Abstract
Background and Purpose
Fingolimod (FTY) inhibits lymphocyte egress from lymphoid organs to cause lymphopenia, but the clinical implications of FTY-induced lymphopenia are not fully understood. We aimed to determine the frequency and severity of lymphopenia during FTY treatment among Korean patients with multiple sclerosis (MS), and its association with infections.
Methods
We retrospectively reviewed the medical records of patients with MS treated using FTY from 12 referral centers in South Korea between March 2013 and June 2021. Patients were classified according to their nadir absolute lymphocyte count (ALC) during treatment: grade 1, 800–999/µL; grade 2, 500–799/µL; grade 3, 200–499/µL; and grade 4, <200/µL.
Results
FTY treatment was administered to 69 patients with a median duration of 18 months (range=1–169 months), with 11 patients being treated for ≥7 years. During FTY treatment, mean ALCs were reduced after the first month (653.0±268.9/µL, mean±standard deviation) (p<0.0001) and remained low during treatment lasting up to 84 months. During follow-up, 41 (59.4%) and 7 (10.1%) patients developed grade-3 and grade-4 lymphopenia, respectively. No significant difference was found in age at FTY initiation, sex, baseline ALC, body mass index, or prior disease-modifying treatment between patients with and without grade-4 lymphopenia. Infections were observed in 11 (15.9%) patients, and the frequencies of patients with and without grade-4 lymphopenia were similar.
Conclusions
FTY treatment induced grade-4 lymphopenia in 10% of South Korean patients with MS, but did not appear to be associated with an increased infection risk.
Keywords: multiple sclerosis, fingolimod, lymphopenia
INTRODUCTION
Fingolimod (FTY) is a sphingosine-1-phosphate receptor modulator that is approved as an oral medication for patients with relapsing-remitting multiple sclerosis (RRMS). The absolute lymphocyte counts (ALCs) of patients with FTY are typically decreased due to the inhibition of the egress of naïve and central memory T cells from lymph nodes.1 This inhibition reduces circulating lymphocytes and cell influx to inflammation sites in the central nervous system, which may have a beneficial effect on multiple sclerosis (MS). Considering the mechanism of FTY, lymphopenia is thought to contribute to its therapeutic effect; however, recent studies found that lymphocyte levels were not associated with therapeutic efficacy.2,3 Receiving FTY may preserve many aspects of the immune function of patients despite FTY-induced lymphopenia being associated with lymphocyte redistribution.4 Nevertheless, a low lymphocyte count during FTY treatment may raise concerns about infection-related adverse effects, including since opportunistic infections such as herpes viruses and cryptococcal infections have been reported in patients who received FTY in the postmarketing experience.5
Grade-4 lymphopenia (ALC <200/µL) is important since it is recommended as a criterion for FTY treatment discontinuation.5,6,7,8 Nevertheless, the information about treatment safety of patients with sustained grade-4 lymphopenia is inadequate since FTY was initially discontinued for patients with repeated low ALC (<100/µL), and later for those with <200/µL in the phase-3 trials.5,6,7 According to the European Medicines Agency8 and information provided on the FTY packaging,9 interruption of FTY treatment until lymphocyte level recovery is recommended if the lymphocyte count is confirmed at <200/µL. However, the USA prescription information does not include a specific recommendation for drug modification, discontinuation, or readministration according to ALC levels.10,11
To better understand the risk–benefit profiles of FTY in the real world, we performed a retrospective cohort analysis of 69 patients who were prescribed FTY at multiple MS centers in South Korea. We assessed the frequency and risk factors of lymphopenia during FTY treatment as well as its association with infection and treatment efficacy.
METHODS
Patients and study design
This multicenter, retrospective cohort study included 69 consecutive patients with RRMS who received FTY between March 2013 and June 2021 at 12 centers in South Korea. The study was approved by the Institutional Review Board of each center.
Clinical, laboratory, and radiological data from the last available assessment of each patient prior to initiating FTY treatment and data from the most recent assessment under FTY were collected.
Data regarding demographics, pre- and posttreatment annualized relapse rates (ARRs), pre- and posttreatment Expanded Disability Status Scale (EDSS) score,12 disease-modifying therapy (DMT) before FTY, FTY dose adjustment history, varicella zoster (VZ) or herpes zoster (HZ) vaccination history, and positivity for varicella zoster virus (VZV) immunoglobulin G (IgG) were collected from the medical records of the patients. Serial ALCs were also measured at baseline and during FTY treatment. Lymphocyte counts were assessed monthly for the first 6 months after FTY initiation and then at 3-month intervals thereafter.
