Abstract
Background
Based primarily on in vitro and animal models, with little data directly addressing patient outcomes, current guidelines recommend treating staphylococcal prosthetic valve endocarditis (PVE) with antibiotic combinations including gentamicin and rifampin. Here, we synthesize the clinical data on adjunctive rifampin and gentamicin in staphylococcal PVE.
Methods
We conducted a systematic review and meta-analysis of PubMed- and Cochrane-indexed studies reporting outcomes of staphylococcal PVE treated with adjunctive rifampin, gentamicin, both agents, or neither (ie, glycopeptide or β-lactam monotherapy). We recorded outcomes including mortality, relapsed infection, length of stay, nephrotoxicity, hepatotoxicity, and important drug–drug interactions (DDIs).
Results
Four relevant studies were identified. Two studies (n = 117) suggested that adding gentamicin to rifampin-containing regimens did not reduce clinical failure (odds ratio [OR], 0.98 [95% confidence interval {CI}, .39–2.46]), and 2 studies (n = 201) suggested that adding rifampin to gentamicin-containing regimens did not reduce clinical failure (OR, 1.29 [95% CI, .71–2.33]). Neither gentamicin nor rifampin was associated with reduced infection relapse; 1 study found that rifampin treatment was associated with longer hospitalizations (mean, 31.3 vs 42.3 days; P < .001). Comparative safety outcomes were rarely reported, but 1 study found rifampin to be associated with hepatoxicity, nephrotoxicity, and DDIs, leading to treatment discontinuation in 31% of patients.
Conclusions
The existing clinical data do not suggest a benefit of either adjunctive gentamicin or rifampin in staphylococcal PVE. Given that other studies also suggest these agents add nephrotoxicity, hepatoxicity, and risk of DDIs without benefit in staphylococcal endovascular infections, we suggest that recommendations for gentamicin and rifampin in PVE be downgraded and primarily be used within the context of clinical trials.
Keywords: endocarditis, gentamicin, prosthetic valve, rifampin, Staphylococcus
A systematic review identified 4 studies suggesting no clinical benefit of either adjunctive gentamicin or rifampin in staphylococcal prosthetic valve endocarditis. Rifampin was associated with longer hospitalizations and frequent adverse events requiring treatment discontinuation.
Prosthetic valve endocarditis (PVE) is a serious infection with 44% of patients requiring surgical treatment and a 37% in-hospital mortality in 1 recent multicenter study [1]. Coagulase-negative staphylococci (CoNS) and Staphylococcus aureus are the most common causes of PVE, representing about a quarter and a sixth of cases, respectively [1]. Despite being common and associated with significant morbidity and mortality, treatment recommendations for staphylococcal PVE have evolved little over the past 4 decades. A prime example of this stagnation is the persistent recommendation to add adjunctive gentamicin and/or rifampin to β-lactam or glycopeptide-based therapy [2–5]. These recommendations, which date back to the 1980s, are considered class I, level B recommendations in both US and European guidelines [2–4]. The supporting data are mainly in vitro and from animal models with extrapolation of studies of CoNS to S aureus infections [6, 7]. There is limited clinical validation. Specifically, the only clinical data the joint American Heart Association/Infectious Diseases Society of America (AHA/IDSA) guidelines cite is a retrospective review of 61 surgically removed prosthetic valves with endocarditis [8]. This study found an increased likelihood of culture-negative valves with combination therapy, but used neither modern culture techniques (eg, sonication) nor describe the impact on important clinical outcomes, such as treatment-related toxicities, microbiologic relapse, or mortality [8].
Despite the lack of supportive clinical evidence, the AHA/IDSA guidelines justify recommendations for combination treatment as due to the high mortality of staphylococcal PVE [3]. However, the “more is better” justification for combination treatment of PVE has not been borne out in other studies of combination treatment with either rifampin or gentamicin for S aureus native valve endocarditis (NVE) or bacteremia [9–13]. Likewise, various other antibiotic combinations have not found a mortality difference in S aureus bacteremia, although the ongoing international Staphylococcus aureus Network Adaptive Platform Trial seeks to answer whether adjunctive cefazolin to vancomycin or daptomycin is beneficial in methicillin-resistant S aureus bacteremia, including endocarditis [14–17]. Rifampin is used frequently in other staphylococcal infections involving prostheses, for example, prosthetic joint infections (PJIs), for its biofilm activity based on similar animal models, inconsistent evidence of benefit in observational studies, and one 33-patient clinical trial comparing rifampin-ciprofloxacin to ciprofloxacin monotherapy [6, 7, 18]. However, for PJIs, rifampin showed limited to no benefit in a larger and more recent randomized clinical trial and a recent meta-analysis [19, 20].
