Abstract
Background
Patient-reported outcomes are increasingly used in the management of patients with multiple sclerosis to understand the patient's perspective of disease and treatment. These measures provide insights into important factors including treatment satisfaction, physical and psychological function, and quality of life.
Objective
To present results from the real-world PRO-ACT study in patients with multiple sclerosis who switched to alemtuzumab from another disease-modifying therapy.
Methods
This 24-month, prospective, multicenter, observational study had a primary endpoint of change in overall satisfaction, measured using the Treatment Satisfaction Questionnaire for Medication (TSQM) version 1.4. Secondary endpoints included the Multiple Sclerosis Impact Scale-29 (MSIS-29), Modified Fatigue Impact Scale-5 (MFIS-5), and the Patient-Determined Disease Steps (PDDS). Safety was monitored with adverse events (AEs).
Results
Of 199 enrolled patients, improvements were observed in mean TSQM scores for overall satisfaction (baseline, 50.3; year 2, + 13.2; p < 0.0001), effectiveness (49.3 and + 12.2; p < 0.0001), and side effects (77.6 and + 4.5; p = 0.04). Improvements were also observed in MSIS-29 physical (52.4 and −6.0; p < 0.0001), MSIS-29 psychological (53.4 and −7.0; p = 0.0003), and MFIS-5 (12.8 and −1.7; p < 0.0001). Most (95.0%) patients experienced ≥ 1 AE (88.4% mild, 67.8% moderate).
Conclusions
The primary endpoint was met; the safety of alemtuzumab was consistent with pivotal studies.
Keywords: Alemtuzumab, disease-modifying therapies, multiple sclerosis, quality of life
Introduction
Relapsing-remitting multiple sclerosis (RRMS) is characterized by discrete episodes of neurological dysfunction that are initially followed by resolution.1 As the disease progresses, inflammation is sustained, and neurologic function worsens.2 Patients often develop chronic sequelae including muscle weakness, impaired gait and mobility, bladder and bowel dysfunction, and cognitive and visual impairments.3 Symptoms and sequelae of MS affect patients’ social and work function and the ability to perform daily activities, in turn reducing the quality of life (QoL) for patients and caregivers.4–7
In the CARE-MS I (NCT00530348) and CARE-MS II (NCT00548405) studies, patients who received two courses of the disease-modifying therapy (DMT) alemtuzumab demonstrated statistically significant QoL improvements from baseline to year 2,8 and significantly greater improvements in clinical and MRI outcomes over 2 years versus subcutaneous interferon (IFN)-β-1a.9,10 In a long-term extension of the CARE-MS studies, treatment efficacy, including QoL outcomes, was maintained over an additional 4 years.11,12
Patient-reported outcomes (PROs) are increasingly used in clinical practice to provide insight into the patient's perspective on disease management, including treatment satisfaction, physical and psychological function, and QoL.13,14 However, little is known about how alemtuzumab treatment influences QoL for patients in real-world settings, particularly among those who switch to alemtuzumab from another DMT. The PRO-ACT study was designed to systematically gather information on PROs from patients with RRMS who switched from prior DMTs to alemtuzumab in clinical practice. The study evaluated changes in treatment satisfaction, QoL, and functional status after switching to alemtuzumab, as well as the safety of alemtuzumab, in a real-world setting.
Materials and methods
Study design
PRO-ACT was a 24-month, prospective, multicenter, noninterventional, single-arm, observational study of patients with RRMS who discontinued prior DMT and were prescribed alemtuzumab in routine clinical practice at neurological clinics in the United States (US) and Canada.
Patients received two courses of intravenous (IV) alemtuzumab 12 mg/day: Course 1 on 5 consecutive days at baseline and Course 2 on 3 consecutive days, 12 months later (Supplemental Figure 1). Patients were monitored from alemtuzumab initiation to 48 months after the last dose, according to the locally approved alemtuzumab label and (at US sites) the Risk Evaluation and Mitigation Strategy. Safety was assessed via ongoing monitoring of treatment-emergent adverse events (TEAEs). Lymphocyte counts were assessed at baseline and every 3 months thereafter until month 24 or end of study (EOS). Patients completed PRO tools at baseline and months 6, 12, 18, and 24 or until EOS.
The study was conducted in accordance with the Declaration of Helsinki and all applicable local, national, and international regulations and guidelines. All patients signed an informed consent form before enrolling.
Participants
Adult patients with RRMS were invited by neurologists to participate in PRO-ACT after the decision had been made to transition from previous DMT to alemtuzumab (Supplemental Table 1). Patients were ineligible to participate if they had previously received alemtuzumab, were participating in another investigational interventional study or had MRI evidence of progressive multifocal leukoencephalopathy. Enrolled patients who discontinued alemtuzumab before month 24 could continue the study and complete assessments through month 24.
