Abstract
The use of chemical fertilizers and pesticides led to a decline in the quality and yield of Bupleurum chinense. The aim of this study was to determine the effects of Trichoderma harzianum biofertilizer on the growth, yield, and quality of radix bupleuri and microbial responses. The results showed that T. harzianum biofertilizer promoted the growth of B. chinense and increased the yield and quality of radix bupleuri. In addition, it increased the contents of NH4 +–N, NO3 −–N, available K, and available P and increased the activities of sucrase and catalase in the rhizosphere soil. High‐throughput analysis showed that the dominant bacteria in the rhizosphere were Proteobacteria (28%), Acidobacteria (23%), and Actinobacteria (17%), whereas the dominant fungi were Ascomycota (49%), Zygomycota (30%), and Basidiomycota (6%). After the application of T. harzianum biofertilizer, the abundance of Proteobacteria and Actinobacteria (relative to total bacteria) and Ascomycota and Basidiomycota (relative to total fungi) increased, but the relative abundance of Acidobacteria decreased. Canonical correlation analysis (CCA) showed that the relative abundance of Pseudarthrobacter, Streptomyces, Rhizobium, Nocardioides, Minimedusa, and Chaetomium were positively correlated with NO3 −–N, NH4 +–N, available K, available P, sucrase, and catalase in microbial communities, whereas Aeromicrobium and Mortierella were positively correlated with soil organic matter and urease. These results suggest that T. harzianum biofertilizer could significantly improve the yield and quality of radix bupleuri by changing the structure of soil microbial flora and soil enzyme activity. Therefore, it could be recommended for commercial scale production of Bupleurum.
Keywords: Bupleurum chinense, enzyme activity, high‐throughput sequencing, quality, Trichoderma harzianum biofertilizer
1. INTRODUCTION
Radix bupleuri is a commonly used Traditional Chinese medicine with many important properties such as antidepressant, antitumor, anti‐inflammatory, and immunomodulatory activities (Yang et al., 2017). The cultivation of Bupleurum chinense in China has a long history, and the demand for radix bupleuri has increased rapidly during the last decade, especially after the new coronavirus outbreak (Zhong et al., 2020). However, the production of Bupleurum in China is still restricted by many factors, such as long‐term excessive application of chemical fertilizers and pesticides and monoculture, which has caused soil acidification, salinization, nutrient imbalance, and degradation of other soil properties together with reduced crop yield and quality (Yan et al., 2018; Zhang, 2020).
Therefore, the amounts of chemical fertilizers should be appropriately reduced to improve the physical and chemical properties of soil as well as eliminate environmental pollution (Wang et al., 2020). In recent years, as society has paid more attention to protection of the agricultural environment, the research on replacing chemical fertilizers with biofertilizers has attracted widespread attention (Patel & Minocheherhomji, 2018).
Biofertilizer contains living microorganisms that can improve soil nutrients and physical and chemical properties, promote plant growth, and control pathogens (Kumar, 2016; Ren et al., 2020). Biofertilizers are considered to be a promising and nontoxic alternative to synthetic agrochemicals, making biofertilizers indispensable in improving the soil environment and plant growth (Suhag, 2016).
Trichoderma species are effective biological control agents able to inhibit soilborne pathogens and promote plant growth (Carro‐Huerga et al., 2020; Sallam et al., 2019). Trichoderma species directly inhibit the growth of pathogenic bacteria by producing various secondary metabolites (Mironenka et al., 2021). On the other hand, they indirectly improve plant disease resistance and promote plant growth by changing soil microbial community structure and increasing the number of beneficial soil microorganisms (Zafra & Cortés‐Espinosa, 2015). Therefore, Trichoderma strains have become a research focus for biological control in recent years. Trichoderma harzianum is one of the most commonly used biocontrol strains, which can antagonize pathogens and promote plant growth (Rubio et al., 2017). Poveda et al. (2019) found that the simultaneous application of T. harzianum strain and arbuscular mycorrhizal fungi inoculum significantly increased the colonization rate in the roots of arabidopsis and rapeseed and increased the yield of these two plants. The application of T. harzianum strain to soil from continuous cucumber culture effectively controlled cucumber Fusarium wilt, enriched soil microbial community, and restored soil biological functions (Chen et al., 2012).
However, to our knowledge, very little is known on the effects of T. harzianum on the soil environment and plant growth during the cultivation of B. chinense. In this study, the effects of T. harzianum biofertilizer on the growth, yield, quality of radix bupleuri, and microbial responses were investigated, which provided essential information for fertilizer management and sustainable development.
2. MATERIALS AND METHODS
2.1. Materials and reagents
T. harzianum biofertilizer was purchased from Shandong Tai'an Nong Bole Biological Fertilizer Company (China). It was composed of T. harzianum T22 strain and organic additives. The biofertilizer contained ≥109 CFU g−1. Organic fertilizer was provided by Shandong Kaoshan Biotechnology Company (China).
Saikosaponins A (P15S11F124709), saikosaponins C (M12O11S126866), and saikosaponins D (P15O10F100354) (with a purity of >98%) were purchased from Shanghai Yuanye Biotechnology (China). Other chromatographic grade reagents were purchased from Sinophenol Reagent Company (China).
2.2. Field experiment design
The experimental work was conducted at Zhangqiu Planting Base in Shandong province from June 2018 to October 2020 (117°22′54″ E 36°35′27″ N, altitude 524 m), where B. chinense was cultivated for 2 years. Plant and row spacings were 5 and 10 cm, respectively. The predominant soil in this area was brown clayey soil.
Three treatments were tested: A, T. harzianum biofertilizer; B, organic fertilizers; and C, control without fertilizer. Each treatment consisted of three replicate blocks. The area of each block was 30 m2. The fertilizer rate and application method were selected based on the manufacturers' instructions. Organic and biofertilizer were applied in the early and full blooming periods of B. chinense. Each fertilizer was applied weekly (four times). The first fertilization was conducted on 13th of July 2020. Samples of medicinal materials were collected on 15th of October 2020.
2.3. Collection of soil samples
The five‐point mixing method was used to collect and process B. chinense radix and the corresponding rhizosphere soil in different fertilization treatments (He et al., 2020). The rhizosphere soil samples were collected into sterile bags, cooled in a portable refrigerator, and brought back to the laboratory where they were screened (2 mm) and stored at −20°C for microbial and soil analyses.
