Abstract
Background:
Many patients in methadone treatment have difficulty achieving or maintaining drug abstinence, and many clinics have policies that lead to discharging these patients. We designed a pilot “Second Chance” (SC) program for patients scheduled to be discharged from other local methadone clinics to be transferred to our clinic.
Aim:
Determine whether SC patients’ retention and opioid use is related to physical or mental health conditions, non-opioid substance use, or treatment features.
Methods:
From December 2012 to December 2014, this program enrolled 70 patients who were discharged from other clinics in the area; we were their last remaining option for methadone treatment. Unlike the clinic’s standard policies, the treatment focus for SC patients was retention rather than abstinence. This program focused on connection to care (eg, psychiatric services) and enabled patients to continue receiving services despite ongoing substance use. Each patient was assessed at treatment entry and followed until June 2016 to evaluate outcomes.
Results:
SC patients receiving disability benefits (n = 37) vs. non-disabled (n = 33) had significantly (P < .05) higher rates of current DSM-IV Axis I psychiatric diagnosis (97% vs 70%), prescriptions for opioids (84% vs 55%) and benzodiazepines (65% vs 27%), and higher methadone doses at admission (58 vs 46 mg) but did not differ significantly in rates of 6-month or 1-year retention (77% and 56%, respectively) or all-drug use (39% positive urine drug screens). Methadone doses >65 mg predicted significantly longer retention and less opioid use, but these effects were not moderated by baseline characteristics.
Conclusions:
Patients in methadone treatment struggling to achieve abstinence may benefit from retention-oriented harm-reduction programs. Higher methadone doses can improve retention and opioid abstinence despite psychiatric comorbidities. Further work is needed to improve program implementation and outcomes in this complex population.
Keywords: Methadone treatment, harm reduction, disability, opioid, benzodiazepines, retention
Introduction
Methadone treatment (MTD), an evidence-based pharmacotherapy for individuals with opioid use disorder (OUD), is a highly effective treatment approach.1,2 Yet opioid agonist treatment (OAT) for OUD is underutilized in the United States.2-7 Moreover, many individuals who enter MTD experience difficulty achieving or maintaining opioid abstinence. Although abstinence is not always the primary goal of treatment, some clinic policies can result in patients being involuntarily discharged from care, a clinical decision that contrasts with managing other chronic conditions (eg, diabetes). It is well-established that longer retention in MTD relates to better long-term outcomes including abstinence and improved health.8-13 Conversely, patients who cease treatment or are unfavorably discharged are at increased risk of death from various factors, especially overdose.14 Unfortunately, factors associated with decreased retention in MTD are often those associated with more severe illness such as concurrent use of non-opioid substances (eg, stimulants),15 comorbid psychiatric illness,16 and inadequate social support.17 Many factors can impact abstinence and retention in MTD and these 2 outcomes are closely related.18-20 One intervening (mediating/moderating) variable in effective MTD is dose because under-dosing is associated with increased likelihood of continued illicit opioid use, and this ongoing opioid use often results in discharge from care. In general, data suggest that MTD doses >60 mg/day and individualized to the patient’s needs are most effective for improving retention and abstinence.8,19
To avoid stigmatizing patients who experience repeated difficulties abstaining from non-medical opioid use, it is preferable to avoid labeling them (or their behavior) as “failures.” Rather, we recognize that treatments have failed these individuals, which re-orients our mission toward determining barriers to recovery and how to improve intervention effectiveness. Here, we describe procedures and outcomes from a “Second Chance” pilot program, founded to explore the effectiveness of enabling patients to remain in MTD despite ongoing substance use. This is critical because OAT retention markedly reduces mortality in patients with OUD.21,22 We hypothesized that placing greater emphasis on treatment retention and assessment (eg, mental health, family/social problems) could enable clinical staff to provide supportive services that gradually change patients’ behaviors. Punitive steps were de-emphasized except for higher-risk behaviors such as diversion or ongoing misuse of sedating drugs that may–in combination with opioids–increase risk of mortality.23-27 This alternative approach could potentially improve outcomes for patients with OUD. This study explores impacts of this program; we primarily focused on predictors of retention but also examined predictors of drug use, and relationships between retention and drug use.
Methods
Setting
This study received exemption from the Wayne State University Institutional Review Board as a retrospective chart review (IRB# 063016M1X) and was conducted in an urban, university-affiliated MTD program that has been described elsewhere.11,28 The SC program was developed as a clinical intervention rather than a research intervention; all data were collected using retrospective chart review thus no explicit patient consent was required. This clinic emphasizes treatment within a chronic care model. Higher doses of methadone (when safe) are used to improve treatment outcomes. Other standard care includes psychosocial interventions and contingency management to promote counseling attendance,29 reduce cocaine use,30 and identify patients’ misuse/diversion of prescribed medications.31 In this pilot project, our clinic was a single-site center for accepting patients transferred from other local MTD programs, as negotiated with the City of Detroit.
“Second Chance” (SC) patient population
All patients were administratively discharged (eg, due to diverting prescribed medications, non-attendance, or ongoing drug use) by their prior MTD clinic; no alternative treatment options were available. In each case, Detroit’s Institute for Population Health Access Management Service (local authority) intervened and transferred the patient to our SC program to avoid loss of care. Patients were enrolled from December 2012 until December 2014, and data were collected until June 2016, at which point data from retained patients were censored.
Clinical care for SC patients
SC patients were treated in the same clinic as our regular MTD patients, due to limited staffing. Our initial protocol attempted to separate these groups by having SC patients attend clinic after lunch because most regular patients attended during morning hours. Ultimately, this was infeasible because many SC patients also needed to dose in the morning. Thus, SC patients were embedded in a clinic where program rules for them differed from those of regular MTD patients. Although all patients were encouraged to reduce their drug use (understanding that abstinence may take longer for some individuals), greater emphasis was placed on drug abstinence for regular MTD patients whereas retention was emphasized for SC patients. This was done by creating separate abstinence policies (see Table 1) and staff training to ensure that individual and group counseling sessions focused on retention rather than abstinence for SC patients. These differences (and appropriately interacting with patients) required staff discussion and adjustments.
Table 1.
Key policies for patients in the Second Chance program and how those policies were similar or different to those for the standard methadone patients.