Patient clinical parameters were also recorded, including 1) clinical relapse, as determined by the physician treating the patients, 2) radiological evidence of disease activity (at least one new or enlarging T2-weighted or gadolinium-enhanced lesion when compared with baseline magnetic resonance imaging), and 3) disability progression according to EDSS score.
To assess FTY safety profiles, the infection history, liver function test (LFT) elevation, cardiac monitoring, malignancy, macular edema, and dermatological evaluations were recorded during treatment. Abnormal LFT was defined as aspartate aminotransferase (AST) >40 U/L or alanine aminotransferase (ALT) >40 U/L.
Patients were graded based on the nadir ALC during follow-up and were classified according to the following common terminology criteria of the National Cancer Institute for adverse events: grade 1, 800–999/µL; grade 2, 500–799/µL; grade 3, 200–499/µL; and grade 4, <200/µL.13
Outcomes
The main outcomes of the study were lymphopenia incidence, lymphocyte-count temporal patterns, and safety profiles, including infection incidence rates according to lymphocyte levels during FTY treatment in patients with RRMS.
Statistical analysis
All statistical analyses were performed using SPSS (version 26.0, IBM Corp., Armonk, NY, USA) or GraphPad Prism (version 9, GraphPad, La Jolla, CA, USA). Mean±standard deviation and 95% confidence interval values were calculated for each value. Differences in patient characteristics were defined using Fisher’s exact test and the Mann–Whitney U test. Wilcoxon matched-pair tests were used to assess differences between baseline and follow-up tests. p<0.05 was considered statistically significant.
RESULTS
Patient characteristics
Table 1 lists the baseline clinical characteristics. The mean age at FTY treatment initiation was 33.1 years (range=19–53 years). The median FTY treatment duration was 18 months (interquartile range=12–27 months), and 11 patients were maintained on FTY treatment over 7 years. Prior to FTY initiation, 50 (72.4%) patients were confirmed as VZV IgG-positive, and 22 (31.8%) had received VZ vaccination. Overall, 59 (85.5%) patients were either VZV IgG-positive or received VZ vaccination prior to FTY therapy. HZ vaccine was administered to 12 patients (17.4%) before FTY treatment.
Table 1. Baseline clinical characteristics of 69 patients treated with FTY.
| Characteristic | All (n=69) | Gr-4 lymphopenia (n=7) | Non-Gr-4 lymphopenia (n=62) | p | |
|---|---|---|---|---|---|
| Age, years | 36.1±9.8 | 31.7±10.4 | 36.6±9.7 | 0.287 | |
| Onset age, years | 26.1±8 | 20.7±5.6 | 26.7±7.9 | 0.066 | |
| Sex, female | 44 (63.8) | 3 (42.9) | 41 (66.1) | 0.245 | |
| Age at initiation of FTY, years | 33.1±9.2 | 29.3±9.4 | 33.6±9.2 | 0.253 | |
| Body mass index, kg/m2 | 23.7±4.6 | 23.2±2.9 | 23.8±4.7 | 0.905 | |
| Body mass index <18.5 kg/m2 | 5 (7.2) | 0 (0) | 5 (8.1) | >0.999 | |
| Varicella zoster vaccination or VZV IgG-positive | 59 (85.5) | 6 (85.7) | 53 (85.5) | 1.000 | |
| Pretreatment ALC, /μL | 1,826±787.1 | 1,709.4±454.2 | 1,839.8±819.2 | 0.639 | |
| Pretreatment EDSS score | 2 [0–6] | 1 [0–4] | 2 [0–6] | 0.07 | |
| Pretreatment ARR | 1.2±1.3 | 1.1±1.1 | 1.2±1.4 | 0.564 | |
| DMT before FTY, numbers | 1.7±0.9 | 1.6±0.5 | 1.7±0.9 | >0.999 | |
| Recent DMT before FTY | |||||
| Interferon-β-Ia or -Ib | 25 (36.2) | 1 (14.3) | 24 (38.7) | 0.199 | |
| Glatiramer acetate | 5 (7.2) | 1 (14.3) | 4 (6.5) | 0.424 | |
| Dimethyl fumarate | 19 (27.5) | 2 (28.6) | 17 (27.4) | >0.999 | |
| Teriflunomide | 15 (21.7) | 3 (42.9) | 12 (19.4) | 0.169 | |
| Natalizumab | 2 (2.9) | 0 (0) | 2 (3.2) | >0.999 | |
| Alemtuzumab | 2 (2.9) | 0 (0) | 2 (3.2) | >0.999 | |
Data are mean±SD, n (%), or median [range] values.
ALC, absolute lymphocyte count; ARR, annualized relapse rate; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; FTY, fingolimod; Gr, grade; IgG, immunoglobulin G; SD, standard deviation; VZV, varicella zoster virus.
