Abstract
Objective
Tripterygium glycoside (TG) is widely used in clinical practice for its multiple bioactivities including anti-inflammatory and immunosuppressive effects. However, emerging studies have frequently reported TG-induced adverse reactions to multiple organs, especially liver. Here, this study aimed to investigate the mechanism of liver damage induced by TG and explore representative components to reflect TG hepatotoxicity.
Methods
Network pharmacology was used to determine the potential targets of bile duct injury caused by TG. Next, the hepatotoxic effects of TG, triptolide (TP) and celastrol (CEL) were investigated and compared in vivo and in vitro. Liver function was determined by measuring serum transaminase and histopathology staining. The cell proliferation and apoptosis were determined by cell viability assay, scratch assay and flow cytometry. The expression of gene of interest was determined by qPCR and Western blot.
Results
Based on the network pharmacological analysis of 12 bioactive ingredients found in TG, a total of 35 targets and 15 pathways related to bile duct injury were obtained. Both TG and TP resulted in cholangiocyte damage and liver injury, as illustrated by increased levels of serum transaminase and oxidative stress, stimulated portal edema and lymphocytic infiltration and decreased expression of cholangiocyte marker, cytoskeletal 19. In addition, TG and TP inhibited cell proliferation and migration, arrested cell cycle and promoted Caspase-dependent apoptosis of cholangiocytes via suppressing the phosphorylation of extracellular regulated protein kinases 1/2 (ERK1/2) and protein kinase B (AKT). While, CEL at equivalent dosage had no obvious hepatotoxicity.
Conclusion
We revealed that TG-stimulated liver injury was specifically characterized by cholangiocyte damage and TP might be the decisive ingredient to reflect TG hepatotoxicity. Our results not only provide novel insights into the mechanism underlying the hepatotoxicity effects of TG but also offer reference for clinical rational use of TG.
Keywords: cell apoptosis, cholangiocyte, liver injury, Tripterygium glycoside, triptolide
1. Introduction
Traditional Chinese medicines (TCM) have been used in clinical practice and makes a great contribution to the management of various ailments throughout history (Ma and Li, 2020, Liu et al., 2019). Tripterygium wilfordii Hook F, one of famous TCM with multiple pharmacological properties, has been widely used for thousands of years in Asian areas (Li, Jiang, & Zhang, 2014). Recently, a considerable amount of bioactive ingredients has been derived from T. wilfordii and widely investigated in treating rheumatoid arthritis, systemic lupus erythematosus and several forms of cancer. As authorized natural drugs with a Chinese patent (Ma et al., 2015), Tripterygium glycoside (TG) is the effective fat-soluble mixture extracted from the roots of T. wilfordii, mainly including but not limited to triptolide (TP), celastrol (CEL), wilforlide, triptonide, wilfordine and tripterygium alkaloid (Jing et al., 2017). Based on recent experimental evidence, TG, with several biological activities including anti-inflammatory, immune-modulatory and anti-tumor, has been used in the treatment of rheumatoid, ankylosing spondylitis and skin disorders (Feng et al., 2021). However, according to National Medical Products Administration (NMPA) statistics, T. wilfordii preparations (mainly Tripterygium glycosides tablets used in clinic) have been reported to cause more than 800 adverse reaction cases and multiple organ damage or even failure (Jing et al., 2017). These adverse reactions, especially hepatotoxicity with a high incidence, have greatly restricted the clinical application of TG (Wang et al., 2018). Thus, the exploration of toxic mechanism of TG and the development of possible intervention strategies would be of great value.
Over the decades, several studies have reported the side effects caused by TG and its preparations, including jaundice, digestive tract symptoms and irregular menstruation. After taken overdose or dose accumulation, TG was reported to stimulate significant liver damage in rats, as illustrated by increased serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), hepatocyte vacuolar damage, lobular inflammation and connective tissue deposited in the portal areas (Wang et al., 2015). Further research indicated that TG also triggered acute liver injury by increasing oxidative stress and promoting inflammatory response (Hu et al., 2020). Until now, most of previous studies only focused on the toxic effects of TG and its preparation on liver in vivo and/or hepatocytes in vitro, while little is known about the toxic mechanism of liver damage induced by TG and which ingredients may reflect its hepatotoxicity.
Recently, more attention has been paid to bile duct injury, which belongs to liver injury and has become one of the leading causes of acute liver failure. Biliary tree provides a place for the bile fluid transportation from liver to intestine and is mainly composed of cholangiocytes (Dobreva & Karagyozov, 2020). In addition to the involvement in bile secretion and liver regeneration, cholangiocytes are also responsible for biliary defense against leaked gut microbiota or accumulated toxicant in liver (Österreicher & Trauner, 2012). Drug-induced bile duct injury as a subcategory of idiosyncratic cholestatic or mixed type injury, is characterized by the disruption to biliary tree architecture and the damage to biliary epitheliums (Visentin, Lenggenhager, Gai, & Kullak-Ublick, 2018). Over the decades, the most common evaluation systems for drug-induced liver injury in vitro are hepatocytes, mainly hepatic cell lines and primary hepatocytes. However, due to the neglect of the role of cholangiocytes played in liver damage, the results of hepatotoxicity in vitro and liver-specific biochemical assays in vivo are not completely consistent in some cases. Multiple drugs without obvious toxic effects on hepatocytes invitro have been reported to trigger severe liver injury in human and mice (Li et al., 2014). Interestingly, despite considerable amounts of efforts have been made to explore the hepatotoxicity of TG, the clinical management of TG is still challenging because of the poor understanding of the TG-induced bile duct injury. Hence, it is of the utmost importance to identify and discover the target hepatic cells and underlying mechanisms of TG-induced liver injury.
Network pharmacology, integrating system biology, pharmacology and computer analysis technology, has been used in the exploration of the complicated relationships among components, targets and diseases (Zhang et al., 2021). Considering the multi-target and multi-pathway functions of TCM, more and more researchers have applied network pharmacology to predict the active components and potential targets of TCM and explore its effects and mechanisms against different diseases. Recent studies have demonstrated the pharmacological effects and mechanism of TCM using network pharmacology, such as the molecular mechanism of Schisandra chinensis (Turcz.) Bail against liver injury (Li et al., 2020, Li et al., 2020, Li et al., 2020), the therapeutic mechanism of Danggui-Chuanxiong herb-pair on liver fibrosis (Cai et al., 2021). On the other hand, network pharmacology provides a new research technique for the analysis of TCM-induced toxicity. Shan Li et al (2021)identified and explored the potential targets underlying the hepatotoxicity of Rheum palmatum L. Dai et al. (2020) validated the network analysis results and demonstrated that hexabromocyclododecane caused obvious hepatotoxicity via stimulating IL-6 signaling. These previous findings contribute to the possibility of predicting the underlying mechanism of bile duct injury caused by TG.
