Abstract
Background and Purpose
Parkinson’s disease (PD) patients present with numerous motor and nonmotor symptoms. Seborrheic dermatitis (SD) is reported in 18.6%–59% of PD patients. However, the etiology of SD in PD patients remains unknown. The aim of this study was to determine how motor and nonmotor symptoms, age, sex, and levodopa-equivalent daily dose (LEDD) influence the appearance and severity of SD in PD patients, and then discuss about SD possible etiology based on the obtained results.
Methods
Motor symptoms were evaluated using the Unified Parkinson’s Disease Rating Scale part III and nonmotor symptoms were evaluated using the Parkinson’s Disease Sleep Scale, Scales for Outcomes in Parkinson’s Disease–Autonomic Dysfunction, and Non-Motor Symptoms Questionnaire. LEDD was calculated and demographic data on age, sex, disease duration, and symptoms of SD prior to a PD diagnosis were collected. A dermatologist evaluated the skin for SD using the Seborrhea Area and Severity Index.
Results
SD was present in 36.1% of the PD patients. There were positive correlations between age, motor-symptoms severity, and SD. After adjusting for age, disease duration, and sex, there remained a positive correlation between the severity of motor symptoms and SD. Patients with moderate-to-severe motor symptoms had more-severe SD symptoms, and their risk of developing SD was 1.8-fold higher. There was no correlation between SD and autonomic dysfunction, sleep disturbances, or other nonmotor symptoms, and no sex difference.
Conclusions
In PD, SD is related to motor symptoms.
Keywords: Parkinson’s disease, seborrheic dermatitis, motor symptoms, nonmotor symptoms, levodopa-equivalent daily dose
INTRODUCTION
Parkinson’s disease (PD) is the second-most-common neurodegenerative disease classified as a synucleinopathy due to the pathological accumulation of α-synuclein and formation of Lewy body and Lewy neurites. The presence of diffuse pathology involving numerous central and peripheral nerve structures results in PD patients presenting with numerous motor and nonmotor symptoms.1 There are reports on sensation abnormalities (olfactory dysfunction, pain, and visual problems), behavioral changes (depression, anxiety, apathy, cognitive impairment, and dementia), autonomic dysfunction (orthostasis hypotension, gastrointestinal dysfunction, urinary symptoms, sexual dysfunction, and thermoregulation impairment), sleep disturbances (e.g., insomnia and rapid eye movement-sleep behavior disorder), fatigue, and skin changes.2,3
Seborrheic dermatitis (SD) is a chronic, inflammatory disorder affecting the skin on the head and trunk at sites where sebaceous glands are most prominent, and manifests with scaling, erythema, pruritus, oily skin, and induration. SD predominantly involves the face (88% of patients) and scalp (70% of patients). The etiology of SD is unknown, but several mechanisms have been proposed for its pathogenesis, including disruption of the skin microbiota, impaired immune reaction to Malassezia spp., epidermal barrier disturbances associated with genetic factors, the role of Malassezia spp. in the degradation of sebum and consumption of saturated fatty acids, elevated unsaturated fatty acids on the skin, disruption of cutaneous neurotransmitters, and abnormal shedding of keratinocytes disrupting the lipid balance on the skin surface.4 Age, male sex, increased sebaceous gland secretion, immunodeficiency, certain neurological and psychiatric diseases (PD, stroke, Alzheimer’s disease, major depression, and autonomic dysfunction), exposure to drug therapy (dopamine antagonists, immunosuppressants, psoralen plus ultraviolet A, and lithium), and low ambient humidity and/or low ambient temperature have been postulated as possible risk factors for SD.4 The prevalence of SD in PD patients has been reported to range from 8.6% to 59%.5 The etiology of this disorder in PD patients remains unknown. Studies have evaluated the effects of autonomic dysfunction, testosterone or androgens, and melanocyte-stimulating hormone and levodopa therapy, as well as the role of Malassezia spp., but these investigations have not led to definite conclusions about its etiology.6,7,8,9,10
The aim of this study was to determine the impacts of motor symptoms, nonmotor symptoms, age, sex, and levodopa-equivalent daily dose (LEDD) on the appearance and severity of SD in PD patients, and to discuss the possible etiology of SD in PD patients based on the obtained results.