Concerningly, some observational studies suggest that the use of adjunctive aminoglycosides and rifampin for staphylococcal endovascular infections may cause harm (eg, nephrotoxicity, significant drug–drug interactions [DDIs], and hepatotoxicity) [9–12] for which this risk may be increased for older adults with preexisting comorbidities and polypharmacy. To better inform clinical decision making, we performed a systematic literature review of the clinical efficacy and safety of staphylococcal PVE treatment with β-lactams or vancomycin given with or without gentamicin and/or rifampin adjunctive therapy.
METHODS
Study Question
We developed the following population, intervention, control, and outcome (PICO) question to guide our literature search:
In adults with PVE due to staphylococci treated with β-lactam or glycopeptide antibiotics, does the addition of gentamicin alone (group 1), rifampin alone (group 2), or gentamicin plus rifampin in combination (group 3) versus β-lactam or glycopeptide monotherapy (group 4) result in lower mortality (any definition), lower rates of repeat surgery, lower rates of endocarditis relapse (any definition), or reduced length of hospitalization or result in higher rates of nephrotoxicity, hepatotoxicity, or significant DDIs?
We included studies with comparative clinical data addressing the PICO question, including observational (case-control and cohort) and randomized trials. We excluded reviews or viewpoint articles without original data, case reports, or studies presenting only in silico, in vitro, or animal data. We also excluded papers that did not provide outcomes stratified by antimicrobial regimen, precluding between-group comparisons. Given the paucity of data identified with our initial search and prior to any meta-analysis, we made the post hoc decision to expand our PICO question to include any studies comparing the addition of rifampin and/or gentamicin to regimens without 1 or both of those agents (ie, any comparison between 2 or more of groups 1–4) [16, 17].
Literature Search
We searched the PubMed and Cochrane Library for studies on use of adjunctive gentamicin or rifampin for staphylococcal PVE from inception to 1 September 2021; we performed an updated search on 1 April 2022. Our search query was [(endocarditis OR valve) AND (staphylococcus OR aureus OR coagulase-negative) AND (rifampin OR rifamycin OR gentamicin OR aminoglycoside) AND (mortality OR surgery OR relapse OR cure OR length of stay OR hospital days OR duration of hospitalization OR acute kidney injury OR nephrotoxicity OR hepatotoxicity OR drug interaction)]. This review was not prospectively registered.
Study Selection
Two independent reviewers (J. H. R. and T. C. L.) screened each title and abstract for initial eligibility based on our selection criteria, then these reviewers performed a full text review to determine final study eligibility. Disagreements were resolved by a third reviewer (N. W. C.-P. or S. Y. C. T.). N. W. C. screened the updated search.
Data Extraction
J. H. R. extracted data from eligible articles including the author, year of publication, number of patients treated with control (β-lactam or glycopeptide monotherapy), number of patients treated with intervention (gentamicin plus rifampin, gentamicin alone, or rifampin alone), number of patients who received surgery, mortality outcomes, repeat surgery, recurrent infection, hospital length of stay, nephrotoxicity, hepatotoxicity, and significant DDIs. We included each of these outcomes through the maximum period of follow-up reported.
Assessment of Bias
J. C. G. assessed risk of study bias with the 9-point Newcastle-Ottawa Scale designed for case-control and cohort studies, as no randomized trials were identified [21]. As there is no defined cutoff for this scale, we used scores of 7–9 as low risk for bias, 4–6 as moderate risk of bias, and 0–3 as high risk of bias, similar to a prior meta-analysis [22].
Data Analysis
N. W. C.-P. performed statistical analyses in Review Manager (RevMan) version 5.4 (Cochrane Collaboration, 2020) and Microsoft Excel software. In the meta-analyses, pooled event rates with 95% confidence interval (CI) were assessed using a random-effects model. Between-study heterogeneity was assessed using the I2 statistic.
