Abstract
Introduction
Asthma exacerbations in pregnancy are associated with adverse perinatal outcomes. We aimed to determine whether fractional exhaled nitric oxide (FENO)-based asthma management improves perinatal outcomes compared to usual care.
Methods
The Breathing for Life Trial was a multicentre, parallel-group, randomised controlled trial conducted in six hospital antenatal clinics, which compared asthma management guided by FENO (adjustment of asthma treatment according to exhaled nitric oxide and symptoms each 6–12 weeks) to usual care (no treatment adjustment as part of the trial). The primary outcome was a composite of adverse perinatal events (preterm birth, small for gestational age (SGA), perinatal mortality or neonatal hospitalisation) assessed using hospital records. Secondary outcomes included maternal asthma exacerbations. Concealed random allocation, stratified by study site and self-reported smoking status was used, with blinded outcome assessment and statistical analysis (intention to treat).
Results
Pregnant women with current asthma were recruited; 599 to the control group (608 infants) and 601 to the intervention (615 infants). There were no significant group differences for the primary composite perinatal outcome (152 (25.6%) out of 594 control, 177 (29.4%) out of 603 intervention; OR 1.21, 95% CI 0.94–1.56; p=0.15), preterm birth (OR 1.14, 95% CI 0.78–1.68), SGA (OR 1.06, 95% CI 0.78–1.68), perinatal mortality (OR 3.62, 95% CI 0.80–16.5), neonatal hospitalisation (OR 1.24, 95% CI 0.89–1.72) or maternal asthma exacerbations requiring hospital admission or emergency department presentation (OR 1.19, 95% CI 0.69–2.05).
Conclusion
FENO-guided asthma pharmacotherapy delivered by a nurse or midwife in the antenatal clinic setting did not improve perinatal outcomes.
Short abstract
Asthma pharmacotherapy guided by fractional exhaled nitric oxide and delivered by a nurse or midwife in the antenatal clinic setting did not improve perinatal outcomes and there was no significant difference in asthma exacerbations between groups https://bit.ly/3LdbJ8V
Introduction
Asthma is one of the most prevalent chronic diseases affecting pregnancy. Worldwide, 8–12% of pregnant women have asthma [1] and exacerbations requiring medical intervention affect 20–45% of these women [2, 3]. Asthma, and exacerbations in particular, are associated with increased risk of adverse perinatal outcomes [4–6]. A meta-analysis showed that women with asthma exacerbations during pregnancy were three times more likely to have a low birthweight baby (relative risk 3.02, 95% CI 1.87–4.89) compared to women with asthma but without exacerbations [4]. Women with asthma were more likely to have a preterm birth than women without asthma [5]; the risk further increased among women using oral corticosteroids (OCS) for exacerbations in pregnancy [4]. There is an increased risk of perinatal mortality and neonatal hospitalisation, with maternal asthma [6, 7]. Active asthma management mitigates adverse outcomes including preterm birth [5], leading us to hypothesise that improvements in asthma management may also improve other perinatal outcomes.
Few studies have tested interventions to improve outcomes among pregnant women with asthma [8]. Our previous trial, the Managing Asthma in Pregnancy (MAP) study, tested asthma management with treatment adjustment using an objective marker of eosinophilic lung inflammation (fractional exhaled nitric oxide (FENO)), among 220 nonsmoking women [9]. Women were randomised by 22 weeks’ gestation to a control algorithm, which adjusted asthma treatment monthly based on symptoms, or a FENO-based algorithm, which adjusted inhaled corticosteroid (ICS) treatment monthly based on FENO, and added long-acting β-agonist (LABA) when symptoms remained uncontrolled. Women in the FENO group had a longer exacerbation-free interval, 50% fewer exacerbations and were more likely to be prescribed ICS (or ICS/LABA), but at a lower mean dose [9]. Perinatal outcomes showed trends towards improvements in the FENO group, e.g. reduced neonatal hospitalisation (8% FENO group, 17% control group), although the trial was not powered for perinatal differences. Follow-up identified reduced parent-reported recurrent bronchiolitis in infancy [10], and less doctor-diagnosed asthma at preschool age in the FENO-group compared to the control group [11].
Prior literature and these data led to our hypothesis that by reducing maternal exacerbations, asthma management using a FENO-guided algorithm would improve perinatal outcomes among women with asthma.
Material and methods
Study subjects and study design
The Breathing for Life Trial (BLT) was a multicentre, parallel-group randomised controlled trial (RCT) (Australian New Zealand Clinical Trials Registry identifier ACTRN12613000202763) [12]. Pregnant women were recruited from six Australian public hospital antenatal clinics (7 March 2013 to 11 June 2019). Participants had doctor-diagnosed asthma as used previously in research trials [9], symptoms of asthma and/or asthma medication use (prior 12 months), were aged ≥18 years and between 12 and <23 completed weeks’ gestation at randomisation. Exclusion criteria were chronic lung disease other than asthma, use of OCS >14 days in the past 3 months, concomitant chronic illness which may affect participation, inability to perform FENO or spirometry (due to medical contraindication), drug or alcohol dependence or inability to attend regular study visits. All women gave written informed consent prior to participation. Ethics approval was from the Hunter New England Human Research Ethics Committee (HREC; 12/10/17/3.04) and Australian Capital Territory Health HREC (ETH.11.15.232).
Concealed random allocation randomised women in equal numbers to the control or intervention (FENO) group using a computer-generated randomisation schedule (blocks of four or six), stratified by study site and self-reported smoking. Participants and research staff could not be blinded, due to the differing number of study visits for each group. However, primary outcome assessment and statistical analysis were conducted blind to treatment allocation.
