Abstract
Allergen-specific immunotherapy (AIT) is considered the only curative treatment for allergic diseases mediated by immunoglobulin E (IgE). Currently, the route of administration depends both on the different types of causal allergens and on its effectiveness and safety profile. Several studies have reported the mechanisms and changes in humoral and cellular response underlying AIT; however, the full picture remains unknown. Knowledge of who can benefit from this type of treatment is urgently needed due to the patient safety risks and costs of AIT. In vivo or in vitro biomarkers have become a strategy to predict clinical outcomes in precision medicine. There are currently no standardized biomarkers that allow determining successful responses to AIT, however, some studies have found differences between responders and nonresponders. In addition, different candidates have been postulated that may have the potential to become biomarkers. In this review, we aim to summarize the findings to date related to biomarkers in different IgE-mediated allergic diseases (respiratory, food, and venom allergy) with the potential to define who will benefit from AIT.
Keywords: Allergen-specific immunotherapy, Allergy, Biomarkers, IgE, Regulatory T cells, regulatory B cells
INTRODUCTION
Allergen-specific immunotherapy (AIT) is the mainstay treatment that can alter the course of allergic diseases, inducing desensitization and/or tolerance by altering the immunological response to allergens. Although AIT has been used in clinical practice for more than a century, knowledge of the mechanisms underlying the clinical response to this type of treatment was largely gained during the past 2 to 3 decades.
The principle of AIT consists of administration of the culprit allergen at high doses to allergic patients with the aim of inducing tolerance to the allergen [1]. Although AIT is a successful and cost-effective way to treat allergies [2,3,4], there is undoubtedly room for improvement. AIT protocols typically last several years to complete and, as a result, are characterized by low treatment adherence and a high rate of drop-out patients [5]. Therefore, the identification of objective biomarkers with predictive value for the response to treatment is urgently needed. This would allow clinicians to decide early during the treatment which patients are most likely to benefit from the continuation of AIT. Moreover, adjustments in allergen preparations (including the addition of tolerogenic adjuvants and structural modification of allergens) and routes of application are being developed, to reduce the duration of the treatment and increase its effectiveness [6].
In this review, we will provide a brief overview of the different types of AIT that are currently in use in clinical practice as well as novel developments in the field of AIT. Moreover, the basic immunological mechanisms that are at play in the response to AIT will be discussed in order to provide insight into the functional role of the potential biomarkers that are being studied in relation to the outcome of AIT. Then we provide an overview of the biomarker candidates that have been reported in AIT for respiratory allergies (allergic rhinitis, AR), food allergy, and venom allergy.
OVERVIEW OF AIT FEATURES
Allergen manufacturing
Since 1911, immunotherapy based on allergen extracts for the treatment of allergies has been used in clinical practice [7]. These extracts are manufactured from one or more natural allergen sources including pollens, arthropods (mites), animal sources, and venom from Hymenoptera species, and related allergens sources are often used as homologous mixtures [8,9]. Manufacturing aspects in the production of extracts such as purity, quality, representativeness of the components as well as their storage are important to ensure effectiveness and safety in AIT [8,10]. Differences in the production of natural allergen extracts frequently occur and this heterogeneity in quality could generate different findings in the clinical and molecular outcomes of AIT [11]. Currently, there is an urgent demand to explore innovative methods for allergen preparation, which has now become possible due to the development of recombinant allergens as a result of the progress that has been made in allergen identification and chemical modifications of allergenic extracts (known as allergoids) which improve the safety profile through hypoallergenic preparations [12,13,14,15,16,17,18].
Routes of application for AIT
AIT can be applied through multiple routes depending on the type of allergic disease and the source of sensitization [19]. Likewise, the selected application route leads to particularities in the antigen-presentation process, reflected in differences in efficacy and safety [20]. Subcutaneous, sublingual, and recently oral immunotherapy (OIT) have received U.S. Food and Drug Administration (FDA) approval for use in the treatment of different allergic diseases, however, experimental approaches with other routes of administration such as epicutaneous, intradermal, intralymphatic and local nasal have shown potential as alternatives [21].
Subcutaneous immunotherapy (SCIT) is the oldest and most frequently used application route of AIT, which has been employed for more than a century [7]. SCIT is regarded as a safe and valid therapy for several allergic diseases including venom allergy, AR, and allergic asthma [19]. While adverse reactions occur in up to 71% of patients, severe side effects are rare [22]. In addition to SCIT, alternative administration routes of AIT are being used and developed to improve the safety and effectiveness of AIT.
Sublingual immunotherapy (SLIT) was approved in 1998 by World Health Organization as an alternative treatment for allergic diseases induced by inhalant allergens [23,24]. SLIT usually places allergen-containing drops or soluble tablets with extracted allergens under the tongue for a few minutes before being swallowed [25].
OIT could become another needle-free alternative method to treat food allergies by daily swallowing tablets or powders with gradually increasing doses of food allergens, such as cow’s milk (CM), egg, and peanuts [26,27]. FDA authorized the first OIT drug for treating peanut allergies in 2020 [26]. Unlike SLIT, the release of the allergen occurs in the digestive tract where there is a large amount of gut-associated lymphoid tissue [19].
Recently, epicutaneous immunotherapy (EPIT) received increased interest after a trial performed by Senti et al. [28] in 2009, which suggested that EPIT could become a promising method for allergic diseases. EPIT involves the application of a noninvasive and painless patch containing an allergen extract to the skin, which avoids the ingestion of oral allergens improving the safety profile [29,30,31].
Intralymphatic immunotherapy (ILIT) is a relatively novel AIT application route, which is designed to have a more robust and rapid immune response by injecting a low dose of allergen directly into lymph nodes compared with intramuscular injection [32]. Unlike years of treatment for SCIT, ILIT only needs 3 times of ultrasound-guided injections spaced one month apart, which allows for shortening the duration period of the treatment [33]. However, up to now, there are few studies on ILIT, and its safety and efficacy need to be further demonstrated.
MECHANISMS UNDERPINNING SUCCESSFUL AIT
Allergic sensitization is initiated when allergens are captured by antigen-presenting cells (APCs) in the skin, respiratory or gastrointestinal mucosa. Skin and mucosal epithelial cells produce alarmins such as interleukin (IL)-25, IL-33, and thymic stromal lymphopoietin as defense mechanisms against allergens. Allergens are processed into peptides and presented by APCs to naïve CD4+ T cells, driving the differentiation into T helper (Th) 2 cells capable of producing type 2 cytokines including IL-4, IL-5, IL-9, and IL-13 [34]. B cells are triggered to undergo IgE class-switching and differentiate into plasma cells secreting allergen-specific IgE (sIgE) antibodies that bind to the high-affinity IgE receptor (FcεRI) on the surface of mast cells and basophils. Subsequent exposure to the same allergen triggers cross-linking of IgE-FcεRI complexes, resulting in the secretion of histamine, leukotrienes, cytokines, and other inflammatory mediators. These mediators drive type 1 hypersensitivity responses and occasionally result in anaphylaxis. Late-phase reactions and numerous allergen exposures, conserve allergic inflammation, and can progress to tissue remodeling, fibrosis, and the chronic phase of allergic diseases [35,36].
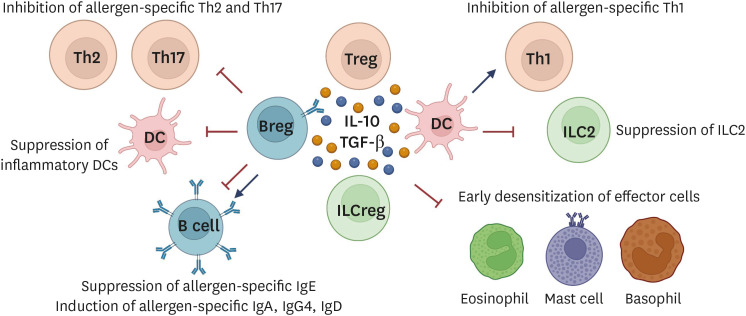
Successful AIT is characterized by development of clinical tolerance to the allergen. Tolerance may wane over time or could persist long-term, processes that are referred the context of food AIT as desensitization and sustained unresponsiveness (SU), respectively and has been associated with Th2-gene networks reprogramming and pathways in the CD4+ T cells [37,38]. The cellular and molecular mechanisms that underly the development of clinical tolerance in response to AIT have been elucidated to a large extent during the past decades. These mechanisms are mediated by an intricate interplay between different immune cells including mast cells, basophils, innate lymphoid cells (ILCs), dendritic cells (DCs), regulatory T cells (Tregs), and regulatory B cells (Bregs). An overview of the changes that occur in response to AIT are described below and illustrated in Fig. 1.
Fig. 1. Mechanisms of immune tolerance induction.
Tregs, Bregs, ILCregs, and tolerogenic DCs produce immunosuppressive cytokines such as IL-10, TGF-β, and IL-35. Tolerogenic DCs express surface molecules that suppress Th2 and Th17 cells as well as inflammatory DCs, and induce allergen-specific Tregs. Tregs suppress early desensitization of effector cells (eosinophils, mast cells, and basophils), effector Th cells (Th1, Th2, and Th17 cells), inflammatory DCs, ILC2, and allergen-sIgE. Through IL-10, TGF-β, and IL-35 cytokines production, allergen-specific IgA, IgG2, IgG4, and IgD antibodies are released and exert IgE-blocking effects. Bregs suppress effector T cells (Th2 and Th17) and induce Treg cell expansion via the release of IL-10, TGF-β, and IL-35 cytokines. Diverse surface molecules on Breg cells including BCR, PDL-1, CD39, CD73, CD80/CD86, CD40, ICOS-L, and AhR are well-expressed and suppress the inflammatory responses. In autoimmune tolerance, Bregs activate iNKT cells with suppressive function. Moreover, Bregs are also the main producer of allergen-specific IgA, IgG2, IgG4, and IgD antibodies that compete with the cross-linking of allergen-sIgE to effector cells. Tregs, regulatory T cells; Bregs, regulatory B cells; ILC, innate lymphoid cell; DC, dendritic cell; IL, interleukin; TGF, transforming growth factor; sIgE, specific IgE; iNKT, Invariant natural killer T; Ig, immunoglobulin.
Mast cells and basophils
Mast cells and basophils play a key role in the effector phase of allergic reactions. The allergen-dependent cross-linking of the IgE bound to its high-affinity receptor (FcεRI) on mast cells and basophils initiates type I hypersensitivity reactions through the secretion of several mediators and type 2 cytokines that drive allergic inflammation. AIT induces very rapid desensitization of mast cells and basophils revealed by low responsiveness to allergens despite the high allergen-sIgE levels observed at the beginning of the treatment. However, the reduction of mast cell and basophil infiltration in the tissues and the decreased mediator release occurs in the late phase of AIT [39]. One possible mechanism is the increased production of allergen-specific IgG4 (sIgG4) and the elevated expression of the low-affinity IgG receptor (FcγRIIa and FcγRIIb) on mast cells and basophils. IgG-mediated inhibition also reduces type 2 cytokine secreting from mast cells and basophils [40].
Another mechanism is the quick upregulation of histamine receptor 2, which displays an inhibitory effect on FcεRI-mediated activation and degranulation of basophils. The decrease in basophil responsiveness was demonstrated during many AIT studies [41]. Importantly, the evaluation of basophil responses before, during, and after AIT can help to identify transient desensitization or SU.
Regulatory T cells
Induction and maintenance of peripheral tolerance to allergens is influenced by the balance between Tregs and effector T cells. Peripheral T-cell tolerance is defined by an increase of Treg cells that can be induced during AIT and a shift of Th2 cell responses to Th1 [39]. Allergen-specific Treg cells can be classified into thymic or natural Treg cells and inducible Treg cells including FOXP3-expressing iTreg cells, IL-10-secreting Tr1 cells, and transforming growth factor (TGF)-β-producing Th3 cells [42,43]. The suppression of different effector cells by Treg cells is required to completely establish cell-mediated tolerance. Treg cells instituted during and after AIT suppress effector cell function and facilitated the production of blocking antibodies by B cells [35]. Four major mechanisms of Treg cell-mediated suppression have been described: (1) secretion of inhibitory cytokines such as IL-10, TGF-β, and IL-35; (2) metabolic disruption mechanisms; (3) mechanisms involving surface molecules including programmed death 1, cytotoxic T-lymphocyte antigen 4, lymphocyte-activation gene 3, or inducible costimulatory molecule (ICOS); and (4) cytolysis [36,39,44]. Increased frequencies of allergen-specific Treg cells and a decrease in Th2 frequencies are associated with natural tolerance in beekeepers during allergen exposure in beekeeping season and AIT-treated allergic patients, respectively [45]. Ex vivo analysis of human peripheral blood mononuclear cells (PBMCs) showed that house dust mite (HDM) AIT leads to an increase of functional allergen-specific Tregs and a decrease of allergen-specific immunoglobulin-like transcript (ILT) 3 Treg cells, which display impaired suppressive function [46]. AIT induces long-term Treg cell development by influencing epigenetic changes (hypomethylation) in FOXP3 regions [47]. IL-10-producing Tregs were induced after birch and grass pollen AIT. In addition, IL-35-inducible Tregs has been identified as a subset of iTregs with the capacity to reduce Th2 inflammation, T-cell proliferation and cytokines produced by ILC2s [48,49]. Recent studies have demonstrated that successful SCIT increases IL-35 and IL-35-induced Treg cell in blood [39]. All these studies have demonstrated the critical role of the tolerance induced by allergen-specific Treg cells in successful AIT in humans.
Regulatory B Cells
Although B cells play a major role in the immune system through the production of specific antibodies, recent evidence clearly showed that B cells can also regulate immune responses via alternative mechanisms beyond antibody production [50]. Breg cells play a crucial role in producing the immune suppressive cytokines including IL-10, IL-35, and TGF-β, and expressing immune suppressive receptors such as cell membrane-bound molecules such as B cell receptor (BCR), PDL-1, CD39, CD73, CD80/CD86, CD40, inducible costimulatory ligand (ICOS-L), and aryl-hydrocarbon receptor (AhR) [51]. Breg cells can be triggered by several factors, including inflammatory cytokines such as IL-6, IL-1b, and interferon (IFN)-α, microbial compounds such as TLR4 or TLR9 ligands, along with CD40 ligation [52]. During AIT, IL-10–secreting B regulatory 1 (Br1) cells specific to bee-venom allergen phospholipase A2 show increased frequencies and are skewed toward IgG4 production [53]. IL-10+Br1 cells increased their frequency in allergic patients receiving AIT and naturally exposed to allergen individuals [54]. Furthermore, HDM allergic patients treated with AIT showed significantly increased frequencies of Dermatophagoides pteronyssinus (Der P) 1-specific B cells, plasmablasts, and IL-10+IL-1RA+ Breg cells [46]. Thus, Breg cells appear to play an important role in achieving immune tolerance during AIT.
Innate lymphoid cells
ILCs are a relatively recently discovered cell type of the innate immune system. These cells are classified into 2 major subsets; cytotoxic and noncytotoxic (helper) ILCs. The cytotoxic ILCs compose of NK cells that display functions of CD8+ cytotoxic T cells and lymphoid tissue inducer cells that are involved in secondary lymphoid structures development. The helper ILCs have been consequently identified into 3 different phenotypes: group 1 ILCs (ILC1s), group 2 ILCs (ILC2s), and group 3 ILCs (ILC3s). These 3 different subsets resemble the functions of Th1, Th2, and Th17 cells, respectively. Interestingly, new discoveries of IL-10–producing ILCs and retinoic acid-mediated pathway of IL-10-producing ILC2s induction has shown the immune regulatory properties of these cells [55,56]. During the course of AIT, a subset of ILC2 cells regained the capacity to produce IL-10 in grass pollen sublingual treated-patients, indicating that IL-10+ ILC2s may have disease-regulating effects in AIT [57].
Dendritic cells
DCs are APCs with the dual capacity to induce and preserve allergic inflammation or tolerance. DCs play a major role during the course of AIT. An increase in plasmacytoid DCs, which is a DC subset involved in the induction of Treg cells and oral tolerance is observed in AIT [58,59]. In addition, reduced frequencies of CD1c+ conventional DCs (cDCs), which support Th2 responses in allergic patients, were also observed after AIT [58]. In mice, CD11b+ cDCs and macrophages transport sublingual allergens to lymph nodes and induce allergen-specific Tregs [39,60]. Tolerogenic DCs represent a heterogeneous population with an immature or mature phenotype that displays an increased capacity to produce IL-10. Allergoid-mannan conjugates generate tolerogenic IL-10-producing DCs and reprogram monocytes and macrophages into tolerogenic phenotypes [61,62,63]. Similarly, a nano-vaccine was produced by coupling PGLA-encapsulated ovalbumin (OVA) with mannan induced In vitro IL-10-producing DCs that generated Tregs [64]. Cannabinoids also induce tolerogenic DCs able to generate Tregs by mechanisms depending on autophagy and metabolic reprogramming [65]. Another in vitro study showed the capacity of IL-27 produced by DCs to suppress grass pollen-stimulated PBMC proliferation. Interestingly, allergic airway inflammation in mice highlighted the important role of IL-10 signaling in DCs to induce allergen-specific tolerance [66].
Allergen-specific antibodies
The secretion of immunoregulatory cytokines such as IL-10 and TGF-β from previously mentioned cells types results in the suppression of Th2 responses and a shift towards the induction of allergen-specific IgA and IgG4 antibodies [67,68,69]. Non-IgE allergen-specific and low-affinity IgE antibodies compete with IgE for allergen binding, which at the cellular level inhibits IgE-mediated cross-linking of FcεRI on basophils and mast cells, leading to reduced mast cell and basophil activation [70,71,72]. While most studies only demonstrated that AIT resulted in increased production of allergen-sIgG4 antibodies, direct evidence for a protective effect against allergic inflammation mediated by allergen-sIgG4 antibodies was recently obtained. Cat-allergic individuals were treated passively immunized with 2 monoclonal anti-Fel d 1 IgG4 antibodies, which resulted in reduced clinical symptoms in response to nasal provocation [73]. Several mechanisms have been described by which IgG4 can regulate the allergic inflammatory response [70]. One of these is its bispecific nature (it has 2 different antigen-binding sites) as a result of a process called Fab-arm exchange which leads to functionally monovalent antibodies and this prevents the formation of immune complexes [74]. In addition, it has a low affinity to activate Fcγ receptors, does not bind to complement, and competes with IgE by blocking its binding to allergens [70]. Similar to IgG4, an IgE-blocking effect has been observed in IgG2. Recent data reveals both IgG2 and IgG4 are induced in patients who received SLIT against grass pollen [75].
Lastly, allergen-specific IgD was recently found to increase in HDM-sensitized asthmatic patients during 30 weeks and more than 2 years of AIT [76]. In addition, allergen-specific IgD was increased in children consuming CM compared to children restricting CM from their daily diet, proposing food antigens may establish humoral IgD responses in humans [77].