METHODS
Adult patients with idiopathic PD treated at the outpatient clinic for movement disorders, Osijek University Hospital Centre were included in the study. Idiopathic PD was diagnosed according to the Movement Disorder Society clinical diagnostic criteria.11 Exclusion criteria were an inability to complete the study questionnaire and to undergo all tests and examinations. The study was approved by the Ethics Committee of Osijek University Hospital Center (Approval number, 25-1:15129-7/2014) and informed consent was obtained from all study patients.
One hundred and one PD patients were surveyed for motor and nonmotor symptoms by a neurologist and then referred for dermatologist examinations. All tests were performed during the ‘ON’ stage, and the dermatological examination was performed within 3 months after the neurological evaluation. Upon completion of the study, we noticed that four of the patients developed symptoms that confirmed a diagnosis other than idiopathic PD (Parkinson plus syndrome), and they were excluded from further analysis. Although all 101 PD patients were referred to a dermatologist, only 61 of them presented for dermatological examinations. Therefore, only these 61 patients who also underwent all tests were included in further statistical analyses.
Demographic data (sex, age, and disease duration) and PD drug dosages were collected. We used the Unified Parkinson’s Disease Rating Scale part III (UPDRS III) to assess motor-symptoms severity. A higher UPDRS III score indicates that motor symptoms are more severe in a patient,12 but there are no official cutoff points for the UPDRS III for grading disease severity. We divided patients according to the severity of motor symptoms into two groups: mild (UPDRS III score <20) and moderate to severe (UPDRS III score ≥20).13 Nonmotor symptoms were evaluated using the Non-Motor Symptoms Questionnaire (NMSQ), which consists of 30 questions answered ‘yes’ or ‘no’ about each nonmotor symptom, with the final score obtained by summing the ‘yes’ answers.14 The Parkinson’s Disease Sleep Scale (PDSS) was used to evaluate night-time sleep problems. The PDSS is a visual analog scale evaluating 15 commonly reported sleep symptoms, with a higher value indicating more-pronounced sleep disturbances.15 The Scales for Outcomes in Parkinson’s Disease–Autonomic Dysfunction (SCOPA-AUT) was used to evaluate autonomic symptoms. This scale is self-completed by patients and consists of 25 items assessing the gastrointestinal, urinary, cardiovascular, thermoregulatory, pupillomotor, and sexual domains. Each subscale score is divided by the maximum subscale score, and the result is presented on a scale from 1 to 100, with a higher score denoting worse symptoms.16 LEDD was calculated using an online calculator as the contribution of each PD drug using the common denominator of an estimate of the levodopa equivalent dose (LED). Adding together all of the LEDs over 1 day gives the LEDD.17
SD has a rather specific clinical appearance that makes the differential diagnosis straightforward, and so it can be diagnosed based only on a dermatological examination (e.g., erythema and desquamation in seborrheic areas such as the scalp, eyebrows, nasolabial folds, and sternal region). The Seborrhea Area and Severity Index (SASI) is a tool introduced by Smith et al.18 in 2002 that involves a numerical assessment of the severity of SD of the face and scalp. It measures the area of skin involved (scored from 0 to 6) and the severities of erythema (from 0 to 4) and scaling (from 0 to 4) separately for the face and scalp, with the final SASI score ranging from 1 to 48. There is no official cutoff value for this scale. All of the patients were also surveyed by a dermatologist for the history of SD prior to making the diagnosis of PD.
Categorical data were analyzed using relative and absolute frequencies. Numerical results were expressed as mean and standard deviation values if normally distributed, and as median and interquartile-range (IQR) values otherwise, with the distribution of numerical variables tested using the Shapiro-Wilk test. Correlations were assessed using Pearson correlation if data conformed to a normal distribution, and Spearman correlation otherwise. Partial correlations were assessed to exclude the influence of certain parameters. The χ2-test was used to assess associations between two categorical variables. Between-group differences were assessed using the Mann-Whitney U test for data that were not normally distributed, and the independent-samples t-test for normally distributed data. The criterion for significance was set at p<0.05. Statistical analyses were performed using SPSS for Windows 10 (version 22, IBM Corp., Armonk, NY, USA).
RESULTS
We evaluated 61 PD patients comprising 39 (63.0%) males and 22 (36.1%) females. The results for age, disease duration, motor and nonmotor symptoms, LEDD, and SASI score are presented in Table 1.