2.4. Growth parameters and yield
At harvest, 10 healthy B. chinense plants were randomly collected from each treatment and used to determine growth index by measuring radix/stem length and diameter. Radix and whole plant weights were determined by an electronic balance. The results were expressed as means ± standard deviation. The average value of bupleurum radix from five quadrats was taken as the yield per treatment, which was then scaled up to yield per hectare.
2.5. Quality analysis
The radices of 10 plants were collected from each treatment, dried at 50°C, mixed, ground with a powder beater, and passed through an 80‐mesh sieve. The contents of saponins A, saponins C, and saponins D in B. chinense were determined by HPLC (National Pharmacopoeia Commission et al., 2020).
2.6. Soil physicochemical properties
After air‐drying, the contents of soil organic matter (SOM), available phosphorus (Ava‐P), and available potassium (Ava‐K) were determined as described by Qu et al. (2019). The contents of NO3 −–N and NH4 +–N in soil were determined as described by Dang et al. (2021).
2.7. Soil enzyme activities
The activities of sucrase, urease, and catalase were determined by using colorimetric method, sodium phenol–sodium hypochlorite colorimetric method, and potassium permanganate titration method, respectively (Guan et al., 1986; Hu et al., 2014; Huang et al., 2016).
2.8. DNA extraction, PCR amplification, and Illumina MiSeq sequencing
Three replicates (0.5 g each) of soil samples were extracted using a Soil DNA Kit (Mo Bio Laboratories, Carlsbad, CA, USA). The V4–V5 region of soil bacteria 16S rDNA and soil fungi ITS1 were amplified by PCR(94°C for 2 min, followed by 22 cycles at 94°C for 30 s, 55°C for 30 s, 72°C for 45 s, and a final extension at 72°C for 10 min), using the primers 515F 5′‐GTGCCAGCMGCCGCGGTAA‐3′, 926R 5′‐CCGTCAATTCMTTTGAGTTT‐3′, ITS1F 5′‐CTTGGTCATTTAGAGGAAGTA, A‐3′, and ITS1R 5′‐GCTGCGTTCTTCATCGATGC‐3′. The target bands were detected by running the PCR products on 2% agarose gels and recovered by a QIAamp DNA Micro Kit (Qiagen, Valencia, CA, USA). Finally, paired‐end sequencing of the amplicon library was performed on an Illumina MiSeq platform (TinyGene Bio‐Tech Co., Ltd., China), according to the standard protocol.
2.9. Processing and analysis of the sequencing data
The raw fastq files were demultiplexed based on the barcodes. Paired‐end reads for all samples were run through Trimmomatic (Version 0.35) to remove the low‐quality base pairs. Flash (Version 1.2.11) software was used to splice the paired reads into a sequence. Mothur soft (Version 1.33.3) was used to control the sequence quality to obtain the optimized sequences. Then, UPARSE software (usearch Version V8.1.1756, http://drive5.com/uparse/) was used for OTU (the operational taxonomic unit) clustering. Based on taxonomic information, statistical analysis of community structure was carried out at the phylum and genus levels.
Diversity analyses were performed using Mothur soft (Version 1.33.3). On the basis of the above analyses, Venn, NMDS, community structure histograms, canonical correlation analysis, and LDA effect size were calculated and visualized by R software (Version 3.6.0).
3. RESULTS
3.1. Effect of T. harzianum biofertilizer on plant growth
The results showed that application of T. harzianum biofertilizer and organic fertilizer both improved the growth and yield of B. chinense (Table 1). However, compared with the organic fertilizer, T. harzianum biofertilizer significantly increased the stem length and thickness, root thickness and whole plant weight.
TABLE 1.
Growth parameters and yield of Bupleurum chinense under different fertilization treatments
| Treatment | Stem length/cm | Root length/cm | Whole plant weight/g | Root weight/g | Stem diameter/cm | Root diameter/cm | Yield/kg |
|---|---|---|---|---|---|---|---|
| A | 100.38 ± 9.10a | 12.21 ± 2.48a | 8.66 ± 3.86a | 1.07 ± .40a | 1.34 ± .34a | 1.01 ± .22a | .27 ± .07a |
| B | 90.08 ± 11.84b | 12.94 ± 3.29a | 5.49 ± 3.049b | 1.05 ± .56a | 1.15 ± .32b | .76 ± .20b | .25 ± .03a |
| C | 83.29 ± 8.30c | 11.34 ± 2.54a | 3.82 ± 2.37c | .79 ± .46b | 1.10 ± .23b | .73 ± 0.18b | .17 ± .04b |
Notes: A, Trichoderma harzianum biofertilizer. B, Organic fertilizer. C, Control. Different lowercase letters in a column indicate significant differences among treatments (P < .05, n = 30).
3.2. Effect of T. harzianum biofertilizer on main active components
Compared with the control, T. harzianum biofertilizer significantly increased the contents of saikosaponins A, C, and D in radix bupleuri. However, in the treatment with organic fertilizer the content of saikosaponins A and D were significantly increased (Figure 1).
FIGURE 1.
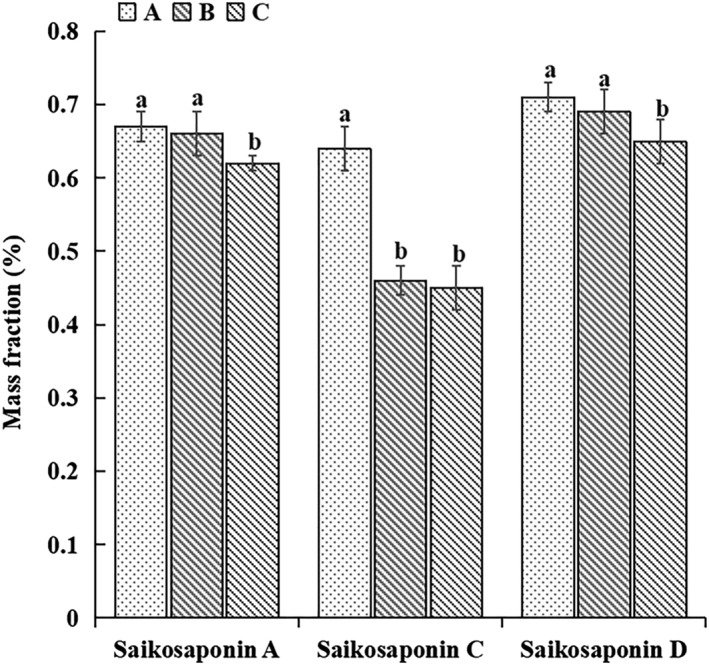
Determination of saikosaponins A, saikosaponins C, and saikosaponins D in Bupleurum chinense samples. A, Trichoderma harzianum biofertilizer. B, organic fertilizer. C, control. Different lowercase letters indicate significant differences among treatments (P < .05, n = 3).