Baseline and outcome measures for all participants
We collected baseline data on maternal age, height, weight, ethnicity, asthma history, symptoms and medication use, lung function by spirometry and exhaled carbon monoxide (ECO), as described previously (supplementary material) [12]. Women were considered current smokers if they self-reported smoking, or ECO ≥10 ppm. Socioeconomic status was determined from residential postcode and the Socio-Economic Indexes for Areas [13], expressed as quintiles; quintile 1 represents areas of most disadvantage. Asthma control was categorised as well controlled, partly controlled or uncontrolled according to Global Initiative for Asthma criteria [14], using data on asthma symptoms and short acting β2-agonist (SABA) use in the previous week.
The primary outcome was a composite of four adverse perinatal events: preterm birth (<37 weeks’ gestation), small for gestational age (SGA; birthweight <10th centile adjusted for sex, parity, maternal height, weight and ethnicity using the Gestation Network calculator (http://gestation.net)), perinatal mortality (stillbirth ≥20 weeks’ gestation, or neonatal death within the first month) or neonatal hospitalisation at birth (neonatal intensive care unit (level III) or special care nursery (level II)). Pregnancies ending in miscarriage (<20 weeks’ gestation) were excluded from analyses. Secondary outcomes included each component of the composite, birthweight and maternal asthma exacerbations (hospitalisation; hospitalisation/emergency department presentation; and exacerbations requiring medical intervention including hospitalisation, emergency department presentation, OCS use, unscheduled doctor visits).
Perinatal outcomes were ascertained from medical records by a blinded outcome assessor. Maternal exacerbations were assessed by self-report (2–6 weeks post-partum) and verified by medical record review where possible. Women reported the date of exacerbation events and treatment changes required. Exacerbations separated by ≥7 days were counted as separate events.
Outcome measures collected in the intervention group only
Additionally in the FENO group, asthma treatment data (current medication use and self-reported adherence to ICS in past week [15]), FENO and Asthma Control Questionnaire (ACQ) score were recorded at all study visits.
Interventions that applied to all participants
In both groups, brief asthma self-management education was provided by the research nurse/midwife, including assessment and correction of inhaler technique, assessment and discussion of medication knowledge, assessment of written asthma action plan and discussion of asthma triggers [15, 16]. A consumer-focused pamphlet on asthma in pregnancy was provided [17]. A letter to the woman's general practitioner informed them of the woman's participation in the trial and women in both groups continued with separate and usual antenatal care.
Women in the control group received no treatment changes as part of the trial.
Intervention in the FENO group
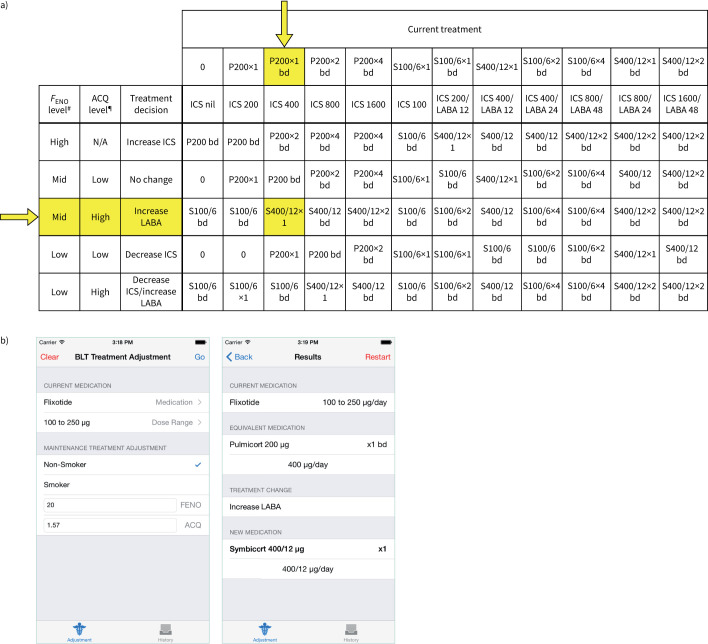
Women randomised to the intervention group also received FENO-based asthma management for the remainder of pregnancy. Women attended visits every 3–6 weeks during pregnancy aligned with antenatal appointments, where self-management education was reinforced, and asthma control, lung function and FENO assessed. Asthma treatment was adjusted at the first visit, and every second visit thereafter (every 6–12 weeks) and medication provided free of charge, dispensed from the hospital pharmacy. Women received an equivalent dose of budesonide or budesonide/eformoterol (supplementary table S1), due to budesonide's better safety rating in pregnancy. FENO was used to adjust ICS dose (when levels were above the high cut-point, ICS dose was increased, while ICS dose was decreased when levels were below the low cut-point), while LABA was added when symptoms based on the ACQ (ACQ7/ACQ6 >1.5) [18, 19] remained uncontrolled, unless FENO was high, when only the ICS dose was adjusted (figure 1) [9, 12], consistent with previous studies [20]. A custom mobile application allowed algorithm application in the clinical setting using an iPad.
FIGURE 1.