Table 1. Demographic data and data on motor and nonmotor symptoms, therapy and seborrheic index.
| Parameter | Value |
|---|---|
| Age (yr) | 69.0 (61.5–73.5) |
| Disease duration (yr) | 7.0 (3.0–10.5) |
| UPDRS III score | 16.00 (11.0–22.5) |
| PDSS score | 99.44±30.58 |
| NMSQ score | 9.70±5.59 |
| SCOPA-AUT score | 14.0 (9.0–22.5) |
| LEDD (mg) | 560.0 (350.0–777.0) |
| SASI score | 0.0 (0.0–6.5) |
| SASI face score | 0.0 (0.0–0.5) |
| SASI scalp score | 0.0 (0.0–3.0) |
Data are mean±standard-deviation or median (IQR) values.
IQR, interquartile range; LEDD, levodopa-equivalent daily dose; NMSQ, Non-Motor Symptoms Questionnaire; PDSS, Parkinson’s Disease Sleep Scale; SASI, Seborrhea Area and Severity Index; SCOPA-AUT, Scales for Outcomes in Parkinson’s Disease–Autonomic Dysfunction; UPDRS III, Unified Parkinson’s Disease Rating Scale part III.
Influence of SD appearance on PD symptoms, LEDD, and demographic parameters
SD was present in 36.1% of patients. An independent-samples t-test was used to determine if SD affected PDSS and NMSQ scores; the Mann-Whitney U test was used to determine if SD varied with age, UPDRS III score, SCOPA-AUT score, and LEDD; and the χ2-test was applied to assess the association between sex and the frequency of SD. Statistically significant difference were found only for the UPDRS III score with more-severe motor symptoms in patients with SD, with no significant differences in SCOPA-AUT score, LEDD, age, PDSS score [95% CI=-27.08 to 6.09, t(58)=-1.267, p=0.208], or NSMQ score [95% CI=-2.83 to 3.32, t(58)=0.160, p=0.874], and no significant association between sex and the frequency of SD [χ2(1)=2.655, p=0.103] (Supplementary Table 1 in the online-only Data Supplement).
Correlations between SD and PD symptoms, LEDD, and demographic parameters
Correlations of the SASI score, SASI-face score, and SASI-scalp score with age, disease duration, LEDD, and motor and nonmotor symptoms are presented in Table 2. A visual inspection of a scatterplot showed the relationship to be monotonic. Significant correlations were noticed only for the UPDRS III score and the SASI score, SASI-face score, and SASI-scalp score, and for age and the SASI score (Table 2). Spearman partial correlation was used to assess the relationships between the UPDRS III score and SASI score, SASI-face score, and SASI-scalp score after adjusting for age, disease duration, and sex. After adjusting for these parameters, significant correlations remained between the UPDRS III score and the SASI score, SASI-face score, and SASI-scalp score (Table 3).
Table 2. Correlations of the SASI score, SASI-face score, and SASI-scalp score with age, disease duration, LEDD, and motor and nonmotor symptoms.
| Parameter | SASI score | SASI-face score | SASI-scalp score | |||
|---|---|---|---|---|---|---|
| rho | p | rho | p | rho | p | |
| Age | 0.258 | 0.044* | 0.142 | 0.275 | 0.246 | 0.056 |
| Disease duration | 0.068 | 0.601 | 0.031 | 0.813 | 0.075 | 0.566 |
| UPDRS III score | 0.316 | 0.013* | 0.307 | 0.016* | 0.255 | 0.047* |
| PDSS score | 0.093 | 0.475 | -0.017 | 0.896 | 0.118 | 0.364 |
| NMSQ score | 0.081 | 0.534 | 0.131 | 0.313 | 0.006 | 0.961 |
| SCOPA-AUT score | 0.006 | 0.965 | -0.011 | 0.932 | 0.064 | 0.624 |
| LEDD | -0.054 | 0.677 | 0.063 | 0.629 | -0.139 | 0.284 |
rho, Spearman correlation coefficient; *p<0.05.