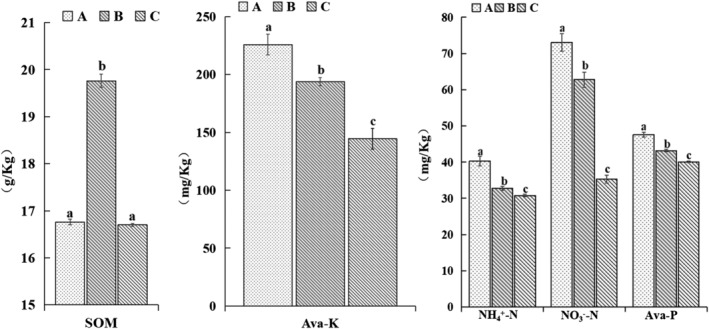
3.3. Effect of T. harzianum biofertilizer on soil physicochemical properties
Compared with the control, the contents of NH4 +–N, NO3 −–N, Ava‐K, and Ava‐P, but not the soil organic matter content, significantly increased after application of T. harzianum biofertilizer (Figure 2).
FIGURE 2.
Effects of different fertilization treatments on physicochemical properties of the Bupleurum chinense rhizosphere soil. Different lowercase letters indicate significant differences among treatments (P < .05, n = 3). Ava‐K, available potassium; Ava‐P, available phosphorus; SOM, soil organic matter.
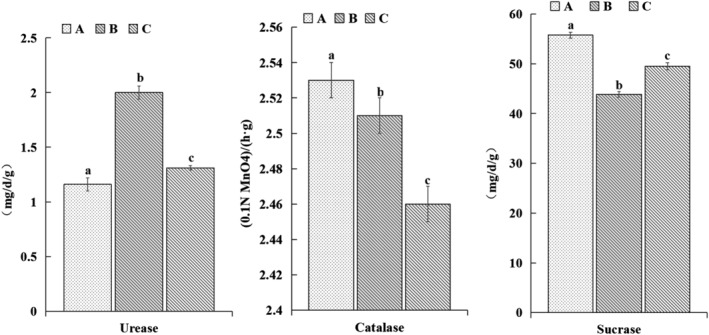
3.4. Effect of T. harzianum biofertilizer on soil enzyme activities
Compared with the control, the activities of sucrase and catalase significantly increased, but the activity of urease significantly decreased after applying T. harzianum biofertilizer. After application of the organic fertilizer, the activities of urease and catalase increased, but the activity of sucrase decreased significantly (Figure 3).
FIGURE 3.
Effects of different fertilization treatments on the activity of enzymes in the Bupleurum chinense rhizosphere soil. Different lowercase letters indicate significant differences among treatments (P < .05, n = 3).
3.5. Analysis of sequencing data
The dilution curves of rhizosphere soil samples are shown in Figure S1. The number of OTUs increased sharply and then gradually leveled off, indicating that the sequencing library has reached saturation. Therefore, the sequencing results can be used for subsequent analysis.
Through classification analysis, the common or unique OTUs of each sample were determined with 97% sequence identity and were displayed in the Venn diagram (Figure 4). The number of soil bacteria‐specific OTUs to which T. harzianum biofertilizer and organic fertilizer were applied increased significantly (Figure 4). Similarly, the number of soil fungi‐specific OTUs with T. harzianum biofertilizer showed a similar trend. However, the number of OTUs specific to soil fungi decreased significantly after applying the organic fertilizer.
FIGURE 4.
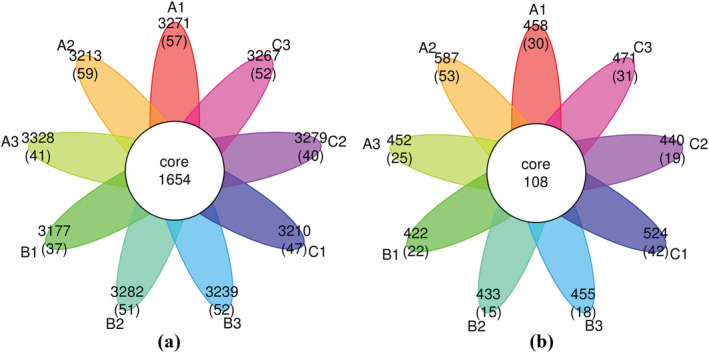
Venn diagram of the similarity and overlap of microbiome operational taxonomic unit (OTU) from different fertilization regimes. (a) Bacteria, (b) fungi
3.6. Alpha diversity of bacterial and fungal communities
The microbial richness (Ace) and community diversity (Shannon and Simpson indices) were measured in different fertilization regimes. The results (Table 2) showed that the bacterial richness in the rhizosphere soil of B. chinense decreased after applying T. harzianum biofertilizer and organic fertilizer. Bacterial diversity showed an upward trend after application of T. harzianum biofertilizer and organic fertilizer. After application of T. harzianum biofertilizer, the abundance of fungi increased, but the diversity decreased. However, the abundance and diversity of fungi decreased after application of organic fertilizer.
TABLE 2.
α‐Diversity index of microorganisms in the rhizosphere soil of Bupleurum chinense under different fertilization treatments
| Treatment | Bacteria | Fungi | ||||
|---|---|---|---|---|---|---|
| Ace | Shannon | Simpson | Ace | Shannon | Simpson | |
| A | 3698.54 ± 58.69 | 6.51 ± .04 | .0045 ± .0002 | 544.88 ± 70.60 | 3.27 ± .45 | .10 ± .05 |
| B | 3686.55 ± 37.31 | 6.54 ± .03 | .0045 ± .0001 | 494.48 ± 18.58 | 3.07 ± .34 | .14 ± .04 |
| C | 3722.48 ± 70.72 | 6.51 ± .02 | .0048 ± .0001 | 519.40 ± 28.40 | 3.46 ± .46 | .09 ± .03 |
Notes: A, Trichoderma harzianum biofertilizer. B, Organic fertilizer. C, Control. Ace represents the abundance of bacteria and fungi. Shannon and Simpson indices represent the diversity of bacteria and fungi.
