Summary
Immunodeficient mice are widely used in human stem cell transplantation research. Recombination activating gene 1 (Rag1) deletion results in immunodeficiency and leads to accelerated aging in zebrafish with increased cytosolic accumulation of lipofuscin (LF). Unlike zebrafish, mammals have two homologs, Rag1 and Rag2, that regulate adaptive immunity. Currently, little is known if and how Rag1−/− and Rag2−/− may impact aging and LF accumulation in immunodeficient mouse brains and how this may confound results in human neural cell transplantation studies. Here, we demonstrate that in Rag2−/− mouse brains, LF appears early, spreads broadly, emits strong autofluorescence, and accumulates with age. LF is found in various types of glial cells, including xenografted human microglia. Surprisingly, in Rag1−/− mouse brains, LF autofluorescence is seen at much older ages compared with Rag2−/− brains. This study provides direct evidence that Rag2−/− expedites LF occurrence and sets a context for studies using aged immunodeficient mice.
Keywords: lipofuscin, transplantation, human induced pluripotent stem cells, aging, disease modeling, immunodeficient mice
Highlights
-
•
Rag2−/− accelerates lipofuscin accumulation in the brain across genetic backgrounds
-
•
Lipofuscin is distributed in host mouse glial cells and xenografted human microglia
-
•
Lipofuscin autofluorescence may confound results in studies using Rag2−/− mice
With immunodeficient mice, Jiang and colleagues demonstrate that in Rag2−/− mouse brains, lipofuscin (LF) appears at a young age and is found in various types of glial cells, including xenografted human microglia. Surprisingly, LF accumulation is seen at much older ages in Rag1−/− mouse brains. This study provides direct evidence that Rag2−/− accelerates LF occurrence and sets a context for future studies utilizing aged immunodeficient mice.
Introduction
Transplantation of stem cells or progenitor cells of human origins into mice provides an exciting opportunity where the development of specific cells and their contribution to disease conditions can be studied under in vivo conditions (Jiang and Alam, 2022). Immunodeficient mice are widely used for transplantation of human cells without concerns of host immune rejections. Particularly, in the human stem cell and neuroscience research fields, immunodeficient mice have long been used as recipients for transplantation of human neural cells and developing human-mouse glial chimeric brain models (Windrem et al., 2004, 2008; Zhang et al., 2001). In recent years, with the development of human induced pluripotent stem cell technologies, immunodeficient mice lacking recombination-activating genes 1 or 2 (Rag1 or Rag2) have been used to create microglial, macroglial (astroglia + oligodendroglia), and neuronal human-mouse brain chimeras (Chen et al., 2016; Espuny-Camacho et al., 2013, 2017; Han et al., 2013; Hasselmann et al., 2019; Linaro et al., 2019; Mancuso et al., 2019; Svoboda et al., 2019; Windrem et al., 2008; Xu et al., 2019, 2020), providing a platform to study the development and function of human neural cells in vivo under normal and diseased conditions.
Interestingly, a recent report in zebrafish showed that Rag1 deletion leads to accelerated aging (Novoa et al., 2019). Zebrafish lacking Rag1 showed an upregulation of genes associated with inflammation and a downregulation of genes involved in DNA damage and repair (Novoa et al., 2019). These immunocompromised fish also showed an increased rate of senescence and an increased cytosolic accumulation of lipofuscin (LF) (Novoa et al., 2019), one of the most consistent features of aging. LF is a complex macromolecule, which is an amalgamate of various cellular metabolites of distinct origins (Hohn and Grune, 2013; Snyder and Crane, 2022). The degree of LF accumulation has been shown to correlate with aging (Singh Kushwaha et al., 2018). The composition of LF varies depending on the stage of aging, with a varying degree of oxidized proteins and sugar molecules being present (Benavides et al., 2002). It is well known that in postmitotic cells, such as neurons, LF accumulates over their lifespan (Jung et al., 2007; Liu et al., 2016). Furthermore, LF has been shown to accumulate in microglia, the resident macrophages of the brain (Xu et al., 2008), and cells of the macroglial lineage—astrocytes and oligodendrocytes (Uranova et al., 2018; Zalfa et al., 2016). Collectively, these observations suggest that Rag1 may play a role in cellular senescence and accumulation of LF within the brain in zebrafish. While it has been reported that mice lacking Rag1 or Rag2 are susceptible to infections (Izadjoo et al., 2000; Wu et al., 2010), the accumulation of this well-known marker of aging, LF, in immunocompromised mice has not been reported.
In this study, we show that Rag2−/− mice across multiple genetic backgrounds share the common phenotype of a premature and age-dependent accumulation of LF, starting from 5 to 6 months old. Unexpectedly, the levels of LF detected in Rag1−/− mice are minimal compared with Rag2−/− mice at the comparable chronological age of 5 months. Thus, although Rag1 and Rag2 serve similar functions in the development of the immune system as a form of V(D)J recombination, we find that Rag1−/− and Rag2−/− have different impacts on the accumulation of LF, suggesting that Rag1 and Rag2 may play differential roles in aging of the mammalian brain. Importantly, our findings have critical implications for studies involving human stem cell transplantation into mouse brains regarding the selection of an appropriate immunodeficient mouse strain as the transplant recipient and the necessity of removing LF autofluorescent aggregates to avoid any potential misinterpretation of results.