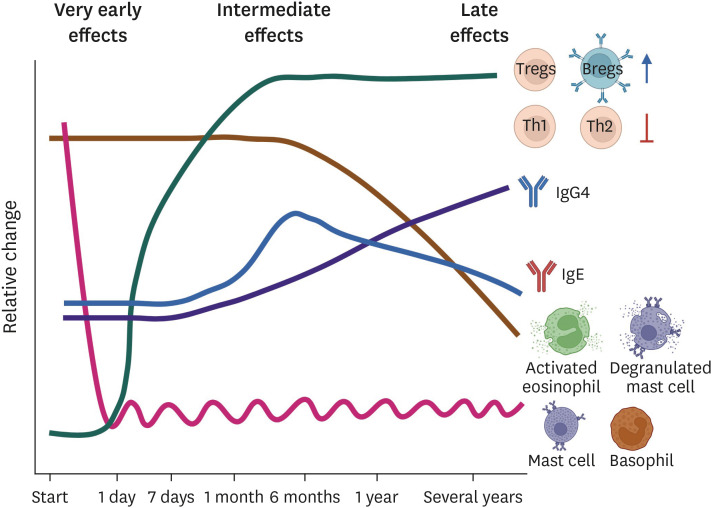
The kinetics of the different cellular and humoral processes involved in immune tolerance induction are summarized in Fig. 2.
Fig. 2. Kinetics of immune tolerance induction.
AIT dominates the skewing of the T-cell population from allergen-specific Th2 to Treg cells and increases the Bregs population. Both of these cells produce immunosuppressive cytokine IL-10 and suppress allergen-sIgE secretion. In parallel, these cells also induce IgG4 production that displays anti-inflammatory properties. Overall, AIT is considered a successful treatment for allergic inflammation. AIT, allergen immunotherapy; Treg, regulatory T cell; Breg, regulatory B cell; IL, interleukin; sIgE, specific IgE; IgG4, immunoglobulin G4.
PREDICTIVE BIOMARKERS FOR AIT OUTCOMES IN ALLERGIC DISEASES
Asthma
Asthma is a heterogeneous disease with varying degrees of airway inflammation and response to treatment. Allergic asthma is triggered by inhaled allergens. The incidence of allergic asthma has been continuously increasing over recent decades [78]. While allergic asthma is the dominant phenotype among asthma diagnosed in childhood, allergic sensitization among asthma patients is decreased with increasing age of asthma onset [79,80]. AIT effectively reduces symptoms and medication needs and improves the quality of life and bronchial hyperresponsiveness in allergic asthma patients [81]. Adolescents and adult asthmatics with AIT exposure are less likely to experience progression of asthma severity [82]. However, a small proportion of patients do not respond to AIT treatments [83]. AIT, either subcutaneously (SCIT) or sublingually (SLIT), with repeated administration for a minimum treatment period of 3 years achieves clinical benefits [84].
Asthma often coexists with rhinitis and is found in up to 38% of patients with AR, which is likely to result in more severe asthma [85,86]. Global Initiative for Asthma Management and Prevention (GINA 2022) considers adding SLIT in adult patients with AR and sensitized to HDM with persisting asthma despite low-medium dose inhaled corticosteroids-containing therapy, provided forced expiratory volume in 1 second is >70% predicted [87]. Post-AIT treatment cessation benefits reduced AR and asthma medication use, and decreased risk of new-onset asthma medication use during treatment [88].
Several immunological biomarkers are applied to measure AIT-induced immune responses and efficacy in asthma (Table 1). Among immune cells as biomarkers in AIT, eosinophil, and basophil cells are inhibited by AIT treatment along with allergen-sIgE that are crucial to type 2 inflammation in the airways [89,90,91,92,93,94].
Table 1. Potential predictive biomarkers for outcome of AIT in asthma with or without rhinitis.
| Bio marker | Type of AIT | Allergen source of study | Study type | Study population | Main finding | Association with effectiveness to AIT | Ref | |
|---|---|---|---|---|---|---|---|---|
| Asthma | ||||||||
| Der p 1-sIgG4, -induced TGF-β, and, -induced IFN-γ | SLIT | HDM (Dermatophagoides spp; Der p 1) | Longitudinal prospective RCT (18-mo follow-up) | 51 Children with mild/moderate asthma (5–12 yr) underwent different AIT and PT | Antigen-specific IgG4, IFN-γ, and TGF-β levels increased in the SCIT and SCIT plus SLIT compared to the pharmacotherapy group and inversely correlate with clinical symptoms. | N/D | [205] | |
| SCIT | ||||||||
| sIgG1 sIgG4 | SCIT | HDM (D. pteronyssinus) | Longitudinal prospective RCT (24-mo follow-up) | 65 Children between 6–17 yr old were randomized for SCIT plus PT vs. PT alone | Increased levels of specific IgG1 and IgG4 in the SCIT+PT compared to PT | N/D | [206] | |
| sIgE, | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective DBCT (24-mo follow-up) | 85 Patients with 7–36 yr old were randomized for SLIT vs. placebo. | Increased sIgE and sIgG4 in the SLIT group compared to the placebo | N/D | [207] | |
| sIgG4 | ||||||||
| sIgE | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective RCT (18-mo follow-up) | 111 Children with mild-to-moderate asthma (5–15 yr) were randomized for SLIT vs. placebo | Increased skin wheal diameter, sIgE, and IgG4 in the SLIT group compared to placebo | N/D | [208] | |
| IgG4 | ||||||||
| SPT | ||||||||
| Th17, Treg | SLIT | HDM (D. farinae) | Longitudinal prospective RCT (12-mo follow-up) | Allergic asthma children (4–18 yr); SLIT (n=30) vs. placebo (n=30) | Mean daily symptom scores and percentages of Th17 cells declined, and percentage of Treg increased in peripheral blood. | N/D | [103] | |
| Blood eosinophils | SCIT | HDM (Dermatophagoides spp.) | Longitudinal prospective | 16 HDM allergic patients (mean, 11 yr) classified according to improvement of lung function (8:8) | The lung function improved group had lower blood eosinophils and higher HDM-sIgE levels than the declined group. | Associated with unsuccessful AIT | [89] | |
| sIgE | Cohort study (>1-yr follow-up) | Associated with successful AIT | ||||||
| sIgG4 | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective RCT (6-mo follow-up) | 20 Children with mildly/moderately asthma (aged 6–12 yr) | Decreased blood eosinophil and increased sIgG4 in the SLIT group | N/D | [90] | |
| Blood eosinophils | ||||||||
| Blood eosinophil | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective cohort study (6- and 12-mo follow-up) | 31 Patients with mean age of 8.27±2.87 yr with a diagnosis of mild-to-moderate persistent asthma | Total eosinophil count and sIgE decreased compared to baseline. | N/D | [91] | |
| sIgE | ||||||||
| ECP | SCIT | HDM (D. pteronyssinus) | Longitudinal prospective DBCT (1-yr follow-up) | 129 Asthmatic patients (6–45 yr) | Serum ECP decreased after AIT. | N/D | [92] | |
| Asthma/AR | ||||||||
| tIgE, sIgE/tIgE | SCIT | HDM (D. pteronyssinus) | Longitudinal retrospective | 185 Asthma and AR children (4–14 yr) | tIgE and sIgE/tIgE ratio associated with clinical response to AIT | Associated with unsuccesful AIT | [110] | |
| Cohort study (3 yr follow-up) | ||||||||
| tIgE, sIgE/tIgE | SCIT | HDM (D. pteronyssinus) | Longitudinal retrospective | 81 Asthma and AR children’s patients with median age of 7 yr | Treatment-effective cases had lower tIgE and a higher ratio of sIgE/tIgE. SPT grade changes to determine efficacy had a high degree of consistency with symptoms and drug score. | Associated with unsuccessful AIT | [111] | |
| SPT | Cohort study (3-yr follow-up) | |||||||
| sIgE/tIgE | SCIT SLIT | HDM (Dermatophagoides spp.) | Longitudinal retrospective Cohort study (4-yr follow-up) | 279 AR with or without asthma (mean, 29.5 yr) | Higher serum sIgE/tIgE ratio (>16.2) associated with an effective clinical response to AIT | Associated with successful AIT | [109] | |
| Grass pollen (P. judaica, O. europea) | ||||||||
| sIgE/tIgE | SCIT | HDM (Dermatophagoides spp.) | Longitudinal prospective | 32 Children asthma and/or AR diagnosed (7–15 yr) | Rhinitis symptom scores improved in children with a higher sIgE/tIgE ratio. sIgE/tIgE inversely correlated with visual analogue scales. | Associated with unsuccessful AIT | [209] | |
| Cohort study (2-yr follow-up) | ||||||||
| sIgE/tIgE | SCIT | HDM (Dermatophagoides spp.) | Longitudinal retrospective | 146 AR patients with/without asthma allergic | Serum sIgE/tIgE ratio significantly increased after SCIT. | N/D | [112] | |
| Cohort study (5-yr follow-up) | ||||||||
| sIgE | SCIT SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective RCT (1-yr follow-up) | 48 Patients (5–10 yr) with mild persistent asthma/rhinitis classified in 3 groups (SCIT, SLIT, PT) | sIgE reduced in SCIT and SLIT groups. | N/D | [210] | |
| sIgG4 | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective RCT (6-mo follow-up) | 693 Patients uncontrolled asthma and rhinitis (mean, 33 yr) | Increase in allergen-specific IgG4. | N/D | [211] | |
| sIgG4 | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective RCT (3-yrs follow-up) | 31 Mild asthmatics ± rhinitis children with a (median age, 10 yr) | Allergen-specific IgG4 was increased in the SCIT group at year 3. | N/D | [108] | |
| SCIT | ||||||||
| sIgG4 | SCIT | HDM (Dermatophagoides spp.) | Longitudinal prospective Cohort study (18-mo follow-up) | 90 Participants: rhinitis including 21 asthma positive patients (mean age, 17 yr) | sIgG4 increased after treatment compared to baseline but sIgG4 to HDM components do not qualify as a biomarker to evaluate the efficacy of AIT. The changes of sIgE, sIgG4, sIgE/sIgG4 ratio and the numbers of positive HDM components were not associated with the improvement of combined symptom and medication scores after AIT. | No association with successful AIT | [113] | |
| sIgG2 | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective RCT (1 yr follow-up) | 100 participants AR ± asthma (mean 27 yrs old) | HDM-specific IgG2 increase in hig responders. In high responders, HDM-specific IgG2 and sIgE is correlated | Association with successful AIT | [150] | |
| IL-10 | SCIT | HDM (D. pteronyssinus) | Longitudinal prospective | 118 Adult patients (18–65 yr) diagnosed with AR ± allergic asthma | Severe patients with poor and no response to treatment had higher baseline IL-13, IL-10, and Th2 cytokines (IL-4 + IL-5 + IL-13). | Associated with unsuccessful AIT | [101] | |
| IL-13 | Observational study (1-yr follow-up) | |||||||
| Basophil IgE | SCIT | Birch pollen (Betula verrucosa) | Longitudinal prospective RCT (18- to 20-mo follow-up) | 21 Patients with AR with or without mild-to-moderate asthma and 21 placebo control patients (mean age, 32 yr) | Inhibition of basophil activation, IgE binding, and IgE-FAP were correlated with AIT. | N/D | [93] | |
| IL-10, eosinophil, IgG4 | SLITSCIT | HDM (Dermatophagoides spp.) | Longitudinal prospective RCT (1-yr follow-up) | 30 Asthmatic + rhinitis patients (mean age,10 yr) | SLIT and SCIT increased serum IL-10 and decreased nasal eosinophils. SCIT reduced sputum eosinophil and increased serum sIgG4. | N/D | [94] | |
| Periostin + FeNO | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective RCT (48-wk follow-up) | Adults with mild/moderate AR and asthma and from 46 SLIT participants 29 were responders and 17 were not responders (20–65 yr) | The proportion of patients with both high periostin and high FeNO levels was significantly higher in responder than in nonresponder. | Associated with successful AIT | [97] | |
| Tregs, IgG4, IFN-γ, TNF-α | SLITSCIT | HDM (Dermatophagoides spp.) | Longitudinal prospective RCT (1-yr follow-up) | Sixty-seven patients (age, 5–55 yr) with moderate-severe AR with or without asthma were randomized SLIT (n=27), SCIT (n=26) and placebo (n=14). | % Tregs in CD4+ T cells increased after treatment in both groups. Serum sIgG4 increased and was 30 times higher in SCIT than SLIT after the treatment. Serum IFN-γ increased in SCIT. Treg (SLIT) and IFN-γ (SCIT) inversely correlated with total rhinitis score. | N/D | [212] | |
| CD66b, MPO | Not specified | HDM (D. pteronyssinus) | Longitudinal prospective | AIT-treated atopic patients with allergic asthma and rhinitis (n=15), non-AIT-treated patients with allergic asthma and rhinitis (n=10), and controls (n=10) aged 20–50 yr | Neutrophil cell-surface expression of CD66b is upregulated in vitro in response to allergens and reduced by AIT. AIT also reduced MPO in nasal lavage fluid. | N/D | [98] | |
| Pollens (D. glomerata, O. europea, A. vulgaris) | Experimental study (3-yr follow-up) | |||||||
| miR-3935, PTGER3 | SCIT | Grass pollen | Longitudinal prospective | Rhinitis –AIT 16, rhinitis +AIT 21, asthma –AIT 21, asthma +AIT 22, healthy controls 44, aged 18–70 yr | AIT induces miR-3935 in induced sputum of allergic asthmatics, while mRNA of its target, prostaglandin EP3 receptor, is downregulated in treated patients. PGE2 correlates with ILC2, sputum IL-13, inflammatory cell load, sputum eosinophils, and symptoms in AIT patients. | N/D | [104] | |
| Experimental study (>1-yr follow-up) | ||||||||
| CD29+ Lol p 1-specific memory B cells | SLIT | Grass pollen (L. perenne) | Longitudinal prospective | 27 AR ± asthma patients, 13 SLIT (median age, 46 yr) and 14 PT (median age, 32 yr) | Associated changes in surface-expressed proteins on memory B cells subsets can be used as early biomarkers for treatment effects. | N/D | [123] | |
| Experimental study (4-mo follow-up) | ||||||||
| AR | ||||||||
| CD23+ B cells | SCIT | HDM (Dermatophagoides spp.) | Longitudinal prospective | Adult AR allergic (n=36) and healthy controls (n=31) | AIT down regulated CD23 expression on memory B cells. Reduction of CD23 expression on switched memory B cells correlated with AIT efficacy in AR patients compared to placebo. | N/D | [122] | |
| Cohort study (1-yr follow-up) | AIT group (n=23), without AIT group (n=21) | |||||||
| ILC2 | SCIT | Grass pollen (P. pratense) | Longitudinal prospective | 12 Participants with mod-severe SAR, 9 with history of SAR with grass pollen SCIT, 11 nonatopic controls | Suppression of peripheral ILC2s during pollen season in AIT patients. AIT subjects reported markedly less seasonal symptoms than untreated subjects with SAR, correlated with ILC2s proportion. | N/D | [136] | |
| Cohort study (8- to 36-mo follow-up) | ||||||||
| Periostin | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective RCT (48 weeks follow-up) | 20 to 65 yr of age with AR | The proportion of patients with both high serum periostin and s-IgE levels was significantly greater in responder than in nonresponder. | Associated with successful AIT | [116] | |
| sIgE | AIT group (n=50), control group (n=46) | |||||||
| MRGPRX2 | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective | Moderate to severe persist AR (n=110) and healthy controls (n=40) | MRGPRX2 & MMP-12 were significantly higher in the AR ineffective than the effective group-Elevated MMP-12 levels were correlated with VAS and TNSS, MRGPRX2 levels were correlated with VAS. | Associated with unsuccessful AIT | [144] | |
| MMP-12 | Cohort study (3-yr follow-up) | AIT patients were classified in effective (n=55) and ineffective (n=45). | ||||||
| ALCAM | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective | Mild AR (n=38), mod-severe AR (n=80), healthy control (n=40) | ALCAM levels were significantly lower in the effective group than in the ineffective group. | Associated with unsuccessful AIT | [127] | |
| Cohort study (3-yr follow-up) | According to response (effective 39, ineffective 29). | |||||||
| CD63 | SLIT | Grass pollen (P. pratense) | Longitudinal prospective | 26 patients with AR sensitized fom at least 2 yr, AIT group (n=16), reference group (n=10) | AIT-treated patients showed a significant decrease in CD63 and CD203c. AIT patients were significant improvement in all symptoms, and no changes in VAS scores were observed in control group. | N/D | [138] | |
| CD203c | Cohort study (1-yr follow-up) | |||||||
| (using CD-Sens) | ||||||||
| IL-33 | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective | 60 AR with HDM children, AIT group (n=30), with aging 120–190 mo, placebo group (n=30) with aging 125–185 mo | Suppression of peripheral ILC2s during pollen season in AIT patients | N/D | [132] | |
| Cohort study (2-yr follow-up) | ||||||||
| Leptin | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective | 40 AR (18–60 yr, without obesity) only allergic to HDM at least 2 yr | Serum and nasal leptin protein levels during AIT were decreased. TNSS score after AIT decreased with significant difference compared to placebo group | N/D | [129] | |
| Cohort study (2-yr follow-up) | ||||||||
| IL-4 | SCIT | HDM (Dermatophagoides spp.) | Longitudinal prospective | 69 of 72 children AR, completed 1-yr follow-up schedule with 46 included in effective group and 23 in ineffective group | Eotaxin, IFN-r, IL-4 levels closely associated with the efficacy of AIT in pediatric HDM-induced AR patients | Associated with successful AIT | [134] | |
| Eotaxin | Cohort study (2-yr follow-up) | Associated with unsuccessful AIT | ||||||
| IFN-γ | ||||||||
| CXCL13 | SCIT | HDM (Dermatophagoides spp.) | Longitudinal prospective | 80 Children with AR treated with AIT were categorized into effective group (n=56), ineffective group (n=24) | CXCL13 in the effective group were higher than those in the ineffective group. | Associated with successful AIT | [124] | |
| Cohort study (6-mo follow-up) | ||||||||
| MIF | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective | 160 Persistent HDM-induced AR, including 48 mild AR (MAR), 112 mod-severe AR (MSAR), 77 healthy controls | MIF were significantly lower in the good response group than in the poor response group. Circulating MF levels were positively correlated with TNSS, VAS, specific IgE, blood eosinophil count. | Associated with unsuccessful AIT | [130] | |
| Cohort study (3-yr follow-up) | MSAR group, 106 receive SLIT for 3 yr → 45 good response, 35 poor responses | |||||||
| LTA4H | Not specified | Grass pollen (Arteminsa spp.) | Longitudinal prospective | Age between 6 and 60, with artemisia pollen allergen AR | LTA4H level in the responders increased significantly after 1yr therapy, that of nonresponders remained unchanged. | Associated with successful AIT | [139] | |
| Cohort study (1-yr follow-up) | After treatment 1 yr, patients were classified as responders (n=30), nonresponses (n=15). | |||||||
| HDM-reactive ST2+CD45RO+CD4+ cells, IL-5+IL-13+CD27-CD161+CD4+ | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective RCT (52-wk follow-up) | 89 HDM-AR patients placebo (43), HDM 300IR (46) | HDM-reactive CD27-CD4+ cells, IL-5+IL-13+CD27-CD161+C4+cells, ST2+CD45RO+CD4+cells were detected in all patients with HDM-AR, were significantly decreased after AIT. | Associated with unsuccessful AIT | [125] | |
| JC-pollen spicific IL-10+Foxp3+ | SLIT | Pollen JC (C. japonica) | Longitudinal prospective RCT (52 weeks follow-up) | 40 Patients age 12–64 yr | JC-pollen specific IL-10+Foxp3+ cells were increased after initiation of SLIT in good responders | Associated with successful AIT | [126] | |
| JC pollen extract AIT group and placebo group 1:1 | ||||||||
| ILC2 | SCIT | HDM (Dermatophagoides spp.) | Transversal Experimental study | 40 Participants, 9 AR untreated group, 24 AR receiving D. p teronyssinus AIT 7 healthy control | Levels of ILC2 in the AIT group were significantly reduced compared with untreated group | N/D | [137] | |
| TAFI | SLIT | Pollen JC (C. japonica) | Longitudinal retrospective | 9 JCP undergoing AIT | Serum levels of TAFI increased gradually over time, a significant change was observed in 2 yr of AIT | N/D | [141] | |
| Cohort study (3-yr follow-up) | Serum C3a levels decreased significantly over the same interval. | |||||||
| Lactic acid, creatinine, sphingosine | SLIT | HDM (Dermatophagoides spp.) | Longitudinal prospective Cohort study (3-yr follow-up) | 72 Assigned to receive AIT, 68 patients completed, 39 into effective group, 29 into ineffective group | Lactic acid, ornithine, sphingosine and creatine levels were elevated in the serum of ineffective group | Associated with unsuccessful AIT | [152] | |
| Arachidonic and linolenic acid | Arachidonic and linolenic acid were higher in effective group | Associated with successful AIT | ||||||
| 13-HODE, 9-HPODE, 5(S)-HETE, 8S(S)-HETE, 11(S)-HETE, 15(S)-HETE, 11-hydro TXB2 | SCIT | HDM (Dermatophagoides spp.) | Longitudinal prospective Cohort study (42-wk follow-up) | 73 AR patients: 35 received Der p. single; 38 received mixed Der p, Der f. | Following the AIT, levels of AA and down-stream metabolic molecules (13-HODE, 9-HPODE, 5(S)-HETE, 8S(S)-HETE, 11(S)-HETE, 15(S)-HETE, 11-hydro TXB2) decreased. | N/D | [153] | |
AIT, allergen immunotherapy; AR, allergic rhinitis; ECP, eosinophil cationic protein; PT, pharmacotherapy; RCT, randomized clinical trial; DBCT, double blinded clinical trial; FAP, CD23-mediated IgE facilitated allergen presentation; HDM, house dust mite; ECP, eosinophil cationic protein; PTGER3, prostaglandin EP3 receptor; SPT, skin prick test; IFN-γ, Interferon γ; GP, grass pollen; MPO, myeloperoxidase; cytotoxic; ALCAM, activated leukocyte cell adhesion molecule; N/D, not determined; MIF, macrophage migration inhibitory factor; LTA4H, leukotriene A4 hydrolase; TAFI, thrombin-activatable fibrinolysis inhibitor; SLIT, sublingual immunotherapy; SCIT, subcutaneous immunotherapy; TGF, transforming growth factor; FeNO, fractional exhaled nitric oxide; SAR, seasonal allergic rhinitis; MMP-12, matrix metalloproteinase-12; MRGPRX2, mas-related G protein-coupled receptor-X2; TAFI, thrombin-activatable fibrinolysis inhibitor; PGE2, prostaglandin E2; VAS, visual analog scale; TNSS, total nasal symptom score; JCP, Japanese cedar pollen.