| Treatment focus* | Second chance | Standard patients |
|---|---|---|
| Retention | Abstinence | |
| Initial dosing* | Dosing started at the dose they were previously receiving | Doses normally began at 35 mg; sometimes a lower starting dose but very rarely higher. |
| Dose adjustments | Dose adjustments were made in 5 to 10 mg increments, not more than 10 mg every other day | Dose adjustments were made in 5 to 10 mg increments, not more than 10 mg every other day |
| Take home dose eligibility* | Were immediately eligible for abstinent contingent take-home
methadone doses because previous time in treatment counted
toward the 90-day requirement. Patients could reduce dosing visits to 3 times weekly after >90 consecutive days of UDS negative for all non-prescribed drugs in addition to adherence with clinic policies and counseling requirements. |
Eligible for abstinent contingent take-home methadone doses
only after 90-day in treatment. Patients could reduce dosing visits to 3 times weekly after >90 consecutive days of UDS negative for all non-prescribed drugs in addition to adherence with clinic policies and counseling requirements. |
| Controlled substances | All prescribed controlled substances (eg, benzodiazepines) required a specialist prescription (eg, psychiatrist) and agreement to weekly monitoring via state prescription drug-monitoring program (PDMP), UDS results, and pill counts | No concurrent controlled substance prescriptions were allowed. |
| Ongoing unauthorized sedative use | Initial warning and offer to meet with psychiatrist for authorized prescription. Followed by methadone dose-reduction and discharge from the program; reinstatement allowed if abstinence demonstrated | Initial warning and offer to meet with psychiatrist for authorized prescription. Followed by methadone dose-reduction and discharge from the program; reinstatement allowed if abstinence demonstrated |
| Ongoing non-sedative substance use* | No impact on care, dosing, or treatment | Minimum abstinence requirements policy (see Appendix 1) |
| UDS Policy | Random, visually monitored UDS approximately 2 to 4 times per month | Random, visually monitored UDS approximately 2 to 4 times per month |
| Response to positive UDS* | None except for change in eligibility for take-home doses | Minimum abstinence requirements policy (see Appendix 1) and changes in eligibility for take-home doses |
| Individual therapy | Two required 1-h counseling sessions every month | Two required 1-h counseling sessions every month |
| Group therapy | One group session required every month, but can attend as many groups as desired | One group session required every month, but can attend as many groups as desired |
| Psychiatric care* | Direct connection to psychiatric care made for all patients entering program | Psychiatric care available but not automatically provided |
| Alternatives* | No remaining alternative treatment options in the Detroit area | Able to leave clinic and transfer to any other Detroit clinic, if desired |
Asterisk (*) indicates policies that differed for the patients in the Second Chance program compared to the patients in the standard methadone program.
At admission, all SC patients underwent psychosocial, medical, psychiatric (Mini-International Neuropsychiatric Interview)32 evaluations, and were oriented to care. All funding was provided by the local authority, which required quarterly authorizations. Similar to regular MTD patients, all SC patients were authorized to receive two 1-h individual counseling sessions and one group session during each month of treatment. Individual counseling attendance was required, and patients could attend as many groups as they wanted. Therapy groups included regular and SC patients and content incorporated educational, cognitive/behavioral, and 12-Step Facilitation approaches. If indicated from the evaluation results, the counselor referred the patient to an onsite mental health practitioner. All patients were strongly encouraged (but not required) to take advantage of onsite mental health treatment.
Each patient’s entry methadone dose (which depended on the dose at the prior MTD program) was titrated based on physician judgment, nursing observations, and counselor input (eg, presence of opioid withdrawal signs/symptoms and ongoing drug use). Dose adjustments were made in 5 to 10 mg increments, not more than 10 mg every other day. Patients were randomly scheduled to provide visually-monitored urine samples for drug screening (UDS); frequency of UDS was based on each patient’s drug-positive history but usually occurred 2 to 4 times/month. This UDS policy was the same for both groups; however, the response to any positive UDS differed between the groups.
One advantage of accommodating SC patients as transfers from other clinics, rather than new patients, is that (using federal regulations33) they immediately became eligible for abstinence-contingent take-home MTD doses. Although retention was the primary outcome, abstinence remained a desired secondary goal; therefore, extra abstinence-contingent take-home doses were offered to encourage abstinence. This was designed to provide patients earlier contact with a reinforcing outcome. Starting at admission, single urine samples testing negative for all non-prescribed drugs earned one take-home bottle (added to the usual Saturday take-home dose). After 90 days in MTD (which included time-in-treatment before transferring to our clinic) with >90 consecutive days of UDS negative for all non-prescribed drugs and adherence with clinic policies for attendance, counseling, and appropriate use of controlled substances, patients could reduce dosing visits to 3 times weekly (clinic policies provided upon request).
Responses to drug-positive UDS in this clinic are generally problem-focused rather than punitive. Nonetheless, due to the risks of using unprescribed sedatives with opioids including methadone, our policies make repeated use of alcohol (breathalyzer testing occurred if this was suspected), benzodiazepines (BZDs), or barbiturates grounds for methadone dose-reduction and possible discharge from the program; however, patients who later demonstrate abstinence from these drugs can reinstate their former status. Some evidence suggests that risks of co-occurring sedative and opioid use are mitigated when sedatives are taken as prescribed.34 Thus, we required patients with an ongoing BZD prescription to receive this from a psychiatrist (ie, program director [CWC], who was not a psychiatrist, would not prescribe a BZD); patients seeking to continue a BZD prescription were referred to onsite psychiatrists for evaluation. Patients with prescribed controlled substances were carefully monitored by comparing data from the state prescription drug-monitoring program (PDMP), UDS results, and pill counts on a weekly basis.31,35 If the PDMP report was positive, the urine sample was positive for the prescribed drug, and pill count matched (within reasonable bounds), the program director exercised clinical judgment that the patient was not diverting medication. If these data points were divergent (suggesting diversion/misuse), a behavioral contract was used to contact the primary care provider, cease additional prescriptions, and allow 1 month (typical length of a prescription) to address the discrepancies in prescription use.
Measures
Clinical measures extracted from each patient’s electronic medical record (Table 2) included demographics, history of substance use, mental health, methadone dosing variables, and treatment outcomes (retention and drug use). Retention was considered as a continuous measure (number of days in treatment) and using binary outcomes (retained or not) at 6 months and 1 year. Drug use was based on UDS results. Urine samples were sent for confirmatory testing at an approval laboratory where they were tested for methadone, opioids, cocaine metabolites, BZDs, and barbiturates (positive cutoffs >300 ng/ml for all of the foregoing drugs), amphetamines (positive cutoffs >1000 ng/ml), oxycodone (positive cutoff >100 ng/ml), and THC (positive cutoff >50 ng/ml). For each patient, extent of drug use was computed as the percentage of all UDS across the patient’s entire treatment episode that were positive for opioids, cocaine metabolites, BZD, THC, or barbiturates without a corresponding, verified prescription. We initially report all measured substances because some literature indicates a relationship between OUD treatment retention and other substances used.36-39
Table 2.
Domains of measures.
| Measurement domain | Variables |
|---|---|
| Demographic | Treatment payer (eg, Medicaid, Medicare, Health Maintenance Plan, Block grant); dates of birth, admission, first and last methadone dose; age, gender, race, education, employment, marital and disability status |
| History of substance use | Number and location of prior treatment episodes; Substance use self-report variables such as injection drug use, number of years using opioids (usually heroin); Participation in group-based treatment for other conditions (eg, cocaine use); Prescriptions for opioid (analgesic) or benzodiazepine (sedative/hypnotic/muscle relaxant) drugs |
| Mental health | Mental health assessments of current and past psychiatric conditions (eg, anxiety, depression, bipolar, PTSD, schizophrenia, adjustment disorder), and whether the patient currently has a psychiatrist and is receiving medication or other treatment for ongoing mental health problems |
| Methadone dosing | Methadone dosing variables including entry dose, minimum and maximum dose, modal and average dose, days until reaching modal dose, number and percent of treatment days on modal dose, and cumulative methadone dose |
| Treatment outcome | Urine drug screen results for opioids, cocaine, benzodiazepines, cannabinoids, barbiturates, and amphetamines; Retention (total days in treatment, and point prevalence at 6-month and 1-year); If discharged from the clinic, reason for discharge (eg, diversion, non-compliance with other clinic policies, dropped out against medical advice, transferred to another clinic) |
Data analyses
Analyses were conducted using SPSS v.23. We first computed descriptive statistics to obtain frequencies (or percentage of patients) for categorical measures and means (SDs) for continuous measures. Correlations (Pearson r for continuous, or Kendall τ for categorical measures) were used to assess the degree of zero-order association among variables.