LEDD, levodopa-equivalent daily dose; NMSQ, Non-Motor Symptoms Questionnaire; PDSS, Parkinson’s Disease Sleep Scale; SASI, Seborrhea Area and Severity Index; SCOPA-AUT, Scales for Outcomes in Parkinson’s Disease–Autonomic Dysfunction; UPDRS III, Unified Parkinson’s Disease Rating Scale part III.
Table 3. Correlation of SASI, SASI face, and SASI scalp with severity of motor symptoms (UPDRS III) after adjusting for age, sex, and disease duration.
| Parameter | UPDRS III | |
|---|---|---|
| rho | p | |
| SASI | 0.351 | 0.007* |
| SASI face | 0.320 | 0.015* |
| SASI scalp | 0.292 | 0.028* |
rho, Spearman correlation coefficient; *p<0.05.
SASI, Seborrhea Area and Severity Index; UPDRS III, Unified Parkinson’s Disease Rating Scale part III.
Influences of motor symptoms and sex on SASI score
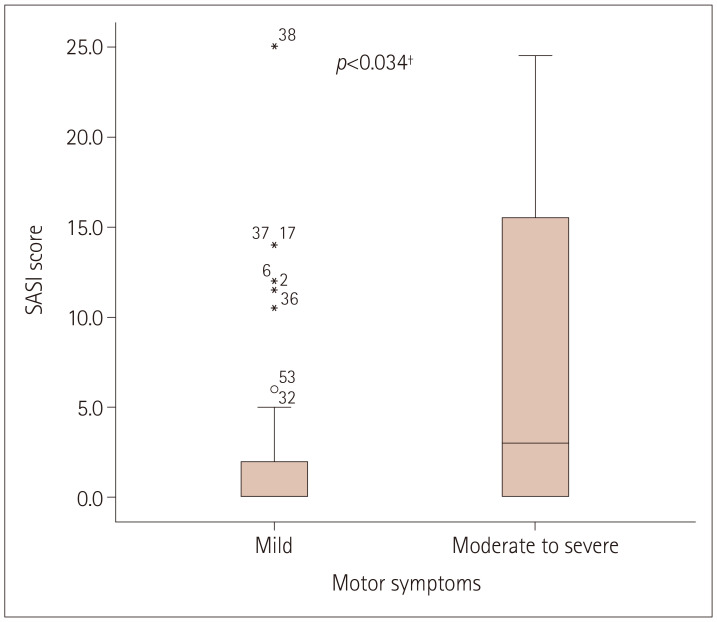
The Mann-Whitney U test was used to determine if there were differences in SASI scores according to the severity of motor symptoms and sex. Visual inspections revealed similar distributions of the SASI score for motor symptoms and sex. The median SASI score was significantly higher in patients with moderate-to-severe motor symptoms (=3.00) than in patients with mild motor symptoms (=0.00) (U=308, z=-2.115, p=0.034) (Fig. 1). The median SASI score for both sexes was the same (=0.00), with no significant difference (U=321, z=-1.888, p=0.059).
Fig. 1. Difference in SASI score according to the severity of motor symptoms. Each box plot shows the median, first and third quartiles and range. †Mann-Whitney U Test. SASI, Seborrhea Area and Severity Index.
Risk ratio for developing SD
Eleven (50%) patients with moderate-to-severe motor symptoms developed SD, compared with 11 (28.2%) of those with mild motor symptoms. The risk ratio for developing SD in patients with moderate-to-severe motor symptoms compared with those with mild motor symptoms was 1.773 (95% CI=0.923 to 3.403).
Influences of age and sex on motor symptoms
The Mann-Whitney U test and the χ2-test were used to determine if motor-symptoms severity differed with age and sex, respectively. A visual inspection revealed a similar distribution of motor symptoms according to age. All of the expected cell frequencies exceeded five. The median score for age did not differ significantly between the patients with mild motor symptoms (68.50, IQR=63.75–72.50) and those with moderate-to-severe motor symptoms (69.50, IQR=59.00–79.25) (U=409.5, z=-0.131, p=0.896). There was also no significant association between sex and motor-symptoms severity [χ2(1)=1.320, p=0.281].