Cellular biomarkers
In an animal model of asthma, AIT treatment significantly decreased eosinophil-predominated inflammatory cells and type 2 cytokines IL-4, IL-5, and IL-9 in bronchoalveolar lavage fluid, and IL-5 and IL-13 production in lung cells [95,96]. Periostin, a biomarker of eosinophilic airway inflammation, was reduced after AIT treatment and was correlated with improved lung function in adults with asthma sensitized to HDM [97]. Interestingly, myeloperoxidase (MPO) released from neutrophil activation was also reduced in nasal lavage fluid and blood after 3-year treatment [98,99]. These findings indicate that AIT actively modifies type 2 airway inflammation and the release of MPO by neutrophils in allergic asthma.
The immunosuppressive role of Tregs is crucial in maintaining the homeostatic balance during dysregulated immune responses of asthma inflammation, and AIT enhances the local functional role of Treg cells. IL-10 produced by Treg cells mediates the changes in sIgE to sIgG4 seen in AIT [100]. Justicia et al. [101] identified increased levels of sIgG4 and sIgE, and IL-10 involved in mechanisms of immune tolerance was induced by AIT specific for HDM. They also found an increased baseline IL-13 in nonresponders which might serve as a biomarker to predict clinical response. In a double-blind placebo-controlled clinical study, Yukselen et al. [94] also found induction of serum IL-10 levels along with improved clinical symptoms in response to SCIT and SLIT. However, SCIT showed a more promising effect with a further decrease in sputum eosinophils and an increase in serum sIgG4. In the airway inflammation model, AIT induced CD4+CD25+Foxp3+ Treg cells in peripheral blood and upregulated the expression of IL-10, TGF-β1, and Foxp3 in lung tissues [102]. Tian et al. [103] reported downregulation of Th17 cells in response to AIT in children with allergic asthma. The analysis revealed significant symptom improvement at 12 weeks and changes in Th17 and CD4+CD25+Treg cells at 24 weeks. Thus, treatment duration should be carefully considered to evaluate the clinical efficacy of AIT, especially when applying T-cell changes as a biomarker.
Numerous publications have described genetic and epigenetic markers as potential biomarkers of allergic asthma. A recent study by Jakwerth et al. [104] investigated epigenetic miR-associated changes in the sputum of grass pollen-allergic patients after one year of AIT. They identified miR-3935 among 4 upregulated miRs in asthmatics, and the mRNA of its target, prostaglandin EP3 receptor, was downregulated in AIT-treated individuals, which might be a therapeutic target for AIT.
Humoral biomarkers
The best-studied predictive biomarkers of humoral immunity including sIgE, sIgG1, sIgG4, and sIgE/total IgE (tIgE) ratio are used to monitor the efficacy of AIT. An initial increment in IgE levels is often observed after AIT treatment followed by a reduction in response to long-term AIT treatment [105,106]. This change is accompanied by induced blocking antibodies (particularly, of the subclass IgG4) [107]. The sIgE/tIgE ratio is often increased following AIT treatment [108,109,110,111,112], however, no changes are observed when evaluated less than 2 years of AIT treatment [113]. The blocking activity of IgG antibodies maintains unchanged for at least 2 years of discontinuation of AIT, despite the reduction in the IgG1 and IgG4 levels [114], suggesting long-term immune tolerance following cessation of AIT treatment.
In vivo biomarkers
Changes in skin prick test (SPT) have a high degree of diagnostic performance in the evaluation of efficacy in HDM-SCIT according to tIgE levels [111]. Recently, one study reported the increased sensitivity and clinical relevance of the intradermal test compared to SPT to predict AIT effectiveness in respiratory allergic diseases [115]. Hoshino et al. [116] report an interesting finding where the proportion of responders to AIT had high periostin and high fractional exhaled nitric oxide levels, which opens a window to explore the combination of in vivo and in vitro biomarkers.
Allergic rhinitis
AR is a highly prevalent health problem in childhood, adolescence, and adult life [117]. Susceptibility to other allergic comorbidities in AR has been described [118,119,120,121]. AIT has been shown to reduce the symptoms of rhinitis and prevent the development of allergic comorbidities, therefore the possibility to reduce pharmacological treatment. Different biomarkers have been proposed for the evaluation of the response to AIT in AR (Table 1).
Cellular biomarkers
The understanding of the role of B and especially, allergen-specific ones, in AR-AIT remains limited. Yao et al. [122] demonstrated that CD23 expression on switched memory B cells, positively correlated with disease severity and clinical efficacy of HDM-SCIT in patients with AR, and also correlated with improvement of the symptom scores. Consistently, grass pollen SLIT induces Lol p 1-specific memory B cells with upregulated expression of surface IgG4, CD23, and CD29 [123]. The serum C-X-C motif ligand 13(CXCL13) but not B cell-activating factor after HDM-SCIT in pediatric AR have clinical values for biomarkers, being CXCL13 level higher in effective AIT group [124].
Regarding T cells, Ihara et al. [125] reported significantly decreased memory Th2 cell subsets (HDM-reactive CD27-CD4+, IL-5+IL-13+CD27-CD161+CD4+, ST2+CD45RO+CD4+) in HDM-AR patients after SLIT. Japanese cedar (JC) (Cryptomeria japonica) pollinosis patients who received SLIT significantly increased JC-specific IL-10+Foxp3+ cells, and good responders of the immunotherapy group had significant suppression of IL-4 and IL-5-producing Th2 cells [126]. Activated leukocyte cell adhesion molecule (ALCAM, also known as CD166) plays an important role in T-cell activation and immune response. Xie et al. [127] demonstrated that moderate to severe HDM-AR received the sublingual AIT at least 3 years, and the ALCAM levels were significantly lower in the effective group and exhibited better for predicting SLIT clinical efficacy. Leptin is known for being involved in the apoptosis and activation of T cells [128]. The leptin concentration in serum and nasal lavage was significantly decreased after SLIT treatment in 40 HDM-AR patients without obesity [129]. The macrophage migration inhibitory factor (MIF) is a T-lymphocyte-derived protein and the serum concentrations of MIFs were significantly lower in HDM-induced AR patient’s good-response sublingual AIT group than in the poor-response group [130].
Proinflammatory cytokines such as IL-4, IL-5, IL-13, eotaxin, and IL-33 are usually expressed at high levels in chronic allergic diseases including AR. They induce inflammation and contribute to the Th2 response [131]. The children who received HDM SLIT decreased significantly both serum and nasal lavage of IL-33 levels after 12 months of treatment compared to the control group, and correlation with symptom score and Th2 cytokines (IL-4, IL-5, IL-13) [132]. Nasr et al. [133] found that AR patients who were treated with SLIT to palm trees for more than 6 months significantly had lower serum IL-33 levels than immunotherapy naïve AR patients. The serum level of IL-33 in patients with intermittent AR (IAR) was correlated with disease severity and could be a part of the pathogenesis of IAR. Periostin is the down-stream molecule of IL-13, and cytokine of type 2 inflammation allergic disease. The age between 20 and 65 with a history of HDM-induced AR, had sublingual administration for alleviated symptoms. The serum periostin level decreased after immunotherapy treatment and it was also found that high serum periostin is an independent factor for improvement in quality of life. Therefore, periostincould be a predictor of clinical response to AIT [116]. Xie et al. [134] prospectively recruited 72 children with moderate to severe persistent HDM-induced AR and treated them with SCIT. Based on treatment efficacy, they found that serum eotaxin, IL-4, and Th1 cytokines IFN-γ levels were closely correlated with the efficacy of AIT in patients.
ILC2 are morphologically similar to lymphocytes, and represent an alternative source of Th2 cytokines in the nasal mucosa [135]. In patients with seasonal AR, sensitized to Phleum pretense, the proportions of ILC2 cells were increased during the pollen season. And they also found that SCIT for 8–36 months can induce a reduction of ILC2 cells, correlation with less seasonal symptoms compared to the subcutaneous AIT [136]. The frequencies of ILC2 cells in HDM-sensitized patients with AR who received immunotherapy were significantly reduced compared with the untreated group [137].
Basophil activation can be studied by monitoring the expression of surface markers such as CD63 and CD203c, using flow cytometry-based analysis [135]. Caruso et al. [138] measured CD63 and CD203c expression in AR patients who were treated with Parietaria officinalis SLIT using CD-Sens, which can detect the minimum dose for activating basophils with increasing doses of allergen. The patients who received SLIT showed a significant decreased in CD-Sens for CD63 and CD203c expression, and found a correspondence in clinical symptoms more with CD203c marker.
Several other chemokines, and arachidonic acid pathway molecules also play roles in allergic inflammation. Patients with Artemisia pollen AR were treated with AIT for 1 year, and AIT responders increased leukotriene A4 hydrolase levels significantly than nonresponders [139]. Thrombin-activatable fibrinolysis inhibitor (TAFI) is known for the regulation of coagulation and fibrinolysis and also inactivates other inflammatory mediators [140,141]. Yoshida et al. [141] demonstrated that in AR patients with allergies to JC pollen who were undergoing SLIT, the serum TAFI levels were increased gradually and significantly changes over the 2 years of SLIT and correlated with improvements in the symptoms-related scores. They also suggested that TAFI could suppress the degranulation of mast cells via an IgE-mediated mechanism.
Mas-related G protein-coupled receptor-X2 (MRGPRX2) is expressed by mast cells, and associated with IgE-independent activation of mast cells [142]. Matrix metalloproteinase-12 (MMP-12) can be induced by M2 macrophages, and promote the proliferation of Th2 cells, and secretion of IL-4 and IL-13 [143]. Zhou et al. [144] found that circulating serum MRGPRX2 and MMP-12 levels were lower in the AR SLIT-treated group and useful for evaluating the disease severity.
Humoral biomarkers
One of the main features of AR is elevated concentrations of allergen-sIgE to clinically suspected allergens [135]. Both SCIT and SLIT are associated with transient increases in serum allergen-sIgE antibody levels that are followed by blunting of seasonal increases in sIgE levels [135,145,146]. Di Lorenzo et al. [109] reported that adult patients with AR and/or asthma symptoms had a significant positive correlation between serum sIgE/tIgE ratio (>16.2) and the clinical response. However, Fujimura et al. [147] found that patients with a low sIgE/tIgE ratio showed a better response to AIT than patients with a higher ratio. Liu et al. [148] found that after 6 months SLIT in HDM-AR children serum tIgE levels, serum sIgE levels, and serum sIgE/tIgE ratios and the levels of IL-10 and IL-35 were correlated with clinical response. Adults and adolescents who underwent SCIT and were monosensitized to birch pollen had a sustained and long-term (5 years) efficacy of the treatment [149]. Bordas-Le Floch et al. [150] report a higher concentration of sIgG2 to HDM in high responders to AIT, assuming a key role of this isotype in immune tolerance. Finally, successful AIT increased allergen sIgG4 levels and correlated with decreased allergen-sIgE levels in grass pollen AIT. Also the pre-existing Phl p 1 and Phl p 5 sIgE levels predict increases in sIgG4 levels after updosing the AIT [151].
Metabolic biomarkers
A recent prospective study of serum metabolomics has demonstrated that after 3 years of Der P and Dermatophagoides farinae SLIT, the effective group exhibited 6 metabolites (lactic acid, ornithine, linolenic acid, creatinine, arachidonic acid, and sphingosine) that showed good performance to evaluate the efficacy of SLIT. These metabolites are mainly involved in glycolysis and fatty acid metabolism pathways, so they may provide a better understanding of the mechanisms of SLIT in AR patients [152]. Interestingly, on metabolic reactions between AIT with single-species mite and double-species mite allergens, 6 metabolites (13-HODE, 9-HPODE, 5(S)-HETE, 8S(S)-HETE, 11(S)-HETE, 15(S)-HETE, 11-hydro TXB2) associated with ω-6 related arachidonic acid and linoleic acid pathway showed significant changes and correlated with symptoms scores. Additionally, a decreased serum 11(S)-HETE level more with single-species mite-SCIT, may serve as a distinguishable marker for options of SCIT [153].
Food allergy
AIT for food allergy is currently under development and OIT is the most-studied approach. OIT is less frequently associated with adverse effects than SCIT [154]. The assessment of food AIT efficacy is typically based on SU evaluation, which is the lack of immune response in subjects treated with AIT after occasional doses of allergen. Different biomarkers have been used to predict the achievement of SU after food AIT, mostly allergen-specific antibodies in serum (Table 2).
Table 2. Potential predictive biomarkers for outcome of AIT in food allergy.