Measures were grouped into 3 categories: independent variables (demographics, substance use history, mental health), intervening variables (methadone dosing which is adaptive for each patient), and outcomes (retention, drug use). Inferential statistics included survival curve or regression analyses (logistic for 6-month and 1-year retention; linear for continuous measures) to determine whether demographics, history of substance use, or mental health measures predicted retention or drug use.
The data-analytic strategy involved assessing correlations between different baseline factors and outcome measures of drug use and retention, as well as assessing correlations between drug use and retention.
Results
Patient characteristics
Seventy patients were enrolled in the SC program (Table 3 lists sample characteristics). Nearly all (97%) self-identified as African-American (compared to ≈ 85% African-American in the rest of our clinic), about half were male, and average age was mid-50s. In most cases (70%), Medicaid funded their treatment. Mean lifetime duration of opioid use exceeded 30 years. Most (70%) had a current prescribed opioid and 40% had a prescribed BZD. Most (84%) had a current psychiatric disorder (typically unipolar depression and/or anxiety). About half (52.9%) reported receiving disability benefits. Patients receiving disability benefits were significantly older, more likely to be Medicaid funded, had higher methadone entry doses, current psychiatric diagnoses, and concurrent prescriptions for opioids and BZDs.
Table 3.
Patient characteristics.a.
| Domain/Measure | Overall Mean (SD) or n (%) | Disability Mean (SD) or n (%) | No disability Mean (SD) or n (%) | t or χ2 (P) |
|---|---|---|---|---|
| N = 70 | n = 37 (52.9%) | n = 33 (47.1%) | ||
| Demographics | ||||
| Race (% African-American) | 67 (97.1%) | 36 (97.3%) | 31 (96.9%) | 0.01 (.92) |
| Gender (% male) | 36 (52.0%) | 20 (54.1%) | 16 (48.5%) | 0.11 (.74) |
| Age (years) | 54.9 (8.1) | 57.3 (7.0) | 52.3 (8.5) | −2.67 (.01) |
| Treatment payer | 7.10 (.01) | |||
| Medicaid | 49 (70.0%) | 31 (83.8%) | 18 (54.5%) | |
| Other | 21 (30.0%) | 6 (16.2%) | 15 (45.5%) | |
| Years using opioids | 33.1 (11.7) | 34.3 (11.0) | 31.7 (12.4) | −0.88 (.38) |
| Injection user | 35 (50.0%) | 21 (56.8%) | 14 (42.4%) | 1.80 (.18) |
| # Previous Tx facilities | 2.1 (1.6) | 2.3 (1.2) | 2.0 (2.0) | −0.84 (0.40) |
| Psychiatric conditions | ||||
| Any dual diagnosis | 59 (84.3%) | 36 (97.3%) | 23 (69.7%) | 10.03 (.002) |
| Depression | 34 (55.7%) | 21 (58.3%) | 13 (52.0%) | 0.24 (.62) |
| Anxiety | 28 (45.9%) | 19 (52.8%) | 9 (36.0%) | 1.67 (.20) |
| Bipolar | 13 (21.3%) | 7 (19.4%) | 6 (24.0%) | 0.18 (.67) |
| PTSD | 6 (9.8%) | 3 (8.3%) | 3 (12.0%) | 0.22 (.64) |
| Schizophrenia | 2 (3.3%) | 2 (5.4%) | 0 (0.0%) | 1.44 (.23) |
| Prescribed medications | ||||
| Opioid | 49 (71.0%) | 31 (83.8%) | 18 (56.3%) | 6.32 (.01) |
| Benzodiazepine | 33 (48.5%) | 24 (66.7%) | 9 (28.1%) | 10.08 (.002) |
| Urinalysis Results | ||||
| Total # UDS | 39.0 (26.8) | 36.0 (22.7) | 41.9 (30.5) | 0.87 (.39) |
| Total % UDS+ any drug | 78.1 (24.1) | 78.4 (23.8) | 78.2 (24.6) | 0.04 (.97) |
| % Opioid+ | 64.6 (28.9) | 64.1 (27.8) | 66.1 (30.6) | 0.40 (.69) |
| % Cocaine+ | 46.5 (37.7) | 52.0 (35.6) | 39.2 (39.2) | −1.49 (.14) |
| % Benzodiazepine+ | 30.5 (32.7) | 32.8 (35.5) | 27.2 (29.1) | −0.65 (.52) |
| % THC+ | 13.7 (27.9) | 6.5 (16.3) | 21.4 (35.3) | 2.18 (.03) |
| % Barbiturates+ | 3.5 (7.9) | 4.3 (8.9) | 3.2 (7.3) | −0.32 (.75) |
| Methadone Dosing | ||||
| Entry dose (mg) | 52.3 (23.6) | 57.4 (25.7) | 46.1 (19.6) | −2.15 (.04) |
| Average dose (mg) | 61.9 (19.1) | 65.7 (20.6) | 57.4 (16.2) | −1.89 (.07) |
| Modal dose (mg) | 65.6 (20.6) | 69.8 (21.6) | 60.9 (18.2) | −1.84 (.07) |
| # Days to modal dose | 100.8 (126.6) | 98.1 (107.7) | 102.8 (145.1) | 0.13 (.90) |
| # Days on modal dose | 159.5 (109.2) | 165.6 (113.4) | 149.2 (105.4) | −0.75 (.46) |
| Maximum dose (mg) | 73.6 (19.3) | 77.7 (19.5) | 68.9 (17.9) | −1.95 (.06) |
| Minimum dose (mg) | 25.4 (24.7) | 24.4 (27.5) | 25.9 (21.4) | 0.16 (.88) |
| Terminal dose (mg) | 33.5 (31.0) | 33.2 (33.9) | 32.9 (27.8) | −0.14 (.89) |
| Cumulative dose (mg) | 26 056 (19 017) | 25 804 (17 823) | 25 964 (20 391) | −0.04 (.97) |
| Treatment Retention | ||||
| Total # days in treatment | 415.4 (259.6) | 372.4 (226.8) | 463.6 (287.9) | 1.48 (.14) |
| Retained at 6 month | 54 (77.1%) | 29 (78.4%) | 25 (75.8%) | 0.07 (.79) |
| Retained at 1 year | 39 (55.7%) | 19 (51.4%) | 20 (60.6%) | 0.61 (.44) |
When a missing value occurred (for a few measures), percentages were computed based on available group size. Italicized values are significantly different from each other, following omnibus testing.
Due to incomplete data for several baseline measures, for example, education, employment, marital status, family history of substance use, and whether the patient had a psychiatrist, we elected not to report or analyze findings related to these measures to avoid problems of potential selection bias.
The different methadone dose-level variables (entry, maximum, minimum, average, modal, terminal, and cumulative amounts) significantly correlated with one another: rs ranged from .31 to .93 (all Ps < .01).