Results
Confounding results in human cell transplantation studies using aged immunodeficient mice
We have previously created human microglial mouse chimeras by transplanting human induced pluripotent stem cell (hiPSC)-derived primitive macrophage progenitors into the brains of postnatal day 0 (P0) Rag2−/− IL2rγ−/− hCSF1KI immunodeficient mice (Xu et al., 2020). In our recent studies (Jin et al., 2022; Xu et al., 2020), we mainly characterized the donor-derived human microglia in chimeric brains at ages of less than 6 months old. In this study, we further examined the distribution of transplanted human microglia in 15-month-old chimeric mice by staining human nuclei (hN) with a specific anti-hN antibody. Numerous hN+ cells were found widely dispersed in the cerebral cortex, the corpus callosum (CC), and the hippocampus (HIP) (Figure 1A). Surprisingly, we observed strong and unknown autofluorescence (AutoF) under the red fluorescent channel—despite not using any red fluorescent antibody—with a pattern resembling the green-stained hN. This red AutoF also exhibited AutoF under the green channel (Figure 1A, arrows). On the other hand, at least some of the hN+ green dots were not fluorescent in the red channel, suggesting that these were the bona fide donor-derived human cells (Figure 1A, arrowheads). The similarity in the AutoF and hN+ staining pattern and the dual emission of the AutoF entity in both the red and green channels challenged our ability to quantitate the number and percentage of the transplanted hN+ human cells in the mouse brain. Given the age of the animals, the intensity of AutoF, and existing literature background, we reasoned that this AutoF is likely attributable to LF.
Figure 1.
Confounding results in chimeric mouse brains
(A and B) Representative images from sagittal brain sections show the distribution of AutoF and transplanted control hiPSC-derived microglia (hN+) at 15 months before and after LF removal. Arrowheads indicate hN+ cells. Arrows indicate AutoF. Scale bars: 500 and 200 μm in the original and enlarged images, respectively.
(C) Quantification of the number of hN+ per mm2 before and after LF removal (n = 3 mice per group). Student’s t test. ∗p < 0.05. Data are presented as mean ± SEM.
(D) Representative images from sagittal brain sections show the distribution of AutoF and transplanted control hiPSC-derived microglia (hCD45+) in the cortex at 15 months. Scale bars: 500 and 200 μm in the original and enlarged images, respectively.
(E) Representative images of human microglia (hTMEM119+/hN+) in 6-month-old chimeras after LF removal. The squared area is enlarged. Scale bars: 20 and 10 μm in the original and enlarged images, respectively.
Previous studies involving aged mice successfully removed LF for downstream immunohistochemical analyses (Chen et al., 2020). Thus, we treated the brain sections from 15-month-old chimeric mice by using TrueBlack LF AutoF Quencher (Biotium). Indeed, this treatment effectively removed all the AutoF signal and allowed for the correct determination of the number of hN+ cells, which was 2- to 3-fold lower than that without removing LF from the brain tissue (Figure 1C). Similarly, as shown in Figure 1D, the AutoF also interfered with the staining of human-specific CD45 (hCD45) in the cerebral cortex, an epitope expressed in all nucleated hematopoietic cells (Carson et al., 1998) and which we used to label donor-derived human microglia in the chimeric mouse brain. After LF removal treatment, the human microglia, labeled by hN and human-specific TMEM119 (hTMEM119) (Bennett et al., 2016), clearly exhibited ramified morphology in 6-month-old chimeric mouse brains (Figure 1E). Altogether, these results showed that the strong AutoF signal in aged Rag2−/− IL2rγ−/− hCSF1KI mice confound immunohistochemical analysis of transplanted human cells in the chimeric mouse brain.
LF starts to accumulate at earlier ages in Rag2−/− mice than in Rag1−/− mice
To characterize the temporal accumulation of AutoF in Rag2−/− IL2rγ−/− hCSF1KI mice, we examined the brains collected from 3- to 12-month-old mice. There was no AutoF in 3-month-old brains. Significant AutoF was first observed in the brains at 5–6 months of age (Figures 2A and 2D). The AutoF signal increased with age and was widely distributed in 12-month-old brains (Figure 2A). In addition, the identity of the AutoF was further affirmed to be LF by staining with oil red O, a widely used method for staining LF (Figure 2B). We further examined the accumulation of LF in Rag1−/− mice. Interestingly, there was no AutoF in 3- to 7-month-old brains in Rag1−/− mice (Figure 2C). Weak AutoF was first seen only in the cerebral cortex at around 7-month-old brains and became significant and broadly spread at the age of 14 months (Figure 2C). Interestingly, in 7-month-old Rag2−/− mice on the same genetic background as the Rag1−/− mice (C57BL/6), very strong and broadly spread AutoF had already been seen (Figure S1A). Taken together, we find that LF accumulates earlier in Rag2−/− than in Rag1−/− mice.
Figure 2.
LF accumulates earlier in Rag2−/−IL2rγ−/− hCSF1KI mice than Rag1−/− mice
(A) Representative images from sagittal brain sections show AutoF distribution at 3, 5–6, and 12 months of Rag2−/−IL2rγ−/− hCSF1KI mice. Scale bars: 500 and 200 μm in the original and enlarged images, respectively.
(B) Bright-field micrographs show oil red O+ staining in the cerebral cortex, corpus callosum (CC), and hippocampus (HIP) of Rag2−/−IL2rγ−/− hCSF1KI mice. Scale bars: 50 and 10 μm in the original (left) and enlarged images (right), respectively.
(C) Representative images from sagittal brain sections show AutoF distribution at 3, 7, and 14 months of Rag1−/− mice. Scale bars: 500 and 200 μm in the original and enlarged images, respectively.