Study endpoints
The primary endpoint was absolute change in overall treatment satisfaction from baseline to month 24/EOS, measured using the Treatment Satisfaction Questionnaire for Medication (TSQM), version 1.4.13 Changes in TSQM subscales for treatment effectiveness, side effects, and convenience were secondary endpoints. Other secondary PRO endpoints included changes from baseline to month 24/EOS in the Multiple Sclerosis Impact Scale-29 (MSIS-29),15,16 Modified Fatigue Impact Scale-5 (MFIS-5),16 Patient-Determined Disease Steps (PDDS),17 and Health-Related Productivity Questionnaire-MS (HRPQ-MS) version 2.18 Tertiary PRO endpoints included changes from baseline to month 24/EOS in the Caregiver Health-Related Quality of Life in MS (CAREQoL-MS) questionnaire.19 Information was also collected on the most recent prior DMT, duration of prior DMT, and reason for switching to alemtuzumab.
Lymphocyte repopulation at 24 months was assessed using total lymphocyte counts and CD4+ T-cell counts. TEAEs were monitored throughout the study and for 24 months after the last alemtuzumab dose.
Sample size and statistical analyses
The study was planned to enroll 200 patients from approximately 45 centers. This sample size provided > 99% power to detect an overall change (improvement) from baseline in TSQM using a null hypothesis of no change versus baseline. The calculation assumed a ≥ 10-point change from baseline with a standard deviation (SD) of 20 using a paired t-test that was 2-sided α of 5%. The study also had a high power to show a change in each TSQM subscale.
All patients who received ≥ 1 alemtuzumab dose and had an evaluable primary endpoint (i.e. who had baseline and ≥ 1 post-baseline evaluation of TSQM overall satisfaction) were included in analyses of PRO endpoints. Safety analyses used data from all patients who received ≥ 1 alemtuzumab dose. Statistical analyses used a 5% significance level and 2-sided t-tests or 95% confidence interval (CI). Descriptive statistics, including mean, SD, median, and 95% CI, were used for continuous variables; count and percentage were used for categorical variables. The primary endpoint of TSQM change from baseline to month 24/EOS was analyzed using a mixed-effect model for repeated measures (MMRM) that used fixed categorical effects of visit as the time axis and was adjusted for the continuous covariate of baseline score. An unstructured correlation matrix was used to model within-person errors. Parameters were estimated using restricted maximum likelihood using the Newton–Raphson algorithm. Model-derived least square (LS) means were reported. Secondary PRO endpoints and lymphocyte counts were assessed similarly to the primary endpoint. A sensitivity analysis was conducted for the primary endpoint using a paired t-test to compare the month 24/EOS value for each patient versus their corresponding baseline value. Descriptive summaries were used to analyze CAREQoL-MS change and information about the most recent DMT. Unless otherwise specified in a PRO scoring method, missing data were not imputed and were not counted in percentages.
Prespecified analyses for all PRO and safety outcomes used subgroups defined according to the last DMT received before alemtuzumab. Group 1 patients had received natalizumab; group 2 received teriflunomide, dimethyl fumarate (DMF), fingolimod, or siponimod; and group 3 received IFN-β-1a, IFN-β-1b, pegylated IFN-β-1a, or glatiramer acetate. Post hoc analyses were also done for subgroups by patient age (< median age, 43 years or ≥ median age), MS duration (< 5 years, ≥ 5 to < 10 years, or ≥ 10 years), and use of assistive devices (unilateral brace, bilateral brace, one cane, two canes, walker, wheelchair/scooter, etc.).
Results
Participants
Of 199 patients who enrolled and received ≥ 1 alemtuzumab dose, 170 (85.4%) completed the 24-month study and were included in the evaluable population (Supplemental Figure 2). Patient demographics and disease characteristics are shown in Table 1. The mean age was 43.7 years (range, 19–73). A majority of participants were women (75.4%) and white (85.9%), with a mean (SD) time since MS diagnosis of 10.2 (7.5) years. Patients had mean (SD) 1.6 (1.6) relapses in the 2 years before study entry. About two-thirds of patients had signs of MRI disease activity at baseline. The mean (SD) follow-up duration was 22.3 (5.0) months.
Table 1.
Patient demographics and baseline disease characteristics, pharmacodynamics parameters, and PRO scores.