RESULTS
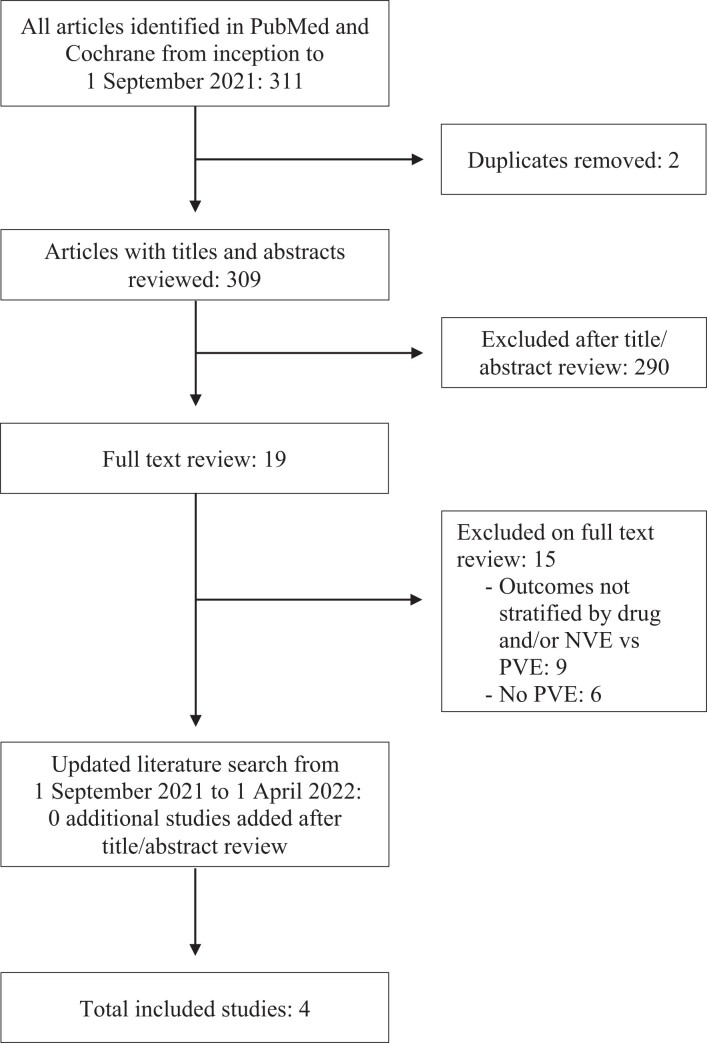
We performed a systematic review of the literature according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23]. Our literature review identified 311 articles, of which 4 met our inclusion criteria (Figure 1). Our search yielded only 1 retrospective study with 46 patients addressing the initial PICO question [24]. The post hoc expansion of the PICO question (ie, to include any study comparing 2 or more of groups 1–4 as defined above) yielded an additional 3 studies totaling 297 patients, of which 1 study was published in the same year by the same authors as the first identified study, with an apparent overlap in patients [25–27]. All 4 included studies are summarized in Table 1. All studies had a moderate risk of bias with scores of 5–6, shown in Table 1.
Figure 1.
Systematic review flowchart. Abbreviations: NVE, native valve endocarditis; PVE, prosthetic valve endocarditis.
Table 1.
Included Studies and Assessment of Study Bias
| Study, First Author | Design | Study Years | Sample Size | SA/MRSA, No. (%) | CoNS/MR-CoNS, No. (%) | Glycopeptide Backbone, No. (%) | β-Lactam Backbone, No. (%) | Received Rifampin, No. (%) | Received Gentamicin, No. (%) | Surgical Management, No. (%) | Newcastle-Ottawa Scale | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort Selection | Cohort Comparability | Outcome Determination | Total Scorea | |||||||||||
| Karchmer et al, 1983 [27] | Retrospective cohort | 1975–1980 | 23 | 0 | 23 (100)/23 (100) | 15 (65.2) | 8 (34.8) | 23 (100) | 8 (34.8) | 14 (60.9) | 4 | 0 | 2 | 6 |
| Karchmer et al, 1983 [24] | Retrospective cohort | 1975–1980 | 46 | 0 | 46 (100)/46 (100) | 26 (56.5) | 20 (43.5) | 23 (50) | 21 (45.7) | 31 (67.4) | 4 | 0 | 2 | 6 |
| Ramos-Martínez et al, 2018 [26] | Prospective registry | 2008–2016 | 94 | 40 (42.5)/NR | 54 (57.4)/NR | 53 (56.4) | 41 (43.6) | 94 (100) | 77 (81.9) | 41 (43.6) | 4 | 0 | 2 | 6 |
| Le Bot et al, 2021 [25] | Retrospective cohort | 2000–2018 | 180 | 114 (63.3)/17 (14.9) | 66 (36.7)/39 (59.1) | NR | NR | 101 (56.1) | 180 (100) | 51 (28.3) | 3 | 0 | 2 | 5 |
Abbreviations: CoNS, coagulase-negative staphylococci; MR-CoNS, methicillin-resistant coagulase-negative staphylococci; MRSA, methicillin-resistant Staphylococcus aureus; NR, not reported; SA, Staphylococcus aureus.