(D) Quantification of AutoF intensity per mm2 at different time points of Rag2−/−IL2rγ−/− hCSF1KI and Rag1−/− mice (n = 3 mice per group). One-way ANOVA test. ∗p < 0.05, ∗∗p < 0.01. Data are presented as mean ± SEM.
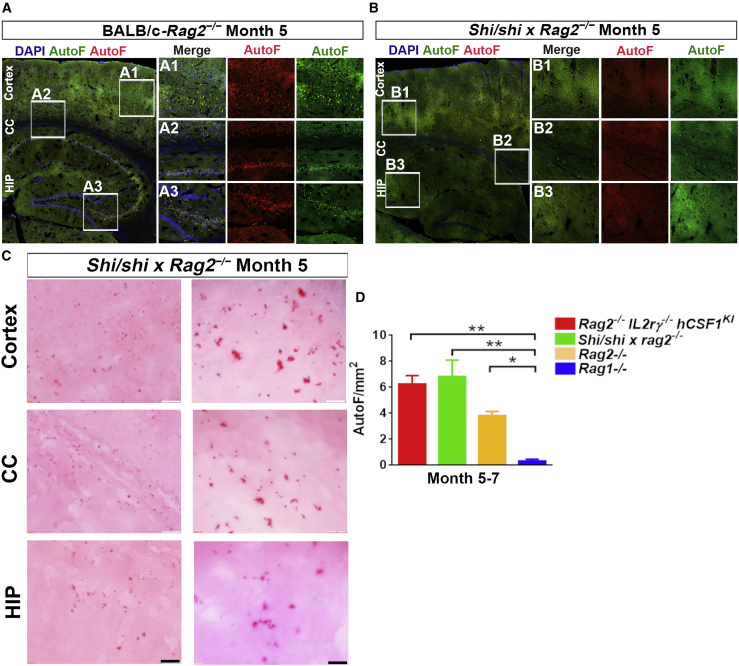
Early accumulation of LF is consistently seen in Rag2−/− mice across different genetic backgrounds
To explore whether the accumulation of LF in Rag2−/− IL2rγ−/− hCSF1KI mice was caused by this particular genetic background and/or the additional knockout and knockin genetic modifications, we further examined the brains of two different Rag2−/− mouse strains: Rag2−/− mice on BALB/c genetic background and shiverer (shi/shi) × Rag2−/− mice on C3H background (Table S1). Consistently, as early as 5 months of age, we observed significant AutoF in both Rag2−/− and shi/shi × Rag2−/− mice (Figures 3A and 3B). Although in shi/shi × Rag2−/− mouse brains, the AutoF appeared to be weaker than that in Rag2−/− mouse brains, the presence of LF was nonetheless confirmed by the oil red O staining (Figure 3C). Furthermore, we integrated quantification of AutoF levels in Rag2−/− IL2rγ−/− hCSF1KI (BALB/c), Rag1−/− (C57BL/6), Rag2−/− (BALB/c), and shi/shi × Rag2−/− (C3H) mice. The results indicated that all three Rag2−/− strains showed accelerated LF accumulation compared with the Rag1−/− strain. In addition, we examined the baseline mouse strains, including C57BL/6, BALB/c, and C3H mice at the ages of 7 months. We found no AutoF in all of these mice (Figures S1B–S1D). Altogether, our data indicate that the early occurrence of LF in Rag2−/− mice is independent of their genetic background.
Figure 3.
LF accumulates in Rag2−/− mice across different genetic backgrounds
(A and B) Representative images from sagittal brain sections showing the distribution of AutoF at 5 months of age in Rag2−/−IL2rγ−/− hCSF1KI and shi/shi × Rag2−/− mice. Scale bars: 500 and 200 μm in the original and enlarged images, respectively.
(C) Bright-field micrographs show oil red O+ staining in the cortex, HIP, and CC of shi/shi × Rag2−/− mice. Scale bars: 50 and 10 μm in the original (left) and enlarged images (right), respectively.
(D) Quantification of AutoF intensity per mm2 at different time points of Rag2−/−IL2rγ−/− hCSF1KI and Rag1−/− mice, as well as Rag2−/− (BALB/c) and shi/shi × Rag2−/− (C3H) (n = 3 mice per group). One-way ANOVA test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are presented as mean ± SEM.