Lymphopenia
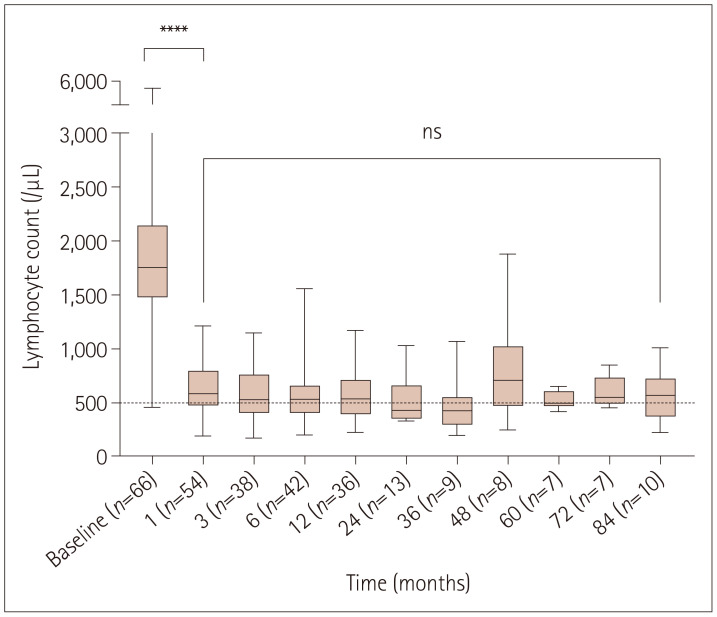
In our cohort, lymphocyte counts were measured an average of 9.3 times per patient during therapy. All patients demonstrated a decrease in ALC after FTY treatment. A significant decrease in mean ALC was observed after the first month (n=53, 653.0±268.9/µL) (p<0.0001), which was approximately 64% of the pretreatment mean value (n=66, 1,826.0±787.1/µL). ALC remained stable and low for up to 84 months during the study (n=10, 723.4±327.2/µL) (Fig. 1).
Fig. 1. Absolute lymphocyte counts during fingolimod therapy. Each box plot shows the median, first and third quartiles, and range. Asterisks indicate the significance level in pairwise comparison. ****p<0.0001. ns, not significant.
During the course of FTY treatment, grade-1, grade-2, grade-3, and grade-4 lymphopenia developed at the nadir ALC in 4 (5.7%), 16 (23.2%), 41 (59.4%), and 7 (10.1%) patients, respectively. Only one (1.4%) patient did not have lymphopenia (ALC >1,000/µL) during 12 months of FTY treatment. The median time to develop the first grade-4 lymphopenia was 6 months (range 1–42 months), and 71% of grade-4 lymphopenias occurred within the first year of treatment. Grade-4 lymphopenia occurred twice in two (2.9%) patients (at 15 months and 36 months, and 42 months and 81 months, respectively) and only once in the remaining patients.
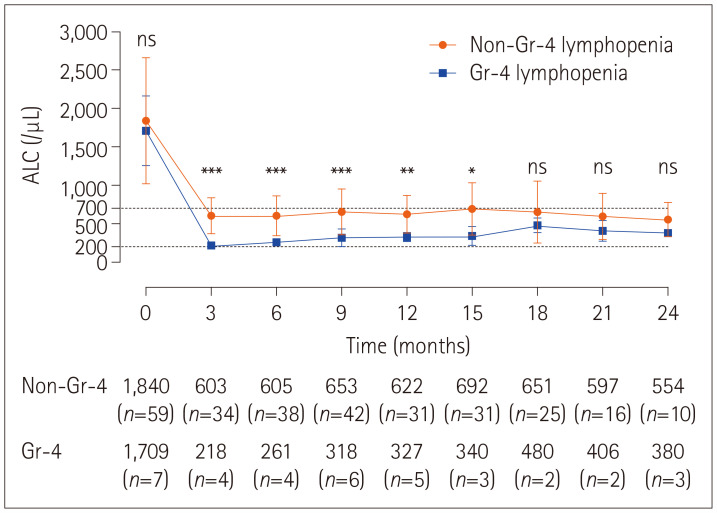
Fig. 2 illustrates the temporal patterns of ALC over the first 24 months. Baseline ALC did not differ significantly between patients with and without grade-4 lymphopenia (1,709.4±454.2/µL vs. 1,839.8±819.2/µL, p=0.639), while mean ALCs were lower and throughout FTY therapy remained lower in patients with than without grade-4 lymphopenia.