Fractional exhaled nitric oxide (FENO) treatment algorithm. The FENO treatment algorithm and FENO cut-points were derived from pilot data from pregnant women with asthma, as described previously [9]. FENO was measured and the Asthma Control Questionnaire (ACQ) score calculated. We used the seven-item questionnaire, or the six-item questionnaire if quality spirometry results could not be obtained (minimum score of 0, maximum score of 6 [18]; scores >1.5 indicate uncontrolled asthma) [19]. Results were entered into an application on an iPad along with smoking status and current treatment. Current treatments were converted into the equivalent doses of Pulmicort or Symbicort (supplementary table S1) and then the algorithm was applied to give the new treatment. a) Current treatment options are presented horizontally and the treatment decision options vertically. Arrows and highlighted cells correspond to the treatment adjustment example in panel b). b) Clinical example showing screenshots from the iPad algorithm. The participant was initially using Flixotide (fluticasone propionate) 100 μg twice per day (total inhaled corticosteroid (ICS) dose 200 μg·day−1). At the first visit, this was theoretically converted to an equivalent of 400 μg·day−1 of Pulmicort (budesonide, initial study drug; yellow arrow). The subject was a nonsmoker, with a FENO of 20 ppb (“mid”), and an ACQ score of 1.57 (“high”). The algorithm suggested a treatment change of “increase LABA”, resulting in a new treatment of Symbicort 400/12 μg·day−1 (400 μg of the ICS budesonide, 12 μg of the LABA e-formoterol, study drug), which was prescribed from the first visit. N/A: not available. #: FENO high: >29 ppb for nonsmokers, >22 ppb for smokers; FENO mid: 19–29 ppb for nonsmokers, 14–22 ppb for smokers; FENO low: <19 ppb for nonsmokers, <14 ppb for smokers. ¶: ACQ high: >1.5, ACQ low: ≤1.5. P: Pulmicort; S: Symbicort; LABA long-acting β-agonist; bd: twice daily.
Analysis
The required sample size was calculated for a likelihood ratio test for two independent proportions [21]. To demonstrate a reduction in the composite adverse perinatal outcome from 35.3% in the control group to 26.2% in the FENO group (90% power, 0.05 significance), 539 women per group were required. Allowing for 10% attrition, we aimed to recruit 600 women per group (supplementary material).
Statistical analysis was conducted using Stata (version 15; StataCorp, College Station, TX, USA) and SAS (version 9.4; SAS Institute, Cary, NC, USA). Data were analysed on an intention-to-treat basis, using two-sided tests, with p<0.05 considered significant. We conducted complete case analyses, assuming data were missing completely at random, due to low missing data rates.
For binary outcomes, intervention effects were estimated as odds ratios with 95% confidence intervals using logistic regression adjusted for study site and baseline maternal smoking. For perinatal mortality, Firth's penalised maximum likelihood was used to estimate the intervention effect due to low event counts. For the continuous, symmetrically distributed outcome of birthweight, intervention effects were estimated as β-coefficients with 95% confidence intervals using linear regression adjusted for study site and baseline smoking status.
For exacerbation outcomes intervention effects were estimated as incidence rate ratios (IRRs) with 95% confidence intervals, using negative binomial regression due to overdispersion. Poisson regression was used to determine group differences in OCS count, unscheduled doctor visits, hospital admission and emergency department presentation. Time to first exacerbation was compared between groups using Kaplan–Meier survival curves and Cox regression. All models adjusted for study site and baseline smoking status.
Medication use, dose and adherence, FENO and ACQ were analysed using generalised linear mixed models with random intercepts. Differences between consecutive visits were determined with post hoc contrast analysis with Bonferroni-adjusted p-values.
Results
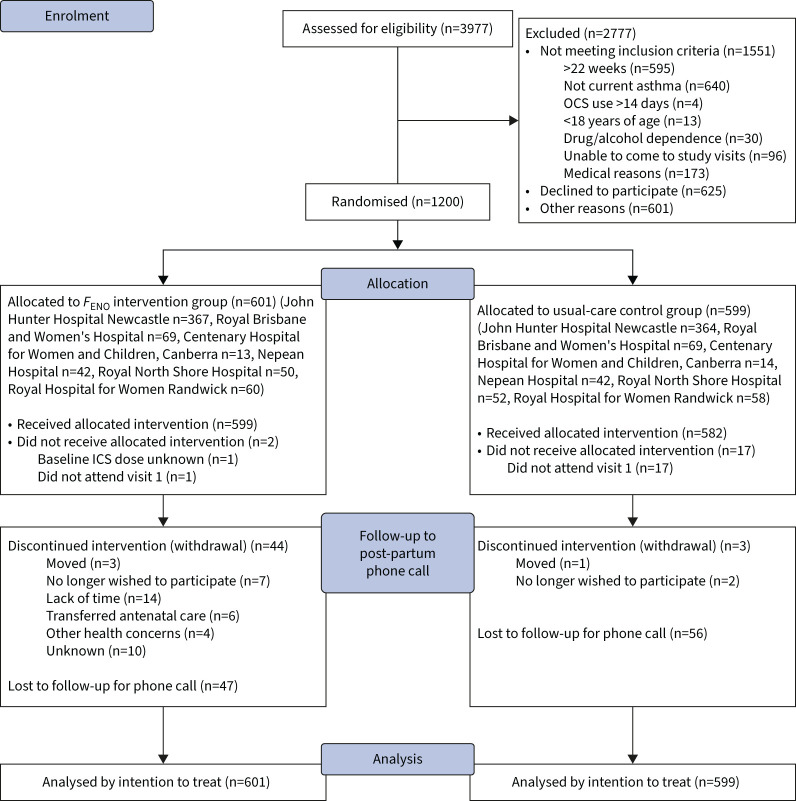
Across six study sites, 599 women were randomised to the control (usual care) group, and 601 to the intervention (FENO) group (figure 2), with baseline characteristics well balanced (table 1). Mean gestational age at recruitment was 18.7 weeks and 12.8% of the women were current smokers. Mean forced expiratory volume at 1 s was 89.5% predicted; 34.6% of the women had uncontrolled asthma; and 42.3% used ICS at a median dose of 400 μg·day−1. Asthma self-management skills were poor; 51.5% of women had inadequate inhaler technique; 14.7% had a written action plan; 27.6% had correct knowledge of ICS; and 29.5% had correct knowledge of reliever medication.
FIGURE 2.