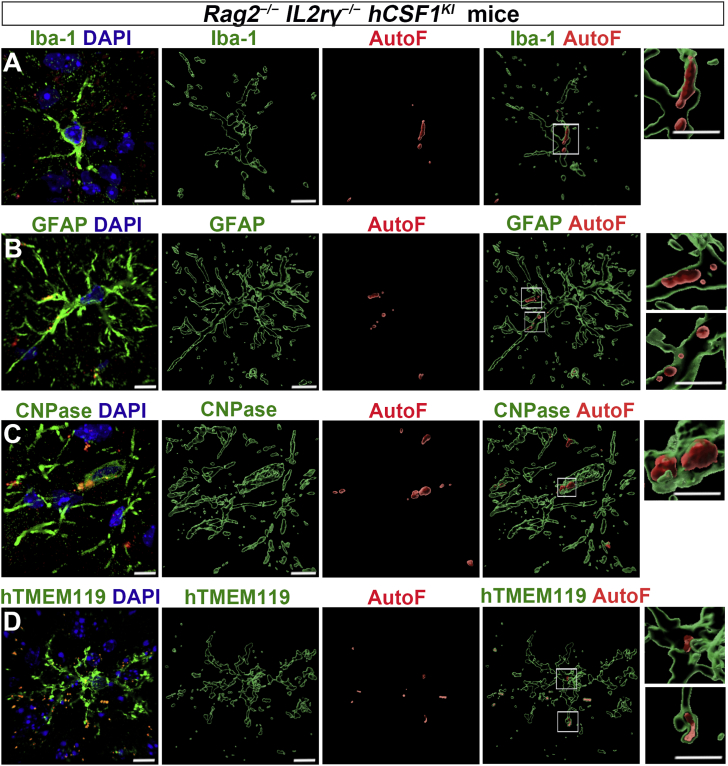
LF accumulates in both human and mouse glial cells in the human microglial chimeric mouse brains
It is well known that LF accumulates in aged neurons (Gilissen et al., 2016). In this study, we observed that LF appeared in different sizes in different brain regions, particularly in the white matter. Here, we thus focused on the accumulation of LF in glial cells in human-mouse chimeric brains. We found that LF accumulated in Iba1+ microglial cells (Figure 4A), in GFAP+ astrocytes (Figure 4B), and in CNPase+ oligodendrocytes (Figure 4C). Since our chimeric mouse contained donor-derived human microglia, we asked if LF accumulation is also seen in xenografted human microglia. As shown in Figure 4D, LF accumulation was indeed found within xenografted microglia labeled by hTMEM119 antibody. Taken together, these results demonstrate that LF is distributed widely and prematurely in host mouse glial cells as well as in xenografted human microglial cells in the Rag2−/− IL2rγ−/− hCSF1KI chimeric mouse brains.
Figure 4.
LF accumulates in both host and transplanted glial cells in Rag2−/−IL2rγ−/− hCSF1KI mouse brains
Representative raw fluorescent super-resolution and 3D surface rendered images showing Iba-1+ staining (A), GFAP+ staining (B), CNPase+ staining (C), and human-specific TMEM119+ (hTMEM119) staining (D) in 5- to 6-month-old Rag2−/−IL2rγ−/− hCSF1KI mice. Scale bars: 5 and 3 μm in the original and enlarged images, respectively.
Discussion
It is not surprising to observe accelerated accumulation of LF in Rag2−/− mice, as immunodeficiency has been linked to accelerated aging (Appay and Rowland-Jones, 2002; Singh Kushwaha et al., 2018), and the accumulation of LF is a well-known index of aging. However, it is unexpected that Rag1−/− mice do not exhibit the premature LF aggregations phenotype as the Rag2−/− mice at comparable chronological ages. Direct evidence in zebrafish shows that zebrafish lacking Rag1 exhibit accelerated aging and a higher LF accumulation compared with control zebrafish (Mittelbrunn and Kroemer, 2021; Novoa et al., 2019). One of the plausible explanations is that there is an enhanced need for upregulation of innate immunity due to a lack of adaptive immunity, which leads to an upregulation of inflammation and radical oxygen species, promoting senescence and aging (Novoa et al., 2019). Zebrafish only has Rag1, which regulates adaptive immunity, whereas mammals, including rodents and humans, possess two such genes: Rag1 and Rag2. Rag1−/− and Rag2−/− mice are both considered immunodeficient. The fact that the early LF accumulation is only observed in the brains of multiple Rag2−/− mouse strains suggests that early LF accumulation in Rag2−/− mice may not solely result from immunodeficiency. Rag1 and Rag2 in the brain do show differential functions. Rag1 is expressed in the HIP, and Rag1−/− mice show impaired social recognition memory, suggesting that Rag1 plays a role in cognitive functions (McGowan et al., 2011). On the other hand, Rag2−/− mice do not show such memory loss (McGowan et al., 2011). Rag2, but not Rag1, is required for axonal growth and retinal ganglionic cell development, whereas Rag1 is thought to be more involved in specifying neuronal identity (Alvarez-Lindo et al., 2019; Feng et al., 2005). Another study has demonstrated that Rag2−/− mice harboring SOD1 mutation show a remarkably shorter life span compared with their littermate control animals with SOD mutation only (Beers et al., 2008), which supports the idea that Rag2 may have yet-to-be-uncovered novel, neuroprotective functions in the brain. It would be interesting and significant in future studies to identify the Rag2-specific, aging-related functions in the brain independent of its role in adaptive immunity.
Our findings have important implications for the studies involving the use of aged Rag2−/− mice. For instance, two studies published in 2015–2016 aimed to examine the role of the adaptive immune system in the beta-amyloid (Aβ) pathology of Alzheimer’s disease but drew completely opposite conclusions (Marsh et al., 2016; Spani et al., 2015). Both studies used Alzheimer’s disease (AD) mouse models that were crossed with immunodeficient Rag2−/− mice to generate AD mouse models lacking the adaptive immune system. Marsh et al. showed that immunodeficient AD mice exhibited greater Aβ pathology compared with immunocompetent AD mice with functional immune systems, suggesting that adaptive immune cell populations help restrain Aβ pathology (Marsh et al., 2016). On the other hand, Späni et al. showed that Aβ pathology was greatly reduced in immunodeficient AD mice lacking functional adaptive immune cells (Spani et al., 2015). What could explain the discrepancy? Both studies used Rag2−/− mice of comparable chronological age (7–12 months). Perhaps some of the puncta-like “Aβ plaques” in these studies could have indeed been LF aggregates that were misinterpreted as Aβ plaques, since there is no information in the studies regarding whether LF removal treatment was performed. As both Aβ plaques and LF do not have a generic stereotypical morphology, it can be difficult to determine from immunostaining data alone what staining signals are authentic Aβ and what may be attributable to LF AutoF. It is noteworthy that LF AutoF has wide excitation (360–647 nm) and emission (500–640 nm) ranges of wavelength (Cho and Hwang, 2011), and LF aggregates can appear as both green and red under the microscope (Haralampus-Grynaviski et al., 2003). In addition, the potential Rag2-specific neuroprotective function in the brain cannot be neglected. Taken together, due to AutoF of LF having very broad excitation and emission spectra, for immunohistochemical analyses in old Rag2−/− mouse brains (i.e., >6 months), precautions must be taken, and LF removal should be performed, particularly when examining proteins with puncta-like immunostaining patterns, such as synaptic markers, e.g., synapsin-1, PSD95, etc., and protein aggregates, e.g., soluble Aβ, soluble hyperphosphorylated tau, etc.