Collectively, applying network pharmacology analysis and experimental verification, we identified that TG significantly inhibited the cell proliferation and migration, arrested cell cycle and promoted cell apoptosis of cholangiocytes via inhibiting the phosphorylation of extracellular regulated protein kinases 1/2 (ERK1/2) and protein kinase B (AKT), and activating Bax and cleaved-Caspase 3 in vivo and in vitro. Notably, we evaluated and compared the toxic effects of TG, TP and CEL on cholangiocytes, and provided experimental evidence that TP may be the main contributor to TG-induced cholangiocyte-related liver injury.
2. Methods and materials
2.1. Materials and chemicals
TG was purchased from Sanling Biotech (Guilin, China). TP and CEL were purchased from Innochem Technology Co., Ltd. (Beijing, China). iQTM SYBR Green Supermix was purchased from Bio-Rad (Hercules, CA). Formaldehyde (FPA), phosphate buffer saline (PBS) powder, and other reagents for cell culture were purchased from Sigma-Aldrich (St. Louis, MO). A antibody against phosphorylated ERK1/2 (p-ERK1/2) (sc-7383) was purchased from Santa Cruz Biotechnology (CA, USA). Antibodies against ERK1/2 (#4695), p-AKT (#4060), AKT (#4685), Caspase 3 (#9662), Bax (#5023), Bcl2 (#3498), CK-7 (#4465) and β-actin (#3180247) were purchased from Cell Signaling Technology (MA, USA).
2.2. Network pharmacology-based prediction of effects of TG on bile duct injury
First of all, all natural ingredients of TG were obtained from TCM system pharmacology database and literatures (TCMSP, https://tcmspw.com/tcmsp.php). Secondly, oral bioavailability (OB) and drug-likeness (DL) were used to select out the potential active compounds, of which threshold values were set to be: OB > 30 % and DL > 0.18 as previously described. Only components meeting the threshold value were considered as potential active ingredients. The most probable biological targets were obtained from Swiss Target Prediction (https://swisstargetprediction.ch) and further screened by searching Uniprot (https://www.uniprot.org) for human normalization. The targets related to ‘bile duct injury’ were obtained from Online Mendelian Inheritance in Man database (OMIM, https://omim.org) and Gene Cards (https://www.genecards.org). Common targets were selected but other targets were removed. In this study, targets obtained from network pharmacology were introduced into String data (https://string-db.org) and Cytoscape software, then a protein–protein interaction (PPI) network was constructed to analyze the relationship between these targets. Gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis were carried out using the cluster profiler software package.
2.3. Animal studies
Male and female BALB/c mice (8 weeks old, license number SCXK(Beijing)2021-0006) were purchased from Vital River Laboratory Animal Technology Co., ltd. (Beijing, China). Mice were housed in a temperature-controlled room [(22 ± 2)°C] with a humidity of (40 ± 10)% and 12:12-h light/dark cycle and were provided with standard chow and tap water ad libitum. TG, TP and CEL weredissolved in 0.5 % CMC-Na solution. Previous studies reported that TG at 150–200 mg/kg stimulated acute liver injury (Wang, Miao, Qu, Cui, & Zhang, 2016), and the contents of TP and CEL in TG were 0.08 % and 0.25 %, respectively (Du et al., 2019). Therefore, mice were randomly divided into seven groups (n = 6): (1) Control group; (2) TGL (150 mg/kg); (3) TGH (200 mg/kg); (4) TPL (12 μg/kg); (5) TPH (16 μg/kg); (6) CELL (37.5 μg/kg); (7) CELH (50 μg/kg). At the end of the experiment, all mice were weighed, anesthetized and sacrificed. Blood was collected for measuring liver functional enzyme activities and livers were prepared for sections or frozen in liquid nitrogen for total RNA and protein isolation. All animal studies and procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Beijing University of Chinese Medicine.
2.4. Measurement of liver functional enzyme activities, malondialdehyde and superoxide dismutase
ALT, AST and alkaline phosphatase (ALP) were measured by ALT assay kit (C009-2-1), AST assay kit (C010-2-1) and ALP assay kit (A059-2-2). Liver tissues were collected and lysed by RIPA lysis buffer. Liver malondialdehyde (MDA) and superoxide dismutase (SOD) were measured using MDA assay kit and SOD assay kit following manufacturer’s instruction, respectively. All above assay kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.5. Histopathology staining
Mice were sacrificed and the livers were immobilized with 4% FPA and embedded in paraffin. Tissues were cut into 4.5-um sections and stained with hematoxylin and eosin (H&E) as previously described (Li, Liu, Wu, & Li, 2020). The images of all slides were captured by Aperio Versa (Leica, Wetzlar, Germany).
2.6. Cell culture of HIBECs
Human biliary epithelial cells (HIBECs) were obtained from ATCC and were cultured with Minimum eagle's medium (MEM) containing 10% fatal bovine serum (FBS), penicillin G (100 U/mL) and streptomycin (100 μg/mL) in an incubator with a humidified atmosphere of 5% CO2 at 37 °C for further experiments.
2.7. Isolation and culture of mouse primary hepatocyte (MPH)
MPHs were isolated by a two-step collagenase perfusion method and cultured with Williams’ Medium E supplemented with dexamethasone (0.1%) and l-Thyroxine (0.1%) according to previous report (Liu et al., 2019).
2.8. Cell viability assay
MPH and HIBECs were seeded in 96-well plates and treated with different concentrations of TG and TP for 24 h. At the end of treatment, 10% CCK-8 working solution was added at 100 μL/well and incubated for 4 h at 37 °C. The absorbency at 450 nm was measured by micro plate spectrophotometer (Spectramax i3x). Growth (%) = [OD (treated) − OD (blank)]/[OD (control) − OD (blank) ].
2.9. Measurement of cell migration by scratch assay
HIBECs were seeded in 6-well plate and treated with TG (20, 40 and 80 μg/mL) or TP (5, 10 and 20 nmol/L) for different time points. When the cells reached a confluent state, a single scratch was made using a 100 μL sterile pipette tip. The cells were washed with PBS and photographed with a microscope. Wound closure was calculated according to the ratio of areas uncovered by cells at 0, 12 and 24 h after wound scratching.
2.10. Cell cycle analysis
After seeded, HIBECs were treated with TG (20, 40 and 80 μg/mL) or TP (5, 10, 20 nmol/L) for 24 h. After being washed twice with ice PBS, cells were fixed in 70 % ethanol at 4 °C for 2 h before staining with 500 μL PI/RNase A for 20 min at room temperature. The cell cycle was analyzed by flow cytometry (Beckman Coulter, USA).
2.11. Annexin V/PI staining
After treated with different concentrations of TG and TP for 24 h, cells were harvested by trypsin, washed with PBS and then re-suspended by 200 μL 1 × Binding Buffer with 5 μL FITC Annexin V and PI for 20 min in the dark at room temperature. After re-suspending by 400 μL 1 × Binding Buffer, cellular apoptosis was analyzed with Annexin V-FITC Apoptosis Detection Kit I by flow cytometry (Beckman Coulter, USA).