Lower scores on a 9-point scale suggest a higher risk of bias.
The 4 included studies were conducted between 1975 and 2018 with no studies collecting data between 1980 and 2000 [24–27]. One study (n = 46) addressed the comparison of treatment groups 1–4 [24]. Two studies (n = 23 and n = 94) evaluated a comparison of treatment group 2 to 3 [26, 27]. One study (n = 180) compared treatment group 1 to 3 [25]. In total, only 1 study reported outcomes of staphylococcal PVE treatment without rifampin or gentamicin, with just 10 patients receiving β-lactam or vancomycin monotherapy [24].
Of the 343 isolates reported in the 4 studies, 154 (44.9%) were S aureus and 189 (55.1%) CoNS. Three studies (n = 249) reported methicillin resistance in S aureus or CoNS in 125 (50.2%) isolates [24, 25, 27]. Among 3 studies (n = 163) reporting the glycopeptide or β-lactam backbone therapy used in each regimen, 94 (57.7%) received a glycopeptide and 69 (42.3%) received a β-lactam [24, 26, 27]. Rifampin was used in 242 of 343 (70.3%) patients in the 4 studies [24–27]. Gentamicin was given in 286 of 343 (83.4%) patients among 4 studies reporting this agent [24–27]. Surgery was performed in 137 of 343 patients (39.9%) across the 4 studies; timing of surgery was after a mean/median 2 weeks of antibiotic therapy in 2 studies, after a median of 1 week of antibiotics in 1 study, and unreported in 1 study [24–27].
Definitions for diagnosis of PVE varied between studies. The 2 papers by Karchmer [24, 27], which predate the modified Duke criteria [28], defined PVE as at least 2 of 3 features: compatible clinical illness, 2 or more positive blood cultures, and/or histopathologic evidence of endocarditis. The 2 more contemporary studies [25, 26] defined possible or definitive PVE via modified Duke criteria. Assessments of treatment failure and mortality were also heterogeneous. Two studies used a composite “treatment failure” primary outcome that included recurrence, all-cause mortality during PVE treatment, and PVE-related mortality at 3 months [24, 27]. The other 2 studies reported all-cause in-hospital and 1-year mortality [25, 26].
Prespecified efficacy outcomes are given in Table 2. Meta-analyses using 1-year mortality as the primary outcome had wide confidence intervals (CIs) including both benefit and harm when comparing both adjunctive gentamicin and rifampin (group 3) versus adjunctive rifampin alone (group 2) (2 studies; n = 117; odds ratio [OR], 0.98 [95% CI, .39–2.46]); both adjunctive gentamicin and rifampin (group 3) versus adjunctive gentamicin alone (group 1) (2 studies; n = 201; OR, 1.29 [95% CI, .71–2.33]); and both adjunctive gentamicin and rifampin (group 3) versus single or no adjunctive antibiotics (groups 1, 2, and 4) (3 studies; n = 320; OR, 1.18 [95% CI, .7–1.96]). Forest plots of these comparisons are given in Supplementary Figures 1–3. All comparisons were without significant heterogeneity (I2 = 0%). No studies reported rates of repeat surgery as an outcome. Two studies found no difference in rates of relapse or recurrence; in the first, no patient experienced relapse or recurrence rate either with or without addition of gentamicin, and in the second, 7 of 79 patients not receiving rifampin experienced relapse or recurrence versus 6 of 101 who received rifampin (5.9% vs 8.9%; P = .45) [25, 26]. Only 1 study reported length of stay as an outcome, finding that rifampin compared to no rifampin use resulted in a significantly longer length of stay (mean, 42.3 vs 31.3 days; P < .001) [25].
Table 2.