Immunodeficient mice are widely used for stem cell transplantation studies. Our current findings also provide useful guidance regarding the selection of appropriate immunodeficient mouse strains as transplant recipients of human neural cells. Recently, we developed a chimeric mouse model by transplanting hiPSC-derived microglia into the brain of neonatal Rag2−/− IL2rγ−/− hCSF1KI mice (Xu et al., 2020). The engrafted hiPSC-derived microglia proliferate, migrate, and widely disperse in the adult mouse brain. At 6 months posttransplantation, xenografted hiPSC-derived microglia recapitulate features of adult human microglia at the transcriptomic and functional levels (Xu et al., 2020). We think that maturation of donor-derived human microglia is accelerated in the much faster-developing mouse brain relative to the human brain, particularly in the Rag2−/− mouse brains with accelerated aging. It is challenging to model aging-related neurodegenerative diseases with hiPSCs because hiPSC-derived neural cells grown in two-dimensional (2D) cultures or 3D organoid/spheroid cultures exhibit limited cellular maturation even after long-term culture, and they mostly approximate brain cells in prenatal or early postnatal stages (Di Lullo and Kriegstein, 2017; Patterson et al., 2012). Thus, engrafting those hiPSC-derived neural cells into the brains of Rag2−/− mouse strains may facilitate analysis of aging-dependent pathological changes in human neural cells. hiPSCs have also been generated from disorders that show accelerated aging, such as Down syndrome (Chen et al., 2014; Cuadrado and Barrena, 1996; Horvath et al., 2015; Lott and Head, 2005; Teipel and Hampel, 2006). In addition, a previous study demonstrates that hiPSC-derived neurons that express progerin, a truncated form of lamin A associated with premature aging, exhibit aging-related markers and characteristics (Miller et al., 2013). Transplantation of these and related hiPSC-derived neural cells with premature genetic makeup into the brains of Rag2−/− mice that confer a premature aging host environment would greatly facilitate analysis of the interplay of intrinsic/genetic versus extrinsic/environmental factors in driving age-related pathologies in neurodegenerative diseases. Our recent study showcasing Down syndrome microglia are prone to pathologic tau-associated senescence provides an example of such possibilities (Jin et al., 2022).
Experimental procedures
Animals
All animal work was performed without gender bias with the approval of the Rutgers University Institutional Animal Care and Use Committee. The animals used in this study are summarized in Table S1.
hiPSC culture
One control hiPSC line was used in this study. The hiPSCs were cultured on dishes coated with hESC-qualified Matrigel (Corning) in mTeSR plus media (STEMCELL Technologies) under a feeder-free condition. The hiPSCs were passaged with ReLeSR media (STEMCELL Technologies) once per week.
Differentiation and culture of PMPs
Primitive macrophage progenitors (PMPs) were generated from the control hiPSC cell line using a previously established protocol (Haenseler et al., 2017). The yolk sac embryoid bodies (YS-EBs) were generated by treating the YS-EBs with mTeSR 1 media (STEMCELL Technologies) supplemented with bone morphogenetic protein 4 (BMP4; 50 ng/mL), vascular endothelial growth factor (VEGF; 50 ng/mL), and stem cell factor (SCF; 20 ng/mL) for 6 days. To stimulate myeloid differentiation, the YS-EBs were plated on dishes with X-VIVO 15 medium (Lonza) supplemented with interleukin-3 (IL-3; 25 ng/mL) and macrophage colony-stimulating factor (M-CSF; 100 ng/mL). At 4–6 weeks after plating, human PMPs emerged into the supernatant and were continuously produced for more than 3 months.
Cell transplantation
PMPs were collected from the supernatant and suspended at a concentration of 100,000 cells/μL in PBS. Cells were then injected into the brains of P0 Rag2−/− IL2rγ−/− hCSF1KI immunodeficient mice (C; 129S4-Rag2tm1.1Flv Csf1tm1(CSF1)Flv Il2rgtm1.1Flv/J, The Jackson Laboratory; stock no. 017708). The transplantation sites were bilateral from the midline = ±1.0 mm, posterior from bregma = −2.0 mm, and dorsoventral depths = −1.5 and −1.2 mm (Xu et al., 2020). The pups were placed in ice for 4 to 5 min to anesthetize them and then injected with 0.5 μL of cells into each site (four sites total) using a digital stereotaxic device (David KOPF Instruments) that was equipped with a neonatal mouse adapter (Stoelting).
Tissue immunostaining, image acquisition, and analysis
Mouse brains were fixed with 4% paraformaldehyde. The brains were then placed in 20%, and later in 30%, sucrose for dehydration. Following dehydration, brain tissues were immersed in optimal cutting temperature (O.C.T) compound and frozen for sectioning. The frozen tissues were cryo-sectioned with 30 μm thickness for immunofluorescence staining. The tissues were blocked with a blocking solution (5% goat serum in PBS with 0.8% Triton X-100) at room temperature (RT) for 1 h. The primary antibodies were diluted in the same blocking solution and incubated with the tissues at 4°C overnight (all the primary antibodies are listed in Table S1). The sections were washed with PBS and incubated with secondary antibodies for 1 h at RT. After washing with PBS, the slides were mounted with anti-fade Fluoromount-G medium containing DAPI (1, 40,6-diamidino-2-phenylindole dihydrochloride) (Southern Biotechnology).
