Abstract
Trypanosoma cruzi, a parasitic protozoan, is the etiological agent of Chagas’ disease. Despite the many immune system disorders recognized in this infection and the crucial role played by dendritic cells (DC) in acquired immune responses, it was not known whether these cells could be infected by T. cruzi trypomastigotes and the consequences of such an infection on their immune functions. We now provide evidence that human monocyte-derived DC can be infected by T. cruzi and can support its intracellular multiplication. Interestingly, this infection has functional consequences on immature DC and on their maturation induced by lipopolysaccharide (LPS). First, after T. cruzi infection, the basal synthesis of interleukin-12 (IL-12) and tumor necrosis factor alpha (TNF-α) was impaired. Furthermore, the process of maturation of DC induced by LPS was drastically affected by T. cruzi infection. Indeed, secretion of cytokines such as IL-12, TNF-α, and IL-6, which are released normally at high levels by LPS-activated DC, as well as the up-regulation of HLA-DR and CD40 molecules, was significantly reduced after this infection. The same effects could be induced by T. cruzi-conditioned medium, indicating that at least these inhibitory effects were mediated by soluble factors released by T. cruzi. Taken together, these results provide new insights into a novel efficient mechanism, directly involving the alteration of DC function, which might be used by T. cruzi to escape the host immune responses in Chagas’ disease and thus might favor persistent infection.
Trypanosoma cruzi, the etiological agent of Chagas’ disease, is a hemoflagellate protozoan parasite that infects humans as well as domestic and wild mammals (39). This parasite exists in two forms in the vertebrate host: the trypomastigote, which is the blood form and infects several cells (macrophages, fibroblasts, nerve cells, and muscle cells), and the amastigote, which replicates in the host cell cytosol (9). Cell invasion and intracellular replication are essential for the induction of the disease and the continuation of the parasite life cycle. In human infection, most patients survive the initial acute phase but some develop the chronic manifestations of the disease, characterized by long-lasting inflammatory lesions and immune system disorders, years later (14, 39). The use of experimental murine models has shown that host resistance to T. cruzi depends at least partially on natural killer (NK) cells, T cells, and macrophages, which produce various cytokines, such as interleukin-12 (IL-12), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α), involved in the control of the disease (1, 3, 11, 25, 37, 40). However, an efficient protective immunity is seldom or never achieved so that viable parasites and focal chronic inflammation can be detected in host tissue for life.
The role of dendritic cells (DC) in T. cruzi infection has never been investigated, despite their unique and essential function in the initiation of the acquired immune response (6). Immature DC, which reside in most tissues and organs, actively capture and process antigens (12). Upon activation by whole bacteria, the microbial cell wall component lipopolysaccharide (LPS), or cytokines such as IL-1β, granulocyte-macrophage colony-stimulating factor (GM-CSF), or TNF-α, they migrate to lymph nodes and the spleen, where they activate naive antigen-specific T cells. During this migration, they undergo a process of maturation, which is a crucial step in the development of DC into fully potent antigen-presenting cells (APC). During maturation, DC lose their ability to capture and process antigens, increase their expression of major histocompatibility complex (MHC) class II costimulatory (CD40, CD80, CD86) and adhesion (CD54) molecules, and up-regulate their production of cytokines such as IL-12. This cytokine plays a key role in the induction of cell-mediated immunity to intracellular pathogens by triggering the production of IFN-γ from NK and T cells (35). In the case of T. cruzi infection, this cytokine is required for both innate and acquired immunity (1, 24). Indeed, in murine models, the induction of IL-12 early in infection with T. cruzi initiates innate resistance which is dependent on IFN-γ and TNF-α (3, 25) while ensuring the induction of an efficient adaptive host response.
Accordingly, we investigated the relationship between DC and T. cruzi trypomastigotes by using DC obtained from human blood monocytes incubated with IL-4 and GM-CSF. We first assessed whether DC could be infected by T. cruzi, as is the case with several viruses (7, 21, 28) and bacteria (15, 22–24, 26, 33) and at least one parasitic protozoan, Leishmania (32). We next evaluated the influence of DC infection by T. cruzi on the capacity of these cells to secrete cytokines (IL-6, IL-8, IL-10, IL-12, and TNF-α) and to express MHC class II costimulatory and adhesion molecules both at the immature stage and upon maturation induced by LPS.
Finally, we tested whether the observed effects on human DC could be also attributed to soluble factors released by T. cruzi (termed T. cruzi-conditioned medium [TCM]).
MATERIALS AND METHODS
Culture medium and reagents.
The culture medium consisted of RPMI 1640 (Biowhittaker Europe, Verviers, Belgium) supplemented with l-glutamine (2 mM), gentamicin (20 μg/ml), 2 × 10−5 M 2-mercaptoethanol, 1% nonessential amino acids (GIBCO, Grand Island, N.Y.), and 10% fetal bovine serum (Biowhittaker). Recombinant IL-4 was kindly provided by Schering-Plough (Kenilworth, N.J.). Recombinant GM-CSF was obtained from Novartis (Basel, Switzerland). LPS (Escherichia coli 0128:B12), phosphate-buffered saline (PBS), and bovine serum albumin were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Human DC.
Human DC were generated from peripheral blood mononuclear cells as previously described (36). Briefly, peripheral blood mononuclear cells from healthy volunteers were isolated by density centrifugation of heparinized blood on Lymphoprep (Nycomed, Oslo, Norway), resuspended in culture medium, and allowed to adhere to culture flasks. After 2 h at 37°C, nonadherent cells were removed and adherent cells were cultured in medium containing GM-CSF (800 U/ml) and IL-4 (500 U/ml). Every 2 days, GM-CSF and IL-4 were added. After 7 days of culture, nonadherent cells corresponding, to the DC-enriched fraction, were harvested, washed, and used for subsequent experiments. As previously reported (8), the DC-enriched fraction obtained by this method routinely contains more than 95% DC as assessed by morphology and flow cytometry analysis.