Efficacy Outcomes
| Study [Reference] | Primary Outcome Definition | Primary Outcome | Relapse/Recurrence, No. (%) | Hospital LOS, d | |||
|---|---|---|---|---|---|---|---|
| Group 4: BL/Gly Alone, No. (%) | Group 1: Gent + BL/Gly, No. (%) | Group 2: Rif + BL/Gly, No. (%) | Group 3: Rif + Gent + BL/Gly, No. (%) | ||||
| Karchmer et al, 1983 [27] | Failure | NR | NR | 5/15 (33.3) | 2/8 (25) | NR | NR |
| Karchmer et al, 1983 [24] | Failure | 5/10 (50) | 3/13 (23.1) | 5/15 (33.3) | 2/8 (25) | NR | NR |
| Ramos-Martínez et al, 2018 [26] | 1-y all-cause mortality | NR | NR | 8/17 (47.1) | 38/77 (49.4) | No Gent: 0/17 (0) Gent: 0/77 (0) | NR |
| Le Bot et al, 2021 [25] | 1-y all-cause mortality | NR | 25/79 (31.6) | NR | 38/101 (37.6) | No Rif: 7/79 (8.9) Rif: 6/101 (5.9) | No Rif: 31.3a Rif: 42.3a |
No study reported repeat surgery outcomes. “Failure” was defined as composite of recurrence and death at up to 3 months. Abbreviations: BL, β-lactam; Gent, gentamicin; Gly, glycopeptide; LOS, length of stay; NR, not reported; Rif, rifampin.
This outcome was significantly different between the 2 groups (P < .001 by exact t test).
Safety outcomes were reported in 2 studies [25, 26]. For nephrotoxicity, 1 study compared adjunctive gentamicin and rifampin (group 3) versus rifampin alone (group 2), which was similar between groups (54.5% vs 52.9%; P = .904) [26]. This same study also reported a single patient with hepatotoxicity attributed to rifampin requiring treatment discontinuation. A different study comparing rifampin and gentamicin (group 3) to gentamicin alone (group 1) found rifampin had to be discontinued in 31 of 101 (30.7%) patients, including 11 due to hepatotoxicity and 2 for nephrotoxicity [25]. Rates of DDIs, including effects on anticoagulation, were reported in 1 study comparing rifampin and gentamicin (group 3) to gentamicin alone (group 1), finding 15 of 35 (42.9%) patients treated with rifampin and a vitamin K antagonist to have vitamin K antagonist imbalances compared to 6 of 27 (22.2%) in the gentamicin only arm (P = .15) [25]. Four additional patients had other DDIs with rifampin [25]. Other reports of toxicity were not comparative in nature, as they only reported rates of the event in the additional adjunctive antibiotic arm.
DISCUSSION
Our systematic review highlights the paucity of clinical data to support the current guideline-recommended treatment regimens for staphylococcal PVE. We identified only 4 relevant studies, all retrospective and observational in design. The 2 more recent, larger studies reported no difference in outcomes for patients receiving rifampin or no rifampin (both groups received gentamicin) [25] or for patients receiving gentamicin or no gentamicin (both groups received rifampin) [26] The 2 older studies were published in 1983 and included 23 and 46 patients with methicillin-resistant Staphylococcus epidermidis (MRSE) infections only and also did not demonstrate statistically significant differences in outcome for any comparison [24, 27] Moreover, it is possible that the 23 patients in the first study are a subset of the 46 patients reported in the second study, as both were derived from a cohort of 70 patients with S epidermidis PVE between 1975 and 1980 recruited at the same 3 hospitals by the same authors. The 46 patients included all those with MRSE infections and the 23 patients appear to be a subset treated with rifampin. Ten of these 46 patients received monotherapy without rifampin or gentamicin, and in fact, 4 of these 10 patients received ineffective therapy, in that they were treated with a β-lactam agent alone for a MRSE infection. Therefore, it appears the initial 1989 AHA guideline recommendations for and subsequent practice of combination therapy in staphylococcal PVE might be based on retrospective comparisons involving only 6 patients who received appropriate monotherapy without an adjunctive agent [4].
In addition to the finding that none of these studies clearly demonstrate a clinical benefit to combination therapy, they have several significant limitations: their observational nature, small sample sizes, inconsistent stratification of outcomes with S aureus versus CoNS and methicillin-susceptible versus methicillin-resistant isolates, heterogeneous definitions of PVE and mortality outcomes, and incomplete reporting of toxicities. Additionally, these studies span over 40 years, during which other aspects of PVE treatment, including diagnosis, antibiotic options, dosing regimens, surgical management (eg, types of mechanical valves and techniques), and critical/supportive care, have greatly evolved.