3.7. Beta diversity of bacterial and fungal communities
To further investigate the differences in microbial community structure, we performed NMDS analysis based on the weighted UniFrac distance (Figure 5). The NMDS results showed that the soil samples to which T. harzianum biofertilizer was applied were clearly separated from the soils of the other treatments. In the heat map, when the sample distance was greater than 0.2, the samples could be divided into three groups (Figure 6). The microbial community structure within groups was similar, but the differences among the groups were significant, indicating that fertilization regimes have a great influence on the microbial community composition in the rhizosphere soil of B. chinense.
FIGURE 5.
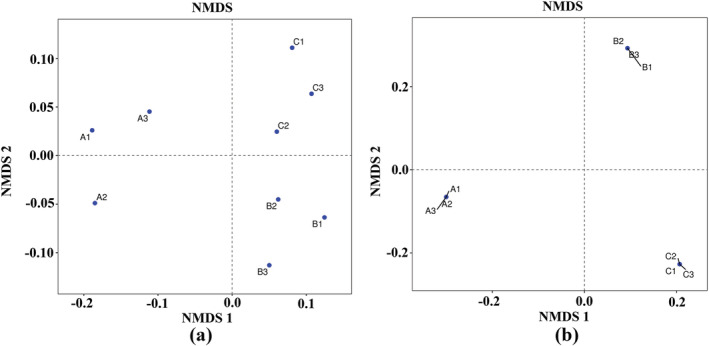
The non‐metric multidimensional scale (NMDS) map of the unweighted UniFrac distance represents the microbial community structure. (a) Bacteria, (b) fungi
FIGURE 6.
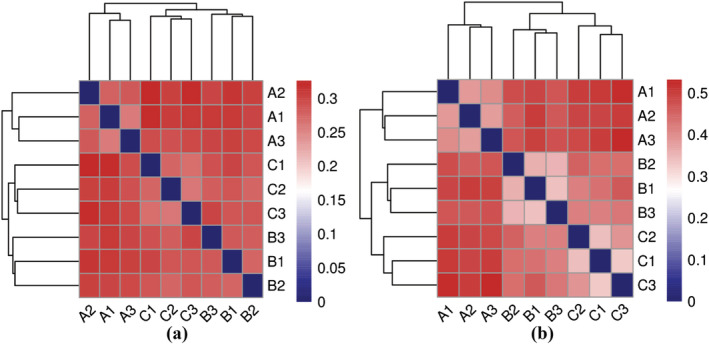
Based on the unweighted UniFrac distance, the heat map analysis of the microbiome community structure in the rhizosphere soil of Bupleurum chinense from different fertilization regimes. (a) Bacteria, (b) fungi
3.8. Composition and structure of the bacterial community
To clarify the microbial community structure in the B. chinense rhizosphere after fertilization, two taxonomic levels (phyla and genera) were analyzed. As shown in Figure 7a, 13 bacterial phyla were detected in the soil from different fertilization treatments. The dominant bacterial phyla in the B. chinense rhizosphere were Proteobacteria (28%), followed by Acidobacteria (23%) and Actinobacteria (17%). The relative abundance of Proteobacteria and Actinobacteria increased after application of T. harzianum biofertilizer. The relative abundance of Acidobacteria decreased after organic fertilizer addition but increased when no fertilization was applied.
FIGURE 7.
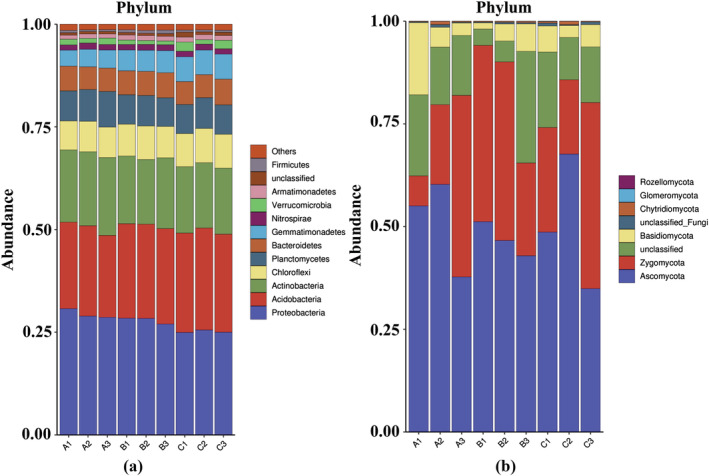
Relative abundance of microbial phyla under different fertilization regimes. (a) Bacteria, (b) fungi
Thirty bacterial genera (Figure 8a) were detected in the soil from different fertilization treatments. The dominant genera were Pseudarthrobacter (2%), Streptomyces (2%), Rhizobium (1%), Nocardioides (1%), Aeromicrobium (1%), and Pirellula (1%). The relative abundance of other dominant genera (except Aeromicrobium and Opitutus) increased after application of T. harzianum biofertilizer. The relative abundance of Aeromicrobium increased only after application of organic fertilizer, whereas the relative abundance of Opitutus increased only after application of T. harzianum biofertilizer.
FIGURE 8.
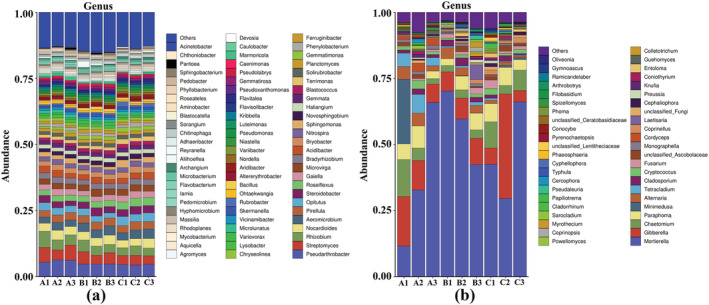
Relative abundance of microbial genera with significant differences under different fertilization regimes. (a) Bacteria, (b) fungi
3.9. Composition and structure of the fungal community
As shown in Figure 7b, eight fungal phyla were detected in the soil samples from different fertilization treatments. The dominant fungal phyla were Ascomycota (49%), Zygomycota (30%), and Basidiomycota (6%). The relative abundance of Ascomycota and Basidiomycota increased after application of T. harzianum biofertilizer, whereas the relative abundance of Ascomycota and Basidiomycota decreased after application of organic manure. The relative abundance of Zygomycota increased after application of the organic fertilizer but decreased after application of T. harzianum biofertilizer.
