compounds, targets and pathways are input into the Excel table, and then the Excel data are imported into Cytoscape 3.7.2 software for visual processing.
3.1.6. Molecular docking verification
Download the core target protein structures from the PDB database (http://www.rcsb.Org/), use PyMOL 2.3.4 software to remove water from the core protein and separate the original ligand. After saving, import Autodock 4.2.6 software for hydrogenation, set the atomic type, and save it in “pdbqt” format. Download the small molecular structure in the tcmsp database, save it as a “mol2” structure, and use autodock for molecular docking of core components.
3.2. Research results
3.2.1. Zedoary tumeric pharmacodynamic group target screening results
In combination with the above screened pharmacodynamic substances, we obtained the targets of curcumol, zedoarondiol, curcumenol, curzerenone, curdione, curcumin, germacrone, β-elemene (Fig. 2) through SwissTargetPrediction and Tcmsp databases, and 228 common targets were obtained after integration.
Fig. 2.
Chemical composition structures of zedoary tumeric pharmacodynamic substance.
3.2.2. Prediction of component targets and disease targets of liver cancer
A total of 16730 targets related to liver cancer were retrieved from the Genecards database. After Wayne’s analysis of their potential target genes, it was found that most of the targets in zedoary tumeric pharmacodynamic substance coincided with the disease targets of liver cancer, indicating that these components have an important targeted therapeutic effect on liver cancer to a certain extent. There are 219 targets in the two sets, that is, the potential target of zedoary tumeric pharmacodynamic substance for the treatment of liver cancer. The Wayne diagram is shown in Fig. 3.
Fig. 3.

Wayne diagram of zedoary tumeric pharmacodynamic substances and liver cancer targets.
3.2.3. Protein protein interaction (PPI) network analysis of potential targets of liver cancer treated by zedoary tumeric pharmacodynamic group
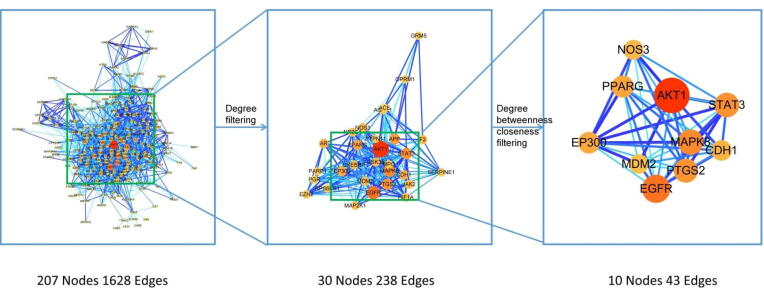
The PPI network in zedoary tumeric anti liver cancer involves 207 nodes and 1628 edges, and the average degree is 15.2. In Fig. 4, the node degree value is taken as the evaluation parameter. The higher the node degree is, the larger the target and the darker the connecting line color is, which also shows that it is more important in the PPI network and may play an important role in playing biological functions. In order to find out the core target of zedoary tumeric pharmacodynamic substance, firstly, a PPI network with 30 nodes and 238 edges is screened according to twice the degree value, and then a PPI network with 10 nodes and 43 edges is screened according to the conditions that the degree is greater than 34, the betweenness is greater than 7.543 and the closeness is greater than 0.674. The core targets involved include AKT1, PTGS2, CDH1, STAT3, MAPK8, MDM2, EGFR, PPARG, EP300 and NOS3. The screening process is shown in Fig. 4, and Table 1 for specific target and topology parameters.
Fig. 4.
Screening of core targets in PPI network of zedoary turmeric pharmacodynamic substance for treatment of liver cancer.
Table 1.
Core targets and topology parameters.
| Genes | Degree | Betweenness | Closeness |
|---|---|---|---|
| AKT1 | 107 | 83.78585304 | 0.966666667 |
| PTGS2 | 56 | 26.30701521 | 0.805555556 |
| CDH1 | 40 | 9.702425352 | 0.743589744 |
| STAT3 | 58 | 39.42779443 | 0.878787879 |
| MAPK8 | 62 | 48.46911422 | 0.90625 |
| MDM2 | 35 | 8.162692863 | 0.743589744 |
| EGFR | 74 | 25.76998002 | 0.828571429 |
| PPARG | 47 | 22.61500167 | 0.783783784 |
| EP300 | 45 | 9.955427905 | 0.763157895 |
| NOS3 | 37 | 13.11762682 | 0.707317073 |
3.2.4. GO biological function annotation and KEGG pathway enrichment
The intersection target information of zedoary tumeric pharmacodynamic substance and liver cancer disease was imported into the Metascape platform for function and signal pathway analysis. According to P < 0.01, the number of targets involved ≥3 and enrichment factor >1.5, 20 key pathways were screened (Table 2, Fig. 5). KEGG analysis results showed that the target enrichment pathways were mainly concentrated in neuroactive live receiver interaction, pathways in cancer, nitrogen metabolism, HIF-1 signaling pathway, etc, It is suggested that these classical pathways may play an important role in the treatment of anti liver cancer by zedoary tumeric pharmacodynamic substance. Take the cancer pathway and draw the pathway map (Fig. 6). Each node represents the gene/protein/enzyme, the arrow represents the relationship between upstream and downstream, the yellow background node represents the direct regulation site of the gene, the red node indicates that the gene appears in the regulation network or is related to the pathway, and the bold arrow indicates the specific pathway of its role, The graph can express the specific ways and means of its anti liver cancer effect.
Table 2.