All fluorescent images were captured with a Zeiss 800 confocal microscope. Large scale images in Figures 1A, 2A, 2B, 4A–4D, and S1A–S1D were obtained by confocal tile scan by the Zen software (Zeiss). To obtain a 3D reconstruction, images were processed by the Zen software (Zeiss).
For each animal, whole-brain tile images were taken from 3–5 brain sections and used for quantification. LF AutoF intensity under red channel was analyzed and quantified using ImageJ (NIH).
Lipid staining
Stock solution of oil red O stain was prepared by dissolving 50 mg oil red O powder (Sigma; O-0625) in 10 mL isopropanol. A 37°C water bath was used to gently heat the solution to facilitate dissolution of the solid. Working solution of oil red O stain was prepared by diluting 3 mL of the stock solution with 2 mL distilled water for a total volume of 5 mL. The solution was then filtered. Working solution was prepared fresh each time. Frozen tissue sections were washed in PBS to remove OCT compound. Tissue sections were then immersed in 60% isopropanol for 30 s and stained with freshly prepared oil red O working solution for 15 min. Stained tissue sections were then immersed in 60% isopropanol for 30 s and mounted using mounting media. All the images were captured by a wide-field microscope (Olympus BX63).
TrueBlack treatment
TrueBlack (1×) solution was prepared by diluting 50× TrueBlack (Biotium) stock solution in 70% ethanol, according to the manufacturer’s instructions. The solution was vortexed to mix well and shielded from light. Following immunostaining, brain slices were removed from PBS. The sections were covered with 1× TrueBlack solution for 20 s. After that, the 1× TrueBlack solution was discarded, and the sections were rinsed three times with PBS.
Statistical analysis
All data are represented as mean ± SEM. When only two independent groups were compared, significance was determined by using two-tailed unpaired t test with Welch’s correction or paired t test. When three or more groups were compared, one-way ANOVA with Bonferroni post-hoc test was used. A p value of <0.05 was considered significant. All the analyses were done in GraphPad Prism v.9.
Author contributions
M.J. and P.J. designed experiments and interpreted data; M.J. carried out most experiments with technical assistance from M.M.A.; A.Y.-C.L. provided study materials and reagents and provided critical suggestions to the overall research direction. P.J. directed the project and wrote the manuscript together with M.J., M.M.A., and A.Y.-C.L.
Acknowledgments
This work was supported in part by grants from the NIH (R01NS102382, R01NS122108, and R01AG073779 to P.J.). M.M.A. is supported by the Rutgers PhD Training Program in Biotechnology funded by the NIH (T32 GM135141).
Conflict of interests
The authors declare no competing financial interests.
Published: October 20, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2022.09.012.
Supplemental information
References
- Alvarez-Lindo N., Baleriola J., de Los Rios V., Suarez T., de la Rosa E.J. RAG-2 deficiency results in fewer phosphorylated histone H2AX foci, but increased retinal ganglion cell death and altered axonal growth. Sci. Rep. 2019;9:18486. doi: 10.1038/s41598-019-54873-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V., Rowland-Jones S.L. Premature ageing of the immune system: the cause of AIDS? Trends Immunol. 2002;23:580–585. doi: 10.1016/s1471-4906(02)02338-4. [DOI] [PubMed] [Google Scholar]
- Beers D.R., Henkel J.S., Zhao W., Wang J., Appel S.H. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc. Natl. Acad. Sci. USA. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides S.H., Monserrat A.J., Farina S., Porta E.A. Sequential histochemical studies of neuronal lipofuscin in human cerebral cortex from the first to the ninth decade of life. Arch. Gerontol. Geriatr. 2002;34:219–231. doi: 10.1016/s0167-4943(01)00223-0. [DOI] [PubMed] [Google Scholar]
- Bennett M.L., Bennett F.C., Liddelow S.A., Ajami B., Zamanian J.L., Fernhoff N.B., Mulinyawe S.B., Bohlen C.J., Adil A., Tucker A., et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA. 2016;113:E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson M.J., Reilly C.R., Sutcliffe J.G., Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Chen C., Jiang P., Xue H., Peterson S.E., Tran H.T., McCann A.E., Parast M.M., Li S., Pleasure D.E., Laurent L.C., et al. Role of astroglia in Down's syndrome revealed by patient-derived human-induced pluripotent stem cells. Nat. Commun. 2014;5:4430. doi: 10.1038/ncomms5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Kim W.Y., Jiang P. Humanized neuronal chimeric mouse brain generated by neonatally engrafted human iPSC-derived primitive neural progenitor cells. JCI Insight. 2016;1:e88632. doi: 10.1172/jci.insight.88632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.T., Lu A., Craessaerts K., Pavie B., Sala Frigerio C., Corthout N., Qian X., Lalakova J., Kuhnemund M., Voytyuk I., et al. Spatial transcriptomics and in situ sequencing to study Alzheimer's disease. Cell. 2020;182:976–991. doi: 10.1016/j.cell.2020.06.038. [DOI] [PubMed] [Google Scholar]