Fig. 2. Serial changes in mean absolute lymphocyte count (ALC) during follow-up in patients with and without grade (Gr)-4 lymphopenia, and the comparison between these two groups during 24 months of fingolimod (FTY) therapy. Patients whose lymphocyte count reduced below 200/µL at least once during FTY therapy were classified as grade-4 lymphopenia. There was no significant difference in mean ALC at baseline, while the mean ALC was lower and remained low throughout FTY therapy in patients with than without grade-4 lymphopenia. *p<0.05; **p<0.01; ***p<0.001.
No significant differences in sex ratio, age at FTY initiation, body mass index (BMI), and prior DMT were found between patients with and without grade-4 lymphopenia (Table 1).
In the analysis of treatment responses, 85.5% of patients remained on FTY, 94.2% remained free from relapse, and 79.7% did not show evidence of MS disease activity for a mean of 32 months. No significant differences were observed between the two groups in posttreatment ARR, number of patients with worsening EDSS scores, radiological activity, and evidence of disease activity (Table 2).
Table 2. Comparison of characteristics at last follow-up after FTY treatment between Gr-4 lymphopenia and non-Gr-4 lymphopenia.
| Characteristic | All (n=69) | Gr-4 lymphopenia (n=7) | Non-Gr-4 lymphopenia (n=62) | p | |
|---|---|---|---|---|---|
| Duration of FTY treatment, months | 32.3±4.7 | 28.5±29.6 | 32.7±40.9 | 0.921 | |
| Posttreatment ALC, /μL | 669.4±304.7 | 330±84.7 | 690.0±263.9 | <0.001 | |
| Posttreatment EDSS score | 2 [0–6.5] | 1 [0–3] | 2 [0–6.5] | 0.077 | |
| Patients with worsening EDSS score | 9 (13) | 0 (0) | 9 (14.5) | 0.582 | |
| Posttreatment ARR | 0.04±0.30 | 0.06±0.20 | 0.04±0.30 | 0.303 | |
| Patients with no radiological activity | 56 (81.2) | 5 (71.4) | 51 (82.3) | 0.609 | |
| Patients without clinical relapse | 65 (94.2) | 6 (85.7) | 59 (95.2) | 0.355 | |
| Patient with NEDA | 55 (79.7) | 5 (71.4) | 50 (80.6) | 0.624 | |
| Patients with cessation of FTY | 10 (14.5) | 1 (14.3) | 9 (14.5) | >0.999 | |
| Dose | |||||
| Full dose | 49 (71) | 4 (57.1) | 45 (72.6) | ||
| Transient EOD | 5 (7.2) | 1 (14.3) | 4 (6.5) | ||
| Transient suspension | 2 (2.9) | 0 (0) | 2 (3.2) | ||
| Maintained EOD | 3 (4.3) | 1 (14.3) | 2 (3.2) | ||
| Interrupting FTY and switching to other DMT | 10 (14.5) | 1 (14.3) | 9 (14.5) | ||
Data are mean±SD, n (%), or median [range] values.
ALC, absolute lymphocyte count; ARR, annualized relapse rate; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; EOD, every other day; FTY, fingolimod; Gr, grade; IgG, immunoglobulin G; NEDA, no evidence of disease activity; SD, standard deviation; VZV, varicella zoster virus.
Infection
Infection during FTY treatment was observed in 11 patients (15.9%) (Table 3). There were no significant differences in the overall infection incidence rate between patients with and without grade-4 lymphopenia (p=0.309). Upper respiratory tract infection was reported in four patients (5.8%). Otitis media developed in two patients. Nasopharyngitis, fever of unknown origin, and urinary tract infection each occurred in one patient. HZ infection was reported in one (1.4%) patient with grade-2 lymphopenia at the nadir ALC. This patient was treated using an antiviral agent and transient FTY suspension (<1 month). All infection events were successfully treated without complications and did not lead to FTY interruption except HZ infection. No progressive multifocal leukoencephalopathy or other serious adverse infection, death, life-threatening event, prolonged hospitalization, or adverse event requiring intervention was reported.
Table 3. Incidence of adverse events in patients treated with FTY according to nadir ALC.