| Parameter | Number (%) of patients contributing data (N = 199)a | Mean (SD) or n (%) |
|---|---|---|
| Demographics | ||
| Age, years, mean (SD) | 43.7 (10.6) | |
| Female, n (%) | 150 (75.4) | |
| White, n (%) | 171 (85.9) | |
| Smoking statusb | ||
| Current smoker, n (%) | 198 (99.5) | 42 (21.2) |
| Former smoker, n (%) | 198 (99.5) | 49 (24.7) |
| Never smoker, n (%) | 198 (99.5) | 107 (54.0) |
| Time smoking, years, mean (SD) | 86 (85.6) | 17.5 (10.9) |
| Number of cigarettes/cigars/pipes per day, mean (SD) | 86 (85.6) | 12.7 (9.7) |
| Baseline disease characteristics | ||
| Duration of MS, years, mean (SD) | 10.2 (7.5) | |
| Number of relapses in the last 2 years, mean (SD) | 195 (98.0) | 1.6 (1.6) |
| Time since the last relapse, years, mean (SD) | 172 (86.4) | 1.2 (2.0) |
| Patients using any assistive devices, n (%)c | 82 (41.2) | |
| One cane, n (%) | 40 (20.1) | |
| Wheelchair/scooter, n (%) | 29 (14.6) | |
| Walker or two canes, n (%) | 29 (14.6) | |
| Unilateral brace, n (%) | 2 (1.0) | |
| Bilateral brace, n (%) | 2 (1.0) | |
| Other, n (%) | 1 (0.5) | |
| Patients with MRI disease activity, n (%) | 195 (98.0) | 136 (69.7) |
| EDSS score, mean (SD) | 66 (33.2) | 3.6 (1.9) |
| EDSS score ≥ 5, n (%) | 66 (33.2) | 21 (31.8) |
| Patients who received prior DMTs, n (%) | 198 (99.5)d | |
| Years on most recent prior DMT, mean (SD) | 2.5 (2.7) | |
| Months since discontinuation from most recent prior DMT, mean (SD) | 4.0 (8.9) | |
| Baseline pharmacodynamics | ||
| Total lymphocyte count (109/L), mean (SD) | 186 (93.5) | 2.2 (1.3) |
| CD4+ T-cell count (109/L), mean (SD) | 79 (39.7) | 1.0 (0.5) |
| Baseline PRO scores | ||
| TSQM V1.4 | ||
| Overall satisfaction score, mean (SD) | 179 (89.9) | 49.9 (28.6) |
| Effectiveness score, mean (SD) | 176 (88.4) | 49.6 (24.5) |
| Side effects score, mean (SD) | 177 (88.9) | 77.4 (31.9) |
| Convenience score, mean (SD) | 179 (89.9) | 70.0 (21.8) |
| MSIS-29 | ||
| Physical subscale score, mean (SD) | 194 (97.5) | 51.6 (25.4) |
| Psychological subscale score, mean (SD) | 189 (95.0) | 52.3 (24.7) |
| MFIS-5, mean (SD) | 193 (97.0) | 12.7 (5.0) |
| PDDS, mean (SD) | 188 (94.5) | 3.1 (2.1) |
The number of patients contributing data to a parameter is indicated if it differs from the overall study population (N = 199).
Data related to smoking are from current or former smokers.
Patients could choose > 1 option.
One patient received methylprednisolone which was incorrectly recorded in the electronic case report form as a prior DMT.
A total of 169 (99.4%) evaluable patients received a prior DMT. For one patient, methylprednisolone was incorrectly recorded as a prior DMT. Among evaluable patients, 35.9% received natalizumab; 39.4% received either teriflunomide, DMF, fingolimod, or siponimod; and 20% received IFN-β-1a, IFN-β-1b, pegylated IFN-β-1a, or glatiramer acetate as most recent DMT (Supplemental Table 2).
The median duration of the most recent DMT was 1.4 years, and the last DMT has discontinued at a median of 1.8 months before entering the study. Three-quarters (75.4%) of patients switched to alemtuzumab due to lack of efficacy of the previous DMT and 15.6% switched for safety reasons.
Patient-reported outcomes
Primary endpoint
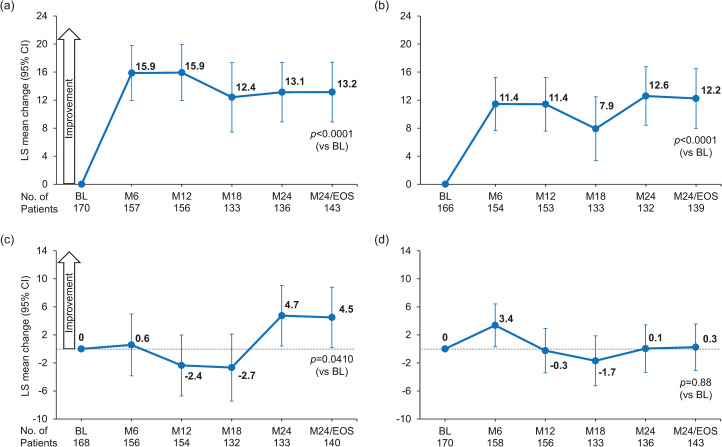
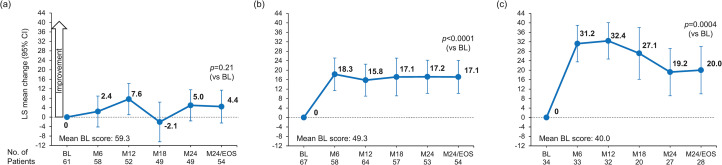
Patients reported a significant increase in TSQM overall treatment satisfaction score from baseline (mean [SD], 50.32 [28.65]) to month 24/EOS (64.34 [26.50]) with alemtuzumab (LS mean change, 13.15 [95% CI, 8.88–17.42]; p < 0.0001) (Figure 1a). TSQM global satisfaction scores increased from baseline to month 6 (15.87, 95% CI: 11.95–19.80) and remained stable through month 24/EOS. In the sensitivity analysis, the mean (SD) change from baseline to month 24/EOS was 13.13 (36.77; p < 0.001). Results from subgroups according to the most recent prior DMT were consistent with the overall population except for group 1 (prior natalizumab), for whom no statistically significant improvement over baseline was found (Figure 2).