2.12. Quantitative real-time PCR (qPCR)
Total RNA in HIBECs and liver tissues in mice was extracted with Fast Pure Cell/Tissue Total RNA isolation kit (RC101-01, Vazyme Biotech) and reverse-transcribed with HiScript III RT SuperMix (R323-01, Vazyme Biotech). qPCR was used to detect the mRNA expression of targeted genes using AceQ Universal SYBR qPCR Master Mix (Q511-02, Vazyme Biotech). The mRNA levels of targeted genes were determined by qPCR normalized with Hprt1. Further inquiries of primers for qPCR can be directed to the corresponding author.
2.13. Western blotting
Total protein of livers and cell samples was extracted with RIPA buffer (Beyotime, China). After being resolved in 10% SDS-PAGE gel, equal proteins were transferred to the PVDF membrane, blocked 5% milk at room temperature for 1 h, and incubated in specific primary antibodies at 4 °C overnight. After rinsing with TBST for threetimes, proteins were incubated with corresponding secondary antibody, detected on a Bio-Rad Gel Doc XR+ Imaging System (CA, USA) and analyzed by Quantity-One software.
2.14. Statistical analysis
All results were repeated at least three independent times and were expressed as mean ± SEM. One-way ANOVA was employed to compare the differences between multiple groups using GraphPad Prism 8 (Graph-Pad, San Diego, CA). P value < 0.05 was defined statistically significant.
3. Results
3.1. Screened active compounds of TG and putative targets for bile duct injury
In this study, a total of 54 ingredients of TG were extracted from TCMSP and 12 candidate components satisfied the rules of OB > 30% and DL > 0.18 (Table 1). In addition, 44 known targets associated with bile duct injury were screened from DrugBank database and 206 targets were derived from OMIM database. After eliminating the redundancy, 228 targets related to bile duct injury were retained. As shown in Fig. 1A, we obtained 35 overlap targets by merging the candidates targets of TG and bile duct injury. Notably, TP- and CEL-related targets were respectively accounted for 46% and 29% of 35 overlap targets, while other ingredients-related targets were only accounted for 25%. These results preliminarily suggested that the toxic effects of TG on cholangiocytes may be more likely related to TP and CEL. After imported the 35 overlap targets into STRING database, the “compound-target-pathway interaction network’ was established (Fig. 1B). To scientifically unveil the underlying mechanism of TG on cholangiocyte toxicity, PPI network was obtained from Cytoscape software (Fig. 1C). Total 29 nodes and 128 edges were embodied in the network. As shown in Fig. 1C, Caspase 3, Bax, Bcl2, AKT, ERK1/2, mTOR, c-Myc, PIK3CG, TP53, JUN were the top 10 targets obtained from the Degree’s network, which were mainly involved in the molecular processes of cell migration, survival and apoptosis.
Table 1.
List of 12 ingredients selected as candidate compounds of TG.
| No. | Compounds | Molecular formula | OB (%) | DL |
|---|---|---|---|---|
| 1 | Triptolide | C20H24O6 | 51.29 | 0.68 |
| 2 | Celastrol | C29H38O4 | 37.84 | 0.78 |
| 3 | Triptonide | C20H22O6 | 31.45 | 0.68 |
| 4 | Wilforlide A | C30H4603 | 34.32 | 0.64 |
| 5 | Wilforine | C41H47NO19 | 30.51 | 0.18 |
| 6 | Wilfortrine | C41H47NO20 | 33.57 | 0.18 |
| 7 | Celafurine | C21H27N3O3 | 31.26 | 0.22 |
| 8 | Celabenzine | C23H29N3O2 | 31.54 | 0.18 |
| 9 | Celacinnine | C25H31N3O2 | 34.23 | 0.34 |
| 10 | Triptoquinone A | C20H24O4 | 30.52 | 0.46 |
| 11 | Wilfornine A | C45H41NO20 | 30.13 | 0.18 |
| 12 | Tripterinin | C20H30O4 | 30.64 | 0.18 |
Fig. 1.
Network pharmacological analysis. (A) Venn diagrams of potential targets of ‘TG’ or different ingredients and ‘bile duct injury’; (B) ‘Compound-target-pathway’ network diagram; (C) Network of potential targets of ‘TG’ and ‘bile duct injury’ analyzed by STRING. The node size and color represented the connectivity degree, and thickness of the edges represented the combine score between targets.
3.2. Network pharmacology prediction
In order to further clarified the categories of major hubs in the biological process (BP), molecular function (MF) and cellular components (CC), GO enrichment analysis was carried out on the major proteins, and the top 20 terms (P < 0.05) were selected as significant entries based on the P value. As shown in Fig. 2A, the GO categorical results indicated that the majority of hubs were enriched in the BP, mainly including apoptotic signaling, extrinsic apoptosis, amino acid starvation, necrotic cell death, organ growth, protein deacetylation, protein translocation, metabolic process, mechanical stimulus, cell proliferation, cell differentiation, biosynthetic process and immunity. To deeply verify whether aforementioned biological functions were associated with the bile duct injury and corresponding potential pathways affected by TG, KEGG pathway enrichment analysis was performed. Subsequently, 20 remarkable pathways were presented in Fig. 2B, including but not limited to apoptosis, tumor necrosis factor alpha (TNF), vascular endothelial growth factor, T helper cell 17 cell differentiation, erythroblastic leukemia viral oncogene homolog (ErbB) and B cell receptor signaling pathways. Based on the GO enrichment analysis, we further established ‘target-pathway’ network to demonstrate the relationship between overlap targets and potential pathways through polychrome lines (Fig. 2C). Consistent with GO enrichment analysis results, the target proteins or genes involved in cell apoptosis, proliferation and inflammatory response were identified, and among these targets, Caspase 3, Bax, Bcl2, ERK1/2, AKT and TNF were considered as the central link of TG-induced cholangiocyte toxicity.
Fig. 2.
GO and KEGG analysis of potential targets of ‘TG’ and ‘bile duct injury’. (A) GO analysis of potential targets of ‘TG’ and ‘bile duct injury’ by R software. (B) KEGG analysis of potential targets of ‘TG’ and ‘bile duct injury’. (C) ‘Target-pathway’ network diagram.