- Cho S., Hwang E.S. Fluorescence-based detection and quantification of features of cellular senescence. Methods Cell Biol. 2011;103:149–188. doi: 10.1016/b978-0-12-385493-3.00007-3. [DOI] [PubMed] [Google Scholar]
- Cuadrado E., Barrena M.J. Immune dysfunction in Down's syndrome: primary immune deficiency or early senescence of the immune system? Clin. Immunol. Immunopathol. 1996;78:209–214. doi: 10.1006/clin.1996.0031. [DOI] [PubMed] [Google Scholar]
- Di Lullo E., Kriegstein A.R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 2017;18:573–584. doi: 10.1038/nrn.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espuny-Camacho I., Arranz A.M., Fiers M., Snellinx A., Ando K., Munck S., Bonnefont J., Lambot L., Corthout N., Omodho L., et al. Hallmarks of Alzheimer's disease in stem-cell-derived human neurons transplanted into mouse brain. Neuron. 2017;93:1066–1081.e8. doi: 10.1016/j.neuron.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Espuny-Camacho I., Michelsen K.A., Gall D., Linaro D., Hasche A., Bonnefont J., Bali C., Orduz D., Bilheu A., Herpoel A., et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Feng B., Bulchand S., Yaksi E., Friedrich R.W., Jesuthasan S. The recombination activation gene 1 (Rag1) is expressed in a subset of zebrafish olfactory neurons but is not essential for axon targeting or amino acid detection. BMC Neurosci. 2005;6:46. doi: 10.1186/1471-2202-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen E.P., Leroy K., Yilmaz Z., Kovari E., Bouras C., Boom A., Poncelet L., Erwin J.M., Sherwood C.C., Hof P.R., Brion J.P. A neuronal aging pattern unique to humans and common chimpanzees. Brain Struct. Funct. 2016;221:647–664. doi: 10.1007/s00429-014-0931-5. [DOI] [PubMed] [Google Scholar]
- Haenseler W., Sansom S.N., Buchrieser J., Newey S.E., Moore C.S., Nicholls F.J., Chintawar S., Schnell C., Antel J.P., Allen N.D., et al. A highly efficient human pluripotent stem cell microglia model displays a neuronal-Co-culture-Specific expression profile and inflammatory response. Stem Cell Rep. 2017;8:1727–1742. doi: 10.1016/j.stemcr.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Chen M., Wang F., Windrem M., Wang S., Shanz S., Xu Q., Oberheim N.A., Bekar L., Betstadt S., et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralampus-Grynaviski N.M., Lamb L.E., Clancy C.M.R., Skumatz C., Burke J.M., Sarna T., Simon J.D. Spectroscopic and morphological studies of human retinal lipofuscin granules. Proc. Natl. Acad. Sci. USA. 2003;100:3179–3184. doi: 10.1073/pnas.0630280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmann J., Coburn M.A., England W., Figueroa Velez D.X., Kiani Shabestari S., Tu C.H., McQuade A., Kolahdouzan M., Echeverria K., Claes C., et al. Development of a chimeric model to study and manipulate human microglia in vivo. Neuron. 2019;103:1016–1033.e10. doi: 10.1016/j.neuron.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn A., Grune T. Lipofuscin: formation, effects and role of macroautophagy. Redox Biol. 2013;1:140–144. doi: 10.1016/j.redox.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S., Garagnani P., Bacalini M.G., Pirazzini C., Salvioli S., Gentilini D., Di Blasio A.M., Giuliani C., Tung S., Vinters H.V., Franceschi C. Accelerated epigenetic aging in Down syndrome. Aging Cell. 2015;14:491–495. doi: 10.1111/acel.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadjoo M.J., Polotsky Y., Mense M.G., Bhattacharjee A.K., Paranavitana C.M., Hadfield T.L., Hoover D.L. Impaired control of Brucella melitensis infection in Rag1-deficient mice. Infect. Immun. 2000;68:5314–5320. doi: 10.1128/iai.68.9.5314-5320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P., Alam M.M. Rise of the human-mouse chimeric brain models. Cell Regen. 2022;11:32. doi: 10.1186/s13619-022-00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M., Xu R., Wang L., Alam M.M., Ma Z., Zhu S., Martini A.C., Jadali A., Bernabucci M., Xie P., et al. Type-I-interferon signaling drives microglial dysfunction and senescence in human iPSC models of Down syndrome and Alzheimer's disease. Cell Stem Cell. 2022;29:1135–1153.e8. doi: 10.1016/j.stem.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T., Bader N., Grune T. Lipofuscin: formation, distribution, and metabolic consequences. Ann. N. Y. Acad. Sci. 2007;1119:97–111. doi: 10.1196/annals.1404.008. [DOI] [PubMed] [Google Scholar]
- Linaro D., Vermaercke B., Iwata R., Ramaswamy A., Libe-Philippot B., Boubakar L., Davis B.A., Wierda K., Davie K., Poovathingal S., et al. Xenotransplanted human cortical neurons reveal species-specific development and functional integration into mouse visual circuits. Neuron. 2019;104:972–986.e6. doi: 10.1016/j.neuron.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.Y.W., Reeves C., Diehl B., Coppola A., Al-Hajri A., Hoskote C., Mughairy S.A., Tachrount M., Groves M., Michalak Z., et al. Early lipofuscin accumulation in frontal lobe epilepsy. Ann. Neurol. 2016;80:882–895. doi: 10.1002/ana.24803. [DOI] [PubMed] [Google Scholar]