T. cruzi trypomastigotes and TCM.
T. cruzi trypomastigotes (Tehuantepec strain, Mexico) were maintained by weekly intraperitoneal inoculations to BALB/c mice. To obtain large quantities of parasites, trypomastigotes (2.5 × 105 parasites/rat) were inoculated into F344 Fischer rats (Iffa Credo, Brussels, Belgium) irradiated with X rays (700 rads). Trypomastigotes were obtained from the blood (containing 10 U of heparin/ml) of infected rats by ion-exchange chromatography on DEAE-cellulose (Whatman DE 52) equilibrated with phosphate saline glucose buffer at pH 7.4 (30, 34). Trypomastigotes were centrifuged (15 min at 1,800 × g and 4°C) and resuspended in endotoxin-free PBS.
TCM was prepared by the method described by Kierszenbaum et al. (27) to obtain trypanosomal immunosuppressive factor. Briefly, suspensions of T. cruzi (2 × 107 trypomastigotes/ml in RPMI 1640 medium) were incubated at 37°C and 5% CO2 for 24 h. The parasites were then removed by filtration through a sterile 0.22-μm-pore-size filter (Millipore Corp., Bedford, Mass.). This TCM was aliquoted and stored at −20°C until used. When necessary, it was diluted in culture medium to obtain a final concentration of 12.5, 25, 50, or 75%.
Effects of T. cruzi infection and TCM on DC.
To assess whether T. cruzi infects human DC, 5 × 105 DC/ml were cultured in 24-well plates with trypomastigotes at parasite-to-cell ratios of 0.1:1, 0.5:1, 1:1, 2:1, 5:1, 10:1, and 20:1. After 24 h, the DC were washed to remove free parasites, fixed with methanol, and then stained with Giemsa stain. In some experiments, DC were not fixed but were further incubated for a total of 48 or 72 h to assess the intracellular multiplication of amastigotes. The percentage of infected DC and the mean number of amastigotes per infected DC were recorded after microscopic examination of at least 300 cells (34).
The effects of T. cruzi on cytokine production and surface molecule expression of DC were investigated by using DC incubated either with trypomastigotes (parasite-to-cell ratios of 5:1, 10:1, and 20:1) or with TCM (12.5 to 50% [vol/vol] of the culture medium) for 24 h. In some experiments, DC were cultured in the bottom of wells and separated by a cell-impermeable filter from trypomastigotes placed in a transwell (clear, 6.5-mm diameter, 0.4-μm pore size, 24-well size [Corning Costar Corp., Cambridge, Mass.]). Similar experiments were performed with DC incubated in medium containing 10 ng of LPS per ml together with trypomastigotes (parasite-to-cell ratios, 0.1:1, 0.5:1, 1:1, 2:1, 5:1, 10:1, 20:1, and 30:1) or TCM (3, 6.25, 12.5, 25, 50, and 75%).
Cytokine assays.
Culture supernatants of DC were harvested, and TNF-α, IL-6, IL-8, IL-10, and IL-12 (p40) levels were assayed with enzyme-linked immunosorbent assay (ELISA) kits from Biosource Europe (Nivelles, Belgium). The IL-12 (p40) assay detects both the heterodimeric (p70) and homodimeric (p40-p40) forms of human IL-12. IL-12 (p70) production was measured with an ELISA kit provided by Genzyme (Leuven, Belgium). The absorbances were measured in a microplate ELISA reader (Spectracount Microplate Photometer; Packard, Meriden, Conn.).
Flow cytometry analysis.
For immunophenotyping, 105 DC were harvested and washed in PBS supplemented with 0.5% bovine serum albumin and 10 mM NaN3 and incubated for 30 min at 4°C with one of the following phycoerythrin- or fluorescein isothiocyanate (FITC)-conjugated murine monoclonal antibodies (MAb): anti-HLA-DR immunoglobulin G2a (IgG2a) MAb (Becton Dickinson, San Jose, Calif.), anti-CD80 (B7-1) IgG1 MAb (Becton Dickinson), anti-CD54 (ICAM-1) IgG2b MAb (Becton Dickinson), anti-CD14 IgG2b MAb (Becton Dickinson), anti-CD86 (B7-2) IgG2b MAb (PharMingen, San Diego, Calif.), and anti-CD40 IgG1 MAb (Biosource Europe). As controls, DC were stained with corresponding isotype-matched MAbs. The DC were fixed with 1% paraformaldehyde before being subjected to flow cytometry analysis (FACSCalibur; Becton Dickinson).
Analysis of cell viability.
Double staining for annexin V-FITC and propidium iodide (PI) was performed for analysis of cell viability, as described elsewhere (5). An annexin V kit (Bender Medsystems, Vienna, Austria) was used. Briefly, DC (105) were washed with PBS and resuspended in 200 μl of annexin V binding buffer (10 mM HEPES-NaOH [pH 7.4], 140 mM NaCl, 2.5 mM CaCl2) containing 5 μl of annexin V-FITC. After 10 min of incubation at 0°C, the cells were washed before the addition of 1 μg of PI per ml and analyzed by flow cytometry.
TEM.
T. cruzi-infected DC were pelleted and fixed in 2.5% glutaraldehyde for 1 h, washed twice in PBS and then fixed in 0.1 M sodium cacodylate buffer (pH 7.4) for 24 h, and postfixed in a 4% aqueous solution of osmium tetroxide for 1 h. The DC were then dehydrated in a graded series of ethanol and embedded in Epon 812. Thin sections were stained with uranyl acetate and lead citrate for examination by transmission electron microscopy (TEM).