Treatment retention
Fifty-four of the 70 patients (77.1%) were retained in the SC program over 6 months and 39 (55.7%) were retained over 1 year. Twenty-six patients (37.1%) were administratively discharged: 15 of these 26 (57.7%) for suspected drug diversion; 6 (23.1%) for other non-adherence violations (non-attendance, threatening behavior, tampering with urine sample); 3 (11.5%) for transferring to another clinic (due to their work schedule or closer proximity to home); and 2 (7.7%) for unknown reasons. Baseline/demographic variables were not significantly related to treatment retention.
Of all methadone-dosing variables, higher maximum methadone doses most strongly related to total days in treatment (Pearson r = .30, P = .013), and retention at 6 months (Kendall r = .41, P = .001) and 1 year (Kendall r = .32, P = .007). Significant, but slightly weaker, correlations were observed for average methadone dose with these retention measures. Using the distribution of maximum methadone doses, tertiles were formed to illustrate these findings (Figure 1): Higher Doses (n = 23, 85-125 mg), Moderate Doses (n = 23, 66-84 mg), and Lower Doses (n = 24, 35-65 mg).
Figure 1.
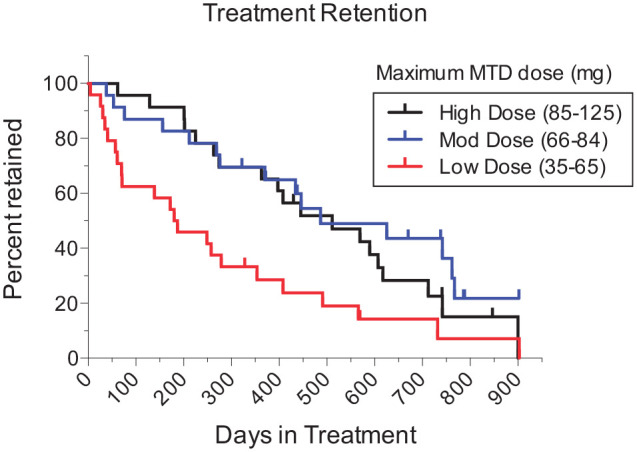
Treatment retention (days) as a function of maximum methadone maintenance dose: higher [85-125 mg], n = 23; moderate [66-84 mg], n = 23; and lower [35-65 mg], n = 24. As 13 enrolled patients (2 Lower Dose, 7 Moderate Dose, and 4 Higher Dose) remained in treatment when data were analyzed, their retention data were censored at the date of electronic medical record extraction (ie, included until that point); censored patients’ data appear as upward ticks.
Survival analysis yielded a significant tertile group effect, log-rank Mantel-Cox χ2[2] = 10.02, P = .007. Median retention was 183.5, 487, and 511 days for Lower, Moderate and Higher Dose groups, respectively. In pairwise tests, the Lower Dose group significantly differed from both the Moderate Dose (χ2[1] = 7.51, P = .006) and Higher Dose group (χ2[1] = 5.15, P = .023), and the 2 latter groups did not differ from one another (χ2[1] = 0.92, P = .338).
Drug use
Table 4 presents relationships between measures of drug use (percentage UDS + overall during each patient’s treatment episode), methadone dosing variables, and retention. In bivariate analyses, higher maximum methadone doses significantly correlated with lower rates of UDS + results for all major drug types measured: opioids, cocaine metabolites, BZDs, and THC. Positivity rates were low for barbiturates (4%) and near-zero for other drugs (oxycodone [hydrocodone use was much more common than oxycodone in the Detroit area] and amphetamines) and are not discussed further. This study was conducted prior to increased fentanyl use and contamination of the illicit drug supply in the USA, which explains why fentanyl was not tested for in this sample.40 Average and modal methadone doses showed similar but slightly weaker associations with UDS results. In contrast, clinic-entry methadone dose did not correlate with any UDS measure. Of the 70 SC patients, 10% (n = 7) achieved 90-day abstinence that qualified them for methadone take-home doses.
Table 4.
Pearson correlations among the percentages of positive urine drug screen results, methadone dosing variables, and treatment retention measures.
| % Any+ | % Opioidi+ | % Cocaine+ | % BZD+ | % THC+ | |
|---|---|---|---|---|---|
| % Opioid + | .685** | ||||
| % Cocaine + | .501** | .033 | |||
| % BZD + | .409** | .320* | .306* | ||
| % THC + | .325** | .182 | −.033 | .189 | |
| Entry dose | −.154 | −.134 | −.129 | −.148 | −.092 |
| Maximum dose | −.301* | −.287* | −.255* | −.438** | −.272* |
| Minimum dose | −.425** | −.062 | −.378** | −.062 | −.084 |
| Average dose | −.314** | −.318** | −.234 | −.388** | −.211 |
| Modal dose | −.285* | −.379** | −.147 | −.332* | −.210 |
| # Days to reach modal dose | −.341** | −.207 | −.188 | −.314* | −.102 |
| # Days on modal dose | −.470** | −.520** | −.273* | −.428** | −.235 |
| % Days on modal dose | .122 | −.031 | .183 | .239 | .160 |
| Terminal dose | −.417** | −.081 | −.376** | −.133 | −.151 |
| Cumulative MTD dose | −.566** | −.510** | −.310** | −.425** | −.342** |
| Treatment duration days | −.483** | −.478** | −.267* | −.377** | −.306* |
| Retained 6 month | −.397** | −.458** | −.227 | −.473** | −.277* |
| Retained 1 year | −.402** | −.415** | −.152 | −.324* | −.248* |
P < .01; *P < .05.
Individuals enrolled in clinic groups that addressed polysubstance use had significantly greater rates of all-drug abstinence (r = −.36). Current injection heroin use at clinic entry was associated with significantly higher rates of cocaine + UDS (r = .26) but not opioid + UDS. Demographic and psychiatric factors were not significantly related to opioid use. However, older age (r = −.31) and receiving disability benefits (r = −.27) were significantly associated with lower proportions of THC + UDS results, whereas presence of bipolar disorder was positively related to THC + rates (r = .29).
To describe the time course of drug use during treatment, we computed the proportion of UDS + results for opioids, cocaine metabolites, BZDs, and THC for each patient across 4-week blocks from months 1 to 6 (ie, weeks 1-4, 5-8, . . . 21-24). Some patients dropped out of treatment but, as noted above, most of the sample was retained during this period. Figure 2 shows that rates of non-prescribed opioid and BZD use decreased after the first month and leveled off, whereas rates of cocaine and cannabis use did not change over time.
Figure 2.
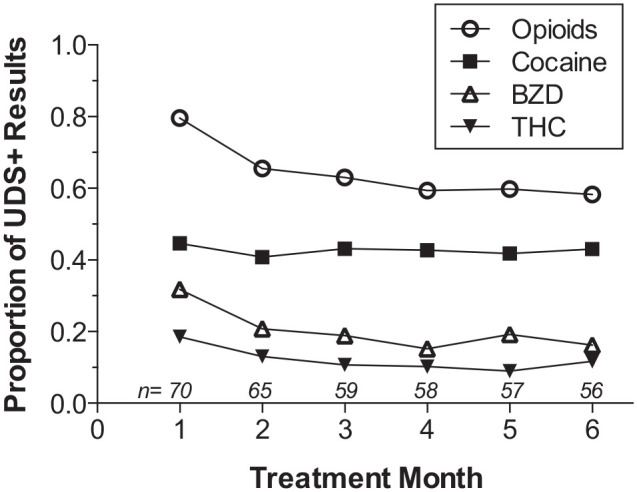
Time course of drug use during treatment. Proportions of monthly UDS + results for all drugs (numbers above X-axis reflect sample size).