| All (n=69) | Non-lymphopenia (n=1) | Gr-1 lymphopenia (n=4) | Gr-2 lymphopenia (n=16) | Gr-3 lymphopenia (n=41) | Gr-4 lymphopenia (n=7) | ||
|---|---|---|---|---|---|---|---|
| Any infection | 11 (15.9) | 0 (0.0) | 0 (0.0) | 5 (31.3) | 4 (9.8) | 2 (28.6) | |
| Nasopharyngitis | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | |
| Otitis media | 2 (2.9) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 1 (14.3) | |
| FUO | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.4) | 0 (0.0) | |
| URI | 4 (5.8) | 0 (0.0) | 0 (0.0) | 2 (12.5) | 2 (4.9) | 0 (0.0) | |
| UTI | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0 (0.0) | |
| Prostatitis | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.4) | 0 (0.0) | |
| Herpes zoster | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0 (0.0) | |
| Elevated LFT (>3 times ULN) | 6 (8.7) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 4 (9.8) | 1 (14.3) | |
| Skin rash | 2 (2.9) | 0 (0.0) | 0 (0.0) | 2 (12.5) | 0 (0.0) | 0 (0.0) | |
| Neoplasm (benign) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0 (0.0) | |
| Bradycardia | 2 (2.9) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 1 (2.4) | 0 (0.0) | |
Data are n (%) values.
ALC, absolute lymphocyte count; FTY, fingolimod; FUO, fever of unknown origin; LFT, liver function test; ULN, upper limit of normal; URI, upper respiratory tract infection; UTI, urinary tract infection.
Other safety profiles
ALT or AST levels that were at three times the upper limit of the normal range were observed in six patients (8.7%). Mild elevations of ALT or AST (from >40 U/L to <3 times the upper limit of the normal range) were observed in 18 patients (26.1%). Of the patients with abnormal LFTs, mean ALT and AST were 113.8 U/L (range 50–284 U/L) and 66 U/L (range 41–110 U/L), respectively. FTY was discontinued in two patients due to persistently increased LFTs. Mild LFT elevation continued for a mean of 25 months in three patients. In the remaining patients, increased LFTs returned to normal after a mean period of 4.6 months (range 0.5–16 months).
Two cases of electrocardiogram abnormalities, including bradycardia or prolonged QTc, were observed when the first dose was administered. Those patients remained stable during follow-up.
Ophthalmological monitoring was performed on 53.6% of the patients in our cohort, and macular edema was not reported. One case of a benign tumor around the parotid gland and two cases with skin rash were reported. In one of the patients who developed a skin rash, the FTY dose was transiently reduced to every other day (EOD). FTY was discontinued in the other, and treatment was switched to dimethyl fumarate, but was switched back to FTY using dose titration due to disease activity. All patients remained stable during follow-up.
Dose adjustment
A full FTY dose was administered to 49 patients (71%), including 4 with transient grade-4 lymphopenia.
FTY administration was reduced to EOD in eight patients (11.5%) due to grade-3 lymphopenia (n=3), grade-4 lymphopenia (n=2), bradycardia (n=1), neutropenia (n=1), and skin rash (n=1). Of those, FTY remained at EOD in three patients, while it was escalated to daily dosing in the other five patients during follow-up. Of the two patients whose dose was reduced due to grade-4 lymphopenia, one remained on EOD administration continuously, whereas the other patient with grade-4 lymphopenia on two occasions was managed by reducing FTY to EOD for 3 months each time and then returned to daily dosing.
FTY was transiently suspended in 2 (2.9%) patients due to bradycardia (n=1) and HZ infection (n=1), but was resumed after 1 month.
FTY was discontinued and treatment was switched to other DMTs in ten (14.5%) patients due to uncontrolled disease activity (n=3), LFT elevation (n=2), grade-4 lymphopenia (n=1), leukopenia (white blood cell count=1,900/µL) (n=1), skin rash (n=1), and personal request (n=2). One of these patients whose FTY was discontinued due to grade-4 lymphopenia was switched to dimethyl fumarate, but then switched back due to disease activity after 1 year of treatment. Grade-4 lymphopenia did not recur during the following one year of FTY treatment.
DISCUSSION
In this current study, lymphopenia (grade-1–4) developed in 68 (98.6%) patients after FTY treatment. FTY led to grade-4 lymphopenia in 10% of patients, which was transient for most patients and was not significantly related to infection rate, disease activity, or serious adverse events compared with in patients with grade-1–3 lymphopenia.
According to early real-world data, 15%–20% of patients with MS in Western populations develop grade-4 lymphopenia during FTY therapy.5,9,14 An early retrospective study of 41 Japanese patients with MS identified that grade-4 lymphopenia had an incidence of 29% after FTY therapy.15 In the current study, grade-4 lymphopenia associated with FTY treatment was less common (10%) than in Western and Japanese populations. This finding might be explained by adjusted doses even for some patients with grade-3 lymphopenia, as this could have contributed to preventing the development of grade-4 lymphopenia.
According to a study of long-term FTY treatment in primary progressive MS (INFORMS), the proportion of patients with ALC progressing to grade-4 lymphopenia did not increase with FTY exposure duration over 4 years of treatment (2.5%–6.8%).11 We consistently found no significant reduction in mean ALC levels after several years of treatment, although only 16% of patients received FTY for over 7 years (Fig. 1). Even so, further studies with large samples are required to clarify the changes in ALC levels according to FTY treatment duration.