Nearly 40% of patients included in the studies in this review were managed surgically. The theoretical benefit of rifampin is believed to be strongest in the setting of retained hardware given the biofilm activity demonstrated in animal models. With removal of the infected valve and corresponding vegetation, adjunctive antibiotics for biofilm activity are likely not biologically plausible or necessary. One study including surgically managed staphylococcal NVE and PVE (52.4% PVE) found no benefit to adjunct rifampin for reoperation-free survival, although outcomes were not stratified by NVE versus PVE [29]. Our review does not address this question, but future studies should further attempt to stratify outcomes between surgically managed and medically treated populations, as the benefit, if any, is most likely to be apparent in the medically treated population.
We have found that the history of combination therapy recommendations in the AHA/IDSA and European Society of Cardiology guidelines are based on expert opinion, foreign-body infection animal models with culture-based outcomes, or clinical studies that do not evaluate clinically relevant outcomes (eg, mortality and toxicity) [2, 3, 6–8]. Nonetheless, these guideline recommendations have significant uptake, as a 2020 survey of 557 infectious diseases physicians found 66% used aminoglycosides and 93% used rifampin for staphylococcal PVE [30]. The AHA/IDSA guidelines, which were published prior to the 2 largest retrospective studies in this review, neither demonstrating benefit of combination therapy with gentamicin or rifampin, give combination therapy for staphylococcal PVE a class I (benefits much greater than risks) recommendation with a level of evidence of B (data derived from observational studies or a single randomized or nonrandomized trial). However, the supporting data do not support these classifications of recommendations. Rather, the recommendation likely fits within class III-B (no clear benefit or harm in observational studies). A Grading of Recommendations Assessment, Development, and Evaluation (GRADE) assessment would rate this certainty of evidence as very low (observational studies downgraded for study limitations, indirectness of evidence, and imprecision of results); modern IDSA guidelines for other infections examining therapeutics with similarly uncertain data for benefit and known potential harms have recommended against use of such agents “outside the context of a clinical trial” [31, 32].
Guideline recommendations based on low-quality evidence, especially nonclinical evidence, should heed caution, as the history of low-quality guideline-based recommendations is fraught with examples of resultant harm [33]. Furthermore, prior randomized controlled trials of S aureus bacteremia for rifampin and gentamicin combination therapy have not demonstrated superiority to monotherapy [11, 34]. Undoubtedly, recommendations to use rifampin and gentamicin could be seen more favorably if neither agent was associated with harms. However, several studies have documented the potential for serious adverse events attributable to these agents, namely nephrotoxicity, ototoxicity, hepatotoxicity, and significant DDIs (notably, anticoagulation in the setting of prosthetic valves and methadone use for treatment of concomitant opioid use disorder) [9–12, 34].
Limitations of this systematic review include the ability to draw conclusions with a small number of identified studies with heterogeneous outcomes. No randomized controlled trials were identified, and the overall quality of evidence included is very low with a moderate risk of bias. Additionally, we did not request data from authors of reports that did not stratify their outcomes by drug or by separating NVE or PVE, so there may exist further unpublished data in particular subgroups. Strengths of this review include the systematic nature and the focus on staphylococcal PVE combination therapy, as prior reviews are either narrative in approach or included NVE and nonstaphylococcal endocarditis [35, 36].
We believe that equipoise exists for future clinical trials investigating whether adjunctive rifampin or gentamicin should be given in staphylococcal PVE. The widespread adoption of this practice despite the extant data demonstrating potential harm of combination therapy likely requires a well-designed trial to understand whether a mortality benefit is worth the associated risks. Carefully designed retrospective studies with large sample sizes from multicenter databases could also advance the science on this topic, as there is no clear retrospective evidence of a benefit to combination therapy in small and flawed studies, and small sample sizes have hampered assessment of the combination therapy's potential harms. However, retrospective studies are likely to be hampered by biases, in particular, immortal time bias and confounding by indication. Future studies should include a control arm (no gentamicin or rifampin) and describe a clear mortality outcome at relevant time points (eg, all-cause in-hospital, 90-day mortality, and 1-year mortality). Additional data points should include recurrent infection rates, rates of unplanned cardiac surgery, and evaluation of toxicities in both control and treatment groups, eg, acute kidney injury, need for hemodialysis, DDIs (especially anticoagulation and methadone), hepatotoxicity, ototoxicity, and early cessation of therapy. Last, outcomes should be stratified by surgical management.