| Biomarker | Type of AIT | Allergen source of study | Study type | Study population | Main finding | Association with with effectiveness to AIT | Ref | |
|---|---|---|---|---|---|---|---|---|
| Food Allergy | ||||||||
| sIgA for EW and OVA | OIT | Whole egg | Longitudinal prospective | 26 Egg-allergic patients divided in a high-desensitization group (n=21, 5–12 yr old) and a low-desensitization/failure group (n=5, 5–11 yr old) | Baseline IgA levels specific for EW and OVA were higher in the high-desensitization group than in the low-desensitization/failure group. | Associated with successful AIT | [160] | |
| Cohort study (12-mo follow-up) | ||||||||
| sIgA for EW, OVA and OVM | OIT | Solid EW | Longitudinal prospective | 37 Egg-allergic patients that underwent OIT divided in clinical responders (n=19) and nonresponders (n=18) | No significant differences in baseline sIgA1 and sIgA2 between responders achieving SU and nonresponders were observed. | No association with successful AIT | [161] | |
| sIgE for EW and OVM sIgG4/sIgE for OVM | Phase II trial (4-yr follow-up) | In children that received egg OIT and obtained SU after 4 yr, lower baseline IgE levels were measured specific for EW and OVM. | Associated with unsuccessful AIT | |||||
| Children who developed SU had increased sIgG4/sIgE for ovomucoid at baseline compared to nonresponders. | Associated with successful AIT | |||||||
| sIgA to CM, casein and β-lactoglobulin | OIT | CM | Longitudinal prospective | 40 CM-allergic patients (6–17 yrs old) that underwent CM OIT (32 achieved desensitization) | High sIgA prior to OIT predicts failure to achieve desensitization. | Associated with unsuccessful AIT | [213] | |
| Phase I trial (3 months follow-up) | ||||||||
| sIgA for peanut | OIT | Peanut | Longitudinal prospective | 69 Peanut-allergic patients (1–4 yr) completed OIT. Patients were divided into 3 categories: desensitized/remission (n=19), desensitized/no remission (n=40), and not desensitized/no remission (n=10) | In the not desensitized/no remission group the baseline sIgA levels were higher than in the desensitized/remission group. | Associated with unsuccessful AIT | [162] | |
| Phase II trial (160-wk follow-up) | ||||||||
| IgE for CM and casein SPT wheal diameter | OIT | CM | Transversal, analytic study (2-yr follow-up) | 70 CM-allergic patients (1–5 yr), 18 of them underwent OIT, 44 underwent accelerated milk introduction, and 8 had no intervention | High baseline levels of IgE specific to milk and casein are associated with persistence of CMA. | Associated with unsuccessful AIT | [163] | |
| SPT wheal diameter was not a predictive marker for AIT outcome. | No association with successful AIT | |||||||
| sIgE for CM, casein, and β-lactoglobulin | OIT | CM | Longitudinal prospective | 85 CM-allergic children (6–18 yr) divided in OIT group (n=48) and control group (n=37) | Showed a reduced likelihood of reaching a maintenance dose in children (9–15 yr) with elevated baseline levels of sIgE. | Associated with unsuccessful AIT | [164] | |
| Cohort study expansion on clinical trial (12-mo follow-up) | ||||||||
| sIgE for peanut | OIT | Peanut | Longitudinal prospective | 146 Children (1–3 yr) divided in OIT group (n=96) and control group (n=50) | The predictive role of lower baseline sIgE in remission was shown by use of multivariable regression analysis. | Associated with unsuccessful AIT | [166] | |
| sIgG4/sIgE ratio for peanut | Phase II trial (2-yr follow-up) | The desensitization/remission group showed highest peanut sIgG4/sIgE ratio at baseline. | Associated with successful AIT | |||||
| sIgE/total IgE ratio for Ara h 1 and h 2 | OIT | Peanut | Longitudinal prospective Pilot clinical trial (3- to 56-mo follow-up) | 24 Children (1–16 yr) (12 achieved SU) | Achievement of SU was associated with lower IgE baseline levels specific for Ara h 1 and Ara h 2. | Associated with unsuccessful AIT | [167] | |
| sIgG4/sIgE ratio for peanut | Higher baseline sIgG4/sIgE ratios were found in children achieving SU. | Associated with succesful AIT | ||||||
| SPT wheal diameter | Smaller SPT wheal diameter was associated with successful OIT outcome. | Associated with unsuccessful AIT | ||||||
| sIgE/total IgE for CM | OIT | CM | Longitudinal prospective | 57 CM-allergic patients (7–35 yr old) that underwent OIT (combined with omalizumab) were divided in treatment group with omalizumab (n=28) and placebo group (n=29). 13 Patients of the treatment group and 10 of the placebo group achieved SU | Lower baseline sIgE/total IgE correlated with the likelihood of achieving SU in the treatment group. | Associated with unsuccessful AIT | [159] | |
| Phase II trial (32 months follow-up) | ||||||||
| Ara h 2-specific IgG, IgG4 and IgE | OIT | Peanut | Longitudinal prospective single-center, open-label trial (13-mo follow-up) | 30 Subjects with peanut allergy (7- to 13-yr old) | Ara h 2-specific IgG and IgG4 showed no correlation with OIT outcome; sIgE is greater in unsuccessful OIT patients compared to those that reached SU; basophil sensitivity decreases in patients with SU. | sIgG and IgG4: N/D | [157] | |
| Basophil sensitivity to Ara h 2 | sIgE associated with unsuccessful OIT | |||||||
| Basophil sensitivity associated with successful OIT | ||||||||
| Peanut- and Ara h 2-specific IgE | OIT | Peanut | Longitudinal prospective | 49 subjects with suspected or known peanut allergy (9–36 mo old) | Measurement of peanut-specific IgE and IgE binding to Ara h 2 at baseline may allow prediction of OIT success. | Associated with successful OIT | [168] | |
| Cohort study | ||||||||
| (Not specified time of follow-up) | ||||||||
| IgE- and IgG4-binding peptides | CM-OIT | CM allergens | Longitudinal prospective cohort study (2-yr follow-up) | 25 CM-allergic children who underwent CM-OIT and 7 nontreated CM-allergic children as control (>4 yr old) | CM-OIT induces increase of IgG4-binding epitopes and decrease in IgE-binding epitopes; 2 sets of IgE-binding peptides can predict efficacy and safety of OIT | N/D | [169] | |
| IgE- and IgG4-binding peptides | CM-OIT | CM | Longitudinal prospective cohort study (28-mo follow-up) | 55 Patients with milk allergy (7–35 yr old) randomized 1:1 to receive omalizumab or placebo during CM-OIT | IgE and IgG4 binding to epitopes were altered by OIT regardless of outcome; SU is associated with lower levels of baseline epitope-specific IgE | No association IgE and IgG4 binding to epitopes | [170] | |
| Epitope-specific IgE associated with unsuccessful OIT | ||||||||
| IgE- and IgG-binding peptides | OIT | Soy (rBet v 1) | Secondary analysis of a double-blind placebo-controlled trial (not specified time of follow-up) | 34 Patients (mean age, 38 yr) | Binding to allergen epitopes is clinically relevant in sub-groups of patients only; IgE-binding to one of the epitopes was correlated with stronger symptoms after ingesting soya | Associated with unsuccessful OIT | [171] | |
| Basophil activation response, sIgE | OIT | Peanut | Longitudinal prospective RCT phase II study (approximately 2-yr follow-up) | 120 Patients (7–55 yr old) with peanut allergy, with a positive result from a double-blind, placebo-controlled, food challenge (≤500 mg of peanut protein), a positive SPT result (≥5-mm wheal diameter above the negative control), and peanut-specific IgE concentration of more than 4 kU/L | Higher IgE, higher risk of OIT failure; low basophil activation response, high success rate | Associated with unsuccessful OIT | [155] | |
| Basophil activation, peanut-specific IgE, IgA and IgG4 | OIT | Peanut | Double-blind, placebo-controlled food challenge (9-mo follow-up) | 20 Patients (4–12 yr old), with peanut-specific IgE ≥ 0.35 kU/L, peanut SPT wheal ≥ 3 mm compared to the negative control, and a positive double-blind, placebo-controlled food challenge | Suppression of basophil activation occurs within the first 90 days of OIT; sIgG4 and IgA increased throughout therapy | Not associated with successful OIT | [156] | |
| CM-specific IgE and IgG4; CD63 and CD203c expression; basophil histamine release | OIT and SLIT | CM | Longitudinal prospective RCT (around 60-wk follow-up) | 30 Subjects (6–17 yr) with CM allergy | OIT was more efficacious for desensitization to CM than SLIT alone, but it had more side effects; CD63 and CD203c expression decreased after therapy; CM-specific IgG4 levels increased in all groups; CM-specific IgE and histamine release decreased in the OIT group | N/D | [158] | |
| Hazelnut-specific IgE, | OIT | Hazelnut | Longitudinal | 100 Subjects (3–9 yr old) who underwent at least 6 months of hazelnut OIT for IgE-mediated allergy; 6-mo follow-up | Hazelnut OIT is associated with hazelnut desensitization. IgE negatively correlated with OIT success; smaller SPT wheal diameter at baseline positively correlated with OIT success | Associated to unsuccessful OIT | [172] | |
| SPT wheal diameter | Retrospective | |||||||
| Single-center study | ||||||||
| (at least 6-mo follow-up) | ||||||||
| CM-specific IgE, SPT wheal diameter | OIT | CM | Longitudinal prospective cohort study (5 yrs) | 74 Subjects (2–18 yr old) with CM allergy; | Lower sIgE and smaller SPT wheal diameter at baseline correlated with complete desensitization. IgE negatively correlated with OIT success; smaller SPT wheal diameter at baseline positively correlated with OIT success | Associated to unsuccessful OIT | [173] | |
| Baseline sIg | OIT | CM (casein and α and β-lactoglobulin) | Longitudinal prospective open label study (median 6 yrs follow-up) | Children (n=168) with CM allergy | Baseline and sIgG4/IgE ratio to casein, and ß-lactoglobulin sIgE concentrations were higher in the group that had a positive challenge. | Associated to unsuccessful OIT | [165] | |
| sIgG4 /IgE ratio | Higher casein sIgE remains in reactive patients. Casein sIgG4/IgE ratio is important to define the outcome of milk OIT | Associated to successful OIT | ||||||
AIT, allergen immunotherapy; CM, cow’s milk; CMA, cow’s milk allergy; EW, egg white; N/D, not determined; OIT, oral immunotherapy; OVA, ovalbumin; OVM, ovomucoid; RCT, randomized clinical trial; sIgA, specific IgA; sIgE, specific IgE; sIgG4, specific IgG4; SPT, skin prick test; SU, sustained unresponsiveness; tIgE, total IgE.
Cellular biomarkers
Contradictory results have been reported for the usefulness of basophil activation as a predictor for the response to food AIT. In a phase II clinical trial, achievement of SU against peanut was associated with lower basophil activation response [155]. More recently, it was reported that grouping participants based on the clinical outcome at any oral food challenge moment over time were possible, indicating that basophil activation does not correlate with AIT success [156]. Another study observed similar basophil sensitivities at baseline in subjects with SU and those with transient desensitization, but also found that an early decrease in basophil sensitivity correlates with SU [157]. In addition to peanut AIT, CM AIT studies have assessed basophil activity as a biomarker. A CM-OIT study with 30 children showed that baseline expression of the basophil markers CD63 and CD203c as well as histamine release at baseline could not predict tolerance [158]. Nevertheless, a CM-omalizumab OIT study found that baseline basophil activation might still help identify subjects in whom omalizumab can enhance SU development, but in combination with other serological markers [159].
Humoral biomarkers
Serum sIgA has been studied as a potential predictive biomarker of food AIT in different studies, although with contradicting results. In one study with egg-allergic patients undergoing OIT, baseline sIgA levels against egg white (EW) and OVA were higher in high-desensitized patients that tolerated one hard-boiled egg after one year compared to those who could not (low-desensitized/failure group). This indicated that high sIgA levels before OIT could predict treatment effectiveness [160]. In contrast, another study showed that there were no significant differences in baseline sIgA1 and sIgA2 between responders and nonresponders [161]. sIgA levels may also be predictive of therapy failure, being higher in patients not achieving SU to CM and peanut after OIT [161,162].
The role of sIgE as a potential predictive biomarker has been investigated in several food AIT studies. A recent study with 70 allergic children to CM showed that high baseline levels of sIgE to CM and casein are associated with persistence of CM allergy despite AIT [163]. This is consistent with the findings of Cohen et al. [164] and Kauppila et al. [165], demonstrating that elevated baseline levels of sIgE to total CM and its allergens are associated with a lower chance of reaching a maintenance dose of 200 mL of CM due to adverse reactions. In line with these findings, lower baseline sIgE levels for EW and ovomucoid (OVM) were measured in children that received egg AIT and obtained SU after 4 years [161] and lower sIgE to total peanut extract and Ara h 2 had a predictive role in peanut-allergic children [166]. Furthermore, achievement of SU in children that underwent peanut AIT was associated with lower sIgE baseline levels for Ara h1 and Ara h 2 [167]. Additionally, these children had a lower sIgE/tIgE ratio at baseline, suggesting that this ratio can also be used as a predictive biomarker for treatment response. This was also observed in subjects enrolled in a trial of CM AIT combined with omalizumab, but only for subjects treated with omalizumab and not for placebo-treated subjects [159]. In contrast, another study showed that, even though Ara h 2-sIgE was lower in subjects that reached SU, the difference in the ratio of Ara h 2-sIgE/tIgE was not significant between the groups [157]. Overall, there is a consensus in the literature that lower sIgE and sIgE/tIgE ratios at the start of AIT are associated with treatment success.
In addition to the levels of sIgE at baseline and the ratio between sIgE and tIgE, analysis of antibody epitope binding may contribute to predicting the outcome of AIT. In an AIT study with 49 peanut-allergic children, Dreskin et al. [168] revealed that the binding of IgE to specific linear Ara h 2 epitopes at baseline can be useful in predicting the success of SU. Furthermore, through bioinformatic analysis, 2 baseline sets of 16 IgE-binding peptides were identified for predicting the safety and efficacy of CM-OIT before treatment [169]. Another CM-OIT study developed predictive models based on epitope-specific antibody binding and showed that having lower diversity and lower binding of IgE at baseline to specific epitopes can predict SU [170]. Lastly, a study investigating the efficacy of subcutaneous injection of birch pollen allergen Bet v 1 in soya allergy reported the potential of IgE- and IgG-binding to epitopes as a biomarker for successful AIT [171].
As the levels of both sIgG4 and sIgE play an important role in AIT, the ratio of sIgG4/sIgE is also of interest as a potential predictive biomarker [165]. Several peanut AIT studies found a higher sIgG4/sIgE ratio at baseline in subjects that achieved desensitization or SU compared with subjects that did not [155,157,166,167], and this was more recently confirmed in a phase II clinical trial with 146 participating children [166]. Furthermore, similar findings have been observed in an egg AIT study, in which children who developed SU had increased sIgG4/sIgE for OVMat baseline compared to nonresponders [161].
In vivo biomarkers
The association between smaller SPT wheal diameter at baseline and successful OIT outcome has been reported in studies using peanut, hazelnut, and CM [167,172,173]. However, this association was not found in a peanut AIT study with 120 participants, which could be due to the recruitment criterium of SPT wheal diameter of ≥5 mm above the negative control instead of the conventional ≥3 mm [155]. Furthermore, the SPT wheal diameter was not a predictive marker for AIT outcome in a recent study with 70 CM-allergic children undergoing AIT or a more rapid introduction of baked milk [163]. Collectively, the contradictory results of these studies suggest that more work is needed to confirm the role of SPT wheal diameter as a predictive biomarker in AIT.
Venom allergy
Allergy to the venom of Hymenoptera species presents with severe manifestations and is potentially life-threatening. Similarly, Hymenoptera venom immunotherapy (VIT) is one of the riskiest since there is a high probability of severe adverse events (8%–20%) [174,175,176], however, it is the only treatment that can change the course of the disease by inducing immunomodulation and tolerance in most patients with effectiveness around 80%–95% depending on the allergen source [177,178,179]. According to the geographical location and environmental factors, different insects of this order may be the cause of the reaction [180] and endotypes and phenotypes have been described, not always mediated by IgE [181]. VIT is recommended in sensitized adults and children with severe reactions to the stings as well as in adults with generalized skin symptoms if the quality of life is impaired [177]. For VIT, subcutaneous injection is the only route of administration that is used in clinical practice, compared to aeroallergen and food-allergen IT which can be administered SC but also sublingually [182].
To date, no complete overview exists of the mechanisms by which VIT induces tolerance, and information that explores predictive biomarkers of response to VIT is scarce [183]. Associations have been described between changes in some components of the immune system and the tolerance to stings or effectiveness of Hymenoptera VIT. However, many of the reported mechanisms also have been studied in beekeepers, who are exposed to high doses of bee venom as a result of their occupation. Few studies have carried out designs to identify biomarkers of successful VIT (Table 3).
Table 3. Potential predictive biomarkers for outcome of AIT in venom allergy.
| Bio marker | Type of AIT | Allergen source of study | Study type | Study population | Main finding | Association with with effectiveness to AIT | Ref | |
|---|---|---|---|---|---|---|---|---|
| Venom allergy | ||||||||
| Kynurenine | SCIT | A. mellifera | Longitudinal prospective | 20 BV allergic patients older than 18 yr and 20 beekeepers as tolerant group | Markers studied have proven helpful for monitoring the early response to VIT, such as IgG4 to Api m 1. | Associated with successful AIT | [198] | |
| Api m 1-sIgG4 | Cohort study (12-mo follow-up) | In contrast, others, such as kynurenine, the basophil degranulation test, or the Treg Helios- cells, are useful for the late phase. | Associated with unsuccessful AIT | |||||
| IL-10 | ||||||||
| Helios- Tregs | ||||||||
| basophil degranulation | ||||||||
| sCD30 | SCIT | Polistes, Apis, and Vespula spp. | Longitudinal prospective | 14 Patients with Hymenoptera venom allergy who had undergone VIT and 61 healthy controls | sCD30 levels are higher in patients with Hymenoptera venom allergy and expression decreases during VIT. | N/D | [196] | |
| Cohort study (12-mo follow-up) | ||||||||
| PLA and peptide specific T cells | SCIT | A. mellifera and phospholipase A2 (group 1 allergen) | Longitudinal prospective | Five BV allergic individuals (mean age, 47 yr) | VIT for 2 months of individuals suffering from bee sting allergy is associated with a significant reduction in T-cell proliferative response and cytokine secretion to the epitopes of the major BV allergen, PLA. | N/D | [45] | |
| anti-PLA IgE | Experimental study (2-mo follow-up) | |||||||
| anti-PLA IgG4 | ||||||||
| BTC | SCIT | Vespula and Apis spp. | Longitudinal retrospective | 302 Patients diagnosed with Hymenoptera venom allergy and undergone VIT | Significant decrease of BTC by 2.5% per year after VIT. | N/D | [186] | |
| Cohort study (4.7 yr mean of follow-up) | ||||||||
| Basophils surface markers | SCIT | Vespula spp. | Longitudinal prospective | Forty-eight patients whom had been treated with rush VIT | Decreased in the expression of CD11c, CD32, CD35, CD63, CD116, CD122, CD124, CD130, and CD132 surface markers in basophils after one week after rush VIT | N/D | [185] | |
| Experimental study (2 wk) | ||||||||
| Mast cells tryptase | SCIT | A. mellifera | Transversal | 58 Patients hyposensitized with bee venom were challenged with the sting of a living bee | Patients which not react to the sting challenge had lower histamine and tryptase levels compared to still reactive ones | Associated with unsuccessful AIT | [184] | |
| Histame by leucocytes | Experimental study | |||||||
| Api m 10-sIgE | SCIT | A. mellifera | Longitudinal retrospective | VIT patients (n=115) were included; responders (n=79) or treatment failure (n=36) | Allergic patients with dominant sensitization to Api m 10 are at increased risk for treatment failure in VIT | Associated with unsuccessful AIT | [204] | |
| Experimental study (at least 6 months of VIT) | ||||||||
| Helios- Tregs | SCIT | A. mellifera | Transversal comparative study | Allergic patients with reactions after bee stings; tolerant beekeepers receiving ≥50 stings/yr; and healthy nonexposed controls | Tolerant Beekepers showed higher levels of sIgG4 and IL-10 as well as an enhanced CTLA-4+ and Helios- Treg, and reduced Th1, Th2 and Th17 subsets. Helios- Treg population could be a novel candidate biomarker useful for monitoring tolerance. | N/D | [214] | |
| sCD30 | SCIT | Vespula and Apis spp. | Transversal comparative study | 21 Patients allergic to wasp and/or honey bee venom and 42 healthy participants | sCD30(TNFRSF8), and sTNF-R1 may be considered as effective prognostic factors | N/D | [197] | |
| sTNF-R1 | ||||||||
| CD30 | SCIT | A. mellifera, Polistes, and Vespula spp. | Longitudinal prospective | Seven patients underwent VIT | Downregulation of the markers in CD4+ T cells after 4 months of VIT | N/D | [183] | |
| CD154 | Experimental study (4-mo follow-up) | |||||||
| CD152 | ||||||||
AIT, allergen immunotherapy; BV, bee venom; BTC, baseline tryptase concentration; IL, interleukin; N/D, not determined; SCIT, subcutaneous immunotherapy; sIgE, specific IgE; sTNF-R1, soluble tumor necrosis factor receptor 1; sCD30, soluble CD30; PLA, phospholipase A2; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; VIT, venom immunotherapy.
Cellular biomarkers
Changes in basophil markers, tryptase levels, and histamine release were among the first approaches to determine the effectiveness of immunotherapy [184,185]. VIT could modulate the mast cell function where it has been reported a decreased baseline serum tryptase concentration over time after VIT [186]. Basophils also downregulate the expression of surface activation markers (CD11c, CD32, CD35, CD63, CD116, CD122, CD124, CD130, and CD132) and histamine release after VIT [184,185,187]. However, monitoring these cells as an in vitro method does not offer an alternative for determining successful immunotherapy when compared to the sting challenge [188].
VIT also has effects on the adaptive response mediated by T and B cells. The directing of the Th2 response of allergic patients towards a Th1 profile after immunotherapy is one of the most recognized mechanisms of action, where the levels of VIT are increased. IFN-γ in parallel with the decrease of IL-4 and IL-13 [189,190]. Akdis et al. showed how the development of epitope-specific T cells (Th1 and Tregs) to bee major allergen, phospholipase A2, in VIT which are capable to suppress cells of the T2 allergic response [45,191]. VIT was found to be related to the progressive expansion of circulating CD4+CD25+Foxp3+ Treg cell numbers [192]. IL-10-producing antigen-specific Tregs also appear in the context of high-dose exposure to allergens; this was demonstrated in beekeepers whose cells were measured in and out of beekeeping season [193].
In a study conducted on patients in VIT rush, samples were taken at 5 days to analyze markers of immune tolerance. Among the findings, tryptophan degradation was observed, which is related to suppression in T cells and the induction of tolerance. In addition, elevated ILT 3 and ILT4, which are inhibitory receptors expressed on monocytes, and elevated levels of IL-10 by monocytes and CD3+ T cells were found [194]. These mechanisms may explain the early response to VIT.