Consolidated Standards of Reporting Trials diagram. OCS: oral corticosteroid; FENO: fractional exhaled nitric oxide; ICS: inhaled corticosteroid.
TABLE 1.
Baseline characteristics
| Control group | FENO group | |
| Subjects | 599 | 601 |
| Age, years | 30.4±5.5 | 30.2±5.4 |
| Gestational age at randomisation, weeks | 18.7 (16.6–20.7) | 18.7 (16.0–20.7) |
| Current smoker | 74 (12%) | 80 (13%) |
| Multiple birth | 11 (1.8%) | 15 (2.5%) |
| Exhaled carbon monoxide, ppm | 2 (1–3) | 2 (2–4) |
| Primiparous | 291 (49%) | 309 (52%) |
| Weight, kg | 79.9±20.9 | 80.6±21.2 |
| BMI, kg·m−2 | 29.3±7.3 | 29.6±7.6 |
| BMI category | ||
| <18.5 kg·m−2 | 4 (0.7%) | 4 (0.7%) |
| 18.5–24.9 kg·m−2 | 181 (32%) | 187 (31%) |
| 25–29.9 kg·m−2 | 170 (30%) | 183 (31%) |
| 30–39.9 kg·m−2 | 152 (27%) | 159 (27%) |
| ≥40 kg·m−2 | 55 (9.8%) | 67 (11%) |
| Ethnicity | ||
| European | 473 (82%) | 477 (80%) |
| Aboriginal/Torres Strait Islander | 27 (4.7%) | 31 (5.2%) |
| Maori/Polynesian | 11 (1.9%) | 11 (1.9%) |
| Indian/Pakistani | 2 (0.3%) | 9 (1.5%) |
| Asian | 20 (3.4%) | 21 (3.5%) |
| African | 3 (0.5%) | 4 (0.7%) |
| Other | 44 (7.6%) | 41 (6.9%) |
| Socioeconomic status (quintiles)# | ||
| Quintile 1 | 59 (9.9%) | 60 (10.0%) |
| Quintile 2 | 69 (11.6%) | 68 (11.3%) |
| Quintile 3 | 194 (32.7%) | 206 (34.3%) |
| Quintile 4 | 171 (28.8%) | 161 (26.8%) |
| Quintile 5 | 101 (17%) | 106 (17.6%) |
| FEV1, % predicted | 89.3±14.1 | 89.6±13.4 |
| FEV1/FVC | 80.82±8.22 | 81.16±7.19 |
| Age at asthma diagnosis, years | 9 (8) | 8 (8) |
| ED visits in past year¶ | 0 (0–4) | 0 (0–4) |
| Hospital admissions in past year¶ | 0 (0–2) | 0 (0–2) |
| OCS courses in past year¶ | 0 (0–6) | 0 (0–10) |
| OCS use in past year | 109/573 (19%) | 114/599 (19%) |
| β2-agonist use in past week, days | 2 (0–5) | 2 (0–7) |
| ICS use | 251 (42%) | 257 (43%) |
| ICS/LABA use | 193 (32%) | 205 (34%) |
| BDP-equivalent ICS dose, μg·day−1 | 400 (250–800) | 400 (200–500) |
| ICS nonadherence | 95 (41%) | 86 (35%) |
| ICS missed doses in past week, % | 14.3 (0.0–50.0) | 14.3 (0.0–28.6) |
| Morning symptoms in past week, days | 1 (0–5) | 2 (0–4) |
| Night symptoms in past week, days | 2 (0–5) | 2 (0–5) |
| Activity limitation in past week, days | 0 (0–1) | 0 (0–2) |
| GINA asthma control classification | ||
| Well controlled | 132 (23%) | 116 (19%) |
| Partly controlled | 253 (44%) | 262 (44%) |
| Uncontrolled | 184 (32%) | 219 (37%) |
| pMDI technique: adequate/optimal | 252 (51%) | 241 (46%) |
| Turbuhaler technique: adequate/optimal | 114 (76%) | 130 (80%) |
| Written action plan | 80 (14%) | 92 (15%) |
| Correct maintenance ICS knowledge | 75 (31%) | 60 (24%) |
| Correct β2-agonist rescue knowledge | 191 (34%) | 151 (25%) |
| FENO, ppb | NA | 16 (10–29) |
| ACQ | NA | 1.14 (0.57–2.00) |
Data are presented as n, mean±sd, median (interquartile range), n (%) or median (range). FENO: fractional exhaled nitric oxide; BMI: body mass index; FEV1: forced expiratory volume in 1 s, FVC: forced vital capacity; ED: emergency department; OCS: oral corticosteroid; ICS: inhaled corticosteroid; LABA: long-acting β-agonist; BDP: beclomethasone dipropionate; GINA: Global Initiative for Asthma; pMDI: pressurised metered dose inhaler; ACQ: Asthma Control Questionnaire; NA: not available. #: quintile 1 represents the most disadvantaged; ¶: median (range).
Excluding four miscarriages (three controls, one FENO participant), 608 infants were born to mothers in the control group (including nine twin pairs, one set of triplets), and 615 infants were born to mothers in the FENO group (15 twin pairs).