Figure 1.
Least squares mean changes in TSQM scores for (a) global satisfaction, (b) effectiveness, (c) side effects, and (d) convenience. The TSQM version 1.4 scale is 0-100. Statistical significance was tested only at M24/EOS. One patient was excluded from the MMRM analysis because methylprednisolone was incorrectly recorded in the electronic case report form as a prior DMT. LS mean changes are estimated from mixed effects models with study visit as the time axis with adjustment for baseline scores; an unstructured correlation matrix was used to model within-person correlations.
BL: baseline; M: month; TSQM: Treatment Satisfaction Questionnaire for Medication; MMRM: mixed-effect model for repeated measures; DMT: disease-modifying therapy; EOS: end of study; LS: least square.
Figure 2.
Least squares mean changes in TSQM global treatment satisfaction by a subgroup of most recent prior disease-modifying therapy. Prior therapies were (a) natalizumab; (b) teriflunomide, dimethyl fumarate, fingolimod, or siponimod; or (c) IFN-β-1a, IFN-β-1b, pegylated IFN-β-1a, or glatiramer acetate (MMRM analysis). The TSQM version 1.4 scale is 0-100. One patient was excluded from the MMRM analysis because methylprednisolone was incorrectly recorded in the electronic case report form as a prior DMT. LS mean changes are estimated from mixed effects models with study visit as the time axis with adjustment for baseline scores; an unstructured correlation matrix was used to model within-person correlations.
BL: baseline; EOS: end of study; M: month; TSQM: Treatment Satisfaction Questionnaire for Medication; IFN: interferon; MMRM: mixed-effect model for repeated measures; DMT: disease-modifying therapy; LS: least square.
Secondary and tertiary endpoints
TSQM subscales
Scores for the TSQM treatment effectiveness subscale increased from a mean (SD) of 49.33 (24.84) at baseline to 61.40 (26.79) at month 24/EOS. By month 24/EOS, LS mean change from baseline was 12.24 (95% CI, 7.99–16.50; p < 0.0001) (Figure 1b). Improvements were observed at 6 months, with scores remaining stable thereafter. In subgroup analyses by prior DMT, results for groups 2 and 3 were consistent with the overall population, but no change was observed for group 1 (Supplemental Figure 3).
On the treatment-related side effects subscale, patients in the overall population reported improvements from baseline (mean [SD], 77.60 [31.92]) to month 24/EOS (82.57 [26.63]), with a significant LS mean change to month 24/EOS of 4.5 (95% CI, 0.19–8.81; p = 0.0410) (Figure 1c). Subgroup analyses by most recent prior DMT found statistically significant improvements for patients in group 3, but not groups 1 and 2 (Supplemental Figure 3).
The treatment convenience subscale showed improvement from baseline (mean [SD], 70.26 [22.31]) to month 6 (LS mean change, 3.4 [95% CI, 0.31–6.43]), but by month 24/EOS convenience scores had returned to near baseline (mean [SD], 70.90 [21.29]; LS mean change, 0.3 [95% CI, −3.07 to 3.56]; p = 0.88) (Figure 1d). Subgroup results were consistent with the overall population (Supplemental Figure 3).
MSIS-29
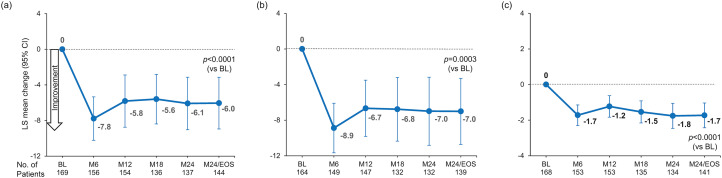
Physical functioning (MSIS-29 physical subscale) improved, with scores reduced from the mean (SD) 52.42 (25.54) at baseline to 46.07 (26.43) at month 24/EOS (LS mean change, −6.04 [95% CI, −8.94 to −3.14]; p < 0.0001) (Figure 3a). In subgroup analyses by prior DMT, groups 2 and 3 had results consistent with the overall population, but no significant improvement was found for group 1 (Supplemental Figure 4).
Figure 3.
Least squares mean change in (a) MSIS-29 physical subscale, (b) MSIS-29 psychological subscale, and (c) MFIS-5 (MMRM analysis). One patient was excluded from the MMRM analysis because methylprednisolone was incorrectly recorded in the electronic case report form as a prior DMT. LS mean changes are estimated from mixed effects models with study visit as the time axis with adjustment for baseline scores; an unstructured correlation matrix was used to model within-person correlations.