Previous Western studies suggested that underweight females (BMI <18.5 kg/m2) and patients with low baseline lymphocyte counts (<1,600/µL) have an increased risk of developing grade-4 lymphopenia.14 In a Japanese cohort, low baseline ALC and history of treatment with interferon-β were suggested as risk factors for FTY-induced lymphopenia.15 However, none of the known risk factors including baseline ALC, BMI, and previous treatment history were associated with lymphopenia in the current study. There is a recent report of no significant association between baseline ALC and lymphocyte levels during FTY treatment, which was consistent with the current data.16 However, the inclusion of an insufficient number of patients with grade-4 lymphopenia might have resulted in poor statistical power in the current study. Further research is therefore required to confirm these findings and to identify the risk factors for lymphopenia.
This study found no significant difference in treatment response according to lymphopenia status. These results are consistent with previous studies finding that the lymphocyte count in peripheral blood was not associated with the level of clinical response to FTY treatment.2,3 Recent studies suggested that analyzing lymphocyte subpopulations, such as CD4-positive T cells16 and the percentage of recent thymic emigrants,17 provides better predictions of treatment responses.
The overall infection incidence was lower in our study (15.9%) than in previous clinical trials (51%–53%),7 but was similar to those of real-world studies (10.7%–11.6%).3,18 In the INFORMS study,11 safety data indicated that infection risk was not significantly higher in the FTY treatment group than in the placebo group over a 5-year follow-up; moreover, there was no apparent association of the nadir or mean ALC with infection incidence. A postmarketing study demonstrated that FTY treatment did not induce higher infection rates than receiving placebo and that the incidence of serious infection was low (approximately 2%), suggesting that FTY therapy is not associated with increased rates of serious infection.5 The current study also found that overall infection rates were not higher in patients with grade-4 lymphopenia than in those with lymphopenia of grades.1,2,3
Nonetheless, HZ infection was more common in patients who received FTY at 0.5 mg/day (1.6%–3%) than in those who received placebo (1.0%) in clinical trial studies,19,20 and the overall HZ incidence rate was 7 per 1,000 patient-years in a postmarketing study of FTY.21 In the current study, HZ infection was identified in one patient (1.4%). These low infection rates were consistent with those observed previously.19,20 HZ infection was not associated with grade-4 lymphopenia in this study. The INFORMS study found no apparent association of nadir or mean ALCs with herpes infection,11 implying that the small increase in HZ infection incidence due to FTY might not be associated with low ALC levels. Reactivation of VZV has been reported, even in patients with a high VZ IgG titer before FTY treatment.18 In our study, 17.4% of patients were vaccinated for HZ, since boosting the anti-VZV adaptive immune responses before FTY treatment may impact the prevention of HZ infection during FTY treatment.22
In the current study, the ALC levels of four patients who presented with transient grade-4 lymphopenia were restored to a grade-3 lymphopenia level with no infection events despite FTY dose being maintained at 0.5 mg/day. Moreover, there are previous reports of withdrawal of FTY treatment being a risk factor for disease activity rebound,23,24,25 and that a half dose of FTY increased the relapse risk.26 Considering these results, confirmation of persistent grade-4 lymphopenia through frequent monitoring of ALC levels may be a more-prudent approach than prompt FTY treatment discontinuation if grade-4 lymphopenia occurs.
The prevalence of ALT or AST levels increasing to at least three times the upper limit of the normal range in the current study (8.7%) was similar to those in clinical trials (8%–8.5%).6,19 Acceptable tolerance was observed in the current study. The discontinuation rate was quite low in the current cohort (14.5%) compared with those in previous studies (10.6%–43.8%),27,28,29 and grade-4 lymphopenia was not considered the main reason for discontinuation.
The current findings should be interpreted in the context of the potential study limitations. Due to its retrospective design, not all blood samples were taken at regular intervals. In addition, the smallness of the sample, particularly the small number of patients with grade-4 lymphopenia, may have affected the statistical power.
In conclusion, grade-4 lymphopenia occurred in approximately 10% of Korean patients with MS who received FTY treatment. However, grade-4 lymphopenia recovered in most patients, and no serious infections occurred during follow-up. The safety profiles of FTY were consistent with the known risk–benefit profiles as observed in both clinical trials and real-world patients treated using FTY, regardless of the lymphopenia status. Nevertheless, adequate lymphocyte monitoring and infection surveillance are necessary to ensure safety during FTY therapy.