Thirty‐three fungal genera (Figure 8b) were detected in the soil from different fertilization treatments. The dominant fungal genera in the rhizosphere were Mortierella (30%), Gibberella (8%), Chaetomium (3%), Paraphoma (3%), Minimedusa (2%), Alternaria (2%), Tetracladium (1%), and Cladosporium (1%). Among them, after the application of T. harzianum biofertilizer, the relative abundance of Gibberella, Chaetomium, and Paraphoma decreased, but that of Alternaria, Tetracladium, and Cladosporium increased. Other fungi showed different responses to fertilization treatments. For example, the relative abundance of Mortierella increased when the organic fertilizer was applied but decreased in response to T. harzianum biofertilizer. The relative abundance of Minimedusa increased significantly when T. harzianum biofertilizer was applied but decreased significantly in the organic fertilizer treatment.
3.10. Correlation analysis of dominant bacteria and soil properties
To study the correlation between the B. chinense rhizosphere microorganisms, soil nutrients, and enzyme activities under different fertilization treatments, we used canonical correlation analysis (CCA). As shown in (Figure 9), the correlations between rhizosphere microorganisms and soil nutrients and enzyme activities were different under different fertilization treatments. The relative abundance of Pseudarthrobacter, Streptomyces, Rhizobium, and Nocardioides was positively correlated with NO3 −–N, NH4 +–N, Ava‐K, Ava‐P, sucrase, and catalase, whereas the relative abundance of Aeromicrobium was positively correlated with SOM and urease. For fungi, the relative abundance of Minimedusa and Chaetomium were positively correlated with NO3 −–N, NH4 +–N, Ava‐K, Ava‐P, sucrase, and catalase, whereas that of Mortierella was positively correlated with SOM and urease. After the application of T. harzianum biofertilizer, NO3 −–N, NH4 +–N, Ava‐K, Ava‐P, sucrase, and catalase had a large influence on the structure of rhizosphere microbial communities, whereas after the application of organic fertilizer, the most influential factories were SOM and urease.
FIGURE 9.
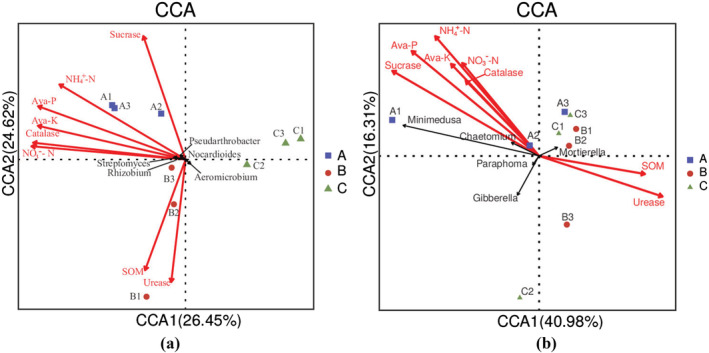
Canonical correlation analysis (CCA) of soil chemical properties and dominant bacterial (a) and fungal genera (b)
3.11. Biomarker analysis
To identify the dominant microbial biomarkers in the rhizosphere soil of B. chinense under different fertilization treatments, LDA effect size (LEfSe) was used for analysis (Figure 10). The LDA results identified 42, 26, and 25 bacterial biomarkers in the T. harzianum biofertilizer, organic manure, and the unfertilized treatments, respectively (Figure 10a). Alpha‐Proteobacteria and Proteobacteria were the most prevailing types of bacteria in the soil in the T. harzianum biofertilizer treatment. Burkholderiales and Comamonadaceae were abundant in the rhizosphere in the organic fertilizer treatment, and Acidobacteria were significantly abundant in the rhizosphere in the control without fertilization.
FIGURE 10.
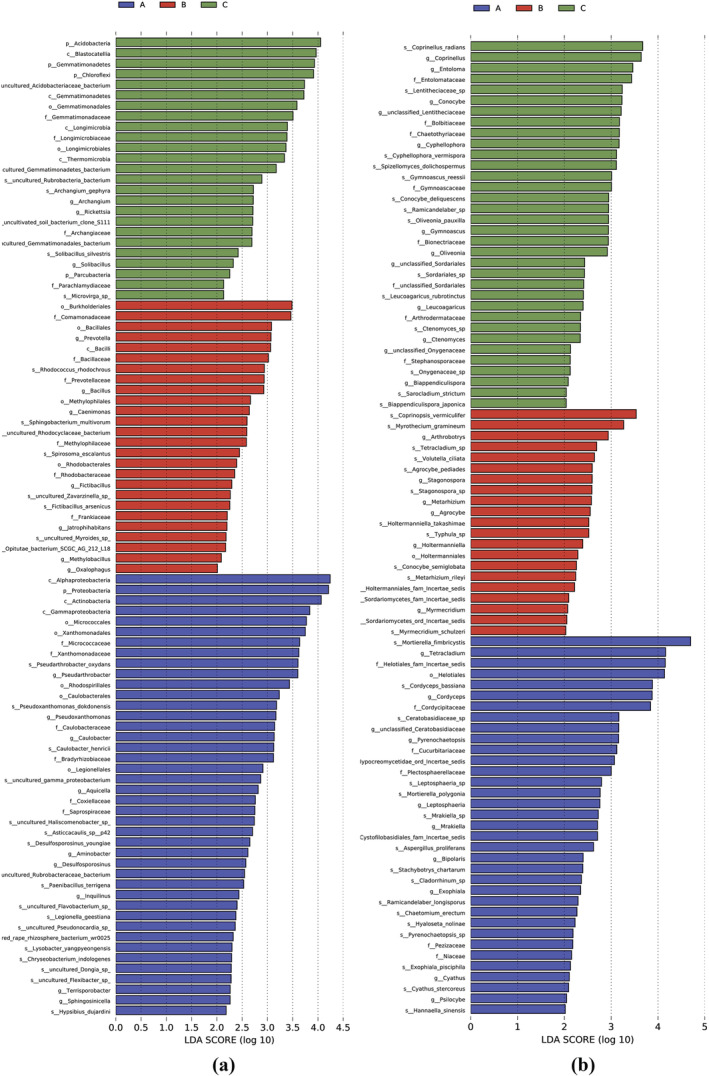
LDA effect size analysis of microbial community differences in the Bupleurum chinense rhizosphere soil in different fertilization treatments. (a) Bacteria, (b) fungi
In the fungal community, 35, 20, and 34 fungal biomarkers were identified in the T. harzianum biofertilizer, organic fertilizer, and unfertilized treatments, respectively (Figure 10b). The relatively abundant biomarker fungal groups included Mortierella fimbricystis and Pleosporales in the T. harzianum biofertilizer treatment; Coprinopsis vermiculifer, Myrothecium gramineum, Mortierella, and Zygomycota in the organic fertilizer; and Coprinellus radians and Coprinellus sp. in the unfertilized control.