BL: baseline; EOS: end of study; M: month; MSIS-29: Multiple Sclerosis Impact Scale-29; MFIS-5: Modified Fatigue Impact Scale-5; MMRM: mixed-effect model for repeated measures; DMT: disease-modifying therapy; LS: least square.
Psychological functioning (MSIS-29 psychological subscale) improved, with scores reduced from a mean (SD) of 53.37 (25.02) at baseline to 44.83 (26.91) at month 24/EOS (LS mean change, −7.01 [95% CI, −10.72 to −3.29]; p = 0.0003) (Figure 3b). Improvements in psychological functioning were consistent in the prior DMT subgroups (Supplemental Figure 4).
MFIS-5
Patient-reported fatigue, measured using the MFIS-5, decreased from a mean (SD) of 12.79 (4.98) at baseline to 10.96 (5.07) at month 24 (LS mean change, −1.7 [95% CI, −2.43 to −1.04]; p < 0.0001) (Figure 3c). Subgroup analyses by prior DMT were consistent with the overall population (Supplemental Figure 5).
PDDS
Mean (SD) PDDS score, an assessment of disability progression, remained constant throughout the study (baseline, 3.09 [2.12]; month 24/EOS, 3.27 [2.25]; LS mean change, 0.12 [95% CI, −0.12 to 0.36]; p = 0.32). Subgroup analyses by prior DMT were consistent with the overall population (Supplemental Figure 5).
HRPQ-MS
Patients reported a significant decrease in the amount of work productivity lost per week (LS mean change, −4.04 h ([95% CI, −6.348 to −1.735]; p = 0.0010), from mean (SD) 11.4 (12.96) hours at baseline to 8.0 (12.20) hours at month 24/EOS (Supplemental Table 3). In subgroup analyses by prior DMT, group 3 had a statistically significant LS mean reduction in lost productivity (−7.43 h [95% CI, −10.935 to −3.929]; p = 0.0009), compared to −2.2 and −2.4 h for groups 1 and 2, respectively (Supplemental Table 4). Mean (SD) hours of household productivity lost per week decreased from 5.9 (7.63) at baseline to 5.1 (6.08) at month 24/EOS (LS mean change, −1.27 h [95% CI, −2.397, −0.145]; p = 0.0273).
CAREQoL-MS
We observed minimal changes in caregiver burden, although sample sizes for these analyses were small (Supplemental Table 5).
PRO results from additional subgroup analyses
The results for subgroups defined by the use of assistive devices, MS duration, and patient age are shown in Supplemental Figures 6 to 14. Improvements from baseline to year 2 were observed for the TSQM overall treatment satisfaction score and TSQM effectiveness subscale for all patient subgroups, except for patients who used wheelchairs or scooters, or patients who had MS for ≥ 5 to < 10 years. The TSQM side effects subscale improved from baseline to year 2 in patients who did not use assistive devices, who had MS for < 5 years or ≥ 10 years, and who were ≥ the median age of 43 years. Similar to the overall population, no subgroup demonstrated improvement in the TSQM convenience subscale.
For the MSIS-29 physical subscale, improvement was observed from baseline to year 2 in all subgroups except patients who used wheelchairs or scooters and those who had MS for < 5 years or ≥ 5 to < 10 years. Improvements in the MSIS-29 psychological subscale were found for all subgroups except patients who used any assistive devices, who used wheelchairs or scooters, and who had MS for > 5 to ≤ 10 years. For the MFIS-5, improvements from baseline to year 2 were found for all subgroups except patients who used wheelchairs or scooters. No subgroup demonstrated improvement over time in the PDDS scale.
Lymphocyte counts
The mean (SD) lymphocyte counts reduced sharply from baseline (2.22 [1.27] × 109/L) to month 3 (0.58 [0.30] × 109/L) before partial recovery; levels then remained relatively stable through month 24/EOS (1.12 [0.54] × 109/L) (Supplemental Figure 15). A similar pattern was observed for the mean (SD) CD4+ T-lymphocyte counts, with a baseline level of 0.96 (0.55) × 109/L reduced to 0.08 (0.06) × 109/L at month 3 and 0.30 (0.24) × 109/L at month 24/EOS.