3.3. Hepatotoxicity of TG, TP and CEL in mice
Based on the predication of network analysis, TP and CEL were considered to be the possible toxic components in TG. Thus, we further verified the results of network pharmacology through multifarious experiments and improved our understanding of the main ingredients that contribute to TG toxicity. TG has been reported to stimulate acute liver injury in mice (Wang et al., 2015), and liquid chromatography assay confirmed the contents of TP (0.008%) and CEL (0.025%) in TG, respectively (Miao et al., 2019). According to above literatures, mice were orally administration with TG (150 mg/kg, 200 mg/kg), TP (12 μg/kg, 16 μg/kg) and CEL (37.5 μg/kg, 50 μg/kg) for 24 h, respectively. Compared with control group, histological analysis revealed the portal edema and lymphocytic infiltration caused by TG at low and high doses (Fig. 3A–C). Meanwhile, compared with high dose of TG, TP at low and high doses induced fewer lymphocytes in the portal area beside normal hepatic parenchyma in the liver (Fig. 3D and E). In addition, no obvious liver damage was observed in all CEL groups (Fig. 3F and G).
Fig. 3.
Photomicrographs of liver tissues revealed histopathological examination after different treatments stained by H&E. (A) Control, (B) TGL (150 mg/kg), (C) TGH (200 mg/kg), (D) TPL (12 μg/kg), (E) TPH (16 μg/kg), (F) CELL (37.5 μg/kg), (G) CELH (50 μg/kg) (n = 6). Scale bar = 100 µm.
Consequently, we investigated and compared the liver injury caused by TG, TP and CEL by detecting different serum transaminases. As shown in Fig. 4A, increased serum levels of ALT and AST were observed in mice treated with low and high doses of TG and TP compared to that in control group, but not in all CEL groups. Notably, the increased serum ALP level indicated the bile duct injury and cholestasis stimulated by both TG and TP (Fig. 4A, right panel). Consistently, the mRNA level of albumin (Alb), a specific marker of liver function, was slightly downregulated by TG and TP rather than CEL (Fig. 4B). Oxidative stress has been recognized as a vital factor that contributes to multiple types of liver injury. As shown in Fig. 4C, TG and TP significantly stimulated oxidative stress in the liver as demonstrated by increased hepatic MDA and decreased SOD content. In addition to continuous oxidative stress, inflammatory response also functions as a critical driver for promoting the progression of liver damage. Based on network pharmacology results, we further elucidated the effects of TG and its active components on pro-inflammatory pathways. The mRNA levels of genes associated with inflammatory reactions were determined by qPCR analysis. As shown in Fig. 4D, TG and TP dramatically increased the mRNA levels of interleukin-6 (IL-6), IL-1β and TNF-α in the liver compared to that in control group. Again, CEL didn’t cause significant changes. Collectively, these results suggested that the main contributor to cholangiocyte-related liver injury of TG was probably TP, but not CEL.
Fig. 4.
Effects of TG, TP and CEL on liver function and inflammatory response (mean ± SEM, n = 6). (A) Serum levels of ALT, AST and ALP. Relative mRNA level of (B) ALB, (D) IL-6, IL-1β and TNF-α was determined by q-PCR and normalized using HPRT1 as an internal control. (C) MDA and SOD levels in livers. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001 compared with control group. One-way ANOVA with Tukey’s post-hoc tests.
3.4. TG and TP triggered cell apoptosis via suppressing ERK1/2 and AKT pathway
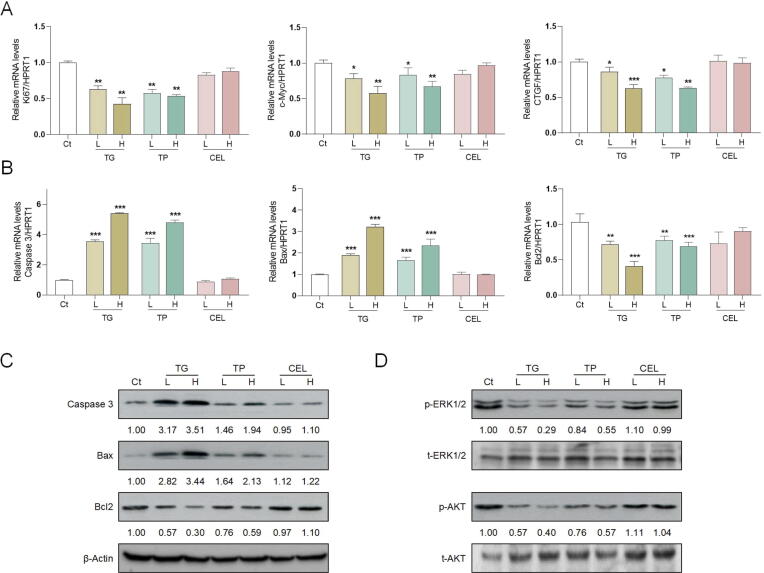
Persistent oxidative stress damage and inflammatory stimulation inevitably trigger the apoptosis and necrosis of hepatic cells (Yu, Li, Wang, & Li, 2020). Consistently, our GO and KEGG analyses showed that apoptosis pathway and cell proliferation signaling may be the most probable mechanism of bile duct injury caused by TG. Bcl2 and three Bax subtypes competitively function as typical pro- and anti-apoptosis Bcl2 family members (Estaphan, Abdel-Malek, Rashed, & Mohamed, 2020). Caspase-3 is the most abundant member of the executioner Caspases family stimulated by Bax, cleaved and activated to form cleaved Caspase-3, which plays a decisive role in the regulation of cell death (Beroske, Van den Wyngaert, Stroobants, Van der Veken, & Elvas, 2021). As shown in Fig. 5A, TG and TP downregulated the mRNA levels of proliferation marker protein Ki67 (Ki67), c-Myc and CCCTC-binding factor (CTGF), indicating a significant toxicity characterized by the inhibition of cell proliferation. Additionally, TG and TP significantly increased the mRNA and protein levels of genes associated with pro-apoptosis, including Caspase-3 (cleaved Caspase-3) and Bax, and decreased the mRNA and protein levels of anti-apoptosis gene Bcl2 (Fig. 5B and C). These results further indicated that TG and TP stimulated cholangiocyte apoptosis and inhibited cell proliferation in the liver. Our previous studies reported that the activation of ERK1/2 and AKT signaling pathways were responsible for conjugated bile acids-mediated bile duct proliferation and cholestatic liver injury (Li et al., 2018, Xiao et al., 2019). It has been reported that the phosphorylation of AKT was required for Bcl2 activation (S. Li et al., 2020) and the phosphorylation of ERK1/2 signaling pathway was associated with the development of various liver diseases (Chen, Wang, Zong, & Yang, 2021). Furthermore, we explored whether the phosphorylation of AKT and ERK1/2 were involved in the effects of TG and TP on bile duct injury. As shown in Fig. 5D, both low and high doses of TG and TP significantly inhibited the phosphorylation of ERK1/2 and AKT but didn’t decrease the expression of total ERK1/2 and AKT. Consistent with in vivo results, CEL didn’t exert obvious influence on the phosphorylation and expression of ERK1/2 and AKT. Collectively, our results demonstrated that accompanied with inhibiting the phosphorylation of ERK1/2 and AKT, TG and TP obviously inhibited cholangiocyte proliferation and promoted cell apoptosis.