Statistical analysis.
Data were compared by using the nonparametric Wilcoxon’s paired one-tailed test.
RESULTS
T. cruzi infects human DC.
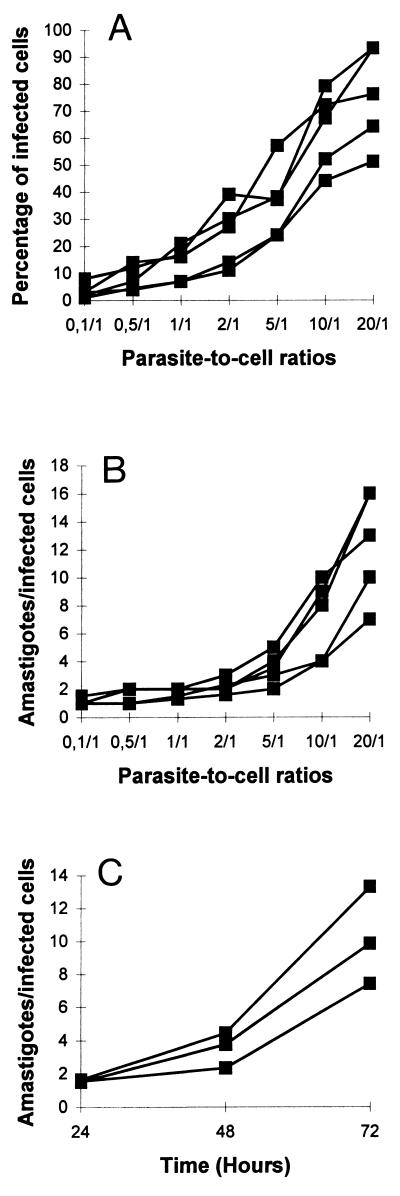
In a first set of experiments, we investigated whether T. cruzi infects human DC. For this purpose, we evaluated by light microscopy the infection rate of human DC cocultured with T. cruzi trypomastigotes for 24 h. The percentage of infected cells increased with increasing parasite-to-cell ratios (Fig. 1A) as well as the mean number of amastigotes per infected DC (Fig. 1B). To assess the intracellular multiplication of amastigotes, DC were infected with T. cruzi trypomastigotes (parasite-to-cell ratio, 5:1) for 24 h and then excess parasites were removed by extensive washing. Cultures of DC were incubated for an additional 24 or 48 h, fixed, and stained with Giemsa stain. The mean number of amastigotes per cell increased five- to eightfold between 24 and 72 h of culture (Fig. 1C).
FIG. 1.
T. cruzi-infected human DC. (A and B) DC were incubated with T. cruzi trypomastigotes at different parasite-to-cell ratios (0.1:1 to 20:1). After 24 h, the cultures were washed to remove free parasites and DC were fixed with methanol and stained with Giemsa stain. The percentages of infected DC (A) and mean numbers of amastigotes per infected DC (B) were recorded after microscopy examination of at least 300 cells. Data are from four independent experiments with four different blood donors. (C) In another set of experiments, DC were first incubated with T. cruzi trypomastigotes at a parasite-to-cell ratio of 5:1 for 24 h and then excess parasites were removed by extensive washing. Cultures of DC were continued for an additional incubation time of 24 or 48 h before the cells were fixed and stained with Giemsa stain. Mean numbers of amastigotes per infected DC were recorded after microscopy examination of at least 300 cells. Data are from three independent experiments with three different blood donors.
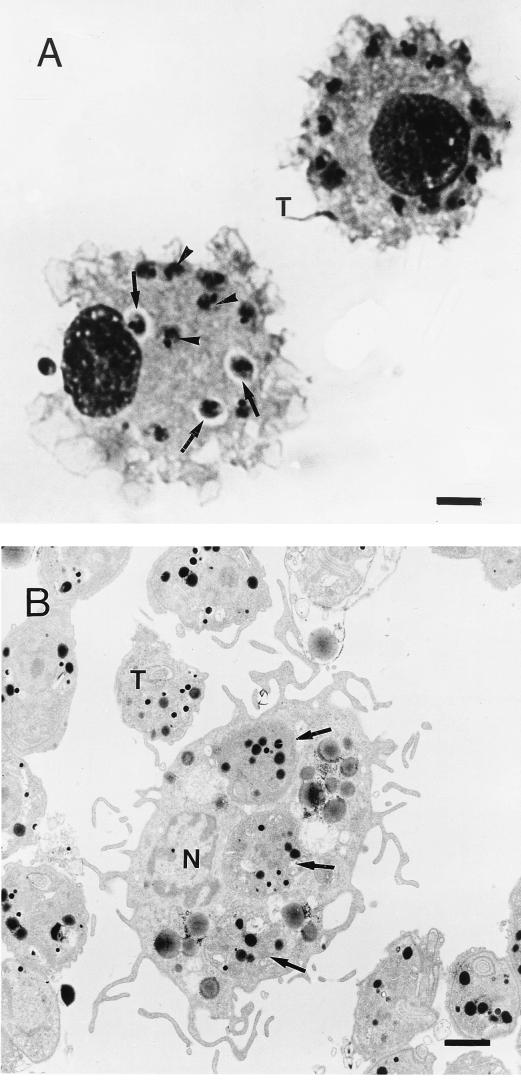
The intracellular location of the parasites was determined by using both light microscopy and TEM. At the beginning of the infection, parasites were seen in the parasitophorous vacuole (Fig. 2A), from which they escaped into the cytosol (Fig. 2B). We also observed that DC viability was over 95%, as assessed by the trypan blue test, and was not affected by T. cruzi infection (data not shown).