Among the most-studied mechanisms of VIT is the increase in IL-10, which can be produced by different cell types. In PBMCs from VIT patients, IL-10 was produced initially by activated CD4+CD25+, allergen-specific T cells, followed by B cells and monocytes [195]. Recently, human IL-10-secreting Br1 cells (identified by surface markers CD73-CD25+CD71+) were described in beekeepers, healthy donors, and allergic patients. Among their functions is the production of high levels of IL-10, suppressed antigen-specific CD4+ T-cell proliferation, and the production of IgG4 [53].
Soluble CD30 (sCD30) is a glycoprotein that is expressed and secreted by Th2 lymphocytes. In patients with an allergy to Hymenoptera venom, sCD30 concentrations are elevated and their levels decrease during AIT [196,197]. Additionally, Packi et al. [197] proposed that a decrease in the level of both TNFR1 and sCD30 markers in patients suffering from Hymenoptera venom allergy may be considered an effective prognostic factor for VIT. In CD4+ cells, downregulated markers have been described after 4 months with VIT, such as CD30, CD154, and CD152. In this same study, VIT was shown to be effective, with an increase in IFNγ-producing CD4+ cells and a decrease in basophil degranulation [183]. Recently, tolerance status in bee-venom-allergic patients after immunotherapy was invariably associated with a significant regulatory response characterized by the expansion of Helios- Tregs subpopulation and increased IL-10, sIgG4, and kynurenine levels [198].
Humoral biomarkers
Increased levels of serum sIgE during the beginning of the VIT is a common finding, however, this decreases after a year [198,199,200,201]. On the other hand, in natural exposure to high doses of hymenopteran venom, as is the case in beekeepers, IgG4 levels are over one hundred times higher than in subjects allergic to the venom and even in unexposed subjects [202]. In the case of VIT, the persistent role of IgG4 antibodies has been discussed for many decades, although successful desensitization is accompanied by increases in the inhibitory activity of both IgG4 and IgE, these return to baseline within months upon discontinuation of treatment, however, subsequent follow-up revealed a more sustained decline in venom-sIgE levels [203]. This also proposes alternative memory mechanisms other than the humoral response that may be involved in the persistence of tolerance.
In contrast, dominant sensitization to some specific allergens, like Api m 10, in bee-venom allergy may be a risk factor for immunotherapy failure showing the importance of component resolved profiles in the prediction of treatment results [204].
CONCLUSION
Biomarkers with predictive value for the success of AIT are highly sought after because their identification will allow a personalized assessment on a per-patient basis of the likelihood that AIT will result in amelioration of the disease. While many biomarker candidates have been identified, as outlined in this review article, none of these have thus far been validated and proven sufficiently accurate predictors of response to treatment. Currently, studies designed to determine biomarkers for a successful response to AIT remain limited in food and venom allergies. The humoral response (assessed levels of sIgE and sIgG4, and ratios with tIgE) seems to be promising (especially in respiratory allergies) however, there are still contradictory findings. It is necessary to propose strategies where in vitro and in vivo markers are combined.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Yoon-Seok Chang, Cezmi A Akdis, Willem van de Veen.
- Formal analysis: Yoon-Seok Chang, Cezmi A Akdis, Willem van de Veen.
- Investigation: Juan-Felipe López, Manal Bel imam, Pattraporn Satitsuksanoa, Sophieke Lems, Minglin Yang, Manal Bel imam, Yu-Kyoung Hwang, Purevsuren Losol.
- Project administration: Willem van de Veen, Yoon-Seok Chang.
- Writing - original draft: Juan-Felipe López, Yu-Kyoung Hwang, Purevsuren Losol, Yoon-Seok Chang, Willem van de Veen.
- Writing - review & editing: Juan-Felipe López, Manal Bel imam , Pattraporn Satitsuksanoa, Sophieke Lems, Minglin Yang, Yu-Kyoung Hwang, Purevsuren Losol, Jun-Pyo Choi, Sae-Hoon Kim, Mübeccel Akdis, Yoon-Seok Chang, Cezmi A Akdis, Willem van de Veen.
References
- 1.Komlósi ZI, Kovács N, Sokolowska M, van de Veen W, Akdis M, Akdis CA. Mechanisms of subcutaneous and sublingual aeroallergen immunotherapy: what is new? Immunol Allergy Clin North Am. 2020;40:1–14. doi: 10.1016/j.iac.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Farraia M, Paciência I, Castro Mendes F, Cavaleiro Rufo J, H Shamji M, Agache I, Moreira A. Cost-effectiveness analysis of house dust mite allergen immunotherapy in children with allergic asthma. Allergy. 2022;77:2688–2698. doi: 10.1111/all.15321. [DOI] [PubMed] [Google Scholar]
- 3.Parra-Padilla D, Zakzuk J, Carrasquilla M, Alvis-Guzmán N, Dennis R, Rojas MX, Rondón M, Pérez A, Peñaranda A, Barragán AM, Caraballo L, García E. Cost-effectiveness of the subcutaneous house dust mite allergen immunotherapy plus pharmacotherapy for allergic asthma: a mathematical model. Allergy. 2021;76:2229–2233. doi: 10.1111/all.14723. [DOI] [PubMed] [Google Scholar]
- 4.Farraia M, Paciência I, Castro Mendes F, Cavaleiro Rufo J, Shamji M, Agache I, Moreira A. Allergen immunotherapy for asthma prevention: a systematic review and meta-analysis of randomized and non-randomized controlled studies. Allergy. 2022;77:1719–1735. doi: 10.1111/all.15295. [DOI] [PubMed] [Google Scholar]
- 5.Senna G, Caminati M, Lockey RF. Allergen immunotherapy adherence in the real world: How bad is it and how can it be improved? Curr Treat Options Allergy. 2015;2:39–53. [Google Scholar]
- 6.Komlósi ZI, Kovács N, Sokolowska M, van de Veen W, Akdis M, Akdis CA. Highlights of novel vaccination strategies in allergen immunotherapy. Immunol Allergy Clin North Am. 2020;40:15–24. doi: 10.1016/j.iac.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;1:1572–1573. [Google Scholar]
- 8.Ezhuthachan ID, Banks TA, Cerise JE, Wong SC, Ponda P. Allergen immunotherapy extract shortages and their effects on clinical care: a work group report of the AAAAI Immunotherapy, Allergen Standardization, and Allergy Diagnostics Committee. J Allergy Clin Immunol Pract. 2022;10:444–452. doi: 10.1016/j.jaip.2021.10.057. [DOI] [PubMed] [Google Scholar]
- 9.Nony E, Martelet A, Jain K, Moingeon P. Allergen extracts for immunotherapy: to mix or not to mix? Expert Rev Clin Pharmacol. 2016;9:401–408. doi: 10.1586/17512433.2015.1131122. [DOI] [PubMed] [Google Scholar]
- 10.Biedermann T, Couroux P, Greve TM, Mäkelä M. Safety of the standardized quality tree sublingual immunotherapy tablet: pooled safety analysis of clinical trials. Allergy. 2021;76:3733–3742. doi: 10.1111/all.14882. [DOI] [PubMed] [Google Scholar]
- 11.Valenta R, Karaulov A, Niederberger V, Zhernov Y, Elisyutina O, Campana R, Focke-Tejkl M, Curin M, Namazova-Baranova L, Wang JY, Pawankar R, Khaitov M. Allergen extracts for in vivo diagnosis and treatment of allergy: is there a future? J Allergy Clin Immunol Pract. 2018;6:1845–55.e2. doi: 10.1016/j.jaip.2018.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhernov Y, Curin M, Khaitov M, Karaulov A, Valenta R. Recombinant allergens for immunotherapy: state of the art. Curr Opin Allergy Clin Immunol. 2019;19:402–414. doi: 10.1097/ACI.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieto A, Mazón Á, Nieto M, Ibáñez E, Jang DT, Calaforra S, Alba P, Pérez-Francés C, Llusar R, Montoro J, de Mateo A, Alamar R, El-Qutob D, Fernández J, Moral L, Toral T, Antón M, Andreu C, Ferrer Á, Flores IM, Cerdá N, Del Pozo S, Caballero R, Subiza JL, Casanovas M. First-in-human phase 2 trial with mite allergoids coupled to mannan in subcutaneous and sublingual immunotherapy. Allergy. 2022;77:3096–3107. doi: 10.1111/all.15374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heldner A, Alessandrini F, Russkamp D, Heine S, Schnautz B, Chaker A, Mwange J, Carreno Velazquez TL, Heath MD, Skinner MA, Kramer MF, Zissler UM, Schmidt-Weber CB, Blank S. Immunological effects of adjuvanted low-dose allergoid allergen-specific immunotherapy in experimental murine house dust mite allergy. Allergy. 2022;77:907–919. doi: 10.1111/all.15012. [DOI] [PubMed] [Google Scholar]
- 15.Zimmer J, Schmidt S, Kaul S, Costanzo A, Buchheit KH, Brown S, Carnés J, Chapman M, Chen A, De Neergaard M, Döring S, Hindley JP, Holzhauser T, Jorajuria S, Le Tallec D, Lombardero M, Iacovacci P, Reese G, Sander I, Smith B, Strecker D, van Ree R, Zebina M, Vieths S. Standardisation of allergen products: 4. Validation of a candidate European Pharmacopoeia standard method for quantification of major grass pollen allergen Phl p 5. Allergy. 2022;77:633–642. doi: 10.1111/all.15003. [DOI] [PubMed] [Google Scholar]
- 16.Jutel M, Brüggenjürgen B, Richter H, Vogelberg C. Real-world evidence of subcutaneous allergoid immunotherapy in house dust mite-induced allergic rhinitis and asthma. Allergy. 2020;75:2050–2058. doi: 10.1111/all.14240. [DOI] [PubMed] [Google Scholar]
- 17.Araujo GR, Aglas L, Vaz ER, Machado Y, Huber S, Himly M, Duschl A, Goulart LR, Ferreira F. TGFβ1 mimetic peptide modulates immune response to grass pollen allergens in mice. Allergy. 2020;75:882–891. doi: 10.1111/all.14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benito-Villalvilla C, Soria I, Pérez-Diego M, Fernández-Caldas E, Subiza JL, Palomares O. Alum impairs tolerogenic properties induced by allergoid-mannan conjugates inhibiting mTOR and metabolic reprogramming in human DCs. Allergy. 2020;75:648–659. doi: 10.1111/all.14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durham SR, Shamji MH. Allergen immunotherapy: past, present and future. Nat Rev Immunol. 2022 Oct 17;:1–12. doi: 10.1038/s41577-022-00786-1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kucuksezer UC, Ozdemir C, Cevhertas L, Ogulur I, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol Int. 2020;69:549–560. doi: 10.1016/j.alit.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Pfützner W, Möbs C. AIT: new avenues in allergen immunotherapy. Handb Exp Pharmacol. 2022;268:135–149. doi: 10.1007/164_2021_514. [DOI] [PubMed] [Google Scholar]
- 22.Roche AM, Wise SK. Subcutaneous immunotherapy. Int Forum Allergy Rhinol. 2014;4(Suppl 2):S51–S54. doi: 10.1002/alr.21382. [DOI] [PubMed] [Google Scholar]
- 23.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102(4 Pt 1):558–562. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 24.Barker-Tejeda TC, Bazire R, Obeso D, Mera-Berriatua L, Rosace D, Vazquez-Cortes S, Ramos T, Rico MDP, Chivato T, Barbas C, Villaseñor A, Escribese MM, Fernández-Rivas M, Blanco C, Barber D. Exploring novel systemic biomarker approaches in grass-pollen sublingual immunotherapy using omics. Allergy. 2021;76:1199–1212. doi: 10.1111/all.14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passalacqua G, Bagnasco D, Canonica GW. 30 years of sublingual immunotherapy. Allergy. 2020;75:1107–1120. doi: 10.1111/all.14113. [DOI] [PubMed] [Google Scholar]
- 26.Leonard SA, Laubach S, Wang J. Integrating oral immunotherapy into clinical practice. J Allergy Clin Immunol. 2021;147:1–13. doi: 10.1016/j.jaci.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Epstein-Rigbi N, Levy MB, Nachshon L, Koren Y, Katz Y, Goldberg MR, Elizur A. Efficacy and safety of food allergy oral immunotherapy in adults. Allergy. 2022 Oct 3; doi: 10.1111/all.15537. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Senti G, Graf N, Haug S, Rüedi N, von Moos S, Sonderegger T, Johansen P, Kündig TM. Epicutaneous allergen administration as a novel method of allergen-specific immunotherapy. J Allergy Clin Immunol. 2009;124:997–1002. doi: 10.1016/j.jaci.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Xiong L, Lin J, Luo Y, Chen W, Dai J. The Efficacy and safety of epicutaneous immunotherapy for allergic diseases: a systematic review and meta-analysis. Int Arch Allergy Immunol. 2020;181:170–182. doi: 10.1159/000504366. [DOI] [PubMed] [Google Scholar]
- 30.Pelletier B, Perrin A, Assoun N, Plaquet C, Oreal N, Gaulme L, Bouzereau A, Labernardière JL, Ligouis M, Dioszeghy V, Wavrin S, Matthews K, Porcheray F, Sampson HA, Hervé PL. Epicutaneous immunotherapy protects cashew-sensitized mice from anaphylaxis. Allergy. 2021;76:1213–1222. doi: 10.1111/all.14605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korotchenko E, Schießl V, Scheiblhofer S, Schubert M, Dall E, Joubert IA, Strandt H, Neuper T, Sarajlic M, Bauer R, Geppert M, Joedicke D, Wildner S, Schaller S, Winkler S, Gadermaier G, Horejs-Hoeck J, Weiss R. Laser-facilitated epicutaneous immunotherapy with hypoallergenic beta-glucan neoglycoconjugates suppresses lung inflammation and avoids local side effects in a mouse model of allergic asthma. Allergy. 2021;76:210–222. doi: 10.1111/all.14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senti G, Prinz Vavricka BM, Erdmann I, Diaz MI, Markus R, McCormack SJ, Simard JJ, Wüthrich B, Crameri R, Graf N, Johansen P, Kündig TM. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci U S A. 2008;105:17908–17912. doi: 10.1073/pnas.0803725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senti G, Freiburghaus AU, Larenas-Linnemann D, Hoffmann HJ, Patterson AM, Klimek L, Di Bona D, Pfaar O, Ahlbeck L, Akdis M, Weinfeld D, Contreras-Verduzco FA, Pedroza-Melendez A, Skaarup SH, Lee SM, Cardell LO, Schmid JM, Westin U, Dollner R, Kündig TM. Intralymphatic immunotherapy: update and unmet needs. Int Arch Allergy Immunol. 2019;178:141–149. doi: 10.1159/000493647. [DOI] [PubMed] [Google Scholar]
- 34.van de Veen W, Akdis M. The use of biologics for immune modulation in allergic disease. J Clin Invest. 2019;129:1452–1462. doi: 10.1172/JCI124607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Veen W, Akdis M. Tolerance mechanisms of allergen immunotherapy. Allergy. 2020;75:1017–1018. doi: 10.1111/all.14126. [DOI] [PubMed] [Google Scholar]
- 36.Głobińska A, Boonpiyathad T, Satitsuksanoa P, Kleuskens M, van de Veen W, Sokolowska M, Akdis M. Mechanisms of allergen-specific immunotherapy: Diverse mechanisms of immune tolerance to allergens. Ann Allergy Asthma Immunol. 2018;121:306–312. doi: 10.1016/j.anai.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Ashley SE, Jones AC, Anderson D, Holt PG, Bosco A, Tang MLK. Remission of peanut allergy is associated with rewiring of allergen-driven T helper 2-related gene networks. Allergy. 2022;77:3015–3027. doi: 10.1111/all.15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manohar M, Dunham D, Gupta S, Yan Z, Zhang W, Minnicozzi S, Kirkey M, Bunning B, Roy Chowdhury R, Galli SJ, Boyd SD, Kost LE, Chinthrajah RS, Desai M, Oettgen HC, Maecker HT, Yu W, DeKruyff RH, Andorf S, Nadeau KC. Immune changes beyond Th2 pathways during rapid multifood immunotherapy enabled with omalizumab. Allergy. 2021;76:2809–2826. doi: 10.1111/all.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celebi Sözener Z, Mungan D, Cevhertas L, Ogulur I, Akdis M, Akdis C. Tolerance mechanisms in allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2020;20:591–601. doi: 10.1097/ACI.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 40.Kanagaratham C, El Ansari YS, Lewis OL, Oettgen HC. IgE and IgG antibodies as regulators of mast cell and basophil functions in food allergy. Front Immunol. 2020;11:603050. doi: 10.3389/fimmu.2020.603050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmid JM, Würtzen PA, Siddhuraj P, Jogdand P, Petersen CG, Dahl R, Erjefält JS, Hoffmann HJ. Basophil sensitivity reflects long-term clinical outcome of subcutaneous immunotherapy in grass pollen-allergic patients. Allergy. 2021;76:1528–1538. doi: 10.1111/all.14264. [DOI] [PubMed] [Google Scholar]
- 42.Zemmour D, Zilionis R, Kiner E, Klein AM, Mathis D, Benoist C. Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nat Immunol. 2018;19:291–301. doi: 10.1038/s41590-018-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scadding GW, Shamji MH, Jacobson MR, Lee DI, Wilson D, Lima MT, Pitkin L, Pilette C, Nouri-Aria K, Durham SR. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40:598–606. doi: 10.1111/j.1365-2222.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- 44.Verhagen J, Blaser K, Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy: T-regulatory cells and more. Immunol Allergy Clin North Am. 2006;26:207–231. doi: 10.1016/j.iac.2006.02.008. vi. [DOI] [PubMed] [Google Scholar]
- 45.Akdis CA, Akdis M, Blesken T, Wymann D, Alkan SS, Müller U, Blaser K. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J Clin Invest. 1996;98:1676–1683. doi: 10.1172/JCI118963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boonpiyathad T, van de Veen W, Wirz O, Sokolowska M, Rückert B, Tan G, Sangasapaviliya A, Pradubpongsa P, Fuengthong R, Thantiworasit P, Sirivichayakul S, Ruxrungtham K, Akdis CA, Akdis M. Role of Der p 1-specific B cells in immune tolerance during 2 years of house dust mite-specific immunotherapy. J Allergy Clin Immunol. 2019;143:1077–1086.e10. doi: 10.1016/j.jaci.2018.10.061. [DOI] [PubMed] [Google Scholar]