Top 20 targets of degree value in KEGG relationship network.
| Number | Pathways | Target number | Log10(P) value |
|---|---|---|---|
| hsa04080 | Neuroactive ligand-receptor interaction | 36 | −32.66 |
| hsa05200 | Pathways in cancer | 30 | −20.29 |
| hsa00910 | Nitrogen metabolism | 11 | −19.26 |
| hsa04066 | HIF-1 signaling pathway | 17 | −16.25 |
| hsa04726 | Serotonergic synapse | 15 | −13.57 |
| hsa00140 | Steroid hormone biosynthesis | 10 | −10.63 |
| hsa04330 | Notch signaling pathway | 9 | −9.96 |
| ko05206 | MicroRNAs in cancer | 17 | −9.66 |
| ko04723 | Retrograde endocannabinoid signaling | 11 | −9.4 |
| hsa04371 | Apelin signaling pathway | 12 | −8.77 |
| hsa03320 | PPAR signaling pathway | 9 | −8.33 |
| hsa05219 | Bladder cancer | 6 | −6.14 |
| hsa00350 | Tyrosine metabolism | 5 | −5.15 |
| hsa00590 | Arachidonic acid metabolism | 6 | −5.07 |
| hsa04720 | Long-term potentiation | 6 | −4.87 |
| hsa05120 | Epithelial cell signaling in Helicobacter pylori infection | 6 | −4.83 |
| hsa00330 | Arginine and proline metabolism | 5 | −4.37 |
| hsa04750 | inflammatory mediator regulation of trp channels | 6 | −3.79 |
| hsa04142 | Lysosome | 6 | −3.4 |
| hsa04340 | Hedgehog signaling pathway | 4 | −3.32 |
Fig. 5.
Bubble diagram of KEGG enrichment analysis.
Fig. 6.
Cancer pathways of zedoary tumeric pharmacodynamic substance.
Set the same screening conditions to obtain the GO analysis results. The GO analysis shows that zedoary tumeric pharmacodynamic substance plays the role of treating liver cancer through multiple locations and biological processes in the body (Fig. 7). Molecular function (MF) analysis shows that the target may play a role through carbon dehydrase activity, neurotransmitter receiver activity, phosphotransfer activity, alcohol group as acceptor, oxidoreductase activity, etc; Biological process (BP) analysis showed that cellular response to nitrogen compound, synaptic signaling, and informational response was important in the anti-living cancer of zedoary tumeric pharmacodynamic substance; Cell component (CC) analysis showed that the products of the target may be mainly in the postsynapse, membrane raft, gamma-secretase complex and so on.
Fig. 7.
GO analysis of anti liver cancer targets of zedoary turmeric pharmacodynamic substances.
3.2.5. Construction of “drug component-target-pathway” network
Import drugs, compounds, key targets, and pathways into Cytoscape 3.7.2 database to build a “drug component-key target-pathway” network, as shown in Fig. 8. The network includes 247 nodes (eight pharmacodynamic substances, 219 targets, and 20 paths) and 522 edges, the figure clearly shows that the zedoary tumeric pharmacodynamic group plays an anti hepatoma role through multi-target and multi-channel regulation.
Fig. 8.
“Pharmacodynamic substance-target-pathway” network of zedoary tumeric.
3.2.6. Molecular docking verification
Using autodock 4.2.6 software, the main pharmacodynamic components curcumin, curzerenone, beta-elemene, curcumenol, curcumol, curcudione, germacrone and zedoarondiol in zedoary tumeric were connected with the corresponding core targets of AKT1, EGFR and MAPK8. It is generally believed that the binding energy < 0 indicates that the small molecular components of the ligand can bind independently with the receptor target protein. The lower the binding energy is, the more stable the molecular conformation is, the greater the possibility of action is, and the greater the absolute value of the binding energy is, that is, the binding between the ligand and the receptor is better. The docking results are shown in Table 3 and Fig. 9, Fig. 10. The results showed that the active components of zedoary tumeric had good docking activity with the core target. Among them, the two groups of molecules with a strong affinity for AKT1 and EGFR are curcumenol and curcudione, while curcumenol and curcumol have the lowest binding energy with MAPK8, which are stably docked in the active pocket of the receptor protein and form hydrogen bonds. The charge number of AKT1 is 7.9713, the charge number of EGFR is −5.0633 and the charge number of MAPK8 is 5.9674.
Table 3.
Molecular docking results of zedoary tumeric pharmacodynamic substance and core targets.
| Active ingredients | Binding free energy (kcal/mol) |
||
|---|---|---|---|
| AKT1 | EGFR | MAPK8 | |
| Curcumol | −7.12 | −6.34 | −7.1 |
| Curzerenone | −7.28 | −4.6 | −6.52 |
| Beta-elemene | −7.1 | −5.12 | −5.99 |
| Curcumenol | −8.07 | −6.49 | −7.49 |
| Curcumin | −5.01 | −4.46 | −6.13 |
| Curdione | −7.4 | −6.48 | −6.63 |
| Germacrone | −6.65 | −5.82 | −6.86 |
| Zedoarondiol | −7.33 | −6.38 | −6.51 |
Fig. 9.
Molecular docking heat map.
Fig. 10.
Molecular docking verification of “pharmacodynamic substances and targets”.
Based on the above network pharmacological analysis, we can know that the core targets of zedoary tumeric pharmacodynamic group in the treatment of liver cancer are AKT1, PTGS2, STAT3, MAPK8, EGFR, etc., and the core pathways are neuroactive live receiver interaction, nitrogen metabolism, HIF-1 signaling pathway, etc, This is also similar to the previous anti hepatoma mechanism of zedoary tumeric pharmacodynamic substance. The development of modern network pharmacology technology provides a feasible method for the analysis and research of the pharmacodynamic group. This research can provide a certain reference value for the further development of zedoary tumeric and its active components.