Relationships between drug use and retention
Table 4 indicates that higher UDS + rates significantly correlated with shorter treatment retention. This was observed across all-drugs, and for opioids, cocaine, BZDs, and THC independently. Drug use and retention can be independent outcomes; however, our clinic policies could have made these outcomes less independent. Although many clients were prescribed opioids and BZDs (making it more likely they would test positive for these drugs), our analyses focused only on positive UDS that did not have a corresponding, verified prescription. Nonetheless, our clinic policies surrounding ongoing non-prescription use of BZDs most likely explain the findings that higher rates of BZD use correlated with lower methadone doses and number of days on modal dose (dose-reduction policy), and shorter retention (discharge policy). On the other hand, higher rates of UDS + opioid, cocaine and THC results were also significantly associated with lower methadone doses and shorter retention, though ongoing use of these drugs did not impact treatment policies. Nonetheless, there may have been an indirect relationship, as rates of BZD + UDS correlated significantly with rates of opioid and cocaine but not cannabis (Table 3).
Discussion
MTD improves outcomes for many, but not all, people with OUD. In this pilot study we developed a “Second Chance” approach to treat patients administratively transferred into our program (who would have otherwise lost care). Table 1 shows key policy differences and similarities for SC and standard care groups. SC patients were intermingled with regular MTD patients, creating several complications. Implementing this novel program required educating staff, as the treatment philosophy for this group of clients challenged clinic norms. Previous studies have noted the importance of addressing staff attitudes when providing treatment and harm-reduction services.41-43
Our policies for these transfer patients were more flexible regarding ongoing drug use (except for high-risk use of sedatives or suspected diversion of controlled substances) than for regular MTD patients. Ideally, we preferred not to mix these subgroups; however, this was not feasible and the situation led to “lessons learned.” Unsolicited feedback from regular clinic patients indicated they often felt SC patients were more privileged due to the more forgiving drug-use policy, and some regular patients resented being held to a higher standard. As noted, many SC patients entered the clinic with prescribed opioids and BZDs due to comorbid disorders (eg, pain, depression, anxiety). Despite education, clinic staff often found it difficult to overcome their traditional attitudes and to manage conflicts between SC and regular patients. Although it may be possible to accommodate patients with differing histories and expectations during the same clinic hours in an OAT program, it creates challenges and illustrates the need for increased education and training of clinic staff. This experience also highlights the insidious effects of stigma—both between clinic staff and patients, and between patient groups—and demonstrates potential risks of including multiple subgroups of patients with differing policies and privileges in the same clinic.
Retention time, a key outcome for OAT programs, averaged about 14.5 months in this sample. Probability of retention at 6 and 12 months was 77% and 57% respectively, which resembles findings from other studies of flexible methadone dosing.8,44,45 What makes this outcome remarkable is that prior treatment had failed these patients, and without this program they would have left treatment entirely; this highlights the importance of patient-focused goals in treatment success. Retention time positively correlated with methadone dose level: survival curve analysis revealed that higher maximum methadone doses (>65 mg/day) were associated with a doubling of median retention time, and decreased drug use. This replicates results of previous studies, confirming that relatively higher methadone doses (>60 mg/day) usually produce better outcomes than lower doses (<30 mg/day).46-49 Our analyses used empirically-derived tertiles; however, our empirically-determined division into 3 equal groups of low (35-65 mg/day), moderate (66-84/day), and high (85-125 mg/day) doses is not inconsistent with the literature regarding standard methadone dosing. To our knowledge, there is no absolute consensus on what doses constitute “high” vs. “moderate” vs. low” and these definitions can be affected by study population. A recent systematic review of studies from 2001 to 2019 included dose as a treatment factor across 26 cohorts in MTD in relation to retention and found wide variability in dosing standards.19 Nonetheless, previous reviews suggest that the methadone doses are considered “high” when greater than between 60 mg and 100 mg,19,46,50 suggesting that our dosing divisions are in line with accepted standards.
We observed that the rate of ongoing non-prescription opioid use among SC patients was significantly higher for those receiving lower methadone doses. Lower methadone doses also correlated significantly with ongoing cocaine use, the latter factor being reliably related to worse MTD outcomes in prior studies.51-55 Rate of cocaine use was also higher among injection heroin users, which is congruent with other studies.56-58 Such correlation implies the need for an additional treatment focus on cocaine use. Thus, injection heroin use and cocaine use during MTD might be useful clinical markers of the need to titrate toward higher methadone doses. Some data suggest higher methadone doses might reduce cocaine use in patients with concurrent opioid and cocaine use disorders.59
Notably, entry methadone dose did not significantly relate to treatment outcomes, which differs from the other methadone dosing variables. Entry doses for patients transferred from other clinics closely corresponded to doses in their former program. Our findings suggest these patients’ prior doses were not adequately titrated. These findings clearly illustrate the importance of personalizing a patient’s MTD dose to their needs and demonstrate the efficacy of titrating OAT to the appropriate dose for each patient.
Opioid-negative UDS results are another indicator of treatment efficacy, although we predicted that patients in this program might take longer to achieve drug abstinence. As expected, rates of opioid abstinence were consistently lower for SC than regular MTD patients. At weeks 13, 26, and 52 after entry into treatment, 40%, 40%, and 61% of retained SC patients provided opioid-free UDS whereas 60%, 73%, and 80% of retained regular MTD patients provided opioid-free UDS during those same weeks.
Our favorable treatment retention results are notable given the high rate of psychiatric problems in this sample, diagnoses that can impact retention in MTD treatment.53,60,61 The majority of our patients (84.3%) had at least one current psychiatric condition, especially major depressive disorder (55.7%) and anxiety disorders (45.9%). Similarly, a study on prevalence of psychiatric comorbidity among MTD patients found that at least one anxiety disorder, one affective disorder, and a comorbid anxiety and depressive disorder were diagnosed in 55%, 58%, and 36% of the total sample.62 Additionally, most patients entering the program had a prescribed opioid and almost half had a prescribed BZD; although we could not access the indications for these prescriptions, their presence suggests this population has a complicated medical history. These findings identify a need to carefully assess for psychopathology in clients entering OAT programs, particularly those struggling to meet program requirements. This takes on further importance in our sample, as patients receiving disability benefits had higher rates of DSM-IV Axis I diagnoses than patients without (97% vs 70%). As previously described,63 the negative impact of co-occurring psychopathology on quality of life is a compelling reason to offer accessible (preferably onsite) psychiatric services for patients in MTD; prior efforts in this regard have shown improvements in treatment engagement, adherence, and mental health.64,65 In our study and elsewhere, psychiatric comorbidity did not significantly affect MTD retention,66-68 although other studies have found an association.53,60,61 Negative findings, as in this study, suggest that addressing mental health problems presented by MTD patients could equalize their opportunities for recovery from OUD. Yet, given mixed findings in the treatment literature, further study is needed to establish which psychiatric problems or associated factors pose the greatest barriers to recovery; for example, whether psychiatric severity or its detrimental effects on coping self-efficacy69 shorten MTD retention or exacerbate ongoing drug use, recognizing that utilization of integrated psychiatric services with MTD could be a mediating factor. Furthermore, it is possible that comorbidities (eg, psychiatric conditions and disability) may impact MTD dosing due to safety concerns, which may further impact outcomes. Future research efforts should also explore reasons behind hesitation to receive care from integrated psychiatric services to ensure that lack of access to care does not remain a barrier to recovery.