Fig. 5.
Effects of TG, TP and CEL on apoptosis- and proliferation-related targets and ERK1/2 and AKT signaling pathways in liver (mean ± SEM, n = 6). Relative mRNA levels of (A) Ki67, c-Myc, CTGF, (B) Caspase 3, Bax and Bcl2 were determined by q-PCR and normalized using HPRT1 as an internal control. Representative immunoblots against (C) cleaved-Caspase 3, Bax, Bcl2, (D) p-ERK1/2, t-ERK1/2, p-AKT, t-AKT and β-Actin were shown. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001 vs control group..
3.5. TG and TP stimulated cholangiocyte-related liver injury
Considering the network analysis and in vivo results, TG and TP might induce cholangiocyte-related liver injury, as evidenced by increased ALP level and disrupted bile duct architecture. We then supposed that the hepatotoxicity of TG and TP may be associated with cholangiocyte damage and subsequently explored the influences of TG, TP and CEL on the expression of hepatocyte marker, hepatocyte nuclear factor 4-alpha (HNF-4α) and cholangiocyte marker, cytoskeletal 19 (CK-19). Consist with our assumption, the mRNA level of HNF-4α was not altered by TG, TP or CEL (Fig. 6A). Interestingly, compared with CEL group, both low and high doses of TG and TP significantly downregulated the mRNA level of CK-19 in livers, indicating the possible causal association between cholangiocyte damage and liver injury stimulated by TG and TP. In addition, we evaluated the influences of TG on the cell proliferation of MPHs and HIBECs, a selected biliary epithelial cell line. As shown in Fig. 6B, the CCK-8 results showed that the IC50 of TG on MPHs and HIBECs were 120.31 μg/mL (Log10 = 2.08) and 40.10 μg/mL (Log10 = 1.34), respectively, indicating a higher sensitivity of cholangiocyte to TG toxicity. In addition, we identified that the IC50 of TP on HIBECs was 12.57 nmol/L (Log10 = 1.10) and less than that IC50 of TG, suggesting a more obvious cytotoxicity of TP on the proliferation and survival of cholangiocytes (Fig. 6C). While, the IC50 of CEL on HIBECs was 821.52 nmol/L (data not shown) and was 37 and 65-fold higher than that of TG and TP, indicating that CEL didn’t cause cholangiocyte damage at the equivalent dose of TG. Therefore, doses below IC50 were selected to evaluate the influences of TG and TP on cholangiocyte migration. As shown in Fig. 6D, TG and TP significantly inhibited cell migration, as illustrated by increased migration distance of HIBECs at 12 and 24 h. Again, these results confirmed that TP may be the main contributor to the toxic effects of TG on cholangiocytes.
Fig. 6.
Effects of TG and TP on expression of hepatic cell markers, cell vitality and migration in HIBECs (mean ± SEM, n = 3). (A) Relative mRNA levels of HNF-4α and CK-19 were determined by q-PCR and normalized using HPRT1 as an internal control. (B) Cell vitality of TG on MPHs and HIEBCs was detected by CCK-8 assay. (C) Cell vitality of TP on HIEBCs was detected by CCK-8 assay. (D) Cell migration was detected by cell scratch assay. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001 vs control group (0 h). $P < 0.05, $$P < 0.01, $$$P < 0.001 vs control group (12 h). #P < 0.05, #P < 0.01, ###P < 0.001 vs control group (24 h).
3.6. TG and TP arrested cell cycle at G0/G1 and s phase and promoted cholangiocyte apoptosis
To examine the molecular mechanism of TG- and TP-mediated cell apoptosis, cell cycle progression was evaluated by flow cytometry analysis after treated with different concentrations of TG or TP for 24 h. As shown in Fig. 7A, compared to the control group, 80 μg/mL TG significantly elevated the cell population at G0/G1 and S phase and suppressed the cell percentage in the G2 phase. On the other hand, 10 and 20 nmol/L TP markedly upregulated the cell percentage in the G0/G1 and S phases and downregulated the population size of cells at G2 phase. To further confirm the effects of TG and TP on cell growth arrest, cell apoptosis was assessed by Annexin V and PI staining. Flow cytometry studies in Fig. 7B confirmed that TG and TP dose-dependently increased the populations of early and late apoptotic HIBECs. Therefore, our data provided evidence supporting that TG and TP induced cholangiocyte injury involving arresting cell cycle and driving apoptosis, and the toxicity of TP on cholangiocytes was more obvious than that of TG.
Fig. 7.
Effects of TG and TP on cell cycle and apoptosis in HIBECs (mean ± SEM, n = 3). (A) The cell cycle was detected by PI staining and flow cytometry. (B) The cell apoptosis was detected by AV/PI staining and flow cytometry. Representative images were shown. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001 vs control group. One-way ANOVA with Tukey’s post-hoc tests.
3.7. TG and TP stimulated apoptosis and inhibited proliferation via abrogating ERK1/2 and AKT signaling pathway in cholangiocytes
To further verify the inhibitory effects of TG and TP on ERK1/2 and AKT signaling observed in vivo, the phosphorylation of ERK1/2 and AKT were determined in HIEBC cells upon TG and TP treatments at different dosages. As shown in Fig. 8A, the phosphorylation of ERK1/2 and AKT was dramatically decreased after TG and TP treatments, suggesting that TG and TP may mediate MAPK/PI3K signaling pathways in cholangiocytes. We further investigated the effects of TG and TP at different concentrations on proliferation-associated genes. As shown in Fig. 8B, TG and TP significantly downregulated the mRNA levels of Ki67, c-Myc and CTGF in HIBECs. In support of the results from Annexin V-PI staining, qPCR results showed that TG and TP markedly upregulated the mRNA levels of several pro-apoptosis genes, like Caspase 3 and Bax, and downregulated the mRNA level of anti-apoptosis gene, Bcl2 (Fig. 8C). Additionally, TG and TP markedly enhanced the protein levels of cleaved-Caspase 3 and Bax, and downregulated Bcl2 protein levels (Fig. 8D). These results suggested that TG and TP promoted cholangiocyte apoptosis and inhibited cell proliferation by decreasing the phosphorylation of ERK1/2 and AKT and activating Caspase-dependent apoptotic pathways (Fig. 9).
Fig. 8.
Effects of TG and TP on apoptosis- and proliferation-related targets and ERK1/2 and AKT signaling pathways in HIBECs (mean ± SEM, n = 3). Representative immunoblots against (A) p-ERK1/2, t-ERK1/2, p-AKT, t-ERK1/2, (D) Caspase-3, Bax, Bcl2 and β-actin were shown. Relative mRNA levels of (B) Ki67, c-Myc, CTGF, (C) cleaved-Caspase 3, Bax and Bcl2 were determined by q-PCR and normalized using HPRT1 as an internal control. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001 vs control group.