4. Discussion and analysis on relationship between pharmacodynamic substance and pharmacodynamic group mechanism
The chemical composition of traditional Chinese medicine is complex, and its efficacy often depends on the role of the “pharmacodynamic group”. What is the relationship between the joint action of pharmacodynamic substance and the action of a single component needs to be further discussed. In zedoary tumeric, curcuol, zedoarondiol, curcumenol, curdione, curcumin, germacrone β-elemene has been proved to have the effect of anti-liver cancer. It can be used as the pharmacodynamic substance of zedoary tumeric anti liver cancer. Curcumol, curcumin, and other components have been proved to play the role of treating liver cancer through “multi-target, multi-channel”. Later, the “pharmacodynamic group” was screened by network pharmacological means. The ten core target proteins for the treatment of liver cancer are AKT1, PTGS2, CDH1, STAT3, MAPK8, MDM2, EGFR, PPARG, EP300, and NOS3. The corresponding bioconcentration functions and pathways are also analyzed and verified by molecular docking. It is not difficult for us to find that most of the core targets AKT1, MAPK, and STAT3 of the “pharmacodynamic group” have been proved to be the regulatory proteins of curcumin against liver cancer, which indicates that curcumin may play a leading role in the anti liver cancer effect of the “pharmacodynamic group”, which is consistent with the statements of “King medicine” and “minister medicine” in the theory of traditional Chinese medicine. This also proves that these pharmacodynamic substances have great potential in the study of unverified target proteins such as CDH1, MDM2, EP300, and NOS3. Of course, the anti-liver cancer mechanisms of these “pharmacodynamic groups” must also have interaction effects. For example, it is the interaction of these pharmacodynamic substances that leads to the existence of these new core target proteins, CDH1 and MDM2; For example, the gene database shows that curcumol does not act on MAPK, but the gene database and experimental studies have proved the existence of MAPK target in curcumin, resulting in strong interaction between a variety of target proteins in curcumol and MAPK. By analyzing the relationship between pharmacodynamic substance and “pharmacodynamic group”, it also has great reference significance for curdione, curzerenone, zedoarondiol, and curcumenol, which have less research on efficacy and mechanism, and can promote their in-depth development and research. In addition, the interaction between pharmacodynamic substances of traditional Chinese medicine has the characteristics of increasing curative effect, which is also the advantage of traditional Chinese medicine different from monomer components. However, there is still a lack of in-depth research on the specific interaction mechanism between these pharmacodynamic substances and whether the combined action will produce side effects. There is a great reference between the specific mechanisms of pharmacodynamic substance and “pharmacodynamic group” in the treatment of diseases, and the study of the relationship between the single and combined effects of pharmacodynamic substance is of great significance for better exertion of curative effect and in-depth mechanism research (Fig. 11).
Fig. 11.
Reference significance between pharmacodynamic substance and pharmacodynamic group mechanism.
5. Summary
From the perspective of traditional Chinese medicine, the generation of liver cancer is due to the deficiency of healthy Qi caused by dampness, heat and toxin, improper diet, and emotional disorder. At the same time, phlegm and dampness are endogenous, which is intertwined with phlegm and blood stasis in the liver to form liver cancer. Deficiency of vital Qi and stagnation of liver Qi is the basic pathogenesis of liver cancer. Zedoary tumeric is good at “eliminating accumulation” and has a strong effect of promoting Qi and dispersing stagnation. It is commonly used in Qi stagnation, symptoms, and masses, and has a strong effect of eliminating accumulation and relieving pain. Therefore, it has basic theoretical support of traditional Chinese medicine for the treatment of liver cancer. Zedoary tumeric can play an active role in the treatment of liver cancer. The injection preparation of zedoary tumeric pharmacodynamic substance has been used in the clinic. By integrating the target genes of zedoary tumeric and liver cancer diseases, it is found that most target genes (>95%) of zedoary tumeric pharmacodynamic substances coincide with liver cancer disease genes, which also shows the specificity and pertinence of these pharmacodynamic substances against liver cancer. Through the establishment of the PPI network, ten core targets, including AKT1, PTGS2, STAT3, MAPK8, EGFR, etc., were screened through degree, betweenness, and closeness indicators. The molecular docking results in this study preliminarily show that zedoary tumeric pharmacodynamic substance has a good affinity with important targets AKT1, EGFR,and MAPK8, suggesting that its main active components have good binding energy with potential targets.
AKT1 protein kinase B is a key regulator and important downstream target of PI3K/Akt pathway. After being phosphorylated and activated, AKT1 protein kinase B mainly participates in cell process, controls cell growth and survival (Hinz, & Jücker, 2019), and plays a role in a variety of biological processes such as glucose metabolism, cell proliferation and apoptosis, cell migration and so on (Martíne z-Rodríguez, Thompson-Bonilla, & Jaramillo-Flores, 2020). Studies have shown that AKT1 inhibitor can effectively prevent liver fibrosis (Reyes-Gordillo et al., 2019), so zedoary tumeric pharmacodynamic substance is likely to play the role of liver protection and anti liver cancer by downregulating AKT1 gene. EGFR plays a key role in hepatocyte proliferation and liver regeneration, and is related to hepatocyte carcinogenesis (Michalo Poulos, 2013, Michalo Poulos, 2007). PTGS2 can metabolize arachidonic acid into various prostaglandin products and is an important rate-limiting enzyme for prostaglandin synthesis. Some studies have reported the role of PTGS2 in tumor occurrence and development, angiogenesis, and metastasis. It is found that PTGS2 is closely related to chemoresistance. Ablation of PTGS2 will reverse T cell rejection and make the tumor sensitive to immunotherapy (Lin et al., 2019, Markosyan et al., 2019). Some experiments have confirmed that overexpression of PTGS2 can reduce Tet1 induced promoter hypermethylation and induce liver cancer (Chen et al., 2017). PTGS2 gene plays an important role in redox regulation, which also shows that energy metabolism regulation is an important regulation mode of zedoary tumeric pharmacodynamic substance combined with anti liver cancer. MAPK family genes can show strong protein expression in tumor tissues. The activation of MAPK pathway is the target of its anti liver cancer effect. In liver cancer, this pathway is also highly activated (Westphal et al., 2021). MAPK is involved in tumor progression, including cell proliferation and migration (Akrami et al., 2016), apoptosis (Lu et al., 2007), survival, and angiogenesis (Gaundar & Bendall, 2010). In addition, the MAPK is also closely related to liver cancer and is involved in the proliferation, migration, invasion, metastasis, autophagy, apoptosis, and drug resistance of liver cancer cells. STAT3 is one of the main members of the signal transduction and transcription factor family. It is abnormally activated in liver cancer. It can not only affect the expression of proto-oncogenes as a transcription factor, but also promote the transduction of multiple carcinogenic signal pathways. The Latest research (Wang et al., 2019) found that lipopolysaccharide mainly promotes the proliferation of liver cancer cells through Stat3. In conclusion, these core targets can play an anti liver cancer role through multiple mechanisms.