Influence of SD symptoms prior to the PD diagnosis established based on PD symptoms
Symptoms of SD were present in 32.8% of the 61 patients prior to being diagnosed with PD. We also analyzed whether patients having reported to have SD prior to the PD diagnosis differed in their clinical presentation of motor and nonmotor symptoms and LEDD. The Mann-Whitney U test was used to determine if there were differences in the UPDRS III score, LEDD, and SCOPA-AUT score according to the presence of SD prior to the PD diagnosis, while the independent-samples t-test was used to determine if there were differences in NMSQ and PDSS scores. Visual inspections revealed that the distributions of the UPDRS III score, LEDD, and SCOPA-AUT score for SD were similar. There were no significant differences in the UPDRS III score, LEDD, and SCOPA-AUT score according to SD (Table 4). We also did not observed difference in the PDSS score between patients with SD (109.03±29.83, mean±standard deviation) and without SD (94.75±30.19) [95% CI=-30.69 to 2.14; t(38.201)=-1.748, p=0.089], neither there was difference in NMSQ score between patients without SD (9.93±5.74) and with SD (9.25±5.37) [95% CI=-2.39 to 3.75, t(40.17)=0.452, p=0.654] (Table 4).
Table 4. Differences in UPDRS III, LEDD, SCOPA-AUT, NMSQ, and PDSS scores according to presence of symptoms of SD (patients anamnestic data) prior to Parkinson’s disease diagnose.
| Parameter | SD symptoms | U | Z | p | |
|---|---|---|---|---|---|
| No | Yes | ||||
| UPDRS III score | 16.00 (9.50–22.50) | 17.00 (13.00–23.50) | 343 | -1.031 | 0.303* |
| LEDD (mg) | 14.00 (9.50–24.50) | 13.00 (6.75–21.75) | 365 | -0.685 | 0.494* |
| SCOPA-AUT score | 570.00 (400.00–826.50) | 495.00 (300.00–736.50) | 366 | -0.668 | 0.504* |
| NMSQ score | 9.93±5.74 | 9.25±5.37 | - | - | 0.654† |
| PDSS score | 94.75±30.19 | 109.03±29.83 | - | - | 0.089† |
Data are mean±standard-deviation or median (IQR) values.
*Mann-Whitney U test; †Student’s t-test.
IQR, interquartile range; LEDD, levodopa-equivalent daily dose; NMSQ, Non-Motor Symptoms Questionnaire; PDSS, Parkinson’s Disease Sleep Scale; SCOPA-AUT, Scales for Outcomes in Parkinson’s Disease-Autonomic Dysfunction; SD, seborrheic dermatitis; UPDRS III, Unified Parkinson’s Disease Rating Scale part III.
DISCUSSION
This study evaluated 61 PD patients for the prevalence and severity of SD and its relationship with motor and nonmotor symptoms and LEDD. SD was found in 36.1% of the patients, which is consistent with previous reports of the SD prevalence ranging from 18.6% to 59%.5 SD was correlated positively with the severity of motor symptoms and age. After adjusting for age, disease duration, and sex, we still observed a positive correlation between motor-symptoms severity and SD. There were no differences in demographic characteristics (age, disease duration, and sex) between the two groups of motor-symptoms severity. Patients with moderate-to-severe motor symptoms had more-severe symptoms as assessed using the SASI score. The risk of developing SD was 1.8-fold higher in those with moderate-to-severe symptoms than in those with mild symptoms.
Our search of the literature revealed only a few papers on this topic, and all of them reported that levodopa was associated with decreased sebum secretion or improvement of SD in PD patients.19,20,21 An interesting explanation for the occurrence of SD in PD patients has been offered by Mastrolonardo et al.,22 who proposed that levodopa indirectly improves SD symptomatology through the improvement of motor symptoms, rather than directly. This observation was based on the hypothesis of Cowley et al.23 that rigidity and amimia reduce or abolish mobility involving the skin. They suggested that PD patients do not exhibit an absolute increase in sebum production, with instead the static pool of already secreted sebum increasing due to immobility and muscular paralysis. This hypothesis was also supported by Bolognia et al.24 reporting that hyperproduction of sebum is not necessary for SD development and that a person with normal sebum production can also develop SD. Cowley et al.23 further proposed that hypomimia itself could cause higher sebum production, which then can enhance the growth of Malassezia yeasts and permit SD development. Malassezia spp. have been reported to colonize skin during the first week of life.25 This requires an exogenous source of specific lipids to increase, and a link between sebum overproduction and Malassezia spp. has been reported.26 Evidence that Malassezia spp. play at least a partial role in the development of SD is confirmed by the antifungal treatment response, with a reduction of Malassezia spp. correlated with marked visible and symptomatic improvement of SD after antifungal treatment.25,26,27 The hypothesis on motor symptoms influencing SD development is consistent with findings in psychiatric patients treated with neuroleptics. Maietta et al.28 found a higher prevalence of SD in psychiatric patients (25 of 42 chronic patients) with neuroleptic-induced parkinsonism. Binder and Jonelis29 compared the prevalence of SD between psychiatric patients who developed extrapyramidal side effects and those without motor side effects, and found a higher prevalence of SD in the former group (59.5% vs. 15%).