Fig. 9.
Schematic diagram of proposed mechanisms by which TG and TP stimulated cholangiocyte-related liver injury.
4. Discussion
Drug-induced liver injury is the leading cause of acute liver failure throughout the global, which may further trigger multiple liver diseases, including cholestasis, hepatitis and liver fibrosis (Kaliyaperumal et al., 2018). In current study, we systematically explored the underlying mechanisms of TG and its representative ingredients on cholangiocyte-related liver injury through network pharmacology analysis and experimental verification. After screened out 54 compounds with standard-compliant OB, DL or activities and retained 228 targets related to bile duct injury, we further obtained 35 overlap targets and in particular, found that TP and CEL occupied the majority of TG-related targets of bile duct injury (Fig. 1). The complex and interlaced PPI network indicated that several typical targets and pathways involved in cell apoptosis, proliferation and inflammatory response have high probabilities of combination with TG ingredients (Fig. 2). Finally, these mechanisms were verified by in vivo and in vitro experiments. By comparing the toxic effects of TG, TP and CEL in vivo, we first demonstrated that only TG and TP at equivalent dosage rapidly caused cholangiocyte damage and triggered liver injury (Figs. 3–5). Among different liver cells (IC50 of TG on Kupffer cells and hepatic stellate cells were not shown), cholangiocytes were more sensitive to TG- and TP-induced inhibition of cell proliferation and migration, cell cycle arrest at G0/G1 and S phase and cell apoptosis, which was correlated with the downregulation of ERK1/2 and AKT and upregulation of Bax and cleaved-Caspase 3 (Figs. 6–8).
TG has been widely used in the treatment of immune-related diseases for thousands of years. In recent years, TG are highly selective for the treatment of several diseases, including rheumatoid arthritis and systemic lupus erythematous (Li, Huang, Liu, Yuan, & Zhao, 2021). However, significant challenges remain to overcome before TG can reach its clinical potential. A growing number of studies havedemonstrated that TG-induced hepatotoxicity depends on dosage and administration times and is also associated with the activation of oxidative stress, cell cycle arrest, apoptosis and mitochondrial damage (Wang et al., 2016). Several ingredients in TG, including TP, CEL and wilforgine, may contribute to its pharmacological effects and even in some cases of hepatotoxicity. Thus, the identification of specific ingredients associated with TG toxicity may improve the safety of TG usage in clinical practice. TP, the most reported ingredient isolated from TG, was identified as a potential therapeutic against several autoimmune diseases (Huang et al., 2019). While, TP was also reported to stimulate liver injury via inducing oxidative stress in the liver and these effects were aggravated by abolishing the hepatic CYP450 enzymes (Yuan et al., 2019). As another representative ingredient isolated from TG, CEL exhibited anti-inflammatory and anti-tumor effects against many diseases including nonalcoholic fatty liver disease and cirrhosis (Yan et al., 2021). Unlike TP, the toxic effects of CEL are rarely reported, and only few of them revealed it-induced cytotoxicity on primary rat hepatic cells via increasing ROS production and stimulating early apoptosis (Jin, Wu, Wang, Kanai, & He, 2019).In the current study, TG and TP at equivalent dose rather than CEL stimulated cholangiocyte-related liver injury both in vitro and in vivo.
Cholangiocyte, secreting HCO3– rich fluid for protecting luminal, are responsible for maintaining the bile acid balance in bile duct, indicating the strong connection between cholangiocytes and bile acid homeostasis (Matton et al., 2019). Bile acids, the major amphipathic components in bile, participate in regulating cholangiocyte proliferation, and lipid and glucose metabolism (Armstrong & Guo, 2017). TG was reported to trigger excessive bile acid accumulation and metabolic dysfunction in liver, and interrupt the bile flow from liver to intestine, subsequently resulting in fibrotic liver injury (Peng, Dai, Bao, Zhu, & Li, 2021). Meanwhile, TP caused the bile acid accumulation in the liver by decreasing the expressions of bile acid transporter and CYP7A1, suggesting a potential damage to cholangiocytes (Yang et al., 2017). However, existing accounts fail to confirm the causal relationship between TG- and TP-induced cholangiocyte injury and bile acid homeostasis disruption. We previously identified that after leaking from damaged cholangiocytes under cholestatic condition, exosomal long non-coding RNA H19 (lncRNA H19) contributed to activate Kupffer cells and drive inflammatory response and liver damage (Li, R. Liu, et al., 2020). In current study, we first verified the specificity and sensitivity of cholangiocytes in TG-stimulated liver injury via comparing toxic effects of TG on hepatocytes, cholangiocytes (Fig. 6), Kupffer cells and hepatic stellate cells (data not shown), respectively. Additionally, TG- and TP-induced cholangiocyte-related liver injury were also accompanied with elevated levels of IL-6, IL-1β and TNF-α (Fig. 4), suggesting that injured cholangiocyte may further trigger inflammatory reactions to aggravate liver injury. Thus, these results innovatively proposed a novel perspective to describe the hepatotoxicity of TG, focused on cholangiocyte-related liver injury.
Notably, TG showed more significant liver damage than TP in vivo, as indicated by a greater degree of increased serum levels of ALT and AST and more severe pathological destruction of the liver (Fig. 3A–E and Fig. 4A). However, TP more obviously inhibited cholangiocyte proliferation and migration, retard cell cycle and promoted cell apoptosis, indicating the more severe cytotoxic effects than TG in vitro. Mechanistically speaking, Hu et al. (2020) applied a novel metabolomics analysis and first identified 10 metabolites of TG, including cytosine, 5-methyluridine, nordeoxycholic acid and isodeoxycholic acid. Among these metabolites, increased nordeoxycholic acid and isodeoxycholic acid might further alter BA composition, aggravate hepatotoxicity and result in a positive feedback cycle of cholangiocyte injury. In contrast to TG, TP was extensively eliminated in rat and hydroxylation was the major phase I metabolic pathway (Du, Liu, Li, & Xing, 2014). These studies suggest that the gentler toxicity of TP is more likely attributable to its metabolism and provide a possible explanation for the difference between TG- and TP-induced cholangiocyte-related liver injury in vivo. On the other hand, Wei et al. (2019) demonstrated that TP directly stimulated cytotoxic and induced oxidative stress by increasing autophagosome formation in human normal hepatocytes, suggesting that TP may not only induce bile duct injury by itself but also trigger cholangiocyte damage through intercellular communication.
5. Conclusion
Collectively, we applied network and experimental pharmacology to investigate the toxic effects and mechanism of TG-stimulated cholangiocyte-related liver injury. TG and its toxic ingredient, TP, markedly increased levels of serum transaminase and oxidative stress, inhibited cell proliferation and migration, blocked cell cycle and promoted cell apoptosis of cholangiocytes via suppressing phosphorylation of ERK1/2 and AKT and activating Bax and cleaved Caspase-3 signalings both in vivo and in vitro. Overall, our results provide a new perspective for TG-induced liver injury and offer novel insights for the importance of cholangiocyte in the evaluation of drug-induced liver injury.