4. DISCUSSION
Reducing the use of chemical fertilizers and pesticides without loss of productivity is feasible, but there are huge challenges. Numerous studies have shown that application of biofertilizers can effectively reduce the use of chemical fertilizers and pesticides. Jia et al. (2020) found that biofertilizers can replace 25% of chemical fertilizers without a detectable impact on the yield and quality of Chinese cabbage. Other studies have shown that inoculation with T. harzianum strains significantly increased the biomass and nutrient uptake of tomato seedlings grown in low‐nutrient soils (Li et al., 2015). Our study showed that application of T. harzianum biofertilizer increased the yield of B. chinense. However, application of organic fertilizer alone reduced the yield of B. chinense, which is consistent with the results of Ye et al. (2020).
The biofertilizer industry is developing rapidly in China. Biofertilizers are widely recognized by most crop growers for their ability to improve crop growth parameters, increase yield, and control soilborne diseases (Zhou et al., 2020). Our study showed that T. harzianum biofertilizer could improve the growth parameters, quality, and yield of radix bupleuri, mainly due to improvement of soil fertility and abundance of beneficial bacteria (Wang et al., 2017).
Fertilization is one of the main factors affecting soil nutrient content and enzyme activity in the rhizosphere soil of B. chinense. The contents of available nutrients reflect the dynamic balance between soil mineralization and plant absorption (Ling et al., 2014). Soil enzyme activity can reflect the nutrient absorption, utilization, and growth of crops to a certain extent and is also an important index of soil fertility, soil quality, and soil health (Yu et al., 2019). This study showed that T. harzianum biofertilizer significantly improved soil available nutrient contents and sucrase and catalase activities, but did not increase the activities of urease and organic matter in soil. However, an appropriate amount of organic fertilizer can be used to increase the content of soil organic matter (Liu et al., 2021).
Soil microbial diversity, abundance, and community structure are related to the functions of microbial community and soil health (Ruiz Gómez et al., 2019). Microbial communities participate in soil functions including nutrient cycling, formation of soil aggregates, and suppression of soilborne diseases. This study showed that T. harzianum biofertilizer significantly improved the diversity of bacteria and the abundance of fungi. The relative abundance of beneficial bacteria increased significantly, which included Streptomyces (Heinsch et al., 2019), Rhizobium (Naamala et al., 2016), Steroidobacter (Alcaraz et al., 2018), and Gaiella (Lazcano et al., 2021). By contrast, the abundance of harmful fungi was significantly reduced, including Gibberella (Zhao et al., 2021), Paraphoma (Marin‐Felix et al., 2019), and Fusarium (Tong et al., 2021). The microbial changes were more obvious in soil bacteria than fungi, suggesting that soil bacterial communities are more sensitive to planting systems (Zhao et al., 2017).
The dominant bacterial groups in rhizosphere mainly included Proteobacteria, Acidobacteria, and Actinomycetes, most of which are involved in decomposition of organic matter. This study showed that the rhizosphere microbial community structure dominated by these bacterial groups was significantly correlated with the contents of soil available nutrients and the activities of urease, catalase, and invertase, indicating that the rhizosphere environment had an important effect on the bacterial and fungal community structures (Lauber et al., 2009).
5. CONCLUSION
This study demonstrated that T. harzianum biofertilizer promoted the growth of B. chinense, increased yield, improved its quality, and increased the contents of NH4 +–N, NO3 −–N, Ava‐K, and Ava‐P and the activities of sucrase and catalase in the rhizosphere soil. Moreover, these parameters showed positive correlation with the relative abundance of beneficial bacteria in soil, such as Pseudarthrobacter, Streptomyces, Rhizobia, and Nocardia. Therefore, T. harzianum biofertilizer can be used in Bupleurum production. Further research will be conducted to investigate the effects of T. harzianum biofertilizer on dynamics of various functional bacteria during bupleuri production.
CONFLICT OF INTEREST
The authors declare they have no competing interests.
AUTHOR CONTRIBUTIONS
Professor Gao Demin designed the experiments and revised the manuscript. Liu Li conducted field experiments, analyzed data, and wrote manuscripts. Professor Cao Hailu, Fan Ya, and Du Kan collected the rhizosphere soil of Bupleurum chinense. Professor Yuansong Xu and Bu Xun assisted in microbial sequencing. All authors read the final manuscript.
Supporting information
Figure S1 Rarefaction curves of Bupleurum chinense samples in different fertilization regimes. (A) Bacteria, (B) Fungi.
ACKNOWLEDGMENTS
This work was supported by grants from the National Science and Technology to Boost Economy 2020 (SQ2020YFF0426286 and SQ2020YFF0426541). We especially thank EditSprings (https://www.editsprings.cn/) for the expert linguistic services provided.
Liu, L. , Xu, Y. , Cao, H. , Fan, Y. , Du, K. , Bu, X. , & Gao, D. (2022). Effects of Trichoderma harzianum biofertilizer on growth, yield, and quality of Bupleurum chinense . Plant Direct, 6(11), e461. 10.1002/pld3.461
DATA AVAILABILITY STATEMENT
The data generated for this study are available in the NCBI Sequence Read Archive database under accession number SRP34470.