Zedoary tumeric pharmacodynamic substance cooperates to play the role of anti liver cancer at multiple levels through many genes and multiple pathways, which is consistent with the characteristics of multi-target and multi-pathway of traditional Chinese medicine in the treatment of diseases. Taking the currently validated zedoary tumeric pharmacodynamic substance as the research object, this paper first summarizes the current research progress of zedoary tumeric single component anti liver cancer mechanism, and then analyzes the potential targets and signal pathways of these pharmacodynamic substances combined with anti liver cancer through network pharmacology technology. Combined with the molecular docking method, it is verified that these components can interact with the target of liver cancer, and the internal mechanism of zedoary tumeric pharmacodynamic substance combined with anti liver cancer is clarified, which provides a direction and reference for the follow-up in-depth verification of the anti liver cancer mechanism of zedoary tumeric and its better application in clinic. At the same time, by analyzing the relationship between zedoary tumeric single component pharmacodynamic substance and “pharmacodynamic group” anti liver cancer, it is found that the relationship between them lies in the determination of target, pathway, biological function and core pharmacodynamic components, the formation of core target protein interaction, in-depth study of single pharmacodynamic substance. Therefore, this paper can also provide some ideas for the relationship between pharmacodynamic substance and “pharmacodynamic group”.
6. Conclusion
Zedoary tumeric has a significant effect in treating liver cancer. Its various pharmacodynamic substances can play an anti liver cancer role by regulating multiple genes and acting on multiple pathways. The core targets of the anti-liver cancer effect of the “pharmacodynamic group” are AKT1, EGFR, MAPK8, etc., and the core pathways are neuroactive live receiver interaction, pathways in cancer Nitrogen metabolism, HIF-1 signaling pathway, etc. Taking zedoary tumeric as an example, this study also found that there is a valuable relationship between the traditional Chinese medicine pharmacodynamic substance and the “pharmacodynamic group” in the action mechanism of target, pathway, and biological function. By comparing the relationship between them, we can determine the core pharmacodynamic components, the formation of core target protein interaction. It is of great significance and value to determine the mechanism of a single pharmacodynamic substance and increase the curative effect.
Authors’ contributions
JGD conceived the original idea. ZYL and XTH wrote the manuscript.RC and ZCD prepared the figures. EWH, LHZ, TYH and SL proofread and revised the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 82060762); China ASEAN Joint Laboratory for International Cooperation in Traditional Medicine Research (phase II) New Center Construction Project (Grant No. cicar2017-z1); and Guangxi Innovation-driven Major Project (Grant No. guike aa181180492 and aa192540334).
Contributor Information
Xiaotao Hou, Email: xthou@126.com.
Jiagang Deng, Email: dengjg53@126.com.
References
- Ahmed Hamdi O.A., Syed Abdul Rahman S.N., Awang K., Abdul Wahab N., Looi C.Y., Thomas N.F., Abd Malek S.N. Cytotoxic constituents from the rhizomes of Curcuma zedoaria. The Scientific World Journal. 2014;321943 doi: 10.1155/2014/321943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akrami H., Mahmoodi F., Havasi S., Sharifi A. PlGF knockdown inhibited tumor survival and migration in gastric cancer cell via PI3K/Akt and p38MAPK pathways. Cell Biochemistry and Function. 2016;34(3):173–180. doi: 10.1002/cbf.3176. [DOI] [PubMed] [Google Scholar]
- Bai J., Wu J., Tang R., Sun C., Ji J., Yin Z.…Yang W. Emodin, a natural anthraquinone, suppresses liver cancer in vitro and in vivo by regulating VEGFR2 and miR-34a. Investigational New Drugs. 2020;38(2):229–245. doi: 10.1007/s10637-019-00777-5. [DOI] [PubMed] [Google Scholar]
- Cao C., Huang G., Huang Z. Effects of curcumin on proliferation and adriamycin resistance of hepatoma drug resistant cells HepG2 / ADM. Guangxi Medical Journal. 2020;42(8):976–980. [Google Scholar]
- Chen X., Zeng J., Dai P. Analysis of chemical components of volatile oil from zedoary tumeric in Guangxi. Pharmaceutical Biotechnology. 2008;15(4):293–295. [Google Scholar]
- Chen C., Xu Q., Zhao H. Intervention of curcumin on the expression of mir-29 and VEGF in hepatoma cells. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine. 2020;30(10):785–790. [Google Scholar]
- Chen H., Cai W., Chu E., Tang J., Wong C.C., Wong S.H., Yu J. Hepatic cyclooxygenase-2 overexpression induced spontaneous hepatocellular carcinoma formation in mice. Oncogene. 2017;36(31):4415–4426. doi: 10.1038/onc.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di J., Gao K., Qu D., Yang J., Zheng J. Rap2B promotes angiogenesis via PI3K/AKT/VEGF signaling pathway in human renal cell carcinoma. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine. 2017;39(7):10104283. doi: 10.1177/1010428317701653. [DOI] [PubMed] [Google Scholar]
- Fang, B. (2013). Study on the mechanism of germacrone inhibiting the proliferation of hepatoma HepG2 cells. Wuhan: Huazhong University of Science and Technology. Thesis of Master Degree.