The present study found no correlation between SD and autonomic dysfunction, sleep disturbances, or other nonmotor symptoms. Although impairment of autonomic function is a possible explanation for SD in PD patients, contradictory results have been reported.3 Martignoni et al.6 also found no correlation, although they evaluated sebum hypersecretion rather than SD, which they found to be increased in male PD patients. Male sex is described as a risk factor.4 However, we found no between-sex difference in SASI score or frequency of SD, although this might have been due to the smallness of the sample.
We also evaluated whether the presence of SD prior to a PD diagnosis could influence the clinical presentation and therapy. One-third of patients (32.8%) reported having symptoms of SD prior to being diagnosed with PD. In the literature, SD is reported to be one of the premotor symptoms appearing in PD patients, which authors have related to autonomic dysfunction.30,31 We did not find that prior SD influenced to the severity of motor symptoms, the appearance or severity of nonmotor symptoms, or LEDD. This also supports our theory that motor symptoms are responsible for the development of SD, but not vice versa.
Our results should be interpreted with caution. One of the limitations of our study was the smallness of the sample. Moreover, the patients reporting having SD prior to a PD diagnosis could cast doubt on our conclusion about the relationship with motor symptoms. We could explain this by not evaluating how long before the diagnosis of PD that the patients had noticed SD symptoms, and we could not exclude that motor symptoms had already been present. PD is a slowly progressive disease with symptoms potentially being present for a long period prior to a diagnosis. Therefore, a prospective study involving a larger sample is needed to improve the understanding of SD in PD patients.
In conclusion, SD was present in 36.1% of our PD patients, and was correlated positively with the severity of motor symptoms and age. After adjusting for age, disease duration, and sex, the correlation remained between the severity of motor symptoms and SD. Patients with moderate-to-severe motor symptoms had more-severe symptoms of SD, and the risk of developing SD was 1.8-fold higher in those with moderate-to-severe symptoms than in those with mild symptoms. There was no correlation between SD and autonomic dysfunction, sleep disturbances, or other nonmotor symptoms. There was also no between-sex difference. The occurrence of SD prior to a PD diagnosis did not influence the severity of motor symptoms, the onset and severity of nonmotor symptoms, or LEDD.
Footnotes
- Conceptualisation: Svetlana Tomic, Igor Kuric, Tihana Gilman Kuric.
- Data curation: Svetlana Tomic, Igor Kuric, Ines Rajkovaca, Sara Matosa, Jagoda Kragujevic.
- Formal analysis: Svetlana Tomic, Zvonimir Popovic.
- Investigation: Igor Kuric, Tihana Gilman Kuric.
- Methodology: Svetlana Tomic, Igor Kuric.
- Project administration: Tea Mirosevic Zubonja.
- Resources: Svetlana Tomic, Igor Kuric.
- Software: Zvonimir Popovic.
- Supervision: Svetlana Tomic.
- Validation: Igor Kuric.
- Visualization: Svetlana Tomic.
- Writing—original draft: Svetlana Tomic.
- Writing—review & eiting: Igor Kuric.