- 47.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, Berglund JP, Tsai M, Maecker H, O’Riordan G, Galli SJ, Nadeau KC. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shamji MH, Layhadi JA, Achkova D, Kouser L, Perera-Webb A, Couto-Francisco NC, Parkin RV, Matsuoka T, Scadding G, Ashton-Rickardt PG, Durham SR. Role of IL-35 in sublingual allergen immunotherapy. J Allergy Clin Immunol. 2019;143:1131–1142.e4. doi: 10.1016/j.jaci.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 49.Rigas D, Lewis G, Aron JL, Wang B, Banie H, Sankaranarayanan I, Galle-Treger L, Maazi H, Lo R, Freeman GJ, Sharpe AH, Soroosh P, Akbari O. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator-inducible T-cell costimulator ligand interaction. J Allergy Clin Immunol. 2017;139:1468–1477.e2. doi: 10.1016/j.jaci.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satitsuksanoa P, Daanje M, Akdis M, Boyd SD, van de Veen W. Biology and dynamics of B cells in the context of IgE-mediated food allergy. Allergy. 2021;76:1707–1717. doi: 10.1111/all.14684. [DOI] [PubMed] [Google Scholar]
- 51.Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, van de Veen W. Regulatory B cells, A to Z. Allergy. 2021;76:2699–2715. doi: 10.1111/all.14763. [DOI] [PubMed] [Google Scholar]
- 52.Ma S, Satitsuksanoa P, Jansen K, Cevhertas L, van de Veen W, Akdis M. B regulatory cells in allergy. Immunol Rev. 2021;299:10–30. doi: 10.1111/imr.12937. [DOI] [PubMed] [Google Scholar]
- 53.van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Söllner S, Akdis DG, Rückert B, Akdis CA, Akdis M. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131:1204–1212. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Boonpiyathad T, Meyer N, Moniuszko M, Sokolowska M, Eljaszewicz A, Wirz OF, Tomasiak-Lozowska MM, Bodzenta-Lukaszyk A, Ruxrungtham K, van de Veen W. High-dose bee venom exposure induces similar tolerogenic B-cell responses in allergic patients and healthy beekeepers. Allergy. 2017;72:407–415. doi: 10.1111/all.12966. [DOI] [PubMed] [Google Scholar]
- 55.Wang S, Xia P, Chen Y, Qu Y, Xiong Z, Ye B, Du Y, Tian Y, Yin Z, Xu Z, Fan Z. Regulatory innate lymphoid cells control innate intestinal inflammation. Cell. 2017;171:201–216.e18. doi: 10.1016/j.cell.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 56.Morita H, Kubo T, Rückert B, Ravindran A, Soyka MB, Rinaldi AO, Sugita K, Wawrzyniak M, Wawrzyniak P, Motomura K, Tamari M, Orimo K, Okada N, Arae K, Saito K, Altunbulakli C, Castro-Giner F, Tan G, Neumann A, Sudo K, O’Mahony L, Honda K, Nakae S, Saito H, Mjösberg J, Nilsson G, Matsumoto K, Akdis M, Akdis CA. Induction of human regulatory innate lymphoid cells from group 2 innate lymphoid cells by retinoic acid. J Allergy Clin Immunol. 2019;143:2190–2201.e9. doi: 10.1016/j.jaci.2018.12.1018. [DOI] [PubMed] [Google Scholar]
- 57.Golebski K, Layhadi JA, Sahiner U, Steveling-Klein EH, Lenormand MM, Li RCY, Bal SM, Heesters BA, Vilà-Nadal G, Hunewald O, Montamat G, He FQ, Ollert M, Fedina O, Lao-Araya M, Vijverberg SJH, Maitland-van der Zee AH, van Drunen CM, Fokkens WJ, Durham SR, Spits H, Shamji MH. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity. 2021;54:291–307.e7. doi: 10.1016/j.immuni.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 58.Eljaszewicz A, Ruchti F, Radzikowska U, Globinska A, Boonpiyathad T, Gschwend A, Morita H, Helbling A, Arasi S, Kahlert H, Berek N, Nandy A, Akdis M, Willers C, Moniuszko M, Akdis CA, Sokolowska M. Trained immunity and tolerance in innate lymphoid cells, monocytes, and dendritic cells during allergen-specific immunotherapy. J Allergy Clin Immunol. 2021;147:1865–1877. doi: 10.1016/j.jaci.2020.08.042. [DOI] [PubMed] [Google Scholar]
- 59.López-Abente J, Benito-Villalvilla C, Jaumont X, Pfister P, Tassinari P, Palomares O. Omalizumab restores the ability of human plasmacytoid dendritic cells to induce Foxp3+Tregs. Eur Respir J. 2021;57:2000751. doi: 10.1183/13993003.00751-2020. [DOI] [PubMed] [Google Scholar]
- 60.Soria I, López-Relaño J, Viñuela M, Tudela JI, Angelina A, Benito-Villalvilla C, Díez-Rivero CM, Cases B, Manzano AI, Fernández-Caldas E, Casanovas M, Palomares O, Subiza JL. Oral myeloid cells uptake allergoids coupled to mannan driving Th1/Treg responses upon sublingual delivery in mice. Allergy. 2018;73:875–884. doi: 10.1111/all.13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benito-Villalvilla C, Pérez-Diego M, Angelina A, Kisand K, Rebane A, Subiza JL, Palomares O. Allergoid-mannan conjugates reprogram monocytes into tolerogenic dendritic cells via epigenetic and metabolic rewiring. J Allergy Clin Immunol. 2022;149:212–222.e9. doi: 10.1016/j.jaci.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 62.Sirvent S, Soria I, Cirauqui C, Cases B, Manzano AI, Diez-Rivero CM, Reche PA, López-Relaño J, Martínez-Naves E, Cañada FJ, Jiménez-Barbero J, Subiza J, Casanovas M, Fernández-Caldas E, Subiza JL, Palomares O. Novel vaccines targeting dendritic cells by coupling allergoids to nonoxidized mannan enhance allergen uptake and induce functional regulatory T cells through programmed death ligand 1. J Allergy Clin Immunol. 2016;138:558–567.e11. doi: 10.1016/j.jaci.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 63.Benito-Villalvilla C, Pérez-Diego M, Subiza JL, Palomares O. Allergoid-mannan conjugates imprint tolerogenic features in human macrophages. Allergy. 2022;77:320–323. doi: 10.1111/all.15118. [DOI] [PubMed] [Google Scholar]
- 64.Wen H, Qu L, Zhang Y, Xu B, Ling S, Liu X, Luo Y, Huo D, Li W, Yao X. A dendritic cells-targeting nano-vaccine by coupling polylactic-co-glycolic acid-encapsulated allergen with mannan induces regulatory T cells. Int Arch Allergy Immunol. 2021;182:777–787. doi: 10.1159/000512872. [DOI] [PubMed] [Google Scholar]
- 65.Angelina A, Pérez-Diego M, López-Abente J, Rückert B, Nombela I, Akdis M, Martín-Fontecha M, Akdis C, Palomares O. Cannabinoids induce functional Tregs by promoting tolerogenic DCs via autophagy and metabolic reprograming. Mucosal Immunol. 2022;15:96–108. doi: 10.1038/s41385-021-00455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dolch A, Kunz S, Dorn B, Alessandrini F, Müller W, Jack RS, Martin SF, Roers A, Jakob T. IL-10 signaling in dendritic cells is required for tolerance induction in a murine model of allergic airway inflammation. Eur J Immunol. 2019;49:302–312. doi: 10.1002/eji.201847883. [DOI] [PubMed] [Google Scholar]
- 67.Matsuoka T, Shamji MH, Durham SR. Allergen immunotherapy and tolerance. Allergol Int. 2013;62:403–413. doi: 10.2332/allergolint.13-RAI-0650. [DOI] [PubMed] [Google Scholar]
- 68.Shamji MH, Larson D, Eifan A, Scadding GW, Qin T, Lawson K, Sever ML, Macfarlane E, Layhadi JA, Würtzen PA, Parkin RV, Sanda S, Harris KM, Nepom GT, Togias A, Durham SR. Differential induction of allergen-specific IgA responses following timothy grass subcutaneous and sublingual immunotherapy. J Allergy Clin Immunol. 2021;148:1061–1071.e11. doi: 10.1016/j.jaci.2021.03.030. [DOI] [PubMed] [Google Scholar]
- 69.Shamji MH, Valenta R, Jardetzky T, Verhasselt V, Durham SR, Würtzen PA, van Neerven RJJ. The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy. 2021;76:3627–3641. doi: 10.1111/all.14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van de Veen W, Akdis M. Role of IgG4 in IgE-mediated allergic responses. J Allergy Clin Immunol. 2016;138:1434–1435. doi: 10.1016/j.jaci.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 71.Chang X, Zha L, Wallimann A, Mohsen MO, Krenger P, Liu X, Vogel M, Bachmann MF. Low-affinity but high-avidity interactions may offer an explanation for IgE-mediated allergen cross-reactivity. Allergy. 2021;76:2565–2574. doi: 10.1111/all.14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grilo JR, Kitzmüller C, Aglas L, Sánchez Acosta G, Vollmann U, Ebner C, Horak F, Kinaciyan T, Radauer C, Ferreira F, Jahn-Schmid B, Bohle B. IgE-cross-blocking antibodies to Fagales following sublingual immunotherapy with recombinant Bet v 1. Allergy. 2021;76:2555–2564. doi: 10.1111/all.14817. [DOI] [PubMed] [Google Scholar]
- 73.Orengo JM, Radin AR, Kamat V, Badithe A, Ben LH, Bennett BL, Zhong S, Birchard D, Limnander A, Rafique A, Bautista J, Kostic A, Newell D, Duan X, Franklin MC, Olson W, Huang T, Gandhi NA, Lipsich L, Stahl N, Papadopoulos NJ, Murphy AJ, Yancopoulos GD. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat Commun. 2018;9:1421. doi: 10.1038/s41467-018-03636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martínez-Martínez P, Vermeulen E, den Bleker TH, Wiegman L, Vink T, Aarden LA, De Baets MH, van de Winkel JG, Aalberse RC, Parren PW. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 75.Heeringa JJ, McKenzie CI, Varese N, Hew M, Bakx ATCM, Aui PM, Rolland JM, O’Hehir RE, van Zelm MC. Induction of IgG2 and IgG4 B-cell memory following sublingual immunotherapy for ryegrass pollen allergy. Allergy. 2020;75:1121–1132. doi: 10.1111/all.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boonpiyathad T, Pradubpongsa P, Mitthamsiri W, Satitsuksanoa P, Jacquet A, Sangasapaviliya A. Allergen-specific immunotherapy boosts allergen-specific IgD production in house dust mite-sensitized asthmatic patients. Allergy. 2020;75:1457–1460. doi: 10.1111/all.14133. [DOI] [PubMed] [Google Scholar]
- 77.Shan M, Carrillo J, Yeste A, Gutzeit C, Segura-Garzón D, Walland AC, Pybus M, Grasset EK, Yeiser JR, Matthews DB, van de Veen W, Comerma L, He B, Boonpiyathad T, Lee H, Blanco J, Osborne LC, Siracusa MC, Akdis M, Artis D, Mehandru S, Sampson HA, Berin MC, Chen K, Cerutti A. Secreted IgD amplifies humoral T helper 2 cell responses by binding basophils via galectin-9 and CD44. Immunity. 2018;49:709–724.e8. doi: 10.1016/j.immuni.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Backman H, Räisänen P, Hedman L, Stridsman C, Andersson M, Lindberg A, Lundbäck B, Rönmark E. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016-results from three population surveys. Clin Exp Allergy. 2017;47:1426–1435. doi: 10.1111/cea.12963. [DOI] [PubMed] [Google Scholar]
- 79.Pakkasela J, Ilmarinen P, Honkamäki J, Tuomisto LE, Andersén H, Piirilä P, Hisinger-Mölkänen H, Sovijärvi A, Backman H, Lundbäck B, Rönmark E, Kankaanranta H, Lehtimäki L. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm Med. 2020;20:9. doi: 10.1186/s12890-019-1040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Warm K, Hedman L, Lindberg A, Lötvall J, Lundbäck B, Rönmark E. Allergic sensitization is age-dependently associated with rhinitis, but less so with asthma. J Allergy Clin Immunol. 2015;136:1559–1565.e2. doi: 10.1016/j.jaci.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 81.Papapostolou N, Makris M. Allergic asthma in the era of personalized medicine. J Pers Med. 2022;12:1162. doi: 10.3390/jpm12071162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmitt J, Wüstenberg E, Küster D, Mücke V, Serup-Hansen N, Tesch F. The moderating role of allergy immunotherapy in asthma progression: Results of a population-based cohort study. Allergy. 2020;75:596–602. doi: 10.1111/all.14020. [DOI] [PubMed] [Google Scholar]
- 83.Dhami S, Kakourou A, Asamoah F, Agache I, Lau S, Jutel M, Muraro A, Roberts G, Akdis CA, Bonini M, Cavkaytar O, Flood B, Gajdanowicz P, Izuhara K, Kalayci Ö, Mosges R, Palomares O, Pfaar O, Smolinska S, Sokolowska M, Asaria M, Netuveli G, Zaman H, Akhlaq A, Sheikh A. Allergen immunotherapy for allergic asthma: a systematic review and meta-analysis. Allergy. 2017;72:1825–1848. doi: 10.1111/all.13208. [DOI] [PubMed] [Google Scholar]
- 84.Scadding GW, Calderon MA, Shamji MH, Eifan AO, Penagos M, Dumitru F, Sever ML, Bahnson HT, Lawson K, Harris KM, Plough AG, Panza JL, Qin T, Lim N, Tchao NK, Togias A, Durham SR Immune Tolerance Network GRASS Study Team. Effect of 2 years of treatment with sublingual grass pollen immunotherapy on nasal response to allergen challenge at 3 years among patients with moderate to severe seasonal allergic rhinitis: the GRASS randomized clinical trial. JAMA. 2017;317:615–625. doi: 10.1001/jama.2016.21040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, Brignardello-Petersen R, Canonica GW, Casale T, Chavannes NH, Correia de Sousa J, Cruz AA, Cuello-Garcia CA, Demoly P, Dykewicz M, Etxeandia-Ikobaltzeta I, Florez ID, Fokkens W, Fonseca J, Hellings PW, Klimek L, Kowalski S, Kuna P, Laisaar KT, Larenas-Linnemann DE, Lødrup Carlsen KC, Manning PJ, Meltzer E, Mullol J, Muraro A, O’Hehir R, Ohta K, Panzner P, Papadopoulos N, Park HS, Passalacqua G, Pawankar R, Price D, Riva JJ, Roldán Y, Ryan D, Sadeghirad B, Samolinski B, Schmid-Grendelmeier P, Sheikh A, Togias A, Valero A, Valiulis A, Valovirta E, Ventresca M, Wallace D, Waserman S, Wickman M, Wiercioch W, Yepes-Nuñez JJ, Zhang L, Zhang Y, Zidarn M, Zuberbier T, Schünemann HJ. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140:950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 86.Pongracic JA, Krouse RZ, Babineau DC, Zoratti EM, Cohen RT, Wood RA, Khurana Hershey GK, Kercsmar CM, Gruchalla RS, Kattan M, Teach SJ, Johnson CC, Bacharier LB, Gern JE, Sigelman SM, Gergen PJ, Togias A, Visness CM, Busse WW, Liu AH. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol. 2016;138:1030–1041. doi: 10.1016/j.jaci.2016.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, Cruz AA, Duijts L, Drazen JM, FitzGerald JM, Fleming LJ, Inoue H, Ko FW, Krishnan JA, Levy ML, Lin J, Mortimer K, Pitrez PM, Sheikh A, Yorgancioglu AA, Boulet LP. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. J Allergy Clin Immunol Pract. 2022;10(1S):S1–S18. doi: 10.1016/j.jaip.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 88.Wahn U, Bachert C, Heinrich J, Richter H, Zielen S. Real-world benefits of allergen immunotherapy for birch pollen-associated allergic rhinitis and asthma. Allergy. 2019;74:594–604. doi: 10.1111/all.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nogami K, Nagao M, Takase T, Yasuda Y, Yamada S, Matsunaga M, Hoshi M, Hamada K, Kuwabara Y, Tsugawa T, Fujisawa T. House dust mite subcutaneous immunotherapy and lung function trajectory in children and adolescents with asthma. Children (Basel) 2022;9:487. doi: 10.3390/children9040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lue KH, Lin YH, Sun HL, Lu KH, Hsieh JC, Chou MC. Clinical and immunologic effects of sublingual immunotherapy in asthmatic children sensitized to mites: a double-blind, randomized, placebo-controlled study. Pediatr Allergy Immunol. 2006;17:408–415. doi: 10.1111/j.1399-3038.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 91.Bahceciler NN, Arikan C, Taylor A, Akdis M, Blaser K, Barlan IB, Akdis CA. Impact of sublingual immunotherapy on specific antibody levels in asthmatic children allergic to house dust mites. Int Arch Allergy Immunol. 2005;136:287–294. doi: 10.1159/000083956. [DOI] [PubMed] [Google Scholar]
- 92.Wang H, Lin X, Hao C, Zhang C, Sun B, Zheng J, Chen P, Sheng J, Wu A, Zhong N. A double-blind, placebo-controlled study of house dust mite immunotherapy in Chinese asthmatic patients. Allergy. 2006;61:191–197. doi: 10.1111/j.1398-9995.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 93.Würtzen PA, Lund G, Lund K, Arvidsson M, Rak S, Ipsen H. A double-blind placebo-controlled birch allergy vaccination study II: correlation between inhibition of IgE binding, histamine release and facilitated allergen presentation. Clin Exp Allergy. 2008;38:1290–1301. doi: 10.1111/j.1365-2222.2008.03020.x. [DOI] [PubMed] [Google Scholar]
- 94.Yukselen A, Kendirli SG, Yilmaz M, Altintas DU, Karakoc GB. Effect of one-year subcutaneous and sublingual immunotherapy on clinical and laboratory parameters in children with rhinitis and asthma: a randomized, placebo-controlled, double-blind, double-dummy study. Int Arch Allergy Immunol. 2012;157:288–298. doi: 10.1159/000327566. [DOI] [PubMed] [Google Scholar]
- 95.Wang SH, Zissler UM, Buettner M, Heine S, Heldner A, Kotz S, Pechtold L, Kau J, Plaschke M, Ullmann JT, Guerth F, Oelsner M, Alessandrini F, Blank S, Chaker AM, Schmidt-Weber CB, Jakwerth CA. An exhausted phenotype of TH 2 cells is primed by allergen exposure, but not reinforced by allergen-specific immunotherapy. Allergy. 2021;76:2827–2839. doi: 10.1111/all.14896. [DOI] [PubMed] [Google Scholar]
- 96.Hesse L, Brouwer U, Petersen AH, Gras R, Bosman L, Brimnes J, Oude Elberink JNG, van Oosterhout AJM, Nawijn MC. Subcutaneous immunotherapy suppresses Th2 inflammation and induces neutralizing antibodies, but sublingual immunotherapy suppresses airway hyperresponsiveness in grass pollen mouse models for allergic asthma. Clin Exp Allergy. 2018;48:1035–1049. doi: 10.1111/cea.13169. [DOI] [PubMed] [Google Scholar]
- 97.Hoshino M, Akitsu K, Kubota K, Ohtawa J. Association between biomarkers and house dust mite sublingual immunotherapy in allergic asthma. Clin Exp Allergy. 2020;50:1035–1043. doi: 10.1111/cea.13686. [DOI] [PubMed] [Google Scholar]
- 98.Aroca R, Chamorro C, Vega A, Ventura I, Gómez E, Pérez-Cano R, Blanca M, Monteseirín J. Immunotherapy reduces allergen-mediated CD66b expression and myeloperoxidase levels on human neutrophils from allergic patients. PLoS One. 2014;9:e94558. doi: 10.1371/journal.pone.0094558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Monteseirín J, Bonilla I, Camacho J, Conde J, Sobrino F. Elevated secretion of myeloperoxidase by neutrophils from asthmatic patients: the effect of immunotherapy. J Allergy Clin Immunol. 2001;107:623–626. doi: 10.1067/mai.2001.113566. [DOI] [PubMed] [Google Scholar]