- Feng Y., Zhao Y., Li Y., Peng T., Kuang Y., Shi X.…Yu C. Inhibition of fibroblast activation in uterine leiomyoma by components of Rhizoma Curcumae and Rhizoma Sparganii. Frontiers in Public Health. 2021;9 doi: 10.3389/fpubh.2021.650022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H., Wang C., Yang D., Wei Z., Xu J., Hu Z.…Cai Q. Curcumin regulates proliferation, autophagy, and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling. Journal of Cellular Physiology. 2018;233(6):4634–4642. doi: 10.1002/jcp.26190. [DOI] [PubMed] [Google Scholar]
- Gao L., Yang X., Li Y., Wang Z., Wang S., Tan S.…Zheng S. Curcumol inhibits KLF5-dependent angiogenesis by blocking the ROS/ERK signaling in liver sinusoidal endothelial cells. Life Sciences. 2021;264 doi: 10.1016/j.lfs.2020.118696. [DOI] [PubMed] [Google Scholar]
- Gao S., Xia G., Wang L., Zhou L., Zhao F., Huang J., Chen L. Sesquiterpenes from Curcuma wenyujin with their inhibitory activities on nitric oxide production in RAW 264.7 cells. Natural Product Research. 2017;31(5):548–554. doi: 10.1080/14786419.2016.1205053. [DOI] [PubMed] [Google Scholar]
- GBD. 2013 Mortality and Causes of Death Collaborators, Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013 Lancet 385 9963 2015 117 171. [DOI] [PMC free article] [PubMed]
- Guo, F. (2018). Curcumol regulates Raf / MEK / ERK signaling pathway to induce apoptosis of hepatoma cells. Guangdong Pharmaceutical University. Thesis of Master Degree.
- Gaundar S.S., Bendall L.J. The potential and limitations of p38MAPK as a drug target for the treatment of hematological malignancies. Current Drug Targets. 2010;11(7):823–833. doi: 10.2174/138945010791320854. [DOI] [PubMed] [Google Scholar]
- He Y., Tang C., Huang M. Effect of curcumin on the proliferation of hepatoma HepG2 cells through glyoxase 1. Pharmacology and Clinic of Traditional Chinese Medicine. 2020;36(2):122–126. [Google Scholar]
- Hassan B., Akcakanat A., Holder A.M., Meric-Bernstam F. Targeting the PI3-kinase/Akt/mTOR signaling pathway. Surgical Oncology Clinics of North America. 2013;22(4):641–664. doi: 10.1016/j.soc.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G., Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Research. 2011;21(1):159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz N., Jücker M. Distinct functions of AKT isoforms in breast cancer: A comprehensive review. Cell Communication and Signaling. 2019;17(1):154. doi: 10.1186/s12964-019-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Yang F., Yang J. Aging of human hepatoma HepG2 cells induced by curcumol and its mechanism. Guangxi Plant. 2018;38(07):894–902. [Google Scholar]
- Huang L., Li A., Liao G., Yang F., Yang J., Chen X., Jiang X. Curcumol triggers apoptosis of p53 mutant triple-negative human breast cancer MDA-MB 231 cells via activation of p73 and PUMA. Oncology Letters. 2017;14(1):1080–1088. doi: 10.3892/ol.2017.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Wang J., Lu F. Mechanism of curcumol inhibiting proliferation of human hepatoma cell line HepG2. Chinese Journal of Traditional Chinese Medicine. 2013;38(11):1812–1815. [Google Scholar]
- Jeddi F., Soozangar N., Sadeghi M.R., Somi M.H., Samadi N. Contradictory roles of Nrf2/Keap1 signaling pathway in cancer prevention/promotion and chemoresistance. DNA Repair. 2017;54:13–21. doi: 10.1016/j.dnarep.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Jiang E., Wang Z. Study on the effects of curdione on proliferation, apoptosis and invasion of human cervical cancer HeLa cells. Jilin Medical Journal. 2021;42(5):1032–1034. [Google Scholar]
- Jun S.Y., Jeon S.J., Yoon J.Y., Lee J.J., Yoon H.R., Choi M.H., Kim N.S. The positive correlation of TIPRL with LC3 and CD133 contributes to cancer aggressiveness: Potential biomarkers for early liver cancer. Scientific Report. 2019;9(1):16802. doi: 10.1038/s41598-019-53191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei B., Chai W., Wang Z., Liu R. Highly expressed UNC119 promotes hepatocellular carcinoma cell proliferation through Wnt/β-catenin signaling and predicts a poor prognosis. American Journal of Cancer Research. 2015;5(10):3123–3134. [PMC free article] [PubMed] [Google Scholar]
- Li Y. Investigation and Study on TCM syndromes and mental health of 140 patients with hepatitis B related primary liver cancer. University of Chinese Medicine. Thesis of Master Degree; Beijing: 2016. [Google Scholar]
- Li Z., Cao R., Hao E. Research progress on chemical constituents and pharmacological effects of zedoary tumeric in Guangxi and prediction and analysis of quality markers (Q-marker) Chinese Traditional and Herbal Drugs. 2021;52(15):4687–4699. [Google Scholar]
- Li G., Lin J., Peng Y., Qin K., Wen L., Zhao T., Feng Q. Curcumol may reverse early and advanced liver fibrogenesis through downregulating the uPA/uPAR pathway. Phytotherapy Research. 2020;34(6):1421–1435. doi: 10.1002/ptr.6616. [DOI] [PubMed] [Google Scholar]
- Li H., Mo C., Zhao C. Antitumor effect of zedoary tumeric extract elemene on hepatoma H22 bearing mice. Chinese Journal of Clinical Pharmacology. 2018;34(11):1345–1348. [Google Scholar]
- Li J., Wei H., Liu Y., Li Q., Guo H., Guo Y., Chang Z. Curcumin inhibits hepatocellular carcinoma via regulating miR-21/TIMP3 axis. Evidence-based Complementary and Alternative Medicine. 2020;2892917 doi: 10.1155/2020/2892917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wang Y., Song Y., Xu L., Zhao J., Fang B. A preliminary study of the effect of curcumin on the expression of p53 protein in a human multiple myeloma cell line. Oncology Letters. 2015;9(4):1719–1724. doi: 10.3892/ol.2015.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhuang J. β-Experimental study of elemene on invasion, metastasis and related mechanisms of human hepatoma cells. Fujian Journal of Medicine. 2012;34(3):70–73. [Google Scholar]
- Li Z., Peng Y., Li J., Chen Z., Chen F., Tu J.…Wang H. N6-methyladenosine regulates glycolysis of cancer cells through PDK4. Nature Communnation. 2020;11(1):2578. doi: 10.1038/s41467-020-16306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.O., Li C.W., Xia W., Cha J.H., Chan L.C., Wu Y., Hung M.C. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30(6):925–939. doi: 10.1016/j.ccell.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X.M., Li S., Zhou C., Li R.Z., Wang H., Luo W., Wu X.P. Cisplatin induces chemoresistance through the PTGS2-mediated anti-apoptosis in gastric cancer. The International Journal of Biochemistry & Cell Biology. 2019;116 doi: 10.1016/j.biocel.2019.105610. [DOI] [PubMed] [Google Scholar]
- Liu X., Ling Z. The role of IDH1 and idh2 gene mutations in tumors. International Journal of Oncology. 2015;42(5):351–354. [Google Scholar]
- Liu Y., Wang W., Fang B., Ma F., Zheng Q., Deng P.…He G. Anti-tumor effect of germacrone on human hepatoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. European Journal of Pharmacology. 2013;698(1–3):95–102. doi: 10.1016/j.ejphar.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Liu, Y. (2013). Inhibitory effect of germacrone on hepatoma cells and its mechanism. Wuhan: Huazhong University of Science and Technology. Thesis of Doctor Degree.