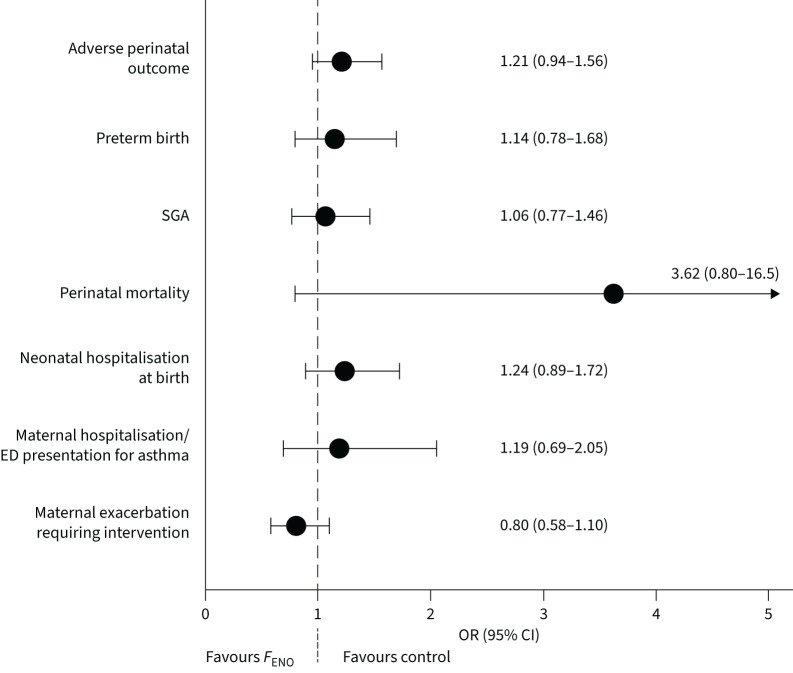
The primary adverse perinatal outcome occurred in 25.6% of infants in the control group, and 29.4% of infants in the FENO group (OR 1.21, 95% CI 0.94–1.56; p=0.15). There were no significant group differences for any component of the composite adverse perinatal outcome, or birthweight (figure 3, table 2). Perinatal deaths, and additional perinatal outcomes are detailed in the supplementary material.
FIGURE 3.
Primary and secondary outcome results. SGA: small for gestational age; ED: emergency department; FENO: fractional exhaled nitric oxide.
TABLE 2.
Primary and secondary outcome results
| Control | F ENO | OR (95% CI) FENO versus control | Coefficient (95% CI) FENO–control | p-value | |
| Infant outcome | (n=608) | (n=615) | |||
| Adverse perinatal outcome (primary) | 152/594 (25.6) | 177/603 (29.4) | 1.21 (0.94–1.56) | 0.15 | |
| Preterm birth | 54/597 (9.0) | 62/606 (10.2) | 1.14 (0.78–1.68) | 0.50 | |
| Small for gestational age | 87/596 (14.6) | 93/603 (15.4) | 1.06 (0.77–1.46) | 0.70 | |
| Perinatal mortality | 1/597 (0.2) | 5/605 (0.8) | 3.62 (0.80–16.5) | 0.10 | |
| Neonatal hospitalisation at birth | 76/595 (12.8) | 92/601 (15.3) | 1.24 (0.89–1.72) | 0.21 | |
| Birthweight, g | 3322.3±603.9 | 3309.6±623.9 | −11.2 (−79.8–57.5) | 0.75 | |
| Maternal outcome | (n=599) | (n=601) | |||
| Maternal hospitalisation for asthma exacerbation | 6/543 (1.1) | 6/554 (1.1) | 1.02 (0.32–3.20) | 0.98 | |
| Maternal hospitalisation/ED presentation | 25/543 (4.6) | 30/554 (5.4) | 1.19 (0.69–2.05) | 0.54 | |
| Maternal exacerbations requiring intervention | 104/543 (19.2) | 89/554 (16.1) | 0.80 (0.58–1.10) | 0.17 |
Data are presented as n, n/N (%) or mean±sd, unless otherwise stated. FENO: fractional exhaled nitric oxide; ED: emergency department.
Maternal exacerbations requiring medical intervention occurred in 19.2% of women in the control group and 16.1% of women in the FENO group (OR 0.80, 95% CI 0.58–1.10; p=0.17; figure 3, table 2). Maternal hospitalisation/emergency department presentation for asthma occurred in 4.6% of the control group and 5.4% of the FENO group (OR 1.19, 95% CI 0.69–2.05; p=0.54). Most women with exacerbations had only one exacerbation (supplementary table S5). Compared to usual care, mothers receiving FENO-based management showed no difference in exacerbation rate (IRR 0.85, 95% CI 0.63–1.14; p=0.27) or OCS use rate during pregnancy (IRR 0.99, 95% CI 0.63–1.55; p=0.96). The number of unscheduled doctor visits per pregnancy was significantly lower in the FENO group compared to the control group (supplementary table S6). There was no difference between groups in time to first exacerbation (hazard ratio 0.86, 95% CI 0.64–1.15; p=0.32).
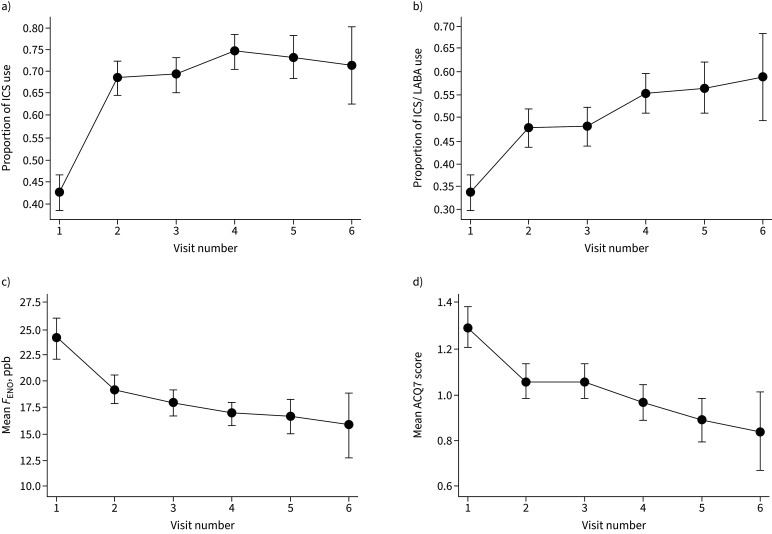
In the intervention group, significant reductions were observed in the traits that were being targeted by the treatment algorithm: FENO (median 16 ppb visit 1 to 15 ppb visit 2, 13 ppb visit 6) and ACQ7 score (1.14 visit 1 to 0.86 visit 2, 0.57 visit 6; all p<0.0001; figure 4). At visit 2, 35% of women had a clinically significant improvement in asthma control (change in ACQ score of ≥0.5). The proportion of women using ICS and ICS/LABA increased significantly during the study (figure 4).