- Lott I.T., Head E. Alzheimer disease and Down syndrome: factors in pathogenesis. Neurobiol. Aging. 2005;26:383–389. doi: 10.1016/j.neurobiolaging.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Mancuso R., Van Den Daele J., Fattorelli N., Wolfs L., Balusu S., Burton O., Liston A., Sierksma A., Fourne Y., Poovathingal S., et al. Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nat. Neurosci. 2019;22:2111–2116. doi: 10.1038/s41593-019-0525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S.E., Abud E.M., Lakatos A., Karimzadeh A., Yeung S.T., Davtyan H., Fote G.M., Lau L., Weinger J.G., Lane T.E., et al. The adaptive immune system restrains Alzheimer's disease pathogenesis by modulating microglial function. Proc. Natl. Acad. Sci. USA. 2016;113:E1316–E1325. doi: 10.1073/pnas.1525466113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan P.O., Hope T.A., Meck W.H., Kelsoe G., Williams C.L. Impaired social recognition memory in recombination activating gene 1-deficient mice. Brain Res. 2011;1383:187–195. doi: 10.1016/j.brainres.2011.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.D., Ganat Y.M., Kishinevsky S., Bowman R.L., Liu B., Tu E.Y., Mandal P.K., Vera E., Shim J.W., Kriks S., et al. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell. 2013;13:691–705. doi: 10.1016/j.stem.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M., Kroemer G. Hallmarks of T cell aging. Nat. Immunol. 2021;22:687–698. doi: 10.1038/s41590-021-00927-z. [DOI] [PubMed] [Google Scholar]
- Novoa B., Pereiro P., Lopez-Munoz A., Varela M., Forn-Cuni G., Anchelin M., Dios S., Romero A., Martinez-Lopez A., Medina-Gali R.M., et al. Rag1 immunodeficiency-induced early aging and senescence in zebrafish are dependent on chronic inflammation and oxidative stress. Aging Cell. 2019;18:e13020. doi: 10.1111/acel.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M., Chan D.N., Ha I., Case D., Cui Y., Handel B.V., Mikkola H.K., Lowry W.E. Defining the nature of human pluripotent stem cell progeny. Cell Res. 2012;22:178–193. doi: 10.1038/cr.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Kushwaha S., Patro N., Kumar Patro I. A sequential study of age-related lipofuscin accumulation in Hippocampus and striate cortex of rats. Ann. Neurosci. 2018;25:223–233. doi: 10.1159/000490908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A.N., Crane J.S. StatPearls; 2022. Histology, Lipofuscin. [PubMed] [Google Scholar]
- Spani C., Suter T., Derungs R., Ferretti M.T., Welt T., Wirth F., Gericke C., Nitsch R.M., Kulic L. Reduced beta-amyloid pathology in an APP transgenic mouse model of Alzheimer's disease lacking functional B and T cells. Acta neuropathol. Commun. 2015;3:71. doi: 10.1186/s40478-015-0251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda D.S., Barrasa M.I., Shu J., Rietjens R., Zhang S., Mitalipova M., Berube P., Fu D., Shultz L.D., Bell G.W., Jaenisch R. Human iPSC-derived microglia assume a primary microglia-like state after transplantation into the neonatal mouse brain. Proc. Natl. Acad. Sci. USA. 2019;116:25293–25303. doi: 10.1073/pnas.1913541116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel S.J., Hampel H. Neuroanatomy of Down syndrome in vivo: a model of preclinical Alzheimer's disease. Behav. Genet. 2006;36:405–415. doi: 10.1007/s10519-006-9047-x. [DOI] [PubMed] [Google Scholar]
- Uranova N.A., Vikhreva O.V., Rakhmanova V.I., Orlovskaya D.D. Ultrastructural pathology of oligodendrocytes adjacent to microglia in prefrontal white matter in schizophrenia. NPJ Schizophr. 2018;4:26. doi: 10.1038/s41537-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem M.S., Nunes M.C., Rashbaum W.K., Schwartz T.H., Goodman R.A., McKhann G., 2nd, Roy N.S., Goldman S.A. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat. Med. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- Windrem M.S., Schanz S.J., Guo M., Tian G.F., Washco V., Stanwood N., Rasband M., Roy N.S., Nedergaard M., Havton L.A., et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Haist V., Baumgartner W., Schughart K. Sustained viral load and late death in Rag2-/- mice after influenza A virus infection. Virol. J. 2010;7:172. doi: 10.1186/1743-422x-7-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Chen M., Manivannan A., Lois N., Forrester J.V. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell. 2008;7:58–68. doi: 10.1111/j.1474-9726.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- Xu R., Brawner A.T., Li S., Liu J.J., Kim H., Xue H., Pang Z.P., Kim W.Y., Hart R.P., Liu Y., Jiang P. OLIG2 drives abnormal neurodevelopmental phenotypes in human iPSC-based organoid and chimeric mouse models of Down syndrome. Cell Stem Cell. 2019;24:908–926.e8. doi: 10.1016/j.stem.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Li X., Boreland A.J., Posyton A., Kwan K., Hart R.P., Jiang P. Human iPSC-derived mature microglia retain their identity and functionally integrate in the chimeric mouse brain. Nat. Commun. 2020;11:1577. doi: 10.1038/s41467-020-15411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa C., Verpelli C., D'Avanzo F., Tomanin R., Vicidomini C., Cajola L., Manara R., Sala C., Scarpa M., Vescovi A.L., De Filippis L. Glial degeneration with oxidative damage drives neuronal demise in MPSII disease. Cell Death Dis. 2016;7:e2331. doi: 10.1038/cddis.2016.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.C., Wernig M., Duncan I.D., Brustle O., Thomson J.A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.