Conflicts of Interest: Svetlana Tomic received honoraria from AbbVie d.o.o., Pliva Hrvatska d.o.o., Phoenix Pharmacia d.o.o., Sanofi-Aventis Croatia d.o.o., Novartis Hrvatska d.o.o., Fresenius Kabi d.o.o., Makpharm d.o.o., Medis Adria d.o.o., Merz Pharmaceuticals GmbH and been sponsored for congress from Medis Adria d.o.o.; Igor Kuric received speaker honorarium from AbbVie d.o.o, Novartis Hrvatska d.o.o. and Elly Lilly d.o.o. and has been sponsored for congress from Jansen Cilag d.o.o, Berlin-Chemie Menarini and Merck; Tihana Gilman Kuric received honoraria from Novartis Hrvatska d.o.o. and been sponsored for congress from Medis Adria d.o.o. and Makpharm d.o.o; Zvonimir Popovic received honoraria from Novartis Hrvatska d.o.o; Tea Mirosevic Zubonja received honoraria from Novartis Hrvatska d.o.o, Roche d.o.o., Pliva Hrvatska d.o.o., Merck d.o.o.; Jagoda Kragujevic, Ines Rajkovaca and Sara Matosa have nothing to declare.
Funding Statement: None
Availability of Data and Material
All data generated or analyzed during the study are included in this published article and its supplementary information files.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2022.18.6.628.
Difference in age, sex, disease duration, UPDRS III, NMSQ, PDSS, SCOPA-AUT, and LEDD in regard to seborrheic dermatitis
References
- 1.Beitz JM. Parkinson’s disease: a review. Front Biosci (Schol Ed) 2014;6:65–74. doi: 10.2741/s415. [DOI] [PubMed] [Google Scholar]
- 2.Pfeiffer RF. Non-motor symptoms in Parkinson’s disease. Parkinson ism Relat Disord. 2016;22 Suppl 1:S119–S122. doi: 10.1016/j.parkreldis.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Skorvanek M, Bhatia KP. The skin and Parkinson’s disease: review of clinical, diagnostic, and therapeutic issues. Mov Disord Clin Pract. 2016;4:21–31. doi: 10.1002/mdc3.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tucker D, Masood S. StatPearls [Internet] Treasure Island, FL: StatPearls Publishing; 2021. [cited 2021 Oct 1]. Seborrheic dermatitis. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551707/ [Google Scholar]
- 5.Fischer M, Gemende I, Marsch WC, Fischer PA. Skin function and skin disorders in Parkinson’s disease. J Neural Transm (Vienna) 2001;108:205–213. doi: 10.1007/s007020170088. [DOI] [PubMed] [Google Scholar]
- 6.Martignoni E, Godi L, Pacchetti C, Berardesca E, Vignoli GP, Albani G, et al. Is seborrhea a sign of autonomic impairment in Parkinson’s disease? J Neural Transm (Vienna) 1997;104:1295–1304. doi: 10.1007/BF01294730. [DOI] [PubMed] [Google Scholar]
- 7.Sariahmetoglu H, Soysal A, Sen A, Yuksel B, Celiker S, Ciftci-Kavaklioglu B, et al. Forehead sympathetic skin responses in determining autonomic involvement in Parkinson’s disease. Clin Neurophysiol. 2014;125:2436–2440. doi: 10.1016/j.clinph.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Shuster S, Thody AJ, Goolamali SK, Burton JL, Plummer N, Bates D. Melanocyte-stimulating hormone and parkinsonism. Lancet. 1973;1:463–464. doi: 10.1016/s0140-6736(73)91885-0. [DOI] [PubMed] [Google Scholar]
- 9.Burton JL, Cartlidge M, Shuster S. Effect of L-dopa on the seborrhoea of Parkinsonism. Br J Dermatol. 1973;88:475–479. doi: 10.1111/j.1365-2133.1973.tb15453.x. [DOI] [PubMed] [Google Scholar]
- 10.Arsic Arsenijevic VS, Milobratovic D, Barac AM, Vekic B, Marinkovic J, Kostic VS. A laboratory-based study on patients with Parkinson’s disease and seborrheic dermatitis: the presence and density of Malassezia yeasts, their different species and enzymes production. BMC Dermatol. 2014;14:5. doi: 10.1186/1471-5945-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Dis ord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 12.Fahn S, Elton R Members of the UPDRS Development Committee. In: Recent Developments in Parkinson’s Disease. Vol 2. Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Florham Park: Macmillan Health Care Information; 1987. The unified Parkinson’s disease rating scale; pp. 153–163. [Google Scholar]