Footnotes
Ethics Statement: The Institutional Review Board of Kosin University Hospital (2021-07-057), National Cancer Center (2019-0259), Seoul National University Hospital (H-1902-079-1011), Yonsei University College of Medicine (4-2019-0576), Samsung Medical Center (2020-04-119), Asan Medical Center (2019-1382), Dongguk University Ilsan Hospital (2018-05-003), Jeju National University School of Medicine (2021-09-042), Chonnam National University Hospital (2019-202), Konkuk University Medical Center (2021-07-071), Busan Paik Hospital (2021-06-026), and Chungnam National University Hospital (2021-06-094).
- Conceptualization: So-Young Huh, Su-Hyun Kim, Ho Jin Kim.
- Data collection: all authors.
- Funding acquisition: Ho Jin Kim.
- Supervision: Su-Hyun Kim, Ho Jin Kim.
- Visualization: So-Young Huh.
- Writing—original draft: So-Young Huh.
- Writing—review & editing: all authors.
Conflicts of Interest: Kim SH has lectured, consulted, and received honoraria from Bayer Schering Pharma, Biogen, Genzyme, Merck Serono, and UCB and received a grant from the National Research Foundation of Korea. Min JH has lectured, consulted, and received Honoria from Bayer Schering Pharma, Merck Serono, Biogen Idec, Sanofi Genzyme, Teva-Handok, UCB, Samsung Bioepis, Mitsubishi Tanabe Pharma and Roche; received a grant from the National Research Foundation of Korea and SMC Research and Development Grant. Kwon YN received research support from Eisai; had lectured, consulted, and received honoraria from Celltrion, Eisai, Merck Serono, Roche, and Sanofi Genzyme. Kim HJ received a grant from the National Research Foundation of Korea and research support from Aprilbio and Eisai; received consultancy/speaker fees from Alexion, Aprilbio, Biogen, Celltrion, Daewoong, Eisai, GC Pharma, HanAll BioPharma, MDimune, Merck Serono, Novartis, Roche, Sanofi Genzyme, Teva-Handok, UCB, and Viela Bio; is a co-editor for the Multiple Sclerosis Journal and an associated editor for the Journal of Clinical Neurology. Kim HJ was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement: This work was supported by the National Research Foundation of Korea (Grant No. NRF-2018R1A5A2023127).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1.Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol. 2009;158:1173–1182. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fragoso YD, Spelman T, Boz C, Alroughani R, Lugaresi A, Vucic S, et al. Lymphocyte count in peripheral blood is not associated with the level of clinical response to treatment with fingolimod. Mult Scler Relat Disord. 2018;19:105–108. doi: 10.1016/j.msard.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Boffa G, Bruschi N, Cellerino M, Lapucci C, Novi G, Sbragia E, et al. Fingolimod and dimethyl-fumarate-derived lymphopenia is not associated with short-term treatment response and risk of infections in a real-life MS population. CNS Drugs. 2020;34:425–432. doi: 10.1007/s40263-020-00714-8. [DOI] [PubMed] [Google Scholar]
- 4.Mehling M, Brinkmann V, Antel J, Bar-Or A, Goebels N, Vedrine C, et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008;71:1261–1267. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- 5.Francis G, Kappos L, O’Connor P, Collins W, Tang D, Mercier F, et al. Temporal profile of lymphocyte counts and relationship with infections with fingolimod therapy. Mult Scler. 2014;20:471–480. doi: 10.1177/1352458513500551. [DOI] [PubMed] [Google Scholar]
- 6.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 8.European Medicines Agency. Gilenya, INN-fingolimod. Summary of product characteristics [Internet] Amsterdam: European Medicines Agency; [cited 2021 Jul 20]. Available from: https://www.ema.europa.eu/en/documents/product-information/gilenya-epar-product-information_en.pdf. [Google Scholar]
- 9.Novartis Pharmaceuticals UK Ltd. Gilenya 0.5 mg hard capsules [Internet] London: Novartis; [cited 2021 Jul 20]. Available from: https://www.medicines.org.uk/emc/product/10357/smpc. [Google Scholar]
- 10.Novartis Pharmaceuticals. Gilenya (fingoliimod) prescribing information [Internet] East Hanover: Novartis Pharmaceuticals; [cited 2021 Jul 20]. Available from: https://www.novartis.com/us-en/sites/novartis_us/files/gilenya.pdf. [Google Scholar]