- Liu Y.Y., Zheng Q., Fang B., Wang W., Ma F.Y., Roshan S., He G.Y. Germacrone induces apoptosis in human hepatoma HepG2 cells through inhibition of the JAK2/STAT3 signalling pathway. Journal of Huazhong University of Science and Technology Medicine Science. 2013;33(3):339–345. doi: 10.1007/s11596-013-1121-z. [DOI] [PubMed] [Google Scholar]
- Lou Y., Zhao F., He H., Peng K.F., Zhou X.H., Chen L.X., Qiu F. Guaiane-type sesquiterpenes from Curcuma wenyujin and their inhibitory effects on nitric oxide production. Journal of Asian Natural Products Research. 2009;11(8):737–747. doi: 10.1080/10286020903042358. [DOI] [PubMed] [Google Scholar]
- Lu M., Xiao L., Li Z. The relationship between p38MAPK and apoptosis during paclitaxel resistance of ovarian cancer cells. Journal of Huazhong University of Science and Technology Medical Sciences. 2007;27(6):725–728. doi: 10.1007/s11596-007-0628-6. [DOI] [PubMed] [Google Scholar]
- Ma L., Zhao Q., Chen W., Zhang Y. Oncogene Lin28B increases chemosensitivity of colon cancer cells in a let-7-independent manner. Oncology Letters. 2018;15(5):6975–6981. doi: 10.3892/ol.2018.8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X. (2015). Effect of curcumol combined with fluorouracil on the growth and expression of PCNA and p27 in transplanted gastric cancer in nude mice. Shijiazhuang: Hebei Medical University. Thesis of Master Degree.
- Mao Y., Zhang J., Hou L., Cui X. The effect of beta-elemene on alpha-tubulin polymerization in human hepatoma HepG2 cells. Chinese Journal of Cancer Research = Chung-kuo Yen Cheng Yen Chiu. 2013;25(6):770–776. doi: 10.3978/j.issn.1000-9604.2013.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markosyan N., Li J., Sun Y.H., Richman L.P., Lin J.H., Yan F., Vonderheide R.H. Tumor cell-intrinsic EPHA2 suppresses anti-tumor immunity by regulating PTGS2 (COX-2) The Journal of Clinical Investigation. 2019;129(9):3594–3609. doi: 10.1172/JCI127755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt J.U., Gomez-Quiroz L., Arreguin Camacho L.O., Pinna F., Lee Y.H., Kitade M., Thorgeirsson S.S. Curcumin effectively inhibits oncogenic NF-κB signaling and restrains stemness features in liver cancer. Journal of Hepatology. 2015;63(3):661–669. doi: 10.1016/j.jhep.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martíne z-Rodríguez O.P., Thompson-Bonilla M., Jaramillo-Flores M.E. Association between obesity and breast cancer: Molecular bases and the effect of flavonoids in signaling pathways. Critical Reviews in Food Science and Nutrition. 2020;60(22):3770–3792. doi: 10.1080/10408398.2019.1708262. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Ninomiya K., Morikawa T., Yoshikawa M. Inhibitory effect and action mechanism of sesquiterpenes from Zedoariae Rhizoma on D-galactosamine/lipopolysaccharide-induced liver injury. Bioorganic & Medicinal Chemistry Letters. 1998;8(4):339–344. doi: 10.1016/s0960-894x(98)00021-3. [DOI] [PubMed] [Google Scholar]
- Meng, X. (2010). β- Effects of elemene on the differentiation of rat bone marrow-derived endothelial progenitor cells and Notch signaling pathway during differentiation. Shenyang: China Medical University. Thesis of Master Degree.