In some previous studies, older age correlated with longer MTD retention.61,70-72 Although this could partly account for the reasonably high retention rates of our patients (most were >50 years), our analysis discovered no significant correlations between age (or other demographic variables) and treatment retention. Prior participation in OAT (but not detoxification), which may correlate with age, has also been associated with longer retention rates in subsequent treatment episodes,53,73,74 which may reflect stepwise progress toward recovery.75 Yet, such an association was not observed in this study. Patients’ self-reported number of prior treatment episodes did not correlate with retention or drug use. This could be due to limitations of patient self-reports, characteristics of this sample (eg, psychiatric comorbidity), and/or the greater importance of MTD dose in predicting retention within this sample.
Limitations of this pilot study include: (1) small sample size; (2) use of one treatment site with unique policies that may reduce generalizability of findings; (3) limited number of assessment measures with some missing data; (4) minimal data regarding patient follow-up with mental health treatment, making it difficult to know whether efforts to assess and treat psychiatric problems were effective in contributing to opioid treatment outcomes; (5) difficulties of treating these SC patients in the same setting as regular MTD patients (eg, negative attitudes of staff and regular patients), which may limit effectiveness of interventions with this population; (6) despite more lenient policies regarding ongoing non-prescription drug use, the SC program still required discharge of SC patients for suspected diversion of any controlled medication or ongoing sedative (eg, BZD) use, which highlights a key group of patients lost to care and hampers our abilities to fully interpret certain aspects of the data regarding ongoing drug use in this sample; (7) indication of disability status came from patient’s self-report of whether they were receiving Social Security Disability Insurance (SSDI) or Supplemental Security Income (SSI); this may not accurately represent disability status in this sample because not everyone with a disability may apply for or receive SSDI/SSI; (8) this study occurred from 2012 to 2014, prior to the current opioid epidemic, and attitudes toward methadone dosing have changed in the past decade due to several factors including the use of more potent opioids and a philosophical shift in concerns about medication under-dosing; therefore, it is important that context be considered when evaluating these results.
In conclusion, we found promising initial results in this SC pilot program designed to assist patients in MTD who transferred directly from other clinics rather than being discharged from care. The strongest predictor of treatment retention was methadone dose. Retention was better among patients receiving higher methadone doses (>65 mg/day), which have proven effectiveness in OAT programs, especially among patients with a history of injection heroin use or comorbid cocaine use. Ceasing ongoing use of drugs (opioids, cocaine, BZDs, and THC) was also predictive of treatment retention; however, clinic policies make it difficult to identify whether it is the ongoing substance use that impacts retention or access to certain treatment privileges (eg, take-home doses), which is impacted by ongoing substance use. As psychiatric comorbidity was highly prevalent, it is imperative to provide easily accessible treatment of comorbid affective and anxiety disorders especially in patients receiving disability benefits. Although rates of drug abstinence were lower among SC patients than regular MTD patients in our clinic, rates improved among those retained in treatment for 6 months. This retention-focused program offers a more positive alternative than discharge to the community. Future research should incorporate evidence-based interventions and consider harm reduction strategies (rather than clinic discharge) for high-risk polysubstance use to better address this population’s significant barriers and needs, toward improving retention and overall health, and reducing substance use.
Supplemental Material
Supplemental material, sj-docx-1-sat-10.1177_11782218221138335 for Predictors of Retention and Drug Use Among Patients With Opioid Use Disorder Transferred to a Specialty “Second Chance” Methadone Program by Tabitha E Moses, Gary L Rhodes, Emytis Tavakoli, Carl W Christensen, Alireza Amirsadri and Mark K Greenwald in Substance Abuse: Research and Treatment
Acknowledgments
We thank Dr. Kanzoni Asabigi for his partnership in developing this pilot treatment program, the Tolan Park Research Clinic treatment team and Edward Mischel for their efforts in maintaining the program, Brent Rowden for initiating data extraction from the electronic medical records, and Emily Deal for preliminary descriptive analyses.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIH F30 DA052118 (TEHM), the Gertrude Levin Endowed Chair in Addiction and Pain Biology (MKG), Helene Lycaki/Joe Young, Sr. Funds (State of Michigan) and the Detroit Wayne Mental Health Authority supported this work.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ Contributions: MKG, GLR and AA worked with the City of Detroit to develop this pilot treatment program. AA, CWC, and GLR coordinated and treated patients in this program. GLR entered and managed patient data. MKG, TEHM and ET conducted the data analyses. TEHM, ET, and MKG drafted the manuscript. All authors reviewed and edited the manuscript.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Santo T, Jr, Clark B, Hickman M, et al. Association of opioid agonist treatment with all-cause mortality and specific causes of death among people with opioid dependence: A systematic review and meta-analysis. JAMA Psychiatr. 2021;78:979-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3:e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grant BF, Saha TD, Ruan WJ, et al. Epidemiology of DSM-5 drug use disorder: results from the National Epidemiologic Survey on alcohol and related Conditions-III. JAMA Psychiatr. 2016;73:39-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105:e55-e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krawczyk N, Jent V, Hadland SE, Cerdá M. Utilization of medications for opioid use disorder across US states: relationship to treatment availability and overdose mortality. J Addict Med. 2022;16:114-117. [DOI] [PubMed] [Google Scholar]
- 6. Saloner B, Karthikeyan S. Changes in substance abuse treatment use among individuals with opioid use disorders in the United States, 2004-2013. JAMA. 2015;314:1515-1517. [DOI] [PubMed] [Google Scholar]
- 7. SAMHSA - Substance Abuse and Mental Health Services Administration. Key Substance use and Mental Health Indicators in the United States: Results From the 2020 National Survey on Drug Use and Health. NSDUH Ser H-56; 2021:51-58. [Google Scholar]
- 8. Bao YP, Liu ZM, Epstein DH, Du C, Shi J, Lu L. A meta-analysis of retention in methadone maintenance by dose and dosing strategy. Am J Drug Alcohol Abuse. 2009;35:28-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Booth RE, Corsi KF, Mikulich-Gilbertson SK. Factors associated with methadone maintenance treatment retention among street-recruited injection drug users. Drug Alcohol Depend. 2004;74:177-185. [DOI] [PubMed] [Google Scholar]