FIG. 2.
Light microscopy and TEM of T. cruzi-infected human DC. (A) DC were incubated with T. cruzi trypomastigotes (parasite-to-cell ratio, 20:1) for 24 h, fixed with methanol, and stained with Giemsa stain. T, trypomastigote; arrows, parasite inside a parasitophorous vacuole; arrowheads, parasite in the cytosol. Bar, 2.5 μm. (B) DC were incubated with T. cruzi trypomastigotes (parasite-to-cell ratio, 5:1) for 24 h and treated for TEM. T, trypomastigote; arrow, parasite in the cytosol; N, DC nucleus. Bar, 1.5 μm.
Taken together, these data indicated that T. cruzi trypomastigotes invade, survive, and multiply in the DC.
Effects of T. cruzi infection and TCM medium on cytokine production and surface molecule expression by immature human DC.
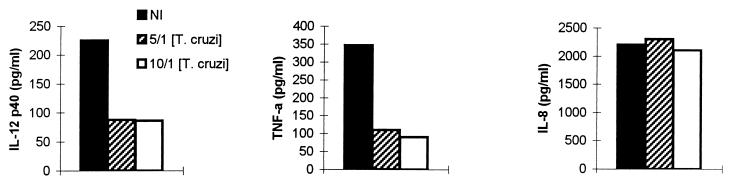
We next investigated whether T. cruzi would affect the production of cytokines by human DC. IL-12 (p40) and TNF-α secretion by DC was significantly decreased after infection with trypomastigotes at almost all parasite-to-cell ratios (5:1, 10:1, and 20:1) tested. In contrast, IL-8 production was not modified (Table 1). IL-6 and IL-10 levels were below the detection limits of the assays in the presence and absence of trypomastigotes (data not shown). To establish whether this inhibitory effect required DC infection, we used a culture system in which DC and trypomastigotes were separated by the presence of a cell-impermeable filter. Inhibition of IL-12 (p40) and TNF-α synthesis was also observed under these conditions, indicating the production of filterable inhibitory soluble factors by T. cruzi (Fig. 3).
TABLE 1.
T. cruzi and TCM inhibit the basal production of IL-12 (p40) and TNF-αa
| Inhibitor | Cytokine level (pg/ml)b
|
||
|---|---|---|---|
| IL-8 | IL-12 (p40) | TNF-α | |
| None (medium alone) | 3,150 ± 1,433 | 244 ± 58 | 383 ± 156 |
| T. cruzi | |||
| 5/1 | 2,518 ± 864 | 80 ± 33* | 129 ± 25 |
| 10/1 | 3,171 ± 1,350 | 79 ± 33* | 105 ± 31 |
| 20/1 | 3,938 ± 2,233 | 69 ± 22* | 194 ± 97* |
| TCM | |||
| 12.5% | 1,987 ± 1,355 | 100 ± 32 | 120 ± 55* |
| 25% | 3,692 ± 2,395 | 96 ± 50* | 124 ± 55* |
| 50% | 3,159 ± 2,450 | 72 ± 33* | 27 ± 24* |
DC (5 × 105) were incubated with or without live T. cruzi trypomastigotes at different parasite-to-cell ratios or TCM at different concentrations. After 24 h, supernatants were collected to determine the cytokine production by ELISA.
Data represent the mean ± standard error of the mean of five independent experiments performed with five different donors. ∗, P ≤ 0.03 by nonparametric Wilcoxon’s paired one-tailed test, compared to cytokine levels in the absence of live T. cruzi trypomastigotes or TCM.
FIG. 3.
Filterable soluble factors released by T. cruzi inhibit the basal production of IL-12 (p40) and TNF-α. DC were cultured in the bottom of wells and separated by a cell-impermeable filter from trypomastigotes placed in a transwell. The parasite-to-cell ratios were 5:1 and 10:1. After 24 h, supernatants were collected to determine the cytokine production by ELISA. Results of one representative experiment of three are shown.
To assess whether this production of inhibitory factors was spontaneous or was induced by the presence of DC or DC products, we used TCM prepared as described by Kierszenbaum et al. (27). A comparable dose-dependent impairment of IL-12 (p40) and TNF-α production was observed when DC were incubated with increasing concentrations of TCM (12.5, 25, and 50%; Table 1). To exclude a cytotoxic effect of TCM on DC, the viability of the DC was evaluated by using annexin V and PI staining. Treatment of DC with TCM at all the concentrations used did not alter cell viability as assessed by annexin V and PI staining (Table 2).
TABLE 2.
DC viability is not affected by TCMa
| TCM concn (%) | % of cells labeledb by:
|
||
|---|---|---|---|
| Annexin V | Annexin V and PI | PI | |
| 0 (control) | 4 ± 2 | 7 ± 2 | 17 ± 5 |
| 12.5 | 3 ± 1 | 8 ± 1 | 23 ± 5 |
| 25 | 3 ± 1 | 10 ± 2 | 23 ± 6 |
| 50 | 3 ± 1 | 11 ± 3 | 22 ± 3 |
| 75 | 3 ± 1 | 9 ± 2 | 22 ± 4 |
DC were incubated for 24 h in medium alone (control) or in the presence of TCM at different concentrations. They were then washed and incubated with annexin V-FITC followed by PI, and staining was analyzed by flow cytometry. Annexin V staining corresponds to early apoptosis, Annexin V PI staining corresponds to late apoptosis, and PI staining alone corresponds to necrotic cells.
Data represent the percentage of positive cells (mean ± standard error of the mean) in each quadrant of dot plot analysis in four independent experiments with four different blood donors.