Safety
Nearly all (95.0%) patients experienced ≥ 1 TEAE (Table 2); 88.4% reported ≥ 1 mild TEAE and 67.8% reported ≥ 1 moderate TEAE. TEAEs occurring in ≥10.0% of patients were headache (45.2%), fatigue (28.6%), nausea (28.6%), urinary tract infection (26.1%), rash (24.6%), MS relapse (19.1%), insomnia (16.6%), pyrexia (16.6%), pain (13.6%), pruritus (12.6%), flushing (12.1%), back pain (12.1%), muscle spasms (11.6%), pain in extremity (11.1%), depression (11.1%), and diarrhea (10.6%). Thirty-five patients (17.6%) had ≥1 serious TEAE, which were primarily infections and infestations (6.5%), and nervous system disorders (3.0%). AEs of special interest were reported for 18.6% of patients, most of which were endocrine disorders (10.1%) (Supplemental Table 6). Four deaths occurred during the study, due to pancytopenia, febrile neutropenia, metabolic encephalopathy, and acute kidney injury (n = 1); MS complications (n = 1); suicide (n = 1); and unknown cause (n = 1). Although none of the deaths were overall considered related to alemtuzumab treatment, the pancytopenia in one of the patients was deemed related to alemtuzumab. The safety profile was generally consistent across subgroups of patients according to the most recent prior DMT. No new or unexpected safety findings were reported during the study.
Table 2.
Incidence of AEs.
| Adverse event | All patients (N = 199) |
|---|---|
| Any treatment-emergent AE | 189 (95.0) |
| Treatment-emergent serious AEa | 35 (17.6) |
| Treatment-emergent AE leading to permanent treatment discontinuation | 1 (0.5) |
| Deathb | 4 (2.0) |
AE: adverse event.
Data are n (%) of patients.
One myocardial infarction occurred approximately 8 months after the first alemtuzumab course and no strokes or arterial dissections were reported.
Causes of death were unknown (n = 1); suicide (n = 1); severe acute kidney injury, febrile neutropenia, metabolic encephalopathy, and pancytopenia 14 months after starting treatment with alemtuzumab (n = 1; pancytopenia was deemed related to alemtuzumab treatment by the investigator); and multiple sclerosis complications 20 months after starting treatment with alemtuzumab (n = 1; deemed unrelated to alemtuzumab treatment by the investigator).
Discussion
Significant increases were reported in overall treatment satisfaction, which were assessed via the TSQM after 24 months of alemtuzumab treatment. The overall population also reported improvements compared with the baseline in aspects of treatment satisfaction (e.g. effectiveness, side effects), physical functioning, fatigue, and work and household productivity. In many cases, the changes were observed at the first follow-up study visit (6 months) following alemtuzumab treatment and were maintained throughout the study. Furthermore, the disability level as assessed by the PDDS remained stable, with no progression occurring over 2 years. These observations occurred in the context of a safety profile for alemtuzumab that was consistent with previous experience. Moderate AEs occurred in most PRO-ACT patients (67.8%), albeit at a lower incidence compared with the CARE-MS studies (92.2% for alemtuzumab 12-mg patients in CARE-MS II who received prior DMT).20 The most common moderate AEs in PRO-ACT included headache, nausea, and fatigue, which were also among the most frequent infusion-associated reactions in the CARE-MS studies,21 as well as urinary tract infections. The PRO-ACT death rate (10.8 per 1000 patient-years) was not higher than expected: the mortality rate in MS patients from a nationally representative US sample was 23.3 per 1000 person-years, although some of this difference likely reflects the higher mean age (56.8 years) in that study compared with PRO-ACT (43.7 years).22
Patients who were previously treated with DMT (group 2: teriflunomide, dimethyl fumarate, fingolimod, and siponimod; group 3: interferons and glatiramer acetate) had improvement that was similar to the overall population. In contrast, patients in group 1 (36.1% of patients), who received natalizumab as the most recent prior DMT, were less likely to report changes in treatment satisfaction or physical functioning. PROs with natalizumab have been largely positive,23 and baseline scores for this subgroup were higher than for groups 2 and 3. These reasons might explain why patients who switched from natalizumab to alemtuzumab did not report further improvements in many PRO domains.
Patients who used wheelchairs or scooters also did not report improvements in PRO scores in our study, although sample sizes in this subgroup were small. In many cases, disease symptoms for such patients are already moderate or severe,24 and a change in DMT might be insufficient to elicit noticeable improvement in the PRO measures assessed in our study. For these patients, the combination of a DMT with rehabilitation interventions may be required to achieve noticeable improvements in QoL, function, and treatment satisfaction.25,26 Patients who had MS for > 5 but ≤ 10 years also showed little improvement in PRO scores, with the exception of fatigue (MFIS-5). Notably, patients in this subgroup had the highest baseline scores for the TSQM; this finding supports the notion that patients who are relatively satisfied with their prior treatment continue to be satisfied after switching.
The primary limitation of our study is the absence of a comparator group. Our study has several notable strengths. To our knowledge, this study is the first to assess PROs for patients who switch to alemtuzumab treatment. Second, we used validated PROs, some of which were specific to MS, to assess patient perspectives of their disease treatment. Third, the multicenter, medical practice setting, with broad inclusion criteria, may more closely reflect a real-world population and increases the generalizability of these results.
In conclusion, patients with RRMS reported increased treatment satisfaction, improvements in physical functioning and fatigue, and less time lost to productivity after switching to alemtuzumab. Improvements were generally seen by 6 months and were maintained for up to 2 years after switching treatment. Findings from PRO-ACT may inform discussions between patients and providers regarding patient expectations for treatment satisfaction, physical and psychological function, and QoL improvements.