REFERENCES
- Alcaraz, L. D. , Peimbert, M. , Barajas, H. R. , Dorantes‐Acosta, A. E. , Bowman, J. L. , & Arteaga‐Vázquez, M. A. (2018). Marchantia liverworts as a proxy to plants' basal microbiomes. Scientific Reports, 8, 12712. 10.1038/s41598-018-31168-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro‐Huerga, G. , Compant, S. , Gorfer, M. , Cardoza, R. E. , Schmoll, M. , Gutiérrez, S. , & Casquero, P. A. (2020). Colonization of Vitis vinifera L. by the endophyte Trichoderma sp. strain T154: Biocontrol activity against Phaeoacremonium minimum . Frontiers in Plant Science, 11, 1170. 10.3389/fpls.2020.01170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. H. , Huang, X. Q. , Zhang, F. G. , Zhao, D. K. , Yang, X. M. , & Shen, Q. R. (2012). Application of Trichoderma harzianum SQR‐T037 bio‐organic fertiliser significantly controls Fusarium wilt and affects the microbial communities of continuously cropped soil of cucumber. Journal of the Science of Food and Agriculture, 92, 2465–2470. 10.1002/jsfa.5653 [DOI] [PubMed] [Google Scholar]
- Dang, H. , Zhang, T. , Wang, Z. , Li, G. , Zhao, W. , Lv, X. , & Zhuang, L. (2021). Differences in the endophytic fungal community and effective ingredients in root of three Glycyrrhiza species in Xinjiang, China. PeerJ, 9, e11047. 10.7717/peerj.11047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, S. Y. , Zhang, D. S. , & Zhang, Z. M. (1986). Soil enzymes and its methodology (pp. 274–338). Agricultural Press. [Google Scholar]
- He, R. , Zeng, J. , Zhao, D. , Huang, R. , Yu, Z. , & Wu, Q. L. (2020). Contrasting patterns in diversity and community assembly of Phragmites australis root‐associated bacterial communities from different seasons. Applied and Environmental Microbiology, 86, e00379–e00320. 10.1128/AEM.00379-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsch, S. C. , Hsu, S. Y. , Otto‐Hanson, L. , Kinkel, L. , & Smanski, M. J. (2019). Complete genome sequences of Streptomyces spp. isolated from disease‐suppressive soils. BMC Genomics, 201, 994. 10.1186/s12864-019-6279-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, B. , Liang, D. L. , Liu, J. J. , Lei, L. M. , & Yu, D. S. (2014). Transformation of heavy metal fractions on soil urease and nitrate reductase activities in copper and selenium cocontaminated soil. Ecotoxicology and Environmental Safety, 110, 41–48. 10.1016/j.ecoenv.2014.08.007 [DOI] [PubMed] [Google Scholar]
- Huang, D. , Xue, W. , Zeng, G. , Wan, J. , Chen, G. , Huang, C. , Zhang, C. , Cheng, M. , & Xu, P. (2016). Immobilization of Cd in river sediments by sodium alginate modified nanoscale zero‐valent iron: Impact on enzyme activities and microbial community diversity. Water Research, 106, 15–25. 10.1016/j.watres.2016.09.050 [DOI] [PubMed] [Google Scholar]
- Jia, Y. , Liao, Z. , Chew, H. , Wang, L. , Lin, B. , Chen, C. , Lu, G. , & Lin, Z. (2020). Effect of Pennisetum giganteum z.x.lin mixed nitrogen‐fixing bacterial fertilizer on the growth, quality, soil fertility and bacterial community of pakchoi (Brassica chinensis L.). PLoS ONE, 15, e0228709. 10.1371/journal.pone.0228709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A. (2016). Phosphate solubilizing bacteria in agriculture biotechnology: Diversity, mechanism and their role in plant growth and crop yield. International Journal of Advanced Research, 4, 116–124. 10.21474/IJAR01/111 [DOI] [Google Scholar]
- Lauber, C. L. , Hamady, M. , Knight, R. , & Fierer, N. (2009). Pyrosequencing‐based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Applied and Environmental Microbiology, 75, 5111–5120. 10.1128/AEM.00335-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazcano, C. , Boyd, E. , Holmes, G. , Hewavitharana, S. , Pasulka, A. , & Ivors, K. (2021). The rhizosphere microbiome plays a role in the resistance to soil‐borne pathogens and nutrient uptake of strawberry cultivars under field conditions. Scientific Reports, 11, 3188. 10.1038/s41598-021-82768-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. X. , Cai, F. , Pang, G. , Shen, Q. R. , Li, R. , & Chen, W. (2015). Solubilisation of phosphate and micronutrients by Trichoderma harzianum and its relationship with the promotion of tomato plant growth. PLoS ONE, 10, e0130081. 10.1371/journal.pone.0130081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, N. , Deng, K. , Song, Y. , Wu, Y. , Zhao, J. , Raza, W. , Huang, Q. , & Shen, Q. (2014). Variation of rhizosphere bacterial community in watermelon continuous mono‐cropping soil by long‐term application of a novel bioorganic fertilizer. Microbiological Research, 169, 570–578. 10.1016/j.micres.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Lv, Z. , Hou, H. , Lan, X. , Ji, J. , & Liu, X. (2021). Long‐term effects of combination of organic and inorganic fertilizer on soil properties and microorganisms in a quaternary red clay. PLoS ONE, 16(12), e0261387. 10.1371/journal.pone.0261387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin‐Felix, Y. , Hernández‐Restrepo, M. , Wingfield, M. J. , Akulov, A. , Carnegie, A. J. , Cheewangkoon, R. , Gramaje, D. , Groenewald, J. Z. , Guarnaccia, V. , Halleen, F. , Lombard, L. , Luangsa‐ard, J. , Marincowitz, S. , Moslemi, A. , Mostert, L. , Quaedvlieg, W. , Schumacher, R. K. , Spies, C. F. J. , Thangavel, R. , … Crous, P. W. (2019). Genera of phytopathogenic fungi: GOPHY 2. Studies in Mycology, 92, 47–133. 10.1016/j.simyco.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironenka, J. , Różalska, S. , Soboń, A. , & Bernat, P. (2021). Trichoderma harzianum metabolites disturb Fusarium culmorum metabolism: Metabolomic and proteomic studies. Microbiological Research, 249, 126770. 10.1016/j.micres.2021.126770 [DOI] [PubMed] [Google Scholar]
- Naamala, J. , Jaiswal, S. K. , & Dakora, F. D. (2016). Antibiotics resistance in Rhizobium: Type, process, mechanism and benefit for agriculture. Current Microbiology, 72, 804–816. 10.1007/s00284-016-1005-0 [DOI] [PubMed] [Google Scholar]
- National Pharmacopoeia Commission . (2020). Chinese pharmacopoeia. Chinese Medicine Science and Technology Press. pp. 293. [Google Scholar]