FIGURE 4.
Changes in the proportion of women using a) inhaled corticosteroid (ICS) and b) ICS/long-acting β-agonist (LABA); and changes in c) mean fractional exhaled nitric oxide (FENO) and d) mean Asthma Control Questionnaire (ACQ)7 at visits during pregnancy. Error bars show the 95% confidence intervals.
Subgroup analysis in smokers/nonsmokers, singleton/multiple pregnancies and by study site are outlined in the supplementary material.
Discussion
This is the first RCT to test the effect of an asthma management intervention, with a perinatal primary outcome. There was no significant difference in the primary outcome or its components (preterm birth, SGA, neonatal hospitalisation or perinatal mortality), or birthweight between groups, which were numerically higher in the FENO group. There was no significant effect of the FENO-based management algorithm on maternal asthma exacerbations, compared to usual care, which were numerically lower in the FENO group.
Women in BLT had a lower incidence of the primary outcome than expected from pilot data, and comparison to national data [22] suggests that further improvements in individual perinatal outcomes may have been difficult. In particular, the rate of adverse perinatal outcomes in the control group was lower than anticipated, despite relatively little contact with this group (one visit only, no treatment recommendations made). In pilot data, 35.3% of women with asthma managed by a symptoms-based algorithm had an adverse perinatal outcome, with BLT being powered to detect a reduction to 26.2% with FENO-based management. However, in this trial, only 25.6% of the control group had the adverse outcome.
The numerically higher rates of adverse outcomes in the FENO group compared to the control group was unexpected. The FENO group had more study visits than the control group, which would be expected to improve health outcomes in a trial context. A significant body of literature indicates that women with asthma are at increased risk of adverse perinatal outcomes and that exacerbations are associated with further increased risks for these outcomes [4]. Data from meta-analyses indicated that active asthma management may mitigate some perinatal risks [5, 6]. Consequently, we hypothesised that an intervention which reduced exacerbations in pregnancy may also improve perinatal outcomes. Since the nonsignificant reduction in exacerbations in BLT was not associated with a reduction in adverse perinatal outcomes (and at one study site a reduction in exacerbations was associated with more adverse perinatal outcomes; supplementary material), additional mediators may be involved in the relationship between exacerbations and perinatal outcomes.
The proportion of pregnant women with asthma exacerbations was nonsignificantly lower in the FENO group compared to the control group, which therefore should not explain the perinatal findings. However, the overall prevalence of 17.6% of women having an exacerbation requiring medical intervention was lower than previously observed in our MAP study (33.2%) [9]. In BLT, exacerbation data were not collected prospectively due to the different number of study visits between groups. Rather, all women were asked to recall exacerbations at a phone call made 2–6 weeks post-partum; this outcome measure was likely subject to recall bias, and more likely to capture severe events.
It is possible that “usual” clinical care in BLT was more effective in controlling asthma exacerbations than asthma management guided by symptoms only in the MAP study [9]. Women in BLT had poor asthma self-management skills, and correction of these, such as improved inhaler technique and adherence, could have led to better asthma control in the whole study population. Information provided to participants indicated that asthma should be reviewed frequently in pregnancy and ICS treatment should not be stopped, which may have influenced the control group's exacerbation rate. The reduction in exacerbations due to the FENO intervention was much less than expected (from 19% in the control group to 16% in the FENO group), compared to our previous prospective observation (MAP Study; from 41% in the control group to 25% in the FENO group) [9].
While the pregnancy-specific FENO cut-points used in BLT were the same as those in MAP, there were differences which may have affected our results, particularly for exacerbations. The MAP control algorithm was probably different from current usual care, and in BLT, the FENO-based treatment algorithm was only applied every 6–12 weeks, rather than monthly (MAP). Pregnancy is a relatively short time period when asthma symptoms are known to be variable [23], and monthly treatment change (particularly the first two treatment changes [24]) may have been crucial to the success of MAP in reducing exacerbations [9]. Differences in asthma symptoms during pregnancy have been noted at 4-weekly intervals regardless of worsening or improvement overall [23]. Pregnancy-specific [25] and general asthma guidelines [14] recommend asthma be monitored every 4–6 weeks due to the variable, unpredictable nature of asthma during pregnancy. Less frequent treatment change in BLT may have resulted in a less effective FENO-based management algorithm. However, asthma guidelines recommend that decreases in ICS dose should only be considered after 3 months of stable asthma [14].
This study does not support the implementation of FENO-based management into antenatal care for the purpose of improving perinatal outcomes. However, there remains a need for improvements in asthma management in pregnancy. We observed poor baseline self-management skills among pregnant women with asthma, including <15% with a written asthma action plan, suggesting that guideline recommendations are not being followed in practice. In addition, pregnant women with asthma have concerns about medication use and their potential effects on the fetus [15], necessitating specific education on medication safety and the importance of ICS adherence for this population.
Particular asthma phenotypes may be influenced more by FENO-based asthma management than others. A primary care study showed that adults with low FENO (<25 ppb) were most likely to benefit, as it was possible to downtitrate their medication without loss of asthma control [26]. In the MAP study, women with noneosinophilic asthma had a significant reduction in exacerbations with FENO-based management [24]. Further work is needed to define the phenotypes or subgroups most responsive to the FENO-based approach in pregnancy, or whether there is the possibility of harm with this approach, and the potential mechanisms involved.