In conclusion, we found no evidence of clinical benefit from combination gentamicin and/or rifampin therapy for staphylococcal PVE within the existing, limited literature, and point out multiple observational and prospective studies suggesting that unnecessary use of these adjunctive agents can cause harm. Given the widespread adoption of this regimen despite the absence of evidence, we suggest prospective, randomized investigation of adjunctive rifampin and/or gentamicin for staphylococcal PVE to determine if in vitro and in vivo benefits translate to patients and to quantify the magnitude of toxicities. Until that study has been published, perhaps the guideline recommendations should be downgraded, and these agents should not continue to be generally used outside the context of a clinical trial.
Supplementary Material
Contributor Information
Jonathan H Ryder, Department of Internal Medicine, Division of Infectious Diseases, University of Nebraska Medical Center, Omaha, Nebraska, USA.
Steven Y C Tong, Victorian Infectious Diseases Service, Royal Melbourne Hospital, Peter Doherty Institute for Infection and Immunity, Melbourne, Australia; Department of Infectious Diseases, University of Melbourne, Peter Doherty Institute for Infection and Immunity, Melbourne, Australia.
Jason C Gallagher, Department of Pharmacy Practice, Temple University, Philadelphia, Pennsylvania, USA.
Emily G McDonald, Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal, Quebec, Canada.
Irani Thevarajan, Victorian Infectious Diseases Service, Royal Melbourne Hospital, Peter Doherty Institute for Infection and Immunity, Melbourne, Australia; Department of Infectious Diseases, University of Melbourne, Peter Doherty Institute for Infection and Immunity, Melbourne, Australia.
Todd C Lee, Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal, Quebec, Canada.
Nicolás W Cortés-Penfield, Department of Internal Medicine, Division of Infectious Diseases, University of Nebraska Medical Center, Omaha, Nebraska, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors give special thanks to Dr Gabriel Vilchez, who helped to inspire this article through conversations over social media and participated in early discussion of the study design.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Muñoz P, Kestler M, de Alarcón A, et al. Current epidemiology and outcome of infective endocarditis: a multicenter, prospective, cohort study. Medicine (Baltimore) 2015; 94:e1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2016; 36:3075–123. [DOI] [PubMed] [Google Scholar]
- 3. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 4. Bisno AL, Dismukes WE, Durack DT, et al. Antimicrobial treatment of infective endocarditis due to viridans streptococci, enterococci, and staphylococci. JAMA 1989; 261:1471–7. [PubMed] [Google Scholar]
- 5. Simmons NA, Ball AP, Eykyn SJ, et al. Antibiotic treatment of streptococcal, enterococcal, and staphylococcal endocarditis. Heart 1998; 79:207–8. [PMC free article] [PubMed] [Google Scholar]
- 6. Chuard C, Herrmann M, Vaudaux P, Waldvogel FA, Lew DP. Successful therapy of experimental chronic foreign-body infection due to methicillin-resistant Staphylococcus aureus by antimicrobial combinations. Antimicrob Agents Chemother 1991; 35:2611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lucet JC, Herrmann M, Rohner P, Auckenthaler R, Waldvogel FA, Lew DP. Treatment of experimental foreign body infection caused by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 1990; 34:2312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drinković D, Morris AJ, Pottumarthy S, MacCulloch D, West T. Bacteriological outcome of combination versus single-agent treatment for staphylococcal endocarditis. J Antimicrob Chemother 2003; 52:820–5. [DOI] [PubMed] [Google Scholar]
- 9. Cosgrove SE, Vigliani GA, Campion M, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis 2009; 48:713–21. [DOI] [PubMed] [Google Scholar]
- 10. Riedel DJ, Weekes E, Forrest GN. Addition of rifampin to standard therapy for treatment of native valve infective endocarditis caused by Staphylococcus aureus. Antimicrob Agents Chemother 2008; 52:2463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thwaites GE, Scarborough M, Szubert A, et al. Adjunctive rifampicin for Staphylococcus aureus bacteraemia (ARREST): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2018; 391:668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts. A prospective study. Ann Intern Med 1982; 97:496–503. [DOI] [PubMed] [Google Scholar]
- 13. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115:674–80. [DOI] [PubMed] [Google Scholar]