Effects of T. cruzi infection or TCM treatment on the expression of HLA-DR, CD40, CD54, CD80, and CD86 molecules, which are involved in the APC function of DC, were also tested. T. cruzi infection or TCM treatment of DC had no effect on the basal expression of these molecules (data not shown and Table 3).
TABLE 3.
TCM inhibits LPS-induced HLA-DR and CD40 up-regulationa
| Condition(s) | Surface molecule expression (MFI)b
|
||||
|---|---|---|---|---|---|
| HLA-DR | CD40 | CD54 | CD80 | CD86 | |
| Medium | 216 ± 3 | 55 ± 4 | 84 ± 31 | 21 ± 5 | 33 ± 16 |
| Medium + TCM | 232 ± 41 | 46 ± 4 | 55 ± 19 | 19 ± 5 | 33 ± 16 |
| LPS | 1,006 ± 419 | 99 ± 11 | 125 ± 35 | 45 ± 6 | 106 ± 20 |
| LPS + TCM | 680 ± 244* | 72 ± 9* | 114 ± 26 | 35 ± 4 | 72 ± 4 |
DC were stimulated with LPS (10 ng/ml) in the presence or absence of TCM (75%). After 2 h, the DC were harvested and washed, and the expression of surface molecules was determined by flow cytometry analysis. As a control, DC were incubated in medium alone in the presence or the absence of TCM (75%).
Results are expressed as median fluorescence intensity (MFI) and represent the mean ± standard error of the mean of six independent experiments with six different donors. Cell labeling with control MAb was not affected by TCM (data not shown). *, P ≤ 0.01 by nonparametric Wilcoxon’s paired one-tailed test, compared to MFI in the absence of TCM.
Effects of T. cruzi infection and TCM on DC maturation induced by LPS stimulation.
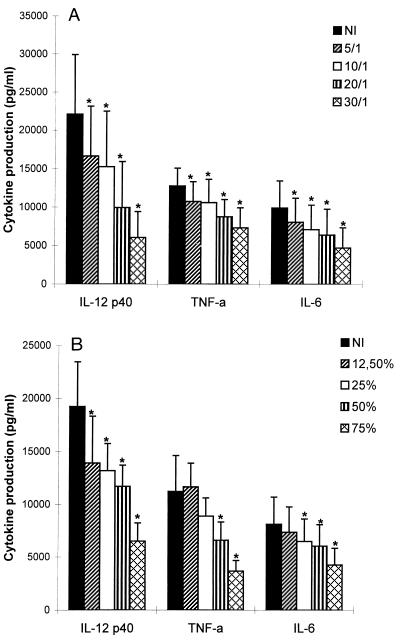
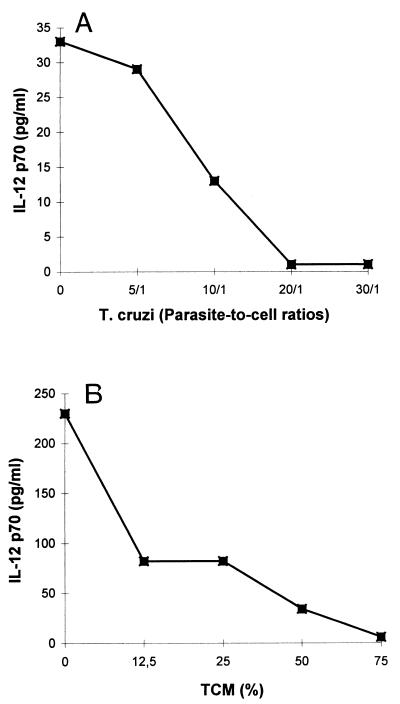
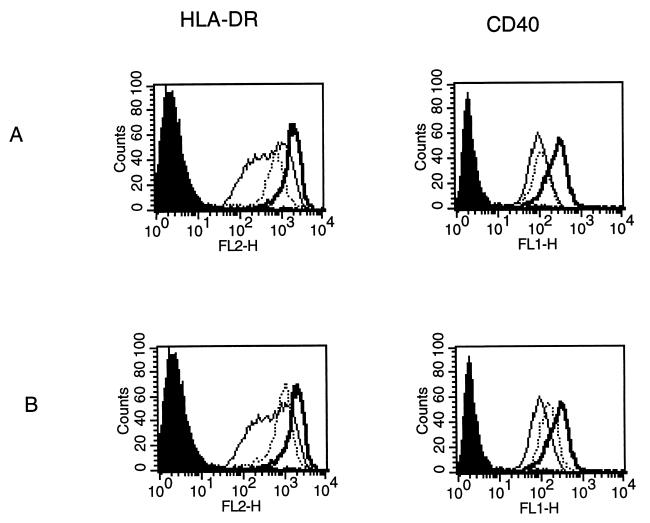
Since DC maturation is an essential process during which DC acquire optimal immunostimulatory properties, we examined the influence of T. cruzi infection on the response of DC to LPS, which has been previously described to be a major stimulus for cytokine production and up-regulation of surface molecule expression (8, 43). Therefore, we performed the same experiments as those described above in the presence of LPS (10 ng/ml). We first observed that DC were infected to the same extent in the presence and in the absence of LPS (data not shown). Furthermore, as shown in Fig. 4, the enhanced secretion of IL-12 (p40), TNF-α, and IL-6 normally induced by LPS was decreased by both T. cruzi infection and TCM in a dose-dependent manner. A maximal inhibitory effect was reached at a parasite-to-cell ratio of 30:1 or when TCM was used instead of 75% of the culture medium. The heterodimeric bioactive p70 form of IL-12 was also inhibited by T. cruzi infection and TCM (Fig. 5). The stimulation of IL-8 and IL-10 production was not modified (data not shown). Finally, we observed that LPS-induced HLA-DR and CD40 up-regulation was significantly inhibited by both trypomastigotes (parasite-to-cell ratio 30:1) and TCM (75%), while no effect was found on CD54, CD80, and CD86 (Fig. 6 and Table 3). DC remained CD14 negative in the presence of trypomastigotes or TCM. This inhibitory effect on LPS-induced IL-12 (p40) production as well as costimulatory-molecule expression (HLA-DR and CD40) was also observed at lower parasite-to-cell ratios (0.1:1, 0.5:1, 1:1, and 2:1) and lower concentrations of TCM (3 and 6.25%) (data not shown).