- 10. Fareed A, Vayalapalli S, Stout S, Casarella J, Drexler K, Bailey SP. Effect of methadone maintenance treatment on heroin craving, a literature review. J Addict Dis. 2011;30:27-38. [DOI] [PubMed] [Google Scholar]
- 11. Levine AR, Lundahl LH, Ledgerwood DM, Lisieski M, Rhodes GL, Greenwald MK. Gender-specific predictors of retention and opioid abstinence during methadone maintenance treatment. J Subst Abuse Treat. 2015;54:37-43. [DOI] [PubMed] [Google Scholar]
- 12. Roux P, Carrieri M, Cohen J, et al. Retention in opioid substitution treatment: a major predictor of long-term virological success for HIV-infected injection drug users receiving antiretroviral treatment. Clin Infect Dis. 2009;49:1433-1440. [DOI] [PubMed] [Google Scholar]
- 13. Cao X, Wu Z, Rou K, et al. Retention and its predictors among methadone maintenance treatment clients in China: a six-year cohort study. Drug Alcohol Depend. 2014;145:87-93. [DOI] [PubMed] [Google Scholar]
- 14. Zanis DA, Woody GE. One-year mortality rates following methadone treatment discharge. Drug Alcohol Depend. 1998;52:257-260. [DOI] [PubMed] [Google Scholar]
- 15. Mackay L, Bach P, Milloy M-J, Cui Z, Kerr T, Hayashi K. The relationship between crystal methamphetamine use and methadone retention in a prospective cohort of people who use drugs. Drug Alcohol Depend. 2021;225:108844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marsh JC, Amaro H, Kong Y, Khachikian T, Guerrero E. Gender disparities in access and retention in outpatient methadone treatment for opioid use disorder in low-income urban communities. J Subst Abuse Treat. 2021;127:108399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pasman E, Kollin R, Broman M, et al. Cumulative barriers to retention in methadone treatment among adults from rural and small urban communities. Addict Sci Clin Pract. 2022;17:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Proctor SL, Copeland AL, Kopak AM, Hoffmann NG, Herschman PL, Polukhina N. Predictors of patient retention in methadone maintenance treatment. Psychol Addict Behav. 2015;29:906-917. [DOI] [PubMed] [Google Scholar]
- 19. O’Connor AM, Cousins G, Durand L, Barry J, Boland F. Retention of patients in opioid substitution treatment: A systematic review. PLoS One. 2020;15:e0232086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lister JJ, Ellis JD, Struble CA, Ledgerwood DM, Greenwald MK. Protective factor predictors of abstinence and retention in patients receiving methadone treatment for opioid use disorder. Int J Ment Health Addict. Published online August 8, 2022. doi: 10.1007/s11469-022-00893-z [DOI] [Google Scholar]
- 21. Degenhardt L, Bucello C, Mathers B, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106:32-51. [DOI] [PubMed] [Google Scholar]
- 22. Degenhardt L, Larney S, Kimber J, et al. The impact of opioid substitution therapy on mortality post-release from prison: Retrospective data linkage study. Addiction. 2014;109:1306-1317. [DOI] [PubMed] [Google Scholar]
- 23. Darke S, Ross J, Mills K, Teesson M, Williamson A, Havard A. Benzodiazepine use among heroin users: baseline use, current use and clinical outcome. Drug Alcohol Rev. 2010;29:250-255. [DOI] [PubMed] [Google Scholar]
- 24. Ghitza UE, Epstein DH, Preston KL. Self-report of illicit benzodiazepine use on the Addiction Severity Index predicts treatment outcome. Drug Alcohol Depend. 2008;97:150-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jann M, Kennedy WK, Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract. 2014;27:5-16. [DOI] [PubMed] [Google Scholar]
- 26. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49:493-501. [DOI] [PubMed] [Google Scholar]
- 27. Moses TEH, Greenwald MK. History of regular nonmedical sedative and/or alcohol use differentiates substance-use patterns and consequences among chronic heroin users. Addict Behav. 2019;97:14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lister JJ, Greenwald MK, Ledgerwood DM. Baseline risk factors for drug use among African-American patients during first-month induction/stabilization on methadone. J Subst Abuse Treat. 2017;78:15-21. [DOI] [PubMed] [Google Scholar]
- 29. Rhodes GL, Saules KK, Helmus TC, et al. Improving On-Time counseling attendance in a methadone treatment program: a contingency management approach. Am J Drug Alcohol Abuse. 2003;29:759-773. [DOI] [PubMed] [Google Scholar]
- 30. Tzilos GK, Rhodes GL, Ledgerwood DM, Greenwald MK. Predicting cocaine group treatment outcome in cocaine-abusing methadone patients. Exp Clin Psychopharmacol. 2009;17:320-325. [DOI] [PubMed] [Google Scholar]
- 31. Christensen C, Rhodes G, Berman M, Bado J, Entenman S, Greenwald MK. Use of the michigan automated prescription system (MAPS) to monitor and manage diversion in an urban methadone clinic. In: Proceedings of the 72nd annual scientific meeting, the College on Problems of Drug Dependence, 2010. [Google Scholar]
- 32. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini-international Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22-33;quiz 34. quiz 34-57. [PubMed] [Google Scholar]
- 33. Substance Abuse and Mental Health Services Administration. Federal Guidelines for Opioid Treatment Programs Federal Guidelines for Opioid Treatment Programs. SAMHSA; 2015. [Google Scholar]
- 34. Moses TEH, Lundahl LH, Greenwald MK. Factors associated with sedative use and misuse among heroin users. Drug Alcohol Depend. 2018;185:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perrone J, Nelson LS. Medication reconciliation for controlled substances—an "ideal" prescription-drug monitoring program. New Engl J Med. 2012;366:2341-2343. [DOI] [PubMed] [Google Scholar]
- 36. DuPont RL. Marijuana and benzodiazepines in patients receiving methadone treatment. JAMA J Am Med Assoc. 1989;261:3409. [PubMed] [Google Scholar]
- 37. Franklyn AM, Eibl JK, Gauthier GJ, Marsh DC. The impact of cannabis use on patients enrolled in opioid agonist therapy in Ontario, Canada. PLoS One. 2017;12:e0187633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yip SW, Carroll KM, Potenza MN. Different patterns of neural activity among cocaine-dependent individuals with and without current methadone treatment: relationship to treatment outcomes. Drug Alcohol Depend. 2015;156:e242-e243. [Google Scholar]
- 39. Zielinski L, Bhatt M, Sanger N, et al. Association between cannabis use and methadone maintenance treatment outcomes: an investigation into sex differences. Biol Sex Differ. 2017;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Althoff KN, Leifheit KM, Park JN, Chandran A, Sherman SG. Opioid-related overdose mortality in the era of fentanyl: monitoring a shifting epidemic by person, place, and time. Drug Alcohol Depend. 2020;216:108321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deren S, Kang S-Y, Mino M, Seewald RM. Attitudes of methadone program staff toward provision of harm-reduction and other services. J Addict Med. 2011;5:289-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gjersing L, Waal H, Caplehorn JR, Gossop M, Clausen T. Staff attitudes and the associations with treatment organisation, clinical practices and outcomes in opioid maintenance treatment. BMC Health Serv Res. 2010;10:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hobden KL, Cunningham JA. Barriers to the dissemination of four harm reduction strategies: a survey of addiction treatment providers in Ontario. Harm Reduct J. 2006;3:35-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarasvita R, Tonkin A, Utomo B, Ali R. Predictive factors for treatment retention in methadone programs in Indonesia. J Subst Abuse Treat. 2012;42:239-246. [DOI] [PubMed] [Google Scholar]