Authors’ contributions
XL conceived the original idea and supervised the study. XL, YL, SL, XX and TW prepared the manuscript and figures. YL conducted all the experiments and performed data analysis. All authors have approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by grants from Beijing Nova Program of Science & Technology (Grant No. Z191100001119088 to XL); Beijing Municipal Science & Technology Commission (Grant No. 7212174 to XL); National Natural Science Foundation of China (Grant No. 82004045 to XL); Young Talents Promotion Project of China Association of Traditional Chinese Medicine (Grant No. 2020-QNRC2-01 to XL); Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (Grant No. ZYYCXTD-C-202006 to XL).
Contributor Information
Ting Wang, Email: wangting1973@sina.com.
Xiaojiaoyang Li, Email: xiaojiaoyang.li@bucm.edu.cn.
References
- Armstrong L.E., Guo G.L. Role of FXR in liver inflammation during nonalcoholic steatohepatitis. Current Pharmacology Reports. 2017;3(2):92–100. doi: 10.1007/s40495-017-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beroske L., Van den Wyngaert T., Stroobants S., Van der Veken P., Elvas F. Molecular imaging of apoptosis: The case of Caspase-3 radiotracers. International Journal of Molecular Sciences. 2021;22(8):3948. doi: 10.3390/ijms22083948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Xu B., Zhou F., Wu J., Li S., Zheng Q.…Liu R. Si-Ni-San ameliorates chronic colitis by modulating type I interferons-mediated inflammation. Phytomedicine. 2021;84 doi: 10.1016/j.phymed.2021.153495. [DOI] [PubMed] [Google Scholar]
- Chen J., Wang D., Zong Y., Yang X. DHA protects hepatocytes from oxidative injury through GPR120/ERK-mediated mitophagy. International Journal of Molecular Sciences. 2021;22(11):5675. doi: 10.3390/ijms22115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W., Tang T., Dai Z., Shi D., Mo L., Zhang Y. Probing the mechanism of hepatotoxicity of hexabromocyclododecanes through toxicological network analysis. Environmental Science & Technology. 2020;54(23):15235–15245. doi: 10.1021/acs.est.0c03998. [DOI] [PubMed] [Google Scholar]
- Dobreva I., Karagyozov P. Drug-induced bile duct injury – ashort review. Current Drug Metabolism. 2020;21(4):256–259. doi: 10.2174/1389200221666200420100129. [DOI] [PubMed] [Google Scholar]
- Du F., Liu Z., Li X., Xing J. Metabolic pathways leading to detoxification of triptolide, a major active component of the herbal medicine Tripterygium wilfordii. Journal of Applied Toxicology. 2014;34(8):878–884. doi: 10.1002/jat.2906. [DOI] [PubMed] [Google Scholar]
- Du X., He X., Huang Y., Fu B., Liang B., Lv C.…Wang Y. Simultaneous determination of seven effective components of tripterygium glycosides in human biological matrices by ultra performance liquid chromatography-triple quadrupole mass spectrometry. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2019;1113:1–13. doi: 10.1016/j.jchromb.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Estaphan S., Abdel-Malek R., Rashed L., Mohamed E.A. Cimetidine a promising radio-protective agent through modulating Bax/Bcl2 ratio: An in vivo study in male rats. Journal of Cellular Physiology. 2020;235(11):8495–8506. doi: 10.1002/jcp.29692. [DOI] [PubMed] [Google Scholar]
- Feng Y., Le F., Tian P., Zhong Y., Zhan F., Huang G.…Tan B. GTW inhibits the epithelial to mesenchymal transition of epithelial ovarian cancer via ILK/AKT/GSK3β/Slug signalling pathway. Journal of Cancer. 2021;12(5):1386–1397. doi: 10.7150/jca.52418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T., Shi C., Liu L., Li P., Sun Y., An Z. A single-injection targeted metabolomics profiling method for determination of biomarkers to reflect tripterygium glycosides efficacy and toxicity. Toxicology and Applied Pharmacology. 2020;389 doi: 10.1016/j.taap.2020.114880. [DOI] [PubMed] [Google Scholar]
- Huang W., Liu C., Xie L., Wang Y., Xu Y., Li Y. Integrated network pharmacology and targeted metabolomics to reveal the mechanism of nephrotoxicity of triptolide. Toxicology Research Camb. 2019;8(6):850–861. doi: 10.1039/c9tx00067d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Wu Z., Wang L., Kanai Y., He X. CYP450s-activity relations of celastrol to interact with triptolide reveal the reasons of hepatotoxicity of Tripterygium wilfordii. Molecules. 2019;24(11):2162. doi: 10.3390/molecules24112162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X., Cheng W., Guo S., Zou Y., Zhang T., He L. Toxic effects of Tripterygium wilfordii Hook F on the reproductive system of adolescent male rats. Biomedicine & Pharmacotherapy. 2017;95:1338–1345. doi: 10.1016/j.biopha.2017.09.038. [DOI] [PubMed] [Google Scholar]
- Kaliyaperumal K., Grove J.I., Delahay R.M., Griffiths W.J.H., Duckworth A., Aithal G.P. pharmacogenomics of drug-induced liver injury (DILI): Molecular biology to clinical applications. Journal of Hepatology. 2018;69(4):948–957. doi: 10.1016/j.jhep.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Li Q., Huang Y., Liu P., Yuan H., Zhao J. effect of Tripterygium wilfordii polyglycoside tablets on serum inflammatory factors and T cells in patients with chronic nephritis. American Journal of Translational Research. 2021;13(7):8385–8390. [PMC free article] [PubMed] [Google Scholar]
- Li S., Qin Q., Luo D., Pan W., Wei Y., Xu Y.…Shang L. Hesperidin ameliorates liver ischemia/reperfusion injury via activation of the Akt pathway. Molecular Medicine Reports. 2020;22(6):4519–4530. doi: 10.3892/mmr.2020.11561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Wang Y., Li C., Yang N., Yu H., Zhou W.…Li Y. Study on hepatotoxicity of rhubarb based on metabolomics and network pharmacology. Drug Design Development and Therapy. 2021;15:1883–1902. doi: 10.2147/DDDT.S301417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Liu R., Huang Z., Gurley E.C., Wang X., Wang J.…Zhou H. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes cholestatic liver injury in mouse and humans. Hepatology. 2018;68(2):599–615. doi: 10.1002/hep.29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Liu R., Wang Y., Zhu W., Zhao D., Wang X.…Zhou H. Cholangiocyte-derived exosomal lncRNA H19 promotes macrophage activation and hepatic inflammation under cholestatic conditions. Cells. 2020;9(1):190. doi: 10.3390/cells9010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yang H., Xiao J., Zhang J., Zhang J., Liu M.…Ma L. Network pharmacology based investigation into the bioactive compounds and molecular mechanisms of Schisandrae Chinensis Fructus against drug-induced liver injury. Bioorganic Chemistry. 2020;96 doi: 10.1016/j.bioorg.2019.103553. [DOI] [PubMed] [Google Scholar]