FIG. 4.
T. cruzi infection and TCM inhibit LPS-induced cytokine release from DC. A total of 5 × 105 DC were incubated with LPS (10 ng/ml) with or without trypomastigotes or TCM for 24 h, and cytokine levels were measured in culture supernatants. (A) Levels of cytokines produced in the absence (NI) or presence of trypomastigotes at different parasite-to-cell ratios. (B) Levels of cytokines produced in the absence (NI) or presence of TCM at different graded concentrations. Data represent the mean and standard error of the mean of five independent experiments performed with five different blood donors. ∗, P ≤ 0.03 by nonparametric Wilcoxon’s paired one-tailed test, compared to cytokine levels in the absence of T. cruzi trypomastigotes or TCM.
FIG. 5.
T. cruzi infection and TCM inhibit LPS-induced IL-12 (p70) release from DC. DC were stimulated with LPS (10 ng/ml) in the presence of trypomastigotes at different parasite-to-cell ratios (A) or in the presence of increasing concentrations of TCM (B). After 24 h, supernatants were collected for determination of IL-12 (p70) levels by ELISA. Results of one representative experiment of two are shown.
FIG. 6.
T. cruzi infection and TCM inhibit LPS-induced HLA-DR and CD40 up-regulation. DC were incubated with LPS (10 ng/ml) with or without trypomastigotes (parasite-to-cell ratio, 30:1) (A) or TCM (75%) (B). DC incubated in the presence of medium alone were used as control. After 24 h, DC were harvested and washed, and the expression of HLA-DR (left) and CD40 (right) was determined by flow cytometry. Thin lines represent FACS profiles obtained for DC incubated with medium alone. Thick lines represent FACS profiles obtained for DC incubated with LPS alone. Dotted lines represent FACS profiles obtained for DC incubated with LPS in the presence of trypomastigotes (A) or TCM (B). The solid histograms represent FACS profiles after staining with the corresponding isotype-matched control MAb. Results of one representative experiment of six are shown.
DISCUSSION
In the present report, we demonstrate for the first time that human DC, the only APC with the ability to initiate a primary immune response, could be infected by T. cruzi and support its intracellular multiplication. T. cruzi is first internalized in a parasitophorous vacuole, from which it escapes into the cytosol. Our observations on human DC infection are in line with those obtained in experiments with human and murine macrophages infected by T. cruzi (4).
Interestingly, T. cruzi infection of human monocytes-derived DC has functional consequences for immature DC and their maturation induced by LPS. Indeed, no increase in the basal production of cytokines is induced by this infection, in contrast to infection by other pathogens, which induce DC maturation (15, 19, 24, 26, 33, 38, 44). Instead, an inhibition of basal production of IL-12 and TNF-α is induced after this infection. The decreased basal production of IL-12 by human DC was also observed with Leishmania donovani promastigotes, Histoplasma capsulatum, Mycobacterium kansasii (2), and viruses (influenza virus, measles virus, and human immunodeficiency virus) (7, 16, 17). Most interestingly, LPS-induced DC maturation was profoundly impaired by T. cruzi infection. Indeed, IL-12, TNF-α, and IL-6 were inhibited in DC activated by LPS in the presence of T. cruzi. Studies performed with mouse models, both in vitro and in vivo, suggested that T. cruzi is a potent stimulator of IL-12 and TNF-α synthesis by macrophages (3, 10, 31). In contrast, and consistent with our results on human DC, LPS-induced IL-12 and TNF-α production was inhibited when human macrophages were infected in vitro with T. cruzi or treated with a mucin-like protein derived from the parasite (13).
Furthermore, in addition to their major IL-12 production upon microbial infection in vivo, the importance of DC in the initiation of acquired immunity is well established (19, 38). Thus, by altering the ability of the DC to secrete IL-12, TNF-α, and IL-6 (cytokines involved in the control of intracellular infection [35, 41]), T. cruzi may use a new mechanism to evade the immune response.
The activity of T. cruzi on DC could be also mediated through transwells, suggesting the production of filterable inhibitory soluble factors by the parasite. Indeed, supernatants derived from suspensions of T. cruzi or TCM have the same inhibitory effect on cytokine release. These results indicate that a close contact between DC and T. cruzi, leading to infection, is not a prerequisite for inhibition of cytokine synthesis. Parasite-derived molecules released into the culture medium seem to be responsible for this inhibition. Moreover, the presence of DC is not required for the spontaneous release of the inhibitory active principle of TCM by the parasite. Preliminary data suggest that the biological activity of TCM on monocyte-derived human DC could be attributed at least to a small (<3,000-Da), heat-stable molecule(s) (data not shown). The effect of TCM on cytokine production is related to trypanosomal immunosuppressive factor, which inhibits lymphoproliferation and IL-2 receptor expression on human T lymphocytes (27). Similarly, in a mouse experimental model, down-regulation of T lymphocyte activation has been demonstrated by using trypomastigotes and parasite-derived glycoinositol-phospholipid (18, 42). These results are also reminiscent of similar disturbances observed in T. cruzi-infected mouse macrophages, such as impairment of APC function (29, 34). These data and our own observations suggest that T. cruzi affects many functions of the host cells, probably by releasing several biologically active molecules, indicating that the infection by itself is not necessary.