- 14. Pujol M, Miró JM, Shaw E, et al. Daptomycin plus fosfomycin versus daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia and endocarditis: a randomized clinical trial. Clin Infect Dis 2021; 72:1517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tong SYC, Lye DC, Yahav D, et al. Effect of vancomycin or daptomycin with vs without an antistaphylococcal β-lactam on mortality, bacteremia, relapse, or treatment failure in patients with MRSA bacteremia: a randomized clinical trial. JAMA 2020; 323:527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng MP, Lawandi A, Butler-Laporte G, de L’Étoile-Morel S, Paquette K, Lee TC. Adjunctive daptomycin in the treatment of methicillin-susceptible Staphylococcus aureus bacteremia: a randomized, controlled trial. Clin Infect Dis 2021; 72:e196–203. [DOI] [PubMed] [Google Scholar]
- 17. Tong SYC, Mora J, Bowen AC, et al. The Staphylococcus aureus network adaptive platform trial protocol: new tools for an old foe [manuscript published online ahead of print 19 June 2022]. Clin Infect Dis 2022. doi: 10.1093/cid/ciac476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant–related staphylococcal infections: a randomized controlled trial. JAMA 1998; 279:1537–41. [DOI] [PubMed] [Google Scholar]
- 19. Aydın O, Ergen P, Ozturan B, Ozkan K, Arslan F, Vahaboglu H. Rifampin-accompanied antibiotic regimens in the treatment of prosthetic joint infections: a frequentist and Bayesian meta-analysis of current evidence. Eur J Clin Microbiol Infect Dis 2021; 40:665–71. [DOI] [PubMed] [Google Scholar]
- 20. Karlsen ØE, Borgen P, Bragnes B, et al. Rifampin combination therapy in staphylococcal prosthetic joint infections: a randomized controlled trial. J Orthop Surg Res 2020; 15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 25 April 2022.
- 22. Koshy L, Jeemon P, Ganapathi S, et al. Association of South Asian-specific MYBPC3Δ25 bp deletion polymorphism and cardiomyopathy: a systematic review and meta-analysis. Meta Gene 2021; 28. [Google Scholar]
- 23. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karchmer AW, Archer GL, Dismukes WE. Staphylococcus epidermidis causing prosthetic valve endocarditis: microbiologic and clinical observations as guides to therapy. Ann Intern Med 1983; 98:447–55. [DOI] [PubMed] [Google Scholar]
- 25. Le Bot A, Lecomte R, Gazeau P, et al. Is rifampin use associated with better outcome in staphylococcal prosthetic valve endocarditis? A multicenter retrospective study. Clin Infect Dis 2021; 72:e249–55. [DOI] [PubMed] [Google Scholar]
- 26. Ramos-Martínez A, Muñoz Serrano A, de Alarcón González A, et al. Gentamicin may have no effect on mortality of staphylococcal prosthetic valve endocarditis. J Infect Chemother 2018; 24:555–62. [DOI] [PubMed] [Google Scholar]
- 27. Karchmer AW, Archer GL, Dismukes WE. Rifampin treatment of prosthetic valve endocarditis due to Staphylococcus epidermidis. Rev Infect Dis 1983; 5(Suppl 3):S543–8. [DOI] [PubMed] [Google Scholar]
- 28. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med 1994; 96:200–9. [DOI] [PubMed] [Google Scholar]
- 29. Shrestha NK, Shah SY, Wang H, et al. Rifampin for surgically treated staphylococcal infective endocarditis: a propensity score-adjusted cohort study. Ann Thorac Surg 2016; 101:2243–50. [DOI] [PubMed] [Google Scholar]
- 30. Huang G, Gupta S, Davis KA, et al. Infective endocarditis guidelines: the challenges of adherence—a survey of infectious diseases clinicians. Open Forum Infect Dis 2020; 7:ofaa342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook. 2013. https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 13 June 2022.
- 32. Bhimraj A, Morgan RL, Shumaker AH, et al. IDSA guidelines on the treatment and management of patients with COVID-19. 2022. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed 13 June 2022.
- 33. Spellberg B, Wright WF, Shaneyfelt T, Centor RM. The future of medical guidelines: standardizing clinical care with the humility of uncertainty. Ann Intern Med 2021; 174:1740–2. [DOI] [PubMed] [Google Scholar]
- 34. Fowler VG, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–65. [DOI] [PubMed] [Google Scholar]
- 35. Lebeaux D, Fernández-Hidalgo N, Pilmis B, Tattevin P, Mainardi JL. Aminoglycosides for infective endocarditis: time to say goodbye? Clin Microbiol Infect 2020; 26:723–8. [DOI] [PubMed] [Google Scholar]
- 36. Sousa JP, Lourenco CN, Teixeira R, Goncalves LM. A case for ending rifampicin use in endocarditis. Circulation 2021; 144(Suppl 1):10302. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.