There were strengths to the BLT's study design. Primary outcome data were obtained for 98% of participants; were validated by an independent, specialist obstetrician; and data collection and analysis were blinded to intervention group. The trial took a pragmatic approach to address potential obstacles to implementation in clinical practice. The trial addressed generalisability of the approach, being conducted in six centres that differed by location, proportion of smoking and obese women, maternal age and education attainment, asthma severity, ethnic background and socioeconomic status. BLT assessed the effectiveness of FENO-based management against usual care, an important comparison to engage relevant stakeholders. The trial addressed cost and feasibility issues by simplifying the management approach in several ways. The number of drug formulations available was reduced, the algorithm results were generated electronically, asthma assessments were aligned with antenatal appointments and there were fewer treatment changes. There were several limitations: we did not collect end-of-study data on asthma outcomes, including medication use in the control group; women in the control group had to buy their own medication, whereas the FENO group received free medication; and we collected exacerbation data retrospectively.
In pregnant women with asthma, FENO-guided pharmacotherapy delivered by a nurse or midwife in the antenatal clinic setting did not improve perinatal outcomes. Further work is needed to optimise the treatment algorithm if it is to be pursued as a management approach in pregnancy.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00298-2022.Supplement (318.1KB, pdf)
Shareable PDF
Acknowledgements
The authors acknowledge the contributions of the many staff members involved at each clinical site. John Hunter Hospital Newcastle, Newcastle, Australia: Kelly Steel, Catherine Delahunty; Royal Hospital for Women Randwick, Randwick, Australia: Anne Lainchbury, Susan Brandrick; Royal Brisbane and Women's Hospital, Brisbane, Australia: Felicity Stalley, Katie Foxcroft; Nepean Hospital, Kingswood, Australia: Sue Downward, Peta Armstrong, Charlene Dunn; The Canberra Hospital, Canberra, Australia: Lori Grlj, Shelley Starkis; Royal North Shore Hospital, Sydney, Australia: Anmaree Wegener, Lyndsey Harvey, Carol Cooke. We thank the following staff, students and volunteers from the University of Newcastle for assistance with data entry and study administration: Amy Cashmore, Heather Powell, Calida Garside, Michelle Gleeson, Soriah Harvey, Amy Gregson. We thank Christian Murphy of ZeroZeroNine Apps (Maitland, Australia) for designing the mobile app for the treatment algorithm.
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.01639-2022
This trial was prospectively registered at www.anzctr.org.au as ACTRN12613000202763. Deidentified participant data along with a data dictionary will be available after publication. A proposal for how the data would be used would need to be submitted to the corresponding author and approved by the trial executive.
Author contributions: V.E. Murphy conceived the study, wrote the manuscript, and was involved in funding acquisition, methodology, project administration, resources and supervision at the central site. M.E. Jensen was involved in funding acquisition, methodology, project administration and supervision at the central site. E.G. Holliday carried out the statistical analysis and was involved in data curation, formal analysis, methodology, validation and visualisation. W.B. Giles was involved in funding acquisition, investigation and supervision at the Royal North Shore Hospital site. H.L. Barrett was involved in funding acquisition and supervision at the Royal Brisbane and Women's Hospital site. L.K. Callaway was involved in funding acquisition and supervision at the Royal Brisbane and Women's Hospital site. A. Bisits was involved in funding acquisition and supervision at the Royal Hospital for Women Randwick site. M.J. Peek was involved in funding acquisition and supervision at the Nepean Hospital and Canberra Hospital sites. S.K. Seeho was involved in funding acquisition and supervision at the Royal North Shore Hospital site. A. Abbott was involved in supervision at the Nepean Hospital site. A.L. Robijn contributed to the statistical analysis, data curation, methodology and visualisation. P.B. Colditz was involved in funding acquisition and supervision at the Royal Brisbane and Women's Hospital site. J. Attia was involved in funding acquisition and methodology. K. McCaffery was involved in funding acquisition. M.J. Hensley was involved in funding acquisition and provided resources. J. Mattes was involved in funding acquisition. P.G. Gibson conceived the study, and was involved in funding acquisition, methodology, resources, and supervision at the John Hunter Hospital site. All co-authors were involved in data interpretation and contributed to editing the final manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of interest: The authors declare no conflict of interest.