Besides their ability to produce large amounts of cytokines while maturing, DC up-regulate their MHC and costimulatory molecules, leading to an efficient stimulation of naive T cells. Our results indicate that T. cruzi infection did not drive any up-regulation of HLA-DR, costimulatory, or adhesion molecules and that parasite-derived molecules had no effect on the basal expression of these molecules. In contrast, both T. cruzi and TCM prevented an optimal maturation of DC by LPS, since a reduced expression of HLA-DR and CD40 molecules was observed. Because of the central roles of HLA-DR molecules and CD40-CD40L pathway in the induction and effector phase of immune system responses (20), the inhibition of these surface molecules by T. cruzi is another strategy to escape specific immune system surveillance and to persist in the host.
Taken together, these data indicate that by altering the maturation of DC, which is a crucial step in induction of a potent cellular immune response, T. cruzi, probably by releasing soluble immunosuppressive factors, may unbalance the immune response to its benefit. If so, this inhibition might be added to the long list of escape mechanisms used by T. cruzi to reduce the effectiveness of the host immune response and could explain the persistence of the parasites together with a long-lasting inflammation process.
ACKNOWLEDGMENTS
We thank V. Vercruysse for valuable technical assistance and I. Mazza for help in preparing the manuscript.
This work was supported by grants from Action de Recherche Concertée de la Communauté Française de Belgique, ULB, 1991 and 1994, and Fondation Emile Defay. L.V.O. is the recipient of a grant from the Fonds pour la Formation à la Recherche dans l’Industrie et dans l’Agriculture (FRIA).
REFERENCES
- 1.Abrahamsohn I A. Cytokines in innate and acquired immunity to Trypanosoma cruzi infection. Braz J Med Biol Res. 1998;31:117–121. doi: 10.1590/s0100-879x1998000100015. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja S S, Mummidi S, Malech H L, Ajuha S K. Human dendritic cell (DC)-based anti-infective therapy: engineering DCs to secrete functional IFN-gamma and IL-12. J Immunol. 1998;16:868–876. [PubMed] [Google Scholar]
- 3.Aliberti J C S, Cardoso M A G, Martins G A, Gazzinelli R T, Vieira L Q, Silva J S. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun. 1996;64:1961–1967. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araujo-Jorge T C. The biology of Trypanosoma cruzi-macrophage interaction. Mem Inst Oswaldo Cruz. 1989;84:441–462. doi: 10.1590/s0074-02761989000400001. [DOI] [PubMed] [Google Scholar]
- 5.Auphan N, DiDonato J A, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Steinman J. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 7.Bender A, Albert M, Reddy A, Feldman M, Sauter B, Kaplan G, Hellman W, Bhardwaj N. The distinctive features of influenza virus infection of dendritic cells. Immunobiology. 1998;198:552–567. doi: 10.1016/S0171-2985(98)80078-8. [DOI] [PubMed] [Google Scholar]
- 8.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Human dendritic cell responses to lipopolysaccharide and CD40 ligation are differentially regulated by interleukin-10. Eur J Immunol. 1997;27:1848–1852. doi: 10.1002/eji.1830270805. [DOI] [PubMed] [Google Scholar]
- 9.Burleigh B A, Andrews N W. The mechanisms of Trypanosoma cruzi invasion of mammalian cells. Annu Rev Microbiol. 1995;49:175–200. doi: 10.1146/annurev.mi.49.100195.001135. [DOI] [PubMed] [Google Scholar]
- 10.Camargo M M, Almeida I C, Pereira M E S, Feguson M A J, Travassos L R, Gazzinelli R T. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J Immunol. 1997;158:5890–5901. [PubMed] [Google Scholar]
- 11.Cardillo F, Voltarelli J C, Reed S G, Silva J S. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin-10: role of NK cells. Infect Immun. 1996;64:128–134. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 13.de Diego J, Punzon C, Duarte M, Fresno M. Alteration of macrophage function by a Trypanosoma cruzi membrane mucin. J Immunol. 1997;159:4983–4989. [PubMed] [Google Scholar]
- 14.DosReis G A. Cell-mediated immunity in experimental Trypanosoma cruzi infection. Parasitol Today. 1997;13:335–342. doi: 10.1016/s0169-4758(97)01073-9. [DOI] [PubMed] [Google Scholar]
- 15.Filgueira L, Nestlé F O, Rittig M, Joller H I, Groscurth P. Human dendritic cells phagocytose and process Borrelia burgdorferi. J Immunol. 1996;157:2998–3005. [PubMed] [Google Scholar]
- 16.Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan M-C, Liu Y-J, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghanekar S, Zheng L, Logar A, Navratil J, Borowski L, Gupta P, Rinaldo C. Cytokine expression by human peripheral blood dendritic cells stimulated in vitro with HIV-1 and herpes simplex virus. J Immunol. 1996;157:4028–4036. [PubMed] [Google Scholar]
- 18.Gomes N A, Previato J O, Zingales B, Mendonça-Previato L, DosReis G A. Down-regulation of T lymphocyte activation in vitro and in vivo induced by glycoinositolphospholipids from Trypanosoma cruzi. J Immunol. 1996;156:628–635. [PubMed] [Google Scholar]
- 19.Gorak P M A, Engwerda C R, Kaye P M. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur J Immunol. 1998;28:687–695. doi: 10.1002/(SICI)1521-4141(199802)28:02<687::AID-IMMU687>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Grewal I S, Borow P, Pamer E G, Oldstone M B A, Flavell R A. The CD40-CD154 system in anti-infective host defense. Curr Opin Immunol. 1997;9:491–497. doi: 10.1016/s0952-7915(97)80100-8. [DOI] [PubMed] [Google Scholar]