- 45. Zhang L, Chow EP, Zhuang X, et al. Methadone maintenance treatment participant retention and behavioural effectiveness in China: a systematic review and meta-analysis. PLoS One. 2013;8:e68906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Faggiano F, Vigna-Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. Published online July 21, 2003. doi: 10.1002/14651858.CD002208 [DOI] [PubMed] [Google Scholar]
- 47. Cousins G, Boland F, Barry J, et al. J-shaped relationship between supervised methadone consumption and retention in methadone maintenance treatment (MMT) in primary care: National cohort study. Drug Alcohol Depend. 2017;173:126-131. [DOI] [PubMed] [Google Scholar]
- 48. Fareed A, Musselman D, Byrd-Sellers J, et al. On-site basic health screening and brief health counseling of chronic medical conditions for veterans in methadone maintenance treatment. J Addict Med. 2010;4:160-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mullen L, Barry J, Long J, et al. A national study of the retention of Irish opiate users in methadone substitution treatment. Am J Drug Alcohol Abuse. 2012;38:551-558. [DOI] [PubMed] [Google Scholar]
- 50. Fareed A, Casarella J, Amar R, Vayalapalli S, Drexler K. Methadone maintenance dosing guideline for opioid dependence, a literature review. J Addict Dis. 2010;29:1-14. [DOI] [PubMed] [Google Scholar]
- 51. DeMaria PA, Jr, Sterling R, Weinstein SP. The effect of stimulant and sedative use on treatment outcome of patients admitted to methadone maintenance treatment. Am J Addict. 2000;9:145-153. [DOI] [PubMed] [Google Scholar]
- 52. Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent poly-drug abusers with contingency management and buprenorphine maintenance. Exp Clin Psychopharmacol. 2000;8:176-184. [DOI] [PubMed] [Google Scholar]
- 53. Pashaei T, Moeeni M, Roshanaei Moghdam B, Heydari H, Turner NE, Razaghi EM. Predictors of treatment retention in a major methadone maintenance treatment program in iran: a survival analysis. J Res Health Sci. 2014;14:291-295. [PubMed] [Google Scholar]
- 54. Roux P, Lions C, Michel L, et al. Predictors of non-adherence to methadone maintenance treatment in opioid-dependent individuals: implications for clinicians. Curr Pharm Des. 2014;20:4097-4105. [DOI] [PubMed] [Google Scholar]
- 55. Sofuoglu M, Gonzalez G, Poling J, Kosten TR. Prediction of treatment outcome by baseline urine cocaine results and self-reported cocaine use for cocaine and opioid dependence. Am J Drug Alcohol Abuse. 2003;29:713-727. [DOI] [PubMed] [Google Scholar]
- 56. Bravo MJ, Llorens N, Barrio G, et al. Methadone maintenance treatment: a protective factor for cocaine injection in a street-recruited cohort of heroin users☆. Drug Alcohol Depend. 2010;112:62-68. [DOI] [PubMed] [Google Scholar]
- 57. Leri F, Stewart J, Tremblay A, Bruneau J. Heroin and cocaine co-use in a group of injection drug users in Montréal. J Psychiatry Neurosci. 2004;29:40-47. [PMC free article] [PubMed] [Google Scholar]
- 58. Luo X, Zhao P, Gong X, et al. Concurrent heroin use and correlates among methadone maintenance treatment clients: A 12-month follow-up study in Guangdong Province, China. Int J Environ Res Public Health. 2016;13:305. doi: 10.3390/ijerph13030305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Peles E, Kreek MJ, Kellogg S, Adelson M. High methadone dose significantly reduces cocaine use in methadone maintenance treatment (MMT) patients. J Addict Dis. 2006;25:43-50. [DOI] [PubMed] [Google Scholar]
- 60. Joe GW, Simpson DD, Broome KM. Retention and patient engagement models for different treatment modalities in DATOS. Drug Alcohol Depend. 1999;57:113-125. [DOI] [PubMed] [Google Scholar]
- 61. Mancino M, Curran G, Han X, Allee E, Humphreys K, Booth BM. Predictors of attrition from a national sample of methadone maintenance patients. Am J Drug Alcohol Abuse. 2010;36:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Milby JB, Sims MK, Khuder S, et al. Psychiatric comorbidity: prevalence in methadone maintenance treatment. Am J Drug Alcohol Abuse. 1996;22:95-107. [DOI] [PubMed] [Google Scholar]
- 63. Carpentier PJ, Krabbe PF, van Gogh MT, Knapen LJ, Buitelaar JK, de Jong CA. Psychiatric comorbidity reduces quality of life in chronic methadone maintained patients. Am J Addict. 2009;18:470-480. [DOI] [PubMed] [Google Scholar]
- 64. Kidorf M, King VL, Peirce J, Gandotra N, Ghazarian S, Brooner RK. Substance use and response to psychiatric treatment in methadone-treated outpatients with comorbid psychiatric disorder. J Subst Abuse Treat. 2015;51:64-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. King VL, Brooner RK, Peirce J, Kolodner K, Kidorf M. Challenges and outcomes of parallel care for patients with co-occurring psychiatric disorder in methadone maintenance treatment. J Dual Diagn. 2014;10:60-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Astals M, Díaz L, Domingo-Salvany A, Martín-Santos R, Bulbena A, Torrens M. Impact of co-occurring psychiatric disorders on retention in a methadone maintenance program: an 18-month follow-up study. Int J Environ Res Public Health. 2009;6:2822-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cacciola JS, Alterman AI, Rutherford MJ, McKay JR, Mulvaney FD. The relationship of psychiatric comorbidity to treatment outcomes in methadone maintained patients. Drug Alcohol Depend. 2001;61:271-280. [DOI] [PubMed] [Google Scholar]
- 68. Verthein U, Degkwitz P, Haasen C, Krausz M. Significance of comorbidity for the long-term course of opiate dependence. Eur Addict Res. 2005;11:15-21. [DOI] [PubMed] [Google Scholar]
- 69. Senbanjo R, Wolff K, Marshall EJ, Strang J. Persistence of heroin use despite methadone treatment: poor coping self-efficacy predicts continued heroin use. Drug Alcohol Rev. 2009;28:608-615. [DOI] [PubMed] [Google Scholar]
- 70. Anderson JF, Warren LD. Client retention in the British Columbia methadone Program, 1996-1999. Can J Public Health. 2004;95:104-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jiang H, Han Y, Du J, et al. Factors associated with one year retention to methadone maintenance treatment program among patients with heroin dependence in China. Subst Abuse Treat Prev Policy. 2014;9:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McHugh RK, Murray HW, Hearon BA, et al. Predictors of dropout from psychosocial treatment in opioid-dependent outpatients. Am J Addict. 2013;22:18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nosyk B, Li L, Evans E, et al. Utilization and outcomes of detoxification and maintenance treatment for opioid dependence in publicly-funded facilities in California, USA: 1991-2012. Drug Alcohol Depend. 2014;143:149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nosyk B, Anglin MD, Brissette S, et al. A call for evidence-based medical treatment of opioid dependence in the United States and Canada. Health Aff. 2013;32:1462-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Volkow ND, Montaner J. The urgency of providing comprehensive and integrated treatment for substance abusers with HIV. Health Aff. 2011;30:1411-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sat-10.1177_11782218221138335 for Predictors of Retention and Drug Use Among Patients With Opioid Use Disorder Transferred to a Specialty “Second Chance” Methadone Program by Tabitha E Moses, Gary L Rhodes, Emytis Tavakoli, Carl W Christensen, Alireza Amirsadri and Mark K Greenwald in Substance Abuse: Research and Treatment