- 11.Fox EJ, Lublin FD, Wolinsky JS, Cohen JA, Williams IM, Meng X, et al. Lymphocyte counts and infection rates: long-term fingolimod treatment in primary progressive MS. Neurol Neuroimmunol Neuroinflamm. 2019;6:e614. doi: 10.1212/NXI.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Common terminology criteria for adverse events (CTCAE), version 4.0 [Internet] Bethesda: National Institutes of Health; [cited 2021 Jul 20]. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. [Google Scholar]
- 14.Warnke C, Dehmel T, Ramanujam R, Holmen C, Nordin N, Wolfram K, et al. Initial lymphocyte count and low BMI may affect fingolimod-induced lymphopenia. Neurology. 2014;83:2153–2157. doi: 10.1212/WNL.0000000000001049. [DOI] [PubMed] [Google Scholar]
- 15.Ohtani R, Mori M, Uchida T, Uzawa A, Masuda H, Liu J, et al. Risk factors for fingolimod-induced lymphopenia in multiple sclerosis. Mult Scler J Exp Transl Clin. 2018;4:2055217318759692. doi: 10.1177/2055217318759692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann M, Haase R, Proschmann U, Ziemssen T, Akgün K. Real world lab data: patterns of lymphocyte counts in fingolimod treated patients. Front Immunol. 2018;9:2669. doi: 10.3389/fimmu.2018.02669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quirant-Sánchez B, Hervás-García JV, Teniente-Serra A, Brieva L, Moral-Torres E, Cano A, et al. Predicting therapeutic response to fingolimod treatment in multiple sclerosis patients. CNS Neurosci Ther. 2018;24:1175–1184. doi: 10.1111/cns.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manni A, Direnzo V, Iaffaldano A, Di Lecce V, Tortorella C, Zoccolella S, et al. Gender differences in safety issues during fingolimod therapy: evidence from a real-life relapsing multiple sclerosis cohort. Brain Behav. 2017;7:e00804. doi: 10.1002/brb3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:545–556. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- 20.Kappos L, Cohen J, Collins W, de Vera A, Zhang-Auberson L, Ritter S, et al. Fingolimod in relapsing multiple sclerosis: an integrated analysis of safety findings. Mult Scler Relat Disord. 2014;3:494–504. doi: 10.1016/j.msard.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Arvin AM, Wolinsky JS, Kappos L, Morris MI, Reder AT, Tornatore C, et al. Varicella-zoster virus infections in patients treated with fingolimod: risk assessment and consensus recommendations for management. JAMA Neurol. 2015;72:31–39. doi: 10.1001/jamaneurol.2014.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winkelmann A, Löbermann M, Zettl UK. Indications for varicella zoster and herpes zoster vaccination in multiple sclerosis: current situation. Nervenarzt. 2019;90:1254–1260. doi: 10.1007/s00115-019-00806-x. [DOI] [PubMed] [Google Scholar]
- 23.Hakiki B, Portaccio E, Giannini M, Razzolini L, Pastò L, Amato MP. Withdrawal of fingolimod treatment for relapsing-remitting multiple sclerosis: report of six cases. Mult Scler. 2012;18:1636–1639. doi: 10.1177/1352458512454773. [DOI] [PubMed] [Google Scholar]
- 24.Havla JB, Pellkofer HL, Meinl I, Gerdes LA, Hohlfeld R, Kümpfel T. Rebound of disease activity after withdrawal of fingolimod (FTY720) treatment. Arch Neurol. 2012;69:262–264. doi: 10.1001/archneurol.2011.1057. [DOI] [PubMed] [Google Scholar]
- 25.Hatcher SE, Waubant E, Nourbakhsh B, Crabtree-Hartman E, Graves JS. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol. 2016;73:790–794. doi: 10.1001/jamaneurol.2016.0826. [DOI] [PubMed] [Google Scholar]
- 26.Zecca C, Merlini A, Disanto G, Rodegher M, Panicari L, Romeo MAL, et al. Half-dose fingolimod for treating relapsing-remitting multiple sclerosis: observational study. Mult Scler. 2018;24:167–174. doi: 10.1177/1352458517694089. [DOI] [PubMed] [Google Scholar]
- 27.Lapierre Y, O’Connor P, Devonshire V, Freedman MS, Kremenchutzky M, Yeung M, et al. Canadian experience with fingolimod: adherence to treatment and monitoring. Can J Neurol Sci. 2016;43:278–283. doi: 10.1017/cjn.2015.325. [DOI] [PubMed] [Google Scholar]
- 28.Harty GT, Wong SL, Tang M, Budhia S. Discontinuation of disease-modifying treatments in relapsing-remitting multiple sclerosis – A systematic literature review of observational studies. Eur Neurol Rev. 2019;14:36–43. [Google Scholar]
- 29.Zecca C, Roth S, Findling O, Perriard G, Bachmann V, Pless ML, et al. Real-life long-term effectiveness of fingolimod in Swiss patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2018;25:762–767. doi: 10.1111/ene.13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.