- 21.Grosjean I, Caux C, Bella C, Berger I, Wild F, Banchereau J, Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186:801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guzman C A, Rohde M, Timmis K N. Mechanisms involved in uptake of Bordetella bronchiseptica by mouse dendritic cells. Infect Immun. 1994;62:5538–5544. doi: 10.1128/iai.62.12.5538-5544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzman C A, Rohde M, Chakraborty T, Domann E, Hudel M, Wehland J, Timmis K N. Interaction of Listeria monocytogenes with mouse dendritic cells. Infect Immun. 1995;63:3665–3673. doi: 10.1128/iai.63.9.3665-3673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson R A, Watkins S C, Flynn J L. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol. 1997;159:635–643. [PubMed] [Google Scholar]
- 25.Hunter C A, Slifer T, Araujo F. Interleukin-12-mediated resistance to Trypanosoma cruzi is dependent on tumor necrosis factor alpha and gamma interferon. Infect Immun. 1996;64:2381–2386. doi: 10.1128/iai.64.7.2381-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaba K, Inaba M, Naito M, Steinman R M. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kierszenbaum F, Majumder S, Paredes P, Tanner M K, Sztein M B. The Trypanosoma cruzi immunosuppressive factor (TIF) targets a lymphocyte activation event subsequent to increased intracellular calcium ion concentration and translocation of protein kinase C but previous to cyclin D2 and cdk4 mRNA accumulation. Mol Biochem Parasitol. 1998;92:133–145. doi: 10.1016/s0166-6851(97)00240-5. [DOI] [PubMed] [Google Scholar]
- 28.Knight S C, Patterson S. Bone marrow-derived dendritic cells, infection with human immunodeficiency virus, and immunopathology. Annu Rev Immunol. 1997;15:593–615. doi: 10.1146/annurev.immunol.15.1.593. [DOI] [PubMed] [Google Scholar]
- 29.La Flamme A C, Kahn S J, Rudensky A Y, Van Voorhis W C. Trypanosoma cruzi-infected macrophages are defective in major histocompatibility complex class II antigen presentation. Eur J Immunol. 1997;27:3085–3094. doi: 10.1002/eji.1830271202. [DOI] [PubMed] [Google Scholar]
- 30.Lanham S M, Godfrey D G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970;28:521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- 31.Meyer zum Büschenfelde C, Cramer S, Trumpfheller C, Fleischer B, Frosch S. Trypanosoma cruzi induces strong IL-12 and IL-18 gene expression in vivo: correlation with interferon-gamma (IFN-γ) production. Clin Exp Immunol. 1997;110:378–385. doi: 10.1046/j.1365-2249.1997.4471463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moll H, Flohé S, Röllinghoff M. Dendritic cells in Leishmania major-immune mice harbor persistent parasites and mediate an antigen-specific T cell immune response. Eur J Immunol. 1995;25:693–699. doi: 10.1002/eji.1830250310. [DOI] [PubMed] [Google Scholar]
- 33.Ojcius D M, Bravo de Alba Y, Kanellopulos J M, Hawkins R A, Kelly K A, Rank R G, Dautry-Varsat A. Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J Immunol. 1998;160:1297–1303. [PubMed] [Google Scholar]
- 34.Plasman N, Guillet J-G, Vray B. Impaired protein catabolism in Trypanosoma cruzi-infected macrophages: possible involvement in antigen presentation. Immunology. 1995;86:636–645. [PMC free article] [PubMed] [Google Scholar]
- 35.Romani L, Puccetti P, Bistoni F. Interleukin-12 in infectious diseases. Clin Microbiol Rev. 1997;10:611–636. doi: 10.1128/cmr.10.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romani N, Gruner S, Brang D, Kämpgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch P O, Steinman R, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva J S, Aliberti J C, Martins G A, Souza M A, Souto J T, Padua M A. The role of IL-12 in experimental Trypanosoma cruzi infection. Braz J Med Biol Res. 1998;31:111–115. doi: 10.1590/s0100-879x1998000100014. [DOI] [PubMed] [Google Scholar]
- 38.Sousa C R, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain R N, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin-12 by dendritic cells and their redistribution in the T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanowitz H B, Simon D, Morris S A, Weiss L M, Wittner M. Chagas’ disease. Clin Microbiol Rev. 1992;5:404–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarleton R L. The role of T cells in Trypanosoma cruzi infections. Parasitol Today. 1995;11:7–9. doi: 10.1016/0169-4758(95)80095-6. [DOI] [PubMed] [Google Scholar]
- 41.Truyens C, Angelo-Barrios A, Torrico F, Van Damme J, Heremans H, Carlier Y. Interleukin-6 (IL-6) production in mice infected with Trypanosoma cruzi: effect of its paradoxical increase by anti-IL-6 monoclonal antibody treatment on infection and acute-phase and humoral immune responses. Infect Immun. 1994;62:692–696. doi: 10.1128/iai.62.2.692-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandekerckhove F, Darij A, Rivera M T, Carlier Y, Vray B, Billiau A, de Baetselier P. Modulation of T-cell responsiveness during Trypanosoma cruzi infection: analysis in different lymphoid compartments. Parasite Immunol. 1994;16:77–85. doi: 10.1111/j.1365-3024.1994.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 43.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–2925. [PubMed] [Google Scholar]
- 44.von Stebut E, Belkaid Y, Jakob T, Sacks D L, Udey M C. Uptake of Leishmania major amastigotes results in activation and interleukin-12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J Exp Med. 1998;188:1547–1552. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]