- Li X.J., Jiang Z.Z., Zhang L.Y. Triptolide: Progress on research in pharmacodynamics and toxicology. Journal of Ethnopharmacology. 2014;155(1):67–79. doi: 10.1016/j.jep.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Li Y., Liu R., Wu J., Li X. Self-eating: Friend or foe? The emerging role of autophagy in fibrotic diseases. Theranostics. 2020;10(18):7993–8017. doi: 10.7150/thno.47826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Shi Y.C., Lee D.Y. Applications of Pueraria lobata in treating diabetics and reducing alcohol drinking. Chinese Herbal Medicines. 2019;11(2):141–149. doi: 10.1016/j.chmed.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Li X., Zhu W., Wang Y., Zhao D., Wang X.…Zhou H. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes hepatic stellate cell activation and cholestatic liver fibrosis. Hepatology. 2019;70(4):1317–1335. doi: 10.1002/hep.30662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Qi H., Li J., Xu H., Chi B., Zhu J.…Zhang Q. Triptolide disrupts fatty acids and peroxisome proliferator-activated receptor (PPAR) levels in male mice testes followed by testicular injury: A GC-MS based metabolomics study. Toxicology. 2015;336:84–95. doi: 10.1016/j.tox.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Ma B.N., Li X.J. Resveratrol extracted from Chinese herbal medicines: A novel therapeutic strategy for lung diseases. Chinese Herbal Medicines. 2020;12(4):349–358. doi: 10.1016/j.chmed.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matton A.P.M., de Vries Y., Burlage L.C., van Rijn R., Fujiyoshi M., de Meijer V.E.…Porte R.J. Biliary bicarbonate, pH, and glucose are suitable biomarkers of biliary viability during ex situ normothermic machine perfusion of human donor livers. Transplantation. 2019;103(7):1405–1413. doi: 10.1097/TP.0000000000002500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y.Y., Luo L., Shu T., Wang H., Jiang Z.Z., Zhang L.Y. Study on difference of liver toxicity and its molecular mechanisms caused by Tripterygium wilfordii multiglycoside and equivalent amount of triptolid in rats. China Journal of Chinese Materia Medica. 2019;44(16):3468–3477. doi: 10.19540/j.cnki.cjcmm.20190301.002. [DOI] [PubMed] [Google Scholar]
- Österreicher C.H., Trauner M. Xenobiotic-induced liver injury and fibrosis. Expert Opinion on Drug Metabolism & Toxicology. 2012;8(5):571–580. doi: 10.1517/17425255.2012.674511. [DOI] [PubMed] [Google Scholar]
- Peng W., Dai M.Y., Bao L.J., Zhu W.F., Li F. FXR activation prevents liver injury induced by Tripterygium wilfordii preparations. Xenobiotica. 2021;51(6):716–727. doi: 10.1080/00498254.2021.1900626. [DOI] [PubMed] [Google Scholar]
- Visentin, M., Lenggenhager, D., Gai, Z., & Kullak-Ublick, G. A. (2018). Drug-induced bile duct injury. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1864(4 Pt B), 1498-1506. [DOI] [PubMed]
- Wang J., Miao M., Qu L., Cui Y., Zhang Y. Protective effects of geniposide against Tripterygium glycosides (TG)-induced liver injury and its mechanisms. Journal of Toxicological Sciences. 2016;41(1):165–173. doi: 10.2131/jts.41.165. [DOI] [PubMed] [Google Scholar]
- Wang J., Miao M., Zhang Y., Liu R., Li X., Cui Y., Qu L. Quercetin ameliorates liver injury induced with Tripterygium glycosides by reducing oxidative stress and inflammation. Canadian Journal of Physiology and Pharmacology. 2015;93(6):427–433. doi: 10.1139/cjpp-2015-0038. [DOI] [PubMed] [Google Scholar]
- Wang X.Z., Xue R.F., Zhang S.Y., Zheng Y.T., Zhang L.Y., Jiang Z.Z. Activation of natural killer T cells contributes to triptolide-induced liver injury in mice. Acta Pharmacologica Sinica. 2018;39(12):1847–1854. doi: 10.1038/s41401-018-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y.M., Luan Z.H., Liu B.W., Wang Y.H., Chang Y.X., Xue H.Q., Ren J.H. Autophagy in triptolide-mediated cytotoxicity in hepatic cells. International Journal of Toxicology. 2019;38(5):436–444. doi: 10.1177/1091581819864518. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Liu R., Li X., Gurley E.C., Hylemon P.B., Lu Y.…Cai W. Long noncoding RNA H19 contributes to cholangiocyte proliferation and cholestatic liver fibrosis in biliary atresia. Hepatology. 2019;70(5):1658–1673. doi: 10.1002/hep.30698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.Y., Ouyang S.H., Wang X., Wu Y.P., Sun W.Y., Duan W.J.…He R.R. Celastrol ameliorates propionibacterium acnes/LPS-induced liver damage and MSU-induced gouty arthritis via inhibiting K63 deubiquitination of NLRP3. Phytomedicine. 2021;80 doi: 10.1016/j.phymed.2020.153398. [DOI] [PubMed] [Google Scholar]
- Yang J., Sun L., Wang L., Hassan H.M., Wang X., Hylemon P.B.…Jiang Z. Activation of Sirt1/FXR signaling pathway attenuates triptolide-induced hepatotoxicity in rats. Frontiers in Pharmacology. 2017;8:260. doi: 10.3389/fphar.2017.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Li Q., Wang Y., Li P. Apotent protective effect of baicalein on liver injury by regulating mitochondria-related apoptosis. Apoptosis. 2020;25(5–6):412–425. doi: 10.1007/s10495-020-01608-2. [DOI] [PubMed] [Google Scholar]
- Yuan Z., Hasnat M., Liang P., Yuan Z., Zhang H., Sun L.…Jiang Z. The role of inflammasome activation in triptolide-induced acute liver toxicity. International Immunopharmacology. 2019;75 doi: 10.1016/j.intimp.2019.105754. [DOI] [PubMed] [Google Scholar]
- Zhang S., Lu Y., Chen W., Shi W., Zhao Q., Zhao J., Li L. Network pharmacology and experimental evidence: PI3K/AKT signaling pathway isinvolved in the antidepressive roles of Chaihu Shugan San. Drug Design Development and Therapy. 2021;15:3425–3441. doi: 10.2147/DDDT.S315060. [DOI] [PMC free article] [PubMed] [Google Scholar]