Supplemental Material
Supplemental material, sj-docx-1-mso-10.1177_20552173221135888 for Satisfaction with alemtuzumab in relapsing multiple sclerosis patients: Results from the real-world PRO-ACT study by Sibyl Wray, Francois Jacques, Tamara A Miller, Jacqueline A Nicholas, Rafael Arroyo, Lori Travis, Bhupendra Khatri, Magdalena Chirieac, Roopali Gandhi, Nora Roesch, Amelie Rodrigues, Lydie Melas-Melt, Andreea M Rawlings and Samuel F Hunter in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Acknowledgements
The authors and Sanofi thank the patients and their caregivers for their participation in the trial. A critical review of the manuscript was provided by Alana T Wong, PhD, of Sanofi. Writing assistance, including assistance drafting and editing of the manuscript text, figures, and tables, as directed by the authors; data checking and incorporation of comments from reviewers; and assisting with the submission process, was provided by Panos Xenopoulos, PhD, and Autumn Kelly, MA, of Elevate Scientific Solutions.
Footnotes
Data sharing: Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.
ORCID iDs: Sibyl Wray https://orcid.org/0000-0001-5116-8776
Francois Jacques https://orcid.org/0000-0002-6530-9370
Previous presentation of the data: Some of the data included in this study have been presented at the 37th Congress of ECTRIMS, which was held virtually from 13 to 15 October 2021.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SW reports receiving consulting, principal investigator, and/or speaking fees (Alkermes, Biogen, Celgene, Genentech, Novartis, Sanofi, and TG Therapeutics). FJ reports receiving honoraria for giving presentations, advisory board participation, research funding, and for an infusion clinic (Biogen, Merck Serono, Novartis, Roche, and Sanofi). TAM reports receiving speaking and/or consulting fees (Abbvie, Amgen, Biogen, Biohaven, BMS, Genentech, Lundbeck, Novartis, Reven, Sanofi, and Teva) and research support (Abbvie, Biogen, BMS, Celgene, Elan, EMD Serono, Genentech, Hoffman-La Roche, Ipsen, Merck, Novartis, Sanofi, and Teva). JAN reports receiving research grants (Biogen Idec, Genentech, Novartis, and PCORI) and consulting and/or speaking fees (Alexion, Bristol Myers Squib, EMD Serono, Genentech, Greenwich Biosciences, Novartis, Sanofi, and Viela Bio). RA reports receiving speaking fees from and advisory board participation for Almirall, Bayer, Biogen, Merck, Novartis, Roche, Sanofi, and Teva. LT reports receiving consulting fees (Acorda, Biogen Idec, EMD Serono, Mallinckrodt, Novartis, Pfizer, and Sanofi), and grant/research support (Biogen, EMD Serono, and Sanofi). BK reports consulting/honorarium (Acorda, Alexion, Biogen, Celgene, Genentech, Novartis, Sanofi, Serono, and Teva); contracted research (Alexion, Biogen, Genentech, Novartis, Ra Pharmaceuticals, and Sanofi). MC, RG, NR, and AMR are employees of Sanofi and may hold shares and/or stock options in the company. AR and LMM were employees of Sanofi at the time the study was conducted, and are currently employees of Ividata Life Sciences. SFH reports receiving consulting agreements, speaker honoraria, and grant/research financial support (AbbVie, Adamas, Alexion, Atara, Avanir, Biogen, Janssen, Mallinckrodt, Novartis, Osmotica, Roche, and Sanofi).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The PRO-ACT study was funded by Sanofi. Editorial support was funded by Sanofi.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Sibyl Wray, Hope Neurology, Knoxville, TN, USA.
Francois Jacques, Clinique Neuro-Outaouais, Gatineau, Canada.
Tamara A Miller, Advanced Neurology of Colorado, Fort Collins, CO, USA.
Jacqueline A Nicholas, OhioHealth Multiple Sclerosis Center, Riverside Methodist Hospital, Columbus, OH, USA.
Rafael Arroyo, Hospital Universitario Quirónsalud Madrid, Madrid, Spain.
Lori Travis, The MS Center of Arizona, Center for Neurology and Spine, Phoenix, AZ, USA.
Bhupendra Khatri, MD Center of Neurological Disorders, Milwaukee, WI, USA.
Andreea M Rawlings, Sanofi, Cambridge, MA, USA.
Samuel F Hunter, Advanced Neuroscience Institute, Franklin, TN, USA.