- 13.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18:738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhuri KR, Schapira AH, Martinez-Martin P, Brown R, Koller W, Sethi K, et al. Can we improve the holistic assessment of Parkinson’s disease? The development of a non-motor symptom questionnaire and scale for Parkinson’s disease. Adv Clin Neurosci Rehabil. 2004;4:20–25. [Google Scholar]
- 15.Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, et al. The Parkinson’s Disease Sleep Scale: a new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2002;73:629–635. doi: 10.1136/jnnp.73.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov Disord. 2004;19:1306–1312. doi: 10.1002/mds.20153. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 18.Smith SA, Baker AE, Williams JH. Effective treatment of seborrheic dermatitis using a low dose, oral homeopathic medication consisting of potassium bromide, sodium bromide, nickel sulfate, and sodium chloride in a double-blind, placebo-controlled study. Altern Med Rev. 2002;7:59–67. [PubMed] [Google Scholar]
- 19.Appenzeller O, Harville D. Effect of L-dopa on seborrhea of Parkinsonism. Lancet. 1970;2:311–312. [PubMed] [Google Scholar]
- 20.Burton JL, Shuster S. Effect of L-dopa on seborrhoea of parkinsonism. Lancet. 1970;2:19–20. doi: 10.1016/s0140-6736(70)92476-1. [DOI] [PubMed] [Google Scholar]
- 21.Kohn SR, Pochi PE, Strauss JS, Sax DS, Feldman RG, Timberlake WH. Sebaceous gland secretion in Parkinson’s disease during L-dopa treatment. J Invest Dermatol. 1973;60:134–136. doi: 10.1111/1523-1747.ep12682040. [DOI] [PubMed] [Google Scholar]
- 22.Mastrolonardo M, Diaferio A, Logroscino G. Seborrheic dermatitis, increased sebum excretion, and Parkinson’s disease: a survey of (im)possible links. Med Hypotheses. 2003;60:907–911. doi: 10.1016/s0306-9877(03)00094-x. [DOI] [PubMed] [Google Scholar]
- 23.Cowley NC, Farr PM, Shuster S. The permissive effect of sebum in seborrhoeic dermatitis: an explanation of the rash in neurological disorders. Br J Dermatol. 1990;122:71–76. doi: 10.1111/j.1365-2133.1990.tb08241.x. [DOI] [PubMed] [Google Scholar]
- 24.Bolognia JL, Jorizzo JL, Rapini RP, Callen JP, Horn TD, Mancini AJ, et al. Dermatology. 2nd ed. St. Louis: Mosby Elsevier; 2008. [Google Scholar]
- 25.Ahtonen P, Lehtonen OP, Kero P, Tunnela E, Havu V. Malassezia furfur colonization of neonates in an intensive care unit. Mycoses. 1990;33:543–547. doi: 10.1111/myc.1990.33.11-12.543. [DOI] [PubMed] [Google Scholar]
- 26.Gupta AK, Bluhm R, Cooper EA, Summerbell RC, Batra R. Seborrheic dermatitis. Dermatol Clin. 2003;21:401–412. doi: 10.1016/s0733-8635(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 27.Elewski BE. Safe and effective treatment of seborrheic dermatitis. Cu tis. 2009;83:333–338. [PubMed] [Google Scholar]
- 28.Maietta G, Fornaro P, Rongioletti F, Rebora A. Patients with mood depression have a high prevalence of seborrhoeic dermatitis. Acta Derm Venereol. 1990;70:432–434. [PubMed] [Google Scholar]
- 29.Binder RL, Jonelis FJ. Seborrheic dermatitis in neuroleptic-induced parkinsonism. Arch Dermatol. 1983;119:473–475. [PubMed] [Google Scholar]
- 30.Ravn AH, Thyssen JP, Egeberg A. Skin disorders in Parkinson’s disease: potential biomarkers and risk factors. Clin Cosmet Investig Dermatol. 2017;10:87–92. doi: 10.2147/CCID.S130319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanner C, Albers K, Goldman S, Fross R, Leimpeter A, Klingman J, et al. Seborrheic dermatitis and risk of future Parkinson’s disease (PD) (S42.001) Neurology. 2012;78(1 Supplement):S42.001 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Difference in age, sex, disease duration, UPDRS III, NMSQ, PDSS, SCOPA-AUT, and LEDD in regard to seborrheic dermatitis
Data Availability Statement
All data generated or analyzed during the study are included in this published article and its supplementary information files.