- Michalo Poulos G.K. Principles of liver regeneration and growth homeostasis. Comprehensive Physiology. 2013;3(1):485–513. doi: 10.1002/cphy.c120014. [DOI] [PubMed] [Google Scholar]
- Michalo Poulos G.K. Liver regeneration. Journal of Cellular Physiology. 2007;213(2):286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M., Zhong Q., Kowluru R.A. Epigenetic modifications of Keap1 regulate its interaction with the protective factor Nrf2 in the development of diabetic retinopathy. Investigative Ophthalmology & Visual Science. 2014;55(11):7256–7265. doi: 10.1167/iovs.14-15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu H., Jin H., Chen X. Effects of curcumin on Keap1 / are signal pathway and bile acid hepatointestinal circulation in liver cancer model mice. Chinese Journal of Comparative Medicine. 2020;30(9):8–14. [Google Scholar]
- Pai P., Sukumar S. HOX genes and the NF-κB pathway: A convergence of developmental biology, inflammation and cancer biology. Biochimica et Biophysica Acta. 2020;1874(2) doi: 10.1016/j.bbcan.2020.188450. [DOI] [PubMed] [Google Scholar]
- Pan Z., Zhuang J., Ji C., Cai Z., Liao W., Huang Z. Curcumin inhibits hepatocellular carcinoma growth by targeting VEGF expression. Oncology Letters. 2018;15(4):4821–4826. doi: 10.3892/ol.2018.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Liu Y., Li W.H., Zhang H.Q., Tian X.X., Fang W.G. P2Y2 receptor promotes the migration and invasion of breast cancer cells via EMT-related genes Snail and E-cadherin. Oncology Reports. 2018;39(1):138–150. doi: 10.3892/or.2017.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que X. β-Research progress on molecular mechanism of elemene antitumor. Traditional Chinese Medicine and Clinic. 2015;6(6):61–64. [Google Scholar]
- Ren B., Luo S., Tian X., Jiang Z., Zou G., Xu F.…Liu J. Curcumin inhibits liver cancer by inhibiting DAMP molecule HSP70 and TLR4 signaling. Oncology Reports. 2018;40(2):895–901. doi: 10.3892/or.2018.6485. [DOI] [PubMed] [Google Scholar]
- Reyes-Gordillo K., Shah R., Arellanes-Robledo J., Cheng Y., Ibrahim J., Tuma P.L. Akt1 and Akt2 isoforms play distinct roles in regulating the development of inflammation and fibrosis associated with alcoholic liver disease. Cells. 2019;8(11):1337. doi: 10.3390/cells8111337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadia R., Liu Y., Zheng Q. Preliminary study on the mechanism of germacrone inhibiting the proliferation of hepatoma BEL7402 cells. Lishizhen Medicine and Materia Medica Research. 2014;25(12):2875–2877. [Google Scholar]
- Scaglione S., Adams W., Caines A., Devlin P., Mittal S., Singal A.G., Parikh N.D. Association between race/ethnicity and insurance status with outcomes in patients with hepatocellular carcinoma. Digestive Diseases and Sciences. 2020;65(6):1669–1678. doi: 10.1007/s10620-019-05890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S., Duan W., Xu Q., Li X., Han L., Li W.…Lei J. Curcumin suppresses hepatic stellate cell-induced hepatocarcinoma angiogenesis and invasion through downregulating CTGF. Oxidative Medicine and Cellular Longevity. 2019;81485 doi: 10.1155/2019/8148510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastri A.A., Saleh A., Savage J.E., DeAngelis T., Camphausen K., Simone N.L. Dietary alterations modulate the microRNA 29/30 and IGF-1/AKT signaling axis in breast cancer liver metastasis. Nutrition & Metabolism. 2020;17:23. doi: 10.1186/s12986-020-00437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Ni F. β-Research progress on antitumor immune effect of elemene. Journal of Molecular Diagnosis and Treatment. 2014;6(1):57–61. [Google Scholar]
- Sun C., Han Z., Wang M. P73 and p63, new members of p53 gene family. Chinese Journal of Oncology. 2001;10(7):403–406. [Google Scholar]
- Sun, X., Jahnke, & Lv, L. (2019). Curdione, effects and mechanisms of migration and invasion on HCC1937 cells in breast cancer. China Journal of Experimental Medicine, 25(3), 66-73.
- Tang Y., Li X. Anticancer effect and mechanism of zedoary tumeric extract on hepatoma cell line HepG2. Chinese Journal of Pharmacology. 2007;23(6):790–794. [Google Scholar]
- Tian N., Shangguan W., Zhou Z., Yao Y., Fan C., Cai L. Lin28b is involved in curcumin-reversed paclitaxel chemoresistance and associated with poor prognosis in hepatocellular carcinoma. Journal of Cancer. 2019;10(24):6074–6087. doi: 10.7150/jca.33421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, E. (2013). Radiosensitization effect of elemene on hypoxic lung cancer cells and mTOR and HIF-1 α/ Study on the correlation of survivin pathway. Dalian: Dalian Medical University. Thesis of Doctor Degree.
- Wagner W., Kania K.D., Blauz A., Ciszewski W.M. The lactate receptor (HCAR1/GPR81) contributes to doxorubicin chemoresistance via ABCB1 transporter up-regulation in human cervical cancer HeLa cells. Journal of Physiology and Pharmacology. 2017;68(4):555–564. [PubMed] [Google Scholar]
- Wang J., Li X.M., Bai Z., Chi B.X., Wei Y., Chen X. Curcumol induces cell cycle arrest in colon cancer cells via reactive oxygen species and Akt/ GSK3β/cyclin D1 pathway. Journal of Ethnopharmacology. 2018;210:1–9. doi: 10.1016/j.jep.2017.06.037. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang C., Bu G. Curcumin inhibits the growth of liver cancer stem cells through the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway. Experimental and Therapeutic Medicine. 2018;15(4):3650–3658. doi: 10.3892/etm.2018.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhou M., Jin X., Li B., Wang C., Zhang Q.…Yang M. Glycochenodeoxycholate induces cell survival and chemoresistance via phosphorylation of STAT3 at Ser727 site in HCC. Journal of Cellular Physiology. 2020;235(3):2557–2568. doi: 10.1002/jcp.29159. [DOI] [PubMed] [Google Scholar]
- Wang, W. (2013). Preliminary study on the mechanism of germacrone inhibiting the proliferation of hepatoma BEL7402 cells. Wuhan: Huazhong University of Science and Technology. Thesis of Master Degree.