- 100.Nikolov G, Todordova Y, Emilova R, Hristova D, Nikolova M, Petrunov B. Allergen-specific IgE and IgG4 as biomarkers for immunologic changes during subcutaneous allergen immunotherapy. Antibodies (Basel) 2021;10:49. doi: 10.3390/antib10040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Justicia JL, Padró C, Roger A, Moreno F, Rial MJ, Parra A, Valero A, Malet A, Teniente A, Boronat A, Torán-Barona C. Immunological parameters as biomarkers of response to MicroCrystalline Tyrosine-adjuvanted mite immunotherapy. World Allergy Organ J. 2021;14:100545. doi: 10.1016/j.waojou.2021.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Su W, Zhong W, Zhang Y, Xia Z. Synthesized OVA323-339MAP octamers mitigate OVA-induced airway inflammation by regulating Foxp3 T regulatory cells. BMC Immunol. 2012;13:34. doi: 10.1186/1471-2172-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tian M, Wang Y, Lu Y, Jiang YH, Zhao DY. Effects of sublingual immunotherapy for Dermatophagoides farinae on Th17 cells and CD4(+) CD25(+) regulatory T cells in peripheral blood of children with allergic asthma. Int Forum Allergy Rhinol. 2014;4:371–375. doi: 10.1002/alr.21305. [DOI] [PubMed] [Google Scholar]
- 104.Jakwerth CA, Chaker AM, Guerth F, Oelsner M, Pechtold L, Zur Bonsen LS, Ullmann JT, Krauss-Etschmann S, Erb A, Kau J, Plaschke M, Winkler M, Kurz A, Kloss A, Esser-von Bieren J, Schmidt-Weber CB, Zissler UM. Sputum microRNA-screening reveals Prostaglandin EP3 receptor as selective target in allergen-specific immunotherapy. Clin Exp Allergy. 2021;51:1577–1591. doi: 10.1111/cea.14013. [DOI] [PubMed] [Google Scholar]
- 105.Gleich GJ, Zimmermann EM, Henderson LL, Yunginger JW. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: a six-year prospective study. J Allergy Clin Immunol. 1982;70:261–271. doi: 10.1016/0091-6749(82)90062-8. [DOI] [PubMed] [Google Scholar]
- 106.Shamji MH, Francis JN, Würtzen PA, Lund K, Durham SR, Till SJ. Cell-free detection of allergen-IgE cross-linking with immobilized phase CD23: inhibition by blocking antibody responses after immunotherapy. J Allergy Clin Immunol. 2013;132:1003–1005.e1-4. doi: 10.1016/j.jaci.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 107.Shamji MH, Sharif H, Layhadi JA, Zhu R, Kishore U, Renz H. Diverse immune mechanisms of allergen immunotherapy for allergic rhinitis with and without asthma. J Allergy Clin Immunol. 2022;149:791–801. doi: 10.1016/j.jaci.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 108.Karakoc-Aydiner E, Eifan AO, Baris S, Gunay E, Akturk E, Akkoc T, Bahceciler NN, Barlan IB. Long-term effect of sublingual and subcutaneous immunotherapy in dust mite-allergic children with asthma/rhinitis: a 3-year prospective randomized controlled trial. J Investig Allergol Clin Immunol. 2015;25:334–342. [PubMed] [Google Scholar]
- 109.Di Lorenzo G, Mansueto P, Pacor ML, Rizzo M, Castello F, Martinelli N, Ditta V, Lo Bianco C, Leto-Barone MS, D’Alcamo A, Di Fede G, Rini GB, Ditto AM. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol. 2009;123:1103–1110. 1110.e1–1104. doi: 10.1016/j.jaci.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 110.Li Q, Li M, Yue W, Zhou J, Li R, Lin J, Li Y. Predictive factors for clinical response to allergy immunotherapy in children with asthma and rhinitis. Int Arch Allergy Immunol. 2014;164:210–217. doi: 10.1159/000365630. [DOI] [PubMed] [Google Scholar]
- 111.Sun W, Pan L, Yu Q, Sun Y, Zeng X, Bai X, Li M. The skin prick test response after allergen immunotherapy in different levels of tIgE children with mite sensitive Asthma/Rhinitis in South China. Hum Vaccin Immunother. 2018;14:2510–2515. doi: 10.1080/21645515.2018.1482171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peng H, Li CW, Lin ZB, Li TY. Long-term efficacy of specific immunotherapy on house dust mite-induced allergic rhinitis in China. Otolaryngol Head Neck Surg. 2013;149:40–46. doi: 10.1177/0194599813485222. [DOI] [PubMed] [Google Scholar]
- 113.Yang L, Yang Y, Xu Q, Zhang W, Jiang Q, Li W, Wang Y, Ma D, Lin X, Sun B, Zhu R. Specific IgE and IgG4 profiles of house dust mite components in allergen-specific immunotherapy. Front Immunol. 2022;12:786738. doi: 10.3389/fimmu.2021.786738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.James LK, Shamji MH, Walker SM, Wilson DR, Wachholz PA, Francis JN, Jacobson MR, Kimber I, Till SJ, Durham SR. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127:509–516.e1-5. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]
- 115.Hurst DS, McDaniel AB. Clinical relevance and advantages of intradermal test results in 371 patients with allergic rhinitis, asthma and/or otitis media with effusion. Cells. 2021;10:3224. doi: 10.3390/cells10113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoshino M, Akitsu K, Kubota K, Ohtawa J. Serum periostin as a biomarker for predicting clinical response to house dust mite sublingual immunotherapy in allergic rhinitis. J Allergy Clin Immunol Pract. 2021;9:1864–1870. doi: 10.1016/j.jaip.2020.11.046. [DOI] [PubMed] [Google Scholar]
- 117.Izquierdo-Domínguez A, Valero AL, Mullol J. Comparative analysis of allergic rhinitis in children and adults. Curr Allergy Asthma Rep. 2013;13:142–151. doi: 10.1007/s11882-012-0331-y. [DOI] [PubMed] [Google Scholar]
- 118.Dennis RJ, Caraballo L, García E, Rojas MX, Rondon MA, Pérez A, Aristizabal G, Peñaranda A, Barragan AM, Ahumada V, Jimenez S. Prevalence of asthma and other allergic conditions in Colombia 2009-2010: a cross-sectional study. BMC Pulm Med. 2012;12:17. doi: 10.1186/1471-2466-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bantz SK, Zhu Z, Zheng T. The atopic March: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 2014;5:202. doi: 10.4172/2155-9899.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu WJ, Ma HX, Cui HY, Lu X, Shao MJ, Li S, Luo YQ, Wang Q, Xu CY, Xu DQ, Liu CH, Chen YZ. Prevalence and treatment of children’s asthma in rural areas compared with urban areas in Beijing. Chin Med J (Engl) 2015;128:2273–2277. doi: 10.4103/0366-6999.163381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vrijheid M, Casas M, Bergström A, Carmichael A, Cordier S, Eggesbø M, Eller E, Fantini MP, Fernández MF, Fernández-Somoano A, Gehring U, Grazuleviciene R, Hohmann C, Karvonen AM, Keil T, Kogevinas M, Koppen G, Krämer U, Kuehni CE, Magnus P, Majewska R, Andersen AM, Patelarou E, Petersen MS, Pierik FH, Polanska K, Porta D, Richiardi L, Santos AC, Slama R, Sram RJ, Thijs C, Tischer C, Toft G, Trnovec T, Vandentorren S, Vrijkotte TG, Wilhelm M, Wright J, Nieuwenhuijsen M. European birth cohorts for environmental health research. Environ Health Perspect. 2012;120:29–37. doi: 10.1289/ehp.1103823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yao Y, Wang N, Chen CL, Pan L, Wang ZC, Yunis J, Chen ZA, Zhang Y, Hu ST, Xu XY, Zhu RF, Yu D, Liu Z. CD23 expression on switched memory B cells bridges T-B cell interaction in allergic rhinitis. Allergy. 2020;75:2599–2612. doi: 10.1111/all.14288. [DOI] [PubMed] [Google Scholar]
- 123.McKenzie CI, Varese N, Aui PM, Reinwald S, Wines BD, Hogarth PM, Thien F, Hew M, Rolland JM, O’Hehir RE, van Zelm MC. RNA sequencing of single allergen-specific memory B cells after grass pollen immunotherapy: two unique cell fates and CD29 as a biomarker for treatment effect. Allergy. 2022 doi: 10.1111/all.15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cheng S, Wen S, Xie S, Zhang C, Zhang H, Gao K, Fan R, Xie Z, Jiang W. Circulating C-X-C motif ligand 13 as a biomarker for early predicting efficacy of subcutaneous immunotherapy in children with chronic allergic rhinitis. Front Pediatr. 2022;10:872152. doi: 10.3389/fped.2022.872152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ihara F, Sakurai D, Yonekura S, Iinuma T, Yagi R, Sakurai T, Ito T, Matsuura A, Morimoto Y, Arai T, Suzuki S, Katayama K, Nakayama T, Okamoto Y. Identification of specifically reduced Th2 cell subsets in allergic rhinitis patients after sublingual immunotherapy. Allergy. 2018;73:1823–1832. doi: 10.1111/all.13436. [DOI] [PubMed] [Google Scholar]
- 126.Sakurai D, Yonekura S, Iinuma T, Sakurai T, Morimoto Y, Mita Y, Arai T, Suzuki S, Okuma Y, Kaneko S, Okamoto Y. Sublingual immunotherapy for allergic rhinitis: subjective versus objective tools to evaluate its success. Rhinology. 2016;54:221–230. doi: 10.4193/Rhino15.223. [DOI] [PubMed] [Google Scholar]
- 127.Xie S, Zhang H, Wang F, Liu Y, Gao K, Zhang J, Fan R, Xie S, Xie Z, Jiang W. Activated leukocyte cell adhesion molecule as a biomarker for disease severity and efficacy of sublingual immunotherapy in allergic rhinitis. Int Immunopharmacol. 2020;88:106975. doi: 10.1016/j.intimp.2020.106975. [DOI] [PubMed] [Google Scholar]
- 128.Cohen S, Danzaki K, MacIver NJ. Nutritional effects on T-cell immunometabolism. Eur J Immunol. 2017;47:225–235. doi: 10.1002/eji.201646423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wen Y, Zhou L, Li Y, Li Z, Deng W, Zhang T. Role of leptin in allergic rhinitis during sublingual immunotherapy. Eur Arch Otorhinolaryngol. 2018;275:2733–2738. doi: 10.1007/s00405-018-5123-0. [DOI] [PubMed] [Google Scholar]
- 130.Xie S, Zhang H, Wang F, Xie Z, Jiang W, Gao K. Circulating MIF associated with disease severity and clinical response of sublingual immunotherapy in house dust mite-induced allergic rhinitis. Front Pharmacol. 2021;12:681724. doi: 10.3389/fphar.2021.681724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Breiteneder H, Diamant Z, Eiwegger T, Fokkens WJ, Traidl-Hoffmann C, Nadeau K, O’Hehir RE, O’Mahony L, Pfaar O, Torres MJ, Wang Y, Zhang L, Akdis CA. Future research trends in understanding the mechanisms underlying allergic diseases for improved patient care. Allergy. 2019;74:2293–2311. doi: 10.1111/all.13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Y, Li C, Xu Y, Xu D, Yang G, Liao F, Luo X. Sublingual immunotherapy decreases expression of interleukin-33 in children with allergic rhinitis. Indian J Pediatr. 2018;85:872–876. doi: 10.1007/s12098-018-2703-3. [DOI] [PubMed] [Google Scholar]
- 133.Nasr WF, Sorour SS, El Bahrawy AT, Boghdadi GS, El Shahaway AA. The role of the level of interleukin-33 in the therapeutic outcomes of immunotherapy in patients with allergic rhinitis. Int Arch Otorhinolaryngol. 2018;22:152–156. doi: 10.1055/s-0037-1605596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xie S, Fan R, Tang Q, Cai X, Zhang H, Wang F, Xie S, Gao K, Zhang J, Xie Z, Jiang W. Identification of robust biomarkers for early predicting efficacy of subcutaneous immunotherapy in children with house dust mite-induced allergic rhinitis by multiple cytokine profiling. Front Immunol. 2022;12:805404. doi: 10.3389/fimmu.2021.805404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shamji MH, Durham SR. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol. 2017;140:1485–1498. doi: 10.1016/j.jaci.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 136.Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134:1193–5.e4. doi: 10.1016/j.jaci.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 137.Fan DC, Wang XD, Wang CS, Wang Y, Cao FF, Zhang L. Suppression of immunotherapy on group 2 innate lymphoid cells in allergic rhinitis. Chin Med J (Engl) 2016;129:2824–2828. doi: 10.4103/0366-6999.194642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Caruso M, Cibella F, Emma R, Campagna D, Tringali G, Amaradio MD, Polosa R. Basophil biomarkers as useful predictors for sublingual immunotherapy in allergic rhinitis. Int Immunopharmacol. 2018;60:50–58. doi: 10.1016/j.intimp.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 139.Ma TT, Cao MD, Yu RL, Shi HY, Yan WJ, Liu JG, Pan C, Sun J, Wei QY, Wang DY, Wei JF, Wang XY, Yin JS. Leukotriene A4 hydrolase is a candidate predictive biomarker for successful allergen immunotherapy. Front Immunol. 2020;11:559746. doi: 10.3389/fimmu.2020.559746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pepler L, Wu C, Dwivedi DJ, Wu C, Kim PY, Liaw PC. The impact of the endothelial protein C receptor on thrombin generation and clot lysis. Thromb Res. 2017;152:30–37. doi: 10.1016/j.thromres.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 141.Yoshida K, Takabayashi T, Imoto Y, Sakashita M, Kato Y, Narita N, Fujieda S. Increased thrombin-activatable fibrinolysis inhibitor in response to sublingual immunotherapy for allergic rhinitis. Laryngoscope. 2021;131:2413–2420. doi: 10.1002/lary.29563. [DOI] [PubMed] [Google Scholar]
- 142.Subramanian H, Gupta K, Ali H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol. 2016;138:700–710. doi: 10.1016/j.jaci.2016.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lagente V, Le Quement C, Boichot E. Macrophage metalloelastase (MMP-12) as a target for inflammatory respiratory diseases. Expert Opin Ther Targets. 2009;13:287–295. doi: 10.1517/14728220902751632. [DOI] [PubMed] [Google Scholar]
- 144.Zhou Y, Xu M, Gong W, Kang X, Guo R, Wen J, Zhou D, Wang M, Shi D, Jing Q. Circulating MMP-12 as potential biomarker in evaluating disease severity and efficacy of sublingual immunotherapy in allergic rhinitis. Mediators Inflamm. 2022;2022:3378035. doi: 10.1155/2022/3378035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shamji MH, Ljørring C, Francis JN, Calderon MA, Larché M, Kimber I, Frew AJ, Ipsen H, Lund K, Würtzen PA, Durham SR. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67:217–226. doi: 10.1111/j.1398-9995.2011.02745.x. [DOI] [PubMed] [Google Scholar]
- 146.Dahl R, Kapp A, Colombo G, de Monchy JG, Rak S, Emminger W, Riis B, Grønager PM, Durham SR. Sublingual grass allergen tablet immunotherapy provides sustained clinical benefit with progressive immunologic changes over 2 years. J Allergy Clin Immunol. 2008;121:512–518.e2. doi: 10.1016/j.jaci.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 147.Fujimura T, Yonekura S, Horiguchi S, Taniguchi Y, Saito A, Yasueda H, Inamine A, Nakayama T, Takemori T, Taniguchi M, Sakaguchi M, Okamoto Y. Increase of regulatory T cells and the ratio of specific IgE to total IgE are candidates for response monitoring or prognostic biomarkers in 2-year sublingual immunotherapy (SLIT) for Japanese cedar pollinosis. Clin Immunol. 2011;139:65–74. doi: 10.1016/j.clim.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 148.Liu W, Zeng Q, Luo R. Predictors for short-term efficacy of allergen-specific sublingual immunotherapy in children with allergic rhinitis. Mediators Inflamm. 2020;2020:1847061. doi: 10.1155/2020/1847061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Novak N, Worm M, Staubach P, Jutel M, Sager A, Pfaar O. Subcutaneous birch pollen allergen immunotherapy with a depigmented polymerized extract shows only sustained and long-term efficacy in a subgroup of monosensitized adults and adolescents with allergic rhinitis. Clin Transl Allergy. 2022;12:e12185. doi: 10.1002/clt2.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bordas-Le Floch V, Berjont N, Batard T, Varese N, O’Hehir RE, Canonica WG, van Zelm MC, Mascarell L. Coordinated IgG2 and IgE responses as a marker of allergen immunotherapy efficacy. Allergy. 2022;77:1263–1273. doi: 10.1111/all.15107. [DOI] [PubMed] [Google Scholar]
- 151.Schmid JM, Würtzen PA, Dahl R, Hoffmann HJ. Pretreatment IgE sensitization patterns determine the molecular profile of the IgG4 response during updosing of subcutaneous immunotherapy with timothy grass pollen extract. J Allergy Clin Immunol. 2016;137:562–570. doi: 10.1016/j.jaci.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 152.Xie S, Jiang S, Zhang H, Wang F, Liu Y, She Y, Jing Q, Gao K, Fan R, Xie S, Xie Z, Jiang W. Prediction of sublingual immunotherapy efficacy in allergic rhinitis by serum metabolomics analysis. Int Immunopharmacol. 2021;90:107211. doi: 10.1016/j.intimp.2020.107211. [DOI] [PubMed] [Google Scholar]
- 153.Zheng P, Yan G, Zhang Y, Huang H, Luo W, Xue M, Li N, Wu JL, Sun B. Metabolomics reveals process of allergic rhinitis patients with single- and double-species mite subcutaneous immunotherapy. Metabolites. 2021;11:613. doi: 10.3390/metabo11090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Renz H, Allen KJ, Sicherer SH, Sampson HA, Lack G, Beyer K, Oettgen HC. Food allergy. Nat Rev Dis Primers. 2018;4:17098. doi: 10.1038/nrdp.2017.98. [DOI] [PubMed] [Google Scholar]