- Patel, T. , & Minocheherhomji, F. (2018). Plant growth promoting rhizobacteria: Blessing to agriculture. Journal of Pure & Applied Biosciences, 6, 481–492. 10.18782/2320-7051.6383 [DOI] [Google Scholar]
- Poveda, J. , Hermosa, R. , Monte, E. , & Nicolás, C. (2019). Trichoderma harzianum favours the access of arbuscular mycorrhizal fungi to non‐host Brassicaceae roots and increases plant productivity. Scientific Reports, 9, 11650. 10.1038/s41598-019-48269-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, B. , Liu, Y. , Sun, X. , Li, S. , Wang, X. , Xiong, K. , Yun, B. , & Zhang, H. (2019). Effect of various mulches on soil physico‐chemical properties and tree growth (Sophora japonica) in urban tree pits. PLoS ONE, 14, e0210777. 10.1371/journal.pone.021077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, H. , Qin, X. , Huang, B. , Fernández‐García, V. , & Lv, C. (2020). Responses of soil enzyme activities and plant growth in a eucalyptus seedling plantation amended with bacterial fertilizers. Archives of Microbiology, 202, 1381–1396. 10.1007/s00203-020-01849-4 [DOI] [PubMed] [Google Scholar]
- Rubio, M. B. , Pardal, A. J. , Cardoza, R. E. , Gutiérrez, S. , Monte, E. , & Hermosa, R. (2017). Involvement of the transcriptional coactivator ThMBF1 in the biocontrol activity of Trichoderma harzianum . Frontiers in Microbiology, 8, 2273. 10.3389/fmicb.2017.02273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz Gómez, F. J. , Navarro‐Cerrillo, R. M. , Pérez‐de‐Luque, A. , Oβwald, W. , Vannini, A. , & Morales‐Rodríguez, C. (2019). Assessment of functional and structural changes of soil fungal and oomycete communities in holm oak declined dehesas through metabarcoding analysis. Scientific Reports, 9, 5315. 10.1038/s41598-019-41804-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam, N. , Eraky, A. , & Sallam, A. (2019). Effect of Trichoderma spp. on Fusarium wilt disease of tomato. Molecular Biology Reports, 46, 4463–4470. 10.1007/s11033-019-04901-9 [DOI] [PubMed] [Google Scholar]
- Suhag, M. (2016). Potential of biofertilizers to replace chemical fertilizers. International Advance Research Journal of Science Engineering Technology, 3, 163–167. 10.17148/IARJSET.2016.3534 [DOI] [Google Scholar]
- Tong, A. Z. , Liu, W. , Liu, Q. , Xia, G. Q. , & Zhu, J. Y. (2021). Diversity and composition of the Panax ginseng rhizosphere microbiome in various cultivation modes and ages. BMC Microbiology, 21, 18. 10.1186/s12866-020-02081-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Li, J. , Yang, F. E. Y. , Raza, W. , Huang, Q. , & Shen, Q. (2017). Application of bioorganic fertilizer significantly increased apple yields and shaped bacterial community structure in orchard soil. Microbial Ecology, 73, 404–416. 10.1007/s00248-016-0849-y [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Geng, Y. , & Liang, T. (2020). Optimization of reduced chemical fertilizer use in tea gardens based on the assessment of related environmental and economic benefits. Science of the Total Environment, 713, 136439. 10.1016/j.scitotenv.2019.136439 [DOI] [PubMed] [Google Scholar]
- Yan, P. , Shen, C. , Fan, L. , Li, X. , Zhang, L. , Zhang, L. , & Han, W. (2018). Tea planting affects soil acidification and nitrogen and phosphorus distribution in soil. Agriculture, Ecosystems and Environment, 254, 20–25. 10.1016/j.agee.2017.11.015 [DOI] [Google Scholar]
- Yang, F. , Dong, X. , Yin, X. , Wang, W. , You, L. , & Ni, J. (2017). Radix Bupleuri: A review of traditional uses, botany, phytochemistry, pharmacology, and toxicology. BioMed Research International, 2017, 7597596. 10.1155/2017/7597596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, L. , Zhao, X. , Bao, E. , Li, J. , Zou, Z. , & Cao, K. (2020). Bio‐organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Scientific Reports, 10, 177. 10.1038/s41598-019-56954-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, P. , Tang, X. , Zhang, A. , Fan, G. , & Liu, S. (2019). Responses of soil specific enzyme activities to short‐term land use conversions in a salt‐affected region, northeastern China. Science Total Environment, 687, 939–945. 10.1016/j.scitotenv.2019.06.171 [DOI] [PubMed] [Google Scholar]
- Zafra, G. , & Cortés‐Espinosa, D. V. (2015). Biodegradation of polycyclic aromatic hydrocarbons by Trichoderma species: A mini review. Environmental Science and Pollution Research International, 22, 19426–19433. 10.1007/s11356-015-5602-4 [DOI] [PubMed] [Google Scholar]
- Zhang, A. M. (2020). Cultivation techniques, existing problems and solutions of bupleuri. Rural Science and Technology, 11, 97–98. 10.19345/j.cnki.1674-7909.2020.27.048 [DOI] [Google Scholar]
- Zhao, T. X. , Mao, X. W. , Cheng, M. , Chen, J. H. , Qin, H. , Li, Y. C. , Liang, C. F. , & Xu, Q. F. (2017). Effects of Phyllostachys edulis cultivation on soil bacterial and fungal community structure and diversity. Chinese Journal of Applied Ecology, 28, 3740–3750. 10.13287/j.1001-9332.201711.033 [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , Fu, W. , Hu, C. , Chen, G. , Xiao, Z. , Chen, Y. , Wang, Z. , & Cheng, H. (2021). Variation of rhizosphere microbial community in continuous mono‐maize seed production. Scientific Reports, 11, 1544. 10.1038/s41598-021-81228-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, L. , Lam, W. C. , Yang, W. , Chan, K. W. , Sze, S. , Miao, J. , Yung, K. , Bian, Z. , & Wong, V. T. (2020). Potential targets for treatment of coronavirus disease 2019 (COVID‐19): A review of Qing‐Fei‐Pai‐Du‐Tang and its major herbs. The American Journal of Chinese Medicine, 48(5), 1051–1071. 10.1142/S0192415X20500512 [DOI] [PubMed] [Google Scholar]
- Zhou, S. , Zeng, X. , Xu, Z. , Bai, Z. , Xu, S. , Jiang, C. , Zhuang, G. , & Xu, S. (2020). Paenibacillus polymyxa biofertilizer application in a tea plantation reduces soil N2O by changing denitrifier communities. Canadian Journal of Microbiology, 66, 214–227. 10.1139/cjm-2019-0511 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Rarefaction curves of Bupleurum chinense samples in different fertilization regimes. (A) Bacteria, (B) Fungi.
Data Availability Statement
The data generated for this study are available in the NCBI Sequence Read Archive database under accession number SRP34470.