- Wang Y., Kuramitsu Y., Ueno T., Suzuki N., Yoshino S., Iizuka N.…Nakamura K. Glyoxalase I (GLO1) is up-regulated in pancreatic cancerous tissues compared with related non-cancerous tissues. Anticancer Research. 2012;32(8):3219–3222. [PubMed] [Google Scholar]
- Wang Z., Yan M., Li J., Long J., Li Y., Zhang H. Dual functions of STAT3 in LPS-induced angiogenesis of hepatocellular carcinoma. Biochimica et Biophysica Acta—Molecular Cell Research. 2019;1866(4):566–574. doi: 10.1016/j.bbamcr.2018.11.016. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Tanahashi M., Suzuki E., Yoshii N., Uchiyama S. Surgical treatment and outcomes for synchronous multiple lung cancer. Kyobu geka. The Japanese Journal of Thoracic Surgery. 2021;74(1):9–15. [PubMed] [Google Scholar]
- Wei W., Liu Y. Curcumin reverses the drug resistance of hepatoma drug resistant cell line BEL7402 / Adr by activating PI3K / Akt signaling pathway. Chinese Journal of Gerontology. 2018;38(12):3015–3019. [Google Scholar]
- Wen B., Jiang L., Fan X. Curcumin inhibits the proliferation of hepatoma cells HepG2 and the expression of stem cell markers. Journal of Hubei Medical College. 2019;38(6):539–543. [Google Scholar]
- Westphal D., Garzarolli M., Sergon M., Horak P., Hutter B., Becker J.C., Meier F. High tumour mutational burden and EGFR/MAPK pathway activation are therapeutic targets in metastatic porocarcinoma. The British Journal of Dermatology. 2021 doi: 10.1111/bjd.20604. [DOI] [PubMed] [Google Scholar]
- Wu H., Li Z., Peng X. Curcumol promotes apoptosis of human hepatoma HepG2 cells by regulating PI3K / Akt pathway. Zhongnan Pharmacy. 2019;17(01):11–14. [Google Scholar]
- Xu J., Ji F., Kang J., Wang H., Li S., Jin D.Q., Guo Y. Absolute configurations and NO inhibitory activities of terpenoids from Curcuma longa. Journal of Agricultural and Food Chemistry. 2015;63(24):5805–5812. doi: 10.1021/acs.jafc.5b01584. [DOI] [PubMed] [Google Scholar]
- Xu X., Zhu Y. Curcumin inhibits human non-small cell lung cancer xenografts by targeting STAT3 pathway. American Journal of Translational Research. 2017;9(8):3633–3641. [PMC free article] [PubMed] [Google Scholar]
- Yan D., Deng S., Gan W., Li S., Li Y. Curcumol attenuates epithelial-mesenchymal transition of nasopharyngeal carcinoma cells via TGF-β1. Molecular Medicine Reports. 2018;17(6):7513–7520. doi: 10.3892/mmr.2018.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong S., Peng M.L. Recent advances in curcumin and its derivatives for treatment of liver diseases. Acta Pharmaceutica Sinica. 2014;49(11):1483. [PubMed] [Google Scholar]
- Yu D., Liu H., Qin J., Huangfu M., Guan X., Li X.…Chen X. Curcumol inhibits the viability and invasion of colorectal cancer cells via miR-30a-5p and Hippo signaling pathway. Oncology Letters. 2021;21(4):299. doi: 10.3892/ol.2021.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Zhang S., Gu X. Role of Yap signaling pathway in curcumin induced apoptosis of HepG2 hepatoma cell line. Journal of Jinzhou Medical University. 2020;41(3):13–18. [Google Scholar]
- Yu X., Zhong J., Yan L., Li J., Wang H., Wen Y., Zhao Y. Curcumin exerts antitumor effects in retinoblastoma cells by regulating the JNK and p38 MAPK pathways. International Journal of Molecular Medicine. 2016;38(3):861–868. doi: 10.3892/ijmm.2016.2676. [DOI] [PubMed] [Google Scholar]
- Yuan J., Zheng L., Wu Y. Discussion on the pathogenesis of primary liver cancer from the pathogenic theory of “phlegm”. Tianjin Traditional Chinese Medicine. 2017;34(5):323–326. [Google Scholar]
- Zang, S. (2017). Study on the anti gastric cancer effect and mechanism of curcumol. Guangzhou: Guangdong Pharmaceutical University. Thesis of Master Degree.
- Zeng H., Chen W., Zheng R., Zhang S., Ji J.S., Zou X., He J. Changing cancer survival in China during 2003–15: A pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6(5):e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- Zeng J., Mo X., Dai P. Study on spectrum effect relationship of antitumor effect of Guangxi zedoary tumeric volatile oil. Chinese Journal of Experimental Prescriptions. 2012;18(13):91–94. [Google Scholar]
- Zhang M., Zhang Y., Shen Z. Endoplasmic reticulum stress mechanism of curcumin regulating STAT3 induced apoptosis of human hepatoma BEL-7404 cells. Lishizhen Medicine and Materia Medica Research. 2020;31(3):594–597. [Google Scholar]
- Zhang K., Lv L., Chen J. Effects of curdione on MDA-MB-231 cell cycle and apoptosis. Chinese Journal of Experimental Prescriptions. 2021;27(12):74–81. [Google Scholar]
- Zhao Z., Malhotra A., Seng W.Y. Curcumin modulates hepatocellular carcinoma by reducing UNC119 Expression. Journal of Environmental Pathology, Toxicology and Oncology: Official Organ of the International Society for Environmental Toxicology and Cancer. 2019;38(3):195–203. doi: 10.1615/JEnvironPatholToxicolOncol.2019029549. [DOI] [PubMed] [Google Scholar]
- Zhou L., Wei E., Zhou B., Bi G., Gao L., Zhang T.…Ge B. Anti-proliferative benefit of curcumol on human bladder cancer cells via inactivating EZH2 effector. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2018;104(8):798–805. doi: 10.1016/j.biopha.2018.05.101. [DOI] [PubMed] [Google Scholar]