Support statement: Funding was received from the following sources: the National Health and Medical Research Council (NHMRC) of Australia (grant 1060983), University of Newcastle, John Hunter Hospital Charitable Trust, Hunter Medical Research Institute, Singleton Foundation and the Woodend Foundation. V.E. Murphy was the recipient of a NHMRC Career Development Fellowship (grant 1084816) and the Gladys M Brawn Memorial Career Development Fellowship from University of Newcastle. P.G. Gibson is a NHRMC Practitioner Fellow (grant 1155810). The funders played no role in data analysis or interpretation. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Sawicki E, Stewart K, Wong S, et al. Medication use for chronic health conditions by pregnant women attending an Australian maternity hospital. Aust N Z J Obstet Gynaecol 2011; 51: 333–338. doi: 10.1111/j.1479-828X.2011.01312.x [DOI] [PubMed] [Google Scholar]
- 2.Schatz M, Dombrowski MP, Wise R, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol 2003; 112: 283–288. doi: 10.1067/mai.2003.1516 [DOI] [PubMed] [Google Scholar]
- 3.Murphy VE, Clifton VL, Gibson PG. The effect of cigarette smoking on asthma control during exacerbations in pregnant women. Thorax 2010; 65: 739–744. doi: 10.1136/thx.2009.124941 [DOI] [PubMed] [Google Scholar]
- 4.Namazy JA, Murphy VE, Powell H, et al. Effects of asthma severity, exacerbations and oral corticosteroids on perinatal outcomes. Eur Respir J 2013; 41: 1082–1090. doi: 10.1183/09031936.00195111 [DOI] [PubMed] [Google Scholar]
- 5.Murphy VE, Namazy JA, Powell H, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG 2011; 118: 1314–1323. doi: 10.1111/j.1471-0528.2011.03055.x [DOI] [PubMed] [Google Scholar]
- 6.Murphy VE, Wang G, Namazy JA, et al. The risk of congenital malformations, perinatal mortality and neonatal hospitalisation among pregnant women with asthma: a systematic review and meta-analysis. BJOG 2013; 120: 812–822. doi: 10.1111/1471-0528.12224 [DOI] [PubMed] [Google Scholar]
- 7.Kemppainen M, Lahesmaa-Korpinen A, Kauppi P, et al. Maternal asthma is associated with increased risk of perinatal mortality. PLoS One 2018; 13: e0197593. doi: 10.1371/journal.pone.0197593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bain E, Pierides KL, Clifton VL, et al. Interventions for managing asthma in pregnancy. Cochrane Database Syst Rev 2014; 10: CD010660. doi: 10.1002/14651858.CD010660.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet 2011; 378: 983–990. doi: 10.1016/S0140-6736(11)60971-9 [DOI] [PubMed] [Google Scholar]
- 10.Mattes J, Murphy VE, Powell H, et al. Prenatal origins of bronchiolitis: protective effect of optimised asthma management during pregnancy. Thorax 2014; 69: 383–384. doi: 10.1136/thoraxjnl-2013-203388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morten M, Collison A, Murphy VE, et al. Managing Asthma in Pregnancy (MAP) trial: FENO levels and childhood asthma. J Allergy Clin Immunol 2018; 142: 1765–1772. doi: 10.1016/j.jaci.2018.02.039 [DOI] [PubMed] [Google Scholar]
- 12.Murphy VE, Jensen ME, Mattes J, et al. The Breathing for Life Trial: a randomised controlled trial of fractional exhaled nitric oxide (FENO)-based management of asthma during pregnancy and its impact on perinatal outcomes and infant and childhood respiratory health. BMC Pregnancy Childbirth 2016; 16: 111. doi: 10.1186/s12884-016-0890-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Australian Bureau of Statistics . Socio-Economic Indexes for Areas 2016. www.abs.gov.au/websitedbs/censushome.nsf/home/seifa/ Date last accessed: 1 May 2020.
- 14.Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention. www.ginasthma.org/ 2020. Date last accessed: 26 October 2020.
- 15.Robijn AL, Jensen ME, Gibson PG, et al. Trends in asthma self-management skills and inhaled corticosteroid use during pregnancy and postpartum from 2004 to 2017. J Asthma 2019; 56: 594–602. doi: 10.1080/02770903.2018.1471709 [DOI] [PubMed] [Google Scholar]
- 16.Murphy VE, Gibson PG, Talbot PI, et al. Asthma self-management skills and the use of asthma education during pregnancy. Eur Respir J 2005; 26: 435–441. doi: 10.1183/09031936.05.00135604 [DOI] [PubMed] [Google Scholar]
- 17.Asthma Australia . Pregnancy and Asthma. 2020. https://asthma.org.au/about-asthma/live-with-asthma/pregnancy-and-asthma/ Date last accessed: 26 October 2020.
- 18.Juniper EF, O'Byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999; 14: 902–907. doi: 10.1034/j.1399-3003.1999.14d29.x [DOI] [PubMed] [Google Scholar]
- 19.Juniper EF, Bousquet J, Abetz L, et al. Identifying ‘well-controlled' and ‘not well-controlled' asthma using the Asthma Control Questionnaire. Respir Med 2006; 100: 616–621. doi: 10.1016/j.rmed.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 20.Petsky HL, Kew KM, Turner C, et al. Exhaled nitric oxide levels to guide treatment for adults with asthma. Cochrane Database Syst Rev 2016; 9: CD011440. doi: 10.1002/14651858.CD011440.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow SC, Shao J, Wang H. Sample Size Calculations in Clinical Research. 3rd Edn. Boca Raton, Chapman & Hall/CRC, 2008. [Google Scholar]
- 22.Australian Institute of Health and Welfare . Australia's Mothers and Babies 2018: in Brief. 2020. Available from: www.aihw.gov.au/reports/mothers-babies/australias-mothers-and-babies-2018-in-brief/summary/
- 23.Schatz M, Harden K, Forsythe A, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol 1988; 81: 509–517. doi: 10.1016/0091-6749(88)90187-X [DOI] [PubMed] [Google Scholar]
- 24.Murphy VE, Porsbjerg CM, Robijn AL, et al. Biomarker-guided management reduces exacerbations in non-eosinophilic asthma in pregnancy: a secondary analysis of a randomised controlled trial. Respirology 2020; 25: 719–725. doi: 10.1111/resp.13713 [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin K, Foureur M, Jensen ME, et al. Review and appraisal of guidelines for the management of asthma during pregnancy. Women Birth 2018; 31: e349–e357. doi: 10.1016/j.wombi.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 26.Boer S, Honkoop PJ, Loijmans RJB, et al. Personalised exhaled nitric oxygen fraction (FENO)-driven asthma management in primary care: a FENO subgroup analysis of the ACCURATE trial. ERJ Open Res 2020; 6: 00351-2019. doi: 10.1183/23120541.00351-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00298-2022.Supplement (318.1KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00298-2022.Shareable (387KB, pdf)