References
- 1.Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron 2018; 97: 742–768. [DOI] [PubMed] [Google Scholar]
- 2.Elliott C, Wolinsky JS, Hauser SL, et al. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler 2019; 25: 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelfand JM. Multiple sclerosis: diagnosis, differential diagnosis, and clinical presentation. Handb Clin Neurol 2014; 122: 269–290. [DOI] [PubMed] [Google Scholar]
- 4.Glanz BI, Degano IR, Rintell DJ, et al. Work productivity in relapsing multiple sclerosis: associations with disability, depression, fatigue, anxiety, cognition, and health-related quality of life. Value Health 2012; 15: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 5.Kwiatkowski A, Marissal JP, Pouyfaucon M, et al. Social participation in patients with multiple sclerosis: correlations between disability and economic burden. BMC Neurol 2014; 14: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oleen-Burkey M, Castelli-Haley J, Lage MJ, et al. Burden of a multiple sclerosis relapse: the patient’s perspective. Patient 2012; 5: 57–69. [DOI] [PubMed] [Google Scholar]
- 7.Williams AE, Vietri JT, Isherwood G, et al. Symptoms and association with health outcomes in relapsing-remitting multiple sclerosis: results of a US patient survey. Mult Scler Int 2014; 2014: 203183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arroyo Gonzalez R, Kita M, Crayton H, et al. Alemtuzumab improves quality-of-life outcomes compared with subcutaneous interferon beta-1a in patients with active relapsing-remitting multiple sclerosis. Mult Scler 2017; 23: 1367–1376. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012; 380: 1819–1828. [DOI] [PubMed] [Google Scholar]
- 10.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012; 380: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 11.Arroyo R, Bury DP, Guo JD, et al. Impact of alemtuzumab on health-related quality of life over 6 years in CARE-MS II trial extension patients with relapsing-remitting multiple sclerosis. Mult Scler 2020; 26: 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coles AJ, Arnold DL, Bass AD, et al. Efficacy and safety of alemtuzumab over 6 years: final results of the 4-year CARE-MS extension trial. Ther Adv Neurol Disord 2021; 14: 1756286420982134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the treatment satisfaction questionnaire for medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004; 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumstarck K, Boyer L, Boucekine M, et al. Measuring the quality of life in patients with multiple sclerosis in clinical practice: a necessary challenge. Mult Scler Int 2013; 2013: 524894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuigan C, Hutchinson M. The multiple sclerosis impact scale (MSIS-29) is a reliable and sensitive measure. J Neurol Neurosurg Psychiatry 2004; 75: 266–269. [PMC free article] [PubMed] [Google Scholar]
- 16.Hobart JC, Riazi A, Lamping DL, et al. Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome. Health Technol Assess 2004; 8(iii): 1–48. [DOI] [PubMed] [Google Scholar]
- 17.Learmonth YC, Motl RW, Sandroff BM, et al. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol 2013; 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tundia N, Hass S, Fuldeore Met al. et al. Validation and U.S. Population Norms of Health-Related Productivity Questionnaire. Poster PRM82. ISPOR 20th Annual International Meeting. Philadelphia, PA, 2015. [Google Scholar]
- 19.Benito-Leon J, Rivera-Navarro J, Guerrero AL, et al. The CAREQOL-MS was a useful instrument to measure caregiver quality of life in multiple sclerosis. J Clin Epidemiol 2011; 64: 675–686. [DOI] [PubMed] [Google Scholar]
- 20.Havrdova E, Arnold DL, Cohen J, et al. Safety of alemtuzumab in relapsing-remitting multiple sclerosis patients who relapsed on prior therapy (CARE-MS II). Mult Scler 2012; 18: P545. [Google Scholar]
- 21.Caon C, Namey M, Meyer C, et al. Prevention and management of infusion-associated reactions in the Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis (CARE-MS) program. Int J MS Care 2015; 17: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Titcomb TJ, Bao W, Du Y, et al. Association of multiple sclerosis with risk of mortality among a nationally representative sample of adults in the United States. Mult Scler J Exp Transl Clin 2022; 8: 20552173221104009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephenson JJ, Kern DM, Agarwal SS, et al. Impact of natalizumab on patient-reported outcomes in multiple sclerosis: a longitudinal study. Health Qual Life Outcomes 2012; 10: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conradsson D, Ytterberg C, von Koch L, et al. Changes in disability in people with multiple sclerosis: a 10-year prospective study. J Neurol 2018; 265: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Backus D. Increasing physical activity and participation in people with multiple sclerosis: a review. Arch Phys Med Rehabil 2016; 97: S210–S217. [DOI] [PubMed] [Google Scholar]
- 26.Beer S, Khan F, Kesselring J. Rehabilitation interventions in multiple sclerosis: an overview. J Neurol 2012; 259: 1994–2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mso-10.1177_20552173221135888 for Satisfaction with alemtuzumab in relapsing multiple sclerosis patients: Results from the real-world PRO-ACT study by Sibyl Wray, Francois Jacques, Tamara A Miller, Jacqueline A Nicholas, Rafael Arroyo, Lori Travis, Bhupendra Khatri, Magdalena Chirieac, Roopali Gandhi, Nora Roesch, Amelie Rodrigues, Lydie Melas-Melt, Andreea M Rawlings and Samuel F Hunter in Multiple Sclerosis Journal – Experimental, Translational and Clinical