- 155.Chinthrajah RS, Purington N, Andorf S, Long A, O’Laughlin KL, Lyu SC, Manohar M, Boyd SD, Tibshirani R, Maecker H, Plaut M, Mukai K, Tsai M, Desai M, Galli SJ, Nadeau KC. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2019;394:1437–1449. doi: 10.1016/S0140-6736(19)31793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kulis MD, Smeekens JM, Burk C, Yue X, Guo R, Orgel KA, Ye P, Herlihy L, Hamilton D, Li Q, Keet C, Shreffler W, Vickery BP, Burks AW, Kim EH. Kinetics of basophil hyporesponsiveness during short-course peanut oral immunotherapy. J Allergy Clin Immunol. 2022;150:S0091-6749(22)00792-8. doi: 10.1016/j.jaci.2022.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Patil SU, Steinbrecher J, Calatroni A, Smith N, Ma A, Ruiter B, Virkud Y, Schneider M, Shreffler WG. Early decrease in basophil sensitivity to Ara h 2 precedes sustained unresponsiveness after peanut oral immunotherapy. J Allergy Clin Immunol. 2019;144:1310–1319.e4. doi: 10.1016/j.jaci.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, Steele P, Driggers S, Burks AW, Wood RA. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129:448–455. 455.e1–445. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Frischmeyer-Guerrerio PA, Masilamani M, Gu W, Brittain E, Wood R, Kim J, Nadeau K, Jarvinen KM, Grishin A, Lindblad R, Sampson HA. Mechanistic correlates of clinical responses to omalizumab in the setting of oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2017;140:1043–1053.e8. doi: 10.1016/j.jaci.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sugimoto M, Kamemura N, Nagao M, Irahara M, Kagami S, Fujisawa T, Kido H. Differential response in allergen-specific IgE, IgGs, and IgA levels for predicting outcome of oral immunotherapy. Pediatr Allergy Immunol. 2016;27:276–282. doi: 10.1111/pai.12535. [DOI] [PubMed] [Google Scholar]
- 161.Wright BL, Kulis M, Orgel KA, Burks AW, Dawson P, Henning AK, Jones SM, Wood RA, Sicherer SH, Lindblad RW, Stablein D, Leung DY, Vickery BP, Sampson HA Consortium of Food Allergy Research. Component-resolved analysis of IgA, IgE, and IgG4 during egg OIT identifies markers associated with sustained unresponsiveness. Allergy. 2016;71:1552–1560. doi: 10.1111/all.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Smeekens JM, Baloh C, Lim N, Larson D, Qin T, Wheatley L, Kim EH, Jones SM, Burks AW, Kulis MD. Peanut-specific IgG4 and IgA in saliva are modulated by peanut oral immunotherapy. J Allergy Clin Immunol Pract. 2022:S2213-2198(22)00800-5. doi: 10.1016/j.jaip.2022.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Muntean IA, Bocsan IC, Wiest LK, Pintea I, Dobrican CT, Duca E, Ureche C, Buzoianu AD, Deleanu D. Predictive factors for oral immune modulation in cow milk allergy. Nutrients. 2022;14:494. doi: 10.3390/nu14030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cohen CG, Zhao WW, Ke D, Beaudette L, Lejtenyi D, McCusker C, Zhang X, Chan ES, Upton JEM, Grunebaum E, Clarke AE, Mazer BD, Ben-Shoshan M. Elevated cow’s milk-specific IgE levels prior to oral immunotherapy decrease the likelihood of reaching the maintenance dose. J Allergy Clin Immunol Pract. 2022;10:215–221.e2. doi: 10.1016/j.jaip.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 165.Kauppila TK, Hinkkanen V, Savinko T, Karisola P, Kukkonen AK, Paassilta M, Pelkonen AS, Mäkelä MJ. Long-term changes in milk component immunoglobulins reflect milk oral immunotherapy outcomes in Finnish children. Allergy. 2022 Aug 15; doi: 10.1111/all.15479. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jones SM, Kim EH, Nadeau KC, Nowak-Wegrzyn A, Wood RA, Sampson HA, Scurlock AM, Chinthrajah S, Wang J, Pesek RD, Sindher SB, Kulis M, Johnson J, Spain K, Babineau DC, Chin H, Laurienzo-Panza J, Yan R, Larson D, Qin T, Whitehouse D, Sever ML, Sanda S, Plaut M, Wheatley LM, Burks AW Immune Tolerance Network. Efficacy and safety of oral immunotherapy in children aged 1-3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): a randomised placebo-controlled study. Lancet. 2022;399:359–371. doi: 10.1016/S0140-6736(21)02390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, Burk C, Hiegel A, Carlisle S, Christie L, Perry TT, Pesek RD, Sheikh S, Virkud Y, Smith PB, Shamji MH, Durham SR, Jones SM, Burks AW. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133:468–475. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dreskin SC, Germinaro M, Reinhold D, Chen X, Vickery BP, Kulis M, Burks AW, Negi SS, Braun W, Chambliss JM, Eglite S, McNulty CMG. IgE binding to linear epitopes of Ara h 2 in peanut allergic preschool children undergoing oral Immunotherapy. Pediatr Allergy Immunol. 2019;30:817–823. doi: 10.1111/pai.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Martínez-Botas J, Rodríguez-Álvarez M, Cerecedo I, Vlaicu C, Diéguez MC, Gómez-Coronado D, Fernández-Rivas M, de la Hoz B. Identification of novel peptide biomarkers to predict safety and efficacy of cow’s milk oral immunotherapy by peptide microarray. Clin Exp Allergy. 2015;45:1071–1084. doi: 10.1111/cea.12528. [DOI] [PubMed] [Google Scholar]
- 170.Suárez-Fariñas M, Suprun M, Chang HL, Gimenez G, Grishina G, Getts R, Nadeau K, Wood RA, Sampson HA. Predicting development of sustained unresponsiveness to milk oral immunotherapy using epitope-specific antibody binding profiles. J Allergy Clin Immunol. 2019;143:1038–1046. doi: 10.1016/j.jaci.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Caballero LR, Treudler R, Delaroque N, Simon JC, Kern K, Szardenings M. Peptide epitopes as biomarkers of soya sensitization in rBet v 1 immunotherapy of birch-related soya allergy. Clin Exp Allergy. 2022 doi: 10.1111/cea.14224. [DOI] [PubMed] [Google Scholar]
- 172.Moraly T, Pelletier de Chambure D, Verdun S, Preda C, Seynave M, Vilain AC, Chenivesse C, Delebarre-Sauvage C. Oral Immunotherapy for Hazelnut Allergy: A Single-Center Retrospective Study on 100 Patients. J Allergy Clin Immunol Pract. 2020;8:704–709.e4. doi: 10.1016/j.jaip.2019.10.045. [DOI] [PubMed] [Google Scholar]
- 173.Mori F, Cianferoni A, Brambilla A, Barni S, Sarti L, Pucci N, de Martino M, Novembre E. Side effects and their impact on the success of milk oral immunotherapy (OIT) in children. Int J Immunopathol Pharmacol. 2017;30:182–187. doi: 10.1177/0394632017697986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lockey RF, Turkeltaub PC, Olive ES, Hubbard JM, Baird-Warren IA, Bukantz SC. The Hymenoptera venom study. III: Safety of venom immunotherapy. J Allergy Clin Immunol. 1990;86:775–780. doi: 10.1016/s0091-6749(05)80182-4. [DOI] [PubMed] [Google Scholar]
- 175.Mosbech H, Müller U. Side-effects of insect venom immunotherapy: results from an EAACI multicenter study. European Academy of Allergology and Clinical Immunology. Allergy. 2000;55:1005–1010. doi: 10.1034/j.1398-9995.2000.00587.x. [DOI] [PubMed] [Google Scholar]
- 176.Ruëff F, Przybilla B, Biló MB, Müller U, Scheipl F, Aberer W, Birnbaum J, Bodzenta-Lukaszyk A, Bonifazi F, Bucher C, Campi P, Darsow U, Egger C, Haeberli G, Hawranek T, Kucharewicz I, Küchenhoff H, Lang R, Quercia O, Reider N, Severino M, Sticherling M, Sturm GJ, Wüthrich B European Academy of Allergy and Clinical Immunology Interest Group. Predictors of side effects during the buildup phase of venom immunotherapy for Hymenoptera venom allergy: the importance of baseline serum tryptase. J Allergy Clin Immunol. 2010;126:105–111.e5. doi: 10.1016/j.jaci.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 177.Sturm GJ, Varga EM, Roberts G, Mosbech H, Bilò MB, Akdis CA, Antolín-Amérigo D, Cichocka-Jarosz E, Gawlik R, Jakob T, Kosnik M, Lange J, Mingomataj E, Mitsias DI, Ollert M, Oude Elberink JNG, Pfaar O, Pitsios C, Pravettoni V, Ruëff F, Sin BA, Agache I, Angier E, Arasi S, Calderón MA, Fernandez-Rivas M, Halken S, Jutel M, Lau S, Pajno GB, van Ree R, Ryan D, Spranger O, van Wijk RG, Dhami S, Zaman H, Sheikh A, Muraro A. EAACI guidelines on allergen immunotherapy: Hymenoptera venom allergy. Allergy. 2018;73:744–764. doi: 10.1111/all.13262. [DOI] [PubMed] [Google Scholar]
- 178.Müller U, Helbling A, Berchtold E. Immunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safety. J Allergy Clin Immunol. 1992;89:529–535. doi: 10.1016/0091-6749(92)90319-w. [DOI] [PubMed] [Google Scholar]
- 179.Ruëff F, Vos B, Oude Elberink J, Bender A, Chatelain R, Dugas-Breit S, Horny HP, Küchenhoff H, Linhardt A, Mastnik S, Sotlar K, Stretz E, Vollrath R, Przybilla B, Flaig M. Predictors of clinical effectiveness of Hymenoptera venom immunotherapy. Clin Exp Allergy. 2014;44:736–746. doi: 10.1111/cea.12275. [DOI] [PubMed] [Google Scholar]
- 180.Golden DB. Anaphylaxis to insect stings. Immunol Allergy Clin North Am. 2015;35:287–302. doi: 10.1016/j.iac.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 181.Blank S, Grosch J, Ollert M, Bilò MB. Precision medicine in hymenoptera venom allergy: diagnostics, biomarkers, and therapy of different endotypes and phenotypes. Front Immunol. 2020;11:579409. doi: 10.3389/fimmu.2020.579409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Feindor M, Heath MD, Hewings SJ, Carreno Velazquez TL, Blank S, Grosch J, Jakob T, Schmid-Grendelmeier P, Klimek L, Golden DBK, Skinner MA, Kramer MF. Venom immunotherapy: from proteins to product to patient protection. Toxins (Basel) 2021;13:616. doi: 10.3390/toxins13090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cabrera CM, Urra JM, Alfaya T, Roca Fde L, Feo-Brito F. Expression of Th1, Th2, lymphocyte trafficking and activation markers on CD4+ T-cells of Hymenoptera allergic subjects and after venom immunotherapy. Mol Immunol. 2014;62:178–185. doi: 10.1016/j.molimm.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 184.Eberlein-König B, Ullmann S, Thomas P, Przybilla B. Tryptase and histamine release due to a sting challenge in bee venom allergic patients treated successfully or unsuccessfully with hyposensitization. Clin Exp Allergy. 1995;25:704–712. doi: 10.1111/j.1365-2222.1995.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 185.Siegmund R, Vogelsang H, Machnik A, Herrmann D. Surface membrane antigen alteration on blood basophils in patients with Hymenoptera venom allergy under immunotherapy. J Allergy Clin Immunol. 2000;106:1190–1195. doi: 10.1067/mai.2000.110928. [DOI] [PubMed] [Google Scholar]
- 186.Dugas-Breit S, Przybilla B, Dugas M, Arnold A, Pfundstein G, Küchenhoff H, Ruëff F. Serum concentration of baseline mast cell tryptase: evidence for a decline during long-term immunotherapy for Hymenoptera venom allergy. Clin Exp Allergy. 2010;40:643–649. doi: 10.1111/j.1365-2222.2009.03436.x. [DOI] [PubMed] [Google Scholar]
- 187.Nullens S, Sabato V, Faber M, Leysen J, Bridts CH, De Clerck LS, Falcone FH, Maurer M, Ebo DG. Basophilic histamine content and release during venom immunotherapy: insights by flow cytometry. Cytometry B Clin Cytom. 2013;84:173–178. doi: 10.1002/cyto.b.21084. [DOI] [PubMed] [Google Scholar]
- 188.Erdmann SM, Sachs B, Kwiecien R, Moll-Slodowy S, Sauer I, Merk HF. The basophil activation test in wasp venom allergy: sensitivity, specificity and monitoring specific immunotherapy. Allergy. 2004;59:1102–1109. doi: 10.1111/j.1398-9995.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- 189.Mamessier E, Birnbaum J, Dupuy P, Vervloet D, Magnan A. Ultra-rush venom immunotherapy induces differential T cell activation and regulatory patterns according to the severity of allergy. Clin Exp Allergy. 2006;36:704–713. doi: 10.1111/j.1365-2222.2006.02487.x. [DOI] [PubMed] [Google Scholar]
- 190.Schuerwegh AJ, De Clerck LS, Bridts CH, Stevens WJ. Wasp venom immunotherapy induces a shift from IL-4-producing towards interferon-gamma-producing CD4+ and CD8+ T lymphocytes. Clin Exp Allergy. 2001;31:740–746. doi: 10.1046/j.1365-2222.2001.01066.x. [DOI] [PubMed] [Google Scholar]
- 191.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J. 2015;8:17. doi: 10.1186/s40413-015-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pereira-Santos MC, Baptista AP, Melo A, Alves RR, Soares RS, Pedro E, Pereira-Barbosa M, Victorino RM, Sousa AE. Expansion of circulating Foxp3+)D25bright CD4+ T cells during specific venom immunotherapy. Clin Exp Allergy. 2008;38:291–297. doi: 10.1111/j.1365-2222.2007.02887.x. [DOI] [PubMed] [Google Scholar]
- 193.Meiler F, Zumkehr J, Klunker S, Rückert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205:2887–2898. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bussmann C, Xia J, Allam JP, Maintz L, Bieber T, Novak N. Early markers for protective mechanisms during rush venom immunotherapy. Allergy. 2010;65:1558–1565. doi: 10.1111/j.1398-9995.2010.02430.x. [DOI] [PubMed] [Google Scholar]
- 195.Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Foschi FG, Emiliani F, Savini S, Quercia O, Stefanini GF. CD30 serum levels and response to hymenoptera venom immunotherapy. J Investig Allergol Clin Immunol. 2008;18:279–283. [PubMed] [Google Scholar]
- 197.Packi K, Matysiak J, Matuszewska E, Bręborowicz A, Kycler Z, Matysiak J. New biomarkers of Hymenoptera venom allergy in a group of inflammation factors. Int J Environ Res Public Health. 2021;18:4011. doi: 10.3390/ijerph18084011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Navas A, Ruiz-Leon B, Serrano P, Martí M, Espinazo ML, Blanco N, Molina J, Alonso C, Jurado A, Moreno-Aguilar C. Natural and induced tolerance to Hymenoptera venom: a single mechanism? Toxins (Basel) 2022;14:426. doi: 10.3390/toxins14070426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Müller U, Helbling A, Bischof M. Predictive value of venom-specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy. 1989;44:412–418. doi: 10.1111/j.1398-9995.1989.tb04172.x. [DOI] [PubMed] [Google Scholar]
- 200.Kemeny DM, Lessof MH, Patel S, Youlten LJ, Williams A, Lambourn E. IgG and IgE antibodies after immunotherapy with bee and wasp venom. Int Arch Allergy Appl Immunol. 1989;88:247–249. doi: 10.1159/000234799. [DOI] [PubMed] [Google Scholar]
- 201.Michils A, Baldassarre S, Ledent C, Mairesse M, Gossart B, Duchateau J. Early effect of ultrarush venom immunotherapy on the IgG antibody response. Allergy. 2000;55:455–462. doi: 10.1034/j.1398-9995.2000.00412.x. [DOI] [PubMed] [Google Scholar]
- 202.Varga EM, Kausar F, Aberer W, Zach M, Eber E, Durham SR, Shamji MH. Tolerant beekeepers display venom-specific functional IgG4 antibodies in the absence of specific IgE. J Allergy Clin Immunol. 2013;131:1419–1421. doi: 10.1016/j.jaci.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 203.Varga EM, Francis JN, Zach MS, Klunker S, Aberer W, Durham SR. Time course of serum inhibitory activity for facilitated allergen-IgE binding during bee venom immunotherapy in children. Clin Exp Allergy. 2009;39:1353–1357. doi: 10.1111/j.1365-2222.2009.03303.x. [DOI] [PubMed] [Google Scholar]
- 204.Frick M, Fischer J, Helbling A, Ruëff F, Wieczorek D, Ollert M, Pfützner W, Müller S, Huss-Marp J, Dorn B, Biedermann T, Lidholm J, Ruecker G, Bantleon F, Miehe M, Spillner E, Jakob T. Predominant Api m 10 sensitization as risk factor for treatment failure in honey bee venom immunotherapy. J Allergy Clin Immunol. 2016;138:1663–71.e9. doi: 10.1016/j.jaci.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 205.Keles S, Karakoc-Aydiner E, Ozen A, Izgi AG, Tevetoglu A, Akkoc T, Bahceciler NN, Barlan I. A novel approach in allergen-specific immunotherapy: combination of sublingual and subcutaneous routes. J Allergy Clin Immunol. 2011;128:808–815.e7. doi: 10.1016/j.jaci.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 206.Zielen S, Kardos P, Madonini E. Steroid-sparing effects with allergen-specific immunotherapy in children with asthma: a randomized controlled trial. J Allergy Clin Immunol. 2010;126:942–949. doi: 10.1016/j.jaci.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 207.Bousquet J, Scheinmann P, Guinnepain MT, Perrin-Fayolle M, Sauvaget J, Tonnel AB, Pauli G, Caillaud D, Dubost R, Leynadier F, Vervloet D, Herman D, Galvain S, André C. Sublingual-swallow immunotherapy (SLIT) in patients with asthma due to house-dust mites: a double-blind, placebo-controlled study. Allergy. 1999;54:249–260. doi: 10.1034/j.1398-9995.1999.00916.x. [DOI] [PubMed] [Google Scholar]
- 208.Pham-Thi N, Scheinmann P, Fadel R, Combebias A, Andre C. Assessment of sublingual immunotherapy efficacy in children with house dust mite-induced allergic asthma optimally controlled by pharmacologic treatment and mite-avoidance measures. Pediatr Allergy Immunol. 2007;18:47–57. doi: 10.1111/j.1399-3038.2006.00475.x. [DOI] [PubMed] [Google Scholar]
- 209.Karakoc GB, Yilmaz M, Altıntaş DU, Kendirli SG. Can serum-specific IgE/total IgE ratio predict clinical response to allergen-specific immunotherapy in children monosensitized to house dust mite? J Allergy (Cairo) 2012;2012:694094. doi: 10.1155/2012/694094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Eifan AO, Akkoc T, Yildiz A, Keles S, Ozdemir C, Bahceciler NN, Barlan IB. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy. 2010;40:922–932. doi: 10.1111/j.1365-2222.2009.03448.x. [DOI] [PubMed] [Google Scholar]
- 211.Virchow JC, Backer V, Kuna P, Prieto L, Nolte H, Villesen HH, Ljørring C, Riis B, de Blay F. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715–1725. doi: 10.1001/jama.2016.3964. [DOI] [PubMed] [Google Scholar]
- 212.Xian M, Feng M, Dong Y, Wei N, Su Q, Li J. Changes in CD4+CD25+FoxP3+ Regulatory T cells and serum cytokines in sublingual and subcutaneous immunotherapy in allergic rhinitis with or without asthma. Int Arch Allergy Immunol. 2020;181:71–80. doi: 10.1159/000503143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Savilahti EM, Kuitunen M, Savilahti E, Mäkelä MJ. Specific antibodies in oral immunotherapy for cow’s milk allergy: kinetics and prediction of clinical outcome. Int Arch Allergy Immunol. 2014;164:32–39. doi: 10.1159/000361023. [DOI] [PubMed] [Google Scholar]
- 214.Ruiz-Leon B, Navas A, Serrano P, Espinazo M, Guler I, Alonso C, Jurado A, Moreno-Aguilar C. Helios negative regulatory T-cells as a key factor of immune tolerance in non-allergic beekeepers. J Investig Allergol Clin Immunol. 2021 Jul 01; doi: 10.18176/jiaci.0722. Epub ahead of print. [DOI] [PubMed] [Google Scholar]